Translate this page into:
Phenolic compound derived from Enteromorpha intestinalis and their bioactivity against bacterial pathogens
⁎Corresponding author at: Department of Life Sciences, Kristu Jayanti College, Autonomous, K. Narayanapura, Bengaluru 560077, Karnataka, India. deepaksiva1252@gmail.com (Paramasivam Deepak), deepak.p@kristujayanti.com (Paramasivam Deepak),
⁎⁎Corresponding author at: Department of Human Genetics and Molecular Biology, Bharathiar University, Coimbatore 641 046, Tamil Nadu, India. avahgmb@buc.edu.in (Vijaya Anand Arumugam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The purpose of the study was to assess a phenolic compound that was extracted from the brown seaweed Enteromorpha intestinalis (Linn.), Nees (1820) for its antibacterial activities on different bacterial pathogens of fish illnesses. Hexane and methanol were utilized to extract bioactive compounds from the seaweed. Phytochemical analysis of the methnaolic extracts have revealed the presence of carbohydrates and glycosides, saponins, alkaloids, amino acids, flavonoids, fixed oil and fat, phenol compounds, tannins, and steroids. The bacterial pathogens of fish, including Providencia vermicola, Aeromonas hydrophila, Vibrio harveyi, and Aeromonas caviae, were targeted for antibacterial assessment. Among them, the methanolic crude extract and fraction-1 of E. intestinalis have shown maximum activity against A. hydrophila (22 ± 0.13 and 33 ± 0.56 µg/mL). Column chromatography was employed to isolate effective seaweed fraction, while FT-IR and HPLC were utilized for characterization. The phytochemical analysis confirmed the presence of various compounds in both extracts. The crude methanolic extract of E. intestinalis have demonstrated the maximum efficacy beside the tested bacterial pathogens. The FT-IR analysis revealed functional groups associated with polymeric structures in the seaweed extracts, including alcohols, phenols, aromatics, alkyl halides, aliphatic amines, alkynes, and alkenes. Overall, the methanolic extract of E. intestinalis exhibited significant antibacterial activity as a result of the phenolic chemicals that are present. The study provides valuable baseline data, emphasizing the need for further research towards developing antibacterial drug formulations.
Keywords
Antibacterial
Fish pathogens
FT-IR
HPLC
Minimal Inhibitory Concentration
1 Introduction
Numerous functional metabolites were extracted from the marine environment for the commercial activities including polyunsaturated fatty acids, different types of polysaccharides, various important minerals, vitamins, antioxidants, industrially important enzymes, and biologically active peptides, were largely extracted from the various marine organisms. A variety of biologically active substances with different molecular makeup that serve a variety of pharmacological purposes in marine creatures can be found in the group of marine algae. Currently, their role and importance of these marine based raw materials as a source of novel biologically active and potent molecules is expanding quickly, and scientists have shown that compounds originating from marine algae have a variety of beneficial biological functions (del Pilar Sánchez-Camargo et al., 2017). Due to their use as a food source and medicinal, seaweeds were greatly reported as a good and alternate raw material for the pharmaceutical industries to produce viable drugs against various pathogenic bacteria and infectious diseases (Lavanya and Veerappan, 2011).
Many biological activities of marine plants have been reported by researches including antialgal, antibacterial, antifungal, mosquitocidal, anticancer, anti-hypercholesterolemic, antiviral, nematicidal, anticoagulant, anti-inflammatory, antifeedant and antioxidant properties, have been assessed for seaweeds collected from different locations (Srivastava et al., 2010). The qualities of seaweeds that have been studied the most globally are their antibacterial and antioxidant capacities (Devi et al., 2012). The people living along the shore have long utilized algal extracts as a preventative and therapeutic measure for a variety of illnesses, including cough cures, antibiotics, anti-helminthics, hypertension drugs, antitumor drugs, and diarrhoea drugs. In recent times, certain scientists have started studying marine algae chemically, specifically focusing on their bioactive characteristics (Tannourya et al., 2016). The control of fish pathogens such as Aeromonas hydrophila, Vibrio harveyi, A. vermicola, and A. caviae is of paramount importance in aquaculture due to their detrimental effects on fish health and productivity. These pathogens are known to cause severe diseases in various fish species, leading to significant economic losses and environmental impacts. A. hydrophila, for instance, can cause Motile Aeromonad Septicaemia, resulting in high mortality rates among cultured fish. Similarly, Vibrio harveyi is notorious for causing vibriosis, characterized by rapid onset and widespread mortality in marine and freshwater fish. And A. vermicola and A. caviae are also emerging as threats in aquaculture, contributing to disease outbreaks that compromise the sustainability of fish farming operations. Addressing these pathogens through effective management strategies, including the development of novel therapeutics or bio-active compounds, not only enhances fish health and welfare but also supports the long-term viability of aquaculture systems and promotes environmental stewardship (Semwal et al., 2023). A study with nine solvent extracts of the seaweed, Ulva lactuca that were recently assessed for their antibacterial activity against five fish and eleven human bacterial pathogens, the acetone extract recorded maximum antibacterial activity (Saritha et al., 2013). Ulvaceae family green alga, Enteromorpha intestinalis (Linnaeus) Nees (1820) is documented for its antioxidant (Akköz et al., 2009), mosquitocidal (Bianco et al., 2013), anti-proliferative (Wang et al., 2014), antibacterial, bioremediation (Naskar et al., 2023), bio-stimulant (Gul et al., 2023) and anti-hemolytic (Soltani et al., 2012). The alga, E. intestinalis have economical important in the preparation of animal feed, fertiliser, cosmetics, pharmaceuticals and food ingredients (Ghallab et al., 2024; Selvam et al., 2024). Identifying a side-effect free natural based bio-active compound from the natural resources to combat fish pathogens encountering in the field of aquaculture has significant implications for the sustainable aquaculture and environmental health. These bacterial fish pathogens were reducing the health of the aquatic animals and shown a great impact on its growth and reproduction (Yildirim-Aksoy and Beck, 2017). Hence, the current study set out to evaluate E. intestinalis's antibacterial activity in relation to bacterial fish pathogens.
2 Materials and methods
2.1 Collection and processing of samples
Freshly collected algae samples were obtained from the coastal area of Ramanathapuram, Tamil Nadu, India (9°22′N,78°52′E). The collected samples were washed in tap and distilled water until all debris and associated biota were removed, and then they were transferred under a plastic cover to the laboratory. After that, they were allowed to dry for three weeks in the shade. Based on its morphological characteristics, the algae were identified using standard keys (Dinabandhu, 2010). The identification was then confirmed by Dr. N. Kaliaperumal, Senior Scientist (Retd.), Central Marine Fisheries Research Institute, Tamil Nadu, India. With the reference specimen number (PU/MBEG/V.026), the reference specimen samples were kept in good condition at the Department of Biotechnology, Periyar University, India. All of the chemicals used in the study were of the highest analytical quality and purity. For the sample preparation initially 500 g of crushed seaweed material is soaked in hexane and methanol for three days while gently stirring it. The hexane extract was taken after three days and kept in a dark, room temperature storage area. After doing the process twice, the extract was gathered and kept for later examination at 4°C. The cold percolation method of extraction avoids the denaturing of the bioactive compounds and protects the biological properties of the seaweed extract (Aina et al., 2022).
2.2 Screening of phytochemicals
Following the previously established methodology, a preliminary phytochemical analysis and the quantification of each phytochemical present in the sample was performed on the freshly prepared sample. For saponin, 50 mg of extract was diluted with distilled water to 20 mL, shaken for 15 min, and a 2 cm foam layer indicated presence. For phenolic compounds, 50 mg of extract was dissolved in 5 ml of water, with 5 % ferric chloride drops added, and dark green color indicated presence. For carbohydrates, 100 mg of extract was dissolved in 5 mL of water, filtered, and boiled with Fehling solutions A and B, forming a red precipitate. For tannins, 0.5 mg of extract was boiled in 20 mL of water, filtered, and 0.1 % ferric chloride was added, with green color indicating presence. For alkaloids, extract stirred with HCl, filtered, and Mayer’s reagent added produced white precipitate (Deepak et al., 2017).
2.3 Isolation, purification and structure elucidation of bioactive compounds
On a silica gel plate, thin layer chromatography (TLC) was conducted. Using a developing solvent system of petroleum ether/ethyl acetate (9:1, 8:2, and 7:3 v/v), a sample was spotted on the silica gel plate. By applying a spraying solution (1 % potassium permanganate in water) to the plates, the spots were visible under the UV light at 254 and 366 nm using a UV TLC viewer (CAMAG UV Cabinet 4, Model: 022.9130; CAMAG; Switzerland). The standard method was used to calculate the Rf value of the several pots that were observed (Athukorala et al., 2007).
After being subjected to column chromatography (60–120 mesh, Merck, Mumbai), the methanol extract of E. intestinalis was eluted using a gradient of petroleum ether/ethyl acetate. First, 100 % petroleum ether was used to treat the eluting solvent. Then, ethyl acetate was added to the mixture at ratios of 90:10, 80:20, 70:30, 60:40, and 50:50 to reduce the polarity. Under horizontal flow, the column was eluted at 10 mL/10 min in designated test tubes (15 mL capacity). After TLC was used to authenticate each test tube fraction, the fractions exhibiting comparable bands were combined and concentrated in a fume hood for later use (Selvam et al., 2024).
The High-Performance Liquid Chromatography (HPLC) analysis of seaweed extracts was carried out according to the standard protocol. About 20 µL of the mixture was injected after one milligram of the substance was dissolved in 1 mL of methanol. Methanol and water were mixed at the ratio of 50:50 to create the mobile phase. The experiment was conducted in Shimadzu LC solution 20 AD, Japan, and SPD 20 A, an apparatus equipped with a UV detector (254 nm), in order to determine the peak of purity. The mobile phase was passed through an LCGC C18 column at a flow rate of 1.0 mL/min in order to achieve isocratic resolution (Qureshi et al., 2016). Seaweed extracts and its fractions were analysed using an fourier transformer infrared spectrophotometer (FT-IR) spectrometer coupled with a TGS (Tri-glycine sulphate) detector (Bruker, D8, Germany model). About 1 mg of each dried sample was mixed with 100 mg of potassium bromide (KBr) and compacted to create a salt-disc (3 mm dia). These discs were recorded using the room resolution of 4 cm−1 and the mid-IR region of 4000–400 cm−1 (Deepak et al., 2017).
2.4 Antibacterial activity
The following fish pathogens such as Providencia vermicola, A. hydrophila, Vibrio harveyi, and A. caviae, were evaluated against the seaweed extracts and compound fractions. The stock bacterial pathogens were obtained from Marine Pharmacology Laboratory, Department of Biotechnology, Periyar University, Salem, India. Specific antibiotics were used to verify the resistance character of these isolates. On Nutrient Agar, all bacterial strains were kept at 4 °C. The agar well diffusion method, as reported by the standard procedure with slight modifications were made and used to evaluate the antibacterial qualities of the compound fractions and seaweed extract. Agar plates containing 15 mL of Muller Hinton Agar (MHA) were swabbed with approximately 0.1 mL of the overnight grown bacterial culture. The wells with a diameter of 7 mm were made on the MHA agar plates using sterilized corkborer, and various concentrations of the samples and standard were added into the well. For a whole day, the plates were incubated at 37°C. The diameters of the inhibitory zones were subsequently determined and they were checked for the presence of the bacterial lawn (Bhagavathy et al., 2011). And the minimal inhibitory concentration of the methanol extract of E. intestinalis and its fraction-1 were analysed by adopting standard protocol (Loganathan et al., 2024).
3 Results and discussion
The findings of initial phytochemical investigations of the methanolic extract of E. intestinalis were presented in Table 1. The findings demonstrated the existence of phytochemical elements such as carbohydrates and glycosides, saponins, alkaloids, amino acids, flavonoids, fixed oil and fat, phenol compounds, tannins, and steroids in the methanol extraction of E. intestinalis. Conversely, all other phytochemicals were found in the extract of hexane, with the exception of tannin and flavonoids. The antibacterial action of seaweeds was previously linked to a phenolic molecule or compounds (Kaur and Geetha, 2006). Seaweed extracts contain phyto-constituents such flavonoids and phenols that have been shown to improve the antibacterial activity, as found in the present study (Verma et al., 2015). Nonetheless, it was noted that the methanol fraction contained all of the resolved phenol materials. The current results are consistent with previous publication on compounds from the families Chlorophyceae, Phaeophyceae and Rhodophyceae, including fucosterol, hydroquinones, sesterpenoids, and brominated phenols (Faulkner, 2001).
Phytochemicals
Methanol
Hexane
Methanol Fraction
Carbohydrates & glycosides
0.8 ± 0.02
0.2 ± 0.01
0.7 ± 0.33
Proteins & amino acids
9.5 ± 0.06
2.56 ± 0.13
6.35 ± 0.56
Saponins
1.68 ± 0.56
0.89 ± 0.00
1.22 ± 0.33
Fixed oil & fat
1.25 ± 0.33
0.24 ± 0.66
0.89 ± 0.25
Tannin
5.65 ± 0.56
-
4.56 ± 0.33
Flavanoids
11.7 ± 1.33
-
8.35 ± 0.45
Alkaloids
12.8 ± 0.33
4.25 ± 1.66
10.45 ± 1.33
Steriods
5.68 ± 0.56
0.58 ± 0.33
4.56 ± 0.06
Phenol compounds
13.56 ± 1.33
2.56 ± 0.5
8.28 ± 1.33
After the incubation period of 24-hrs, the antibacterial activity was detected. In the methanol, E. intestinalis showed the creation of a zone at the minimal concentration level (Table 2 and Fig. 1). The crude hexane extract of E. intestinalis have not shown activity against tested bacterial pathogens. The results of crude methanolic extract of E. intestinalis and its fraction 1 have showed maximum activity 22 ± 0.13 and 33 ± 0.56 µg/mL, respectively which are highest than the positive control, ciproflaxain used in the current study. The minimal inhibitory concentration studies also revealed and confirmed the agar well diffusion study of methanol extract and its fraction. Today, there are about 12,000 marine natural products that have been structurally classified by researchers and every year about several hundred new chemical entities with potential biological activity are being discovered (Wang, 2014). Among them, several important bioactive compounds have been isolated from the family Chlorophyceae, including β-carotene from Dunaliella sp., Xanthophylls from Haematococcus pluvialis and lutein from Caulerpa racemosa are few examples (Talero et al., 2015). Maximum activity was recorded with the compound-1 on the fish pathogen, A. hydrophila are due to the presence of such bioactive compounds in E. intestinalis. Similarly, the Aspergillus niger, A. flavus and Cholococcum humicola have also showed more susceptibility to benzene extract in earlier study (Bhagavathy et al., 2011). The band separation in the petroleum ether/ethyl acetate solvent solution (the ratio of 9:1, 8:2, and 7:3) was analyzed using TLC utilizing the methanol extract of E. intestinalis. The distinct band separation that was seen under UV light was displayed by the 7:3 ratio. The separated fractions' Rf value was computed. Rf = 3.4/5.5 = 0.6181 cm is the fraction one. The fractions were verified as phenolic group compounds based on the Rf values. Footnote: M−Methanol; E- Enteromorpha; A- Aeromonas; P- Providencia; V- Vibrio; F- Fraction
Antibacterial activity of Enteromorpha intestanllis-Methanol extract and its fractions
Sample and bacteria
Zone of Inhibition (millimeter)
Positive Control (Ciproflaxain)
50 µL
100 µL
150 µL
200 µL
MIC (µg/mL)
E. intestanllis (M) – A. hydrophila
17 ± 0.53
16 ± 0.56
17 ± 0.65
18 ± 0.13
22 ± 0.13
24 ± 0.55
E. intestanllis (M) – A. caviae
16 ± 0.23
16 ± 0.85
19 ± 0.16
18 ± 0.58
19 ± 0.33
15 ± 1.67
E. intestanllis (M) – P. vermicola
14 ± 0.16
13 ± 0.13
16 ± 0.23
20 ± 1.33
20 ± 0.12
18 ± 1.33
E. intestanllis (M) – V. harveyi
16 ± 0.45
0 ± 0
16 ± 0.58
19 ± 0.58
18 ± 0.56
13 ± 1.56
E. intestanllis (M) – F1 − A. hydrophila
21 ± 0.56
31 ± 0.25
31 ± 0.75
32 ± 0.13
33 ± 0.56
34 ± 0.33
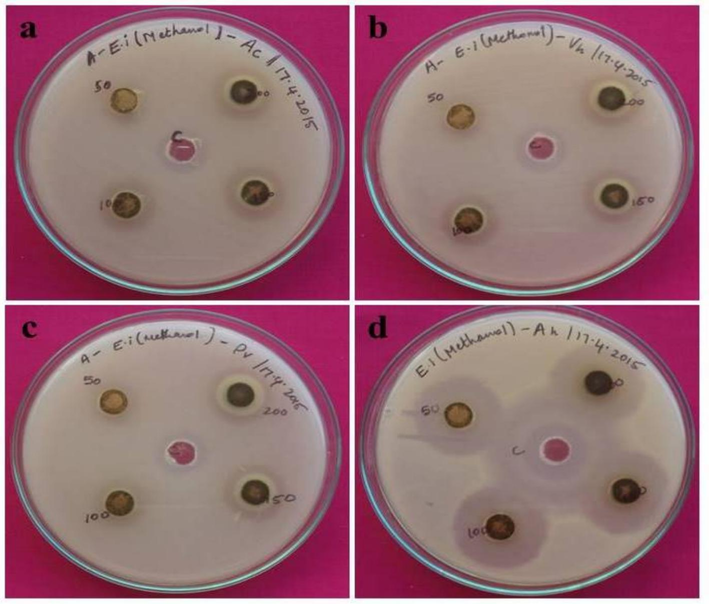
Antibacterial activity of seaweed extract against a) Aeromonas caviae, b) Vibrio harveyi, c) Providencia vermicola, d) Aeromonas hydrophila.
The chemicals were separated from the column chromatography fractions using a mobile phase consisting of petroleum ether and ethyl acetate (7:3) ratio, and the eluted fractions were seen concurrently using precoated analytic TLC aluminum sheets (Fig. 2). KMnOH reagents were used to observe the TLC spots. Eluents with comparable Rf values were combined and treated as a single eluent. The solvent was removed from the eluents that had accumulated, dried, and then weighed. The fractions that had been separated were employed in a later step. A possible correlation between the amount of phenol compounds in E. intestinalis organic extracts and the currently established antibacterial action exists. The current results support the earlier reports (Ozdemir et al., 2004) that suggested phenolic compounds' antibacterial properties depended on concentration. According to previous studies Chondrus crispus extracts have antibacterial activity against every tested bacterium and that activity is dose dependent (Abd El-Baky et al., 2008; Seenivasan et al., 2010). The structures of several isolated and purified marine bioactive compounds were identified as fatty acids and hydroxyl unsaturated fatty acids, glycolipid, steroids, phenolics, and terpenoids have been reported to have antibacterial action (Thillairajasekar et al., 2009). There is evidence that lauric acid, palmitic acid, linolenic acid, oleic acid, and stearic acid may have antimicrobial or antifungal properties (MacMillan et al., 2002). The commercial linolenic acid obtained from linseed/flax seed oil has been effective against Mycobacterium tuberculosis (Choi, 2016). Additionally, our current research demonstrates how vulnerable gram-positive bacteria are more susceptible to the seaweed extracts. The methanol extract of Pithophora oedogonia, has been found to produce the largest zone of inhibition (18.1 mm) against Staphylococcus aureus and Bacillus subtilis, two gram-positive bacteria (Singh and Chaudhary, 2010). In vitro antibacterial assessments against pathogenic microbes, including three gram-positive bacteria, Escherichia coli and Enterobacter aerogenes, revealed that six marine algae belonging to the brown algae (Cystoseria barbata, Dictyota dichotoma, Halopteris filicina and Cladostephus spongiosus), Rhodophyceae (Corallina officinalis), and Chlorophyceae (Vulva rigida) communities possessed antibacterial properties (Taskin et al., 2007). The development and metabolism of bacteria may be impacted by the phenolic chemicals found in seaweeds (Alberto et al., 2001).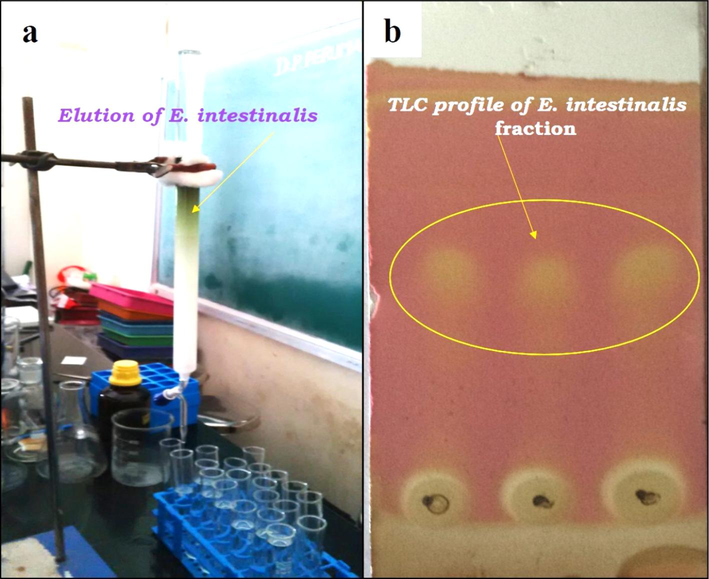
A) column chromatography analysis of E. intestinalis and b) TLC profile of E. intestinalis-fraction.
Two peaks were found in the E. intestinalis methanol extract according to the results of the HPLC analysis, which was conducted under UV absorption. The Rt values are 3.493 (4090674) and 3.182 (5218031). Compound 1′s results revealed the existence of two peaks, with Rt values (area) of 7.068 (109256) and 7.998 (37718). The Rf values correspond to the percentage areas covered by each of the various peaks, as shown in Fig. 3a and b. An abundance of antibacterial chemicals is thought to come from seaweeds. Two peaks were found in the E. intestinalis methanol extract according to the results of the HPLC analysis, which was conducted under UV absorption. Similar outcomes were previously reported in a hexane extract of Turbinaria ornata (Deepak et al., 2017). Previous studies indicated that the fatty acids from Borticoccus braunii, which include linolenic, oleic, linolin, and hexadecanoic acid, exhibit strong antibacterial action (Benkendorff et al., 2005; Athukorale, 2002). The earlier study reported that Ulva lactuca, cultivated in a lab, is rich in non– and moderate-polar chemicals, such as carotenoids, which are generated from chlorophyll and have phenolic compounds, antioxidant, and antibacterial properties (Awad, 2000).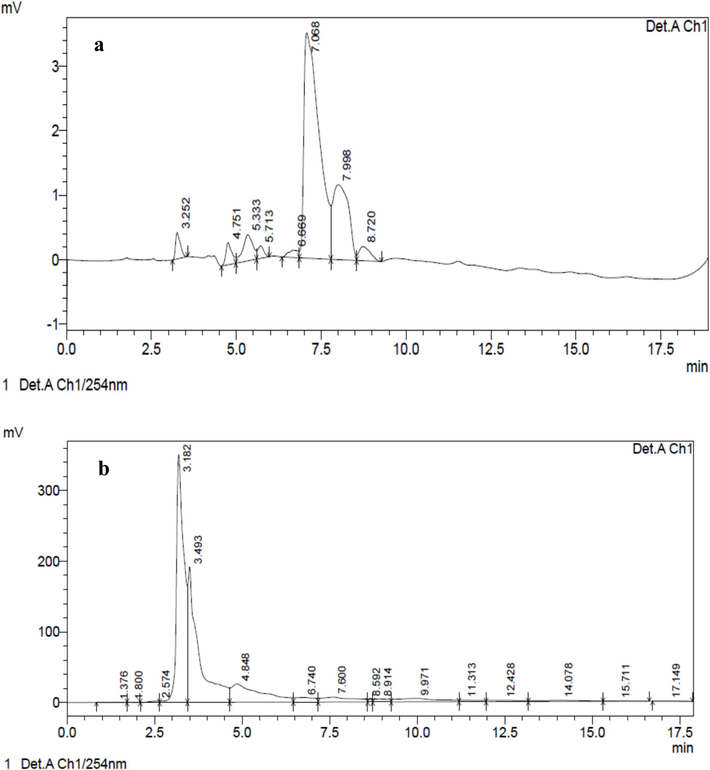
HPLC chromatogram of a) E. intestinalis methanolic crude b) E. intestinalis methanolic fraction- 1.
In accordance with the highest peak of the infrared spectrum, the FT-IR was utilized to determine the functional group of the active compounds. On the basis of peak proportion, the functional compounds of the constituents were distinguished when the three isolated compounds were subjected to FT-IR analysis. The functional groups are displayed in Fig. 4a&b. The molecular makeup of the various functional groups found in the drug and seaweed extract is predicted by the FT-IR. The methanol crude extract had a significant number of absorption peaks, which were identified as 3335.89, 2920.02, 2850.91, 2286.07, 1627.97, 1414.26, 1220.64, 1037.10, 718.62, 634.30, 547.50, and 518.05 cm−1. 2923.21, 1702.87, 1655.23, 1511.02, 1461.03, 1377.40, 1260.90, 1089.38, 1019.22, 800.53, 576.20, and 547.14 cm−1 were the obtained compound peak values. Alcohols, phenols, aromatics, alkyl halides, aliphatic amines, alkynes, and alkenes group with polymeric connection are shown by the peaks (Fig. 4). The presence of functional groups in the seaweed extracts viz, alkenes, aliphatic amines, phenols, and alkyl halides group was confirmed by the already recorded peaks in the methanolic seaweed extract of E. intestinalis (Beula et al., 2011).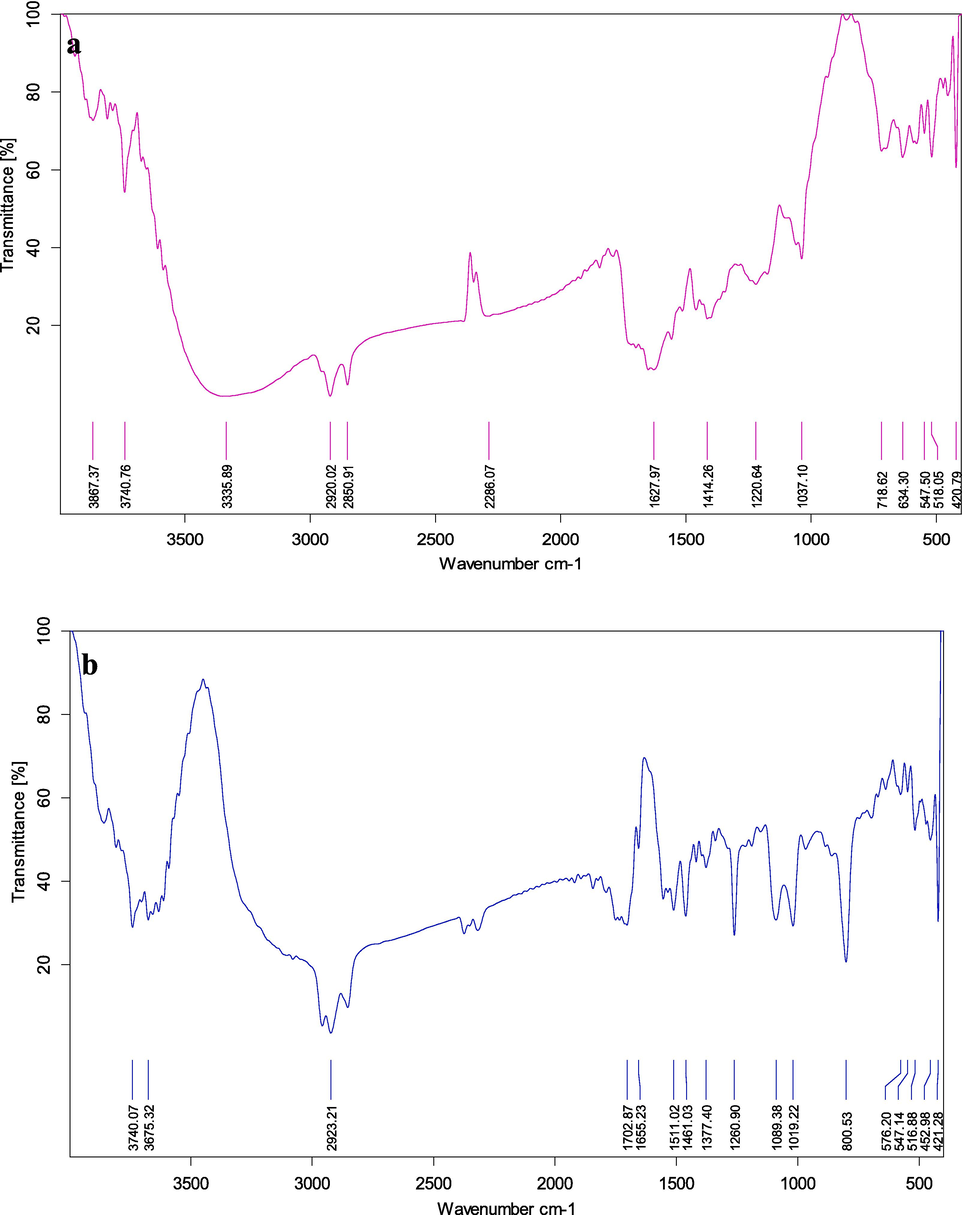
FT-IR analysis of a) E. intestinalis methanolic crude b) E. intestinalis methanolic fraction- 1.
4 Conclusions
Marine algal secondary metabolites are thought to include bioactive substances that could be useful for pharmacological and biological applications. The current work has demonstrated the existence of phenolic chemicals, which may account for the inhibitory impact against fish infections that has been observed. The methanolic crude extract and fraction-1 of E. intestinalis have shown maximum activity against A. hydrophila (22 ± 0.13 and 33 ± 0.56 µg/mL) in the present study. Further research is necessary to fully characterize the bioactive compound found in seaweed that comes from E. intestinalis. This study highlights the potential societal benefit of developing new treatments for fish diseases, which could enhance aquaculture productivity and sustainability.
Institutional Review Board Statement.
Not Applicable.
Informed Consent Statement.
Not Applicable.
Funding.
The authors extend their appreciation to the Researchers supporting project number (RSP2024R419) King Saud University, Riyadh, Saudi Arabia.
CRediT authorship contribution statement
Paramasivam Deepak: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Balasubramanian Balamuralikrishnan: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Formal analysis. Ashraf Atef Hatamleh: Writing – review & editing, Validation, Resources, Data curation. Bassam Khalid Alnafisi: Writing – review & editing, Visualization, Resources, Data curation. Vijaya Anand Arumugam: Writing – review & editing, Supervision, Resources, Project administration, Formal analysis, Conceptualization.
Acknowledgments
The authors are grateful to the authorities of Kristu Jayanti College, Bengaluru, Karnataka, India for providing a lab facility and necessary tools needed to complete this work. The authors extend their appreciation to the Researchers supporting project number (RSP2024R419) King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Characterization of nutraceutical compounds in blue green alga Spirulina maxima. J. Medicinal Plants Res.. 2008;2(10):292-300.
- [Google Scholar]
- Seaweed-derived phenolic compounds in growth promotion and stress alleviation in plants. Life. 2022;12(10):1548.
- [Google Scholar]
- In vitro antioxidant activity of Enteromorpha intestinalis (Linnaeus) Nees. Asian J. Chem.. 2009;21(8):6525-6528.
- [Google Scholar]
- Effect of gallic acid and catechin on Lactobacillus hilgardii growth and metabolism of organic compounds. J. Agric. Food Chem.. 2001;49(9):4359-4363.
- [Google Scholar]
- Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Technol.. 2007;98(9):1711-1716.
- [Google Scholar]
- Biologically active steroid from the green alga Ulva lactuca. Phytother. Res.An Int. J. Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2000;14(8):641-643.
- [Google Scholar]
- Free fatty acids and sterols in the benthic spawn of aquatic molluscs, and their associated antimicrobial properties. J. Exp. Mar. Biol. Ecol.. 2005;316(1):29-44.
- [Google Scholar]
- Mosquito larvicidal efficacy of seaweed extracts against dengue vector of Aedes aegypti. Asian Pac. J. Trop. Biomed.. 2011;1(2):S143-S146.
- [Google Scholar]
- Green algae Chlorococcum humicola-a new source of bioactive compounds with antimicrobial activity. Asian Pac. J. Trop. Biomed.. 2011;1(1):S1-S7.
- [Google Scholar]
- Larvicidal activity of seaweeds from northeastern Brazil and of a halogenated sesquiterpene against the dengue mosquito (Aedes aegypti) Ind. Crop. Prod.. 2013;43:270-275.
- [Google Scholar]
- Evaluation of anti-tubercular activity of linolenic acid and conjugated-linoleic acid as effective inhibitors against Mycobacterium tuberculosis. Asian Pac. J. Trop. Med.. 2016;9(2):125-129.
- [Google Scholar]
- Phytochemical profiling of Turbinaria ornata and its antioxidant and anti-proliferative effects. J. Taibah University Medical Sci.. 2017;12(4):329-337.
- [Google Scholar]
- Bioactives obtained from plants, seaweeds, microalgae and food by-products using pressurized liquid extraction and supercritical fluid extraction. In: Comprehensive Analytical Chemistry. Vol 76. Elsevier; 2017. p. :27-51.
- [Google Scholar]
- Evaluation of Antibacterial and antioxidant properties from Brown Seaweed, Sargassum wightii (Greville, 1848) against Human bacterial pathogens. Academic Sci.. 2012;4(3):143-149.
- [Google Scholar]
- Common seaweeds of India. New Delhi (India): IK International Pvt Ltd; 2010. p. :1-196.
- Marine algae: a treasure trove of bioactive anti-inflammatory compounds. Mar. Pollut. Bull.. 2024;199:116023
- [Google Scholar]
- Seaweed-derived bio-stimulant improves growth and salt tolerance of radish varieties under saline conditions. Biocatal. Agric. Biotechnol.. 2023;52:102822
- [Google Scholar]
- Screening methods for antioxidants-a review. Mini Rev. Med. Chem.. 2006;6(3):305-312.
- [Google Scholar]
- Antibacterial potential of six seaweeds collected from Gulf of Mannar of southeast coast of India. Adv. Biological Res.. 2011;5(1):38-44.
- [Google Scholar]
- Synthesis of anthraquinone-connected coumarin derivatives via grindstone method and their evaluation of antibacterial, antioxidant, tyrosinase inhibitory activities with molecular docking, and DFT calculation studies. Heliyon. 2024;10(3)
- [Google Scholar]
- Lobocyclamides A− C, Lipopeptides from a cryptic cyanobacterial mat containing Lyngbyac onfervoides. J. Org. Chem.. 2002;67(23):8210-8215.
- [Google Scholar]
- The green seaweed, Enteromorpha intestinalis: An efficient inorganic extractive species for environmental remediation and improved performances of fed species in brackishwater integrated multi-trophic aquaculture (BIMTA) system. Aquaculture. 2023;569:739359
- [Google Scholar]
- Antibacterial activity of volatile component and various extracts of Spirulina platensis. Phytotherapy Research: An Int. J. Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2004;18(9):754-757.
- [Google Scholar]
- Phytochemical investigations and evaluation of antidiabetic potential of Prunus dulcis nuts. LWT-Food Science and Technol.. 2016;66:311-317.
- [Google Scholar]
- Antibacterial activity and biochemical constituents of seaweed Ulva lactuca. Global J. Pharmacology. 2013;7(3):276-282.
- [Google Scholar]
- The antibacterial activity of some marine algae from south east coast of India. J. Pharm. Res.. 2010;3(8):1907-1912.
- [Google Scholar]
- Bioprospecting marine microalgae as sustainable bio-factories for value-added compounds. Algal Res. 2024103444
- [Google Scholar]
- A review on pathogenicity of Aeromonas hydrophila and their mitigation through medicinal herbs in aquaculture. Heliyon. 2023;9(3)
- [Google Scholar]
- Analysis and In vitro antibacterial screening of Pethophoraoedogonia (Mont) Wittrock-a preliminary phycochemical freshwater green alga forming mats in the water bodies. J. Algal Biomass Util.. 2010;1:33-41.
- [Google Scholar]
- Antibacterial and antihemolytic activities of Enteromorpha intestinalis in Caspian Sea Coast. Iran. J. Medicinal Plants Res.. 2012;6(3):530-533.
- [Google Scholar]
- Antibacterial potential of macroalgae collected from the Madappam coast, India. Br. J. Pharmacol. Toxicol.. 2010;1(2):72-76.
- [Google Scholar]
- Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs. 2015;13(10):6152-6209.
- [Google Scholar]
- Evaluation of cytotoxic activity of Sargassum vulgare from the Lebanese coast against Jurkat cancer cell line. J. Appl. Pharmaceutical Sci.. 2016;6(6):108-112.
- [Google Scholar]
- Antibacterial activities of some marine algae from the Aegean Sea (Turkey) Afr. J. Biotechnol.. 2007;6(24):2746.
- [Google Scholar]
- Antimicrobial activity of Trichodesmium erythraeum (Ehr) (microalga) from south East coast of Tamil Nadu, India. Int. J. Integrative Biology. 2009;5(3):167-170.
- [Google Scholar]
- Antioxidant activity and DNA damage inhibition in vitro by a methanolic extract of Carissa carandas (Apocynaceae) leaves. J. Taibah University for Sci.. 2015;9(1):34-40.
- [Google Scholar]
- Antitumor activity of a sulfated polysaccharide from Enteromorpha intestinalis targeted against hepatoma through mitochondrial pathway. Tumor Biol.. 2014;35:1641-1647.
- [Google Scholar]
- Antimicrobial activity of chitosan and a chitosan oligomer against bacterial pathogens of warm water fish. J. Appl. Microbiol.. 2017;122(6):1570-1578.
- [Google Scholar]







