Translate this page into:
Precise regulation of Ultra-thin platinum decorated Gold/Graphite carbon nitride photocatalysts by atomic layer deposition for efficient degradation of Rhodamine B under simulated sunlight
⁎Corresponding author. zhanghf_2007@126.com (Hongfen Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Atomic Layer Deposition (ALD) precise controlling ultra-thin platinum (Pt) modified Graphite carbon nitride (g-C3N4) photocatalysts, which had been doped with gold nanoparticles (Au NPs) by photodeposition, were successfully synthesized. The experimental results showed that precise regulation of platinum decorated C3N4-Au(C3N4-Au/nPt (n is the number of Pt ALD cycles, 1 Å per cycle)) exhibited excellent photocatalytic degradation ability for Rhodamine B (RhB). Under simulated sunlight irradiation, the degradation rate of 10 mg/L RhB(100 mL) by 1.5 mg C3N4-Au/10Pt catalysts was 95.8% within 60 min that is much better than other photocatalysts for the degradation of RhB. The efficient degradation mechanism of RhB by C3N4-Au/10Pt photocatalysts was studied and the experiments demonstrated the ·O2– as main active species played an important role in the photocatalytic process. Local surface plasmon resonance (LSPR) of Au NPs and Schottky barrier between Pt clusters and g-C3N4 may be the reasons for enhanced C3N4-Au/10Pt photocatalytic performances. Furthermore, the successive catalytic cycles revealed the excellent stability of C3N4-Au/10Pt photocatalyst.
Keywords
g-C3N4-based photocatalysts
Atomic layer deposition
Cocatalysts
Efficient degradation of RhB
1 Introduction
The global energy crisis and environmental issues have spurred the search for clean and renewable energy source to replace conventional coal, oil and gas. Photocatalysis, using inexhaustible solar energy, is a powerful tool to solve the worldwide energy shortage, environmental contamination and the greenhouse effect (Meng et al., 2016, Jinlong et al., 2019, Eshwar et al., 2020). Among many photocatalysts that have been developed, graphitic carbon nitride (g-C3N4) has become an attractive visible-light catalyst owing to its numerous qualities such as simple preparation, electrochemical properties, abundance, nontoxicity and chemical tenability (Ya-Nan et al., 2017, Taehwan et al., 2019, Mohammed et al., 2020). For example, g-C3N4 has a bandgap of 2.7 eV which can lead itself to be an excellent visible light responsive photocatalyst (Mamba and Mishra 2016, Li et al., 2021, Wenjun et al., 2021). The g-C3N4 materials lead to multiple excitation after single-photon absorption, producing a large number of active substances (such as ·O2–, ·OH, etc.), degrading organic pollutants, killing bacteria and reducing carbon dioxide (Amene et al., 2017, Dilshad et al., 2017, Guohui et al., 2017, Jingrun et al., 2018).
However, the photocatalytic efficiency of g-C3N4 is still not ideal due to its limited visible-light capture ability (about 460 nm with absorption edge), high charge carrier recombination rate and low surface area (Yanchun et al., 2020). Thus, various modification methods were used to improve photocatalytic performance of g-C3N4-based materials, such as elemental and molecular doping to narrow the bandgap for enhancing the absorption of visible light (Wee-Jun et al., 2016, Yanchun et al., 2020), structuring composite with cocatalysts to improve the catalytic kinetics (Juan et al., 2015, Sibo et al., 2019), the formation of heterojunction with other semiconductors to improve light response (Noor Izzati Md et al., 2021, Xu et al., 2021, Yan et al., 2021) and the introduction of substrates with excellent carrier mobility to make better charge extraction efficiency (Min-Ying et al., 2012, Wee-Jun et al., 2015).
Designing the electronic structure of g-C3N4 by doping, loading and hybridizing with metal atoms (e.g., Au, Ag, Zn, Pt and Cu) is an important strategy to improve the performance of g-C3N4 (Xinchen et al., 2009, Zhengxin et al., 2010, Jingqi et al., 2013, Surendar et al., 2014, Trishamoni et al., 2021, Noor Izzati Md et al., 2022). For instance, Sun et al. (Ningyan et al., 2013) reported that the nanohybrids of Au-loaded g-C3N4 nanosheets showed superior photocatalytic activities for the decomposition of methyl orange (MO) under visible-light irradiation to bulk g-C3N4 and g-C3N4 nanosheets. Very recently, novel ZnO/Au/g-C3N4 nanocomposites were prepared by a liquid-phase pulsed laser method followed by calcination (Seung Jun et al., 2020). The ZnO/Au/g-C3N4 nanocomposites showed enhanced catalytic activity for the degradation of methylene blue (MB) under simulated sunlight. A novel plasmonic photocatalyst, Au/Pt/g-C3N4, was prepared by a facile calcination-photodeposition technique. The Au/Pt decorated g-C3N4 heterostructure displayed enhanced photocatalytic activity for antibiotic tetracycline hydrochloride (TC-HCl) degradation. The degradation rate was 3.4 times higher than that of pure g-C3N4 under visible light irradiation (Jinjuan et al., 2015). However, the above methods in the synthetic process of g-C3N4-based nanocomposites are difficult to accurately control the size, distribution and loading capacity of metal atoms. Therefore, it is essential to develop new strategies to accurately design nano-photocatalysts from a single atom to clusters with specific atomic numbers and unique complex structures, in order to improve photocatalytic activity under sunlight.
Atomic layer deposition (ALD) is a thin film preparation technology that originated in Finland in the 1970 s. ALD has the advantages of self-limiting, atom-by-atom growth and a variety of materials that can be deposited. It is an ideal method for the precise design of metal nano-photocatalysts with adjustable atomic numbers (Jie et al., 2021). ALD technology also can control the thickness of monolayer, submonolayer and angstrom level to engineer the formation and growth of surface layer sediments at the atomic level (Zhe and Yong 2017, Jiankang et al., 2019, Ji-Xiao et al., 2020, Jie et al., 2021).
Here, we developed a new strategy to modulate Pt nucleation and growth by ALD to precisely tailor g-C3N4-based nano-photocatalysts (see Schematic diagram 1) for achieving superior photocatalytic performance by downsizing Pt nanocatalysts at the sub-nanometric size (cluster and a single atom). The precise controlling g-C3N4-based nano-photocatalysts as-obtained (expressed as C3N4-Au/nPt, n for the number of Pt ALD cycles) were characterized by transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared spectroscopy (FTIR), UV–vis diffuse reflectance spectra (UV–vis DRS) and electrochemical methods. All the experimental results confirmed that platinum was precisely designed on the surface of Au/C3N4, where g-C3N4 had been doped with Au by photodeposition. The C3N4-Au/10Pt nano-photocatalysts exhibited an outstanding efficient degradation of Rhodamine B (RhB) under simulated sunlight. Theoretical and experimental studies were carried out to investigate the origin of this highly efficient photocatalytic performance. The precisely regulated C3N4-Au/nPt photocatalysts were first synthesized by ALD and successfully applied in the efficient degradation of RhB. The results of this paper provide a hopeful prospect for the application of precisely controlled g-C3N4-based nanomaterials with metal atoms in the degradation of organic pollutants under sunlight.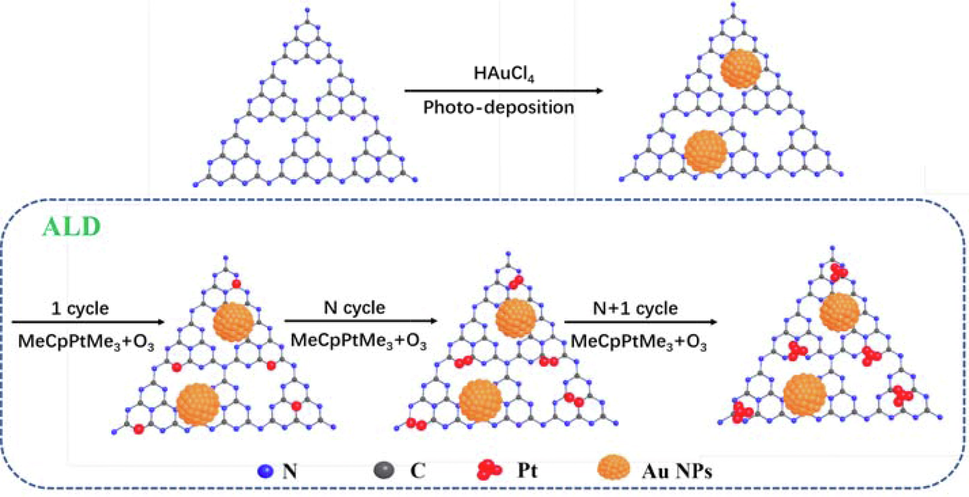
Schematic illustration of synthetic processes for precisely controlled C3N4-Au/nPt nano-photocatalysts by ALD.
2 Experimental details
2.1 Materials
All reagents were analytically pure and can be used without further purification. Deionized water was used in all experiments. Chloroauric acid and Trimethyl (methylcyclopentadienyl) platinum (MeCpPtMe3, 99%) were purchased from Alfa Aesar, USA. Urea was obtained from Sinopharm Chemical Reagent Co., Ltd. Disodium ethylenediaminetetraacetic acid (EDTA-2Na) was purchased from Beijing Suolaibao Technology Co., Ltd. NaOH, NaH2PO4, concentrated sulfuric acid, isopropanol (IPA) and ascorbic acid (AA) were obtained from Tianjin Damao Chemical Reagent Co., Ltd.
2.2 Synthesis of g-C3N4
40 g urea powder was put into a porcelain crucible with a lid and heated from room temperature to 500 °C at a rate of 10 °C min−1 in a Ksl-1100x muffle furnace (Hefei keying Material Technology Co., Ltd), and kept for 4 h, then cooled naturally to room temperature. The bulk g-C3N4 was obtained and ground for 3 min to obtain a fine powder. The g-C3N4 (C3N4 for short) prepared at 600℃ and 700℃ were prepared by the same way. The C3N4 synthesized at 800℃ was stored for 10 min in a muffle furnace, then cooled naturally to room temperature.
2.3 Fabrication of C3N4-Au by photodeposition method
Firstly, 165 μL of chloroauric acid (0.1 g·mL−1), 150 mg of carbon nitride and 15 mL of methanol were added into a photoreactor containing 60 mL of deionized water, respectively. Then, the above-mentioned mixed solution was stirred for 3 h under a CEL-HXF300-T3 xenon lamp (Beijing Zhongjiao Jinyuan Technology Co., Ltd). Lastly, C3N4-Au was prepared after being filtered by suction filtration (Millipore Filter Membrane, Water-system, 0.45 μm, Shanxi mini bio NG-1345 W) and dried at 60℃ for 6 h under the condition of vacuum.
2.4 Fabrication of sub-nanometric photocatalyst (C3N4-Au/nPt) by ALD
Pt was precisely engineered on the as-prepared C3N4-Au, which was dispersed on a quartz surface of 8.0 × 8.0 × 0.2 cm3, using MeCpPtMe3 and O3 generated from a CF-G-3–5 g ozone generator (Qingdao Garden Environmental Protection Technology Co., Ltd) as precursors at 280 °C in the same self-developed atomic layer deposition system (Self-developed by Institute of Shanxi Coal Chemistry, Chinese Academy of Sciences). MeCpPtMe3 was maintained at 68˚C to provide adequate steam pressure. For each ALD cycle, the pulse, exposure, and purge times for MeCpPtMe3 were 1, 15 and 25 s, respectively, and those of O3 were 1, 12 and 20 s, respectively. Nitrogen (99.999%) was used as the carrier and purge gas. Different Pt ALD cycles were performed to obtain a series of catalysts with precisely controlled Pt sizes and loads (expressed as C3N4-Au/nPt, n for the number of Pt ALD cycles). Under the same ALD conditions, different cycles of Pt ALD were carried out on the g-C3N4 substrate (expressed as C3N4/xPt, x is the number of Pt ALD cycles) as the control experiments.
2.5 Characterizations of catalysts
The morphology and crystalline structure of the samples were determined by TEM, high resolution TEM (HRTEM) and EDS profiles on a JEOL-2100F microscope operated at 200 kV. The crystal structure of the samples were confirmed by X-ray diffraction (XRD) with Cu Kα radiation (λ = 1.540 Å, Bruker D8 Advance X-ray diffractometer). The XPS of the samples were measured by ESCALAB250Xi photoelectron spectrometer equipped with Al Kα source (hν = 1486.6 eV). The binding energy is calibrated at 248.8 eV. The functional groups on the surface of the materials were scanned at 400 ∼ 4000 cm−1 by Fourier Transform infrared spectrometer (Nicolet iS5). A Thermo Scientific Evolution 220 spectrophotometer was used to record the diffuse reflection ultraviolet–visible spectra (UV–vis DRS) in the range of 200–800 nm with BaSO4 as the background to characterize the light absorption performance of the samples.
2.6 Photocatalytic experiments
The photocatalytic performances of the as-prepared photocatalysts were evaluated by RhB degradation under simulated sunlight irradiation. Photocatalysts (1.5 mg) were introduced into 100 mL RhB solution (10 mg⋅L-1) and dispersed under ultrasonic dispersion for 30 min. Then, the systems were magnetically stirred in the dark for 20 min to reach the adsorption–desorption equilibrium prior to light irradiation. A 300 W xenon lamp with the current intensity of 14 A was used as the light source. The sampling frequency by a syringe was 5 min and all the samples were filtrated through a 0.45 µm cellulose acetate membrane to remove the catalysts particles. The simulated sunlight irradiation time was 30 min. 2 mL of the clarified solution was analyzed using a U-3900 UV–vis spectrophotometer (Hitachi, Japan) and the maximum absorbance wavelength of RhB was 550 nm. Degradation efficiency and photocatalytic rate kinetics were determined by the following equations (1) and (2), which are:
2.7 Photoelectrochemical measurement
Electrochemical measurements were performed by using the CHI660E electrochemical workstation (Shanghai ChenHua Instrument Co., Ltd.). The electrochemical analyzer has a standard three-electrode system, in which the synthesized samples coated on F-doped SnO2-coated glass (FTO glass) as the working electrodes, a Pt wire was used as the counter electrode and saturated calomel electrode (SCE) used as a reference electrode. The Electrochemical impedance spectroscopy (EIS) frequency range was from 0.005 to 106 Hz with amplitude of 10 mV. Transient photocurrents (TPC) were performed in the same three-electrode system. The preparation of working electrodes was as follows: 0.1 g of the photocatalysts was mixed with 5 mL of ethanol and ultrasonically dispersed for 30 min to form a uniformly dispersed liquid. The 10 μL of the above-mentioned dispersion liquid was coated onto a 2.0 × 2.0 cm2 FTO glass electrode directly. The obtained electrodes were dried in an oven and heated in a nitrogen stream at 300 °C for half an hour. The film diameter of all electrodes used was approximately 6 mm.
3 Results and discussion
3.1 Characterization of synthesized photocatalysts
To confirm the successful loading Au NPs and Pt clusters on the surface of C3N4 via photodeposition and ALD methods, the morphology, composition and chemical states of C3N4-Au/10Pt were investigated. The TEM images of C3N4-Au/10Pt are shown in Fig. 1. The well dispersed Au NPs are closely combined with C3N4 in Fig. 1a, which is beneficial for transferring of photo-induced electrons in Au NPs to C3N4 by Localized Surface Plasmon Resonance (LSPR) (Chuang et al., 2017). In Fig. 1b, the fringe with the interplanar spacing of 0.235 and 0.205 nm is assigned to the (1 1 1) and (1 0 0) crystal plane of Au NPs, respectively (Ji-Xiao et al., 2020). However, Pt clusters could not be observed neither in TEM nor in HRTEM after being decorated on C3N4-Au by ALD, which is due to the high dispersion of ALD method (Zhe and Yong 2017) and low platinum loading (0.26 at.% in Fig. 1c and Table 1). Elemental composition of C3N4-Au/10Pt in Fig. 1c revealed that as-synthesized catalyst was composed of C, N, Au, and Pt elements, providing evidence for the successful formation of C3N4-Au/10Pt photocatalysts.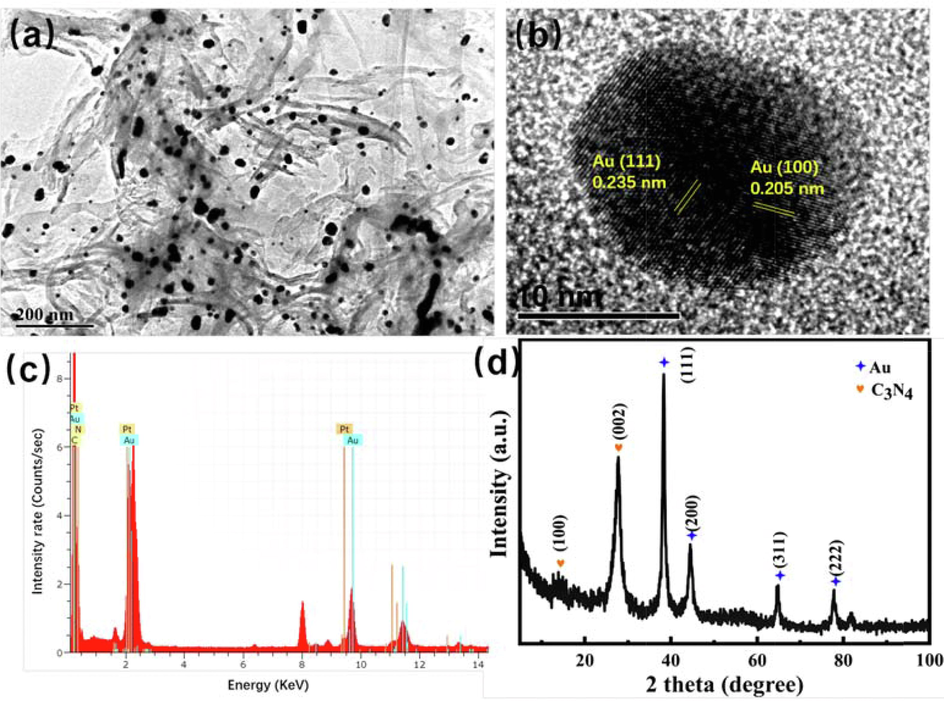
TEM images of C3N4-Au/10Pt (a), HRTEM of Au NPs (b), EDS (c) and XRD pattern of C3N4-Au/10Pt(d).
Photocatalyst
C 1 s
N 1 s
O 1 s
Au 4f
Pt 4f
C3N4
44.47%
55.22%
2.31%
C3N4-Au/10Pt
40.92%
54.31%
4.31%
0.21%
0.26%
The chemical composition and crystal structure of C3N4-Au/10Pt were studied by XRD patterns. In Fig. 1d, two representative diffraction peaks located at 13.7° and 27.8°, which can be indexed as the in-plane periodic repeat structural and conjugated aromatic systems in (1 0 0) and (0 0 2) graphitic plane of carbon nitride, respectively (Ning et al., 2017, Yanchun et al., 2020, Yiping et al., 2020). The diffraction peaks at 38.4°, 44.7°, 64.9°, 77.8° and 81.3° were attributed to the (1 1 1), (2 0 0), (2 2 0), (3 1 1) and (2 2 2) diffraction faces of the Au species, respectively (Xin et al., 2019). However, no peak belonged to Pt, indicating Pt species was too small and highly dispersed. The result was consistent with the TEM results.
The XPS spectra were applied to elucidate the chemical states and the interactions between components in bare C3N4 and C3N4-Au/10Pt. Table 1 and Fig. 2a exhibit that there are C1s, N1s, O1s, Au 4f and Pt 4f in C3N4-Au/10Pt, which are consistent with EDS results. During the deposition of platinum clusters by ALD, these increased oxygen species that oxidized by O3 pulse, namely O-H, C-O, C = O and O-C = O, which further induced the nucleation of Pt on C3N4-Au (Fig. 2b) (Jie et al., 2021). The C1s spectra in Fig. 2c are divided into two peaks located on 284.8 eV and 288.3 eV, which are ascribed to the graphitic carbon and N = C-N bounding in the triazine framework, respectively (Kwun-Han et al., 2017, Yanchun et al., 2020). Moreover, the N1s spectra (Fig. 2d) show three peaks at 398.7, 399.6 and 400.6 eV for C3N4, which are ascribed to the C-N = C, N(C)3 and NHX in the triazine framework, respectively. The C1s and N1s spectra indicate C3N4 is a triazine framework. Compared with C3N4, C3N4-Au/10Pt exhibited new functional groups of N-C = O, indicating that a part of N is replaced by O in C3N4-Au/10Pt, which is consistent with the increase of O contents shown in Fig. 2b and Table 1. Moreover, the peaks for C-N = C, N(C)3 and NHx shift to higher binding energy at 398.8, 400.2 and 401.2 eV after Au NPs and Pt clusters decoration, respectively. This is because the nitrogen groups with high local electron density in C3N4 can provide lone pair electrons to the empty d orbitals of the Pt atoms, thus stabilizing Pt through strong metal-carrier interaction and leading to its high distribution (Shaowen et al., 2016). Meanwhile, two characteristic peaks at 83.3 and 87.5 eV were observed in Fig. 2e, originating from metallic Au 4 f5/2 and Au 4f7/2, respectively (Xin et al., 2019). In Pt 4f spectra of C3N4-Au/10Pt (Fig. 2f), a tremendous amount of Pt exists in the form of high valence state, which indicates a large number of Pt atoms binding with N atoms in C3N4 (Xiaogang et al., 2016).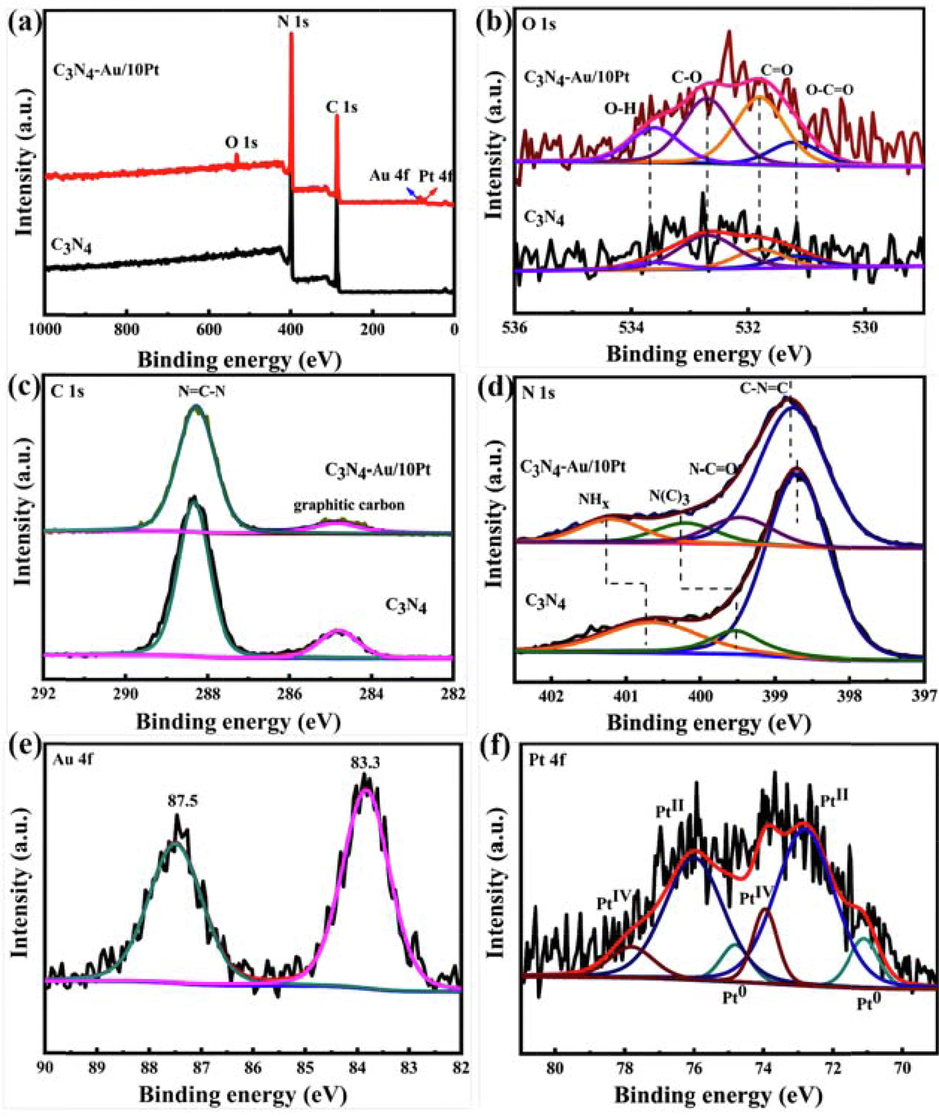
XPS survey spectra (a) and high-resolution XPS spectra of O 1 s (b), C 1 s (c), N 1 s (d), Au 4f (e) and Pt 4f (f) for bare C3N4 and C3N4-Au/10Pt.
FTIR was used to analyze the chemical bonds in the samples. In Fig. 3a, the FTIR spectra of all these samples have similar chemical bond at 811 cm−1 (the breathing smode of the tris-triazine system) and 890 cm−1 (the deformation mode of N-H), 1200–1700 cm−1 (C-N and C = N stretching vibration modes) and 3000–3500 cm−1 (O-H and N-H stretching vibration modes) (Kwun-Han et al., 2017, Yanchun et al., 2020). These results indicate that triazine framework structure remained mostly unchanged after the modification of Au NPs and Pt clusters. Fig. 3b illustrates the UV–vis DRS of the samples. The absorption edges of C3N4, C3N4-Au and C3N4-Au/10Pt have a little difference, suggesting that the decoration of Au NPs and Pt clusters modified on C3N4, which are consistent with XPS, XRD and TEM results. Compared with C3N4, strong photo-absorption was observed at approximately 550 nm in C3N4-Au and C3N4-Au/10Pt, which was attributed to the LSPR effect of Au NPs on C3N4 (Zhi Wei et al., 2012, Xin et al., 2019). In addition, the whole increase of light absorption intensity in the relatively wide range (λ > 420 nm) is caused by the scattering of Pt clusters on C3N4 (Shaowen et al., 2016). Moreover, the band gap values of the synthesized samples were calculated as 2.87 eV, 2.94 eV and 2.94 eV, respectively (Fig. 3c) by Kubelka-Munk formula (Yuyang et al., 2015, Yong et al., 2018, Yuan et al., 2020):
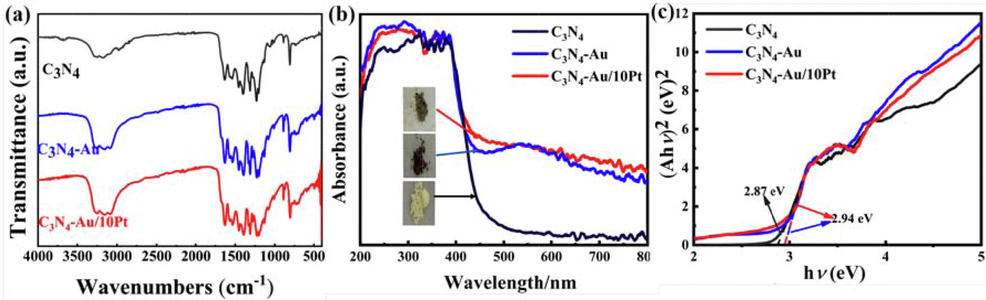
FTIR absorption spectra (a), Diffuse reflectance spectra(b) Tauc plots (c)of C3N4, C3N4-Au and C3N4-Au/10Pt.
The EIS spectra and photocurrent-time response were implemented to investigate the charge separation and transfer performance of C3N4-Au/10Pt. Fig. 4a showed that the arc radii of the samples increase in the order C3N4-Au/10Pt < C3N4-Au < C3N4, which indicated C3N4-Au/10Pt owns the smallest impedance in the photocatalytic process. Compared with C3N4 and C3N4-Au, C3N4-Au/10Pt showed a significantly enhanced photocurrent density, further verifying C3N4-Au/10Pt exhibited higher charge separation and transfer efficiency (see Fig. 4b). These results indicated that the Au NPs and Pt clusters could synergistically attribute the separation and transfer of carriers.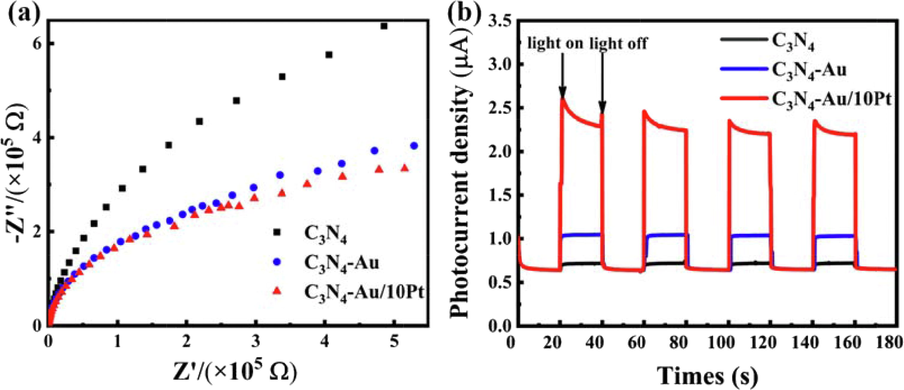
EIS spectra (a) and photocurrent responses(b) of C3N4, C3N4-Au and C3N4-Au/10Pt.
3.2 Photocatalytic activity and stability
The photocatalytic activities of the as-prepared samples were evaluated by degrading RhB dye in simulated sunlight. As can be seen from Fig. 5a, the degradation rate of RhB dye without catalyst was almost unchanged after simulated sunlight irradiation for 30 min. However, when the synthesized C3N4 photocatalysts were added, the degradation efficiency of RhB was higher than that without photocatalysts (see Fig. 5), which demonstrated that the as-prepared C3N4 catalysts had good photocatalytic activities. In order to find out the optimal synthesis temperature of C3N4, the effects of different synthesis temperatures such as 500 ℃, 600 ℃, 700 ℃ and 800 ℃ on the degradation of RhB under simulated sunlight irradiation were studied. The experimental results showed that the activities of synthesized photocatalysts were all good apart from 800 ℃. It was reported that a large amount of ammonia was released during the pyrolysis of urea (Liu et al., 2011). The released ammonia gas filled and eroded the system of carbon nitride, forming a microporous structure and increasing the specific surface area of the carbon nitride. The larger specific surface area and more mesoporous structure will be favorable for C3N4 to adsorb organic dyes and sunlight (Fan et al., 2013). However, 800℃ was maybe too high for urea to break down into ammonia and H2O, which led to the poor photocatalytic activity of C3N4. And more defeats in C3N4 under 800℃ calcination increase non-radiative recombination rates of the carriers (Po et al., 2014). According to the experimental results (see Fig. 5b), photocatalysts of C3N4 synthesized at 500℃ was finally used for the subsequent tests.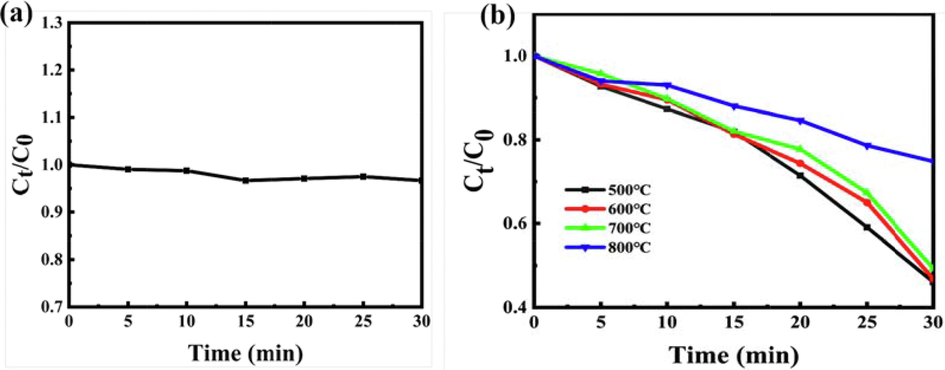
Degradation efficiency of RhB without (a) and present (b) catalysts with different calcinated temperature under sunlight irradiation.
By controlling the number of Pt ALD cycles on the C3N4-Au, a series of precisely regulated Pt sub-nano catalysts were synthesized (see Schematic diagram 1). As a model reaction, the photocatalytic degradation of RhB aqueous solution by C3N4, C3N4-Au, xPt/C3N4 and C3N4-Au/nPt photocatalysts was further studied. As shown in Fig. 6a, during the 30-minute photoreaction process, different photocatalysts have different photocatalytic degradation rates for RhB. Among them, C3N4-Au/10Pt has the fastest degradation rate of RhB with a degradation rate of 86.3%, followed by C3N4-Au with a degradation rate of 81.1%, C3N4/10Pt with a degradation rate of 58.53% and C3N4 with a degradation rate of 53.50%. Au NPs as light absorber in C3N4-Au and C3N4-Au/10Pt can generate a large amount of high-energy hot e- due to the plasma resonance effect and transfer them to the conduction band of C3N4 (Xixian et al., 2020, Dandan et al., 2021). The high electron affinity of Pt atoms as reductive cocatalysts in C3N4/10Pt and C3N4-Au/10Pt can promote electron transfer and inhibit e--h+ recombination. In this case, the electrons in the C3N4 conduction band are transferred to Pt through the Pt-N bond, which reduced the transfer distance and improved the charge separation (Yidong et al., 2021). In purpose to explore the effect of Pt amounts on photocatalyst degradation rate, 5, 10, 15 cycles of Pt precisely controlled by ALD were deposited on C3N4-Au surface, respectively. As illustrated in Fig.6b C3N4-Au/10Pt (86.3%) has a faster degradation rate than C3N4-Au-5Pt (49.86%) and C3N4-Au/15Pt (34.04%). The Pt clusters have optimal particle size through the competing effects of the Schottky barrier and Coulomb barrier charging energy on the charge transfer efficiency at the C3N4-Pt interface. This is because the Schottky barrier increases with the Pt cluster size, while the Coulomb blocking charge energy is decreasing (Yifat et al., 2017). The experimental results showed that C3N4-Au/10Pt photocatalysts doped with 0.21% gold and loaded with 0.26% platinum on C3N4 by photodeposition and ALD, respectively, had the most excellent photocatalytic degradation ability for RhB (see Fig. 6a, b, c, d and Table 1).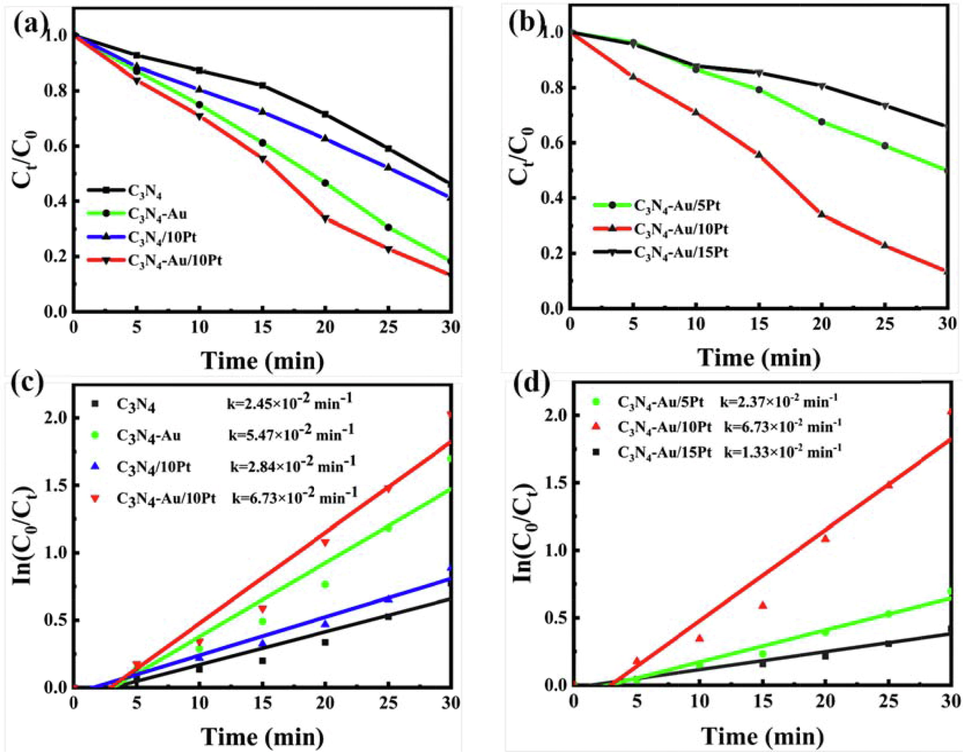
RhB degradation curves (a, b) and ratios (c, d) by as-prepared different photocatalysts under visible light irradiation.
In order to further study the properties of the synthesized photocatalysts and compare their photocatalytic activity differences, the kinetic curves of various photocatalysts for the photocatalytic degradation of RhB were fitted and the first-order reaction rate constants (k) were calculated (as shown in Fig. 6c and Fig. 6d). Fig. 6c shown the fitted kinetic curves of degradation of RhB by four different photocatalysts within 0–30 min under simulated sunlight irradiation. Among them, C3N4-Au/10Pt photocatalyst had the largest slope of all the fitted curves, indicating an excellent reaction kinetics of its photocatalytic activity. The k values of C3N4-Au/10Pt, C3N4-Au, g-C3N4/10Pt and C3N4 were 6.73 × 10-2, 5.47 × 10-2, 2.84 × 10-2 and 2.45 × 10-2 min−1, respectively (Fig. 6c). The precisely controlled C3N4-Au/10Pt exhibited the highest kinetic constant k (6.73 × 10-2 min−1), 2.75 times as high as that of C3N4 (2.45 × 10-2 min−1). Fig. 6d indicated the k values of C3N4-Au/5Pt, C3N4-Au/10Pt, C3N4-Au/15Pt were 2.37 × 10-2, 6.73 × 10-2 and 1.33 × 10-2 min−1, respectively. With the Pt ALD cycle numbers increasing from 5 to 10, the active sites and the catalytic activity of C3N4-Au/nPt increased. But the catalytic site reached saturation when Pt ALD cycle numbers were 10. So, the 10 cycle numbers of Pt ALD is dispersed on C3N4 well, the catalytic activity of C3N4-Au/10Pt reached the best. However, with the Pt ALD cycles number further increasing, Pt accumulates on the surface of C3N4-Au and its catalytic activity decreased (Chien-Te et al., 2013). C3N4-Au/10Pt synthesized by ALD technology had the homogeneity dispersion, which increased the adsorption capacity of RhB and maximized the active sites.
The results indicated that the precisely controlled sub-nanometric C3N4-Au/10Pt photocatalysts with high photocatalytic activity have been successfully prepared by ALD, which may be ascribed to the synergistic effect of C3N4, Pt and Au NPs (Jia et al., 2019, Wei et al., 2020, Dandan et al., 2021). By comparing with the reported photocatalytic activities of most C3N4-based photocatalysts for degradation of RhB under sunlight, the C3N4-Au/10Pt photocatalysts precisely tailored by ALD in this research showed quite more excellent photocatalytic activities. The specific characteristics of photocatalytic activity of C3N4-Au/10Pt are shown in Table S1. In order to investigate the stability of photocatalytic degradation of RhB by C3N4-Au/10Pt, four photocatalytic degradation cycles were repeated under the same conditions. The results showed the degradation rate of RhB by C3N4-Au/10Pt after four cycles was still comparable to that of after the first cycle, which proved that the C3N4-Au/10Pt photocatalysts had high photocatalytic stability (see Fig. 7a). To further investigate the reusability and stability of C3N4-Au/10Pt photocatalysts, the XRD patterns of them before and after the 4th recycling test were also studied. From Fig. 7b, we can see there were no difference between before and after the 4th recycling tests, indicating excellent recyclability and reusability of C3N4-Au/10Pt photocatalysts.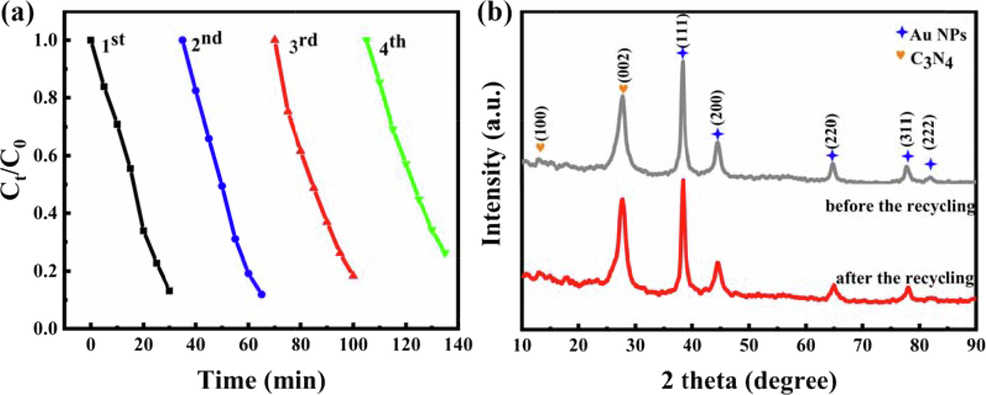
Degradation stability of RhB by photocatalysts (a) and XRD patterns of C3N4-Au/10Pt before and after the 4th recycling (b).
3.3 Mechanism of photocatalytic degradation
Under the sunlight irradiation, photogenerated electrons (e-) and holes (h+) on the surface of the photocatalysts participate in the degradation reaction which leading to different photocatalytic degradation paths of RhB. In order to study the photocatalytic mechanisms of C3N4-Au/10Pt photocatalysts for RhB under the sunlight irradiation, AA, EDTA-2Na and IPA were used as scavengers of superoxide radicals(⋅O2–), h+ and hydroxyl radicals(⋅OH), respectively. As shown in Fig. 8a, after adding of AA, the photocatalytic degradation rate of RhB decreased significantly and the reactions were inhibited, indicating that ⋅O2– was the main active species in the degradation process. Interestingly, when EDTA-2Na was added, the degradation efficiency of RhB was slightly improved. This may be because EDTA-2Na trapped the holes, which reduced the recombination of electrons and holes and allowed more electrons to react with oxygen to produce ⋅O2– active species (Xixian et al., 2020). In addition, the addition of IPA had little effect on the degradation of RhB. As a whole, ⋅O2– is the main active species in the RhB degradation process by C3N4-Au/10Pt photocatalysts under the sunlight irradiation.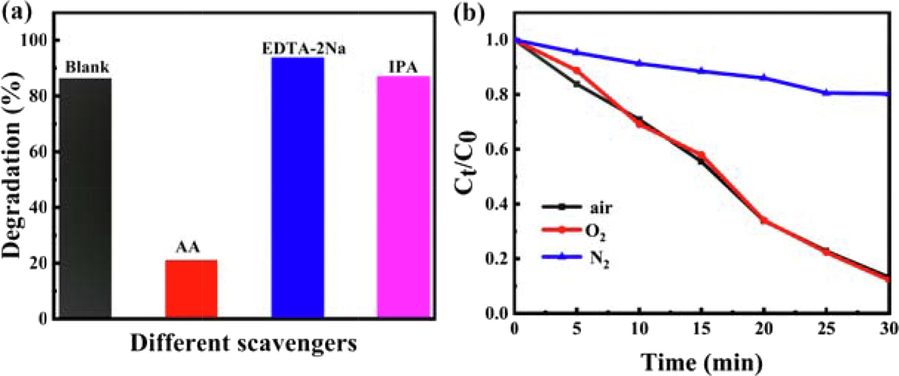
Radicals trapping experiments (a) and degradation experiments of different gases (b) in C3N4-Au/10Pt/sunlight system.
The degradation effect of C3N4-Au/10Pt under different conditions was studied in Fig. 8b. When N2 was introduced in the reaction system, the degradation rate of RhB was only 19.62% after 30 min, indicating that the degradation of RhB was inhibited under N2 condition. When O2 was passed into RhB solution, the degradation rate of RhB was 87.2%, which is similar to the degradation rate in air. It is further proved that ⋅O2– is the main active species in the C3N4-Au/10Pt catalytic system under the sunlight irradiation and the oxygen content in the system (air) is enough for RhB degradation.
By studying the electronic band structure of C3N4 and C3N4-Au/10Pt, the catalytic degradation mechanism of C3N4-Au/10Pt photocatalysts can be further explained. The following empirical formulas are used to estimate the EVB and ECB of photocatalytic materials (Zhixiao et al., 2017, Taiping et al., 2018):
Based on the conclusions above mentioned, the photocatalytic mechanism of C3N4-Au/10Pt photocatalysts for efficient degradation of RhB under sunlight is shown in Fig. 9. When C3N4-Au/10Pt under the irradiation of sunlight, the electrons in the EVB of C3N4 are excited and jump to the ECB, leaving h+ in the EVB. At the same time, Au NPs in C3N4-Au/10Pt can generate a large amount of high-energy hot e- due to the plasma resonance effect and transfer them to the conduction band of C3N4 (Xixian et al., 2020, Dandan et al., 2021). The high electron affinity of Pt atoms in C3N4-Au/10Pt can promote electron transfer and inhibit e--h+ recombination. In this case, the electrons in the C3N4 conduction band are transferred to Pt through the Pt-N bond, which reduced the transfer distance and improved the charge separation (Yidong et al., 2021). Furthermore, the carrier separation efficiency is improved by forming Schottky Barrier in the contact between Pt and g-C3N4. Because the C3N4-Au/10Pt conduction potential (-0.71 eV) is more negative than that of O2/O2– (-0.33 eV) reduction potential, a large number of photogenerated electrons react with O2 absorbed on the C3N4-Au/10Pt surface to generate the active radical ⋅O2–, which leads to the efficient degradation of RhB (Mingjuan et al., 2019). Since the value of EVB in C3N4 (+1.17 eV) is more negative than the potentials of OH−/⋅OH (+1.99 eV) and H2O/⋅OH (+2.27 eV) (Chao et al., 2020, Bo et al., 2021), the h+ generated by C3N4-Au/10Pt cannot react with H2O to form ⋅OH. So, the degradation mechanism of RhB by C3N4-Au/10Pt photocatalysts are shown as follows:
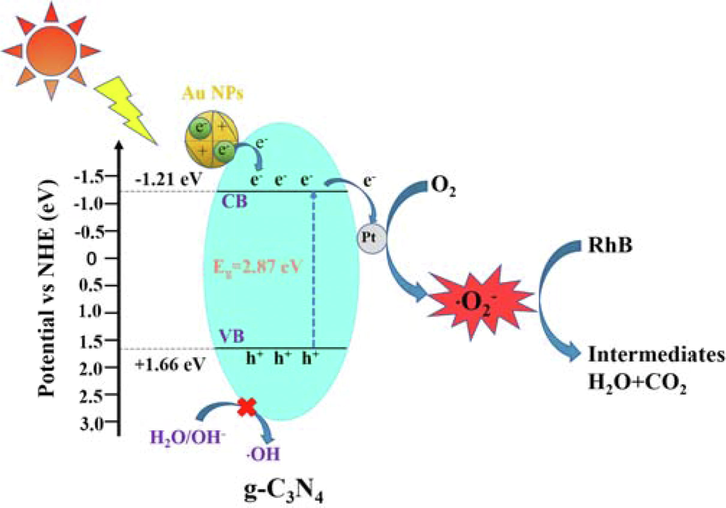
The mechanism of photocatalytic degradation of RhB by C3N4-Au/10Pt photocatalysts under sunlight.
4 Conclusion
In summary, the new precisely regulated C3N4-Au/nPt photocatalysts were successfully synthesized by ALD. Under simulated sunlight irradiation, the degradation rate of 10 mg/L RhB (100 mL) by 1.5 mg C3N4-Au/10Pt catalysts was 100% within 65 min, which is much better than other photocatalysts for the degradation of RhB. Pt clusters and Au NPs synergistically promoted the photocatalytic degradation of C3N4 by Schottky barrier and LSPR, respectively. The degradation mechanism of RhB by C3N4-Au/10Pt was demonstrated that ·O2– was the main active species in the photocatalytic process. This work highlights that an easy and controllable method can be used to prepare a “two in one” high-efficiency photocatalysts by ALD, which open up a new method to tailor and fabricate highly active photocatalysts in solving the energy crisis and environmental pollution.
Acknowledgments
This research was sponsored by Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (20210013), the Startup Foundation for Doctors of Shanxi Medical University (1C052017040, 3C322021041).
References
- Graphitic carbon nitride (g-C3N4)-based photocatalysts for solar hydrogen generation: recent advances and future development directions. J. Mater. Chem. A. 2017;5:23406-23433.
- [Google Scholar]
- Self-photoreduced Ag0-doped Ag(I)–organic frameworks with efficient visible-light-driven photocatalytic performance. CrystEngComm. 2021;23:7496-7501.
- [Google Scholar]
- Ultrathin Z-scheme 2D/2D N-doped HTiNbO5 nanosheets/g-C3N4 porous composites for efficient photocatalytic degradation and H2 generation under visible light. J. Colloid Interface Sci. 2020:58358-58370.
- [Google Scholar]
- Platinum electrocatalysts attached to carbon nanotubes by atomic layer deposition with different cycle numbers. J. Taiwan Inst. Chem. Eng.. 2013;45:186-191.
- [Google Scholar]
- Progressive Design of Plasmonic Metal-Semiconductor Ensemble toward Regulated Charge Flow and Improved Vis–NIR-Driven Solar-to-Chemical Conversion. Small.. 2017;13:1602947.
- [Google Scholar]
- Improved visible-light driven photocatalysis by loading Au onto C3N4 nanorods for degradation of RhB and reduction of CO2. Adv. Powder Technol.. 2021;32:1653-1662.
- [Google Scholar]
- Dilshad, M., M. Yuanyu and R. Sohrab, 2017. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B Environm. 206556–206588.
- Achieving Net Zero Energy Greenhouses by Integrating Semitransparent Organic Solar Cells. Joule.. 2020;4:490-506.
- [Google Scholar]
- In Situ Construction of g-C3N4/g-C3N4 Metal-Free Heterojunction for Enhanced Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces. 2013;5:11392-11401.
- [Google Scholar]
- Guohui, D., L. J. Daniel, Z. Ling, et al., 2017. Carbon vacancy regulated photoreduction of NO to N2 over ultrathin g-C3N4 nanosheets. Appl. Catal. B Environm. 218515–218524.
- In situ fabrication of ultrathin-g-C3N4/AgI heterojunctions with improved catalytic performance for photodegrading rhodamine B solution. Appl. Surf. Sci.. 2021;538:148132
- [Google Scholar]
- Selectivity Regulation in Au-Catalyzed Nitroaromatic Hydrogenation by Anchoring Single-Site Metal Oxide Promoters. ACS Catal.. 2020;10:2837-2844.
- [Google Scholar]
- Pt nanoparticles decorated heterostructured g-C3N4/Bi2MoO6 microplates with highly enhanced photocatalytic activities under visible light. Sci. Rep.. 2019;9:7636.
- [Google Scholar]
- Origin of synergistic effects in bicomponent cobalt oxide-platinum catalysts for selective hydrogenation reaction. Nat. Commun.. 2019;10:4166.
- [Google Scholar]
- Atomic Design and Fine-Tuning of Subnanometric Pt Catalysts to Tame Hydrogen Generation. ACS Catal.. 2021;11:4146-4156.
- [Google Scholar]
- Ultrathin graphitic carbon nitride nanosheets: a novel peroxidase mimetic, Fe doping-mediated catalytic performance enhancement and application to rapid, highly sensitive optical detection of glucose†. Nanoscale.. 2013;5:11604-11609.
- [Google Scholar]
- Metal-Free 2D/2D Phosphorene/g-C3N4 Van der Waals Heterojunction for Highly Enhanced Visible-Light Photocatalytic H2 Production. Adv. Mater.. 2018;30:e1800128
- [Google Scholar]
- Facile Photochemical Synthesis of Au/Pt/g-C3N4 with Plasmon-Enhanced Photocatalytic Activity for Antibiotic Degradation. ACS Appl. Mater. Interfaces. 2015;7:9630-9637.
- [Google Scholar]
- Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science. 2015;46:970-974.
- [Google Scholar]
- Oligomer-Incorporated Polymeric Layer Framework of Graphitic Carbon Nitride for Effective Photocatalytic Hydrogen Evolution. Part. Part. Syst. Char.. 2017;35:1700221.
- [Google Scholar]
- Tremella-like integrated carbon nitride with polyvinylimine-doped for enhancing photocatalytic degradation and hydrogen evolution performances. Sep. Purif. Technol.. 2021;279:119766
- [Google Scholar]
- Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. A. 2011;21:14398-14401.
- [Google Scholar]
- Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Applied Catalysis B: Environmental; 2016. p. :198347-198377.
- Meng, X., L. Liu, S. Ouyang, et al., 2016. Nanometals for Solar‐to‐Chemical Energy Conversion: From Semiconductor‐Based Photocatalysis to Plasmon‐Mediated Photocatalysis and Photo‐Thermocatalysis Advanced Materials. 28, 6781–6803.
- Enhanced Photocatalytic Hydrogen Generation Using Polymorphic Macroporous TaON. Adv. Mater.. 2012;24:3406-3409.
- [Google Scholar]
- Ultrathin Bi2WO6 nanosheets loaded g-C3N4 quantum dots: A direct Z-scheme photocatalyst with enhanced photocatalytic activity towards degradation of organic pollutants under wide spectrum light irradiation. J. Colloid Interface Sci. 2019:539654-539664.
- [Google Scholar]
- Emerging Chemical Functionalization of g-C3N4: Covalent/Noncovalent Modifications and Applications. ACS Nano. 2020;14:12390-12469.
- [Google Scholar]
- Enhanced photocatalytic activity of mesoporous carbon/C3N4 composite photocatalysts. J. Colloid Interface Sci.. 2017;512:474-479.
- [Google Scholar]
- Au-Nanoparticle-Loaded Graphitic Carbon Nitride Nanosheets: Green Photocatalytic Synthesis and Application toward the Degradation of Organic Pollutants. ACS Appl. Mater. Interfaces. 2013;5:6815-6819.
- [Google Scholar]
- Noor Izzati Md, R., L. Sze-Mun, S. Jin-Chung, et al., 2021. Fabrication of Z-scheme rod-like Ag2Mo2O7/g-C3N4 for phenol degradation under IO4¯/visible light system. Mater. Lett. 294, 129791.
- Noor Izzati Md, R., L. Sze-Mun, S. Jin-Chung, et al., 2022. Comparative study of g-C3N4/Ag-based metals (V, Mo, and Fe) composites for degradation of Reactive Black 5 (RB5) under simulated solar light irradiation. J. Environ. Chem. Eng. 10, 107308.
- Structure defects in g-C3N4 limit visible light driven hydrogen evolution and photovoltage†. J. Mater. Chem. A. 2014;2:20338-20344.
- [Google Scholar]
- Plasmonic ZnO/Au/g-C3N4 nanocomposites as solar light active photocatalysts for degradation of organic contaminants in wastewater. Chemosphere. 2020;263:128262
- [Google Scholar]
- Shape-dependent photocatalytic hydrogen evolution activity over a Pt nanoparticle coupled g-C3N4 photocatalyst†. PCCP. 2016;19457
- [Google Scholar]
- Sibo, C., Y. Siyuan, S. Xiaolin, et al., 2019. Carbon‐Coated Cu nanoparticles as a Cocatalyst of g‐C3N4 for Enhanced Photocatalytic H2 Evolution Activity under Visible‐Light Irradiation. Energy Technol. 7, 1800846–1800846.
- Fe-doped and -mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight†. J. Mater. Chem. A. 2014;2:6772-6780.
- [Google Scholar]
- Atomic and electronic structures of graphene-decorated graphitic carbon nitride (g-C3N4) as a metal-free photocatalyst under visible-light. Appl. Catal. B. 2019;256:117850
- [Google Scholar]
- Highly efficient direct Z-scheme WO3 /CdS-diethylenetriamine photocatalyst and its enhanced photocatalytic H2 evolution under visible light irradiation. Appl. Surf. Sci.. 2018;442:20-29.
- [Google Scholar]
- Plasmon Activation versus Plasmon Quenching on the Overall Photocatalytic Performance of Ag/Au Bimetal Decorated g-C3N4 Nanosheets Under Selective Photoexcitation: A Mechanistic Understanding with Experiment and Theory. Appl. Catal. B. 2021;298:120614
- [Google Scholar]
- Surface charge modification via protonation of graphitic carbon nitride (g-C3N4) for electrostatic self-assembly construction of 2D/2D reduced graphene oxide (rGO)/g-C3N4 nanostructures toward enhanced photocatalytic reduction of carbon dioxide to methane. Nano Energy. 2015;13:757-770.
- [Google Scholar]
- Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev.. 2016;116:7159-7329.
- [Google Scholar]
- Z-scheme Au decorated carbon nitride/cobalt tetroxide plasmonic heterojunction photocatalyst for catalytic reduction of hexavalent chromium and oxidation of Bisphenol A. J. Hazard. Mater.. 2020;124539
- [Google Scholar]
- AgCl/Au/g-C3N4 ternary composites: Efficient photocatalysts for degradation of anionic dyes. J. Alloy. Compd.. 2021;868:159266
- [Google Scholar]
- Single-Atom Pt as Co-Catalyst for Enhanced Photocatalytic H2 Evolution. Adv. Mater.. 2016;28:2427-2431.
- [Google Scholar]
- Improved charge transfer by size-dependent plasmonic Au on C3N4 for efficient photocatalytic oxidation of RhB and CO2 reduction. Chin. J. Catal.. 2019;40:928-939.
- [Google Scholar]
- Metal-Containing Carbon Nitride Compounds: A New Functional Organic-Metal Hybrid Material. Adv. Mater.. 2009;21:1609-1612.
- [Google Scholar]
- Novel Au@C modified g-C3N4 (Au@C/g-C3N4) as efficient visible-light photocatalyst for toxic organic pollutant degradation: Synthesis, performance and mechanism insight. Sep. Purif. Technol.. 2020;252:117485
- [Google Scholar]
- Boosting interfacial charge separation and photocatalytic activity of 2D/2D g-C3N4/ZnIn2S4 S-scheme heterojunction under visible light irradiation. J. Alloy. Compd.. 2021;162209
- [Google Scholar]
- g-C3N4 Hydrogen-Bonding Viologen for Significantly Enhanced Visible-Light Photocatalytic H2 Evolution. ACS Catal.. 2017;7:8228-8234.
- [Google Scholar]
- Enhanced photocatalytic hydrogen evolution of 2D/2D N-Sn3O4/g-C3N4 S-scheme heterojunction under visible light irradiation. Appl. Surf. Sci.. 2021;567:150903
- [Google Scholar]
- Engineering the Photocatalytic Behaviors of g/C3N4-Based Metal-Free Materials for Degradation of a Representative Antibiotic. Adv. Funct. Mater.. 2020;30:2002353.
- [Google Scholar]
- Single Pt atom-anchored C3N4: A bridging Pt−N bond boosted electron transfer for highly efficient photocatalytic H2 generation. Chem. Eng. J.. 2021;412:128749
- [Google Scholar]
- Size Matters: Cocatalyst Size Effect on Charge Transfer and Photocatalytic Activity. Nano Lett.. 2017;18:357-364.
- [Google Scholar]
- Photochemical transformation of C3N4 under UV irradiation: Implications for environmental fate and photocatalytic activity. J. Hazard. Mater.. 2020;394:122557
- [Google Scholar]
- Visible-light driven oxidative coupling of amines to imines with high selectivity in air over core-shell structured CdS@C3N4. Appl. Catal. B. 2018;236:176-183.
- [Google Scholar]
- Anatase TiO2@MIL-101(Cr) nanocomposite for photocatalytic degradation of bisphenol A. Colloids Surf., A. 2020;596:124745
- [Google Scholar]
- An Amorphous Carbon Nitride Photocatalyst with Greatly Extended Visible-Light-Responsive Range for Photocatalytic Hydrogen Generation. Adv. Mater.. 2015;27:4572-4577.
- [Google Scholar]
- Design and Properties of Confined Nanocatalysts by Atomic Layer Deposition. Acc. Chem. Res.. 2017;50:2309-2316.
- [Google Scholar]
- Synthesis of Transition Metal-Modified Carbon Nitride Polymers for Selective Hydrocarbon Oxidation. ChemSusChem. 2010;4:274-281.
- [Google Scholar]
- Janus Au-TiO2 Photocatalysts with Strong Localization of Plasmonic Near-Fields for Efficient Visible-Light Hydrogen Generation. Adv. Mater.. 2012;24:2310-2314.
- [Google Scholar]
- A bifunctional NiCoP-based core/shell cocatalyst to promote separate photocatalytic hydrogen and oxygen generation over graphitic carbon nitride. J. Mater. Chem. A. 2017;5:19025-19035.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103951.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







