Translate this page into:
Phytochemical and pharmacological studies on the genus Arcangelisia: A mini review
⁎Corresponding authors at: College of Pharmaceutical Engineering of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China. pharmwch@126.com (Chunhua Wang), lizheng@tjutcm.edu.cn (Zheng Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Only 3 plants in the genus Arcangelisia, belonging to the family Menispermaceae, are used as folk medicines for the treatment of various diseases by local residents. Alkaloids are main compounds of berberine analogues found in this genus and they demonstrate a wide range of pharmacological activities. The aim of this review is to compile the phytochemical progress including all the compounds isolated from this genus, their pharmacological activities together with the 13C NMR spectral data of the main bioactive components, which will bring more attention of other researchers to this genus for further study to find new active compounds.
Keywords
Arcangelisia
Alkaloid
Natural medicinal chemistry
Pharmacological activity
Folk medicine
13C NMR data
1 Introduction
The genus Arcangelisia (A.), which belongs to the family Menispermaceae, comprises 3 species (A. gusanlung, A. flava and A. tympanopoda) distributed in southeast Asia and south to Irian island (Zhang et al., 1995; Suzuki et al., 2011). These plants possess various medicinal properties and have wide-ranging applications in traditional and modern medicine. Among them, A. gusanlung H. S. Lo is the sole species of this genus found in the south of China including the provinces of Guangdong, Guangxi, and Hainan (Chinese Pharmacopoeia, 1977). As an important part of traditional Chinese medicine, it has a long history in the Dai and Li (Chinese national minorities) nationalities of China, and the Dai people in China use it to treat acute jaundice hepatitis (Chinese Pharmacopoeia, 1977; Peng et al., 2008). As a folk medicine, it has the functions of clearing away internal heat and detoxification, removing wind and relieving itching, promoting gangrene and reducing jaundice (Zhang et al., 1995; Chinese Pharmacopoeia, 1977; Hu et al., 2013; Li et al., 2019; Yu et al., 2014). However, the Li nationality has not its own character but the national language in history, the information of the herbs was transferred orally, which can lead to misinformation and unfounded rumor easily. To a certain extent, the inheritance and development of Li medicine are influenced (Hu et al., 2013). Another species, A. flava (L.) Merr. has been used as one of folk medicines (Jamu) in Indonesia. The medicinal plant is known locally as ‘Akar kuning’, whose name derived from the yellow sap. Fibraurea tinctoria Lour. and Cosinium fenestratum Colebr. are also called ‘Akar kuning’ in Indonesia, and they are distributed widely throughout Southeast Asia (Suzuki et al., 2011; Verpoorte et al., 1982; Wahyudi et al., 2016). It has been traditionally used by local people for the treatment of several diseases, such as malaria, dysentery, fever, abortion, the healing of hepatitis, indigestion and as atonic agent (Subeki et al., 2005; Niwat et al., 2005; Maryani et al., 2013; Ramadan et al., 2018; Julie et al., 2007). In addition, A. flava was used as an important component of folk medicines for the treatment of jaundice, smallpox, sore eyes, aphtha, water flea and as a stomachic and antihelminthic agent (Subeki et al., 2005; Setyowati et al., 2014; Kiss et al., 1988; Kaharap et al., 2016; Kusuma et al., 2011). A. tympanopoda (Lauberb. and K. Schum.) Diels., which is found only in New Guinea. A. flava and A. tympanopoda are distinguished by the different sizes of the fruits, the latter has larger fruits than the former (Suzuki et al., 2011; Verpoorte et al., 1982). Traditional uses of the genus A. are shown in Table 1.
Plants
Traditional uses
Refs.
A. gusanlung
Acute jaundice hepatitis, clearing away internal heat and detoxification, removing wind and relieving itching, promoting gangrene and reducing jaundice
Peng et al., 2008; Zhang et al., 1995; Chinese Pharmacopoeia, 1977; Hu et al., 2013; Li et al., 2019; Yu et al., 2014
A. flava
Malaria, dysentery, fever, abortion, the healing of hepatitis, indigestion and as atonic agent, jaundice, smallpox , sore eyes, aphtha, water flea and as a stomachic and antihelminthic agent
Subeki et al., 2005; Niwat et al., 2005; Maryani et al., 2013; Ramadan et al., 2018; Julie et al., 2007; Subeki et al., 2005; Setyowati et al., 2014; Kiss et al., 1988; Kaharap et al., 2016; Kusuma et al., 2011
In this review, we compile the phytochemical progress including all the compounds isolated from this genus, their pharmacological activities together with the 13C NMR spectral data of the main biologically active compounds.
2 Morphological features
Since they share morphological similarity, botanical classification is required using different parts such as leaf, stem, flower and fruit. A. gusanlung is a woody rattan and reaches a length of about 30 m after several years of growth. Its root is cylindrical, twisted, occasionally branched, 0.5–3 cm in diameter. The surface is brownish yellow with obvious longitudinal wrinkles, lateral lenticels, root scars. The cork is easy to fall off. The cross section is hard, bright yellow, with chrysanthemum-like texture and cracks. Smell slightly and very bitter. The stem is cylindrical, with a few curved, up to 3 cm in diameter or more progenitor. The surface is dark grayish yellow to grayish green, the section emblem is raised, the section is bright yellow, and the center is pith. The leaves are ovoid or oblong, 11–23 cm long and 5.5–14 cm wide. Dark gray-green to dark yellow–brown, apex shortly pointed, blunt at base, entire, glabrous on both sides, 3–5 veins away from the base, veins protruding on both sides, the underside is obvious; petiole is 5–14 cm long, both ends are swollen, shield-shaped near base. Leathery and brittle. The smell and taste are weak. Inflorescence grow on old stem and peduncle is stout, 0.7–1.5 cm long, 0.5–0.7 cm in diameter, fruit subglobose, slightly flat, 2.5–3 cm wide. Yellow at maturity, black at last, mesocarp fleshy, nucleus nearly bony, oblate, rusty long hairy, without any protuberance. The roots and stems are preferably thick and yellow on the cross sections. (https://baike.sogou.com/v133052.htm?fromTitle = Arcangelisia gusanlung).
A. flava is a vigorous, climbing shrub producing twining stems up to 20 m long and 5 cm in diameter near their base (http://tropical.theferns.info/viewtropical.php?id = Arcangelisia flava). Plant glabrous apart from leaf-domatia. Leaves: inflorescences axillary or cauliflorous, paniculate, slender, 10–50 cm, lateral branches spicate or subspicate, 1–5 cm. Leaves apparently indistinguishable from those of A. flava, except for stomata. The difference between the two forms of infructescence is remarkable ( http://portal.cybertaxonomy.org/flora-malesiana/cdm_dataportal/taxon/609d549e-cca5-4a6d-aa5a-4ffecb0d4005).
A. flava and A. tympanopoda are distinguished by the different sizes of the fruits, the latter has larger fruits than the former (Suzuki et al., 2011; Verpoorte et al., 1982). The pictures of two plants are shown in Fig. 1.
The pictures of the two plants. The left is A. gusanlung and the right is A. flava.
3 Phytochemistry
To the best of our knowledge, the first phytochemical investigation on the genus A. can be traced back to 1919. In 1919, Wells (Verpoorte et al., 1982) reported the presence of berberine (1) in the stem of A. flava. Garcia (Garcia et al., 1970) et al isolated berberine (1), palmatine (4) and jatrorrhizine (3) in the stem and berberine (1) and jatrorrhizine (3) in the roots of A. gusanlung for the first time in 1970. Up to the end of 2020, the total number of identified secondary metabolites from the genus A. amounts to 64, including alkaloids, phenylpropanoids, terpenoids, glycosides and some other components. The chemical structures of these compounds are shown in Figs. 2–6. Their names and the corresponding plant sources are compiled in Table 2.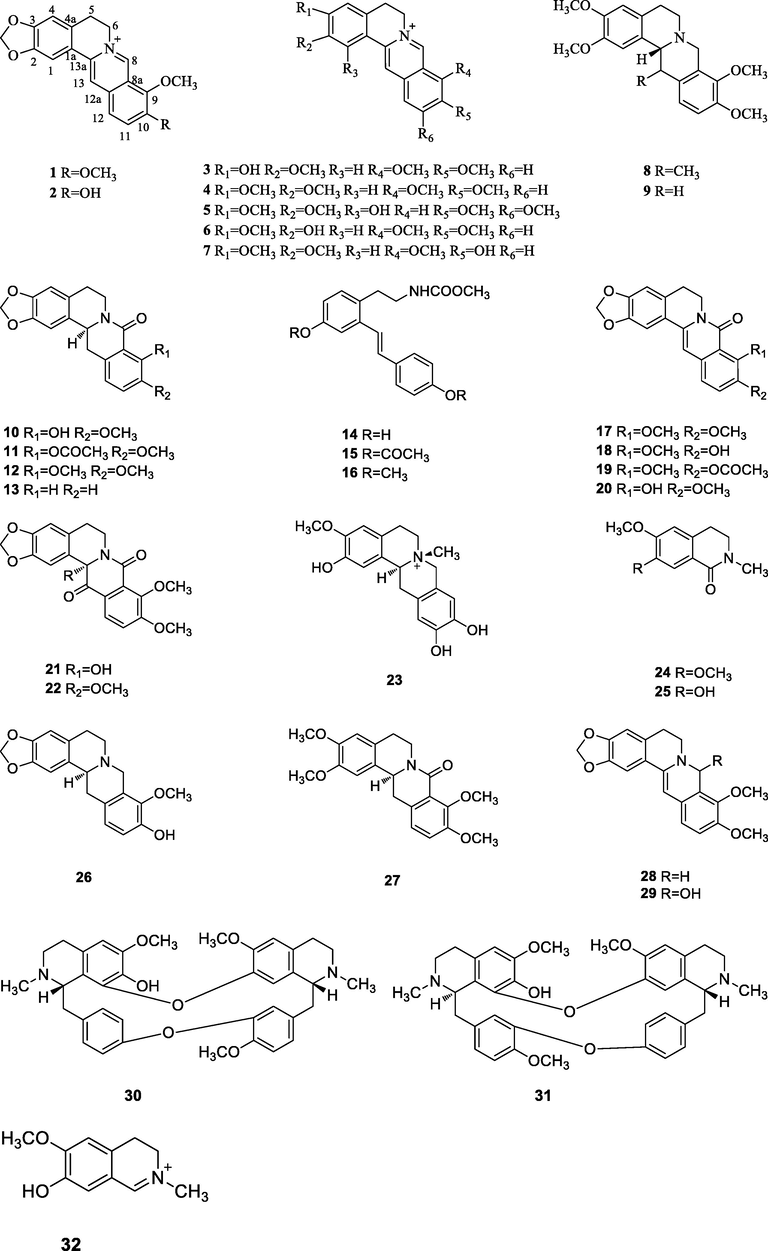
The chemical structures of the isolated alkaloids.
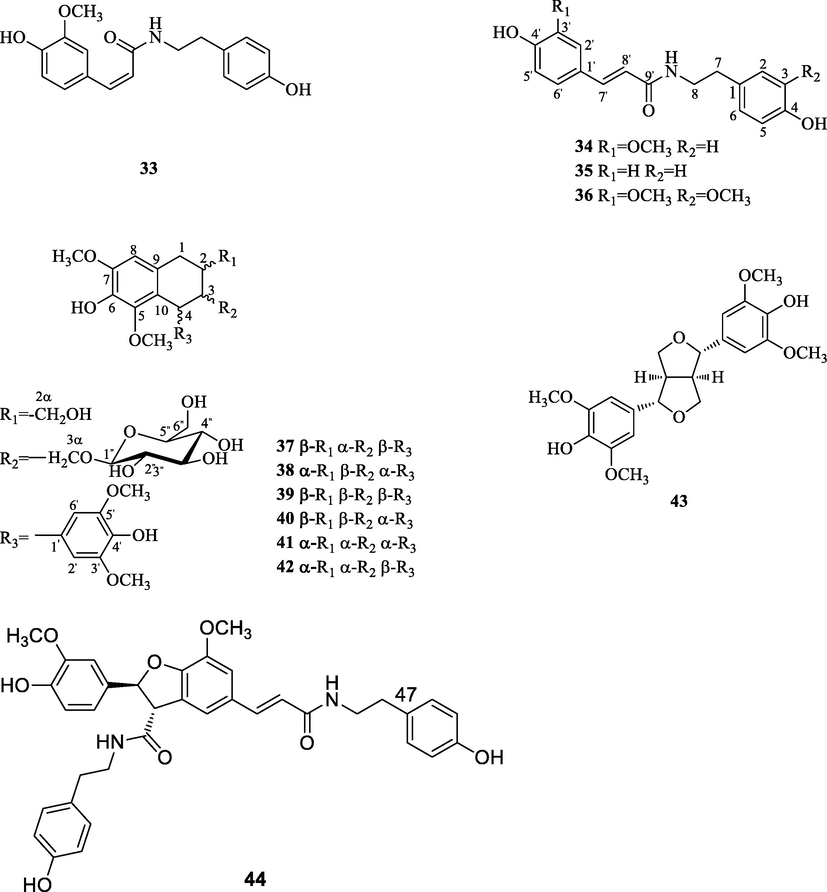
The chemical structures of phenylpropanoids.
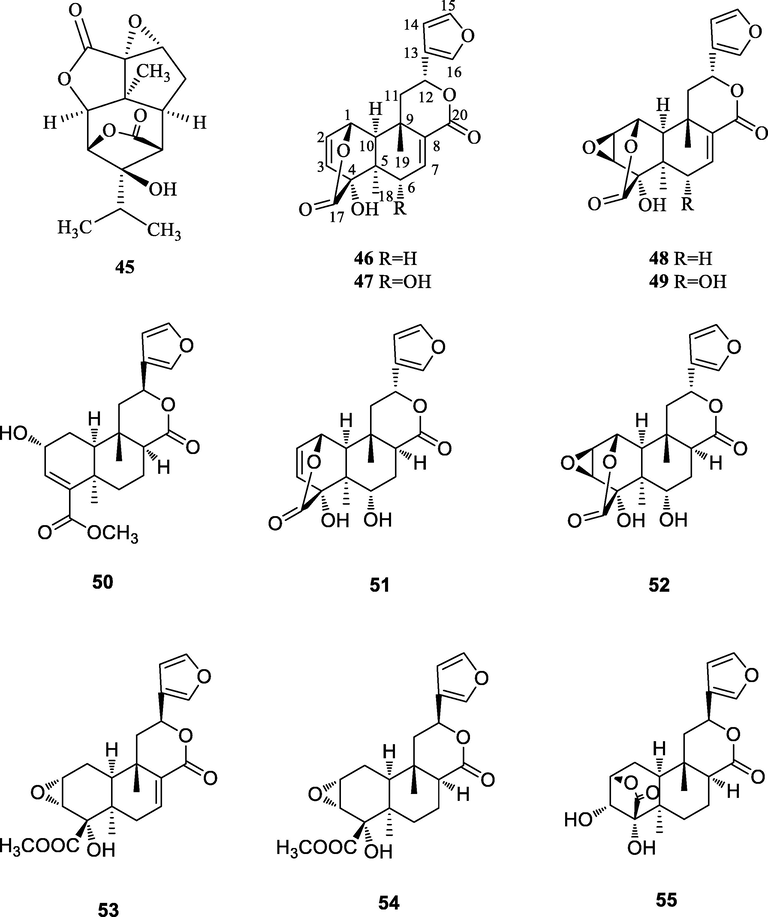
The chemical structures of terpenoids.
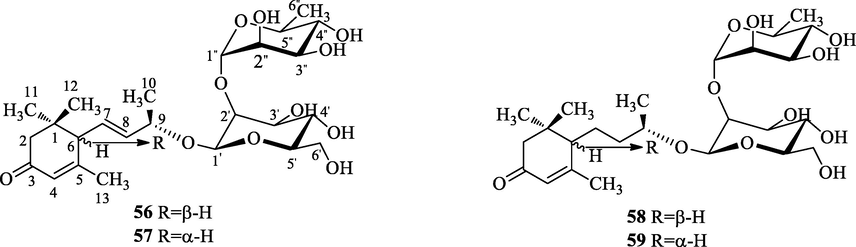
The chemical structures of glycosides.
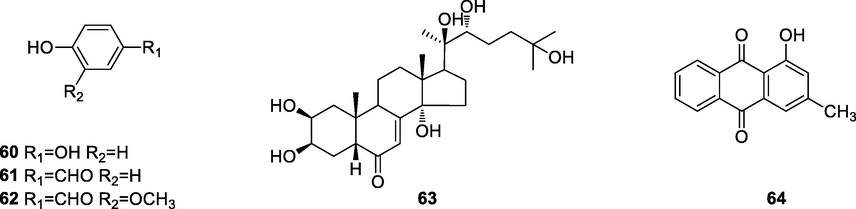
The chenmical structures of other compounds.
3.1 Alkaloids
Previous chemical investigations have indicated that alkaloids are the most frequently occurring constituents in the genus A.. Thirty-two alkaloids, 1∼32, have been isolated and elucidated from the genus A., most of which were isolated from A. gusanlung. Amongst, most alkaloids are protoberberine alkaloids, which belong to the type of isoquinoline alkaloids.
3.2 Phenylpropanoids
Up to date, twelve phenylpropanoids have been found in this genus. Among them, phenylpropanamides are the characteristic compounds, which are composed of a phenylpropionic acid structure and a phenylethylenediamine structure. Four phenylpropanamides were named as N-cis-feruloyltyramine (33), N-trans-feruloyltyramine (34), N-trans-coumaroyltyramine (35) and N-trans-feruloyl-3-methoxytyramine (36). Besides, eight lignans were also obtained from this genus. Take compound 44 as an example, it has a lignan linked to two phenylethylenediamine homologues.
3.3 Terpenoids
One sesquiterpenoid, aduncin (45) and ten furanoditerpenes (46∼55) were identified from the genus A.. These furanoditerpenes are bicyclic diterpenoids, whose molecular skeletons consist of four isoprene units and most of these diterpenes contain ester ring or oxygen ring, which is also a very interesting phenomenon found in this genus.
3.4 Glycosides
Interestingly, four megastigane glycosides (56∼59) have been found in the genus A.. Such compounds are rare in nature, whose aglycones are isomers that appear in pairs.
3.5 Other compounds
Simple phenolic compounds were found in this genus, and sterols and anthraquinones were also found.
3.6 13C NMR spectral data of some active compounds isolated from the genus A.
13C NMR spectroscopy is used to determine the type of carbon atoms in the molecules. Since 13C NMR chemical shifts are widely distributed in the spectrum, there is little signal overlap between carbon atoms except for the chemical and/or magnetic equivalences. 13C NMR signals are more easily discriminated than 1H NMR signals because they generally appear as singlets through the use of proton decoupling. In addition, 13C NMR chemical shifts are quite sensitive to structural features, although structurally similar compounds may have similar chemical shifts.
In order to help the chemical workers find the 13C NMR data of the isolated compounds more quickly, we specially summarized the 13C NMR spectral data of the main biologically active compounds from the genus A. (Tables 3–8). binDMSO-d6. aRecorded in CD3OD.
Positions
1 a (Yu et al., 2014)
2 a (Yu et al., 2014)
3 a (Yu et al., 2014)
4 a (Yu et al., 2014)
6b,c (Grycová et al., 2007)
8 a (Yu et al., 2014)
9c (Blanchfield et al., 2003)
10b (Zhang and Chen, 1991)
12 a (Yu et al., 2014)
1
105.4
106.4
115.5
104.7
114.9
108.7
108.5
106.1
106.5
1a
120.4
122.0
119.8
121.9
121.4
128.4
129.5
129.3
131.0
2
147.6
150.0
148.9
148.9
143.7
147.1
147.4
145.9
146.5
3
149.7
152.1
149.9
149.7
150.4
147.5
147.3
147.7
146.6
4
108.4
109.4
112.5
110.5
109.4
110.8
111.2
107.8
108.5
4a
130.6
131.6
130.3
132.6
133.6
128.4
126.6
129.1
128.7
5
26.4
28.3
26.8
27.3
26.3
29.2
28.9
29.0
29.0
6
55.2
57.1
57.2
56.6
55.6
51.3
51.3
37.8
39.2
8
145.4
145.2
145.3
145.3
144.8
54.0
53.8
161.4
162.4
8a
121.4
124.4
135.0
120.1
117.4
128.4
127.6
122.3
125.9
9
143.6
143.2
151.7
153.7
148.0
145.0
144.9
149.7
153.3
10
150.4
150.8
144.6
144.6
149.9
146.0
150.1
145.7
150.1
11
126.7
132.4
123.8
126.9
126.5
111.1
110.8
118.9
115.8
12
123.5
124.7
123.0
124.4
123.3
123.8
123.7
122.1
121.5
12a
132.9
135.3
122.9
126.9
128.2
134.8
128.5
128.2
128.9
13
120.2
121.7
121.5
121.5
119.8
38.2
36.1
37.7
38.2
13a
137.4
139.4
139.4
138.4
138.3
63.0
59.1
54.4
55.5
2-OCH3
56.5
57.2
55.5
55.9
3-OCH3
57.3
55.6
55.7
9-OCH3
62.0
62.4
62.2
63.0
56.9
60.0
60.0
56.2
10-OCH3
57.1
57.3
61.8
56.0
55.6
60.5
61.5
–OCH2O
102.1
103.6
56.3
100.5
101.5
N-CH3
18.2
Positions
13c (Zhang et al., 1995)
14 a (Yu et al., 2014)
17 a (Yu et al., 2014)
20c (Zhang et al., 1995)
21 a (Yu et al., 2014)
23 a (Yu et al., 2014)
26 a (Yu et al., 2014)
27 a (Yu et al., 2014)
1
107.3
119.7
104.9
104.0
121.8
114.5
106.7
109.5
1a
126.5
128.0
135.9
122.1
135.4
125.8
131.0
130.7
2
135.0
156.7
148.1
141.6
147.9
147.5
144.1
148.0
3
147.0
122.5
148.8
146.4
148.9
150.1
144.0
147.9
4
107.5
116.1
109.6
107.1
118.8
113.3
110.8
111.5
4a
126.5
131.0
129.8
109.6
138.1
120.3
127.8
127.8
5
29.7
36.8
29.8
28.4
31.8
24.3
29.2
29.8
6
42.0
40.2
38.8
39.1
39.5
53.3
51.5
39.2
8
162.0
167.0
160.3
164.0
168.6
65.1
53.0
162.7
8a
117.3
116.0
126.4
129.9
132.6
118.0
125.9
123.0
9
128.7
130.3
153.0
149.0
156.2
114.1
145.0
153.4
10
127.9
149.0
149.6
147.5
153.4
146.7
143.0
150.7
11
127.1
130.3
109.7
114.9
110.7
147.8
111.3
115.5
12
126.8
116.0
124.6
120.0
109.6
115.8
121.7
120.6
12a
124.6
148.6
134.0
128.9
124.5
122.0
127.1
127.3
13
33.5
140.7
103.6
103.6
203.9
35.4
36.2
38.0
13a
49.4
111.6
119.7
133.6
103.9
67.6
59.0
54.5
2-OCH3
56.2
3-OCH3
56.7
56.2
9-OCH3
60.9
62.8
55.1
61.5
10-OCH3
56.2
56.7
57.5
56.2
–OCH2O
100.9
102.3
100.6
92.2
101.3
–COOCH3
56.5
–CH3
50.7
Positions
34 a (Jiang et al., 2015)
1
131.5
2
131.0
3
116.5
4
157.1
5
116.5
6
131.0
7
36.0
8
42.7
1′
128.5
2′
111.8
3′
149.5
4′
150.0
5′
116.7
6′
123.4
7′
142.4
8′
118.9
9′
169.4
3′–OCH3
56.6
Positions
37a (Ohashi et al., 1994)
38 a (Ohashi et al., 1994)
1
33.8
33.9
2
40.7
41.3
3
46.7
46.6
4
42.8
43.3
5
147.7
147.6
6
139.0
139.5
7
148.7
148.7
8
108.0
107.8
9
130.3
130.2
10
126.5
126.3
1′
134.6
134.6
2′,6′
107.1
107.1
3′,5′
149.1
149.0
4′
139.4
138.9
2α
66.4
66.2
3α
71.6
72.0
5-OCH3
60.3
60.1
7-OCH3
56.7
56.6
3′,5′–OCH3
57.0
56.9
1″
104.9
104.3
2″
75.3
75.1
3″
78.3
78.2
4″
71.8
71.6
5″
78.0
78.0
6″
62.9
62.7
Positions
44 a (Yu et al., 2011)
56 a (Yu et al., 2011)
57 a (Yu et al., 2011)
58 a (Yu et al., 2011)
1
37.4
37.3
37.6
37.5
2
48.6
48.6
48.3
48.3
3
202.2
202.2
202.6
202.6
4
126.3
126.4
125.7
125.6
5
166.2
166.0
170.2
170.2
6
57.0
56.9
52.7
52.6
7
128.9
129.0
27.3
26.9
8
138.6
138.4
38.3
38.0
9
76.6
76.9
75.3
75.1
10
21.2
21.2
20.0
20.0
11
27.6
27.8
27.8
27.8
12
28.2
28.3
29.2
29.3
13
24.2
24.0
25.3
25.2
1′
100.9
101.0
100.5
100.5
2′
78.8
78.9
78.6
78.5
3′
79.6
79.7
79.8
79.8
4′
71.9
71.9
72.2
72.2
5′
78.0
78.0
77.9
78.0
6′
62.9
62.9
63.2
63.1
1″
102.1
102.2
102.1
102.0
2″
72.5
72.5
72.5
72.5
3″
72.4
72.4
72.5
72.4
4″
74.1
74.1
74.2
74.1
5″
69.9
69.9
69.8
69.8
6″
18.3
18.3
18.3
18.3
Positions
45b (Kunii et al., 1985)
46b (Kunii et al., 1985)
47b (Kunii et al., 1985)
48b (Kunii et al., 1985)
49b (Kunii et al., 1985)
50b (Kunii et al., 1985)
51b (Kunii et al., 1985)
52c (Suzuki et al,. 2011)
1
73.8
73.5
69.7
69.9
28.6
72.9
69.2
23.7
2
130.6
131.0
49.5
49.4
62.5
131.2
49.1
52.8
3
137.0
135.9
51.6
51.1
139.3
135.0
50.5
57.2
4
80.3
81.9
80.0
82.0
138.7
82.8
82.7
80.5
5
35.6
35.6
35.2
35.2
36.0
35.5
34.8
36.7
6
31.1
71.1
31.2
70.2
43.9
70.0
69.3
25.8
7
142.1
146.6
142.1
145.8
19.7
28.2
27.3
15.9
8
134.3
132.1
133.3
131.1
47.0
44.3
43.1
40.9
9
42.4
46.5
44.8
48.7
36.9
40.2
43.4
43.0
10
55.9
57.3
54.0
55.1
54.2
57.5
55.5
48.8
11
42.1
41.8
42.0
42.0
34.8
43.7
43.2
47.2
12
69.7
69.9
70.6
71.0
69.4
71.9
71.3
70.0
13
125.0
124.8
125.0
124.8
124.6
125.4
125.2
123.9
14
109.1
109.1
109.1
109.0
109.2
109.5
109.1
108.5
15
140.4
140.5
140.4
140.4
140.1
140.7
140.4
143.7
16
143.8
143.8
143.9
143.9
143.8
144.1
143.8
139.6
17
163.2
162.4
163.2
168.4
168.0
172.4
171.4
174.0
18
26.4
20.8
25.0
21.3
33.2
18.1
17.2
28.9
19
20.4
19.4
20.7
18.4
22.7
18.6
18.4
23.4
20
174.6
174.2
171.5
171.1
174.2
174.9
171.7
174.0
4-OCH3
51.5
–COOCH3
53.2
First of all, we take berberine (1) as an example to analyze the carbon spectral data of alkaloids. Its 13C NMR spectrum gives twenty carbon signals, including fifteen olefinic carbon signals at δC 108.4 (C-4), 105.4 (C-1), 120.2 (C-13), 120.4 (C-1a), 121.4 (C-8a), 123.5 (C-12), 126.7 (C-11), 130.6 (C-4a), 132.9 (C-12a), 137.4 (C-13a), 143.6 (C-9), 145.4 (C-8), 147.6 (C-2), 149.7 (C-3), 150.4 (C-10); a methylene carbon signal (-OCH2O-) at δC 102.1; two methylene carbon signals at δC 26.4 (C-5) and 55.2 (C-6) and two methoxy carbon signals at δC 62.0 (9-OCH3) and 57.1 (10-OCH3).
Then, we take N-trans-feruloyltyramine (34) and (+)-lyoniresinol 3α-O-β-D-glucopyranoside (37) as examples to analyze the carbons of phenylpropanoids, the 13C NMR spectrum of N-trans-feruloyltyramine (34) gives eighteen carbon signals, include fifteen olefinic carbon signals at δC 111.8 (C-2′), 116.5 (C-3, 5), 116.7 (C-5′), 118.9 (C-8′), 123.4 (C-6′), 128.5 (C-1′), 131.0 (C-2, 6), 131.5 (C-1), 142.4 (C-7′), 149.5 (C-3′), 150.0 (C-4′), 157.1 (C-4), 169.4 (C-9′); two methylene carbon signals at δC 36.0 (C-7) and 42.7 (C-8) and a methoxy carbon signal at δC 56.6 (3′-OCH3). (+)-lyoniresinol 3α-O-β-D-glucopyranoside (37) gives twenty-eight carbon signals, include twelve olefinic carbon signals at δC 107.1 (C-2′, 6′), 108.0 (C-8), 126.5 (C-10),130.3 (C-9), 134.6 (C-1′), 139.0 (C-6), 139.4 (C-4′), 147.7 (C-5), 148.7 (C-7), 149.1 (C-3′, 5′); an anomeric carbon signal at δC 104.9 (Glu-1″); six methylene carbon signals at δC 33.8 (C-1), 40.7 (C-2), 42.8 (C-4), 46.7 (C-3), 66.4 (C-2α) and 71.6 (C-3α) and four methoxy carbon signals at δC 56.7 (7-OCH3), 57.0 (3′, 5′-OCH3) and 60.3 (5-OCH3).
In addition, we take fibleucin (46) as an example to analyze the carbons of terpenoids, its 13C NMR spectrum gives twenty carbon signals, include two carbonyl group signals at δC 162.4 (C-17), 174.2 (C-20); eight olefinic carbon signals at δC 109.1 (C-14), 124.8 (C-13), 131.0 (C-2), 132.1 (C-8), 135.9 (C-3), 140.5 (C-15), 143.8 (C-16), 146.6 (C-7); two methyl carbon signals at δC 19.4 (C-19) and 20.8 (C-18); two methylene carbon signals at δC 41.8 (C-11) and 71.1 (C-6); three tertiary carbon signals at δC 57.3 (C-10), 69.9 (C-12) and 73.5 (C-1) and three quaternary carbon signals at δC 35.6 (C-5), 46.5 (C-9) and 81.9 (C-4).
Finally, we take gusanlungionoside A (56) as an example to analyze the carbon signals of glycosides, its 13C NMR spectrum gives twenty-five carbon signals, include a carbonyl group signals at δC 202.2 (C-3); four olefinic carbon signals at δC 126.4 (C-4), 129.0 (C-7), 138.4 (C-8) and 166.0 (C-5); two anomeric carbon signal at δC 101.0 (Glu-1′) and 102.2 (Rha-1″); four methyl carbon signals at δC 21.2 (C-10), 24.0 (C-13), 27.8 (C-11) and 28.3 (C-12); a methylene carbon signals at δC 48.6 (C-2); two tertiary carbon signals at δC 56.9 (C-6) and 76.9 (C-9) and a quaternary carbon signals at δC 37.3 (C-1).
4 Pharmacological activities
According to the above chemical constituents, this genus is rich in alkaloids, diterpenoids and other chemical components, so their biological activities are also quite rich, such as antimicrobial, anti-inflammatory, antimalarial, antidiabetic, antibabesial, antioxidant, antitumor, cardiotonic and antihypertensive activities, et al. Consequently, their main pharmacological activities are summarized as Table 9.
Biological activity
Object of study
Bioactive compound
Study model and mechanism
Refs.
Antimicrobial activity
A. flava, stem, water extract
–
Candida albicans: inhibition zone 16.65 ± 4.52 mm, MIC value 10 mg/ml, MBC value 40 mg/ml; Trichophyton mentagrophytes: inhibition zone 6.55 ± 0.05 mm, MIC value 10 mg/ml, MBC value 50 mg/ml
Setyowati et al., 2014
A. flava, leaf, 96% methanol extract
–
Pseudomonas fluorescens: MIC value concentration of 1.5%, inhibition zone 7.18 mm; MBC value concentration of 3.5%, inhibition zone 14.17 mm
Maryani et al., 2018
A. flava, stem, water extract
–
Inhibition zone: Salmonella typhii 19.35 mm, Staphylococcus aureus 16.98 mm, Trichophyton rubrum 19.78 mm, respectively at application level of 2% (v/v)
Hesty et al., 2015
A. flava
berberine (1)
Molecular docking (inhibition of protein and cell wall synthesis)
Pratama et al, 2018
A. flava, chloroform extract
–
Aeromonas hydrophila: widest preventive zone 17.25 mm
Maryani et al., 2013
A. flava, oneendophytic filamentous fungus
pachybasin (64)
Escherichia coli, Bacillus subtilis, Micrococcus luteus, Candida albicans, Saccharomyces cerevisiae, Aspergillus niger, Aspergillus flavus with MIC values of 64.0 µg/ml, Staphylococcus aureus: MIC 32.0 µg/ml, Fusarium oxysporum: MIC 16.0 µg/ml
Wulansari et al., 2014
A. flava, root, 80% ethanol extract
–
Staphylococcus aureus, Bacillus cereus
Soonthornchareonnon et al., 2012
A. flava, root, acetone and methanol extract
2β, 3α-dihydroxy-2,3,7,8α-tetrahydropenianthic acid-2,17-lactone (54)
Trametes versicolor, Fomitopsis palustris
Suzuki, et al., 2011
Anti-inflammatory activity
A. gusanlung, rhizome, 70% ethanol extract
–
Ear swelling induced by xylene in mice, paw edema induced by carrageenan and cotton pellet granuloma in rats
Hu et al., 2013
A. gusanlung, stem, methanol extract
N-trans-feruloyltyramine (34)
Downregulation of COX-2 and iNOS via suppression of AP-1 and the JNK signaling pathway in RAW 264.7 macrophages
Jiang et al., 2015
A. flava
berberine (1), thalifendine (2), jatrorrhizine (3), palmatine (4), columbamine (6), dehydrocorydalmine (7), dihydroberberine (28), limacine (31), pycnarrhine (32), fibraurin (48), 20-hydroxyecdysone (63)
Molecular docking (iNOS inhibitors)
Levita et al., 2018
Antimalarial activity
A. flava
–
Antiplasmodial activities
IC50 = 0.4– 8.6 µg/mlKaur et al., 2009
A. flava
berberine (1)
Plasmodium falciparum: dose-dependent range 30–300 µM
Sriwilaijareon et al., 2002
Antidiabetic activity
A. flava, leaf, hexane and ethyl acetate extract
–
α-amylase and α-glucosidase inhibition assays
Wahyudi et al., 2016
Antibabesial activity
A. flava, stem, water extract
berberine (1), jatrorrhizine (3), palmatine (4) and dihydroberberine (28)20-hydroxyecdysone (63)
Against babesia gibsoni in cultureberberine (1), jatrorrhizine (3), palmatine (4) and dihydroberberine (28) inhibition concentration: 100–1.0 µg/ml, 20-hydroxyecdysone (63): 100 µg/ml
Subeki et al., 2005
A. flava, water extract
–
IC50 values: 5.3–49.3 µg/ml
Subeki et al., 2004
Antioxidant activity
A. flava, stem, methanol extract
–
DPPH, EC50 values 25–55 µg/ml
Niwat et al., 2005
A. flava, leaf, hexane, ethyl acetate and methanol extract
–
Methanol extract: highest scavenging activity on superoxide and hydroxyl radical. ethyl acetate extract: highest scavenging activity on DPPH radical
Wahyudi et al., 2016
Antitumor activity
A. flava
berberine (1)
Molecular docking (EGFR inhibitor especially EGFR-2)
Pratama, 2016
A. flava, stem, chloroform extract
berberine (1), jatrorrhizine (3), palmatine (4)
Cytotoxic activity against brine shrimp and MCF-7 cells Chloroform extracts: LC50 values: 210–278 µg/ml, IC50 values: 8–12 µg/ml,berberine (1), jatrorrhizine (3), palmatine (4): LC50 values on brine shrimp 37–206 µg/ml; IC50 values against MCF-7 cells 1–4 µg/ml
Niwat et al., 2005
A. gusanlung, stem, methanol extract
gusanlung E (23)
Cancer cell line SGC 7901: IC50 value, 85.1 µM
Yu et al., 2014
Cardiotonic and antihypertensive activities
A. flava, ethanol extract
–
Exhibited cardiotonic activity on turtle heart (perfusion) and hypotensive activity in dog
Niwat et al.,2005, Estrada et al., 1963
Neuraminidase inhibitive activity
A. flava
fibleucin (45)
Antiinfluenza particularly to H5N1 with oseltamivir resistance
Mohammad et al., 2017
Tyrosinase inhibitive activity
A. gusanlung, stem, methanol extract
gusanlungionosides A-D (56∼59)
On the mushroom tyrosinase activity in vitro but also on melanogenesis in cells
Yu et al., 2011
Analgesic effect
A. gusanlung, 70% ethanol extract
–
Hot-plate test and peripheral analgesia model of the acetic acid injection in mice
Hu et al., 2013
Antipyretic effect
A. gusanlung, 70% ethanol extract
–
Rat fever model induced by yeast
Hu et al., 2013
Antitussive effect
A. gusanlung, 70% ethanol extract
–
Sequential method
Hu et al., 2013
Expectorant action
A. gusanlung, 70% ethanol extract
–
Tracheal excretion of phenol red
Hu et al., 2013
Antidiarrheal function
A. gusanlung, 70% ethanol extract
–
Observed on normal intestinal propulsion of mouse model of diarrhea induced by decoction of Sennae Folium
Hu et al., 2013
Antidepressant activity
A. flava, stem, water extract
–
312 mg/kg BW on white mice balb-c strain in terms of immobility time in the forced swim test method
Tiara et al., 2015
4.1 Antimicrobial activity
Serveral studies have shown that the ethanol extract of A. flava stems has excellent antimicrobial activity against various microbes both bacteria and fungi (Setyowati et al., 2014; Pratama, 2016). The antimicrobial spectrum of A. flava extracts is even quite extensive including some Gram-positive and Gram-negative bacteria (Maryani et al., 2018; Hesty et al., 2015). This activity is mainly associated with the ability of secondary metabolites as antimicrobials through various pathways including inhibition of cell wall, protein, nucleic acid synthesis and antimetabolites (Alves et al., 2014; Cheng et al., 2009; Dharani et al., 2016). Maryani (Maryani et al., 2018) et al showed that A. flava leaf possessed inhibition activity on the growth of bacteria Pseudomonas fluorescens. In addition, Pratama (Pratama et al, 2018) et al found that the main mechanism of action for the selected secondary metabolites of A. flava was the inhibition of protein and cell wall synthesis, which was shown by berberine (1). Berberine (1) has been claimed to be therapeutically useful for the treatment of malaria and as an antimicrobial agent (Iwasa et al., 1997; Sarma et al., 1999; Singh et al., 2010; Subeki et al., 2005; Vennerstrom and Klayman, 1988). And its mechanism of berberine (1) as antimicrobial agent could be changing the arrangement of amino acid chain on DNA that rises balances changes of genetics on DNA, so that the DNA of microbial will be defeat, this causes a core of microbial cell to be defeat and dead (Setyowati et al., 2014). Yu (Yu et al., 2005) et al reported that berberine (1) showed antimicrobial activity against all tested strains of MRSA (methicillin-resistant Staphylococcus aureus). Futhermore, A. flava had antibacterial capability against Aeromonas hydrophila (Maryani et al., 2013). Pachybasin (64), a major metabolite from culture broth of endophytic Coelomyceteous AFKR-18 fungus, was isolated from A. flava (Wulansari et al., 2014). Crude extract of A. flava was also active against Staphylococcus aureus and Bacillus cereus (Soonthornchareonnon et al., 2012). And Suzuki (Suzuki, et al., 2011) et al found that 2β, 3α-dihydroxy-2,3,7,8α-tetrahydropenianthic acid-2,17-lactone (54) showed the highest antifungal activity of the five isolated furanoditerpenes against a white-rot fungus (Trametes versicolor) and a brown-rot fungus (Fomitopsis palustris). Hesty (Hesty et al., 2015) et al found that water extract of A. flava exhibited a positive respond against Salmonella typhii, Staphylococcus aureus, and Trichophyton rubrum. Furthermore, it is reported that (+)-lyoniresinol-3α-O-β-D-glucopyranoside (37) has excellent potential as a leading compound for the development of antibiotic agents (Lee et al., 2005).
In a short conclusion of this part, there are many studies on the antimicrobial activity of A. flava, but there are few studies on A. gusanlung and A. tympanopoda. In the study of A. flava, berberine (1) is the main active component, and its mechanism may be the inhibition of protein and cell wall synthesis.
4.2 Anti-inflammatory activity
It is reported that A. gusanlung has anti-inflammatory activity (Chinese Pharmacopoeia, 1977). Hu (Hu et al., 2013) et al used various inflammatory models including ear swelling induced by xylene in mice, paw edema induced by carrageenan and cotton pellet granuloma in rats. The results indicated that A. gusanlung had effect on acute and chronic inflammatory. Jiang (Yu et al., 2011) et al found that the anti-inflammatory effects of N-trans-feruloyltyramine (34) might be attributed to downregulation of COX-2 (Cyclooxygenase-2) and iNOS (Inducible Nitric Oxide Synthase) via suppression of AP-1 (Activator Protein-1) and the JNK (c-JunN-terminalkinase) signaling pathway in RAW 264.7 macrophages (Mouse Monocyte Macrophage Leukemia Cells). Levita (Levita et al., 2018) et al thought that phytoconstituents in A. flava fit the pharmacophore features generated from AT2 (PDB code: 3E7G) or SEITU (PDB code: 4NOS) complex with iNOS, therefore, they might be potential as iNOS inhibitors. Besides, dihydroberberine (28) was proved to be one of the anti-inflammatory ingredients and the anti-inflammatory mechanism might be associated with dual modulation of NF-κB (Nuclear Transcription Factor-κB) and MAPK (Mitogen-activated Protein Kinase) signaling pathways and regulation of inflammatory mediators (Tan et al., 2019). Futhermore, Singh (Singh et al., 2010) et al found that berberine (1) has anti-inflammatory activity. In additiion, Choi (Choi et al., 2009) et al found that the anti-inflammatory effect of berberine (1) is not mediated by the inhibition of leptin signal transduction. Moreover, they have also found that berberine (1) can down-regulate the NF-κB signaling pathway.
In general, there are few studies on the anti-inflammatory activities of A. flava and A. gusanlung, only the mechanism of action of N-trans-feruloyltyramine (34) isolated from A. gusanlung is clear, and the mechanism of action of other compounds still needs further study.
4.3 Antimalarial activity
It is also reported that A. flava has antimalarial activity (Kaur et al., 2009; Lovin et al., 2012). The active components are protoberberine alkaloids such as berberine (1), jatrorrhizine (3), palmatine (4) and columbamine (6), which possess strong antiplasmodial activity (Iwasa et al., 1998) and inhibit Plasmodium falciparum telomerase activity (Sriwilaijareon et al., 2002). Besides, berberine (1) in combination with pyrimethamine, demonstrated the best parasite clearance when compared with treatment using a combination of pyrimethamine and tetracycline or pyrimethamine and cotrimoxazole in patients with chloroquine-resistant malaria (Sheng et al., 1997).
At present, malaria is a major global public health problem and is responsible for the death of over one million people annually, with more than 90% of cases found in sub-Saharan Africa. The increasing global spread of drug resistance to most of the available and affordable antimalarial drugs is a major concern and requires innovative strategies to combat. A. flava is expected to play a key role in the treatment of malaria.
4.4 Antidiabetic activity
It is reported that the ethyl acetate and hexane extracts of A. flava leaves possessed the potential antidiabetic activity which was comparable that of the standard acarbose. And the ethyl acetate extract possesessed the most potential antidiabetic effect because it has a high inhibitory activity on α-amylase and α-glucosidase (Wahyudi et al., 2016). In addition, It is reported that dihydroberberine (28) possessed the antidiabetic effect (Turner et al., 2008). Furthermore, Pan (Pan et al., 2003) et al found that the anti-hyperglycaemic activity of berberine (1) was at least partly due to its ability to inhibit α-glucosidase and decrease glucose transport through the intestinal epithelium. Besides, Chang (Chang, 2017) found that berberine (1) has shown promise as an anti-hyperglycaemic, anti-hyperlipidaemic agent against type 2 diabetes.
In our opinion, we can further isolate the A. flava to identify its antidiabetic ingredients and further study its mechanism.
4.5 Antibabesial activity
Subeki (Subeki et al., 2005) et al tested the compounds isolated from A. flava for antibabesial activity against babesia gibsoni in culture. Among them, berberine (1), jatrorrhizine (3), palmatine (4) and dihydroberberine (28) showed significant inhibitions at concentrations from 100 to 1.0 μg/ml, while 20-hydroxyecdysone (63) at a concentration of 100 μg/ml. In addition, Subeki (Subeki et al., 2004) et al reported that the extract of A. flava displayed very high antibabesial activity with IC50 (Half Maximal Inhibitory Concentration) of 5.3 μg/ml.
In our view, A. flava has a good inhibitory effect against babesia gibsoni, the range of action is clear, and its mechanism needs further study.
4.6 Antioxidant activity
Keawpwadub (Niwat et al., 2005) et al reported that methanol extract of A. flava exhibited moderate antioxidant activity. In addition, phenolic extract of A. flava leaves possessed the potential antioxidant activity which is comparable that of the standard vitamin C. Furthermore, the methanol extract exhibited the strongest DPPH radical scavenging ability and hydroxyl radical scavenging ability. Moreover, the ethyl acetate extract exhibited the strongest superoxide anion radical scavenging ability (Wahyudi et al., 2016). Besides, Singh (Singh et al., 2010) et al found that berberine (1) has antioxidant activity.
In our opinion, we can further isolate the A. flava to identify its antioxidant ingredients and further study its mechanism.
4.7 Antitumor activity
Several secondary metabolites of A. flava showed antiproliferative activity towards cancer cells (Pratama, 2016). Moreover, berberine (1) was also found to have antiproliferative activities against human cervix HeLa adenocarcinoma, human lung A549 adenocarcinoma, murine colon 26-L5 carcinoma, murine LLC (Lewis lung carcinoma), and murine B16-BL6 melanoma cells (Ueda et al., 2002). In addition, an experiment using berberine (1) to treat HepG2 (Hcuman Hepatoellular Carcinomas) cells indicated that the viability was increased to more than 90% after treatment and the secretion of alpha-fetoprotein by HepG2 cells was also inhibited by berberine (1) (Watthanachaiyingcharoen et al., 2010; Sun et al., 2009). Singh (Singh et al., 2010) et al found that berberine (1) has antitumor activity. And Ho (Ho et al., 2009) et al found that berberine (1) induced apoptosis via promoting the expression of Caspase-8, −9 and −3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Futhermore, chloroform extracts of A. flava showed pronounced cytotoxic activity against brine shrimp and MCF-7 cells with LC50 (lethal concentration 50) and IC50 values of 210–278 and 8–12 µg/ml, respectively. And berberine (1), palmatine (4), and jatrorrhizine (3) isolated from A. flava showed cytotoxic activity against MCF-7 (Michigan Cancer Foundation-7) cells (Niwat et al., 2005). Gusanlung E (23) showed weak cytotoxicity against cancer cell line SGC 7901 with IC50 value of 85.1 µM (Yu et al., 2014). It is reported that protoberberine alkaloids are highly effective as cytotoxic and antileukemic agents against human fibroblast and promyelocytic leukemic cells (Kuo et al., 1995; Orfila et al., 2000).
Cancer is one of the major causes of death in developed countries, together with cardiac and cerebrovascular diseases (World Health Organization, 1998). Cancer is clinically treated by surgery, radiotherapy and chemotherapy. After surgical ablation of progressive cancer, however, metastasized tumor cells continue to progress, and this is one of the causes making cancer treatment difficult (Fidler and Kripke, 1977). Thus, more effective anticancer drugs with high selectivity against only malignant cells and with ability to repress tumor metastasis are desired, as candidates for such drugs, A. flava and A. gusanlung may become as the key drugs.
4.8 Cardiotonic and antihypertensive activities
Ethanol extract of A. flava exhibited cardiotonic activity on turtle heart (perfusion) and hypotensive activity in dog (Keawpradub et al., 2005; Estrada et al., 1963). In addition, berberine (1) has been reported that it reduced heart disease or NAFLD (non alchololic fatty liver disease). It has a role in heart steatosis recovery, lipid metabolism disturbance, inflammation relieve, and insulin resistance (Singh et al., 2010; Pramono et al., 2019; Xing et al., 2011).
4.9 Neuraminidase inhibitive activity
A. flava contains many secondary metabolites which potentially have effects as neuraminidase inhibitor (Mohammad et al., 2017), and some literatures have reported that fibleucin (45) could be considered as neuraminidase inhibitor and should be potential to be developed as antiinfluenza particularly to H5N1 (Influenza A Virus Subtype H5N1) with oseltamivir resistance (Mohammad et al., 2017; Shen et al., 2015).
At present, only molecular docking technology is used to preliminarily infer that fibleucin (45) is a neuraminidase inhibitor and the mechanism of action and the property of patent medicine need to be further studied.
4.10 Tyrosinase inhibitive activity
Gusanlungionosides A-D (56∼59) exhibited strong inhibitory effects not only on the mushroom tyrosinase activity in vitro but also on melanogenesis in cells (Yu et al., 2011). And it is reported that melanogenesis was inhibited by N-trans-feruloyltyramine (34) in a dose-dependent manner (Efdi et al., 2007).
In our opinion, we can further study the mechanisms of gusanlungionosides A-D (56∼59) as tyrosinase inhibitors.
4.11 Other activities
Studies have shown that by means of hot-plate test and peripheral analgesia model of the acetic acid injection in mice, Hu (Hu et al., 2013) et al found that A. gusanlung had analgesic effect on the central nervous system, but its peripheral analgesic effect was greater. And they used in rat fever model induced by yeast observed antipyretic effect and its effect was obvious, the greater of the dosage was, the longer the effect lasted. Besides, Hu (Hu et al., 2013) et al used sequential method tested antitussive effect in mice, and the result was that A. gusanlung H. S. Lo could prolonged the latency of cough (Hu et al., 2013; Liu et al., 2009). Futhermore, the expectorant action was evaluated by tracheal excretion of phenol red, and the result showed that A. gusanlung H. S. Lo obviously increased the tracheal excretion of phenol red (Hu et al., 2013). Additionally, the antidiarrheal function was observed on normal intestinal propulsion of mouse model of diarrhea induced by decoction of Sennae Folium (Hu et al., 2013). And it is reported that berberine (1) and palmatine (4) have common antidiarrheal effects. However, the antidiarrhoeal mechanisms are different. Berberine (1) blocked basolateral K+ (Potassium ion) channels, while palmatine (4) inhibited on both Ca2+-activated (Calcium Ion-activated) and Camp-activated pathways (Taylor et al., 1999; Wu et al., 2008; Mcnamara et al., 1999; Albano et al., 2005). Even more impressively, antidepressant effect of A. flava has also been reported (Tiara et al., 2015). And Singh (Singh et al., 2010) et al found that berberine (1) has antidepressant activity.
5 Conclusion
In this review, we reported the phytochemical progress including all the isolated from the genus A., and their pharmacological activities together with the 13C NMR spectral data of the main biologically active compounds. Compounds isolated from the genus A. including alkaloids, phenylpropanoids, terpenoids, glycosides and some other components, and alkaloids are the main compounds found in this genus. Meanwhile, we have summarized a wide range of biological properties, such as antimicrobial, anti-inflammatory, antimalarial, antidiabetic, antibabesial, antioxidant, antitumor, cardiotonic and antihypertensive activities, et al. At present, there are relatively few studies on the genus A., especially for A. tympanopoda, and the related mechanism of A. flava is still the focus in the future’s research, while the most research on A. gusanlung is still the chemical separation.
Although there are only three species in this genus, the chemical composition of this genus is rich and diverse, and mainly contains alkaloids with a variety of activities. The pharmacological activity and mechanism of action of its main components are still very superficial. Therefore, the further study of the chemical constituents of the genus and the bioactivity of the isolated compounds still needs the attention and efforts of medical researchers. Consequently, we hope this review will provide a reference for further investigation and application of the genus A.
Acknowledgements
This work was supported by the National Key R&D Program of China, Synthetic Biology Research (No. 2019YFA0905300) and the National Natural Science Foundation of China (No. 82074276).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Guanylin and E. coli heat-stable enterotoxin induce chloride secretion through direct interaction with basolateral compartment of rat and human colonic cells. Pediatr. Res.. 2005;58(1):159-163.
- [CrossRef] [Google Scholar]
- Docking studies in target proteins involved in antibacterial action mechanisms: extending the knowledge on standard antibiotics to antimicrobial mushroom compounds. Molecules. 2014;19:1672-1684.
- [CrossRef] [Google Scholar]
- Characterisation of alkaloids from some Australian Stephania (Menispermaceae) species. Phytochem.. 2003;63(6):711-720.
- [CrossRef] [Google Scholar]
- Non-coding RNAs and berberine: a new mechanism of its anti-diabetic activities. Eur. J. Pharm.. 2017;795:8-12.
- [CrossRef] [Google Scholar]
- Synthesis, antibacterial activities and molecular docking studies of peptide and Schiff bases as targeted antibiotics. Bioorg. Med. Chem.. 2009;17(23):7861-7871.
- [CrossRef] [Google Scholar]
- Chinese Pharmacopoeia, 1977. People’s Medical Publishing House: Beijing, China, p. 521.
- The inhibition of inflammatory molecule expression on 3T3-L1 adipocytes by berberine is not mediated by leptin signaling. Nutr. Res. Pract.. 2009;3(2):84-88.
- [CrossRef] [Google Scholar]
- Docking studies in target proteins involved in antibacterial action mechanisms: Alkaloids isolated from Scutellaria genus. Asian. J. Pharm. Clin. Res.. 2016;9(5):121-125.
- [CrossRef] [Google Scholar]
- N-trans-feruloyltyramine as a melanin biosynthesis inhibitor. Biol. Pharm. Bull.. 2007;30(10):1972-1974.
- [CrossRef] [Google Scholar]
- Some pharmacologic effects of Arcangelisia flava (linn.) Merr. extract. Acta. Med. Philipp.. 1963;19:11-20.
- [Google Scholar]
- Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197(4306):893-895.
- [CrossRef] [Google Scholar]
- Absolute configuration of fibaruretin B. Acta. Crystallogr. Sect. E Struct. Rep. Online.. 2011;67(5):o1246-o1247.
- [CrossRef] [Google Scholar]
- The alkaloids of Arcangelisia loureirii and Coscinium wallichianum. Phytochem.. 1970;9(3):663-664.
- [CrossRef] [Google Scholar]
- Study of yellow root (Arcangelisia flava Merr) as a natural food additive with antimicrobial and acidity-stabilizing effects in the production process of palm sugar. Procedia. Environ. Sci.. 2015;23(2):346-350.
- [CrossRef] [Google Scholar]
- Berberine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res.. 2009;29(10):4063-4070.
- [Google Scholar]
- Pharmacology and toxicology of extract from Arcangelisia gusanlung. Chin. Herb Med.. 2013;5(2):109-115.
- [Google Scholar]
- Antimalarial activity and structure-activity relationships of protoberberine alkaloids. Eur. J. Immunol.. 1998;33(1):65-69.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of some 13-alkyl substituted protoberberinium salts. Planta. Med.. 1997;63(3):196-198.
- [CrossRef] [Google Scholar]
- N-trans-feruloyltyramine inhibits LPS-induced NO and PGE2 production in RAW 264.7 macrophages: Involvement of AP-1 and MAP kinase signalling pathways. Chem. Biol. Interact.. 2015;235:56-62.
- [CrossRef] [Google Scholar]
- Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J. Ethnopharmacol.. 2007;109(3):417-427.
- [CrossRef] [Google Scholar]
- Kaharap, A.D., Mambo, C., Nangoy, E., 2016. Uji efek antibakteri ekstrak batang Akar Kuning (Arcangelisia flava Merr.) terhadap bakteri Staphylococcus aureus dan Escherichia coli. Jurn. e-Biomedik. 4 (1). http://dx.doi.org/10.35790/ebm.4.1.2016.12138.
- Indonesian medicinal plants. I. new furanoditerpenes from Arcangelisia flava Merr. (2). stereostructure of furanoditerpenes determined by nuclear magnetic resonance analysis. Chem. Pharm. Bull.. 1987;35(12):4839-4845.
- [CrossRef] [Google Scholar]
- Kiss, T., Kis, M., Steffen, A., Ferenc, S., 1988. Nucleotide sequence of the 17S-25S spacer region from tomato rDNA. Nuclc Acids Res. 16 (14), 7179-7179. http://dx.doi.org/10.1093/nar/16.14.7179.
- Indonesian medicinal plants. I. new furanoditerpenes from Arcangelisia flava Merr. (1) Chem. Pharm. Bull.. 1985;33(2):479-487.
- [CrossRef] [Google Scholar]
- Berberine complexes with DNA in the berberine-induced apoptosis in human leukemic HL-60 cells. Cancer Lett.. 1995;93(2):193-200.
- [CrossRef] [Google Scholar]
- Kajian ilmiah air rebusan batang katola (Arcangelisia flava Merr) obat tradisional diare berdarah masyarakat kabupaten muna sulawesi tenggara. Majalah Farmasi Indones.. 2011;21(4):283-289.
- [Google Scholar]
- Antimicrobial property of (+)-lyoniresinol-3α-O-β-D-glucopyranoside isolated from the root bark of Lycium chinense Miller against human pathogenic microorganisms. Arch. Pharm. Res.. 2005;28(9):1031-1036.
- [CrossRef] [Google Scholar]
- Pharmacophore modeling and molecular docking of phytoconstituents in Morus sp. and Arcangelisia flava against nitric oxide synthase for antiinflammatory discovery. J. Appl Pharm. Sci.. 2018;8(12):53-59.
- [CrossRef] [Google Scholar]
- Comparative study of ethnic medicine Arcangelisia gusanlung and traditional Chinese medicine Phellodendri Cortex on the Baogantuihuang effect. Pharm. Clin. Chin. Mater. Med.. 2019;35(2):101-104.
- [Google Scholar]
- Sequential study on the antitussive effect of diammonium glycyrrhizinate on mice. Chin. J. Hosp. Pharm.. 2009;29(10):820-822.
- [Google Scholar]
- In vitro intraerythrocytic antimalarial activity of Akar Kuning (Arcangelisia flava (L.) Merr) stem aqueous extract in Plasmodium falciparum. Folia. Med. Indones.. 2012;48(3):90-95.
- [Google Scholar]
- Maryani, Marsoedi., Nursyam, H., Maftuch., 2013. The Phytochemistry and the anti-bacterial activity of yellow root (Arcangelisia flava Merr.) against Aeromonas hydrophila. J. Biol. Life Sci. 4 (2), 180-190. http://dx.doi.org/10.5296/jbls.v4i2.3683.
- Maryani, Rosita., Monalisa, S.S., Rozik, M., 2018. In vitro test of natural antibacterial activity of yellow-fruit moonseed Arcangelisia flava Merr. Leaf on bacterium Pseudomonas fluorescens under different doses. AACL. Bioflux. 11 (1), 288-294
- Basolateral K+ channel involvement in forskolin-activated chloride secretion in human colon. J. Physiol.. 1999;519(1):251-260.
- [CrossRef] [Google Scholar]
- Mohammad, R.F.P., 2017. Akar Kuning (Arcangelisia flava) as neuraminidase inhibitor: molecular docking and pharmacophore optimization approach. 2nd Sari Mulia International Conference on Health and Sciences, December 1, one health to address the problem of tropical infectious diseases in Indonesia. 6, 502-511.
- Antioxidant and cytotoxic activities of Thai medicinal plants named Khaminkhruea: Arcangelisia flava, Coscinium blumeanum and Fibraurea tinctoria. Songklanakarin J. Sci. Technol.. 2005;27(Suppl. 2):455-467.
- [Google Scholar]
- Indonesian medicinal plants. XII. Four isomeric lignan-glucosides from the bark of Aegle marmelos (Rutaceae) Chem. Pharm. Bull.. 1994;42(9):1924-1926.
- [CrossRef] [Google Scholar]
- Structural modification of berberine alkaloids in relation to cytotoxic activity in vitro. J. Ethnopharmacol.. 2000;71(3):449-456.
- [CrossRef] [Google Scholar]
- The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta. Med.. 2003;69(7):632-636.
- [CrossRef] [Google Scholar]
- Methods and common prescriptions for prevention and treatment of liver and gallbladder diseases in Dai nationality (in chinese) J. Med. Pharm. Chin. Minor.. 2008;2:39-41.
- [Google Scholar]
- Pramono, S., Paramawidhita, R.Y.T., Marini., Bahri, S., 2019. Subchronic effect of yellow root (Arcangelisia flava (L) Merr.) containing berberine boiled with water and brackish water on hematological,blood biochemical and histopathological parameters of liver function in female Wistar Rats. Intern. J. Trend. Sci. Res. Dev. 3 (5), 2456-6470.
- Akar Kunning (Arcangelisia flava) as EGFR inhibitor: in silico study. J Farmagazine.. 2016;3(1):6-16.
- [Google Scholar]
- Antibacterial activity of Akar Kuning (Arcangelisia flava) secondary metabolites: molecular docking approach. Asian. J. Pharm. Clin. Res.. 2018;11(11):447-451.
- [CrossRef] [Google Scholar]
- Antiprotozoal properties of indonesian medicinal plant extracts. J. Herb. Med.. 2018;11:46-52.
- [CrossRef] [Google Scholar]
- Antifungal activity of berberine iodide, a constituent of Fumaria indica. Folia. Microbiol.. 1999;44(2):164-166.
- [CrossRef] [Google Scholar]
- Setyowati, R., Sudarsono., Setyowati, E.P., 2014. The effect of water-soluble stem extract “Kayu Kuning” (Arcangelisia flava Merr) on the growth inhibition of Candida albicans ATCC 10231 and Trichophyton mentagrophytes in vitro. Biol. Med. Nat. Prod. Chem. 4 (1), 22-28. http://dx.doi.org/10.14421/biomedich.2014.31.15-19.
- New small-molecule drug design strategies for fighting resistant influenza A. Acta. Pharm. Sin. B.. 2015;5(5):419-430.
- [CrossRef] [Google Scholar]
- Treatment of chloroquine-resistant malaria using pyrimetnamine in combination with berberine, tetracycline or cotrimoxazole. East Afr. Med. J.. 1997;74(5):283-284.
- [CrossRef] [Google Scholar]
- Berberine: alkaloid with wide spectrum of pharmacological activities. J. Nat. Prod.. 2010;3:64-75.
- [CrossRef] [Google Scholar]
- Biological activities of medicinal plants from mangrove and beach forests. Mahidol. Univ. J. Pharm. Sci.. 2012;39(1):9-18.
- [Google Scholar]
- Stage specificity of Plasmodium falciparum telomerase and its inhibition by berberine. Parasitol. Int.. 2002;51(1):99-103.
- [CrossRef] [Google Scholar]
- Antibabesial activity of protoberberine alkaloids and 20-hydroxyecdysone from Arcangelisia flava against Babesia gibsoni in culture. J. Vet. Med. Sci.. 2005;67(2):223-227.
- [CrossRef] [Google Scholar]
- Effects of central Kalimantan plant extracts on intraerythrocytic Babesia gibsoni in culture. Intern. Med.. 2004;66(7):871-874.
- [CrossRef] [Google Scholar]
- A systematic review of the anticancer properties of berberine, a natural product from Chinese herb. Anticancer Drugs. 2009;20(9):757-769.
- [CrossRef] [Google Scholar]
- Furanoditerpenes from Arcangelisia flava (l.) M. and their antifungal activity. Phytochem. Lett.. 2011;4(3):333-336.
- [CrossRef] [Google Scholar]
- Dihydroberberine, a hydrogenated derivative of berberine firstly identified in Phellodendri Chinese Cortex, exerts anti-inflammatory effect via dual modulation of NF-κB and MAPK signaling pathways. Int. Immunopharmacol.. 2019;75:105802.
- [CrossRef] [Google Scholar]
- Berberine inhibits ion transport in human colonic epithelia. Eur. J. Pharm.. 1999;368(1):111-118.
- [CrossRef] [Google Scholar]
- Tiara, A., Arief, R.H., Sudarsono, S., 2015. The antidepressant effects of (Arcangelisia flava (l.) Merr) water-soluble extract in Balb-C mice reviewed from immobility time by forced. Biol. Med. Nat. Prod. Chem. 3 (2), 65. http://dx.doi.org/10.14421/biomedich.2014.32.65-67.
- Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57(5):1414-1418.
- [CrossRef] [Google Scholar]
- Antiproliferative activity of vietnamese medicinal plants. Biol. Pharm. Bull.. 2002;25(6):753-760.
- [CrossRef] [Google Scholar]
- Protoberberine alkaloids as antimalarials. J. Med. Chem.. 1988;31(6):1084-1087.
- [CrossRef] [Google Scholar]
- Studies on indonesian medicinal plants. vii. alkaloids of Arcangelisia flava. J. Nat. Prod.. 1982;45(5):229-238.
- [CrossRef] [Google Scholar]
- Potential antioxidant and antidiabetic activities of Kayu Kuning (Arcangelisia flava) Agric. Agric. Sci. Procedia.. 2016;9:396-402.
- [CrossRef] [Google Scholar]
- Authentication of Coscinium fenestratum among the other Menispermaceae plants prescribed in Thai folk medicines. Biol. Pharm. Bull.. 2010;33(1):91-94.
- [CrossRef] [Google Scholar]
- World Health Statistics Annual 1996. Geneva: World Health Organization; 1998.
- Palmatine, a protoberberine alkaloid, inhibits both Ca2+ and cAMP-activated Cl- secretion in isolated rat distal colon. Br. J. Pharm.. 2008;153(6):1203-1213.
- [CrossRef] [Google Scholar]
- Pachybasin, a major metabolite from culture broth of Endophytic Coelomyceteous AFKR-18 fungus isolated from a yellow moonsheed plant, Arcangelisia flava (L.) Merr. Hayati. J. Biosci.. 2014;21(2):95-100.
- [CrossRef] [Google Scholar]
- Berberine reducing insulin resistance by up-regulating IRS-2 mRNA expression in nonalcoholic fatty liver disease (NAFLD) rat liver. Eur. J. Pharm.. 2011;668(3):467-471.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J. Med. Food.. 2005;8(4):454-461.
- [CrossRef] [Google Scholar]
- Gusanlungionosides A-D, potential tyrosinase inhibitors from Arcangelisia gusanlung. J. Nat. Prod.. 2011;74(5):1009-1014.
- [CrossRef] [Google Scholar]
- Protoberberine isoquinoline alkaloids from Arcangelisia gusanlung. Molecules. 2014;19(9):13332-13341.
- [CrossRef] [Google Scholar]
- Yu, L.L., Zou, Z.M., 2012. New megastigane glucosides stereoisomers of Arcangelisia gusanlung H.S.Lo from hainan province. The 8th annual conference of doctoral students. Hohhot, Inner Mongolia, China. 168.
- Zhang, J.S., Chen, Z.L., 1991. Two new 8-oxotetrahydroprotoberberine alkaloids, gusanlung A and B, from Acangelisia gusanlung. Planta Med. 57 (5), 457-459. http://dx.doi.org/10.1055/s-2006-960150.
- Isoquinoline alkaloids from Acangelisia gusanlung. Phytochem.. 1995;39(2):439-442.
- [CrossRef] [Google Scholar]







