Translate this page into:
Photoprotective nanoemulsions containing microbial carotenoids and buriti oil: Efficacy and safety study
⁎Correspondence author at: LADEG, Federal University of Rio de Janeiro, Brazil, Avenida Carlos Chagas Filho, 373, Centro de Ciências da Saúde – CCS, Cidade Universitária, Ilha do Fundão, CEP 21941-902, Brazil. maria.cristina.reis@hotmail.com (Maria Cristina Pinheiro Pereira Reis Mansur)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Photoprotective nanoemulsions are able to attenuate skin damage from overexposure to the sun, thus avoiding the immediate effects caused by ultraviolet radiation. The global cosmetics market understands that there is a demand and greater acceptance by consumers for formulations containing natural products compatible with the skin. Consequently, there is an increasingly need to develop such products that are safe and effective. Furthermore, there is a growing interest in nanoemulsions (NE) in the pharmaceutical industry, due the versatility of incorporating lipophilic substances into cosmetic formulations. In the present work, oil-in-water photoprotective nanoemulsions containing microbial carotenoids, buriti oil and chemical filters were developed and characterized. The essential physical properties of the droplets, the transmission electronic microscopy (TEM), the sun protection factor (SPF) as well as the stability of the formulations were determined. In vitro phototoxicity was evaluated using Balb 3 T3 with relative cell viability estimated by Neutral Red Uptake, with the Photo Irritation Factor (PIF) and the Medium Photo Effect Factor (MPF) as the measurement parameters. Nanoemulsion 3 (NE3) showed spherical morphology with an average droplet size of 142.11 ± 0.92 nm and polydispersity index (PDI) of 0.198 ± 0.017. This nanoemulsion containing microbial carotenoids and buriti oil exhibited a SPF of 36 ± 1.5. Neutral Red Uptake revealed that the cells kept their viability even after irradiation and those nanoemulsions containing the microbial carotenoids and buriti oil were not phototoxic. The addition of microbial carotenoids and buriti oil in nanoemulsions was positive in increasing the mean SPF values compared to the control formulation.
Keywords
Photoprotection
Nanoemulsions
Microbial-carotenoids
Buriti oil
SPF
1 Introduction
Careless exposure to sunlight can be harmful to the skin due to ultraviolet (UV) radiation. Therefore, application of cosmetic formulations containing chemical filters and antioxidants can attenuate these harmful effects (Shaath, 2005). Chemical filters can absorb most of the UV-A and UV-B radiation, although a significant portion of these rays may still reach the skin, generating reactive oxygen species (ROS) (Ferreira et al., 2007, Mansur et al., 2012). Nevertheless, the skin possesses antioxidant defense mechanisms that can prevent and eliminate alterations caused by free radicals and ROS. Sun overexposure and, consequently, to UV radiation, may cause an imbalance in the pro-oxidant substances, leading to oxidative stress that produces lesions to skin cells such as erythema, stains, photoaging and skin cancer (Mansur et al., 2016). The cosmetic industries associate chemical filters to different antioxidants to optimize skin protection that act together with endogenous antioxidants to reestablish the skin redox equilibrium (Vinardel and Mitjans, 2015).
A number of synthetic substances used as sunscreens were removed from the market because they proved to be toxic and constituted a real danger to human health (Scott et al., 2010). Furthermore, according to the US Environmental Protection Agency (EPA) these substances act as pollutants entering the marine trophic chain and also as endocrine disruptors (Trenholm et al., 2006). Therefore, the development of formulations containing multifunctional actives from natural sources, and lower concentrations of chemical filters are providing satisfactory results in preventing photocarcinogenesis, photoaging of the skin (Mansur et al., 2016) and environnmental damages.
During the last decades, nanosciences have been through great advances, especially in the pharmaceutical and cosmetic fields, enabling the encapsulation of actives to clarify problems of solubility and stability. Nanocarriers form colloidal dispersions of nanoparticles or droplets with sizes below 1000 nm (Li et al., 2011, Montenegro et al., 2016). Nanocarriers such as polymeric nanoparticles, liposomes, niosomes and nanoemulsions are widely used to disperse lipophilic actives in formulations for cutaneous administration (Montenegro et al., 2016, Puglia et al., 2014).
Nanoemulsions consist of oil in water (o/w) or water in oil (w/o) dispersions with droplet diameters generally in the range of 10–200 nm. Their advantages, such as improved stability when compared to traditional emulsions, low toxicity due the employment of non-ionic surfactants, high biocompatibility and potential to incorporate hydrophilic and lipophilic actives in the same formulation are remarkable (Clares et al., 2014, Ricci-Junior et al., 2018). Moreover, they are viable and innovative alternatives to traditional dermocosmetics, also exhibiting other interesting advantages for the cosmetic industry, such as low viscosity which facilitates the application on the skin. Their smooth texture and nanometer size improve skin coverage and formation of the occlusive film. Large-scale production and applying of simple, fast, and cheap methods, controlled-release of active ingredients, modulate their sun protection factor, and reduction of side effects on the organism as well (Campos et al., 2017).
Recent studies have demonstrated that the antioxidant actives of plant extracts, algae and bacteria are able of scavenge UV- induced ROS. However, active substances obtained by biotechnological processes are unstable when exposed to sunlight due to photodegradation or even during their storage. A viable alternative to improve the stability of lipophilic biotechnologically-obtained actives can be overcome by encapsulation in nanocarriers such as nanoemulsions. The simultaneous encapsulation of sunscreens, antioxidants, and actives in nanoemulsions can generate efficient and stable photoprotective cosmetic formulation as well (Vinardel and Mitjans, 2015, Pesek et al.,2011).
As such, carotenoids are natural ROS-scavenging antioxidant substances synthesized by bacteria, fungi, algae, and plants that can protect the skin from solar radiation (Reis-Mansur et al., 2019). They knockdown peroxide radicals in the human skin, preventing lipidic peroxidation, which is the most aggressive reaction in cellular membranes (Ashikhmin et al., 2014, Mortensen, 2002). Carotenoids are known as lipophilic pigments and have been used in the textile, food, cosmetic and pharmaceutical industries. They can be dispersed in aqueous vehicles when encapsulated in nanoemulsions (Han et al., 2016). Some studies have demonstrated that the encapsulation of carotenoids is important to protect them from degradation caused by sunlight, oxygen and enzymes, preserving their antioxidant properties and keeping their activity in pharmaceutical formulations and dermocosmetic products (Soukoulis and Bohn, 2018).
Microbacterium sp. LEMMJ01 is a new bacterium species that was isolated from soil samples near a penguin colony (Pygoscelis adeliae) located on King George Island, Northwest of the Antarctic Peninsula, a region with a high incidence of ultraviolet radiation. This Microbacterium sp. is a mesophilic actinomycete resistant to UVA, UVB and UVC radiations. This species synthesizes a group of carotenoids, which are natural pigments with antioxidant activity (Reis-Mansur et al., 2019, Schultz et al., 2017, Han et al., 2016).
The association of antioxidant substances and carotenoids in photoprotective formulations is interesting and beneficial due the synergic effect observed in preventing skin erythema during solar exposure (Gaspar and Campos, 2007, Stahl and Sies, 2002). Nanoemulsions are promising release systems capable of dispersing chemical filters, buriti oil and carotenoids into physiologically acceptable vehicles for cutaneous administration, being considered for the development of innovative photoprotective formulations (Reis-Mansur et al., 2019, Montenegro et al., 2016, Clares et al., 2014). Buriti (Mauritia flexuosa L.) oil contains high concentration of monounsaturated fatty acids, tocopherols and carotenes with antioxidant and pro-vitamin A function. It has been administrated under the form of o/w emulsions with moisturizing, emollient and antioxidant action to the skin (Aquino et al., 2012, Podda and Grundmann-Kollmann, 2001).
In the present work, microbial carotenoids were produced and isolated from the crude biomass of Microbacterium sp. LEMMJ01 (Reis-Mansur et al., 2019). Photoprotective nanoemulsions with antioxidant and moisturizing properties were produced containing an association of chemical filters, microbial carotenoids and buriti oil. The formulations were characterized in relation to their pH, organoleptic characteristics, droplet size, polydispersity index, and morphology by transmission electronic microscopy (TEM). Furthermore, the Sun Protection Factor (SPF) was evaluated, a stability study of the photoprotective formulations was performed as were safety studies using cell culture.
Researchers in Microbiology have pointed out a variety of bioproducts that can be used in different industrial segments, as well as in photoprotective formulations. Furthermore, the search for innovative photoprotective product, originated from renewable natural resources and is not only effective but safe, biocompatible and harmless to the environment from its production to its disposal is fully justified.
In this research, a preliminary study of the efficacy and safety of the photoprotective nanoemulsions containing microbial carotenoids, buriti oil, and chemical filters was carried out. The formulations were developed with a goal to increase the SPF and improve product stability as an innovative and viable alternative.
2 Material and methods
2.1 Material
Buriti oil is the volatile oil obtained from the fruit of Mauritia flexuosa L. It was purchased from Plantus da Amazônia (Brazil). The following materials were purchased from the respective companies: Conserve Novamit MF (methylisothiazolinone and phenoxyethanol) - Ipel Itibanyl Produtos Especiais Ltda. (Brazil); Octyl methoxycinnamate (OMC) (Eusolex®) - Pharmanostra (Germany); Ethylhexyl methoxycrylene (EHMC) (Eusolex) - Ocr (Spain); Benzofenone-3 (BZF-3) (Eusolex 4360-Benz-3®) - Merck (Germany); Vitamin E - Sarfam (Switzerland); Propyleneglycol, Tween® 80 and Span® 80 - Tedia (USA); Aristoflex®AVC (sodium polyacryloyldimethyl taurate) - Clariant (Spain).
2.2 Obtainment of Microbacterium sp. LEMMJ01 biomass, extraction, and characterization of the microbial carotenoids
The Microbacterium sp. LEMMJ01 was isolated from Antarctic ornithogenic soil that was collected at the Arctowski Polish Station (62°09′790″S; 58°27′687″W), in Admiralty Bay, in the central region of the King George Island during the austral summer of 2009/2010, with the support of the Brazilian Antarctic Program. The island belongs to the South Shetland Archipelago, located Northwest of the Antarctic Peninsula. The temperatures range between −3°C and 8 °C with glaciers covering 90% of the island (Schultz et al., 2017).
The Microbacterium sp. isolate LEMMJ01 biomass was produced in a bioreactor for 48 h at 28 °C and 160 rpm. After obtaining the biomass of orange color, the crude carotenoid fraction was extracted and chemically characterized by high performance liquid chromatography (HPLC) coupled to a mass spectrophotometer, and carotenes were found in its chemical composition. The production methodology, extraction and chemical characterization of the microbial carotenoids was made by our research group and has been reported in the literature (Reis-Mansur et al., 2019).
2.3 Preparation of the photoprotective nanoemulsions
The description of the generated oil-in-water (o/w) nanoemulsions is shown in Table 1. The aqueous phase (AP) was prepared by mixing Tween® 80, Span® 80, propyleneglycol, preservative and purified water. The oil phase (OP) was prepared by mixing the chemical filters, microbial carotenoids, buriti oil and vitamin E. The OP was added to the AP under constant homogenization using an ultrasonic processor (UP100H, Ultrasonic Processor, Hielscher, Teltow, Germany) containing a 7 mm titanium probe. The experimental conditions were continuous cycle, 100% amplitude, temperature controlled by an ice bath at 5 °C (Fig. 1). The formulation was homogenized for 4 periods of 5 min with 2 min of rest between homogenizations. Aristoflex® was added to the formulation to adjust the viscosity (Mansur et al., 2012, Cerqueira-Coutinho et al., 2015). OMC = octyl methoxycinnamate; EHMC = ethylhexyl methoxycrylene; BZF-3 = Benzofenone-3.
Components
Function
Formulations
NE1
NE2
NE3
Oil phase (OP)
OMC
Chemical filter
10%
8%
10%
EHMC
Chemical filter
3%
3%
3%
BZF-3
Chemical filter
3%
3%
3%
Buriti Oil
Antioxidant/Moisturizer
–
3%
3%
Vitamin E
Antioxidant
0.1%
0.1%
0.1%
Microbial carotenoids
Antioxidant
–
0.2%
0.2%
Aqueous phase (AP)
Tween® 80
Surfactant
12%
12%
12%
Span® 80
Surfactant
3%
3%
3%
Conserve Novamit MF
Preservative
0.3%
0.3%
0.3%
Propyleneglycol
Humectant
2%
2%
2%
Aristoflex®
Viscosity agent
1%
1%
1%
Purified Water
Vehicle
qsp. 100 g
qsp. 100 g
qsp. 100 g

Scheme of production of the oil-in-water (o/w) NE.
Three nanoemulsions were prepared, the control nanoemulsion was NE1, containing only the chemical filters, while NE2 and NE3 containing in addition to filters the microbial carotenoids and buriti oil. The nanoemulsions were transferred to tubes and stored in the dark at 25˚ C until further use.
2.4 Nanoemulsions characterization
2.4.1 Determination of pH and organoleptic characteristics
The nanoemulsions were inspected for their pH and organoleptic characteristics. The pH was measured using a pHmeter (Cherker portable pHmeter, Hanna Instruments, Woonsocket, RI, USA). The organoleptic characteristics such as aspect, color, homogeneity, and phase separation were evaluated visually by analysts.
2.4.2 Determination of droplet size and polydispersity index (PDI)
Droplet size and polydispersity index (PDI) were measured using a NanoSizer® model 90S (Malvern, UK). Nanoemulsions were diluted in distilled water at 1:100 and analyzed in a cell with 1 cm optical path at 25 °C. These analyses were conducted in three runs with fifteen readings. The values shown (Table 2) represent the mean ± standard deviation of three independent measurements for each formulation. The PDI parameter reflects the sample quality in terms of homogeneity of the droplet diameter. The values for PDI results below 1 are considered satisfactory (Malvern Instruments, 2004, Cerqueira-Coutinho et al., 2015). Results are expressed as average ± standard deviation of n = 3 independent measurements.
Formulation
Size (nm)
PDI
Aspect
pH
NE1
111.35 ± 0.95
0.235 ± 0.019
Bright yellow, Homogenous, no phase separation
5.27 ± 0.32
NE2
157.03 ± 3.32
0.165 ± 0.018
Bright yellow, Homogenous, no phase separation
5.18 ± 0.27
NE3
142.11 ± 0.92
0.198 ± 0.017
Bright yellow, Homogenous, no phase separation
5.32 ± 0.19
2.4.3 Transmission electronic microscopy (TEM)
The droplet morphology of NE3 was evaluated by Transmission Electronic Microscopy (TEM) using a Morgagni 265 (FEI Company, Netherlands) electronic microscope. A NE3 stock solution was diluted to 1:5 with purified water. A volume of 5 µL was placed on a copper grid previously covered with Formvar®. The grid was placed in a desiccator for 1 h to eliminate any water excess, and then placed under the transmission beam for inspection of droplet’s morphology.
2.5 Efficacy assays
2.5.1 Sun protection Factor (SPF) assessment
In vitro SPF measurements were performed using a UV transmittance analyzer (Labsphere® UV-2000S, North Sutton, NH, USA) and polymethylmethacrylate (PMMA) plates. A 50 mg (2.0 mg/cm2) sample of each formulation was pipetted and manually spread with circular movements on the surface of a PMMA plate until form a homogeneous film. The PMMA plates were stored in a dark chamber at 25 °C and dried for 15 min before being analyzed. Glycerin was also spread on the surfaces of clean PMMA plates and used as reference for 100% transmittance (COLIPA, 2009, MOTA et al., 2013). SPF, UVA/UVB ratio, and critical wavelength (λc) for each formulation were determined in triplicates.
2.6 Stability study
The nanoemulsions were produced and stored at 25 °C. Droplet diameter and PDI were determined by Dynamic Light Scattering (DLS) technique using a NanoSizer® model 90S (Malvern, UK) at intervals of 0, 7, 15, and 30 days, in order to evaluate the stability of the formulations. The droplet diameter and PDI values were determined in triplicates and represented as average ± standard deviation for each one.
2.7 Safety assay
2.7.1 The in vitro phototoxicity test
2.7.1.1 Cell culture
Balb 3T3 clone A31 (ATCC CCl-163) cells were cultivated and expanded in DMEM (BioWhittaker, Lonza, USA) with 10% fetal bovine serum (FBS; Gibco, Life technologies, USA), 4.5 mg/mL glucose, 4 mM l-glutamine, 1 mM sodium pyruvate, 100 UI/mL penicillin and 100 µg/mL streptomycin (Sigma-Aldrich, USA). Cells were incubated at 37 °C ± 1 °C in a humidified atmosphere (90% ± 10%) with 5% ± 0.5% CO2. The cultures were monitored the presence of microbiological contaminants using microbiological tests, PCR and bioluminescence culture probes. Only bacteria-free cultures (including mycoplasma) and fungi were used. All experiments were done with previously qualified cells without any significant cytotoxicity induced by exposure to spurious light (cell viability > 80%).
2.7.1.2 Solar simulation and irradiations conditions
The Q-Sun XE-1-BC Xenon Test Chamber equipped with a xenon arc lamp and daylight optical filter was used to simulate the outdoor solar light exposure. Irradiation was performed at 25 °C, with an irradiance of 1.7 mW/cm2 for 50 min to achieve a dose of 5 J/cm2. The irradiance was monitored in real time by the Solar Eye irradiance control light output system, which was calibrated to the standards of the National Metrology Institute (National Institute of Standards and Technology – NIST, USA), ensuring accuracy, reliability and traceability. Q-Lab Corporation (USA) provided all solar simulation equipment.
2.7.1.3 In vitro phototoxicity of 3T3 cells by Neutral Red Uptake (NRU)
The phototoxicity was evaluated according to the Organization for Economic Co-operation and Development (OECD) Test Guideline n° 432 (described in Mota et al., 2013). Briefly, Balb 3 T3 cells were seeded in two 96-well plates with recommended cell seeding density of 1.0 × 104 per well and incubated for 24 h ± 2 h before subjected to the tests. A logarithmic series (1:10) of seven dilutions were prepared in Hank’s Balanced Salt Solution (HBSS; BioWhittaker, Lonza, USA) containing a start solution of 1 mg/mL of NE1 or NE3 in HBSS. The range of cytotoxicity was split in smaller decimal geometric series of seven dilutions in HBSS using the dilution factors 3.16 and 2.15, respectively. Chlorpromazine was used as the positive control (CAS 50-53-3, Santa Cruz Biotechnologies, USA), and a stock solution of 7.5 mg/mL in DMSO was initially diluted 100-fold in HBSS. A decimal geometric series of seven dilutions in HBSS (1:3.16) was performed from the chlorpromazine solution in the range of 75 to 2.4 × 10-2 µg/mL. The buriti oil starting stock solution of 100 mg/mL in DMSO was diluted 100-fold in HBSS. A logarithmic series (1:10) of seven dilutions in HBSS was performed from the stock solution of buriti oil.
The 96-well plates were washed twice with 150 µL HBSS and then two plates treated with 100 µL of eight concentrations of each test chemical were prepared in duplicates. One plate was irradiated and its duplicate other kept in the dark at 25 °C. Afterwards, both plates were washed twice with HBSS and incubated in complete medium for 20 h ± 2 h to allow cell growth and recuperation. The relative cell viability was estimated by Neutral Red Uptake (NRU). The plates were washed once with 250 µL of Dulbecco's Phosphate-Buffered Saline without Ca2+ and Mg2+ (DPBA-A) (BioWhittaker, Lonza, USA), and then 250 µL of 25 µg/mL Neutral Red (NR) solution in DMEM + 5% FBS was added and finally the plates were incubated for 3 h ± 10 min. The NR solution was then decanted, and the plates were washed with 250 µL of DPBS-A. The absorbed NR by the Balb 3 T3 cells was extracted with 100 µL of ethanol:distillated water:acetic acid solution (50%: 49%: 1%) and shaking for 20 min using an orbital shaker protected from light. The absorbance of the NR solution was measured at a 540 nm wavelength using a spectrophotometer. The IC50, Photo Irritation Factor (PIF) and Mean Photo Effect (MPE) values were calculated by means of the Phototox 2.0, a validated and freely available software provided by the OECD (2004).
The IC50 is the concentration of the test compound that decreases cell viability by 50% (OECD, 2004, Spielmann et al., 1998). PIF is the ratio between non-irradiated IC50 value and the IC50 obtained for the irradiated sample. The MPE is calculated by a complex mathematical model that compares the displacement of the irradiated dose-response curve relatively to the non-irradiated curve.
2.7.2 Model of the in vivo phototoxicity prediction
According to the validation study (Spielmann et al., 1998), a chemical is classified as phototoxic if the score of the PIF or the MPE are, respectively, above 5 or 0.15. If PIF < 2 or MPE < 0.1, a chemical is considered non-phototoxic. If 2 < PIF < 5 and 0.1 < MPE < 0.15, a given test compound is classified as probably phototoxic by this methodology. Statistically, there is no significant difference between the PIF and MPE models, and this method is often used when IC50 cannot be calculated (Ceridono et al., 2012).
2.8 Statistical analysis
Experimental data are presented as the mean ± SD or standard error of the mean calculated using Origin Pro 8 (OriginLab, USA) software and p < 0.05 was considered to be statistically significant.
3 Results and discussion
3.1 Preparation and characterization of the nanoemulsions (NE)
After several ratios between the surfactants were tested to verify which provided greater stability to the formulations, the proportion of 12% Tween® 80 and 3% Span® 80 turned out to be the ideal mixture for the proposed NE.This proportion of surfactants has already been used in a previous work and proven to be efficient to obtain a stable nanoemulsion (Cerqueira-Coutinho et al., 2015). NE were made using a high-energy method that utilizes an ultrasonic processor. The concentration of the chemical filter OMC from 8% (the most common concentration) has been changed to 10% (maximum permitted concentration in Brazil) to check the effect of natural substances on the SPF. The usual concentration of buriti oil in cosmetics is 1 to 5%. The concentration of 3% was chosen because it allows the formation of an adequate nanoemulsion. The concentration of the microbial carotenoids was 0.2% as it is the concentration normally used for antioxidants in cosmetics.
On Table 2, it is possible to observe the medium diameter and the PDI of the developed formulations, as well as the pH and organoleptic characteristics. They were produced successfully, presenting nanometric size and low PDI (Lademann et al., 2011; Puglia et al., 2014; Larese Filon et al., 2015). In terms of the organoleptic characteristics, the nanoemulsions were bright yellow in color, homogenous and there was no phase separation. Human skin has a pH of approximately 5.0–6.0. The pH values of NE1, NE2 and NE3 were shown in Table 2. The pH values of NE1, NE2 and NE3 are shown in Table 2, and, thus, compatible with the pH range of the human skin (also 5.0–6.0). This later fact is beneficial for homeostasis when applied to the skin surface (Lambers et al., 2006; Segger et al., 2008; Zanela da Silva Marques et al., 2018).
3.2 Transmission electronic microscopy (TEM)
TEM result of NE3 can be observed in Fig. 2, where Fig. 2A is a panoramic image in which the homogeneous dispersion of the nanoemulsion droplets can be visualized. The same field was amplified in Fig. 2B and it is possible to observe the round shapes of the NE droplets with dark grey color that contrast with the light gray background of the grid. The droplets appear as a homogeneous dispersion on the grid surface, and there are no agglomerations. Siqueira et al. (2019) developed antimicrobial formulation with nanometric size droplets containing clove oil, also seen without agglomerations under TEM. This technique developed by our research group that involves dilution and fixation of the sample on the microscopy grid provides excellent high-resolution images of the nanoemulsion droplets. NE droplets suffered negligible deformation from their original spherical state due to the drying process. The dark grey dots in the image refers to the oil phase containing microbial carotenoids, buriti oil, chemical filters, and vitamin E. A magnification with greater detail of the morphology of the nanoemulsion droplets is shown in Fig. 2C.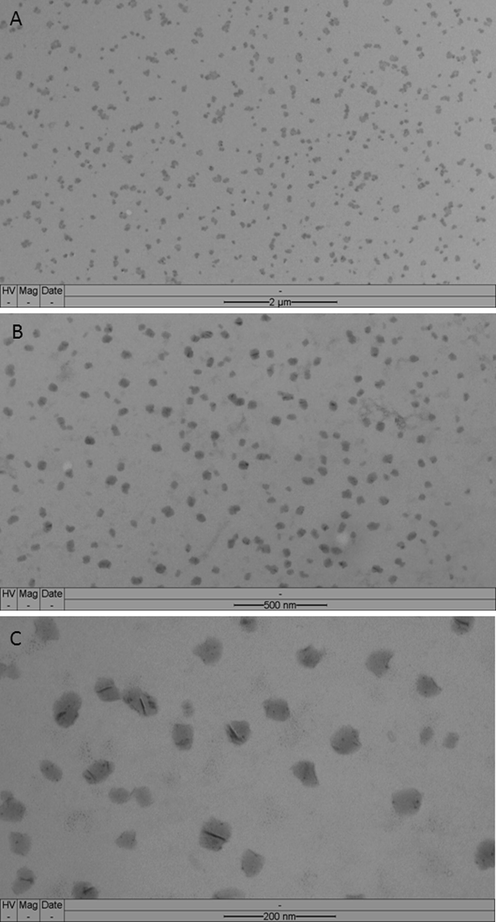
Microphotographs of a sample of the NE3: (A) panoramic image, in (B) and (C) are shown magnified images.
3.3 Efficacy assay: Sun protection Factor (SPF) assessment
The in vitro SPF measurement only allows an estimate of the actual value, which can only assess by in vivo tests with humans. The determination of the efficacy of the photoprotective nanoemulsions was based on the SPF of the three formulations on the day of preparation (t0) and after 30 days of storage (t30). Measurements were done in triplicates at 25 °C. There was no significant difference in the values measured at t0 and t30.
The SPF values after 30 days of storage (t30) are shown in Table 3. The average SPF values of NE2 and NE3 were superior to the average SPF value of NE1, and the greatest average SPF value was for NE3. The average SPF values for NE2 and NE3 were significantly different when compared to NE1 (p < 0.05) (Table 3). However, no significant statistical difference between the average SPF values of NE2 and NE3 (p > 0.05). NE2 and NE3 contains microbial carotenoids in their composition UV-absorbing radicals and, thus, exhibited higher average SPF values than NE1 without any microbial carotenoids. NE1- 10% OMC, 3% EHMC and 3% BZF-3; NE2- 8% OMC, 3% EHMC, 3% BZF-3, 3% buriti oil and 0.2% microbial carotenoids; NE3- 10% OMC, 3% EHMC, 3% BZF-3, 3% buriti oil and 0.2% microbial carotenoids. Average ± standard deviation of n = 3 independent experiments. *SPF with significant statistical difference in relation to NE1 (p < 0.05).
Formulation
SPF
UV-A/UV-B ratio
λc
NE1
21 ± 1.2
0.37 ± 0.042
358 ± 1.5
NE2
32 ± 2.0*
0.67 ± 0.023
368 ± 2.1
NE3
36 ± 1.5*
0.61 ± 0.015
360 ± 1.6
The Boots Star Rating System classifies a product according to the UV-A/UV-B ratio. How higher the ratio is, the better the product's protection against UV-A radiation. NE2 and NE3 showed a UV-A/UV-B ratio higher than 0.7, which according to the Boots Star Rating System, means that they can be awarded as good UV-A photoprotective formulations (Boots the Chemists Ltd, 2004, Levy, 2007, Cerqueira-Coutinho et al., 2015). However, NE1 presented an UV-A/UV-B ratio below 0.59 with no rating but NE2 and NE3 were scored with three stars as products with moderate UV-A protection. The presence of buriti oil and microbial carotenoids improved the photoprotective characteristics of the NE2 and NE3 in terms of their protection properties against UV-A radiation.
According to COLIPA (2009) and FDA (2011), a photoprotective product with a λc of 370 nm or higher is considered a broad-spectrum product. On the other hand, a range from 340 to 370 nm is considered a product with intermediate photoprotection against UVA. All NE developed showed a λc within this range and, for this reason, can be classified as good products, providing an intermediate protection against UV-A radiation (Couteau et al., 2011, Polefka et al., 2012, Butler, 2013, Donglikar and Deore, 2016).
4 Stability study
NE were produced using an ultrasonic processor and the average size and distribution of the droplets were determined by DLS (Dynamic Light Scattering). The stability investigation was a preliminary study that carried out the test with reduced duration in the initial phase of the development of the product stored at room temperature on the shelves. The goal of this test was to assist and guide the choice of the formulations being developed. NE2 and NE3 were stable during the stability study, presenting nanometric size and low PDI (Table 4). All nanoemulsions were composed of droplets with an average particle size under 200 nm and were classified as monodisperse systems with PDI < 0.3 (Table 4). The size distribution profile of nanoemulsions, NE2 and NE3 during the stability study was shown in Fig. 3B and C, respectively. NE1 exhibited an increase in droplet size in the order of 55 nm from T0 until T30 (Table 4) with alterations in the size distribution profile (Fig. 3A). On Table 4, the results showed small changes in the droplet size for NE2 and NE3, however, these increases were not statistically significant (Table 5). Results are expressed an average ± standard deviation of n = 3 independent measurements.
Time (Days)
NE1
NE2
NE3
Size
PDI
Size
PDI
Size
PDI
0
111.35 ± 0.95
0.235 ± 0.019
157.00 ± 3.32
0.165 ± 0.018
142.11 ± 0.92
0.198 ± 0.161
7
135.71 ± 2.57
0.257 ± 0.009
159.53 ± 1.72
0.132 ± 0.007
146.73 ± 2.32
0.177 ± 0.019
15
153.47 ± 3.81
0.246 ± 0.014
161.52 ± 1.40
0.146 ± 0.019
148.47 ± 2.68
0.133 ± 0.018
30
166.21 ± 1.08
0.212 ± 0.012
165.21 ± 2.08
0.145 ± 0.014
147.72 ± 1.63
0.092 ± 0.011
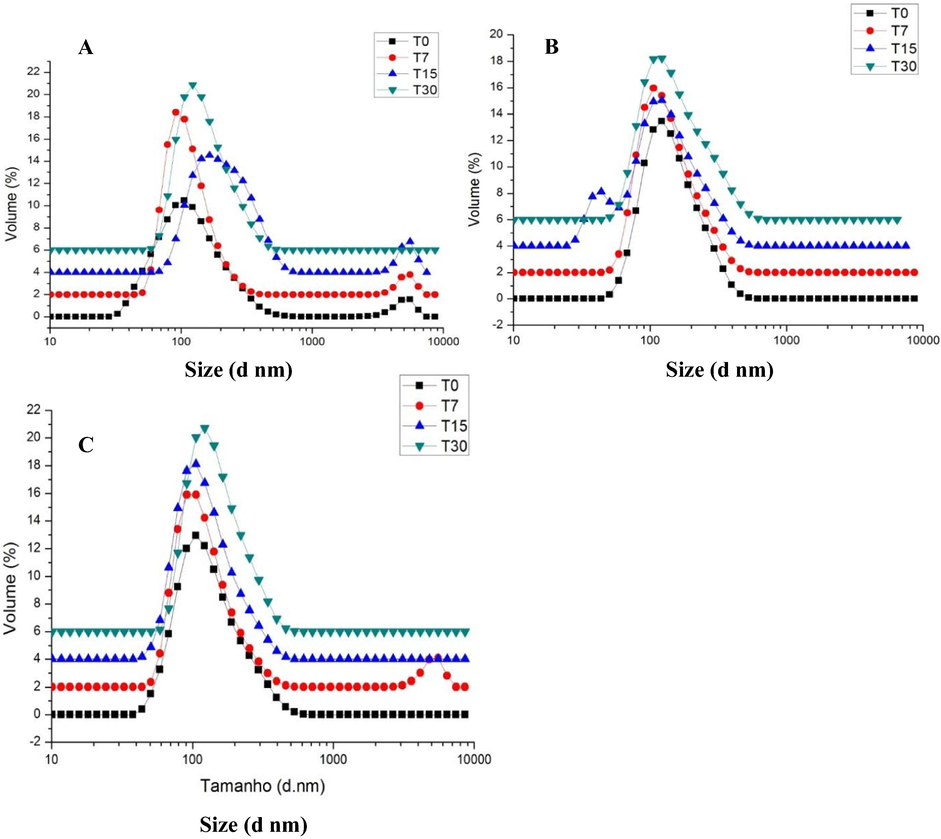
Size distribution of the three NE observed along the stability studied period: (A) NE 1, (B) NE2 and (C) NE3. Results are expressed as an average of n = 3 independent measurements.
The percentage (%) of droplets growing observed along the stability studied period are shown in Table 5. The results demonstrated that the size alterations of NE2 and NE3 were smaller in comparison to NE1 (49.3% of increase). There was a small growth in the average size of the NE2 and NE3 droplets from T0 (beginning of the stability study) to T30 (end of the stability study), although not statistically relevant (p > 0.05) (Table 5). Note that the addition of buriti oil and microbial carotenoids helped to maintain the droplet size and stability of the NE2 and NE3 products, which are missing in NE1 formulation. The increased percentage was calculated taking into consideration the average droplet size at T0 and the average size at T30.
5 Safety assay
5.1 In vitro phototoxicity test
The results of the phototoxicity evaluation assays are shown in Fig. 4.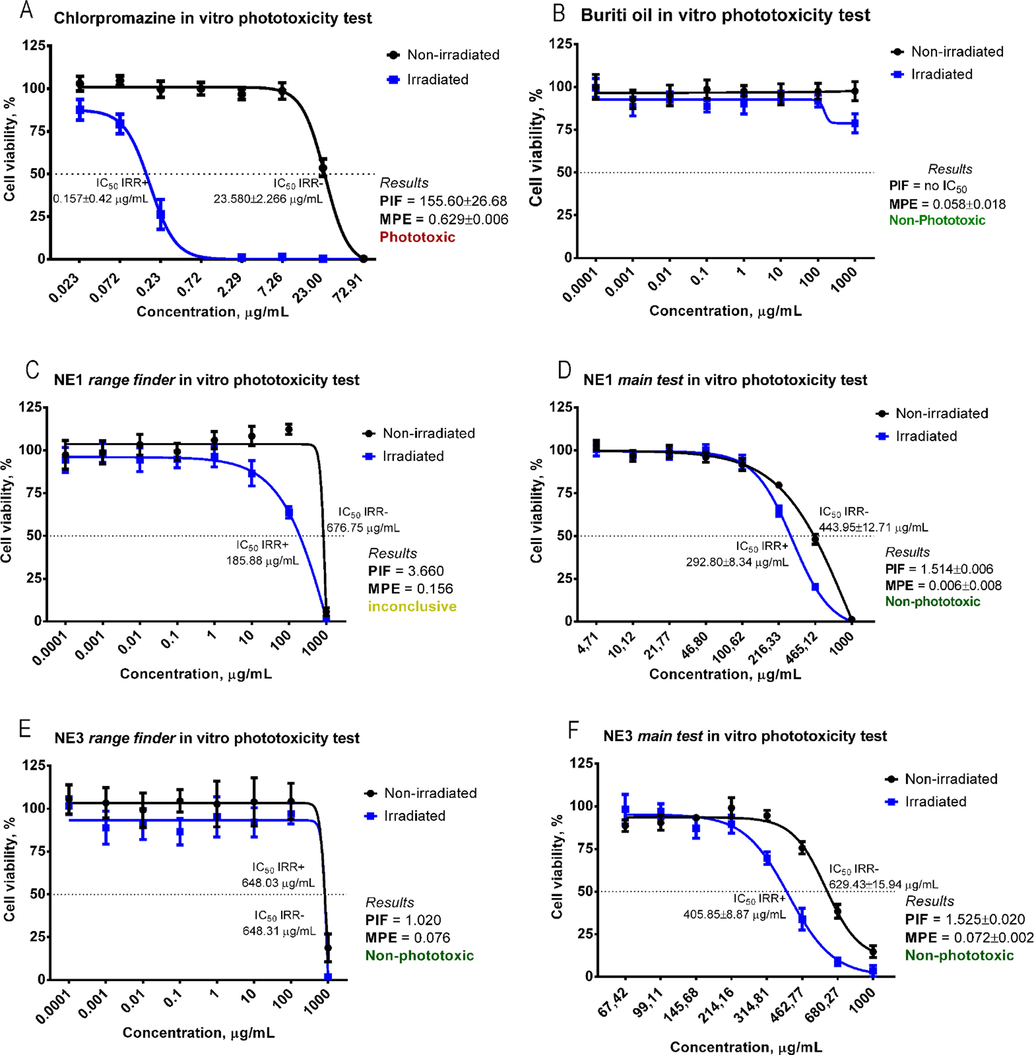
Dose-response curves and classification of the phototoxicity assays according to the PIF and MPE models: (A) chlorpromazine (positive control), (B) buriti oil (active), (C) NE1 range finder, (D) NE1 main test, (E) NE3 range finder and (F) NE3 main test.
The exposure to light intensifies the cell death in the presence of the positive control chlorpromazine (Fig. 4A), that is classified as phototoxic, and the results are in agreement with the parameters established by the OECD TG 432: IC50 (IRR +) = 0.1 to 2.0 µg/mL, IC50 (IRR-) = 7.0 to 90.0 µg/mL and PIF > 6. Buriti oil (Fig. 4B) was classified as non-phototoxic (MPE < 0.1), although a decrease in cell viability was observed with the largest concentration in the presence of light. The cytotoxicity stripes reported on the range finder of NE1 (Fig. 4C) and NE3 (Fig. 4E), 1000–10 µg/mL and 1000–100 µg/mL, respectively, were used in the phototoxicity assay (Fig. 4D and F). Neither NE showed any evidence of induced phototoxicity, according to the results obtained with the PIF and MPE models (PIF < 2 and MPE < 0.1).
Images displayed in Fig. 5A and B represent the uptake of the neutral vital red pigment in a concentration of 7.26 µg/mL of chlorpromazine. Notably in the irradiated condition (B), the pigment was not retained in the cells, meanwhile in the non-irradiated condition (A), the cells accumulate the neutral red in lysosomes of the cytoplasm. For NE3, the image representing the NRU by the lysosomes can be seen in Fig. 5C and D, respectively, in the absence and presence of light. The NRU shows that the cells kept their viability even after irradiation, and that cells exposed to NE3 did not exhibit phototoxicity.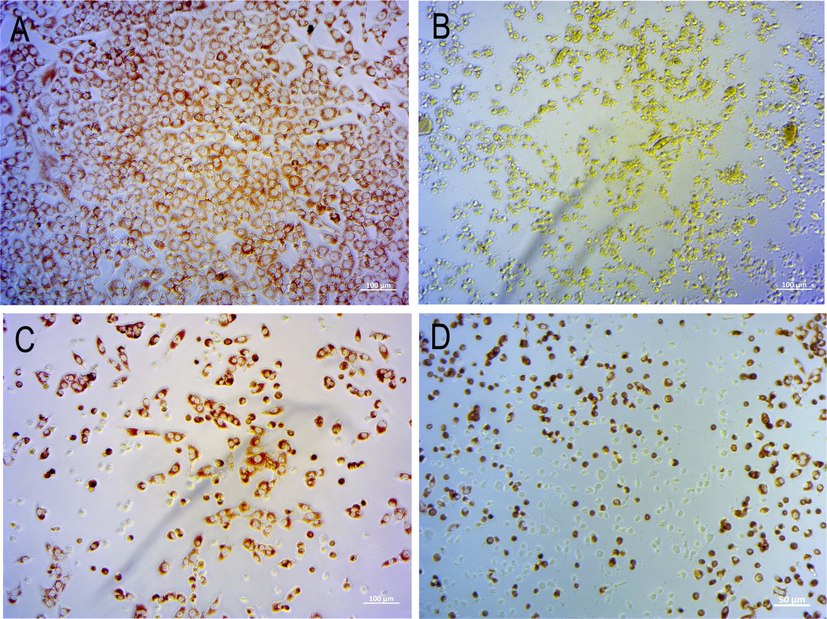
Microphotographs representative of NRU by cells after exposure to: (A) chlorpromazine (positive control) of non-irradiated cell culture; (B) chlorpromazine of irradiated cell culture; (C) NE3 in non-irradiated culture; (D) NE3 in irradiated culture; NE3 was non-phototoxic in both conditions.
6 Conclusion
The cosmetics industry is under continuous pressure to use finishing processes that are more environmentally friendly and to find new methods to make cosmetic products more competitive on the global market. The use of bioproducts produced by microorganisms is a sustainable and non-toxic bioprocess. In the present work, microbial carotenoids presented excellent properties for applications in photoprotection. Furthermore, these microbial carotenoids improved the stability and increased the sun protection factor in the photoprotective nanoemulsions that were successfully produced and presented nanometric size with a narrow size distribution, which also showed a pH compatible with the physiological pH of the human skin. Our results showed the formulations containing microbial carotenoids and buriti oil were more stable than the control formulation according to the preliminary stability study, and also exhibited higher SPF than the control formulation lacking these actives. In addition, nanoemulsions containing microbial carotenoids did not exhibit phototoxicity by the specific test accredited by the OECD. Thus, these nanoemulsions are promising photoprotective formulations and complementary studies of in vivo SPF, cytotoxicity in human cell cultures (fibroblasts and keratinocytes) and the possible anti-inflammatory potential of these compounds will be performed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Refining of buriti oil (Mauritia flexuosa) originated from the brazillian cerrado: physicochemical, thermal-oxidative and nutritional implications. J. Braz. Chem. Soc.. 2012;23:212-219.
- [CrossRef] [Google Scholar]
- Distribution of colored carotenoids between light-harvesting complexes in the process of recovering carotenoid biosynthesis in Ectothiorhodospira haloalkaliphila cells. J. Photochem. Photobiol. B. 2014;141:59-66.
- [CrossRef] [Google Scholar]
- The Revised Guidelines to the Practical Measurement of UVA:UVB Ratios According to The Boots Star Rating System. Notthingham, UK: The Boots Co. PLC; 2004.
- Butler, H., 2013. Poucher’s Perfumes, Cosmetics and Soaps. 10th. ed. Springer Science + Business Media Dordrecht, Netherlands. In: Part 3: Quality, Stability and Safety Assurance, Roberts, M. E. Efficacy testing of cosmetics, 555–601.
- Development and in vitro assessment of nanoemulsion for delivery of ketoconazole against Candida albicans. J. Nanosci. Nanotech.. 2017;17:4623-4630.
- [CrossRef] [Google Scholar]
- The 3T3 neutral red uptake phototoxicity test: practical experience and implications for phototoxicity testing - the report of an ECVAM-EFPIA workshop. Regul. Toxicol. Pharmacol.. 2012;63:480-488.
- [CrossRef] [Google Scholar]
- Development of a photoprotective and antioxidant nanoemulsion containing chitosan as an agent for improving skin retention. Eng. Life Sci.. 2015;15:593-604.
- [CrossRef] [Google Scholar]
- Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: Effect on skin permeation. Int. J. Pharm.. 2014;473:591-598.
- [CrossRef] [Google Scholar]
- COLIPA, 2009. The European cosmetic, toiletry and perfumery association. In vitro method for the determination of the UVA Protection Factor and “Critical Wavelength” Values of Sunscreen Products. https://doi.org/10.1067/mjd.2000.109291.
- Sunscreens products: What do they protect us from? Int. J. Pharmaceut.. 2011;415:181-184.
- [CrossRef] [Google Scholar]
- FDA, 2011. Sunscreen Drug Products for Over-the-Counter Human Use; Request for Data and Information Regarding Dosage Forms. Department of Health and Human Services 76, 35669-35672. https://www.federalregister.gov/ Docket No. FDA-1978-N-0018 formerly Docket No. 1978N-0038.
- Doxorubicin as an antioxidant: Maintenance of myocardial levels of lycopene under doxorubicin treatment. Free Radic. Biol. Med.. 2007;43:740-751.
- [CrossRef] [Google Scholar]
- Photostability and efficacy studies of topical formulations containing UV-filters combination and vitamins A, C and E. Int. J. Pharm.. 2007;343:181-189.
- [CrossRef] [Google Scholar]
- Complete genome sequence of carotenoid-producing Microbacterium sp. strain PAMC28756 isolated from an Antarctic lichen. J. Biotechnol.. 2016;226:18-19.
- [CrossRef] [Google Scholar]
- Penetration and storage of particles in human skin: Perspectives and safety aspects. Eur. J. Pharm. Biopharm.. 2011;77:465-468.
- [CrossRef] [Google Scholar]
- Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci.. 2006;28(5):359-370.
- [CrossRef] [Google Scholar]
- Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol.. 2015;72:310-322.
- [CrossRef] [Google Scholar]
- Levy, S.B., 2007. Sunscreens. 2nd. ed. Philadelphia: Saunders Elsevier. In: Wolverton, S. E., editor. Comprehensive dermatologic drug therapy, 703–718.
- Advanced carrier systems in cosmetics and cosmeceuticals: A review. J. Cosmet. Sci.. 2011;62:549-563.
- [Google Scholar]
- Malvern Instruments Ltda., 2004. Zetasizer nano series user manual Worcestershire.
- Evaluation of the antioxidant and phototoxic potentials of Bauhinia microstachya var. massambabensis Vaz Leaf Extracts. Lat. Am. J. Pharm.. 2012;31:200-206.
- [Google Scholar]
- In vitro and in vivo evaluation of efficacy and safety of photoprotective formulations containing antioxidant extracts. Braz. J. Pharmacog.. 2016;26:251-258.
- [CrossRef] [Google Scholar]
- From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol.. 2016;32:100-112.
- [CrossRef] [Google Scholar]
- Scavenging of benzylperoxyl radicals by carotenoids. Free Radic. Res.. 2002;36:211-216.
- [CrossRef] [Google Scholar]
- In vivo and in vitro evaluation of octylmethoxycinnamate liposomes. Int. J. Nanomed.. 2013;8:4689-4701.
- [CrossRef] [Google Scholar]
- OECD, 2004. Test No. 432: In Vitro 3T3 NRU Phototoxicity Test. Paris OECD Publishing.
- Structure and mechanism of iron translocation by a DPS protein from Microbacterium arborescens. J. Biol. Chem.. 2011;286:34872-34882.
- [Google Scholar]
- Low molecular weight antioxidants and their role in skin aging. Clin. Exp. Dermatol.. 2001;26:578-582.
- [CrossRef] [Google Scholar]
- Effects of solar radiation on the skin. J. Cosmet. Dermatol.. 2012;11 329–329
- [CrossRef] [Google Scholar]
- Evaluation of nanostructured lipid carriers (NLC) and nanoemulsions as carriers for UV-filters: Characterization, in vitro penetration and photostability studies. Eur. J. Pharm. Sci.. 2014;51:211-217.
- [CrossRef] [Google Scholar]
- Carotenoids from UV-resistant Antarctic Microbacterium sp. LEMMJ01. Sci. Rep.. 2019;9(9554):1-14.
- [CrossRef] [Google Scholar]
- Ricci-Junior, E., de Oliveira de Siqueira, L.B., Rodrigues, R.A.S., Sancenón, F., Martínez-Máñez, R., de Moraes, J.A., Santos-Oliveira, R., 2018. Nanocarriers as phototherapeutic drug delivery system: Appraisal of three different nanosystems in an in vivo and in vitro exploratory study. Photodiag. Photod. Ther. 21, 43–49. https://doi.org/10.1016/j.pdpdt.2017.11.003.
- Draft Genome Sequence of Microbacterium sp. LEMMJ01 sp. Isolated from Antarctic Ornithogenic Soil. Genome Announc.. 2017;5:672-717.
- [CrossRef] [Google Scholar]
- A proposed eye irritation testing strategy to reduce and replace in vivo studies using bottom-up and top-down approaches. Toxicol. In Vitro.. 2010;24:1-9.
- [CrossRef] [Google Scholar]
- Multicenter study on measurement of the natural pH of the skin surface. Int. J. Cosmet. Sci.. 2008;30:75-76.
- [CrossRef] [Google Scholar]
- Shaath, N.A., 2005. Sunscreens: Regulations and Commercial Development, 3rd ed. CRC Press, https://doi.org/10.1201/b14170.
- Clove oil nanoemulsion showed potent inhibitory effect against Candida spp. Nanotechnology. 2019;30(42):425101
- [CrossRef] [Google Scholar]
- Comprehensive overview on the micro and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr.. 2018;58:1-36.
- [CrossRef] [Google Scholar]
- The international EU/COLIPA in vitro phototoxicity validation study: Results of phase II (blind trial). Part 1: The 3T3 NRU phototoxicity test. Toxicol. Vitr.. 1998;12:305-327.
- [CrossRef] [Google Scholar]
- Carotenoids and protection against solar UV radiation. Skin Pharmacol. Appl. Skin Physiol.. 2002;15:291-296.
- [CrossRef] [Google Scholar]
- Nanocarriers for Delivery of antioxidants on the Skin. Cosmetics. 2015;2:342-354.
- [CrossRef] [Google Scholar]
- Broad range analysis of endocrine disruptors and pharmaceuticals using gas chromatography and liquid chromatography tandem mass spectrometry. Chemosphere. 2006;65(11):1990-1998.
- [CrossRef] [Google Scholar]
- Zanela da Silva Marques, T., Santos-Oliveira, R., Betzler de Oliveira de Siqueira, L., Cardoso, V., de Freitas, Z., Barros, R., Villa, A., Monteiro, M., Dos Santos, E. P., Ricci-Junior, E., 2018. Development and characterization of a nanoemulsion containing propranolol for topical delivery. Int. J. Nanomed., 13, 2827–2837. https://doi.org/10.2147/IJN.S164404.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.06.028.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







