Translate this page into:
Phytochemical, Antimicrobial, Antidiabetic, Thrombolytic, anticancer Activities, and in silico studies of Ficus palmata Forssk
⁎Corresponding authors. jalqahtani@ksu.edu.sa (Jawaher Al-Qahtani), kashifur.rahman@iub.edu.pk (Kashif-ur-Rehman Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Ficus palmata Forssk. (Moraceae family) is medicinally valuable plant that is mostly used as folk medicine for the treatment of different diseases. Phytochemical composition was evaluated by preliminary phytochemical investigation, GCMS analysis, and total bioactive contents (TPC and TFC). The antioxidant, enzyme inhibition, antimicrobial, thrombolytic and anticancer activities were performed for biological evaluation. The extract exhibited the maximum total phenolic (49.24 ± 1.21 mg GAE/g) and total flavonoid contents (29.9 ± 1.13 mg QE/g) which may be correlated to higher antioxidant potential of extract. The GCMS investigation identified the presence of 27 phytocompounds of different classes related to aldehydes, esters of fatty acids, triterpenes, steroids, triterpenoid. The extract possessed the strong α-glucosidase (73.4 ± 4.65 %) and moderate α-amylase inhibition activity (47.1 ± 3.29 %). Significant results were observed in case of antiviral, antifungal, and antibacterial activities. F. palmata extract inhibited the growth of HepG2 cancer cells in a dose-dependent manner. The extract also exhibited moderate in vitro thrombolytic activity. In addition, the phytocompounds identified by GCMS were subjected to in silico molecular docking studies to analyze the binding affinity between phytocompounds and enzymes (α-glucosidase and α-amylase). Moreover, the best docked compounds were selected for ADMET studies which provide information about pharmacokinetics, physicochemical properties, drug-likeness, and toxicity of identified phytocompounds. The outcome of our research revealed that ethanolic extract of F. palmata possessed good antidiabetic, antimicrobial, thrombolytic and anticancer potential. This plant should be further explored to isolate the bioactive compounds for new drug development.
Keywords
Ficus palmata Forssk
GCMS
Thrombolytic
Anticancer HepG2 cell line
Molecular docking
ADME
1 Introduction
Ethnopharmacology and health sciences have observed a fast growing pharmaceutical and pharmacological studies towards the use of medicinal flora for potential therapeutic purposes (Ramalingum and Mahomoodally 2014). For many years, plant species have been medicinally utilized for therapeutic reasons (Hussain and Kumaresan 2014).
Despite the increasing prevalence of synthetic and semisynthetic derivatives for the treatment of infectious disorders, the number of resistant bacterial strains towards antibiotic drugs has been increasing day by day (Saklani and Chandra 2011). It continues to pose a threat to both underdeveloped and industrialized countries. Gram-positive bacteria produce resistance against antibacterial agents, has been a major international problem. A few effective antimicrobial agents are available for bacterial infections caused by these bacteria (Patel et al., 2012). Staphylococci that are multidrug-resistant (MDR) are becoming a bigger threat for the human physical and mental health. The resistant pathogenic Staphylococcus aureus strain, notably methicillin-resistant Staphylococcus aureus (MRSA), has posed severe difficulty in the treatment and control of Staphylococcal infections (Kaur and Chate 2015). Methicillin-resistant Staphylococci (MRS) have induced illnesses that are difficult to treat. MRSA has arisen simultaneous resistance to routinely used antibiotics, including macrolides, fluoroquinolones, tetracycline, aminoglycosides and chloramphenicol (Clinical and Institute 2009). In recent literature, plant polyphenols have been proved to exert antibacterial activity by inducing morphological and physiological damage to the bacterial cell membrane. Different studies have revealed that polyphenol rich plants possess many activities chiefly antioxidant and antibacterial activities (Takó et al., 2020). Additionally, plants polyphenols were reported to work by different mechanisms against viruses, including preventing virus entrance and affecting virus replication (Montenegro-Landívar et al., 2021). Due to these reasons, plants and their metabolites should be studied thoroughly for their potentials to treat and prevent bacterial and viral infections, and this was one of the aims of our current study.
Diabetes is a metabolic disease caused by decrease in production or impaired action of insulin (Chigurupati et al., 2022). Inhibiting the diabetic enzymes α-glucosidase and α-amylase is the first step for reducing postprandial hyperglycemia (Anjum and Tripathi 2019). Both of these diabetic enzymes can also be inhibited by antioxidants which help in reducing hyperglycemic conditions and these antioxidants include N-acetylcysteine, vitamin C and α-lipoic acid and some others (Bajaj and Khan 2012, Alqahtani et al., 2019). In this context, we selected a plant that is traditionally used in Saudi Arabia to treat diabetes with hope of supporting global efforts to battle diabetes and its complications.
Ficus palmata belongs to family Moraceae which comprises very high tree, shrubs and rarely herbs (Galil 2019). The plant of this family are traditionally used in asthma, scabies, gonorrhea, diabetes, diarrhea, and as antiseptic (Khan et al., 2022). The traditional use of this genus include in variety of diseases such as diabetes, ulcer, fungal infections, tumor, anti-inflammatory, hepatoprotective, nephroprotective and anticoagulant activities (Kitajima et al., 1999). F. palmata is distributed in some countries of South Asia, Somalia, South Egypt, Iran, Sudan, Ethiopia and Saudi Arabia (Khan et al., 2011). Ficus palmata is used for the treatment of various diseases such as tumor, diabetes, ulcer, gastrointestinal and fungal diseases (Sati et al., 2020). In numerous studies the important pharmacological activities of different parts of F. palmata have been reported; F. palmata latex have antibacterial activity while stem bark of this plant possessed antioxidant, cardioprotective and antimicrobial activity in addition to several in vivo therapeutic effects like hepatoprotective, nephroprotective, antiulcer and anticoagulant activities (Khajuria et al., 2018). In literature, phytochemical investigation of F. palmata revealed the presence of terpenes, coumarins, sterols, furanocoumarin glycosides, isoflavones, lignans, chromone, triterpene, bergapten, vanillic acid, psoralen and flavone glycosides (Alqasoumi et al., 2014).
The present research aims to evaluate numerous in vitro biological activities and in silico characteristics of ethanolic extract of F. palmata. Phytochemical tests were performed by qualitative phytochemical evaluation, determination of total phenolic and flavonoid contents and GCMS analysis. Additionally, in vitro biological assays including antioxidant (DPPH, ABTS, FRAP, CUPRAC), antibacterial (Gram-negative and Gram-positive bacterial strains), antiviral (H9, IBV and NDV strains), cytotoxicity and enzyme inhibition (α-glucosidase and α-amylase) activities were performed. The phytocompounds identified by GCMS in ethanolic extract of F. palmata were studied for in silico studies.
2 Materials and methods
2.1 Collection and Extraction of F. palmata aerial parts
Plant was collected from Jazan province in September 2020, Fayfa Mountains, Saudi Arabia. Plant was identified by botanist, King Saud University, Riyadh, Saudi Arabia with voucher number (24558), submitted specimen in college of science, king Saud University. The collected aerial part of plant (1 kg) was dried in shade and grounded to fine powder. The grounded plant powder was soaked in 80 % EtOH (3 L) (Javed et al., 2020). The aqueous alcoholic solution have better properties for extraction of polyphenols (Ghalloo et al., 2022). The extract was concentrated using a rotary evaporator at 40 °C, to yield a dark brown residue of 98.5 g.
2.2 Phytochemical analysis of extract
2.2.1 Preliminary phytochemical investigation
The F. palmata extract was investigated for qualitative phytochemical analysis for the determination of metabolites (primary and secondary) like carbohydrates, glycosides, amino acids, starch, lipids, proteins, saponins, steroids, terpenoids, phenols, flavonoids and tannins according to methods described in literature (Velavan 2015).
2.2.2 Quantification of total bioactive contents
2.2.2.1 Determination of total phenolic content
The total phenolic content was determined by Folin–Ciocalteu method adapted from literature with slight modification (Sembiring et al., 2018, Ghalloo et al., 2022). 0.5 mg/mL of sample was prepared; 0.1 mL of Folin-ciocalteu reagent was mixed with 0.1 mL of sample in a 96-well microplate. Sodium carbonates 10 % (2.8 mL) was prepared then added into the mixture and leave it at room temperature for 2 h.Absorbance was taken by BioTek synergy HT (Winooski, VT, USA) microplate reader at 765 nm in a 96-well microplate. As a standard, Gallic acid dilutions (0.05–0.5 mg/mL) used for calibration curve.
2.2.3 Determination of total flavonoid content
The total flavonoid contents was determined by using Aluminum calorimetric assay from the literature (Sembiring et al., 2018) by made some modifications. Standard solution with different concentrations 30, 40, 50, 60, 70, 80, 90, 100 µg/mL was prepared in ethanol. 50 µL of extract was diluted from 1 mg/mL and added in 10 µL of Aluminum chloride (10 %) solution then add 150 µL of ethanol added. Sodium acetate (1 M) 10 µL also added in the mixture in a 96-well microplate. The entire mixture was incubated for 30 min at room temperature. Ethanol was taken as blank and Quercetin was used as standard. Absorbance was taken by using a BioTek Synergy HT microplate reader at 415 nm.
2.2.4 Gas chromatography-Mass spectrometry (GCMS) Analysis
The extract of F. palmata was analyzed by GCMS analysis with a specification of instrument was Agilent 7890B with Mass Hunter acquisition software. The GCMS (instrument) has a HP-5MS ultra inert capillary with non-polar column 30 m × 0.25 mm ID × 0.25 µm film, containing inert gas Helium which runs at a speed of 1 mL/min. Sample injector started at 250 °C, and oven temperature was adjusted in this manner that for 5 min at 50 °C, then progressively rise to 250 °C at 100 °C/minute and at the end, goes to 3000◦C at 70 °C /minute for 10 min. The identification of phytocompounds was determined by evaluation with the NIST library data and the % peak area was calculated from chromatogram total peak area (Dilshad et al., 2022).
2.2.5 Phenolic acids quantification by HPLC
The sample extract was quantified by HPLC Shimadzu, Japan. Briefly, dried sample (0.1 g) was mixed in a 1 mL methanol, and 10 µL volume was injected in the HPLC system for quantification of the phenolic profile. Shim-pack CLC-ODS C-18 column (5 cm × 4.5 mm, 5 µm) Shimadzu was used for sample analysis and a mixture of glacial acetic acid and distilled water was utilized as a mobile phase with the ratio of 56:44 v/v.
2.3 In vitro biological activity
2.3.1 Antioxidant assay
2.3.1.1 Dpph radical scavenging Activity
DPPH assay was used to determine the anti-oxidant activity of plant extract was performed according to literature with slight modification (Ratshilivha et al., 2014). DPPH solution 0.1 mM was prepared then sample solution (plant extract) was also prepared in methanol at 5 mg/mL. Inoculated 90 µL of DPPH solution and 10 µL of sample solution in 96-well microplate. The reaction mixture was incubated at 37 °C for 30 min. Ascorbic acid was used as standard. The absorbance was taken at 517 nm with a microplate reader synergy HT Biotek, USA. The following formula was used for determination of Radical scavenging activity
2.3.1.2 Frap assay
Ferric-reducing anti-oxidant power assay was performed according to literature with little modification (Akinrinde et al., 2018). 100 µL of F.palmata(1 mg/mL) and 2 mL FRAP reagent was mixed and incubated for 30 min. Absorbance was taken at 593 nm by using BioTek Synergy HT reader.
2.3.1.3 Cuprac
Cupric reducing antioxidant capacity was determined by method adopted in literature with slight modification (Benarfa et al., 2019). Firstly, 100 µL of F. palmata extract was added into 10 mM (200 µL) CuCl2, 7.5 mM (200 µL) neocuprine and 1 M (200 µL) ammonium acetate buffer (pH 7.5) reaction mixture. After incubation for 30 min, absorbance was taken at 450 nm by using microplate reader.
2.3.1.4 ABTS
ABTS activity was carried out by the modified method in literature (Ghalloo et al., 2022) in which 100 µL of sample solution was added with 200 µL of ABTS solution. The mixture was incubated for 30 min at room temperature. The absorbance was measured at 417 nm using a BioTek Synergy HT microplate reader.
2.3.2 Antibacterial assay
Antibacterial activity was performed according to literature (Ahmad et al., 2019).
2.3.2.1 Test organism
Micrococcus luteus (ATCC 10240), Staphylococcus epidermidis (ATTC 12228), Bordetella bronchiseptica (ATCC 4617), Bacillus subtilis (ATCC 6633), Bacillus pumilus (ATCC 14884), Staphylococcus aureus (ATTC 6538), Escherichia coli (ATCC 10536). These 7 bacterial strains were procured from microbiology laboratory of the Islamia University of Bahawalpur.
2.3.2.2 Agar well diffusion method
Fresh Inoculum was prepared from fresh bacterial cultures by picking the little amount of colonies and inoculates in sterile Muller Hilton nutrient broth and places it in incubator for 24 h at 37 °C for bacterial growth. Standardize inoculated bacterial nutrient broth with 0.5Macforland by taken absorbance in UV. Agar media was poured in petri plates and left it to solidify. Wells were formed in each petri plates respective to concentrations of plant extract used after diluting it in DMSO (1 mL for each) 25 mg/mL, 50 mg/mL, 75 mg/mL, 100 mg/mL. Petri plated were Incubate for18- 24 h at 37 °C, After 24 h, zone of inhibition was measured. Ceftriaxone (250 mg) was used as standard. Dilution of standard was made 1 mg/mL in sterilized distilled water. The zone of Inhibition of standard and sample were noted to determine the antibacterial activity of plant.
2.3.3 Enzymes inhibition assay (α-glucosidase and α-amylase)
2.3.3.1 α-Glucosidase inhibitory activity
α-Glucosidase inhibition activity was performed according to literature (Rehman et al., 2018) with minor modification by changing the absorbance at 400 nm. Phosphate buffer solution 0.1 M of pH 6.8 was prepared. Ficus palmata extract (sample) 1 mg was diluted in 1 mL of methanol. Added 10 µL of sample, 70 µL of phosphate buffer solution, 10 µL of α-glucosidase in each well of 96-well microplate. The plate was incubated for 15 min at 30 °C. Added 10 µL of substrate ρ-Nitrophenyl-α-d-glucopyranoside and incubate for 30 min further. Absorbance was measured in %inhibition through the HT BioTek microplate reader. Methanol set as control and acarbose (100 µg/mL) as a positive control. The enzyme inhibitory activity of sample was measured as
2.3.3.2 α-Amylase inhibitory activity
α-Amylase inhibitory activity of Ficus palmate was performed according to literature (Anyanwu et al., 2019) with slight modification by changing the incubation time. Assay was performed in 96-well microplate. Sodium phosphate buffer (contained 0.6 M NaCl) was prepared with maintained pH 6.9. The volume of 25 µL Sodium phosphate buffer, 20 µL of soluble starch (1 %W/V 20 mg/2mL), 20 µL of plant extract (1 mg/mL), 20 µL of Acarbose was added in each well of 96-well microplate and incubate at 37 °C for 7 min. After incubation, 15 µL of α-amylase (12 mg/2mL) was added in reaction mixture and incubate at 37 °C for 17 min for further. The volume of 20 µL of HCl (1 M) and 100 µL of iodine reagent was added. The volume of 20 µL methanol and acabose (100 µg/mL) as negative and positive control in separate wells were used. Absorbance was measured at 620 nm by HT BioTek microplate reader.
2.3.4 Evaluation of antiviral activity
2.3.4.1 Viral strains
Three viral strains that was Infectious bronchitis virus, Newcastle disease virus, Avian Influenza A.
2.3.4.2 Inoculation of viruses
The protocol followed from literature (Shahzad et al., 2020). Eggs were brought from the government poultry farm, Model town A Bahawalpur. Eggs were free of pathogens. Chick embryonated eggs were taken which was 9–11 days old, were injected with viruses in chorioallantoic fluid. Before and after inoculations, eggs were candled. The wider side of eggs was swabbed with 70 % ethanol and moves it in tray place in Biosafety cabinet II. The viral inoculum injected through the 3 cc sterile needle along the wider side of eggs. The viral inoculum and drug was in same quantity. Molten wax was used to seal the hole after it, eggs were incubated at 37 °C. After 72 h of inoculation, allantoic fluids were obtained from the harvested eggs and check for haemagglutination test (HA). The changes in HA titer was noted as the virus particles in sample increases the HA titer quantity increase. For negative control, Normal saline was used; DMSO used as solvent control, virus without drug as virus control. The standard used was acyclovir (Musaddiq et al., 2020).
2.3.4.3 Hemagglutination Test (HA)
Test usually performs for quantification of titer of viruses except IBDV(Elizondo-Gonzalez et al., 2012) All steps followed for HA test was according to world organization of animal health manual of diagnostic test and vaccine for terrestrial animal (Stear 2005) 96-well flat bottom microplate was used. 50 µL of 7.4 pH phosphate buffer solution was added in each well. Same quantity of allantoic fluid(from infected eggs) was added in first wells and then mix it and add 50 µL from well 1 to well 2 then serially diluted it and mix it till 11 th well. Well 12th remain as negative control. 50 µL of chicken RBC solution (1 %) put in each well. Afterthat the plate set at 37 °C for 1 h and note the HA variations.
2.3.5 Antifungal assay
2.3.5.1 Fungal strains
Three fungal strains Aspergillus niger, Fusarium avenaceum, Fusarium brachygibbosum were used to identify antifungal activity of extract.
2.3.5.2 Agar tube dilution method
Antifungal activity was conducted through agar tube dilution method according to method in literature with slight modification (Andleeb et al., 2020). Agar, dose of extract and fungi were inoculated in tubes and then incubated at 37 °C for 24 h. Terbinafine as standard and DMSO as control (negative) was used in this assay.
2.3.6 2.3.6. Evaluation of thrombolytic assay
The thrombolytic assay of extract was determined according to the method reported in literature(Ahmed et al., 2022). The blood sample was taken out without affecting the blood clot in eppendorf tube and incubated at 37 °C for 45 min. After incubation, serum was carefully removed from the blood sample without affecting the blood clot then again weighed the tube. 1 mg/mL of plant extract was added to each pre-weight tube containing blood clot which was then incubated for 90 min at 37 °C. The Eppendorf tubes thrombolytic activity was observed after 90 min. The Eppendorf tubes released fluids which was taken out and weight again. The weight difference between eppendorf tubes was observed which demonstrated the extract antithrombotic activity. Streptokinase (standard) and distilled water (negative control) was used.
Where;
Wr = released clot weight.
Wc = clot weight.
2.3.7 Determination of Cytotoxic activity of extract
Cytotoxicity of extract was determined by MTT assay on HepG2 liver cancer cells which was performed according to literature with slight modification in the absorbance at 490 nm (Khan et al., 2013). Dulbecco’s Modified Eagle’s Medium (DMEM) was used as medium for cancer cell growth. Penicillin and streptomycin set as standard. The Dulbecco's Modified Eagle's Medium (DMEM) was added in a 75 cm2 flask along with 10 % foetal bovine serum (FBS), 100 IU/mL penicillin, and 100 µg/mL streptomycin. The cells were then kept in an incubator at 37 °C with 5 % CO2. Extracts diluted in DMSO (0.05 % concentration) were used to treat cells.
2.3.7.1 Determination of cell viability
HepG2 cell line was treated with different concentration of sample extract 20,40,60 and 100 µg/mL for 48 h. After treatment, a 10 µL MTT reagent was added to each well and incubated for an additional 4 h. After that, 150 µL of DMSO was added to dissolve the formazan crystals, and the absorbance was measured at 490 nm. The percentage of cell viability was determined by the following equation;
Where;
A sample = absorbance of sample.
A blank = absorbance of blank.
A control = absorbance of control.
A blank = absorbance of blank.
2.4 In Silico activities
2.4.1 Molecular docking studies
The binding affinities between the phytocompounds (by GCMS) with enzymes 5zcb (α-glucosidase) and 4w93 (α-amylase) were assessed by using PyRx software. The 3D shapes of proteins were obtained from the RCSB Protein Data Bank ( https://www.rcsb.org/) (Majid et al., 2022). First, the proteins were prepared using the Discovery studio by removing water and ligands, inserting polar hydrogen atoms, and saving them in PDB format. Then download the ligand in SDF format through PubChem (https://pubchem.ncbi.nlm.nih.gov/). The Open babel software converted the ligands to PDB files. The prepared ligands were docked blindly in the protein's grid box to allow them to find any suitable binding location (Khursheed et al., 2022). The molecular docking was confirmed by redocking α-glucosidase, α-amylase, and chosen ligands with Autodock 1.5.6. Furthermore, the binding affinity values yielded the same results. The Ligplot software was used for visualization of 2D structures of ligand–protein interactions (Aati et al., 2022).
2.4.2 ADMET studies
The best docked compounds were analyzed for ADMET studies by using the online Swiss ADME ( https://www.swissadme.ch/) tool (Majid et al., 2022). The toxicity of docked compounds (best docked) were assessed through the online software ProTox-II ( https://tox-new.charite.de/ ) (Banerjee et al., 2018).
2.5 Statistical analysis
The findings were all presented as mean standard deviation (mean ± SD). The information obtained through quantitative analysis IBMSPSS (v20, Chicago, IL, USA) was used to perform ANOVA (one-way analysis of variance) followed by post-hoc test. Significant values were defined as p values less than 0.05.
3 Results
3.1 Phytochemical profiling of extract
3.1.1 Preliminary phytochemical investigation
Phytochemical tests of Ficus palmata ethanolic extract revealed the presence of carbohydrates, lipids, saponins and tannins as well as phenols, flavonoid, steroids/terpenes, alkaloids and cardiac glycoside while the resins, proteins and amino acids were absent in extract of F. palmata as confirmed by the various tests and presented in Table 1. “+” present; “-“ absent.
Sr. No.
Metabolites
Tests
Results
1.
Carbohydrates
Molisch’s Test
+
Iodine Test
+
2.
Glycoside
Erdmann’s Test
+
3.
Phenols
Ferric chloride test
+
4.
Proteins
Biurette Test
–
5.
Alkaloids
Hager’s Test
+
6.
Tannins
Lead Acetate Test
+
7.
Lipids
Saponification Test
+
8.
Amino acids
Ninhydrin Test
–
9.
Saponins
Froth Test
+
10.
Flavonoids
Reaction with NaOH
+
11.
Resins
Acetic anhydride
–
12.
Steroids/
TerpenesSalkowaski’s test
+
3.1.2 Total bioactive contents
The results of total phenolic and flavonoid contents are shown in Table 2. The total phenolic content in the extract of Ficus palmata was 49.24 mg GAE/g and the total flavonoid content was 29.9 mg QE/g. “TPC” Total phenolic content, and “TFC” Total flavonoid content.
Total bioactive content
Antioxidant (mg TE/g)
TPC
(mg GAE/g)
TFC
(mg QE/g)
DPPH
FRAP
ABTS
CUPRAC
49.24 ± 1.21
29.9 ± 1.13
75.62 ± 2.31
110.39 ± 4.53
92.89 ± 3.92
195.62 ± 7.72
3.1.3 Determination of phytochemicals by GCMS
GCMS analysis was conducted to determine the major and minor compounds in extract of Ficus palmata ethanolic extract showed various peaks in GCMS chromatogram as shown in Fig. 1 which were determined on fused silica capillary column. The total 27 tentative compounds were identified and the major compounds were recognized by their peak % Area which was determined by the total area of peaks. Major compounds of GCMS are 12-Oleanen-3-yl acetate (5.28 %), 11, 14, 17-eicosatrienoic acid methyl ester (1.99 %), Bergapten (1.82 %), Acetic acid, 4,4,6a,6b,8a,11,11,14b-octamethyl-13oxodocosahydropicen-3-yl ester (1.77 %), 9,12-Octadecadienoic acid (1.63 %), n-Hexadecenoic acid (1.57 %), Taraxasterol (1.56 %), Stigmasterol, 22,23-dihydro-(1.30 %), Phytol (1.03 %), 3-Hydroxy-4-methoxybenzoic acid (1.02 %), 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (0.98 %), and 2,7-Naphthalenediol (0.89 %) in addition to many other minor compounds. Most of the detected compounds belong to triterpenoid, fatty ester, aldehyde, organic acid, aromatic substances, diterpene alcohol, and sterols classes (Table 3).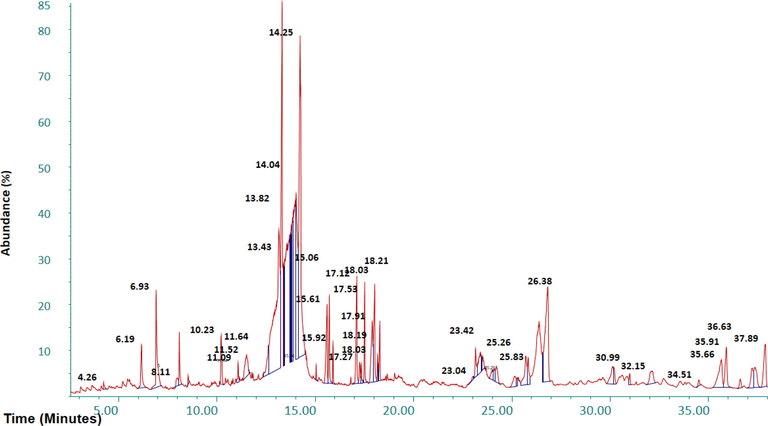
GCMS chromatogram of ethanolic extract of F. palmata.
Retention time (minutes)
Peak area (%)
Phytochemical compounds identified
Molecular formula
Molecular weight (g/mol)
Chemical class
6.19
0.98
3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one
C6H8O4
144.1
Organic acid
8.00
0.25
Hydroquinone
C6H6O2
110.1
Aromatic organic
8.11
0.58
2-Methoxy-4-vinylphenol
C9H10O
150.1
Phenolic compound
10.23
0.89
2,7-Naphthalenediol
C10H8O2
160.1
Aromatic substance
11.52
1.02
3-Hydroxy-4-methoxybenzoic acid
C8H8O4
168.1
phenolic acid
11.64
0.03
4-Hydroxy-3-methylbenzoic acid
C8H8O3
152.1
Monohydroxybenzoic acid
15.06
0.12
Pentadecanoic acid, 14-methyl-, methyl ester
C17H34O2
270.5
Fatty acid methyl ester
15.62
1.57
n-Hexadecenoic acid
C16H30O2
254.4
Palmitic acid
15.91
0.38
Hexadecenoic acid, ethyl ester
C18H36O2
284.5
Palmitic acid ester
17.12
1.82
Bergapten
C12H8O4
216.1
Furocoumarin
17.35
0.25
9,12,15-Octadecatrienoic acid, methyl ester
C19H32O2
292.5
Fatty acid
17.52
1.03
Phytol
C20H40O
296.5
Diterpene alcohol
17.91
1.63
9,12-Octadecadienoic acid
C18H32O2
280.4
Fatty acid
18.03
1.99
11, 14, 17-Eicosatrienoic acid methyl ester
C21H36O2
320.5
Fatty acid methyl ester
18.19
0.29
9, 12-Octadecadienoic acid ethyl ester
C20H36O2
308.5
Fatty acid ester
18.22
0.14
Octadecanoic acid
C18H36O2
284.5
Fatty acid
23.03
0.09
Urs-12-en-24-oic acid, 3-oxo-, methyl ester
C31H48O3
468.7
Triterpenes
23.44
0.26
β-Amyrin
C30H50O
426.7
Triterpenes
25.26
0.14
Lupeol
C30H50O
426.7
Triterpenoid
25.83
0.64
cis,cis,cis-7,10,13-Hexadecatrienal
C16H26O
234.38
Fatty aldehyde
26.38
5.28
12-Oleanen-3-yl acetate, (3.α.)
C32H52O2
468.8
Triterpenoid
31.00
0.19
Brucine
C23H26N2O4
394.5
Monoterpenoid indole alkaloid
34.51
0.12
Stigmasterol
C29H48O
412.7
Sterol
35.66
1.77
Acetic acid, 4,4,6a,6b,8a,11,11,14b-octamethyl-13-oxodocosahydropicen-3-yl ester
C32H52O3
484.8
Ester of acetic acid/ picene
35.91
1.30
Stigmasterol, 22,23-dihydro-
C29H50O
414.7
Steroid
36.63
0.23
α-Amyrin
C30H50O
426.7
Triterpenoid
37.89
1.56
Taraxasterol
C30H50O
426.7
Triterpenoid
3.1.4 Phenolic Acids Quantification by HPLC
In the HPLC quantification, six compounds; p-Coumeric acid, Kaempherol, Quercetin, Ferrulic Acid, Benzoic acid, and Rutin were quantified (Fig. 2 and Table 4).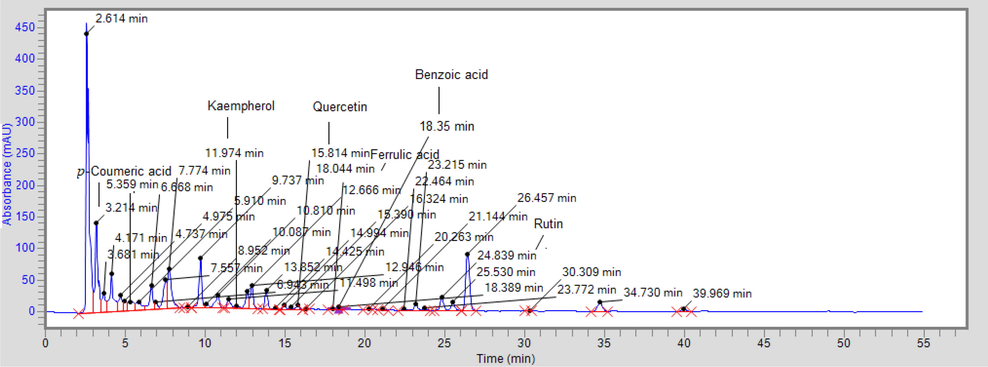
HPLC chromatogram in Ficus palmata.
Compound Name
RT (Min)
Area
k-factor
Conc. (µg/g)
p-Coumeric acid
3.214
1,673,213.2
0.0003
207.47
Kaempherol
11.974
2,347,633.2
0.0000408
95.78
Quercetin
15.814
103,407.0
0.000696
71.97
Ferrulic acid
12.666
394,200.0
0.0000767
30.26
Benzoic acid
18.394
77,093.7
0.0000345
2.66
Rutin
24.839
332,476.7
0.000113
37.57
3.2 In vitro biological activies
Ficus palmata extract was evaluated in vitro biologically for its antioxidant, enzyme-inhibiting, antimicrobial, anticancer, and thrombolytic potentials.
3.2.1 Antioxidant activities
Ficus palmata showed strong antioxidant results due to the presence of compounds such as flavonoids, diterpenes, triterpenes and phenolics (Karatoprak et al., 2020). These antioxidant activities are responsible for the antidiabetic potential of plant. Antioxidant activities were determined by DPPH, ABTS, FRAP and CUPRAC. Results are shown in Table 2. DPPH and ABTS scavenging activities of ethanolic extract of F. palmata were 75.62 mg TE/g, 92.89 mg TE/g respectively while the FRAP and CUPRAC results were 110.39 mg TE/g, 195.62 mg TE/g respectively.
3.2.2 In vitro enzyme inhibition assay
Antidiabetic activity was performed to confirm its traditional use in diabetes mellitus which our results also supported its use by indicating the strong antidiabetic potential against diabetic enzymes. The extract possessed α-glucosidase inhibitory activity of 73.4 % at a concentration of 1 mg/mL as shown in Fig. 3. The inhibitory effect of F. palmata extract against α-amylase was 47.1 % at 1 mg/mL (Fig. 3). The inhibitory effect of F. palmata against α-glucosidase enzyme was much higher than against α-amylase while the acarbose showed 89.91 % inhibition against α-glucosidase and 77.41 % against α-amylase. Acarbose was used as a positive control while methanol was used as a negative control.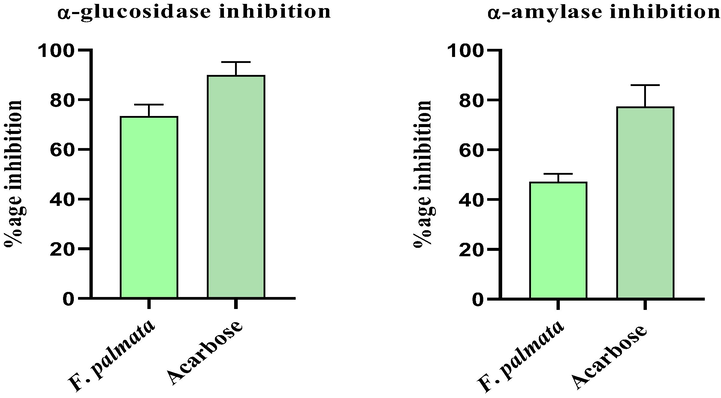
α-Glucosidase and α-Amylase % inhibition assay.
3.2.3 Antibacterial assay of ethanolic extract of F. palmata
Antibacterial activity was performed to evaluate the potential of F.palmata extract against seven bacterial strains and our report confirmed that the F. palmata extract was active against many bacterial strains and could be employed to reduce different bacterial infections. The F. palmata possess many secondary metabolites like triterpenes, alkaloids, sterols, polyphenols, flavonoids, and saponins, which showed their antibacterial potential and make the plant significant for therapeutic purposes i.e. dysentery, diarrhea, skin diseases, lung complications, ulcers, cough, heart disease (Santos et al., 2013). The extract showed highest zone of inhibition (23 mm) against Staphylococcus aureus at a concentration of 100 mg/mL and showed minimum activity (15 mm) at concentration 100 mg/mL against Bordetella bronchiseptica. The extract showed least activity at concentration of 25 mg against few bacteria but little more for some other bacteria. The results revealed the direct relation between zone of inhibition (mm) and different concentrations of F. palmata extract. The detailed results of antibacterial activity are shown in Table 5 and Fig. S1.
Bacterial strains
Zone of inhibition (mm)
Conc. 25 mg/mL
Conc.
50 mg/mL
Conc.
75 mg/mL
Conc.
100 mg/mL
Standard
Ceftriaxone 1 mg/mL
Staphylococcus aureus
17
20
21
23
26
Staphylococcus epidermidis
14
16
18
21
26
Escherichia coli
10
12
15
22
25
Bordetella bronchiseptica
12
13
14
15
20
Bacillus subtilis
15
16
17
18
22
Bacillus pumilus
12
13
14
16
21
Micrococcus luteus
11
13
15
17
21
3.2.4 Antiviral activity
The purpose to perform this experiment was that the extract possess different phytochemicals like flavonoids, polyphenolics, terpenoids, lignans, coumarins, sulphides, alkaloids, saponins, furyl compounds, as determined by GCMS, which have antiviral activity and our results confirm the antiviral potential,antiviral activity was performed (Bouazzi et al., 2018). Purpose of this study, Synthetic antiviral agents was used for the treatment of various viruses have reported adverse effects so less toxic agents are required, so the antiviral assay was performed to evaluate its activity against some specific viral strains (Fernandez et al., 1998). The results demonstrated a significant antiviral activity of F. palmata ethanolic extract of aerial parts against IBV, NDV and H9 with respective titer count 04, 04 and 00. Ethanol extract showed highly strong inhibitory effect against H9 virus and strong activity against IBV and NDV as depicted in Table 6. “IBV” Avian Infectious Bronchitis Virus, “H9” Influenza virus, and “NDV” Newcastle disease virus, HA titer 0–8; highly strong, 16–32; strong, 64–128; moderate, and 256–2048; not active (Musaddiq et al., 2020).
Strains
Titer count
in control
Titer count
with Acyclovir
Titer count
with F. palmata
IBV
1024
00
04
NDV
2048
00
04
H9
2048
00
00
3.2.5 In vitro thrombolytic assay
This activity was intended to be performed due to its excellent medicinal relevance and result which demonstrated its great thrombolytic potential and potential for use as an antiplatelet or thrombolytic agent.The thrombolytic potential of the plant F. palmata extract was evaluated, which are shown in Fig. 4. The percentage of clot lysis by ethanolic extract of F. palmata was 37.17 % at 1 mg/mL while Streptokinase showed 76.98 % clot lysis.
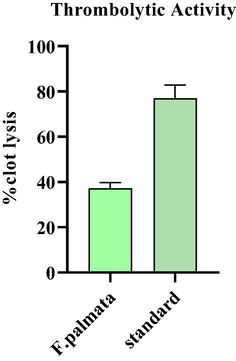
Thrombolytic activity of ethanol extract of F. palmata.at 1 mg/mL.
3.2.6 Antifungal activity
Ficus palmata traditionally used as antifungal agent and to confirm its antifungal potential, the antifungal activity was performed. Our results also confirmed the antifungal potential of plant because it possesses antimicrobial compounds likely 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (Igwe et al., 2016), Octadecanoic acid (Jasim et al., 2015), and Urs-12-en-24-oic acid, 3-oxo-, methyl ester (Chenniappan et al., 2020). Table 7 represented the antifungal activity of F. palmata extract. The results of this assay showed the maximum antifungal activity of extract against fungal strains Fusarium avenaceum (58.6 %) followed by Fusarium brachygibbosum (45.45 %) and Aspergillus niger (41.66 %).
Sample
strains
growth
test (mm)
Growth
control (mm)
% inhibition
F. palmate
A. niger
40
96
41.66 ± 4.29
F. avenaceum
51
87
58.6 ± 5.67
F. brachygibbosum
45
99
45.45 ± 5.23
3.2.7 Cytotoxic effect of F. Palmata extract
Because of its folkoric use, this activity was performed to confirm its potential as anticancer agent and great results of anticancer activity also justify its anticancer/antitumor phytocompounds in this plant. Results of cytotoxicity of Ficus palmata extract against HepG2 cell line are shown in Fig. 5. The plant extract showed significant variations in % cell viability at different concentrations. At 100 µg/mL, extract showed 17.46 % viability on a cell line HepG2. However, at 20 µg/mL concentration of extract showed 57.67 % viability.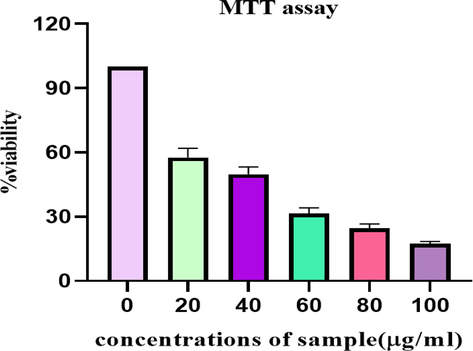
Cytotoxic effect of F. palmata ethanolic extract on HepG2 cell line.
3.3 In silico studies
3.3.1 Molecular docking studies of phytocompounds against enzymes
Molecular docking of compounds identified by the GCMS was performed against both α-glucosidase and α-amylase. Binding affinity of 9 compounds with α-glucosidase and 8 compounds with α-amylase were greater than the binding affinity of acarbose (standard). Maximum binding affinity of phytocompound with α-glucosidase was by urs-12-en-24-oic acid, 3-oxo-, methyl ester with a value of −9.8 Kcal/mol by making no hydrogen bond and hydrophobic interactions Leu287, Gly286, Asn258, Met229, Phe225, Pro223, Val222, Leu219, and binding affinity of taxasterol with α-amylase with a value −10.8 Kcal/mol was maximum by making hydrogen bond Glu233, Asp197 (Table 8, Fig. 6, Fig. 7, S2, S3, S4). The molecular docking for the compounds quantified by the HPLC was also performed. Amongst these compounds, Rutin showed best binding affinity with binding affinity −9.0 Kcal/mol against α-amylase and −8.9 Kcal/mol again
Sr. no
Ligand
Structures
α-glucosidase
α-amylase
Binding energy (Kcal/mol)
H-bond interactions
Hydro-phobic interactions
Binding energy
(Kcal/mol)
H-bond interactions
Hydro-
phobic interactions
1.
urs-12-en-24-oic acid, 3-oxo-, methyl ester
−9.8
–
Leu287,Gly286, Asn258,Met229,Phe225, Pro223, Val222, Leu219
−9.2
–
Gln63, Trp59, Trp58, Asp300, Ile235, Glu233, Asp197, Leu162, Tyr62, Thr163, Leu165
2.
β-Amyrin
−8.4
–
Leu287, Met229, Glu226, Phe225,
Pro223, Val222
−10.1
–
Asp300, His299, Glu233,, Thr163, Tyr62, Leu162, Asp197, Trp58, Trp59
3.
acetic acid, 4,4,6a,6b,8a,11,11,14b-octamethyl-13-oxodocosahydropicen-3-yl ester
−8.3
Asn371, Lys334
Glu408, Asn333, Ile329, Leu301, Glu300, Leu296, His284
–
–
–
4.
α-Amyrin
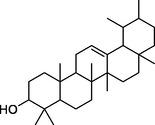
−8.1
Asn275, Thr253
Asn277, Phe276, Gly274, Glu271, Ile251, Ala247, Phe246, Lys242, Lys7, Trp6, Lys4
−10.2
Asp197, Glu233
His305, Asp300, His299, Thr163, Leu162, Tyr62, Trp59, Trp58
5.
taraxasterol
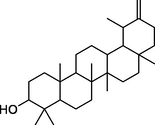
−7.8
Glu408
Phe335, Lys334, Ile304, Leu301, Asp297, Leu296, His284
−10.8
Glu233, Asp197
Asp356, Asp300, His299, Thr163, Leu162, Tyr62,, Trp59, Trp58,
6.
stigmasterol
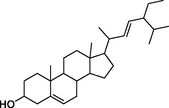
−7.6
–
Lys290, Trp288, Leu287, Gly286, Gly259, Asn258, Phe225, Pro223, Ile143, Glu141,
−9
Ile235, Lys200
His201, Asp197, Tyr62, Leu165, Gln63, Thr163, Trp59, Leu162, Asp300, Tyr151
7.
lupeol
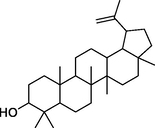
−7.5
Phe282, Asn258,
Gly286, Gly259, Met229, Glu226, Phe225, Pro223
−10
Asp197
Asp356, His305, Asp300, Thr163, Leu162, Tyr62, Trp59, Trp58,
8.
brucine
−7.5
–
Asn316, Asn277, Glu271, Val269, Tyr249, Ala247, Phe246, Lys242, Lys7, Trp6
−9.2
–
Tyr151, Ile235, Leu162, Trp58, Trp59, Tyr62, Asp300, Asp197, His201, Glu233
9.
stigmasterol, 22,23-dihydro-
−7.1
Lys205
Phe210, Gly209, Ala208, Glu173, Asn171, Glu157, Glu130, His129, Trp128, Ile127, Lys118
−9.3
Glu240
Ile235, Tyr151, Trp59, His299, Leu162, Arg195, Asp197, Ty62, Trp58, Glu233, Ala198, His201, Lys200
10.
Acarbose
−6.9
Asn316, Asn277, Phe276, Glu271, Ala270, Thr253, Tyr249, Lys248, Phe246, Lys7, Trp6
Asn275, Val269, Ile251, Asp250, Lys242,
−7.7
Gly403, Arg421, Arg398, Gly334, Thr11, Ser3, Thr6, Arg252, Gln404
Pro405, Asp402, Gln8, Phe335, Pro332
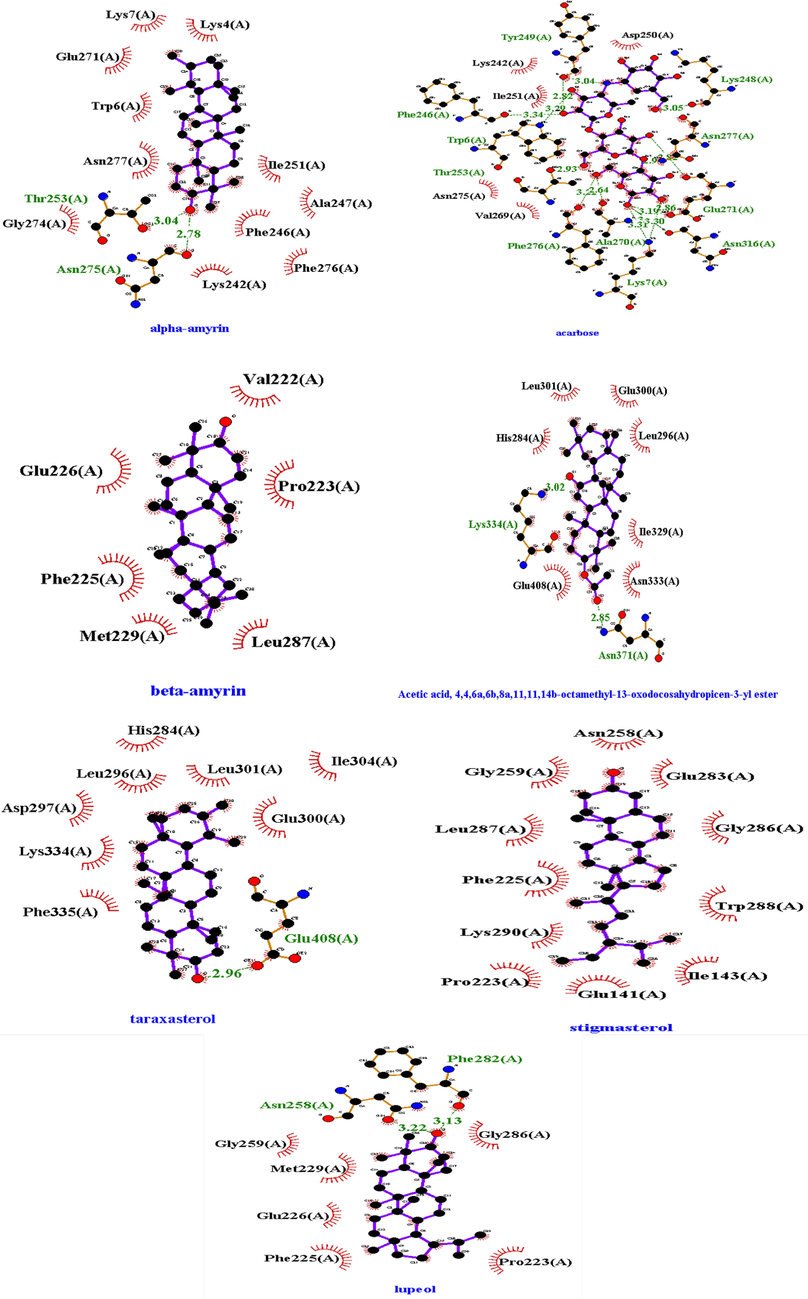
2D structures of best docked compounds with illustrated interactions (hydrogen and hydrophobic interactions) to α-glucosidase. Red color (interactions) represented the hydrophobic interactions and Green dotted line showed the hydrophilic interactions.
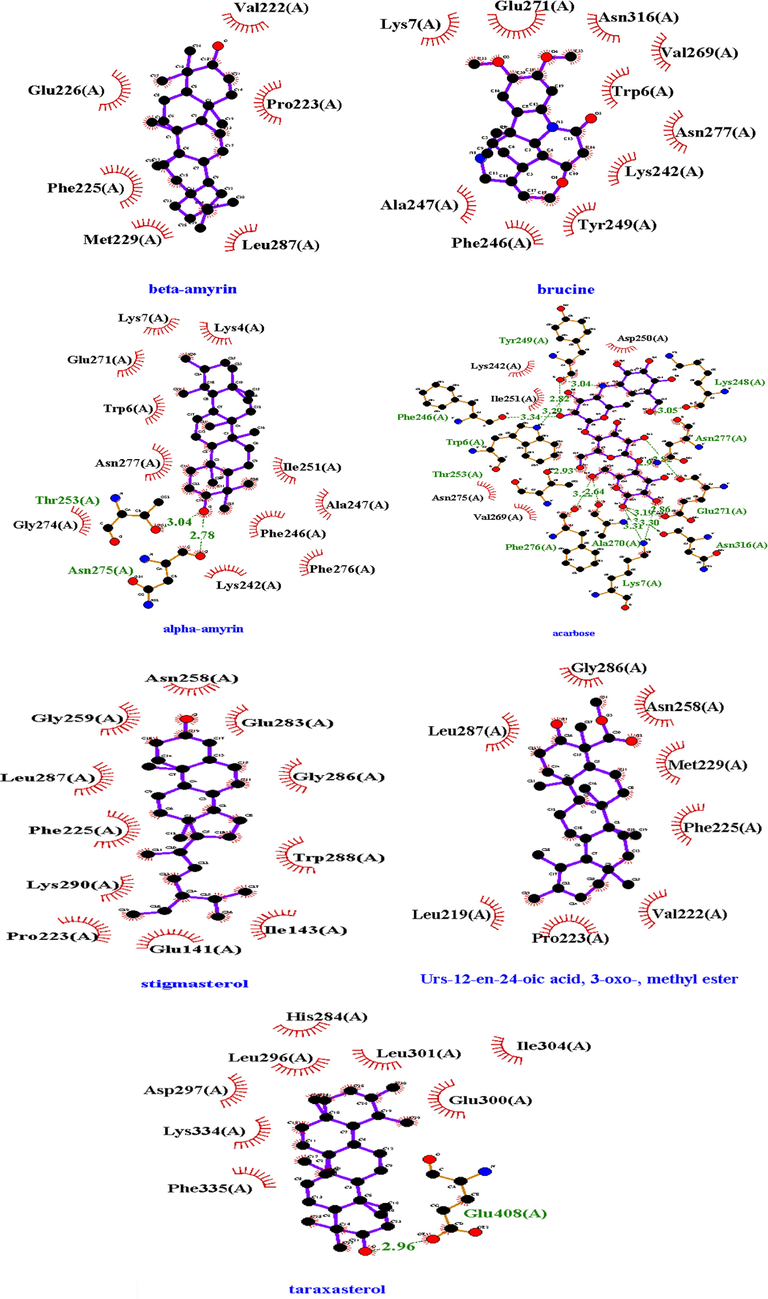
2D structures of best docked compounds with illustrated interactions (hydrogen and hydrophobic interactions) to α-amylase. Red color (interactions) represented the hydrophobic interactions and Green (dotted) line showed the hydrophilic interactions.
st α-glucosidase (Figs. S5 and S6, and Table S1). This revealed the potential of F. palmata extract to inhibit diabetic α-amylase and α-glucosidase.
3.3.2 ADMET analysis
The compounds (best docked) were further investigated using online software SwissADME which provide more knowledge about pharmacokinetic, physiochemical behavior and drug-likeness. In case of Lipinski’s rule of five, nearly all compounds violate the lipinski’s rule except brucine while stigmasterol violate the lipophilicity rule and acetic acid, 4,4,6a,6b,8a,11,11,14b-octamethyl-13-oxodocosahydropicen-3-yl ester violate the molar refractory rule. When any drug fails to meet two or more criteria of Lipinski’s rule that drug considered as non-orally drug. All compounds violate one rule except brucine (follows all rules) indicated that these compounds are bioavailable or orally available. All the best docked compounds possessed orally active likeness characteristics. Due to the many benefits it offers, the oral administration route is favoured over the many other medication delivery methods. These benefits include dependability to accommodate a range of medications, safety, excellent patient compliance, convenience of swallowing, pain avoidance, and simplicity of use. Table S2 and S3 represents various characteristics of best docked compounds including lipophilicity, pharmacokinetic behavior, molecular weight, no. of rotatable bond, no. of hydrogen bond acceptor and donor, Lipinski rule and other properties while Fig. 8 presented the bioavailability radar of compounds (best docked). Table S4 represented the compounds with predicted toxicity and LD50 performed by software ProTOX-II. In this, one compound predicted as carcinogenic and immunotoxic agent as urs-12-en-24-oic acid, 3-oxo-, methyl ester while other compounds showed low toxicity level.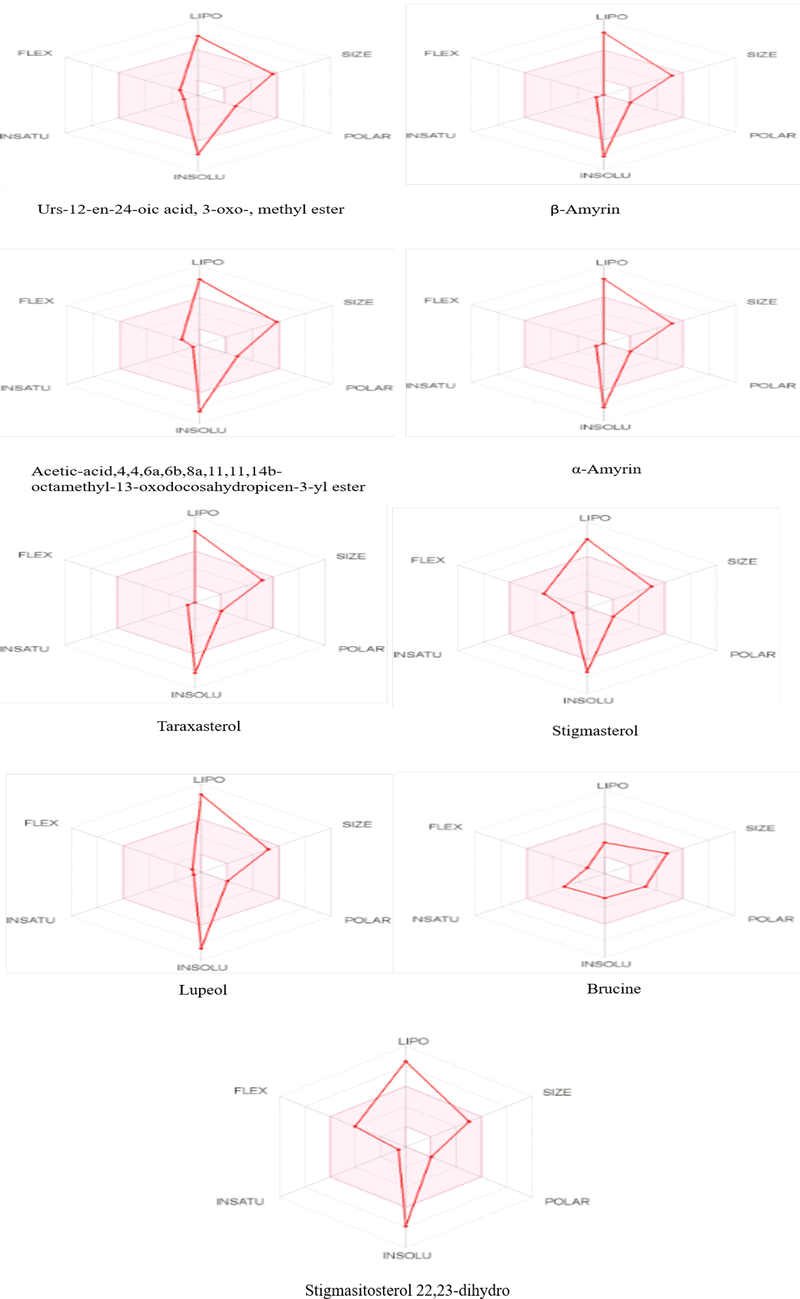
Bioavailability RADAR of best docked compounds.
4 Discussion
Phytocompounds investigation of extract of F. palmata exhibited phytocompounds such as carbohydrate, amino acids, lipids, tannins, phenols, flavonoid, saponins, steroid/ terpenes and alkaloids. Our results were in agreement with previous studies which have reported the presence of phytochemicals including carbohydrates, alkaloids, flavonoids saponins, tannins, resins and phenolic compounds in F. palmata extract while protein and amino acids were absent in all extract (Saklani and Chandra 2012). Most of these phytochemicals possessed pharmacological properties; for example alkaloids were found to possess analgesic and antimicrobial activities; flavonoids act as antioxidant and antibacterial while saponins possessed antibacterial, anticancer, and antidiabetic activities (Ghalloo et al., 2022). Thus, the presence of these phytochemicals in extract of F. palmata makes it likely to possess promising various therapeutical activities.
In literature, total bioactive components of the F. palmata plant parts were previously reported (Abbasi et al., 2015, Tewari et al., 2021). Our study revealed that the ethanolic extract of F. palmata possessed total phenolic content (49.24 ± 1.21 mg GAE/g extract) and total flavonoid content (29.9 ± 1.13 mg QE/g extract). The presence of high phenolic contents may increase its antioxidant potential (Balamurugan et al., 2020). From the vast literature review of whole plant of F. palmata which confirmed that there was no comprehensive data available about total bioactive components.
Antioxidants play a key role in the defense mechanism of organism against various pathological diseases (Litescu et al., 2011). Antioxidant activities of plants are attributed mainly to phenolic compounds which are necessary plant secondary metabolites. Basic mechanism of antioxidants is to neutralize reactive oxygen species and prevent conversion to free radicals. In our study, the plant F. palmata showed a significant antioxidant activities due to the presence of some polyphenolic acids which have antioxidant activities. These compounds are p-Coumeric acid (Boz 2015), Ferulic acid (Graf 1992), and Quercetin (Azeem et al., 2022). These results support of previous data reported in literature (Tewari et al., 2021).
The antidiabetic effect of F. palmata was previously reported and was found to happen through the inhibition of α-amylase and α-glucosidase enzymes leading to reduction of blood glucose level (Tewari et al., 2021). Our study confirmed the antidiabetic property of F. palmata extract which was more effective against α-glucosidase as compared to α-amylase; this is also in support of previous literature (Singh et al., 2014, Anjum and Tripathi 2019, Sati et al., 2020). This activity could be explained by antioxidant effect of flavonoid and triterpenoids (Singh et al., 2014), and also may be due to the compounds Kaempherol (Imran et al., 2019), Quercetin (Bule et al., 2019), and Rutin (Mel et al., 2020).
In our study, determination of antimicrobial potential of F. palmata extract was conducted by agar tube dilution and agar well diffusion method for antifungal and antibacterial activity respectively, against three fungal strains and seven bacterial strains. The results revealed maximum inhibition against fungal strains and significant inhibition zone (≥10) towards many bacterial strains used in assay. Phytochemicals identified tentatively by GCMS and HPLC quantified compounds namely Hydroquinone, 2-Methoxy-4-vinylphenol, Bergapten, 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one, Phytol, Octadecanoic acid, 9, 12-octadecadienoic acid ethyl ester, Urs-12-en-24-oic acid, 3-oxo-, methyl ester, Ferulic acid, Benzoic acid, and Quercetin (Formica and Regelson 1995) are likely to be responsible for this antimicrobial activity. Phytochemical compounds (phenolic compounds, alkaloids, terpenoids, carotenoids, and some sulfur-containing compounds) are known to inhibit pathogenic strains. Such phytochemical compounds could be involved in the discovery of new antibacterial medications to combat various multidrug-resistant bacterial infections (Barbieri et al., 2017). Previous studies revealed the antibacterial potential of F. palmata bark, leaf, root and fruit extracts which showed significant zone of inhibition (Saklani and Chandra 2011, Kothiyal and Saklani 2017).
Most of phytochemicals as flavonoid, polyphenols, saponins, coumarins, alkaloids, proteins and peptides have been reported to possessed antiviral activity against different viruses (Jassim and Naji 2003, Shahzad et al., 2019). There is no literature data found about the antiviral activity of F. palmata extract. Results of our study showed for the first time that ethanolic extract of F. palmata is strongly effective against viruses H9, NDV and IBV. These findings are promising and can be further investigated to develop new antiviral agent.
Thrombosis or blood clot is formed by the deposition of fibrin, tissue factors and platelets at the site of injury in endothelial cell surface. Platelets is a key factor in the formation of clot, as the thrombosis process begins when active platelets establish platelet-to-platelet linkages which leads in the development of plaque (Bhowmick et al., 2014). Streptokinase is a major thrombolytic agent with severe side effects that emphasize the researcher in the discovery of an alternative antithrombotic agent. Several studies reported that the tannins, saponins and alkaloids are involved in clot lysis and these phytochemicals are responsible for disrupting the fibrin and fibrinogen which leads to fibrinolysis (Uddin et al., 2020). In this present study, compounds detected by GCMS and quantified by LCMS; mainly 11, 14, 17-Eicosatrienoic acid methyl ester (Hansch and Von Kaulla 1970), α-amyrin, β-amyrin, lupeol (Mohammad et al., 2015), Kaempherol (Formica and Regelson 1995), Rutin (Dar and Tabassum 2012), Benzoic acid (Hansch and Von Kaulla 1970) are likely to be responsible for thrombolytic activity. There is no data available in literature about thrombolytic activity of F. palmata extract. Our study reported a significant thrombolytic potential of ethanolic extract of aerial part of F. palmata.
To best of our knowledge, there is no literature found about the cytotoxic activity of F. palmata extract against HepG2 cell line. We are reporting cytotoxicity of F. palmata extract against HepG2 cell line for the first time, in which we observed remarkable decline in the viability of cancer cells. This effect could be attributed to the high phenolic content of F. palmata with antioxidant potential. These antioxidants work by scavenging free radicals which are responsible for many pathological diseases including cancer, heart disease, aging, alzheimer’s disease, inflammation, neurodegenerative disorder (Menaga et al., 2021).
In literature, there is no in silico studies data available on F. palmata. The docking study of F. palmata have been employed for the prediction of binding affinity and to find the best pose of the respective compounds to bind within the active sites of diabetic enzymes. For better understanding about the inhibition ability of the studied bioactive phytochemical compounds, 9 phytochemical compounds of ethanolic extract (Taraxasterol, α-amyrin, β-amyrin, stigmasterol 22,23-dihydro, urs-12-en-24-oic acid, 3-oxo-methyl ester, lupeol, brucine, acetic acid, 4,4,6a,6b,8a,11,11,14b-octamethyl-13-oxodocosahydropicen-3-yl ester and stigmasterol) along with acarbose were docked against α-glucosidase and 8 compounds (Taraxasterol, α-amyrin, β-amyrin, stigmasterol 22,23-dihydro, urs-12-en-24-oic acid, 3-oxo-, methyl ester, lupeol, brucine, stigmasterol) along with acarbose were docked against α-amylase enzyme. ADME and toxicity studies were also performed for the evaluation of its pharmacokinetic behavior and toxicity level of major compounds analyzed by GCMS.
5 Conclusions
The present research exploring the in vitro and in silico activities, GCMS and phytochemical composition of F. palmata extract. The extract exhibited the high total phytochemical compounds which support the high antioxidant and significant enzyme (α-amylase and α-glucosidase) inhibition activities. The extract was evaluated for antibacterial activity (against seven bacterial strains) which, due to certain antibacterial phytochemicals; revealed good antibacterial activity. Strong antiviral and anticancer activities were exerted by the plant against certain viral strains and cell line (HepG2) because of presence of some important phytocompounds in the extract, justifying pharmacological activities of plant. The inhibition activity of F. palmata extract against diabetic enzymes were further evaluated by in silico studies such as molecular docking of all GCMS phytocompounds, from which the selected compounds (best docked) as Taraxasterol, α-amyrin, β-amyrin, stigmasterol 22,23-dihydro, urs-12-en-24-oic acid, 3-oxo-, methyl ester, lupeol, acetic acid, 4,4,6a,6b,8a,11,11,14b-octamethyl-13- oxo docosahydropicen −3-yl ester, brucine, and stigmasterol, undergo ADME and toxicity studies. ADME and toxicity study indicated pharmacokinetic behavior, drug likeness and toxicity level of best docked compounds. The results of biological assays of F. palmata ethanolic extract presented this plant to be uncovered treasure for the discovery of some lead compounds that can be applied against several disorders. In-depth in vivo studies to evaluate the therapeutic potentials of this plant are highly recommended.
Funding
The authors are grateful to the King Saud University, Riyadh, Saudi Arabia for funding this study through Project number RSP2022R504.
Acknowledgements
The authors acknowledge to the King Saud University, Riyadh, Saudi Arabia for funding this study through Project number RSP2022R504.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aati, H. Y., M. Anwar, J. Al-Qahtani, et al., 2022. Phytochemical profiling, in vitro biological activities, and in-silico studies of ficus vasta forssk.: an unexplored plant. antibiotics. 11, 1155.
- Ethnomedicinal values, phenolic contents and antioxidant properties of wild culinary vegetables. J. Ethnopharmacol.. 2015;162:333-345.
- [Google Scholar]
- In vitro bioactivity of extracts from seeds of Cassia absus L. growing in Pakistan. J. Herbal Med.. 2019;16:100258
- [CrossRef] [Google Scholar]
- Comprehensive phytochemical profiling, biological activities, and molecular docking studies of Pleurospermum candollei: an insight into potential for natural products development. Molecules. 2022;27:4113.
- [Google Scholar]
- Phytochemical composition and antioxidant activities of Dianthus Thunbergii hooper and Hypoxis Argentea harv ex baker: Plants used for the management of diabetes mellitus in Eastern Cape, South Africa. Pharmacognosy Mag.. 2018;14:195.
- [Google Scholar]
- Alpha-amylase and alpha-glucosidase enzyme inhibition and antioxidant potential of 3-oxolupenal and katononic acid isolated from Nuxia oppositifolia. Biomolecules. 2019;10:61.
- [Google Scholar]
- Phytochemical and pharmacological study of Ficus palmata growing in Saudi Arabia. Saudi Pharmaceutical J.. 2014;22:460-471.
- [Google Scholar]
- In-vitro antibacterial and antifungal properties of the organic solvent extract of Argemone mexicana L. J. King Saud Univ. - Sci.. 2020;32:2053-2058.
- [Google Scholar]
- Phytochemical screening and in vitro evaluation of antidiabetic activity of Ficus palmata fruits. Eur. J. Pharm. Med. Res.. 2019;6:251-258.
- [Google Scholar]
- Pharmacological activities of a novel phthalic acid ester and iridoid glycoside isolated from the root bark of Anthocleista vogelii Planch. Trop. Biomed.. 2019;36:35-43.
- [Google Scholar]
- An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: a review. Polym. Bull. 2022:1-22.
- [Google Scholar]
- Phytochemical screening, antioxidant, anti-diabetic and cytotoxic activity of leaves of Pandanus canaranus Warb. Mater. Today: Proc. 2020
- [Google Scholar]
- ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res.. 2018;46:W257-W263.
- [Google Scholar]
- Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res.. 2017;196:44-68.
- [Google Scholar]
- Effect of seasonal and regional variations on phenolic compounds of Deverra scoparia (flowers/seeds) methanolic extract and the evaluation of its in vitro antioxidant activity. Chem. Biodivers.. 2019;16:e1900420.
- [Google Scholar]
- In vivo analgesic, antipyretic, and anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic extract from Litsea glutinosa leaves. Biol. Res.. 2014;47:1-8.
- [Google Scholar]
- Cytotoxic and antiviral activities of the essential oils from Tunisian Fern, Osmunda regalis. S. Afr. J. Bot.. 2018;118:52-57.
- [Google Scholar]
- p-Coumaric acid in cereals: presence, antioxidant and antimicrobial effects. Int. J. Food Sci. Technol.. 2015;50:2323-2328.
- [Google Scholar]
- Antidiabetic effect of quercetin: a systematic review and meta-analysis of animal studies. Food Chem. Toxicol.. 2019;125:494-502.
- [Google Scholar]
- Evaluation of antimicrobial activity of Cissus quadrangularis L. stem extracts against Avian pathogens and determination of its bioactive constituents using GC-MS. J. Sci. Res.. 2020;64:90-96.
- [Google Scholar]
- Molecular docking of phenolic compounds and screening of antioxidant and antidiabetic potential of Moringa oleifera ethanolic leaves extract from Qassim region, Saudi Arabia. Saudi J. Biol. Sci.. 2022;29:854-859.
- [Google Scholar]
- Clinical and L. S. Institute, 2009. Performance standards for antimicrobial susceptibility testing of anaerobic bacteria: informational supplement, Clinical and Laboratory Standards Institute (CLSI).
- Chemical composition and biological evaluation of Typha domingensis pers. to ameliorate health pathologies: in vitro and in silico approaches. Biomed Res. Int.. 2022;2022:8010395.
- [CrossRef] [Google Scholar]
- In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virol. J.. 2012;9:1-9.
- [Google Scholar]
- Anti-inflammatory activity in rats and mice of phenolic acids isolated from Scrophularia frutescens. J. Pharm. Pharmacol.. 1998;50:1183-1186.
- [Google Scholar]
- Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol.. 1995;33:1061-1080.
- [Google Scholar]
- Ficus: En. Fig; Fr. Figuier; Ge. Feigenbaum; Sp. Higo. CRC PressCRC Handbook of Flowering; 2019. p. :331-350.
- Phytochemical profiling, in vitro biological activities, and in silico molecular docking studies of Dracaena reflexa. Molecules. 2022;27:913.
- [Google Scholar]
- Fibrinolytic congeners of benzoic and salicylic acid: a mathematical analysis of correlation between structure and activity. Biochem. Pharmacol.. 1970;19:2193-2200.
- [Google Scholar]
- GC-MS analysis and antimicrobial evaluation of Oldenlandia corymbosa. J. Environ. Nanotechnol.. 2014;3:161-167.
- [Google Scholar]
- Screening for secondary metabolites in Huru crepitans bark ethanol extract using GCMS analysis: a preliminary study approach. J. Sci. Technol. Adv.. 2016;1:64-71.
- [Google Scholar]
- Characterization of alkaloid constitution and evaluation of antimicrobial activity of Solanum nigrum using gas chromatography mass spectrometry (GC-MS) J. Pharmacogn. Phytother.. 2015;7:56-72.
- [Google Scholar]
- Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol.. 2003;95:412-427.
- [Google Scholar]
- Pharmacological evaluation of analgesic, anti-inflammatory and antipyretic activities of ethanolic extract of Indigofera argentea Burm. f. J. Ethnopharmacol.. 2020;259:112966
- [Google Scholar]
- Potential antioxidant and enzyme inhibitory effects of nanoliposomal formulation prepared from Salvia aramiensis Rech. f. extract. Antioxidants.. 2020;9:293.
- [Google Scholar]
- Study of antibiotic resistance pattern in methicillin resistant Staphylococcus aureus with special reference to newer antibiotic. J. Glob. Infect.. 2015;7:78.
- [Google Scholar]
- In-vitro assessment of cytotoxicity, antioxidant and anti-inflammatory activities of Ficus palmata. J. Herbal Med.. 2018;13:71-75.
- [Google Scholar]
- Alantolactone induces apoptosis in HepG2 cells through GSH depletion, inhibition of STAT3 activation, and mitochondrial dysfunction. BioMed Res. Int. 2013
- [Google Scholar]
- Foliar epidermal anatomy of some ethnobotanically important species of genus Ficus Linn. J. Medicinal Plants Res.. 2011;5:1627-1638.
- [Google Scholar]
- Evaluation of the Characteristics of Native Wild Himalayan Fig (Ficus palmata Forsk.) from Pakistan as a Potential Species for Sustainable Fruit Production. Sustainability. 2022;14:468.
- [Google Scholar]
- Efficacy of Phytochemicals Derived from Roots of Rondeletia odorata as Antioxidant, Antiulcer, Diuretic, Skin Brightening and Hemolytic Agents—A Comprehensive Biochemical and In Silico Study. Molecules. 2022;27:4204.
- [Google Scholar]
- New dammarane-type acetylated triterpenoids and their related compounds of Ficus pumila fruit. Chem. Pharm. Bull.. 1999;47:1138-1140.
- [Google Scholar]
- Isolation and identification of Ficus palmata leaves and their antimicrobial activities. J. Sci. Res.. 2017;9:193-200.
- [Google Scholar]
- Biosensors applications on assessment of reactive oxygen species and antioxidants. Environ. Biosensors. 2011;1:35-40.
- [Google Scholar]
- An Extensive Pharmacological Evaluation of New Anti-Cancer Triterpenoid (Nummularic Acid) from Ipomoea batatas through In Vitro, In Silico, and In Vivo Studies. Molecules. 2022;27:2474.
- [Google Scholar]
- Formulation of microencapsulated rutin and evaluation of bioactivity and stability upon in vitro digestive and dialysis conditions. Int. J. Biol. Macromol.. 2020;159:316-323.
- [Google Scholar]
- Antioxidant and Cytotoxic Activities of A Novel Isomeric Molecule (PF5) Obtained from Methanolic Extract of Pleurotus Florida Mushroom. J. Bioresources Bioproducts.. 2021;6:338-349.
- [Google Scholar]
- Phytochemical and Biological Investigations of Methanol Extract of Leaves of Ziziphus mauritiana Lam. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas.. 2015;14:179-189.
- [Google Scholar]
- Polyphenols and their potential role to fight viral diseases: an overview. Sci. Total Environ.. 2021;801:149719
- [Google Scholar]
- Thiazolidines: Potential anti-viral agents against avian influenza and infectious bronchitis viruses. Urmia, Iran: Veterinary Research Forum, Faculty of Veterinary Medicine, Urmia University; 2020.
- Antibiotic-resistant Staphylococcus aureus: a challenge to researchers and clinicians. Bacteriol. J.. 2012;2:23-45.
- [Google Scholar]
- Ramalingum, N., Mahomoodally, M.F., 2014. The therapeutic potential of medicinal foods. Advances in pharmacological sciences.
- The variation in antimicrobial and antioxidant activities of acetone leaf extracts of 12 Moringa oleifera (Moringaceae) trees enables the selection of trees with additional uses. S. Afr. J. Bot.. 2014;92:59-64.
- [Google Scholar]
- Evaluation of α-Glucosidase inhibitory potential of some homeopathic mother tinctures. RADS J. Pharm. Pharm. Sci.. 2018;6:190-193.
- [Google Scholar]
- Antimicrobial activity, nutritional profile and quantitative study of different fractions of Ficus palmata. Int. Res. J. Plant Sci.. 2011;2:332-337.
- [Google Scholar]
- Phytochemical screening of Garhwal Himalaya wild edible fruit Ficuspalmata. Int. J. Pharm Tech Res.. 2012;4:1185-1191.
- [Google Scholar]
- Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2013
- [Google Scholar]
- Biosynthesis of metal nanoparticles from leaves of Ficus palmata and evaluation of their anti-inflammatory and anti-diabetic activities. Biochemistry. 2020;59:3019-3025.
- [Google Scholar]
- Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacognosy J.. 2018;10
- [Google Scholar]
- Antiviral activities of Cholistani plants against common poultry viruses. Trop. Biomed.. 2020;37:1129-1140.
- [Google Scholar]
- Study of antiviral potential of cholistani plants against new castle disease virus. Pakistan. J. Zool.. 2019;51
- [Google Scholar]
- Anti-diabetic effect of hydroalcoholic extract of Ficus palmata Forsk leaves in streptozotocin-induced diabetic rats. Int. J. Green Pharmacy (IJGP). 2014;8
- [Google Scholar]
- Stear, M. J., 2005. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees), fifth ed. Volumes 1 & 2. World Organization for Animal Health 2004. ISBN 92 9044 622 6.€ 140. Parasitology. 130, 727-727.
- Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants.. 2020;9:165.
- [Google Scholar]
- Phenolic profiling, antioxidants, multivariate, and enzyme inhibitory properties of wild Himalayan Fig (Ficus palmata Forssk.): a potential candidate for designing innovative nutraceuticals and related products. Anal. Lett.. 2021;54:1439-1456.
- [Google Scholar]
- Exploration of in vitro thrombolytic, anthelminthic, cytotoxic and in vivo anxiolytic potentials with phytochemical screening of flowers of Brassica nigra. Future J. Pharm. Sci.. 2020;6:1-9.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104455.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







