Translate this page into:
Plant polyacetylenoids: Phytochemical, analytical and pharmacological updates
⁎Corresponding author at: Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, 10 Poyanghu Road, West Area, Tuanbo New Town, Jinghai District, Tianjin, 301617, People's Republic of China. wuhonghua2011@tjutcm.edu.cn (Hong-Hua Wu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
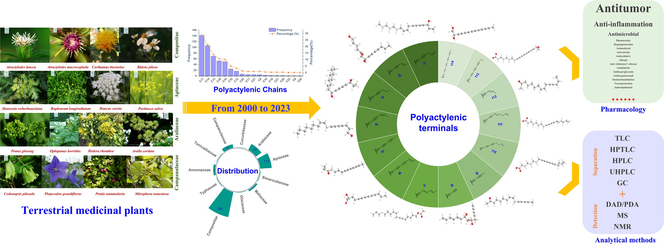
In terrestrial medicinal plants, polyacetylenoids have been isolated from almost 110 species belonging to 11 families including Compositae, Apiaceae, Araliaceae, Campanulaceae, Annonaceae, and Meliaceae. They are a class of natural products derived from fatty acids with carbon chain lengths of C8–19, C21, C23–25, C27, C29, and C33, possessing a pleiotropic profile of bioactivities such as anti-tumor, anti-inflammatory activities. Herein, this review aims at summarizing the inventory of polyacetylenoids occurring in terrestrial medicinal plants during the last two decades from 2000 to 2023, the NMR characteristics, and the progress on analytical methods and pharmacological investigation of the well-known plant polyacetylenoids.
Keywords
Plant polyacetylenoids
Phytochemicals
Analytical methods
Pharmacology
- TCM
-
Traditional Chinese medicine
- CMs
-
Chinese medicines
- ChP
-
Chinese Pharmacopoeia
- TLC
-
Thin-layer chromatography
- PTLC
-
Preparative thin-layer chromatography
- HPTLC
-
High-performance thin-layer chromatography
- MPLC
-
Medium pressure liquid chromatography
- HPLC
-
High performance liquid chromatography
- UHPLC
-
Ultra-high performance liquid chromatography
- UV
-
Ultraviolet
- PDA
-
Photo-diode array
- DAD
-
Diode array detector
- FID
-
Flame ionization detector
- MS
-
Mass spectrometry
- LC-MS
-
Liquid chromatography-mass spectrometry
- HPLC-UV
-
High performance liquid chromatography-ultraviolet
- GC–MS
-
Gas chromatography-mass spectrometry
- Q-TOF
-
Quadrupole-time of flight
- Q-Q-Q
-
Triple quadrupole tandem
- ESI
-
Electrospray ionization
- APCI
-
Atmospheric pressure chemical ionization
- SFE
-
Supercritical fluid extraction
- HR-MS
-
High resolution mass spectrometry
- FaOH
-
Falcarinol
- FaDOH
-
Falcarindiol
- FaDOAc
-
Falcarindiol-3-acetate
Abbreviations
1 Introduction
According to the fossil records, the human history of using terrestrial medicinal plants as remedies dates back at least 60,000 years. Terrestrial medicinal plants produce constitutive metabolites (primary or secondary) for the purpose of reproduction and survival. It's precisely these metabolites, well-known as phytochemicals, with qualities of a definite chemical diversity, a wide range of biological activities and drug-likeness, give us the possibility to protect against a variety of diseases such as malaria, gastrointestinal disorders, traumatic infection, fever, liver disorders, hypertension, tumor, and cancer. (Sanchez-Ramos et al., 2021). In today's world, the potential of this chemical arsenal has been well-recognized by chemists and pharmacologists to discover molecules functional as lead structures in the search and development of new drugs or as biological probes for physiological investigation (Yeboah et al., 2022).
Polyacetylenoids are a class of compounds derived from polyacetylenes (or acetylenes) that may be biosynthesized from fatty acids, featuring two or more acetylenic bonds in their nucleus scaffolds (Konovalov 2014; Xie and Wang 2022). So far, naturally occurring polyacetylenoids have been isolated from a wide range of biomasses such as plants, animals, fungi, and marine sponges (Kuklev and Dembitsky, 2014; Negri 2015; Christensen 2020). Until now, more than 1400 plant polyacetylenoids and their relevant derivatives have been isolated, mainly from the higher plants of Compositae, Apiaceae and Araliaceae families and sporadically from the plants of other families (Christensen and Brandt 2006; Patil et al., 2012). And numerous researches have been devoted into the pharmacological and biological properties of polyacetylenoids, such as antitumoral (Kobaek-Larsen et al., 2017), anti-inflammatory (Christensen 2020; Redl et al., 1994), antimicrobial, (Marčetić et al., 2014), hepatoprotective (Utrilla et al., 1995), phototoxic (Chobot et al., 2006), antimalarial (Tobinaga et al., 2009), anti-obesity(Jiao et al., 2014), antioxidative (Lee et al., 2013), allergic (Hansen et al., 1986), anti-Alzheimer’s disease (Hao et al., 2005), antidiabetic (Chien et al., 2009), immunoregulatory (Song et al., 2019), neuroprotective (Wang et al., 2016) and insecticidal (Herrmann et al., 2011) activities. Notably, the successful drug development of the polyacetylenoids is exemplified by the case of allyl enediyne antibiotics, which have been the most active antitumoral agents to date. Benefiting from the special molecular structure, the novel mechanism of action, and the broad development prospects (Thorson et al., 2000), enediyne antibiotics have been reported to be highly effective in killing tumor cells, and are very likely to be exploited as novel and highly effective antitumoral drugs.
As far as the literature we can reach, before 2000, people mainly reported the antitumoral (Matsunaga et al., 1990), phototoxic (Towers et al., 1979, Wat et al., 1979), and allergic properties (Hansen et al., 1986, Towers 1986, Gafner et al., 1988) of plant polyacetylenoids, followed by their anti-inflammatory, antioxidative (Redl et al., 1994) and hepatoprotective (Utrilla et al., 1995) activities. For examples, there were clinical trial and in vitro study convincing us the contact allergic action of falcarinol (Gafner et al., 1988). Several in vivo studies revealed the antioxidative (Cavin. et al., 1998) and hepatoprotective (Utrilla et al., 1995) effects of santolindiacetylene, and the antitumoral efficacy of falcarinol oxylipin in a LOX melanoma mouse xenograft model (Bernart et al., 1996). Since 2000, most studies have focused on their antitumoral, anti-inflammatory, and antimicrobial effects, followed by the bioactivities of antimalarial, anti-obesity, hepatoprotection, and anti-Alzheimer’s disease. And numerous in vivo studies have illustrated the above-mentioned biological functions of plant polyactylenoids.
Since 1972, several reviews summarized the studies on polyacetylenoids that occurred in families of Araliaceae and Apiaceae, and furtherly the genus of Bupleurum and Echinacea, focusing on their distribution, phytochemistry, biosynthesis and the bioactive chemicals and their pharmacological properties, as well as the analytical methods of the polyacetylenoids in Bupleurum species (Hansen and Boll 1986; Pellati et al., 2012; Chen et al., 2015; Lin et al., 2016). At the same time, biosynthesis progress on the natural polyacetylenoid products and their glycosidal derivatives has also been reviewed by Minto et al. (Minto and Blacklock 2008; Gung 2009; Pan et al., 2009; Dawid et al., 2015; Santos et al., 2022). Furthermore, the cytotoxic, anticancer, and anti-inflammatory bioactivities of several specific polyacetylenoids, including natural and synthetic acetylenic lipids, C17 and C18 acetylenic oxylipins, lobetyolin and its structural analogs, were collected by Dembitsky et al. (Bailly 2020; Christensen 2020; Dembitsky 2006). And recently, two reviews covering the advances on bioactivity properties of those polyacetylenoids originated from the terrestrial eukaryotic organisms, especially the herbal medicines, during 2000–2015 and 2014–2021, respectively (Negri 2015; Xie and Wang 2022).
However, there were few reviews focusing on a global classification and the structural spectroscopic characteristics of those terrestrial polyacetylenoids, which intuitively unveiling their molecular shapes and structural characteristics in aid of their structural elucidation. Further, there were few concerns on summarizing the analytical methods that have been developed for those representative terrestrial plant polyacetylenoids, as quality control of polyacetylenoid products plays a more and more important role in their future development and utilization. Under this background, the terrestrial plant polyacetylenoids were re-classified based on their intrinsic molecular characteristics in this Review. And herein, recent research progresses on their phytochemistry, plant origins, NMR characteristics and determination methods of their configurations, analytical methods, and the pharmacological benefits as exemplified by some typical polyacetylenoids (such as falcarinol, falcarindiol and lobetylolin) were updated.
In this Review, 'polyacetylenoids' is introduced to represent the polyacetylenes and their derivatives including polyacetylenic ethers, esters, glycosides, and the thio-products. For better clearness and ease for readers, the polyacetylenoid molecules described in this Review have been divided into two sections (linear polyacetylenoids and cyclic polyacetylenoids) based on their structural features (cyclic or not). And within each section, they have been presented in an order based on the general types of carbon skeletons in the clue of the substitution patterns of the 'acetylenic terminal' concentrated with triple and double bonds. Considering the main differences between polyacetylenoids' structures are the chain length and the cyclic pattern and that C17, C14, C18, C10, C13 and C15-polyacetylenoids are the most common polyacetylenoids in terrestrial medicinal plants, the linear polyacetylenoid structures are then divided into seven main classes, i.e., those with 17 carbons, 14 carbons, 18 carbons, 10 carbons, 13 carbons, and 15 carbons, and others. The clyclic polyacetylenoids have been furtherly separated into monocyclic, bicyclic, and polycyclic based on the complexity of ring systems in their structures. Summary tables reporting, for each group of molecules, the names, and sources of the presented structures, as well as their literature references, are embedded in the text (Table 1). a The compound with a letter (a, b, c, or d) in the compound number represents a different configurational isomer of the compound.
No.
Name
Formula
Origin
Part
Reference
1
(3R,8S)-Heptadec-1-ene-4,6-diyne-3,8-diol
C17H26O2
L. tenuissimum
Roots
(Choi et al., 2016)
2
8-Hydroxyheptadec-1-ene-4,6-diyn-3-yl acetate
C19H28O3
C. zimmermannii
Roots
(Baur et al., 2005; Senn et al., 2007)
3
10-Chloro-1-heptadecene-4,6-diyne-3,8,9-triol
C17H25ClO3
N. ternata
Aerial parts
(Nakagawa et al., 2004)
4
Panaxydol chlorohydrin
C17H25ClO2
P. ginseng
Roots
(Suzuki et al., 2017)
5
Baisanqisaponin B
C63H96O20
P. japonicus
Roots
(Liu et al., 2016)
6
Baisanqisaponin A
C59H90O16
P. japonicus
Roots
(Liu et al., 2016)
7
Baisanqisaponin C
C59H90O16
P. japonicus
Roots
(Liu et al., 2016)
8
(3R,9R,10R)-Panaxytriol
C17H26O3
P. ginseng
Roots
(Yang et al., 2008; Herrmann et al., 2013; Suzuki et al., 2017)
9
10-Methoxyheptadec-1-ene-4, 6-diyne-3,9-diol
C18H28O3
P. ginseng
Roots
(Yang et al., 2008)
10
Ac-panaxytriol
C23H32O6
P. ginseng
Roots
(Suzuki et al., 2017)
11
cis-Panaxydiol
C17H24O2
E. triquetrum
Aerial parts
(Bouzergoune et al., 2016)
12
Panaxydiol
C17H24O2
A. graveolens
Roots
(Zidorn et al., 2005)
G. littoralis
Whole plant
(Um et al., 2010)
O. horridus
Root barks
(Resetar et al., 2020)
P. sativa
Roots
(Roman et al., 2011)
S. divaricata
Roots and rhizomes
(Yokosuka et al., 2017)
13
(8E)-10-Hydroperoxy-1,8-heptadecadiene-4,6-diyn-3-ol
C17H24O3
S. divaricata
Roots and rhizomes
(Yokosuka et al., 2017)
14
1,8-Heptadeca-diene-4,6-diyne-3,10-diol
C17H24O2
P. ginseng
Roots
(Washida and Kitanaka 2003; Suzuki et al., 2017)
15
(8E)-Heptadeca-1,8-diene-4,6-diyne-(3S),10-diol
C17H24O2
D. morbiferus
Leaves
(Chung et al., 2011)
E. triquetrum
Aerial parts
(Bouzergoune et al., 2016)
16
(8E)-1,8-Heptadecadiene-4,6-diyne-3,10-diol
C17H24O2
G. littoralis
Roots
(Zhang et al., 2020)
17
Heptadeca-1,8-diene-4,6-diyne-3-ol-10-one
C17H22O2
E. triquetrum
Aerial parts
(Bouzergoune et al., 2016)
18
Cadiyenol
C24H36O6
C. asiatica
Aerial parts
(Govindan et al., 2007)
19
Falcarinol
C17H24O
P. pseudoginseng
Roots and rhizomes
(Tanaka et al., 2000)
N. ternata
Aerial parts
(Nakagawa et al., 2004)
C. aureum
Aerial parts
(Rollinger et al., 2003)
A. graveolens
Roots
(Zidorn et al., 2005)
E. yuccifolium
Aerial parts
(Ayoub et al., 2006)
L. officinale
Roots
(Zloh et al., 2007; Schinkovitz et al., 2008)
P. ginseng
Roots
(Washida and Kitanaka 2003; Liu et al., 2007; Yang et al., 2008; Qian et al., 2009; Herrmann et al., 2013; Suzuki et al., 2017)
G. littoralis
Whole plant
(Um et al., 2010; Zhang et al., 2020)
N. incisum
Roots
(Blunder et al., 2014)
S. divaricata
Roots and rhizomes
(Yokosuka et al., 2017)
E. triquetrum
Aerial parts
(Bouzergoune et al., 2016)
E. platyloba
Aerial parts
(Chianese et al., 2018)
A. furcijuga
Roots
(Yoshikawa et al., 2006)
C. pilosula
Roots
(Bailly 2020)
P. quinquefolius
Roots
(Baranska et al., 2006; Christensen et al., 2006)
D. carota
Roots
(Purup et al., 2009; Kramer et al., 2011; Killeen et al., 2013)
P. sativa
\
(Rawson et al., 2010; Roman et al., 2011; Corell et al., 2013)
P. quinquefolius
Roots
(Wang et al., 2010)
19aa
(R)-Falcarinol
C17H24O
D. guatemalense
Flowers, leaves, and twigs
(Grant et al., 2020)
O. horridus
Root barks
(Resetar et al., 2020)
20
Falcarindiol
C17H24O2
N. ternata
Aerial parts
(Nakagawa et al., 2004)
S. taiwaniana
Leaves
(Kuo et al., 2002)
L. mutellina
Roots
(Renate et al., 2002)
S. yunnanensis
Roots
(Wang et al., 2003)
C. barteri
Leaves
(Kraus 2003)
A. cordata
Roots
(Dang et al., 2005)
H. rhombea
Leaves
(Yamazoe et al., 2006; Yamazoe et al., 2007)
C. zimmermannii
Roots
(Senn et al., 2007)
A. sylvestris
Roots
(Jeong et al., 2007)
C. maritimum
Leaves
(Meot-Duros et al., 2010)
G. littoralis
Whole plant
(Um et al., 2010; Zhang et al., 2020)
O. horridus
Root barks
(Sun et al., 2010; Resetar et al., 2020)
O. elatus
Stems
(Yang et al., 2010)
N. incisum
Roots
(Blunder et al., 2014; Zheng et al., 2019)
P. praeruptorum
Roots
(Lee et al., 2015)
S. divaricata
Roots and rhizomes
(Yokosuka et al., 2017)
B. chinense
Roots
(Liu et al., 2017)
H. dissectum
Roots
(Gao et al., 2019)
A. furcijuga
Roots
(Yoshikawa et al., 2006)
P. ginseng
Roots
(Herrmann et al., 2013)
C. officinale
Rhizomes
(Venkatesan et al., 2018)
C. pilosula
Roots
(Bailly 2020)
D. carota
Roots
(Purup et al., 2009; Kramer et al., 2011; Koidis et al., 2012; Killeen et al., 2013)
P. sativa
\
(Rawson et al., 2010; Roman et al., 2011)
D. guatemalense
Flowers, leaves, and twigs
(Grant et al., 2020)
L. tenuissimum
Roots
(Choi et al., 2016)
20a
(+)-Falcarindiol
C17H24O2
A. scott-thomsonii
Subaerial parts
(Perry et al., 2001)
20b
(3R,8S)-Falcarindiol
C17H24O2
A. graveolens
Roots
(Zidorn et al., 2005)
L. officinale
Roots
(Zloh et al., 2007; Schinkovitz et al., 2008)
H. maximum
Roots
(Johnson et al., 2013)
O. fistulosa
Underground parts
(Appendino et al., 2009)
20c
(3S,8S)-Falcarindiol
C17H24O2
E. tricuspidatum
Aerial parts
(Djebara et al., 2019)
D. morbiferus
Leaves
(Chung et al., 2011)
21
8-Acetoxyfalcarinol
C20H28O2
N. ternata
Aerial parts
(Nakagawa et al., 2004)
H. rhombea
Leaves
(Yamazoe et al., 2006)
N. incisum
Roots
(Blunder et al., 2014)
22
8-O-Methylfalcarindiol
C18H26O2
P. sativa
Roots
(Roman et al., 2011)
A. graveolens
Roots
(Zidorn et al., 2005)
23
Falcarindiol 8-acetate
C19H26O3
A. cordata
Roots
(Dang et al., 2005)
24
Notoether D
C32H50O4
N. incisum
Roots and rhizomes
(Liu et al., 2014)
25
Notoether F
C32H50O4
N. incisum
Roots and rhizomes
(Liu et al., 2014)
26
Notoether B
C32H50O3
N. incisum
Roots and rhizomes
(Liu et al., 2014)
27
Notoether H
C32H50O4
N. incisum
Roots and rhizomes
(Liu et al., 2014)
28
(Z)-3-Hydroxyheptadeca-1,9-diene-4,6-diyn-8-yl-11-(1H-indol-3-yl) acetate
C27H31NO3
H. rhombea
Flower buds
(Yamazoe et al., 2007)
29
Notoincisol A
C27H32O5
N. incisum
Roots and rhizomes
(Liu et al., 2014)
30
Japoangelol A
C34H40O8
L. officinale
Roots
(Zloh et al., 2007)
31
Japoangelol C
C33H38O7
L. officinale
Roots
(Zloh et al., 2007)
32
Falcarindiol 3-O-acetate
C19H26O3
L. mutellina
Roots
(Renate et al., 2002)
33
Notoether C
C32H50O4
N. incisum
Roots and rhizomes
(Liu et al., 2014)
34
Notoether E
C32H50O4
N. incisum
Roots and rhizomes
(Liu et al., 2014)
35
Notoether A
C32H50O3
N. incisum
Roots and rhizomes
(Liu et al., 2014)
36
Notoether G
C32H50O4
N. incisum
Roots and rhizomes
(Liu et al., 2014)
37
Japoangelol B
C34H40O8
L. officinale
Roots
(Zloh et al., 2007)
38
Japoangelol D
C33H38O7
L. officinale
Roots
(Zloh et al., 2007)
39
Falcarinaiol 3-acetate
C19H26O3
D. carota
Roots
(Kramer et al., 2011; Koidis et al., 2012; Killeen et al., 2013)
P. sativa
Roots
(Roman et al., 2011)
40
(3S,8S)-Falcarindiol-3,8-diacetate
C21H28O4
E. tricuspidatum
Aerial parts
(Djebara et al., 2019)
41
11-Hydroxyfalcarindiol
C17H24O3
O. horridus
Root barks
(Resetar et al., 2020)
42
(3S,8S)-11-Acetoxyfalcarindiol
C19H26O4
E. tricuspidatum
Aerial parts
(Djebara et al., 2019)
43
Falcarinone
C17H22O
E. yuccifolium
The parts
(Ayoub et al., 2006)
P. ginseng
Roots
(Murata et al., 2017)
D. carota
Roots
(Purup et al., 2009)
P. sativa
Roots
(Roman et al., 2011)
44
Falcarinolone
C17H22O2
P. sativa
\
(Rawson et al., 2010; Roman et al., 2011)
45
(Z)-8-Acetoxy-3-oxoheptadeca-1,9-diene-4,6-diyne
C19H24O3
H. rhombea
Leaves
(Yamazoe et al., 2006)
46
Falcarindione
C17H20O2
P. sativa
\
(Rawson et al., 2010)
47
8-Acetoxy-heptadeca-1,9-diene-4,6-diyn-8-ol
C19H26O3
L. officinale
Roots
(Zloh et al., 2007)
48
Triquetridiol
C17H24O2
E. triquetrum
Aerial parts
(Bouzergoune et al., 2016)
49
Arteordoyn A
C17H24O2
A. ordosica
Aerial parts
(Wang et al., 2020)
50
(3R,8S)-Heptadeca-1,16-diene-4,6-diyne-3,8-diol
C17H24O2
A. halodendron
\
(Jin et al., 2021)
51
Ginsenoyne C
C17H24O3
P. ginseng
Roots
(Yang et al., 2008)
52
11,12-Dehydrofalcarinol
C17H22O
H. rhombea
Leaves
(Yamazoe et al., 2007)
53
(9Z,11Z)-Heptadeca-1,9,11-triene-4,6-diyne-3,8-diol
C17H22O2
H. rhombea
Flower buds
(Yamazoe et al., 2007)
54
Dendroarboreol B
C17H22O2
A. capillaris
Aerial parts
(Zhao et al., 2014)
D. guatemalense
Flowers, leaves, and twigs
(Grant et al., 2020)
T. procumbens
Aerial parts
(Chen et al., 2008)
55
2,9,16-heptadecatriene-4,6-diyn-8-ol
C17H22O
G. koraiensis
Roots
(Jung et al., 2002)
56
Dehydrofalcarindiol
C17H22O2
A. cordata
Roots
(Dang et al., 2005)
56a
(3R,8R)-Dehydrofalcarindiol
C17H22O2
A. ordosica
Aerial parts
(Wang et al., 2020)
A. monosperma
Aerial parts
(Stavri et al., 2005)
L. officinale
Roots
(Zloh et al., 2007; Schinkovitz et al., 2008)
57
Dehydrofalcarinol
C17H22O
A. capillaris
Aerial parts
(Zhao et al., 2014)
A. ordosica
Aerial parts
(Wang et al., 2020)
P. sativa
\
(Rawson et al., 2010)
D. guatemalense
Flowers, leaves, and twigs
(Grant et al., 2020)
T. procumbens
Roots
(Larque-Garcia et al., 2020)
58
Dehydrofalcarindiol 8-acetate
C19H24O3
A. cordata
Roots
(Dang et al., 2005)
59
1,9,16-Heptadecatriene-4,6-diyne-3,8-diol
C17H22O2
G. koraiensis
Roots
(Jung et al., 2002)
60
Gymnasterkoreayne C
C19H24O3
G. koraiensis
Roots
(Jung et al., 2002)
61
Dehydrofalcarinone
C17H20O
P. sativa
\
(Rawson et al., 2010)
62
10-Methoxyheptadeca-4,6-diyne-3,9-diol
C18H30O3
S. macrophylla
Roots
(Mi et al., 2019)
63
Dihydropanaxacol
C17H28O3
P. ginseng
Roots
(Fukuyama et al., 2012; Suzuki et al., 2017)
64
Oploxyne B
C18H30O4
O. elatus
Stems
(Yang et al., 2010)
65
8-Hydroxyheptadeca-4,6-diyn-3-yl acetate
C19H30O3
C. zimmermannii
Roots
(Baur et al., 2005; Senn et al., 2007)
66
1-Hydroxydihydropanaxacol
C17H28O4
P. ginseng
Roots
(Fukuyama et al., 2012)
67
Toonasindiyne A
C17H26O2
T. sinensis
Root barks
(Xu et al., 2020)
C. pilosula
Roots
(Bailly 2020)
68
Herpecaudene A
C17H26O3
H. caudigerum
Fruits
(Feng et al., 2017)
69
Herpecaudene B
C17H26O3
H. caudigerum
Fruits
(Feng et al., 2017)
70
Heptadec-8-ene-4,6-diyne-3,10-diol
C17H26O2
P. stipuleanatus
Roots
(Tuyen et al., 2018)
70a
(3R,8E,10S)-Heptadec-8-ene-4,6-diyne-3,10-diol
C17H26O2
S. macrophylla
Roots
(Mi et al., 2019)
T. sinensis
Root barks
(Xu et al., 2020)
70b
Panaxjapyne B
C17H26O2
E. triquetrum
Aerial parts
(Bouzergoune et al., 2016)
70c
1,2-Dihydropanaxydiol
C17H26O2
O. horridus
Root barks
(Resetar et al., 2020)
71
(E)-Heptadec-8-ene-4,6-diyne-3,10,11-triol
C17H26O3
S. macrophylla
Roots
(Mi et al., 2019)
72
Toonasindiyne F
C17H26O3
T. sinensis
Root barks
(Xu et al., 2020)
73
Toonasindiyne C
C17H24O2
T. sinensis
Root barks
(Xu et al., 2020)
C17H24O2
C. pilosula
Roots
(Bailly 2020)
74
Sadivaethyne D
C17H26O4
S. divaricata
Roots
(Sun et al., 2022)
75
Sadivaethyne C
C17H26O4
S. divaricata
Roots
(Sun et al., 2022)
76
1,2-Dihydrofalcarinol
C17H26O
O. horridus
Inner stem barks
(Cheung et al., 2019)
77
(9Z)-1,9-Heptadecadiene-4,6-diyne-3,8,11-triol
C17H26O3
L. officinale
Roots
(Zloh et al., 2007)
78
Panaxjapyne A
C17H26O
T. sinensis
Root barks
(Xu et al., 2020)
79
Oplopandiol
C17H26O2
O. horridus
Root barks
(Sun et al., 2010; Resetar et al., 2020)
O. elatus
Stems
(Yang et al., 2010)
L. tenuissimum
Roots
(Choi et al., 2016)
80
(3S,8S)-1,2-Dihydro-11-acetoxy-falcarindiol
C19H28O4
E. tricuspidatum
Aerial parts
(Djebara et al., 2019)
81
Sadivaethyne B
C17H26O4
S. divaricata
Roots
(Sun et al., 2022)
82
(9Z)-Heptadecene-4,6-diyn-1-ol
C17H26O
N. incisum
Roots
(Blunder et al., 2014)
83
Hederyne A
C17H26O3
H. rhombea
Leaves
(Yamazoe et al., 2007)
O. horridus
Root barks
(Resetar et al., 2020)
84
(9Z)-1-Methoxy-9-heptadecene-4,6-diyn-3-ol
C18H26O2
S. divaricata
Roots and rhizomes
(Yokosuka et al., 2017)
85
(Z)-8-Acetoxy-1-methoxy-3-oxoheptadec-9-ene-4,6-diyne
C20H28O4
H. rhombea
Leaves
(Yamazoe et al., 2006)
86
Heptadec-9-ene-4,6-diyne-3,11-diol
C17H26O2
S. macrophylla
Roots
(Mi et al., 2019)
87
Cirussuryne C
C17H28O3
C. japonicum
Roots
(Lee et al., 2022)
88
Ciryneol C
C17H25ClO2
C. rhinoceros
Whole plant
(Yim et al., 2003)
89
Cirussuryne G
C18H26O3
C. japonicum
Roots
(Lee et al., 2022)
90
Cirussuryne D
C18H26O2
C. japonicum
Roots
(Lee et al., 2022)
91
1,2-Dihydrodendroarboreol B
C17H24O2
D. guatemalense
Flowers, leaves, and twigs
(Grant et al., 2020)
T. procumbens
Aerial parts
(Chen et al., 2008)
92
1,2-Dihydro-16,17-dehydrofalcarinol
C17H24O
D. guatemalense
Flowers, leaves, and twigs
(Grant et al., 2020)
93
Dendroarboreol A
C17H24O2
T. procumbens
Aerial parts
(Chen et al., 2008)
94
Gymnasterkoreayne G
C17H24O3
G. koraiensis
Leaves
(Dat et al., 2005)
95
Gymnasterkoreayne E
C17H24O3
G. koraiensis
Roots
(Jung et al., 2002)
96
Cirussuryne H
C18H28O3
C. japonicum
Roots
(Lee et al., 2022)
97
Dihydrooenanthotoxin
C17H24O2
O. fistulosa
Underground parts
(Appendino et al., 2009)
98
Cicutol
C17H22O
Cicuta maculata
The tuber or seed
(Panter et al., 2011)
99
Cicutoxin
C17H22O2
O. fistulosa
Underground parts
(Appendino et al., 2009)
C. maculata
The tubers or seeds
(Panter et al., 2011)
100
(2Z,9Z)-Heptadecadiene-4,6-diyn-1-ol
C17H24O
B. longiradiatum
Whole plant
(Huang et al., 2009)
N. incisum
Roots
(Blunder et al., 2014)
101
Cirussuryne A
C17H24O3
C. japonicum
Roots
(Lee et al., 2022)
102
Cirussuryne F
C18H26O3
C. japonicum
Roots
(Lee et al., 2022)
103
Cirussuryne E
C19H26O2
C. japonicum
Roots
(Lee et al., 2022)
104
(2Z,8Z,10E)-Heptadecatriene-4,6-diyn-1-ol
C17H22O
B. scorzonerifolium
Roots
(Liu et al., 2015)
105
(2Z,8Z,10E)-Heptadecatriene-4,6-diyne-1,14-diol
C17H22O2
B. longiradiatum
Roots
(Huang et al., 2011)
106
(2Z,8Z,10E)-1-Hydroxyheptadecatriene-4,6-diyn-14-yl acetate
C19H24O3
B. longiradiatum
Roots
(Huang et al., 2011)
107
Bupleurynol
C17H22O
B. longiradiatum
Whole plant
(Huang et al., 2009; Huang et al., 2011)
B. scorzonerifolium
Roots
(Liu et al., 2015)
108
Acetylbupleurotoxin
C19H24O3
B. longiradiatum
Roots
(Huang et al., 2009; Huang et al., 2011)
109
Bupleurotoxin
C17H22O2
B. longiradiatum
Whole plant
(Huang et al., 2009; Huang et al., 2011; Zhang et al., 2014)
110
Bupleuronol
C17H20O2
B. longiradiatum
Whole plant
(Huang et al., 2009)
111
(2Z,8E,10E)-14S-Hydroxyheptadecatriene-4,6-diyn-1-yl acetate
C19H24O3
B. longiradiatum
Whole plant
(Huang et al., 2009)
112
(2Z,8E)-Heptadecadiene-10-oxo-4,6-diyn-1-ol
C17H22O2
B. chinense
Roots
(Cao et al., 2020)
113
Oenanthotoxin
C17H22O2
O. fistulosa
Underground parts
(Appendino et al., 2009)
E. platyloba
Aerial parts
(Chianese et al., 2018)
114
Gymnasterkoreayne F
C17H22O2
G. koraiensis
Roots
(Jung et al., 2002)
115
Gymnasterkoreayne D
C19H24O3
G. koraiensis
Roots
(Jung et al., 2002)
116
(8E,15E)-Heptadeca-8,15-diene-11,13-diynoic acid
C17H22O2
A. japonica
Rhizomes
(Rui and Chou 2022)
117
4,5-Dihydropanaxynol
C17H26O
N. incisum
Roots
(Blunder et al., 2014)
118
Crithmumdiol
C17H26O2
N. incisum
Roots
(Blunder et al., 2014)
119
(2E,4E,9Z)-Heptadecatrien-6-yn-1-yl acetate
C19H28O2
B. longiradiatum
Whole plant
(Huang et al., 2009; Huang et al., 2011)
120
(2E,4E,8E,10E)-Heptadecatetraen-6-yn-1-yl acetate
C19H26O2
B. longiradiatum
Whole plant
(Huang et al., 2009)
121
Panaxacol
C17H26O3
P. ginseng
Roots
(Fukuyama et al., 2012)
122
17-Hydroxypanaxacol
C17H26O4
P. ginseng
Roots
(Fukuyama et al., 2012)
123
Cirussuryne B
C17H24O3
C. japonicum
Roots
(Lee et al., 2022)
124
(2E)-10R-Tetradecaene-4,6-diyne-1,10,14-triol-1-O-β-D-glucopyranoside
C20H30O8
C. tinctorius
Florets
(He et al., 2011; Baek et al., 2021)
125
(2E,8Z)-12R-Tetradecadiene-4,6-diyne-1,12,14-triol-1-O-β-D-glucopyranoside
C20H28O8
C. tinctorius
Florets
(He et al., 2011)
126
Ritroyne A
C21H30O6S
E. ritro
Whole plant
(Li et al., 2019)
127
Coreoside E
C21H30O7
C. tinctoria
Fresh buds
(Guo et al., 2017)
128
Coreoside F
C26H38O11
C. tinctoria
Fresh buds
(Guo et al., 2017)
129
(6E,12E)-6,12-Tetradecadiene-8,10-diyne-1,3-diol
C14H18O2
A. japonica
Rhizomes
(Rui and Chou 2022)
130
(6E,12E)-1-Acetoxytetradeca-6,12-diene-8,10-diyn-3-ol
C16H20O3
A. japonica
Rhizomes
(Rui and Chou 2022)
A. macrocephala
Rhizomes
(Jeong et al., 2019)
131
Bidensyneoside E
C20H28O7
L. capitata
Aerial parts
(Emad et al., 2020)
132
1,3-Diacetoxy-tetradeca-(6E,12E)-diene-8,10-diyne
C18H22O4
A. japonica
Rhizomes
(Rui and Chou 2022)
A. macrocephala
Rhizomes
(Kim et al., 2018; Jeong et al., 2019)
133
Bidensyneoside F
C23H30O10
L. capitata
Aerial parts
(Emad et al., 2020)
134
Coreoside A
C25H36O11
C. tinctoria
Capitula
(Zhang et al., 2013)
135
(6E,12E)-3-Oxo-tetradeca-6,12-diene-8,10-diyn-1-ol
C14H16O2
B. pilosa
Aerial parts
(Wang et al., 2010)
136
Codonopilodiynoside E
C26H38O12
C. pilosula
Roots
(Jiang et al., 2015)
137
(6E,12E)-Tetradecadiene-8,10-diyne-1,3,14-triol
C14H18O3
B. bipinnata
Whole plant
(Wang et al., 2013)
137a
(2E,8E)-Tetradecadiene-4,6-diyne-1,11,14-triol
C14H18O3
C. tinctorius
Florets
(He et al., 2011)
138
3-O-β-D-Glucosyl-tetradeca-(6E,12E)-diene-8,10-diyne-1,14-diol
C20H28O8
B. bipinnata
Whole plant
(Wang et al., 2013)
B. gardneri
Leaves and stems
(Silva et al., 2015)
C. tinctoria
Capitula
(Zhang et al., 2013)
139
Coreoside B
C25H36O12
C. tinctoria
Capitula
(Zhang et al., 2013)
140
(6E,12E)-3-Oxo-tetradecadiene-8,10-diyne-14-hydroxy-1-O-β-D-glucopyranoside
C20H26O8
B. bipinnata
Whole plant
(Wang et al., 2013)
B. gardneri
Leaves and stems
(Silva et al., 2015)
141
Cordifolioidyne B
C20H28O8
C. cordifolioidea
Roots
(Mei et al., 2008)
C. tangshen
Roots
(Sun et al., 2016)
B. chinense
Roots
(Phan et al., 2022)
C. pilosula
Roots
(He et al., 2014; He et al., 2014; Bailly 2020)
141a
Codonopilodiynoside A
C20H28O8
C. pilosula
Roots
(Jiang et al., 2015)
142
Codonopilodiynoside B
C26H38O13
C. pilosula
Roots
(Jiang et al., 2015)
C. lanceolata
Roots
(Hu et al., 2018)
143
Coreoside C
C27H38O13
C. tinctoria
Capitula
(Zhang et al., 2013)
144
(2E,8E)-12R-Tetradecadiene-4,6-diyne-1,12,14-triol-1-O-β-D-glucopyranoside
C20H28O8
C. tinctorius
Florets
(He et al., 2011; Baek et al., 2021)
145
Codonopilodiynoside C
C26H38O13
C. pilosula
Roots
(Jiang et al., 2015)
146
Cordifolioidyne C
C20H28O7
C. cordifolioidea
Roots
(Mei et al., 2008)
147
Codonopilodiynoside F
C26H38O12
C. pilosula
Roots
(Jiang et al., 2015)
148
Lobetyol
C14H18O3
P. nummularia
Callus and hairy root
(Ishimaru et al., 2003)
C. cordifolioidea
Roots
(Mei et al., 2008)
L. chinensis
Aerial part
(Yang et al., 2014)
C. tangshen
Roots
(Sun et al., 2016)
P. grandiflorus
Roots
(Li 2022)
B. chinense
Roots
(Phan et al., 2022)
C. pilosula
Roots
(He et al., 2014; He et al., 2014; Bailly 2020)
149
Lobetyolin
C20H28O8
P. nummularia
Callus and hairy roots
(Ishimaru et al., 2003)
C. cordifolioidea
Roots
(Mei et al., 2008)
C. tangshen
Roots
(Sun et al., 2016)
P. grandiflorus
Roots
(Chen et al., 2018; Li 2022)
C. lanceolata
Roots
(Hu et al., 2018)
B. chinense
Roots
(Phan et al., 2022)
C. pilosula
Roots
(He et al., 2014; He et al., 2014; Bailly 2020)
L. inflata
Roots
(Bálványos et al., 2004)
150
Tangshenyne B
C26H38O13
C. tangshen
Roots
(Sun et al., 2016)
C. pilosula
Roots
(Bailly 2020)
151
Lobetyolinin
C26H38O13
P. nummularia
Callus and hairy roots
(Ishimaru et al., 2003)
C. tangshen
Roots
(Sun et al., 2016)
B. chinense
Roots
(Phan et al., 2022)
C. pilosula
Roots
(He et al., 2014; He et al., 2014; Bailly 2020)
L. inflata
Roots
(Bálványos et al., 2004)
152
Pratialin B
C32H48O18
P. nummularia
Callus and hairy roots
(Ishimaru et al., 2003)
C. pilosula
Roots
(Bailly 2020)
153
Pratialin A
C26H38O12
P. nummularia
Callus and hairy roots
(Ishimaru et al., 2003)
C. pilosula
Roots
(Bailly 2020)
154
Tangshenyne A
C20H28O9
C. tangshen
Roots
(Sun et al., 2016)
C. pilosula
Roots
(Bailly 2020)
155
Choushenpilosulyne A
C36H58O9
C. pilosula
Roots
(Bailly 2020)
156
threo-Tetradeca-2,10-diene-4,6-diyne-1,8,9,14-tetraol
C14H18O4
C. cordifolioidea
Roots
(Mei et al., 2008)
156a
(4E,12Z)-threo-Tetradeca-4,12-diene-8,10-diyne-1,6,7,14-tetraol
C14H18O4
C. lanceolata
Roots
(Hu et al., 2018)
157
Cordifolioidyne A
C20H28O9
C. cordifolioidea
Roots
(Mei et al., 2008)
158
Atractylodemayne A
C19H22O3
A. macrocephala
Rhizomes
(Yao and Yang 2014)
159
14-Acetoxy-12-α-methylbutyryltetradeca-(2E,8Z,10E)-triene-4,6-diyn-1-ol
C21H26O5
A. macrocephala
Rhizomes
(Yao and Yang 2014)
160
14-Acetoxy-12-senecioyloxytetradeca-(2E,8Z,10E)-triene-4,6-diyn-1-ol
C21H24O5
A. macrocephala
Rhizomes
(Yao and Yang 2014)
161
Atractylodemayne F
C20H24O5
A. macrocephala
Rhizomes
(Yao and Yang 2014)
162
Atractylodemayne D
C23H28O6
A. macrocephala
Rhizomes
(Yao and Yang 2014)
163
Atractylodemayne C
C23H26O6
A. macrocephala
Rhizomes
(Yao and Yang 2014)
164
(4E,6E,12E)-Tetradecatriene-8,10-diyn-1-ol
C14H16O
A. japonica
Rhizomes
(Rui and Chou 2022)
165
(4E,6E,12E)-1-Acetoxytetradecatriene-8,10-diyne
C16H18O2
A. japonica
Rhizomes
(Rui and Chou 2022)
166
1-O-Feruloyl-(4E,6E,12E)-tetradecatriene-8,10-diyne
C24H24O4
A. japonica
Rhizomes
(Rui and Chou 2022)
167
1-O-Feruloyl-(4E,6E,12E)-tetradecatriene-8,10-diyne-1,3-diol
C24H24O5
A. japonica
Rhizomes
(Rui and Chou 2022)
168
(4E,6E,12E)-Tetradecatriene-8,10-diyne-1,3-diyl diacetate
C18H20O4
A. lancea
Rhizomes
(Resch et al., 2001; Jiao et al., 2014)
169
1-O-Feruloyl-(4E,6E,12E)-tetradecatriene-8,10-diyne-1,3,14-triol
C24H24O5
A. japonica
Rhizomes
(Rui and Chou 2022)
170
Atractylodemayne B
C19H24O3
A. macrocephala
Rhizomes
(Yao and Yang 2014)
171
14-α-Methylbutyryltetradeca-(2E,8E,10E)-triene-4,6-diyn-1-ol
C19H24O4
A. macrocephala
Rhizomes
(Yao and Yang 2014)
172
14-β-Methylbutyryltetradeca-(2E,8E,10E)-triene-4,6-diyn-1-ol
C19H24O4
A. macrocephala
Rhizomes
(Yao and Yang 2014)
173
12-Senecioyloxytetradeca-(2E,8E,10E)-triene-4,6-diyn-1-ol
C19H22O4
A. macrocephala
Rhizomes
(Yao and Yang 2014)
174
14-Acetoxy-12-α-methylbutyl-(2E,8E,10E)-triene-4,6-diyn-1-ol
C21H26O5
A. lancea
Rhizomes
(Nur et al., 2020)
A. macrocephala
Rhizomes
(Yao and Yang 2014)
175
14-Acetoxy-12-senecioyloxytetradeca-(2E,8E,10E)-triene-4,6-diyn-1-ol
C21H24O5
A. lancea
Rhizomes
(Yao and Yang 2014)
A. macrocephala
Rhizomes
(Yao and Yang 2014)
176
Atractylodemayne G
C20H24O5
A. macrocephala
Rhizomes
(Yao and Yang 2014)
177
14-Acetoxy-12-β-methylbutyl-(2E,8E,10E)-triene-4,6-diyn-1-ol
C21H26O5
A. lancea
Rhizomes
(Yao and Yang 2014)
A. macrocephala
Rhizomes
(Yao and Yang 2014)
178
(2E,8E,10E,12R)-Tetradeca-2,8,10-triene-4,6-diyne-1,12,14-triol-1-O-β-D-glucopyranoside
C20H26O8
A. lancea
Rhizomes
(Xu et al., 2017)
179
(2E,8E,10E,12R)-Tetradeca-2,8,10-triene-4,6-diyne-1,12,14-triol-1-O-β-D-apiofuranosyl-(1–6)-β-D-glucopyranoside
C25H34O12
A. lancea
Rhizomes
(Xu et al., 2017)
180
Atractylodemayne E
C23H26O6
A. macrocephala
Rhizomes
(Yao and Yang 2014)
181
(5R)-5-Acetoxy-8,10,12-tetradecatriyne-1-O-β-D-glucopyranoside
C22H30O8
C. tinctorius
Florets
(Li et al., 2021)
182
1,3-Dihydroxy-6(E)-tetradecene-8,10,12-triyne
C14H16O2
B. pilosa
Whole plant; leaves
(Wu et al., 2004; Chen et al., 2020; Chung et al., 2021)
183
3-β-D-Glucopyranosyloxy-1-hydroxy-6(E)-tetradecene-8,10,12-triyne
C20H26O7
B. pilosa
Leaves
(Chien et al., 2009; Wang et al., 2010; Wen-Chin et al., 2013; Wei et al., 2016; Chen et al., 2021; Chung et al., 2021)
184
Artemisiaketone
C14H14O
T. vulgare
Flowers
(Moricz et al., 2018)
185
(5R,6E)-tetradecene-8,10,12-triyne-1-ol-5-O-β-glucoside
C20H26O7
C. lanceolata
Flowers
(Kim et al., 2020)
186
Platetyolin A
C20H26O8
P. grandiflorus
Roots
(Chen et al., 2018)
187
1-Acetoxy-3-angeloyloxy-(4E,6Z)-tetradeca-4,6-diene-8,10,12-triyne
C21H22O4
L. alpinum
Sub aerial parts
(Schwaiger et al., 2004)
188
3-O-β-D-Glucopyranosyloxy-1-hydroxy-(4E,6E)-tetradecene-8,10,12-triyne
C20H24O7
E. prostrata
Aerial parts
(Xi et al., 2014)
189
1-Acetoxy-3-angeloyloxy-(4E,6E)-tetradeca-4,6-diene-8,10,12-triyne
C21H22O4
L. alpinum
Sub aerial parts
(Schwaiger et al., 2004)
190
Tetradeca-1,3-diyne-6,7,8-triol
C14H22O3
S. macrophylla
Roots
(Mi et al., 2019)
191
6,7,8,9-Tetraacetoxytetradeca-1,3-diyne
C23H32O7
S. macrophylla
Roots
(Mi et al., 2019)
192
Panaxyne
C14H20O2
P. ginseng
Roots
(Yang et al., 2008)
193
8-Hydroxy-tetradec-(9E)-ene-11,13-diyn-2-one
C14H18O2
E. pallida
Roots
(Pellati et al., 2007; Tacchini et al., 2017)
194
Tetradec-(8Z)-ene-11,13-diyn-2-one
C14H18O
E. pallida
Roots
(Pellati et al., 2007; Pellati et al., 2012; Tacchini et al., 2017)
195
(2Z,8Z)-12R-Tetradecadiene-4,6-diyne-1,12,14-triol-1-O-β-D-glucopyranoside
C20H28O8
C. tinctorius
Florets
(He et al., 2011)
196
(2Z,8E)-12R-Tetradecadiene-4,6-diyne-1,12,14-triol-1-O-β-D-glucopyranoside
C20H28O8
C. tinctorius
Florets
(He et al., 2011)
197
Platetyolin B
C20H28O8
P. grandiflorus
Roots
(Chen et al., 2018)
198
(2E,4E,12Z)-N-Isobutyltetradeca-2,4,12-triene-8,10-diynamide
C18H23NO
C. zawadskii
Roots
(Rahman et al., 2007)
199
Pilosulyne G
C14H20O4
C. lanceolata
Roots
(Hu et al., 2018)
200
Codonopilodiynoside D
C20H30O8
C. pilosula
Roots
(Jiang et al., 2015)
201
16-Acetoxy-11-hydroxyoctadec-17-ene-12,14-diynyl acetate
C22H32O5
C. zimmermannii
Roots
(Senn et al., 2007)
201
16-Acetoxy-11-hydroxyoctadec-17-ene-12,14-diynyl acetate
C22H32O5
C. zimmermannii
Whole plant
(Baur et al., 2005)
202
11,16-Diacetoxyoctadec-17-ene-12,14-diynyl acetate
C24H34O6
C. zimmermannii
Roots
(Senn et al., 2007)
203
(8E)-Octadeca-1,8-diene-4,6-diyne-3,10-diol
C18H26O2
P. ginseng
Roots
(Murata et al., 2017)
203a
Stipudiol
C18H26O2
C. pilosula
Roots
(Bailly 2020)
204
Fruticotriol
C18H26O3
B. fruticosum
Aerial parts
(Fois et al., 2017)
204a
Oplopantriol C
C18H26O3
O. horridus
Root barks
(Resetar et al., 2020)
205
Oplopantriol C 18-acetate
C20H28O4
O. horridus
Root barks
(Resetar et al., 2020)
206
Octadeca-1,9-diene-4,6-diyne-3,8,18-triol
C18H26O3
A. gigas
Roots
(Choi et al., 2000)
206a
Oplopantriol A
C18H26O3
C. barteri
Leaves
(Kraus 2003)
C. zimmermannii
Roots
(Senn et al., 2007)
O. horridus
Root barks
(Resetar et al., 2020)
206b
(+)-9(Z),17-Octadecadiene-12,14-diyne-1,11,16-triol
C18H26O3
A. scott-thomsonii
Sub aerial parts
(Perry et al., 2001)
207
(Z)-11,16-Dihydroxyoctadeca-9,17-dien-12,14-diyn-1-yl acetate
C20H28O4
A. gigas
Roots
(Choi et al., 2000)
S. taiwaniana
Leaves
(Kuo et al., 2002)
207a
(9Z,11S,16S)-Octadeca-9,17-diene-12,14-diyne-1,11,16-triol 1-Acetate
C20H28O4
C. zimmermannii
Roots
(Senn et al., 2007)
C20H28O4
O. horridus
Root barks
(Sun et al., 2010; Resetar et al., 2020)
207b
(11R,16R,Z)-11,16-Dihydroxyoctadeca-9,17-dien-12,14-diyn-1-yl acetate
C20H28O4
O. horridus
inner stem bark
(Tai et al., 2014; Cheung et al., 2019)
207c
(+)-9(Z),17-Octadecadiene-12,14-diyne-1,11,16-triol 1-acetate
C20H28O4
A. scott-thomsonii
Leaves
(Perry et al., 2001)
208
Homopanaxynol
C18H26O
P. ginseng
Roots
(Murata et al., 2017)
209
Dendrotrifidiol
C18H26O2
O. horridus
Root barks
(Resetar et al., 2020)
210
Dendrotrifidiol 18-acetate
C20H28O3
O. horridus
Root barks
(Resetar et al., 2020)
211
cis-9,17-Octadecadiene-12,14-diyne-1,16-diol
C18H26O2
B. fruticosum
Aerial parts
(Fois et al., 2017)
212
(9Z,16S)-16-Hydroxyl-9,17-octadecadiene-12,14-diynoic acid
C18H24O3
D. morbiferus
Leaves
(Park et al., 2004; Kang et al., 2018)
213
cis-16-Oxo-octadeca-9,17-diene-12,14-diyn-1-al
C18H22O2
P. sativa
Roots
(Roman et al., 2011)
214
3,8,18-Triacetoxyloctadeca-1,9-diene-4,6-diyne
C24H32O6
A. gigas
Roots
(Choi et al., 2000)
215
1-Acetoxymethylfalcarindiol
C24H32O6
C. barteri
Leaves
(Kraus 2003)
216
Toonasindiyne B
C18H28O3
T. sinensis
Root barks
(Xu et al., 2020)
C. pilosula
Roots
(Bailly 2020)
217
(3R,10S,8E)-8-Octadecene-4,6-diyne-3,10,18-triol
C18H28O3
O. horridus
Root barks
(Resetar et al., 2020)
218
(3R,10S,8E)-8-Octadecene-4,6-diyne-3,10,18-triol 18-acetate
C20H30O4
O. horridus
Root barks
(Resetar et al., 2020)
219
Octadec-10-ene-12,14-diyne-1,9,16-triol
C18H28O3
T. sinensis
Root barks
(Xu et al., 2020)
220
9,16-Dihydroxyoctadec-10-ene-12,14-diyn-1-yl acetate
C20H30O4
T. sinensis
Root barks
(Xu et al., 2020)
221
Toonapolyyne A
C18H28O4
T. sinensis
Root barks
(Xu et al., 2020)
222
Toonasindiyne D
C18H26O3
T. sinensis
Root barks
(Xu et al., 2020)
C. pilosula
Roots
(Bailly 2020)
223
Toonasindiyne E
C20H28O4
T. sinensis
Root barks
(Xu et al., 2020)
C. pilosula
Roots
(Bailly 2020)
224
Toonapolyyne C
C18H28O2
T. sinensis
Root barks
(Xu et al., 2020)
225
Oplopandiol acetate
C20H30O4
O. horridus
Root barks
(Sun et al., 2010; Resetar et al., 2020)
226
Oplopantriol B
C18H28O3
O. horridus
Root barks
(Resetar et al., 2020)
227
1-Hydroxyoplopantriol B
C18H28O4
O. horridus
Root barks
(Resetar et al., 2020)
228
1-Hydroxyoplopantriol B 18-acetate
C20H30O5
O. horridus
Root barks
(Resetar et al., 2020)
229
13,14-Dihydrooropheic acid
C18H24O2
M. tomentosa
Leaves and twigs
(Wongsomboon et al., 2021)
230
(-)-(7R,8S)-Mitrephentosin B
C41H50O8
M. tomentosa
Leaves and twigs
(Wongsomboon et al., 2021)
231
(-)-(7R,8S)-Mitrephentosin A
C39H48O7
M. tomentosa
Leaves and twigs
(Wongsomboon et al., 2021)
232
(-)-(7R,8S)-Mitrephentosin C
C39H48O7
M. tomentosa
Leaves and twigs
(Wongsomboon et al., 2021)
233
Oropheic acid
C18H22O2
M. glabra
The stem bark
(Li et al., 2009)
M. tomentosa
Leaves and twigs
(Wongsomboon et al., 2021)
234
Methyloropheate
C19H24O2
M. glabra
The stem bark
(Li et al., 2009)
235
(-)-(7R,8S)-Mitrephentosin E
C41H48O8
M. tomentosa
Leaves and twigs
(Wongsomboon et al., 2021)
236
(-)-(7R,8S)-Mitrephentosin D
C39H46O7
M. tomentosa
Leaves and twigs
(Wongsomboon et al., 2021)
237
(2E,4E,9Z)-Octadecatrien-6-yne-1,18-diol
C18H28O2
B. longiradiatum
Whole plant
(Huang et al., 2009)
238
(2E,4E,9Z)-1-Hydroxyoctadecatrien-6-yn-18-yl acetate
C20H30O3
B. longiradiatum
Whole plant
(Huang et al., 2009)
239
(2E,4E,9Z)-Octadecatrien-6-yne-1,18-diyl diacetate
C22H32O4
B. longiradiatum
Whole plant
(Huang et al., 2009; Huang et al., 2011)
240
(2Z,9Z)-Octadecadiene-4,6-diyne-1,18-diol
C18H26O2
B. longiradiatum
Whole plant
(Huang et al., 2009)
241
Octadec-17-ene-9,11-diynoate ethyl
C20H30O2
O. gore
The seeds
(Ntumba et al., 2018)
242
(-)-17-Hydroxy-9,11,13,15-octadecatetraynoic acid/Minquartynoic acid
C18H20O3
O. amentacea
The branches
(Rashid et al., 2001)
243
Minquartynoic acid methyl ester
C19H22O3
O. amentacea
The branches
(Rashid et al., 2001)
244
(S)-17,18-Dihydroxy-9,11,13,15-octadecatetraynoic acid
C18H20O4
O. amentacea
The twigs
(Ito et al., 2001)
245
8-Hydroxy-octadec-13-ene-9,11-diynoate ethyl
C20H30O3
O. gore
The seeds
(Ntumba et al., 2018)
246
(-)-(7R,8S)-Mitrephentosin F
C39H50O7
M. tomentosa
Leaves and twigs
(Wongsomboon et al., 2021)
247
8-Hydroxy-octadeca-13,17-diene-9,11-diynoate ethyl
C20H28O3
O. gore
The seeds
(Ntumba et al., 2018)
248
Octadeca-9,11,13-triynoic acid
C18H24O2
M. glabra
The stem barks
(Li et al., 2009)
249
(E)-15,16-Dihydrominquartynoic acid\(S)-17-Hydroxy-15E-octadecene-9,11,13-triynoic acid
C18H22O3
O. amentacea
The dried twigs
(Ito et al., 2001)
250
(8E)-Decene-4,6-diyne-1-ol-1-O-β-D-glucopyranoside
C16H22O6
C. tinctorius
Florets
(He et al., 2011; Baek et al., 2021)
251
(8E)-Decaene-4,6-diyne-1-O-β-D-glucopyranosyl-(1–2)-β-D-glucopyranoside
C22H32O11
C. morifolium
Flowers
(Li et al., 2021)
252
(8E)-Decaene-4,6-diyne-1-O-β-D-glucopyranosyl-(1–6)-β-D-glucopyranosyl-(1–2)-β-D-glucopyranoside
C28H42O16
H. dissectum
Roots
(Gao et al., 2019)
253
Gymnasterkoreayne A
C10H12O2
G. koraiensis
Roots
(Jung et al., 2002; Park et al., 2002)
254
Bidensyneoside A1
C16H22O7
B. parviflora
Whole plant
(Wang et al., 2001)
C. pilosula
Roots
(Bailly 2020)
L. capitata
Aerial parts
(Emad et al., 2020)
255
6́-O-Acetylbidensyneoside A1
C18H24O8
L. capitata
Aerial parts
(Emad et al., 2020)
256
(E,E)-Matricarianol
C10H10O
T. angulata
Roots
(Pan et al., 2006)
257
(2E,8E)-Decadiene-4,6-diyne-1-O-β-D-glucopyranoside
C16H20O6
C. tinctorius
Florets
(Li et al., 2021)
258
(3R,8E)-Decene-4,6-diyne-1,3,10-triol
C10H12O3
B. parviflora
Whole plant material
(Li et al., 2008)
259
3-Deoxybidensyneoside B
C16H22O7
B. parviflora
Whole plant
(Wang et al., 2001)
260
(E)-Dec-2-ene-4,6-diyne-1,10-diol-1-O-β-D-apiofuranosyl-(1–6)-β-D-glucopyranoside
C21H30O11
A. lancea
Rhizomes
(Xu et al., 2016)
261
(E)-Dec-2-ene-4,6-diyne-1,10-diol-1-O-β-D-glucopyranoside
C16H22O7
A. lancea
Rhizomes
(Xu et al., 2016)
262
(2E,8E)-Deca-2,8-diene-4,6-diyne-1,10-diol-1-O-β-D-apiofuranosyl-(1–6)-β-D-glucopyranoside
C21H28O11
A. lancea
Rhizomes
(Xu et al., 2016)
263
(2Z,8E)-Deca-2,8-diene-4,6-diyne-1,10-diol-1-O-β-D-glucopyranoside
C16H20O7
A. lancea
Rhizomes
(Xu et al., 2016)
264
Bidensyneoside C
C16H22O8
B. parviflora
Whole plant
(Wang et al., 2001)
C. pilosula
Roots
(Bailly 2020)
265
(2E)-Decaene-4,6-diyne-1-O-β-D-glucopyranoside
C16H22O6
C. tinctorius
Florets
(Li et al., 2021)
266
(8R)-Deca-2-trans-2-ene-4,6-diyne-1,8-diol
C10H12O2
T. angulata
Roots
(Pan et al., 2006)
267
(2E,8R)-Decene-4,6-diyne-1,8-diol-8-O-β-D-glucopyranoside
C16H22O7
A. lancea
Rhizomes
(Xu et al., 2017)
268
(2E,8R)-Decene-4,6-diyne-1,8-diol-O-di-β-D-glucopyranoside
C22H32O12
A. lancea
Rhizomes
(Xu et al., 2017)
269
(2E,8S)-Decene-4,6-diyne-1,8-diol-8-O-β-D-glucopyranoside
C16H22O7
A. lancea
Rhizomes
(Xu et al., 2017)
270
Bidenoside C
C16H22O6
C. tinctorius
Florets
(He et al., 2011)
C. tinctorius
Florets
(Baek et al., 2021; Li et al., 2021; Ngo et al., 2021)
C. lanceolata
Flowers
(Kim et al., 2020)
271
Kamiohnoyneoside B
C22H32O11
C. morifolium
Flowers
(Kurimoto et al., 2021; Li et al., 2021)
272
(8Z)-Decene-4,6-diyne-1-O-β-D-glucopyranosyl-(1–6)-β-D-glucopyranosyl-(1–2)-β-D-glucopyranoside
C28H42O16
H. dissectum
Roots
(Gao et al., 2019)
273
(8Z)-8-Decene-4,6-diyn-1-yl 2-O-β-D-glucopyranuronosyl-β-D-glucopyranoside
C22H32O11
C. tinctorius
Flowers
(Ngo et al., 2021)
274
(8Z)-Decene-4,6-diyn-1-ol-1-O-β-D-glucuronyl-(1′'-2′)-β-D-glucopyranoside
C22H30O12
C. tinctorius
Florets
(He et al., 2011)
275
(8Z)-1-[(3-O-β-D-Glucosyl)-isovaleroyloxy]-8-decene-4,6-diyne
C21H30O8
C. tinctorius
Florets
(Li et al., 2021)
276
Bidensyneoside A2
C16H22O7
B. parviflora
Whole plant
(Wang et al., 2001)
277
(8Z)-Decene-4,6-diyne-1,10-diol-1-O-β-D-glucopyranoside
C16H22O7
C. tinctorius
Florets
(Baek et al., 2021)
278
(8Z)-Decene-4,6-diyne-1,3,10-triol
C10H12O3
A. capillaris
Aerial parts
(Zhao et al., 2014)
279
(8Z)-Decen-1-isovaleroyloxy-4,6-diyne-10-O-β-D-glucopyranoside
C21H30O8
C. tinctorius
Florets
(Li et al., 2021)
280
(2E,8Z)-Deca-2,8-diene-4,6-diyne-1,10-diol-1-O-β-D-glucopyranoside
C16H20O7
A. lancea
Rhizomes
(Xu et al., 2016)
281
(2E,8Z)-Deca-2,8-diene-4,6-diyne-1,10-diol-1-O-β-D-apiofuranosyl-(1–6)-β-D-glucopyranoside
C21H28O11
A. lancea
Rhizomes
(Xu et al., 2017)
282
(2E,8Z)-Decadiene-4,6-diyne-1-ol-1-O-β-D-glucopyranoside
C16H20O6
C. tinctorius
Florets
(He et al., 2011)
283
Echinacetylene
C11H12O3
E. purpurea
Roots
(Chang et al., 2020)
284
(8S)-Deca-2-trans-2,9-diene-4,6-diyne-1,8-diol
C10H10O2
T. angulata
Roots
(Pan et al., 2006)
285
3, 8-Dihydroxydec-9-en-4, 6-yne-1-O-β-D-glucopyranoside
C16H22O8
A. monosperma
Aerial parts
(Stavri et al., 2005)
286
(1,3R,8R)-Trihydroxydec-9-en-4,6-yne
C10H12O3
A. monosperma
Aerial parts
(Stavri et al., 2005)
L. officinale
Roots
(Zloh et al., 2007)
287
(1,3S,8S)-Trihydroxydec-9-en-4,6-yne
C10H12O3
A. capillaris
Aerial parts
(Zhao et al., 2014)
288
(3S,8S)-Dihydroxydec-9-en-4,6-yne
C25H28O11
A. scoparia
Aerial parts
(Geng et al., 2015)
289
(3S,8S)-Dihydroxydec-9-en-4,6-yne-1-O-(6′-O-caffeoyl)-β-D-glucopyranoside
C25H28O11
A. scoparia
Aerial parts
(Geng et al., 2015)
290
4,6-Decadiyne-1-O-β-D-glucopyranoside
C16H24O6
C. tinctorius
Florets
(Li et al., 2021)
291
4,6-Decadiyne-1-O-β-D–glucopyranosyl-(1–6)-β-D-glucopyranosyl-(1–2)-β-D-glucopyranoside
C28H44O16
H. dissectum
Roots
(Gao et al., 2019)
292
(8S)-Deca-4,6-diyne-1,8-diol-1-O-β-D-glucopyranoside
C16H24O7
C. tinctorius
Florets
(Baek et al., 2021)
293
(8S)-Deca-4,6-diyne-1,8-diol-8-O-β-D-glucopyranoside
C16H24O7
C. tinctorius
Florets
(Baek et al., 2021)
294
(8S)-Deca-4,6-diyne-1,8-di-O-β-D-glucopyranoside
C22H34O12
C. tinctorius
Flowers
(Ngo et al., 2021)
295
(2Z)-Decene-4,6,8-triyne-1-O-β-D-glucopyranoside
C16H18O6
C. tinctorius
Florets
(Li et al., 2021)
296
Dehydromatricaria ester
C11H8O2
A. ordosica
Aerial parts
(Wang et al., 2020)
297
Kamiohnoyneoside A
C22H30O11
C. morifolium
Flowers
(Kurimoto et al., 2021)
298
Bidensyneoside B
C16H20O7
B. parviflora
Whole plant
(Wang et al., 2001)
C. pilosula
Roots
(Bailly 2020)
299
(2S) (5E,11E)-Tridecadiene-7,9-diyne-1,2,13-triol
C13H16O3
B. bipinnata
Whole plant
(Wang et al., 2013)
300
(12R)-Trideca-(2E,8E)-diene-4,6-diyne-1,14-diol 12-O-β-D-glucopyranoside
C19H26O8
B. parviflora
Whole plant
(Zhu et al., 2021)
301
(3Z,5E,11E)-Tridecatriene-7,9-diynyl 1-O-(E)-ferulate
C23H22O4
A. lancea
Rhizomes
(Resch et al., 2001)
302
(3Z,5E,11E)-3,5,11-Tridecatriene-7,9-diyne 1-O-acetate
C15H16O2
A. lancea
Rhizomes
(Jiao et al., 2014)
303
(3Z,5E,11E)-3,5,11-Tridecatriene-7,9-diyne 1,2-diacetate
C17H18O4
A. lancea
Rhizomes
(Jiao et al., 2014)
304
(3E,5E,11E)-Tridecatriene-7,9-diyne-1,2,13-triol-2-O-β-D-glucopyranoside
C19H24O8
B. frondosa
Aerial parts
(Le et al., 2015)
305
(2E,8E,10E)-12R-Tridecatriene-4,6-diyne-1,12,13-triol-1-O-β-D-glucopyranoside
C19H24O8
C. tinctorius
Flowers
(He et al., 2011; Ngo et al., 2021)
306
syn-(5E,11E)-3-Acetoxy-4-O-(3-methylbutanoyl)-1,5,11-tridecatriene-7,9-diyne-3,4-diol
C20H24O4
A. lancea
Rhizomes
(Jiao et al., 2014)
307
(5E,11E)-1,5,11-Tridecatriene-7,9-diyne 3,4-diacetate
C17H18O4
A. lancea
Rhizomes
(Jiao et al., 2014)
308
(1,3Z,11E)-Tridecatriene-7,9-diyne-5-hydroxyl 6-O-β-D-glucopyranoside
C19H24O7
A. lancea
Rhizomes
(Ji et al., 2010)
309
erythro-(1,3Z,11E)-Tridecatriene-7,9-diyne-5,6-diyl diacetate
C17H18O4
A. lancea
Rhizomes
(Resch et al., 2001)
310
1,2-Dihydroxy-(5E)-tridecene-7,9,11-triyne
C13H14O2
B. pilosa
Leaves
(Chen et al., 2020; Chen et al., 2021; Chung et al., 2021)
311
2-β-D-Glucopyranosyloxy-1-hydroxy-5(E)-tridecene-7,9,11-triyne
C19H24O7
B. pilosa
Leaves
(Chien et al., 2009; Wen-Chin et al., 2013; Wei et al., 2016; Chen et al., 2020; Chen et al., 2021; Chung et al., 2021)
312
(+)-threo-(5E)-Trideca-1,5-diene-7,9,11-triyne-3,4-diol
C13H12O2
A. annua
Aerial parts
(Ivarsen et al., 2014)
313
(5E)-Trideca-1,5-diene-7,9,11-triyne-3,4-diol-4-O-β-D-glucopyranoside
C19H22O7
B. bipinnata
Whole plant
(Hu et al., 2018)
E. prostrata
Aerial parts
(Xi et al., 2014)
313a
(3S,4S,5E)-Trideca-1,5-diene-7,9,11-triyne-3,4-diol-4-O-β-glucopyranoside
C19H22O7
C. lanceolata
Flowers
(Kim et al., 2020)
314
erythro-(5E)-1,5-Didecadiene-7,9,11-triyne 3,4-diacetate
C17H16O4
P. tatsienense
Stems and leaves
(Lu et al., 2020)
315
(2S,3Z,11E)-Decadiene-5,7,9-triyne-1,2-diol
C13H12O2
C. tinctoria
Capitula
(Liu et al., 2015)
316
(2S,3E,11E)-Decadiene-5,7,9-triyne-1,2-diol
C13H12O2
C. tinctoria
Capitula
(Liu et al., 2015)
317
(3E,11E)-Tridecadiene-6,8,10-triyne-1,2,13-triol
C13H12O3
B. bipinnata
Whole plant
(Wang et al., 2013)
318
(3E,11E)-Tridecadiene-6,8,10-triyne-1,13-diol-2-O-β-D-glucopyranoside
C19H22O8
B. bipinnata
Whole plant
(Wang et al., 2013)
B. gardneri
Leaves and stems
(Silva et al., 2015)
319
2-O-β-D-Glucosyl-13-acetyltrideca-(3E,11E)-diene-5,7,9-triyn-1-ol
C21H24O9
B. gardneri
Leaves and stems
(Silva et al., 2015)
320
(10S)-Tridecane-2E,12-diene-4,6,8-triyne-1-ol 10-O-β-D-glucopyranoside
C19H22O7
B. parviflora
Whole plant
(Zhu et al., 2021)
321
1,2-Dihydroxytrideca-5,7,9,11-tetrayne
C13H12O2
B. pilosa
Whole plant
(Wu et al., 2004; Chung et al., 2021)
322
2-β-D-Glucopyranosyloxy-1-hydroxytrideca-5,7,9,11-tetrayne
C19H22O7
B. pilosa
Leaves
(Chien et al., 2009; Wen-Chin et al., 2013; Wei et al., 2016; Chen et al., 2020; Chung et al., 2021)
323
2-O-β-D-Glucosyltridec-(11E)-ene-3,5,7,9-tetrayne-1,13-diol
C19H20O8
B. gardneri
Leaves and stems
(Silva et al., 2015)
324
2-O-β-D-Glucosyl-13-acetyltridec-(11E)-ene-3,5,7,9-tetrayn-1-ol
C21H22O9
B. gardneri
Leaves and stems
(Silva et al., 2015)
325
(R)-1,2-Dihydroxytrideca-3,5,7,9,11-pentayne
C13H8O2
B. pilosa
Aerial parts
(Tobinaga et al., 2009)
326
2-β-D-Glycopyrasyloxy-1-hydroxytrideca-3,5,7,9,11-pentayne
C19H18O7
B. pilosa
Aerial parts
(Tobinaga et al., 2009; Chen et al., 2021)
327
(5E)-1,5-Tridecadiene-7,9-diyne-3,4,12-triol
C13H16O3
B. pilosa
Aerial parts
(Wang et al., 2010)
328
(2R,3E,11Z)-Decadiene-5,7,9-triyne-1,2-diol
C13H12O2
C. tinctoria
Capitula
(Liu et al., 2015)
329
Pentaynene
C13H6
A. maritima
Roots
(AbouZid et al., 2007)
330
8-Acetoxycentellynol
C17H22O3
C. asiatica
Leaves
(Randriamampionona et al., 2007)
331
(E)-Pentadeca-1,9-diene-4,6-diyne-3,8-diol
C15H20O2
D. carota
Roots
(Ahmed et al., 2005)
332
Cofalcarinol D
C15H18O
D. guatemalense
Flowers, leaves, and twigs
(Grant et al., 2020)
333
(3R,Z)-3-Hydroxypentadeca-1,9,14-triene-4,6-diyn-8-yl acetate
C17H20O3
D. guatemalense
Flowers, leaves, and twigs
(Grant et al., 2020)
333a
(Z)-8-Acetoxy-3-hydroxy-1,9,14-pentadecatriene-4,6-diyne
C17H20O3
H. annuus
Seeds of sunflower
(Shigemori et al., 2011)
334
(Z)-3,8-Dihydroxy-1,9,14-pentadecatriene-4,6-diyne
C15H18O2
H. annuus
Seeds of sunflower
(Shigemori et al., 2011)
335
(3R,8S,Z)-Pentadeca-1,9,14-triene-4,6-diyne-3,8-diol
C15H20O2
D. guatemalense
Flowers, leaves, and twigs
(Grant et al., 2020)
336
(Z)-3,8-Diacetoxy-1,9,14-pentadecatriene-4,6-diyne
C19H22O4
H. annuus
Seeds of sunflower
(Shigemori et al., 2011)
337
Saikodiyne A
C15H20O2
B. chinense
Roots
(Liu et al., 2017)
338
(2Z,9Z)-Pentadecadiene-4,6-diyn-1-ol
C15H20O
B. longiradiatum
Roots
(Huang et al., 2011)
339
Saikodiyne F
C15H20O2
B. chinense
Roots
(Liu et al., 2017)
340
(2Z,8Z,10E)-Pentadecatriene-4,6-diyn-1-ol
C15H18O
B. scorzonerifolium
Roots
(Liu et al., 2015)
341
(2Z,8E,10E)-Pentadecatriene-4,6-diyn-1-ol
C15H18O
B. longiradiatum
Roots
(Huang et al., 2009; Huang et al., 2011)
B. scorzonerifolium
Roots
(Liu et al., 2015)
342
8-Hydroxy-pentadeca-(9E,13Z)-dien-11-yn-2-one
C15H22O2
E. pallida
Roots
(Pellati et al., 2006; Pellati et al., 2007; Pellati et al., 2012; Tacchini et al., 2017)
343
Pentadeca-(9E,13Z)-dien-11-yne-2,8-dione
C15H20O2
E. pallida
Roots
(Pellati et al., 2006; Pellati et al., 2007; Pellati et al., 2012; Tacchini et al., 2017)
344
Pentadeca-(8Z,13Z)-dien-11-yn-2-one
C15H22O
E. pallida
Roots
(Pellati et al., 2006; Pellati et al., 2007; Pellati et al., 2012; Tacchini et al., 2017)
345
8-Hydroxy-pentadec-(9E)-ene-11,13-diyn-2-one
C15H20O2
E. pallida
Roots
(Pellati et al., 2006; Pellati et al., 2007; Pellati et al., 2012; Tacchini et al., 2017)
346
Pentadec-(9E)-ene-11,13-diyne-2,8-dione
C15H18O2
E. pallida
Roots
(Pellati et al., 2006; Pellati et al., 2007; Pellati et al., 2012)
347
Pentadec-(8Z)-ene-11,13-diyn-2-one
C15H20O
E. pallida
Roots
(Pellati et al., 2006; Pellati et al., 2007; Pellati et al., 2012)
348
Saikodiyne E
C15H22O2
B. chinense
Roots
(Liu et al., 2017)
349
(Z)-3-Hydroxy-9,14-pentadecatriene-4,6-diyne
C15H20O
H. annuus
Seeds of sunflower
(Shigemori et al., 2011)
350
Saikodiyne D
C15H20O2
B. chinense
Roots
(Liu et al., 2017)
351
Nona-3,5-diyne
C9H12
S. tenuifolium
Roots
(Chauhan et al., 2012)
352
Nona-3,5-diyn-2-ol
C9H12O
S. tenuifolium
Roots
(Chauhan et al., 2012)
353
Nona-3,5-diyn-2-one
C9H10O
S. tenuifolium
Roots
(Chauhan et al., 2012)
354
Nona-4,6-diyn-3-ol
C9H12O
S. tenuifolium
Roots
(Chauhan et al., 2012)
355
Nona-4,6-diyn-3-one
C9H10O
S. tenuifolium
Roots
(Chauhan et al., 2012)
356
Sadivaethyne A
C12H16O5
S. divaricata
Roots
(Sun et al., 2022)
357
Cofalcarinol C
C16H22O2
D. guatemalense
Flowers, leaves, and twigs
(Grant et al., 2020)
358
Cofalcarinol B
C16H20O2
D. guatemalense
Flowers, leaves, and twigs
(Grant et al., 2020)
359
Cofalcarinol A
C16H20O
D. guatemalense
Flowers, leaves, and twigs
(Grant et al., 2020)
360
Eprostrata Ⅰ
C17H22O7
E. prostrata
Stems
(Meng et al., 2019)
361
Echinalkamide
C22H31NO7
E. purpurea
Roots
(Chang et al., 2020)
362
Yuccifolol (nonadeca-1,11-diene-4,6,8-triyne-3,10-diol)
C19H24O2
E. yuccifolium
Aerial parts
(Ayoub et al., 2006)
363
Aciphyllal
C34H42O5
A. scott-thomsonii
Sub aerial parts
(Perry et al., 2001)
364
Pontica epoxide
C13H10O
T. vulgare
Flowers
(Moricz et al., 2018)
364a
Pontica epoxide
C13H10O
A. annua
Aerial parts
(Zhai and Zhong 2010; Ivarsen et al., 2014)
365
Panaxydol
C17H24O2
N. ternata
Aerial parts
(Nakagawa et al., 2004)
P. ginseng
Roots
(Washida and Kitanaka 2003; Liu et al., 2007; Yang et al., 2008; Qian et al., 2009; Herrmann et al., 2013; Suzuki et al., 2017)
O. horridus
Root barks
(Resetar et al., 2020)
P. quinquefolius
Roots
(Baranska et al., 2006; Christensen et al., 2006)
D. carota
Roots
(Purup et al., 2009)
P. quinquefolius
Roots
(Wang et al., 2010)
P. sativa
Roots
(Roman et al., 2011)
366
Homopanaxydol
C18H26O2
P. ginseng
Roots
(Murata et al., 2017)
367
Ginsenoyne A
C17H22O2
P. ginseng
Roots
(Yang et al., 2008)
368
9-Epoxyfalcarindiol
C17H24O3
F. campestris
Roots
(Dall'Acqua et al., 2010)
N. incisum
Roots
(Blunder et al., 2014)
O. fistulosa
Underground parts
(Appendino et al., 2009)
369
trans-Epoxytriquetrol
C17H24O3
E. triquetrum
Aerial parts
(Bouzergoune et al., 2016)
370
α-Hexy-3-(6-hydroxy-2,4-ocadiynyl)oxiranemethanol
C17H26O3
S. macrophylla
Roots
(Mi et al., 2019)
371
Oploxyne A
C17H26O3
O. elatus
Stems
(Yang et al., 2010)
372
Ginsenoyne D
C17H26O2
P. ginseng
Roots
(Fukuyama et al., 2012)
373
(9R,10S)-Epoxyheptadeca-4,6-diyn-3-one
C17H24O2
P. ginseng
Roots
(Lee et al., 2004)
O. horridus
Root barks
(Resetar et al., 2020)
374
(9R,10S)-Epoxy-16-heptadecene-4,6-diyn-3-one
C17H22O2
O. horridus
Root barks
(Resetar et al., 2020)
375
1-Methoxy-(9R,10S)-epoxyheptadeca-4,6-diyn-3-one
C18H26O3
P. ginseng
Roots
(Lee et al., 2004)
376
(Z)-8-Acetoxyl-1,2-epoxy-9,14-pentadecatriene-4,6-diyne
C17H18O4
H. annuus
Seeds of sunflower
(Shigemori et al., 2011)
377
(Z)-8-Acetoxy-1,2-epoxy-3-oxoheptadec-9-ene-4,6-diyne
C19H24O4
H. rhombea
Leaves
(Yamazoe et al., 2006)
378
Gymnasterkoreayne B
C17H22O2
G. koraiensis
The plant material
(Butler et al., 2013)
378a
(5S,Z)-1-(3-methyloxiran-2-yl)tetradeca-6,13-diene-1,3-diyn-5-ol
C17H22O2
G. koraiensis
Roots
(Jung et al., 2002; Park et al., 2002)
379
19-(2-furyl)nonadec-5-ynoic acid
C21H32O3
P. evecta
Roots
(Kanokmedhakul et al., 2006)
380
Notopolyenol A
C17H20O2
N. incisum
Roots and rhizomes
(Zheng et al., 2019)
381
(1Z)-Atractylodin
C13H10O
A. lancea
Rhizomes
(Jiao et al., 2014)
382
1-(2-Furanyl)-7-nonene-3,5-diyne 1,2-diacetate
C17H16O5
A. lancea
Rhizomes
(Jiao et al., 2014)
383
(1Z)-Atractylodinol
C13H10O2
A. lancea
Rhizomes
(Resch et al., 2001; Jiang et al., 2015)
384
(1Z)-Acetylatractylodinol
C15H12O3
A. lancea
Rhizomes
(Resch et al., 2001)
385
19-(2-Furyl)nonadeca-5,7-diynoic acid
C23H32O3
P. evecta
Roots
(Resch et al., 2001)
386
1-(2- furyl)pentacosa-7,9-diyne
C29H46O
P. evecta
Roots
(Kanokmedhakul et al., 2006)
387
Panaxfurayne A
C23H32O12
P. ginseng
Roots
(Lee et al., 2009)
388
Panaxfurayne B
C23H32O12
P. ginseng
Roots
(Lee et al., 2009)
389
2-[(1E,8E)-Deca-1,8-diene-4,6-diyn-1-yl]tetrahydrofuran
C14H16O
C. cordifolioidea
Roots
(Mei et al., 2008)
390
3,4-Dihydrovernoniyne-4-O-β-glucoside
C18H20O8
V. scorpioides
Aerial parts
(Pollo et al., 2018)
391
4-β-Hydroxy-3,4-dihydrovernoniyne
C12H10O3
V. scorpioides
Leaves
(Pollo et al., 2013)
392
5-Octa-2,4,6-triynyl-furan-2(5H)-one
C12H8O2
V. scorpioides
Flowers and leave
(Buskuhl et al., 2009)
392a
Vernoniyne
C12H8O2
V. scorpioides
Leaves
(Klein et al., 2013; Pollo et al., 2013)
393
8′-Hydroxy-3,4-dihydrovernoniyne
C12H10O3
V. scorpioides
Leaves
(Klein et al., 2013; Pollo et al., 2013)
394
3,4-Dihydrovernoniyne-8′-O-β-glucoside
C18H20O8
V. scorpioides
Leaves
(Pollo et al., 2013)
395
1′,8′-Dihydroxy-3,4-dihydrovernoniyne
C12H10O4
V. scorpioides
Leaves
(Pollo et al., 2013)
396
4,8′-Dihydroxy-3,4-dihydrovernoniyne
C12H10O4
V. scorpioides
Leaves
(Pollo et al., 2013)
397
4-β-Hydroxy-3,4-dihydrovernoniyne-8′-O-β-glucoside
C18H20O9
V. scorpioides
Leaves
(Pollo et al., 2013)
398
8′-Hydroxy-3,4-dihydrovernoniyne-4-O-β-glucoside
C18H20O9
V. scorpioides
Leaves
(Pollo et al., 2013)
399
Junipic acid
C8H6O2S
E. ritro
Whole plant
(Li et al., 2019)
400
Atracthioenyneside B
C17H22O8S
A. lancea
Rhizomes
(Feng et al., 2018)
401
4-(5-(Penta-1,3-diyn-1-yl)thiophen-2-yl)but-3-yne-1,2-diol
C13H10O2S
A. repens
Roots
(Quintana et al., 2008)
E. ritro
Whole plant
(Li et al., 2019)
E. transiliensis
Roots
(Nakano et al., 2011)
E. prostrata
Aerial parts
(Xi et al., 2014)
402
3′-Chloro-1′-(5-penta-1,3-diyn-1-yl-2-thienyl)-but-2′-yn-4′-ol
C13H9ClOS
A. repens
Roots
(Quintana et al., 2008)
403
4′-Chloro-1′-(5-penta-1,3-diyn-1-yl-2-thienyl)-but-2′-yn-3′-ol
C13H9ClOS
A. repens
Roots
(Quintana et al., 2008)
404
Echinopsacetylene B
C31H40O3S
E. transiliensis
Roots
(Nakano et al., 2011)
405
(E)-1-[5-(Hept-5-ene-1,3-diynyl)-2-thienyl]ethan-1,2-diol
C13H12O2S
L. carthamoides
Roots
(Chobot et al., 2006)
406
Atracthioenyneside A
C19H26O8S
A. lancea
Rhizomes
(Feng et al., 2018)
407
5′-Methyl-1′-(5-prop-1-yn-1-yl-2-thienyl)-hexa-2′,4′-diyn-6′-yl acetate
C16H14O3S
A. repens
Roots
(Quintana et al., 2008)
408
Thiarubrine A diol
C13H10O2S2
A. maritima
Roots
(AbouZid et al., 2007)
409
Thiarubrine A
C13H8S2
A. maritima
Roots
(AbouZid et al., 2007)
410
(3R,5S)-5-(Hydroxymethyl)-3-(tetradec-13-en-5-yn-1-yl)dihydrofuran-2(3H)-one
C19H30O3
P. debilis
Roots
(Panthama et al., 2010)
411
9,10-Dihydrooropheolide
C21H28O3
M. glabra
The stem bark
(Li et al., 2009)
412
Oropheolide
C21H26O3
M. glabra
The stem bark
(Li et al., 2009)
413
Debilisone B
C25H40O4
P. debilis
Roots
(Panthama et al., 2010)
414
Saccopetrin A
C25H42O3
P. debilis
Roots
(Panthama et al., 2010)
415
Debilisone A
C25H40O3
P. debilis
Roots
(Panthama et al., 2010)
416
Debilisone C
C25H38O3
P. debilis
Roots
(Panthama et al., 2010)
417
Debilisone D
C25H36O3
P. debilis
Roots
(Panthama et al., 2010)
418
Debilisone E
C25H36O3
P. debilis
Roots
(Panthama et al., 2010)
419
Longifolione A
C25H38O3
E. longifolia
Roots
(Wang et al., 2017)
420
Debilisone F
C27H42O3
P. debilis
Roots
(Panthama et al., 2010)
421
Longifolione C
C27H42O3
E. longifolia
Roots
(Wang et al., 2017)
422
Longifolione B
C27H40O3
E. longifolia
Roots
(Wang et al., 2017)
423
Longifolione E
C29H46O3
E. longifolia
Roots
(Wang et al., 2017)
424
Longifolione D
C29H44O3
E. longifolia
Roots
(Wang et al., 2017)
425
Echinophorin C
C12H10O2
E. cinerea
Aerial parts
(Jelodarian et al., 2017)
426
Echinophorin A
C14H14O2
E. cinerea
Aerial parts
(Jelodarian et al., 2017)
E. platyloba
Aerial parts
(Chianese et al., 2018)
427
Echinophorin B
C14H12O2
E. cinerea
Aerial parts
(Jelodarian et al., 2017)
E. platyloba
Aerial parts
(Chianese et al., 2018)
428
Echinophorin D
C14H10O2
E. platyloba
Aerial parts
(Chianese et al., 2018)
429
Ichthyothereol
C14H14O2
P. tatsienense
Stems and leaves
(Lu et al., 2020)
430
Icthyothereol acetate
C17H18O3
B. pilosa
Whole plant
(Chen et al., 2021)
431
Codojavanyol
C14H18O3
B. chinense
Roots
(Phan et al., 2022)
432
Isolobetyol
C14H20O4
P. grandiflorus
Roots
(Li 2022)
433
9-(Tetrahydropyran-2-yl)–non-trans-8-en-4,6-yn-l-ol
C14H18O2
C. tangshen
Roots
(Sun et al., 2016)
434
(2E,8E)-9-(Tetrahydro-2H-pyran-2-yl)nona-2,8-diene-4,6-diyn-1-ol
C14H16O2
C. cordifolioidea
Roots
(Mei et al., 2008)
C. tangshen
Roots
(Sun et al., 2016)
B. chinense
Roots
(Phan et al., 2022)
435
Codonopilodiynoside G
C26H38O12
C. pilosula
Roots
(Jiang et al., 2015)
436
Artemisidiyne A
C14H16O4
A. lactiflora
Aerial parts
(Xiao et al., 2014)
437
o-Hydroxycapillene
C12H10O
A. ordosica
Aerial parts
(Wang et al., 2020)
438
1-Phenyl-hepta-1,3,5-triyne
C13H8
B. pilosa
Aerial parts
(Wang et al., 2010; Chen et al., 2020; Chung et al., 2021)
439
4-Chloro-2-(hepta-1,3,5-triyn-1-yl)-phenol
C13H7ClO
H. aureonitens
Aerial parts
(Ziaratnia et al., 2009)
440
7-Phenyl-hepta-2,4,6-triyn-2-ol
C13H8O
B. pilosa
Aerial parts
(Wang et al., 2010)
441
7-Phenyl-2-heptene-4,6-diyn-1-ol
C13H10O
B. pilosa
Aerial parts
(Wang et al., 2010)
C. tinctoria
Capitula
(Liu et al., 2015)
442
7-Phenyl-hepta-4,6-diyn-2-ol
C13H12O
B. pilosa
Aerial parts
(Wang et al., 2010)
443
7-Phenyl-hepta-4,6-diyne-1,2-diol
C13H12O2
B. pilosa
Aerial parts
(Wang et al., 2010)
444
Arteordoyn B
C13H12O3
A. ordosica
Aerial parts
(Wang et al., 2020)
445
Arctic acid
C12H8O2S2
E. ritro
Whole plant
(Li et al., 2019)
446
Arctinol A
C12H10OS2
E. ritro
Whole plant
(Li et al., 2019)
447
Arctinal
C12H8OS2
E. ritro
Whole plant
(Li et al., 2019)
448
Arctinol B
C13H12O2S2
E. ritro
Whole plant
(Li et al., 2019)
449
4-(5′-Methyl-[2,2′-bithiophen]-5-yl)but-3-yn-1-ol
C13H12OS2
E. ritro
Whole plant
(Li et al., 2019)
450
4-([2,2′-Bithiophen]-5-yl)but-3-yn-1-ol
C12H10OS2
E. ritro
Whole plant
(Li et al., 2019)
451
5′-Isovaleryloxymethyl-5-(4-isovaleryloxy-but-1-ynyl)-2,2′-bithiophene
C23H28O4S2
E. prostrata
Aerial parts
(Xi et al., 2014)
452
4-([2,2′-Bithiophen]-5-yl) but-3-yne-1,2-diol
C12H10O2S2
E. ritro
Whole plant
(Li et al., 2019)
453
5′-(3,4-Dihydroxybut-1-yn-1-yl)-[2,2′-bithiophene]-5-carboxylic acid
C13H10O4S2
E. ritro
Whole plant
(Li et al., 2019)
454
5′-(3,4-Dihydroxybut-1-yn-1-yl)-[2,2′-bithiophene]-5-carbaldehyde
C13H10O3S2
E. ritro
Whole plant
(Li et al., 2019)
455
5-(But-3-yne-1,2-diol)-5′-hydroxymethyl-2,2′-bithiophene
C13H12O3S2
E. prostrata
Aerial parts
(Xi et al., 2014)
456
Thiarubrine A epoxide
C13H8OS2
A. maritima
Roots
(Quintana et al., 2008)
457
3′-(5-Penta-1,3-diynylthiophen-2-ylethynyl)-oxirane
C13H8OS
A. repens
Roots
(Quintana et al., 2008)
458
5-(2-Phenylethynyl)-2-thiophene methanol
C13H10OS
B. pilosa
Aerial parts
(Wang et al., 2010)
459
5-(2-Phenylethynyl)-2-β-glucosylmethyl-thiophene
C19H20O6S
B. pilosa
Aerial parts
(Wang et al., 2010)
460
(Z)-1,6-Dioxaspiro[4.4]non-3-ene
C13H12O2
C. indicum
Flowers
(Liu et al., 2011)
461
(E)-1,6-Dioxas-piro[4.4]non-3-ene
C13H12O2
C. indicum
Flowers
(Liu et al., 2011)
462
Dendrazawayne A
C14H14O3
C. zawadskii
Roots
(Rahman et al., 2007)
463
Tonghaosu
C13H12O2
M. chamomilla
Flower heads
(Avula et al., 2014; Avonto et al., 2017)
464
Dendrazawayne B
C14H14O3
C. zawadskii
Roots
(Rahman et al., 2007)
465
trans-2-(2,4-Hexadiynylidene)-1,6-dioxaspiro[4.5]dec-3-ene
C14H14O2
T. vulgare
Flowers
(Moricz et al., 2018)
466
cis-2-(2,4-Hexadiynylidene)-1,6-dioxaspiro[4.5]dec-3-ene
C14H14O2
T. vulgare
Flowers
(Moricz et al., 2018)
467
cis-Spiroketalenolether polyyne
C14H14O2
C. indicum
Flowers
(Liu et al., 2011)
468
Chrysindin A
C13H12O2
C. indicum
Flowers
(Liu et al., 2011)
469
2-(Hexa-2,4-diyn-1-ylidene)-1,6-dioxaspiro[4.5]decane
C14H16O2
C. zawadskii
Roots
(Rahman et al., 2007)
470
2-(Hexa-2,4-diyn-1-ylidene)-1,6-dioxaspiro[4.5]decan-8-ol
C14H16O3
C. zawadskii
Roots
(Rahman et al., 2007)
471
(+)-(3S,4S,5R)-(E)-4-Hydroxyl-3-isovaleroyloxy-2-(hexa-2,4-diynyliden)-1,6-dioxaspiro[4, 5]decane
C19H24O5
C. indicum
Flowers
(Liu et al., 2011)
472
(−)-(3S,4S,5R)-(E)-3,4-Diacetoxy-2-(hexa-2,4-diynyliden)-1,6-dioxa-spiro[4, 5]decane
C18H20O6
C. indicum
Flowers
(Liu et al., 2011)
473
Chrysindin C
C16H18O5
C. indicum
Flowers
(Liu et al., 2011)
474
Chrysindin D
C14H16O4
C. indicum
Flowers
(Liu et al., 2011)
475
Artemiselenol C
C14H16O5
A. selengensis
Whole plant
(Wang et al., 2016)
476
Artemiselenol B
C19H24O6
A. selengensis
Whole plant
(Wang et al., 2016)
477
(+)-(3S,4S,5R,8S)-(E)-8-Acetoxy-4-hydroxyl-3-isovaleroyloxy-2-(hexa-2,4-diynyliden)-1,6-dioxaspiro[4, 5]decane
C21H26O7
C. indicum
Flowers
(Liu et al., 2011)
478
Artemiselenol A
C19H23ClO5
A. selengensis
Whole plant
(Wang et al., 2016)
479
Carlina oxide
C13H10O
C. acaulis
Roots
(Herrmann et al., 2011; Strzemski et al., 2019)
480
Ester 21-(2-furyl)heneicosa-14,16-diyne-19-(2-furyl)nonadeca-5,7-diynoate
C48H66O4
P. evecta
Roots
(Kanokmedhakul et al., 2006)
481
Chrysindin B
C13H12O3
C. indicum
Flowers
(Zhang et al., 2013)
482
2-(2,4-Hexadiynylidene)-3,4-epoxy-1,6-dioxaspiro-[4.5]-decane
C14H14O3
T. vulgare
Flowers
(Moricz et al., 2018)
483
Notoincisol C
C27H32O4
N. incisum
Roots and rhizomes
(Liu et al., 2014)
484
Notoincisol B
C27H32O4
N. incisum
Roots and rhizomes
(Liu et al., 2014)
485
Echinopsacetylene A
C25H16OS4
E. transiliensis
Roots
(Nakano et al., 2011)
2 Plant polyacetylenoids and their distribution
In the last twenty years, more than 485 polyacetylenoids, with chain lengths of C8–19, C21, C23–25, C27, C29 and C33, have been isolated from the terrestrial medicinal plants. Almost all of them were isolated from the species in families of Compositae, Apiaceae, Araliaceae, Campanulaceae, Annonaceae and Meliaceae. The top 7 chain lengths of polyacetylenoids, occurring mostly in the terrestrial plants, were 17, 14, 13, 18, 10, 15 and 12. Among them, 363 linear polyacetylenoids (C9–19 and C33) and 122 cyclic ones (including 78 monocyclic, 35 bicyclic, 4 tricyclic and 1 tetracyclic; C8, C11–19, C21, C23–25, C27, and C29) have been isolated from almost 110 species of terrestrial medicinal plants as listed in Supplementary Table S1.
2.1 Linear polyacetylenoids
Linear polyacetylenoids accounted for about 80% of all the polyacetylenoids isolated from terrestrial medicinal plants in the last two decades. As summarized in Table 1, the linear polyacetylenoids mainly consist of C17, C14, C13, C18, C10, and C15 polyacetylenoids. And based on the numbers and substitution sites of triple and double bonds, the polyacetylenic terminals of linear polyacetylenoids can be mainly sorted into 14 types, as shown in Fig. 1. In most linear polyacetylenoids, carbon–carbon triple bonds prefer to be situated at C-4,5 or C-6,7, and carbon–carbon double bonds tend to be at C-1,2 or C-2,3. In the last two decades, liner polyacetylenoids were mainly isolated from the plants of Compositae, Apiaceae, Araliaceae, Campanulaceae, Annonaceae and Meliaceae families, among which, 251 and 113 polyacetylenoids, were obtained from plants of the top 2 families, Compositae (45 species) and Apiaceae (34 species), respectively.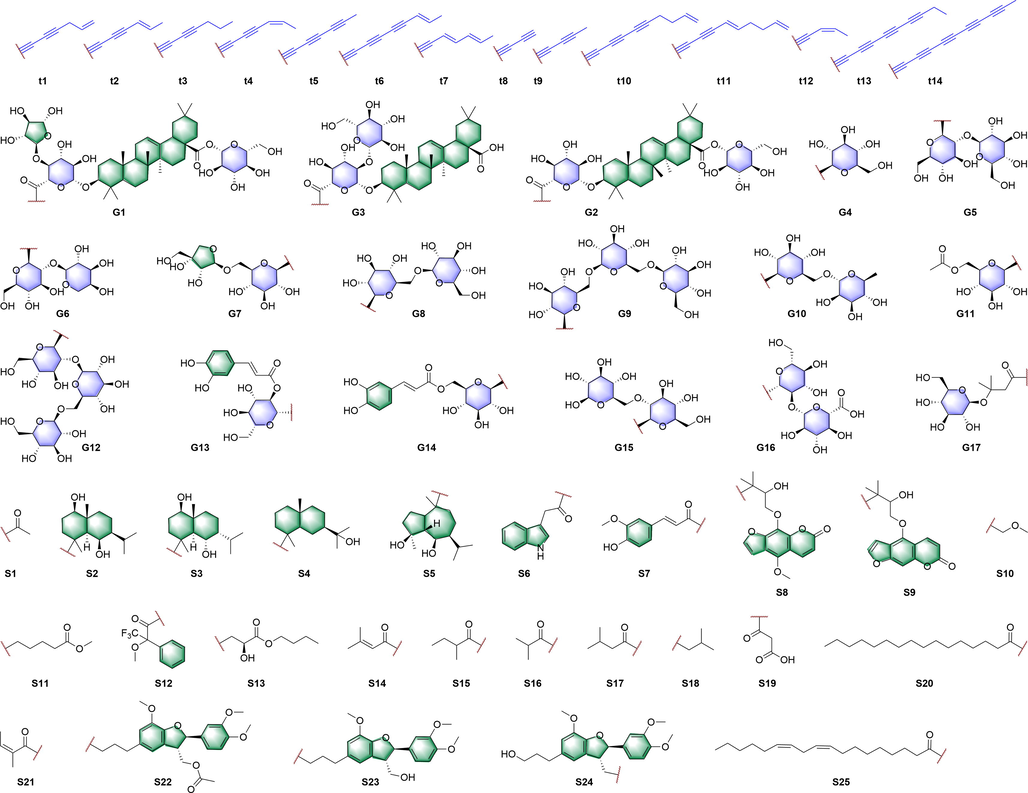
The acetylenic terminal types of polyacetylenic phytochemicals and the glycosyl, acyl and ether substituents.
2.1.1 C17 polyacetylenoids
C17 polyacetylenoids (Fig. 2), such as falcarinol (19) and falcarindiol (20), are the most common linear polyacetylenoids that have ever been discovered from terrestrial medicinal plants (Hansen and Boll 1986). A hepta-4,6-diyne moiety is normally embedded in their polyacetylenic terminals, whose styles include mainly t1, t3, t4, t5, t6 and t7. C17 Polyacetylenoids are widely distributed in plants of Apiaceae (eg. Notopterygium incisum), Araliaceae (eg. Panax ginseng) and Compositae families. There were few glycosylated C17 polyacetylenoids except baisanqisaponins A–C (5–7), which have been isolated from Panax japonicus (Araliaceae) by Liu et al (Liu et al., 2016).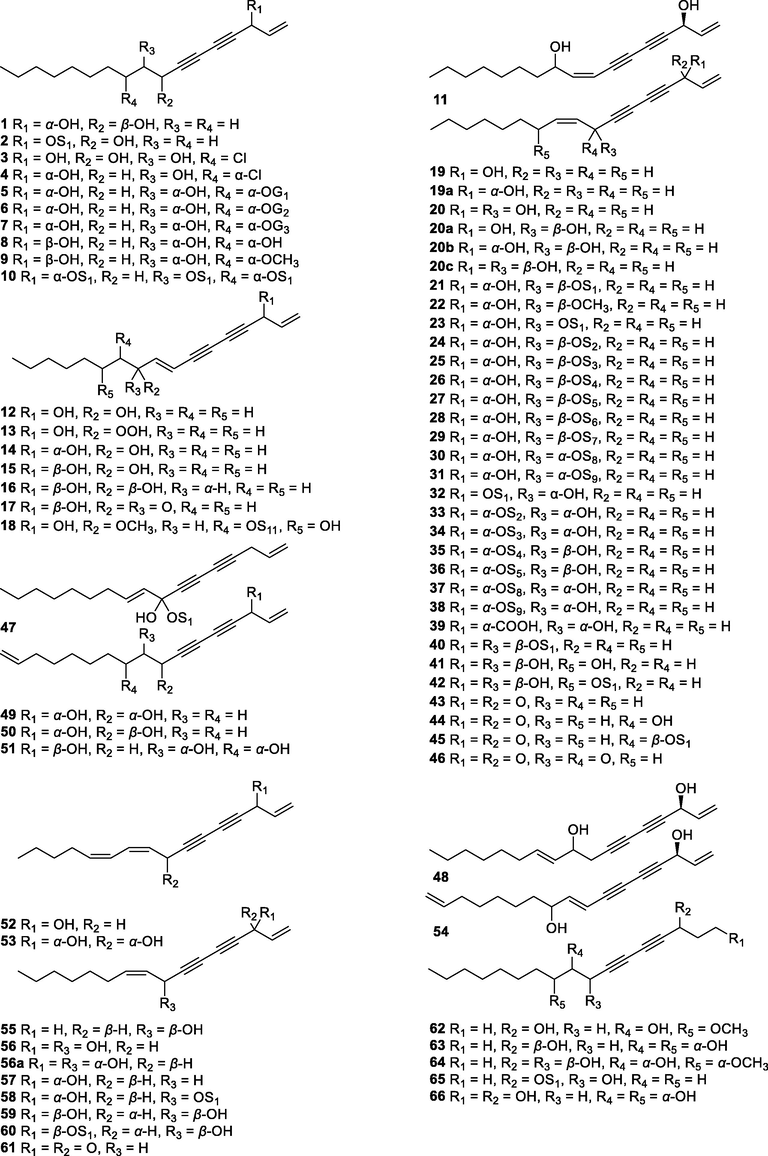
Chemical structures of the linear C17-polyacetylenoids.
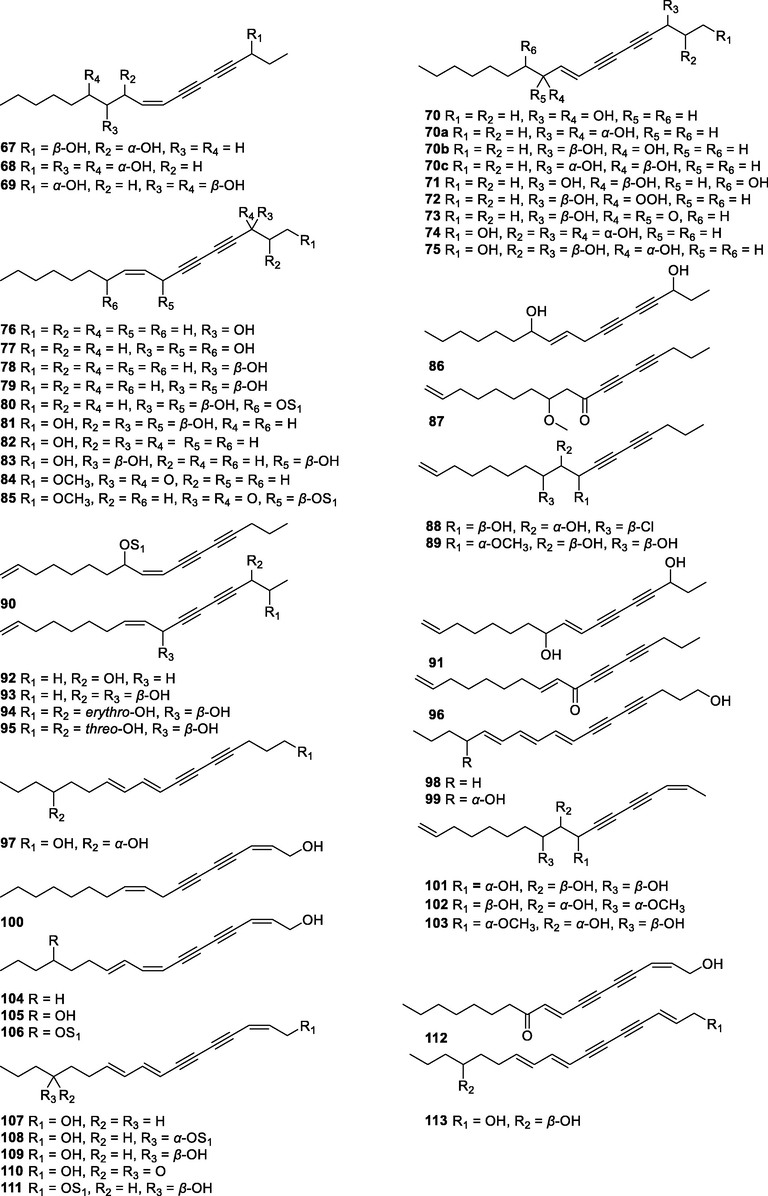
Chemical structures of the linear C17-polyacetylenoids.
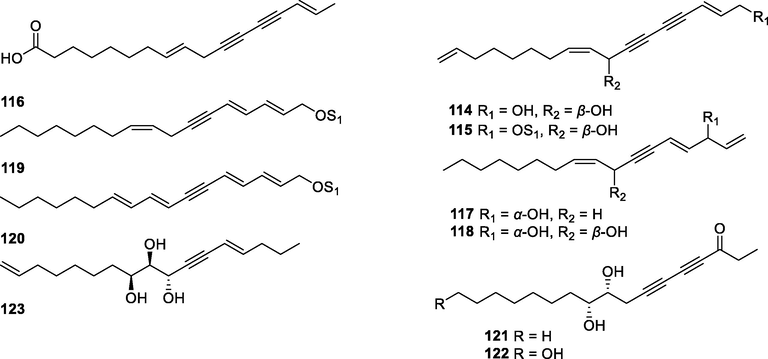
Chemical structures of the linear C17-polyacetylenoids.
2.1.2 C14 polyacetylenoids
Lobetyol (1 4 8), lobetyolin (1 4 9), lobetyolinin (1 5 1) and cordifolioidyne B (1 4 1) are the representative C14 polyacetylenoids originated from terrestrial medicinal plants, whose polyacetylenic terminals are grouped into the styles of t2, t5, t8 and t4. The C14 polyacetylenoids are extremely abundant in Compositae (eg. Atractylodes macrocephala) and Campanulaceae (eg. Codonopsis pilosula) species. Unlike C17 polyacetylenoids, nearly half of the known C14 polyacetylenoids (Fig. 3) are glycosylated.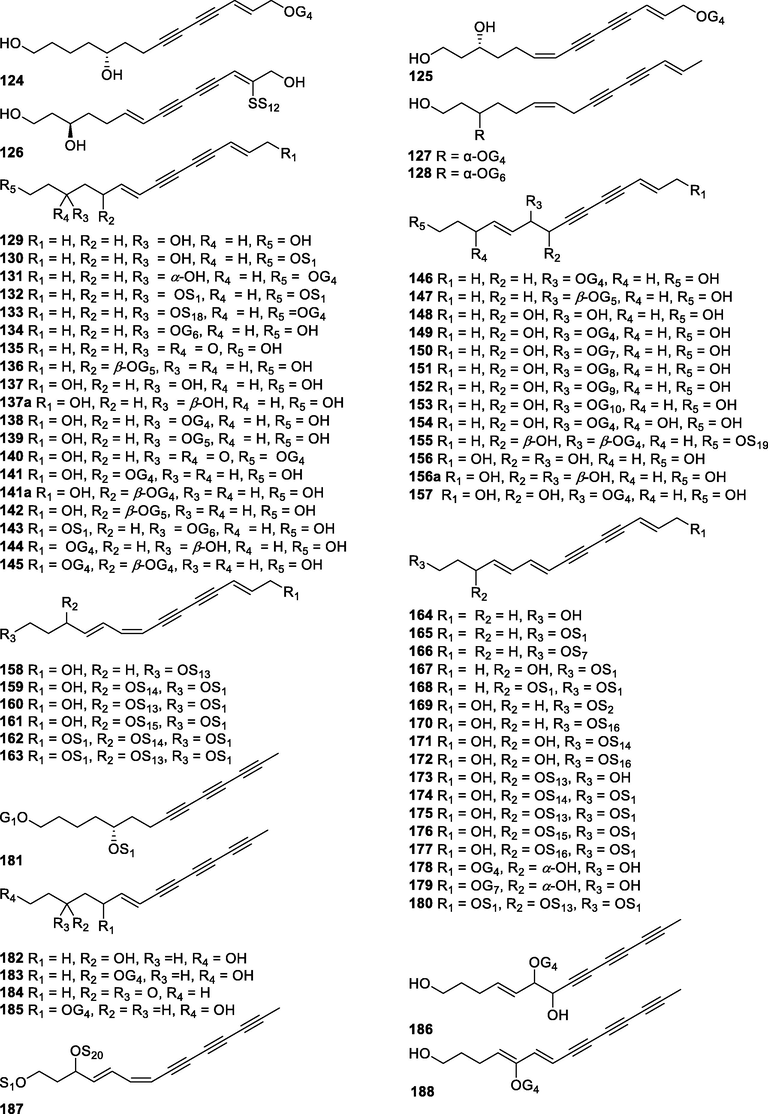
Chemical structures of the linear C14-polyacetylenoids.
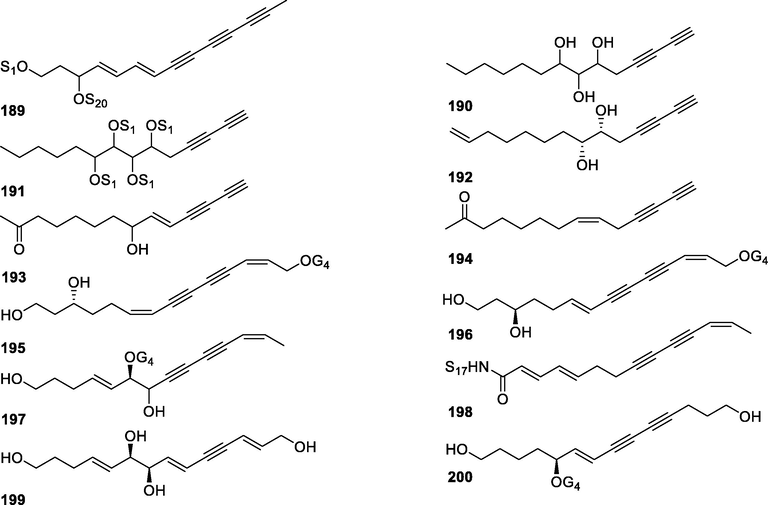
Chemical structures of the linear C14-polyacetylenoids.
2.1.3 C18 polyacetylenoids
Similar as C17 linear polyacetylenoids, the polyacetylenic terminal styles of C18 linear polyacetylenoids are mainly t1, t3, t11, t12, t8 and t14, featuring with a same hepta-4,6-diyne moiety as C17 polyacetylenoids. Till now, C18 polyacetylenoids were most frequently isolated from Araliaceae plants, such as Oplopanax horridus. In the last two decades, no glycosylated C18 polyacetylenoids (Fig. 4) have been isolated from terrestrial medicinal plants.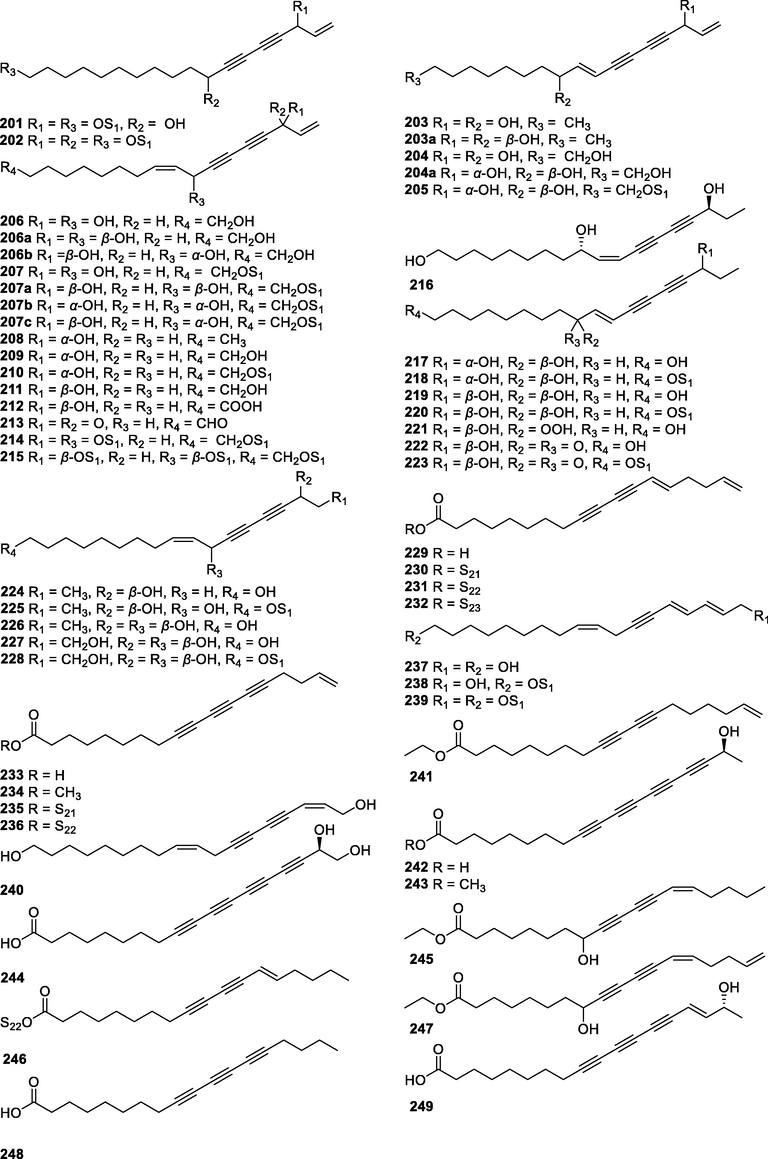
Chemical structures of the linear C18-polyacetylenoids.
2.1.4 C10 polyacetylenoids
Deca-4,6-diyne is the basic skeleton of C10 linear polyacetylenoids, as exemplified by bidensyneosides A1 (2 5 4), B (2 9 8) and C (2 6 4), that have been isolated from terrestrial medicinal plants in the last two decades. As shown in Fig. 5, the styles of polyacetylenic terminals of C10 linear polyacetylenoids are mainly t2, t4, t3 and t1. Most of the C10 linear polyacetylenoids are glycosylated at C-1, C-8 or C-10 of the skeleton, and C-10 glycosylated C10 linear polyacetylenoids are the most common ones. In the last two decades, C10 polyacetylenoids have been mostly afforded by Compositae plants, such as Carthamus tinctorius.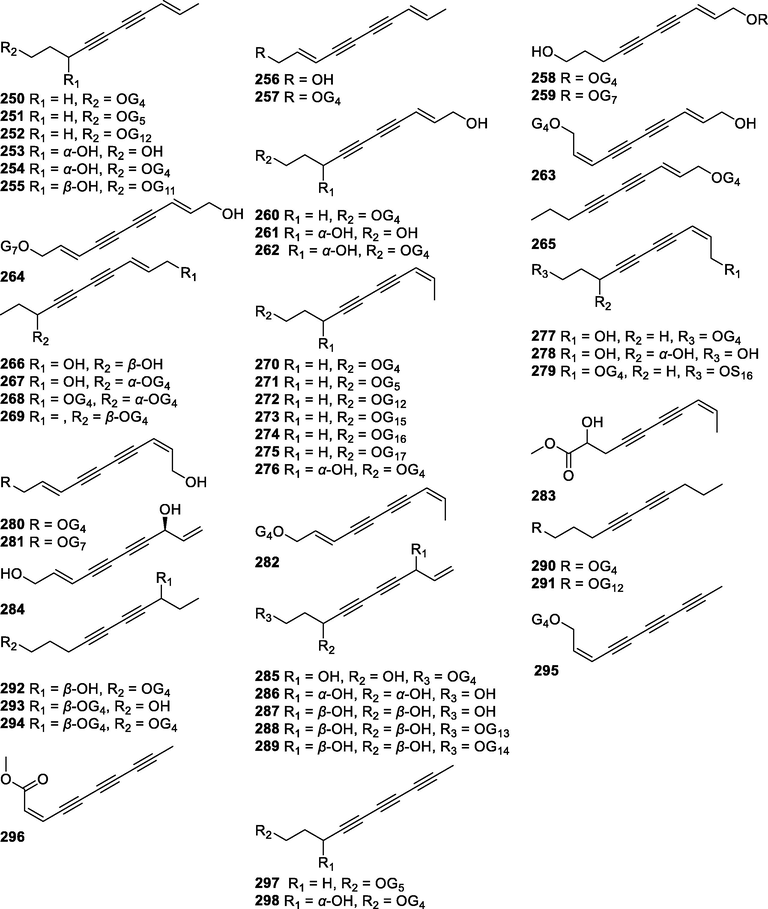
Chemical structures of the linear C10-polyacetylenoids.
2.1.5 C13 polyacetylenoids
The polyacetylenic terminal styles of C13 linear polyacetylenoids are mainly t2, t3, t5, and t6. As shown in Fig. 6, the structural frameworks of C13 linear polyacetylenoids are quite diverse, including the classic 4,6-diyne moiety, the hepta-2,4,6-triayne and the non-2-ene-4,6,8-triyne. Nearly half of the known C13 linear polyacetylenoids are glycosylated at the C-1, C-12, or C-13 of the skeleton. Notably, C13 linear polyacetylenoids have only been discovered from Compositae plants, mostly from the Bidens and Atractylodes species.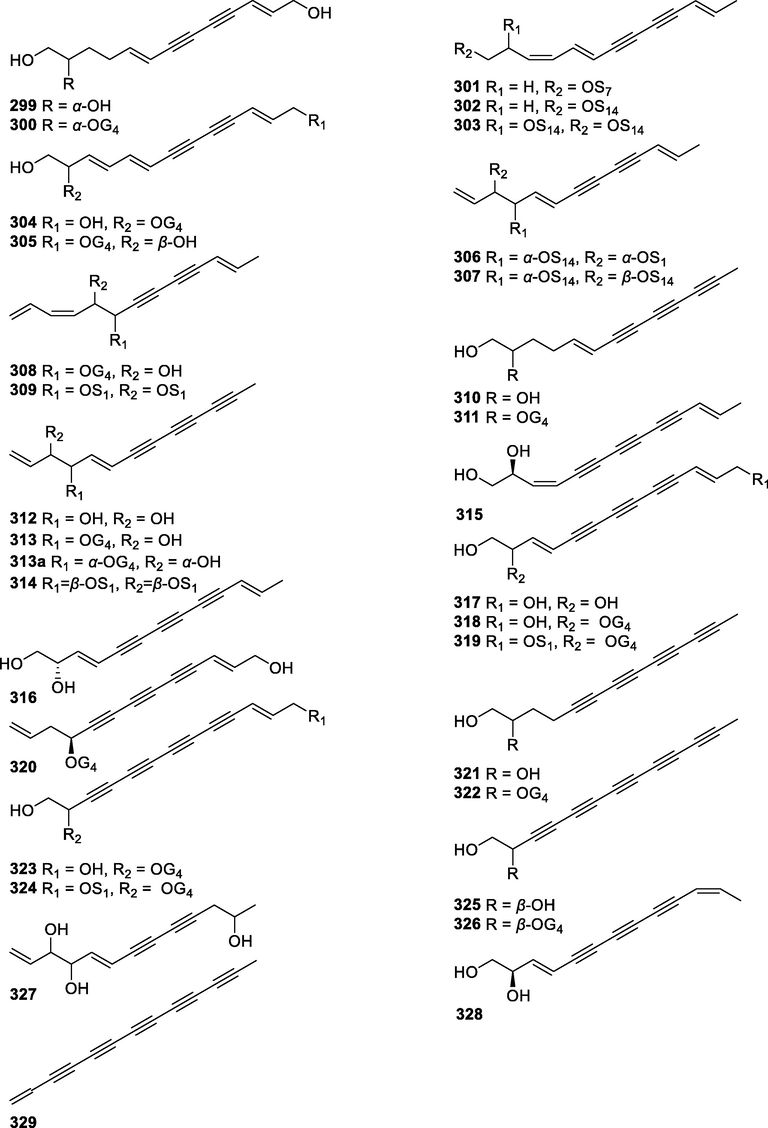
Chemical structures of the linear C13-polyacetylenoids.
2.1.6 C15 polyacetylenoids
As shown in Fig. 7, more than twenty unglycosylated C15 polyacetylenoids, with polyacetylenic terminal styles of t1, t3, t4, t9, and t13, have been isolated from Compositae and Apiaceae plants in the last twenty years.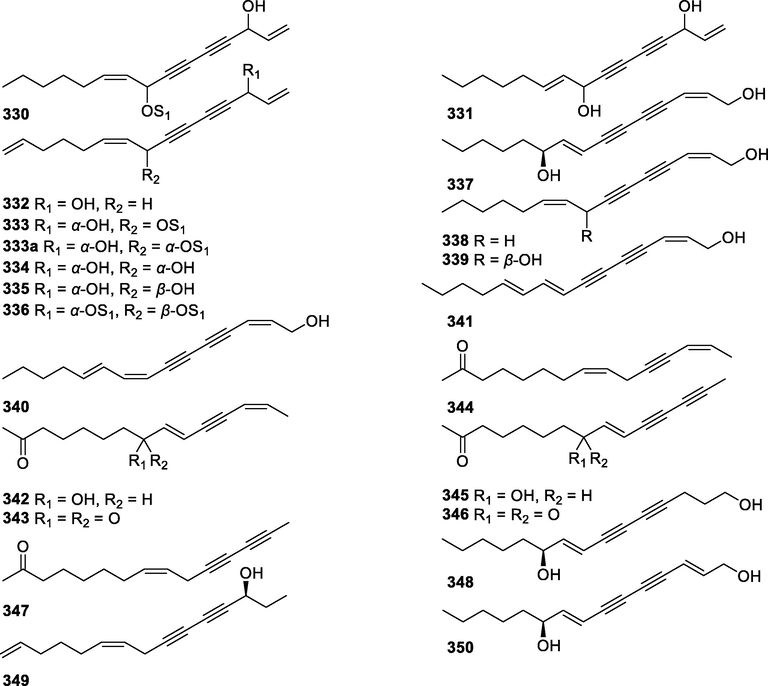
Chemical structures of the linear C15-polyacetylenoids.
2.1.7 Other linear polyacetylenoids
In addition to the above linear polyacetylenoids, there were more than 10 linear polyacetylenoids with other carbon chains of C9, C11, C16, C19, and C34 isolated from terrestrial medicinal plants in the last two decades. Among them (Fig. 8), five C9 linear polyacetylenoids came from Selinum tenuifolium (Chauhan et al., 2012), three C16 polyacetylenoids originated from Desmanthodium (Grant et al., 2020), while a C19 polyacetylenoid (yuccifolol, 362) was isolated from Eryngium yuccifolium (Ayoub et al., 2006). Notably, aciphyllal (3 6 3) isolated from a New Zealand plant Aciphylla scott-thomsonii with a chain length of C33, was a linear polyacetylenoid with the longest carbon chain ever found in terrestrial medicinal plants (Perry et al., 2001).(See Fig. 8).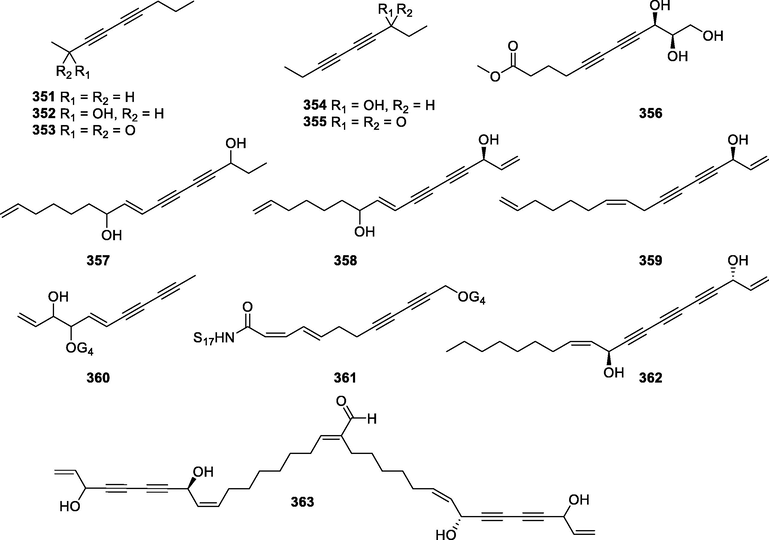
Chemical structures of other linear polyacetylenoids.
2.2 Cyclic polyacetylenoids
2.2.1 Monocyclic polyacetylenoids
Monocyclic polyacetylenoids are those cyclic polyacetylenoids with one oxygen or sulfur heterocycle, or a carbon ring system in their structures, possessing a chain length of C8–C29. Till now, there are oxirane, furan or α-furanone, thiofuran, pyran or α-pyrone, cyclopentanone and benzene rings that have ever been reported to be existied in the monocyclic polyacetylenoids.
In those polyacetylenoids with an orian ring in their structures, the expoxidation always occurs between C-9 and C-10, occasionally between C-10 and C-11 (3 6 4), between C-2 and C-3 (3 7 8), or between C-1 and C-2 (376 and 377). They all possess a hepta-4,6-diyne moiety at the polyacetylenic terminal. And the majority of monocyclic polyacetylenoids is C17 polyacetylenoid, followed by C13, C15 and C18 polyacetylenoids, mainly obtained from the plants of Apiaceae, Araliaceae (eg. Panax ginseng), Compositae and Meliaceae.
Furan or α-furanone-type monocyclic polyacetylenoids, with chain lengths of C11–14, C17, C19, C21, C25, C27 and C29, were isolated from species of Panax ginseng, Vernonia scorpioides, Atractylodes lancea, Codonopsis cordifolioidea, Notopterygium incisum, Polyalthia debilis, Mitrephora glabra and Eurycoma longifolia. Most of them contain a hepta-4,6-diyne or a heptyl-2,4,6-triyne moiety at their polyacetylenic terminals. For example, notopolyenol A (3 8 0) may be derived from falcarindiol by C-8,11 epoxidation in view of the biogenetic pathway (Zheng et al., 2019).
Further, in the last two decades, nine thiofuran-type monocyclic polyacetylenoids (Fig. 9), exemplified by 4-(5-(penta-1,3-diyn-1-yl)thiophen-2-yl)but-3-yne-1,2-diol (4 0 1), have been isolated from Compositae plants including Echinops ritro, Acroptilon repens, Atractylodes lancea, Eclipata prostrata, Echinops transiliensis, and Leuzea carthamoides. And eleven pyran or α-pyrone-type monocyclic polyacetylenoids (Fig. 9), with hepta-4,6-diyne, hepta-2,4,6-triyne or penta-2,4-diyne moiety in their structures, was isolated from Bidens pilosa, Pyrethrum tatsienense, Bupleurum chinense, Codonopsis cordifolioidea, Codonopsis tangshen, Echinophora cinerea, Echinophora platyloba and Codonopsis pilosula, belonging to Compositae, Apiaceae and Campanulaceae families. Pyran or α-pyrone-type monocyclic polyacetylenoids are mainly C14 polyacetylenoids and mostly unglycosylated).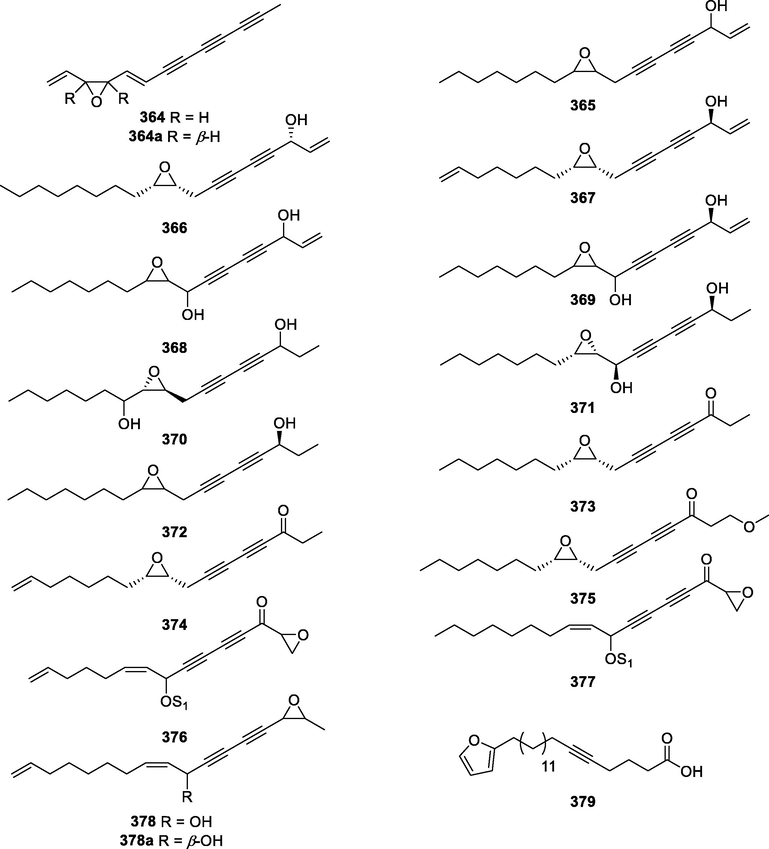
Chemical structures of the monocyclic polyacetylenoids.
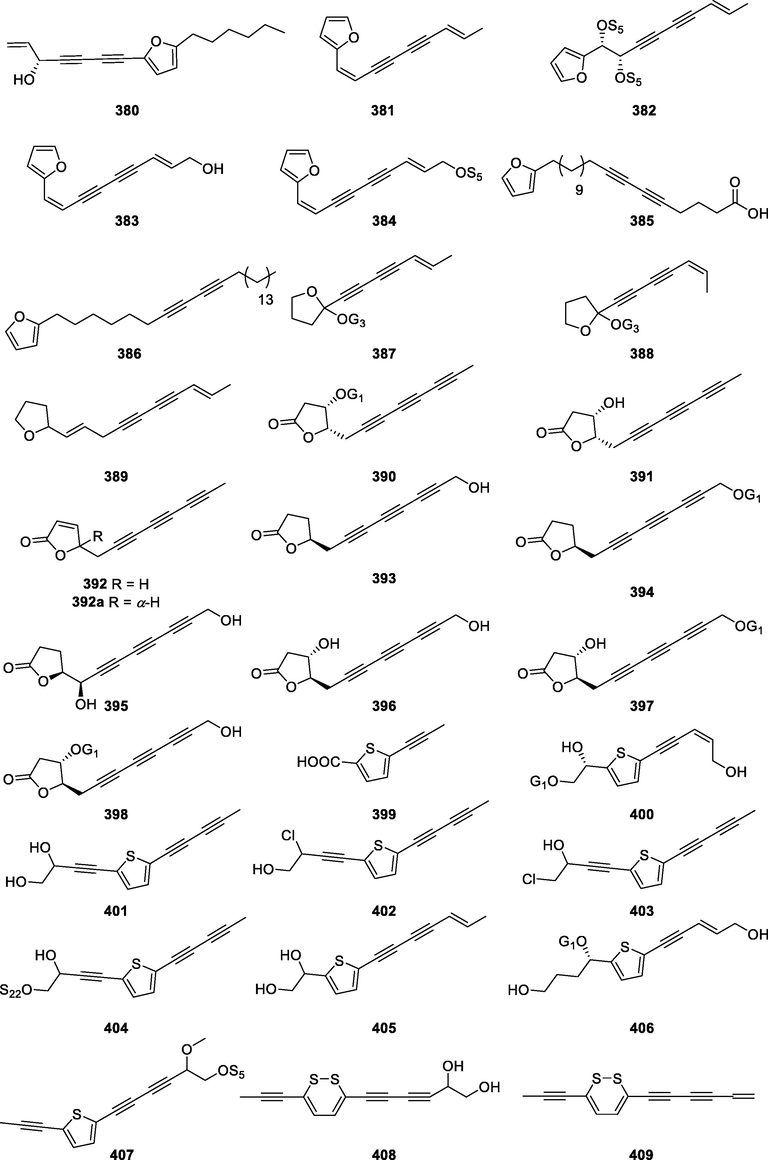
Chemical structures of the monocyclic polyacetylenoids.
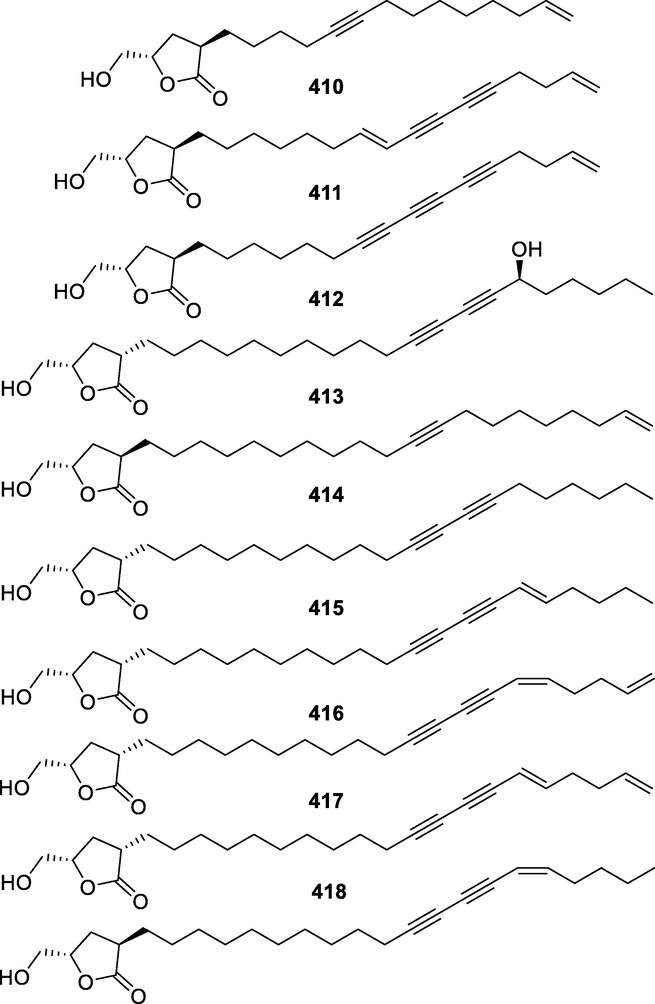
Chemical structures of the monocyclic polyacetylenoids.
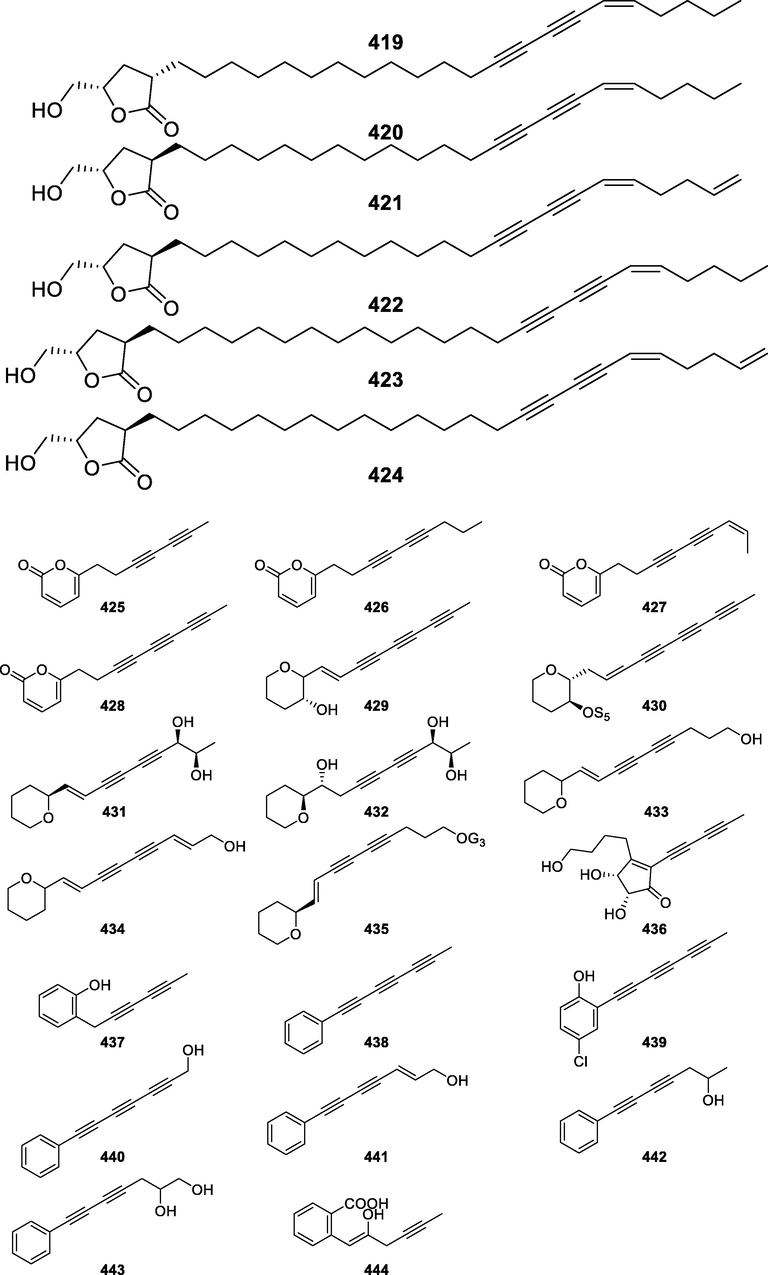
Chemical structures of the monocyclic polyacetylenoids.
The above-mentioned monocyclic polyacetylenoids are heterocyclic compounds, mostly oxygen heterocyclic compounds. It is worth mentioning that some non-heterocyclic polyacetylenoids, containing benzene rings, have been found to be distributed in Compositae plants, including Bidens pilosa, Coreopsis tinctoria, Artemisia ordosica and Helichrysum aureonitens. In the last two decades, non-heterobicyclic or non-heteromonocyclic polyacetylenoids were only found to be distributed in these Compositae plants, with a hepta-2,4,6-triyne, a hepta-4,6-diyne or a penta-2,4-diyne at their polyacetylenic terminals. What’s more, there occurs compound 436 (artemisidiyne A) featuring one cyclopentanone ring, possessing a penta-2,4-diyne moiety at its polyacetylenic terminal (Xiao et al., 2014).
2.2.2 Bicyclic polyacetylenoids
In the past twenty years, bicyclic polyacetylenoids with chain lengths of C12, C13 and C14, were mostly isolated from Echinops ritro, Bidens pilosa, Chrysanthemum indicum, Eclipata prostrata, Acroptilon repens, Ambrosia maritima, Matricaria chamomilla, Carlina acaulis, Tanacetum vulgare, Artemisia selengensis and Chrysanthemum zawadskii in Compositae family. As shown in Fig. 10, compounds 445–455 are a series of 2,2′-bithiofurphen-type bicyclic polyacetylenoids that distributed mostly in plants of Echinops ritro (Li et al., 2019) and Eclipata prostrate (Xi et al., 2014). And compounds 460–478 come to be a series of dioxaspirocyclic polyacetylenoids isolated with beneficial antibacterial activities (Li et al., 2019), mostly from the Chrysanthemum and Artemisia plants.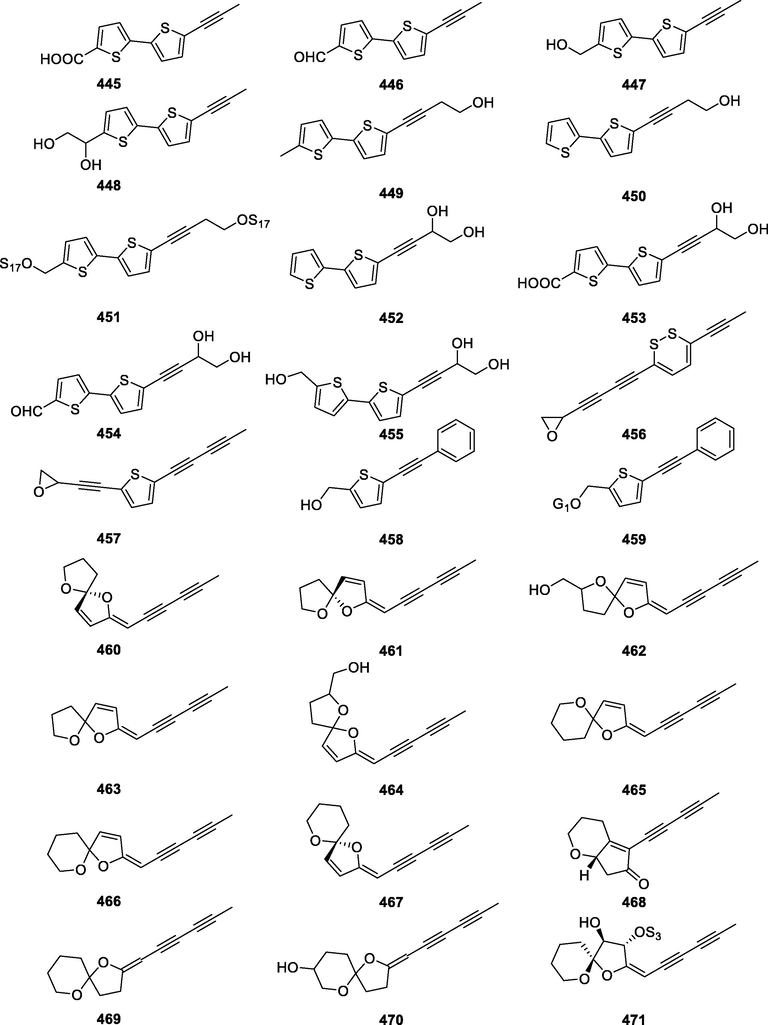
Chemical structures of the bicyclic polyacetylenoids.
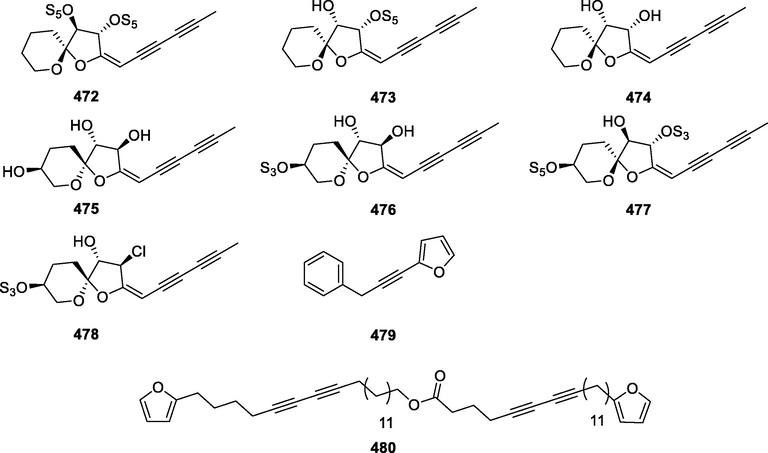
Chemical structures of the bicyclic polyacetylenoids.
2.2.3 Other cyclic polyacetylenoids
Polycyclic polyacetylenoids (481–485) have also been found to be existed in terrestrial medicinal plants. For examples, two naphthyl tricyclic polyacetylenoids, notoincisols B (4 8 4) and C (4 8 3), have been isolated from Notopterygium incisum (family Apiaceae) (Liu et al., 2014), and one tetracyclic polyacetylenoid with four thiofuran rings, echinopsacetylene A (4 8 5), was isolated from Echinops transiliensis (Nakano et al., 2011).(See Fig. 11)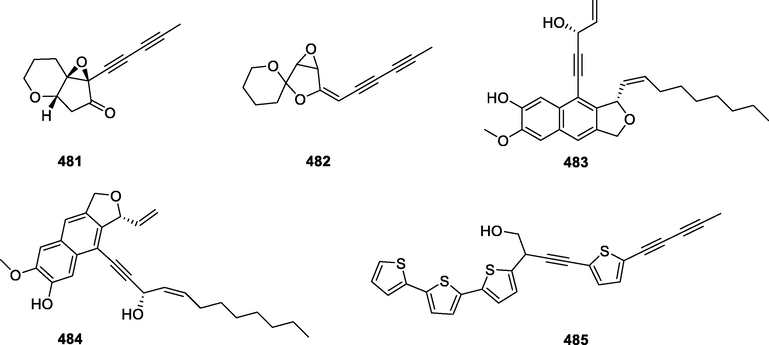
Chemical structures of the other cyclic polyacetylenoids.
2.3 NMR characteristics and assignment of their configurations
2.3.1 1H and 13C chemical shift behaviors of the linear polyacetylenoids
In general, polyacetylenic phytochemicals were commonly afforded as colorless oils or white amorphous powder, with planar structures and the concerned relative configurations established by analysis of their extensive spectroscopic and spectrometric data (UV, IR, 1D and 2D NMR, and HRESIMS). As far as we know, NMR analysis plays a vital role in the elucidation of the chemical structures of polyacetylenoids. The common deuterated solvents used are CDCl3, CD3OD, and DMSO‑d6 followed by D2O, C6D6 and so on. As summarized in Tables S1 and S2, in NMR spectra of plant polyacetylenoids, acetylenic carbons often exhibited quaternary carbon resonances at δC 58.9–91.0, which easily and frequently overlap with those carbon resonances of sugars and saturated alcohol carbons, making the assignments of these acetylenic carbons more challenging. In CDCl3, the terminal methyl adjacent to an acetylenic bond showed a proton resonance at δH 1.93–2.07 (3H, s) and a highly shielded carbon resonance at δC 3.7–4.8, while a terminal methyl adjacent to an olefinic bond exhibited a proton resonance at δH 1.83–1.95 (3H, d, br d, or m, 3JHH ≈ 7.0 Hz and 4JHH ≈ 1.8 Hz) and a carbon resonance at δC 14.0–19.0. Interestingly, in common plant polyacetylenoids, the terminal hydroxymethyl group was only found to be adjacent to an olefinic bond rather than an acetylenic bond, with NMR resonances at δH 4.17–4.66 (2H, m), and δC 61.1–61.3 (cis-olefinic bond) or 62.6–64.4 (trans-olefinic bond). Acetylation (acylation) of the saturated tertiary carbinol adjacent to an acetylenic bond, as exemplified by t1- and t3- types of polyacetylenic terminals, will cause down-field proton and carbon shifts with ΔδH 0.86–1.01 and ΔδC 0.7–1.1, respectively. Glycosylation of the saturated tertiary and secondary carbinols in polyacetylenoids perturbs the chemical shifts of their protons and carbons following the common glycosidation chemical shift rules.
2.3.2 Determination of the configurations of polyacetylenoids
It can be found from Table 1, the saturated acyclic tertiary carbinols and/or vicinal diol groups (74, 75, 81, 94, 95, 356) occur frequently in natural polyacetylenoids originating from terrestrial medicinal plants, due to biological oxidation of their conjugated olefinic bonds. For typical cyclic polyacetylenoids with three- to six- membered rings displaying predictable conformational behavior, the relative configurations can be deduced using simple NMR parameters such as the 3JHH values and/or the nuclear overhauser effect (NOE) intensities (Guo et al., 2017). However, to determine the relative configurations of conformationally flexible linear polyacetylenic chains are significantly more challenging. The NMR profiles (δH, δC, δH(OH), 3JHH, and 2,3JHC) of acyclic threo and erythro diols in achiral solvents were found to be very similar to each other. So, in the last two decades, optimization of the NMR experiment (such as recording solvent, NMR parameters, pulse sequence, and use of a higher-resolution NMR spectrometer) (Wongsomboon et al., 2021; Zhu et al., 2021), and chemical derivatization of the test sample with chiral reagents (Kanokmedhakul et al., 2006; Xu et al., 2020), were two main approaches to amplify their Δδ behavior and/or J-value differences among the various potential configurational isomers, for assigning their relative or absolute configurations.
Optimization of the NMR experiment, without chemical derivatization of the given compounds, is a direct, convenient, and reliable approach for discriminating the threo and erythro configurations of the acyclic vicinal diols in polyacetylenoids (Lee et al., 2022). The 3JHH values of these acyclic vicinal diol groups in DMSO‑d6 were not so interpretable to distinguish the threo and erythro configurations, but they seem to follow an empirical rule in CDCl3: a relatively larger value (more than 6.0 Hz) corresponds to a threo configuration, whereas a smaller value (<5.0 Hz) corresponds to an erythro configuration. However, the poor solubility of polyacetylenoid glycosides in CDCl3 limited the direct application of this rule. Furthermore, preparation of the aglycones by acid hydrolysis of the minor polyacetylenoids containing more than one sugar moiety was difficult due to structural instability. Higashibayashi and Kishi found that the Δδ (Δδ = δ(R,R) - δ(S,S)) behaviors of acyclic secondary 1,2-diols in chiral bidentate NMR solvent [eg. (R,R)- and (S,S)-BMBA-p-Me] were significantly different, and accordingly developed a method to predict both the relative and absolute configurations of acyclic secondary 1,2-diols (Xu et al., 2017). And recently, Pei-Cheng Zhang's group found an excellent deuterated solvent, aceticacid‑d4, enabling the collection of quality spectra that display a similar spectroscopic tendency as the nonpolar CDCl3 solvent. Hence, the relative configurations of polyacetylenoid glycosides containing an acyclic vicinal diol group adjacent to an olefinic bond, an acetylenic bond, a thiophene ring, or a furan ring could be conveniently and reliably determined by 1H NMR spectroscopy using aceticacid‑d4/D2O as the solvent. A relatively larger 3JHH value (7.0 Hz) was assigned to the threo configuration, whereas a smaller value (3.5 Hz) was assigned to the erythro configuration(Guo et al., 2017). what is more important, the proportions of aceticacid‑d4 can be adjusted slightly based on the solubility of the samples to be tested.
Mosher's method provides another stereochemical solution but requires a certain amount of the tested compounds sufficient for hydrolysis and/or chemical derivatizations before further NMR analysis and/or ECD simulation and comparison. The common hydrolysis methods of polyacetylenoid glycosides include: 1) Enzymatic hydrolysis, such as cellulase (Rücker et al., 1992), β-glucosidase (Wang et al., 2001), snailase (Jiang et al., 2015; Xu et al., 2017) and 2) Acid Hydrolysis, such as HCl/MeOH (Mei et al., 2008; Guo et al., 2017). And in the last two decades, the reagents dedicated to the chemical derivatization of saturated acyclic tertiary carbinols in the determination of relative and absolute stereochemistry of polyacetylenoids was mostly (S)-(+)- and (R)-(-)-α-methoxy-α-trifluoromethyl phenylacetyl chloride (MTPA) (Wang et al., 2001), occasionally (S)-(+)- and (R)-(-)-α-methoxyphenylacetic acid (MPA) (Jiang et al., 2015).
3 Botanic origins
As retrieved in the recent literature dating from 2000 to 2022, more than 485 polyacetylenoids, with a broad array of biological properties (Zhou et al., 2015), have been isolated from almost 110 species belonging to 11 families including Compositae, Apiaceae, Araliaceae, Campanulaceae, Annonaceae, Meliaceae, Simaroubaceae, Typhaceae, Olacaceae, Torricelliaceae and Cucurbitaceae, as summarized in Table 1. These 110 terrestrial species cover 45 Compositae species (251 polyacetylenoids), 34 Apiaceae species (113 polyacetylenoids), 13 Araliaceae species (67 polyacetylenoids), 8 Campanulaceae species (36 polyacetylenoids), 3 Annonaceae species (20 polyacetylenoids), 2 Meliaceae species (18 polyacetylenoids), and other 5 species belonging to the remaining 5 families (17 polyacetylenoids) (Fig. 12).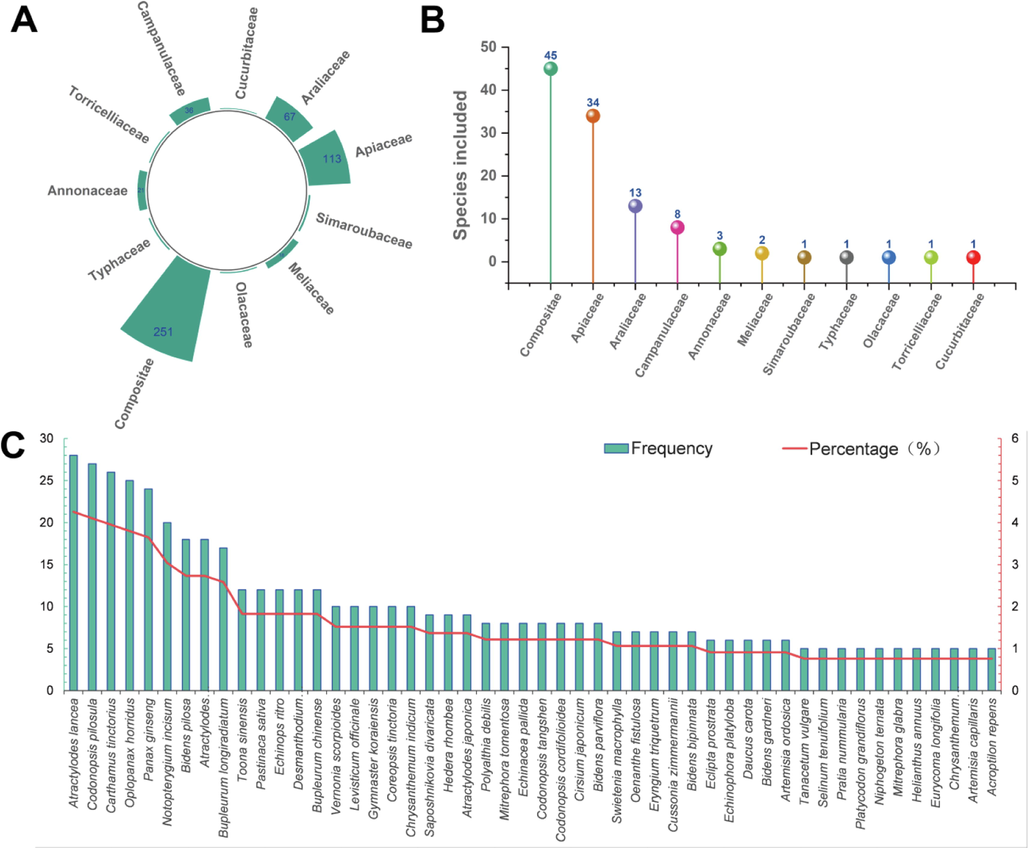
Distribution of polyacetylenoids in different families of the terrestrial medicinal plants (A); The number of plant species in the major families containing polyacetylenoids (B); Frequency and the percentage of polyacetylenoids occurring in the main plant species (C).
3.1 Compositae
Compositae is the largest source of polyacetylenoids that has ever been discovered so far. Since 2000, more than 250 polyacetylenoids have been isolated from Compositae species, among which, the top 6 polyacetylenoid-producing Compositae species ranked from most to least are Atractylodes lancea, Carthamus tinctorius, Atractylodes macrocephala, Bidens pilosa, Desmanthodium guatemalense and Echinops ritro. Most of them were C10, C13, and C14 polyacetylenoids. And, glycosylated polyacetylenoids account for nearly one-third of all these isolated polyacetylenoids. As far as we know, most of the polyacetylenoids embedded with thiophene (thiofuran) ring(s) are originated from the plants in the Compositae family, in which C17 polyacetylenoids happen normally to be falcarinol-type.
3.2 Apiaceae
Some of the most bioactive (eg. antifungal, anti-inflammatory, anti-platelet-aggregatory, or antibacterial) polyacetylenoids have been found in plants of the Apiaceae family, including the well-known daily vegetables such as carrot, celery, and parsley (Christensen and Brandt 2006). As is reported, such genera as Bupleurum (Bupleurum longiradiatum), Notopterygium (eg. Notopterygium incisum), Eryngium, and Pastinaca in the Apiaceae family are rich in polyacetylenoid compounds, and falcarinol-type polyacetylenoids (C17) represent the most widespread and representative polyacetylenoids in Apiaceae (Chen et al., 2015).
3.3 Araliaceae
In Araliaceae plants, Ginseng and Oplopanax genera are found to be rich in polyacetylenoids. Since last twenty years, 73 C17 and C18 predominating polyacetylenoids have been isolated from Araliaceae plants, and among them, falcarindiol, falcarinol and panaxydol occurred frequently with a wide range of biological activities, including anti-tumor (Sun et al., 2010), anti-pathogenic microbial (Yamazoe et al., 2007), and hair growth promoting effects (Suzuki et al., 2017).
3.4 Campanulaceae
From 2010 to 2022, phytochemistry studies on plants of the genera Codonopsis (eg. Codonopsis pilosula), Lobelia (eg. Lobelia nummularia Lam) and Platycodon (eg. Platycodon grandiflorus) led to the isolation of 38 polyacetylenoids, mainly C14 polyacetylenoids and most of which are glycosylated. Notably, lobetyol, lobetyolinin, lobetyolin occurring frequently in Campanulaceae plants, were found to possess beneficial anticancer properties (Bailly, 2020).
3.5 Species in other families
The Mitrephora and Polyalthia plants (Annonaceae family) contain mostly C18 polyacetylenoids in the form of polyacetylenoid ester or acid, following with C16, C22, and C24 polyacetylenoids. Few polyacetylenoids have been isolated from the plants of Meliaceae family, including Toona ciliate and Swietenia mahagoni, amongst which, T. ciliate mainly produces C17 and C18 polyacetylenoids while S. mahagoni affords mostly C14 and C17 polyacetylenoids, sharing similar skeletons as those from T. ciliate.
In addition to the above, some plants of other families, including Simaroubaceae, Olacaceae, Torricelliaceae and Cucurbitaceae, also occasionally biosynthesize polyacetylenoid metabolites during their growth cycle. Among them, the Eurycoma longifolia in family Simaroubaceae has been found to be able to produce the rare α-furanone-type monocyclic polyacetylenoids (Wang et al., 2017).
4 Analytical methods
So far, various separation-based and combined detection-based methods have been developed for qualitative and/or quantitative analyses of the polyacetylenoid compositions in terrestrial medicinal plants. Polyacetylenoids are usually extracted from fresh or dried plant material by an organic solvent such as n-hexane (Pellati et al., 2012), ethyl acetate (Moricz et al., 2018), chloroform (Marčetić et al., 2014), ethanol (Silva et al., 2015), and methanol (He et al., 2014; Chen et al., 2018). Extraction methods for polyacetylenoid typically include reflux (Wang et al., 2017; Kim et al., 2018), sonication (He et al., 2014; Chen et al., 2018), supercritical fluid extraction (Tacchini et al., 2017), percolation (Silva et al., 2015), and hydrodistillation (Marcetic et al., 2013). Before analysis, polyacetylenoid samples are normally accumulated or prepared by repeated solvent extraction and/or thin-layer chromatography (TLC) on silica gel or by using a combination of silica gel CC and gel permeation CC on Sephadex LH-20 (Huang et al., 2011; Silva et al., 2015). Also, other special techniques such as centrifugal partition chromatography (CPC), counter-current chromatography (CCC) and elution-extrusion counter-current chromatography (EECCC), have been developed and adopted for the isolation of natural polyacetylenoids (Chen et al., 2018; Chen et al., 2021).
As summarized in Table 2, analysis of the main polyacetylenoid components is usually performed using thin-layer chromatography (TLC), high-performance thin-layer chromatography (HPTLC), high performance liquid chromatography (HPLC), ultra-high performance liquid chromatography (UHPLC), gas chromatography (GC), and effective detection of the marker components are always accomplished by using ultraviolet (UV), photo-diode array (PDA), diode array detector (DAD), mass spectrometry (MS), and flame ionization detector (FID) alone or in combinations. Generally, to obtain supplementary information, different types of detectors are often combined in one run, such as liquid chromatography-mass spectrometry (LC-MS) and the high-performance liquid chromatography-ultraviolet (HPLC-UV). Gas chromatography-mass spectrometry (GC–MS) is dedicated to the qualitative or semi-quantitative analysis of the polyacetylenoids in essential oils or nonpolar extracts of terrestrial medicinal plants (Liu et al., 2007). a FaDOAc, Falcarindiol-3-acetate; b FaOH, Falcarinol; c DW, dry weight; d FaDOH, Falcarindiol; e FW, fresh weight; f TP, Total polyacetylenoids.
Source
Method
Extract solvent
Column
Mobile phase
Detection condition
Plant part
Remark
Reference
D. carota
HPLC
EtOAc
Luna C18 column (100 mm × 4.6 mm, 5 μm)
H2O (A) and MeCN (B)
205 nm
Roots
Fresh carrot juice: FaDOAca 73 μg/L, FaOHb 233 μg/L
(Aguilo-Aguayo et al., 2014)
HPLC-DAD
MeOH
Phenomenex Luna C18(2) column (100 mm × 3 mm, 3 µm)
MeCN (A) and H2O (B) (70:30 v/v)
205 nm
Roots
/
(Kjellenberg et al., 2012)
HPLC-DAD
EtOAc
Zorbax RX-C18 column (12.5 mm × 4.6 mm, 5 μm)
H2O (A) and MeCN (B)
/
Roots
Carrot: FaOHb 85.13–244.85 μg/g DWc
(Hinds et al., 2017)
HPLC-DAD-MS
MeOH
Phenomenex Aqua C18 column (250 mm × 21.2 mm, 5 μm)
H2O (A) and MeCN (B)
205 nm
Roots
A. sylvestris: TPf 3.8 g/kg DWc; Celeriac: TPf < 0.1 g/kg DWc
(Kramer et al., 2011)
HPLC-DAD
15% Aqueous EtOAc
Phenomenex Luna C18(2) column (150 mm × 4.6 mm, 3.0 µm)
H2O (A) and MeCN (B)
205 nm
Roots
Peeled carrot: FaDOHd 6.4–30.4 mg/kg FWe, FaDOAca 10.2–21.6 mg/kg FWe, FaOHb 35.2–67.0 mg/kg FWe
(Christensen and Kreutzmann 2007)
HPLC-DAD and UPLC-TOFMS
EtOAc
Prodigy RP-C18 column (250 mm × 4.6 mm, 5 μm)
H2O (A) and MeCN (B)
205 nm
Roots
Carrot: FaDOHd 222 μg/g DWc, FaDOAca 30 μg/g DWc and FaOHb 94 μg/g DWc in year 1, and 3–15% lower in year 2
(Soltoft et al., 2010)
UPLC-PDA-MS
DCM + 0.1 % BHT
Waters Acquity UPLC® HSS SB-C18 column (2.1 mm × 100 mm, 1.8 µm)
H2O + 5 mM NH4OAc:MeOH:MeCN:EtOAc (A) (50:22.5:22.5:5) and MeCN: EtOAc (B) (50:50))
PDA: 190–800 nm; m/z 100–1400
/
Whole raw small carrots: FaOHb 18 mg/kg FWe, FaDOHd 35 mg/kg FWe; Peeled blanched round carrots: FaOHb 2.9 mg/kg FWe, FaDOHd 13 mg/kg FWe
(Bijttebier et al., 2014)
B. pilosa
HPLC
n-Hexane
Aglient ZORBAX SB-C18 column (150 mm × 4.6 mm, 5 μm)
H2O (A) and MeCN (B)
254 nm
Whole grass
/
(Chen et al., 2021)
HPLC
70% aqueous EtOH
Phenomenex Luna C18 column (250 mm × 4.6 mm, 5 µm)
H2O (A) and MeCN (B)
245 nm
Leaves
/
(Chen et al., 2020)
HPLC-DAD-MS
EtOAc
Phenomenex Luna C18 (5 μm)
H2O (A) and MeCN (B) both containing 0.05% trifluoroacetic acid
240 nm
Flowers
TPf 15.9–76.9 mg/g in methanolic extract, 25.8–100.9 mg/g in ethyl acetate fractionate
(Lee et al., 2013)
B. pilosa L. var. radiata (BPR), B. pilosa L. var. pilosa (BPP), and B. pilosa L. var. minor (BPM)
HPLC
MeOH
Phenomenex Luna C18(2) column (150 mm × 2.0 mm, 3 µm)
H2O (A) and MeCN (B) both containing 0.05% trifluoroacetic acid
240 nm
Leaves
2-β-D-glucopyranosyloxy-1-hydroxy-5(E)-tridecene-7,9,11-triyne, cytopiloyne, 3-β-D-glucopyranosyloxy-1-hydro-
xy-6(E)-tetradecene-8,10,12-triyne 0.61 ± 0.02%, 0.44 ± 0.02%, and
0.32 ± 0.01% in BPR, 0.17 ± 0.01%, 0.19 ± 0.01%, and 0.27 ± 0.01% in
BPP and 0.16 ± 0.02%, 0.15 ± 0.02%, and 0.27 ± 0.01% in BPM,
respectively(Chien et al., 2009)
GC-EI-MS
Supercritical CO2
RTX-5 MS capillary column (30 m × 0.25 mm, 0.25 μm)
He
/
Whole plants
/
(Chien et al., 2009)
P. ginseng
HPLC-DAD-MS
MeOH
Prevail C18 rocket column (33 mm × 7 mm, 3.0 µm)
H2O (A) and MeCN (B)
203 nm
Leaves, roots
Polyacetylenoids 1.93–2.72 mg/g
(Qian et al., 2009)
UPLC-PDA
15% aqueous EtOH
Acquity UPLC BEH-C18 column (50 mm × 2.1 mm, 1.7 µm)
MeCN (16%) in H2O
265 nm
Roots
Panaxfuraynes A and B < 3 and 2 ng/g, respectively
(Lee et al., 2010)
GC–MS
70% aqueous MeOH
DB-5 column (5% phenyl-methylpolysiloxane, 30 m × 0.25 mm, 0.25 µm)
He
/
Roots
/
(Park et al., 2013)
GC–MS
Hexane
OV-1 capiliary column (30 m × 0.25 mm, 0.25 mm)
He
m/z 40–500
Roots
Nona-3,5-diyne 85.6%, nona-3,5-diyn-2-one 3.0%, nona-4,6-diyn-3-one 2.5%, nona-3,5-diyn-2-ol 2.2%, and nona-4,6-diyn-3-ol 3.1% in the total volatiles
(Herrmann et al., 2013)
E. pallida
HPLC
n-Hexane
Chromolith performance RP-18e column (100 mm × 4.6 mm)
H2O (A) and MeCN (B)
210 nm
Roots
8-Hydroxy-tetradec-(9E)-ene-11,13-diyn-2-one, 8-Hydroxy-pentadec-(9E)-ene-11,13-diyn-2-one, 8-Hydroxy-pentadeca-(9E,13Z)-dien-11-yn-2-one, Pentadec-(9E)-ene-11,13-diyne-2,8-dione, Pentadeca-(9E,13Z)-dien-11-yne-2,8-dione, Tetradec-(8Z)-ene-11,13-diyn-2-one, Pentadec-(8Z)-ene-11,13-diyn-2-one, Pentadeca-(8Z,13Z)-dien-11-yn-2-one 0.09–1.13 mg/g
(Pellati et al., 2007)
HPLC-DAD-(ESI)MS
MeOH with 0.1% HCOOH
Ascentis C18 column (250 mm × 4.6 mm, 5 μm)
H2O (A) and MeCN (B) both containing 0.1% formic acid
DAD:190–450 nm
Roots
TPf 5.46 ± 0.23 mg/g
(Pellati et al., 2012)
HPLC-UV/DAD and ESI-MS
n-Hexane
Ascentis Express C18 column (150 mm × 3.0 mm, 2.7 µm)
H2O (A) and MeCN (B)
DAD:190–500 nm, 210 nm; m/z 100–1700
Roots
/
(Tacchini et al., 2017)
A. annua
HPLC-DAD
MeOH
Hypersil ODS2 RP-18 column (250 mm × 4.6 mm, 5.0 µm)
H2O (A) and MeCN (B)
190–400 nm
Hairy roots
/
(Zhai and Zhong 2010)
LC-DAD/APCI-MS
n-Hexane
Eclipse C18 (150 mm × 4.6 mm, 5 µm)
0.05% TFA in H2O (A) and MeCN (B)
DAD: 200–600 nm, 220, 254, 280, 354 nm
Aerial parts
/
(Ivarsen et al., 2014)
B. longiradiatum
HPLC-DAD-MS
CH2Cl2
TSKgel ODS-100 V C18 column (150 mm × 4.6 mm, 3 µm)
H2O (A) and MeCN (B)
DAD: 190–400 nm, 254 nm
Whole plants
Bupleurotoxin 2.25–0.18 mg/g, acetylbupleurotoxin 3.91–0.02 mg/g, and bupleurynol 1.00–0.03 mg/g
(Huang et al., 2011)
LC-Q-TOF-MS
Serum metabolites
Eclipse plus C18 column (100 mm × 3.6 mm, 1.8 μm)
MeCN (A) and 0.1% formic acid H2O (B)
m/z 80–1000
Roots
/
(Zhang et al., 2014)
C. Radix
HPLC-UV
MeOH
YMC-Pack ODS-A column (20 mm × 250 mm, S-5 μm, 12 nm)
MeCN (A) and 0.1% phosphoric acid H2O (B)
215 nm
Roots
/
(He et al., 2014)
UHPLC-Q- TOF-MS and UHPLC-MS/MS
Plasma and tissue
Acquity UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 µm)
H2O (A) and MeCN (B) both containing 0.1% formic acid
m/z 100–1200
Roots
/
(Xie et al., 2023)
P. quinquefolium
HPLC
100% Aqueous MeOH − 80% aqueous MeOH
Purospher STAR RP-18 column (250 mm × 4 mm, 5 µm)
H2O (A) and MeCN (B)
203 nm
Roots
TPf 2560 mg/kg FWe in root hairs, 910 mg/kg FWe in lateral roots and 570 mg/kg FWe in main roots
(Christensen et al., 2006)
GC–MS
Hexane
DM-1 capiliary column (methyl siloxane, 30 mm × 0.25 mm, 0.25 mm)
He
m/z 159
Roots
Type I: FaOHb 156.07 mg/kg, Panaxydol 556.74 mg/kg
Type Ⅱ: FaOHb 372.10 mg/kg, Panaxydol 503.41 mg/kg(Wang et al., 2010)
T. vulgare
HPTLC-UV/Vis/FLD-EDA-HRMS
EtOAc
/
MeCN (A) and H2O both containing 0.1% formic acid
254, 366 nm
Roots
/
(Moricz et al., 2018)
A. podagraria
HPLC
MeOH
Nucleosil-3C-18 column (100 mm × 4.6 mm)
MeCN (A) and H2O (B) both containing 0.1% formic acid
/
Roots, leaves, stems, flowers
FaOHb 88 mg/g in flowers
(Prior et al., 2007)
A. maritima
HPLC
CHCl3
Inertsil ODS-column (250 mm × 4.6 mm, 3.5 μm)
H2O:MeOH (20:80)
480 nm
Roots
Pentayeneene 3.3%, thiarubrine A 2.6%, thiarubrine A epoxide 1.0%, and thiarubrine A diol 0.2%
(AbouZid et al., 2007)
C. asiatica
HPLC
90% Aqueous MeOH
Symmetry 300™ C18 (150 mm × 3.9 mm, 5 μm)
H2O (A) and MeCN (B)
/
Leaves
(Randriamampionona et al., 2007)
L. inflata
HPLC
HCl: MeOH (1:1, v/v)
Hypersil MOS column (250 mm × 4 mm)
H2O (A) and MeCN (B).
270 nm
Hairy roots
Lobetyolin 3.6% and lobetyolinin 0.8–1.6%
(Bálványos et al., 2004)
P. pseudo-ginseng subsp. pseudo-ginseng
HPLC
EtOAc
YMC-Pack A-612 (NH2) (150 mm × 6 mm)
MeOH:H2O (80:20)
202 nm
Roots, rhizomes
FaOHb 0.028%
(Tanaka et al., 2000)
Panax species (white ginseng; red ginseng; P. Japonicus; P. Noteginseng)
HPLC
MeOH
LiChrosorb RP-18 (250 mm × 4.6 mm)
H2O (A) and MeCN / MeOH (B)
254 nm
Roots
TPf content of white ginseng
0.020–0.073%, red ginseng 0.019–0.055%, P. quinquefolium 0.067–0.080%, P. japonicus 0.004–0.006%, and P. noteginseng 0.045–0.056%(Washida and Kitanaka 2003)
D. carota; P. satica
HPLC-UV
MeCN
Luna C18 column (4.6 mm, 5 μm)
H2O (A) and MeCN (B)
205 nm
Roots
Carrots: FaDOHd 158.3 mg/kg, FaDOAca 55.5 mg/kg, FaOHc 277.5 mg/kg;
Parsnips: FaDOHd 252.1 mg/kg, FaOHb 330.7 mg/kg in parsnips(Koidis et al., 2012)
C. taxa; C. pilosula; C. pilosula var. modesta and C. tangshen
HPLC-DAD
MeOH
YMC-Pack Pro-C18 column (250 mm × 4.6 mm, 5 μm)
MeCN (A) and 0.1 % (v/v) phosphoric acid H2O (B)
215 nm
Roots
C. pilosula: lobetyolin 0.034–0.720 mg/g;
C. pilosula var. modesta: lobetyolin 0.008–1.302 mg/g(He et al., 2014)
A. graveolens
HPLC-DAD
CH2Cl2
Zorbax Rx-C18 (150 mm × 4.6 mm, 3.5 µm)
H2O (A) and MeCN (B)
205 nm
Roots
/
(Zidorn et al., 2005)
P. sativa
HPLC/LC-Q-TOF-MS
MeCN
Phenomenex Luna C18 columns (100 mm × 4.6 mm, 5 μm; 100 mm × 2 mm, 2.5 μm)
H2O (A) and MeCN (B)
205 nm; m/z 100–1000
Roots
/
(Rawson et al., 2010)
A. macrocephala or A. japonica
HPLC/ESI-MS
MeOH
INNO C18 column (250 mm × 4.6 mm, 5 µm)
MeCN (A) and H2O (B) both containing 0.1% formic acid
254 nm
Roots
/
(Kim et al., 2018)
Radix Bupleuri
UHPLC-DAD/ESI-MS
Serum samples
Acquity BEH C18 column (50 mm × 2.1 mm, 1.7 μm)
0.1% formic acid H2O (A) and MeCN (B)
ESI probe in both positive and negative ion modes
Roots
/
(Gao et al., 2020)
A. membranaceus and C. pilosula
UPLC-Q-TOF-MS
H2O
Acquity BEH C18 (100 mm × 2.1 mm, 1.7 µm)
H2O (A) and MeCN (B) both containing 0.1% (v/v) formic acid
m/z 100–1700 in negative mode and 50–1600 in positive mode
Roots
/
(Chau et al., 2016)
O. horridus
UPLC/Q-TOF-MS
Human fecal specimens
Waters Acquity UPLC HSS C8 column (100 mm × 2.1 mm, 1.7 μm)
0.1% formic acid H2O (A) and MeCN (B)
Dual electrospray ionization (ESI) source
Root bark
/
(Wang et al., 2020)
C. pilosula
UPLC-MS/MS
Rat plasma
ZORBAX Extend-C18 column (100 mm × 2.1 mm, 1.8 μm)
MeCN (A) and H2O (B) both containing 0.1% formic acid
278 nm
Roots
/
(Dong et al., 2021)
M. chamomilla
UHPLC-PDA-Q-TOF-MS
MeOH
Acquity UPLCTM BEH Shield RP18 column (100 mm × 2.1 mm, 1.7 µm)
H2O (A) and MeCN (B) both containing 0.05% formic acid
m/z 50–1500; 190–600 nm
Whole plant
/
(Avula et al., 2014)
B. gardneri
LC-ESI-MS
CH2Cl2 and EtOAc
Onyx C18 (15 mm × 4.6 mm)
H2O (A) and MeCN (B) both added 1% (v/v) acetic acid
/
Leaves, stems
/
(Silva et al., 2015)
P. grandiflorum
HR-TOF-MS
MeOH
Agilent ZORBAX® SB-C18 column (150 mm × 4.6 mm, 5 μm)
H2O (A) and MeCN (B)
DAD:210 nm
Roots
/
(Chen et al., 2018)
T. procumbens
GC-FID
Hexane
HP-5 capiliary columns (5% phenylmethylsiloxane, 30 m × 0.320 mm, 0.25 μm)
N2
/
Roots, stems, leaves, flowers, fruits
/
(Larque-Garcia et al., 2020)
C. acaulis
GC-FID
EtOH
HP-5 capillary column (30 m × 0.32 mm, 0.25 μm)
He
/
Plant material
/
(Strzemski et al., 2019)
E. bannaticus and E. sphaerocephalus
GC–MS
Et2O
DB-5MS capiliary columns (5% phenylmethylsiloxane, 30 m × 0.25 mm, 0.25 mm)
He
RI: C7–C40 alkanes
Roots
/
(Radulovic and Denic 2013)
E. yuccifolium
GC–MS
Essential oil
Chrompack CPSil 5 CB capillary column (polydimethylsiloxane, 25 m)
He
/
Whole plants
Leaves oil: FaOHb 9.6%; stalk oil: FaOHb 3.2%
(Ayoub et al., 2006)
S. africana
GC–MS
Essential oil
BP-1 capillary column (polydimethylsiloxane, 50 m × 0.22 mm, 0.25 µm); BP-20 capillary column (polyethylene glycol, 50 m × 0.22 mm, 0.25 µm)
GC: H2; MS: He
/
Aerial parts
(E)-2-(2′,4′-Hexadiynylidene)-1,6-dioxaspiro [4.4]-nona-3,7-diene (7.3%)
(E)-tonghaosu 3.8%(Malti et al., 2019)
P. ginseng, ginseng, notoginseng, American ginseng
GC-EI-MS
n-Hexane
DM-1 MS column (30 mm × 0.25 mm, 0.25 µm)
He
SIM m/z: 159, 121
Roots
FaOHb 84.2–261.8 µg/g and panaxydol 102.7–247.4 µg/g
(Liu et al., 2007)
E. palmatum
GC-FID and GC–MS
CH2Cl2
HP-5 MS column (30 m × 0.25 mm, 0.25 μm)
He
m/z 35–550, RI: n-alkanes (C8–C20 and C21–C40)
Aerial parts, roots
/
(Marčetić et al., 2014)
S. tenuifolium
GC-FID and GC–MS
Essential oil
Elite-5 MS capillary column (30 m × 0.32 mm, 0.32 mm)
He
RI: n-alkane series (C6–C32)
Underground parts
/
(Chauhan et al., 2012)
4.1 Detection method
DAD and MS detectors are commonly used for qualitative and quantitative analysis of plant polyacetylenoids. The wavelength range for UV detection is 200–400 nm. Generally, the detection sensitivity above 230 nm is extremely low in the clue of the existence of few conjugated unsaturated bonds in their structures, and the excitation coefficients (ε) of these compounds (normally two triple bonds in conjugation) were commonly below 6000 at their characteristic UV-maxima. Instead, the UV sensitivity of the polyacetylenoids is improved approximately 10 times when detected at 205 nm and hence the detection of these compounds were commonly conducted at 205 nm (Christensen and Brandt 2006). However, additional co-eluting peaks may interfere with the analytes at 205 nm because no selective detection is possible in plants, such as carrot genotype (Pferschy-Wenzig et al., 2009). Therefore, more elaborate sample preparation is necessary. In general, two types of mass spectrometers are applied, quadrupole-time of flight (Q-TOF) and Q-Q-Q mass spectrometers, dedicated to qualitative and quantitative analysis of the polyacetylenoid constituents of plant origins, respectively. The most frequently used ion source is electrospray ionization (ESI).
4.2 TLC and HPTLC analyses
TLC and HPTLC are rapid separation and qualitative analytical techniques that can be used for routine chemical analysis and identification of the polyacetylenoid components in plants. For HPTLC and TLC analyses of polyacetylenoids, silica gel 60 F254 plates were often eluted with the following developing solvents: Petrol ether–ethyl acetate (3:1, v/v), hexane–ethyl acetate (1:1, v/v) (Prior et al., 2007), and n-hexane–isopropyl acetate (9:1, v/v) (Moricz et al., 2018). Normally, polyacetylenoids can be detected as dark black spots by spraying with vanillin (1% in MeOH) or sulfuric acid (5% in MeOH) before heating (Zidorn et al., 2005), or dark blue spots after spraying phosphomolybdic acid reagent and heating (Larque-Garcia et al., 2020). Recently, a situ effect-directed analysis by integrating HPTLC, chemical derivatization, and high-resolution mass spectrometry (HRMS) detection (HPTLC-UV/Vis/FLD-EDA-HRMS) was developed to profile and identify the antibacterial polyacetylenoids in root extracts of tansy (Moricz et al., 2018).
4.3 HPLC and UHPLC analyses
4.3.1 In vitro analysis
Till now, HPLC and UHPLC have been two of the most common techniques for quality assessment of the plant-originated polyacetylenoids, due to their availability, ease of operation, high sensitivity and reproducibility, good resolution and linearity, and the ability to analyze multiple components in a single run. For HPLC and UHPLC analyses of these polyacetylenoids, the most frequently equipped column for the separation is the RP-C18 columns, including Onyx C18 (Silva et al., 2015), Acquity BEH C18 (Chau et al., 2016), Ascentis Express C18 (Tacchini et al., 2017), Zorbax RX-C18 (Hinds et al., 2017), INNO C18 (Kim et al., 2018), Phenomenex Luna C18 (Chen et al., 2020), and Agilent ZORBAX SB-C18 (Chen et al., 2021), with methanol–water or acetonitrile–water containing 0.1–0.3% formic acid, acetic acid or phosphoric acid as the mobile phases. Notably, the use of acetic acid or phosphoric acid in the mobile phase not only afforded a satisfactory baseline that led to MS spectra of higher quality, but also produced many clusters, which were used for internal calibration to allow the molecular formulae of polyacetylenoids to be easily found (Silva et al., 2015). PDA and/or MS were used as the common detectors, and ESI and atmospheric pressure chemical ionization (APCI) was commonly used as the ion sources for MS detection. However, UV detection seems more suitable than ESI-MS for polyacetylenoids, because of the lack of low ionization by ESI, which requires high concentrations of the target compound in the samples to produce satisfactory results.
In order to improve the ionization for ESI detection of polyacetylenoids, a post-column sodiation is often used to show a good MS spectrum. Since ion suppression can happen in the ESI source, the added sodium must be low and carefully controlled. Polyacetylenoids present the electron pairs (non-bounded) of oxygen in their chemical structures that can interact with metals hence they show preferentially sodiated ions. The coordination reactions of polyacetylenoids with sodium can take place by oxygen of sugar, or even the oxygen from the polyacetylenic aglycone (Silva et al., 2015). Actually, polyacetylenoids are non-detectable among the deprotonated molecular ions [M - H]-, thus, the electrospray ionization in positive ion mode (ESI+) seems a good choice for the detection of the polyacetylenoids (Soltoft et al., 2010; Wen-Chin et al., 2013), which showed [M + H]+ and [M + Na]+ ions, as well as some other fragment ions, such as [M - H2O + H]+ and [M + H - CH3COOH]+ (Huang et al., 2011). Amongst them, the [M + H]+ ions showed lower abundance than the [M - H2O + H]+ ions due to the easy dehydration of the protonated molecular ion for those hydroxylated polyacetylenoids, such as falcarinol (FaOH), falcarindiol (FaDOH) and falcarindiol-3-acetate (FaDOAc). For example, the relative abundances of the [FaDOH + H]+ ion and the [FaDOAc + H]+ ion were only 2 and 9%, respectively, while the [FaOH + H]+ ion couldn't be observed in the (+)-ESI mode. Instead, [M - H2O + H]+ ions of FaDOH and FaDOAc showed abundances of 75–100% and a more modest 21% for that of FaOH (Soltoft et al., 2010). Interestingly, dimeric ion species were predominant in the ESI spectrum of falcarinol, recorded with the mobile phase containing MeOH. From the foregoing, a positive ESI detection with a mobile phase of MeCN-H2O-0.1% HCOOH was found to be most appropriate for the determination of FaOH, with the ion adduct at m/z 268 [FaOH + H - H2O + MeCN]+ in 100% relevant abundance, together with those at m/z 309 [FaOH + H - H2O + 2MeCN]+, 182 [FaOH + H - H2O - C6H14 + MeCN]+, and etc. in lower relevant abundances (Pferschy-Wenzig et al., 2009).
Nevertheless, the MS spectrometric behaviour of FaOH, FaDOH and FaDOAc was comprehensively studied by ionization using an APCI interface in the positive ionization mode, with methanol–water as the mobile phase. For FaOH, no ionization at all was observed in APCI detection with MeCN-containing mobile phases, while a MeOH eluted APCI detection yielded rather complex MS spectra, with m/z 259 [FaOH + H - H2O + MeOH]+ and 227 [FaOH + H - H2O]+ as the most abundant ion adducts, accompanied with a considerable degree of the usually concentration-dependent dimeric ion species. Therefore, a linear calibration curve over a wide concentration range is quite difficult to obtain (Pferschy-Wenzig et al., 2009). Incredibly, FaOH generated an ion at m/z 243 [M − 1]+, suggesting either a hydride subtraction [M - H]+ or a dehydrogenation of the protonated molecule ion [(M + H) - H2]+ (Kramer et al., 2011).
4.3.2 Pharmacokinetics analysis
For those active polyacetylenoids, finding out their half-life, bioavailability, toxicity, and other pharmacokinetic (PK) properties would help us understand how the body may respond to the intake of the polyacetylenoids. Recently, UPLC-PDA, UHPLC-Q Extractive Orbitrap MS/MS, or UPLC/Q-TOF-MS method has been successfully applied to study the pharmacokinetic and biotransformation properties of plant polyacetylenoids (Lee et al., 2010; Avula et al., 2014; Dong et al., 2021; Xie et al., 2023). Four polyacetylenoids, including compounds 341 [(2Z,8E,10E)-pentadecatriene-4,6-diyn-1-ol], 107 (bupleurynol), 340 [(2Z,8Z,10E)-pentadecatriene-4,6-diyne-1-ol], and 104 [(2Z,8Z,10E)-heptadecatriene-4,6-diyne-1-ol], could be detected in rat serum after a single intragastric (i.g.) administration of the petroleum ether extract (22.5, 45.0, 90.0 g/kg) of Radix Bupleuri to rats. Among them, 341 and 107 showed a fast distribution phase followed by a relatively slow elimination phase (t1/2z, 4–7 h), however, 340 and 104 could not be detected in rat serum. The reason for this may be either the existence of the less stable Z-configuration of C8/C9 in their structures, which may undergo a trans-isomerism in vivo, or the limitation of their original contents in Radix Bupleuri (Liu et al., 2015). The major biotransformation routes of 341 and 107 involved oxidation and glucuronidation as indicated by the combined analytical results of UHPLC-Q Extractive Orbitrap MS/MS and Compound Discoverer 2.0, which can facilitate us the understanding of an individual's response and accomplishing the drug–drug interaction prediction for us (Gao et al., 2020).
Furthermore, by applying UPLC/Q-TOF-MS analysis, 37 metabolites could be identified from the biotransformed Oplopanax horridus extract (OHE) with the enteric microbiome of healthy human subjects. Among the seven polyacetylenoids originally identified in OHE, only oplopantriol A (206a) was detected to be extensively biotransformed (94.7%), and demethylation and dehydroxylation may be the two major metabolic pathways. All these microbial metabolites are more hydrophobic than the parent oplopantriol A, and are expected to show more potent anticancer activity than their parent compound (Wang et al., 2020). Another, Xie et al. established a rapid, sensitive, and selective UPLC-MS/MS method for the simultaneous quantitative and semi-quantitative determination of the rat metabolites of lobetyol (1 4 8). As a result, a total of 47, 30 and 34 metabolites of lobetyol, lobetyolin (1 4 9) and lobetyolinin (1 5 1), respectively, were found in all the rat liver microsomes (RLMs), human liver microsomes (HLMs), and rat plasma, bile, feces, and urine samples, concerning mainly the metabolic pathways of oxidation, glucuronidation and glutathione conjugation (Xie et al., 2023).
UPLC–MS/MS Method has also been developed to evaluate the pharmacokinetic properties of polyacetylenoid monomers in vivo. In rat plasma, after administration of lobetyolin and Codonopsis pilosula extract (CPE), the elimination half-times (t1/2) and the areas under the concentration–time curve were statistically different between the two treatments, but there was no significant difference between them in the time to reach the maximum plasma concentration (Tmax) and the maximum concentration (Cmax). Notably, the bioavailability of lobetyolin (3.90%) was lower than CPE's (6.97%), indicating that this compound may either be absorbed poorly or metabolized extensively in rats. Therefore, the methods to enhance its oral absorption should be further studied (Dong et al., 2021). Using the similar method, the pharmacokinetics of falcarinol (19) was elucidated in vivo. Falcarinol had a half-life of 1.5 hr. after an intravenous injection (5 mg/kg), while 5.9 hr. after an oral administration (20 mg/kg) with a moderate bioavailability of 50.4%. Falcarinol also shows a low metabolism and slow absorption in the clue of its highly lipid-soluble property (Tashkandi et al., 2020).
4.4 GC analysis
Gas chromatography (GC), coupled with a FID and/or a EI-MS detection, has been most frequently adopted to detect the polyacetylenic components in volatile (essential) oils or nonpolar extracts of the terrestrial medicinal plants. Separation of the polyacetylenoids can be achieved on various types of fused silica gel capillary GC columns including DB-5MS (Park et al., 2013; Radulovic and Denic 2013; Wnorowski et al., 2020), DM-1 (Liu et al., 2007; Wang et al., 2010), RTX-5 (Chien et al., 2009), Elite-5 (Chauhan et al., 2012), SPB-1 (de Carvalho Augusto et al., 2020) and HP-5 (Benelli et al., 2020; Larque-Garcia et al., 2020), coated with 5% phenylmethylsiloxane (Benelli et al., 2020) or cross-linked methylsiloxane (Wang et al., 2010). Compared with HPLC/UHPLC-UV method, GC–MS has much lower LOD and LOQ, assuring its suitability for the analysis of such polyacetylenoids with extremely low abundance in plants as exemplified by ginseng (Liu et al., 2007). Quantitative analysis of them was carried out in selected ion monitoring (SIM) mode, in whose chromatograms seldom interference was found near the peaks of compounds, allowing accurate determination. Then, ions at m/z 159 and m/z 121 were selected for scanning with the aim of the detection of falcarinol and panaxydol, respectively (Liu et al., 2007; Wang et al., 2010).
In addition to the above, Strzemski et al. established a fast and low-cost voltammetric methodology for determination of carlina oxide (4 7 9), one major volatile constituent of Carlina plants. The results were quite similar to those obtained using HPLC and GC methods, with the differences in mean contents < 8.51%, and the values of relative standard deviation obtained for all analyzed samples were 1.5–3.1% (<5%), revealing excellent repeatability of the method (Strzemski et al., 2019).
5 Pharmacology
Falcarinol-type polyacetylenoids are widely present in vegetables including carrots and parsley, and some food-medicine herbs as exemplified by P. ginseng and C. pilosula. They exert diverse pharmacological actions and hold potential as health-promoting and therapeutic agents (P Christensen 2011). For example, falcarinol and falcarindiol, both exhibit anti-inflammatory, anti-tumor, hepatoprotective and some other interesting bioactivities. Besides, other types of polyacetylenoids including lobetylolin, lobetyol and panaxytriol were frequently reported to possess beneficial pharmacological activities against cancer (colorectal cancer, lung cancer, gastric cancer, etc.) (Bailly, 2020; Kobaek-Larsen et al., 2017; Liu et al., 2022). Herein, we try to draw a brief outline of the latest pharmacological achievements on the fore-mentioned falcarinol- and lobetyol-types of polyacetylenoids.
5.1 Falcarinol and falcarindiol
Falcarinol could upregulate intestinal heme oxygenase-1 and modify plasma cytokine IL-4, IL-13, IL-9 and IL-10 profile in late-phase lipopolysaccharide (LPS)-induced acute inflammation in C57BL/6 mice. Hence, a diet rich in falcarinol is anti-inflammatory, immunomodulatory and can have beneficial effects on inflammatory gastrointestinal as well as other inflammatory disorders (Stefanson and Bakovic 2020).
Notably, dietary supplements with falcarinol and falcarindiol reduced the number of neoplastic lesions as well as the growth rate of the polyps, suggesting a preventive effect of falcarinol and falcarindiol on the development of colorectal cancer (Kobaek-Larsen et al., 2017). And, falcarinol and falcarindiol isolated from Daucus carota could prevent the formation of colon tumor induced by azomethane in rats (Kobaek-Larsen et al., 2017). Furthermore, orally administered falcarinol significantly reduced lung tumorigenesis in KrasG12D/þ transgenic mice and mice carrying NSCLC xenografts without detectable toxicity, as a novel natural Hsp90 inhibitor that effectively eliminates both non-CSC (cancer stem-like cells) and CSC populations of NSCLC (Non-small cell lung cancer) by blocking N- and C-terminal ATP pockets without inducing Hsp70 expression (Le et al., 2018).
5.2 Lobetylolin, lobetyol and panaxytriol
Lobetyolin (LBT) suppressed lung cancer in a mouse model by inhibiting epithelial-mesenchymal transition (Liu et al., 2022). Xanthine oxidase (XO) catalyzes the formation of uric acid from xanthine, concerning a critical metabolic pathway related to hyperuricemia and gout. Oral administration of LBT at 50 mg/kg significantly reduced the activity of hepatic XO in vivo (Yoon and Cho 2021).
A recent study has revealed a key aspect of the mode of action of LBT, with the discovery of the capacity of the compound to inhibit glutamine metabolism and specifically, to down-regulate the amino acid transporter ASCT2, in a p53-dependent manner. Human ASCT2 is a trimeric protein (also known as SLC1A5) acting as a sodium-dependent neutral amino acid antiporter. Its transport activity can be modulated by lipophilic molecules, like the antagonist V-9302 which is a potent anticancer agent, sulfonamide/sulfonic acid esters linked to a hydrophobic group, and cholesterol. Given the diverse functional roles of hASCT2, the blockade of this transporter can have multiple implications in human diseases, not only in cancer. Interestingly, LBT-containing extracts and TCM prescriptions (eg. Weikang Keli), lobetyol, and other polyacetylenoids structurally close to lobetyol, such as 4,6,12-tetradecatriene-8,10-diyne-1,3,14-triol and panaxytriol, were demonstrated to show significant inhibitory activities against gastric cancer lines. Nowadays, efficient treatments are lacking for advanced gastric cancer. Thus, it would be worth a lot to investigate the further therapeutic potential of such polyacetylenoid glycosides in this indication (Bailly, 2020; He et al., 2020).
6 Conclusion and discussion
6.1 Conclusion
This review covers the research updates on the polyacetylenic phytochemicals and their distribution, botanic origins, NMR characteristics and analytical methods, as well as a brief bioactivity sketch of some representative polyacetylenoids in terrestrial medicinal plants by excavating various literature dating from 2000 to 2023. Herein, 363 linear polyacetylenoids and 122 cyclic ones with chain lengths of C8–19, C21, C23–25, C27, C29, and C33, have been collated from the terrestrial medicinal plants, belonging mostly to the families of Compositae, Apiaceae, Araliaceae, Campanulaceae, Annonaceae, and Meliaceae. Their molecular scaffolds occurred mainly to be the polyacetylenoids with carbon lengths of C17, C14, C18, C10, C13, C15, with 14 main types of polyacetylenic terminals. And their representative NMR characteristics and the determination methods of the related configurations have also been included. Further, the analytical methods of polyacetylenoid compounds and/or extracts are summarized. HPLC, UHPLC and GC analysis, as well as the co-location technology, have been widely used for in vitro or in vivo determination and quantification of the polyacetylenoids. And finally, the plant origins and a brief outline of the bioactivities of several representative polyacetylenoids were also referred to in this Review.
6.2 Discussion
6.2.1 Polyacetylenic phytochemicals and their distribution
In the last two decades, more than 485 polyacetylenoids have been isolated from almost 110 kinds of terrestrial herbs. Linear polyacetylenoids dominate about 85% of all the 485 polyacetylenoids, with C17-polyacetylenoids ranked in the top 1. The moiety of hepta-4,6-diyne is normally embedded in their polyacetylenic terminals. As is known, plant polyacetylenoids are widely distributed in plants of families Compositae and Apiaceae. Not very early, due to the low contents in their botanical origins, they come to people's attention on account of their intrinsic special triple-bond functionalities and fatty acid-like carbon chains. In view of biosynthesis pathways, most of them are falciferol-derived, showing anticancer and other diverse pharmacological activities. It is worth noting that polyacetylenoid glycosides hold important potential as one of the anti-tumor active ingredients for future anti-tumor compounds and traditional Chinese medicine preparations.
6.2.2 Analytical methods and quality control
As shown in Table 2, common analytical methods of plant polyacetylenoids were summarized and HPLC-MS, UHPLC-MS and GC–MS represented the main methods ever reported. Several well-known active polyacetylenoids including falcarinol, falcarindiol, falcarindiol-3-acetate, lobetyol, lobetyolin, and lobetyolinin, have been frequently selected out as the maker components for both qualitative determination and quantitative analysis of those specific herbs, considering their valuable bioactivities in vitro and/or in vivo. Several studies had revealed that the ratio of polyacetylenoids such as panaxydol/falcarinol can be proposed as an important marker for differentiating Asian ginseng, Notoginseng, and cultivated American ginseng, indicating that analysis of the ratios of the major polyacetylenoid constituents, to some extent, may supply a guideline for distinguishing certified herbs from their counterfeit species (Wang et al., 2010).
Unfortunately, they are unstable and highly lipophilic, their low metabolism and slow absorption in vivo as exemplified by falcarinol and lobetyolin are key problems to be overcome for future in-depth exploitation (Tashkandi et al., 2020; Dong et al., 2021). Hence, there is still an urgent need for highly rapid, convenient, and cost-effective methods, artificial synthesis, biosynthesis, and phytochemical separation for high production of active and structurally stable polyacetylenoids to light their pharmacological effects. Moreover, their plasma concentrations for exerting biological functions in vivo are still unclear.
6.2.3 Pharmacology of polyacetylenoids
As reviewed by Negri, Xie and Wang (Negri 2015; Xie and Wang 2022), at the current stage, in-vivo preclinical studies are still needed as they play essential and vital roles in the assessment and supervision of the clinical functions of those polyacetylenoids. Up-to-date laboratory researches, especially in vitro evaluations at the cellular level, on the pharmacological properties of polyacetylenoids are extensive. However, there are few in-vivo scale pharmacological and pharmacokinetic studies on the active plant polyacetylenoids, as bioavailability and metabolism of the polyacetylenoids are critically important for future research and development of such drugs.
Studies have shown that a generous intake of fruits and vegetables can promote people's health. And aliphatic C17 polyacetylenoids, represented by the common falcarinol-type polyacetylenoids abundant in foods, fruits and vegetables of the family Apiaceae, may act as the potential functional molecules promoting the consumer's health. As far as we know, there are still numerous falcarinol-type polyacetylenoids that have not been fully studied due to their unavailable production in larger amounts, although some of them have been tested out to show favorable anticancer or anti-tumor activities. Therefore, in addition to conducting more and more in-vivo preclinical studies and even trials in the clinic to achieve the ideal intake dosage for health-promoting effects, there is also a necessary to improve their bioavailability by structural modification or synthesize methods, as well as their yield in plants (foods, fruits, or vegetables) by developing new genotypes and/or processing techniques (eg. large-scale extraction and isolation) (Christensen 2011).
Currently, polyacetylenoids in red ginseng extract composed of panaxynol and panaxydol has furtherly been found to possess beauty-related bioactivities such as skin care and hair generation, by alleviating even eliminating the symptoms of acne (Hou et al., 2019). And, hydroxylated polyacetylenoids isolated from P. ginseng may provide therapeutic benefits for hair growth disorders (eg. alopecia) by inhibiting neurotrophins from binding to the receptors (Suzuki et al., 2017). Therefore, in addition to the abovementioned health-promoting, skin care, and hair generation potentials, it is speculated that polyacetylenoids will possess good research prospect and important value in applications as perfume, appetite enhancer and natural preservative (Xie and Wang 2022). And, polyacetylenoids from terrestrial medicinal plants show great future potentials to be developed into health care products and new drugs in treatment of a wide array of pathologies.
Author contributions
The idea of putting forward innovative ideas and summarizing the whole review were done by Hong-Hua Wu. Jia-Xin Lai and Su-Fang Dai collated documents and summarize objective laws cooperatively. Jia-Xin Lai was responsible for drafting the manuscript with critical suggestions from Hong-Hua Wu. Li-Hua Zhang, Yan-Xu. Chang and Wen-Zhi. Yang assisted with the revision of the manuscript.
Acknowledgments
This work was financially supported by grants (No. 21ZYJDJC00080 and 22ZYJDSS00040) from the Tianjin Committee of Science and Technology of China and a grant (No. 62231025) from the Major Program of National Natural Science Foundation of China.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Molluscicidal activity of polyacetylenes from Ambrosia maritima hairy roots. Nat. Prod. Commun.. 2007;2:177-180.
- [CrossRef] [Google Scholar]
- Polyacetylene levels in carrot juice, effect of pH and thermal processing. Food Chem.. 2014;152:370-377.
- [CrossRef] [Google Scholar]
- Rare trisubstituted sesquiterpenes daucanes from the wild Daucus carota. Phytochemistry. 2005;66:1680-1684.
- [CrossRef] [Google Scholar]
- Polyacetylenes from sardinian Oenanthe fistulosa: a molecular clue to risus sardonicus. J. Nat. Prod.. 2009;72:962-965.
- [CrossRef] [Google Scholar]
- Identification of a compound isolated from German chamomile (Matricaria chamomilla) with dermal sensitization potential. Toxicol. Appl. Pharmacol.. 2017;318:16-22.
- [CrossRef] [Google Scholar]
- Quantitative determination of phenolic compounds by UHPLC-UV-MS and use of partial least-square discriminant analysis to differentiate chemo-types of Chamomile/Chrysanthemum flower heads. J. Pharm. Biomed. Anal.. 2014;88:278-288.
- [CrossRef] [Google Scholar]
- Essential oils and a novel polyacetylene from Eryngium yuccifolium Michaux. (Apiaceae) Flavour Fragrance J.. 2006;21:864-868.
- [CrossRef] [Google Scholar]
- Anti-adipogenic polyacetylene glycosides from the florets of Safflower (Carthamus tinctorius) Biomedicines. 2021;9
- [CrossRef] [Google Scholar]
- Anticancer properties of lobetyolin, an essential component of radix Codonopsis (Dangshen) Nat. Prod. Bioprospect.. 2020;11:143-153.
- [CrossRef] [Google Scholar]
- Analysis of polyacetylenes by HPLC in hairy root cultures of Lobelia inflata cultivated in bioreactor. Chromatographia. 2004;60:S235-S238.
- [CrossRef] [Google Scholar]
- Structural changes of polyacetylenes in American ginseng root can be observed in situ by using Raman spectroscopy. J. Agric. Food Chem.. 2006;54:3629-3635.
- [CrossRef] [Google Scholar]
- Novel plant substances acting as beta subunit isoform-selective positive allosteric modulators of GABAA receptors. Mol. Pharmacol.. 2005;68:787-792.
- [CrossRef] [Google Scholar]
- Developing a highly stable carlina acaulis essential oil nanoemulsion for managing Lobesia botrana. Nanomater. (Basel). 2020;10
- [CrossRef] [Google Scholar]
- Automated analytical standard production with supercritical fluid chromatography for the quantification of bioactive C17-polyacetylenes: A case study on food processing waste. Food Chem.. 2014;165:371-378.
- [CrossRef] [Google Scholar]
- Polyacetylenes from radix et rhizoma Notopterygii incisi with an inhibitory effect on nitric oxide production in vitro. Planta Med.. 2014;80:415-418.
- [CrossRef] [Google Scholar]
- Phytochemical study of Eryngium triquetrum: isolation of polyacetylenes and lignans. Planta Med.. 2016;82:1438-1445.
- [CrossRef] [Google Scholar]
- A new polyacetylene from Vernonia scorpioides (Lam.) Pers. (Asteraceae) and its in vitro antitumoral activity. J. Braz. Chem. Soc.. 2009;20:1327-1333.
- [CrossRef] [Google Scholar]
- A polyacetylene-rich extract from Gymnaster koraiensis strongly inhibits colitis-associated colon cancer in mice. Food Chem. Toxicol.. 2013;53:235-239.
- [CrossRef] [Google Scholar]
- New polyacetylene and other compounds from Bupleurum chinense and their chemotaxonomic significance. Biochem. Syst. Ecol.. 2020;92
- [CrossRef] [Google Scholar]
- Use of on-flow LC/1H NMR for the study of an antioxidant fraction from Orophea enneandra and isolation of a polyacetylene, lignans, and a tocopherol derivative. J. Nat. Prod.. 1998;61(12):1497-1501.
- [CrossRef] [Google Scholar]
- Effect of echinalkamide identified from Echinacea purpurea (L.) Moench on the inhibition of osteoclastogenesis and bone resorption. Sci. Rep.. 2020;10:10914.
- [CrossRef] [Google Scholar]
- Chemical composition of the volatile oil from the roots of Selinum tenuifolium Wall. Helvetica Chim. Acta. 2012;95:780-787.
- [CrossRef] [Google Scholar]
- Transformation and characterization of delta12-fatty acid acetylenase and delta12-oleate desaturase potentially involved in the polyacetylene biosynthetic pathway from Bidens pilosa. Plants (Basel). 2020;9
- [CrossRef] [Google Scholar]
- Application of high-speed counter-current chromatography and HPLC to separate and purify of three polyacetylenes from Platycodon grandiflorum. J. Sep. Sci.. 2018;41:789-796.
- [CrossRef] [Google Scholar]
- Chemical-constituent diversity of Tridax procumbens. Can. J. Biochem.. 2008;86:892-898.
- [CrossRef] [Google Scholar]
- Chemical and pharmacological progress on polyacetylenes isolated from the family apiaceae. Chem. Biodivers.. 2015;12:474-502.
- [CrossRef] [Google Scholar]
- Separation of chemical constituents in Bidens pilosa Linn. var. radiata Sch. Bip. by elution-extrusion counter-current chromatography using two new three-phase solvent systems. J. Sep. Sci.. 2021;44:3540-3550.
- [CrossRef] [Google Scholar]
- Devil's club falcarinol-type polyacetylenes inhibit pancreatic cancer cell proliferation. Nutr. Cancer. 2019;71:301-311.
- [CrossRef] [Google Scholar]
- TRPA1 modulating C(14) polyacetylenes from the iranian endemic plant Echinophora platyloba. Molecules. 2018;23
- [CrossRef] [Google Scholar]
- Anti-diabetic properties of three common Bidens pilosa variants in Taiwan. Phytochemistry. 2009;70:1246-1254.
- [CrossRef] [Google Scholar]
- Phototoxic activity of a thiophene polyacetylene from Leuzea carthamoides. Fitoterapia. 2006;77:194-198.
- [CrossRef] [Google Scholar]
- Polyacetylenes from angelica gigas and their inhibitory activity on nitric oxide synthesis in activated macrophages. Biol. Pharm. Bull.. 2000;23:884-886.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of a novel acetylene isolated from the roots of Angelica tenuissima Nakai. Helvetica Chim. Acta. 2016;99:447-451.
- [CrossRef] [Google Scholar]
- Aliphatic C(17)-polyacetylenes of the falcarinol type as potential health promoting compounds in food plants of the Apiaceae family. Recent Pat. Food Nutr. Agric.. 2011;3:64-77.
- [CrossRef] [Google Scholar]
- Bioactive C17 and C18 acetylenic oxylipins from terrestrial plants as potential lead compounds for anticancer drug development. Molecules. 2020;25:2568.
- [CrossRef] [Google Scholar]
- Bioactive polyacetylenes in food plants of the Apiaceae family: occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal.. 2006;41:683-693.
- [CrossRef] [Google Scholar]
- Determination of polyacetylenes in carrot roots (Daucus carota L.) by high-performance liquid chromatography coupled with diode array detection. J. Sep. Sci.. 2007;30:483-490.
- [CrossRef] [Google Scholar]
- Simultaneous determination of ginsenosides and polyacetylenes in American ginseng root (Panax quinquefolium L.) by high-performance liquid chromatography. J. Agric. Food Chem.. 2006;54:8995-9003.
- [CrossRef] [Google Scholar]
- P Christensen, L., 2011. Aliphatic C17-polyacetylenes of the falcarinol type as potential health promoting compounds in food plants of the Apiaceae family. Recent Pat. Food, Nutr. Agric. 3, 64-77. https://doi.org/10.2174/2212798411103010064
- Chau, S. L., Huang, Z. B., Song, Y. G., et al., 2016. Comprehensive quantitative analysis of SQ injection using multiple chromatographic technologies. molecules. 21, https://doi.org/10.3390/molecules21081092.
- Anticomplement activity of polyacetylenes from leaves of Dendropanax morbifera Leveille. Phytother. Res.. 2011;25:784-786.
- [CrossRef] [Google Scholar]
- Elucidation of enzymes involved in the biosynthetic pathway of bioactive polyacetylenes in Bidens pilosa using integrated omics approaches. J. Exp. Bot.. 2021;72:525-541.
- [CrossRef] [Google Scholar]
- Absolute configuration of falcarinol (9Z-heptadeca-1,9-diene-4,6-diyn-3-ol) from Pastinaca Sativa. Nat. Prod. Commun.. 2013;8
- [CrossRef] [Google Scholar]
- Identification of non-alkaloid acetylcholinesterase inhibitors from Ferulago campestris (Besser) Grecescu (Apiaceae) Fitoterapia. 2010;81:1208-1212.
- [CrossRef] [Google Scholar]
- Inhibitory constituents against cyclooxygenases from Aralia cordata Thunb. Arch. Pharm. Res.. 2005;28:28-33.
- [CrossRef] [Google Scholar]
- Gymnasterkoreayne G, a new inhibitory polyacetylene against NFAT transcription factor from Gymnaster koraiensis. Chem. Pharm. Bull. (Tokyo). 2005;53:1194-1196.
- [CrossRef] [Google Scholar]
- Bioactive C17-polyacetylenes in carrots (Daucus carota L.): current knowledge and future perspectives. J. Agric. Food Chem.. 2015;63:9211-9222.
- [CrossRef] [Google Scholar]
- Molluscicidal and parasiticidal activities of Eryngium triquetrum essential oil on Schistosoma mansoni and its intermediate snail host Biomphalaria glabrata, a double impact. Parasit. Vectors. 2020;13:486.
- [CrossRef] [Google Scholar]
- Anticancer activity of natural and synthetic acetylenic lipids. Lipids. 2006;41:883-924.
- [CrossRef] [Google Scholar]
- Oxygenated C(17) polyacetylene metabolites from Algerian Eryngium tricuspidatum L. roots: structure and biological activity. Fitoterapia. 2019;138:104355
- [CrossRef] [Google Scholar]
- Comparative pharmacokinetic and bioavailability study of lobetyolin in rats after administration of lobetyolin and Codonopsis pilosula extract by ultra-performance LC-tandem mass spectrometry. Biomed. Chromatogr.. 2021;35:e5125.
- [CrossRef] [Google Scholar]
- Three new polyacetylene glycosides (PAGs) from the aerial part of Launaea capitata (Asteraceae) with anti-biofilm activity against Staphylococcus aureus. Fitoterapia. 2020;143:104548
- [CrossRef] [Google Scholar]
- Two new thiophene polyacetylene glycosides from Atractylodes lancea. J. Asian Nat. Prod. Res.. 2018;20:531-537.
- [CrossRef] [Google Scholar]
- Two new polyhydroxyl polyacetylenes from fruits of Herpetospermum caudigerum. J. Nat. Med.. 2017;71:574-577.
- [CrossRef] [Google Scholar]
- Phenylpropenoids from Bupleurum fruticosum as anti-human rhinovirus species a selective capsid binders. J. Nat. Prod.. 2017;80:2799-2806.
- [CrossRef] [Google Scholar]
- Antimicrobial polyacetylenes from Panax ginseng hairy root culture. Chem. Pharm. Bull. (Tokyo). 2012;60:377-380.
- [CrossRef] [Google Scholar]
- Human maximization test of falcarinol, the principal contact allergen of English ivy and Algerian ivy (Hedera helix, H. canariensis) Contact Dermatitis. 1988;19:125-128.
- [CrossRef] [Google Scholar]
- Three new polyacetylene glycosides from the roots of Heracleum dissectum and their triglyceride accumulating activities in 3T3-L1 cells. Chem. Biodivers.. 2019;16:e1800424.
- [Google Scholar]
- Metabolic profiling of RB-2 and RB-4, two analogs of polyacetylene from Bupleurum. J. Asian Nat. Prod. Res.. 2020;22:1045-1064.
- [CrossRef] [Google Scholar]
- Three new anti-HBV active constituents from the traditional Chinese herb of Yin-Chen (Artemisia scoparia) J. Ethnopharmacol.. 2015;176:109-117.
- [CrossRef] [Google Scholar]
- A bioactive polyacetylene compound isolated from Centella asiatica. Planta Med.. 2007;73:597-599.
- [CrossRef] [Google Scholar]
- CRISPR-Cas9 genome-wide knockout screen identifies mechanism of selective activity of dehydrofalcarinol in mesenchymal stem-like triple-negative breast cancer cells. J. Nat. Prod.. 2020;83:3080-3092.
- [CrossRef] [Google Scholar]
- Total synthesis of polyyne natural products. Comptes Rendus Chim.. 2009;12:489-505.
- [CrossRef] [Google Scholar]
- Isolation, characterization and antimicrobial activities of polyacetylene glycosides from Coreopsis tinctoria Nutt. Phytochemistry. 2017;136:65-69.
- [CrossRef] [Google Scholar]
- Polyacetylenes in Araliaceae: their chemistry, biosynthesis and biological significance. Phytochemistry. 1986;25:285-293.
- [CrossRef] [Google Scholar]
- Allergic contact dermatitis from falcarinol isolated from Schefflera arboricola. Contact Dermatitis. 1986;14:91-93.
- [CrossRef] [Google Scholar]
- Up-regulation of M1 muscarinic receptors expressed in CHOm1 cells by panaxynol via cAMP pathway. Neurosci. Lett.. 2005;383:121-126.
- [CrossRef] [Google Scholar]
- New polyacetylene glucosides from the florets of Carthamus tinctorius and their weak anti-inflammatory activities. Carbohydr Res.. 2011;346:1903-1908.
- [CrossRef] [Google Scholar]
- Lobetyolin induces apoptosis of colon cancer cells by inhibiting glutamine metabolism. J. Cell Mol. Med.. 2020;24:3359-3369.
- [CrossRef] [Google Scholar]
- Quality evaluation of medicinally-used Codonopsis species and Codonopsis Radix based on the contents of pyrrolidine alkaloids, phenylpropanoid and polyacetylenes. J. Nat. Med.. 2014;68:326-339.
- [CrossRef] [Google Scholar]
- HPLC/UV analysis of polyacetylenes, phenylpropanoid and pyrrolidine alkaloids in medicinally used Codonopsis species. Phytochem. Anal.. 2014;25:213-219.
- [CrossRef] [Google Scholar]
- Carlina oxide–a natural polyacetylene from Carlina acaulis (Asteraceae) with potent antitrypanosomal and antimicrobial properties. Planta Med.. 2011;77:1905-1911.
- [CrossRef] [Google Scholar]
- Antitrypanosomal properties of Panax ginseng C. A. Meyer: new possibilities for a remarkable traditional drug. Phytother. Res.. 2013;27:86-98.
- [CrossRef] [Google Scholar]
- Evaluating the antibacterial properties of polyacetylene and glucosinolate compounds with further identification of their presence within various carrot (Daucus carota) and broccoli (Brassica oleracea) cultivars using high-performance liquid chromatography with a diode array detector and ultra performance liquid chromatography-tandem mass spectrometry analyses. J. Agric. Food Chem.. 2017;65:7186-7191.
- [CrossRef] [Google Scholar]
- Anti-acne properties of hydrophobic fraction of red ginseng (Panax ginseng CA Meyer) and its active components. Phytother. Res.. 2019;33:584-590.
- [CrossRef] [Google Scholar]
- Chemical constituents from Bidens bipinnata Linn. Biochem. Syst. Ecol.. 2018;79:44-49.
- [CrossRef] [Google Scholar]
- Qualitative and quantitative determination of polyacetylenes in different Bupleurum species by high performance liquid chromatography with diode array detector and mass spectrometry. J. Chromatogr. A. 2011;1218:1131-1138.
- [CrossRef] [Google Scholar]
- Polyacetylenes from Bupleurum longiradiatum. J. Nat. Prod.. 2009;72:2153-2157.
- [CrossRef] [Google Scholar]
- Polyacetylene glycosides from Pratia nummularia cultures. Phytochemistry. 2003;62:643-646.
- [CrossRef] [Google Scholar]
- Cytotoxic polyacetylenes from the twigs of Ochanostachys amentacea. J. Nat. Prod.. 2001;64:246-248.
- [CrossRef] [Google Scholar]
- Bioassay-guided chromatographic isolation and identification of antibacterial compounds from Artemisia annua L. that inhibit Clostridium perfringens growth. J. AOAC Int.. 2014;97:1282-1290.
- [CrossRef] [Google Scholar]
- New polyacetylenes from Echinophora cinerea (Boiss.) Hedge et Lamond. Nat. Prod. Res.. 2017;31:2256-2263.
- [CrossRef] [Google Scholar]
- Anti-inflammatory compounds from Atractylodes macrocephala. Molecules. 2019;24
- [CrossRef] [Google Scholar]
- Lignans and coumarins from the roots of Anthriscus sylvestris and their increase of caspase-3 activity in HL-60 cells. Biol. Pharm. Bull.. 2007;30:1340-1343.
- [CrossRef] [Google Scholar]
- A new polyacetylene glycoside from the rhizomes of Atractylodes lancea. Chin. Chem. Lett.. 2010;21:850-852.
- [CrossRef] [Google Scholar]
- C14-Polyacetylene glucosides from Codonopsis pilosula. J. Asian Nat. Prod. Res.. 2015;17:601-614.
- [CrossRef] [Google Scholar]
- Lipase inhibition and antiobesity effect of Atractylodes lancea. Planta Med.. 2014;80:577-582.
- [CrossRef] [Google Scholar]
- A new polyacetylene and other constituents with anti-inflammatory activity from Artemisia halodendron. Nat. Prod. Res.. 2021;35:1010-1013.
- [CrossRef] [Google Scholar]
- The Canadian medicinal plant Heracleum maximum contains antimycobacterial diynes and furanocoumarins. J. Ethnopharmacol.. 2013;147:232-237.
- [CrossRef] [Google Scholar]
- Gymnasterkoreaynes A-F, cytotoxic polyacetylenes from Gymnaster koraiensis. J. Nat. Prod.. 2002;65:897-901.
- [CrossRef] [Google Scholar]
- Polyacetylene from Dendropanax morbifera alleviates diet-induced obesity and hepatic steatosis by activating AMPK signaling pathway. Front. Pharmacol.. 2018;9:537.
- [CrossRef] [Google Scholar]
- 2-substituted furans from the roots of Polyalthia evecta. J. Nat. Prod.. 2006;69:68-72.
- [CrossRef] [Google Scholar]
- Quantitative Raman spectroscopy for the analysis of carrot bioactives. J. Agric. Food Chem.. 2013;61:2701-2708.
- [CrossRef] [Google Scholar]
- Application of ccentrifugal partition chromatography for bioactivity-guided purification of antioxidant-response-element-inducing constituents from Atractylodis Rhizoma Alba. Molecules. 2018;23
- [CrossRef] [Google Scholar]
- Constituents of Coreopsis lanceolata flower and their dipeptidyl peptidase IV inhibitory effects. Molecules. 2020;25
- [CrossRef] [Google Scholar]
- Polyacetylenes in fresh and stored carrots (Daucus carota): relations to root morphology and sugar content. J. Sci. Food Agric.. 2012;92:1748-1754.
- [CrossRef] [Google Scholar]
- Cytotoxic, antitumour and antimetastatic activity of two new polyacetylenes isolated from Vernonia scorpioides (Lam.) Pers. Basic Clin. Pharmacol. Toxicol.. 2013;113:307-315.
- [CrossRef] [Google Scholar]
- Dietary polyacetylenes, falcarinol and falcarindiol, isolated from carrots prevents the formation of neoplastic lesions in the colon of azoxymethane-induced rats. Food Funct.. 2017;8:964-974.
- [CrossRef] [Google Scholar]
- Influence of unit operations on the levels of polyacetylenes in minimally processed carrots and parsnips: An industrial trial. Food Chem.. 2012;132:1406-1412.
- [CrossRef] [Google Scholar]
- Polyacetylene compounds of plants of the Asteraceae family. Pharm. Chem. J.. 2014;48:613-631.
- [CrossRef] [Google Scholar]
- Quantification of polyacetylenes in apiaceous plants by high-performance liquid chromatography coupled with diode array detection. Z. Naturforsch. C. J. Biosci.. 2011;66:319-327.
- [CrossRef] [Google Scholar]
- Investigation of biologically active natural products using online LC-bioassay, LC-NMR, and LC-MS techniques. J. Toxicol. Toxin Rev.. 2003;22:495-508.
- [CrossRef] [Google Scholar]
- Epoxy acetylenic lipids: their analogues and derivatives. Prog. Lipid Res.. 2014;56:67-91.
- [CrossRef] [Google Scholar]
- Cytotoxic components from the leaves of Schefflerataiwaniana. J. Chin. Chem. Soc.. 2002;49:427-431.
- [CrossRef] [Google Scholar]
- Kamiohnoyneosides A and B, two new polyacetylene glycosides from flowers of edible Chrysanthemum “Kamiohno”. J. Nat. Med.. 2021;75:167-172.
- [CrossRef] [Google Scholar]
- Quantitative seasonal variation of the falcarinol-type polyacetylene (3S)-16,17-didehydrofalcarinol and its spatial tissue distribution in Tridax procumbens. Phytochem. Anal.. 2020;31:183-190.
- [CrossRef] [Google Scholar]
- Anti-inflammatory constituents from Bidens frondosa. Molecules. 2015;20:18496-18510.
- [CrossRef] [Google Scholar]
- Panaxynol, a natural Hsp90 inhibitor, effectively targets both lung cancer stem and non-stem cells. Cancer Lett.. 2018;412:297-307.
- [CrossRef] [Google Scholar]
- Panaxfuraynes A and B, two new tetrahydrofuranic polyacetylene glycosides from Panax ginseng C. A. Meyer. Tetrahedron Lett.. 2009;50:416-418.
- [CrossRef] [Google Scholar]
- New Polyacetylenes, DGAT inhibitors from the roots of Panax ginseng. Planta Med.. 2004;70:197-200.
- [CrossRef] [Google Scholar]
- Polyacetylenes from the roots of Cirsium japonicum var. ussuriense. Phytochemistry. 2022;202:113319
- [CrossRef] [Google Scholar]
- A convenience UPLC/PDA method for the quantitative analysis of panaxfuraynes A and B from Panax ginseng. Food Chem.. 2010;123:955-958.
- [CrossRef] [Google Scholar]
- Pyranocoumarins from root extracts of Peucedanum praeruptorum dunn with multidrug resistance reversal and anti-inflammatory activities. Molecules. 2015;20:20967-20978.
- [CrossRef] [Google Scholar]
- Extraction of antioxidant components from Bidens pilosa flowers and their uptake by human intestinal Caco-2 cells. Molecules. 2013;18:1582-1601.
- [CrossRef] [Google Scholar]
- Isolobetyol, a new polyacetylene derivative from Platycodon grandiflorum root. Nat. Prod. Res.. 2022;36:466-469.
- [CrossRef] [Google Scholar]
- Bioactive constituents of the stem bark of Mitrephora glabra. J. Nat. Prod.. 2009;72:1949-1953.
- [CrossRef] [Google Scholar]
- Flavonoids and a new polyacetylene from Bidens parviflora Willd. Molecules. 2008;13:1931-1941.
- [CrossRef] [Google Scholar]
- Polyacetylene glucosides from the florets of Carthamus tinctorius and their anti-inflammatory activity. Phytochemistry. 2021;187:112770
- [CrossRef] [Google Scholar]
- Novel substituted thiophenes and sulf-polyacetylene ester from Echinops ritro L. Molecules. 2019;24:805.
- [CrossRef] [Google Scholar]
- Anti-inflammatory dendranacetylene A, a new polyacetylene glucoside from the flower of Chrysanthemum morifolium Ramat. Nat. Prod. Res.. 2021;35:5692-5698.
- [CrossRef] [Google Scholar]
- Toxic polyacetylenes in the genus Bupleurum (Apiaceae)–distribution, toxicity, molecular mechanism and analysis. J. Ethnopharmacol.. 2016;193:566-573.
- [CrossRef] [Google Scholar]
- Novel polyacetylenes from Coreopsis tinctoria Nutt. J. Asian Nat. Prod. Res.. 2015;17:744-749.
- [CrossRef] [Google Scholar]
- A qualitative, and quantitative determination and pharmacokinetic study of four polyacetylenes from Radix Bupleuri by UPLC-PDA-MS. J. Pharm. Biomed. Anal.. 2015;111:257-265.
- [CrossRef] [Google Scholar]
- Polyyne hybrid compounds from Notopterygium incisum with peroxisome proliferator-activated receptor gamma agonistic effects. J. Nat. Prod.. 2014;77:2513-2521.
- [CrossRef] [Google Scholar]
- Bupleurum chinense roots: a bioactivity-guided approach toward saponin-type NF-kappaB inhibitors. Planta Med.. 2017;83:1242-1250.
- [CrossRef] [Google Scholar]
- Quantification of two polyacetylenes in radix ginseng and roots of related Panax species using a gas chromatography–mass spectrometric method. J. Agric. Food Chem.. 2007;55:8830-8835.
- [CrossRef] [Google Scholar]
- Lobetyolin suppressed lung cancer in a mouse model by inhibiting epithelial-mesenchymal transition. Eur. J. Histochem.. 2022;66(3):3423.
- [CrossRef] [Google Scholar]
- Chrysindins A-D, Polyacetylenes from the flowers of Chrysanthemum indicum. Planta Med.. 2011;77:1806-1810.
- [CrossRef] [Google Scholar]
- Polyacetylenic oleanane-type triterpene saponins from the roots of Panax japonicus. J. Nat. Prod.. 2016;79:3079-3085.
- [CrossRef] [Google Scholar]
- Polyacetylenes and flavonoids from the stems and leaves of Pyrethrum tatsienense. Phytochem. Lett.. 2020;40:130-134.
- [CrossRef] [Google Scholar]
- Biological activities and chemical composition of Santolina Africana Jord. et Fourr. aerial part essential oil from Algeria: occurrence of polyacetylene derivatives. Molecules. 2019;24
- [CrossRef] [Google Scholar]
- Variability of the root essential oils of Seseli rigidum Waldst. & Kit. (Apiaceae) from different populations in Serbia. Chem. Biodivers.. 2013;10:1653-1666.
- [CrossRef] [Google Scholar]
- Composition, antimicrobial and antioxidant activity of the extracts of Eryngium palmatum Pančić and Vis. (Apiaceae) Open Life Sci.. 2014;9:149-155.
- [CrossRef] [Google Scholar]
- Cytotoxic activity of polyacetylene compounds in Panax ginseng CA Meyer. Chem. Pharm. Bull.. 1990;38:3480-3482.
- [CrossRef] [Google Scholar]
- Three new polyyne (= polyacetylene) glucosides from the edible roots of Codonopsis cordifolioidea. Helvetica Chim. Acta. 2008;91:90-96.
- [CrossRef] [Google Scholar]
- New polyacetylenes glycoside from Eclipta prostrate with DGAT inhibitory activity. J. Asian Nat. Prod. Res.. 2019;21:501-506.
- [CrossRef] [Google Scholar]
- New antibacterial and cytotoxic activities of falcarindiol isolated in Crithmum maritimum L. leaf extract. Food Chem. Toxicol.. 2010;48:553-557.
- [CrossRef] [Google Scholar]
- Polyacetylenes from the roots of Swietenia macrophylla King. Molecules. 2019;24
- [CrossRef] [Google Scholar]
- Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res.. 2008;47:233-306.
- [CrossRef] [Google Scholar]
- Discovered acetylcholinesterase inhibition and antibacterial activity of polyacetylenes in tansy root extract via effect-directed chromatographic fingerprints. J. Chromatogr. A. 2018;1543:73-80.
- [CrossRef] [Google Scholar]
- Novel polyacetylene derivatives and their inhibitory activities on acetylcholinesterase obtained from Panax ginseng roots. J. Nat. Med.. 2017;71:114-122.
- [CrossRef] [Google Scholar]
- Chemical constituents from the Colombian medicinal plant Maytenus laevis. J. Nat. Prod.. 2004;67:1919-1924.
- [CrossRef] [Google Scholar]
- Echinopsacetylenes A and B, new thiophenes from Echinops transiliensis. Org. Lett.. 2011;13:6228-6231.
- [CrossRef] [Google Scholar]
- Polyacetylenes from terrestrial plants and fungi: recent phytochemical and biological advances. Fitoterapia. 2015;106:92-109.
- [CrossRef] [Google Scholar]
- Polyacetylenes and flavonoids isolated from flowers of Carthamus tinctorius. Chem. Nat. Compd.. 2021;57:635-640.
- [CrossRef] [Google Scholar]
- Effective antimalarial activities of alpha-hydroxy diynes isolated from Ongokea gore. Planta Med.. 2018;84:806-812.
- [CrossRef] [Google Scholar]
- Inhibition of cholesteryl ester synthesis by polyacetylenes from Atractylodes rhizome. Bioorg. Med. Chem. Lett.. 2020;30:126997
- [CrossRef] [Google Scholar]
- Naturally occurring and synthetic polyyne glycosides. Can. J. Chem.. 2009;87:1565-1582.
- [CrossRef] [Google Scholar]
- Two new naturally occurring optical polyacetylene compounds from Torricellia angulata var intermedia and the determination of their absolute configurations. Nat. Prod. Res.. 2006;20:1098-1104.
- [CrossRef] [Google Scholar]
- Water hemlock poisoning in cattle: Ingestion of immature Cicuta maculata seed as the probable cause. Toxicon. 2011;57:157-161.
- [CrossRef] [Google Scholar]
- Polyacetylenes from the roots of Polyalthia debilis. J. Nat. Prod.. 2010;73:1366-1369.
- [CrossRef] [Google Scholar]
- Gas chromatography/mass spectrometry-based metabolic profiling and differentiation of ginseng roots according to cultivation age using variable selection. J. AOAC Int.. 2013;96:1266-1272.
- [CrossRef] [Google Scholar]
- Polyacetylene glycosides from Gymnaster koraiensis. Chem. Pharm. Bull. (Tokyo). 2002;50:685-687.
- [CrossRef] [Google Scholar]
- Isolation and anticomplement activity of compounds from Dendropanax morbifera. J. Ethnopharmacol.. 2004;90:403-408.
- [CrossRef] [Google Scholar]
- Emerging trends in dietary components for preventing and combating disease. ACS Publications 2012
- [CrossRef] [Google Scholar]
- Isolation and structure elucidation of cytotoxic polyacetylenes and polyenes from Echinacea pallida. Phytochemistry. 2006;67:1359-1364.
- [CrossRef] [Google Scholar]
- High-performance liquid chromatography analysis of polyacetylenes and polyenes in Echinacea pallida by using a monolithic reversed-phase silica column. J. Chromatogr. A. 2007;1149:56-65.
- [CrossRef] [Google Scholar]
- Simultaneous metabolite fingerprinting of hydrophilic and lipophilic compounds in Echinacea pallida by high-performance liquid chromatography with diode array and electrospray ionization-mass spectrometry detection. J. Chromatogr. A. 2012;1242:43-58.
- [CrossRef] [Google Scholar]
- Aciphyllal—a C34-polyacetylene from Aciphylla scott-thomsonii (Apiaceae) Tetrahedron Lett.. 2001;42:4325-4328.
- [CrossRef] [Google Scholar]
- Determination of falcarinol in carrot (Daucus carota L.) genotypes using liquid chromatography/mass spectrometry. Food Chem.. 2009;114:1083-1090.
- [CrossRef] [Google Scholar]
- Polyacetylene and phenolic constituents from the roots of Codonopsis javanica. Nat. Prod. Res.. 2022;36:2314-2320.
- [CrossRef] [Google Scholar]
- Polyacetylenes from the leaves of Vernonia scorpioides (Asteraceae) and their antiproliferative and antiherpetic activities. Phytochemistry. 2013;95:375-383.
- [CrossRef] [Google Scholar]
- A new polyacetylene glucoside from Vernonia scorpioides and its potential antihyperglycemic effect. Chem. Biol Interact.. 2018;279:95-101.
- [CrossRef] [Google Scholar]
- The polyacetylene falcarindiol with COX-1 activity isolated from Aegopodium podagraria L. J. Ethnopharmacol.. 2007;113:176-178.
- [CrossRef] [Google Scholar]
- Differential effects of falcarinol and related aliphatic C(17)-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem.. 2009;57:8290-8296.
- [CrossRef] [Google Scholar]
- Rapid method for simultaneous determination of flavonoid, saponins and polyacetylenes in folium ginseng and radix ginseng by pressurized liquid extraction and high-performance liquid chromatography coupled with diode array detection and mass spectrometry. J. Chromatogr. A. 2009;1216:3825-3830.
- [CrossRef] [Google Scholar]
- Phytotoxic polyacetylenes from roots of Russian knapweed (Acroptilon repens (L.) DC.) Phytochemistry. 2008;69:2572-2578.
- [CrossRef] [Google Scholar]
- Essential oils from the roots of Echinops bannaticus Rochel ex Schrad. and Echinops sphaerocephalus L. (Asteraceae): chemotaxonomic and biosynthetic aspects. Chem. Biodivers.. 2013;10:658-676.
- [CrossRef] [Google Scholar]
- Dendrazawaynes A and B, antifungal polyacetylenes from Dendranthema zawadskii (Asteraceae) Planta Med.. 2007;73:1089-1094.
- [CrossRef] [Google Scholar]
- Comparative analysis of active constituents in Centella asiatica samples from Madagascar: Application for ex situ conservation and clonal propagation. Fitoterapia. 2007;78:482-489.
- [CrossRef] [Google Scholar]
- Absolute stereochemistry and anti-HIV activity of minquartynoic acid, a polyacetylene from Ochanostachys amentacea. Nat. Prod. Lett.. 2001;15:21-26.
- [CrossRef] [Google Scholar]
- Influence of Sous Vide and water immersion processing on polyacetylene content and instrumental color of parsnip (Pastinaca sativa) disks. J. Agric. Food Chem.. 2010;58:7740-7747.
- [CrossRef] [Google Scholar]
- Anti-inflammatory active polyacetylenes from Bidens campylotheca. Planta Med.. 1994;60:58-62.
- [CrossRef] [Google Scholar]
- Phenylpropanoids and polyacetylenes from Ligusticum mutellina (Apiaceae) of tyrolean origin. Sci. Pharm.. 2002;70:101-109.
- [CrossRef] [Google Scholar]
- Further phenols and polyacetylenes from the rhizomes of Atractylodes lancea and their anti-inflammatory activity. Planta Med.. 2001;67:437-442.
- [CrossRef] [Google Scholar]
- Polyacetylenes from Oplopanax horridus and Panax ginseng: Relationship between structure and PPARγ activation. J. Nat. Prod.. 2020;83:918-926.
- [CrossRef] [Google Scholar]
- Lignans, phenylpropanoids and polyacetylenes from Chaerophyllum aureum L. (Apiaceae) Z. Naturforsch. C. J. Biosci.. 2003;58:553-557.
- [CrossRef] [Google Scholar]
- Nondestructive Raman analysis of polyacetylenes in Apiaceae vegetables. J. Agric. Food Chem.. 2011;59:7647-7653.
- [CrossRef] [Google Scholar]
- Acetylenic glucosides from Microglossa pyrifolia. Planta Med.. 1992;58:266-269.
- [CrossRef] [Google Scholar]
- Three new polyacetylenes from Atractylodes japonica Koidz.ez Kitam. Nat. Prod. Res.. 2022;36:2063-2070.
- [CrossRef] [Google Scholar]
- Phytochemical, pharmacological, and biotechnological study of Ageratina pichinchensis: A native species of Mexico. Plants (Basel). 2021;10
- [CrossRef] [Google Scholar]
- Structural diversity, biosynthesis, and function of plant falcarin-type polyacetylenic lipids. J. Exp. Bot.. 2022;73:2889-2904.
- [CrossRef] [Google Scholar]
- Antimycobacterial polyacetylenes from Levisticum officinale. Phytother. Res.. 2008;22:681-684.
- [CrossRef] [Google Scholar]
- New constituents of Leontopodium alpinum and their in vitro leukotriene biosynthesis inhibitory activity. Planta Med.. 2004;70:978-985.
- [CrossRef] [Google Scholar]
- Antiprotozoal polyacetylenes from the Tanzanian medicinal plant Cussonia zimmermannii. J. Nat. Prod.. 2007;70:1565-1569.
- [CrossRef] [Google Scholar]
- New antibacterial polyacetylenes from sunflower (Helianthus annuus L.) seedlings. Heterocycles. 2011;83
- [CrossRef] [Google Scholar]
- Post-column sodiation to enhance the detection of polyacetylene glycosides in LC-DAD-MS analyses: an example from Bidens gardneri (Asteraceae) Talanta. 2015;135:87-93.
- [CrossRef] [Google Scholar]
- Comparison of polyacetylene content in organically and conventionally grown carrots using a fast ultrasonic liquid extraction method. J. Agric. Food Chem.. 2010;58:7673-7679.
- [CrossRef] [Google Scholar]
- Dendropanax morbifera branch water extract increases the immunostimulatory activity of RAW264.7 macrophages and primary mouse splenocytes. J. Med. Food. 2019;22:1136-1145.
- [CrossRef] [Google Scholar]
- Bioactive constituents of Artemisia monosperma. Phytochemistry. 2005;66:233-239.
- [CrossRef] [Google Scholar]
- Dietary polyacetylene falcarinol upregulated intestinal heme oxygenase-1 and modified plasma cytokine profile in late phase lipopolysaccharide-induced acute inflammation in CB57BL/6 mice. Nutr. Res.. 2020;80:89-105.
- [CrossRef] [Google Scholar]
- Methodological approach to determine carlina oxide - a main volatile constituent of Carlina acaulis L. essential oil. Talanta. 2019;191:504-508.
- [CrossRef] [Google Scholar]
- Hydrophobic constituents and their potential anticancer activities from Devil's Club (Oplopanax horridus Miq.) J. Ethnopharmacol.. 2010;132:280-285.
- [CrossRef] [Google Scholar]
- Four new polyacetylenes from the roots of Saposhnikovia divaricata. Nat. Prod. Res.. 2022;36:3579-3586.
- [CrossRef] [Google Scholar]
- Two new polyacetylene glycosides from the roots of Codonopsis tangshen Oliv. Nat. Prod. Res.. 2016;30:2338-2343.
- [CrossRef] [Google Scholar]
- Inhibitory effects of polyacetylene compounds from Panax ginseng on Neurotrophin. Biol. Pharm. Bull.. 2017;40:1784-1788.
- [CrossRef] [Google Scholar]
- Inhibitory effects of polyacetylene compounds from Panax ginseng on neurotrophin receptor-mediated hair growth. Biol. Pharm. Bull.. 2017;40:1784-1788.
- [CrossRef] [Google Scholar]
- A new method based on supercritical fluid extraction for polyacetylenes and polyenes from Echinacea pallida (Nutt.) Nutt. roots. J. Pharm. Biomed. Anal.. 2017;146:1-6.
- [CrossRef] [Google Scholar]
- Antiproliferation activity of Devil's club (Oplopanax horridus) and anticancer agents on human pancreatic cancer multicellular spheroids. Phytomedicine. 2014;21:506-514.
- [CrossRef] [Google Scholar]
- Saponins of plants of Panax species collected in central Nepal, and their chemotaxonomical significance. III. Chem. Pharm. Bull.. 2000;48:889-892.
- [CrossRef] [Google Scholar]
- Pharmacokinetics of panaxynol in mice. J. Cancer Sci. Clin. Ther.. 2020;4:133-143.
- [CrossRef] [Google Scholar]
- Understanding and exploiting nature's chemical arsenal: the past, present and future of calicheamicin research. Curr. Pharm. Des.. 2000;6:1841-1879.
- [CrossRef] [Google Scholar]
- Isolation and identification of a potent antimalarial and antibacterial polyacetylene from Bidens pilosa. Planta Med.. 2009;75:624-628.
- [CrossRef] [Google Scholar]
- Significance of phototoxic phytochemicals in insect herbivory. J. Chem. Ecol.. 1986;12:813-821.
- [CrossRef] [Google Scholar]
- Phototoxic polyacetylenes and their thiophene derivatives [effects on human skin] Contact Dermatitis. 1979;5:140-144.
- [CrossRef] [Google Scholar]
- Heptadeca-8-en-4,6-diyne-3,10-diol – A new cytotoxic polyacetylene from Vietnamese Panax stipuleanatus. Chem. Nat. Compd.. 2018;54:156-157.
- [CrossRef] [Google Scholar]
- Evaluation of chemical constituents from Glehnia littoralis for antiproliferative activity against HT-29 human colon cancer cells. Process Biochem.. 2010;45:114-119.
- [CrossRef] [Google Scholar]
- Santolindiacetylene, a polyacetylene derivative isolated from the essential oil of Santolina canescens. J. Nat. Prod.. 1995;58:1749-1752.
- [CrossRef] [Google Scholar]
- Falcarindiol inhibits LPS-induced inflammation via attenuating MAPK and JAK-STAT signaling pathways in murine macrophage RAW 264.7 cells. Mol. Cell Biochem.. 2018;445:169-178.
- [CrossRef] [Google Scholar]
- Isolation and structure elucidation of two new compounds from artemisia Ordosica Krasch. Nat. Prod. Res.. 2020;34:1862-1867.
- [CrossRef] [Google Scholar]
- Quantitative comparison of ginsenosides and polyacetylenes in wild and cultivated American ginseng. Chem. Biodiversity. 2010;7:975-983.
- [CrossRef] [Google Scholar]
- Four guaianolides from Sinodielsia yunnanensis. Chem. Pharm. Bull. (Tokyo). 2003;51:68-70.
- [CrossRef] [Google Scholar]
- Human intestinal microbiota derived metabolism signature from a North American native botanical Oplopanax horridus with UPLC/Q-TOF-MS analysis. Biomed. Chromatogr.. 2020;34:e4911.
- [Google Scholar]
- New naturally occurring diacetylenic spiroacetal enol ethers from Artemisia selengensis. Tetrahedron Lett.. 2016;57:32-34.
- [CrossRef] [Google Scholar]
- Polyacetylenes and flavonoids from the aerial parts of Bidens pilosa. Planta Med.. 2010;76:893-896.
- [CrossRef] [Google Scholar]
- Isolation and identification of constituents with activity of inhibiting nitric oxide production in RAW 264.7 macrophages from Gentiana triflora. Planta Med.. 2013;79:680-686.
- [CrossRef] [Google Scholar]
- Antiallergic agents from natural sources. 3. structures and inhibitory effects on nitric oxide production and histamine release of five novel polyacetylene glucosides from Bidens parviflora WILLD. Chem. Pharm. Bull. (Tokyo). 2001;49:938-942.
- [CrossRef] [Google Scholar]
- Isolation of new polyacetylenes from the roots of Eurycoma longifolia via high-speed counter-current chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci.. 2017;1055–1056:39-44.
- [CrossRef] [Google Scholar]
- Determination of polyacetylenes and ginsenosides in Panax species using high performance liquid chromatography. Chem. Pharm. Bull.. 2003;51:1314-1317.
- [CrossRef] [Google Scholar]
- Ultraviolet-mediated cytotoxic activity of phenylheptatriyne from Bidens pilosa L. J. Nat. Prod.. 1979;42:103-111.
- [CrossRef] [Google Scholar]
- Inhibiting MDSC differentiation from bone marrow with phytochemical polyacetylenes drastically impairs tumor metastasis. Sci. Rep.. 2016;6:36663.
- [CrossRef] [Google Scholar]
- Extraction of antioxidant components from Bidens pilosa flowers and their uptake by human intestinal Caco-2 cells. Molecules. 2013;18:1582-1601.
- [CrossRef] [Google Scholar]
- Toxicity of carlina oxide-a natural polyacetylene from the Carlina acaulis roots-in vitro and in vivo study. Toxins (Basel). 2020;12
- [CrossRef] [Google Scholar]
- Unique polyacetylenic ester-neolignan derivatives from Mitrephora tomentosa and their antimalarial activities. Phytochemistry. 2021;183:112615
- [CrossRef] [Google Scholar]
- Polyacetylenes function as anti-angiogenic agents. Pharm. Res.. 2004;21:2112-2119.
- [CrossRef] [Google Scholar]
- Thiophenes, polyacetylenes and terpenes from the aerial parts of Eclipata prostrate. Bioorg. Med. Chem.. 2014;22:6515-6522.
- [CrossRef] [Google Scholar]
- A novel polyacetylene from the aerial parts of Artemisia lactiflora. Phytochem. Lett.. 2014;8:52-54.
- [CrossRef] [Google Scholar]
- Polyacetylenes in herbal medicine: A comprehensive review of its occurrence, pharmacology, toxicology, and pharmacokinetics (2014–2021) Phytochemistry 2022:113288.
- [CrossRef] [Google Scholar]
- The in vitro/in vivo metabolic pathways analysis of lobetyol, lobetyolin, and lobetyolinin, three polyacetylenes from Codonopsis Radix, by UHPLC-Q/TOF-MS and UHPLC-MS/MS. J. Pharm. Biomed. Anal.. 2023;223:115140
- [CrossRef] [Google Scholar]
- Bioactive sesquiterpenoid and polyacetylene glycosides from Atractylodes lancea. J. Nat. Prod.. 2016;79:1567-1575.
- [CrossRef] [Google Scholar]
- Sesquiterpenoid and C 14 -polyacetylene glycosides from the rhizomes of Atractylodes lancea. Chin. Chem. Lett.. 2017;28:597-601.
- [CrossRef] [Google Scholar]
- Four new C(10)-polyacetylene glycosides from the rhizomes of Atractylodes lancea. J. Asian Nat. Prod. Res.. 2017;19:121-127.
- [CrossRef] [Google Scholar]
- Toonasindiynes A-F, new polyacetylenes from Toona sinensis with cytotoxic and anti-inflammatory activities. Fitoterapia. 2020;146:104667
- [CrossRef] [Google Scholar]
- Direct assignment of the threo and erythro configurations in polyacetylene glycosides by 1H NMR spectroscopy. Org. Lett.. 2017;19:686-689.
- [CrossRef] [Google Scholar]
- Growth inhibitory polyacetylenes from galls of Hedera rhombea bean. Nat. Prod. Commun.. 2006;1:87-94.
- [CrossRef] [Google Scholar]
- Hederyne A, a new antimicrobial polyacetylene from galls of Hedera rhombea Bean. J. Asian Nat. Prod. Res.. 2007;9:537-540.
- [CrossRef] [Google Scholar]
- Growth inhibitory indole acetic acid polyacetylenic ester from Japanese ivy (Hedera rhombea Bean) Phytochemistry. 2007;68:1706-1711.
- [CrossRef] [Google Scholar]
- Polyacetylenes from the roots of cultivated-wild ginseng and their cytotoxicity in vitro. Arch. Pharm. Res.. 2008;31:154-159.
- [CrossRef] [Google Scholar]
- Oploxynes A and B, polyacetylenes from the stems of Oplopanax elatus. J. Nat. Prod.. 2010;73:801-805.
- [CrossRef] [Google Scholar]
- Chemical constituents of Lobelia chinensis. Fitoterapia. 2014;93:168-174.
- [CrossRef] [Google Scholar]
- Bioactivity-guided isolation of polyacetylenes with inhibitory activity against NO production in LPS-activated RAW264.7 macrophages from the rhizomes of Atractylodes macrocephala. J. Ethnopharmacol.. 2014;151:791-799.
- [CrossRef] [Google Scholar]
- Bridelia ferruginea Benth.; An ethnomedicinal, phytochemical, pharmacological and toxicological review. Heliyon. 2022;8:e10366.
- [CrossRef] [Google Scholar]
- A polyacetylene and flavonoids from Cirsium rhinoceros. Arch. Pharm. Res.. 2003;26:128-131.
- [CrossRef] [Google Scholar]
- Chemical constituents of the roots and rhizomes of Saposhnikovia divaricata and their cytotoxic activity. Nat. Prod. Commun.. 2017;12:255-258.
- [CrossRef] [Google Scholar]
- Effects of lobetyolin on xanthine oxidase activity in vitro and in vivo: weak and mixed inhibition. Nat. Prod. Res.. 2021;35:1667-1670.
- [CrossRef] [Google Scholar]
- Inhibitory effects of coumarin and acetylene constituents from the roots of Angelica furcijuga on D-galactosamine/lipopolysaccharide-induced liver injury in mice and on nitric oxide production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg. Med. Chem.. 2006;14:456-463.
- [CrossRef] [Google Scholar]
- Simultaneous analysis of three bioactive compounds in Artemisia annua hairy root cultures by reversed-phase high-performance liquid chromatography-diode array detector. Phytochem. Anal.. 2010;21:524-530.
- [CrossRef] [Google Scholar]
- Chemical constituents from Glehnia littoralis and their chemotaxonomic significance. Nat. Prod. Res.. 2020;34:2822-2827.
- [CrossRef] [Google Scholar]
- Global and targeted metabolomics reveal that Bupleurotoxin, a toxic type of polyacetylene, induces cerebral lesion by inhibiting GABA receptor in mice. J. Proteome Res.. 2014;13:925-933.
- [CrossRef] [Google Scholar]
- Coreosides A-D, C14-polyacetylene glycosides from the capitula of Coreopsis tinctoria and its anti-inflammatory activity against COX-2. Fitoterapia. 2013;87:93-97.
- [CrossRef] [Google Scholar]
- Polyacetylenes and anti-hepatitis B virus active constituents from Artemisia capillaris. Fitoterapia. 2014;95:187-193.
- [CrossRef] [Google Scholar]
- Cytotoxic polyacetylenes isolated from the roots and rhizomes of Notopterygium incisum. Chin. Chem. Lett.. 2019;30:428-430.
- [CrossRef] [Google Scholar]
- Polyacetylenes of marine origin: chemistry and bioactivity. Chem. Rev.. 2015;115:1543-1596.
- [CrossRef] [Google Scholar]
- Two new polyacetylene glucosides and a new caffeoyl derivative with angiogenic activity from Bidens parviflora Willd. Phytochem. Lett.. 2021;42:82-86.
- [CrossRef] [Google Scholar]
- Isolation and identification of a novel chlorophenol from a cell suspension culture of Helichrysum aureonitens. Chem. Pharm. Bull.. 2009;57:1282-1283.
- [CrossRef] [Google Scholar]
- Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem.. 2005;53:2518-2523.
- [CrossRef] [Google Scholar]
- Quantum chemical studies on structure activity relationship of natural product polyacetylenes. Theor. Chem. Acc.. 2007;117:247-252.
- [CrossRef] [Google Scholar]
- Bernart., M. W., J. H. Cardellina., M. S. Balaschak., et al., 1996. Cytotoxic falcarinol oxylipins from Dendropanax arboreus. J. Nat. Prod. 59, 748-753. https://doi.org/10.1021/np960224o
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105137.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







