Translate this page into:
Process optimization and effect of different extraction methods on the characteristics and activities of Herba Patriniae polysaccharides: A correlation analysis
⁎Corresponding authors. lzdxhui@163.com (Heping Hui), 707507995@qq.com (Xuejun Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The process of papain assisted hot water (WP), cellulase assisted hot water (WC), papain mixed cellulase assisted hot water (WPC), ultrasonic assisted papain (PU), and ultrasonic assisted cellulase (CU) for Patriniae polysaccharides (HPs) extraction were optimized using the indicate of polysaccharide yield, respectively. Then, the physicochemical characteristics, antioxidant and hypoglycemic activities of HPs were compared, with an additional correlation analysis between the characteristics and activities of HPs. The results showed that CU demonstrated the highest extraction rate at 30.50 % among all methods. Compared to previous hot water extraction (HW), the enzyme-assisted extraction increased molecular weight, reducing sugar, mannose, and glucose content of HPs, while ultrasound-assisted extraction lowered mannose and glucose content of HPs, and increased reducing sugar content of HPs. All HPs showed concentration-dependent hypoglycemic and antioxidant activities. Correlation analysis revealed that HPs with moderate galactose content, higher reducing sugar, protein, glucose, mannose, xylose, and molecular weight polysaccharide content would exhibit superior hypoglycemic and antioxidant activities, with CU-HPs expressing these excellent characteristics. Together with the exceptional polysaccharide yield, CU was a promising strategy to extract HPs, whose optimum process were: enzyme addition 2.5 %, solid–liquid ratio 1:25, ultrasonic power 150 W, ultrasonic time of 50 min.
Keywords
Herba Patriniae polysaccharide
Process optimization
Physicochemical characteristics
Antioxidant and hypoglycemic activity
Correlation analysis
- WP
-
papain assisted hot water extraction
- WC
-
cellulase assisted hot water extraction
- WPC
-
papain mixed cellulase assisted hot water extraction
- PU
-
ultrasonic assisted papain extraction
- CU
-
ultrasonic assisted cellulase extraction
- PCU
-
ultrasonic assisted papain mixed cellulase extraction
- WU
-
ultrasonic assisted hot water extraction
- HW
-
hot water extraction
- HPs
-
Patriniae polysaccharides
Abbreviations
1 Introduction
Herba Patriniae belonging to the Patrinia villosa family, widely distributed in Northwest of China, whose unique alpine and arid climate cultivated the rich nutrients and physiological active substances of Herba Patriniae, such as flavonoids and polysaccharides. The theory of traditional Chinese medicine believed that the whole grass of Herba Patriniae can be used as medicine for positive clearing heat and detoxification, eliminating phlegm and discharging pus (Gong, Zou, Zhang, Shi, & Liu, 2021; Yang et al., 2023; Su et al., 2022), especially for the treatment of intestinal carbuncle, lung carbuncle, postpartum blood stasis and abdominal pain caused by heat toxin (Su et al., 2022; Zheng et al., 2019; Chen, & Han, 2017).
Recently, accumulating studies confirmed that plant polysaccharides showed significant bioactivities in anti-tumor, anti-hepatitis and regulating glucose metabolism (Yang et al., 2023; Tang, Wang, Chen, et al., 2023; Qi et al., 2023), which led to the plant polysaccharides becoming a research hotspot (Xu et al., 2023; Shi et al., 2023; Gong, Li, Sun, et al., 2022). Patriniae contained many polysaccharides, which had rich bioactivities, such as anti-fatigue, anti-virus, anti-oxidation, anti-tumor and immune regulation, and possessed a high medical value and widespread application (Hui, & Gao, 2022). However, as one of the crucial factors affecting the bioactivity of polysaccharides, a suitable extraction method became a key step for polysaccharide preparation, which not only influenced the yield of polysaccharide, but also changed its physicochemical characteristics, such as total polysaccharide and protein contents, monosaccharide composition and molecular weight distribution (Fernandes et al., 2023; Tang, He, Liu, et al., 2023; Bai et al., 2023; Guo et al., 2020; Yi et al., 2020; Song et al., 2023).
As far as we know, several extraction methods have been applied for Pariniae polysaccharides, such as hot water extraction, ultrasonic-assisted extraction, and ultrasonic composite-enzyme extraction (Hui, & Gao, 2022; Zhang et al., 2022; Hui, Jin, Zhao, et al., 2022). However, the hot water extraction was simple but time-consuming and a lower extraction rate, and ultrasonic-assisted extraction was efficient but high costs and severe loss, while ultrasonic composite-enzyme extraction produced a high extraction rate but costed expensively. Generally, the extraction of interesting plant polysaccharides, which can be ingested or used for the purpose of functional products, depended on compatible methods with economically viable yields (Tang, He, Liu, et al., 2023; Gong, Li, Sun, et al., 2022). As an alternative, enzyme-assisted extraction emerged as a promising approach, with effective cell walls degradation, quick polysaccharide release, high yield, environment-friend and easy to operate (Guo et al., 2020; Yi et al., 2020; Wang et al., 2021). Whereas, the extraction methods for Pariniae polysaccharide including enzyme-assisted hot water and ultrasonic-assisted enzyme extraction have not been reported so far. Additionally, there was limited information in comparison with the physicochemical characteristics and bioactivity of HPs extracted by different methods, as well as their correlations.
Thus, the current study was aimed to evaluate the effects of different extraction methods on the yield, physicochemical characteristics, hypoglycemic and antioxidant activities of HPs, and firstly obtained the optimal extraction process of HPs by the polysaccharide yields of single factor and orthogonal test, then compared the physicochemical characteristics, molecular weight, constituent monosaccharides and activities of HPs extracted by different extraction methods, finally analyzed the correlations of activities of HPs and their physicochemical characteristics for further understanding the structure and bioactive characteristics of HPs, as well as providing the reference for potential high value utilization of Patriniae polysaccharide in pharmaceutical and healthcare industry.
2 Materials and methods
2.1 Materials
Herba Patriniae, purchased from the Yellow River Market in Lanzhou, Gansu Province, which was identified as Patrinia villosa(Thunb.)Juss. by Professor Ma Zhigang from School of Pharmacy, Lanzhou University; DPPH reagent (sigma, USA), ABTS reagent, acarbose α-amylase, p-nitrophenyl-β-D-glucopyranoside (Yuanye company), 95 % ethanol (Yantai Shuangshuang Chemical Co., Ltd.), sulfuric acid (Chengdu Cologne Chemical Co., Ltd.), anhydrous sodium acetate (Yantai Shuangshuang Chemical Co., Ltd.), acetic acid (Yantai shuangshuangshuang Chemical Co., Ltd), α-glucosidase (Solarbio, China), rhamnose (Rha), mannose (Man), glucose (Glu), galactose (Gal), xylose (Xyl) and arabinose (Ara) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other reagents and chemicals used were of analytical grade.
2.2 Extraction procedure
2.2.1 Pretreatment of raw materials
According to reference (Hui, & Gao, 2022), the raw material of Herba Patriniae was powdered and passed through a 60-mesh sieve. 200 g of Herba Patriniae powder was taken accurately, added with 3 times the amount of 80 % ethanol, and refluxed to degrease for 3 times (2 h/time). The supernatant was removed and the residues were collected and placed in the ventilation cabinet dry standby.
2.2.2 Hot water extraction (HW)
Taken the above treated Patriniae powder, added 20 times its volume of distilled water to boil extraction for 3 times (2 h/time), then filtered by multi-layer gauze, collected the filtrate, centrifuged at 3000 rpm for 5 min, and the supernatant was concentrated under reduced pressure to 1/10 of the original volume, added the ethanol with a final concentration of 80 % to precipitate at 4 °C, allowed to stand for 24 h, followed by centrifugation. Then the precipitation was collected and freeze-dried with a freeze drier (Labconco, FreeZone®, American) to obtain Patriniae polysaccharide (Hui, & Gao, 2022). The polysaccharide extraction yield (%) was calculated as the following formula:
Polysaccharide extraction yield (%) = (W1/W0) × C × 100 (1).
Where W0 is the quality of weight of pretreated Patriniae powder, g; W1 is the weight of the extracted polysaccharide after freeze-drying, g; C is the content of total polysaccharide, mg/mL.
2.2.3 Ultrasonic assisted hot water extraction (WU)
According to the literature method (Zhang et al., 2022), the treated Patriniae powder was mixed with 15 times its volume of distilled water, ultrasonically treated at 70 °C, 120 W power for 90 min, followed by filtered. The resulting filtrate was concentrated under reduced pressure to 1/10 of the original volume, and then precipitated by ethanol with a final concentration of 80 % at 4 °C, standing overnight and following centrifugation (5000 rpm, 5 min). The obtained precipitation was collected and further freeze-dried with a freeze drier (Labconco, FreeZone®, American) to obtain Patriniae polysaccharide, whose extraction yield was calculated according to formula 1.
2.2.4 Ultrasonic assisted papain mixed cellulase extraction (PCU)
The treated Patriniae powder was mixed with 20 times its volume of sodium acetate buffer (pH 6.5), then added a 2.0 % composite enzyme solution (papain: cellulase = 1:1/ m:m), shaken vigorously and left to stand for 30 min. Subsequently, the mixture was ultrasonically extracted at 90 W power and 55 °C for 20 min, then quickly transferred the mixture to a 95 °C oven and heated for 10 min, taken out, cooled to room temperature, and filtered, added ethanol with a final concentration of 80 % to the filtrate, placed in a refrigerator overnight at 4 °C and then centrifuged (3000 rpm, 5 min). The resulting precipitate was collected, washed with anhydrous ethanol and acetone in turn, and freeze-dried to obtain Patriniae polysaccharide (Hui, Jin, Zhao, et al., 2022), whose extraction yield was calculated according to Formula 1.
2.2.5 Ultrasonic assisted cellulase extraction (CU)
2.2.5.1 Extraction process of Patriniae polysaccharide by CU
Refer to the literature method with slight modification (Zhang et al., 2022; Hui, Jin, Zhao, et al., 2022; Song et al., 2023), accurately weighed 1.6 g of treated Patriniae powder, added a certain amount of sodium acetate buffer (pH 6.5) according to the ratio of material to liquid, and then mixed with amount of cellulase, shaken thoroughly, and stood for 30 min, subsequently, transferred the mixture to the ultrasonic instrument and ultrasonically extracted by the set parameters. After completion of extraction, the operation methods of inactivating enzyme, filtration, alcohol precipitation, centrifugation and freeze-drying were the same as the PCU treatment method of 2.2.4, and the Pariniae polysaccharide extracted by CU method was finally obtained.
2.2.5.2 Single factor test of CU
The single factor tests were performed according to the reference and made some modification (Zhang et al., 2022; Hui, Jin, Zhao, et al., 2022). Choosing the optimal pH value and temperature of the cellulase, 1.6 g of treated Patriniae powders were taken to investigate the effects of four factors (ultrasonic time, ultrasonic power, solid–liquid ratio, and cellulase addition) on the extraction yield of Pariniae polysaccharides. The default conditions of single factor test were as follows: cellulase addition 2.0 %, solid–liquid ratio 1:20, ultrasonic power 120 W, ultrasonic time 50 min. Parallel operation 3 times, and the extraction yield of polysaccharides was calculated using formula 1, while the statistical software was employed to analyze the results and determine the experimental levels of four factors: cellulase dosage, solid–liquid ratio, ultrasonic power, and ultrasonic time.
2.2.5.3 Orthogonal test of CU
A four-factor orthogonal test including liquid-to-material ratio, cellulase addition, ultrasonic power, and ultrasonic time was conducted by the factor-level table (Table 2) and the L9(34) orthogonal test table (Table 3). A: Extraction time. B: Extraction temperature. C: The amount of enzyme added. D: Ratio of solid to liquid. A: Ultrasonic time. B: Ultrasonic power. C: The amount of enzyme added. D: Ratio of solid to liquid.
Level
Factor
A /min
B /℃
C /%
D /g: mL
WP
WC
WPC
WP
WC
WPC
WP
WC
WPC
WP
WC
WPC
1
40
20
60
35
35
45
2.0
2.0
2.0
1:10
1:20
1:10
2
60
40
80
45
45
55
2.5
2.5
2.5
1:15
1:25
1:15
3
80
60
100
55
55
65
3.0
3.0
3.0
1:20
1:30
1:20
Level
Factor
A /min
B /W
C /%
D /g: mL
PU
CU
PU
CU
PU
CU
PU
CU
1
30
30
60
90
1.5
1.5
1:20
1:15
2
40
40
90
120
2.0
2.0
1:25
1:20
3
50
50
120
150
2.5
2.5
1:30
1:25
No.
A
B
C
D
Polysaccharide yield/%
WP
WC
WPC
PU
CU
WP
WC
WPC
PU
CU
WP
WC
WPC
PU
CU
WP
WC
WPC
PU
CU
WP
WC
WPC
PU
CU
1
1
1
1
1
9.7
12.7
11.4
12.8
11.3
2
1
2
2
2
13.7
14.5
15.1
16.6
15.7
3
1
3
3
3
13.8
19.9
12.85
13.7
17.7
4
2
1
2
3
10.1
16.7
9.19
8.4
16.2
5
2
2
3
1
12.7
19.8
12.89
20.3
14.5
6
2
3
1
2
17.1
19.6
15.61
20.5
17.6
7
3
1
3
2
9.9
15.4
4.83
24.4
15.7
8
3
2
1
3
12.8
23.1
14.99
19.7
24.2
9
3
3
2
1
20.9
20.8
15.09
21.0
25.5
K1
12.5
15.8
11.7
14.5
15.1
10.0
15.1
8.5
15.3
14.5
12.3
17.4
10.2
15.4
16.1
12.3
16.6
11.9
14.1
16.4
K2
13.4
18.8
12.2
16.5
16.2
13.2
19.2
14.4
18.5
18.2
13.3
18.5
13.2
17.8
17.8
13.7
17.9
12.4
18.2
17.2
K3
14.6
19.9
13.2
21.8
21.9
17.4
20.2
14.6
19.0
20.3
15.0
18.6
14.1
19.6
19.2
14.5
20.0
13.2
20.6
19.5
R
2.1
4.1
1.5
7.3
6.8
7.4
5.1
6.1
3.7
5.8
2.7
1.2
3.9
4.2
3.1
2.2
3.4
1.3
6.5
3.1
Optimal
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
2.2.6 Ultrasonic assisted papain extraction (PU)
2.2.6.1 Extraction process of Patriniae polysaccharide by PU
Like the methods of 2.2.5.1. 1.6 g of treated Patriniae powder was mixed with a certain amount of sodium acetate buffer (pH 6.5) according to the ratio of material to liquid, then added an amount of papain, shaken thoroughly, left to stand for 30 min. Subsequently, placed the mixture into the ultrasonic extraction instrument, and started ultrasonic extraction according to the set parameters. After that, the operation methods of inactivating enzyme, filtration, alcohol precipitation, centrifugation and freeze-drying were the same as the PCU treatment method of 2.2.4, and the Pariniae polysaccharide extracted by PU method was finally obtained.
2.2.6.2 Single factor test of PU
Likely, weighted 1.6 g of treated Patriniae powder, the optimal pH value and temperature of the papain were selected to investigate the effects of four factors (ultrasonic time, ultrasonic power, solid–liquid ratio, and papain addition) on the extraction yield of Pariniae polysaccharides. The default conditions of single factor test were set as follows: papain addition 2.0 %, solid–liquid ratio 1:20, ultrasonic power 90 W, and ultrasonic time 40 min. The experiments were conducted three parallels.
2.2.6.3 Orthogonal test of PU
Based on the single-factor tests, a four-factor orthogonal test was carried out to investigate the impact of liquid-to-material ratio, papain addition, ultrasonic power, and ultrasonic time on the polysaccharide extraction yield (calculated from formula 1) in accordance with the factor-level table (Table 2) and L9(34) orthogonal test table (Table 3).
2.2.7 Papain mixed cellulase assisted hot water extraction (WPC)
2.2.7.1 Extraction process of Patriniae polysaccharide by WPC
According to the reference with slight modification (Hui, Jin, Zhao, et al., 2022; Guo et al., 2020), 1.6 g of treated Patriniae powder was mixed with a certain amount of sodium acetate buffer (pH 6.5) according to the material liquid ratio, then added to an amount of compound enzyme (Papain: Cellulase = 1:1/ m:m), fully shaken, allowed to stand for 30 min. Subsequently, the mixture was transferred to hot water at set temperature to extract a certain time. After completion of extraction, the operation methods of inactivating enzyme, filtration, alcohol precipitation, centrifugation and freeze-drying were the same as the PCU treatment method of 2.2.4, and the Pariniae polysaccharide extracted by WPC method was finally obtained.
2.2.7.2 Single factor test of WPC
It was performed according to the reference and made some modification (Hui, Jin, Zhao, et al., 2022; Guo et al., 2020). Taken 1.6 g of treated Patriniae powders, the optimal pH value of the compound enzyme was chosen to investigate the effects of four factors (time, temperature, solid–liquid ratio, and compound enzyme addition) on the extraction yield of Pariniae polysaccharides. The default conditions of single factor test were as follows: compound enzyme addition 2.0 %, solid–liquid ratio 1:10, extraction temperature 45 °C and extraction time 60 min, test parallel operation 3 times.
2.2.7.3 Orthogonal test of WPC
Following the guidelines from the factor-level table (Table 1) and L9(34) orthogonal test table (Table 3), a four-factor orthogonal test was performed to investigate the effects of compound enzyme addition, liquid-to-material ratio, extraction temperature, and time on the Pariniae polysaccharide extraction yield (calculated from formula 1).
2.2.8 Cellulase assisted hot water extraction (WC)
2.2.8.1 Extraction process of Patriniae polysaccharide by WC
Same as the method of 2.2.7.1. Briefly, mixed a certain amount of sodium acetate buffer (pH 6.5) with 1.6 g of treated Patriniae powder according to the ratio of material to liquid, then added to amount of cellulase, shaken and stood for 30 min. After that, the operation methods of inactivating enzyme, filtration, alcohol precipitation, centrifugation and freeze-drying were the same as the PCU treatment method of 2.2.4, and the Pariniae polysaccharide extracted by WC method was finally obtained.
2.2.8.2 Single factor test of WC
Like the methods of 2.2.7.2. Taken 1.6 g of treated Patriniae powders, the optimal pH value of the cellulase were chosen to investigate the effects of four factors (time, temperature, solid–liquid ratio, and cellulase addition) on the extraction yield of Pariniae polysaccharides. The default conditions of single factor test were as follows: cellulase addition 2.0 %, solid–liquid ratio 1:20, extraction temperature 35 °C and extraction time 20 min, parallel operation 3 times.
2.2.8.3 Orthogonal test of WC
It was conducted a four-factor orthogonal test for liquid-to-material ratio, cellulase addition, extraction temperature and time according to the factor-level table (Table 1) and L9(34) orthogonal test table (Table 3).
2.2.9 Papain assisted hot water extraction (WP)
2.2.9.1 Extraction process of Patriniae polysaccharide by WP
Same as the method of 2.2.8.1, mixed a certain amount of sodium acetate buffer (pH 6.5) with 1.6 g of treated Patriniae powder according to the ratio of material to liquid, afterward added to amount of papain, shaken completely, and stood for 30 min, then transferred the mixture to the hot water at set temperature to extract a certain time. After that, the operation methods of inactivating enzyme, filtration, alcohol precipitation, centrifugation and freeze-drying were the same as the PCU treatment method of 2.2.4, and the Pariniae polysaccharide extracted by WP method was finally obtained.
2.2.9.2 Single factor test of WP
Like the methods of 2.2.8.2. choosing the optimal pH value of the papain, the effects of four factors (time, temperature, solid–liquid ratio, and papain addition) on the extraction yield of Pariniae polysaccharides were investigated, and the default conditions of single factor test were as follows: papain addition 2.0 %, solid–liquid ratio 1:10, extraction temperature 45 °C and extraction time 40 min. The test was operated in parallel for 3 times.
2.2.9.3 Orthogonal test of WP
Similarly, a four-factor orthogonal test for liquid-to-material ratio, papain addition, extraction temperature and time were demonstrated by the factor-level table (Table 1) and L9(34) orthogonal test table (Table 3).
2.3 Determination of physicochemical characteristics of HPs
2.3.1 Determination of chemical composition of HPs
The contents of reducing sugar were determined by the 3,5-dinitrosalicylic acid method (DNS), using glucose as standard (Hui, Wang, Zhang, et al., 2022), and the protein contents were determined by Bradford’s method, using bovine serum albumin as the standard (Hui, & Gao, 2022), while the total polysaccharide contents were determined by phenol–sulfuric acid method with glucose as standard (Hui, Jin, Zhao, et al., 2022).
2.3.2 Determination of molecular weight of HPs
According to the previous method with slight modifications (Hui et al., 2019), the absolute molecular weight (Mw) and polydispersity (Mw/Mn) of Pariniae polysaccharides were measured by high performance size exclusion chromatography coupled with multi-angle laser light scattering and refractive index detector (HPSEC-MALLS-RID). HPSEC-MALLS-RID measurements were carried out on a multi-angle laser light scattering detector with an Agilent 1260 series LC system. TSK-Gel G5000PWXL (300 mm × 7.8 mm, i.d.) and TSK-Gel G3000PWXL (300 mm × 7.8 mm, i.d.) were used in series at 30℃. The MALLS instrument was equipped with a He-Ne laser (λ = 658 nm), and an Optilab rEX refractometer was simultaneously connected. The sample concentration was about 1.0 mg/mL with an injection volume 5 μL, and the mobile phase was distilled water at a flow rate of 0.8 mL/min. The Mw was calculated by the Zimm method of static light scattering based on the basic light scattering equation.
2.3.3 Determination of monosaccharide composition of HPs
Monosaccharides composition of Pariniae polysaccharides were measured by GC (Hui, & Gao, 2022). Briefly, each sample (5.0 mg) was hydrolyzed with 2.0 M trifluoracetic acid (2.0 mL) at 110 °C for 6 h, then mixed with 10 mg of hydroxylamine hydrochloride and 1 mL of pyridine in the reaction bottle, continued to react in an oven at 90 °C for 0.5 h. After that, removed from the oven and cooled, added 1 mL acetic anhydride to the mixture and continued to react at 90 °C for 0.5 h. Subsequently, 1 mL of distilled water and chloroformed were added into the reaction solution, shaken evenly, and stood for layering, and then transferred the chloroform layer to the evaporating dish and evaporated to dryness. The residue was dissolved into 0.5 mL of chloroform for GC analysis. A mixed standard solution, containing rhamnose, mannose, glucose, galactose, xylose, and arabinose, was derivatized by the same method as described above.
All the hydrolysates were modified to acetate derivative and analyzed by Agilent 6890n gas chromatograph (Agilent, Santa Clara, CA, USA) in flame ionization detector (FID) and quartz capillary column DB-1 (30 m × 0.32 mm × 0.3 μm). The temperatures of injection port and detector were 250 °C and 260 °C, respectively. The column temperature was programmed to rise from 100 °C (2 min) to 220 °C (5 min) at a rate of 3 mL/min, while the diffusion ratio was 50:1, and the carrier gas was N2 at a flow rate of 2 mL/min (Hui, Jin, Zhao, et al., 2022).
2.4 Determination of HPs antioxidant activities in vitro
2.4.1 The clearance activity of DPPH of HPs
The DPPH clearance activity of Pariniae polysaccharides was measured according to the previous method with minor modification (Xiong et al., 2022; Hui, & Gao, 2022). Briefly, 2 mL of polysaccharide solutions with different concentrations was mixed with 2 mL of DPPH solution (0.1 mM with 50 % ethanol), shaken vigorously, and incubated in the dark at room temperature for 30 min. Then, the absorbance value of the reaction solution was quickly measured at 517 nm. Each concentration gradient was operated in parallel three times, and the DPPH radical clearance capacity of polysaccharide was calculated as the following formula:
Where: As is the absorbance of 2 mL of polysaccharide solution mixed with 2 mL of DPPH solution, Ab is the absorbance of 2 mL of water mixed with 2 mL of DPPH solution, Ac is the absorbance of 2 mL of polysaccharide solution mixed with 2 mL of anhydrous 50 % ethanol.
2.4.2 The clearance activity of hydroxyl radical of HPs
Hydroxyl radical clearance capacity was carried out as reference with slight modifications (Sun et al., 2022; Hui, & Gao, 2022). In short, 1 mL of polysaccharide solution with different concentrations was fully mixed with 1 mL of FeSO4·7H2O solution (9 mM) and 1 mL of H2O2 solution (10 mM), and incubated at 37 °C for 10 min. Then, 1 mL of salicylic acid solution (9 mM) was added, mixed well, and continued to incubate at 37 °C for 30 min. Thereafter, the absorbance value was measured at 510 nm. Each concentration gradient was operated in parallel three times, and the hydroxyl radical clearance capacity of polysaccharide was calculated as the following formula:
Where: As is the absorbance of polysaccharide solution with different concentrations mixed with FeSO4·7H2O solution and salicylic acid solution; Ac is the absorbance value of polysaccharide solution with different concentrations mixed with FeSO4·7H2O solution and distilled water; Ab is the absorbance value of distilled water mixed with FeSO4·7H2O solution and salicylic acid solution.
2.5 Determination of HPs hypoglycemic activity in vitro
2.5.1 The HPs inhibitory effect of α-amylase
α-amylase inhibitory effect of Pariniae polysaccharides was measured according to a previous report (Hui, & Gao, 2022a). In brief, 0.04 mL of polysaccharide with the concentrations of 0.125, 0.25, 0.5, 1, 2 and 4 mg/mL were transferred into 96 well plates, added 0.04 mL of phosphate buffer (0.1 mol/L, pH 6.8) and 0.02 mL of α-amylase solution, mixed thoroughly. After 15 min of warm bath at 37 °C, 0.04 mL of PNPG substrate (10 mmol/L) was added and mixed well again. After water bath at 37 °C for 20 min, the absorbance value was measured at 405 nm. The experiment was repeated three times, and the calculation formula was as follows:
Where: A1 is the absorption of sample; A2 is the absorption of sample mixed with substrate; A3 is the absorption of enzyme mixed with substrate; A0 is the absorption value of substrate.
2.5.2 The HPs inhibitory effect of α-glucosidase
α-glucosidase inhibitory effect of Pariniae polysaccharides was measured by the reported method with slight modifications (Sun et al., 2022). In brief, 0.03 mL of polysaccharide with the concentration of 0.125, 0.25, 0.5, 1, 2 and 4 mg/mL were transferred into 96 well plates, added 0.03 mL of α-glucosidase (10 U/mL) and mixed completely. After 15 min of warm bath at 37 °C, 0.03 mL of soluble starch (1 %) was added and mixed well again, then heated in a water bath at 37 °C for 15 min, added 0.05 mL of DNS. After boiling water bath for 15 min, 0.11 mL of distilled water was added, and the absorbance was measured at 660 nm. Repeat the test three times, and the calculation formula was as follows:
Where: A1 is the absorption of sample; A2 is the absorption of sample mixed with substrate; A3 is the absorption of enzyme mixed with substrate; A0 is the absorption value of substrate.
2.6 Statistical analysis
All experiments were carried out in triplicate. The data and the correlation analysis were analyzed by SPSS software (version 18.0 for Windows, SPSS Inc., Chicago, IL, USA), while differences between the groups were analyzed by one-way analysis of variance (ANOVA), and p ≤ 0.05 was considered statistically significant.
3 Results and discussion
3.1 Single factor test analysis of WP, WC, WPC, PU, and CU
As shown in the Fig. 1a, the extraction yield of polysaccharide first increased and then decreased with the increase of enzyme addition. When the enzyme addition was 2.0 %, the extraction yield reached the maximum. Under certain conditions, the increase of enzyme amount accelerated the dissolution of cell wall, which was conductive to the dissolution of polysaccharides. However, an excessive addition of enzymes could lead to the complete saturation of the substrate with enzyme molecules, which resulted in protease autolysis and hydrolysis of other enzymes, ultimately leading to a reduction in the substrate's hydrolysis rate (Hui, Jin, Zhao, et al., 2022; Guo et al., 2020). Therefore, the amount of enzyme added for PW was selected as 2 %, 2.5 %, and 3 %. Similarly, for WC, and WPC, the enzyme amounts were chosen as 2 %, 2.5 %, and 3 %, for PU, and CU, the enzyme amounts were chosen as 1.5 %, 2 %, and 2.5 %.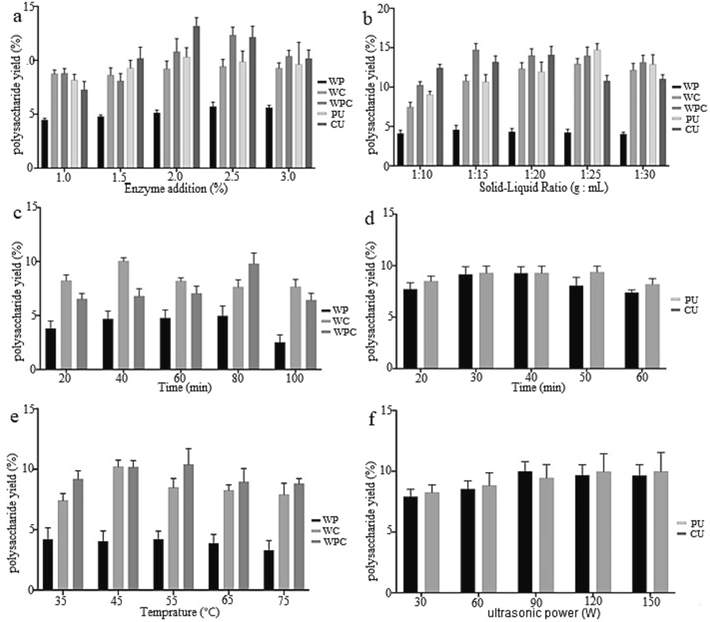
Effects of enzyme addition (a), solid–liquid ratio (b), time (c, d), temperature (e) and ultrasonic power (f) on the extraction rate of Patriniae polysaccharides with different extraction methods.
As the increase of the solid–liquid ratio, the extraction yield of polysaccharide first increased and then decreased (Fig. 1b). Polysaccharide extraction could be achieved effectively with an appropriate solid–liquid ratio, nevertheless, continuous increasing this ratio would lead to higher difficulty of concentration and energy wastage. Thus, the solid–liquid ratios of 1:10, 1:15, and 1:20 were chosen for WP and WPC's orthogonal test level, respectively. In the case of the orthogonal test level for WC and PU, the selected ratios were 1:20, 1:25, and 1:30, while the orthogonal test level of CU's solid–liquid ratios were selected for 1:15, 1:20, and 1:25, respectively.
Likewise, the time factors of 60 min, 80 min, and 100 min were selected for the orthogonal test level of WPC, while WP's orthogonal test level had the time factors of 40 min, 60 min, and 80 min. WC possessed its time level of orthogonal test as 20 min, 40 min, and 60 min, whereas, PU and CU owned its time level of orthogonal test as 30 min, 40 min, and 50 min, respectively, as illustrated in Fig. 1c and 1d. Additionally, for WPC, the temperature level of orthogonal test were chosen as 45 °C, 55 °C, and 65 °C, while WP and WC had its temperature level of orthogonal test 35 °C, 45 °C, and 55 °C, as shown in Fig. 1e.
Moreover, as depicted in Fig. 1f, the extraction yield of PU-HPs initially increased and then decreased with the rise of ultrasonic power. Subsequently, the extraction yield continued to increase, but the growth trend tended to level off, and no significant difference was observed. Therefore, the ultrasonic power level of orthogonal test for PU were selected as 60 W, 90 W, and 120 W, while the selected level of orthogonal test for CU were 90 W, 120 W, and 150 W.
3.2 Orthogonal test analysis of WP, WC, WPC, PU and CU
Based on the analysis of the orthogonal test results presented in Table 3 and the variance analysis in Table S1 and S2, the four factors A, B, C, and D in WP, WC, WPC, PU, and CU significantly influenced the polysaccharide yield. Therefore, the four factors in WP, WC, WPC, PU, and CU were selected at test level 3, indicating that the optimal process for WP, WC, WPC, PU, and CU was A3B3C3D3. Subsequent verification revealed that the polysaccharide yields of WP, WC, WPC, PU, and CU under optimum extraction processes were 20.97 %, 23.18 %, 15.61 %, 27.47 % and 30.50 %, respectively, which were significantly higher than that of HW (approximately around 3.42 %). Considering previous reports (Hui, Jin, Zhao, et al., 2022; Hui, & Gao, 2022), the yields of PCU-HPs and WU-HPs were 25.01 % and 22.63 %, respectively. It can be concluded that the combination of ultrasonic treatment with papain or cellulase extraction exhibited high efficiency, with CU showing the highest polysaccharide yield among all test methods so far. Therefore, CU was identified as the optimal method for preparing HPs from the perspective of polysaccharide yield, whose optimal process were solid–liquid ratio of 1:25, ultrasonic power of 150 W, ultrasonic time of 50 min, enzyme addition of 2.5 %.
3.3 Physicochemical characteristics analysis of HPs
3.3.1 Chemical composition analysis of HPs
The results were shown in Table S3. It was found that the reducing sugar content of polysaccharides extracted by ultrasonic or enzyme treatment was significantly increased. Among them, the PCU-HPs exhibited the highest reducing sugar content, reaching up to 8.8 %, which was nearly 30 times that of HW-HPs. This might be due to the degradation of some bound proteins and lipids by ultrasound or enzyme, which promoted the depolymerization of polysaccharide molecular structure, so that more hydroxyl groups at the reducing end were freed, resulting in an increase in reducing sugar content (Al-Shawi et al., 2021; Wu et al., 2019; Fu et al., 2019). Whereas, the protein content of polysaccharides extracted by different methods basically kept except for PCU extraction, which was due to the synergistic degradation of cellulase and papain into small molecular proteins (Feng et al., 2022).
3.3.2 Monosaccharide composition analysis of HPs
The results were showed in Fig. 2. and Table 4. All HPs extracted by different methods were mainly composed of arabinose, glucose, mannose, and galactose with varying molar ratios. Whereas, the WP-HPs, CU-HPs and HW-HPs contained a small amount of xylose. This might be related with the degradation or conversion of xylose during processing, suggesting that xylose was mainly located at the end of the branch or main chain of the skeleton of Patriniae polysaccharide. Compared with the HW-HPs, enzyme assisted ultrasound method increased the content of mannose and glucose in HPs, and decreased the content of galactose and xylose. While the ultrasonic method reduced the content of mannose, glucose, and xylose in HPs, but increased the content of galactose. This might be the rearrangement of atoms caused by ultrasound and conversion of some mannoses and glucoses into galactose (Hu et al., 2020). In contrast, the enzymatic method generally increased the content of mannose and glucose in HPs, and reduced the content of galactose except for the cellulase method. In general, mannose was derived from cellulose and hemicellulose in plant cell walls. The cellulase used in current experiment mainly contained endoglucanase, which randomly cleaved the β-1,4 glycosidic bonds within cellulose molecules in the cell wall to generate cellobiose and cellooligosaccharides, which caused them to dissolve in the solvent with the polysaccharides, thereby altering the composition ratio of monosaccharides in the polysaccharides. (Huang et al., 2019; Chen et al., 2018).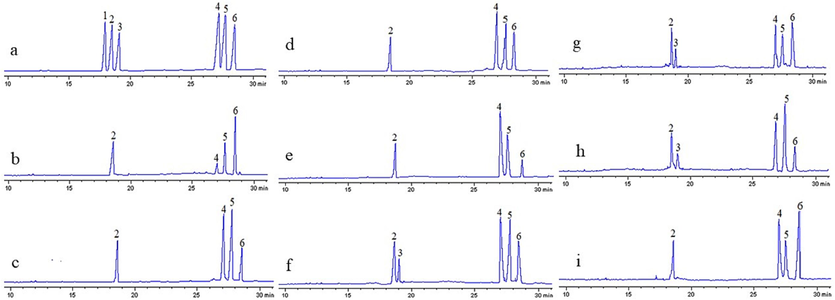
(a): GC profile of monosaccharide standards (1. Rha, 2. Ara, 3. Xyl, 4. Man, 5. Glc, 6. Gal). (b–i): GC profiles of the Patriniae polysaccharides extracted by WU, PCU, WPC, PU, WP, HW, CU, and WC methods.
Monosaccharide and molar ratios /%
Ara
Rha
Xyl
Man
Glu
Gal
WU
1.00
–
–
0.12
0.53
2.48
PCU
1.00
–
–
2.58
4.1
0.52
WPC
1.00
–
–
3.41
2.78
1.59
PU
1.00
-a
–
3.18
1.34
0.24
WP
1.00
–
0.32
4.1
3.78
1.01
HW
1.00
–
0.49
1.47
1.18
1.59
CU
1.00
–
0.15
4.89
5.1
0.81
WC
1.00
–
–
2.1
1.19
3.01
3.3.3 Molecular weight analysis of HPs
Generally, the bioactivities of polysaccharides are closely related to their molecular weight (Hui et al., 2019; Feng et al., 2022; Hui et al., 2023). As shown in Fig. 3a-h. and Table S4, the polysaccharides extracted by different methods contained about three kinds of polysaccharides with different molecular weights, indicating that these polysaccharides were heterogeneous polysaccharides. The proportion of high molecular weight polysaccharides in PU, HW and WU were basically the same as that of low and medium molecular weight polysaccharides. Whereas, the high molecular weight polysaccharides contained in WP, CU, WC, WPC and PCU were dominant, accounting for 88.56 %, 79.93 %, 74.30 %, 73.27 %, 67.61 %, respectively, while low and medium molecular weight polysaccharides were contained secondarily. This might be due to the different depolymerization and degradation ability of enzyme and ultrasound. The depolymerization and degradation ability of papain for HPs was weaker than that of cellulase, which resulted in the higher content of high molecular weight HPs. The order of average molecular weight was PCU-HPs > CU-HPs > WPC-HPs > WC-HPs > HW-HPs > WP-HPs > WU-HPs > PU-HPs.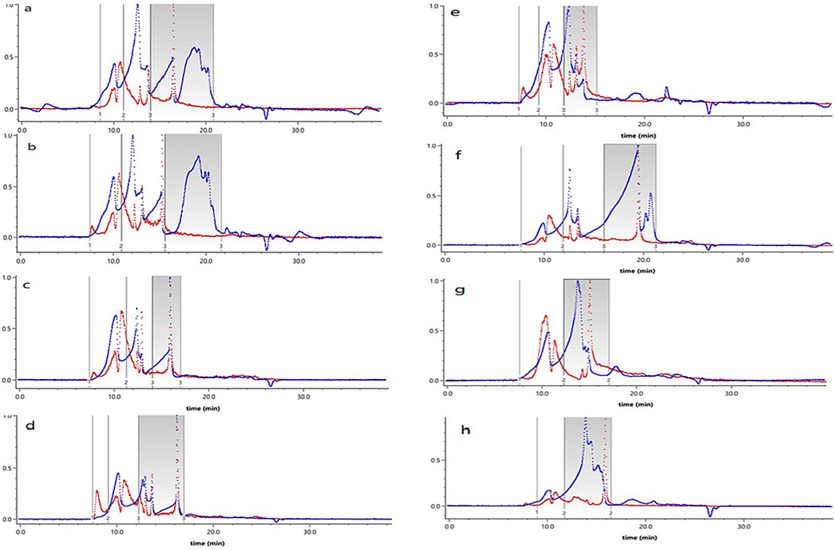
Profile of SEC-MALLS assay of Patriniae polysaccharides from different extraction methods (a. PU; b. WP; c. CU; d. WC; e. WPC; f. PCU; g. HW; h. WU). Light scattering signal (red), Refractive index signal (blue). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In addition, the ultrasonic and enzymatic extraction increased the molecular weight distribution range (DR) of HPs, further showing that enzymes and ultrasound had different depolymerization and degradation abilities on HPs. The DR value of polysaccharides was close to 1, which belonged to the narrow distribution of width sample, and the DR value was greater than 2, which belonged to the wide distribution of width sample (Tang, He, Liu, et al., 2023; Bai et al., 2023; Sheng et al., 2014). Looking from the Table S4, WP-HPs was a wide distribution of width sample, and the HPs extracted by other methods was a narrow distribution of width sample. Enzymatic method, ultrasonic method or their combination can cause the degradation, grafting and crosslinking reaction of polysaccharides, and then change the physicochemical characteristics of polysaccharides, such as molecular weight and its distribution as well as monosaccharide composition, thus affecting the biological activity of polysaccharides (Ji, et al.,2023; Xue,et al., 2022).
3.4 In vitro antioxidant activities of HPs
3.4.1 DPPH free radical clearance activity
DPPH as a stable nitrogen-centered free radical and is widely employed to screen various antioxidant products. As shown in Fig. 4a, all polysaccharides extracted by different methods presented a good effect on DPPH free radical, which was much better than that of comfrey root and Ginkgo biloba seed polysaccharides (Chen et al., 2018; Hu et al., 2020). With the increase of concentration, the DPPH free radical clearance ability of CU-HPs and PU-HPs initially demonstrated a decrease as the concentrations < 0.5 mg/mL, followed by an increase trend within the range of 0.5–4 mg/mL. In contrast, the DPPH free radical clearance ability of WPC-HPs exhibited an initial increase with concentrations < 2 mg/mL, but decreased within the 2–4 mg/mL range. However, the WC-HPs showed a positive concentration-dependent manner for DPPH free radical clearance ability, and the WP-HPs showed a negative concentration-dependent pattern for DPPH free radical clearance ability. This positive or negative trend gradually leveled off after the polysaccharide concentration reaching to 2 mg/mL. At a concentration of 0.25 mg/mL, the DPPH free radical clearance rate of all HPs was approximately 60 %. When the concentration climbed 4 mg/mL, the DPPH free radical clearance rate of WP-HPs, WC-HPs and CU-HPs exceeded 80 %. Conversely, the PU-HPs and WPC-HPs yielded clearance rate of 62 % and 78 % at 4 mg/mL, respectively. Compared the findings with prior literature (Hui, & Gao, 2022; Hui, Jin, Zhao, et al., 2022) and current data, cellulase-assisted extraction improved much more clearance ability of HPs on DPPH free radical than that of papain-assisted extraction. Interestingly, ultrasound-assisted extraction without enzyme addition led to a reduction of the clearance ability of HPs on DPPH free radical. In terms of DPPH free radical clearance ability, the orders were as follows: WC-HPs > WP-HPs > CU-HPs > WPC-HPs > PCU-HPs > HW-HPs > WU-HPs > PU-HPs.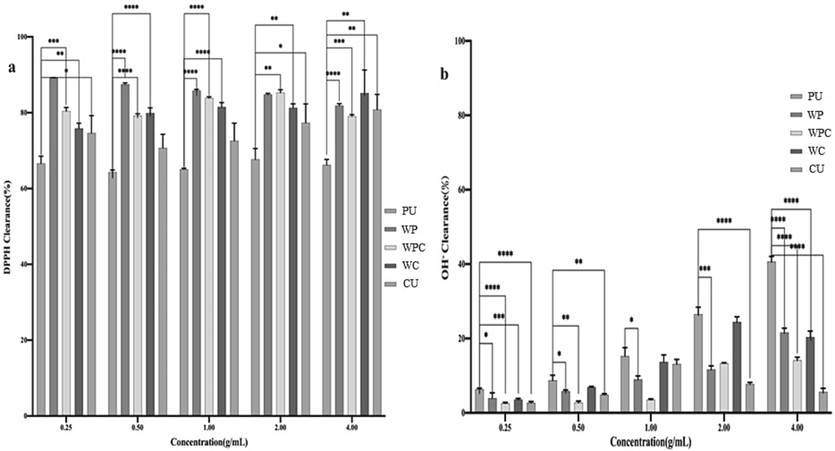
Clearence ability of Patriniae polysaccharides with different extraction methods on DPPH (a) and hydroxyl radical (b).
3.4.2 Hydroxyl radical clearance activity
To date, hydroxyl radicals is the most potent oxidant, capable of reacting with biomacromolecules such as proteins, lipoproteins, and nucleic acids within living cells. This reaction triggers tissue and cellular damage, leading to various pathological and physiological phenomena. As depicted in Fig. 4b, the hydroxyl radical clearance ability of HPs extracted by different methods in current study was weaker than that of HW-HPs and WU-HPs (Hui, & Gao, 2022; Zhang et al., 2022). At a concentration 4 mg/mL, the hydroxyl radical clearance rate of HW-HPs and WU-HPs reached 90 % and 82 %, respectively. Whereas, the PU-HPs, WP-HPs, WPC-HPs, WC-HPs, and CU-HPs showed weak clearance effect on hydroxyl radicals, with clearance rate of 40 %, 21 %, 14 %, 20 %, and 6 % at 4 mg/mL, respectively. In view of the above analysis, the ultrasound and enzyme assisted extraction were did not significantly enhance the inhibitory ability of HPs on hydroxyl radicals. However, it was observed that the hydroxyl radical clearance ability of HPs extracted by different methods gradually increased with the increasing concentration, which was in accordance with the earlier reports (Feng et al., 2022; Bai et al., 2023; Hui, & Gao, 2022; Tang, He, Liu, et al., 2023). In terms of hydroxyl radical clearance ability, the orders were: HW-HPs > WU-HPs > PCU-HPs > PU-HPs > WP-HPs > WPC-HPs > WC-HPs > CU-HPs. Combined with DPPH free radical clearance ability, CU-HPs exhibiting a moderate and mild antioxidant activity compared to other extraction methods.
3.5 In vitro hypoglycemic activity of HPs
3.5.1 α-amylase inhibition activity
As shown in Fig. 5a, it could be seen that the rate of inhibition on α-amylase by WPC-HPs decreased with the increase of concentration. In contrast, WP-HPs, WC-HPs, and CU-HPs showed an increasing concentration-dependent inhibitory trend, while PU-HPs exhibited the highest inhibition rate at 2 mg/mL, with a value of 68.51 %. Notably, at a concentration of 0.0625 mg/mL, the inhibition rate of WPC-HPs was close to 100 %, then reduced to 48.88 % at 4 mg/mL. However, the WP-HPs, WC-HPs, and CU-HPs at 4 mg/mL presented inhibition rates of 76.36 %, 82.78 %, and 62.21 %, respectively. Preliminary analysis suggested that the mixed enzyme method for inhibiting α-amylase was more effective than the single enzyme method, and the α-amylase inhibitory activity of HPs using papain and cellulase-assisted extraction were superior to that of water extraction alone. These findings were consistent with the results of previous research (Bai et al., 2023; Guo et al., 2020; Feng et al., 2022; Tang, He, Liu, et al., 2023; Hu et al., 2020).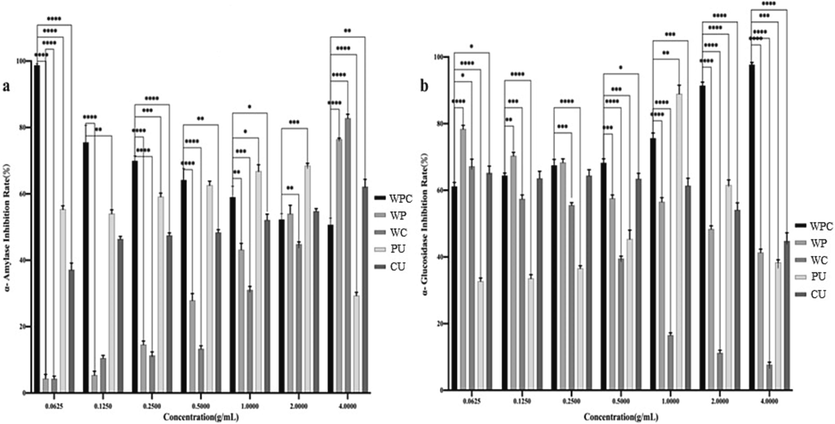
Effect of Patriniae polysaccharides with different extraction methods on inhibition of α- amylase (a) and α- glucosidase (b).
3.5.2 α-glucosidase inhibition activity
The results presented in Fig. 5b demonstrated the inhibition rates of HPs extracted by various methods. Specifically, the inhibition rates of WPC-HPs and HW-HPs for α-glucosidase increased with increasing concentration, while the inhibition rates of WP-HPs, WC-HPs, and CU-HPs decreased. Within the test concentration range, the maximum inhibition rates of WPC-HPs, HW-HPs, WP-HPs, WC-HPs, and CU-HPs on α-glucosidase were 97.69 %, 96.87 %, 78.38 %, 67.23 % and 66.29 %, respectively. Apparently, the α-glucosidase inhibition rate of HPs extracted using the compound enzyme was superior to that of a single enzyme extraction, suggesting that papain and cellulase extraction had a synergistic effect on the inhibition of α-glucosidase by HPs. Intriguingly, the inhibition rates of α-glucosidase by PCU-HPs and PU-HPs reached their maximum at a concentration of 1 mg/mL, with values of 86.33 % and 88.95 %, respectively, indicating that ultrasound-assisted extraction had a promoting inhibitory effect on α-glucosidase by HPs. These results implied that different extraction methods might influence the physicochemical characteristics of HPs, such as total polysaccharide content, protein content, reducing sugar content, molecular weight, and monosaccharide composition, thereby impacting their inhibitory effect on α-glucosidase (Xiong et al., 2022; Sun et al., 2022), and the specific relationship will be discussed later. Combined with the ability of inhibition on α-amylase, CU-HPs exhibited a mild hypoglycemic activity compared to other extraction methods.
3.6 Correlation analysis of HPs
Correlation analysis was carried out to understanding the relationship between the characteristics and activities of the HPs. Many factors impacted the hypoglycemic and antioxidant activities of polysaccharides, such as their reducing sugar content, protein content, molecular weight, and monosaccharide composition (Fernandes et al., 2023; Yi et al., 2020; Wang et al., 2021; Ji et al., 2023; Shi et al., 2023). As shown in Table 5, the content of reducing sugar and protein in HPs showed significantly positive correlations with α-glucosidase (p < 0.01) and α-amylase (p < 0.05) inhibition, respectively. As shown in Table 6, the content of reducing sugar was positively correlated with glucose content (p < 0.01), arabinose content (p < 0.05), and molecular weight (p < 0.01), respectively, but negatively correlated with galactose content (p < 0.05). In addition, the content protein was significantly positively correlated with mannose (p < 0.01), glucose (p < 0.05) and xylose content (p < 0.05). Accordingly, it was deduced that the content of galactose was positively correlated with hydroxyl radical inhibition (p < 0.05) and negatively correlated with α-glucosidase inhibition (p < 0.05), while the content of glucose was positively correlated with α-glucosidase inhibition (p < 0.05). Similarly, it was inferred that the content of mannose, glucose and xylose was positively correlated with α-amylase inhibition (p < 0.05), and the molecular weight was positively correlated with α-glucosidase inhibition (p < 0.05). Obviously, the hypoglycemic and antioxidant activities of HPs were not a function of a single factor but rather a combination of several factors. However, the correlation analysis results suggested that the HPs with higher content of reducing sugar, protein, glucose, mannose, xylose, and lower content of galactose had stronger hypoglycemic activities, while HPs with higher content of galactose had superior inhibition on hydroxyl radical, but weaker inhibition on DPPH free radical. What’ more, HPs with a higher content of the high molecular weight polysaccharide had stronger α-glucosidase inhibition, which was consistent with previous reports (Xiong et al., 2022; Sun et al., 2022; Wen et al.,2023; Adil Gani et al., 2022). Generally, the glucose adsorption is the key factor to evaluate the hypoglycemic effect of polysaccharide (Tang, He, Liu, et al., 2023; Ji et al., 2023). The HPs with high molecular weight probably had more surface charges and larger particles to bind to glucose, which further inhibited the α-glucosidase activity.
Reducing sugar
Rotal sugar
Protein
DPPH
Hydroxyl radical
α-amylase inhibition
α-glucosidase inhibition
Reducing sugar
1
0.102
−0.009
0.291
−0.112
0.198
0.606**
Total sugar
0.102
1
0.226
−0.401
−0.233
0.032
−0.173
Protein
−0.009
0.226
1
−0.031
−0.191
0.457*
0.157
DPPH
0.291
−0.401
−0.031
1
−0.241
0.06
0.317
Hydroxyl radical
−0.112
−0.233
−0.191
−0.241
1
−0.23
0.336
α-amylase inhibition
0.198
0.032
0.457*
0.06
−0.23
1
0.543**
α-glucosidase inhibition
0.606**
−0.173
0.157
0.317
0.336
0.543**
1
Reducing sugar
Rotal sugar
Protein
Ara
Xyl
Man
Glu
Gal
Molecular weight
Reducing sugar
1
0.102
−0.009
0.449*
−0.229
0.249
0.576**
-0.467*
0.779**
Total sugar
0.102
1
0.226
−0.035
−0.03
0.058
0.072
−0.056
0.169
Protein
−0.009
0.226
1
−0.338
0.413*
0.562**
0.475*
−0.304
0.064
Ara
0.449*
−0.035
−0.338
1
−0.207
0.115
0.143
−0.185
0.254
Xyl
−0.229
−0.03
0.413*
−0.207
1
0.072
0.069
−0.099
−0.261
Man
0.249
0.058
0.562**
0.115
0.072
1
0.812**
-0.604**
0.352
Glu
0.576***
0.072
0.475*
0.143
0.069
0.812***
1
-0.579**
0.728**
Gal
-0.467*
−0.056
−0.304
−0.185
−0.099
-0.604**
-0.579**
1
−0.312
Molecular weight
0.779**
0.169
0.064
0.254
−0.261
0.352
0.728**
−0.312
1
Combined the above analysis, the moderate content of galactose, the higher content of reducing sugar, protein, glucose, mannose, and xylose, as well as higher molecular weight polysaccharides were more conducive to the hypoglycemic and antioxidant activity of HPs. The content of galactose in WPC-HPs, WC-HPs, and WU-HPs were too high to contain xylose. Interestingly, CU-HPs had the highest content of protein, glucose, and mannose, moderate content of reducing sugar and galactose, and a certain amount of xylose. Therefore, it included that the process of CU was more suitable for Patriniae polysaccharide extraction because of its highest yield and excellent physicochemical characteristics.
4 Conclusions
This study adopted single-factor and orthogonal tests to optimize the extraction process of HPs by using WP, WC, WPC, PU, and CU methods, then compared the physicochemical characteristics, antioxidant, and hypoglycemic activities of HPs. The results showed that enzyme-assisted extraction increased the molecular weight distribution, reducing sugar, mannose, and glucose content of HPs, while ultrasound-assisted extraction increased the content of reducing sugar in HPs and reduced the content of protein, mannose, and glucose in HPs. All HPs exhibited concentration-dependent antioxidant and hypoglycemic activities. Correlation analysis indicated that HPs with moderate galactose content, higher content of reducing sugar, protein, glucose, mannose, xylose, and molecular weight polysaccharide would exhibit superior hypoglycemic and antioxidant activities. Interestingly, CU-HPs possessed these favorable characteristics. Combined its highest polysaccharide yield, CU was a promising candidate for the best extraction method of HPs, whose optimal process was: enzyme dosage of 2.5 %, liquid-to-material ratio of 1:25, ultrasonic power of 150 W, and ultrasonic time of 50 min. The findings provide a foundational understanding of the structure–activity relationship and high valuable utilization of Patriniae polysaccharides. However, multiple factors, such as purification methods, glycosidic bond types and their linkage pattern much influenced the activities of polysaccharide except extraction methods. Thus, the next step will involve researching the purification and fine structure of Patriniae polysaccharides and their activities in vivo.
Acknowledgments
This work was supported by Dr. Kim of Shangluo University (22SKY106), Shaanxi Qinling Industry Technology Research Institute of Special Biological Resources Co. Ltd. 2022 Annual Open Fund Project (23HKY035).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Extraction of polysaccharide from Althea rosea and its physicochemical, anti-diabetic, anti-hypertensive and antioxidant properties. Sci. Rep.. 2022;12:17116.
- [Google Scholar]
- Review on the “Biological applications of Okra polysaccharides and prospective research”. Future J. Pharma. Sci.. 2021;7:102.
- [Google Scholar]
- Comparison in structural, physicochemical and functional properties of sweet potato stems and leaves polysaccharide conjugates from different technologies. Int. J. Biol. Macromol.. 2023;247:125730
- [Google Scholar]
- Modern research progress on Patriniae. J. Guangdong Med. Univ.. 2017;33(06):816-821.
- [Google Scholar]
- Effects of different extraction techniques on physicochemical properties and activities of polysaccharides from comfrey (Symphytum officinale L.) root. Ind. Crop. Prod.. 2018;121:18-25.
- [Google Scholar]
- Physicochemical properties and in vitro bioactivities of polysaccharides from lotus leaves extracted by different techniques and solvents. J. Food Meas. Charact.. 2022;16:1583-1594.
- [Google Scholar]
- The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym.. 2023;314:120965
- [Google Scholar]
- Physicochemical characteristics and biological activities of polysaccharides from the leaves of different loquat (Eriobotrya japonica) cultivars. Int. J. Biol. Macromol.. 2019;135:274-281.
- [Google Scholar]
- A review on plant polysaccharide based on drug delivery system for construction and application, with emphasis on traditional Chinese medicine polysaccharide. Int. J. Biol. Macromol.. 2022;211:711-728.
- [Google Scholar]
- The Herba Patriniae (Caprifoliaceae): A review on traditional uses, phytochemistry, pharmacology and quality control. J. Ethnopharmacol.. 2021;265:113264
- [Google Scholar]
- Enzyme-assisted extraction of a cup plant (Silphium perfoliatum L.) polysaccharide and its antioxidant and hypoglycemic activities. Process Biochem.. 2020;92:17-28.
- [Google Scholar]
- Comparison in bioactivity and characteristics of Ginkgo biloba seed polysaccharides from four extract pathways. Int. J. Biol. Macromol.. 2020;159:1156-1164.
- [Google Scholar]
- Physicochemical properties and prebiotic activities of polysaccharides from longan pulp based on different extraction techniques. Carbohydr. Polym.. 2019;206:344-351.
- [Google Scholar]
- Hui, H. P., Jin, H., Li, X. Z., Yang, X. Y., Cui, H. Y., Xin, A. Y., et al. 2019. Purification, characterization and antioxidant activities of a polysaccharide from the roots of Lilium davidii var. unicolor Cotton. International Journal of Biological Macromolecules, 2019, 135, 1208-1216.
- Physicochemical features and antioxidant activity of polysaccharides from Herba Patriniae by gradient ethanol precipitation. Arab. J. Chem.. 2022;15:103770
- [Google Scholar]
- Structure characterization, antioxidant and hypoglycemic activity of an arabinogalactoglucan from Scutellaria baicalensis Georgi. Int. J. Biol. Macromol.. 2022;207:346-357.
- [Google Scholar]
- Studies on the ultrasonic composite-enzyme extraction, structure and antibacterial activity of Herba Patriniae polysaccharide. Chinese J. Mod. Appl. Pharma.. 2022;39(11):1412-1418.
- [Google Scholar]
- Study on the enzymatic hydrolysis of lanzhou lily polysaccharide and its antioxidant activity in vitro. Farm Prod. Process.. 2022;12(566):23-28.
- [Google Scholar]
- The structure and antioxidant activities of three high molecular weight polysaccharides purified from the bulbs of Lanzhou lily. J. Food Meas. Charact.. 2023;17:800-812.
- [Google Scholar]
- Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Human Wellness. 2023;12(6):1969-1980.
- [Google Scholar]
- Ginseng polysaccharide reduces autoimmune hepatitis inflammatory response by inhibiting PI3K/AKT and TLRs/NF-κB signaling pathways. Phytomedicine. 2023;116:154859
- [Google Scholar]
- Antioxidant properties of different molecular weight polysaccharides from Athyrium multidentatum (Doll.) Ching. Carbohydr. Polym.. 2014;108:41-45.
- [Google Scholar]
- Bioactive effects advances of natural polysaccharides. J. Future Foods. 2023;3(3):234-239.
- [Google Scholar]
- Ultrasound-assisted extraction and characteristics of maize polysaccharides from different sites. Ultrason. Sonochem.. 2023;95:106416
- [Google Scholar]
- Study on the new anti-atherosclerosis activity of different Herba patriniae through down-regulating lysophosphatidylcholine of the glycerophospholipid metabolism pathway. Phytomedicine. 2022;94:153833
- [Google Scholar]
- Preparation, structure and α-glucosidase inhibitory of oligosaccharides by enzymatic hydrolysis from Annona squamosa polysaccharide. Ind. Crop. Prod.. 2022;177:114468
- [Google Scholar]
- Effects of different extraction methods on the structural, antioxidant and hypoglycemic properties of red pitaya stem polysaccharide. Food Chem.. 2023;405:134804
- [Google Scholar]
- Natural polysaccharides protect against diet-induced obesity by improving lipid metabolism and regulating the immune system. Food Res. Int.. 2023;172:113192
- [Google Scholar]
- Advances in the extraction, purification, structural-property relationships and bioactive molecular mechanism of Flammulina velutipes polysaccharides: A review. Int. J. Biol. Macromol.. 2021;167:528-538.
- [Google Scholar]
- Wen, L., Wu, Z. W., Lin, L. W., AI-Romaima, A., Peng, X. R., & Qiu, M. H. 2023. Structural characterizations and α-glucosidase inhibitory activities of four Lepidium meyenii polysaccharides with diferent molecular weights. Natural Products and Bioprospecting, 13, 18.
- Physicochemical characteristics and antioxidant activities of non-starch polysaccharides from different kiwifruits. Int. J. Biol. Macromol.. 2019;136:891-900.
- [Google Scholar]
- Physicochemical properties, antioxidant activities and α-glucosidase inhibitory effects of polysaccharides from Evodiae fructus extracted by different solvents. Int. J. Biol. Macromol.. 2022;194:484-498.
- [Google Scholar]
- Rheological properties, gel properties and 3D printing performance of soy protein isolate gel inks added with different types of apricot polysaccharides. Int. J. Biol. Macromol.. 2023;242:124624
- [Google Scholar]
- Recent advances in medicinal and edible homologous polysaccharides: Extraction, purification, structure, modification, and biological activities. Int. J. Biol. Macromol.. 2022;222:1110-1126.
- [Google Scholar]
- Mechanistic insights into the anti-tumor and anti-metastatic effects of Patrinia villosa aqueous extract in colon cancer via modulation of TGF-β R1-smad2/3-E-cadherin and FAK-RhoA-cofilin pathways. Phytomedicine. 2023;117:154900
- [Google Scholar]
- Natural polysaccharides experience physiochemical and functional changes during preparation: A review. Carbohydr. Polym.. 2020;234:115896
- [Google Scholar]
- Optimization of ultrasonic extraction technology and in vitro antioxidant activity of polysaccharide from Herba Patriniae using response surface methodology. Chinese Chem. Res. Appl.. 2022;34(9):2074-2081.
- [Google Scholar]
- Zheng, C. J. (2019). Study on chemical constituents of dichloromethane layer of Herba Patriniae and its bioactivity of promoting intestinal peristalsis. Journal of Tianjin Medical University.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105460.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







