Translate this page into:
Phthalazine-triones: Calix[4]arene-assisted synthesis using green solvents and their anticancer activities against human cancer cells
⁎Corresponding author. Tel.: +55 31 3409 6373; fax: +55 31 3409 5700. adefatima@qui.ufmg.br (Ângelo de Fátima)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Fourteen phthalazine-triones bearing different substituents at C-4 position were synthesized through multicomponent reactions (MCR) by using phthalhydrazide, dimedone and diferent aldehydes as starting materials, p-sulfonic acid calix[4]arene as catalyst and ethyl lactate as solvent under microwave irradiation. Compounds 7–16 were obtained in excellent to moderate yields (94–51%) in only 10 min of reaction using this methodology. The antiproliferative activity against cancer cells was disclosed, for the first time, for synthesized compounds. The capacity of all compounds to inhibit cancer cells growth was dependent on the histological origin of cells. Compound 20 was active against more than one strain.
Keywords
Catalysis
Green chemistry
Microwave assisted synthesis
Antiproliferative activity
p-Sulfonic acid calix[4]arene
1 Introduction
Phthalazine derivatives (Fig. 1) are nitrogen heterocycle compounds constituting a bridgehead hydrazine (Khurana and Magoo, 2009). This class of compounds has been shown to possess a range of biological and pharmacological properties such as anticonvulsant (Grasso et al., 2000), cardiotonic (Nomoto et al., 1990), vasorelaxant (Watanabe et al., 1998) and antiproliferative (Scott et al., 2007), as well as their unique luminescence properties (Wu et al., 2009).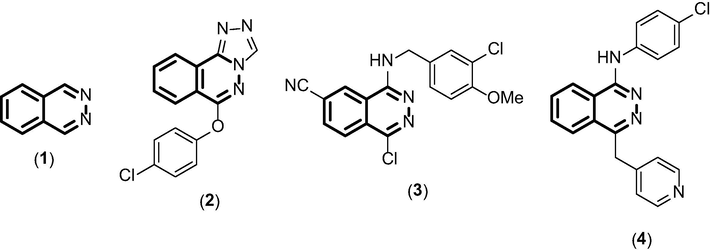
Phthalazine moiety (1) and chemical structures of bioactive phthalazine derivatives (2–4).
The phthalazine 2 (Fig. 1) was described by Grasso and coworkers (Grasso et al., 2000) as a potent anticonvulsant agent, while compound 1-chloro-4-(3-chloro-4-methoxybenzylamino)-6-phthalazine carbonitrile, 3, (Fig. 1) has vasorelaxant activity via inhibition of cyclic nucleotide phosphodiesterase 5 (PDE5). This compound was able to inhibit the activity of PDE5 at level of 50% when used at only 3.5 nM (Watanabe et al., 1998). Antiproliferative activities were also described for phthalazines, being the most notable example is vatalanib (4), an inhibitor of vascular growth factor, which is in clinical phase III for metastatic colorectal cancer (Fig. 1) (Scott et al., 2007). Despite the diverse biological profile exhibited by phthalazines derivatives, phthalazine-triones have their biological activities poorly explored. In the only reports, Berber et al., demonstrated that urea- and β-lactam-phthalazine-trione derivatives inhibit the human carbon anhydrase (Berber et al., 2013, 2015).
These compounds can be obtained using multicomponent reactions (MCR). This approach is based on three-component condensations with aldehydes, 5,5-dimethylcyclohexane-1,3-dione (dimedone) and 2,3-dihydro-1,4-phthalazinedione (phthalhydrazide) and employs different catalysts such as p-toluenesulfonic acid (PTSA) (Sayyafi et al., 2008), Ce(SO4)2·4H2O (Mosaddegh and Hassankhani, 2011), dodecylphosphonic acid (DPA) (Kidwai et al., 2012), camphorsulfonic acid (CSA) (Shukla et al., 2011), silica supported polyphosphoric acid (Shaterian et al., 2009) and N-halosulfonamides (Ghorbani-Vaghei et al., 2011). However, so far there are no reports of the use of calix[n]arenes as catalysts in the synthesis of phthalazine derivatives.
Calix[n]arenes are macrocyclic cavity-shaped molecules obtained from the ortho-condensation of para-substituted phenols and formaldehyde in a basic medium (de Fátima et al., 2009; Simoes et al., 2012). Over the last few decades, these supramolecules have received increasing attention, particularly because of their applications as molecular hosts and organocatalysts (Gutsche, 2008; Varejão et al., 2013). Among all of the calix[n]arenes known so far, p-sulfonic acid calix[4]arene (5) and p-sulfonic acid calix[6]arene (6) (Fig. 2) have been shown to be the most efficient catalysts for different multicomponent reactions (da Silva et al., 2011, 2015; Simões et al., 2013, 2014).![Chemical structures of p-sulfonic acid calix[4]arene (5) and calix[6]arene (6).](/content/184/2019/12/8/img/10.1016_j.arabjc.2016.04.007-fig3.png)
Chemical structures of p-sulfonic acid calix[4]arene (5) and calix[6]arene (6).
Solvents are responsible for a large share of the environmental impact of the production processes of the chemical industry and are directly related to factors such as cost, safety and performance (Capello et al., 2007). In this context, the search for “green solvents” aims to reduce the environmental impact resulting from the use of solvents in chemical production processes (Capello et al., 2007). These solvents have been characterized for their low toxicity, easy biodegradability under environmental conditions, high boiling point and easy recyclability after use (Capello et al., 2007). Ethyl lactate is a “green solvent” derived from processing raw biomass material. This compound is the ester of lactic acid, and some lactate esters are solvents commonly used in the paints and coatings industry and have numerous attractive advantages, including being 100% biodegradable, easy to recycle, non-corrosive, non-carcinogenic and non-ozone depleting (Pereira et al., 2011). Dimethyl and diethyl carbonate also have properties that can lead to them being considered “green solvents”, such as their very low solubility in water, high boiling point and low vapor pressure.
In this paper, we report a facile synthesis of 2H-indazolo[2, 1-b]phthalazine-trione derivatives by the microwave-assisted one-pot three-component condensation of aromatic or aliphatic aldehydes, 5,5-dimethylcyclohexane-1,3-dione and phthalhydrazide in the presence of a catalytic amount of p-sulfonic acid calix[4]arene using ethanol, ethyl lactate, dimethyl carbonate and diethyl carbonate as “green solvents”. This procedure is very interesting because it combines a MCR synthetic protocol using microwave irradiation in the presence of green solvents. We also report the biological evaluation of fourteen phthalazine-triones as inhibitors of cancer cells proliferation.
2 Materials and methods
All of the chemicals were obtained from commercially available sources and used without further purification. Melting points (uncorrected) were determined on a Mettler FP 80 HT apparatus. Infrared spectra were recorded on a Perkin Elmer Spectrum One spectrometer (KBr). 1H and 13C NMR spectra were recorded on a Bruker AVANCE DPX-200 spectrometer in CDCl3 or DMSO-d6. High resolution mass spectra were acquired on a Shimadzu LCMS-IT-TOF instrument. Single crystal X-ray diffraction measurements were carried out on an Oxford-Diffraction GEMINI-Ultra diffractometer (LabCri-UFMG) using graphite-Enhance Source Mo Kα radiation (λ = 0.71073 Å) at 150(2) K. The data collection, cell refinements, and data reduction were performed using the CrysAlisPro software package (Oxford Diffraction, 2010). An absorption correction based on a multi-scan method was applied (Oxford Diffraction, 2010). The crystal structure was solved by direct methods using SHELXS-97 (Sheldrick, 2008). A full-matrix least-squares refinement procedure on F2 with anisotropic thermal parameters was carried on using SHELXL (Sheldrick, 2008). Positional and anisotropic atomic displacement parameters were refined for all non-hydrogen atoms. Hydrogen atoms were placed geometrically, and their positional parameters were refined using a riding model. PLATON was used to prepare the molecular graphics (Spek, 2009).
2.1 General procedure for the synthesis of phthalazine-triones 7–20
2,3-Dihydrophthalazine-1,4-dione (1.0 mmol), 5,5-dimethylcyclohexane-1,3-dione (1.2 mmol), aldehyde (1.5 mmol) and p-sulfonic acid calix[4]arene (1.5 mol%) were dissolved in 1 mL of ethyl lactate. The mixture was irradiated in a DiscoverCem® reactor for 10 min at 130 °C. The reaction was cooled to room temperature and water was added. The mixture was placed in a freezer at −20 °C to form the precipitate. The solid was isolated by filtration and purified by chromatography on a silica gel column using hexane/ethyl acetate as the eluent to obtain the phthalazine-trione of interest.
2.1.1 3,3-Dimethyl-13-phenyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (7) (Sayyafi et al., 2008)
Yellow solid. mp: 189–190 °C. IR (KBr) (νmax/cm−1): 3086, 3026, 2960, 2926, 1666, 1628, 1600, 1468, 1420, 1358, 1314, 1268, 1168, 1142, 1100, 1082, 798, 702, 560. 1H NMR (200 MHz, CDCl3): δ 1.11 (bs, 6H, 2x CH3), 2.23 (bs, 2H, CH2), 3.14 (d, 1H, J = 18.0 Hz, CH2), 3.33 (d, 1H, J = 18.0 Hz, CH2), 6.34 (s, 1H, CH), 7.09–7.46 (m, 5H, ArH), 7.66–7.85 (m, 2H, ArH), 8.08–8.36 (m, 2H, ArH); 13C NMR (50 MHz, CDCl3): δ 28.3 (CH3), 28.5 (CH3), 34.5 (C), 37.8 (CH2), 50.7 (CH2), 64.8 (CH), 118.4 (C), 127.0 (CH), 127.5 (CH), 127.8 (CH), 128.5 (CH), 128.7 (C), 128.8 (C), 133.4 (CH), 134.4 (CH), 136.2 (C), 150.7 (C), 154.1 (C), 155.8 (C), 192.0 (C). HRMS (ESI, IT-TOF) calculated for C23H21N2O3+: 373.1547; found: 373.1596.
2.1.2 13-(Benzo[d][1,3]dioxol-5-yl)-3,3-dimethyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (8) (Veeranarayana Reddy et al., 2012)
Yellow solid. mp: 256–257 °C. IR (KBr) (νmax/cm−1): 2962, 2898, 1662, 1624, 1490, 1364, 1314, 1268, 1244, 1100, 1034, 924, 788, 700. 1H NMR (200 MHz, CDCl3): δ 1.21 (s, 3H, CH3), 1.22 (s, 3H, CH3), 2.34 (bs, 2H, CH2), 3.22 (d, 1H, J = 18.0 Hz, CH2), 3.41 (d, 1H, J = 18.0 Hz, CH2), 5.90 (s, 2H, CH2), 6.36 (s, 1H, CH), 6.75 (d, 1H, J = 8.0 Hz, ArH), 6.84 (s, 1H, ArH), 6.94 (d, 1H, J = 8.0 Hz, ArH), 7.76–7.95 (m, 2H, ArH), 8.19–8.42 (m, 2H, ArH); 13C NMR (50 MHz, CDCl3): δ 28.5 (CH3), 28.6 (CH3), 34.6 (C), 38.0 (CH2), 50.9 (CH2), 64.7 (CH), 101.2 (CH2), 107.4 (CH), 108.4 (CH), 118.4 (C), 121.2 (CH), 127.6 (CH), 127.9 (CH), 128.9 (C), 129.1 (C), 130.1 (C), 133.5 (CH), 134.5 (CH), 147.9 (C), 150.8 (C), 154.3 (C), 156.0 (C), 192.1 (C). HRMS (ESI, IT-TOF) calculated for C24H21N2O5+: 417.1445; found: 417.1442.
2.1.3 13-(4-Chlorophenyl)-3,3-dimethyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (9) (Sayyafi et al., 2008)
Yellow solid. mp: 227–229 °C. IR (KBr) (νmax/cm−1): 3166, 3032, 2958, 2932, 2894, 1654, 1624, 1492, 1468, 1362, 1312, 1268, 1148, 1106, 1090, 1014, 840, 794, 698, 530. 1H NMR (200 MHz, CDCl3): δ 1.20 (bs, 6H, 2x CH3), 2.33 (bs, 2H, CH2), 3.23 (d, 1H, J = 19.0 Hz, CH2), 3.41 (d, 1H, J = 19.0 Hz, CH2), 6.41 (s, 1H, CH), 7.29 (d, 2H, J = 8.5 Hz, ArH), 7.37 (d, 2H, J = 8.5 Hz, ArH), 7.78–7.90 (m, 2H, ArH), 8.18–8.41 (m, 2H, ArH); 13C NMR (50 MHz, CDCl3): δ 28.3 (CH3), 28.6 (CH3), 34.6 (C), 37.9 (CH2), 50.7 (CH2), 64.2 (CH), 117.9 (C), 127.6 (CH), 127.9 (CH), 128.4 (CH), 128.7 (C), 128.8 (C, CH), 133.6 (CH), 134.4 (C), 134.5 (CH), 134.8 (C), 150.1 (C), 154.3 (C), 155.9 (C), 192.0 (C). HRMS (ESI, IT-TOF) calculated for C23H20ClN2O3+: 407.1157; found: 407.1144.
2.1.4 13-(4-Fluorophenyl)-3,3-dimethyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (10) (Sayyafi et al., 2008)
Yellow solid. mp: 196–198 °C. IR (KBr) (νmax/cm−1): 3070, 2960, 2926, 2878, 1666, 1628, 1602, 1508, 1468, 1420, 1360, 1314, 1268, 1220, 1144, 1098, 1080, 1028, 850, 798, 702, 528. 1H NMR (200 MHz, CDCl3): δ 1.21 (bs, 6H, 2x CH3), 2.34 (bs, 2H, CH2), 3.23 (d, 1H, J = 19.2 Hz, CH2), 3.42 (d, 1H, J = 19.2 Hz, CH2), 6.43 (s, 1H, CH), 6.92–7.11 (m, 2H, ArH), 7.32–7.49 (m, 2H, ArH), 7.78–7.93 (m, 2H, ArH), 8.20–8.42 (m, 2H, ArH); 13C NMR (50 MHz, CDCl3): δ 28.4 (CH3), 28.6 (CH3), 34.6 (C), 38.0 (CH2), 50.9 (CH2), 64.2 (CH), 115.7 (d, JC–F = 21.8 Hz, CH), 118.2 (C),127.7 (CH), 128.0 (CH), 128.9 (C), 129.0 (d, JC–F = 8.6 Hz, CH), 132.2 (C), 133.6 (CH), 134.6 (CH), 151.0 (C), 154.3 (C), 156.0 (C), 192.2 (C). HRMS (ESI, IT-TOF) calculated for C23H20FN2O3+: 391.1452; found: 391.1466.
Crystals suitable for single-crystal X-ray diffractometry were obtained from slow evaporation of a saturated methanolic solution of the product.
2.1.5 13-(4-Nitrophenyl)-3,3-dimethyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (11) (Sayyafi et al., 2008)
Yellow solid. mp: 205–206 °C. IR (KBr) (νmax/cm−1): 3076, 2970, 2956, 1694, 1660, 1618, 1522, 1470, 1366, 1312, 1276, 1256, 1146, 1018, 856, 792, 696, 578. 1H NMR (200 MHz, CDCl3): δ 1.20 (s, 3H, CH3), 1.22 (s, 3H, CH3), 2.34 (bs, 2H, CH2), 3.26 (d, 1H, J = 18.8 Hz, CH2), 3.43 (d, 1H, J = 18.8 Hz, CH2), 6.51 (s, 1H, CH), 7.61 (d, 2H, J = 8.0 Hz, ArH), 7.82–8.00 (m, 2H, ArH), 8.12–8.50 (m, 4H, ArH); 13C NMR (50 MHz, CDCl3): δ 28.3 (CH3), 28.6 (CH3), 34.7 (C), 38.0 (CH2), 50.7 (CH2), 64.1 (CH), 117.2 (C), 124.0 (CH), 127.7 (CH), 128.0 (CH), 128.2 (CH), 128.5 (C), 128.9 (C), 133.9 (CH), 134.8 (CH), 143.4 (C), 147.8 (C), 151.7 (C), 154.5 (C), 155.9 (C), 192.1 (C). HRMS (ESI, IT-TOF) calculated for C23H20N3O5+: 418.1397; found: 418.1388.
2.1.6 13-(3-Hydroxyphenyl)-3,3-dimethyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (12) (Ghorbani-Vaghei et al., 2011)
White solid. mp: 260–261 °C. IR (KBr) (νmax/cm−1): 3356, 3116, 2952, 2872, 1662, 1630, 1592, 1470, 1432, 1360, 1314, 1288, 1266, 1234, 1144, 1102, 1084, 784, 700, 686, 532. 1H NMR (200 MHz, DMSO-d6): δ 1.11 (s, 3H, CH3), 1.12 (s, 3H, CH3), 2.27 (bs, 2H, CH2), 3.16 (dd, 1H, J = 18.8 Hz, J = 2.1 Hz, CH2), 3,27–3.37 (m, 1H, CH2), 6.18 (s, 1H, CH), 6.62–6.68 (m, 1H, ArH), 6.79–6.85 (m, 2H, ArH), 7.08 (t, 1H, J = 8.1 Hz, ArH), 7.93–8.01 (m, 2H, ArH), 8.08–8.15 (m, 1H, ArH), 8.23–8.30 (m, 1H, ArH), 9.39 (s, 1H, OH); 13C NMR (75 MHz, DMSO-d6): δ 27.8 (CH3), 28.0 (CH3), 34.3 (C), 37.2 (CH2), 50.3 (CH2), 64.2 (CH), 114.4 (CH), 114.9 (CH), 117.6 (C), 117.8 (CH), 126.8 (CH), 127.6 (CH), 128.7 (C), 128.9 (C), 129.1 (CH), 133.7 (CH), 134.6 (CH),138.8 (C), 151.0 (C), 153.7 (C), 155.3 (C),157.2 (C), 191.8 (C). HRMS (ESI, IT-TOF) calculated for C23H21N2O4+: 389.1496; found: 389.1472.
2.1.7 13-(4-Cyanophenyl)-3,3-dimethyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (13) (Hasaninejed et al., 2012)
Yellow solid. mp: 213–215 °C. IR (KBr) (νmax/cm−1): 3130, 3040, 2958, 2932, 2230, 1654, 1620, 1502, 1468, 1390, 1362, 1312, 1270, 1150, 1106, 1026, 844, 792, 700, 558, 498. 1H NMR (200 MHz, CDCl3): δ 1.19 (s, 3H, CH3), 1.22 (s, 3H, CH3), 2.34 (bs, 2H, CH2), 3.25 (d, 1H, J = 19.2 Hz, CH2), 3.41 (d, 1H, J = 19.2 Hz, CH2), 6.45 (s, 1H, CH), 7.55 (d, 2H, J = 8.1 Hz, ArH), 7.63 (d, 2H, J = 8.1 Hz, ArH), 7.81–7.96 (m, 2H, ArH), 8.19–8.43 (m, 2H, ArH); 13C NMR (50 MHz, CDCl3): δ 28.3 (CH3), 28.6 (CH3), 34.6 (C), 37.9 (CH2), 50.7 (CH2), 64.3 (CH), 112.3 (C), 117.2 (C), 118.3 (C), 127.6 (CH), 127.8 (CH), 128.1 (CH), 128.5 (C), 128.8 (C), 132.5 (CH), 133.8 (CH), 134.7 (CH), 141.5 (C), 151.5 (C), 154.4 (C), 155.8 (C), 192.0 (C). HRMS (ESI, IT-TOF) calculated for C24H20N3O3+: 398.1499; found: 398.1471.
2.1.8 13-(3-Methoxyphenyl)-3,3-dimethyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine -1,6,11-trione (14) (Hasaninejed et al., 2012)
Yellow solid. mp: 221–222 °C. IR (KBr) (νmax/cm−1): 3030, 3022, 2971, 2937, 1654, 1600, 1502, 1372, 1221, 1180, 1031, 744, 535, 496. 1H NMR (200 MHz, CDCl3): δ 1.21 (bs, 6H, 2x CH3), 2.33 (bs, 2H, CH2), 3,22 (d, 1H, J = 19.3 Hz, CH2), 3,41 (d, 1H, J = 19.3 Hz, CH2), 3.78 (s, 3H, OCH3), 6.41 (s, 1H, CH), 6.82 (d, 1H, J = 8.0 Hz, ArH), 6.95 (s, 1H, ArH), 7.00 (d, 1H, J = 8.0 Hz, ArH), 7.25 (t, 1H, J = 8.0 Hz, ArH), 7.77–7.94 (m, 2H, ArH), 8.20–8.43 (m, 2H, ArH); 13C NMR (50 MHz, CDCl3): δ 28.4 (CH3), 28.6 (CH3), 34.6 (C), 38.0 (CH2), 50.9 (CH2), 55.2 (CH3), 64.7 (CH), 113.1 (CH), 113.8 (CH), 118.5 (C), 119.4 (CH), 127.7 (CH), 127.9 (CH), 128.9 (C), 129.0 (C), 129.7 (CH), 133.5 (CH), 134.5 (CH), 137.9 (C), 150.8 (C), 154.2 (C), 156.0 (C), 159.7 (C), 192.1 (C). HRMS (ESI, IT-TOF) calculated for C24H23N2O4+: 403.1652; found: 403.1626.
2.1.9 13-(4-Hydroxy-3,5-dimethoxyphenyl)-3,3-dimethyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (15)
Yellow solid. mp: 210–212 °C. IR (KBr) (νmax/cm−1): 3510, 3168, 3018, 2962, 1656, 1624, 1518, 1492, 1466, 1432, 1362, 1314, 1266, 1218, 1146, 1112, 1082, 826, 792, 700, 628. 1H NMR (200 MHz, CDCl3): δ 1.24 (bs, 6H, 2x CH3), 2.36 (bs, 2H, CH2), 3.21 (d, 1H, J = 19.1 Hz, CH2), 3.47 (d, 1H, J = 19.1 Hz, CH2), 3.85 (bs, 6H, 2x OCH3), 5.68 (bs, 1H, OH), 6.38 (s, 1H, CH), 6.65 (s, 2H, ArH), 7.79–7.95 (m, 2H, ArH), 8.21–8.43 (m, 2H, ArH); 13C NMR (50 MHz, CDCl3): δ 28.0 (CH3), 28.9 (CH3), 34.5 (C), 38.0 (CH2), 50.8 (CH2), 56.3 (2x CH3), 65.0 (CH), 104.4 (CH), 118.3 (C), 127.2 (C), 127.6 (CH), 127.9 (CH), 128.8 (C), 129.0 (C), 133.5 (CH), 134.5 (CH), 136.2 (C), 147.0 (C), 150.7 (C), 154.4 (C), 156.1 (C), 192.2 (C). HRMS (ESI, IT-TOF) calculated for C25H25N2O6+: 449.1707; found: 449.1691.
2.1.10 3,3-Dimethyl-13-(4-(trifluoromethyl)phenyl)-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (16) (Wang et al., 2010)
Yellow solid. mp: 248–250 °C. IR (KBr) (νmax/cm−1): 2960, 2934, 2874, 1658, 1624, 1468, 1424, 1360, 1310, 1266, 1162, 1130, 1066, 854, 702. 1H NMR (200 MHz, CDCl3): δ 1.20 (s, 3H, CH3), 1.22 (s, 3H, CH3), 2.34 (bs, 2H, CH2), 3.25 (dd, 1H, J = 19.2 Hz, J = 2.2 Hz, CH2), 3.41 (dd, 1H, J = 19.2 Hz, J = 1.0 Hz, CH2), 6.49 (s, 1H, CH), 7.54 (d, 2H, J = 8.4 Hz, ArH), 7.61 (d, 2H, J = 8.4 Hz, ArH), 7.84–7.93 (m, 2H, ArH), 8.22–8.42 (m, 2H, ArH); 13C NMR (50 MHz, CDCl3): δ 28.4 (CH3), 28.7 (CH3), 34.7 (C), 38.0 (CH2), 50.8 (CH2), 64.4 (CH), 117.8 (C), 125.8 (q, J = 3.9 Hz, CH), 127.4 (CH), 127.8 (CH), 128.1 (CH), 128.8 (C), 128.9 (C), 130.7 (q, J = 32.5 Hz, C), 133.8 (CH), 134.7 (CH), 140.3 (C), 151.3 (C), 154.4 (C), 156.0 (C), 192.1 (C). HRMS (ESI, IT-TOF) calculated for C24H20F3N2O3+: 441.1421; found: 441.1441.
2.1.11 3,3-Dimethyl-13-(4-(methylthio)phenyl)-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (17) (Ghorbani-Vaghei et al., 2011)
Yellow solid. mp: 216–218 °C. IR (KBr) (νmax/cm−1): 3024, 2960, 2946, 2924, 1656, 1630, 1466, 1360, 1312, 1268, 1148, 1104, 1092, 704. 1H NMR (200 MHz, CDCl3): δ 1.20 (bs, 6H, 2x CH3), 2.33 (bs, 2H, CH2), 2.42 (s, 3H, CH3), 3.22 (dd, 1H, J = 19.1 Hz, J = 1.8 Hz, CH2), 3.42 (d, 1H, J = 19.1 Hz, CH2), 6.40 (s, 1H, CH), 7.19 (d, 2H, J = 8.3 Hz, ArH), 7.33 (d, 2H, J = 8.3 Hz, ArH), 7.77–7.89 (m, 2H, ArH), 8.19–8.40 (m, 2H, ArH); 13C NMR (50 MHz, CDCl3): δ 15.6 (CH3), 28.4 (CH3), 28.6 (CH3), 34.5 (C), 38.1 (CH2), 51.0 (CH2), 64.5 (CH), 118.4 (C), 126.1 (CH), 127.5 (CH), 127.6 (CH), 127.9 (CH), 129.0 (C), 129.1 (C), 133.2 (C), 133.4 (CH), 134.4 (CH), 139.2 (C), 150.8 (C), 154.3 (C), 156.0 (C), 191.9 (C). HRMS (ESI, IT-TOF) calculated for C24H23N2O3S+: 419.1424; found: 419.1424.
2.1.12 3,3-Dimethyl-13-propyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (18) (Ghorbani-Vaghei et al., 2011)
Yellow solid. mp: 186–187 °C. IR (KBr) (νmax/cm−1): 3158, 3016, 1646, 1622, 1515, 1490, 1456, 1422, 1382, 1310, 1260, 1141, 1110, 1081, 824, 790, 666. 1H NMR (200 MHz, CDCl3): δ 0.87 (t, 3H, J = 7.2 Hz, CH3), 1.06–1.30 (m, 8H, CH2, 2x CH3), 1.90–2.53 (m, 4H, 2x CH2), 3.13 (d, 1H, J = 19.3 Hz, CH2), 3.34 (d, 1H, J = 19.3 Hz, CH2), 5.70 (s, 1H, CH), 7.79–7.97 (m, 2H, ArH), 8.28–8.42 (m, 2H, ArH); 13C NMR (50 MHz, CDCl3): δ 13.7 (CH3), 16.7 (CH2), 28.5 (CH3), 28.7 (CH3), 31.5 (CH2), 34.5 (C), 38.0 (CH2), 51.0 (CH2), 62.8 (CH), 117.3 (C), 127.5 (CH), 127.9 (CH), 128.9 (C), 129.0 (C), 133.4 (CH), 134.5 (CH), 151.6 (C), 154.7 (C), 156.1 (C), 193.1 (C). HRMS (ESI, IT-TOF) calculated for C20H23N2O3+: 339.1703; found: 339.1691.
2.1.13 13-Butyl-3,3-dimethyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (19)
Yellow solid. mp: 146–148 °C. IR (KBr) (νmax/cm−1): 3078, 2962, 2928, 2856, 1654, 1626, 1468, 1434, 1366, 1294, 1274, 1152, 1086, 788, 696. 1H NMR (200 MHz, CDCl3): δ 0.81 (t, 3H, J = 7.1 Hz, CH3), 1.01–1.37 (m, 10H, 2x CH2, 2x CH3), 2.01–2.55 (m, 4H, 2x CH2), 3.13 (dd, 1H, J = 19.2 Hz, J = 2.3 Hz, CH2), 3.35 (d, 1H, J = 19.2 Hz, CH2), 5.70 (s, 1H, CH), 7.76–7.96 (m, 2H, ArH), 8.23–8.43 (m, 2H, ArH); 13C NMR (50 MHz, CDCl3): δ 13.8 (CH3), 22.3 (CH2), 25.5 (CH2), 28.4 (CH3), 28.7 (CH3), 29.3 (CH2), 34.4 (C), 38.1 (CH2), 51.1 (CH2), 62.9 (CH), 117.4 (C), 127.5 (CH), 127.8 (CH), 129.0 (C), 129.1 (C), 133.3 (CH), 134.3 (CH), 151.5 (C), 154.7 (C), 156.1 (C), 192.7 (C). HRMS (ESI, IT-TOF) calculated for C21H25N2O3+: 353.1860; found: 353.1797.
2.1.14 3,3-Dimethyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (20)
Yellow solid. mp: 150–152 °C. IR (KBr) (νmax/cm−1): 3168, 3022, 2958, 2892, 1656, 1494, 1360, 1270, 1082, 848, 792, 700. 1H NMR (200 MHz, CDCl3): δ 1.18 (br, 6H, 2x CH3), 2.37 (bs, 2H, CH2), 3.20 (br, 2H, CH2), 4.93 (bs, 2H, CH2), 7.73–7.96 (m, 2H, ArH), 8.22–8.39 (m, 2H, ArH); 13C NMR (50 MHz, CDCl3): δ 28.5 (2x CH3), 34.6 (C), 38.0 (CH2), 49.5 (CH2), 50.7 (CH2), 114.9 (C), 127.4 (CH), 127.9 (CH), 128.6 (C), 129.0 (C), 133.4 (CH), 134.3 (CH), 152.3 (C), 154.5 (C), 155.9 (C), 192.7 (C). HRMS (ESI, IT-TOF) calculated for C17H17N2O3+: 297.1234; found: 297.1506.
2.2 Antiproliferative assay
Human tumor cell lines U251 (glioma), MCF7 (breast) NCI-ADR/RES (multiple drug-resistant ovarian), 786-0 (renal), NCI-H460 (lung, non-small cells), PC-3 (prostate), OVCAR-03 (ovarian) HT-29 (colon) and K562 (leukemia) were kindly provided by the Frederick Cancer Research & Development Center, National Cancer Institute (Frederick, MA, USA). Stock cultures were grown in RPMI 1640 (GIBCO BRL, Life Technologies) supplemented with 5% of fetal bovine serum and penicillin (final concentration of 1 mg/mL) and streptomycin (final concentration of 200 U/mL). Cells in 96-well plates (100 μL cells/well) were exposed to phthalazine-triones (0.25–250 μg/mL) for 48 h at 37 °C and 5% of CO2. Afterward cells were fixed with 50% trichloroacetic acid, submitted to sulforhodamine B assay for cell proliferation quantification at 540 nm (Monks et al., 1991). The concentration of compound that inhibits cell growth by 50% (GI50) was determined through non-linear regression analysis using software ORIGIN 7.5 (OriginLab Corporation). Doxorubicin was used as a reference drug.
3 Results and discussion
3.1 Synthesis of phthalazine-triones
Sulfonic acid calix[n]arenes have proven to be efficient catalysts in different types of organic reactions (Fernandes et al., 2012; Natalino et al., 2014; Liu et al., 2008; Shimizu et al., 2006). Recently, we demonstrated that these macrocycles are effective in promoting synthesis of xanthenones by multicomponent reactions (da Silva et al., 2015). Thus, in the present study, we evaluated the efficiency of p-sulfonic acid calix[4]arene 5 in the synthesis of phthalazine-triones.
A preliminary investigation aiming to optimize the reaction conditions was performed. For a model reaction, benzaldehyde, 5,5-dimethylcyclohexane-1,3-dione and phthalhydrazide were employed (Table 1). The reactions were performed in a DiscoverCem® reactor for a period of 10 min using ethanol as the solvent. Initially, we evaluated the catalytic activity of p-sulfonic acid calix[4]arene (5) in promoting the reaction. This catalyst was tested in ratios ranging from 2.5 to 0.5 mol% (Table 1, entries 2–6). The best yield was obtained when 5 was used at a concentration of 1.5 mol% (Table 1, entry 4). Interestingly, the yields decreased with an increase in the molar ratio of the catalyst (Table 1, entries 2 and 3). The use of p-sulfonic acid calix[6]arene (6) as the catalyst furnished the desired products in low yields (<40%) (data not shown). To explore the role of the spatial arrangement imposed by the calix[4]arene 5 on the efficiency of the catalysis process, we used p-hydroxybenzenesulfonic acid (PHSA) as a catalyst. Because each molecule of p-sulfonic acid calix[4]arene corresponds to four units of PHSA, this catalyst was used at a concentration of 6.0 mol% (Table 1, entry 7). At this concentration, PHSA led to the formation of phthalazine-trione 7 in only a 12% yield. Even at a concentration of 9.0 mol%, PHSA was still less effective than p-sulfonic acid calix[4]arene (Table 1, entry 8). These results suggest that the spatial organization of the macrocycle is important for the catalytic activity. No product formation was observed in the reaction carried out without any catalyst under the tested experimental conditions (Table 1, entry 1).
Entry
Catalyst (mol%)
Solvent
Temperature (°C)
Time (min)
Yield (%)b
1
–
Ethanol
Reflux
10
0
2
5 (2.5)
Ethanol
Reflux
10
41
3
5 (2.0)
Ethanol
Reflux
10
22
4
5 (1.5)
Ethanol
Reflux
10
75
5
5 (1.0)
Ethanol
Reflux
10
45
6
5 (0.5)
Ethanol
Reflux
10
39
7
PHSAc (6.0)
Ethanol
Reflux
10
12
8
PHSA (9.0)
Ethanol
Reflux
10
60
9
5 (1.5)
Ethanol
Reflux
5
35
10
5 (1.5)
Dimethyl carbonate
Reflux
10
5
11
5 (1.5)
Diethyl carbonate
Reflux
10
6
12
5 (1.5)
Ethyl lactate
130
10
81
Once the optimal concentration of p-sulfonic acid calix[4]arene was obtained, we evaluated the effect of reducing the reaction time. Thus, a new test reaction was carried out for a period of 5 min. However, the reduction in the reaction time led to a significant decrease in the yield (Table 1, entry 9).
The promising results obtained with calix[4]arene 5 prompted us to further investigate the effect of solvents in the synthesis of phthalazine-trione 7 catalyzed by this macrocyclic compound. Dimethyl carbonate, diethyl carbonate, and ethyl lactate were evaluated. Dimethyl and diethyl carbonate furnished 7 in low yields (5% and 6%, respectively) (Table 1, entries 10 and 11). The best yield, 81%, was achieved using ethyl lactate as the solvent (Table 1, entry 12). Reactions carried out without solvents furnished phthalazine-trione 7 in yields lower than 40% (data not shown).
After establishing the best solvent, the scope of this protocol was further investigated. Aliphatic and aromatic aldehydes bearing electron-donating or electron-withdrawing groups were employed in the synthesis of a series of phthalazine-trione derivatives, and the expected products were obtained, mainly, in good to moderate yields (Table 2).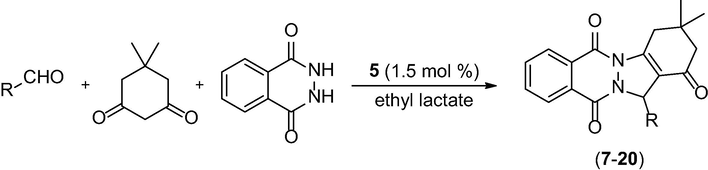
Entry
R
Compound
Yield (%)b
1
Phenyl
7
81
2
3,4-OCH2O-phenyl
8
51
3
4-Cl-phenyl
9
85
4
4-F-phenyl
10
83
5
4-NO2-phenyl
11
83
6
3-OH-phenyl
12
67
7
4-CN-phenyl
13
87
8
3-OCH3-phenyl
14
75
9
3,5-OCH3, 4-OH-phenyl
15
61
10
4-CF3-phenyl
16
94
11b
4-SCH3-phenyl
17
31
12b
n-propyl
18
30
13b
n-butyl
19
35
14b
H
20
11
In general, aromatic aldehydes led to good yields, except for piperonal and 4-(methylthio)benzaldehyde (Table 2, entries 2 and 11, respectively). Aldehydes with electron-withdrawing groups led to higher yields (83–94%) than do those with electron-donating groups. However, non-aromatic aldehydes were less reactive than aromatic aldehydes and afforded only low yields (Table 2, entries 12–14).
Once synthesized, the structural features of the compounds were determined from the corresponding IR, NMR, and ESI-HRMS data. Crystals of 10 were formed, and its crystal structure was determined using single-crystal X-ray diffractometry. The atom arrangements and atom numbering scheme for 10 are shown in Fig. 3 while the crystal data and refinement results are listed in Table 3.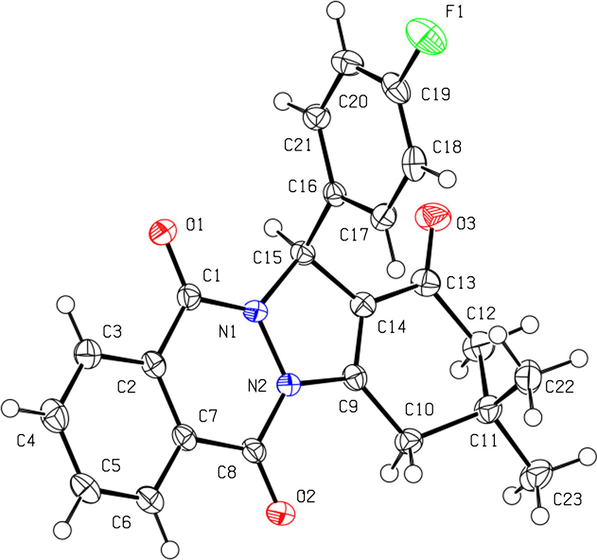
Molecular plot of 10 showing the labeling scheme of the non-H atoms and their displacement ellipsoids at a 50% probability level. The hydrogen atoms are shown as circles of arbitrary radii.
CCDC number
1,021,277
Empirical formula
C23H19FN2O3
Molecular weight (g mol−1)
390.40
Crystal system
Monoclinic
Space group
P21/n
Unit cell dimensions
a (Å)
8.5083(2)
b (Å)
20.3729(4)
c (Å)
11.3768(3)
α = γ (°)
90
β (°)
111.220(3)
V (Å3)
1838.33(7)
Z
4
Density calculated (g cm−3)
1.411
Absorption coefficient (mm−1)
0.101
F(0 0 0)
816
Crystal size (mm3)
0.64 × 0.59 × 0.25
θ range for data collection (°)
2.00–26.37
Index ranges
−10 ⩽ h ⩽ 10
−25 ⩽ k ⩽ 25
−14 ⩽ l ⩽ 14
Reflections collected
36,936
Independent reflections
3762 [R(int) = 0.0351]
Completeness to θ = 26.37° (%)
100.0
Max. and min. transmission
1.00000 and 0.83326
Data/restraints/parameters
3762/0/264
Goodness-of-fit on F2
1.059
Final R indices [I > 2σ(I)]
R1 = 0.0362, wR2 = 0.0902
R indices (all data)
R1 = 0.0409, wR2 = 0.0938
Largest diff. peak and hole (eÅ−3)
0.235 and −0.314
Phthalazine-trione 10 crystallized in the monoclinic space group P21/n with one independent molecule in the asymmetric unit. Although the molecule shown in Fig. 3 has a chiral center located on atom C15, the compound crystallized as a racemate because it crystallizes in a centrosymmetric space group. The geometrical parameters for the indazolophthalazine-1,6,11-trione skeleton of 10 are very similar to those reported for 13-(4-bromophenyl)-3,3-dimethyl-2,3,4,13-tetrahydro-1H-indazolo[1, 2-b]phthalazine-1,6,11-trione (Sayyafi et al., 2008).
3.2 Antiproliferative activities
The effect of synthesized phthalazine-triones (0.25–250 μg/mL) on glioma (U251), breast (MCF-7), adriamycin-resistant ovarian cancer (NCI-ADR/RES), kidney cancer (786-0), lung non-small cancer (NCI-H460), prostate cancer (PC-3), ovarian cancer (OVCAR-03), colon cancer (HT-29) and leukemia (K562) cells was also studied. Cell proliferation was determined by the sulforhodamine B method using doxorubicin (Dox) as a positive control. The concentration of phthalazine-triones that elicited the inhibition of cell growth by 50% (GI50) is summarized in Table 4. The selectivity index (SI), herein is defined as the ratio of the GI50 of pure compound in HaCaT cell line to the GI50 of the same pure compound in a cancer cell line, for the most promising compounds and doxorubicin.
Compound
Cell Line
U251
MCF7
NCI-ADR/RES
786-0
NCI-H460
PC-3
OVCAR-3
HT-29
K-562
HaCaT
7
115.1
>250
>250
131.0
>250
17.5
136.6
>250
>250
16.8
8
>250
>250
>250
140.9
>250
>250
>250
>250
>250
>250
9
>250
>250
>250
>250
>250
204.7
>250
>250
>250
>250
10
90.3
243.5
41.5
74.0
83.7
29.2
26.8
>250
175.1
201.4
11
>250
>250
>250
>250
>250
>250
>250
>250
>250
>250
12
27.0
21.3
9.1
39.6
40.5
23.8
15.6
70.7
54.1
25.4
13
>250
225.4
129.8
247.1
>250
>250
51.0
>250
>250
152.1
14
139.8
101.4
>250
79.4
>250
52.2
95.2
>250
>250
>250
15
147.5
>250
210.0
128.8
>250
203.7
72.4
>250
>250
>250
16
>250
>250
>250
>250
>250
>250
93.5
>250
7.4
>250
17
127.8
>250
>250
58.9
>250
>250
119.9
>250
28.6
>250
18
48.8
132.9
10.5
42.1
122.2
26.2
23.5
60.5
41.4
43.2
19
>250
>250
51.7
>250
>250
138.8
>250
>250
2.6
>250
20
171.4
124.0
>250
>250
>250
5.3
186.2
>250
9.4
>250
Doxb
0.03
0.06
0.05
0.03
0.01
0.03
0.24
0.08
0.03
0.2
Compounds 12 and 18 were the most active compounds against NCI-ADR/RES cell line (Table 4) with GI50 values lower than 15 μg/mL. Compound 20 had the most promising activity against PC-3 cancer cells, with GI50 value of 5.3 μg/mL (Table 4) and SI of over 47, 15-fold more than required to define a highly selective compound (Prayong et al., 2008). For K562 cells, compounds 16, 19 and 20 were active with GI50 values of 7.4, 2.6 and 9.4 μg/mL (Table 4) and SI greater than 33.8, 96.2 and 26.6, respectively. It is noteworthy that doxorubicin’s SI for PC-3 and K562 cell lineages was equal to 0.2, demonstrating no differentiation of normal cells and cancerous cells.
Among the cell lines tested K562 was the most sensitive, and three phthalazine-triones were active against these cells. For U251, MCF7, 786-0, NCI-H460, OVCAR-3 and HT29 cell lines, none of the compounds tested proved to be active. Compound 20 was the only active against more than one strain. Overall, the potency of phthalazine-triones adducts was dependent on the histological origin of cancer cells.
4 Conclusion
A new and efficient green methodology for obtaining 2H-indazolo[2,1-b]phthalazine-trione derivatives was developed using p-sulfonic acid calix[4]arene as a catalyst and ethyl lactate as the solvent. Fourteen compounds were obtained in moderate to good yields in only 10 min of reaction time. This procedure is of great interest because it allows the synthesis of compounds of biological and technological interest through an environmentally friendly methodology.
Acknowledgments
The authors are thankful for the financial support provided by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). ADF and SAF are supported by CNPq research fellowships.
References
- Synthesis and evaluation of new phthalazine substituted β-lactam derivatives as carbonic anhydrase inhibitors 1. Russ. J. Bioorgan. Chem.. 2015;41:414-420.
- [Google Scholar]
- Synthesis and evaluation of new phthalazine urea and thiourea derivatives as carbonic anhydrase inhibitors. J. Chem.. 2013;2013:1-8.
- [Google Scholar]
- What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem.. 2007;9:927-934.
- [Google Scholar]
- p-Sulfonic acid calixarenes as efficient and reusable organocatalysts for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/-thiones. Tetrahedron Lett.. 2011;52:6328-6330.
- [Google Scholar]
- Xanthenones: calixarenes-catalyzed syntheses, anticancer activity and QSAR studies. Org. Biomol. Chem.. 2015;13:3280-3287.
- [Google Scholar]
- Calixarenes as new platforms for drug design. Curr. Drug Discov. Technol.. 2009;6:151-170.
- [Google Scholar]
- p-Sulfonic acid calix[n]arenes as homogeneous and recyclable organocatalysts for esterification reactions. Tetrahedron Lett.. 2012;53:1630-1633.
- [Google Scholar]
- One-pot synthesis of aliphatic and aromatic 2H-indazolo[2, 1-b]phthalazine-triones catalyzed by N-halosulfonamides under solvent-free conditions. Tetrahedron. 2011;67:1930-1937.
- [Google Scholar]
- Synthesis and anticonvulsant activity of novel and potent 6,7-methylenedioxyphthalazin-1(2H)-ones. J. Med. Chem.. 2000;43:2851-2859.
- [Google Scholar]
- Calixarenes: An Introduction (second ed.). The Royal Society of Chemistry; 2008.
- Solvent-free, one-pot, four-component synthesis of 2H-indazolo[2, 1-b] phthalazine-triones using sulfuric acid-modified PEG-6000 as a green recyclable and biodegradable polymeric catalyst. Catal. Today. 2012;196:148-155.
- [Google Scholar]
- Efficient one-pot syntheses of 2H-indazolo[2, 1-b] phthalazine-triones by catalytic H2SO4 in water–ethanol or ionic liquid. Tetrahedron Lett.. 2009;50:7300-7303.
- [Google Scholar]
- Dodecylphosphonic acid (DPA): a highly efficient catalyst for the synthesis of 2H-indazolo[2, 1-b]phthalazine-triones under solvent-free conditions. Tetrahedron Lett.. 2012;53:1728-1731.
- [Google Scholar]
- Calix[n]arene sulfonic acids bearing pendant aliphatic chains as recyclable surfactant-type Brönsted acid catalysts for allylic alkylation with allyl alcohols in water. Green Chem.. 2008;10:635-640.
- [Google Scholar]
- Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Nat. Cancer Inst.. 1991;83:757-766.
- [Google Scholar]
- A rapid, one-pot, four-component route to 2H-indazolo[2, 1-b]phthalazine-triones. Tetrahedron Lett.. 2011;52:488-490.
- [Google Scholar]
- P-sulfonic acid calix[n]arenes: the most active and water tolerant organocatalysts in esterification reactions. Catal. Sci. Technol.. 2014;4:1369-1375.
- [Google Scholar]
- Studies on cardiotonic agents. II. Synthesis of novel phthalazine and 1,2,3-benzotriazine derivatives. Chem. Pharm. Bull.. 1990;38:2179-2183.
- [Google Scholar]
- Ethyl lactate as a solvent: properties, applications and production processes – a review. Green Chem.. 2011;13:2658-2671.
- [Google Scholar]
- Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia. 2008;79:598-601.
- [Google Scholar]
- One-pot, three-component route to 2H-indazolo[2, 1-b]phthalazine-triones. Tetrahedron. 2008;64:2375-2378.
- [Google Scholar]
- Vatalanib: the clinical development of a tyrosine kinase inhibitor of angiogenesis in solid tumours. Expert Opin. Investig. Drugs. 2007;16:367-379.
- [Google Scholar]
- Reusable silica supported poly phosphoric acid catalyzed three-component synthesis of 2H-indazolo [2, 1-b] phthalazine-trione derivatives. Arkivoc. 2009;2:59-67.
- [Google Scholar]
- Mannich-type reactions in water using anionic water-soluble calixarenes as recoverable and reusable catalysts. Green Chem.. 2006;8:608-614.
- [Google Scholar]
- Solvent-free sonochemical one-pot three-component synthesis of 2H-indazolo[2, 1-b]phthalazine-1,6,11-triones and 1H-pyrazolo[1, 2-b]phthalazine-5,10-diones. Tetrahedron Lett.. 2011;52:7195-7198.
- [Google Scholar]
- Calix[n]arenes in action: useful host-guest catalysis in organic chemistry. Curr. Org. Chem.. 2012;16:949-971.
- [Google Scholar]
- Organocatalysis in the three-component Povarov reaction and investigation by mass spectrometry. Org. Biomol. Chem.. 2013;11:5069-5073.
- [Google Scholar]
- Efficient synthesis of 2,4-disubstituted quinolines: calix[n]arene-catalyzed Povarov-hydrogen-transfer reaction cascade. RSC Adv.. 2014;4:18612-18615.
- [Google Scholar]
- Structure validation in chemical crystallography. Acta Crystallogr. D Biol. Crystallogr.. 2009;65:148-155.
- [Google Scholar]
- Calix[n]arenes as goldmines for the development of chemical entities of pharmaceutical interest. Curr. Pharm. Des.. 2013;19:6507-6521.
- [Google Scholar]
- Microwave-assisted, montmorillonite K-10 catalyzed three-component synthesis of 2H-indazolo[2, 1-b]phthalazine-triones under solvent-free conditions. Tetrahedron. 2012;68:6820-6828.
- [Google Scholar]
- Highly efficient three-component synthesis of 1H-indazolo[1, 2-b]phthalazinetrione derivatives catalyzed by heteropolyacids. Monatsh. für Chem. – Chem. Mon.. 2010;141:425-430.
- [Google Scholar]
- 4-Benzylamino-1-chloro-6-substituted phthalazines: synthesis and inhibitory activity toward phosphodiesterase 5. J. Med. Chem.. 1998;41:3367-3372.
- [Google Scholar]
- Synthesis and luminescence of 7-amino-2H-indazolo [2, 1-b]phthalazine-1,6,11(13H)-triones catalyzed by silica sulfuric acid. Luminescence. 2009;11:219-223.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.arabjc.2016.04.007.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







