Translate this page into:
Polyphenylsulfone/multiwalled carbon nanotubes mixed ultrafiltration membranes: Fabrication, characterization and removal of heavy metals Pb2+, Hg2+, and Cd2+ from aqueous solutions
⁎Corresponding authors at: Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia (Inamuddin). isloor@yahoo.com (Arun M. Isloor), inamuddin@rediffmail.com (Inamuddin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Polyphenylsulfone/multiwalled carbon nanotubes/polyvinylpyrrolidone/1-methyl-2-pyrrolidone mixed matrix ultrafiltration flat-sheet membranes were fabricated via phase inversion process to inspect the heavy metals separation efficacy from aqueous media. Fabricated membranes cross-sectional morphological changes and the topographical alterations were assessed with Scanning electron microscopy (SEM) and atomic force microscopy (AFM). Particularly, MWCNTs assisted membranes exhibited better permeability ability as well as heavy metal removal enactment than virgin membrane. The dead-end filter unit was engaged in current research to examine the permeability and heavy metal removal competence of membranes. With the continuous enhancement of MWCNTs wt% in a polymer matrix, significant enhancement was observed with pure water flux study, from 41.69 L/m2 h to >185 L/m2 h as well as with the heavy metals separation study. Added additive MWCNTs can impact the pore sizes in membranes. The heavy metal separation results achieved, the membrane with 0.3 wt% of MWCNTs (PCNT-3) exhibited >98%, >76% and >72% for Pb2+, Hg2+ and Cd2+ ions, respectively. Overall, MWCNTs introduced PPSU membranes exposed best outcomes with heavy metals contained wastewater treatment.
Keywords
Polyphenylsulfone
MWCNTs
Ultrafiltration membranes
Dead-end filtration
Heavy metal rejection
1 Introduction
As a result of the increased industrial activities, huge quantities of heavy metals are contaminating our nearby environment. These pollutants are mainly released by leather, textile, paint, wood processing, pigment & dyes, petroleum refining industries by various processes, i.e. electroplating, surface treatment process, and other chemical treatments. These heavy metals cause a harmful effect on the environment and may cause physical discomfort, illness and irreversible damage to a vital body system of human beings (Zhou et al., 2013). These industrial wastes create the most important cause of various kinds of metal pollution in the water ecosystem (Aziz et al., 2015). In earlier, researchers were invented few methods for decreasing the heavy metal concentration in wastewater, i.e. precipitation, ion exchange method, reverse osmosis, oxidation, reduction, electrodialysis, and adsorption methods reported by various authors. Removal of Ni2+, Zn2+, Cr+4, and Cu2+ from electroplating industrial wastewater by using Kyanite as an adsorbent has been demonstrated by Ajmal and group members (Ajmal et al., 2001). Cadmium desorption in the sand by flow-through and batch methods (Tran et al., 2002), removal of Pb, Cd, Ni, Cu, Mn, Mg, and Cr by sequential adsorbent treatment (Maria and de Oliveira, 2003) was reported by the researchers. The effect of mercury on ecological health is related to the level of local and worldwide discharges, depositions are potential for crises (Munthe et al., 2007; Driscoll et al., 2007).
In recent years, various types of membrane separation methods such as Reverse osmosis (RO), Nanofiltration (NF), Microfiltration (MF), Ultrafiltration (UF) and distillation technologies have gained significant commercial importance. Many of the physical processes were involved in membrane preparation are by sintering, track etching, and phase inversion processes. Thermally-induced phase separation (TIPS) and immersion precipitation methods are discussed in detail by Witte and co-workers (van de Witte et al., 1996). The Majority of the polymeric membranes are fabricated via the Phase inversion process due to its convenience. Membrane filtration together with carbon nanotubes (CNT) can make better water desalination and the decontamination approaches were more effectual and cost-effective (Elimelech and Phillip, 2011). Polymeric membranes were mechanized for diverse industrial applications (Lonsdale, 1982; Pusch and Walch, 1982; Mulder, 1992). CNT based membrane applications are relevant to many areas, such as heavy metal rejection (Anitha et al., 2015), dye removal (Ashish et al., 2010) salt rejection (Michael and Ben, 2015), etc. Functionalized CNTs embedded polymeric membranes revealed several enhanced properties i.e. hydrophilicity, fouling resistance, mechanical and thermal stability possessions (Qu et al., 2013).
Nowadays, CNT based nanomaterials are frequently using in wastewater treatments due to its huge availability and lesser manufacture cost compare with other nanomaterials (Thines et al., 2017). In particular, there are enormous amounts of studies have been executed by the investigators concerning the rejection of heavy metals from the polluted water. On the other hand, functionalized MWCNTs developed to assess the permeability enhancement characteristics of membranes (Arockiasamy et al., 2013). Shen et al. suggested the new sewage action method by engaging polymer surfactant complexation and flocculation approaches to distinct the certain heavy metals such as Cr3+, Zn2+ and Cd2+ from aqueous media (Shen et al., 2015). Magnetic adsorbents synthesized from agricultural wastes and used for choosy heavy metals separation such as nickel, cadmium, lead, and arsenic from wastewater (Noor et al., 2017). A favorable hydrothermally altered Circulating fluidized bed (CFB) fly ash (HM-CFB-FA) cost-effective adsorbent was used for the deletion of heavy metal Cd2+ from contaminated water (Qiu et al., 2018). So far, insufficient journal reports were stated towards heavy metals separation possessions by CNTs enclosed mixed matrix membranes (Ismail et al., 2008; Ismail et al., 2009). Polyethersulfone based ultrafiltration membranes engaged to investigate the separation efficiency of polyvinyl amine complexed selective heavy metals such as Co2+, Cu2+, Ni2+, Pb2+, Fe3+, Cd2+, Zn2+, and Mn2+ from aqueous media via dead-end filtration procedure (Huang et al., 2016). Afshar et al. described a novel technique for the removal of Pb (II) from aqueous media, via magnetic Fe3O4 coated polypyrrole-polyaniline nanocomposites (Ahmad et al., 2018). Polysulfone and nano nickel-iron oxide mixed hollow fiber membranes fabricated via dry-wet process, to examine the adsorption efficacy of selected heavy metals such as Pb2+, Cu2+, Zn2+, Cd2+, Ni2+ and Cr3+ ions from aqueous media (Mondal et al., 2017). In polymeric membranes, the integrated CNTs are enhanced the hydrophilic nature and forms a repulsive border barricade, and that enhances anti-fouling stuff for palm oil mill waste management (Moideen et al., 2016). Bushra et al. described the preparation of composite material Polyaniline-Zr (IV) phosphor borate (PZPB) through a sol-gel method, used for the removal of Hg2+ ions from aqueous media and studied the antibacterial activity of PZPB in contradiction of E. coli (Ho et al., 2017). Polyethylenimine altered graphene oxide calcium alginate (GOCA) composites were synthesized, to assess the separation capacity of Pb+2, Cd+2, and Hg+2 heavy metals from wastewater (Arshad et al., 2018). MWCNTs were chemically modified with thorium oxide nanocomposites to examine the adsorption conductance of Pb2+ ions from the aqueous systems (Naushad et al., 2015). Novel Fe3O4@TAS nanocomposites were effectively invented and used as an influential adsorbent for the rejection of Co2+, Cd2+ and Cr3+ metal ions from aqueous media (Mittal et al., 2016). Ruthiraan et al. described the comparative study between functionalized multiwall carbon nanotube (FMWCNTs) and magnetic biochar to determine the adsorption capability of Cd2+ ions from wastewater (Alqadami et al., 2017). MWCNTs were amended with 8-hydroxyquinoline and used for the separation of Cu2+, Pb2+, Cd2+ and Zn2+ ions from aqueous solutions (Samia et al., 2012). MWCNTs prepared by decomposition of acetylene gas in the presence of ferrocene catalysts, to assess the Ni2+ ions deletion study (Kandah and Meunier, 2007). The piperazine based polyamide thin film composite nanofiltration membranes fabricated via the incorporation of oxidized MWCNTs, to advance the Na2SO4 salt rejection efficacy from wastewater (Reza Mahdavi et al., 2017). A hydroxyapatite-carbon adsorbent with the categorised porous microstructure of sugarcane stalks (SS-HAP/C) was utilized to detect the Pb2+ ions from wastewater (Zhu et al., 2018).
In current research choose PPSU as a key polymer to fabricate the membranes, owing to its specific characteristics such as good dimensional stability, good mechanical property, chemical resistance, etc. PPSU is a high-performance polymer containings aromatic rings with sulfone (SO2) group. Sulfone containing polymers are extensively useful for the preparation of UF, MF and NF membranes with extremely manageable pore sizes and because of wide distribution, which allows their extensive worth in various membrane separation developments (Anonymous, 2009).
The above survey, reports conclude very fewer reports can found towards the deletion of heavy metals from aqueous media through the usage of MWCNTs. According to our knowledge, heavy metals such as Pb2+, Hg2+ and Cd2+ ions removal using PPSU/MWCNTs polymeric membranes has not been reported as yet. In the current research, the PPSU/MWCNTs mixed UF membranes fabricated via the phase inversion process. The characterization, surface morphological changes, pure water flux performance, fouling ability and polyethyleneimine (PEI) complexed heavy metals removal ability of membranes were systematically studied.
2 Experimental
2.1 Materials
PPSU (Radel R-5000) (MW ∼ 50,000 g/mol) provided through Solvay Adv. Polymers, Belgium. N-methyl-2-pyrrolidone (NMP) was obtained since Merck India, Limited. Bovine Serum Albumin (BSA) (MW ∼ 69 KDa) procured from CDH Chemicals, India. MWCNTs (>98%), Polyvinylpyrrolidone (PVP), lead (II) nitrate (≥99.0%), cadmium nitrate tetrahydrate (98%), and mercury (II) chloride (>99.50%) were acquired from Sigma-Aldrich. Polyethyleneimine (PEI) 50 wt% aq. Solution acquired since New Jersey, USA.
2.2 PPSU-MWCNT membrane compositions
PPSU/MWCNT mixed membranes fabricated via phase inversion process (Shenvi et al., 2014). MWCNTs in the polymeric matrix were diverse as 0 g, 0.018 g, 0.036 g, and 0.054 g, and categorized as PCNT-0, PCNT-1, PCNT-2, and PCNT-3, respectively. Additive MWCNTs dissolved in an appropriate volume of NMP solvent and sonicated 30 min for the proper dispersion of MWCNTs in NMP. Further, added 18 g PPSU and 2 g PVP, preserved for stirring at 60 °C and continued up to 18 hrs to acquire the clear dope solution. Afterward, membranes prepared via the casting method by the K-202 control coater instrument. Later, the glass plate immersed in a water bath for phase inversion mechanism (Kumar et al., 2013; Valeen Rashmi et al., 2016) (see Table 1).
Membranes
PPSU (g)
NMP (g)
PVP (g)
CNT (wt%)
CNT (g)
PCNT-0
18
80
2
0
0
PCNT-1
18
79.982
2
0.1
0.018
PCNT-2
18
79.964
2
0.2
0.036
PCNT-3
18
79.946
2
0.3
0.054
2.3 Characterization methods
2.3.1 Contact angle (CA)
Fabricated membranes surface hydrophilic property was assessed by FTA-200 Dynamic contact angle analyzer by sessile droplet technique (Liu and Rui, 2013; Nayak et al., 2018). The membranes were cut into small pieces and affixed on a glass slide. Water droplet was introduced using a microsyringe and then measured CA values. To diminish the tentative errors, every membrane evaluated four times and then stated the standard values.
2.3.2 Porosity and water uptake
The gravimetric method was employed to calculate the porosity characteristic of membranes (Guoliang et al., 2013).
In the water uptake test, each membrane sample cut into 1 cmx1 cm size, then immersed in water up to 24 h. After this, the membranes separated and smeared with tissue paper, then recorded the wet weight and kept for dry in the oven at 60 °C up to 5–6 h. Once more recorded the dry weight, and Eq. (2) employed to assess the water uptake results.
2.3.3 Membrane morphology
2.3.3.1 Scanning electron microscope (SEM)
MWCNTs surface morphology and laboratory fabricated membranes cross-sectional morphological changes were examined using SEM instrument (Model No. JEOL JSM-6380LA). The membranes were immersed and cracked in liquid nitrogen, and then carefully fixed on the sample holder. After that, performed gold sputtering and then cross-sectional results of membrane samples analyzed using SEM (Shenvi et al., 2015).
2.3.3.2 Atomic force microscope (AFM)
Innova SPM atomic force microscope was employed to assess the surface roughness limits of membranes. In AFM analysis dry membrane samples were used. Operational condition: tapping mode and maintained with scan area 3 μm2. The surface roughness of membranes stated in terms of average roughness (Ra), root mean square roughness (Rq) and roughness range (Rz) was measured.
2.3.4 Pure water flux (PWF)
Lab-scale dead-end filter unit was employed to revise the PWF of membranes. 5 cm2 membrane area was engaged to study the permeation studies. After fixing the membrane in the holder, the membranes preserved for compaction up to 40 min at 0.4 MPa at room temperature. Further, transmembrane pressure (TMP) was condensed to 0.3 MPa TMP, and performed time-bound PWF analysis. Preserved two min time gap and continued up to 60 min.
2.3.5 Antifouling property
BSA flux studies attained to examine the antifouling ability of membranes. 800 ppm concentrated BSA solution hired. The membranes preserved for compaction up to 30 min at 0.3 MPa TMP. Supplementary, TMP was reduced to 0.3 MPa and then calculated the BSA flux at every two min time gap for each of the membrane permeation. Thereafter, the membranes were cleaned carefully with water under continuous tap flow, and again PWF ‘Jw2’ (L/m2 h) study performed at similar conditions (Rajesh et al., 2012). The antifouling performance of membranes was measured using the following equation,
The fouling conductance of membranes was advance analyzed with total fouling ratio (Rt), reversible fouling (Rr) and irreversible fouling ratio (Rir) calculations with Eqs. (5)–(7) (Shao et al., 2014).
‘Jw1’ PWF, ‘Jp’ BSA protein flux, and ‘Jw2′ PWF after BSA protein flux.
2.3.6 Rejection study of toxic heavy metal ions
The lab-scale dead-end filter unit (Fig. 1) was engaged to assess the heavy metal ions rejection ability through membranes. The procedure was followed as per the literature (Hebbar et al., 2016). A proper amount of aqueous solutions of lead nitrate, mercuric chloride, and cadmium nitrate were equipped in the manifestation of 1 wt% of PEI. In the current research, the aqueous solutions of both lead nitrate, cadmium nitrate were prepared in 500 ppm, and mercuric chloride prepared in 50 ppm, individually. The pH of the prepared solutions was balanced to 6 ± 0.2 with 0.1 M HCl/0.1 M NaOH solution. These aqueous solutions were stirred up to 5 continuous days at 25 °C to take the proper interactions among the metal ions and complexing agent PEI. Then, the aqueous solutions transferred into the feed tank and fixed the 5 cm2 active area membrane samples in the membrane module cell, thereafter the heavy metals contained aqueous solutions filtered through the membranes. After the permeation study, the permeated samples were assessed using the atomic absorption spectrometer (AAS). Throughout the study maintained 0.3 MPa TMP, and 40 min time duration was maintained to every membrane sample (PCNT-0 to PCNT-3). Eq. (8) employed to assess the heavy metals rejection results.
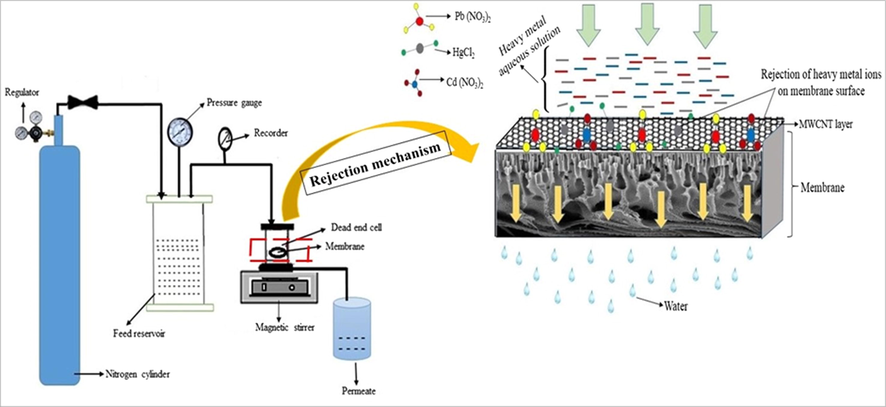
Graphical illustration of heavy metals rejection by lab-scale dead-end filter unit.
After the rejection experiment, the dried membrane sample surface was analyzed by SEM-EDS to detect the elemental mapping analysis of heavy metals on the membrane surface.
3 Results and discussions
3.1 Contact angle (CA)
The surface hydrophilic aptitude of membranes was measured by a contact angle study. In general, if the contact angle value is low, the surface hydrophilicity will be more. The plain membrane (PCNT-0) revealed 65.73°CA due to its lower hydrophilic characteristics of PPSU polymer. MWCNTs can impact the pore sizes of membranes, the morphological changes were able to observe from Fig. 5. Due to the effect of added MWCNTs, the water-absorbing nature of membranes better than before. So, the CA results diminished by increasing the MWCNTs wt% on PPSU polymer and the obtained CA values were specified in Table 2. The contact angle results arising from PCNT-0 to PCNT-3 due to the fact through the phase inversion method the MWCNTs move impulsively to membrane/liquid partition to decrease the interfacial energy and greater to extend the hydrophilic nature on hydrophobic PPSU polymeric membranes (Fig. 2).
Membranes
Water uptake (%)
Porosity (%)
Contact angle (°)
PCNT-0
66.03 ± 1.02
41.17 ± 0.92
65.73 ± 1.25
PCNT-1
68.18 ± 0.65
37.03 ± 1.26
64.76 ± 0.94
PCNT-2
70.16 ± 0.32
46.77 ± 0.54
62.03 ± 1.63
PCNT-3
70.32 ± 0.72
50.93 ± 0.69
61.79 ± 1.98
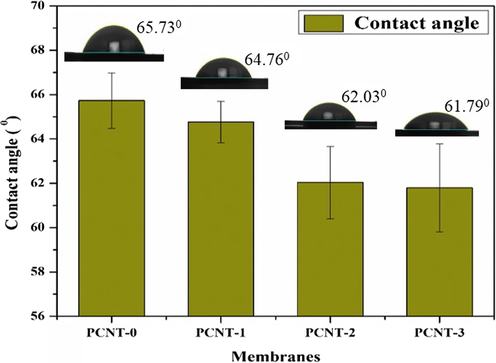
Contact angle results of membranes.
3.2 Water uptake and porosity effects
Water uptake is a specific characteristic to examine the wettability property of membranes. Continuous enhancement was observed by the continuous enhancement of MWCNTs wt% in the polymer matrix (Fig. 3). The water uptake ability of the membranes were determined by SEM results, i.e. the attendance of macro-voids, pores in sub-layer (Ganesh et al., 2013). Initially, the water uptake capacity of the PCNT-0 membrane was about 66.03% and it was improved up to 70.32% by raising the MWCNTs wt% in PPSU polymer and the results are stated in Table 2.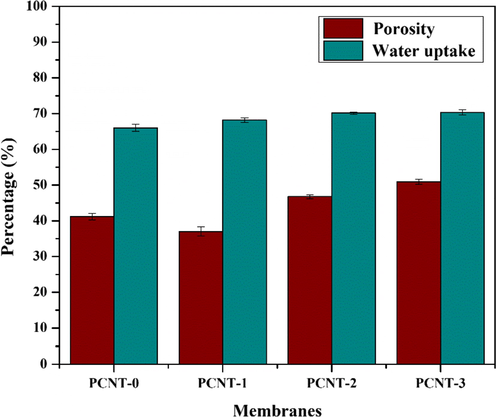
Porosity and water uptake results of membranes.
In porosity results, PCNT-1 membrane porosity was decreased, while for PCNT-2 and PCNT-3 membrane porosity increased (Fig. 3). This is because, the increase in additive MWCNTs wt% in the PPSU polymer matrix, the viscosity of the polymer solution tends to increase. Due to which diffusion of solvent is more favored than in diffusion of non-solvent, leading to decreased porosity. The porosity was increased progressively due to the observable developed finger-like cavities and the macro voids in membranes, which could be observed from Fig. 5 SEM cross-sectional images. Also, the percentage of PVP drain out during the solvent-nonsolvent exchange process, so the membrane porosity is increasing (Song et al., 2012).
3.3 Membrane morphology
3.3.1 SEM outcomes
The morphology of the MWCNTs and structural changes in prepared membranes was assessed based on the obtained SEM images. Fig. 4 shows the images of MWCNTs outlook (A) and magnified SEM images (B). MWCNTs show a high specific surface area with better antifouling ability and exhibited good rejection performance with heavy metals. Fig. 4 (A&B) reveals the MWCNTs SEM investigational results and noticed that the MWCNTs diameter range was between 96.0 nm and 97.7 nm. The cross-sectional SEM results of fabricated membranes shown heterogeneous layers consist of dense skin layer on top and a porous supportive sub-layer. With increasing the MWCNTs wt% in the polymer matrix, the sub-layer effectively gets improved. The PCNT-0 membrane-enclosed with finger-like projections and few pear-like small pores as shown in Fig. 5a. By the addition of additive MWCNTs on membranes, the asymmetric structure consisting of a dense skin layer on top and a thick porous layer with finger-like channels and macro voids in bottommost were developed excellently (Fig. 5b-d). With increasing the MWCNTs wt% on the polymer, the finger-like channels, macro voids are expanding significantly. The additive contained membranes (Fig. 5b-d) exposed a few of the horizontal channels, which can progress the water permeability of membranes (Zinadini et al., 2014; Wang et al., 2012). Moreover, additional PVP can affect the pore assembly of membranes (Morihama and Mierzwa, 2014; Nayak et al., 2017).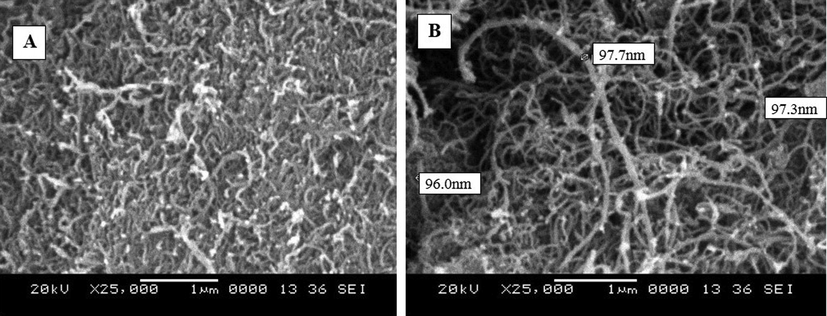
(A) SEM outlook appearance of MWCNTs, (B) magnified SEM image of MWCNTs.
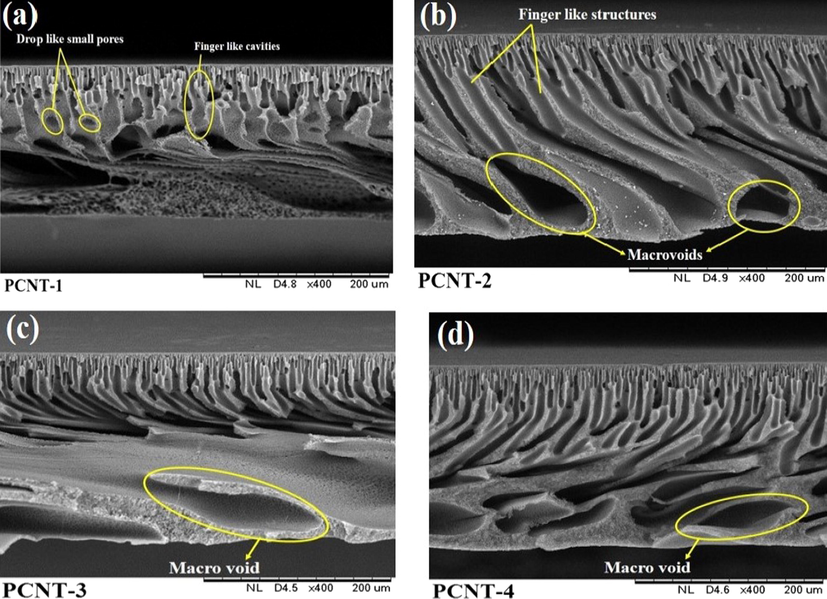
SEM Cross sectional micrographs of PPSU/MWCNTs membranes.
3.3.2 AFM results
AFM technique was engaged to define the surface topography of fabricated membranes, and the acquired Ra, Rq, and Rz values were specified in Table 3. Fig. 6 reveals the outline of three dimensional (3D) AFM descriptions of plane and MWCNTs contained membranes. The obtained surface roughness values of composite membranes were greater than the virgin (PCNT-0) membrane. In results, PCNT-3 membrane expressed higher Ra, Rq, and Rz values (21.7 nm, 27.7 nm, and 334 nm). The progressive improvement of surface roughness parameters of membranes due to the continuous enhancement of MWCNTs wt% on PPSU polymer. The surface roughness of polymeric membranes remarkably and conceptually explains the fouling performance of membranes, which directed to increase the PWF ultimately (Yu et al., 2015).
Membrane
Roughness
Surface area
Ra (nm)
Rq (nm)
RZ (nm)
(μm2)
PCNT-0
10.3
13.2
84.3
9.03
PCNT-1
12.7
17.4
146
9.12
PCNT-2
14.8
20.0
204
9.25
PCNT-3
21.7
27.7
334
27.2
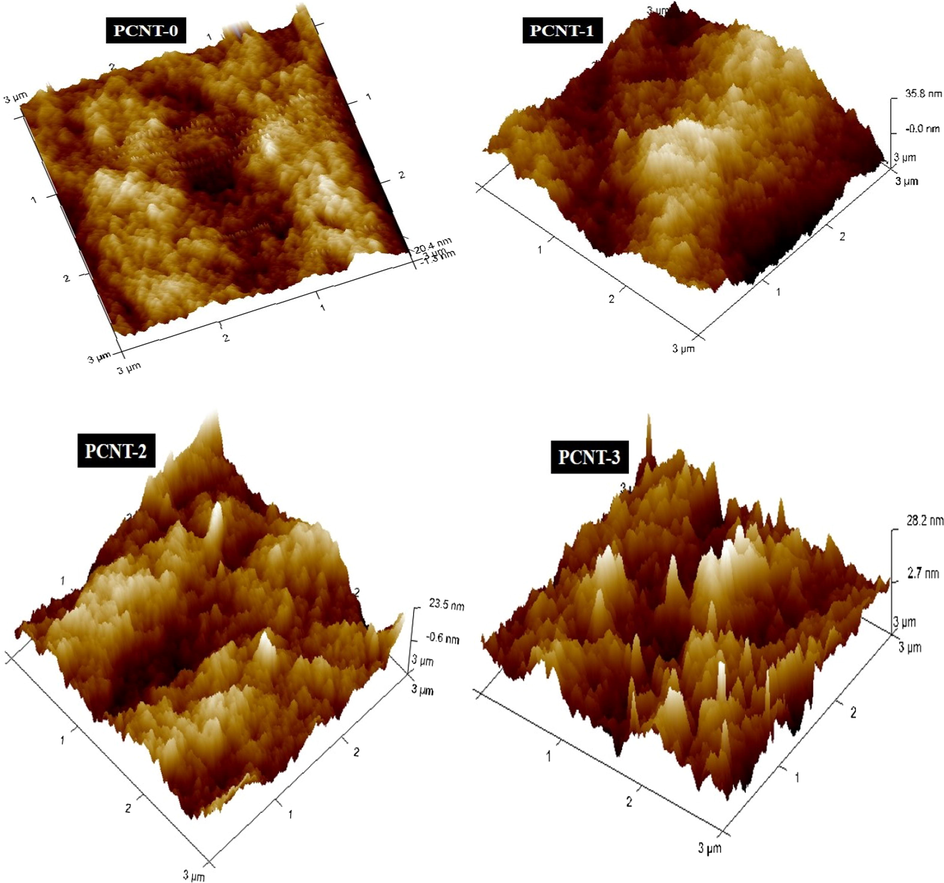
3D AFM analysis images of membranes.
3.4 Pure water flux
Fig. 7 reveals the PWF outcomes of a virgin (PCNT-0) and MWCNTs contained (PCNT-1 to PCNT-3) membranes. As can be understood, the PWF performance of PCNT-1 to PCNT-3 membranes was greater than the virgin membrane (PCNT-0). With increasing the MWCNTs wt% in the polymer matrix, the noticeable enhancement was observed with PWF. Incorporated MWCNTs can impact the pore sizes of polymeric membranes, due to that the permeability ability of membranes greater than before, the enhanced PWF fallouts can be observed from Fig. 7. Initially, the PWF was 41.69 L/m2 h with PCNT-0, and it extended up to >185 L/m2 h with PCNT-3 membrane, it’s almost four times greater than the PCNT-0 membrane. Water-soluble PVP can impact the pore sizes, this is also one of the reasons for flux improvement.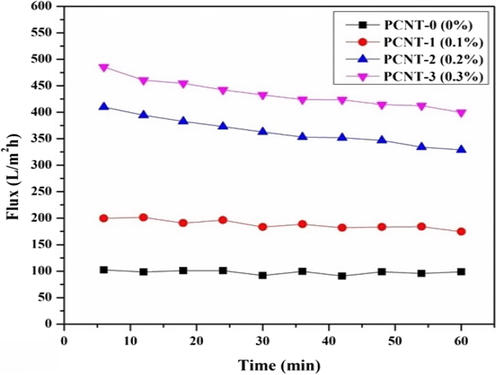
PWF outline of plane and additive membranes.
3.5 Antifouling results
Antifouling ability membranes was assessed with BSA protein flux. After the BSA flux, the membranes were washed away systematically under continuous water flow to decrease the fouling effect and again performed PWF under the same operational conditions (Fig. 8(a)). With the BSA filtration test observed less flux compare with PWF, this is because of the larger size effect of BSA molecules, cannot pass easily through the membrane pores and causes fouling on the membrane surface. Initially, the BSA flux (Jp) was 6.80 L/m2 h (PCNT-0) and it reached >9 L/m2 h (PCNT-3). PCNT-3 membrane shown improved anti-fouling results due to the authentic membrane preparation. The higher antifouling competence of the membranes was caused by the greater hydrophilic nature of membranes. The anti-fouling ability of membranes was inspected with FRR (flux recovery ratio), and the Rt, Rr, Rir results (Fig. 8(b)) of membranes were detailed in Table 4. The FRR values are slowly decreasing from membranes PCNT-0 to PCNT-3 due to increasing the membrane fouling ability. Jw1-PWF; Jp-BSA flux; Jw2-PWF (after cleaning); Rr-reversible fouling; Rir-irreversible fouling; FRR-flux recovery ratio.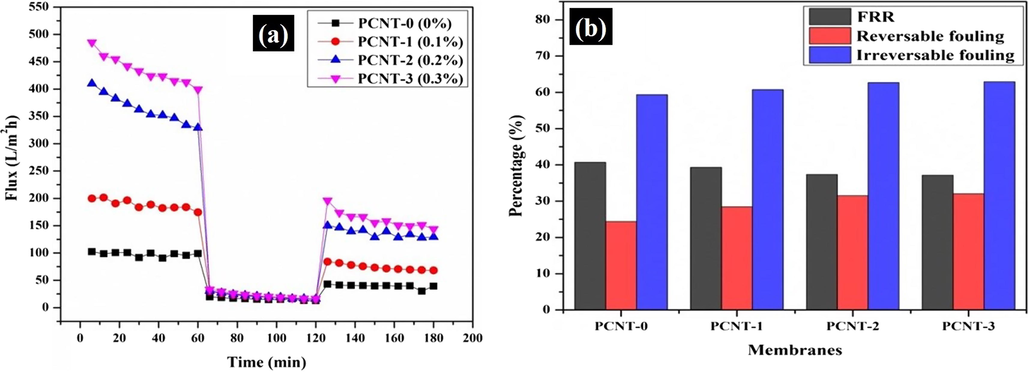
(a) Flux V/s time for membranes at 0.3 MPa under three conditions: PWF; BSA flux; and PWF after clean with water, (b) FRR and anti-fouling performance of membranes.
Permeate flux (L/m2 h)
Fouling performance (%)
Membrane code
Jw1
Jp
Jw2
FRR
Rt
Rr
Rir
PCNT-0
41.69
6.80
16.96
40.68
83.68
24.37
59.31
PCNT-1
80.64
8.76
31.68
39.28
89.13
28.42
60.71
PCNT-2
155.98
9.13
58.24
37.33
94.13
31.48
62.66
PCNT-3
185.84
9.52
69
37.12
94.87
32
62.87
3.6 Heavy metal ions removal efficiency of membranes
Heavy metals are highly toxic and are able to cause serious environmental problems (Zou et al., 2009). In the current research, polymer enhanced UF process employed to revise the rejection behavior of selected heavy metals such as Pb2+, Hg2+, and Cd2+ from aqueous media. Added PEI as a strong, heavy metal complexing agent (Bolto, 1995). The sieving mechanism was engaged in current research (Schaep et al., 1998). After the addition of a complexing agent to the heavy metal solution, obviously, the size of the metal ions will enhance due to the close interactions between the metal ions and a complexing agent. According to the sieving effect, the larger complexed metal ions will reject primarily. Fig. 9(a) reveals the schematic representation of heavy metals complexation with additive PEI. Takagishi et al. proposed a complexation mechanism between various divalent metal cations and polyetherimide (PEI) complexing agent (Toru et al., 1985). In results, the rejection conductance of Pb2+ ions is more as compared with Cd2+ ions, because the Pb2+ ions are formed larger complexes with PEI molecules as compared to Cd2+ ions (Liang et al., 2012). For Hg2+ ions rejection study 50 ppm aqueous solutions were prepared because at lower concentrations only mercury forms favorable sites with PEI. As the mercury concentration increases, then all the available sites get bound and filled with the mercury ions. This results in lesser rejection (Uludag et al., 1997). Table 5 discloses the results of MWCNTs separation efficacy towards selected various hazardous heavy metal ions.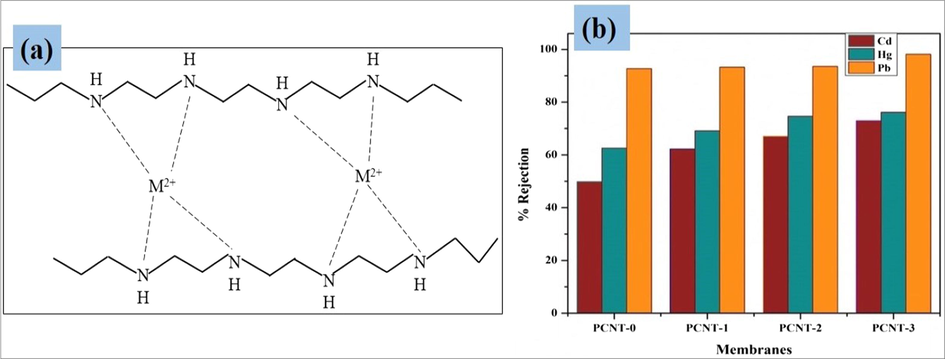
Graphical representation of heavy metals complexation with PEI (a), Heavy metal ions rejection results of membranes (b).
Sl. No.
Polymer
Nanoadditive
Heavy metals
Mechanism
Reference
1
Polyvinylidene fluoride (PVDF)
MWCNTs
Ni2+
Adsorption
Zeng et al. (2016)
2
Polysulfone (PSf)
NH2-MWCNTs
Cr+6, Cd2+
Adsorption
Shah and Murthy (2013)
3
Poly (amido amine) (PAMAM)
CNT
Ni2+, Zn2+, As3+, Co2+
Adsorption
Hayati et al. (2016)
4
Poly(acrylic acid) (PAA)
MWCNTs
Co2+
Sorption
Chen et al. (2012)
5
Poly vinyl alcohol (PVA)/Chitosan (CS)
NH2-MWCNTs
Cu2+
Adsorption
Salehi et al. (2013)
6
Polyaniline (PANI)
MWCNT
Pb2+
Adsorption
Shao et al. (2012)
7
Chitosan
SWCNT/MWCNTs
Hg2+
Adsorption
Shawky et al. (2012)
8
Poly(2-amino thio phenol)
MWCNT
Pb2+, Cd2+
Adsorption
Nabid et al. (2012)
9
Polyphenylsulfone (PPSU)
MWCNTs
Pb2+, Hg2+, Cd2+
Sieving mechanism
Current research
Apart from the heavy metals rejection test, adsorption studies also performed with all fabricated membranes. From the adsorption results, the prepared membranes showed a very limited affinity for the adsorption of metal ions. The fabricated membranes adsorption results can found in given supplemental file-1. It concludes that the heavy metal rejection enhancement observed during the rejection studies could be mainly due to the sieving mechanism. The investigation results displayed that, the removal efficacies of metal ions improved progressively with increasing the MWCNTs wt% on PPSU polymer. The best rejection results were acquired with PCNT-3 membrane, and exhibited >98%, >76%, and >72% for Pb2+, Hg2+ and Cd2+ ions, respectively (Fig. 9(b)).
After the heavy metals rejection experiment, the membrane surface was assessed with SEM-EDS analysis to evaluate the presence of heavy metals. Fig. 10 reveals the information about SEM-EDS analysis results (A, C and E) and elemental mapping results (B, D, and F) of PCNT-3 membranes after the rejection of Pb2+, Hg2+ and Cd2+ ions.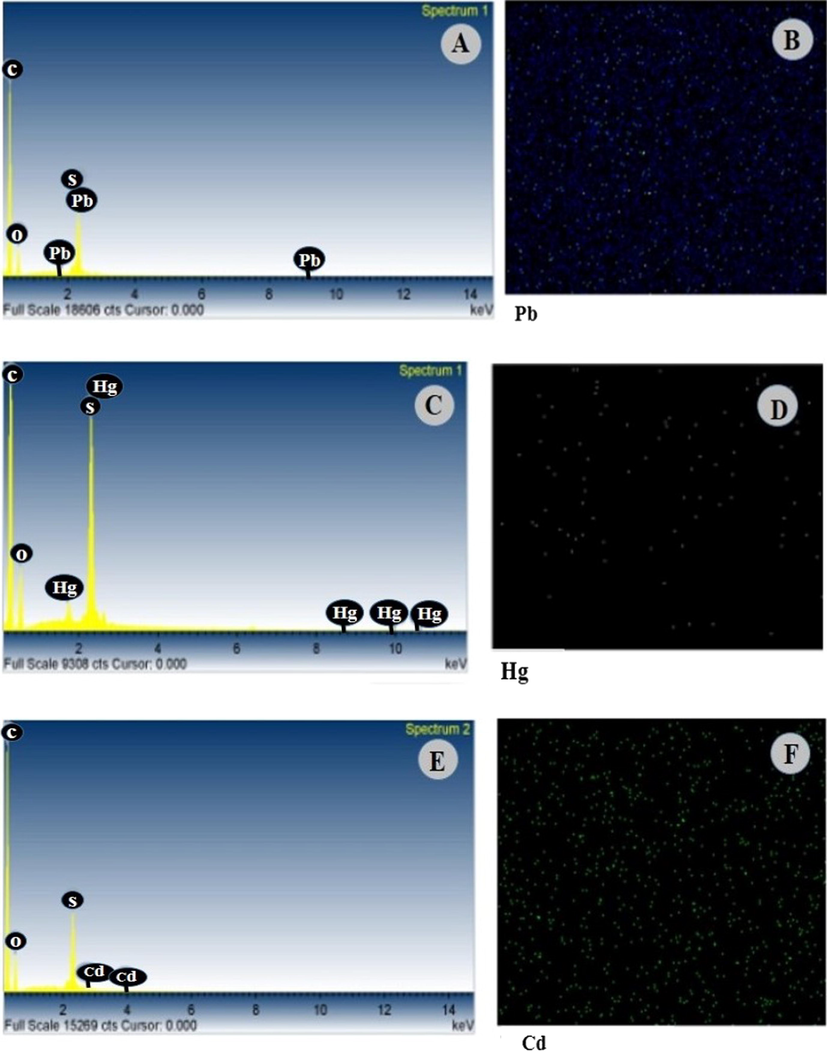
A, C and E are the EDS scan analysis results of PCNT-3 membranes and B, D, and F are the elemental mapping descriptions of Pb2+, Hg2+, and Cd2+ ions.
4 Conclusions
In summary, the PPSU/MWCNT/PVP/NMP mixed matrix membranes were fabricated via the phase inversion process, to investigate the heavy metals removal efficacy of membranes via a dead-end filtration unit. Several characterization techniques were employed to assess the various properties of membranes such as SEM-EDS, AFM, contact angle, water uptake, porosity, PWF, anti-fouling, rejection test, etc. Experimental results showed enhanced permeability and good rejection ability with increasing the MWCNTs wt% on PPSU polymer. In end, the PCNT-3 membranes were exhibited best heavy metal rejection results of >98% for Pb2+, >76% for Hg2+ and >72% for Cd2+ ions, respectively. We hope the greatest future of CNTs in membrane technology for wastewater treatments nearby the future.
Acknowledgement
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. KEP-54-130-38. The authors, therefore, acknowledge with thanks DSR for technical and financial support. Prof. AMI thank the Director, NITK Surathkal for providing research facilities. The authors thank to Prof. K. Narayan Prabhu, Dept. of MME, NITK for providing Contact angle facilities. The authors would like to express their thanks to Head, Dept. of Chemical Engineering, NITK Surathkal, India and DST-PURSE, Mangalore University for providing the technical assistance.
References
- Removal of Cu (II), Cd (II) and Pb (II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod.. 2018;180:437-449.
- [Google Scholar]
- Removal and recovery of heavy metals from electroplating wastewater by using Kyanite as an adsorbent. J. Hazard. Mater.. 2001;87:127-137.
- [Google Scholar]
- Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: a study of adsorption parameters and interaction mechanism. J. Clean Prod.. 2017;156:426-436.
- [Google Scholar]
- Removal of heavy metal ions using a functionalized single-walled carbon nanotube: a molecular dynamics study. J. Phys. Chem. A. 2015;119:8349-8358.
- [Google Scholar]
- Anonymous, 2009. Design guide for Radel polymers, Solvay Advanced Polymers. Alpharetta, GA, USA.
- Carbon nanotubes-blended poly(phenylene sulfone) membranes for ultrafiltration applications. Appl. Water Sci.. 2013;3:93-103.
- [Google Scholar]
- Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep. Purif. Technol. 2018
- [CrossRef] [Google Scholar]
- Study of removal of azo dye by functionalized multi walled carbon nanotubes. Chem. Eng. J.. 2010;162:1026-1034.
- [Google Scholar]
- Removal of Ni, Cd, Pb, Zn and colour from aqua solution using potential low cost adsorbent. Indian J. Eng. Mater. Sci.. 2015;12:248-258.
- [Google Scholar]
- Poly (acrylic acid) grafted multiwall carbon nanotubes by plasma techniques for Co (II) removal from aqueous solution. Chem. Eng. J.. 2012;210:475-481.
- [Google Scholar]
- Mercury contamination in forest and freshwater ecosystems in the North eastern United States. Biosci.. 2007;57:17-28.
- [Google Scholar]
- The future of seawater desalination: energy, technology, and the environment. Sci.. 2011;333:712-717.
- [Google Scholar]
- Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination. 2013;313:199-207.
- [Google Scholar]
- Novel polysulfone hybrid ultrafiltration membrane prepared with TiO2-g HEMA and its antifouling characteristics. J. Membr. Sci.. 2013;436:163-173.
- [Google Scholar]
- Synthesis and characterization of PAMAM/CNT nanocomposite as a super-capacity adsorbent for heavy metal (Ni2+, Zn2+, As3+, Co2+) removal from wastewater. J. Mol. Liq.. 2016;224:1032-1040.
- [Google Scholar]
- Fabrication of polydopamine functionalized halloysite nanotube/polyetherimide membranes for heavy metal removal. J. Mater. Chem. A. 2016;4:764-774.
- [Google Scholar]
- Novel GO/OMWCNTs mixed-matrix membrane with enhanced antifouling property for palm oil mill effluent treatment. Sep. Purif. Technol.. 2017;117:337-349.
- [Google Scholar]
- Removal of heavy metals from water using polyvinylamine by polymer-enhanced ultrafiltration and flocculation. Sep. Purif. Technol.. 2016;158:124-136.
- [Google Scholar]
- Transport and separation properties of carbon nanotube-mixed matrix membrane. Sep. Purif. Technol.. 2009;70:12-26.
- [Google Scholar]
- Removal of Ni2+ from water by multi-walled carbon nanotubes. J. Hazard. Mater.. 2007;146:283-288.
- [Google Scholar]
- Polysulfone-Chitosan blend ultrafiltration membranes: preparation, characterization, permeation and antifouling properties. RSC Adv.. 2013;3:7855-7861.
- [Google Scholar]
- Separation and recovery of lead from a low concentration solution of lead(II) and zinc(II) using the hydrolysis production of poly styrene-co-maleic anhydride. J. Hazard. Mater.. 2012;203:183-187.
- [Google Scholar]
- Effect of annealing conditions on crystallization behavior and mechanical properties of NIPS poly(vinylidene fluoride) hollow fiber membranes. J. Appl. Polym. Sci.. 2013;129:1417-1425.
- [Google Scholar]
- Heavy metals removal in industrial effluents by sequential adsorbent treatment. Adv. Environ. Res.. 2003;7:263-272.
- [Google Scholar]
- A computational assessment of the permeability and salt rejection of carbon nanotube membranes and their application to water desalination. Phil. Trans. R. Soc. A.. 2015;374:1-20.
- [Google Scholar]
- Fabrication of MWCNTs/ThO2 nanocomposite and its adsorption behavior for the removal of Pb2+ metal from aqueous medium. Desalin. Water Treat.. 2016;57(46):21863-21869.
- [Google Scholar]
- Fabrication and characterization of new PSF/PPSU UF blend membrane for heavy metal rejection. Desalin. Water Treat.. 2016;57(42):19810-19819.
- [Google Scholar]
- A novel ultrafiltration grade nickel iron oxide doped hollow fiber mixed matrix membrane: spinning, characterization and application in heavy metal removal. Sep. Purif. Technol.. 2017;188:155-166.
- [Google Scholar]
- Clay Nanoparticles effects on performance and morphology of poly(vinylidene fluoride) membranes. Braz. J. Chem. Eng.. 2014;31:79-93.
- [Google Scholar]
- Basic Principles of Membrane Technology. Dordrecht. The Netherlands: Kluwer Academic; 1992.
- Preparation and application of poly (2-amino thiophenol)/MWCNTs nanocomposite for adsorption and separation of cadmium and lead ions via solid phase extraction. J. Hazard. Mater.. 2012;203:93-100.
- [Google Scholar]
- Synthesis, characterization and application of curcumin formaldehyde resin for the removal of Cd2+ from wastewater: Kinetics, isotherms and thermodynamic studies. J. Ind. Eng. Chem.. 2015;29:78-86.
- [Google Scholar]
- Preparation and characterization of PPSU membranes with BiOCl nanowafers loaded on activated charcoal for oil in water separation. J. Taiwan Inst. Chem. Eng.. 2017;77:293-301.
- [Google Scholar]
- Fabrication of novel PPSU/ZSM-5 ultrafiltration hollow fiber membranes for separation of proteins and hazardous reactive dyes. J. Taiwan Inst. Chem. Eng.. 2018;82:342-350.
- [Google Scholar]
- Agricultural biomass-derived magnetic adsorbents: Preparation and application for heavy metals removal. J. Taiwan Inst. Chem. Eng.. 2017;78:168-177.
- [Google Scholar]
- Synthetic membranes-preparation, structure and application. Angew. Chem. Int. Ed. Engl.. 1982;21:660-685.
- [Google Scholar]
- Removal of Cd2+ from aqueous solution using hydrothermally modified circulating fluidized bed fly ash resulting from coal gangue power plant. J. Clean Prod.. 2018;172:1918-1927.
- [Google Scholar]
- Applications of nanotechnology in water and wastewater treatment. Water Res.. 2013;47:3931-3946.
- [Google Scholar]
- Structure-property interplay of poly (amide-imide) and TiO2 nanoparticles impregnated poly (ether-sulfone) asymmetric nanofiltration membranes. RSC Adv.. 2012;2(17):6854-6870.
- [Google Scholar]
- Fabrication and water desalination performance of piperazine–polyamide nanocomposite nanofiltration membranes embedded with raw and oxidized MWCNTs. J. Taiwan Inst. Chem. Eng.. 2017;75:189-198.
- [Google Scholar]
- Static and dynamic adsorption of copper ions on chitosan/polyvinyl alcohol thin adsorptive membranes: Combined effect of polyethylene glycol and aminated multi-walled carbon nanotubes. Chem. Eng. J.. 2013;215:791-801.
- [Google Scholar]
- Removal of heavy metals from aqueous solutions by multiwalled carbon nanotubes modified with 8-hydroxyquinoline. Chem. Eng. J.. 2012;181:159-168.
- [Google Scholar]
- Influence of ion size and charge in nanofiltration. Sep. Purif. Technol.. 1998;14:155-162.
- [Google Scholar]
- Studies on the porosity control of MWCNT/polysulfone composite membrane and its effect on metal removal. J. Memb. Sci.. 2013;437:90-98.
- [Google Scholar]
- Application of polyaniline and multiwalled carbon nanotube magnetic composites for removal of Pb (II) Chem. Eng. J.. 2012;185:144-150.
- [Google Scholar]
- A facile strategy to enhance PVDF ultrafiltration membrane performance via self-polymerized polydopamine followed by hydrolysis of ammonium fluotitanate. J. Membr. Sci.. 2014;461:10-21.
- [Google Scholar]
- Chitosan/carbon nanotube composite beads: Preparation, characterization, and cost evaluation for mercury removal from wastewater of some industrial cities in Egypt. J. Appl. Polym. Sci.. 2012;125:93-101.
- [Google Scholar]
- Removal of heavy metal ions from dilute aqueous solutions by polymer–surfactant aggregates: A novel effluent treatment process. Sep. Purif. Technol.. 2015;152:101-107.
- [Google Scholar]
- Enhanced permeation performance of cellulose acetate ultrafiltration membranes by incorporation of sulfonated Poly(1,4-phenylene ether ether sulfone) and poly(styrene-co-maleic anhydride) Ind. Eng. Chem. Res.. 2014;53:13820-13827.
- [Google Scholar]
- Humic acid based biopolymeric membrane for effective removal of methylene blue and rhodamine B. Ind. Eng. Chem. Res.. 2015;54:4965-4975.
- [Google Scholar]
- Performance Improvement of Polysulfone ultrafiltration membrane using well-dispersed Polyaniline–poly(vinylpyrrolidone) nanocomposite as the additive. Ind. Eng. Chem. Res.. 2012;51:4661-4672.
- [Google Scholar]
- Application potential of carbon nanomaterials in water and wastewater treatment: a review. J. Taiwan Inst. Chem. Eng.. 2017;72:116-133.
- [Google Scholar]
- Binding of metal ions by polyethyleneimine and its derivatives. J. Polym. Sci.. 1985;23:2109-2116.
- [Google Scholar]
- Removal of mercury from aqueous solutions via polymer-enhanced ultrafiltration. J. Membr. Sci.. 1997;129:93-99.
- [Google Scholar]
- Preparation of polysulfone-based PANI–TiO2 nanocomposite hollow fiber membranes for industrial dye rejection applications. RSC Adv.. 2016;6:99764-99773.
- [Google Scholar]
- Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci.. 1996;117:1-31.
- [Google Scholar]
- Effect of functionalized multi-walled carbon nanotubes on the microstructure and performances of PVDF membranes. RSC Adv.. 2015;5:75998-76006.
- [Google Scholar]
- Preparation of novel high copper ions removal membranes by embedding organosilane-functionalized multi-walled carbon nanotube. J. Chem. Technol. Biotechnol.. 2016;91(8):2322-2330.
- [Google Scholar]
- Bio-removal of cadmium by growing deep-sea bacterium Pseudoalteromonas sp. SCSE709-6. Extremophiles. 2013;17(5):723-731.
- [Google Scholar]
- Preparation of a porous hydroxyapatite-carbon composite with the bio-template of sugarcane top stems and its use for the Pb (II) removal. J. Clean. Prod.. 2018;187:650-661.
- [Google Scholar]
- Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci.. 2014;453:292-301.
- [Google Scholar]
- Effective heavy metal removal through porous stainless-steel-net supported low siliceous zeolite ZSM-5 membrane. Microporous Mesoporous Mater.. 2009;124:70-75.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.10.007.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







