Translate this page into:
Protective effect of polysaccharide from the artificially cultivated Sanghuangporus vaninii against H2O2-induced toxicity in vitro and in zebrafish models
⁎Corresponding author. zzf2050@163.com (Zhang Zuofa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
PFSV-2 was a purified polysaccharide from Sanghuangporus vaninii. The phytochemical properties of PFSV-2 were characterized. PFSV-2 could reduce H2O2-induced cytotoxicity in L929 cells. PFSV-2 exhibited effective protection against H2O2-stimulated oxidative stress in zebrafish.
Abstract
PFSV-2 is a purified polysaccharide with good hypolipidemic activity from artificially cultivated Sanghuangporus vaninii. In this study, PFSV-2 was characterized by scanning electron microscopy, atomic force microscopy, X-ray diffraction, zeta potential, thermogravimetric analysis and the Congo red test. To assess the protective effect of PFSV-2, H2O2-induced oxidant injury on L929 cells and larval zebrafish models was employed. PFSV-2 significantly reduced H2O2-induced cytotoxicity in L929 cells by decreasing intracellular ROS levels and cell apoptosis. Furthermore, PFSV-2 had an obvious protective effect against H2O2-stimulated oxidative stress in larval zebrafish by decreasing heart rate and reducing ROS generation and cells death. Additionally, PFSV-2 decreased the production of MDA and increased the activity of antioxidant enzymes SOD and CAT in both L929 cells and larval zebrafish. These results suggested that PFSV-2 may be useful as an antioxidant in the medical and cosmetic industries.
Keywords
Sanghuangporus vaninii
Purified polysaccharide
Antioxidant activity
Zebrafish
L929 cells
1 Introduction
Reactive oxygen species (ROS) are attracting considerable attention due to their important role in the biological functions of organisms (Kim and Jiang, 2010). At low concentrations, ROS mainly act as intercellular signaling molecules and growth factors (Wang et al., 2016). However, excessive accumulation of ROS can have deleterious effects on biological macromolecules and induce the disorganization of cell structures (Barzilai and Yamamoto, 2004). Indeed, the fundamental pathogenic mechanisms of many diseases have been found to originate from oxidative damage (Poprac et al., 2017). Searching for antioxidants as functional foods or medicines has attracted considerable attention (Hui and Gao, 2022).
In recent years, many natural antioxidants with no side effects have been employed to help protect the human body from oxidative damage. Epidemiological studies have revealed that intake of fresh vegetable, fruits, tea and wines (good source of natural antioxidants) is related to the reduction of the incidence of disease (Davovobasha et al., 2018). Dietary antioxidants include numerous phytochemicals such as vitamin A, vitamin C and vitamin E, α-tocopherol, β-carotene and phenolic compounds (Chandra et al., 2019). Mushrooms have been documented as a source of potentially safer natural antioxidants (Sánchez, 2017). Various antioxidant compounds such as polysaccharide, phenolic compounds, ascorbic acid, tocopherols and ergosterol have been isolated from mushrooms (Trznadel et al., 1990; Tsai et al., 2007).
The use of Sanghuangporus as a traditional medicine can be traced back 2000 years in China. As one of the most important species in Sanghuangporus, Sanghuangporus vaninii can be artificially cultivated, thus it occupies the largest share of the ‘Sanghuang’ application market (Zhou et al., 2022). A variety of active components including polysaccharides, terpenoids, flavonoids, polyphenols, etc., have been found in S. vaninii (Zhang et al., 2019). Polysaccharides, the major components of S. vaninii, have been the subject of several previous studies (Zhang et al., 2019).
Previously, our research group extracted and purified a novel polysaccharide (PFSV-2) with a molecular weight of 20.377 kDa from the fruiting body of S. vaninii; this was a heteropolysaccharide that was rich in fucose, galactose and mannose (Zhang et al., 2023). The backbone of PFSV-2 was composed of an → 6)-α-Galp (1→, →2, 6)-β-Manp(1 → and → 2)-α-Fucp (1 → and was branched at the O-2 position of the t-α-Manp(1→, the primary structure of PFSV-2 was exhibited in supplementary Fig. 1. It was found that PFSV-2 had outstanding lipid-lowering activity through decreasing lipid accumulation and levels of TC and TG in larval zebrafish. There is no doubt that the polysaccharides from S. vaninii are valuable for functional foods. However, the antioxidant activity of PFSV-2 has not been investigated. Accordingly, the present study investigated the antioxidant activity of PFSV-2 in L929 cells and zebrafish models.
2 Materials
2.1 Materials and reagents
PFSV-2 was extracted and purified from S. vaninii by our research group. Trypsin, H2O2, and the BCA protein test kit were obtained from Shanghai Baoman Biotech. Co., Ltd. Superoxide dismutase (SOD), catalse (CAT) and malondialdehyde (MDA) test kits were bought from the Nanjing Jiancheng Bioengineering Research Institute. L929 cells were obtained from China Infrastructure of Cell Line Resources (Beijing China). 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl(MTT), acridine orange, 2,7- dichlorofluorescein diacetate (DCFH-DA) and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO, USA).All other reagents were analytic reagents.
2.2 Characterization of PFSV-2
The surface morphology of PFSV-2 was observed by scanning electron microscopy (SEM, Philips XL 30 ESEM, Philips, Holland). The samples were fixed onto the sample stand and then sputter-coated with platinum powder for observation.
Atomic force microscopy (AFM, Multimode 8, Brooke Corporation, USA) of PFSV-2 was conducted according to the reported methods (Jin et al., 2006). Polysaccharide solution (1 μg/mL, 1 mL) was filtered through a 0.45 μm membrane and then dropped onto a mica sheet for AFM analysis. A Si3N4 probe was used to determine the micromorphological characteristic of PFSV-2.
X-ray diffraction (XRD) analysis of PFSV-2 was determined using an X-ray diffractometer (Siemens D 5000, Bruker, Germany). Patterns were recorded with a scanning speed of 1° /min and an incident current of 40 mA over a diffraction angle (2θ) range of 5–90.
A Zetasizer Nano S90 instrument (Malvern Instruments, UK) was used to determine the zeta potential of PFSV-2. Briefly, PFSV-2 was dissolved in distilled water (1 mg/mL) and analyzed in triplicate at 25 °C.
The thermal stability and mass loss of PFSV-2 during thermal treatment were monitored by differential scanning calorimetery (Discovery series DSC, TA Instruments, USA) and thermogravimetric analyzer (Discovery SDT 650, TA Instruments, USA). PFSV-2 powder (10 mg) was weighed and heated from 20 to 800 °C at a heat rate of 10 °C/min.
The conformation of PFSV-2 was determined by the Congo red test. Various concentrations of NaOH were added to a mixture of PSV (2 mg·mL−1) and Congo red (80 μM) and the mixtures were left to stand for 10 min. The maximum absorption wavelength of the samples was recorded using a spectrophotometer (Shanghai Precision and Scientific Instrument Co., Ltd, Shanghai, China).
2.3 Investigation of the protection of PFSV-2 on L929 cells against H2O2-induced oxidative damage
2.3.1 Cell culture
L929 cells were cultured in a 5% CO2 humidified atmosphere (37 °C). The cells were cultured using DMEM supplemented with fetal bovine serum (10%), of sodium pyruvate (110 μg/mL), streptomycin (100 μg/mL) and penicillin (100 unit/mL). The cells were incubated for 2 days and then seeded on a 96-well plate with a density of 1 × 104 cells/well.
2.3.2 Assessment of cell viability
After the medium was changed to serum-free medium for the L929 cells, different doses of PFSV-2 (100 and 200 μg/mL) and H2O2 (300 μM) were added to each well. After 24 h, the medium was removed and the cells were treated with MTT-containing medium (100 μg /mL) for 3 h.The medium was then aspirated and DMSO (100 μL) was added to the wells. The absorbance at 540 nm was recorded using a microplate reader (Model 680, Bio-Rad, USA).
2.3.3 Intracellular ROS generation
After the L929 cells medium was changed to the serum-free medium, the cells were pretreated with different concentrations of PFSV-2 (100 and 200 μg/mL) and incubated for 1 h. Then, H2O2 (300 μM) was added to the cells and allowed to incubate for 1 h. Then, the cells were treated with DCFH-DA solution (1.0 μg/mL). After 30 min, the fluorescence was measured with a microplate reader at 485 nm (excitation)/535 nm (emission).
2.3.4 Nuclear staining with propidium iodide (PI)
L929 cells were seeded on 24-well cell imaging plates with of 5 × 104 cells/well for 24 h. Then, the cells were pretreated with PFSV-2 (100 and 200 μg/mL). After 24 h, the cells were treated with H2O2 (300 μM). After 30 min, cells were stained with PI (100 μg/mL, 1.0 mL) and incubated for 20 min at 37 °C. Finally, the cells were washed twice with PBS and observed by fluorescence microscopy (Nikon Instruments Co., Ltd, Tokyo, Japan) at 535 nm (excitation)/615 nm (emission).
2.3.5 Analysis of mitochondrial membrane potential (MMP)
Rh123, a cell-permeant cationic dye, was used to monitor the mitochondrial membrane. MMP depolarisation results in the loss of Rh123 from the mitochondria and a decrease in intracellular fluorescence (Satoh et al., 1997). After the cells were treated with PFSV-2 plus H2O2 as described above, Rh123 (10 μM) was added to the cell culture and allowed to incubate for 30 min at 37 °C. The cells were then washed twice with PBS and then observed by flow cytometry. The laser emission was at 480 nm, and a 530 nm long-pass filter was used (Shimizu et al., 1996).
2.3.6 Detection of apoptotic cells with acridine orange/ethidium bromide (AO/EB) staining
The AO/EB double staining method was used for the morphological assessment of apoptotic cells. L929 cells were seeded in 24-well microtiter plates (1 × 104 cells per well) for 24 h. The cells were pretreated with PFSV-2 for 3 h, then incubated with H2O2 (300 μM) for 3 h at 37 °C before dye solution (AO and EB 100 μg/mL, 4 μL) was added to each well. The cells were observed and photographed with a fluorescence light microscope.
2.3.7 Flow cytometric analysis of apoptotic cells
L929 cells were treated with PFSV-2 (100 or 200 μg/mL) for 3 h prior to exposure to H2O2 (300 μM). After 3 h, the cells were collected, washed and then resuspended in binding buffer (1 × 106 cells/mL). Then, annexin V-FITC (5 μL) and PI (5 μL) were added, and the cells were incubated for 15 min in the dark. Finally, the apoptotic cells were quantified and analyzed by flow cytometry.
2.3.8 Evaluation of MDA levels, and SOD and CAT activities of L929 cells
The L929 cells were pretreated with PFSV-2 (100 or 200 μg/mL) for 3 h and then exposed to H2O2 (300 μM). After 3 h, the L929 cells were harvested, washed and lysed with lysis buffer. The supernatant was utilised for the SOD, CAT and MDA analyses.
2.4 In vivo antioxidant activity against H2O2-induced oxidative stress in zebrafish model
2.4.1 Maintenance of zebrafish
This study was conducted in conformity with the guide for the Care and Use of Laboratory Animals. AB-type zebrafish were purchased from Suzhou Murui Biotech. Co. Ltd. The zebrafish were raised in a 3 L acrylic tank at 28.5 °C, with a 14/10 h light/dark cycle. Embryos were collected within 30 min after natural spawning.
2.4.2 Application of PFSV-2 and H2O2 to zebrafish embryos
Approximately 8 h post-fertilization (hpf), embryos were transferred to individual wells and maintained in embryo medium containing PFSV-2. After 1 h of incubation, the embryos were treated with H2O2 (5 mM) until 24 hpf. The surviving fish were then used for further experiments.
2.4.3 Measurement of heart rate, ROS generation and cell death in larval zebrafish
The zebrafish heart rates were measured according to the method of Kim et al. (2016). The zebrafish heart rates in all the groups were recorded at 2 days post-fertilization (dpf) under a microscope. Intracellular ROS generation and cell death were measured in live embryos using DCFH-DA staining and acridine orange according to the method of Kim et al. (2016). The zebrafish were observed and photographed under a fluorescence microscope. The Image J program was used to quantify the fluorescence intensity.
2.4.4 Evaluation of MDA levels, and SOD and CAT activity in larval zebrafish
The treated zebrafish larvae were used to prepare a homogenate with halogenation buffer (pH 7.8, 1 mL) at 4 °C. The homogenate was centrifuged (9000 rpm, 15 min) and then the supernatant was utilized for the SOD, CAT and MDA analyses.
2.5 Statistical analysis
All the results are expressed as mean ± standard deviation. Statistical analyses were performed using one-way analysis of variance (ANOVA). The statistical significance of differences between the groups was assessed using the Student’s t-test and a level of P < 0.05 was considered to be statistically significant.
3 Results and discussion
3.1 Characteristics of PFSV-2
The surface morphology of PSFV-2 was determined by SEM, with the results shown in Fig. 1a. A relatively complete lamellar structure with some holes was observed, indicating the entanglement and aggregation of PFSV-2 induced by the polysaccharide chains and electrical charges (Wan et al., 2022).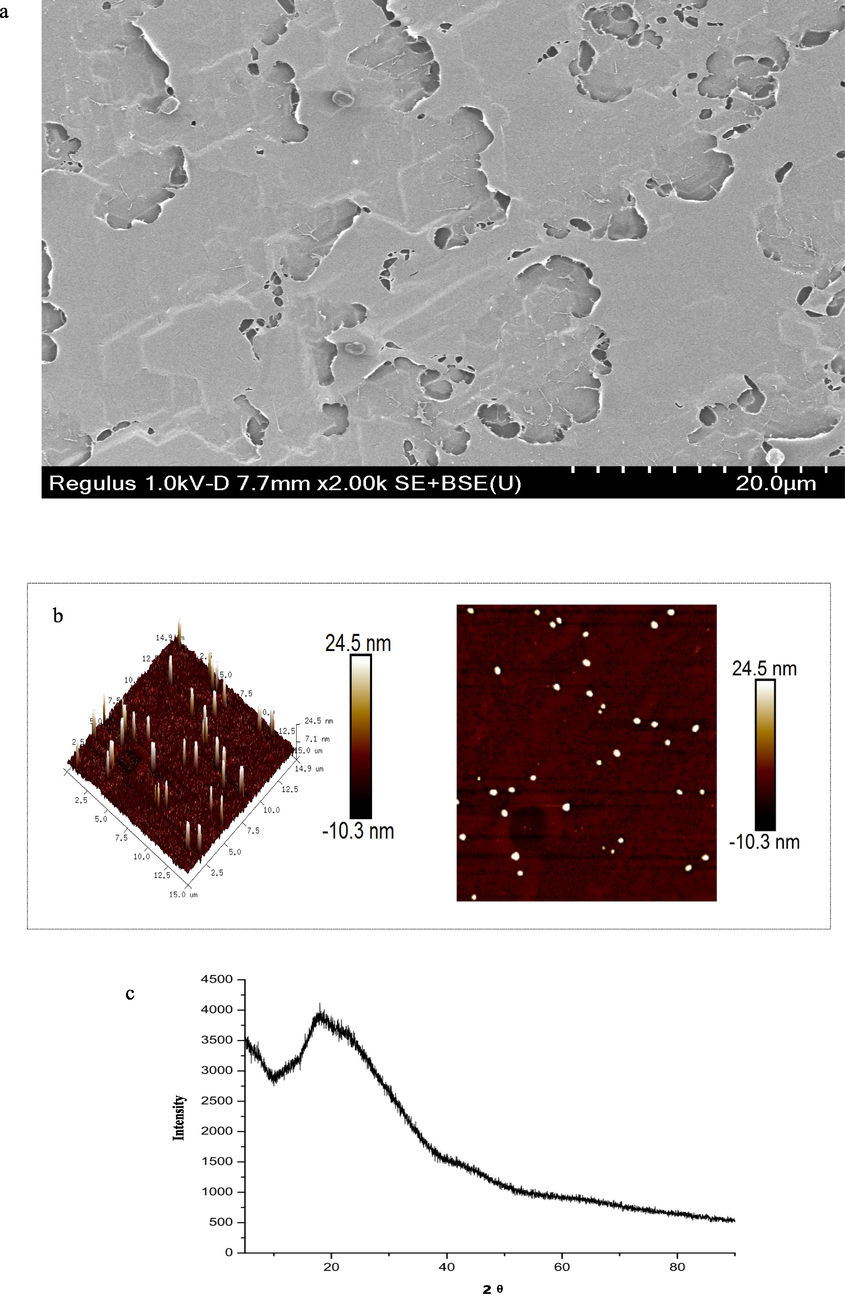
Characterization of PFSV-2. (a) SEM, (b) AFM, (c) XRD pattern, (d) TGA trace, XRD pattern,(e) zeta potential, and (f) Maximum absorption wavelengths of Congo red and the Congo red/ PFSV-2 complex as functions of NaOH concentrations.
Characterization of PFSV-2. (a) SEM, (b) AFM, (c) XRD pattern, (d) TGA trace, XRD pattern,(e) zeta potential, and (f) Maximum absorption wavelengths of Congo red and the Congo red/ PFSV-2 complex as functions of NaOH concentrations.
AFM was used to determine the molecular morphology of PSFV-2with the results shown in Fig. 1b. The polysaccharide chain formed flame-like aggregates of uniform size and regularity. The height of PSFV-2 reached 24.5 nm, which was much higher than that of a single polysaccharide (0.1–1.0 nm) and suggested that the molecular aggregation of PFSV-2 was involved (Yang et al., 2022). The hydroxyl groups in the polysaccharide chains and hydrogen bond interactions caused the chains to entangle with each other and form nonlinear ellipsoids or cylindrical structures (Henriksen et al., 2004).
XRD is a highly efficacious method for revealing the crystalline or amorphous nature of a polysaccharides. As shown in Fig. 1c, the XRD profile of PFSV-2 was characteristic, with one major reflection observed in the 5–90° 2θ region at 19.6°. This indicated that PFSV-2 contained one main crystalline component; hence, it was concluded that PFSV-2 was a semicrystalline polymer (Ren and Liu, 2020).
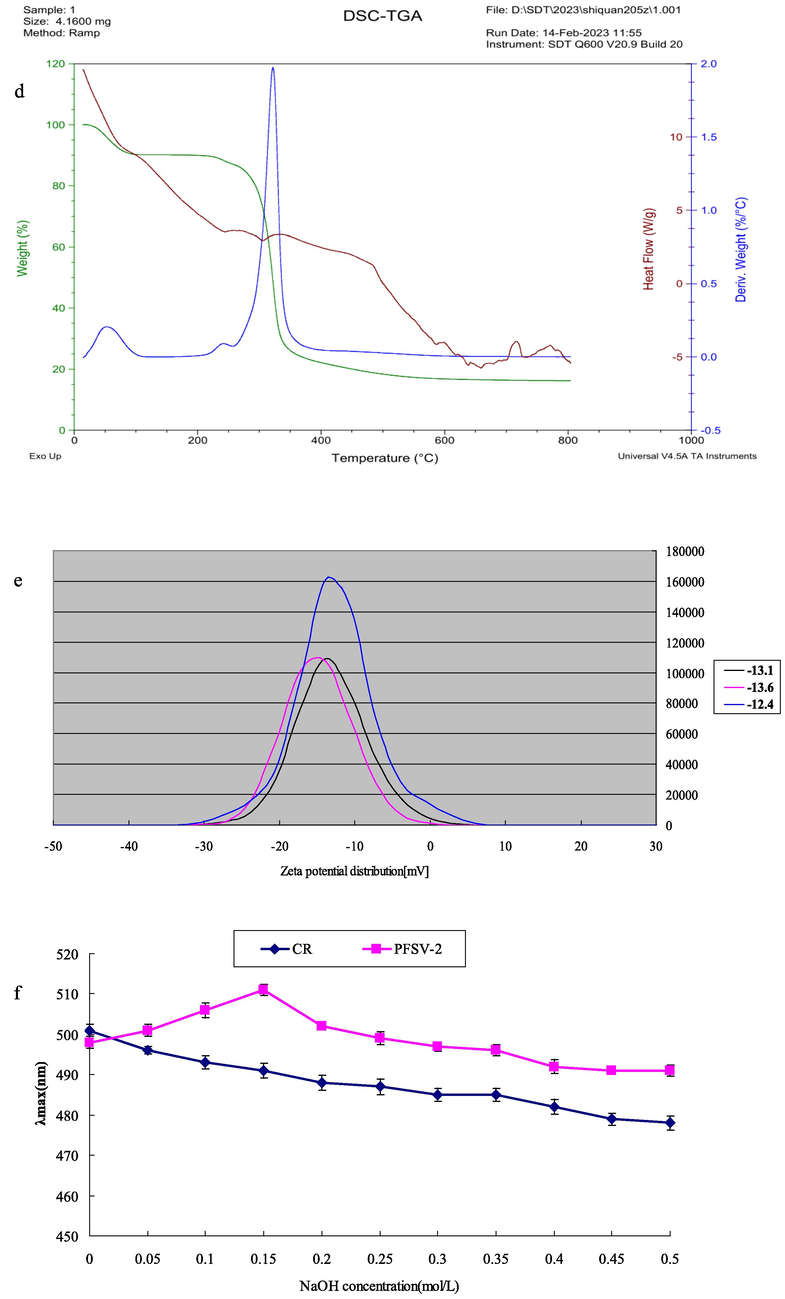
Stability is an important property when considering the biological applications of polysaccharides. In this study, the stability of PFSV-2 was examined by TGA. As shown in Fig. 1d, three main steps were involved in the decomposition of PFSV-2. In the first stage, 10% weight loss occurred below 220 °C, which was attributed to the loss of absorbed or hydrogen-bound water in the polysaccharide pores and the volatilization of a small amount of organic solvent (Archana et al., 2013). This indicated that PFSV-2 was stable at relatively low temperatures. The main weight-loss peak was observed at 250 °C, where the weight of PFSV-2 began decreasing sharply: approximately 70% of the initial weight was lost as the temperature was increased from 250 to 350 °C, which was mainly due to depolymerization reactions and the degradation of volatile components (Xie et al., 2013). During this stage, PFSV-2 underwent strong thermal cracking and its backbone began to break. Similar to the first stage, slow weight loss was observed during the third stage, during which the polysaccharide completely decomposed. Approximately 5% of the weight of PFSV-2 was lost during this stage (Ballesteros et al., 2015).
The charge of a polysaccharide can affect its stability, which directly determines its potential applications (Chen et al., 2021). As shown in Fig. 1e, the PFSV-2 solution had a charge of −13.03 mV, which indicated the strong electron-donating capacity and ease of coagulation of PFSV-2 (Li and Wang, 2016). For a polysaccharide, factors such as the charge density, physicochemical environment, molecular weight and type of wet-end additive are important factors that influence its zeta potential. The above results revealed the relative instability of the PFSV-2 solution (Wang et al., 2019a, 2019b). The polydispersity index (PDI) can reflect the distribution of individual molecular masses in a batch of polymers (Deng et al., 2021). According to our previous results, the PDI for PFSV-2 was 1.10, indicating that this polysaccharide had a narrow distribution of molecular weight without large aggregates (Zhang et al., 2023).
The conformation of the polysaccharide was analyzed by the Congo red test (Wang et al., 2019a, 2019b). Triple-helix polysaccharides react with Congo red, shifting to longer wavelengths at low concentrations and shorter wavelengths at higher concentrations of NaOH (Guo et al., 2018). As shown in Fig. 1F, the maximum absorption wavelength of PFSV-2 first increased and then decreased with increasing NaOH concentration. These results suggested that PFSV-2 had a triple-helix conformation.
3.2 Cell experiments
3.2.1 Influence of PFSV-2 on the survival rate of L929 cells
To evaluate the protective effect of PFSV-2 against H2O2-induced injury in L929 cells, the MTT assay was used to measure the viability of the L929 cells. As shown in Fig. 3a, the decreased cell viability induced by H2O2 (45.35 ± 3.87%) was significantly attenuated by PFSV-2 pretreatment in a concentration-dependent manner (100 μg/mL and 200 μg/mL PFSV-2remarkable increased cell viability to 67.28 ± 4.25% and 70.82 ± 3.62%, respectively). PFSV-2 These observations suggested that PFSV-2 protected L929 cells from H2O2-induced cell injury.
3.2.2 Effects of PFSV-2 on the ROS levels in L929
The DCFH-DA probe was used to evaluate the ROS levels in H2O2-treated L929 cells. As shown in Fig. 2a, a significant increase in DCF fluorescence was observed after H2O2 treatment, indicating high ROS levels (p < 0.05). The pretreatments of L929 cells with PFSV-2 (100 or 200 μg/mL) suppressed the increase in ROS in a dose-dependent manner (100 μg/mL and 200 μg/mL PFSV-2 inhibited intracellular ROS generation by up to 6.47 ± 0.67% and 4.28 ± 1.02%, respectively).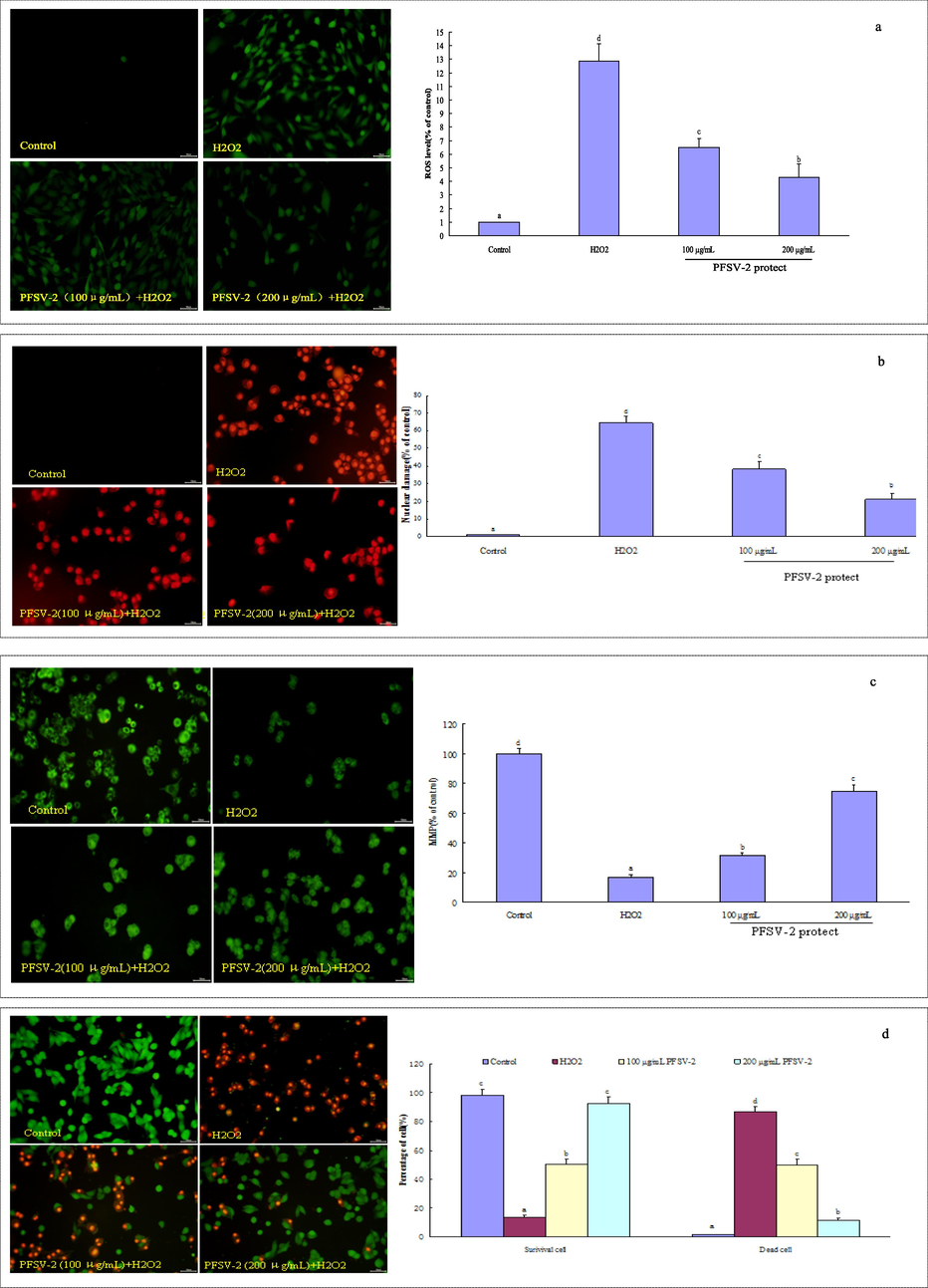
PFSV-2 protects L929 cells against H2O2-induced oxidative damage. (a) ROS level using DCFH-DA staining, (b)nuclear damage using PI staining, (c) mitochondrial membrane potential detection using Rh123 staining, (d) morpholgical apoptotic cells using AO/EB staining, and (f) flow cytometric analysis of apoptotic cells using annexin V-FITC and PI staining.
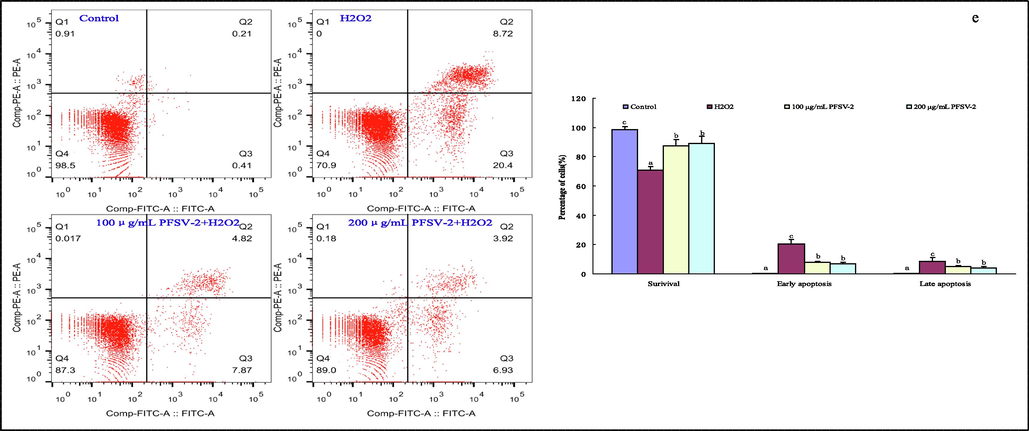
PFSV-2 protects L929 cells against H2O2-induced oxidative damage. (a) ROS level using DCFH-DA staining, (b)nuclear damage using PI staining, (c) mitochondrial membrane potential detection using Rh123 staining, (d) morpholgical apoptotic cells using AO/EB staining, and (f) flow cytometric analysis of apoptotic cells using annexin V-FITC and PI staining.
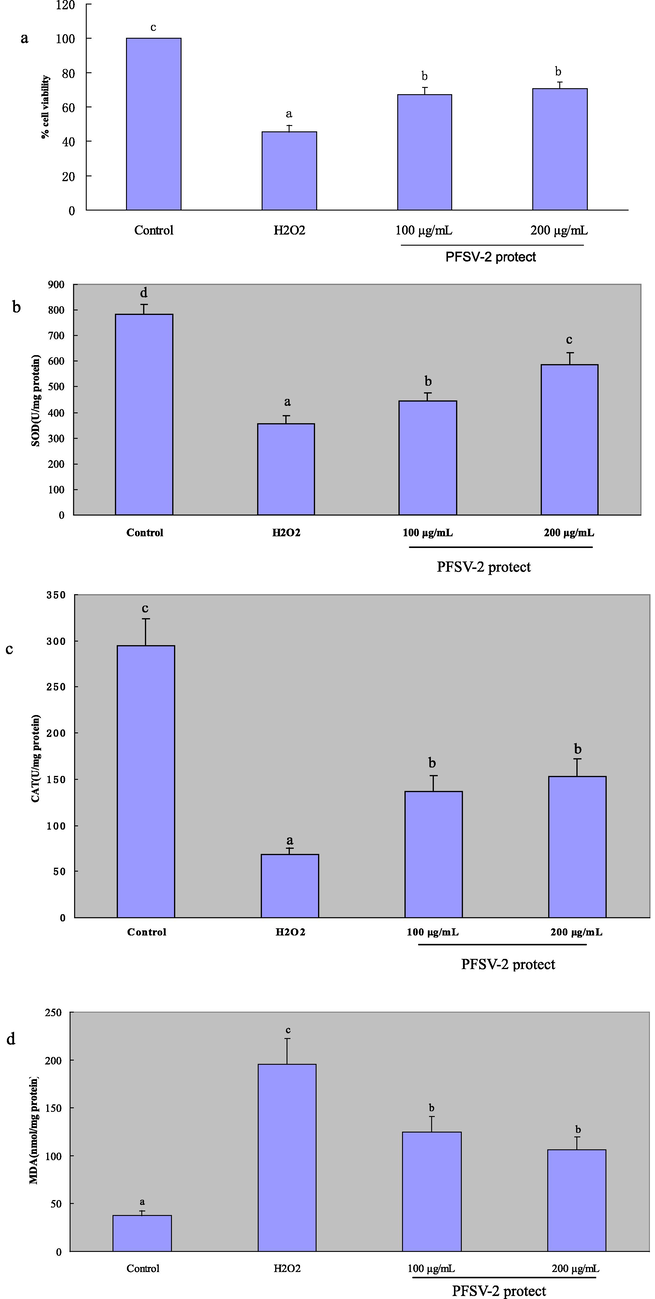
(a) The protective effects of PFSV-2 against H2O2-induced cell death in L929 cells; (b)SOD activity of each treated cells; (c) CAT activity of each treated cells; (d) MDA content of each treated cells. Bar indicates means ± SD. Bars without the same superscripts (a-d) denote significant difference (p < 0.05).
3.2.3 Protection of PFSV-2 against H2O2-induced nuclear damage in L929 cells
As shown in Fig. 2b, the red fluorescence of the H2O2-treated cells was significantly higher than that of the control groups. Pretreatment with PFSV-2 significantly reduced the red fluorescence (100 μg/mL and 200 μg/mL PFSV-2 reduced the nuclear damage to 38.16 ± 4.28% and 21.35 ± 3.16%, respectively). H2O2 treatment disrupted the cell membrane of L929, which resulted in more PI passing through the damaged cell membrane and improved the fluorescence intensity (Fan et al., 2022).
3.2.4 PFSV-2 prevented the loss of MMP in L929 cells
To assess the effect of PFSV-2 on the changes in MMP induced by H2O2 in L929 cells, flow cytometric analyses were carried out using Rh123. After the incubation of L929 cells with 300 μM H2O2 for 30 min, the MMP level was 16.72 ± 2.04% that of the control (Fig. 2c). Pretreatment with PFSV-2 protected cells against the H2O2-induced lowering of MMP: compared with the MMP in the control, the MMP level in cells incubated with 300 μM H2O2 was 31.50 ± 1.89% and 74.52 ± 4.42% in the presence of 100 and 200 μg/mL PFSV-2, respectively.
3.2.5 Effect of PFSV-2 on the nuclei of L929 cells
The morphological changes in L929 cells, as revealed by AO/EB staining, are shown in Fig. 2d. In the control group, the nucleus was arranged closely, chromatin was distributed uniformly and most of the cells emitted green light. After incubation with 300 μM H2O2, most of the L929 cells were in a state of apoptosis (orange and red, the ratio of dead cells reached 86.59 ± 3.86%). Pretreatment with PFSV-2 could reduce the apoptosis of cells in a dose-dependent manner (the ratios of dead cell reduced to 49.64 ± 4.18% and 11.41 ± 1.64% of 100 μg/mL and 200 μg/mL PFSV-2, respectively). This result indicated the PFSV-2 could diminish the ratio of apoptosis cells in L929 cells.
3.2.6 Effects of PFSV-2 on H2O2-induced apoptosis in L929 cells by flow cytometry
To verify the protective effect of PFSV-2 against H2O2-induced apoptosis in L929 cells, the annexin V-FITC/PI double staining method was used to measure the cell apoptosis rates in different groups (Fig. 2e). In the scatter plot of flow cytometry, the Q4 quadrant shows living cells; the Q3 quadrant represents early apoptotic cells; the Q2 quadrant stands for late apoptotic cells and the Q1 quadrant acts for the dead cells. As displayed in Fig. 2F, the percentage of apoptotic cells was higher in the H2O2 group (20.41% early apoptosis and 8.72% late apoptosis) than that in the control group (0.41% early apoptosis and 0.21% late apoptosis). But a marked dose-dependent decrease in both the early and late stages of apoptosis occurred in the L929 cells after 100 and 200 μg/mL PFSV-2 treatments when compared with the H2O2 treated group.
3.2.7 Evaluation of MDA levels, and SOD and CAT activity
As shown in Fig. 3b, c and d, compared with the control group, H2O2 (300 μM) treatment could induced a significant decrease in the activity of SOD and CAT and an increase in the MDA levels in the L929 cells. However, the pretreatment of cells with PFSV-2 (100 and 200 μg/mL) distinctly ameliorated the decreased activity of SOD and CAT as well as markedly reducing the increased MDA levels induced by H2O2, in a concentration-dependent manner.
3.3 Protective effect of PFSV-2 against H2O2 in zebrafish
3.3.1 Influence of PFSV-2 on heart rate of zebrafish
The close relationship between oxidative stress and the heart rate of zebrafish has previously been reported (Kim et al., 2014). As shown in Fig. 4a, an elevation of the heart rate in zebrafish was observed after challenge with H2O2 when compared to the control. In contrast, pretreatment with different PFSV-2 concentrations significantly lowered the heart rate in a concentration-dependent mode. This result showed that PFSV-2 can alleviate H2O2-induced oxidative damage in the zebrafish model.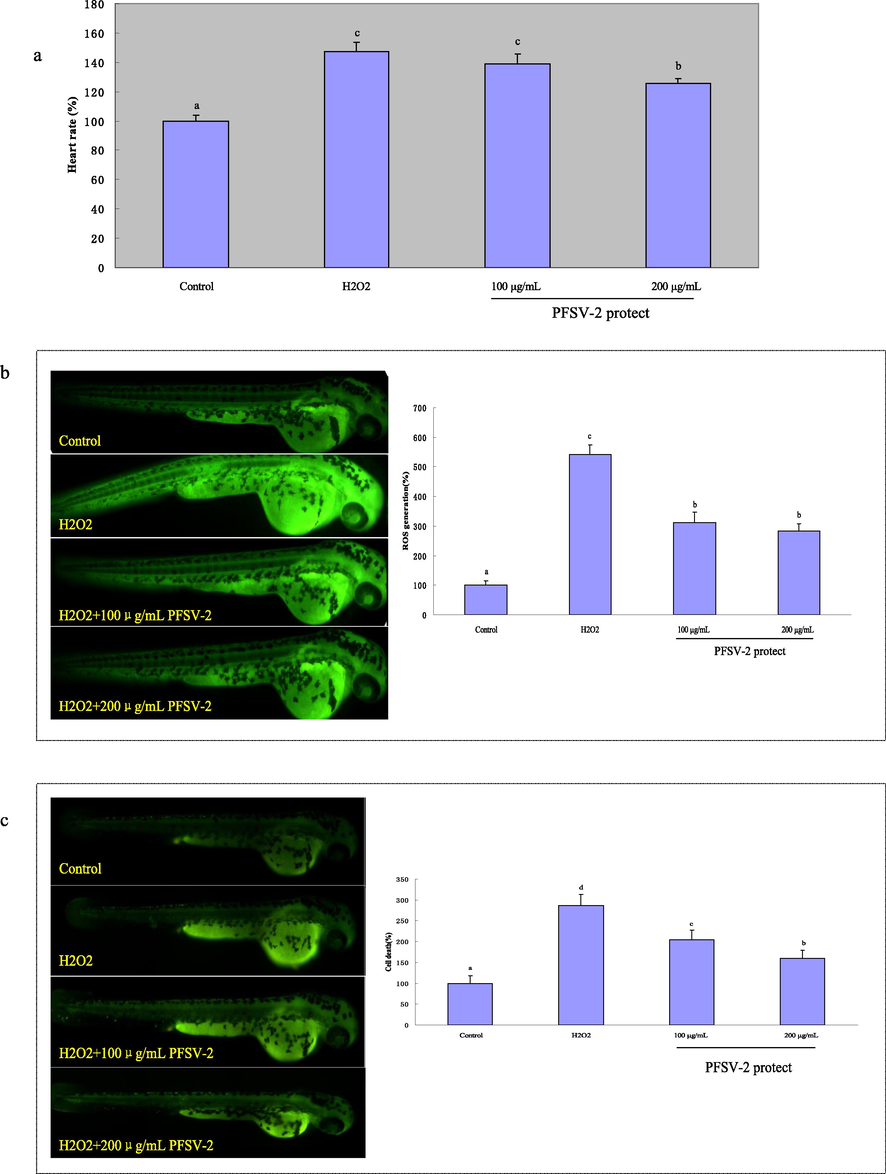
The heart rates, ROS generation and cell death of zebrafish after pretreatment with PFSV-2 and/or treated with H2O2: (a) heart rate; (b) ROS generation, and (c) cell death. ROS and cell death levels were measured by Image J software. Bar indicates means ± SD. Bars without the same superscripts (a-d) denote significant difference (p < 0.05).
3.3.2 Effect of PFSV-2 on ROS production and cell death in zebrafish
DCFH-DA was used to measure the ROS production induced by H2O2 in zebrafish. As shown in Fig. 4b, the ROS levels in zebrafish treated with H2O2 increased significantly to 542.35 ± 31.96 % compared to the control group. In contrast, the zebrafish that were pretreated with different concentrations of PFSV-2 (100 and 200 μg/mL) had significantly decreased ROS levels of 312.42 ± 35.31 % and 283.57 ± 23.44 %, respectively. These results suggested the intracellular ROS abatement potential of PFSV-2 in zebrafish. Acridine orange, as a nucleic acid-selective fluorescent dye, was used to detect cell death in zebrafish. As shown in Fig. 4c, the cell death levels increased significantly to 286.36 ± 26.96% in the H2O2-treated group. However, in the presence of PFSV-2, the incidence of H2O2-induced cell death was significantly decreased.
3.3.3 Evaluation of MDA levels, and SOD and CAT activities in zebrafish
To further confirm the antioxidant effect of PFSV-2, the effects of H2O2 and PFSV-2 on the larval zebrafish were tested. As shown in Fig. 5.a, b and c, H2O2 reduced the activity of SOD and CAT, and PFSV-2 pretreatment resulted in the significant enhancement of SOD and CAT activity. Furthermore, compared with the control group, the H2O2-treated group showed significantly higher MDA levels and the PFSV-2 treatment exhibited a concentration-dependent decrease in MDA levels. All of the above results indicated that PFSV-2 could ameliorate the oxidative stress condition in zebrafish.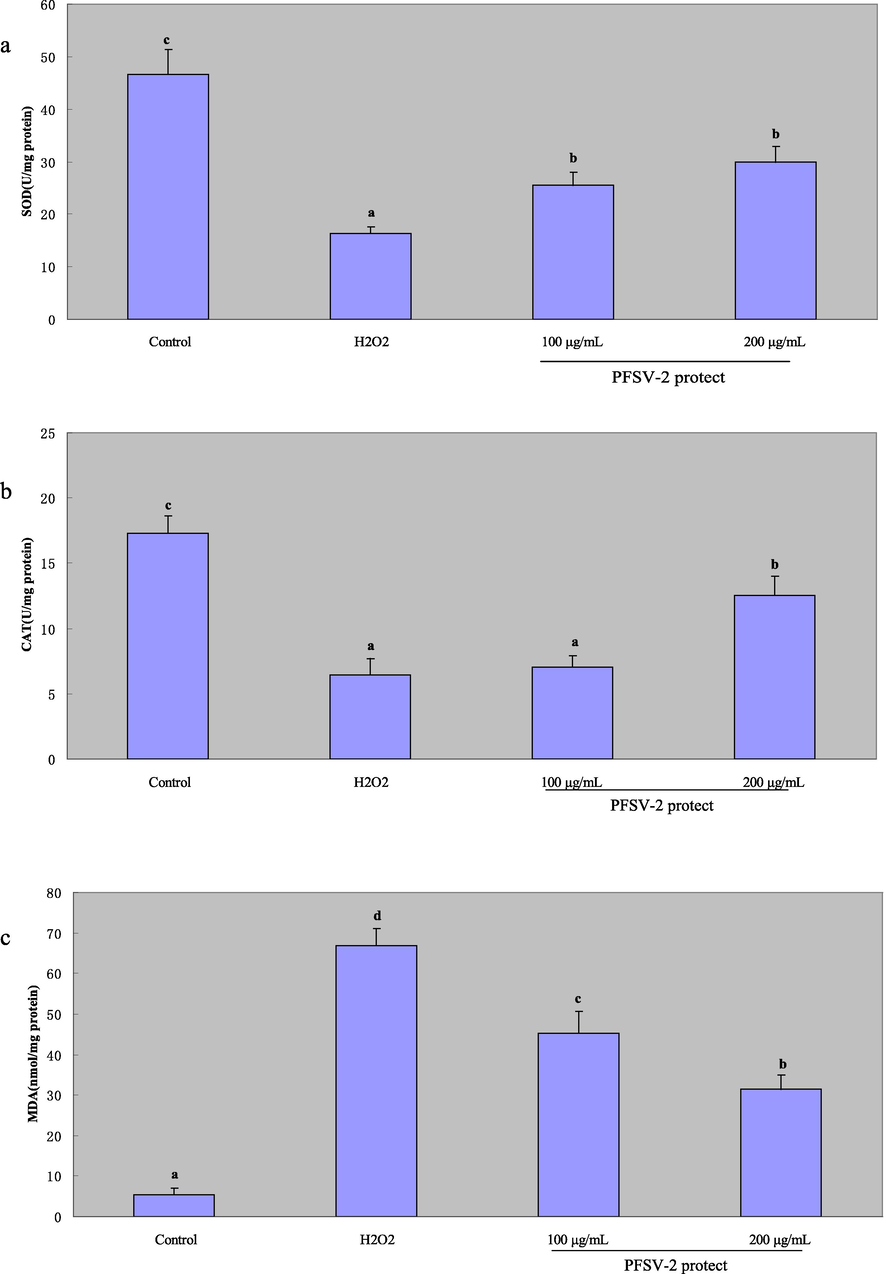
The protective effects of PFSV-2 against H2O2-induced damage in zebrafish. (a) SOD activity of each treated zebrafish; (b) CAT activity of each treated zebrafish; and (c) MDA content of each treated zebrafish. Bar indicates means ± SD. Bars without the same superscripts (a-d) denote significant difference (p < 0.05).
4 Discussion
As a type of ROS with non-free radical form, H2O2 can easily cross the cell membrane and injure the cell (Akhtar et al., 2017). Currently, H2O2 is used widely as an inducer of oxidative stress injury in the body (Fang et al., 2018). To explore whether PFSV-2 could protect cells and larval zebrafish from H2O2-induced oxidative damage, L929 cells and larval zebrafish were pretreated with PFSV-2 at two different concentrations and then treated with H2O2.
After exposure to H2O2, the survival rate of the cells that were pretreated with PFSV-2 was higher than that of the injury group, indicating that PFSV-2 exerted a protective effect on L929 cells against H2O2. In this study, H2O2 induced the rapid accumulation of ROS in L929 cells and larval zebrafish but PFSV-2 significantly decreased the ROS levels compared with the injury group. It is widely shared that excessive ROS generation can damage the mitochondrial membrane and result in cell death (Kroemer and Galluzzi, 2007). PFSV-2 could alleviate the damage induced by ROS by decreasing the aggravation of the MMP.
As an analogue of ethidium bromide, PI is a DNA staining reagent. PI can pass through the damaged cell membrane but not the intact living cell membrane (Kobayashi and Odake, 2017). Therefore, PI staining can be used to determine the integrity of the cell membrane. In the present study, the red fluorescence of the H2O2-treated groups was significantly higher than that of the control groups, indicating that H2O2 treatment disrupted the cell membrane. However, pretreatment with PFSV-2 significantly decreased the red fluorescence, indicating that PFSV-2 could alleviate the cell membrane damage induced by ROS.
AO/EB staining was used to distinguish between living and apoptotic cells (Xiang et al., 2020). Compared to the control, after L929 cell were exposed to H2O2, a higher percentage of apoptotic cells were detected. Pretreatment with PFSV-2 decreased the ratio of apoptotic L929 cells. This result indicated that PFSV-2 could protect the L929 cells against apoptosis.
Annexin V-FITC/PI labeling, followed by flow cytometry analysis, was used to confirm cell apoptosis. This assay could distinguish the viable cells, necrotic cells, and cells in early and late apoptosis. It was found that PFSV-2 pretreatment could decrease in both early- and late-stages apoptosis in L929 cells.
Over exposure to ROS can result in lipid peroxidation of the unsaturated fatty acids in the cell membrane (Amara et al., 2022). In this study, the MDA content was ascertained, which is the end product of lipid peroxidation and a biological marker of oxidative damage (Ferrer et al., 2009). PFSV-2 decreased the MDA levels in both cell and larval zebrafish models, exhibiting its protective effect. These results suggested that PFSV-2 could decrease the effects of oxidative stress and exhibit protective effects on L929 cells and larval zebrafish (Fig. 2C, Fig. 3C).
To further confirm the protective effect of PFSV-2 against oxidative stress, the activity of SOD and CAT (key antioxidant enzymes that scavenge ROS) following H2O2-induced oxidative injury in L929 cells and larval zebrafish models was assessed. The results showed that H2O2 treatment decreased the activity of SOD and CAT in both cells and larval zebrafish, while PFSV-2 could restore the activity of both enzymes in the H2O2-induced injury model in vitro and in vivo. Thus, PFSV-2 could restore the antioxidant system, which was a possible reason for the scavenging of ROS from injured cells and larval zebrafish. In a word, PFSV-2 could reduce the oxidative damage of H2O2 through scavenging intracellular ROS, protecting cell membrane, increasing the activities of antioxidant enzymes and reducing the cell apoptosis (Hu et al., 2022).
The antioxidant properties of polysaccharides are a result of the synergistic effects of various factors (Liu et al., 2021). Molecular weight, glycosidic bond type, monosaccharide composition and uronic acid content are the key factors that influence the antioxidant properties of polysaccharides (Ji et al., 2022). The antioxidant activity of polysaccharides is related to their electron- or hydrogen-donating capacity. Low molecular weight polysaccharides with good water solubility, higher surface areas and numerous exposed reducing ends have a higher chance of counteracting free radicals, granting them better antioxidant activity (Shang et al., 2021). Uronic acid can react with the hydrogen atom on the anomeric caribon, so the presence of uronic acid is considered to be another factor that is indicative of the antioxidant activity of polysaccharides (Wang et al., 2010).
The antioxidant activity of polysaccharides may be affected by the monosaccharide composition, which is due to the effects of the monosaccharide composition on the chain structure of polysaccharides (Chen et al., 2010). Lo et al. (2011) have reported that polysaccharides with a high proportion of mannose and rhamonse exhibited higher antioxidant activity. Additionally, polysaccharides with low glucose content are reported to have good antioxidant capacity (Chen et al., 2019). Recent studies have also found that polysaccharides with triple-helical structural integrity possess high antioxidant activity (He et al., 2016). Taking together, the strong antioxidant activity of PFSV-2 may be attributed to its low glucose content, low molecular weight and triple-helical structures. Further, research on the structure-effect relationship will inform the development of polysaccharide products with potential functional effects. Therefore, further studies are urgently needed to clarify the structure–activity relationship of PFSV-2.
5 Conclusion
The study indicated that the PFSV-2 purified from S. vaninii exhibited pronounced antioxidant activity in vitro and in vivo. PFSV-2 exhibited cytoprotective activity through increasing cell viability and the MMP, while decreasing ROS levels and L929 apoptosis. Additionally, PFSV-2 counteracted ROS generation and cell death and reduced the heart rate in the H2O2-induced oxidative stress in vivo zebrafish model. Furthermore, FPSV-2 decreased the production of MDA and increased the activity of antioxidant enzymes SOD and CAT in both the L929 cells and larval zebrafish. The above results open the possibility of exploitation of PFSV-2 as a novel therapeutic agent for oxidative stress diseases.
CRediT authorship contribution statement
Zhang Zuofa: Funding acquisition. Lv Guoying: Project administration, Investigation. Shen Meng: Project administration, Investigation. Song Tingting: Data curation. Peng Juan: Validation.
Acknowledgements
The authors are grateful to Shanghai Sanshu Biotechnology Co., LTD for their help in characterization of PFSV-2. This research was funded by the New Variety Breeding Project of Science Technology Department of Zhejiang Province (2021C02073) and Agriculture, Rural areas and Farmers and nine-party Project of Zhejiang Province (2022SNJF047).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mechanism of ROS scavenging and antioxidant signaling by redox metallic and fullerene nanomaterials: potential implications in ROS associated degenerative disorders. BBA-Gen. Subjects. 2017;1861:802-813.
- [Google Scholar]
- The protective effects of thymol and carvacrol against di(2-ethylhexyl) phthalateinduced cytotoxicity in HEK-293 cells. J. Biochem. Mol. Toxicol.. 2022;36:e23092.
- [Google Scholar]
- Preparation and characterization of mucilage polysaccharide for biomedical for biomedical applications. Carbohyd. Polym.. 2013;98:89-94.
- [Google Scholar]
- Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohyd. Polym.. 2015;127:347-354.
- [Google Scholar]
- Antioxidant compounds from microbial sources: a review. Food Res. Int.. 2019;129:108849
- [Google Scholar]
- Comparison of different extraction methods for polysaccharides from bamboo shoots (Chimonobambusa quadrangularis) processing by-products. Int. J. Biol. Macromol.. 2019;2019(130):903-914.
- [Google Scholar]
- Disruption of metabolic function and redox homeostasis as antibacterial mechanism of Lindera glauca fruit essential oli against Shigella flexneri. Food Control. 2021;130:108282
- [Google Scholar]
- Optimization of hydroxyl radical scavenging activity of exo-polysaccharides from Inonotus obliquus in submerged fermentation using response surface methodology. J. Microbiol. Biotechnol.. 2010;20:835-843.
- [Google Scholar]
- Antioxidant potentials of annoceria synthesized by solution plasma process and its biocompatibility study. Arch. Biochem. Biophys.. 2018;645:42-49.
- [Google Scholar]
- Extraction optimization of polysaccharides from Gougunao tea and assessment of the antioxidant and hypoglycemic activities of its fractions in vitro. Bioact. Carbohyd. Dietary Fibre. 2021;26:100287
- [Google Scholar]
- Epinecidin-1, a marine antifungal peptide, inhibits Botrytis cinerea and delays gray mold in postharvest peaches. Food Chem.. 2022;403:134419
- [Google Scholar]
- Characterization of purified red cabbage anthocyanins: improvement in HPLC separation and protective effect against H2O2-induced oxidative stress in HepG2 cells. Molecules. 2018;24:124.
- [Google Scholar]
- Reactive oxygen species induced by beauvericin, patulin and zearalenone in CHO-K1 cells. Toxicol. Vitro. 2009;23:1504-1509.
- [Google Scholar]
- The combination between cations and sulfated polysaccharide from abalong gonad (Haliotis discus hannai Ino) Carbohyd. Polym.. 2018;188:54-59.
- [Google Scholar]
- Characterization and antioxidation study of different weight of soluble soybean polysaccharides. Soybean Sci.. 2016;35:805-809.
- [Google Scholar]
- Biological control on calcite crystallization: AFM investigation of coccolith polysaccharide function. Am. Mineral.. 2004;89:1709-1716.
- [Google Scholar]
- Protective effect of antioxidant peptides from grass carp scale gelatin on the H2O2-mediated oxidative injured HepG2 cells. Food Chem.. 2022;373:131539
- [Google Scholar]
- Physicochemical features and antioxidant activity of polysaccharides from Herba Patriniae by gradient ethanol precipitation. Arab. J. Chem.. 2022;15:103770
- [Google Scholar]
- Structural characterization and antioxidant activity of a novel high-molecular-weight polysaccharide from Ziziphus Jujuba cv. Muzao. J. Food Meas. Charact.. 2022;16:2191-2200.
- [Google Scholar]
- Comparison of curdlan and its carboxymethylated derivative by means of rheology, DSC, and AFM. Carbohyd. Res.. 2006;341:90-99.
- [Google Scholar]
- Protective mechanism of quercetin and rutin using glutathione metabolism on ho-induced oxidative stress in HepG2 cells. Ann. Ny. Acad. Sci.. 2010;1171:530-537.
- [Google Scholar]
- Protective effects of polysaccharides from Psidium guajava leaves against oxidative stresses. Int. J. Biol. Macromol.. 2016;91:804-811.
- [Google Scholar]
- Protective effect of fucoidan aganist AAPH-induced oxidative stress in zebrafish model. Carbohyd. Polym.. 2014;102:185-191.
- [Google Scholar]
- Intracellualr acidification and damage of cellular membrane of Saccharomyces pastorianus by low-pressure carbon dioxide microbubbles. Food Control. 2017;71:365-370.
- [Google Scholar]
- Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev.. 2007;87:99-163.
- [Google Scholar]
- Effect of extraction method on structure and antioxidant activity of Hohenbuehelia serotina polysaccharides. Int. J. Bio. Macromol.. 2016;83:270-276.
- [Google Scholar]
- Structural properties and antioxidant activities of polysaccharides isolated from sunflower meal after oil extraction. Arab. J. Chem.. 2021;14:103420
- [Google Scholar]
- Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohyd. Polym.. 2011;86:320-327.
- [Google Scholar]
- Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci.. 2017;38:592-607.
- [Google Scholar]
- Effects of separation and purification on structural characteristics of polysaccharide from quinoa (Chenopodium quinoa willd) Biochem. Biophys. Res. Commun.. 2020;522:286-291.
- [Google Scholar]
- Reactive oxygen species and antioxidant properties from mushrooms. Syn. Syst. Biotechno.. 2017;2:13-22.
- [Google Scholar]
- Changes in mitochondrial membrane potential during oxidative stress-induced apoptosis in PC12 cells. J. Neurosci. Res.. 1997;50:413-420.
- [Google Scholar]
- Physicochemical characterization and in vitro biological activities of polysaccharides from alfalfa (Medicago sativa L.) as affected by different drying methods. Process Biochem.. 2021;103:39-49.
- [Google Scholar]
- Bcl-2 blocks loss of mitochondrial membrane potential with ICE inhibitors acts at a different step during inhibition of death induced by respiratory chain inhibitors. Oncogene. 1996;12:21-29.
- [Google Scholar]
- Superoxide anion generation and lipid peroxidation processes during hemodialysis with reused cuprophan dialyzers. Free Radic. Biol. Med.. 1990;8:429-432.
- [Google Scholar]
- Antioxidant properties of Agaricus balzei, Agrocybe cylindracea, and Boletus edulis. LWT Food Sci. Technol.. 2007;40:1392-1402.
- [Google Scholar]
- Purification, physico-chemical properties and antioxidant activity of polysaccharides from Sargassum fusiforme by hydrogen peroxide/ascorbic acid-assisted extraction. Int. J. Biol. Macromol.. 2022;223:490-499.
- [Google Scholar]
- Structural characterization, physicochemical properties and α-glucosidase inhibitory activity of polysaccharide from the fruits of wax apple. Carbohyd. Polym.. 2019;211:227-236.
- [Google Scholar]
- Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Research Int.. 2016;90:33-41.
- [Google Scholar]
- Sulfated modification, characterization and structure-antioxidant relationships of Artemisia sphaerocephala polysaccharides. Carbohyd. Polym.. 2010;81:897-905.
- [Google Scholar]
- Nanostructures assembly and the property of polysaccharide extracted from Tremella Fuciformis fruiting body. Int. J. Bio. Macromol.. 2019;37:751-760.
- [Google Scholar]
- Antitumor effects of crucumin on the proliferation migration and apoptosis of human colorectal carcinoma HCT-116 cells. Oncol. Rep.. 2020;44:1997-2008.
- [Google Scholar]
- Purification, physicochemical characterisation and anticancer activity of a polysaccharde from Cyclocarya paliurus leaves. Food Chem.. 2013;136:1453-1460.
- [Google Scholar]
- Isolation, purification, structural characterization, and hypoglycemic activity assessment of polysaccharides from Hovenia dulcis (Guai Zao) Int. J. Biol. Macromol.. 2022;208:1106-1115.
- [Google Scholar]
- Characterization and biological activities of polysaccharides from artificially cultivated Phellinus baumii. Int. J. Biol. Macromol.. 2019;129:861-868.
- [Google Scholar]
- Recovery of a hypolipidemic polysaccharide from artificially cultivated Sanghuangporus vaninii with an effective method. Front. Nutr.. 2023;9:1095556.
- [Google Scholar]
- Current status of ‘Sanghuang’ as a group of medicinal mushrooms and their perspective in industry development. Food Rev. Int.. 2022;38:589-607.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105115.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







