Translate this page into:
Preparation and application of an environmentally friendly compound lubricant based biological oil for drilling fluids
⁎Corresponding author. zhoufs@cugb.edu.cn (Fengshan Zhou)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
As the basic raw material of bio-oil based eco-friendly lubricant, a special selected by-product vegetable acidified oil (AO) was modified by vulcanization, esterification, or vulcanization followed by esterification. The optimized vulcanization process conditions are 1% sulfur powder catalyzed, temperature 130 ℃, reaction time 2 h, while the optimized esterification process requires 20% glycerol and 1% H2SO4 catalysis, reaction temperature 220 ℃, reaction time 3 h. We compounded modified AO, diluent, pour point depressant and emulsifier into an advanced drilling lubricant F-1. F-1 has excellent performances in bentonite drilling fluids, the extreme pressure lubrication coefficient reduction rate (Δf) in fresh water mud is 86.84%, and 85.76% in 4% NaCl salt water mud. After aging at 150 ℃ for 16 h, its Δf is improved compared with room temperature. Adding F-1 to the basic bentonite mud system, the filtration loss of drilling fluids decreased from 10 mL to 6.5 mL, the apparent viscosity and plastic viscosity experienced little change before and after aging. The new bio-oil compound lubricant has an excellent temperature resistance, a high salt contamination resistance and cost-effective. Vulcanization and esterification processes help to improve the lubricity and reduce foaming rates.
Keywords
Drilling fluid
By-product bio-oil
Lubricant
Temperature tolerance
Salt contamination
Lubrication coefficient
1 Introduction
Drilling fluids are essential for oil & gas drilling engineering. Drilling fluids are complex system that consists of liquids, solids, and chemicals (Agwu and Akpabio, 2018; Long et al., 2020). The base of the drilling fluid can be water, oil, or in some cases both (Davoodi et al., 2020). Several types of chemicals and polymers are added to the base fluid to meet the required properties of the drilling fluid such as viscosity, density, fluid loss control, and chemical composition (Al-Hameedi, A.T. et al., 2019; Ghaderi et al., 2020; Moslemizadeh et al., 2017). These fluids may vary in composition but their functions remain largely the same. Drilling fluids are circulating fluid in a drilling operation to fulfil (Davoodi et al., 2018). The functions of transporting cuttings to the surface, controlling the pressure of formations, cooling and lubricating the drilling bit, stabilizing the wellbore and supporting the weight of drill string (Ali and Ahmad, 2020; Moslemizadeh and Shadizadeh, 2017; Zhou et al., 2013).
Lubricants are added to the drilling fluid as an additive to improve the lubricating effect and minimize the friction (Kania et al., 2015). Poor lubrication can lead to drill bit bearing wear, casing wear, over-pulls in trip-outs and drag, torque problem, and differential sticking (Brandon et al., 1993). It is often necessary to add lubricant to the drilling fluid to avoid sticking accidents and increase the drilling speed.
The most commonly used drilling fluid lubricants are mineral oil and vegetable oil (Naidu et al., 1984). Mineral oil is difficult to biodegrade, causing serious pollution to the environment, and the high level of fluorescence is not conducive to geological logging (Willing, 1999, 2001). This situation has also forced the lubricant industry to move towards environmentally friendly products, and to find the biodegradable lubricants with superior lubrication performances than mineral oil (Amanullah et al., 2020). Therefore, the research of vegetable oil-based lubricants is one of the main directions of environmentally friendly lubricants (Faes et al., 2020; Ma et al., 2021). Vegetable oil has good biodegradability (Fox and Stachowiak, 2007; Liu et al., 2019). The molecule of vegetable oil contains many polar groups, which makes vegetable oil form a layer of adsorption film on the surface of metal and rock, so as to play a lubricating role (Martín-Alfonso et al., 2021; Wang et al., 2020; Yin et al., 2021).
However, a single lubricating grease sample often fails to meet the requirements of drilling industry standards, and it is necessary to add diluents, pour point depressants and defoamers to adjust and optimize the overall performance (Al-Hameedi et al., 2019; Chang et al., 2019; Marquez-Alvarez et al., 2004). An excellent lubricant product not only meeting the drilling industry standard specifications, but also making complete use of the advantages of vegetable oils, while improving some of their performance deficiencies (Corma et al., 2008; Jie et al., 2015; Li et al., 2016; Roy and Karak, 2012; Zha et al., 2018). This paper tries to solve the potential problems in existing products through choosing some excellent biological oil by-products, modifying them by vulcanization and esterification, and then intermixing them with other functional additives, so as to enhance the comprehensive performances and price competitiveness of the environmentally friendly compound lubricant based biological oil in water-based drilling fluids.
2 Materials and methods
2.1 Experimental materials
Vegetable acidified oil (AO), cottonseed oil (CSO) and white mineral oil (WO) are all of industrial grade and were purchased from Jingkai Chemical Factory of Renqiu. Black vegetable soybean oil (BO) and oleic acid (OA) are all of industrial grade and were purchased from Tianjin Huafu Oilfield Chemical Co., Ltd. Other reagents include methanol (MT), glycerol (GL), sulphur (S) and non-ionic surfactant (QT) and Span-80 (SP-80), which are of analytical purity and were purchased from Beijing Chemical Factory.
2.2 Experimental apparatus
Extreme Pressure (E-P) Lubrication Device, OFI, USA. F-4600 Fluorescence Spectrophotometer, Hitachi, Japan. ZNN-D6S Six Speed Rotary Viscometer and ZNS-2A-Low Pressure Filter Press, Qingdao Haitongda Special Instruments Company. Infrared Spectrometer, Spectrum 100, Perkin Elmer, USA. XGRL-4 High Temperature Roller Heating Furnace, Qingdao ChuangMeng Instrument Company. GHYSZ Automatic Acid Value Detector, Wuhan State Testing & Huaneng Company. BM-6000 luminescent bacteria toxicity detector, Qindao Loobo JianYe Environmental Protection Technology Co., Ltd. Inverter high-speed mixer, Qindao ChuangMeng Instrument Co., Ltd, China.
2.3 Preparation method
2.3.1 Preparation of freshwater slurry
The preparation method is according to a special enterprise standard formulated by China National Petroleum Corporation (CNPC) Liquid Lubricants for Drilling Fluids Q/SY 17088–2016 (CNPC, 2016) and Bentonite, National Standards of the People's Republic of China, Standards Press of China (GB/T 20973, 2020). Take four high-speed mixing cups, add 400 mL of fresh water, 0.6 g (accurate to 0.01 g) Na2CO3, 20 g (accurate to 0.01 g) calcium-based bentonite, stir in a high-speed mixer (Inverter high-speed mixer, Qindao ChuangMeng Instrument Co., Ltd, China) 11000 rpm for 20 min, the mixture was in an airtight curing condition at room temperature for 16 h.
2.3.2 Preparation of salt-water slurry
The preparation method is according to Liquid lubricants for drilling fluids Q/SY 17088–2016 (CNPC, 2016), the salt-water base slurry is prepared on the basis of fresh water slurry as mentioned previously. For a 4% NaCl slurry, add 16 g (accurate to 0.01 g) NaCl to 400 mL fresh water slurry and stir stir in a high-speed mixer (Inverter high-speed mixer, Qindao ChuangMeng Instrument Co., Ltd, China) 11000 rpm for 20 min, the mixture was in an airtight curing condition at room temperature for 16 h.
2.4 Evaluation methods
2.4.1 Lubricity performance test
The lubrication inclination angle (α) and lubrication coefficient reduced rate (Δf) are evaluation indicators of lubricity. The evaluation methods are according to Liquid Lubricants for Drilling Fluids Q/SY 17088–2016 (CNPC, 2016), that is also the test method commonly used in the world oil drilling industry. Lubrication inclination angle (α) is a relatively simple method to evaluate lubricity, and the repeatability of the experiment is poor and the error of the experimental data is large. It is only used for simple lubrication performance evaluation in the drilling field laboratory. Therefore, we use the extreme pressure lubrication coefficient reduction rate (Δf) to evaluate the lubrication performance, the evaluation is divided into the following steps: step 1, turn on the extreme pressure lubricator (Extreme Pressure Lubrication Device, OFI, USA) and preheat for 15 min. Step 2, fix the slider to the holder with the concave side outwards, against the test ring. Step 3, adjust speed to 60 rpm and adjust torque zero knob to zero torque. Step 4, take the cured fresh water base slurry, pour it into a high mixing cup, and stir for 10 min at 11000 rpm, then pour it into tray, press the lubricator to 16.95 N·m, and test the base slurry lubrication coefficient, read after 10 min and record as T1. Step 5, clean lubrication test rings and test blocks, then take 400 mL of fresh water base slurry and add 2 mL of lubricant sample to it, then continue with the same operation in step 4, take the reading after ten minutes, and record it as T2. Then calculate its Δf, calculated as:
In the formula:
Δf - reduction rate of extreme pressure lubrication coefficient, %;
T1 - Extreme pressure lubrication coefficient of the base paste;
T2 - The extreme pressure lubrication coefficient after the base slurry is added to the specimen.
2.4.2 Rheological performance
Since bentonite drilling fluids belongs to a kind of pseudoplastic fluid, pseudoplastic fluid obeys power law equation: τ = Kγn, where τ is shear stress, γ is shear rate, n is fluidity index and K is consistency coefficient, and they are two important parameters of pseudoplastic fluid (Huang, 2016). The performance of drilling fluids is evaluated by rheological data and filtration loss, followed by API standard (API 13B-1, 2009). The usual parameters are apparent viscosity (AV), plastic viscosity (PV), yield point (YP), filtration loss (FL), and friction coefficient Kf.
In the formula:
AV -Apparent viscosity (mPa·s).
PV-Plastic viscosity (mPa·s).
YP-Yield point (Pa).
α-Lubrication inclination angle.
Φ600-Viscometer dial reading at 600 r/min (dia).
Φ300-Viscometer dial reading at 300 r/min (dia).
FL-Filtration volume at ambient temperature under API
standard (mL);
V-Filtrate volume between 7.5 min and 30 min (mL).
F-Friction force (N).
P-Positive pressure (Pa).
2.4.3 Biological toxicity test
Refer to Biotoxicity Classification and Detection Methods of Oilfield Chemicals and Drilling Fluids: Luminescent Bacteria Method (Q/SY 111-2007, 2007)“, using BM-6000 luminescent bacteria toxicity detector to determine the biological toxicity of samples.
3 Result and discussion
3.1 Physical properties of vegetable oil by-products
Evaluation of the pour point and acid value of the tested oil samples according to Petroleum Products-Determination of Pour Point GB/T 3585–2006 (CNSC, 2006) and National Food Safety Standard-Determination of Acid Value in Food GB 5009.229-2016(National Standards of the People's Republic of China, 2016). The fluorescence intensity grade, density, pour point and acid value of the tested oil samples in the paper are shown in Table 1.
Oil samples
Fluoresce
Density (g/cm3)
Pour Point (℃)
Acid Value (mg KOH/g)
Price (CNY/ton)
Vegetable acidified oil (AO)
3.0
0.95
18
184.34
4500
cottonseed oil (CSO)
3.0
0.92
15
103.51
7000
black vegetable soybean oil (BO)
3.0
0.88
12
157.04
5500
edible vegetable oil (VO)
2.0
0.89
10
4.15
12,000
oleic acid (OA)
1.0
0.86
8
223.16
8000
mineral white oil (WO)
0
0.86
−42
0.06
5500
From Table 1, we can find that: of all the oil samples, the fluorescence level is lower than 5 levels. The density of grease is around 0.9 g/cm3. The pour point of WO reaches −42 ℃, can be used as a good pour point depressant. The acid value of AO also reaches 180 mg KOH/g or more, can be used as a good lubricant base oil.
The rate of reduction of the extreme pressure lubrication coefficient for various oils after the addition of the Calcium-based bentonite slurry is shown in Fig. 1.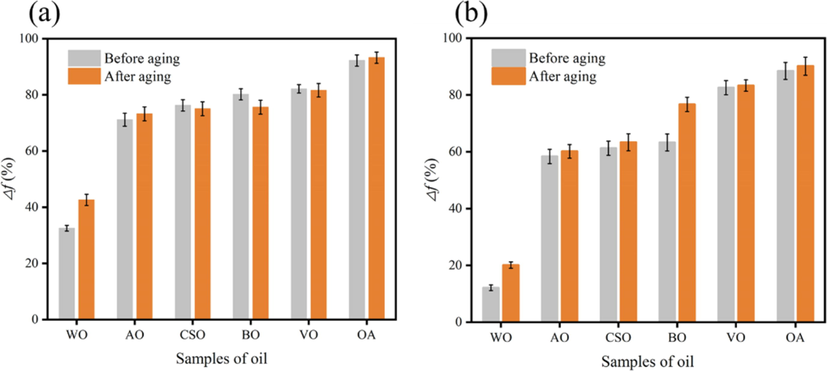
Comparison of lubricity of different oils before and after hot aging. (a): Fresh water-based slurry; (b): Salt water-based slurry.
From Table 1 and Fig. 1, the acid value of AO is not much different from that of OA, but its lubricating performance is quite different, which may be due to high foaming. Therefore, considering vulcanization and esterification modification to reduce its foaming rate, the cheap AO modified may be comparable to OA as a good lubricating base oil.
3.2 Vulcanization of oils
Vulcanization modification aims to open the double -C = C- bond which introduces extreme pressure element sulfur (S), so as to improve the grease stability and extreme pressure anti-wear. The reaction equation is shown in Fig. 2.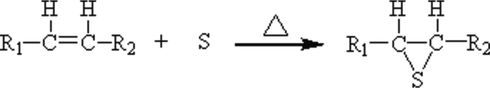
Schematic diagram of vulcanization reaction.
The vulcanization experiments are designed according to a three-factor, three-level orthogonal experiment. At room temperature, Δf of fresh-water based slurry is used as the evaluation target. The main influencing factors of the experiments are selected as reaction temperature (A), reaction time (B) and sulfur powder addition (C) to explore the influence of the three factors on the vulcanization performance, and the results are shown in Table 2. Note: (1) K1, K2 and K3 in the table are the sums of the indicators examined at the first, second and third levels of the corresponding factors, respectively. (2) The table k1, k2 and k3 are the means of the indicators examined at the first, second and third levels of the corresponding factors, respectively. (3) The extreme difference is the maximum difference between k1, k2 and k3.
Run
Combinations
Experimental factors
Δf (%)
A
Temperature (℃)B
Times (h)C
Dosage of S (wt/%)
1
A1B1C1
120
1
1
83.12
2
A1B2C2
120
2
2
86.58
3
A1B3C3
120
3
3
78.35
4
A2B1C2
130
1
2
85.28
5
A2B2C3
130
2
3
90.04
6
A2B3C1
130
3
1
90.91
7
A3B1C3
140
1
3
88.74
8
A3B2C1
140
2
1
87.45
9
A3B3C2
140
3
2
81.82
Δf experimental factors analysis
K1
248.05
257.14
261.48
K2
266.23
264.07
253.68
K3
258.01
251.08
257.13
k1
82.68
85.71
87.16
k2
88.74
88.02
84.56
k3
86.01
83.69
85.71
R
6.06
4.33
2.60
Influence factor A > B > C Optimal term A2 B2 C1
In Table 2, K1, K2, and K3 are the sum of the inspection indicators corresponding to the first, second, and third levels of the corresponding factors. k1, k2, and k3 are the average values of the inspection indicators at the first, second, and third levels of the corresponding factors. The range is the maximum difference between k1, k2, and k3. According to the size of the polar difference we can get the degree of influence of the three factors on Δf : A > B > C; from K1, K2, K3 and k1, k2, k3 of the total value selected the largest, and finally selected the best conditions for the three factors as A2B2C1, that is, the best reaction conditions are: reaction temperature 130 ℃, reaction time 2 h, and sulfur catalyst 1%.
3.3 Esterification of oils
When AO is added to the base slurry, there will be much foaming after high stirring, and it will not be eliminated for a long time. Esterification of vegetable oils can effectively reduce the acid value of oils, thus achieving the effect of defoaming. The equation of esterification reaction is shown in Fig. 3.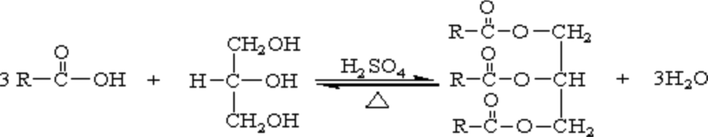
Esterification of vegetable oil.
The esterification reaction conditions include the amount of catalyst (Glycerol + H2SO4) added, reaction temperature and reaction time. The reaction temperature is 200 ∼ 280℃, the reaction time is 2 ∼ 8 h, and the amount of Glycerol (GL) is 10 ∼ 20% of raw material.
Esterification rate = (pre-reaction acid value - post-reaction acid value)/pre-reaction acid value × 100% (Marchetti et al., 2007; Pasias et al., 2006)
Calculation method of theoretical accession GL volume.
In the formula:
S-acid value of oil and grease (mg KOH/g)
M - mass of grease (g)
M1 - GL molar mass (g/mol)
M2 - KOH molar mass (g/mol)
Taking 100 g of AO, and the acid value of AO is 184.34 mg KOH/g, the molar mass of GL is 92 g/mol, and the molar mass of KOH is 56 g/mol. The addition of GL can be calculated as 10.1 g, and taking the whole 10.0 g, and the theoretical amount of GL added is about 10% of the mass of AO. Since the catalyst NaOH addition has less effect on the esterification, the fixed addition amount is 1% of the GL amount.
3.3.1 Effect of glycerol addition on esterification
The effect of glycerol addition on esterification was investigated at 220 °C for 3 h. The results are shown in Fig. 4.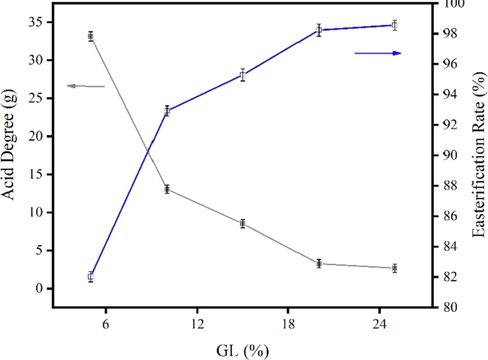
Effect of GL addition on the acid value of esterification products.
From Fig. 4, the amount of GL addition has a significant effect on the esterification process, especially the acid value which decreases when the amount of GL addition increases from 5% to 10%. When adding GL at 20–25%, the acid value is only about 3 mg KOH/g, and the esterification rate rises to more than 98%, and the reaction tends to slow down. However, the excessive amount of GL does not only increase the production cost, but also easily cause harmful reactions, so the final choice of GL addition amount is 20% of the oil sample.
3.3.2 Effect of reaction temperature on esterification
The effect of reaction temperature on esterification was investigated, and the experimental conditions are chosen to be 20% of GL addition and 3 h. The results are shown in Fig. 5.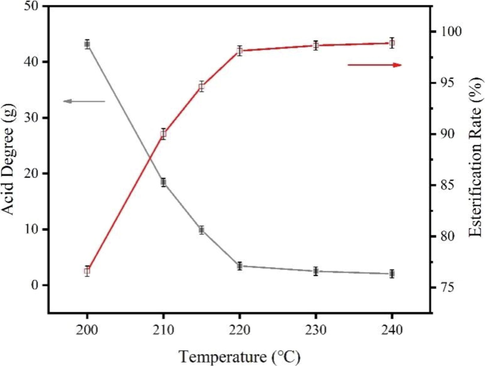
Effect of reaction temperature on acid value of esterification products.
From Fig. 5, the acid value decreases and sands the esterification rate increases as the temperature increased. The acid value was already 3.44 mg KOH/g and the esterification rate reached more than 98% when the temperature is 220 ℃, so the optimized reaction temperature is 220 ℃.
3.3.3 Effect of reaction time on esterification
The effect of reaction time on esterification was investigated, and the experimental condition is chosen to have a GL addition of 20% and a reaction temperature of 220 °C. The results are shown in Fig. 6.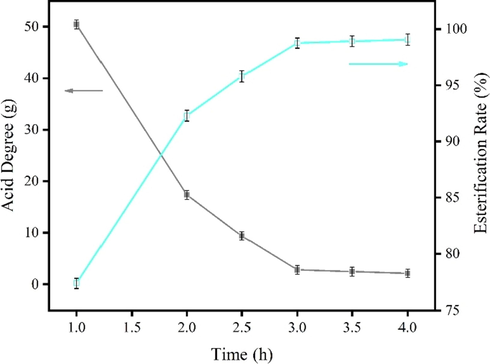
The effect of reaction time on acid value of esterification products.
From Fig. 6, the acid value decreased and the esterification rate increased with the increase of reaction time. At a reaction time of 3 h, the acid value was only 3.18 mg KOH/g, and the esterification rate reached more than 98%. At this time, increasing the reaction time cannot effectively improve the esterification effect. Moreover, if the reaction is maintained too long at 220 ℃, it invariably increases the danger and consumed energy, which also lead to the blackening and viscosity of the reactants. Therefore, the optimized reaction time is 3 h.
Optimal process conditions for GL esterification are finally determined as follows: 100 g AO is added with 20% GL (20 g), 1% NaOH (0.2 g), and the reaction is carried out at 220 °C for 3 h.
3.4 Vulcanization and esterification of acidified oils
The vulcanization of AO alone will take 2 h at 130 ℃, and esterification alone will take 3 h at 220 ℃. Considering that it takes a lot of time to heat up to the reaction temperature, the actual production will take 1 h to add materials, 1 h to heat up to 130 ℃, 1 h to heat up to 220 ℃, and 1 h to cool down after the reaction, it will take 1 h to cool down from 220 ℃ to 130 ℃, and 1 h to cool down to room temperature. Therefore, the whole process of vulcanization will take 5 h, and esterification will take 8 h. Non-stop reaction for 24 h can only do 4 groups vulcanization or 3 groups esterification, not to mention that AO needs both vulcanization and esterification to be carried out, which has low production efficiency and huge production cost.
Considering the reaction of vulcanization and esterification in one step, we first add material to proceed esterification. When the temperature is lowered to 130 ℃, add sulfur powder, then add MT when the temperature is lowered below 60 ℃ followed by stirring room temperature, so that the time will be shortened from 13 h to 10 h, shortening 3 h. The results are shown in Table 3.
Influence factor
Vulcanization
Esterification
Vulcanization and esterification
Temperature (℃)
130
220
220 ∼ 130
Reaction time (h)
2
3
2 + 3
Heating and cooling time (h)
3
5
5
Total time (h)
5
8
10
3.5 Performance evaluation of modified acidified oils
3.5.1 Characterization of modification results
Fig. 7 shows the FT-IR of AO modified by three modification methods. There are three groups in AO, –CH2, –COOH and -C = CH. –CH2 and –COOH correspond to the strong absorption peaks of 2928 cm−1, 2856 cm-1 and 1711 cm−1 in FT-IR, -C = C-H corresponds to the peak at 3007 cm−1. AO is recorded as V-AO after vulcanization, and the disappearance of the peak at 3007 cm−1 (-C = C-H) indicates the occurrence of this process. After AO esterification, it is denoted as E-AO. The disappearance of the peak at 2928 cm-1 (–CH2-) and the peak at 1711 cm−1 (–COOH) indicates the conversion of –CH2 to –CH3 during the esterification process, the appearance of absorption peaks at 1041 cm−1 and 1087 cm−1 also indicates that this process is taking place, with both the formation of -C-O-C-. The AO that is first vulcanized and then esterified is recorded as VE-AO, and the disappearance of the peak at 3007 cm−1 (-C = C-H) and the appearance of absorption peaks at 1041 cm−1 and 1087 cm−1 indicates the occurrence of this process. For VE-AO, the peak at 1742 cm−1 may be caused by water.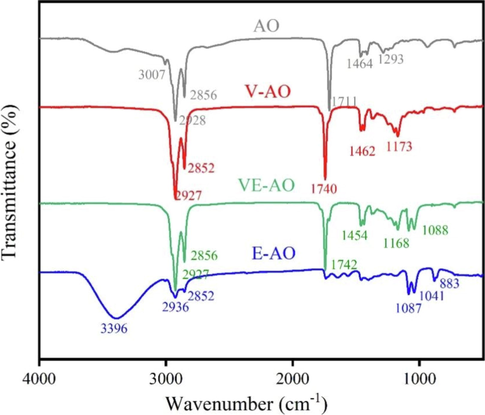
Fourier transform infrared spectroscopy (FT-IR) of samples of oil.
3.5.2 Comparison of the performance of several modification methods
AO modification experiments are compared, and the experimental results are shown in Table 4 and Fig. 8.
Performances
AO
Different modification methods
Vulcanization
Esterification
Vulcanization and esterification
Fluorescence (level)
3.0
4.0
4.0
4.0
AV (mPa·s)
8.0
6.5
4.5
3.5
Δρ (g/cm3)
0.12
0.10
0.05
0.06
α (°)
40.5
34.5
26.5
30.5
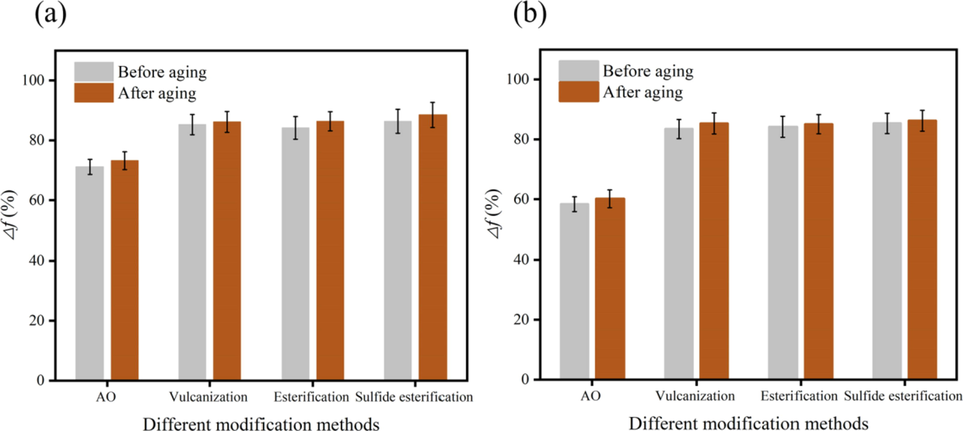
Evaluation the lubricity performance of AO modified by different methods. (a): Fresh water-based slurry; (b): Salt water-based slurry.
Comparing the results of modification, it is found that different modification methods improved the lubricity of various oils to a great extent, especially in the salt-water-based slurry. In saltwater -based slurry, the lubricity Δf of AO is increased from 58.33% to 85.32%, which exceeded the industry required liquid lubricant index and could be used as liquid lubricant base oil.
3.5.3 Adjustment and optimization
AO modified by sulfide esterification (denoted as VEAO) exhibits excellent performance in lubricity and can be used as a base oil, and then diluents, wetting agents and emulsifiers are added to adjust and optimize the comprehensive performances. Therefore, the design of tempering program F-1 is as follows.
F-1: VEAO + WO + SP-80 + QT. The results of the performance tests on F-1 are shown in Table 5. Where, Δffb: fresh-water slurry before aging, Δffa: fresh-water slurry after aging, Δfsb: salt-water slurry before aging, Δfsa: salt-water slurry before aging.
Performances
Test Data
Fluorescence (level)
4.0
AV (mPa·s)
4.0
Δρ (g/cm3)
0.06
Δffb (%)
86.84
Δffa (%)
88.03
Δfsb (%)
85.76
Δfsa (%)
86.36
3.5.4 Temperature tolerance and salt resistance
For the sample F-1, the test evaluation of Δf is performed at different temperatures (150 ℃ and 180℃) after ageing for 16 h and different salinities (4% NaCl, 15% NaCl, 36% NaCl, and 7% KCl). The freshwater base slurry is prepared by: 400 mL water + 20 g standard calcium-based bentonite + 0.6 g Na2CO3, high stirring for 20 min and then maintained at room temperature for 16 h. For making a saltwater base slurry, a specified quantified NaCl/KCl is added to the well-maintained freshwater base slurry. The specified amount of lubricant specimen added to the base slurry, and then to evaluate its lubricity. The test results are shown in Fig. 9.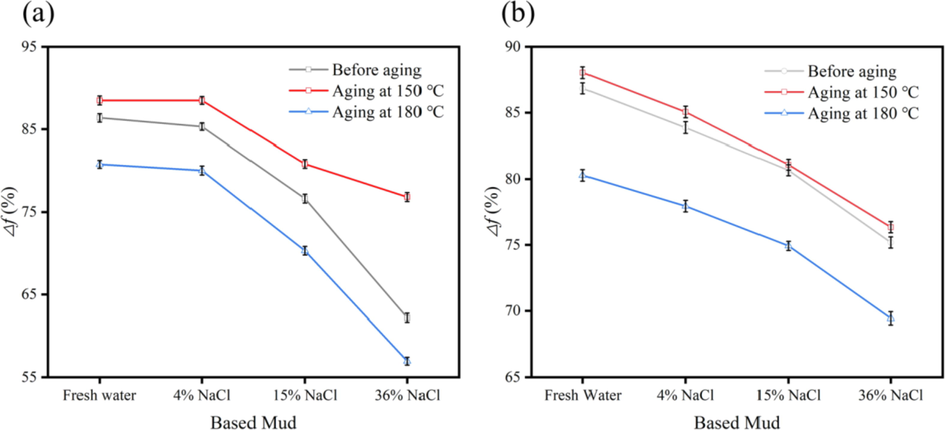
Performance evaluation of different mud. (a): AO; (b): F-1.
From the above test results, we can find that AO itself has poor lubrication anti-salt performance, and the temperature has a large effect on its Δf. F-1 is better than AO, because of dilution, surfactant addition making lubrication effective and molecules dispersed even.
In terms of salt resistance, its Δf is 83.87% in 4% NaCl based mud, which also still reach 75.19% in 36% NaCl based mud and 76.35% after aging at 150 ℃, as the aging temperature rises from 150 ℃ to 180 ℃, its Δf decreases about 8%, In general, F-1 has excellent temperature and salt resistance, and is a good temperature and salt resistant lubricant.
3.5.5 Pilot test performance
The pilot production is selected in an oilfield auxiliary production enterprise in Karamay, Xinjiang. A lot of lubricants are consumed yearly in oil wells of the Xinjiang oilfield, and there are a lot of by-products AO in the local cotton production, so it will be beneficial for production and sales to make use of local conditions and take local materials. According to the laboratory formula, 12 t products are formulated and produced. Samples are then taken for evaluation and testing respectively after production. Table 6 shown the performance of four batches industrial samples F-1 (noted as F-1a ∼ F-1d).
Performances
Industrial Samples
F-1a
F-1b
F-1c
F-1d
Fluorescence (level)
4.0
4.0
4.0
4.0
AV (mPa·s)
4.5
4.5
4.5
5.0
Δρ (g/cm3)
0.07
0.08
0.07
0.07
Δffb (%)
83.12
83.62
84.11
83.57
Δffa (%)
81.21
82.37
82.38
83.41
Δfsb (%)
83.18
82.82
81.89
81.34
Δfsa (%)
82.66
83.53
82.62
83.39
α (°)
20.3
20
21.0
20.2
From the test results, we can observe that the F-1 pilot production product fully meets the requirements of the standard indicators and is a vegetable oil lubricant with excellent lubrication performance and high-cost performance.
3.6 Industrial applications
3.6.1 Comparison with different lubricants
The F-1 optimized sample was evaluated in Xinjiang Tuha Oilfield Technical Service, and compared the basic performances with their common-used better commercial lubricant products such as LU-99, PGCS-1, LUSXR and HLB. Comparison of test results are shown in Table 7.
Performances
Comparison Samples
F-1
PGCS-1
LUSXR
LU-99
HLB
Fluorescence (level)
4.5
4.5
4.0
4.0
4.0
AV (mPa·s)
4.5
4.0
3.5
4.5
5.0
Δρ (g/cm3)
0.07
0.06
0.05
0.07
0.08
Δffb (%)
85.08
86.16
84.69
84.34
85.09
Δffa (%)
86.27
87.26
85.47
85.22
86.13
Δfsb (%)
88.61
87.21
86.39
86.11
87.06
Δfsa (%)
89.18
88.64
87.63
86.55
87.78
α (°)
19.5
11.3
7.6
8.7
6.6
Price (CNY/ton)
6000
8000
6500
7000
7000
From the test results in Table 7, we can find that F-1 is not much different from LU-99 and HLB in all performance comparisons, and they are all excellent drilling fluid lubricants. Compared with their samples, F-1 is more inexpensive and vegetable oil-based, which is more environmentally friendly.
3.6.2 Effect on rheological performances of drilling fluids
The effect of the lubricant sample on the rheological performances of the drilling fluids system are evaluated. The base drilling fluid system (Mud-1) formula is: 4% calcium-based bentonite slurry + 0.5% NH4PAN + 1% SPNH + 0.3% FA-367 + 0.5% SP-80 + 0.5% NaOH + 10% KCl, the dosage of all the evaluated lubricant samples in Mud-1 is 0.5%, and the rheological properties are evaluated.
According to the above experimental method, prepare Mud-1, add lubricant samples respectively, subject to high stirring for 30 min, pour into an aging tank, after aging at 150 ℃×16 h, put to room temperature, high stirring for 5 min, and test the rheological performances and filtration loss after aging. The test results are shown in Figs. 10, 11. Several most common drilling fluid additives in China are used in this Mud-1 formula including hydrolyzed polyacrylonitrile ammonium (NH4PAN) filtrate reducer, sulfonated lignite phenol formaldehyde resin (SPNH) high temperature filtrate reducer, copolymer coating inhibitor (FA-367) and spartan emulsifier (SP-80).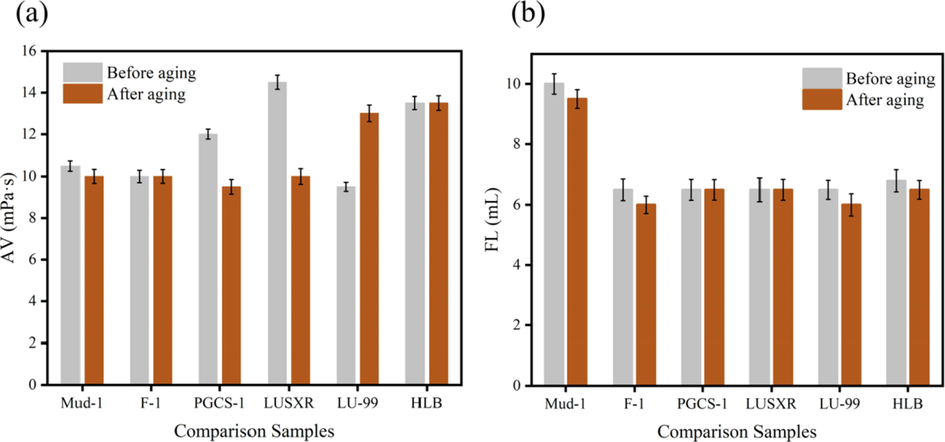
Test results of rheological properties. (a): AV; (b): FL.
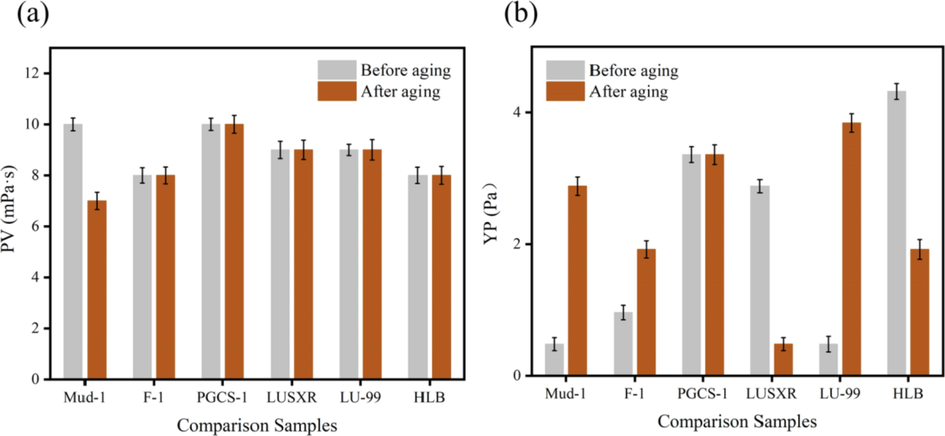
Test results of rheological properties. (a): YP; (b): PV.
From the test results in Fig. 10 and Fig. 11, we can observe that after adding 0.5% (2 g) lubricant sample to Mud-1, the filtration loss of suspension decreased from 10 mL to 6.5 mL, the apparent viscosity and plastic viscosity have little change before and after aging at high temperature, which indicated that the stability of the suspension has been strengthened. These data can show that F-1 does not only improve the apparent and plastic viscosity of the bentonite suspension at room temperature as well as high temperature, but also reduce the filtration loss of bentonite suspension. As a result, the fluidity of the suspension has been improved and the cutting carrying capacity has been greatly improved.
3.6.3 Evaluation of on-site well slurry
Field well-site drilling mud slurry test: well Hongtai 23-P12-2 in CNPC Dagang Oilfield, well depth 2614 m, drilling fluid density 1.36 g/cm3, sand content 0.3%, bentonite content 50.05 g/L, friction coefficient Kf 3°/0.0524. The test results are shown in Table 8.
Rheological properties
Samples
Well-site Mud
F-1
LU-99
AV (mPa·s)
28
26
28
PV (mPa·s)
14
13
12
PY (Pa)
14
11
15
Gel (mPa·s)
9/14
10/15
11/14
FL (mL)
13.2
10.4
9.6
α (°)
3.0
1.5
1.5
From the results of the well-site drilling mud slurry test in Table 8, we can observe that F-1 has a little effect on the rheological performances after it is added to the well-site slurry, it has good lubricating properties because of its α of mud cake is only 1.5°, and is an environment-friendly compound lubricant with higher price competitiveness suitable for the water-based drilling fluids system.
3.7 Biotoxicity
The biological toxicity of the lubricant F-1 was evaluated according to the biological toxicity test method. The results showed that the EC50 value of the lubricant F-1 was 7.5 × 104 mg/L, which was much higher than the standard requirement of 2.5 × 104 mg/L. Therefore, the lubricant F-1 can be considered as non-biotoxic and environmentally friendly.
4 Conclusion
By exploring the modification schemes of biological acidified oil vulcanization and esterification, the optimized vulcanization process entails: adding 1% sulfur powder and reacting at 130 °C for 2 h; the optimized esterification process entails: adding 20% glycerol and 1% H2SO4 and reacting at 220 °C for 3 h.
The extreme pressure element sulphur (S) has improved the stability and extreme pressure anti-wear properties of AO. The esterification modification reduces the foaming rate of AO in the base slurry and improves the lubricating properties of AO.
For the advanced compound drilling lubricant F-1, its Δf is 86.84% in fresh water slurry, after aging at 150 ℃ for 16 h is 88.03%. In terms of high salt contamination resistances, its Δf is 85.76% in 4% NaCl base slurry, which also still reach 75.19% in 36% NaCl base slurry. In general, the compound lubricant has excellent temperature resistance and high salt contamination resistance.
When lubricant F-1 is added to the basic mud system (Mud-1), the fluid loss (FL) of the drilling fluids decreased from 10 mL to 6.5 mL, and the apparent viscosity (AV) and plastic viscosity (PV) changed a little before and after aging. Therefore, F-1 can improve the fluidity of drilling fluid, and can also increase its sand-carrying capacity.
The new lubricant is not only eco-friendly and non-biologically toxic, but also higher cost-effective compared with the common lubricants in-used in the petroleum drilling field in China.
CRediT authorship contribution statement
Fengshan Zhou participated in the design of this study. Cunfa Ma carried out the study, collected important background information and drafted the manuscript. Ruo Wen, Hongxing Zhao, Xincheng Bao, Wenjun Long, Zhongjin Wei and Amutenya Evelina carried out the concepts, design, definition of intellectual content, literature search, data acquisition, data analysis and manuscript preparation. Cunfa Ma, Ruo Wen, Fengshan Zhou, Hong xing Zhao, Xincheng Bao, Amutenya Evelina, Wenjun Long, Zhongjin Wei, Liang Ma, Jinliang Liu and Sinan Chen read and approved the final manuscript. All authors should have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for intellectual content, (3) final approval of the version to be submitted. All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Acknowledgments
Funding: This work was supported by the Fundamental Research Funds for the Central Universities (No. 2-9-2019-141)
Declaration of Competing Interest
The authors declare no conflicts of interest regarding this article.
References
- Using agro-waste materials as possible filter loss control agents in drilling muds: a review. J. Pet. Sci. Eng.. 2018;163:185-198.
- [CrossRef] [Google Scholar]
- Long, W., Leo, H., Yan, Z., Zhang, C., Cha, R., 2020. Synthesis of filtrate reducer from biogas residue and its application in drilling fluid. Tappi J. 19(3), 151–158. https://doi.org/10.32964/TJ19.3.153.
- Insights into application of acorn shell powder in drilling fluid as environmentally friendly additive: filtration and rheology. Int. J. Environ. Sci. Technol.. 2020;18(4):835-848.
- [CrossRef] [Google Scholar]
- Al-Hameedi, A. T. T., Alkinani, H. H., Dunn-Norman, S., Alashwak, N. A., Alsaba, M. T., 2019. Environmentally friendly drilling fluid additives: can food waste products be used as thinners and fluid loss control agents for drilling fluid, SPE Symposium: Asia pacific health, safety, security, environment and social responsibility. One Petro. https://doi.org/10.2118/195410-ms.
- Application of sustainable saffron purple petals as an eco-friendly green additive for drilling fluids: A rheological, filtration, morphological, and corrosion inhibition study. J. Mol. Liq.. 2020;315
- [CrossRef] [Google Scholar]
- A natural dye in water-based drilling fluids: Swelling inhibitive characteristic and side effects. Pet.. 2017;3(3):355-366.
- [CrossRef] [Google Scholar]
- Davoodi, S., Ramazani S.A, A., Jamshidi, S., Fellah J. A., 2018. A novel field applicable mud formula with enhanced fluid loss properties in High Pressure-High Temperature well condition containing pistachio shell powder. J. Pet. Sci. Eng. 162, 378-385.https://doi.org/10.1016/j.petrol.2017.12.059.
- Mesoscopic rheological modeling of drilling fluids: Effects of the electrolyte. J. Pet. Sci. Eng.. 2020;195
- [CrossRef] [Google Scholar]
- Effect of nitric acid on the low fluorescing performance of drilling fluid lubricant-based animal and vegetable oils. J. Spectrosc.. 2013;1–7
- [CrossRef] [Google Scholar]
- A review of bio-lubricants in drilling fluids: Recent research, performance, and applications. J. Pet. Sci. Eng.. 2015;135:177-184.
- [CrossRef] [Google Scholar]
- The effect of cathodic currents on friction and stuck pipe. J. Pet. Sci. Eng.. 1993;10(2):75-82.
- [CrossRef] [Google Scholar]
- Evaluation of liquid phase oxidation products of ester and mineral oil lubricants. Ind. Eng. Chem. Prod. R&D.. 1984;23(4):613-619.
- [CrossRef] [Google Scholar]
- Oleochemical esters – environmentally compatible raw materials for oils and lubricants from renewable resources. Lipid/Fett.. 1999;101(6):192-198.
- [CrossRef] [Google Scholar]
- Lubricants based on renewable resources-an environmentally. Chemosphere.. 2001;43(1):89-98.
- [CrossRef] [Google Scholar]
- Amanullah, M., Ramasamy, J., Alouhali, R., Aramco S., 2020. HSE-friendly lubricants to safeguard environment and enhance operational excellence. Int. Pet. Technol. Conf. https://doi.org/10.2523/IPTC-19926-MS.
- Friction, wear and corrosion behavior of environmentally-friendly fatty acid ionic liquids. Coat.. 2020;11(1)
- [CrossRef] [Google Scholar]
- Novel environmentally friendly lubricants for drilling fluids applied in shale formation. Energy Fuels.. 2021;35(9):8153-8162.
- [CrossRef] [Google Scholar]
- Vegetable oil-based lubricants—a review of oxidation. Tribol. Int.. 2007;40(7):1035-1046.
- [CrossRef] [Google Scholar]
- Liu, Y., Qiu, Z., Zhong, H., Meng M., 2019. Development of a novel anti-temperature, anti-wear and ecofriendly lubricant SDL-1 for water-based drilling fluid. Int. Pet. Technol. Conf. Beijing, China. https://doi.org/10.2523/IPTC-19406-MS.
- Rheological characterization of sepiolite-vegetable oil suspensions at high pressures. Appl. Clay Sci.. 2021;212
- [CrossRef] [Google Scholar]
- Vegetable oil-based nanofluid minimum quantity lubrication turning: Academic review and perspectives. J. Manuf. Processes.. 2020;59:76-97.
- [CrossRef] [Google Scholar]
- Effects of physicochemical properties of different base oils on friction coefficient and surface roughness in MQL Milling AISI 1045. Int. J. Precis. Eng. Manuf. Technol.. 2021;8(6):1629-1647.
- [CrossRef] [Google Scholar]
- Synthesis of a novel environment-friendly filtration reducer and its application in water-based drilling fluids. Colloids Surf. A.. 2019;568:284-293.
- [CrossRef] [Google Scholar]
- Solid catalysts for the synthesis of fatty esters of glycerol, polyglycerols and sorbitol from renewable resources. Top. Catal.. 2004;27(1):105-117.
- [CrossRef] [Google Scholar]
- Surfactants from biomass: a two-step cascade reaction for the synthesis of sorbitol fatty acid esters using solid acid catalysts. ChemSusChem: Chem. Sustainability Energy Mater.. 2008;1(1-2):85-90.
- [CrossRef] [Google Scholar]
- Modification and application of a plant gum as eco-friendly drilling fluid additive. Iran. J. Chem. Chem. Eng.. 2015;34(2):102-108.
- [Google Scholar]
- Modification of hyperbranched epoxy by vegetable oil-based highly branched polyester resin. Polym. Bull.. 2012;68(9):2299-2312.
- [CrossRef] [Google Scholar]
- Turning waste drilling fluids into a new, sustainable soil resources for landscaping. Ecol. Eng.. 2018;121:130-136.
- [CrossRef] [Google Scholar]
- A novel environmentally friendly lubricant for water-based drilling fluids as a new application of biodiesel. J. Pet. Explor. Prod. Technol.. 2016;6(3):505-517.
- [CrossRef] [Google Scholar]
- Q/SY 17088-2016. Liquid lubricants for drilling fluids. China National Petroleum Corporation. Effective on August 1,2016.
- GB/T 20973-2020. Bentonite. National Standards of the People's Republic of China, Standards Press of China, Effective on April 1,2021.
- API 13B-1-2009, Recommended practice for field testing water-based drilling fluids, 4th Edition,2009.
- The principles and technology of drilling fluids. Beijing China: Petroleum industry press; 2016.
- Q/SY 111-2007, 2007. Biotoxicity classification and detection methods of oilfield chemicals and drilling fluids: luminescent bacteria method.
- GB/T 3585, 2006. Petroleum products-determination of pour point. China National Standardization Committee, effective October 1, 2006.
- GB 5009.229-2016, 2016. National food safety standard-determination of acid value in food. National Standards of the People's Republic of China.
- Heterogeneous esterification of oil with high amount of free fatty acids. Fuel. 2007;86(5–6):906-910.
- [CrossRef] [Google Scholar]
- Heterogeneously catalyzed esterification of FFAs in vegetable oils. Chem. Eng. Technol.. 2006;29(11):1365-1371.
- [CrossRef] [Google Scholar]







