Translate this page into:
Photocatalytic decolourization of a new water-insoluble organic dye based on phenothiazine by ZnO and TiO2 nanoparticles
⁎Corresponding author at: Chemistry Department, Faculty of Science, King Abdulaziz University, P.O. Box 80203, Jeddah 21589, Saudi Arabia. elshishtawy@hotmail.com (Reda M. El-Shishtawy) relshishtawy@kau.edu.sa (Reda M. El-Shishtawy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
A new water-insoluble organic dye, namely, 2-((10-decyl-10H-phenothiazin-3-yl)methylene)malononitrile, was synthesized and fully characterized. It was envisioned that photocatalytic decolourization of a dye-containing long chain would pave the way for the photocatalytic remediation of wastewater containing toxic hydrophobic organic pollutants. Two commercially available nanoparticles, ZnO and TiO2, were selected, and their photocatalytic decolourization of the dye from aqueous medium were compared. The black UV light irradiation of the colored samples in the presence of TiO2 (P25) or ZnO resulted in their decolourization and the photocatalytic activity observed for TiO2 (P25) was better than that of ZnO. The kinetic of decolourization indicated that the process was first-order from which the rate constant was calculated. Also, the effect of pHs on the kinetic of decolourization revealed a negligible effect, indicating that the pH, although it affects the catalysts but has no effect on the organic-based hydrophobic dye and thus no effect on the photocatalytic process.
Keywords
Phenothiazine
Photocatalysis
Water-insoluble dye
Photocatalytic decolourization
TiO2
ZnO
1 Introduction
Dyes are conventionally used in textile coloration and coating of polymers. Hi-tech functional dyes, on the other hand, are being manufactured for optoelectronic and medicinal applications (El-Shishtawy, 2009; El-Shishtawy et al., 2017). Parallel to this growing interest in dyes and their application, a severe environmental impact is being a challenge for scientists due to the waste of toxic organic dyes in industrial effluents that damage the aquatic life. It is estimated that about 105 of various commercial dyes and pigments amounted to 7 × 105 tons are produced annually worldwide (Zollinger, 2001). Ultimately, about 10–15% of these chemicals are wasted into the effluents of the textile industry (Pagga and Taeger, 1994). Considerable research activities have been paid for the decolorisation of organic dyes from industrial effluents. These activities include decolorisation by sonocatalytic degradation (Salavati et al., 2012; Li et al., 2014; Taghizadeh and Seifi-Aghjekohal, 2015), ozonation (Hu et al., 2016), chemical oxidation (Esteves et al., 2016; Al Angari et al., 2019), adsorption (Hashem and El-Shishtawy, 2001; El-Shishtawy and Melegy, 2001; El-Shishtawy and Soltan, 2001; Sathian et al., 2014; El-Zahhar and Awwad, 2016; Patra et al., 2016), photocatalytic degradation (Cai et al., 2015; Chandra et al., 2016; Elango and Roopan, 2016; Kumar et al., 2016.), coagulation (Wei et al., 2015; Huang et al., 2015], microbiological decomposition (Kumar et al., 2016.), and adsorption processes (Hao et al., 2000). Each process has its advantages and disadvantages, as summarized in the literature (Pekkuz et al., 2008). However, in some of these, dye pollutants are only transferred from one phase to another, leaving the problem mostly unsolved.
Photocatalytic degradation is generally believed to proceed by the excitation with photons of energy higher than the bandgap of a semiconductor. This process results in the formation of a hole- electron pair in which the hole presents the valence band and the electron in the conduction band and. The generation of such an exciton pair would degrade the organic pollutants by a redox mechanism (Rochkind et al., 2015).
Zinc oxide, with its characteristic features such as low toxicity, abundance, chemical, and photochemical stability, renders this semiconductor as one of the most photocatalysts. However, ZnO has a high bandgap (3.37 ev), and its photocatalytic efficiency remains low as a result of electron-hole pair recombination (Becker et al., 2011; Umar et al., 2011; Li and Wang, 2010; Zhang et al., 2015). Titanium dioxide (TiO2) has several advantages similar to ZnO but with more efficiency as photocatalyst as its bandgap energy (3.20 ev) is lower than that of ZnO (Hoffmann et al., 1995; Chen and Mao, 2007; Wang et al., 2014).
The above information concluded some successes in the degradation of water-soluble dyes; however, water-insoluble dyes that hazard the environment are scarcely investigated. Water-insoluble dyes are class of dyestuffs that are widely used in the coloration of textile industries as well as in the recent application of functional dyes. The use of an electron donor such as phenothiazine moiety in the construction of optoelectronics and dye-sensitized solar cells has been growing (Luo et al., 2016; El-Shishtawy et al., 2018; El-Shishtawy et al., 2016; Kafafy et al., 2014). It was envisioned that paving the way towards photocatalytic decolourization of such an important class of water-insoluble organic dyes would inspire scientists for further developments in this field. In this paper, a new water-insoluble dye derived from phenothiazine as electron donor and malononitrile as electron accepter as a model dye of functional dyes was synthesized. The photocatalytic decolourization of aqueous solutions of the dye using TiO2 and ZnO and at different pHs was investigated.
2 Experimental
2.1 General
Reagent grade chemicals purchased from Sigma-Aldrich and used without further purification. Aqueous solutions of the dye were prepared by using distilled water. 1H and 13C NMR spectra were measured on a Bruker Avance 600 MHz spectrometer using in CDCl3-d6 solution. Infrared spectra and mass spectrometry were measured on a PerkinElmer spectrum 100 FTIR spectrometer and Agilent GC 7000 mass spectrometer, respectively. LC-mass spectrometry was performed using Agilent LC 6320 Ion Trap Mass Spectrometer. UV–Vis absorption spectra were determined in DMF/water (90/10, v/v) on Shimadzu UV–Vis Spectrophotometer. The melting points were measured with a Stuart Scientific melting point apparatus and uncorrected. ZnO nanoparticle (particle size < 100 nm) and TiO2 (P25) of particle size < 25 nm were purchased from Sigma-Aldrich.
2.2 Synthesis of compound I
Into a round-bottomed flask containing a mixture of phenothiazine (2.4 g, 12 mmol), 1-bromodecane (4.48 g, 18 mmol), and KI (catalytic amount) in 50 ml DMSO, KOH (2 g, 35.71 mmol) was added portion-wise with stirring at room temperature. The reaction was completed after 5 h, as judged by the TLC, then 250 ml of water was added, and the organic product gets extracted with CHCl3 (4 × 40 ml). The combined extracts were collected, washed with 20% (w/w) NH4Cl (50 ml), and water. Drying the combined extracts with anhydrous Na2SO4, filtrated and finally dried in vacuo. Column chromatography (SiO2) of the crude product using hexane as eluent gives I as oil product, TLC System: Petroleum ether: Ethyl acetate (PE/EtOAc, 8/2), Yield 78%, 1H NMR (600 MHz, CDCl3) δ 0.90 (3H, t, J = 7.2 Hz, CH3) ,1.3 (12H, m, CH2), 1.45 (2H, m, CH2), 1.82 (2H, m ,CH2), 3.86 (2H, t, J = Hz, N-CH2), 6.90 (2H, d, J = 7.8 Hz, Ar-H), 6.93 (2H, t, Ar-H), 7.16 (2H, d, J = 7.2 Hz, Ar-H), 7.18 (2H, t, H-Ar). 13C NMR (125 MHz, CDCl3) δ 14.33, 22.89, 27.12, 27.18, 29.47, 29.50, 29.70, 29.76, 29.93, 32.10, 47.73, 115.68, 122.57, 125.12, 127.38, 127.63, 145.47 FTIR, , cm−1: Aliphatic and olefinic C–H appears at 2922, 2852 and 3064, respectively. The peaks at 1594, 1572, 1457 are due to C⚌C stretch vibrations.
2.3 Synthesis of compound II
A cooled POCl3 (15 ml) was dropwise into an ice-cooled DMF (40 ml) at 0 °C with stirring. Afterward, the reaction temperature raised gradually to room temperature, where the reaction continued for 90 min with stirring to be completed. The flask of the reaction was cooled to 0 °C, and compound I (66 mmol) was added, followed by removing the ice-bath to allow the reaction mixture to get warm gradually to reach 80 °C, where it kept running with stirring for 2 h. The mixture was then quenched after being cooled and mixed with ice–water and basified (sat. aqueous K2CO3 solution). The product was extracted from the mixture with CHCl3 (4 × 40 ml) washed with brine and water. Drying the combined extracts with anhydrous Na2SO4, filtrated and finally dried in vacuo. Column chromatography (SiO2) of the crude product (eluent: PE/EtOAc, 8:2) on silica gel gives a yellow solid product. Yield 91%. 1H NMR (600 MHz, CDCl3) δ 3.91 (t, 3H, N-CH2), 6.91 (d, 1H, J = 8.4 Hz, Ar-H), 6.92 (d, 1H, J = 9.0 Hz, Ar-H) , 6.99 (td, 1H, J = 7.2 Hz, Ar-H) , 7.14 (dd, H, J = 7.8, 1.2 Hz) ,7.19 (td, 1H, J = 7.5, 1.2 Hz, Ar-H) , 7.61 (d,1H, J = 1.8 Hz, Ar-H) , 7.67 (dd, 1H, J = 8.4, 1.8 Hz, Ar-H) , 9.82 (s, 1H, CHO). 13C NMR (125 MHz, CDCl3) δ 14.30, 22.82, 26.96, 27.03, 29.35, 29.39, 48.23, 115.01, 116.16, 123.78, 124.04, 125.25, 127.76, 127.78, 128.64, 130.27, 131.26, 143.67, 150.99, 190.26. FTIR, , cm−1: Aliphatic and olefinic C—H bands appear at 2923, 2852 and 3061, respectively. The peaks at 2724 and 1686 are due to C—H and C⚌O stretching vibration of aldehyde group, respectively.
2.4 Synthesis of 2-((10-decyl-10H-phenothiazin-3-yl)methylene)malononitrile III
A mixture of malononitrile (0.2 g, 3 mmol) and compound II (1.1 g, 3 mmol) and in 7 ml of basic ethanol solution (composed from mixing 1:3:96 by volume of piperidine, glacial acetic acid, and absolute ethanol, respectively) was reacted overnight with stirring at room temperature, filtered off and recrystallized from ethanol to produce dye III, Yield 52%, M.P. 65–57 °C. 1H NMR (600 MHz, CDCl3) δ 3.91 (t, 2H, N-CH2), 6.88 (d, 1H, J = 9.0 Hz, Ar-H), 6.91 (d, J = 8.4 Hz, Ar-H), 7.01 (td, 1H, J = 7.8 Hz, Ar-H), 7.11 (dd, 1H, J = 7.5, 1.2 Hz, Ar-H) , 7.20 (td, 1H, J = 7.8, 1.8 Hz, Ar-H) , 7.51 (s, 1H, H-C⚌C), 7.57 (d, 1H, J = 2.4 Hz, Ar-H), 7.78 (dd, 1H, J = 9.0, 2.4 Hz, Ar-H) 13C NMR δ 14.33, 22.88, 26.90, 26.98, 29.36, 29.47, 29.66, 29.72, 32.08, 48.42, 113.76, 114.88, 115.12, 116.28, 123.21, 124.38, 125.20, 125.39, 127.84, 128.00, 129.75, 131.55, 142.69, 151.05, 157.54.
FTIR, , cm−1: Aliphatic C—H bands appear at 2923, 2851. The peak at 2224 is due to CN group and the bands at 1600, 1581, 1563 are due to C⚌C. MS (ESI): M+; found: 415.1, C26H29SN3 requires 415.6.
2.5 The photocatalytic method
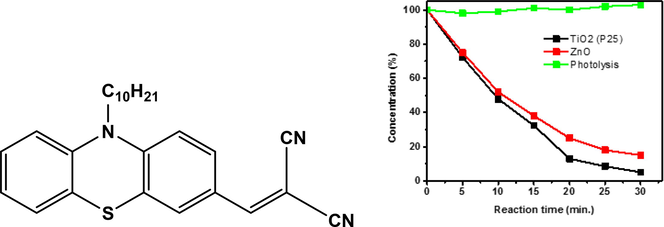
The photocatalytic decolorization of the dye was made following the method reported in the literature (Hamdy et al., 2014). In this method, black UV light (λ > 367 nm) was used as the light source for the irradiation of the sample. The dye sample aqueous solution (50 ml, 8.02 × 10−5 M) was prepared by dilution of a concentrated DMF solution of the dye in DMF/water, 1/9 v/v. Two catalysts, namely TiO2 (P25) and ZnO, were used for the photocatalytic decolorization of the dye. Typically, the catalyst (TiO2 (P25) or ZnO, 100 mg) and 50 ml of the aqueous dye solution (8.02 × 10−5 M) were mixed in a beaker. The sample was subjected to sonication for about 10 min and then placed into the photoreactor. Dark chemisorption of the catalyst was firstly allowed by stirring the samples (700 rounds per minute (rpm)) for 15 min at room temperature. Afterward, an aliquot of the dye sample was taken, and the remaining dye solution was subjected to the irradiation. For the kinetic studies, several aliquots were taken every 10 min, filtered (0.2 mm PTFE Millipore membrane filter) to remove the catalyst, and finally analyzed optically at the maximum wavelength of absorption of the dye at 538 nm to determine the remaining concentration of the dye (Fig. 1-a). Assuming that the photocatalysis proceeds via first-order kinetics, then the reaction rate constant (K) can be obtained from the following equation: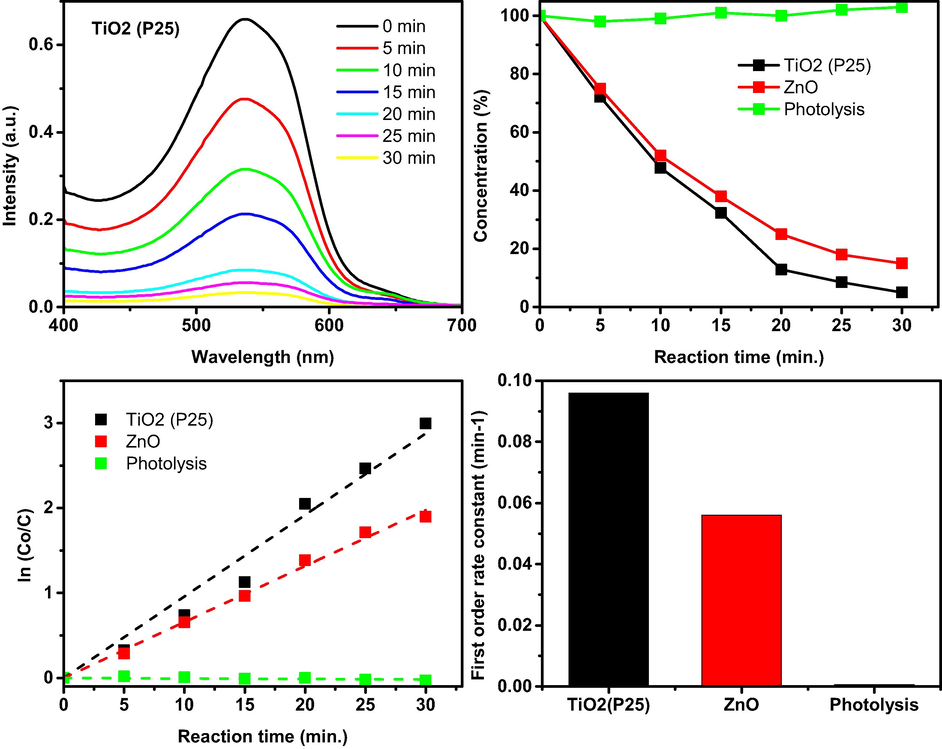
(Top left) the UV–Vis spectra obtained as a results of 2-((10-decyl-10H-phenothiazin-3-yl)methylene)malononitrile dye degradation over TiO2 (P25) under UV–Vis illumination. (Top right) the degradation profile of 2-((10-decyl-10H-phenothiazin-3-yl)methylene)malononitrile dye over TiO2 (P25), ZnO, and without catalyst (photolysis). Bottom left) the kinetics of the 2-((10-decyl-10H-phenothiazin-3-yl)methylene)malononitrile dye degradation. Bottom right) the first order rate constants of the 2-((10-decyl-10H-phenothiazin-3-yl)methylene)malononitrile dye degradation.
ln(C0/C) = −Kt, where C0 and C are the dark chemisorption concentration and the concentration at time t, respectively. K is the reaction rate constant. Thus, plotting ln(C0/C) versus t results in a straight line with a slope equal –K. Two or three runs of photocatalysis were repeated using a fresh catalyst, and the standard deviation obtained was about 5%.
3 Results and discussion
3.1 Synthesis
The synthesis of dye III was made, as shown in Scheme 1. Thus, the N-alkylation of phenothiazine via SN2 reaction afforded the corresponding product I, which underwent Vilsmeier formylation reaction to give the corresponding aldehyde II in high yield. Condensation of compound II with malononitrile via Knoevenagel reaction afforded the corresponding dye III in good yield. The chemical structure of these synthesized compounds was confirmed by IR, NMR, and high-resolution mass spectra (see supplementary file).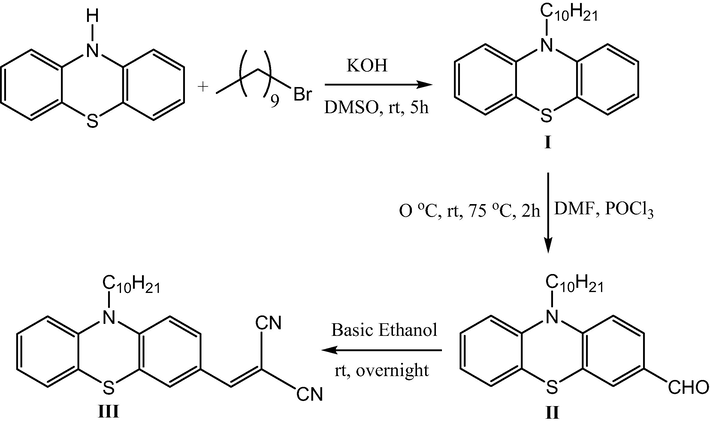
The synthetic route of the dye.
3.2 Photocatalytic studies
The photocatalytic decolourization of water-insoluble dyes is not common, and to the best of our knowledge, as yet, no report for such an approach has appeared. It is believed that the success in the decolorizing organic-based hydrophobic dye, such as the presented one, would pave the way for the decontamination of water-insoluble organic pollutants. Thus, the title dye was subjected to photodecolorization from aqueous solution (1:9, DMF/water v/v). It was observed that at zero time (dark chemisorption), the dye concentration remains almost constant, indicating the absence of chemisorption. Having been informed by that, it was necessary to study whether TiO2 (p25) would catalyze the decolourization of the dye. Fig. 1 top (right) reveals the gradual decreases in the dye absorption upon irradiation time to reach almost complete decolourization after 30 min. Fig. 1 top (left) reveals the time curve of the decolourization of the dye in the absence of catalyst and in the presence of TiO2 (p25) and ZnO. It is clearly observed that the dye is almost stable during all tested irradiation time in the absence of catalysts, whereas in the presence of catalysts, TiO2 (p25) shows superior efficiency at a higher rate than ZnO. Analyzing the data, as shown in Fig. 1 bottom (left) and bottom (right), the rate using TiO2 (0.095 min−1) is higher than the rate constant using ZnO (0.055 min−1). The effect of solution pH on the rate of photocatalytic decolourization of the dye was performed at three different pHs of 5, 7, and 9. The colour of the dye did not change, and the obtained first-order ate constant did not change a lot (only negligible difference), indicating that the pH, although it affects the catalysts but has no effect on the organic-based hydrophobic dye and thus no effect on the photocatalytic process. The results of pH effect are presented in the supplementary file.
4 Conclusion
Heterogeneous photocatalytic declourization of water-insoluble dye present in an aqueous solution made from 1:9, DMF: water was successfully made in a short time. The title dye showed photostability without catalysts and could be decolourized in a short time using both catalysts (TiO2 (p25) and ZnO), although with a faster rate using TiO2 (P25) compared with ZnO. In addition, the results of pH effect on the photocatalytic decolourization indicate that the process is independent of the pH as the dye used is an organic-based hydrophobic dye. These results suggest that the photocatalytic decolorization of water-insoluble organic pollutants is a viable process for environmental remediation and will inspire further work using different oxide nanoparticles for the photocatalytic decontamination of organic pollutants.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through research groups program under grant number R.G.P.1/8/38.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- Kinetics and mechanism of the oxidative decolorization of direct violet 31 in the presence of peroxodisulfate-silver (I) as a redox system. Transition Met. Chem.. 2019;44:57-64.
- [Google Scholar]
- Tuning of the crystallite and particle sizes of ZnO nanocrystalline materials in solvothermal synthesis and their photocatalytic activity for dye degradation. J. Phys. Chem. C. 2011;115:13844-13850.
- [Google Scholar]
- Highly efficient photocatalytic treatment of mixed dyes wastewater via visible-light-driven AgI–Ag3PO4/MWCNTs. Mater. Sci. Semicond. Process. 2015;37:19-28.
- [Google Scholar]
- TiO2@ZIF-8: A novel approach of modifying micro-environment for enhanced photo-catalytic dye degradation and high usability of TiO2 nanoparticles. Mater. Lett.. 2016;164:571-574.
- [Google Scholar]
- Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev.. 2007;107:2891-2959.
- [Google Scholar]
- Efficacy of SnO2 nanoparticles toward photocatalytic degradation of methylene blue dye. J. Photochem. Photobiol. B. 2016;155:34-38.
- [Google Scholar]
- Functional dyes and some hi-tech applications. Int. J. Photoenergy. 2009;2009:434897
- [Google Scholar]
- Synthesis, linear and nonlinear optical properties of a new dimethine cyanine dye derived from phenothiazine. RSC Adv.. 2016;6:91546-91556.
- [Google Scholar]
- Synthesis of a new fluorescent cyanide chemosensor based on phenothiazine derivative. Sens. Actuators B. 2017;240(2):288-296.
- [Google Scholar]
- New J. Chem.. 2018;42:9045-9050.
- Geochemistry and utilization of montmorillonitic soil for cationic dye removal. Adsorpt. Sci. Technol.. 2001;19:609-620.
- [Google Scholar]
- Bypass kiln dust as adsorbent for anionic dye and heavy metal ions removal from aqueous solution. Toxicol. Environ. Chem.. 2001;82:1-10.
- [Google Scholar]
- Removal of malachite green dye from aqueous solutions using organically modified. J. Environ. Chem. Eng.. 2016;4:633-638.
- [Google Scholar]
- Coupling of acrylic dyeing wastewater treatment by heterogeneous Fenton oxidation in a continuous stirred tank reactor with biological degradation in a sequential batch reactor. Environ. Manage.. 2016;166:193-203.
- [Google Scholar]
- A novel TiO2 composite for photocatalytic wastewater treatment. J. Catal.. 2014;310:75-83.
- [Google Scholar]
- Preparation and characterization of cationized cellulose for the removal of anionic dyes. Adsorpt. Sci. Technol.. 2001;19:197-210.
- [Google Scholar]
- Environmental applications of semiconductor photocatalysis. Chem. Rev.. 1995;95:69-96.
- [Google Scholar]
- Catalytic ozonation of simulated textile dyeing wastewater using mesoporous carbon aerogel supported copper oxide catalyst. J. Clean. Prod.. 2016;112:4710-4718.
- [Google Scholar]
- Compound bioflocculant used as a coagulation aid in synthetic dye wastewater treatment: The effect of solution pH. Sep. Purif. Technol.. 2015;154:108-114.
- [Google Scholar]
- Steric and solvent effect in dye-sensitized solar cells utilizing phenothiazine-based dyes. Int. J. Photoenergy. 2014;2014:548914
- [Google Scholar]
- Kumar, R., El-Shishtawy, R.M., Barakat, M.A., 2016. Synthesis and characterization of Ag-Ag2O/TiO2@polypyrrole heterojunction for enhanced photocatalytic degradation of methylene blue. Catalysts 6, Article number 76.
- Bioinformatics aided microbial approach for bioremediation of wastewater containing textile dyes. Ecol. Inf.. 2016;31:112-121.
- [Google Scholar]
- Facile Synthesis and enhanced photocatalytic performance of flower-like ZnO hierarchical microstructures. J. Phys. Chem. C. 2010;114:890-896.
- [Google Scholar]
- Sonocatalytic activity of Yb, B, Ga-codoped Er3+:Y3Al5O12/TiO2 in degradation of organic dyes. Mater. Sci. Semicond. Process.. 2014;26:438-447.
- [Google Scholar]
- Recent advances in phenothiazine-based dyes for dye-sensitized solar cells. Chin. Chem. Lett.. 2016;27:1304-1318.
- [Google Scholar]
- Development of a method for adsorption of dyestuffs on activated sludge. Water Res.. 1994;28(1):1051-1057.
- [Google Scholar]
- Agar based bimetallic nanoparticles as high-performance renewable adsorbent for removal and degradation of cationic organic dyes. J. Ind. Eng. Chem.. 2016;33:226-238.
- [Google Scholar]
- Kinetics and thermodynamics of the adsorption of some dyestuffs from aqueous solution by poplar sawdust. Bioresour. Technol.. 2008;99:2009-2017.
- [Google Scholar]
- Using dyes for evaluating photocatalytic properties: a critical review. Molecules. 2015;20:88-110.
- [Google Scholar]
- Preparation and characterization of polyphosphotungstate/ZrO2 nanocomposite and their sonocatalytic and photocatalytic activity under UV light illumination. Ultrason. Sonochem.. 2012;19(3):546-553.
- [Google Scholar]
- Performance evaluation of SBR for the treatment of dyeing wastewater by simultaneous biological and adsorption processes. J. Water Process Eng.. 2014;4:82-90.
- [Google Scholar]
- Sonocatalytic degradation of 2-hydroxyethyl cellulose in the presence of some nanoparticles. Sonochem.. 2015;26:265-272.
- [Google Scholar]
- Large-scale synthesis of ZnO balls made of fluffy thin nanosheets by simple solution process: Structural, optical and photocatalytic properties. J. Colloid Interface Sci.. 2011;363:521-528.
- [Google Scholar]
- Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem. Soc. Rev.. 2014;43:5234-5244.
- [Google Scholar]
- Characterisation and coagulation performance of an inorganic coagulant—poly-magnesium-silicate-chloride in treatment of simulated dyeing wastewater. Colloids Surf. A Physicochem. Eng. Aspects. 2015;470:137-141.
- [Google Scholar]
- Carbon-doped ZnO nanostructures: facile synthesis and visible light photocatalytic applications. J. Phys. Chem. C. 2015;119:20544-20554.
- [Google Scholar]
- Color Chemistry—Synthesis, Properties, and Applications of Organic Dyes and Pigments (third ed.). Weinheim-Federal Republic of Germany: Wiley-VCH; 2001.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.12.007.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







