Translate this page into:
Qualitative and quantitative analysis of multi-components in Xing-Su-Ning Capsules for quality improvement
⁎Corresponding authors at: Tianjin University of Traditional Chinese Medicine, 10 Poyanghu Road, West Area, Tuanbo New Town, Jinghai District, Tianjin 301617, China. renming2008@126.com (Ming Ren), miaomiaojiang@126.com (Miaomiao Jiang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Xin-Su-Ning Capsules (XSNC) is an effective prescription for the treatment of arrhythmia composed of eleven Chinese herbs. With the wide application of XSNC in clinic, its quality control issues have also received increasing attention. Based on the multi-components characteristics of Chinese herbal compound, there is an urgent need to establish a quality evaluation system.
Methods
Gas chromatography-mass spectrometry (GC-MS) and ultra high-performance liquid chromatography quadrupole electrostatic orbitrap high resolution mass spectrometry (UHPLC-Q-Exactive-Orbitrap-MS) were performed to identify the preliminary chemical profile of XSNC. Subsequently, a rapid ultra high-performance liquid chromatography coupled with electrospray ionization triple-quadrupole mass spectrometry (UHPLC-QQQ-MS/MS) method was developed to evaluate the quality of XSNC through a simultaneous determination of 16 components.
Results
A total of 21 volatile components and 59 non-volatile compounds were tentatively identified from the XSNC, each identified compound is marked on the corresponding chromatogram. Moreover, sixteen chemical constituents (sophocarpine, matrine, febrifugine, berberine, palmatine, Tangeratin, nobiletin, liensinine, neferine, scopoletin, isoliquiritigenin, liquiritigenin, naringenin, naringin, hesperidin and glycyrrhizic acid) were quantified by the developed UHPLC-QQQ-MS/MS method. The method validation of the sixteen compounds was performed with acceptable linearity (R2, 0.9990-1.0000), precision (RSD, 0.25-2.06%), repeatability (RSD, 0.93-2.90%) and recovery (99.65%-104.03%, RSD≤4.35%).
Conclusions
This qualitative analysis method sensitive and reliable for searching the volatile and non-volatile compounds from XSNC. The linearity, accuracy and precision of the quantitative analysis method were satisfactory. It is proposed that the methods described here can be applied for rapid evaluation, quality control and authenticity establishment of XSNC.
Keywords
Xing-Su-Ning Capsules
Constituent identification
Quantitative analysis
UHPLC-Q-Exactive-Orbitrap-MS
UHPLC-QQQ-MS/MS
- ESI
-
electrospray ionization
- GC-MS
-
gas chromatography-mass spectrometry
- TCM
-
Traditional Chinese medicine
- UHPLC-Q-Exactive-Orbitrap-MS
-
ultra performance liquid chromatography tandem quadrupole orbitrap mass spectrometer
- UHPLC-QQQ-MS/MS
-
ultra high-performance liquid chromatography coupled with electrospray ionization triple-quadrupole mass spectrometry
- XSNC
-
Xin-Su-Ning Capsules
Abbreviations
1 Introduction
Chinese herbal compound has definite curative effect and low side effects. It is the main drug for the clinical treatment of some complex diseases, chronic diseases and other diseases (Li and Du, 2015). Arrhythmia is a common and extremely dangerous cardiovascular diseases, it can not only aggravate the pre-existing heart disease, but also cause sudden death of patients (James and Calkins, 2016; Sossalla and Vollmann, 2018) With the limitation of anti-arrhythmic effect of chemical drugs, Chinese herbal compound represented by Xin-Su-Ning capsules (XSNC) has been more and more recognized in clinical practice because of its remarkable anti-arrhythmic effect in terms of multi-ion channel block and non-ion channel regulation(Li et al., 2019; Sun, 2017; Yao and Fang, 2017). XSNC consists of 11 Chinese herbs, including Coptidis Rhizoma, Pinelliae Rhizoma, Poria, Aurantii fructus Immaturus, Dichroae Radix, Nelumbinis Plumula, Sophorae flavescentis Radix, Artemisiae annuae Herba, Ginseng Radix et Rhizoma, Ophiopogonis Radix, Glycyrrhizae Radix et Rhizoma, it is a good prescription for the treatment of phlegm heat disturbance arrhythmia (Ma et al., 2006). Evidence-based medicine studies carried out from 2014 to 2017 have confirmed that XSNC has a definite clinical effect in treating cardiac arrhythmias caused by phlegm-heat (Zhaiet al., 2017). However, its chemical composition and quality control research are not in-depth, according to the instruction of Committee for the Pharmacopoeia of China in 2015, only berberine was indicated to be a index of XSNC for qualitative identification and quantitative analysis. In view of the complexity of chemical components of traditional Chinese medicine (TCM), its single qualitative and quantitative index is not enough to show the overall quality information of TCM, and it is difficult to fully reflect the effectiveness and safety of TCM. As a result, the comprehensive identification method of chemical composition is of great significance to the research of chemical composition of TCM. A new analytical method is needed to quantitatively determine various active components in XSNC.
In recent years, with the development of analytical technology, ultra high-performance liquid chromatography quadrupole electrostatic orbitrap high resolution mass spectrometry (UHPLC-Q-Exactive-Orbitrap-MS) is widely used in the analysis of TCM and compound prescription due to its fast separation speed, high sensitivity and strong determination accuracy (Eliuk and Makarov, 2015; Yang et al., 2020). Ultra high-performance liquid chromatography coupled with triple-quadrupole tandem mass spectrometry (UHPLC-QQQ-MS/MS) could provide simultaneous quantification of multiple components in the analysis of TCM (Liu et al., 2017, 2017; He et al., 2015).
To achieve the comprehensive chemical characterization of XSNC, we developed methods based on GC-MS and LC-MS to effectively analyze the chemical composition of XSNC. Then, an approach based on UHPLC-QQQ-MS/MS was developed to investigate the content of multi-components in XSNC. The quality of XSNC was comprehensively evaluated by quantifying the content of 16 compounds, which provided a reference for its quality evaluation, and laid a foundation for the later-stage drug-effective material basic research and clinical application.
2 Materials and methods
2.1 Materials and reagents
Methanol and acetonitrile (chromatographic purity) was purchased from Fisher company (USA), formic acid (MS grade) was purchased from ACS company (USA), and distilled water was purchased from Guangzhou Watsonsfood and beverage company (Guangzhou, China). Reference standards of sophocarpine, matrine, febrifugine, berberine, palmatine, Tangeratin, nobiletin, liensinine, neferine, scopoletin, isoliquiritigenin, liquiritigenin, naringenin, naringin, hesperidin and glycyrrhizic acid, jatrorrhizine were purchased from Sichuan Weikeqi Biotechnology Co., Ltd. or Shanghai Yuanye Biotechnology Co., Ltd. XSNC were supplied by Shanxi Momentum Pharmaceutical Co., Ltd (Shanxi, China).
2.2 Sample solutions preparation
XSNC were completely removed the capsule and weighed 1.77 g of the powder precisely. The powder was placed in a headspace sample bottle and sealed with an aluminum cap. Then, it was injected in the headspace sampler for GC-MS analysis.
The contents of XSNC were extracted by cold leaching with methanol for 24 h and repeated three times, then ultrasonically extracted for three times with methanol, the extracts was combined, concentrated and freeze-dried to obtain freeze-dried powder of non-volatile components. The lyophilized powder was weighed and dissolved in methanol a concentration of 5 mg/mL, then the solution was centrifuged at about 14000 rpm for 10 min. The supernatant was filtered through a 0.22 μm syringe filter and the filtrate was stored at 4 ◦C ready for UHPLC-ESI-Q-Exactive-Orbitrap-MS qualitative analysis.
A total of 0.2 g powder of XSNC was accurately weighed, ultrasound (25 kHz, 35 ◦C, 300 W) for 30 min with 20 mL methanol room temperature. After cooling down, the lost volume of methanol was complemented. Then the extracted solution was centrifuged at 14000 rpm for 10 min and the supernatant was taken. The supernatant 1 mL was accurately measure, diluted it with methanol and constant volume to obtain a test solution diluted 500 times with XSNC extract. The solution was filtered through a 0.22 μm syringe filter and the filtrate was stored at 4 ◦C ready for UHPLC-QQQ-MS/MS quantitative analysis.
2.3 Standard solutions preparation
The standards for matrine, sophocarpine, jatrorrhizine, palmatine, berberine, liquiritigenin, isoliquiritigenin, Tangeratin, nobiletin, and scopoletin were weighed accurately and dissolved in methanol for preparation of 1 mg/mL single reference solutions. Each reference solution was drew 0.1 mL and obtained a 100 µg/mL mixed standard solution. The solutions were filtered with 0.22 μm syringe filters before UHPLC-ESI-Q-Exactive-Orbitrap analysis.
The standards for sophocarpine, matrine, febrifugine, berberine, palmatine, Tangeratin, nobiletin, liensinine, neferine, scopoletin, isoliquiritigenin, liquiritigenin, naringenin, naringin, hesperidin and glycyrrhizic acid were weighed accurately and dissolved in methanol for preparation of single reference substance mother solution. The concentrations of reference substance mother solutions were as follows: sophocarpine 20 µg/mL, matrine 20 µg/mL, febrifugine 1 µg/mL, berberine 20 µg/mL, palmatine 20 µg/mL, Tangeratin 1 µg/mL, nobiletin 1 µg/mL, liensinine 2 µg/mL, neferine 10 µg/mL, scopoletin 5 µg/mL, isoliquiritigenin 2 µg/mL, liquiritigenin 2 µg/mL, naringenin 10 µg/mL, naringin 300 µg/mL, hesperidin 20 µg/mL, and glycyrrhizic acid 40 µg/mL.
Preparation of the standard curve: The above-mentioned reference substance mother solution was taken 50 µL each and the volume was made up to 1 mL with methanol. It contains sophocarpine 1 µg/mL, matrine 1 µg/mL, and febrifugine 50 ng/mL, berberine 1 µg/mL, palmatine 1 µg/mL, Tangeratin 50 ng/mL, nobiletin 50 ng/mL, liensinine 100 ng/mL, neferine 500 ng/ mL, scopoletin 250 ng/mL, isoliquiritigenin 100 ng/mL, liquiritigenin 100 ng/mL, naringenin 500 ng/mL, naringin 15 µg/mL, hesperidin 1 µg/mL and glycyrrhizic acid 2 µg/mL the highest concentration of mixed standard solution. The highest concentration of mixed reference solution was diluted with methanol 1:1 (v: v) by 2, 4, 8, 16, 32, 64 times to obtain a series of mixed reference solution.
2.4 GC-MS analysis
Chromatographic analysis was performed on an Agilent 7890B gas chromatograph (American, Agilent). A HP-5 MS quartz capillary column was used for chromatographic separation. Injections were performed in a split mode (ratio 5:1). High-purity nitrogen was used as a carrier gas and injector temperature was 240 ◦C. The initial column temperature was maintained at 50 ◦C for 2 min, then raised to 200 ◦C at a rate of 4 ◦C/min and held isothermally for 2 min. The column flow was 10 ml/min. Mass spectrometry analysis was performed on an Agilent 5977B mass spectrometer (American, Agilent). EI ionization method was adopted; the ion source temperature and quadrupole temperature were 230 ◦C and 150 ◦C respectively; the full scan mode range m/z 40∼400.
2.5 UHPLC-Q-exactive-orbitrap-MS qualitative analysis
Chromatographic analysis was performed on a Thermo Scientific UltiMate 3000 Ultra Performance Liquid Chromatograph (Thermo Fisher Scientific, USA); A Waters ACQUITY UPLC BEH C18 (1.7 μm, 2.1×100 mm) maintained at 35 ◦C was used for chromatographic separation. The mobile phase consisted of water acidified with 0.1 % (v/v) formic acid (A) and acetonitrile (B), was delivered at flow rate of 0.2 mL/min using the following gradient program: 0-2 min, 5-10% B; 2-5 min, 10-15% B; 5-10 min, 15-25% B; 10-15 min, 25-30% B; 15-20 min, 30-45%; 20-25 min, 45-65% B; 25-30 min, 65-95% B.
The Q-Exactive-Orbitrap mass spectrometer (Thermo Fisher Scientific, USA) equipped with an electrospray ion source. The atomizing gas was nitrogen; the spraying voltage was 3.5 KV; the flow rate of the sheath gas and the aux gas was 35 L/h and 10 L/h, respectively; the capillary temperature and auxiliary heating temperature were 350 ◦C; the first level spectrum adopted the positive and negative ion full scan mode, the scan range was 100-1500 m/z, the full scan resolution was 70000 FWHM; the second level fragment spectrum used the target ion detection mode, the resolution was 17500 FWHM; the collision induced dissociation energy gradient was set to 30/40/50 V.
2.6 UHPLC-QQQ-MS/MS quantitative analysis
Chromatographic analysis was performed on an ACQUITY UPLC Ultra Performance Liquid Chromatograph (American, waters company); Chromatographic separation was conducted on a Waters UPLC ACQUITY BEH C18 (1.7 μm, 2.1 mm×100 mm) maintained at 35 ◦C, the mobile phase consisted of 0.1% formic acid solution (A) and acetonitrile (B) using a gradient elution as following: 0-2 min, 5-10% B; 2-5 min, 10-20% B; 5-8 min, 20-25% B; 8-10 min, 25-30% B; 10-15 min, 30-45% B; 15-20 min, 45-95% B; the flow rate was kept at 0.3 mL/min.
MS detection was performed on Waters Xevo TQ-S Triple Quadrupole Mass Spectrometer (American, waterscompany). Quantification was performed using multiple reaction monitoring (MRM) mode. The optimized MS conditions for the positive ion mode were as follows: capillary voltage 3.0 KV, cone voltage 30 V, solvent removal temperature 350 ◦C. The optimized MS conditions for the negative ion mode were as follows: capillary voltage 2.0 KV, cone voltage 37 V, desolvation temperature 350 ◦C. The mass spectrometry analysis conditions of the 16 compounds were optimized and summarized in Table 1.
Compound
Formula
Parent
Daughters
CV
CE
Detection mode
Sophocarpine
C15H22N2O
247.14
136.13
82
28
positive
Matrine
C15H24N2O
249.16
148.16
80
26
positive
Febrifugine
C20H18NO4
336.16
320.24
20
28
positive
Berberine
C21H22NO4
352.12
308.13
62
28
positive
Palmatine
C16H19N3O3
302.11
138.13
32
14
positive
Tangeratin
C20H20O7
373.03
343.14
26
26
positive
Nobiletin
C21H22NO8
403.10
373.15
84
26
positive
Liensinine
C37H42N2O6
611.40
206.17
100
34
positive
Neferine
C38H44N2O6
625.42
206.16
100
30
positive
Scopoletin
C10H8O4
191.01
176.03
36
16
negative
Isoliquiritigenin
C15H12O4
255.11
119.15
46
24
negative
Liquiritigenin
C15H12O4
255.17
119.15
40
26
negative
Naringenin
C15H12O5
271.04
151.07
30
20
negative
Naringin
C27H32O14
579.21
151.05
80
46
negative
Hesperidin
C28H34O15
609.22
301.18
48
24
negative
Glycyrrhizic acid
C42H62O16
821.56
351.09
38
42
negative
2.7 Method validation of UHPLC–QQQ–MS/MS
According to “2.6” analysis conditions and “2.2” extraction conditions, the linearity, limit of detection (LOD), limit of quantification (LOQ), precision, repeatability, stability and recovery rate of 16 compound markers were determined. The standard curve was drew with the concentration x (ng/mL) of the reference substance as the abscissa and the corresponding peak area y of each reference substance as the ordinate. Then linear regression was performed on the standard curve to examine the correlation coefficient and linear range of the resulting linear regression equation. LOD and LOQ were determined based on the standard deviation of response value and the slope of standard curve, calculation formula: LOD=3.3δ/S, LOQ=10δ/S (δ, standard deviation; S, slope). XSNC was accurately weighed to test its precision, repeatability and stability. The test solution was continuously injected 6 times within 24 hours to evaluate the accuracy of the instrument, and 16 identical samples were prepared for repeatability analysis. The stability of the samples was studied after being placed at room temperature for 0, 2, 4, 8, 12 and 24 hours. The test solution and the reference solution were added at a ratio of 1:1, and six parts were measured in parallel to calculate the recovery rate of each component.
3 Results
3.1 Identification of chemical composition of Xin-Su-Ning capsules
3.1.1 Analysis of volatile components
The volatile components in XSNC were analyzed by GC-MS. The total ion flow diagram was shown in Fig. 1. A total of 21 volatile components were identified by searching with NIST mass spectrometry database (Table 2).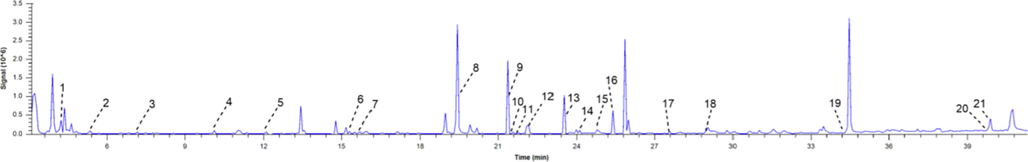
The total ion current diagram of volatile components in Xin-Su-Ning capsules.
No
tR(min)
Compounds
Molecular formula
possibility
Forward match
Reverse match
CAS
1
4.249
2-Methylbutanal
C5H10O
85.02
910
918
96-17-3
2
5.382
Ethylpropenylether
C5H10O
65.08
876
888
928-55-2
3
7.184
2-Ethoxyoxolane
C6H12O2
64.13
808
811
13436-46-9
4
10.114
(Z)-2-Butenoic acid ethyl ester
C6H10O2
78.51
910
911
6776-19-8
5
12.116
Ethyl acetate
C8H16O2
90.07
868
875
123-66-0
6
15.183
Methyl lactate
C4H8O3
95.95
859
883
2155-30-8
7
15.716
Methylheptenone
C8H14O
73.86
778
865
110-93-0
8
19.984
Acetoxy-2-acetone
C5H8O3
87.60
898
935
592-20-1
9
21.380
2-Acetylfuran
C6H6O2
74.57
943
950
1192-62-7
10
21.581
Pyrrole
C4H5N
81.84
939
946
109-97-7
11
21.781
2,4-Dihydroxy-2,5-dimethyl-3
C6H8O4
68.04
740
797
10230-62-3
12
22.113
Propionic acid
C3H6O2
79.50
960
977
137-40-6
13
23.515
5-Methylfuran aldehyde
C6H6O2
85.14
900
913
620-02-0
14
24.116
Hotrienol
C10H16O
73.52
789
811
20053-88-7
15
25.380
γ-Butyrolactone
C4H6O2
60.08
961
966
96-48-0
16
25.849
Furfuryl alcohol
C5H6O2
67.13
916
916
98-00-0
17
27.583
5-Methyl-2-furanmethanol
C6H8O2
86.83
806
806
3857-25-8
18
29.048
2(5H)-Furanone
C4H4O2
64.78
735
934
497-23-4
19
34.450
2-Acetylpyrrole
C6H7NO
77.55
930
935
1072-83-9
20
40.646
Ethyl palmitate
C18H36O2
91.76
901
903
628-97-7
21
40.715
Ethyl hexadecanoate
C18H36O2
71.12
700
721
628-97-7
3.1.2 Analysis of non-volatile components
UHPLC-MS was used to analyze the non-volatile components in XSNC. The total ion flow diagram of the sample solution and the reference solution were shown in Fig. 2. For the compounds with chemical standards, according to the retention time, as well as accurate and high-resolution mass and tandem mass spectra, as a results, 10 compounds (peak 1, 2, 9, 26, 34, 37, 38, 43, 49 and 50) were identified as matrine, sophocarpine,scopoletin, jatrorrhizine, liquiritigenin, berberine,palmatine, isoliquiritigenin, nobiletin and Tangeratin, respectively. For the compounds without chemical standards, based on the retention time, exact mass data, fragment information, and molecular formula reported in the literatures, a total of 49 compounds were detected. Take hesperidin as an example to illustrate the fragmentation process, peak 20 exhibited the precursor ion [M−H]- ion at m/z 609.1822 in the negative mode and [M+H]+ ion at m/z 611.1976 in the positive mode. It was speculated that the relative molecular weight of the compound was 610 and the predicted molecular formula was C28H34O15. In the first-order mass spectrum of positive ion mode, there were fragments of m/z 449.1439 and m/z 465.1389, and in the second-order mass spectrum, there were fragment ions of m/z 303.0861 [M+H–Rha–Glc]+, m/z 153.0182 [M+H–Rha–Glc–C9H10O2]+, which was consistent with the fragments of hesperidin in the literature, and speculated that the compound was hesperidin (Chen et al., 2012). All in all, a total of 59 chemical constituents were tentatively identified including 18 alkaloids, 33 flavonoids, 5 coumarins, 2 alcoholamines and 1 triterpenoid (Table 3). Among these compounds, sophocarpine, matrine, febrifugine, berberine, palmatine, Tangeratin, nobiletin, liensinine, neferine, scopoletin, isoliquiritigenin, liquiritigenin, naringenin, naringin, hesperidin and glycyrrhizic acid were mainly active constituents with reported bioactivities. As a result, the quantitative analysis of these 16 constituents was performed in XSNC extracts. Note: # stands for comparison with standard products.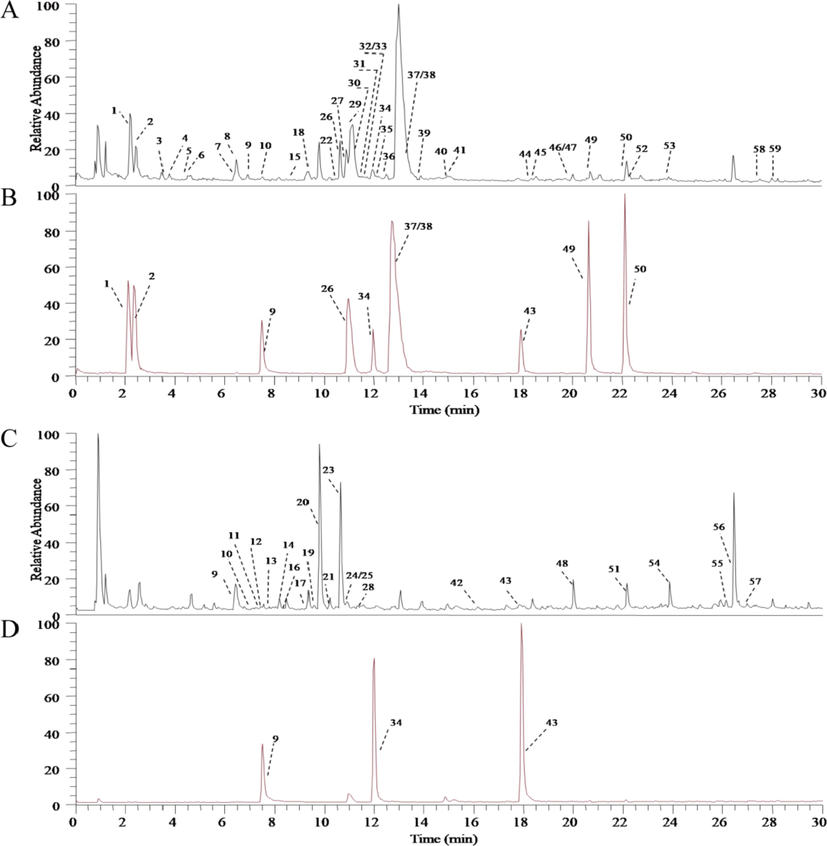
Total ion current diagram of non-volatile components in Xin-Su-Ning capsules (A: positive ion mode Xin-Su-Ning capsules extract TIC; B: positive ion mode reference substance TIC; C: negative ion mode Xin-Su-Ning capsules extract TIC; D: negative ion mode reference substance TIC).
Peak NO.
tR (min)
Formula
Measured (m/z)
Detected (m/z)
Delta (ppm)
Fragments
Identification
class
source
1#
2.21
C15H24N2O
248.1889
249.1959
–0.642
148.1116 150.1271 112.0760
Matrine
Alkaloids
Sophora flavescens
2#
2.48
C15H22N2O
246.1732
247.1804
–0.283
229.1705 179.1541 150.1275 136.1132
Sophocarpine
Alkaloids
Sophora flavescens
3
3.51
C15H22N2O
246.1732
247.1804
–0.525
148.1121 136.1125 112.0763
Sophocarpine Isomers
Alkaloids
Sophora flavescens
4
3.76
C15H24N2O2
264.1838
265.1909
–0.394
150.1278 138.1277
Hydroxylated matrine
Alkaloids
Sophora flavescens
5
4.06
C16H19N3O3
301.1421
302.1497
–0.556
284.1398 203.0816 138.0914
Febrifugine
Alkaloids
Changshan
6
4.52
C19H23NO3
313.1678
314.1748
–0.732
269.1172 237.0911 107.0495
Lotusine
Alkaloids
Lotus Seed Heart
7
6.48
C20H24NO4
342.1705
342.1696
–1.241
265.0858 297.1119
Magnoflorine
Alkaloids
Coptis
8
7.45
C27H30O14
578.1636
579.1706
–0.418
271.0599 195.0287 219.0287 153.0181
Rhoifolin
Flavone
Citrus aurantium
9#
7.53
C10H8O4
192.0423
193.0495
–0.131
178.0261 133.0284 137.0597 145.0958
Scopoletin
Coumarin
Citrus aurantium
10
8
C27H32O15
596.1741
595.1671
0.347
459.1097 287.0555 269.0454 135.0436
Eriocitrin
Flavone
Citrus aurantium
11
8.16
C26H30O13
550.1686
549.1609
–0.754
297.0073 255.0655 153.0180
Glycyrrhizin-4'-apirin
Flavone
Citrus aurantium
12
8.36
C27H30O16
610.1484
609.1465
0.611
301.0341 269.0451 201.0556 151.0022 88.9862
Rutin
Flavone
Citrus aurantium
13
8.45
C27H32O15
596.1741
595.1669
0.146
459.1134 339.0707 287.0556 235.0556
Neoeriocitrin
Flavone
Citrus aurantium
14
8.73
C27H30O15
594.1585
593.1513
0.197
447.0923 327.0599 285.0395
Lonicerin
Flavone
Citrus aurantium
15
9.3
C19H15NO4
322.1079
322.1071
–0.914
307.0837 294.0759
Greenland Xanthine
Alkaloids
Coptis
16
9.32
C27H32O14
580.1792
579.1718
–0.309
339.9276 295.0617 151.0023
Narirutin
Flavone
Citrus aurantium
17
9.75
C27H32O14
580.1792
579.1714
0.162
459.1171 271.0607 151.0022 119.0487
Naringin
Flavone
Citrus aurantium
18
9.8
C15H12O5
272.0685
273.0753
–1.794
153.0182 147.0040 171.0287 177.0546
Naringenin isomers
Flavone
Citrus aurantium
19
9.85
C21H22O10
434.1163
433.1134
–1.432
363.3987 271.0607 151.0022 83.0123
Prunin
Flavone
Citrus aurantium
20
10.17
C28H34O15
610.0898
609.1822
–0.498
325.0722 301.0710 164.0102 151.0022
Hesperidin
Flavone
Citrus aurantium
21
10.41
C28H32O15
608.1691
607.167
0.34
329.1388 299.0554 242.0673 164.0103 125.0228
Neogeranin
Flavone
Citrus aurantium
22
10.64
C16H14O6
302.0785
303.0858
–1.764
153.0182 285.0758 322.1056
Hesperetin
Flavone
Citrus aurantium
23
10.7
C28H34O15
610.0898
609.18119
–0.892
555.9461 325.0710 301.0711 286.0476 151.0022
Neohesperidin isomers
Flavone
Citrus aurantium
24
10.81
C22H24O11
464.1319
463.1247
0.227
301.0709 286.0476 242.0575 151.0021
Hesperetin-7-O-β-D-glucoside
Flavone
Citrus aurantium
25
10.89
C28H32O15
608.1691
607.167
0.242
489.1394 343.0817 301.0710 267.0657 151.0023
Geranidin
Flavone
Citrus aurantium
26#
10.9
C20H20NO4
338.1392
338.1383
–1.108
294.1133 97.1016 83.0861
Jatrorrhizine
Alkaloids
Sophora flavescens
27
11.01
C20H18NO4
336.1236
336.1226
–1.232
320.0917 292.0962 292.0966
Dihydroberberine
Alkaloids
Coptis
28
11.08
C26H30O13
550.1686
549.16156
0.357
399.1046 255.0655 153.0179 135.0072
Isoliquiritin glucocelium
Flavone
Licorice
29
11.09
C19H14NO4
320.0923
320.0913
–1.232
277.0729 262.0871
Coptisine
Alkaloids
Coptis
30
11.17
C20H20NO4
338.1392
338.1385
–2.202
323.1144 294.1122 308.0920
Tetrandrine isomers
Alkaloids
Coptis
31
11.48
C21H22O9
418.1264
419.1335
–0.426
257.0806 137.0233 239.0702
Liquiritin
Flavone
Licorice
32
11.71
C20H20NO4
338.1392
338.1385
–0.487
323.1147 294.1121
Tetrandrine isomers
Alkaloids
Coptis
33
11.96
C19H15NO4
322.1079
322.1072
–0.635
307.0838 279.0880
Berberrubine
Alkaloids
Coptis
34#
12
C15H12O4
256.0736
257.0808
–0.332
211.0753 147.0441 137.0234 119.0494
Liquiritigenin
Flavone
Licorice
35
12.23
C21H20NO4
350.1386
350.1387
–0.042
334.1072 322.0706 306.1126
13-methylepiberberine
Alkaloids
Coptis
36
12.8
C16H12O5
284.0679
285.0756
–0.648
270.052 253.0493 225.0546
Calycosin
Flavone
Sophora flavescens
37#
12.96
C20H18NO4
336.1236
336.1227
–1.055
321.0984 292.0966 306.0757
Berberine
Alkaloids
Coptis
38#
13.27
C21H22NO4
352.1549
352.1539
–1.149
337.1296 322.1074 308.1280
Palmatine
Alkaloids
Coptis
39
13.91
C28H34O14
594.1943
595.2021
0.03
287.0911 153.0182
Poncirin
Flavone
Citrus aurantium
40
14.93
C15H12O5
272.0685
273.0756
–0.476
153.0182 147.0440 119.0494
Naringenin
Flavone
Citrus aurantium
41
15.08
C21H20NO4
350.1387
350.1386
–1.358
335.1142 320.0918 306.1123 292.0971 254.0569
13-methylberberine
Alkaloids
Coptis
42
16.18
C16H14O6
302.079
301.0718
0.261
286.0479 257.0841 242.0574 233.0796
Hesperetin
Flavone
Sophora flavescens
43
17.95
C15H12O4
256.0736
255.0661
–0.162
211.0753 135.0072 119.0487
Isoliquiritigenin
Flavone
Licorice
44
18.33
C16H12O4
268.073
269.0805
–1.358
237.0542 137.0233 118.0414
Formononetin
Coumarin
Citrus aurantium
45
18.53
C15H16O4
260.1043
261.1114
–2.74
189.0545 243.1012 159.0440 131.0492
Hesperitone
Coumarin
Citrus aurantium
46
19.67
C26H30O8
470.1935
471.2009
–0.414
425.1963 339.1952 213.0911 161.0598 95.0132
Limonin
Flavone
Citrus aurantium
47
19.64
C19H18O6
342.1098
343.1172
–0.335
313.0704 285.0755 181.0129 373.0918
4',5,7,8-tetramethoxyflavonoid
Flavone
Citrus aurantium
48
19.97
C42H62O16
822.4038
821.3964
–0.108
683.7745 513.6673 443.4119 351.0563 175.0234
Glycyrrhizic acid
Flavone
Citrus aurantium
49#
20.7
C21H22O8
402.1315
403.1383
–0.404
388.1163 373.0916 355.0822
Nobiletin
Flavone
Citrus aurantium
50#
22.17
C20H20O7
372.1209
373.1277
–1.397
343.0811 358.1042 325.0703
Tangeratin
Flavone
Citrus aurantium
51
22.09
C26H30O6
438.2042
437.1969
–0.142
301.1429 151.0386 91.0539
Kurarinone
Flavone
Sophora flavescens
52
22.21
C16H35NO2
273.2662
274.2737
–1.261
256.2634
Cetyl-Dihydrosphingosine
Alcoholamines
Pinellia
53
23.67
C27H32O6
452.2193
453.2268
–0.85
329.1025 303.1590 197.0440
2’-Methoxymatrine
Flavone
Sophora flavescens
54
23.84
C25H30O6
424.1886
423.1816
0.634
261.1491 161.0231 109.0281
Kushenol E isomers
Flavone
Sophora flavescens
55
25.97
C25H30O6
424.1886
423.1967
–0.553
261.1491 161.0231 109.0281
Kushenol E isomers
Flavone
Sophora flavescens
56
26.54
C26H30O6
438.2042
437.1967
–0.553
275.1648 161.0230 109.0277
Kuraridin
Flavone
Sophora flavescens
57
27.18
C30H46O4
470.3391
469.3312
–0.326
425.3423 409.3109
Glycyrrhetinic acid
Triterpene
Licorice
58
27.47
C20H43NO2
329.3288
330.3361
–1.623
312.3257 106.0866 88.0762
2-amino-1,3-eicosanediol
Alcoholamines
Pinellia
59
28.21
C19H22O3
298.1563
299.1633
–0.871
189.0542 163.0389 119.0494
Grapefruit lactone
Coumarin
Citrus aurantium
3.2 Quantitative analysis
Through LC-MS multiple reaction detection mode, the test solution was prepared according to the method under “2.2”, and the prepared test solution was determined under the detection conditions “2.6”. Aiming at the problem of large difference in mass spectrum response and content of various types of compounds in the complex system of XSNC, through the multiple dilution method, the same sample was prepared by preparing a low dilution ratio test solution to detect components with low mass spectrometry response and low content (including febrifugine, liensinine, neferine, scopoletin, isoliquiritigenin, liquiritigenin, naringenin, naringin, hesperidin, and glycyrrhizic acid); then low dilution ratio sample was diluted by times to obtain the test solution with high dilution ratio to detect the components with high mass response and high content (including sophocarpine, matrine, berberine, palmatine, Tangeratin and nobiletin). The rapid detection of various components in the sample was realized by different dilution methods.
3.2.1 Methodology validation
LC-MS was used for the quantitative analysis of sophocarpine, matrine, febrifugine, berberine, palmatine, Tangeratin, nobiletin, liensinine, neferine, scopoletin, isoliquiritigenin, liquiritigenin, naringenin, naringin, hesperidin and glycyrrhizic acid .The 16 index components had a good linear relationship within the corresponding concentration range, and their R2 were all greater than 0.999, the LOD and LOQ were 0.146-60.074 ng/mL and 0.442-182.043 ng/mL, the results were shown in Table 4. The relative standard deviation (RSD) values of accuracy, repeatability, and stability were all less than 2.90 %, indicating that the instrument had good precision, the method had high repeatability, and the sample solution was stable for 24 h at room temperature. The sample recovery rate was between 99.65%-104.03%, and the RSD value was less than 4.35 %, indicating that the recovery rates of the 16 compounds in XSNC were good, and the established method had sufficient reliability and accuracy, the results were summarized in Table 5 (Supporting information Table S1-S5).
Compounds
Regression equation
R2
Linearity range (ng/mL)
LOD (ng/mL)
LOQ (ng/mL)
Sophocarpine
y =8033.50x + 112717.00
R2 = 0.9992
15.625-1000
4.910
14.880
Matrine
y = 3834.20x- 25.18
R2 = 0.9997
15.625-1000
3.362
10.187
Febrifugine
y =2782.70x- 1136.30
R2 = 0.9994
0.781-50
0.146
0.442
Berberine
y = 13757.00x + 16757.00
R2 = 0.9999
15.625-1000
3.722
11.280
Palmatine
y = 16658.00x - 3566.10
R2 = 1.0000
15.625-1000
3.236
9.805
Tangeratin
y = 92881.00x + 5240.10
R2 = 0.9994
0.781-50
0.320
0.971
Nobiletin
y = 73488.00x + 16786.00
R2 = 0.9998
0.781-50
0.498
1.509
Liensinine
y = 721.52x - 757.60
R2 = 0.9990
1.563-100
0.429
1.299
Neferine
y = 1079.90x - 3426.70
R2 = 0.9993
7.813-500
2.296
6.959
Scopoletin
y = 148.42x - 293.17
R2 = 0.9995
3.906-250
1.230
3.729
Isoliquiritigenin
y = 418.49x - 279.76
R2 = 0.9994
1.563-100
0.461
1.395
Liquiritigenin
y = 233.48x - 115.91
R2 = 0.9992
1.563-100
0.158
0.480
Naringenin
y =295.30x - 599.14
R2 = 0.9996
7.813-500
2.435
7.378
Naringin
y = 156.15x + 1022.20
R2 = 1.0000
234.375-15000
60.074
182.043
Hesperidin
y = 433.93x + 109.30
R2 = 0.9992
15.625-1000
4.831
14.641
Glycyrrhizic acid
y = 213.75x - 2960.30
R2 = 0.9993
31.250-2000
5.541
16.792
Compounds
Precision RSD (%)
Repeatability RSD (%)
Stability RSD (%)
Recovery
Mean
RSD (%)
Sophocarpine
0.77
0.93
0.44
100.43
4.35
Matrine
0.28
0.93
0.30
101.14
2.15
Febrifugine
1.94
2.04
2.17
101.58
1.32
Berberine
0.82
1.26
0.81
99.92
0.43
Palmatine
1.65
1.18
1.02
100.35
1.25
Tangeratin
0.77
1.23
0.54
101.65
0.25
Nobiletin
0.49
1.25
0.51
101.19
1.03
Liensinine
1.90
2.34
1.29
101.98
2.79
Neferine
2.06
1.22
1.05
99.65
1.92
Scopoletin
0.45
2.61
0.49
101.81
1.47
Isoliquiritigenin
0.68
2.29
0.73
99.71
0.77
Liquiritigenin
1.98
2.90
1.82
101.50
2.09
Naringenin
1.11
1.90
2.42
99.71
2.50
Naringin
0.37
2.73
0.80
104.03
0.60
Hesperidin
0.25
2.47
0.84
99.89
1.27
Glycyrrhizic acid
0.47
1.90
1.14
101.06
1.27
3.2.2 Determination of sample content
Multiple reaction monitoring (MRM) is a highly specific and sensitive mass spectrometry technique for quantifying predefined compounds of interest. The UHPLC–MS/MS analysis method described above was subsequently used to simultaneously quantify 16 compounds in 10 collected batches. Every sample was analyzed in triplicates to acquire the average contents of the constituents. The results were shown in Table 6. Note: values are expressed as the mean ± SD of three parallel samples.
Sample batch
Sophocarpine (mg/g)
Matrine (mg/g)
Febrifugine (mg/g)
Berberine (mg/g)
Palmatine (mg/g)
Tangeratin (mg/g)
Nobiletin (mg/g)
Liensinine (mg/g)
Neferine (mg/g)
Scopoletin (mg/g)
Isoliquiritigenin (mg/g)
Liquiritigenin (mg/g)
Naringenin (mg/g)
Naringin (mg/g)
Hesperidin (mg/g)
Glycyrrhizic acid (mg/g)
220201
3.06 ± 0.27
11.62 ± 0.75
0.05 ± 0.00
31.05 ± 1.31
7.56 ± 0.32
0.46 ± 0.01
0.55 ± 0.02
0.06 ± 0.01
0.38 ± 0.01
0.36 ± 0.03
0.11 ± 0.01
0.15 ± 0.01
0.43 ± 0.04
28.01 ± 0.01
9.86 ± 0.21
1.66 ± 0.03
220202
2.65 ± 0.10
11.65 ± 0.21
0.06 ± 0.01
31.66 ± 0.64
7.79 ± 0.06
0.46 ± 0.00
0.55 ± 0.01
0.05 ± 0.00
0.37 ± 0.01
0.40 ± 0.01
0.12 ± 0.00
0.17 ± 0.00
0.52 ± 0.01
32.64 ± 0.05
10.99 ± 0.23
1.59 ± 0.04
210701
4.39 ± 0.20
15.90 ± 1.62
0.06 ± 0.00
34.25 ± 1.44
8.77 ± 0.21
0.45 ± 0.01
0.55 ± 0.01
0.07 ± 0.00
0.45 ± 0.11
0.69 ± 0.02
0.12 ± 0.00
0.24 ± 0.01
0.77 ± 0.02
47.02 ± 0.94
13.24 ± 0.13
1.60 ± 0.04
220101
2.52 ± 0.29
10.38 ± 0.99
0.17 ± 0.02
34.96 ± 0.93
8.82 ± 0.03
0.36 ± 0.00
0.43 ± 0.01
0.06 ± 0.01
0.41 ± 0.03
0.42 ± 0.02
0.20 ± 0.01
0.23 ± 0.01
0.44 ± 0.01
34.38 ± 0.36
13.46 ± 0.08
1.25 ± 0.04
210901
3.15 ± 0.05
12.16 ± 0.07
0.15 ± 0.01
25.35 ± 1.21
6.31 ± 0.26
0.27 ± 0.00
0.30 ± 0.01
0.05 ± 0.00
0.33 ± 0.03
0.53 ± 0.02
0.10 ± 0.00
0.16 ± 0.01
0.37 ± 0.02
24.66 ± 0.49
6.77 ± 0.10
1.66 ± 0.04
210502
4.05 ± 0.03
14.39 ± 0.77
0.10 ± 0.01
37.07 ± 0.54
8.79 ± 0.13
0.59 ± 0.02
0.58 ± 0.01
0.07 ± 0.01
0.55 ± 0.02
0.45 ± 0.01
0.19 ± 0.00
0.20 ± 0.01
0.42 ± 0.03
26.34 ± 0.84
7.57 ± 0.21
1.59 ± 0.04
210301
1.72 ± 0.08
9.20 ± 0.39
0.04 ± 0.01
20.60 ± 0.62
5.18 ± 0.17
0.37 ± 0.01
0.41 ± 0.01
0.05 ± 0.00
0.40 ± 0.01
0.32 ± 0.01
0.12 ± 0.01
0.15 ± 0.01
0.36 ± 0.01
19.03 ± 0.01
5.11 ± 0.05
1.72 ± 0.03
210501
3.96 ± 0.05
11.79 ± 0.03
0.07 ± 0.01
30.90 ± 1.30
7.50 ± 0.22
0.46 ± 0.01
0.53 ± 0.01
0.08 ± 0.01
0.68 ± 0.01
0.29 ± 0.01
0.12 ± 0.01
0.18 ± 0.00
0.61 ± 0.02
35.63 ± 0.40
9.46 ± 0.03
2.97 ± 0.02
201001
2.86 ± 0.06
10.56 ± 0.76
0.02 ± 0.00
17.13 ± 0.78
4.46 ± 0.15
0.35 ± 0.01
0.39 ± 0.01
0.04 ± 0.01
0.32 ± 0.01
0.35 ± 0.02
0.05 ± 0.00
0.09 ± 0.00
0.32 ± 0.02
17.33 ± 0.33
3.31 ± 0.05
0.55 ± 0.01
211101
2.16 ± 0.03
9.40 ± 0.03
0.05 ± 0.00
26.73 ± 0.49
6.34 ± 0.26
0.34 ± 0.01
0.37 ± 0.00
0.07 ± 0.01
0.66 ± 0.01
0.37 ± 0.00
0.09 ± 0.00
0.16 ± 0.01
0.40 ± 0.02
23.38 ± 0.34
6.40 ± 0.14
1.89 ± 0.09
4 Discussion
How to combine the basic requirements of “safe, effective and controllable quality” with the characteristics of TCM is the key problem of TCM quality research and control. In this context, Academician Liu Changxiao and his team put forward the concept of Chinese medicine quality marker (Q-Marker) (Liu, 2019; Liu et al., 2016). In view of this, a method for simultaneous quantitative analysis of 16 chemical components in XSNC was established. Berberine and palmatine are derived from the prince drug Coptidis Rhizoma. A large number of studies have shown that berberine mainly exerts anti-arrhythmic effects by affecting potassium ion channels (Chen et al., 2018); Palmatine and berberine have similar structures and have higher content in Coptidis Rhizoma, it also has better anti-arrhythmic activity (Liu et al., 2017, 2017). Sophocarpine and matrine are derived from the official medicine Sophorae flavescentis Radix, and liensinine, neferine come from the central medicine Nelumbinis Plumula. These alkaloids can exert their anti-arrhythmic effects by influencing myocardial cell ion channels and prolonging APD (Jain and Parmar, 2011). Hesperidin is derived from the adjuvant Aurantii Fructus Immaturus, and isoliquiritin is derived from the drug Glycyrrhizae Radix et Rhizoma. These ingredients are all antiarrhythmic active ingredients (Ojha et al., 2013). Another research report glycyrrhizic acid has a protective effect on the heart (Ding et al., 2018). Dichroae Radix has small poison and febrifugine is the active component of Dichroae Radix, the content of febrifugine is determined to ensure the safety of TCM compound preparation. The active ingredients and characteristic ingredients contained in each component of the TCM are selected as indicators for quantitative analysis, which can provide a better reference for the quality evaluation and the material basis of the medicinal effect of the traditional Chinese medicine compound.
The chemical components of XSNC are complex and it is difficult to achieve baseline separation by liquid chromatography. In this experiment, multi reaction monitoring technology (MRM) in LC-MS technology was selected for quantitative analysis of the selected 16 chemical components. MRM monitoring mode can detect and analyze specific compounds with strong specificity, high sensitivity and high accuracy. Different compounds have different mass spectrometric responses, and the content of each component is completely different. To solve this problem, a multiple dilution method was constructed to realize the simultaneous quantitative analysis of 16 components in XSNC.
In this study, qualitative analysis of the chemical components of XSNC was carried out by GC-MS and LC-MS techniques. A total of 21 volatile components and 59 non-volatile components were identified, which further clarified the chemical composition of XSNC. It provides a reference for the characterization of chemical components of other TCM preparations; on the basis of LC-MS technology, considering the active components and characteristic components contained in various components of XSNC, the quantitative analysis of 16 chemical components with great content difference in XSNC was realized by constructing the double ratio dilution method. The method has high sensitivity and good selectivity, which can provide experimental basis for formulating a comprehensive quality control method of XSNC.
Acknowledgements
This work was supported by the National Key Research and Development Plan of China (No.2018YFC1707403); the Science and Technology Program of Tianjin (No.20ZYJDJC00120 and 21ZYJDJC00080).
Funding information
National Key Research and Development Plan of China, Grant Number: 2018YFC1707403; Science and Technology Program of Tianjin, Grant Numbers: 20ZYJDJC00120 and 21ZYJDJC00080.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Simultaneous quantification of polymethoxylated flavones and coumarins in Fructus aurantii and Fructus aurantii immaturus using HPLC-ESI-MS/MS. Pharm. Biomed. Anal.. 2012;10:0456-0463.
- [Google Scholar]
- Protective effect of berberine on aconite–induced myocardial injury and the associated mechanisms. Mol. Med. Rep.. 2018;18:4468-4476.
- [Google Scholar]
- Inheritance and innovation of traditional Chinese medicine from the development of Xinsuning capsule. Chin. Traditional Patent Med.. 2018;40:1875-1877.
- [Google Scholar]
- Evolution of orbitrap mass spectrometry instrumentation. Annu. Rev. Anal. Chem.. 2015;8:61-80.
- [Google Scholar]
- Qualitative and quantitative analysis on chemical constituents from Curculigo orchioides using ultra high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal.. 2015;102:236-245.
- [Google Scholar]
- Further progress in predicting life-threatening arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy. J. Am. Coll. Cardiol.. 2016;68:2551-2553.
- [Google Scholar]
- Evaluation of antioxidative and anti-inflammatory potential of hesperidin and naringin on the rat air pouch model of inflammation. Inflamm. Res.. 2011;60:483-491.
- [Google Scholar]
- Li, Y., Du, Z., 2015. Overview on method and strategy of therapeutic material basisin traditional Chinese medicine by multidisciplinary approach.Zhongguo Zhongyao Zazhi. 40, 1644–1648.
- Li, Y., Wang, J., Li, X., Ma, X., Li, Z., 2019. Mechanism of bradyarrhythmia and Current Traditional Chinese and Western Medicine Treatment. Med. Rev. 25, 3221-3226+3231.
- Q-Marker: A New Concept for Quality Control of Chinese Medicine Products. Chin. Herbal Med.. 2016;47:1443-1457.
- [Google Scholar]
- Developmentof Q-marker theory, methods and strategies, and research to improve the level of science and technology of Chinese medicine. Acta Pharm. Sin.. 2019;54:185-186.
- [Google Scholar]
- Liu, D., Cao, G., Si, X., Chen, Q., Sun, H., 2017. Summary of the research on anti-arrhythmia of the alkaloids from Coptidis Rhizoma. Shandong J. Traditional Chin. Med., 36, 164-166+171.
- Qualitative and quantitative analysis of major constituents from Dazhu Hongjingtian capsule by UPLC/Q-TOF-MS/MS combined with UPLC/QQQ-MS/MS. Biomed. Chromatogr.. 2017;31:1-10.
- [Google Scholar]
- The effects of paeonol on the electrophysiological properties of cardiac ventricular myocytes. Eur. J. Pharmacol.. 2006;545:87-92.
- [Google Scholar]
- Glycyrrhiza glabra protects from myocardial ischemia-reperfusion injury by improving hemodynamic, biochemical, histopathological and ventricular function. Exp. Toxicol. Pathol.. 2013;65:219-227.
- [Google Scholar]
- On the research progress of antiarrhythmic effects of traditional Chinese medicines. China Modern Med. Appl.. 2017;11:194-195.
- [Google Scholar]
- Rapid identificationof chemical compositions in callicarpa kwangtungensisChun byultrahigh-performance liquid chromatography with Q Exactive hybrid quadrupole orbitrap highresolution accurate mass spectrometry. J. Sep. Sci.. 2020;43:2487-2494.
- [Google Scholar]
- Research progress of antiarrhythmic Chinese medicines acting on ion channels of cardiomyocytes. Chin. J. Integr. Med. Cardio-/Cerebrovasc. Dis.. 2017;15:1057-1059.
- [Google Scholar]
- Xinsuning capsule for the treatment of premature ventricualr contraction: a multicenter randomised clinical trial. The Lancet.. 2017;390:61.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103825.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







