Translate this page into:
Preparation and characterization of dicarboxylic acid modified starch-clay composites as carriers for pesticide delivery
⁎Corresponding author. iamshakil@gmail.com (Najam A. Shakil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Modified starch based composites were prepared with bentonite/organobentonite for their potential use as carriers for pesticide delivery. Soluble starch was esterified with dicarboxylic acid chlorides. Mean degree of substitution of modified starches varied from 0.101 to 0.353. Composites were prepared by solution intercalation method using these esterified starches and bentonite or cetyltrimethylammonium bentonite as filler materials. TEM analysis of composites showed well exfoliated/intercalated structures. Atrazine as a model pesticide was encapsulated in them and encapsulation was found to be 83.52–92.83%. These composites were also found to be acted as controlled release matrices for atrazine and release pattern was diffusion controlled. As compared to the commercially available wettable powder formulation, the composites were able to release encapsulated atrazine in water for a longer period. The research showed that the prepared composites could be further investigated as carriers for controlled release formulations of pesticides to minimize dose and number of applications.
Keywords
Polymer-clay composites
Esterified starch
Controlled release formulation
Atrazine
Organobentonites
Release kinetics
1 Introduction
For the last few decades, polymeric composites are being widely explored for their unique properties over the simple polymers. Reinforcement of neat polymers with specific filler materials has made them suitable for various need based applications (Ray and Okamoto, 2003; Hussain et al., 2006; Borsacchi et al., 2013). Successful preparation of customized composites depends on better interaction between polymers and filler materials. Thus, choice of polymer as well as the filler is very important for preparation of a composite for defined purpose. Clay minerals, the naturally occurring phyllosilicates, have been used widely as filler materials for preparation of such composites (Chung et al., 2010; Giannakas et al., 2014; Hanif et al., 2016). Properties like expandable inter-layers, high aspect ratio and high surface area have made bentonite as the most suitable clay for this purpose (Cyras et al., 2008). A general way of preparing the clay-polymer composites is by intercalation of polymer through the clay interlayers. However, due to inherent hydrophilic nature of the clay and limited scope to separate the aluminosilicate layers, intercalation becomes difficult for different type of polymers, especially the hydrophobic ones (Borsacchi et al., 2013; Zhu et al., 2019). In order to generate intercalated structure and hydrophobic nature, clays are modified by organic cations for improved intercalation of polymers between interlayer spaces (Sharif et al., 2019). Long chain quaternary ammonium cations are widely used for modification of bentonite to prepare organoclays with desired properties (Favre and Lagaly, 1991; Dutta and Singh, 2015).
For mutual compatibility with organoclays, polymer should be hydrophobic in nature or either of them should be functionalized (Borsacchi et al., 2013). Starch is a naturally occurring, nontoxic biopolymer that exists in higher plants as food reserve in seeds, roots, tubers, piths and fruits. It has numerous applications, like in food packaging, due to ability for film formation, elasticity and gas barrier properties (Arvanitoyannis et al., 1998; Liu and Han, 2005). The properties of starch have been altered through modifications by various means such as physical (Le Thanh-Blicharz et al., 2012) chemical (Namazi et al., 2011) and enzymatic (van der Maarel and Leemhuis, 2013) methods. Starch contains three hydroxyl groups in each glucose unit on C-2, C-3, and C-6 carbon which can be substituted by esterification reaction and hence maximum degree of substitution for starch is three. Grafted modified starch has been synthesized by reacting with various organic reagents such as acid anhydrides (Bartz et al., 2015; Bhosale and Singhal, 2006), acids and acid chlorides (Aburto et al., 1999; Namazi and Dadkhah, 2010), vinyl laurate or vinyl stearate (Junistia et al., 2008), epichlorohydrin (Ačkar et al., 2010), hexamethylene diisocyanate (Wilpiszewska and Spychaj, 2007), benzyl chloride (Olu-Owolabi et al., 2014), alkyl and alkenyl ketene dimers (Qiao et al., 2006) and carboxylic acid imidazolides (Neumann et al., 2002) etc. Earlier attempts were made to make dicarboxylic acid complexes or cross-linked starch with short chain dicarboxylic acids such as oxalic, malonic, succinic, glutamic, adipic, azelaic acid in water medium in presence of catalyst like acetic anhydride. It was observed that dicarboxylic acids form surface complexes and inclusions but reaction was found to be time consuming (Tomasik et al., 1995; John and Raja, 1999; Šubarić et al., 2014). Starch esterification reactions were performed in various solvents likes pyridine, dimethyl acetamide (Sagar and Merrill,1995; Kapusniak and Siemion, 2007), toluene (Thiebaud et al., 1997), dimethyl sulphoxide (Junistia et al., 2008), dimethyl formamide (Rajan et al., 2008), water (Namazi and Dadkhah, 2010), super critical carbon dioxide (Muljana et al., 2010), ionic liquids (Biswas et al., 2008) and ionic liquid-organic solvent mixtures (Desalegn et al., 2014). Available literatures suggest that either organic solvents or ionic liquids were primarily used as a medium for modification of starch. However, both suffer from drawbacks as organic solvents are toxic and non-environment friendly whereas, ionic liquids, though safe and environmentally benign, are not economical. In addition, these methods were either time consuming or required high reaction temperature. In present study, starch has been esterified with dicarboxylic acid chlorides in water medium that is environmentally benign, to alter its hydrophilic property.
One of the most trending applications of clay-polymer composites is to use them as carriers for controlled release formulations of pesticides (Gerstl et al., 1998; Li et al., 2009; Singh et al., 2009; Wilpiszewska et al., 2016). The polymers act as reservoir for the pesticides, whereas clays as filler materials control the release of pesticides from the matrix. The advantages of using such composites are to minimize the dose of pesticides, prolong persistence at the required rate, reduce the load of pesticides on the environment and thereby to decrease environmental pollutions (Radian and Mishael, 2008; Li et al., 2012). Although the preparation of polymer-clay composites as carriers for pesticide delivery was reported in many literatures, to the best of our knowledge the potential of dicarboxylic acid modified starch-organobentonite composite as carrier for pesticide has not been reported yet. Recently, few researchers have tried to develop novel controlled release formulation of atrazine by employing polymer composites (de Oliveira et al., 2015; Oliveira et al., 2015; Taverna et al., 2018; de Andrade et al., 2019) to reduce its impact on the environment. However, there is still a need to optimize the preparation of a customized matrix for controlled release of the pesticide.
The objective of this research was therefore to prepare and characterize dicarboxylic acid esterified starch and organobentonite based composites along with the normal starch and bentonite based composites and their application in preparation of controlled release formulation of atrazine as a model pesticide.
2 Materials and methods
2.1 Reagents and chemicals
Soluble starch (average molecular weight of 82 kDa) was procured from Thomas Baker (Chemicals) Pvt. Ltd., Mumbai, India. Sebacic acid dichloride and dodecanedioic acid dichloride were synthesized in laboratory by reacting corresponding dicarboxylic acids (each of 99% purity purchased from Sigma-Aldrich India) with thionyl chloride. Terephthaloyl chloride (99% purity) was purchased from Spectrochem Pvt. Ltd., Mumbai, India. Commercial bentonite powder was purchased from Merck India Ltd., Mumbai having cation exchange capacity (CEC) 95.75 cmol(p+) kg−1 and basal spacing 14.73 Å (reported earlier by Jain et al., 2017). Cetyltrimethylammonium bentonite (CTA-ben), prepared (with cetyltrimethylammonium bromide (CTAB) equivalent to CEC of bentonite) in laboratory by ion exchange process reported elsewhere (Dutta and Singh, 2015), had d001 value of 22.29 Å and percent exchanged cation 87.77. CTAB and HPLC grade methanol were procured from HiMedia Laboratories Pvt. Ltd., Mumbai, India. Technical grade atrazine (94.3% purity) was obtained from the Rallis India Limited, Bangalore, India.
2.2 Preparation of dicarboxylic acid modified starch
Starch modification was carried out in two steps according to reported method by Fang et al. (2004) and as adopted by Namazi and Dadkhah (2010) with some modifications. In the first step, 6.48 g starch (0.04 M) was solubilised in 0.25 N NaOH (65 mL) solution at room temperature with continuous stirring for 15–20 min. In the second step, acid dichloride, at molar ratio of 1:1 with respect to anhydroglucose unit in starch, was added drop wise and the reaction mixture was stirred on a magnetic stirrer until the product was precipitated. The mixture was neutralized to pH 7 with dilute acid (0.01 N HCl) at the completion of the reaction and the modified starch was isolated by methanol precipitation (150 mL). The product was filtered through sintered funnel and the solid was washed with methanol with continuous stirring for 1 h to remove excess acid and acid chloride, moisture etc. This step was repeated twice. The filtrates were dried in vacuum oven at 60 °C for 10–12 h, weighed and sealed in glass vials. Probable reaction scheme is shown in Fig. 1 and Supplementary 1.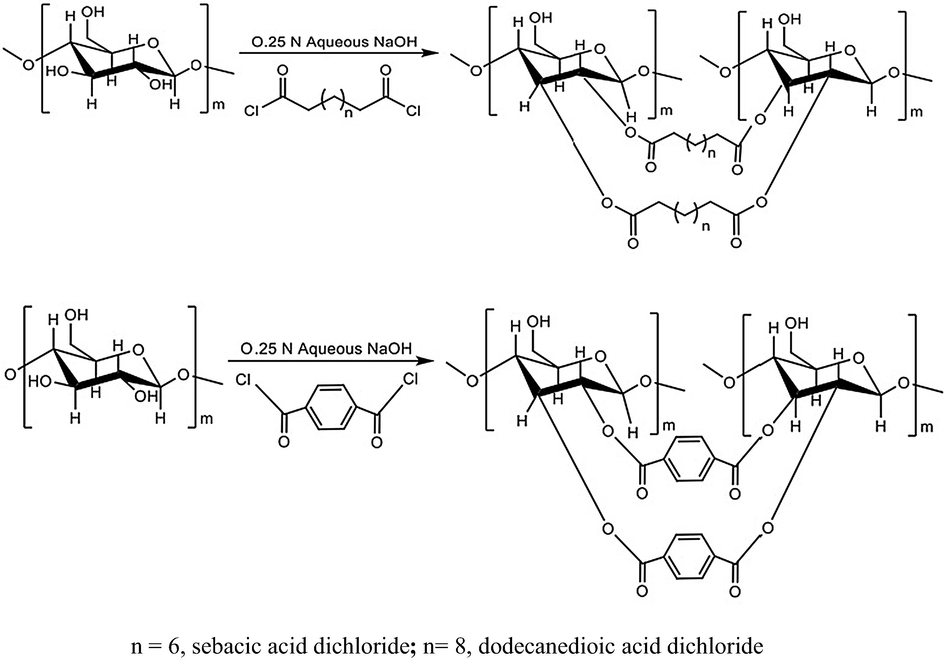
Probable reaction scheme of starch esterification with dicarboxylic acid chloride at 1:1 molar ratios with anhydroglucose unit.
2.3 Characterization of modified starch
Normal starch (M.W. 82 kDa) and dicarboxylic acid modified starches were characterized with Fourier Transform Infra-Red (FT-IR) spectroscopy, High Resolution X-Ray Diffraction (HR-XRD) analysis, CHNS elemental analyser and 1H NMR (Nuclear Magnetic Resonance) spectroscopy. Using FT-IR (Alpha-T, Bruker) instrument, the variability of functional groups of starch/modified starch was analyzed by making KBr pellets. Typically, 24 scans with 4 cm−1 resolutions were recorded for spectra and samples were scanned in 4000–600 cm−1 region. Powder XRD patterns for modified starch along with normal starch have been recorded using a Rigaku Smartlab X-Ray Diffractometer equipped with Automated Powder Diffraction (APD) software. The following parameters were used: Cu Kα radiation (λ = 1.54 Å); generator voltage 40 kV and current 30 mA; start angle (2θ) 3.00, end angle (2θ) 35.00; scanning rate 5° min−1. Percentage change in carbon content in modified starch with respect to normal starch was determined by elemental analysis using Vario EL CHNS Element Analyzer, GmbH, Germany with moisture trap. 1H NMR spectra were recorded on Bruker Avance II-400 MHz (Ultrashield TM) instrument. For 1H NMR spectra, starch or modified starch (10 mg) was dissolved in 0.6 mL DMSO at 70 °C. The spectra were obtained at 25 °C with a flip angle of 30°, an acquisition time of 3.98 s and no. of scans were 16. All chemical shifts were reported in δ (ppm).
2.4 Determination of degree of substitution (DS) of modified starch
Degree of substitution was determined by percent carbon content (CHNS elemental analysis) and 1H NMR by comparing peak area and number of protons. Each starch anhydroglucose unit contains three free hydroxyl groups that can be substituted, so the average degree of substitution (DS) can be between 0 and 3.0. Prior to analysis, the samples were dried at 60 °C for 12 h.
2.4.1 Degree of substitution (DS) determination by CHNS elemental analysis
The DS values of modified starches were calculated using equation (1) based on measured percentage C from the results of elemental analysis (Vaca-Garcia et al., 2001).
2.4.2 Degree of substitution (DS) determination by 1H NMR
DS values were also obtained from the ratio of the total peak area of the hydroxyl group proton of anhydroglucose unit to proton peak of substituent. In case of substituents, for dicarboxylic acid modified starch, it was the methylene group peak adjacent to carbonyl group, whereas, for terephthaloyl chloride modified starch, it was the aromatic ring protons. Degree of substitution was calculated by using equation (2) (Namazi et al., 2011).
2.5 Preparation of normal bentonite/modified bentonite and starch/modified starch based composites
To prepare starch based composites (scheme presented in Supplemenatry 1), starch (20 g) was suspended in 100 mL deionized water and kept on oil bath at 70 °C for gelatinization with continuous stirring for 1 h. Two gram bentonite clay/CTA-ben (10% of starch) was dispersed in 100 mL of deionized water and dispersion was poured in gelatinized starch solution. Similarly, for preparation of modified starch based composites, dicarboxylic acid modified starch i.e. starch sebacate, starch dodacanedioate and starch terephthalate (20 g), was suspended individually in 100 mL deionized water with 0.4 g (2% of modified starch) cationic surfactant CTAB and kept on oil bath at 70 °C for gelatinization with continuous stirring for 1 h. CTAB was used as phase transfer catalyst. Two gram CTA-ben (10% of modified starch) was dispersed in 100 mL deionized water and dispersion was poured in gelatinized modified starch solution. For all the preparations, the whole mass was kept on oil bath at 70 °C with continuous stirring for further 6 h. Slurry was poured on Petri dish and kept at 50 °C for 36 h in a vacuum oven. Dried material was crushed and powdered using planetary ball mill (Retsch, PM-400 MA-Type), sieved with 100 BSS sieve.
2.6 Characterization of starch-clay composites
Starch-bentonite, starch-CTA-ben, modified starch-bentonite and modified starch-CTA-ben based composites were characterized by FT-IR spectroscopy, HR-XRD and TEM (Transmission Electron Microscopy). Functional group variability of samples was analyzed using FT-IR (Alpha-T, Brucker) by making KBr pellets as described in Section 2.3. Powder X-Ray Diffraction (XRD) patterns for composite materials were recorded using a Rigaku Smartlab High Resolution X-Ray Diffractometer equipped with Automated Powder Diffraction (APD) software as described in Section 2.3. For TEM analysis, HRTEM–FEI, 300 kV HRTEM TECHNAI G2 30S TWIN was used with, operating condition, 300 kV at room temperature. Samples were prepared by dispersing in methanol, mounted on copper grid to see the internal structure of composite material.
2.7 Encapsulation of atrazine
Solid active ingredient (a.i.) of atrazine was added to clay polymer gelatinized matrix (20% of formulation w/w) while preparing the composites. Whole mass was kept for stirring for 6 h at room temperature. Slurry with technical ingredient was poured into Petri dish and kept at 50 °C for 36 h in a vacuum oven. Dried material was crushed, powdered and sieved with 100 BSS sieve. In powdered material 10% (w/w) moisture was added, mixed with glass rod and extruded with the help of Mini Screw Extruder with aperture pore size 1 mm to prepare granular formulations. Prepared granules were dried in vacuum oven at 50 °C for 6 h and stored. To determine the amount of a.i. present in formulations (encapsulation efficiency), granular formulations of atrazine (50 mg) was crushed, dipped in acetonitrile and ultrasonicated for 10 min, which resulted in complete disintegration of the granules. The mixture was sonicated again for 10 min after keeping for 2 h at room temperature and filtered through a syringe filter (0.45 μm). Extract was diluted with acetonitrile and analysed using Varian, Prostar high performance liquid chromatography (HPLC) system equipped with quaternary pump, Rheodyne injector and UV detector using C-18 stainless steel column [LiChrospher; 250 mm × 4 mm (i.d.)]. Acetonitrile-0.1% aqueous o-phosphoric acid (70:30) mobile phase was used at a flow rate of 0.5 mL min−1 and atrazine was detected at a wavelength of 222 nm.
Encapsulation Efficiency (EE) was calculated by using the equation (3) (Kumar et al., 2010).
2.8 Release study of atrazine from developed and commercial formulations
According to the method adopted by Choudhary et al. (2006), the release study of atrazine from developed clay polymer composites and the commercial formulation in water was performed in laboratory with slight modification. An accurately weighed quantity of developed formulations and commercial formulation (Atrazine 50 WP) containing atrazine (2.5 mg a.i.) was packed individually in empty tea bags and dipped in stoppered conical flasks containing 50 mL double distilled water. The study was conducted in triplicates. All the conical flasks were placed in BOD (biological oxygen demand) incubator at 28 ± 2 °C. At specific intervals, aliquots (2 mL) were drawn from each conical flask using micropipette and replaced by the same volume of fresh double distilled water. Samples were drawn for atrazine analysis at intervals of 0, 1, 3, 7, 10, 14, 21, 28, 35, 42, 49 and 60 days. Aqueous samples were directly analysed by HPLC after filtering through a syringe filter of 0.45 µm.
2.9 Prediction of period of optimum availability (POA) for atrazine
The optimum availability of atrazine from developed as well as commercial formulations was predicted by fitting the release data in the quadratic equation (4):
2.10 Release kinetics
In order to determine the release kinetics parameters, the release study data at different time interval were fitted to Korsmeyer-Peppas model (Ritger and Peppas, 1987) (Equation (5)).
Diffusion exponent (n) is the indicative of the transport mechanism. By taking logarithm on both sides of equation (5), the model was transformed as:
In order to calculate the time taken for release of 50% of initial atrazine (t1/2) in water from developed formulations together with commercial formulation equation (5) was converted as follows:
3 Results and discussion
3.1 Characterization of modified starch
FT-IR spectra of starch (NS) and starch modified with dicarboxylic acid chlorides namely, starch sebacate (NSS), starch dodecanedioate (NSD) and starch terephthalate (NST) are shown in Fig. 2. The characteristic FT-IR spectrum of starch showed a broad band between 3350 and 3450 cm−1 due to stretching vibrations of —OH group of starch, 2922 and 2855 cm−1 due to asymmetric and symmetric stretching vibrations of methylene group associated to starch. The bands at 1650, 1462 and 1369 cm−1 were the bending vibration due to OH group of starch and methylene group (symmetric and asymmetric vibrations), respectively. The stretching vibration of C—O—H and C—O—C group present in the anhydroglucose unit of starch appeared at 1161 and 992 cm−1 while, the C—O—C ring vibration of anhydroglucose unit appeared at 767 cm−1. The FT-IR spectra of modified starch showed the bands of both starch and dicarboxylic acids. The carbonyl absorption band appeared at around 1738–1760 cm−1 showing the ester bond formation between starch and acid. The bands in the region of 1710 cm−1 and 3000 cm−1 were not found which confirms the absence of physically sorbed dicarboxylic acid (Thielemans et al., 2006; Namazi and Dadkhah, 2010). The intensity of C—H stretching band at 2920–2925 cm−1 and 2850–2855 cm−1 increased after crosslinking of dicarboxylic acid into starch. In case of terephthalic acid modified starch (NST), band appeared at around 1550 cm−1 and small intensity band at 3100 cm−1 were due to the stretching and bending vibrations of C⚌H of benzene ring confirming the incorporation of terephthalic acid in starch. Esterification was further confirmed by reduced intensity of band at 3350–3450 cm−1 due to hydroxy group (Luo and Zhou, 2012). Assignment of the bands in the FT-IR spectra has been summarized in supplementary S2.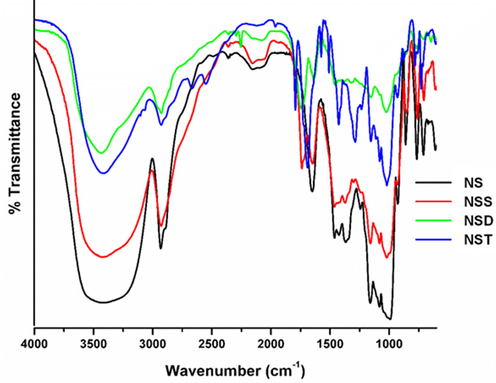
FT-IR spectra of starch (NS), starch sebacate (NSS), starch dodecanedioate (NSD) and starch terephthalate (NST).
The X-ray diffraction pattern of dicarboxylic acid modified starches (NSS, NSD, NST) and normal starch (NS) are shown in Fig. 3. Normal starch showed X-ray diffraction pattern with reflections at around 2θ = 15.3°, 22.6° with unresolved doublet between 2θ = 17.0° and 18.0°, which is a typical A-type pattern (Corradini et al., 2007). Normal starch also showed broad reflections at around 8.4° and 19.6° indicating that amylose-lipid complex was present in it (Zobel, 1988). In dicarboxylic acid modified starches XRD pattern showed new broad reflections at 2θ = 13.5° and 21.8° and disappearance of crystalline peak of A-type of starch. Ester group introduction lead to the disappearance of normal starch reflection which also depends on the degree of substitution of starch. Higher the degree of substitution, lower the intensity of normal starch (Zhang et al., 2014). The reflection intensity at around 2θ = 21.8° also depends on the chain length of substituting group. Similar kinds of results were observed by Namazi and Dadkhah (2010) while modifying the starch with long chain mono acids. In case of chemical modification of starch with terephthalic acid, A-type normal starch reflections disappeared and new reflections were observed around at 2θ = 17.8°, 25.4° and 28.1°, which suggested that new crystalline regions were formed in terephthalic acid modified starch (NST) and terephthalic acid crystallized at the surface. The crystallinity of acid modified starch depends on the degree of substitution (Lin et al., 2015). It was observed that covalently linked acid esters formed the coating around starch which provided a diffusing layer (Thielemans et al., 2006; Namazi and Dadkhah, 2010).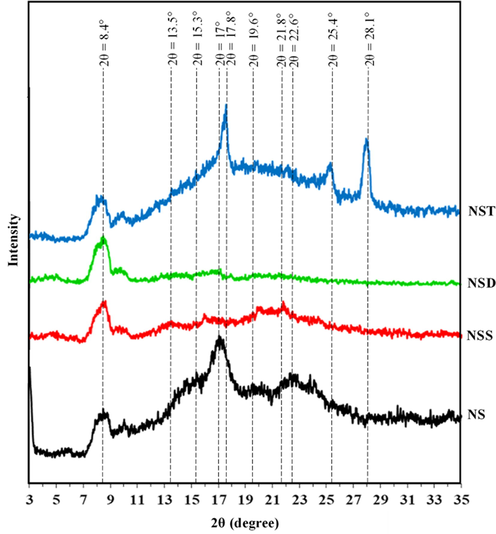
High Resolution X-Ray Diffraction pattern of starch (NS), starch sebacate (NSS), starch dodecanedioate (NSD) and starch terephthalate (NST).
1H NMR spectra of dicarboxylic acid modified starches along with normal starch are shown in Fig. 4. The broad signals ranging from 3.33 to 3.65 ppm were allocated to starch chain protons (H-2, H-3, H-4, H-5 and H-6). The signal at 3.32 ppm was due to residual water associated with DMSO‑d6 and starch (Tizzotti et al., 2011; Zhang et al., 2014). The resonance signal of the anomeric proton (H-1) corresponding to the internal α-1,4 linkages, was noted downfield at 5.10 ppm (Chi et al., 2008; Zhang et al., 2014). The NMR signals observed at 4.58, 5.40 and 5.49 ppm correspond to the three hydroxyl groups i.e. OH-6, OH-3 and OH-2, respectively (Chi et al., 2008; Zhang et al., 2014). After the esterification process, resonance signals of the anhydroglucose unit proton showed some changes (Namazi et al., 2011; Zhang et al., 2014). 1H NMR spectra of dicarboxylic acid modified starches showed new signals apart from anhydroglucose unit signals at 1.24, 1.47 and 2.19 ppm corresponding to alkyl chain of dicarboxylic acids. In case of terephthalic acid modified starch (NST), multiplet at 8.09 ppm, due to symmetrical aromatic protons was observed in addition to anhydroglucose unit protons.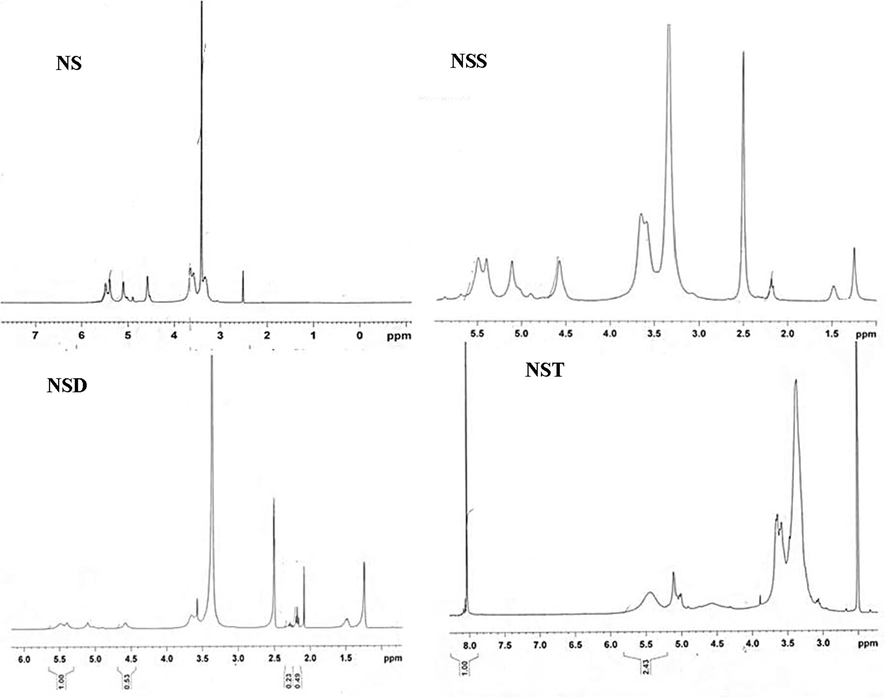
1H NMR spectra of starch (NS), starch sebacate (NSS), starch dodecanedioate (NSD), starch terephthalate (NST) in solvent DMSO‑d6.
3.2 CHNS elemental analysis
Percent carbon contents in normal starch and dicarboxylic acid modified starches are given in Table 1. Normal starch contains 44.60% carbon by weight. The carbon content was higher in all the modified starches which ranged from 45.83 to 49.87%. The higher carbon percentage in all the modified starches indicated successful esterification. Percent carbon content in modified starch depends on the chain length of the modifying reagent (Fang et al., 2004). In the present study similar observation was made as dodecanedioic acid chloride (C12) modified starch (NSD) has more carbon content than sebacic acid chloride (C10) modified starch (NSS) (Table 1). However, the fact that terephthaloyl chloride (C8) modified starch (NST) had more carbon content than starch sebacate (NSS) might be because of better reaction of terephthaloyl chloride with starch as suggested by the respective degrees of substitution.
Code
Starch
Theoretical % C
Measured % C
DSEA
DSNMR
NS
Starch
46.81
44.60
–
–
NSS
Starch sebacate (1:1)
51.74
45.83
0.104
0.098
NSD
Starch dodecanedioate (1:1)
52.81
49.87
0.381
0.353
NST
Starch terephthalate (1:1)
51.09
47.98
0.397
0.308
3.3 Degree of substitution
Degree of substitution was determined on the basis of percent carbon content and 1H NMR (Table 1). The Degree of substitution value obtained from CHNS elemental analysis (DSEA) was higher than the obtained value from 1H NMR (DSNMR). This could be due to incomplete removal of by-products from the samples. Similar kind of result was obtained by Qi et al. (2012) during the calculation of degree of substitution on the basis of bromine content from elemental analysis. Therefore, DSNMR data was considered as more reliable as it also coincided with the carbon content data by elemental analysis. The results suggested the degree of substitution in the following order: starch dodecanedioate > starch terephthalate > starch sebacate.
3.4 Characterization of prepared composites
The FT-IR spectra of normal starch-bentonite (CP1) composite, normal starch-CTA-ben (CP2) composite, representative modified starch-CTA-ben composite (starch dodecanedioate-CTA-ben, CP4) and representative modified starch-bentonite composite (starch sebacate-bentonite, CP6) are presented in Fig. 5. In FT-IR, main bands of distinctive functional groups of starch and bentonite were identical. So, to distinguish them as separate bands was difficult in composites (Namazi and Mosadegh, 2011). In CP1, apart from the common bands of starch and bentonite, new bands of starch in composites were observed at 2925 cm−1 and 2850 cm−1 because of asymmetric and symmetric stretching vibrations of methylene group of starch (Gao et al., 2014), at 1460 and 1337 cm−1 due to symmetric and asymmetric bending vibrations of methylene group of starch, at 1149 cm−1 due to stretching vibrations of C—OH of starch and at 1012 cm−1 due to stretching vibrations of Si—O of bentonite. This indicated the formation of composites having bands of both starch and bentonite. In case of starch-CTA-ben composite (CP2), it was difficult to distinguish and separate bands of starch and CTA-ben. However, band at 1149 cm−1 due to stretching vibrations of C—OH of starch and 1012 cm−1 because of stretching vibrations of Si—O of CTA-ben could be identified. In modified starch and bentonite/CTA-ben based composites (both CP4 and CP6), a new band of modified starch appeared at 1743 cm−1 due to carbonyl group of esterified starch along with the usual bands at 1149 cm−1 and 1012 cm−1 as described earlier. Assignment of the bands in the FT-IR spectra has been summarized in supplementary S2. The FT-IR study validated the incorporation of bentonite/CTA-ben particles in starch/modified starch matrix by solution polymer intercalation method for composites preparation. Similar results were observed by Namazi and Mosadegh, (2011) while preparing the starch/clay and starch/organoclay composites.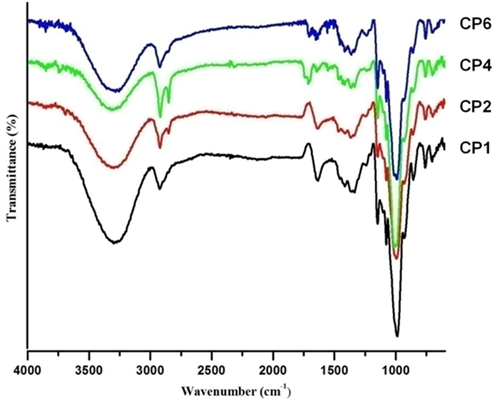
FT-IR spectra of normal starch-bentonite composite (CP1), normal starch-CTA-ben composite (CP2), starch dodecanedioate-CTA-ben composite (CP4) and starch sebacate-bentonite composite (CP6).
High Resolution-XRD pattern of modified starch-CTA-ben composites (starch sebacate-CTA-ben, CP3; starch dodecanedioate-CTA-ben, CP4; starch terephthalate-CTA-ben, CP5) along with starch-bentonite composite (CP1), starch-CTA-ben composite (CP2) and representative modified starch-bentonite composite (starch sebacate-bentonite, CP6) are presented in Fig. 6. X-ray diffraction pattern revealed the expansion of interlayer spacing of bentonite/organobentonite (CTA-ben) due to intercalation of starch/modified starch into them. XRD pattern of normal starch-bentonite composite (CP1) showed a broad reflection with reflection maxima at 2θ = 3.40°, d001 value of 25.96 Å while normal bentonite had distinctive reflection at 2θ = 6.02°, d001 value of 14.73 Å which revealed that the basal spacing of bentonite had increased. XRD pattern of normal starch-CTA-ben composite (CP2) showed a broad reflection with reflection maxima at 2θ = 3.32°, and d001 value of 27.41 Å while CTA-ben had distinctive reflection at 2θ = 3.96°, and d001 value of 22.29 Å which revealed that the basal spacing of CTA-ben layer has also increased but expansion was less as compared to normal starch-bentonite composite and had broader reflection. In case of starch sebacate-bentonite composite (CP6), the reflection appeared at around 2θ = 3.12°, while d001 value of 28.28 Å indicating thereby that bentonite basal spacing has increased. However, in XRD pattern of starch sebacate-CTA-ben and starch dodecadioate-CTA-ben composite (CP3 and CP4), no reflection was observed at or around 2θ = 3° indicated well expansion of silicate layers which might resulted in complete exfoliation (Park et al., 2006; Chen et al., 2008). These observations confirmed the intercalation of bentonite/CTA-ben by starch/modified starch in the interlayer space of bentonite on the basis of expansion of d001 value. In all the clay polymer composites, basal spacing increased due to intercalation of polymer. In case of normal starch-CTA-ben composite (CP2), the layer spacing did not increase like that of modified starch-CTA-ben composites (CP3, CP4, CP5), because less polymer was intercalated in the interlayer space of organobentonite owing to phase dissimilarity. Though in case of modified starch-bentonite composites (CP6), intercalation of polymer was higher despite having phase dissimilarity. This might be due to the amphiphilic nature of modified starch and surfactant used for gelatinization of starch also acted as compatibilizer for bentonite.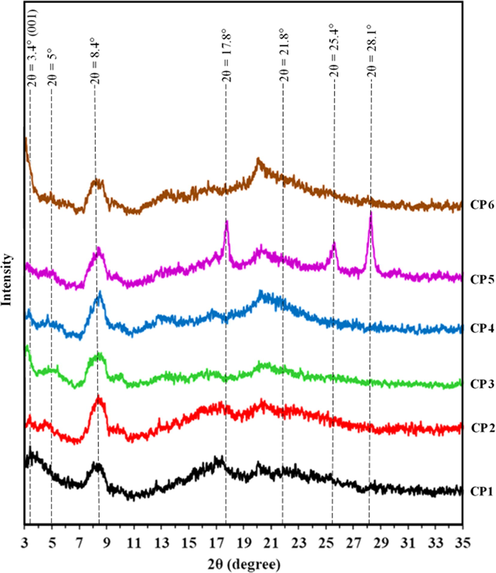
High Resolution X-Ray Diffraction patterns of starch-bentonite composite (CP1), starch-CTA-ben composite (CP2), starch sebacate-CTA-ben composites (CP3), starch dodecanedioate-CTA-ben composite (CP4), starch terephthalate-CTA-ben composite (CP5) and starch sebacate-bentonite composite (CP6).
Generally, polymer enters the clay interlayer space and, owing to intercalation, forces the layers apart. As compared to Fig. 3, it was observed in Fig. 6 that the intensity of reflections of starch (NS) at 2θ = 17.0°, 18.0° and 22.6° decreased while reflection at 2θ = 15.3° disappeared when composites formed (CP1 and CP2). Similarly, the reflection of modified starch at around 2θ = 21.8° remained as such but intensity decreased in composites (CP3-CP6). In terephthalic acid modified starch-CTA-ben composite (CP5), there was no reflection of organobentonite at 2θ = 3° or above, but reflections of terephthalic acid modified starch (NST) remained at 2θ = 17.8°, 25.4° and 28.1° with reduced intensity. The reduced reflection intensity of normal starch and modified starches was attributed due to loss of crystallinity. The reduction in crystallinity was because of the dispersion of clay platelets and gelatinization of starch (Tang et al., 2008; Baishya and Maji, 2016). In all modified starch based composites, reflection at around 2θ = 4.84–5.00° was due to complex formation of cetyltrimethylammonium bromide surfactant with modified starch, which was added to gelatinize modified starch in water medium (Bordbar et al., 2005; Hossain et al., 2014).
3.5 Characterization by transmission electron microscopy
TEM analysis was conducted to confirm the structure of composites. TEM micrographs of composites namely, starch-bentonite (CP1), starch-CTA-ben (CP2), dicarboxylic acid modified starch-CTA-ben (CP3, CP4, CP5) and representative dicarboxylic acid modified starch-bentonite composite (starch sebacate-bentonite, CP6) were shown in Fig. 7. The micrographs represented pictures of layered structure silicates in composites. Starch-bentonite composite (CP1) displayed multi-layered structures which appeared as dark parallel lines, while the composite of starch-CTA-ben (CP2) had less polymer intercalated morphology and more of particle agglomeration (as distinct stacks of clay agglomerates were observed as dark bands). Modified starch-bentonite composite (CP6) also showed intercalated morphology but with particle aggregation due to phase dissimilarity. On the other hand, modified starch-CTA-ben composites (CP3-CP5) showed the fully intercalated and laminar structure because of high amount of intercalation of polymer. The dark lines observed for CP3-CP5 had morphological dissimilarities in appearance from CP1. On close observation, the dark lines were appeared to be more separated than that of CP1 suggested polymer intercalation within clay interlayers. This observation reinforced the conclusion drawn from XRD analysis as the d001 peak of bentonite was found to be less intent in case of CP3-CP5. For the formation of homogeneous intercalated or exfoliated composites, compatibility and optimum interactions between starch matrixes and organic bentonite was crucial (Tang et al., 2008). In the present study, the modified starch and bentonite based composite (CP6) showed little homogeneous condition but the dicarboxylic acid modified starch and organobentonite composites (CP3-CP5) were compatible in nature and having homogeneous condition. On the basis of above results, it can be said that the prepared composites with solution intercalation method had both intercalated and exfoliated structures.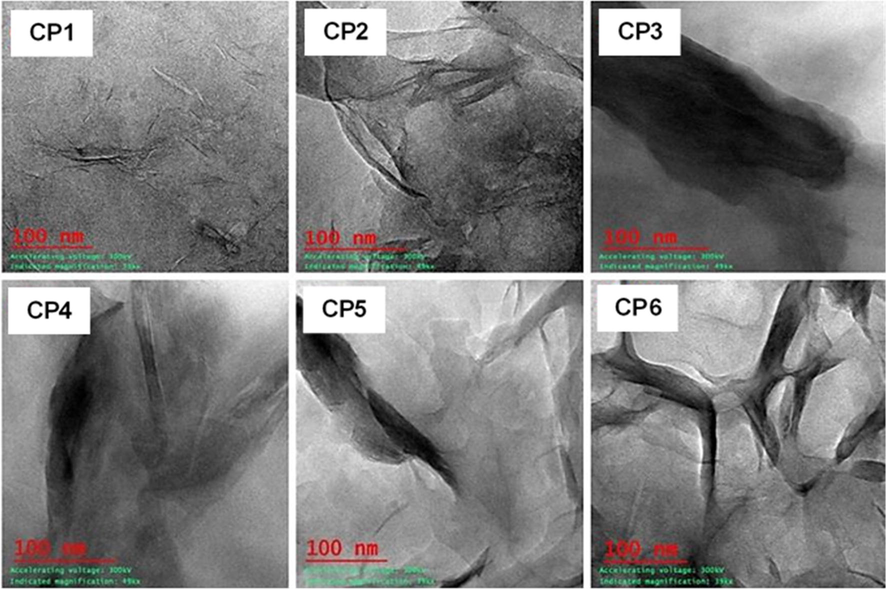
TEM micrographs of starch-bentonite composite (CP1), starch-CTA-ben composite (CP2), starch sebacate-CTA-ben composites (CP3), starch dodecanedioate-CTA-ben composite (CP4), starch terephthalate-CTA-ben composite (CP5) and starch sebacate-bentonite composite (CP6).
3.6 Encapsulation efficiency
Starch-bentonite composite and modified starch-CTA-ben composites were further used to encapsulate atrazine as model molecule. Encapsulation efficiency of all the developed formulations was in the range of 29.06 to 92.83% (Table 2). The order of encapsulation was as follows: SCGA4 > SCGA3 > SCGA2 > SCGA1. Results showed that encapsulation efficiency vary with clay polymer composite matrix composition. Normal starch-bentonite composite (SCGA1) encapsulated least amount of added atrazine because of the hydrophilic nature of the matrix. Whereas, all of the modified starch-CTA-ben composites encapsulated considerably higher amount of atrazine. This was probably because of hydrophobic interaction of atrazine with the composites. As hydrophobicity of the matrix depends on the DS of starch, chain length and distribution of organo-modifier in matrix, encapsulation efficiency varied among the composites.
Formulation
Polymer-clay composite matrix
Encapsulation Efficiency (%)
K
n
R2
POA (days)
t1/2 (days)
SCGA1
Starch-bentonite
29.06
0.280
0.458
0.997
33.4
3.54
SCGA2
starch terephthalate-CTA-ben
83.52
0.247
0.311
0.990
39.8
9.63
SCGA3
starch sebacate-CTA-ben
88.03
0.244
0.396
0.991
35.9
6.14
SCGA4
starch dodecanedioate-CTA-ben
92.83
0.139
0.475
0.978
46.3
14.87
Atrazine 50 WP
–
–
0.462
0.246
0.992
29.7
1.38
3.7 Release study and release kinetics
Fig. 8 represents the cumulative release of atrazine from the developed formulations as well as commercial WP formulation as expressed by Mt/M∞ over time, where Mt indicates the amount of a.i. released at a given time t, and M∞ indicates the theoretical amount of a.i. loaded in the respective formulations. Commercial atrazine 50 WP formulation showed an initial burst release and almost 90% of the available a.i. was found to be released within 14 days of incubation. After 14th day no significant release of a.i. was observed as the release curve achieved a plateau. Similar trend was observed in the case of formulation prepared with normal starch-bentonite composite (SCGA1). After an initial burst release up to 10 days, slow but steady release of a.i. from SCGA1 was observed till 21 days followed by a plateau. However, in case of formulations prepared by modified starch-organobentonite composites, the scenario is completely different. Instead of initial burst release of a.i., the release curves of SCGA2, SCGA3 and SCGA4 showed a slow monotonically increasing pattern that reached the saturation point after 35, 28 and 50 days, respectively. In all the cases the saturation point arrived at 85–90% release of the total loaded a.i. Clearly, modified starch-organobentonite composite based formulations released atrazine in a more controlled pattern than commercial formulation as release pattern is dependent on type of polymer used in composites (Boddeda et al., 2012) and bentonite provides tortuosity due to disruption of the polymer pore network in the presence of clay (Rashidzadeh et al., 2014). However, use of only starch and bentonite in composite matrix may not regulate the release of pesticides (Giroto et al., 2014). Therefore, organomodification of bentonite is a common method to control the release behaviour (Undabeytia et al., 2000). In the present study, starch-bentonite composite based formulation (SCGA1) released atrazine faster because of hydrophilic nature of both bentonite and starch (Giroto et al., 2014). However, in case of the modified starch and organobentonite based composite formulations, various factors were involved in slow release of atrazine viz. tortuosity, high retention capacity of atrazine by organobentonite and hydrophobic nature of modified starch. The formulation SCGA4 has comparatively slower release due to high DS in modified starch and long chain of modifying reagent.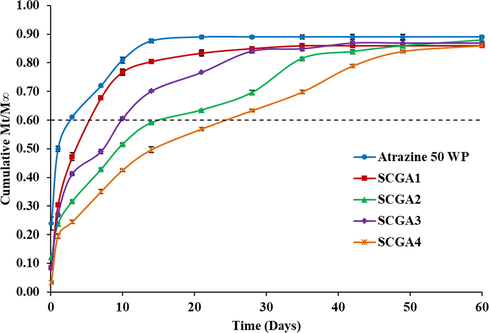
Rate of release of atrazine in water from composite based granular controlled release formulations.
To understand the release mechanism in a better way, the data were fitted to the Korsmeyer-Peppas model (Kadam et al., 2019). However, as the model is valid only for Mt/M∞ < 0.6 (Scaffaro et al., 2019), only for the early portion of the release curves the model is applicable. Therefore, the experimental data were fitted to equation (6) and splitted only up to the time interval where satisfactory R2 values were obtained. The release parameters namely, diffusion exponent (n) and rate constant (K) for all the formulations were calculated by analysing the slope and intercept of the fitted lines (Scaffaro et al., 2020). The n values ranged between 0.31 and 0.47 (Table 2) for all developed controlled release formulations. The n value for commercial formulation was found to be 0.25. According to Ritger and Peppas (1987), if n values are approximated to <0.5, the release mechanism may be Fickian. If n approaches 1, the release mechanism may be zero order and if 0.5 < n < 1, non-Fickian (anomalous) transport could be obtained. In the present study, all the n values were below 0.5 for all the developed formulations indicating that Fickian diffusion was involved in the release of atrazine. The rate constant (K) for all the developed formulations were low and varied from 0.139 to 0.280 but was high (0.462) for commercial WP formulation. Thus, the calculated t1/2 value for the commercial WP formulation was as low as 1.38 days, whereas, for the prepared controlled release formulations t1/2 values were found to be much higher (Table 2).
The period of optimum availability (POA) of atrazine from formulations in water was given in Table 2. It clearly showed that POA values were higher in case of the prepared formulations as compared to the commercial WP formulation. SCGA4 showed highest POA (46.3 days) among the prepared formulation, which implied that SCGA4 could encapsulate atrazine for a longer period of time.
Among the prepared formulations, SCGA4 showed highest t1/2 value (14.87 days) and POA. The release pattern of a.i. from SCGA4 was also found to be more controlled (K = 0.139) than the other formulations. This suggested that dodecanedioic acid modified starch retained atrazine for longer period than the other modified starch. The long chain of the dicarboxylic acid and higher degree of substitution made it a preferable candidate over others for atrazine retention. In general, CTA-ben was found to be suitable as filler material for modified starch based composites to retain more atrazine due to its hydrophobicity and compatibility.
4 Conclusion
In the present study, an environmentally benign method was developed for the preparation of modified starches by reacting with dicarboxylic acid chlorides viz. sebacic acid chloride, dodecanedioic acid chloride and terephthalic chloride in water. Prepared dicarboxylic acid esterified starches were characterized by CHNS elemental analysis, FT-IR, 1H NMR and High Resolution X-ray diffraction techniques. Degree of substitution of esterified starch was calculated on the basis of percent carbon content and 1H NMR. Both normal and modified starch were used for the preparation of composites with bentonite and organobentonite employing water as solvents. Formation of composites was confirmed by High Resolution-X-Ray Diffraction and Transmission Electron Microscopy techniques. Esterified starch and organobentonite based composites showed better expansion of inter layer spacing than normal starch and bentonite due to phase compatibility. Normal starch and organobentonite formed aggregated and less expanded structure due to incompatibility of phases and less intercalation of polymer. Similar was the case of esterified starch and normal bentonite. These prepared composites were utilized for encapsulation of atrazine and it was found that modified starch-organobentonite composites were highly effective in encapsulation of atrazine. Subsequent release study proved that those composites not only retain atrazine better, but also release the encapsulated atrazine in a controlled manner as compared to the commercially available wettable powder formulation. These kind of novel dicarboxylic acid modified starch-organobentonite composites hold a great potential to be used as carrier matrices for development of controlled release formulations of pesticides.
Acknowledgements
Financial assistance provided by the Indian Council of Agricultural Research in terms of Senior Research Fellowship is gratefully acknowledged.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Preparation of long-chain esters of starch using fatty acid chlorides in the absence of an organic solvent. Starch-Stärke. 1999;51:132-135.
- [Google Scholar]
- Isolation of starch from two wheat varieties and their modification with epichlorohydrin. Carbohydr. Polym.. 2010;81:76-82.
- [Google Scholar]
- Edible films made from hydroxypropyl starch and gelatin and plasticized by polyols and water. Carbohydr. Polym.. 1998;36(2):105-119.
- [Google Scholar]
- Studies on the physicochemical properties of modified starch-based wood nanocomposites. Starch-Stärke. 2016;68:249-257.
- [Google Scholar]
- Acetylation of barnyardgrass starch with acetic anhydride under iodine catalysis. Food Chem.. 2015;178:236-242.
- [Google Scholar]
- Process optimization for the synthesis of octenylsuccinyl derivative of waxy corn and amaranth starches. Carbohydr. Polym.. 2006;66(4):521-527.
- [Google Scholar]
- Rapid and environmentally friendly preparation of starch esters. Carbohydr. Polym.. 2008;74(1):137-141.
- [Google Scholar]
- Formulation and evaluation of glipizide sustained release tablets. Int. J. Pharm. Biomed. Res.. 2012;3(1):44-48.
- [Google Scholar]
- Study on interaction of α-amylase from Bacillus subtilis with cetyl trimethylammonium bromide. Colloids Surf. B Biointerfaces. 2005;40(1):67-71.
- [Google Scholar]
- Synthesis, characterization, and solid-state NMR investigation of organically modified bentonites and their composites with LDPE. Langmuir. 2013;29(29):9164-9172.
- [Google Scholar]
- A critical appraisal of polymer–clay nanocomposites. Chem. Soc. Rev.. 2008;37:568-594.
- [Google Scholar]
- Effect of acetylation on the properties of corn starch. Food Chem.. 2008;106(3):923-928.
- [Google Scholar]
- Controlled Release of carbofuran in water from some polymeric matrices. Pestic. Res. J.. 2006;18(1):65-69.
- [Google Scholar]
- Preparation and properties of biodegradable starch–clay nanocomposites. Carbohydr. Polym.. 2010;79(2):391-396.
- [Google Scholar]
- Preparation and characterization of thermoplastic starch/zein blends. Mater. Res.. 2007;10(3):227-231.
- [Google Scholar]
- Physical and mechanical properties of thermoplastic starch/montmorillonite nanocomposite films. Carbohydr. Polym.. 2008;73(1):55-63.
- [Google Scholar]
- Can atrazine loaded nanocapsules reduce the toxic effects of this herbicide on the fish Prochilodus lineatus? A multibiomarker approach. Sci. Total Environ.. 2019;663:548-559.
- [Google Scholar]
- Solid lipid nanoparticles co-loaded with simazine and atrazine: preparation, characterization, and evaluation of herbicidal activity. J. Agric. Food Chem.. 2015;63(2):422-432.
- [Google Scholar]
- Enzymatic synthesis of epoxy fatty acid starch ester in ionic liquid–organic solvent mixture from vernonia oil. Starch-Stärke. 2014;66(3–4):385-392.
- [Google Scholar]
- Surfactant-modified bentonite clays: preparation, characterization, and atrazine removal. Environ. Sci. Pollut. Res.. 2015;22(5):3876-3885.
- [Google Scholar]
- The chemical modification of a range of starches under aqueous reaction conditions. Carbohydr. Polym.. 2004;55(3):283-289.
- [Google Scholar]
- Organo-bentonites with quaternary alkylammonium ions. Clay Miner.. 1991;26(1):19-32.
- [Google Scholar]
- Effects of organic modification of montmorillonite on the performance of starch-based nanocomposite films. Appl. Clay Sci.. 2014;99:201-206.
- [Google Scholar]
- Controlled release of pesticides into water from clay−polymer formulations. J. Agric. Food Chem.. 1998;46(9):3803-3809.
- [Google Scholar]
- Preparation, characterization, mechanical and barrier properties investigation of chitosan–clay nanocomposites. Carbohydr. Polym.. 2014;108:103-111.
- [Google Scholar]
- Study of a nanocomposite starch–clay for slow-release of herbicides: Evidence of synergistic effects between the biodegradable matrix and exfoliated clay on herbicide release control. J. Appl. Polym. Sci.. 2014;131(23):1-9.
- [Google Scholar]
- Halloysite nanotubes as a new drug-delivery system: a review. Clay Miner.. 2016;51:469-477.
- [Google Scholar]
- Structure of starch-ionic surfactant complexes studied by ternary phase, XRD and scanning electron microscopy. Orient. J. Chem.. 2014;30(1):71-79.
- [Google Scholar]
- Review article: polymer-matrix nanocomposites, processing, manufacturing, and application: an overview. J. Compos. Mater.. 2006;40(17):1511-1575.
- [Google Scholar]
- Adsorption of pesticides and heavy metals from water on modified bentonites. Pestic. Res. J.. 2017;29(2):141-152.
- [Google Scholar]
- Properties of cassava starch–dicarboxylic acid complexes. Carbohydr. Polym.. 1999;39(2):181-186.
- [Google Scholar]
- Synthesis of higher fatty acid starch esters using vinyl laurate and stearate as reactants. Starch-Stärke. 2008;60(12):667-675.
- [Google Scholar]
- Sustained release insect repellent microcapsules using modified cellulose nanofibers (mCNF) as pickering emulsifier. Colloids Surf. A. 2019;582:123883
- [Google Scholar]
- Thermal reactions of starch with long-chain unsaturated fatty acids. Part 2. Linoleic acid. J. Food Eng.. 2007;78(1):323-332.
- [Google Scholar]
- Controlled release formulations of metribuzin: Release kinetics in water and soil. J. Environ. Sci. Heal. B. 2010;45(4):330-335.
- [Google Scholar]
- Starch modified by high-pressure homogenisation of the pastes–Some structural and physico-chemical aspects. Food Hydrocoll.. 2012;27(2):347-354.
- [Google Scholar]
- Carboxylmethylcellulose/bentonite composite gels: water sorption behavior and controlled release of herbicide. J. Appl. Polym. Sci.. 2009;112(1):261-268.
- [Google Scholar]
- Controlled release and retarded leaching of pesticides by encapsulating in carboxymethyl chitosan /bentonite composite gel. J. Environ. Sci. Heal. B. 2012;47(8):795-803.
- [Google Scholar]
- Structure and characteristics of lipase-catalyzed rosin acid starch. Food Hydrocoll.. 2015;43:352-359.
- [Google Scholar]
- Homogeneous synthesis and characterization of starch acetates in ionic liquid without catalysts. Starch-Stärke. 2012;64(1):37-44.
- [Google Scholar]
- Synthesis of fatty acid starch esters in supercritical carbon dioxide. Carbohydr. Polym.. 2010;82(2):346-354.
- [Google Scholar]
- Convenient method for preparation of hydrophobically modified starch nanocrystals with using fatty acids. Carbohydr. Polym.. 2010;79(3):731-737.
- [Google Scholar]
- Preparation and properties of starch/nanosilicate layer/polycaprolactone composites. J. Polym. Environ.. 2011;19(4):980-987.
- [Google Scholar]
- Hydrophobically modified starch using long-chain fatty acids for preparation of nanosized starch particles. Sci. Iran.. 2011;18(3):439-445.
- [Google Scholar]
- Synthesis of hydrophobic starch esters by reaction of starch with various carboxylic acid imidazolides. Starch-Stärke. 2002;54(10):449-453.
- [Google Scholar]
- Nanoencapsulation enhances the post-emergence herbicidal activity of atrazine against mustard plants. PLoS ONE. 2015;10(7):e0132971
- [Google Scholar]
- Comparison of functional properties between native and chemically modified starches from acha (Digitaria stapf) grains. Food Nutr. Sci.. 2014;5:222-230.
- [Google Scholar]
- Rheological behavior of polymer/layered silicate nanocomposites under uniaxial extensional flow. Macromol. Res.. 2006;14(3):318-323.
- [Google Scholar]
- Homogenous synthesis of 3-allyloxy-2-hydroxypropyl-cellulose in NaOH/urea aqueous system. Cellulose. 2012;19(3):925-932.
- [Google Scholar]
- Enzyme-catalyzed synthesis of hydrophobically modified starch. Carbohydr. Polym.. 2006;66(1):135-140.
- [Google Scholar]
- Characterizing and designing polycation−clay nanocomposites as a basis for imazapyr controlled release formulations. Environ. Sci. Technol.. 2008;42(5):1511-1516.
- [Google Scholar]
- Enzymatic modification of cassava starch by fungal lipase. Ind. Crops Prod.. 2008;27(1):50-59.
- [Google Scholar]
- On the encapsulation of natural pesticide using polyvinyl alcohol/alginate–montmorillonite nanocomposite for controlled release application. Polym. Eng. Sci.. 2014;54(12):2707-2714.
- [Google Scholar]
- Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog. Polym. Sci.. 2003;28(11):1539-1641.
- [Google Scholar]
- A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release. 1987;5(1):23-36.
- [Google Scholar]
- Properties of fatty-acid esters of starch. J. Appl. Polym. Sci.. 1995;58(9):1647-1656.
- [Google Scholar]
- PLA-based functionally graded laminates for tunable controlled release of carvacrol obtained by combining electrospinning with solvent casting. React. Funct. Polym.. 2020;148:104490
- [Google Scholar]
- Effect of graphene and fabrication technique on the release kinetics of carvacrol from polylactic acid. Compos. Sci. Technol.. 2019;169:60-69.
- [Google Scholar]
- Mucoadhesive micro-composites: Chitosan coated halloysite nanotubes for sustained drug delivery. Colloids Surf. B. 2019;184:110527
- [Google Scholar]
- Controlled release of the fungicide thiram from starch–alginate–clay based formulation. Appl. Clay Sci.. 2009;45:76-82.
- [Google Scholar]
- Modification of wheat starch with succinic acid/acetic anhydride and azelaic acid/acetic anhydride mixtures I. Thermophysical and pasting properties. J. Food Sci. Technol.. 2014;51(10):2616-2623.
- [Google Scholar]
- Barrier and mechanical properties of starch-clay nanocomposite films. Cereal Chem.. 2008;85(3):433-439.
- [Google Scholar]
- Microparticles based on ionic and organosolv lignins for the controlled release of atrazine. J. Hazard. Mater.. 2018;359:139-147.
- [Google Scholar]
- Properties of fatty-acid esters of starch and their blends with LDPE. J. Appl. Polym. Sci.. 1997;65(4):705-721.
- [Google Scholar]
- Starch nanocrystals with large chain surface modifications. Langmuir. 2006;22(10):4804-4810.
- [Google Scholar]
- New 1H NMR procedure for the characterization of native and modified food-grade starches. J. Agric. Food Chem.. 2011;59(13):6913-6919.
- [Google Scholar]
- Organo-clay formulations of the hydrophobic herbicide norflurazon yield reduced leaching. J. Agric. Food Chem.. 2000;48(10):4767-4773.
- [Google Scholar]
- Determination of the degree of substitution (DS) of mixed cellulose esters by elemental analysis. Cellulose. 2001;8(3):225-231.
- [Google Scholar]
- Starch modification with microbial alpha-glucanotransferase enzymes. Carbohydr. Polym.. 2013;93(1):116-121.
- [Google Scholar]
- Chemical modification of starch with hexamethylene diisocyanate derivatives. Carbohydr. Polym.. 2007;70(3):334-340.
- [Google Scholar]
- Carboxymethyl starch/montmorillonite composite microparticles: properties and controlled release of isoproturon. Carbohydr. Polym.. 2016;136:101-106.
- [Google Scholar]
- A green technology for the preparation of high fatty acid starch esters: Solid-phase synthesis of starch laurate assisted by mechanical activation with stirring ball mill as reactor. Ind. Eng. Chem. Res.. 2014;53(6):2114-2120.
- [Google Scholar]
- Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci.. 2019;169:48-66.
- [Google Scholar]
- Molecules to granules: a comprehensive starch review. Starch-Stärke. 1988;40(2):44-50.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.09.028.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







