Translate this page into:
Pulsatilla chinensis: A review of traditional uses, phytochemistry and pharmacology research progress
⁎Corresponding author at: College of Acupuncture & Massage, Shaanxi University of Chinese Medicine, Xixian New Area, Shaanxi Province 712046, PR China. 1511006@sntcm.edu.cn (Haifa Qiao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This paper is intended to review advances in the botanical, traditional uses, phytochemical and pharmacological studies of the Pulsatilla chinensis. Up to date, 68 kinds of chemical constituents have been isolated and identified from P. chinensis. Among these compounds, triterpenoids, flavonoids, lignans and coumarins are the major constituents. Researches about the pharmacological properties of P. chinensis revealed that this plant exhibited therapeutic potential both in vivo and in vitro, including antitumor, anti-inflammatory, anti-microbial and antiviral activities. Further attention should be paid to gathering information about their toxicology data, quality-control measures, and the clinical value of the active compounds from P. chinensis.
Keywords
Pulsatilla chinensis
Triterpenoids
Antitumor
Review
1 Introduction
Pulsatilla chinensis (Bunge) Regel, (a synonym of Anemone chinensis Bunge) belongs to the family of Ranunculaceae, a kind of perennial herbs with rhizomes, being used as a kind of medical herb for a long history. It is called as “Bai Tou Weng/白头翁” in China, はくとうおう (in Japanese), and 할미꽃 (in Korean), also be called as Milkweed, White-headed Grass, Old Aunt Grass. It is a kind of valuable plant with both medicinal potential and ornamental value, planted naturally in gardens to arrange flower beds, road sides, or interspersed in forest spaces (Fig. 1). In traditional Chinese medicine (TCM), the rhizome of P. chinensis is used to clear heat in body and detoxify, to cool blood and stop dysentery such as bacterial dysentery, amebic dysentery, especially good at clearing damp-heat of gastrointestinal tract and blood-heat toxin, in addition, it was reported to treat malaria and relieve spasm and pain as well. Modern pharmacological studies confirmed that the extracts or the active compounds isolated from P. chinensis played important roles in potencies of the P. chinensis including antitumor, anti-inflammatory, anti-microbial and antiviral (Cheng, et al., 2008, Li, et al., 2014, Sun, et al., 2010). Up to now, there is no comprehensive review on P. chinensis, herein, in this paper, we summarize the botany, traditional uses, phytochemistry, pharmacology, toxicity and chemical analysis of P. chinensis based on the research literature across the world over past decades. We expect this paper will make contributions to fill in the gaps in this field and develop potential clinical candidates from the constituents of P. chinensis.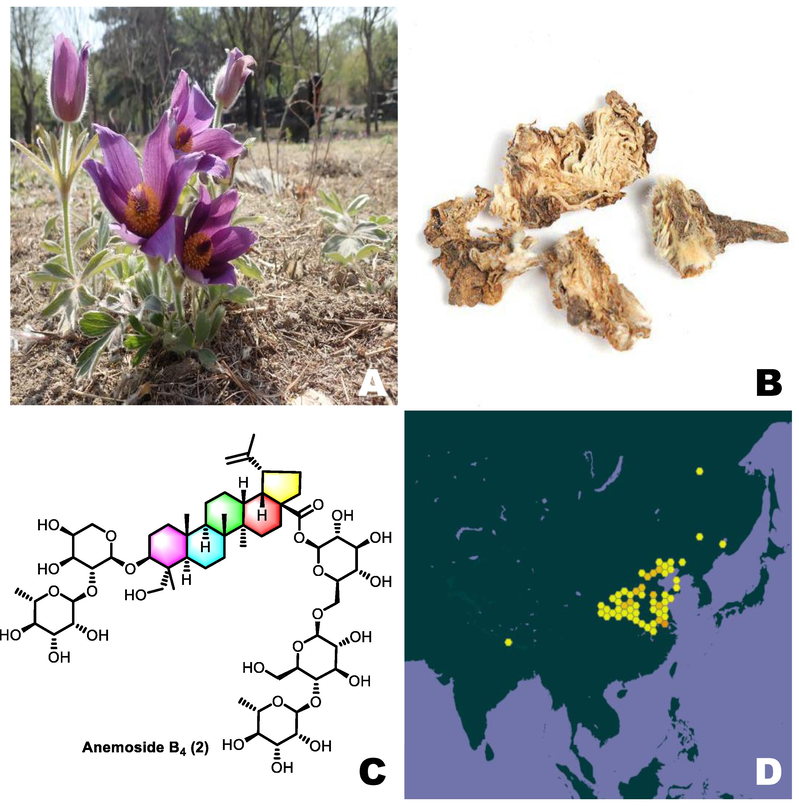
P. chinensis: (A) P. chinensis bushes; (B) dry root of P. chinensis; (C) Chemcal structure of anemoside B4; (D) distribution of P. chinensis (obtained from GBIF database: https://www.gbif.org).
2 Botany
To date, P. chinensis has been reported to distribute in East Asian region, especially in China. In China, P. chinensis is widely distributed in northeastern aera of China including Jilin, Liaoning, Hebei, Shandong, Henan, Shanxi, Shaanxi and Heilongjiang provinces (Fig. 1). The plant commonly prefers to grow on the low mountain slopes surrounded by grass and woodland or dry, stony slopes on plains. The height of P. chinensis is as high as 15–35 cm, with 0.8–1.5 cm rhizome underground in thickness. It grows 4–5 basal leaves with long petioles usually just emerging at flowering period; leaves are wide oval, 4.5–14 cm in length and 6.5–16 cm in width, trilobed, medium-deep lobes form as wedge-shaped inverted-ovate, with few narrow wedges or inverted trapezoids, toothed or not. The flowering stage ranges from March to May, and the fruit phase is typically from June to July.
3 Traditional uses
P. chinensis was first listed in the Chinese medical classic “Shen Nong’s herbal Classic” (created by Shen Nong, 200 CE). It was described as cold and bitter in taste and attributive to the stomach, and large intestine meridians with potential in curing fever and physical trauma. According to “Yao Xing Lun” (created by Quan Zhen, around 700 CE), P. chinensis was listed as an herbal medicine with therapy effects on stomachache, dentalgia and joints pain. Based on “Ri Hua Zi Ben Cao” (created by Ri Hua Zi, around 700 CE), P. chinensis was described as a therapy for wind-dampnes and potential for weight loss and eyesight improvement. P. chinensis has been considered as an herbal medicine according to its wide spectrum of biological activities with a long history, especially its therapeutic effect on the gynopathy. Decocting with water or mashing for external application are the traditional possess methods of P. chinensis. As for the prescription of P. chinensis, the most commonly used formula was “Bai Tou Weng Tang” from “Synopsis of the Golden Chamber”, which was composed of P. chinensis, Coptis chinensis, Phellodendron chinense and Fraxinus chinensis with an equal proportion for the treatment of pyretic dysentery for more than one thousand years. The Chinese Pharmacopoeia recommends a dose of 9–15 g for S. chinensis (China Pharmacopoeia Commission, 2015).
4 Phytochemistry
Till now, at least 68 kinds of constituents have been isolated and identified from the P. chinensis, which are mainly made up of triterpenoids, flavonoids, coumarins and lignans. All compounds are summarized and compiled in Table 1 with their details including name, CAS number and formula, and the chemical structure of listed compounds is shown in Fig. 2 and Fig. 3 (Bahramsoltani et al., 2019). The mostly contained in the plant were triterpenoids and their saponins, among them, anemoside B4 (2, Fig. 1) has been comprehensively studied and used in China and regulated as the standard for quality control of P. chinensis as medicine by Chinese Pharmacopoeia, known as an active substance to treat cancer, nephrotoxicity, and it had other pharmacological effects including anti-inflammatory, antioxidant, antimicrobial, etc (He, et al., 2019, Xue, et al., 2019). Note: type of triterpenoids: A: lupine; B: 23-hydroxybutyric acid; C: ivy; D: oleanolic acid; E: others.
NO
Name
Type
Part/Extract
CAS
Formula
Ref.
Triterpenoids
1
Anemoside A3
A
Root/EtOH
129724–84-1
C41 H66 O12
(Chen, et al., 1990)
2
Anemoside B4
A
Root/EtOH
129741–57-7
C59 H96 O26
(Chen, et al., 1990)
3
Pulchinenoside B
A
Root/EtOH
135247–95-9
C53 H86 O22
(Wu, et al., 1991)
4
Pulsatilloside A
A
Root/MeOH
136684–41-8
C35 H56 O8
(Wen et al., 1996)
5
Pulsatilloside B
A
Root/MeOH
136684–40-7
C30 H46 O4
(Wen et al., 1996)
6
Pulsatilloside C
A
Root/MeOH
162341–28-8
C48 H78 O18
(Ye, et al., 1998)
7
Pulsatilloside D
A
Root/MeOH
439590–38-2
C59 H96 O27
(Ye, et al., 2002)
8
Pulsatilloside E
A
Root/MeOH
366814–43-9
C65 H106 O31
(Ye, et al., 2002)
9
3-[(O-6-deoxy-α-L-mannopyranosyl-(1 → 2)-O-[O-β-D-glucopyranosyl-(1 → 4)-β-D-glucopyranosyl-(1 → 4)]-α-L-arabinopyranosyl)oxy]-23–hydroxy-, O-6-deoxy-α-L-mannopyranosyl-(1 → 4)-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosyl ester
A
Root/MeOH
366814–44-0
C71 H116 O36
(Mimaki, et al., 2001)
10
(3β,4α)-3-[(O-6-Deoxy-α-L-mannopyranosyl-(1 → 2)-O-[β-D-glucopyranosyl-(1 → 4)]-α-L-arabinopyranosyl)oxy]-23–hydroxylup-20(29)-en-28-oic acid
A
Root/EtOH
848784–85-0
C47 H76 O17
(Xu, 2013)
11
3-[(4-O-β-D-glucopyranosyl-α-L-arabinopyranosyl)oxy]-23–hydroxy-, 6-O-β-D-glucopyranosyl-β-D-glucopyranosyl ester
A
Root/EtOH
1476801–07-6
C53 H86 O23
(Xu, 2013)
12
3-[(O-6-deoxy-α-L-mannopyranosyl-(1 → 2)-O-[β-D-glucopyranosyl-(1 → 4)]-α-L-arabinopyranosyl)oxy]-23–hydroxy-,6-O-β-D-glucopyranosyl-β-D-glucopyranosyl ester
A
Root/EtOH
1476801–08-7
C59 H96 O27
(Xu, 2013)
13
(3β,4α)-23–(Acetyloxy)-3-[[3,4-di-O-acetyl-2-O-(2,3,4-tri-O-acetyl-6-deoxy-α-L-mannopyranosyl)-α-L-arabinopyranosyl]oxy]lup-20(29)-en-28-oic acid
A
Root/EtOH
133377–68-1
C53 H78 O18
(Chen, et al., 1990)
14
Daucosterol
B
Root/EtOH
474–58-8
C35 H60 O6
(Wu, et al., 1991)
15
Betulinic acid
A
Root/MeOH
85999–40-2
C30 H48 O4
(Wen et al., 1996)
16
Cussosaponin C
A
Root/MeOH
366814–42-8
C59 H96 O25
(Mimaki, et al., 2001)
17
Betulinic acid 3β-O-α-L-rhamnopyranosyl-(1 → 2)-[β-D-glucopyranosyl-(1 → 4)]-α-L-arabinopyranoside
A
Root/EtOH
848784–87-2
C47 H76 O16
(Xu, 2013)
18
3-[[2-O-(6-deoxy-α-L-mannopyranosyl)-α-L-arabinopyranosyl]oxy]-6-O-β-D-glucopyranosyl-β-D-glucopyranosyl ester
A
Root/EtOH
1257378–27-0
C53 H86 O21
(Xu, 2013)
19
3-[(O-β-D-glucopyranosyl-(1 → 3)-O-6-deoxy-α-L-mannopyranosyl-(1 → 2)-α-L-arabinopyranosyl)oxy]-, 6-O-β-D-glucopyranosyl-β-D-glucopyranosyl ester
A
Root/EtOH
1476801–09-8
C59 H96 O26
(Xu, 2013)
20
3-[(O-β-D-glucopyranosyl-(1 → 3)-O-6-deoxy-α-L-mannopyranosyl-(1 → 2)-α-L-arabinopyranosyl)oxy]-, O-6-deoxy-α-L-β-D-mannopyranosyl-(1 → 4)-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosyl ester
A
Root/EtOH
1476801–10-1
C65 H106 O30
(Xu, 2013)
21
Pulsatillic acid
A
Root/EtOH
136684–40-7
C30 H46 O4
(Xu, 2013)
22
Leontoside B
C
Root/MeOH
17233–22-6
C41 H66 O13
(Mimaki et al., 1999)
23
Pulsatilla saponin A
C
Root/MeOH
27013–91-8
C41 H66 O12
(Mimaki et al., 1999)
24
Pulsatilla saponin D
C
Root/MeOH
68027–15-6
C47 H76 O17
(Mimaki et al., 1999)
25
Kalopanaxsaponin H
C
Root/MeOH
128730–82-5
C47 H76 O17
(Mimaki et al., 1999)
26
3-[(O-β-D-glucopyranosyl-(1 → 3)-O-6-deoxy-α-L-mannopyranosyl-(1 → 2)-α-L-arabinopyranosyl)oxy]-23–hydroxy-, methyl ester
C
Root/MeOH
244202–38-8
C48 H78 O17
(Mimaki et al., 1999)
27
Pulsatilla saponin H
C
Root/EtOH
68027–14-5
C65 H106 O31
(Glebko et al., 2002)
28
3-[[2-O-(6-deoxy-α-L-mannopyranosyl)-α-L-arabinopyranosyl]oxy]-20,23–dihydroxy-, O-6-deoxy-α-L-mannopyranosyl-(1 → 4)-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosyl ester
A
Root/MeOH
366814–41-7
C59 H98 O27
(Mimaki, et al., 2001)
29
Tauroside C
D
Root/MeOH
35790–95-5
C41 H66 O11
(Mimaki et al., 1999)
30
Pulsatilla saponin I
D
Root/MeOH
103956–33-8
C47 H76 O16
(Mimaki et al., 1999)
31
Hederacolchiside A1
D
Root/MeOH
106577–39-3
C47 H76 O16
(Mimaki et al., 1999)
32
(3β)-3-[(O-β-D-Glucopyranosyl-(1 → 4)-O-β-D-glucopyranosyl-(1 → 3)-O-6-deoxy-α-L-mannopyranosyl-(1 → 2)-α-L-arabinopyranosyl)oxy]olean-12-en-28-oic acid
D
Root/MeOH
244202–36-6
C53 H86 O21
(Mimaki et al., 1999)
33
(3β)-3-[(O-β-D-Glucopyranosyl-(1 → 4)-O-[O-β-D-glucopyranosyl-(1 → 4)-O-β-D-glucopyranosyl-(1 → 3)-6-deoxy-α-L-mannopyranosyl-(1 → 2)]-α-L-arabinopyranosyl)oxy]olean-12-en-28-oic acid
D
Root/MeOH
244202–37-7
C59 H96 O26
(Mimaki et al., 1999)
34
3-[(O-β-D-glucopyranosyl-(1 → 4)-O-β-D-glucopyranosyl-(1 → 3)-O-6-deoxy-α-L-mannopyranosyl-(1 → 2)-α-L-arabinopyranosyl)oxy]-methyl ester
D
Root/MeOH
244202–39-9
C54 H88 O21
(Mimaki et al., 1999)
35
Hederacolchiside E
D
Root/EtOH
33783–82-3
C65 H106 O30
(Xu et al., 2012)
36
Beesioside Q
D
Root/EtOH
261767–91-3
C65 H106 O30
(Xu et al., 2012)
37
(3β)-3-[(O-β-D-Glucopyranosyl-(1 → 4)-O-[O-β-D-glucopyranosyl-(1 → 3)-6-deoxy-α-L-mannopyranosyl]-α-L-arabinopyranosyl)oxy]olean-12-en-28-oic acid;
D
Root/EtOH
106577–41-7
C53 H86 O21
(Shu, et al., 2013)
38
Scabiosaponin D
D
Root/EtOH
689257–60-1
C59 H96 O26
(Shu, et al., 2013)
39
Lonimacranthoide II
D
Root/EtOH
1201426–02-9
C65 H106 O31
(Shu, et al., 2013)
40
(3β)-3-[(O-6-Deoxy-α-L-mannopyranosyl-(1 → 6)-O-β-D-glucopyranosyl-(1 → 4)-O-β-D-glucopyranosyl-(1 → 3)-O-6-deoxy-α-L-mannopyranosyl-(1 → 2)-α-L-arabinopyranosyl)oxy]olean-12-en-28-oic acid
D
Root/EtOH
1415553–83-1
C59 H96 O25
(Shu, et al., 2013)
41
Hederasaponin C
C
Root/EtOH
14216–03-6
C59 H96 O26
(Glebko et al., 2002)
42
Pulsatiloside C
C
Root/EtOH
57539–70-5
C48 H78 O18
(Glebko et al., 2002)
43
Hederacoside D
C
Root/EtOH
760961–03-3
C53 H86 O22
(Glebko et al., 2002)
44
2,3,23–trihydroxy-, O-6-deoxy-α-L-mannopyranosyl-(1 → 4)-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosyl ester
C
Root/EtOH
1146973–76-3
C48 H78 O19
(Glebko et al., 2002)
45
Leontoside D
C
Root/EtOH
20830–84-6
C59 H96 O27
(Xu et al., 2012)
46
Ursolic acid
E
Root/EtOH
77–52-1
C30 H48 O3
(Ding, et al., 2010)
47
β-Sitosterol
E
Root/EtOH
83–46-5
C29 H50 O
(Ding, et al., 2010)
48
Hederagonic acid
D
Root/EtOH
466–01-3
C30 H46 O4
(Ding, et al., 2010)
49
23–Hydroxybetulinic acid
E
Root/EtOH
472–15-1
C30 H48 O3
(Ding, et al., 2010)
50
Daucosterin
B
Root/EtOH
474–58-8
C35 H60 O6
(Ding, et al., 2010)
51
Hederasaponin B
D
Root/EtOH
36284–77-2
C59 H96 O25
(Glebko et al., 2002)
52
Oleonolic acid
D
Root/EtOH
508–02-1
C30 H48 O3
(Ding, et al., 2010)
53
Betunolic acid
A
Root/EtOH
4481–62-3
C30 H46 O3
(Ding, et al., 2010)
54
Pulchinenoside E
A
Root/EtOH
1310041–02-1
C65 H106 O31
(Xu et al., 2012)
55
Pulsatilla triterpenic acid A
A
Root/EtOH
1360878–33-6
C35 H50 O7
(Shu, et al., 2011)
56
Pulsatilla triterpenic acid B
D
Root/EtOH
1360878–34-7
C36 H54 O6
(Shu, et al., 2011)
57
Pulsatilla triterpenic acid C
D
Root/EtOH
1360878–35-8
C36 H50 O6
(Shu, et al., 2011)
58
O-6-Deoxy-α-L-mannopyranosyl-(1 → 4)-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosyl(3β,4α)-3-hydroxy-23–(α-D-ribofuranosyloxy) ester
D
Root/EtOH
342613–09-6
C53 H86 O22
(Shu, et al., 2013)
59
(3β,4α)-3-(β-D-Glucopyranosyloxy)-23–(α-D-ribofuranosyloxy)olean-12-en-28-oic acid
D
Root/EtOH
1500092–25-0
C41 H66 O13
(Shu et al., 2013)
Flavonoids
60
Tiliroside
Root/EtOH
20316–62-5
C30 H26 O13
(Zhang, et al., 2008)
61
5-Hydroxy-2-(4-hydroxyphenyl)-7-[[3-O-[(2E)-3-(4-hydroxyphenyl)-1-oxo-2-propen-1-yl]-β-D-glucopyranosyl]oxy]-4H-1-benzopyran-4-one
Root/EtOH
171367–93-4
C30 H26 O12
(Zhang, et al., 2008)
Coumarins
62
Siderin
Root/EtOH
53377–54-1
C12 H12 O4
(Zhang, et al., 2008)
63
4,6,7-Trimethoxy-5-methylcoumarin
Root/EtOH
62615–63-8
C13 H14 O5
(Zhang, et al., 2008)
64
Xanthotoxine
Root/MeOH
298–81-7
C12 H8 O4
(Quan, et al., 2011)
Lignans
65
(+)-Pinoresinol
Root/MeOH
487–36-5
C20 H22 O6
(Mimaki et al., 1999)
66
beta-Podophyllin
Root/MeOH
518–29-6
C22 H22 O8
(Mimaki et al., 1999)
Other compounds
67
Anemonin
Root
508–44-1
C10 H8 O4
(Duan, et al., 2006)
68
trans-Caffeoyltartaric acid
Root/ n-BuOH
70831–56-0
C22 H18 O12
(Zhang, et al., 2008)
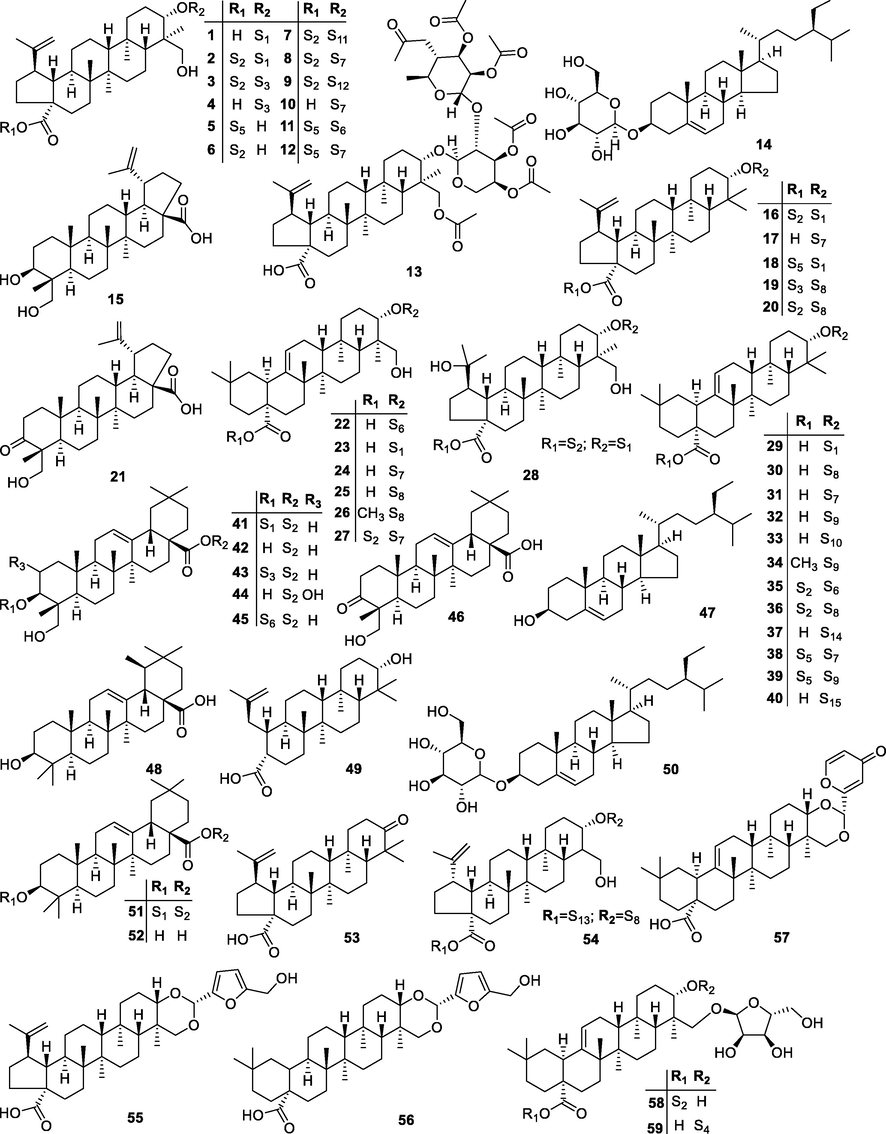
Chemical structures of triterpenoids isolated from P. chinensis.
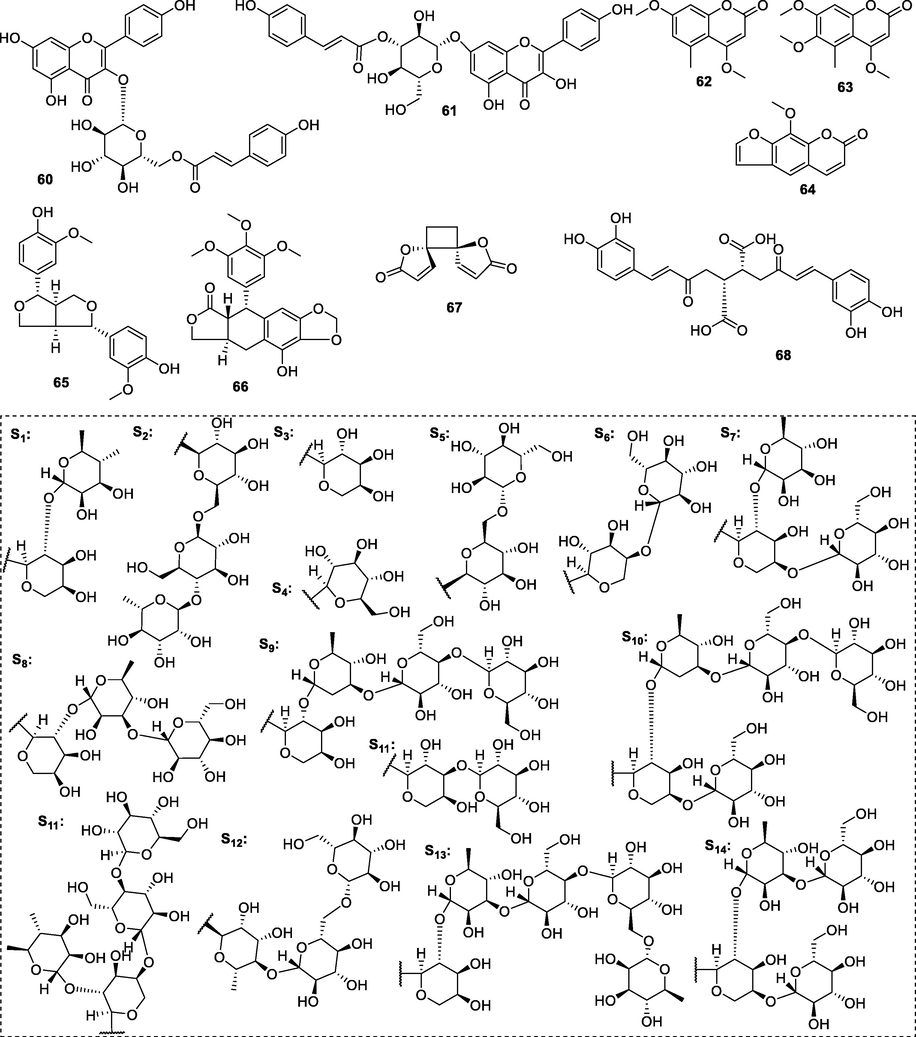
Chemical structures of other constituents isolated from P. chinensis.
4.1 Triterpenoids
Triterpenoid saponins are the main and representative components of P. chinensis. At present, more than dozens of species have been isolated and identified, which belong to oleanolic and lupinane pentacyclic triterpenoids respectively. The main types of aglycones are oleanolic acid saponins (29–40, 48, 51, 52), lupane type saponins (1–13, 15, 21, 28, 16–20, 49, 53–55), ivy saponins (22–27, 41–45) and 23-hydroxybutyric acid saponins (14, 50). The positions of glycosides are mainly 3-hydroxy and 28-carboxyl groups of aglycones. The five types of glycosides are alpha-L-arabinose, alpha-L-rhamnose, beta-D-glucose, beta-D-galactose and beta-D-xylose.
4.2 Flavonoids
Up to date, only two kinds of flavonoid-derived compounds named tiliroside (60) and 5-hydroxy-2-(4-hydroxyphenyl)-7-[[3-O-[(2E)-3-(4-hydroxyphenyl)-1-oxo-2-propen-1-yl]-β-D-glucopyranosyl]oxy]-4H-1-benzopyran-4-one (61) have been characterized from P. chinensis, which are reported by (Zhang, et al., 2008), both the isolated flavonoids are kaempferol analogues, indicating potential research direction of this classes of constituents in P. chinensis.
4.3 Coumarins
Three kinds of benzofurans-type coumarins have been separated from P. chinensis. The structural characterization offer help to confirm their name as siderin (62), 4,6,7-trimethoxy-5-methylcoumarin (63) and xanthotoxine (64). The pharmacological properties of these monomers are still waiting for excavating.
4.4 Lignans
Investigations about lignans from P. chinensis are rare as well. Currently, only two kinds of lignans were separated from P. chinensis. They were (+)–pinoresinol (65) and beta-podophyllin (66). Among them, beta-podophyllin is the first derivative of podophyllotoxin isolated from Ranunculaceae plants.
4.5 Other compounds
Apart from the constituents mentioned above, anemonin (67) and trans-caffeoyltartaric acid (68) have also been isolated and identified from P. chinensis. The potential pharmacological potency of these compounds is a valuable task for researchers in the future.
5 Pharmacology
The traditional medicinal applications of P. chinensis have inspired various pharmacological investigations about it. The extracts and isolated compounds from P. chinensis displayed multiple bioactivities including antitumor, anti-inflammatory, anti-microbial and antiviral activities. The detailed pharmacological reviews were as follows.
5.1 Antitumor activity
5.1.1 Saponin-enriched extracts of P. chinensis as antitumor agents
P. chinensis saponins, composed of 15 kinds of monomers, possessed anticancer effects with non-toxic side effects in human liver tumor 7402 cells in vitro (Xu, et al., 2012). On different concentrations (12.5 to 200 mg/mL), P. chinensis saponins inhibited the proliferation of human liver tumor 7402 cells in vitro by apoptosis. 19 days after administration of P. chinensis saponins (100, 200 mg/kg), the weight of tumor transplanted beneath the underarm skin of mice was markedly decreased in nude mice. The anti-tumor effect of P. chinensis saponins in vivo was associated with a significant increase in the tumor cell apoptosis rate. Although P. chinensis saponins inhibited the weight of mice, the extracts showed almost no effect on leukocyte number, liver and spleen weight index with no specific lesion in organ.
Total saponins from P. chinensis showed the potency to be effective therapy of human chronic myelogenous leukemia, and 23-hydroxybetulinic acid (23-HBA, 49) was speculated to be the active substance (Liu, et al., 2015). As for the potential constituents, compared with other saponins with substituted glucosides on the C-3 or C-28 position, as an aglycone, compound 49 displayed better activities on multiple cancer cell lies including K562, B16, Hela and HUVEC with IC50 values for 18.7, 39.9, 78.5, 80 and 94.8 μM, respectively.
The extracts from P. chinensis were reported to inhibit the growth of hepatocellular carcinoma cells. Furthermore, to investigate the effective substance of P. chinensis extracts, the cytotoxic effect of anemoside B4 (2) was evaluated with significantly inhibitory effects of the growth of SMMC7721 cell. Finally, a conclusion was draw that compound 2 induced apoptosis and autophagy of tumor cell lines through modulating the PI3K/Akt/mTOR signaling pathways Xue, et al. (2019).
5.1.2 Active monomers from P. chinensis as antitumor agents
Numerous previous studies provided evidences for therapeutic effects of active monomers isolated from P. chinensis against tumor cells. As a promising monomer, the privileged antitumor activity of compound 49 was proved again, it was demonstrated to activate cell cycle arrest at S phase to cause the apoptosis of tumor cells, with the selectively regulations of expression levels of significant proteins including Bcl-2, Bax, surviving, cytochrome C and caspase-9.
The 3-(4, 5-dimethylthiazol-2-yl)-2, 5diphenyltetrazoliumbromide (MTT) assay was implemented to test the antitumor potency of compounds isolated from P. chinensis, the results showed that multiple promising constituents including compound 27 (IC50: 7.33 µM for A549, 7.13 µM for SGC-7901, 6.32 µM for HL-7702 cells), compound 37 (IC50: 8.11 µM for A549, 5.41 µM for SGC-7901, 9.42 µM for HL-7702 cells), compound 40 (IC50: 9.89 µM for A549, 7.15 µM for SGC-7901, 8.83 μM for HL-7702 cells) significantly inhibited the growth of cancer cells (Shu et al., 2013). Pulsatilla saponin D (24) was reported to show inhibitory effect on autophagic flux in some cancer cell lines to performed anticancer activities in diverse types of cancer.
Xu, et al. (2013) proposed that different types and structure of saponins induced disequilibrium effects towards the cancer cells including the human cancer cell lines (A549, SGC-7901) as well as the hepatic cell line (HL-7702). Multiple saponins from P. chinensis including compounds 8–11, 23, 29–33, 38, 39, 42, 43 were tested for the cytotoxicity by MTT assay. Among the test compounds, raddeanoside R13 (31) exhibited the strongest cytotoxic potency on multiple cell lines. Additionally, the results showed that a free carboxylic group on C-28 of saponins for example 31 (cytotoxic activity against A549, SGC-7901 cancer cell lines, and HL-7702 hepatic cell lines as IC50 values 5.81, 3.79 mM, and 8.23 mM, respectively) was indispensable for their cytotoxic activity, meanwhile, through the analysis of SAR demonstrated that the oleanane-type saponins had better cytotoxic effect than lupane-type saponins, also, the substituent of C-3 glycolic chain had an exact influence to the activity to kill the cancer cell.
Recently in 2020, pulchinenosides from P. chinensis such as compounds 23 and 24 were disclosed to stimulate P-glycoprotein functional activities and upregulate P-glycoprotein/ABCB1 (Liu et al., 2020). Moreover, both short-term and long-term dosing of pulchinenosides exerted the potential to reduce the oral bioavailability of P-glycoprotein substrates mRNA and protein expression, suggesting promising potential for P. chinensis using as antitumor agents.
Pulsatilla saponin A (23) exhibited expectable potential to develop as a novel differentiation inducer to treat the acute myeloid leukemia. Wang et al. (2016) picked U937 cells, K562 cells and HL-60 cells as well as the primary leukemia cells extracted from the AML patients to investigate the differentiation induced by compound 23, the results illustrated compound 23 modified the differentiation effects of AML cells, possibly by modulating the MEK/ERK signaling pathway. It was reported that compound 23 also exhibited activity to against human colon cancer HT-29 cells, with a 9.5% ratio of apoptosis compared with 2% of control group. Additionally, compound 23 displayed synergy effects of exiting anticancer drug, it enhanced the activity of fluorouracil, a first line chemotherapy drug for colon cancer (Yang, et al., 2017).
5.1.3 Structural optimization of anti-tumor P. chinensis compounds with their SAR
Nowadays, structural optimization of precursors excavating from natural products has become an important task for the development of natural compound chemists, which matters the potential yields for researchers and convenience for patients. Rational design of the synthesis of lead compounds can reduce the cost during the extraction and isolation, and the SAR analysis can provide perspective to both chemists and biologists. To date, the structural optimization investigations were mainly possessed on betulinic acid (15), pulsatilla saponin A (23), pulsatilla saponin D (24), hederacolchiside A1 (31), herein, we introduce the research progress in this field according to this sequence in order to give more evidence to develop clinical candidates from constituents in P. chinensis.
In 2009, Gauthier et al. (Gauthier, et al., 2009) synthesized a sequence of betulinic acid (15) bidesmosidic derivatives as antitumor agents. Among the synthesized derivatives, compound S1 (synthetic derivative 1, Fig. 4.) showed the most significant antitumor potency on cancer cell lines including A549, DLD-1, MCF7, PC-3 and WS1 with IC50 values for 1.9, 1.9, 1.7, 1.8 and 1.3 μM, respectively. The SAR analysis was speculated as that the relative cytotoxicity of bidesmosidic betulin and betulinic acid saponins were strongly influenced by the nature of both the aglycone and the ranosyl moieties at both C-3 and C-28 positions were highly cytotoxic sugar moieties.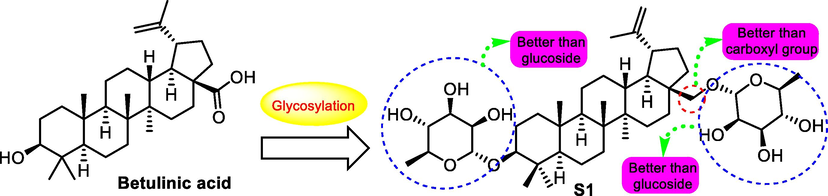
SAR study of betulinic acid analogue S1 as antitumor agent.
In 2015, Shilin Yang task group carried out the structural optimization jobs on pulsatilla saponin A and D and evaluated their antitumor potency (Chen, et al., 2015), among the synthetized derivatives, compound S2 (Fig. 5) exhibited the most significant antitumor activities in vitro against cancer cell lines A-549, Bel-7402 and SMMC-7721 with IC50 values for 1.0, 0.9 and 3.2 μM and good selectivity with the cancer cell lines to normal cell line with IC50 value for more than 100 μM on normal cell lines HL-7702. The concentration inducing fifty percent of erythrocytes haemolysis for S2 (Fig. 5.) was more than 500 μM, indicating a favorable safety with low-risk of haemolysis problem. The antitumor effects on mentioned cell lines were better than both pulsatilla saponin A and D, moreover, in vivo in the acute toxicity test, the administration of S2 was detected to obtain the tolerance dose for more than 160 mg/g in mice, which was 16-folds to the value of pulsatilla saponin A and D.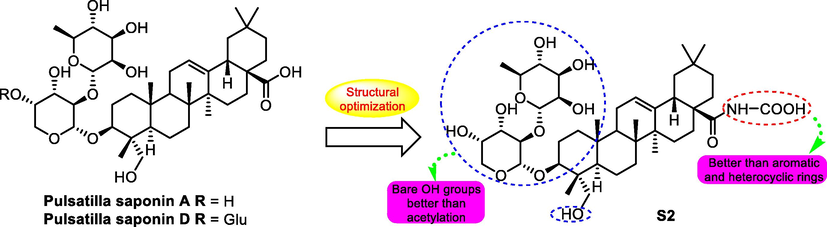
SAR study of pulsatilla saponin A and D analogue S2 as antitumor agent.
In 2016, the same task group published their further research. Based on the structure of hederacolchiside A1 (31), a series of analogues were synthesized as antitumor agents (Fang, et al., 2016), among which most promising compound S3 (Fig. 6) displayed the most remarkable antitumor activity. Compound S3 showed cytotoxicity against six human cancer cell lines including SMMC-7721, NCI-H460, U251, SK-OV-3, HCT-116 and SGC-790 with IC50 values ranging from 1.1 to 4.6 μM after 48 h incubation. In addition, in mice acute toxicity test, S3 promoted the tolerance dose and reduced the death number of test mice compared with the precursor hederacolchiside A1.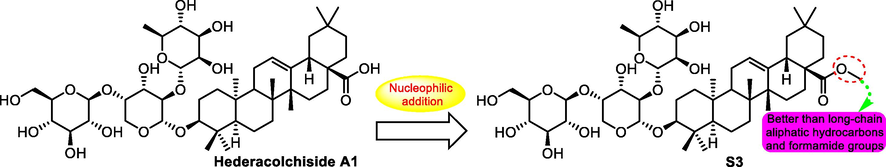
SAR study of hederacolchiside A1 analogue S3 as antitumor agent.
Subsequently, more pulsatilla saponin D-based derivatives were synthesized and determined the antitumor activity (Fang, et al., 2019). Compound S4 (Fig. 7) showed strongest antitumor activity among the productions against cancer cell lines such as SMMC-772, MCF-7, NCI-H460, A549 and HCT-116 with IC50 values for 2.4, 4.5, 3.2, 3.7 and 1.7 μM, respectively. Same as the results of S3 in mice acute toxicity test, S4 promoted the tolerance dose and reduced the death number of test mice compared with the lead compound.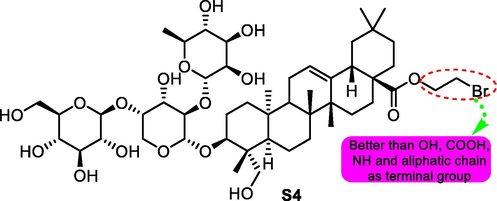
SAR study of pulsatilla saponin D analogue S4 as antitumor agent.
In 2017, Zhong Chen and co-workers (Tong, et al., 2017) were deeply involved with the structural modification of pulsatilla saponin A. Compound S5 (Fig. 8) was considered to be the most potent antitumor derivative on cancer cell lines including A549, MDA-MB-231, KB, KB-VIN and MCF-7 with IC50 values for 4.685, 5.540, 5.075, 5.248 and 10.737 μM. Additionally, compound S5 showed a better balance between haemolytic toxicity (HD50 > 500 μM) and cytotoxicity toward lung cancer cells A549. Molecular studies indicated that S5 was liked to lead to G1 cell cycle arrest.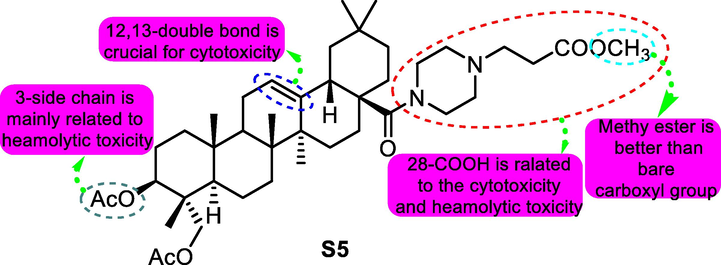
SAR study of pulsatilla saponin A analogue S5 as antitumor agent.
In 2018, they reported new progress in this area. Directing against the strong hemolytic toxicity of pulsatilla saponin D (HD50: 6.3 μM), a cluster of derivatives were designed and prepared (Chen, et al., 2018). Compound S6 (Fig. 9) was selected for its significant antitumor activity (IC50 values for A549, MDA-MB-231, KB, KB-VIN and MCF-7 cells: 2.8–8.6 μM) and weak hemolytic toxicity (HD50 > 500 μM). Molecular studies indicated that S6 induced typical G1 cell cycle arrest and apoptosis in A549 cells, and Western blot assays suggested that both intrinsic and extrinsic apoptosis pathways were activated by S6.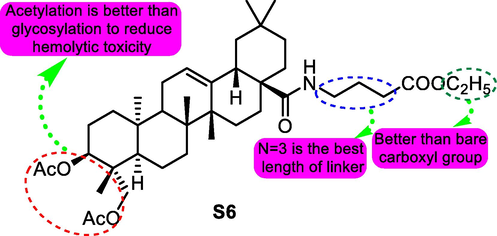
SAR study of pulsatilla saponin D analogue S6 as antitumor agent.
The task group also possessed the structural optimization from the aspect of carbohydrate chemistry. Based on the structure of compound 31 and β-hederin, multiple substituted saponins were synthesized (Wang, et al., 2017), among which compound 30 (Fig. 10) exhibited strongest antitumor potential than other derivatives against cancer cell lines including SMMC-7721, Bel-7402 and A-549 with IC50 values for 3.07, 4.57 and 2.43 μM, respectively. The results showed that type of terminal monosaccharides and linkage position had apparent effects on cytotoxicity and selectivity of the synthesized against cancer cell lines tested.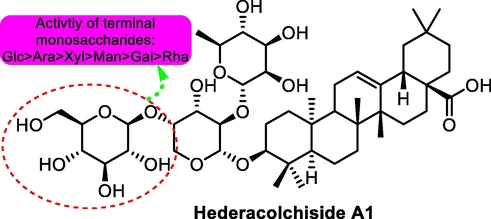
SAR study of hederacolchiside A1 (30) as antitumor agent.
5.1.4 Effective polysaccharides isolated from P. chinensis as antitumor agents
Polysaccharides from P. chinensis were disclosed to be promising antitumor agents as well. P. chinensis polysaccharides (PCPs) showed significant anti-proliferative effect on C6 glioma in vitro (Zhou, et al., 2012), meanwhile P. chinensis polysaccharides inhibited the growth of C6 glioma and prolonged the life survival in vivo, comparable to carmustine administration. In addition, PCPs treatment to tumor bearing mice could not only reduce the body weight loss, but also elevate the thymus and spleen indices. In addition, PCPs administration to tumor bearing mice could relieve the liver and kidney damage with decreased levels of aspartate aminotransferase, alanine aminotransferase and urea, and promote superoxide dismutase (SOD) and catalase enzyme activities.
A water-soluble P. chinensis polysaccharide, which consisted of five different monosaccharides, including rhamnose, arabinose, xylose, galactose, mannose with the molar ratio of 1.00: 7.85: 0.37: 0.65: 3.01 (molecular weight: 7.8 × 105 Da), inhibited the growth of transplantable tumor on bearing mice in vivo Liu, et al. (2013). Moreover, the polysaccharide promoted the concanavalin A, lipopolysaccharide (LPS)-stimulated splenocytes proliferation, the serum lysozyme level and 2,4-dinitrofluorobenzene (DNFB)-induced delayed-type hypersensitivity reactions at the dosage of 100 mg/kg, meanwhile, significant improvements in peripheral blood abnormality and anemia were observed in P. chinensis polysaccharide treatment, indicating both cellular and humoral immune response were improved.
5.2 Anti-inflammatory activity
The anti-inflammation activity of 16 compounds isolated from Pulsatilla koreana was investigated Yang, et al. (2010), almost all the compounds exhibted the activity of anti-inflammation. Thereinto, pulsatilloside E (8) also isolated from P. chinensis, and the results showed that compound 8 exerted strong inhibitory activity against NO production in LPS-stimulated RAW 264.7 cells, the inhibitions were 67.7%, 49.2%, 5.3% respectively at concentrations of 1, 10 and 100 μM. In the isolated compounds, the in monosaccharide or trisaccharide substituted triterpenoids were better than disaccharide substituted triterpenoids in general.
Anemonin (67), isolated from P. chinensis, at the dosage of 10 μg/mL, prevented intestinal microvascular dysfunction and exerted beneficial therapeutic action in intestinal inflammation (Duan, et al., 2006). Compound 67 inhibited LPS-induced rat intestinal microvascular endothelial cells (RIMECs) damage in vitro, the expression of cell adhesion molecules and secretory products in RIMECs were also regulated by compound 67. Compound 67 may also have applications for various other diseases, including cardiovascular diseases and arthritis where ET-1 and NO activation has been shown to mediate pathogenesis.
Anemoside B4 (2), a representative constituent from P. chinensis, was reported to exhibit anti-inflammatory and immune-modulatory activities in vivo in mice models (Kang, et al., 2019). At the dosages 12.5–50 mg/kg, compound 2 suppressed xylene-induced mice ear edema remarkedly. Furthermore, it ameliorated LPS-induced kidney and lung inflammation damage, which inhibited pro-inflammatory response by NF-κB pathway in mice. In addition, compound 2 reduced CD4+/CD8+ ratio, inhibited splenic lymphocyte proliferation and decreased DNFB-induced changes of ear thickness, exerting potential to be a novel natural anti-inflammatory drug candidate for treating inflammatory disorder. Lately in 2020, compound 2 was reported to prevent acute ulcerative colitis through inhibiting of TLR4/NF-κB/MAPK signaling pathway (Ma, et al., 2020). Compound 2 was injected in the C57BL/6 mice model of ulcerative colitis and the DSS-induced colitis mice from losing weight, shortening colon length and improving pathological changes of colon tissues was prevented by compound 2. Moreover, compound 2 significantly reduced levels of inflammatory cytokines interleukin-1 beta (IL-1β), IL-6, and tumor necrosis factor-alpha (TNF-α) in colon tissues. In vitro, compound 2 was almost nontoxic to RAW264.7 cells and could protect cells against LPS. Furthermore, compound 2 significantly inhibited the activation of the TLR4 signaling pathway induced by DSS and down-regulated the expression of key proteins in the TLR4/NF-κB/MAPK signaling pathway in RAW264.7 cells induced by LPS.
5.3 Anti-microbial activity
Infectious diseases induced by microbial trouble both human and animals both in health and economics (Bao, et al., 2017, Dao Ngoc Hien, et al., 2018). The exploration and development of P. chinensis as anti-microbial agent gives inspiration in the development of natural products as health productions. P. chinensis has been recorded to be used in the veterinary medicine for the treatment of enteritis induced by parasite according to the Chinese Veterinary Pharmacopoeia. The dosages of P. chinensis are ranging from 5 g to 100 g based on the body type of animals. The common formula so called Baitouweng oral liquid is composed of P. chinensis, C. chinensis, P. chinense and F. rhynchophylla, which is similar to the composition of the prescription Bai Tou Weng Tang.
P. chinensis extracts were reported to inhibit the growth of Giardia intestinalis Li, et al. (2012). The ethyl acetate fraction was the most effective part on parasite growth, cell viability, adherence and morphology among the sub-fractionsn according to the pharmacological screening results. In addition, the growth of G. intestinalis could also be moderately inhibited by the ethyl acetate fraction (IC50: 257.081 µg/mL). Changes in G. intestinalis morphology induced by P. chinensis were observed to some degree, providing evidence that P. chinensis can be develop potential anti-giardiasis agent.
Schistosomiasis, a parasitic disease mainly caused by Schistosoma japonicum, S. mansoni and S. haematobium, affects public health potentially (Kang, et al., 2018). Pulsatilla saponin A (23) extracted from the P. chinensis was proved to be a potential candidate to schistosomiasis infected by the S. japonicum and S. mansoni. After injecting compound 23 to S. japonicum-infected mice, the female and total worm were decreased by 97.2% and 89.5% in vivo, respectively, as well as in the mice infected by S. mansoni with the reductions of the female and total worms were 88.6% to 80.7%, respectively. In vitro, at the concentration of 8.93 µM, compound 23 killed S. japonicum worms with a ratio of 100% on newly transformed schistosomula, which was equivalent to the currently used drugs praziquantel and artesunate at the doses of 96.03 and 78.04 µM, respectively.
5.4 Antiviral activity
P. chinensis was used as an herbal substitutive therapy for hepatitis B patient, with a great clearance of hepatitis B virus (HBV) Yao, et al. (2009). Betulinic acid (15) extracted from P. chinensis exhibited hopeful inhibitory potency on hepatitis B virus (HBV). Compound 15 downregulated the manganese SOD expression to inhibit the HBV, additionally, compound 15 suppressed the HBV X protein and translocated into the mitochondria followed by cytochrome C release. Both compound 15 and the P. chinensis crude extracts increased the clearance of HBV. Herein, compound 15 was proved to be a potential candidate for the development of anti-HBV drug. It was worth mentioning that as a potential monomer, compound 15 had been reported for antiviral activities not limited in HBV, refractory diseases including human immunodeficiency virus (HIV) and herpes viruses HSV-1 were also introduced into the treatment (Aiken and Chen, 2005, Bildziukevich, et al., 2019, Rios and Manez, 2018), suggesting the feasibility for it to develop as antiviral agent. The antiviral potency of P. chinensis was disclosed to preserve plant against plant virus. After screening for 126 kinds of plants grown in the Qinling region of China for the antiviral effects against infection by Tobacco mosaic virus, the extracts of P. chinensis stand out through the half-leaf method Jing, et al. (2012), the results showed a good activity to against T. mosaic virus with the inhibition rate of 61.25% at the concentration of 20 µg/mL.
5.5 Other biological activities
Apart from the broad bioactivities mentioned above, cardiovascular system activity was also related to the pharmacological potential of P. chinensis. 23-Hydroxybetulinic acid (49) isolated from P. chinensis was reported to prevent heart from cardiotoxicity induced by the doxorubicin with a correlation on the inhibition carbonyl reductase mediated metabolism (Zhou, et al., 2015). Compound 49 alleviated the doxorubicin-induced cardiotoxicity in mice, and this was accompanied by inhibition of the metabolism of doxorubicin and reduced accumulation of doxorubicinol selectively in hearts. In H9c2 cells, the protective effect of compound 49 was shown to be closely associated with a decreased rate and extent of accumulation of doxorubicinol in mitochondria and nuclei. siRNA and docking analysis (Fig. 11) demonstrated that carbonyl reductase 1 has a crucial role in doxorubicin-mediated cardiotoxicity and compound 49 inhibiting this metabolic pathway.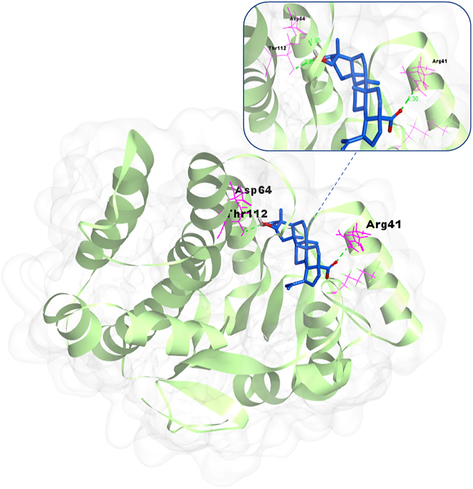
Molecular docking between the potential target carbonyl reductase 1 and active compound 23-hydroxybetulinic acid (48) from P. chinensis.
With the development of molecular biology, more and more investigations have been carried out about the biocative constituents isolated from TCM with their potential molecular mechanism, providing evidence for elucidating the effective substances. Anemoside B4 (2), a representative and major component with a content of up to 10% in root of P. chinensis, was reported to show protecting effects against cisplatin-induced nephrotoxicity through NF-κB and MAPK mediated apoptosis signaling pathways in mice recently (Wang, et al., 2020). Compound 2 mainly act on NF-κB signaling pathway to reduce the levels of TNF-α, IL-1β, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) in the kidney after CP exposure. Furthermore, compound 2 regulated MAPK signaling pathway and its downstream apoptotic factors to inhibit the occurrence of apoptosis including Bax, Bcl-2, caspase-3 and caspase-9. Notably, the activations of caspase-3 induced by cisplatin were strikingly reduced in compound 2-treated mice.
6 Toxicity
Toxicity, especially hemolytic toxicity and liver injury, induced by the constituents from P. chinensis should be vigilant. Chronic liver injury, caused by crude P. chinensis saponin for a long time taking was disclosed along with the elevation of biochemical indexes including alkaline phosphatase (from 30 to 65 U/L), cereal third transaminase (from 90 to 105 U/L) and alkaline phosphatase (from 100 to 115 U/L) in patients, indicating a potential chronic liver toxicity of P. chinensis (Song, et al., 2019). As for the molecular biology level, recently in 2020, (Su, et al., 2020) reported the liver injury induced by P. chinensis saponins through interfering ceramide/sphingomyelin balance that promoted lipid metabolism dysregulation in vivo and apoptosis in vitro. Further investigations revealed that P. chinensis saponins caused lipid metabolism dysregulation and apoptosis through bile acid-mediated sphingolipid pathway which ultimately induced liver injury. Extrinsic and intrinsic apoptosis were observed on hepatocytes.
7 Quality control and metabolism analysis
Quality control of TCM is essential to ensure their efficiency and safety. Advanced analytical techniques provide reliable and efficient methods for the quality control of P. chinensis (Zhao, et al., 2019). According to the 2015 edition of Chinese Pharmacopoeia, the content of anemoside B4 (2) in P. chinensis must be no less than 4.6 % based on HPLC calibration Standard Operating Procedure. The chromatographic separation should be performed on a C18 silica gel column, with methanol and water (65:35, V/V) as the mobile phase, and the wavelength for detection is at 201 nm.
The HPLC-MS fingerprint method has also been prevalently used in the quality control of compounds isolated from P. chinensis. Multiple analysis methods have been developed, such like liquid chromatography-mass spectrometry (LC-MS). Some main compounds including pulsatilla saponin A (23), anemoside B4 (2), anemoside A3 (1) and 23-hydroxybetulinic acid (49) have been quantifed as internal standard substances. Comprehensive methods for the quality control of P. chinensis are being set up. Pulsatilla saponin A (23) were separated by gradient elution with the mobile phase of methanol and water (25–15%) through the HPLC, moreover, the oral bioavailability of the compound 23 was obtained as 1.16 through the calculation between each major pharmacokinetic parameters (Liu, et al., 2013).
As for the metabolism analysis of the effective substances from P. chinensis, several studies have been published, giving inspirations for the further investigations for P. chinensis. Ouyang and co-workers totally identified 18 kinds of metabolites including prototype in plasm, urine and feces of rat after pulsatilla saponin D (24) orally administered to the rat through liquid chromatography-mass spectrometry (Ouyang, et al., 2014). The results of this study indicated that compound 24 was mainly metabolized through deglycosylation, dehydrogenation, hydroxylation and sulfation in rat.
In 2018, an research was published to investigate the dynamic condition of Gouteng-Baitouweng (GB), a pair of traditional Chinese medicine (Tian, et al., 2018). After orally administrated GB with the dosage of 25 g/kg to rats, a sensitive, selective and accurate LC-MS/MS analysis method result revealed that three contents in P. chinensis, anemoside B4 (2), anemoside A3(1) and 23-hydroxybetulinic acid (49) were main effective substances through the brain according to the organ distribution test results, in which compound 49 had the highest values for AUC and Cmax at 3301.8 ng/g ∗ h and 187.2 ng/g, respectively, thus the three saponins should be paid more attention in Parkinson treatments.
8 Conclusion
Numerous plants are traditionally and ethno-pharmacologically used to treat multiple disorders, and have been proven effective (Liu et al., 2020, Zhao, et al., 2018, Zhao, et al., 2017, Zhao, et al., 2020). Therefore, researches in the area of natural medicines have improved opportunities for finding newer and safer alternatives to fight diseases. P. chinensis is excellent medicinal plant rich in bioactive constituents, which have been extensively studied in recent years. The traditional pharmacological activities of P. chinensis focused on the treatment of cool blood and stop dysentery. Modern investigation proved that triterpenoids with the prominent antitumor and anti-inflammatory activities were the main contributors to the traditional pharmacological activities. Constituents from P. chinensis are more promising for the treatment of inflammatory-immune related diseases in the future. While the toxicity on of the plant liver and kindey is notable as well, according to the Chinese pharmacopoeia, the suggested dosage of P. chinensis ranges from 9 to 15 g, thus it is vigilant when the dosage is beyond the safe range. However, there are still unclear issues about this plant. Firstly, the pharmacological activities of only a few potential compounds including typical compounds betulinic acid (15), pulsatilla saponin A (23), pulsatilla saponin D (24), hederacolchiside A1 (31), have been investigated, further studies should pay attention to more constituents in order to discover promising precursors for the clinical drug development. Moreover, the information on randomized clinical trials (RCTs) of P. chinensis is limited, although P. chinensis is frequently used in TCM. Therefore, further studies on the effective substances, toxicity, and clinical investigations of P. chinensis are indispensable to meet the requirements of evidence-based medicine. It is also expected that new skeletons and new active molecules will be found from P. chinensis. This review brings together the most recent studies in the field of the P. chinensis research; therefore, it will help to promote further investigation of P. chinensis for the development of new herbal medicine and health products.
Acknowledgements
This work was supported by the Program for Meridian-Viscera Correlationship Innovative Research Team of Shaanxi University of Chinese Medicine (YL-09) National Natural Science Foundation of China (no. 8210142829).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Betulinic acid derivatives as HIV-1 antivirals. Trends Mol. Med.. 2005;11(1):31-36.
- [Google Scholar]
- Naturally occurring furanoditerpenoids: distribution, chemistry and their pharmacological activities. Phytochem. Rev.. 2017;16:235-270.
- [Google Scholar]
- Recent achievements in medicinal and supramolecular chemistry of betulinic acid and its derivatives (double dagger) Molecules. 2019;24:3546-3565.
- [Google Scholar]
- Saponin of the Chinese drug Bai-Tou-Weng. IV. Structures of anemosides B4 and A3. Huaxue Xuebao. 1990;48:501-505.
- [Google Scholar]
- Cytotoxicity, hemolytic toxicity, and mechanism of action of pulsatilla saponin D and its synthetic derivatives. J. Nat. Prod.. 2018;81(3):465-474.
- [Google Scholar]
- Synthesis, cytotoxicity and haemolytic activity of Pulsatilla saponin A. D derivatives. Bioorg. Med. Chem. Lett.. 2015;25(12):2550-2554.
- [Google Scholar]
- Silver complexation and tandem mass spectrometry for differentiation of triterpenoid saponins from the roots of Pulsatilla chinensis (Bunge) Regel. Rapid Commun. Mass Spectrom.. 2008;22(23):3783-3790.
- [Google Scholar]
- Dao Ngoc Hien, T., Duy Hieu, T., Thi Thanh Hoa, N., Le Nhat, Q., Linh, T., Hong Duong, N., Shamandy, B., Thi Minh Huong, L., Dang Khoa, T., Sayed, D., Van Vinh, V., Mizukami, S., Hirayama, K., Nguyen Tien, H., 2018. Ginsenoside Rh1: A systematic review of its pharmacological properties. Planta Med. 84, 139–152.
- Effect of anemonin on NO, ET-1 and ICAM-1 production in rat intestinal microvascular endothelial cells. J. Ethnopharmacol.. 2006;104(3):362-366.
- [Google Scholar]
- Synthesis, biological evaluation, and mode of action of pulsatilla saponin D derivatives as promising anticancer agents. Front. Pharmacol.. 2019;10:1208-1216.
- [Google Scholar]
- Synthesis and biological evaluation of Hederacolchiside A(1) derivatives as anticancer agents. Bioorg. Med. Chem. Lett.. 2016;26(19):4576-4579.
- [Google Scholar]
- Synthesis and cytotoxicity of bidesmosidic betulin and betulinic acid saponins. J. Nat. Prod.. 2009;72(1):72-81.
- [Google Scholar]
- Glebko, L., N.P., Strigina, L.I., Ulanova, K.P., Denisenko, V.A., Dmitrenok, P.S., 2002. Triterpene glycosides from Pulsatilla chinensis. Russ. Chem. B+ 51, 1945–1950.
- Anemoside B4 attenuates nephrotoxicity of cisplatin without reducing antitumor activity of cisplatin. Phytomedicine. 2019;56:136-146.
- [Google Scholar]
- Evaluation of the antiviral activity of extracts from plants grown in the Qinling region of China against infection by Tobacco mosaic virus (TMV) J. Phytopathol.. 2012;160:181-186.
- [Google Scholar]
- Anti-inflammatory and immune-modulatory properties of anemoside B4 isolated from Pulsatilla chinensis in vivo. Phytomedicine. 2019;64:152934.
- [Google Scholar]
- Antischistosomal properties of hederacolchiside A1 isolated from Pulsatilla chinensis. Molecules. 2018;23:1431-1446.
- [Google Scholar]
- Giardia intestinalis: effects of Pulsatilla chinensis extracts on trophozoites. Parasitol. Res.. 2012;111:1929-1935.
- [Google Scholar]
- Anti-inflammatory and PPAR transactivational effects of oleanane-type triterpenoid saponins from the roots of Pulsatilla koreana. Biomol. Ther.. 2014;22(4):334-340.
- [Google Scholar]
- A review of traditional uses, phytochemistry, and pharmacological properties of the genus Saururus. Am. J. Chin. Med.. 2020;48(01):47-76.
- [Google Scholar]
- Cytotoxicity of the compounds isolated from Pulsatilla chinensis saponins and apoptosis induced by 23-hydroxybetulinic acid. Pharm. Biol.. 2015;53(1):1-9.
- [Google Scholar]
- Immunopontentiating and antitumor activities of a polysaccharide from Pulsatilla chinensis (Bunge) Regel. Int. J. Biol. Macromol.. 2013;54:225-229.
- [Google Scholar]
- Pulchinenosides from Pulsatilla chinensis increase P-glycoprotein activity and induce P-glycoprotein expression. Evid. Based Complement. Alternat. Med.. 2020;2020:4861719.
- [Google Scholar]
- Validated rapid resolution LC-ESI-MS/MS method for simultaneous determination of five pulchinenosides from Pulsatilla chinensis (Bunge) Regel in rat plasma: application to pharmacokinetics and bioavailability studies. J. Chromatorgr. B. 2013;942:141-150.
- [Google Scholar]
- Anemoside B4 prevents acute ulcerative colitis through inhibiting of TLR4/NF-kappa B/MAPK signaling pathway. Int. Immunopharmacol.. 2020;87
- [Google Scholar]
- New bisdesmosidic triterpene saponins from the roots of Pulsatilla chinensis. J. Nat. Prod.. 2001;64(9):1226-1229.
- [Google Scholar]
- Triterpene saponins and lignans from the roots of Pulsatilla chinensis and their cytotoxic activity against HL-60 cells. J. Nat. Prod.. 1999;62(9):1279-1283.
- [Google Scholar]
- Metabolites profiling of Pulsatilla saponin D in rat by ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF-MS/MS) Fitoterapia. 2014;96:152-158.
- [Google Scholar]
- Preparative isolation of 8-methoxypsoralen from the rhizomes of Pulsatilla chinensis using high-speed counter-current chromatograpy. Appl. Biol. Chem.. 2011;54:623-627.
- [Google Scholar]
- The genus Tamarix: Traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol.. 2019;246:112245.
- [Google Scholar]
- New pharmacological opportunities for betulinic acid. Planta Med.. 2018;84(01):8-19.
- [Google Scholar]
- Three new triterpenoids from Pulsatilla chinensis (Bunge) Regel and their cytotoxic activitie. Heterocycles. 2011;83:2365-2372.
- [Google Scholar]
- A new oleanane-type triterpenoidal saponin from Pulsatilla chinensis. Nat. Prod. Res.. 2013;27(23):2196-2201.
- [Google Scholar]
- Safety investigation of Pulsatilla chinensis saponins from chronic metabonomic study of serum biomedical changes in oral treated rat. J. Ethnopharmacol.. 2019;235:435-445.
- [Google Scholar]
- Pulsatilla chinensis saponins cause liver injury through interfering ceramide/sphingomyelin balance that promotes lipid metabolism dysregulation and apoptosis. Phytomedicine. 2020;76:152-158.
- [Google Scholar]
- Isolation and evaluation of immunological adjuvant activities of saponins from the roots of Pulsatilla chinensis with less adverse reactions. Int. Immunopharmacol.. 2010;10(5):584-590.
- [Google Scholar]
- Simultaneous determination of eight bioactive compounds by LC-MS/MS and its application to the pharmacokinetics, liver first-pass effect, liver and brain distribution of orally administrated Gouteng-Baitouweng (GB) in rats. J. Chromatorgr. B. 2018;1084:122-131.
- [Google Scholar]
- The derivatives of Pulsatilla saponin A, a bioactive compound from Pulsatilla chinensis: their synthesis, cytotoxicity, haemolytic toxicity and mechanism of action. Eur. J. Med. Chem.. 2017;129:325-336.
- [Google Scholar]
- Synthesis and cytotoxicity of oleanolic acid trisaccharide saponins. Carbohydr. Res.. 2017;442:9-16.
- [Google Scholar]
- Pulchinenoside B4 exerts the protective effects against cisplatin-induced nephrotoxicity through NF-kappaB and MAPK mediated apoptosis signaling pathways in mice. Chem-Biol. Interact.. 2020;331:109233.
- [Google Scholar]
- Pulsatilla saponin A induces differentiation in acute myeloid leukemia in vitro. Hematology. 2016;21(3):182-186.
- [Google Scholar]
- Glycosides from Pulsatilla chinensis (Bunge) Regel. Zhongguo Yaoke Daxue Xuebao. 1991;22:265-269.
- [Google Scholar]
- Cytotoxic activity of Pulsatilla chinensis saponins and their structure-activity relationship. J. Asian Nat. Prod. Res.. 2013;15(6):680-686.
- [Google Scholar]
- Antitumor activity of Pulsatilla chinensis (Bunge) Regel saponins in human liver tumor 7402 cells in vitro and in vivo. Phytomedicine. 2012;19(3-4):293-300.
- [Google Scholar]
- Lupane-type triterpenoidal saponins from Pulsatilla chinensis and their anticomplement activities through the classical pathway. Planta Med.. 2013;79(06):506-512.
- [Google Scholar]
- Anemoside B4 exerts anti-cancer effect by inducing apoptosis and autophagy through inhibiton of PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Am. J. Transl. Res.. 2019;11:2580-2589.
- [Google Scholar]
- Anti-tumor effect of evodiamine by inducing Akt-mediated apoptosis in hepatocellular carcinoma. Biochem. Biophys. Res. Commun.. 2017;485(1):54-61.
- [Google Scholar]
- Triterpenoidal saponins of Pulsatilla koreana roots. Phytochemistry. 2010;71(16):1892-1899.
- [Google Scholar]
- Betulinic acid-mediated inhibitory effect on hepatitis B virus by suppression of manganese superoxide dismutase expression. FEBS J.. 2009;276:2599-2614.
- [Google Scholar]
- Pulsatilloside C from the Roots of Pulsatilla chinensis. J. Nat. Prod.. 1998;61(5):658-659.
- [Google Scholar]
- New lupane glycosides from Pulsatilla chinensis. Planta Medica. 2002;68(2):183-186.
- [Google Scholar]
- Chemical constituents in aerial parts of Pulsatilla chinensis. Zhongcaoyao. 2008;39:651-653.
- [Google Scholar]
- Genus Tetradium L.: a comprehensive review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol.. 2019;231:337-354.
- [Google Scholar]
- Excavating anticonvulsant compounds from prescriptions of traditional Chinese medicine in the treatment of epilepsy. Am. J. Chin. Med.. 2018;46(04):707-737.
- [Google Scholar]
- Traditional uses, chemical constituents and biological activities of plants from the genus Sanguisorba L. Am. J. Chin. Med.. 2017;45(02):199-224.
- [Google Scholar]
- Rhodomyrtus tomentosa (Aiton.): a review of phytochemistry, pharmacology and industrial applications research progress. Food Chem.. 2020;309:125715.
- [Google Scholar]
- Protective effect of 23-hydroxybetulinic acid on doxorubicin-induced cardiotoxicity: a correlation with the inhibition of carbonyl reductase-mediated metabolism. Br. J. Pharmacol.. 2015;172:5690-5703.
- [Google Scholar]
- Inhibitory effect of Pulsatilla chinensis polysaccharides on glioma. Int. J. Biol. Macromol.. 2012;50(5):1322-1326.
- [Google Scholar]







