Translate this page into:
Preparation of a novel Mixed-Ligand divalent metal complexes from solvent free Synthesized Schiff base derived from 2,6-Diaminopyridine with cinnamaldehyde and 2,2′‐Bipyridine: Characterization and antibacterial activities
⁎Corresponding author. diary.tofiq@univsul.edu.iq (Diary I. Tofiq),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The solvent-free conditions were employed to synthesise symmetrical Schiff base ligand from 2,6-diaminopyridine with cinnamaldehyde in (1 min) with a fair yield utilizing formic acid as a catalyst. Through coordination chemistry, new heteroleptic complexes of Cu(II), Co(II), Ni(II), Pt(II), Pd(II) and Zn(II) were achieved from Schiff base as a primary chelator (L1) and 2,2′‐bipyridine (2,2′-bipy) as a secondary chelator (L2). The prepared compounds have been characterized by elemental analysis, molar conductivity, magnetic susceptibility, FTIR, 1H NMR, UV–visible, mass spectrometry, and thermal gravimetric analysis, and screened in vitro for their potential as antibacterial activity by the agar well diffusion method. The metal complexes were formulated as [M (L1) (L2) (X)] Y⋅nH2O, L1 = Schiff base, L2 = 2,2′-bipy, (M = Cu(II), Co(II), Zn(II), Y = 2NO3, n = 1), (M = Ni(II), X = 2H2O, Y = 2NO3, n = 0) and (M = Pd(II) Pt(II), Y = 2Cl, n = 0). Both L1 and L2 act as a neutral bidentate ligand and coordinates via nitrogen atoms of imine and 2,2′-bipy to metal ions. The metal complexes were found to be electrolytic, with square-planar heteroleptic Cu(II), Co(II), Pt(II), and Pd(II) chelates and octahedral Ni(II) complex. As well as tetrahedral geometry, has been proposed for the complex of Zn(II). Furthermore, the biological activity study revealed that some metal chelates have excellent activity than Schiff base when tested against Gram-negative and Gram-positive strains of Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). Finally, it was found that the Zn(II) and Pd(II) complexes were more effective against both types of bacteria tested than the imine and other metal complexes.
Keywords
Mixed-ligand complexes
2,2′‐bipyridine
Transition metals
Schiff base
Biological activity
Free-solvent condition
1 Introduction
The study of transition metal ions in the coordination chemistry with various types of ligands in the fields of medicine and bioinorganic chemistry has been enhanced by the current developments.
Particularly those formed with transition metals, due to their important roles in biological processes in the human body (Omar et al., 2017; Shoukry and Al-Mhayawi, 2015). Consequently, the preparation of new Schiff bases from any primary amine and active carbonyl compounds is a vital precursor of chelates that can bond with the metal center by imine group which exhibits unique properties and novel reactivity (Yousif et al., 2013). In the past few years, in coordination chemistry azomethine has expanded a vital role like chelating ligands, due to their wide structural variability, stability under different reductive and oxidative situations, and also the Schiff base is the boundary between soft and hard Lewis bases (Anacona and Santaella, 2013; Garnovskii et al., 1993). The C═N linkage in azomethine derivatives are excellent for biological activity due to coordination to transition metal ions to form the stable complexes, numerous Schiff base have been testified to have antifungal, antibacterial, anticancer and antimalarial activities (Selvaganapathy and Raman, 2016). As a subsequent, ability to reversibly bind oxygen to an inhibitor of corrosion (Jones et al., 1979); transfer of an amino group (Dugas, 1996), chelating ability towards some toxic metals (Sawodny and Riederer, 1977) and in the hydrogenation of olefins used like a catalyst (Henrici-Olivé and Olivé, 1984), all of these many vital applications of azomethine and their metals chelates that previously have been reported. Also heteroleptic complexes in the biological field take a crucial situation in which is reported to exhibit good enzyme activation, as well as the storage and transportation of active substances through the membrane (Devi and Batra, 2015). 2,6-diaminopyridine has a symmetrical structure with three nitrogen lone pairs. Pyridine is a heterocyclic molecule that can be found in a variety of pharmaceutical products such as antimicrobial and anticancer (Mahmoud et al., 2016). extraction, enzyme mimics, metal chelation, host–guest systems, antibiotics and natural compounds such as marine alkaloids all benefit from pyridine-based Schiff base systems (Rezaeivala and Keypour, 2014). Condensation of 2,6-diaminopyridine with other compounds, such as 1,7-bis(2-formylphenyl)-1,4,7-trioxaheptane and 1,10-bis(2-formylphenyl)-1,4,7,10-tetraoxadeca ne, can produce different Schiff bases (Ilhan et al., 2007; Ilhan and Temel, 2007). It has been widely researched that cinnamaldehyde antimicrobial substance for use in medicine, food, and wood preservation. However, there are some application limits due to several problems, such as its strong odor and high volatility. Many researchers have attempted via a chemical modification to solve this drawback (Wang et al., 2016). Also, in coordination chemistry, the Schiff bases that contain cinnamaldehyde moieties are a vital class of ligands and were widely reported (Taher and Mohammed, 2008; Adabiardakani et al., 2012).
A large number of mixed-ligand complexes were reported by many researchers that containing heterocyclic bases such as 2,2′-bipyridine and 1,10-phenanthroline due to their bioinorganic applications and thermal stability (Osowole et al., 2008; Maurya et al., 2003). 2,2′-bipy is a strong bidentate ligand that has been used widely in coordination class and mainly in the synthesis of mixed-ligand chelates since its strong redox steady and relative easy of functionalization (Selvaganapathy and Raman, 2016). Mixed-ligand complexes incorporating 2,2′-bipy with other chelators have been studied as anti-bacterial, anti-neoplastic, cytotoxicity anti-tumor and genotoxicity activity (Mihsen and Shareef, 2018).
Various methods and routes have been developed for the synthesis of Schiff bases such as reflux in solution, stirring, grinding in a mortar, also microwave irradiation methods (K. H., J. P., P. P. and H. P., , 2016). Many synthetic approaches have not been completely reasonable due to many weaknesses such as low yields and tedious workup processes. Over the past few decades, significant research has been directed towards the development of new technologies for environmentally benign processes (green chemistry). A potential method under free-solvent is one of the modern green synthetic and efficient methodologies for Schiff bases that avoids using any type of dangerous solution, waste of time and tiresome build-up processes. The benefit from this skill style is a product of good yield, reaction complete in a short time and easy workup procedure. This route is offering an environmentally proactive and attractive economically for the synthesis of Schiff base ligand (Devidas et al., 2011).
Hence in the present research work, we describe a green and efficient method for the synthesis of a Schiff base ligand derived from 2,6-diaminopyridine with cinnamaldehyde, under solvent-free conditions with a short time followed by synthesis of new mixed-ligand transition metal complexes of azomethine in the presence of heterocyclic 2,2′-bipy. The prepared compounds were identified by elemental analysis, molar conductance, magnetic susceptibility, thermogravimetric analysis, and (IR, 1H NMR, Mass and UV–Vis) spectroscopic techniques and evaluated the antibacterial activity of the syntheses compounds in vitro.
2 Materials and methods
2.1 Materials
The compounds included cinnamaldehyde, 2,6-diaminopyridine, Cu(NO3)2·3H2O, Ni(NO3)2·6H2O, Co(NO3)2.6H2O and Zn(NO3)2.6H2O were procured from Merck, and compounds K2[PtCl4] and PdCl2 were purchased from Sigma Aldrich and 2,2′‐bipyridine from (GPR). Anhydrous grade ethanol (Merck), dimethyl sulfoxide (DMSO) (Fisher Scientific Company) diethyl ether (Biochem), and tetrahydrofuran (THF) (Alfa Chemika) were used. All the chemicals were analytically pure and used without further purification.
2.2 Characterization techniques
Melting points were obtained in open glass capillary tubes using electro-thermal digital melting point machine model 1102D. 1H NMR spectra of ligand and metal complexes were taken on Bruker ultra-shield (300 MHz), Switzerland and Varian INOVA (500 MHz) spectrometer, United States instrument respectively in DMSO‑d6 concerning tetramethylsilane (TMS) equally as an internal standard. The elemental (C, H, N) compositions of the compounds were determined using a Euro vector analyzer EA 3000 series. The IR spectra of the synthesized compounds were collected on Perkin–Elmer FTIR spectrophotometer using KBr pellets (νmax in cm−1) in the range 400–4000 cm−1. The mass spectrum of the Schiff base was determined using the liquid chromatography mass spectrometry (LC/MS) Sciex-ABI model 3200 spectrometer. Magnetic susceptibility measurements of the complexes were carried out by the Sherwood Scientific Auto Guoy balance instrument at 20○C, and Pascal’s constants using to calculated diamagnetic corrections. The molar conductance of the complexes in DMSO was measured with Fisher Scientific Multimeter Model XL600 and Electronic transition of the ligands and the chelates were obtained in (DMSO: D.W) 1:9 solutions in the range 200–800 nm using Cary Eclipse UV–Visible Spectrophotometer–Agilent Technologies. Thermal gravimetric analysis (TGA) was conducted using Perkin Elmer Diamond TGA/DTA (SII) thermal analyzer at a heating rate 10 °C/min in an air environment.
2.3 Preparation of Schiff base ligand
The L1 [(N2E, N6E)–N2, N6-bis((E)-3-phenylallylidene)pyridine-2,6-diamine] ligand was synthesized in one-pot under solvent-free conditions. Pyridine-2,6-diamino (10 mmol, 1.0913 g) was dissolved gently with (20 mmol, 2.6432 g) of trans-cinnamaldehyde. Then a few drops of formic acid were added to the mixture in an ice bath and stirred continuously for about 1–2 min until the yellow precipitate was formed as shown in Fig. 1. After completion of reaction ethanol was used to wash the yellow precipitate several times. After filtration, the product was recrystallized in chloroform. The preparation was monitored on TLC. Schiff base compounds are obtained 89 % yield.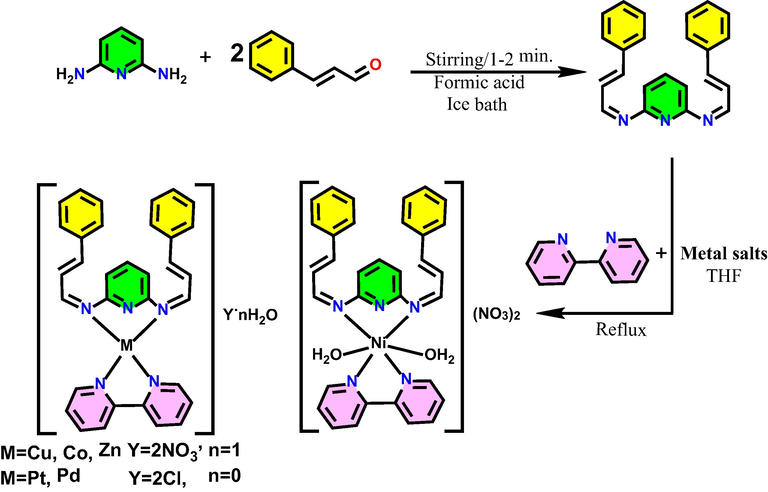
Schematic illustration preparation of the Schiff base and divalent mixed-ligand complexes.
2.4 Preparation of divalent mixed-ligand complexes
The Schiff base (2 mmol, 0.6748 g) dissolved in 15 ml of THF as a primary ligand was mixed with stoichiometric amount of 2,2ʹ-bipy in 5 ml THF as a secondary ligand. Subsequently, (2 mmol, 0.4832 g of Cu(NO3)2·3H2O, 0.4362 g of Ni(NO3)2·6H2O, 0.5821 g of Co(NO3)2·6H2O, 0.5949 g of Zn(NO3)2·6H2O and 0.5884 g of Na2[PdCl4]) in 10 ml of THF were added dropwise to the mixed-ligands with stirring. For Pt(II) complex (1 mmol, 0.3374 g) of Schiff base with 2,2ʹ-bipy solution (1 mmol, 0.1562 g) in THF was mixed and then (1 mmol, 0.4151 g) of K2[PtCl4] was dissolved in a mixed solvent of (8 ml D.W: 2 ml THF) was added dropwise to the mixture as shown in Fig. 1. The mixtures were reflexed for 24 h. After that, the obtained products were filtered using filter paper and washed several times using THF and diethyl ether. Finally, they were dried in a vacuum desiccator.
2.5 Antibacterial activity
The biological characterization of the newly prepared Schiff base, its mixed-ligand chelates, and standard antibiotic were examined for their antibacterial activities in vitro against (E.coli) Gram-negative and (S.aureus) Gram-positive by the agar well diffusion method using Muller Hinton agar medium (Natarajan and Raja, 2007). A bacteria old culture was swabbed uniformly on the agar plate surface. The labeled wells were made in the medium agar plates filled with 40 μL the test solutions were prepared by dissolving 20 mg/ml in DMSO. The DMSO and gentamicin were used as a control of the solvent and standard compound respectively for comparison and incubated the plate for 24 h at 37 °C. After the incubation time, the antibacterial activity of each sample was determined by measuring the diameter inhibition zone around each well in terms of mm by comparing it with the standard drug.
3 Results and discussion
The reactions of prepared Schiff base ligand and 2,2ʹ-bipy with transition metal salts (Cu, Ni, Co, Zn, Pt and Pd) gave a colored complex in good yields (72–89%) that were presented in Table 1 with other physical and analytical data such as yield percent, molecular weight melting /decomposition temperature and conductance. The comparison elemental (C, H, N) analyses results in Table 1 displays that the practical values are close to the calculated values and confirm the proposed chemical composition of the ligand and their complexes. Also, elemental analyses of the mixed-ligand metal complexes indicate the coordination of the ligands (Schiff base and 2,2ʹ-bipy) to metal ions in a 1:1:1 M ratio mean one mole of metal salts reacted with one mole of Schiff base and one mole of 2,2′-bipyridine. The prepared divalent metal complexes have molar conductance values in 10-3 M DMSO in the range of 130–159 Ω−1.cm2.mol−1 which denoted that complexes are electrolytic in nature in a 1:2 ratio. The anions (2Cl- and (NO3)2) are not bound to the metal ion and are outside the coordination sphere, according to the molar conductance values (Usharani et al., 2013). (Dec.Temp.) = decomposition temperature, * (Ω−1 .cm2.mol−1).
Compounds
M.Wt.
Color
%Yield
Dec. Temp.(°C)
% Found (Calculated)
Ʌm*
C
H
N
L1
337
Pale Yellow
89
300d
82.04
(81.87)5.64
(5.68)13.24
(12.45)–
[Cu(L1)(L2)](NO3)2·H2O
699.18
Pale Brown
82
330d
60.30
(56.69)3.55
(4.18)12.06
(14.02)148
[Ni(L1)(L2)(H2O)2](NO3)2
712.35
Deep Brown
78
290d
57.35
(55.64)4.78
(4.39)11.32
(13.76)159
[Co(L1)(L2)](NO3)2·H2O
694.57
Deep Brown
89
290d
59.65
(57.07)5.74
(4.21)11.57
(14.12)151
[Pt(L1)(L2)].2Cl
759.0.60
Yellow
74
230d
53.06
(52.18)4.36
(3.58)8.96
(9.22)130
[Zn(L1)(L2)](NO3)2·H2O
701.02
Brown
74
300d
55.46
(56.54)6.86
(4.17)12.95
(13.99)140
[Pd(L1)(L2)].2Cl
670.93
Pale
Yellow72
∼243d
56.25
(59.08)3.71
(4.06)11.59
(10.44)130
3.1 Mass spectrum
The mass spectrum of Schiff base ligand displayed the parent molecular ion at m/z, 337.2 g/mol which is in agreement with the proposed molecular weight of the new synthesized ligand L1 (C23H19N3). This supports the condensation of 2,6-diaminopyridine and the cinnamaldehyde to produce the L1 ligand. Ions from the breakdown of an aromatic nucleus are of relatively low abundance, but have some characteristic of molecular ions like (m/z 39, 50, 51, 65, and 76, 77, 79) which the most important some of these molecular ions were detected in the mass spectrum of Schiff base ligand Fig. 2 though the metastable ion peaks at m/z (79 and 65) characteristic to ions [C6H7]+ and [C5H5]+ respectively for the fragmentation of the aromatic nucleus must be detectable. The ion peaks at m/z 222, 183, 157 and 130 corresponds to the ion [C14H12N3]+, [C12H11N2]+, [C10H9N2]+ and [C9H8N]+ these showed the fragmentation of none-bonding electrons in the Schiff base. Besides, fragments at m/z, 274, 117 and 103 molecular weight-matched to ion [C18H16N3]+, [C9H9]+ and [C8H7]+ moiety that display the fragmentation of conjugated double bond as shown Fig. 3.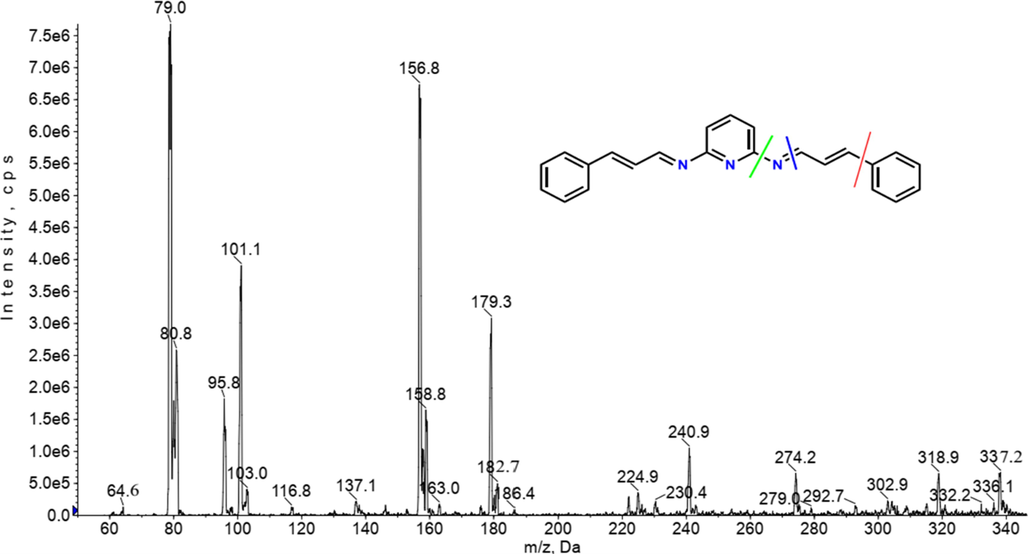
Mass spectrum of Schiff base ligand.
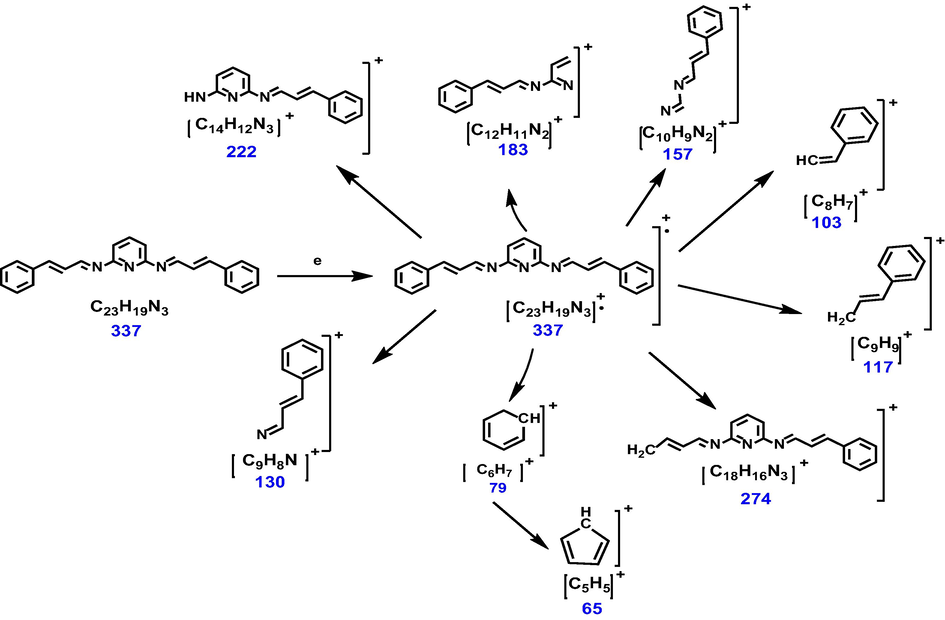
Schematic of the fragmentation pattern of Schiff base ligand.
3.2 Magnetic susceptibility measurements
The measurement of magnetic susceptibility is important to identify the number of unpaired electrons and to determine their geometric structure that are shown in Table 2. The magnetic susceptibility value data suggest octahedral geometry for Ni(II) which is (2.4B.M.), slightly lower than the spin-only value of 2.8B.M. (Omar et al., 2017).
Compounds
Band Absorption
Assignment
µeff (B.M.)
nm
cm−1
L1
252
39,683
π → π*
–
332
30,120
n → π*
L2
285
35,088
π → π*
–
[Cu(L1)(L2)](NO3)2·H2O
254
39,370
C.T
2.1
Square planar
298
33,557
C.T
312
32,051
C.T
657
15,221
2B1g → 2A1g
[Ni(L1)(L2)(H2O)2](NO3)2
251
39,840
C.T
2.4
Octahedral
294
34,014
C.T
305
32,787
C.T
[Co(L1)(L2)](NO3)2·H2O
252
39,682
C.T
2.21
Square planar
297
33,670
C.T
305
32,787
C.T
523
19,121
2A1g →2Eg
642
15,576
2A1g→2B1g
[Zn(L1)(L2)](NO3)2·H2O
252
39,682
C.T
Diamagnetic
Tetrahedral
294
34,014
C.T
305
32,787
C.T
405
24,691
C.T
[Pt(L1)(L2)].2Cl
250
40,000
C.T
Diamagnetic
Square planar
310
32,258
C.T
320
31,250
Pt → π*
[Pd(L1)(L2)].2Cl
255
39,216
C.T
Diamagnetic
Square planar
302
33,113
C.T
312
32,051
Pd → π*
The values of magnetic susceptibility of Cu (II) and Co (II) complexes are (2.1 and 2.21B.M.) respectively which suggest square-planar geometry around the metal ions. The magnetic moment of Cu(II) is expectedly higher than the spin only moment 1.73B.M.; for the unpaired electron due to spin–orbit coupling and orbital contributions. However, this can be attributed to electronic spectra measurements (Osowole et al., 2008) (Abd El-Wahed et al., 2008; Cibian and Hanan, 2015).
The magnetic moments of the Zn (II), Pt (II), Pd (II) complexes were found to be diamagnetic. The Zn(II) complex had a tetrahedral geometry while Pt (II), Pd (II) are suggested to possess a square-planar geometry in their metal complexes (Nair et al., 2012; Leka et al., 2004).
3.3 Electronic spectra measurements
The results of electronic transitions are often used to complement the data obtained from other physical measurements for easy structural investigations. The bands of absorption spectra and their assignments are collected in Table 2.
The absorption spectrum of the free Schiff base (L1) Fig. 4 the peak at 252 nm is consistent to π → π* transitions due to presence π electron in conjugated double bond such as C⚌N and C⚌C for the alkene, aromatic and pyridine ring and the band at 332 nm which is attributed to the n → π* transition due to present nonbonding electron (n) in C⚌N pyridine ring and azomethine moieties. These values shifted slightly to a lower wavelength (blue shift) during the formation of all metal complexes due to coordination of azomethine moieties in metal complexation, which may be explained by the decrease in conjugation due to the transfer of electrons from nitrogen atoms in the ligand to metal ion (Surabhi and Pradeepkuma, 2016). However, the absorption peak at 285 nm in the free 2,2ʹ-bipyridine ligand shifted in all spectra of chelates to higher wavenumber (red shift), due to coordination via two nitrogen atoms to metal ions (Anupama et al., 2017).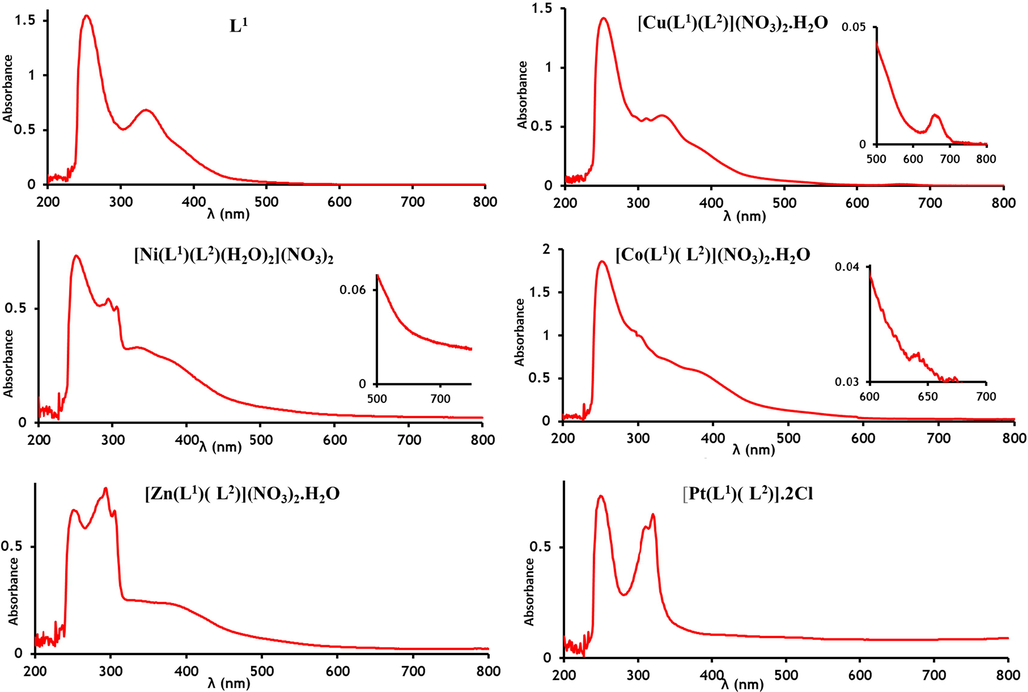
Absorption spectrum for the L1 ligand and mixed-ligand Cu(II), Ni(II), Co(II), Zn(II) and Pt(II) complexes.
The bands at 254 nm, 298 nm 312 nm and 337 nm were observed in the absorption spectrum of the copper complex. These are assignments to the intra-ligand (π → π* and n → π*) transition in ligands and ligand to metal charge transition (transfer of electrons from filled ligand (π-donar) orbital to the sets on the metal) respectively. Also, a broad band was observed at 657 nm elongated to a lower energy attribute to 2B1g-2A1g transition in square-planar geometry around Cu (Kellett, 2012).
In the Ni(II) chelate, the absorption spectrum doesn’t show any d-d transitions, while the intra-ligand and ligand metal charge transfer are appearing at higher intensity at 251 nm, 294 nm and 305 nm respectively since obscure d-d transition. This suggests octahedral geometry around Ni (II) ion with results reported (Mihsen and Shareef, 2018).
In the visible spectrum of the heteroleptic Co(II) chelates showed two bands at 523 nm and 642 nm which were assigned to 2A1g →2Eg and 2A1g →2B1g transitions. Thus, square-planar geometry was proposed for the cobalt complex (Cibian and Hanan, 2015) (Narayana and Galendragad, 1997).
The electronic spectrum of the divalent Zn-complex frequently reveals a single charge transfer band at 405 nm characteristic to M → L transition, which is suitable with tetrahedral geometry for this complex The result was in a good agreement with result reported (Nair et al., 2012) (Galini et al., 2020; Hasan and Salman, 2018).
Electronic spectra of the divalent heteroleptic Platinum and Palladium chelates show three bands in the 250–255 nm 310–302 nm and 320–312 nm. The higher-energy band is an internal π → π* transition of the ligand, while the lower is assigned as a Pt, Pd → π* charge transfer (Gregson et al., 1997; Abdullah and Salh, 2010). In the range 300–400 nm sturdy band that attributed to incorporation d-d transition and L → M and intra-ligand charge transfer are noticeable. Which compatible with the square planar geometry around the metal ions (Matesanz et al., 1999).
3.4 1H NMR spectra
The chemical shifts of the different positions of protons in the 1H NMR spectra are used to elucidate the structure of the Schiff base and its diamagnetic Pd(II), Pt(II), and Zn(II) heteroleptic complexes that are recorded in DMSO‐d6 solution and shown in (Fig. 5) respectively.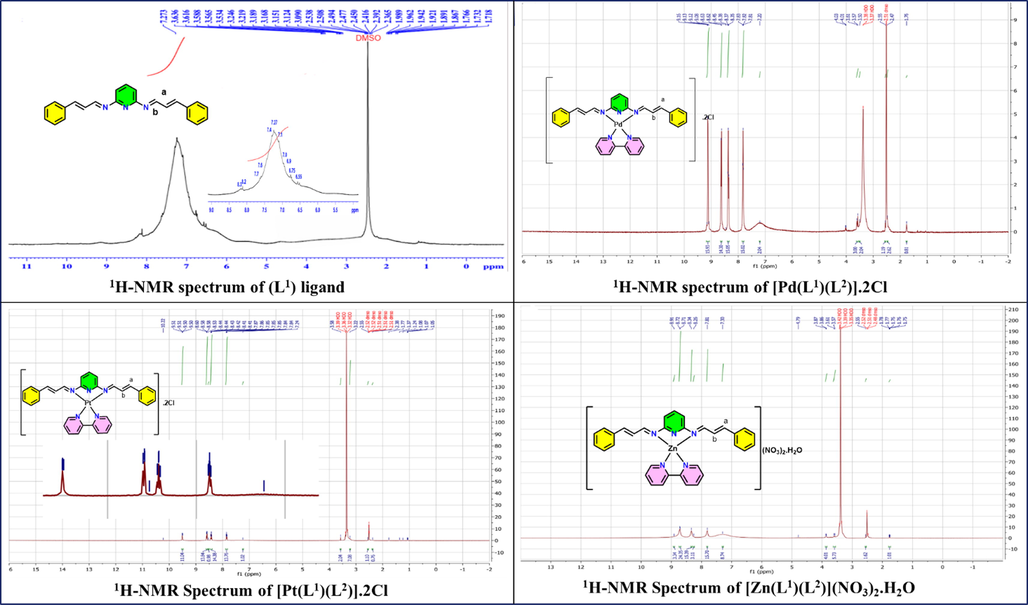
1H NMR spectra of (L1) ligand and Pd(II), Pt(II) and Zn(II) complexes in DMSO‑d6.
The 1H NMR spectrum of the Schiff base Fig. 5 shows the signal at δ (8.2, 8.3 ppm) that is attributed to two protons of (HC = N) imine protons. Also, the ligand reveals a multiplet signals at δ (6.9), (7.0), (7.1), (7.3), (7.4), (7.6), (7.7) ppm are due to aromatic protons (Usharani et al., 2013) (Mohammed and Taha, 2017). Moreover, the C-H proton at position (b) is observed as a doublet and doublet at δ (6.55–6.75) ppm, as it is the combination of both the imine proton and the other alkene proton at position (a). The C-H proton at position (a) should resonate as a doublet as, but it is signal is overlapping with the signal of the aromatic proton (Morshedi et al., 2009). The position of the azomethine signal on complexation is shifted to a downfield region 9.13 ppm, 9.51 ppm, and 8.72 ppm for Pd(II), Pt(II), and Zn(II) complexes, respectively. This is indicated the complexation occurs through azomethine nitrogen atoms comparison with the free ligand (Narayanachar et al., 2013). Also, the Multiples signal in the range 7.2–8.6 ppm appeared which are matched to the proton of aromatic and pyridine ring, this supports the participation of bipyridine nitrogen atoms in the chelation (Omar et al., 2017).
3.5 IR spectral studies
The important IR bands of ligands and their mixed-ligand complexes along with their assignments are given in Table 3. The formation of the ligand as indicated due to the presence of the (CH⚌N) imine stretching band at 1611 cm−1 which not present in precursor reactant. This was combined with the disappearance of the NH2 stretching band (Usharani et al., 2013) (Şenol, 2020) in the IR spectrum of the ligand Fig. 6. In addition, another evidence for the formation of the ligand was obtained through the appearance of a band at (2853–2922 cm−1) which is characteristic of aliphatic C-H stretching of the alkene and imine group, while the aromatic C-H stretching are centered at (3058 cm−1). Also, bands at 1493 and 1450 cm−1 are assigned to (C⚌C) frequencies (Amaral, 2018). Also, the IR spectrum of ligand displayed a band at 1578 cm−1 due to (C⚌N) stretching of pyridyl moiety (Şenol, 2020).
Compounds
OH (H2O)
C⚌N of imine
C⚌N of bipy
δ(C—H) bipy
M—N
M—O
ionic νNO3-
L1
–
1611
–
–
–
–
–
L2
–
–
1579
993–757
–
–
–
[Cu(L1)(L2)](NO3)2·H2O
3371
1604
Overlap with C⚌N group
1029–750
485
1384
[Ni(L1)(L2) (H2O)2](NO3)2
3364
1601
1028–763
493
534
1384
[Co(L1)(L2)](NO3)2·H2O
3358
1601
1029–766
493
–
1384
[Zn(L1)(L2)](NO3)2·H2O
3327
1605
1599
1025–768
479
–
1384
[Pt(L1)(L2)].2Cl
–
1607
1562
1029–762
457
–
[Pd(L1)(L2))].2Cl
–
1604
1567
1038–764
485
–
–
2·H2O complex.](/content/184/2021/14/12/img/10.1016_j.arabjc.2021.103429-fig6.png)
FTIR spectrum for (L1) ligand and [Cu(BPPA)(bipy)](NO3)2·H2O complex.
The band of C⚌N of the azomethine group in the spectra of all mixed-ligand complexes was shifted to a lower frequency and while the band for the pyridine ring is found unchanged. This suggests that the coordination takes place through nitrogen atoms of the azomethine group (Şenol, 2020). The characteristic absorption bands of the free 2,2′‐bipyridine L2 ligand appear at 1579 cm−1 due to stretching C⚌N, and δ(C-H) at (993 cm−1,757 cm−1). The band of C⚌N shifted to lower/higher frequencies and in the same prepared metal complexes overlapped with the band of other C⚌N group and the ring C-H deformation bands shift to higher frequencies (1025–1038 cm−1) and (750–768 cm−1). This suggests that the nitrogen atoms of bipyridine are coordinated to the metal ion (Sun et al., 2015).
Also, the broad band in the infrared spectra all metal complexes except Pt(II) and Pd(II) at (3387–3327 cm−1) suggests the presence of coordinated or hydrated molecules of water which are confirmed due to TG analysis (Ilhan et al., 2007). The IR-spectra of divalent Cu, Co, Ni and Zn complexes showed the high intense peaks at 1384 cm−1 can be assigned to ionic nitrate (Ilhan and Temel, 2007). Further, in the spectra of metal complexes, the new weak bands appear in the range (457–493 cm−1) referred to (M—N) bonding (Jayalakshmi and Rajave, 2015) and the absorption peak due to (M—O) of the water molecules was observed at (534 cm−1) in the Ni(II)-complex (Usharani et al., 2013).
3.6 Thermal gravimetric analysis (TGA)
The thermogravimetric (TG) technique was used to investigate the thermal properties of metal complexes in an air environment over the temperature range of 35–600○C and a heating rate of 10 °C/min. The calculated and estimated mass losses with temperature ranges decomposition for metal complexes are shown in Table 4 and TGA curves are given in Fig. 7.
Compounds
TG
range ○C
Mass loss%
Compound
decomposition
Residue
%
Metallic residue
calc. (est.)
calc.
(est.)
[Cu(L1)(L2)](NO3)2·H2O
40–105
2.57 (2.48)
H2O
11.38 (10.49)
CuO
105–266
17.74 (17.23)
2NO3–
266–314
22.34 (22.36)
C10H8N2
314–420
48.26 (47.73)
C23H19N3
[Ni(L1)(L2)(H2O)2](NO3)2
35–224
5.05 (4.64)
2H2O
10.49 (10.28)
NiO
224–281
17.41 (16.37)
2NO3–
281–316
21.93 (21.85)
C10H8N2
316–421
47.37 (46.99)
C23H19N3
[Co(L1)(L2)](NO3)2·H2O
44–83
2.59 (2.46)
H2O
10.79 (11.3)
CoO
83–251
17.85 (17.32)
2NO3–
251–279
22.48 (22.32)
C10H8N2
279–398
48.58 (47.76)
C23H19N3
[Zn(L1)(L2)](NO3)2·H2O
42–159
2.57 (2.54)
H2O
11.61 (9.5)
ZnO
159–257
17.69 (17.63)
2NO3–
257–278
22.28 (21.83)
C10H8N2
278–395
48.13 (47.60)
C23H19N3
[Pt(L1)(L2)].2Cl
40–335
9.33 (9.26)
2Cl-
27.79 (26.44)
PtO
335–360
20.56 (20.42)
C10H8N2
360–394
44.42 (44.16)
C23H19N3
[Pd(L1)(L2)].2Cl
41–326
10.57 (10.46)
2Cl-
18.25 (17.42)
PdO
326–364
23.28 (23.10)
C10H8N2
364–419
50.29 (49.88)
C23H19N3
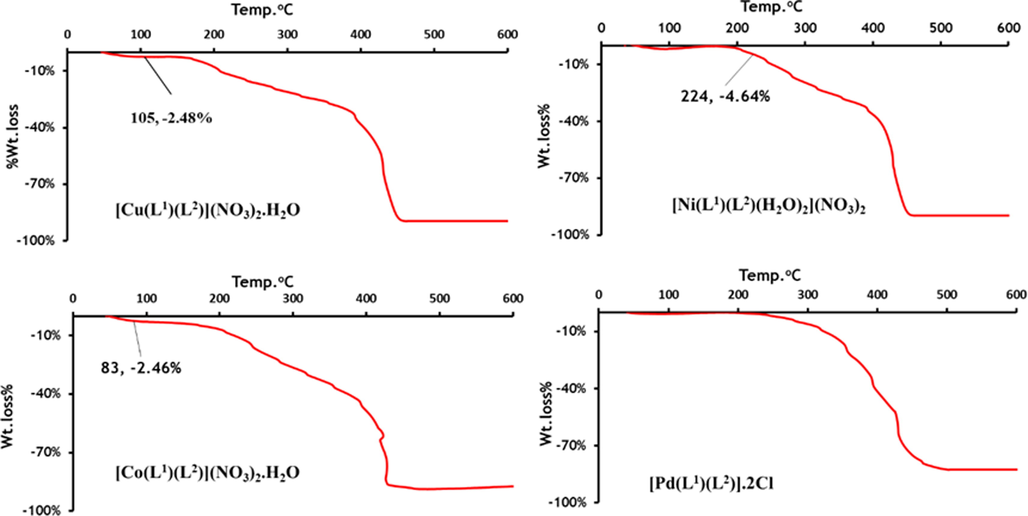
TGA curves of mixed-ligand Cu(II), Ni(II), Co(II) and Pd(II) complexes.
Thermogravimetric analysis is used to identify the position of water molecules in a complex, such as whether they are in the inner or outer coordination sphere. dehydration of hydrated water takes place at temperatures range 50–160 °C, while coordinated water molecules are eliminated at temperatures below 250 °C (El-Ansary et al., 2002).
The TGA data for the studied Co(II), Cu(II), and Zn(II) complexes displayed similar pattern of thermal decomposition. The thermal decomposition curves of Co(II) complex Fig. 7 showed four steps decomposition. The first step between 44˚C and 83˚C corresponds to the loss of a hydrated water molecule (calc.: 2.59%; est.: 2.46%) (El-Ansary et al., 2002). The second step between 83˚C and 251˚C (calc.: 17.85%; est.: 17.32%) corresponding to the loss of a nitrate ions. While the third decomposition step between 251˚C and 279˚C (calc.: 22.48%; est.: 22.32%) and four step between 279˚C and 398˚C (calc.:48.58%; est.:47.76%) corresponds to the loss of the organic moieties (C10H8N2 and C23H19N3) respectively, leaving a residue which corresponds to cobalt oxide, (calc.: 10.79%; est.: 11.3%).
The TG plot of Ni(II) (Fig. 7) displayed four decomposition steps. The first stage takes place in the 35–224 °C range corresponding to the release of two coordinated H2O molecules (calc.: 5.05%; est.: 4.64%) (El-Ansary et al., 2002). The second, third and four decomposition steps involved the removal of two nitrate ions and an organic ligands molecule, respectively, leaving nickel oxide as a residue.
Within the range of 40–419 °C, the TG curves of Pt(II) and Pd(II) complexes show three stages of decomposition. The loss of two chlorine atoms is represented by the first step, while the loss of (C10H8N2 and C23H19N3) molecules is represented by the second and third steps. However, organic constituent degradation continued until stable Pt(II) and Pd(II) oxides were formed as end products, with mass percents of (calc.: 27.79%; est.: 26.44%) and (calc.: 18.25%; est.: 17.42%) respectively. Thermal studies of the metal complexes confirmed the molecular formulae of the studied complexes.
3.7 Antimicrobial activity
The Schiff base ligand and its mixed-ligand chelates were evaluated for antimicrobial activity against Gram‐positive (S. aureus), Gram‐negative bacteria (E. coli) and gentamycin (as a standard compound) as shown in Fig. 8 because resistance bacteria towards available drugs through biochemical and morphological modifications is the most important obstacle in the universal rapidly. Hence today synthesis of the new design compounds one of the most vital branches to interpretation the resistance (Huang et al., 2001).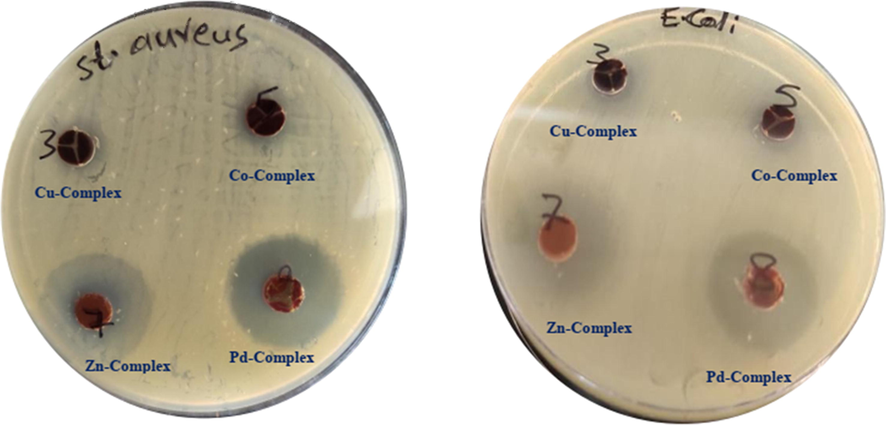
The inhibition zone of mixed-ligand complexes on the plate.
In the comparison of activity results between Schiff base and their heteroleptic complexes against bacteria which are listed in Table 5, it has been clear that the synthesized Schiff base are not antimicrobial activity while Zn(II) and Pd(II) complexes showed significant antimicrobial activities against both Gram-positive (S. Aureus) and Gram-negative (E. coli) and metal complexes of Cu(II) and Co(II) against Gram-positive (S. Aureus). Whereas other complexes such as Cu(II), Ni(II), Co(II), Pt(II) against (E. coli) and Ni(II), Pt(II) complexes against (S. Aureus) are found not exhibit antibacterial activity. Also metal complexes are found to have sensitivity for inhibition of Gram-positive more than Gram-negative bacteria. The studies demonstrations that the Zn(II) and Pd(II) complexes mentioned good activity against both organisms than other heteroleptic complexes and moderately comparable to the activity of bipyridine and standard drug.
Compounds
Inhibition Zone (mm)
Escherichia coli
Gram(-)
Staphylococcus Aureus
Gram(+)
L1
–
–
L2
22
37
[Cu(L1)(L2)](NO3)2·H2O
–
9
[Ni(L1)(L2)(H2O)2](NO3)2
–
–
[Co(L1)(L2)] (NO3)2·H2O
–
10
[Zn(L1)(L2)](NO3)2·H2O
13
25
[Pt(L1)(L2)].2Cl
–
–
[Pd(L1)(L2)].2Cl
12
24
Gentamicin
18
40
DMSO
12
–
The actual mechanism for this is unknown but this may be due to the electronic configuration, solubility, dipole moment, difference in the ribosomes of microbial cells and the impermeability of the microbial cells, mechanism and their enzymatic action may be the possible reason (Singh et al., 2013). Another factors that cause different effect of the compounds against bacteria may be because of the structure of the compound and the solvent used. The diffusion capacity of the compounds varies with the employed solvent, which may be because of the polarity of the solvent (Parekh et al., 2005).
Additionally, the activities of metal complexes might be due to the decline of the polarity of the metal ions as a result of complexation partial positive charge of a metal ion with ligands and increase delocalization of π electron over the heteroleptic complex. The lipophilic nature of metal(II) complexes which afterword favors its permeation through the lipid layer of the bacterial membrane system moreover such mixed-ligand complexes might be inhibiting the enzyme activity of the bacterial that necessary for metabolic pathways in the microbe, thus improving antimicrobial activity (Abd El-Halim et al., 2011). According to recent researches, metal ions cause many types of microbial cell damage as a result of membrane breakdown, protein malfunction, and oxidative stress. These unique modes of action, combined with the wide range of three-dimensional geometries that metal complexes can adopt, make them suitable for the development of new antimicrobial drugs (Claudel et al., 2020).
4 Conclusion
A new symmetrical Schiff base has been synthesized from 2,6-diaminopyridine and cinnamaldehyde within few minutes under the free solvent condition at room temperature with superb yield and its divalent mixed-ligand chelates of Cu, Zn, Ni, Co, Pt, and Pd with 2,2′‐bipyridine were synthesized and characterized based on the analytical and spectroscopic method. The experimental data showed that the Schiff base and 2,2′‐bipy act as a bidentate and matched the metal ions respectively via imine and pyridine nitrogen atoms. Also, the metal complexes are electrolytic and divalent Cu, Co, Pt and Pd complexes have square planer structure while octahedral and tetrahedral geometry has been proposed for the complexes of Ni(II) and Zn(II) respectively. The antimicrobial activities of the ligands and the metal chelates were screened against organisms. From the antibacterial studies, the results revealed that the chelates have higher activity toward S. aureus than E. coli strain. Also, the divalent Zn and Pd complexes presented significant activity than the synthesized Schiff base and other metal complexes and moderately concerning the activity standard drugs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.







