Translate this page into:
Recent progress on catalytic co-pyrolysis of plastic waste and lignocellulosic biomass to liquid fuel: The influence of technical and reaction kinetic parameters
⁎Corresponding author.at: School of Chemical Engineering, Engineering Campus, Universiti Sains Malaysia, 14300 Nibong Tebal, Pulau Pinang, Malaysia. chazam@usm.my (A.T. Mohd Din)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Better product properties could be obtained from microwave and plasma heating systems. Co-pyrolysis of plastic and biomass improves the oil composition and yield in comparison to biomass derived oil. Co-pyrolysis liquids from plastic and biomass can be used as an alternative to fossil fuel. Positive synergistic effect in catalytic co-pyrolysis of plastic and biomass is reported.
Abstract
Discharge of non-biodegradable plastic waste and lignocellulosic biomass into the environment, and the resultant pollution has persistently increased all over the globe. This activity poses a threat not only to mankind, but also to the environment. In addressing this challenge, catalytic co-pyrolysis (CCP) of plastic waste and lignocellulosic biomass is one of the attractive ways used to reduce these types of waste, while simultaneously obtaining an alternative for conventional fossil fuel. This article has critically reviewed the literature on CCP in several areas, especially the impact of technical parameters, such as heating systems, experimental conditions, and synergistic effect of the CCP of plastic and biomass wastes. The kinetics and reaction pathways of CCP of plastic and biomass wastes are also discussed. The analysis and information presented in this review may be used in future studies to develop a sustainable and efficient heating processes in pyrolysis system to re-engineer plastic and biomass wastes.
Keywords
Catalytic co-pyrolysis
Biomass
Plastics waste
Heating rate
Liquid fuel
Kinetic models
- CPP
-
Catalytic co-pyrolysis
- KAS
-
Kissinger-Akahira-Sunose
- FWO
-
Flynn-Wall-Ozawa
- FR
-
Friedman
- ST
-
Starink
- DAEM
-
Distributed Activated Energy Methods
- CR
-
Coats-Redfern
- Vy
-
Vyazovkin
- F1
-
First order reaction
- PE
-
Polyethylene
- PW
-
Plastic waste
- PP
-
Polypropylene
- PVC
-
Polyvinyl chloride
- LPDE
-
Low-density polyethylene
- PET
-
Polyethylene terephthalate
- HDPE
-
High-density polyethylene
- PS
-
Polystyrene
- TGA
-
Thermogravimetric analyser
- A
-
Pre-exponential factor (min−1)
- α
-
Conversion
- β
-
Heating rate (Kmin−1)
- E
-
Activation energy (kJ/mol−1)
- f(α)
-
Function expressing dependence of the reaction rate on the conversion
- g(α)
-
Integrated reaction model
- k
-
Reaction rate constant (min−1)
- n
-
Order of the reaction
- p(x)
-
Integral function
- R
-
Universal gas constant (8.3145 Jmol−1K−1)
- R2
-
Correlation coefficient
- t
-
time (min)
- T
-
Temperature (K)
- Tm
-
The temperature at maximum reaction rate (K)
- X
-
Equivalent form of E/RT
- Cs
-
Starink’s constant,
- K
-
Rate constant
Abbreviations
1 Introduction
Owing to the finite nature of fossil fuel, many nations have begun to explore alternative sources of energy in order to meet the rising demand for clean and sustainable energy. Plastic, which is a petrochemical material and widely used in most activities, will serve as a better and promising alternative to fossil fuel (Mwanza and Mbohwa, 2017). The ease with which plastic is manufactured, as well as the pressure for economic efficiency and the urbanisation of populations in most parts of the world, has resulted in a massive production of this material, which would reach an estimated value of 110 million tonnes annually (Budsaereechai & Hunt, 2019; Biradar & Hebbal, 2020).
Furthermore, due to increased research and development effort into the technology of plastic production, its annual global production has surged from approximately 1.5 million metric tons in 1950 to 359 billion metric tons in 2018. The cumulative production of plastic has already surpassed eight billion metric tons worldwide, with further increases expected in the coming decades (Tiseo, 2020). Plastic has succeeded in replacing metals, wood, and ceramic products due to its outstanding combination of properties, such as mechanical, thermal, and chemical resistance, as well as dimensional stability (Biradar and Hebbal, 2020). Therefore, enormous quantities of new plastic materials are thermoformed, foamed, laminated, and extruded into millions of packages and products.
However, some of these plastic products are designed for single use that become waste within a short time. This emerging development has resulted in grave consequences to the environment, which is generating global concern (Jiang et al., 2018). Currently, most countries are locked in a huge battle over how they should handle the growing number of plastic waste dumps in their country (Dumbili and Henderson, 2020; Marks, 2019; New Straits Times, 2018; Sarpong, 2020). Colossal masses of plastic trash, such as wrecked compact discs, used charging wires, plastic cutlery, foam hot drink cups, plastic milk cartons, and plastic bags have formed large heaps of waste in both developing and developed nations of the world. The packaging industries (GreenPeace International, 2020) are a major source of these types of waste, which end up in landfills, beaches, rivers, and oceans (Bengali, 2018; Cazan et al., 2017). Fig. 1 presents the quantity of plastic waste generated globally between 1950 and 2015, including past trends and the forecast for 2050.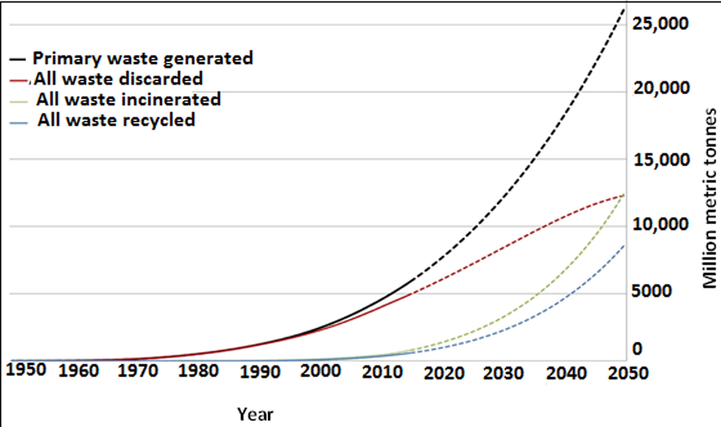
Global plastic waste generation and projected data for 2050 (Geyer et al., 2017).
Recently, the world is coming to realize the dangers of plastic pollution and is responding at an already impressive scale towards achieving substantial reductions in global plastic emissions to the environment. For example, Korea recently implemented the Resource Circulation Act (RCA), which includes the concept of circular economy and resource efficiency policy for overall waste management (Shin et al., 2020). In the European Union countries, the strategy for single-use plastic packaging is being discussed as a response to contemporary challenges and as an element of achieving the goals of sustainable development and circular economy. These strategies include legislation on banning, taxing and limiting the use of single-use bags and increasing recycling rates (Baran, 2020; Borrelle and Law, 2020).
Since 2015, approximately 6,300 metric tonnes of plastic waste generated around the world have been valorised (9%), combusted (12%), and dumped (79%) into the environment (Fig. 1). In 2018, the global plastic recycling volume was 29,438 kilotons, with Asia Pacific having the highest share in the plastic recycling market in terms of value and volume owing to the highest share in plastic waste generation and high-volume of plastic waste import in the region (Dublin, 2020).
There are several techniques of recycling plastics such as pyrolysis, hydrothermal, co-liquefaction, co-gasification and biological conversion. However, recently, ample attention has been given to co-pyrolysis because it does not release harmful substances into the environment. Co-pyrolysis is the simultaneous thermal decomposition of two types of wastes at elevated temperatures in an inert atmosphere. It is commonly used in the treatment of organic materials. Studies have shown that the co-pyrolysis of a mixture of plastic and biomass in the presence of a catalyst results in improved quality and composition of products than in the pyrolysis plastic/biomass alone.
Co-pyrolysis of plastic with biomass allows modifying the composition of the solid, liquid and gaseous products and adapting it as a liquid fuel. In addition, the co-pyrolysis of plastic and biomass waste can contribute to a reduction in production costs, expand waste disposal options, and reduce environmental impacts (Zhang et al., 2018a,b,c). The most suitable plastics for co-pyrolysis with biomass are polyethylene, polypropylene and polystyrene. The properties of these plastics have been comprehensively explained by (Anuar Sharuddin et al., 2016).
Some reviews have been conducted on the catalytic co-pyrolysis of plastic and biomass. Uzoejinwa et al. (2018) reviewed several catalysts that have been used in the pyrolysis and co-pyrolysis of various categories of plastics and biomass. Ryu et al. (2020) reviewed different reaction pathways involved in acid- and/or base-catalysed co-pyrolysis of plastic and biomass wastes. Alternatively, Ahmed et al. (2020) reviewed the development of catalyst to influence and vary the reaction pathways of simultaneous pyrolysis of biomass with plastic, tyre, and scum. Nonetheless, the development of energy efficient, large-scale, and feedstock flexible pyrolysis requires a clear understanding of the influence of heating rates, heating systems, activation energy, reaction rate compositions, and yield of pyrolytic products.
The co-pyrolysis and catalytic co-pyrolysis reactions have been studied using mostly fixed bed reactors. However, there is no review and discussion on the impact of heating systems towards an efficient and effective co-pyrolysis of plastics and biomass to obtain high yield product. To the best of the authors’ knowledge, there is no work in the literature comparing the yield and distribution of products for co-pyrolysis of plastic and biomass in different heating systems. The objective of this article, therefore, is to critically review the literature for the influence of conventional, microwave, and plasma heating systems in the catalytic co-pyrolysis of plastic waste and lignocellulosic biomass. In addition, this article will review the effects of experimental conditions on the yield and composition of liquid fuel production from co-pyrolysis of plastic and biomass wastes. Finally, the various kinetics and reaction models that were used to establish the kinetics and synergy effect on the co-pyrolysis of plastic and biomass wastes using thermogravimetric analyser is discussed.
2 Influence of technical parameters on co-pyrolysis of plastic waste and biomass to liquid fuel
The yield and quality of liquid products obtained during co-pyrolysis of plastics and biomass may be affected by several factors. Additionally, the modern approach for reactor design processes in general is to primarily find suitable heating systems and operating conditions, as well as take the synergistic effects of feedstock materials into consideration. Along these lines, the following sections will review previous research endeavours dealing with heating systems, experimental conditions, and synergistic effects on the co-pyrolysis of plastic and biomass wastes.
2.1 Influence of heating systems on co-pyrolysis of plastic and biomass wastes
Pyrolysis reactors utilise a high amount of external heat in order to transform plastic waste and lignocellulosic biomass into liquid fuel. The general characteristics of conventional, microwave, and plasma-assisted heating systems are summarised in Table 1. Feedstock of various particle sizes can be used in this system to produce retorting gas of high quality Rapid heating of feed stocks is unrealistic and could cause significant loss of absorbed energy Produces relatively low oil yield and high char yield, which triggers coke formation and fouling in the reactor. Offers uniform and excellent heat transfer juxtaposed with conventional heating. Produces enhanced product yield with improved calorific value within a short reaction time. Reaction temperature is difficult to regulate and therefore, no uniformity during the overall pyrolytic run. Can handle complex plastic waste (such as hospital and automobile plastic waste). The high energy of the DC thermal plasma torches fosters its function as catalyst. It involves the use of plasma gun which leads to the rapid consumption of electrodes Extremely fine feedstock particles are required to achieve rapid heating rate. Requires high operating temperatures
Type of heating
Advantages
Limitation
Reference
Conventional system
(Joardder et al., 2017)
Microwave-supported system
(Shiung et al., 2019a,b; Suriapparao et al., 2018; Zhao et al., 2018; Zhou et al., 2018)
Plasma-supported pyrolysis system
(Tamošiunas et al., 2014; Tamošiunas et al., 2016; Tang et al., 2013)
In conventional heating systems, heat is transmitted through the wall of the reactor to the raw materials by conduction and convection heat transfer principles while in the case of microwave heating systems, heat is delivered by the interaction of varying field forces with the magnetic poles and charged ions in the material. In the case of Plasma heating systems, it involves, high-frequency heating with zero emission philosophy. Microwaves (MW) heating systems have great potential for use in pyrolysis reaction processes, through enabling unique reaction fields that cannot be formed by conventional heating systems (Durka et al., 2009). Few studies have investigated the variation of yield and composition of products from the co-pyrolysis of plastic and biomass under different heating systems. In the conventional and microwave-assisted pyrolysis of rapeseed oil for bio-fuel production, Omar and Robinson (2014) remarkably found aromatics in the residual oil after a microwave treatment, whereas none were found with conventional pyrolysis. In terms of product distribution, Dominguez et al. (2007) found that in the conventional and microwave induced pyrolysis of coffee hulls, microwave treatment produced more gas and less oil compared to conventional pyrolysis. These aforementioned findings aligned fully with the outcomes found by Mahmud et al. (2019) and Wu et al. (2014), whereby a higher gas yield was produced using the conventional pyrolysis. However, a divergent outcome was obtained in a recent investigation by Shi et al. (2020), whereby the bio oil produced by conventional pyrolysis was higher in contrast to the yield by microwave pyrolysis. In the pyrolysis of HDPE using both conventional and microwave, (Khaghanikavkani, (2013) found that the microwave heating did not alter product composition in comparison to the conventional pyrolysis of HDPE. The large portion of the produced hydrocarbons were in the range of C8 to C35. The product yields of different plastic and biomass mixtures using different heating systems are presented in Table 2. N/A = Not available.
Feedstock
Heating systems
Heating rate oC/ min
Catalyst
Heating value
Yield (wt%)
Reference
MJ/kg
Liquid
Gas
Solid
PP and saw dust
Conventional
30
N/A
N/A
24–48
N/A
11.6–57
(Ye et al., 2008)
PE + sawmills powders
Conventional
5
N/A
23.25
52.75
N/A
N/A
(Uguz et al., 2017)
Pine sawdust + waste PS foam
Conventional
N/A
N/A
39.65
63.31
N/A
N/A
(Nguyen et al., 2019)
Grape seeds + PS
Conventional
100
N/A
19.5
56
20
20
(Veses et al., 2019)
PE + Rice straw
Conventional
∼ 8
N/A
N/A
80
20.5
N/A
(Hossain et al., 2019)
Rice straw + Sugarcane bagasse + PP
Microwave
10
HZSM-5
43
25–27
2–44
N/A
(Suriapparao et al., 2020)
Used cooking + WS
Microwave
29
N/A
N/A
84
37
6
(Shiung et al., 2019a,b)
Waste cooking oil + Waste Plastic
Microwave
50
N/A
42–49
62
N/A
N/A
(Adibah et al., 2018a)
Bamboo and PP
Microwave
N/A
HZSM-5
N/A
61.6
N/A
N/A
(Zhao et al., 2018)
Lignin + PP
Microwave
n/a
HZSM-5
N/A
47.4
N/A
N/A
(Duan et al., 2017)
Baby diapers (cellulose + polyolefins)
Microwave
43
N/A
N/A
43
29
28
(Shiung et al., 2019a)
Rice straw + LDPE
Microwave
24
ZSM-5
N/A
29.8
46.7
N/A
(Bu et al., 2018)
PS - Biomass blends
Microwave
61.6
N/A
51–60
N/A
N/A
N/A
(Suriapparao et al., 2018)
PP - Biomass blends
Microwave
∼ 61.6
N/A
38–42
N/A
N/A
N/A
(Suriapparao et al., 2018)
LDPE
Plasma
7.8
N/A
N/A
56.9
37.8
minimal
(Gabbar et al., 2017)
Rice straw
Plasma
−263
N/A
N/A
N/A
66.5
N/A
(Tu et al., 2009)
The product distribution presented in Table 2 were obtained under different experimental conditions which makes it difficult to objective quantify the underlying mechanisms. However, one of the fundamental reasons for the variation in the yield and heating value of the products is the heating rate. For conventional heating systems, the heating rate in range of 8–30 °C/min were mostly used in many studies while for microwave systems, heating rate in the range of 10–61 °C/min have been used. In microwave systems, faster heating rates are usually used compared to conventional heating systems. Fast heating rates cause quick fragmentations of plastic and biomass and enhances the yield of volatiles. It will observed that the heating value of the co-pyrolysis bio-oil obtained using microwave heating systems were within the range of 42–61 MJ/kg which is higher than the heating values of pyrolytic oil from pyrolysis biomass (15–20 MJ/kg.) and plastic (30–45 MJ/kg) (Adrados et al., 2012; López et al., 2010). Many of the co-pyrolysis liquids produced have high heating value above 36.6 MJ/kg which indicates that they can be used as an alternative to fossil fuels (Adrados et al., 2012). HHV is the most important characteristic of pyrolytic oil because it gives an indication of the satisfactory combustion of gases.
Most of the studies presented in Table 2 were mostly performed on a bench and discontinuous mode. Very few have been performed in a continuous mode. In the following sub-section, different heating systems are critically discussed in greater detail.
2.1.1 Conventional heating system
The regularly used heating system for pyrolysis of plastic and biomass is the traditional electric furnace. Liquid fuel yield and the efficiency of this system are determined by the heat transfer rate across the reactor wall, reactor material type, and the temperature difference between the reactor surface and innermost core of the material in the reactor.
The furnace layouts of some conventional heating systems are shown in Fig. 2. The use of liquefied petroleum gas (LPG) as the heat source for pyrolysis has been developed and this heating system is presented in Fig. 2 (a). However, the LPG heating system is typically associated with several shortcomings. Since the heating system is in the lower compartment of the reactor, inconsistent and inefficient heat transport from the burner will result in low liquid fuel yield. Additionally, the reactor compartment has a costly insulation which needs periodic maintenance and replacement. Thus, it is important to utilise apparatus for the pyrolysis of plastic and biomass that jettisons the need for heat jacket.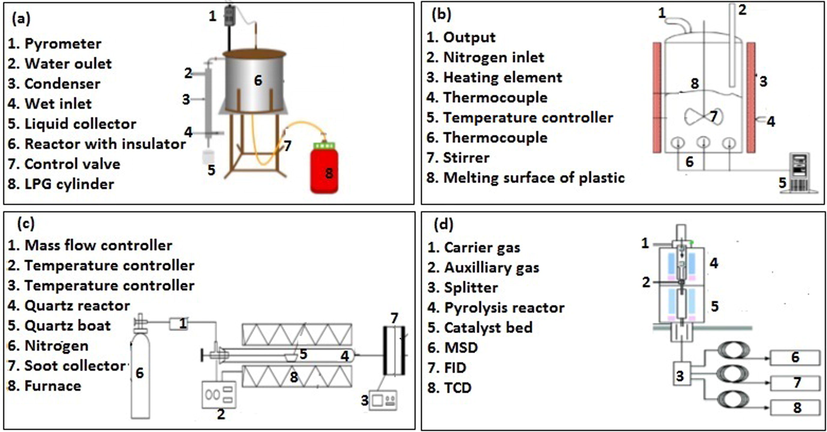
Experimental set up of conventional heating system using (a) liquid petroleum gas (LPG) powered furnace (Hossain et al., 2019) (b) stirred tank heating furnace (Supramono et al., 2020) (c) horizontal electrical furnace (Wang et al., 2019) (d) vertical electrical furnace (Wang et al., 2015).
Fig. 2(c) and (d) show the experimental setup of a horizontal heating furnace and a vertical heating furnace, respectively. The furnace in Fig. 2(d) has two partitions, which is regulated independently to provide the static and rapid temperature changes during co-pyrolysis. Because the reactor in this heating system is a fixed-bed type, the effect of heat and mass transfer in the sample particles cannot be investigated. However, this setup and heating system can measure the temperature-time history accurately. Therefore, it can be used to obtain not only the kinetics of reaction, but possibly get an insight into the mechanism of pyrolysis. Moreover, the effect of catalyst temperature on the oil yield can be studied using this setup.
Catalytic and non-catalytic co-pyrolysis of Corn Stalk (CS) and Polystyrene (PS) was conducted in in-situ and ex-situ setup represented in Fig. 2(c) and (d) with a separate catalyst bed reactor to evaluate the mode of catalyst addition. Mesoporous HZSM-5 catalyst applied in the in-situ and ex-situ modes led to decreased oil and increased gas yields at all catalyst feedstock ratios as compared to non-catalytic co-pyrolysis. However, as compared to ex-situ, in-situ mode showed better results, yielding more liquid oil and mono aromatic hydrocarbon (MAH) content, yet less acids and oxygenates. With regard to MAH selectivity, styrene dealkylation into benzene with increasing catalyst feedstock ratios was noticed in both in-situ and ex-situ (Muneer et al., 2019).
However, there is one limitation faced by setups in Fig. 2(a), (c), and (d), which is the effect of stirring speed on co-pyrolysis cannot be studied. Stirring the plastic-biomass mixture during co-pyrolysis improves the convective heat transfer because it causes the reactor bed to expand, thereby increasing the friction between the melting plastic and the hot reactor. This will induce high convective heat flux to the plastic-biomass feed. A stirred tank heating furnace was used to perform co-pyrolysis of corncobs and polypropylene (PP) granules, as shown in Fig. 2 (b).
In the industry, rotary kiln reactor design is used to achieve mechanical stirring while fluidized bed reactors use fluidization gas to achieve a good mixture of the solids in order to improve heat transfer. Researchers have developed a reactor which does not use any fluidization gas but has a stirrer that provides the required mixing between the injected biomass and the bed material while effectively breaking any possible agglomerate. Performance evaluation of the reactor revealed that stirring was necessary to attain effective heat transfer between the heaters and the bed, and between the bed and the biomass particles. Furthermore, the stirrer geometry played a major role in increasing the efficiency of the reactor (Lago et al., 2015; Villemont et al., 2019).
Recently, a group of researchers have introduced a reactor that can operate in the slow-pyrolysis mode to obtain high-yielding liquids. The concept of the reactor was designed to develop the fluidization condition by using a stirring device. The obtained liquid yield increased remarkably from 38.4 wt%. to 51.6 wt% and comprised a higher proportion of organic phase than aqueous phase when the 50- rpm condition was applied (Qureshi et al., 2019).
On catalysis, few pilot plants studies have been carried on the catalytic pyrolysis of plastics waste and catalytic co-pyrolysis of plastics. On plastic alone, a small-scale pilot research has been conducted to study the effect of zeolite catalyst on liquid oil produced from catalytic pyrolysis of waste polystyrene plastics was found to consist of around 99% aromatic hydrocarbons (Rehan et al., 2017). Miandad et al., (2017) obtained similar result from another pilot test when the same catalyst type was used in the co-pyrolysis of PS mixed with other plastic wastes.
On an industrial scale, catalytic co-pyrolysis of real municipal solid wastes comprising of paper and plastic mixtures was undertaken in an electrically heated furnace under continuous mode. Interesting findings revealed that zeolite-based catalysts with alkali characters resulted in the reduction of oxygenated hydrocarbons and also increasing the saturation and isomerization reactions (Fekhar et al., 2020). The conventional heating systems has been established to operate in industrial scale around the world.
Catalytic co-pyrolysis of plastic and biomass waste has been extensively investigated, with most studies focusing primarily on the use of zeolites. However, the deactivation and high costs of zeolites opens up many opportunities for the development of other inexpensive cracking catalyst. Few researchers have worked on the development of catalysts from industrial waste and renewable organic materials such steel slag, alumina, biomass waste and activated carbon (Hassan et al., 2019; Lin et al., 2020; Mateo et al., 2020; Zhang et al., 2019) for application in the catalytic co-pyrolysis of plastic and biomass. Hydrocarbon yields of the liquid fuel that ranged between 47% and 97.51 % were obtained in these studies which indicate the suitability of these materials as catalyst support in the co-pyrolysis process. Specifically, catalyst developed from sulphonated activated corn cobs having a surface area of 1159.28 m2/g and pore volume of 0.815 cm3/g displayed the capacity to increase the hydrocarbon yield of Douglas fir-LDPE based pyrolytic bio-oil from 66.95% to 94.18–100% (Mateo et al., 2020).
2.1.2 Microwave-supported pyrolysis system
To overcome the shortcomings of conventional heating in pyrolysis reactor systems, microwave (MW) heating pyrolysis has attracted the interest of researchers in the last two decades. Microwave heating is emerging as one of the most attractive alternative technologies in the pyrolysis and co-pyrolysis process. Microwave pyrolysis not only overcomes the disadvantages of conventional pyrolysis methods such as slow heating and necessity of feedstock shredding, but also improves the quality of final pyrolysis products. At the same time, it significantly saves processing time and energy.
Overall, most studies have concluded that microwave pyrolysis is a more energy-efficient process and has immense potential in converting plastic and biomass wastes into valuable pyrolytic products. Some studies have shown that microwave-assisted heating systems and temperature of reaction can affect the characteristics of the liquid oil (Suriapparao et al., 2018; Zhang et al., 2018a,b,c). Nonetheless, insufficient understanding of microwave systems has impeded the development of commercial microwave heating systems and their use in the co-pyrolysis of plastic and biomass wastes.
During microwave heating, the raw material takes in microwave energy from the reactor and in series generates heat right through the bulk of the material. Any organic material that is exposed to electromagnetic radiation will be heated up. Microwaves interact with organic materials and the strength of this interaction depends on the dielectric properties of the materials. Absorption of radiofrequency on microwave energy by these dielectric materials generates heat in the material (Ulloa et al., 2019).
Biomass are the most common types of materials that have been subjected to microwave energy. Plastics cannot absorb microwave energy, as it has a very low dielectric loss factor. Therefore, an absorbent must be mixed with the plastic to aid in heating the plastic in pyrolysis. Materials with high dielectric loss factor are good candidates as absorbents for plastic pyrolysis e.g. tyre shredded, silicon carbide and carbon.
The mechanism of plastic microwave pyrolysis is based on absorbing the microwave energy via absorbent and subsequently transferring thermal heat to the plastic via conduction. The physical properties and the volume ratio of the absorbent affects the uniformity of heating distribution.
It is pertinent to mention that most laboratory studies employed reworked standard microwave ovens as pyrolysis reactors to perform co-pyrolysis of plastic and biomass wastes. The power level functions of most of these setups were by default designed to be varied at wide intervals, which affects the result. Thus, it is imperative to explore co-pyrolysis of plastic and biomass wastes using microwave heating systems that permit power adjustments at reduced intervals. Energy is conserved if a lower optimal power level is discovered. The experimental setups of ex-situ and in-situ microwave heating systems are presented in Fig. 3. Each microwave oven was fabricated into a batch pyrolysis reactor and adopted for the simultaneous pyrolysis of plastic and biomass wastes.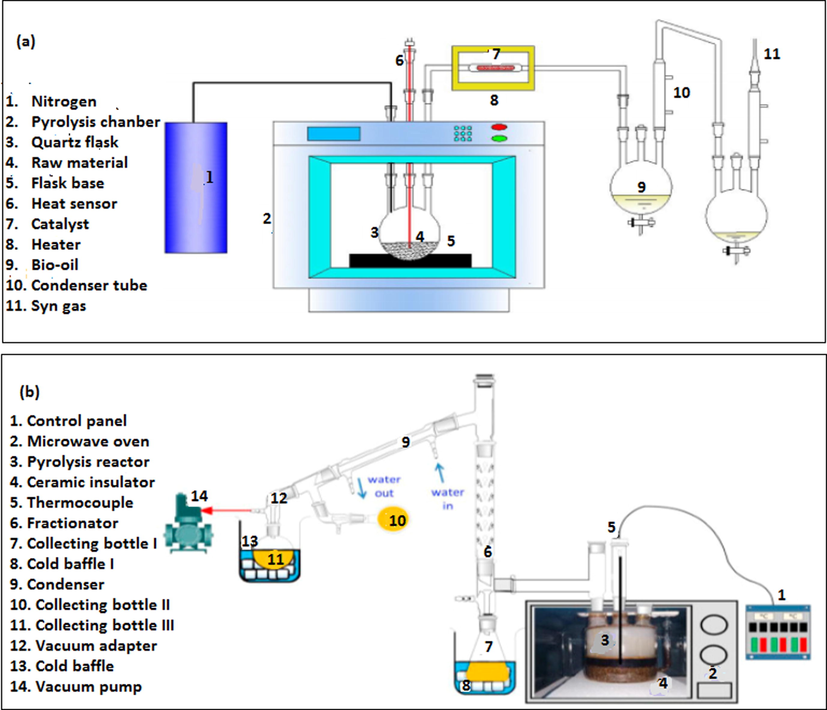
Experimental set up of microwave heating systems with (a) ex-situ (Bu et al., 2018) and (b) in-situ (Shiung et al., 2019a,b) catalytic systems.
Due to the limitation in these setups, the influence of mixing intensity and stirring speed could not be studied. The impacts of these variables are important and should not be ignored if the formation of non-condensable gases at the expense of bio-oil and biochar is desired or the process is being contemplated for a scale-up. These setups could be further modified by inserting an adjustable stainless-steel two-bladed stirrer. However, the influence of catalyst temperature on the yield of bio-oil can be investigated under microwave heating using the setup in Fig. 3 a. The heating set-up shown in Fig. 3b has no separate heating setup for catalytic reactor which reduces the overall capital cost of the system fabrication as well as operating cost. However it is necessary to consider the fact that the catalytic temperature is same as that of pyrolysis temperature and the optimized pyrolysis temperature may not be optimum for catalytic cracking (Wan and Wang, 2014). Provision of separate temperature control for pyrolysis and catalysis is desirable because it helps to improve the performance of the catalyst and prolongs the catalyst lifetime (Duan et al., 2017; Luo and Resende, 2016).
Study has shown that the combined use of microwave vacuum pyrolysis and activated carbon reaction bed produced 84 wt% yield of liquid oil with diesel range hydrocarbons and energy content of 49 MJ/kg (Shiung et al., 2019a,b). When a catalyst was used, synergistic effects were observed with microwave assistance. For instance, during the co-pyrolysis of low-density polyethylene (LDPE) and rice straw using a microwave supported system, synergistic effect between these feedstocks was observed in terms of the yield and composition of the bio-oil. The yield of bio-oil obtained by co-pyrolysis was 22.75% and the addition of the ZSM-5 catalyst was 24.46% (Bu et al., 2018). The literature survey showed that most co-pyrolysis reactions were conducted using microwave power ranging from 800 to 1,000 W.
One of the most problematic aspects of microwave assisted heating is working in a continuous mode as all the references from literature have worked discontinuously or in batch processes. The reason is explained by the difficulties of combining the chemical and electrical engineering technologies to meet the requirements for a high temperature microwave pyrolysis of plastic. Another reason is due to the complex nature of pyrolysis of plastic which needs a detailed design in addition to a robust electromagnetic simulation model (Khaghanikavkani, 2013). The complex nature of microwave heating factors such as microwave frequency, microwave mode, reactor geometry, temperature variation, surrounding medium properties, dielectric properties and cavity geometry can all influence the distribution of electromagnetic waves and the resulting heating patterns (Goodman, 2014). The major limitation which is preventing this microwave heating from being widely employed in continuous mode is uncertainty about the actual costs (Fernandez et al., 2011).
2.1.3 Plasma heating system
Plasma heating system amalgamates the pyrolysis process with the thermo chemical properties of the plasma. The plasma heating system has intense and flexible heat generation capability, which can be used to dispose of all forms of plastic waste (Hikmath and Arachchige, 2020). For laboratory experiments and industrial applications, plasmas are generated through electrode gas discharge, microwave, laser, high-energy particle beam, or electrodeless radiofrequency (RF) methods. In a plasma, the electrons are ripped from atoms to produce freely moving ions. Since ions and electrons are charged, they respond to electric and magnetic forces and interact with each other through these forces as well.
When electromagnetic waves of appropriate frequency are beamed into the plasma, the particles will absorb energy from the wavefield and transfer it to other particles through collisions. As soon as plastic and biomass wastes are fed into a plasma heating system, they are swiftly heated, leading to the discharge of volatile matter. The volatile matter is subsequently cracked to produce gases rich in hydrogen and hydrocarbons. Heating in a limited supply of oxygen will mostly generate water, synthetic gas, and other gaseous hydrocarbons in sizeable quantities, including small amounts of solid products. The gas produced can be upgraded or reformed to conform with a standard for use as a source of power or feedstock for manufacturing chemicals and liquid fuels. An efficient design of thermal plasma heating system is necessary to guarantee energy-effective and cost-effective operation. DC/AC electric discharges, microwave discharges, and laser-induced plasmas are the main approaches for generating plasma for the pyrolysis of plastic and biomass wastes for fuel production (Tang et al., 2013).
The experimental setup of a plasma heating system is shown in Fig. 4. Plasma heating system usually have coaxial configuration containing a dielectric shaped in the same form as common fluorescent tubing. The device is filled at atmospheric pressure with either a rare gas or rare gas-halide mix. For setup in Fig. 4 (a), it is possible to optimise the thermal performance of the heating system using the self-regulating controller, which receives feedback signals from gas analysers. A constant thermal profile in the pyrolysis system increases liquid product yield. Furthermore, the stirring mechanism in the reactor reduce the limitation in heat and mass transfers compared to setup in Fig. 4 (b).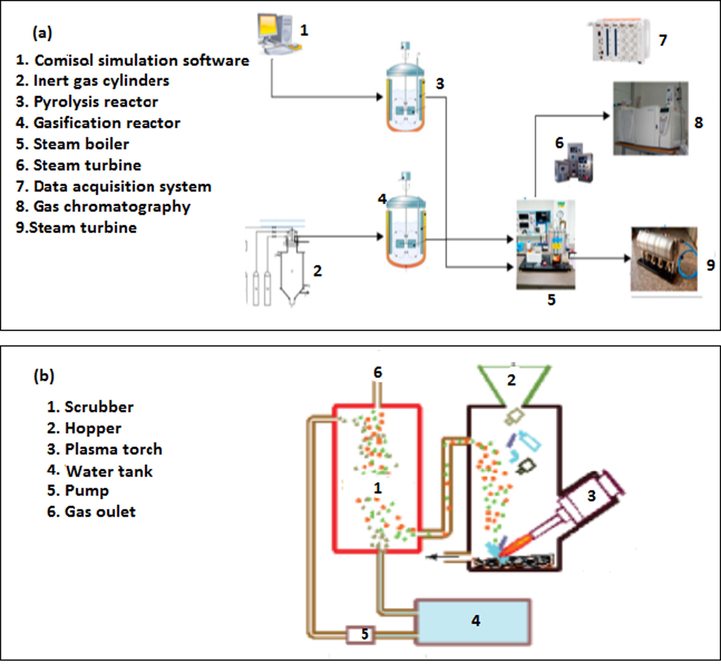
Experimental set up of plasma heating system with (a) steam turbine (Gabbar et al., 2020) (b) scrubber system (Nema et al., 2016).
Plasma assisted heating is quite new and the research is so incipient that only very few laboratory studies have proved the effectiveness of this technique. Recently, a pilot study was conducted to measure the heating efficiency of a plasma system in heating a plastic sample confined in the plasma region. The results showed that the heating efficiency of the system had a lower bound value of about 74 ± 5%, a value which might be very interesting for most industrial applications (Borin et al., 2019). Plasma as a heat source for co-pyrolysis of plastic and biomass would open a new horizon in this topic. Salvia et al., (2019) used biomass derived catalysts for the catalytic pyrolysis of mixed plastic waste using a plasma heating system. The cracking process increased the gas, hydrogen and methane yield up to 85, 3.25 and 55 wt% respectively compared to non-catalytic pyrolysis. The capital costs of plasma heating systems are high, because of the high energy involved and this can hinder the profitability and sustainability of pyrolysis and co-pyrolysis of plastic and biomass waste.
2.2 Influence of experimental conditions on co-pyrolysis of plastic and biomass wastes
Since plastic and biomass wastes are composed of various components, the extent and rate of thermal fragmentations and interaction of these components hinge upon experimental operating conditions. Experimental conditions are important for specifying the yields composition and properties of pyrolysis products (Gulab et al., 2010). The effects of experimental operating conditions, on yield of main products for co-pyrolysis of different plastic and biomass mixtures are summarised in Table 3.
Parameters
Ranges
Feedstocks
Optimum value
Main product
Max yield (wt%)
Reference
Biomass (B)
Plastic (P)
Reaction Time (min)
10–60
Rice husk
PP
60
Liquid
74.0
(Costa et al., 2014)
15–75
Palm shell
PS
45
Liquid
61.6
(Abnisa et al., 2013)
60–120
–
PS
75
Liquid
80.8
(Miandad et al., 2016)
Reaction Temperature (°C)
400–500
–
Mix
440
Liquid
79.3
(López et al., 2011)
300–700
Palm shell
PS
600
Liquid
68.3
(Abnisa et al., 2013)
500–800
Water paper
HDPE
600
Liquid
–
(Chen et al., 2017a,b)
550–600
Sawmill powders
PE
550
Liquid
46.0
(Uguz et al., 2017)
525–675
Red oak
HDPE
625
Liquid
57.6
(Brown et al., 2015)
400–700
Sugarcane bagasse
HDPE
600
Liquid
63.7
(Hassan et al., 2020)
450–700
Juliflora
LPDE
600
Gas
41
(Somasundaram and k, 2019)
700–1250
Wood
PUR
700
Char
43.1
(Wang et al., 2019)
Pressure (Bar)
2–10
Petroleum fraction
LDPE
-.
–
–
(Schubert et al., 2019)
Mass of B-P mixture (g)
250–1000
Rice straw
PP
1000
Liquid
35.7
(Suriapparao et al., 2020)
B-P mixture Catalyst ratio (w/w)
1:30–3:30
Durian shell
–
2:30
Liquid
50.2
(Tan et al., 2020)
1:0–1:5
Corn stover
PP
1:4
Gas
61.2
(Wu et al., 2020a,b)
1:20–4:20
Pine sawdust
HDPE
1:5
Liquid
70.0
(Chen et al., 2020)
1:0–4:1
Lignin
PP
1:2
Liquid
59.4
(Duan et al., 2017)
1:20–2:20
–
PP
2:20.
Liquid
80.0
Kassargy et al., 2019)
B-P mixture ratio (w/w)
1:0–0:1
Lignin
PP
4:1
Liquid
47.7
(Duan et al., 2017)
1:0–1:3
Waste paper
HDPE
1:1
Liquid
–
(Chen et al., 2017a,b)
1:0–0:1
Not specified*
Mix
1:2
Gas
63.2
(Zhang et al., 2019)
4:1–2:3
Palm shell
PS
2:3
Liquid
64.2
(Abnisa et al., 2013)
19:1–3:2
Grape seeds
PS
3:2
Liquid
57.0
(Veses et al., 2019)
3:1–1:3
Cellulose
HDPE
3:1
–
–
(Yuan et al., 2018)
2.2.1 Effect of time
Investigations on the effect of time on pyrolysis of plastic and biomass wastes are very scarce in the literature. This may be due to the fact that the important issue in catalytic co-pyrolysis is to work under continuous mode where the characteristics and yields of the products are stable with time. Nevertheless, according to Tripathi et al. (2016), reaction time affects the properties and yield of liquid fuel. In the presence of a catalyst, the product distribution of liquid fuel can change to a reasonable extent, with changes in composition and structure of the used catalyst with longer reaction time.
2.2.2 Effect of temperature
Temperature has also been proven to influence the yield, distribution and properties of products obtained in the co-pyrolysis process since it controls the cracking reaction of the plastic-biomass mixture. For instance, when the temperature during the co-pyrolysis of low density polyethylene (LDPE) and rice straw was 550 °C, the ketone content in the bio-oil was relatively low, compared to temperatures of 450 °C where the ketone content was higher (Bu et al., 2018). This is similar to the results obtained in the work of Liang et al (2017). It was observed that the change of the reaction temperature resulted in the change of the ketone content. In most pilot studies in the literature, the reactors were operated at safe temperature of up to 600 °C. The results of these studies revealed that liquid oil comprised of hydrocarbon within the conventional diesel range were obtained.
Generally, increasing temperature in a system causes the inner molecules to vibrate, which would evaporate away from the surface of the object. The carbon chain eventually split once the single bond in the chain has an enthalpy lower than the energy activated by Van der Waals force within the plastic and biomass chains (Mustafa, 2019). Generally, a higher temperature facilitates the conversion of plastic and biomass wastes to fuel by providing the additional energy needed to split the bonds in the waste mixture (Guedes et al., 2018). In several co-pyrolysis studies of plastic-biomass mixture, the temperature had ranged between 400 and 1250 °C. Temperature that ranged between 600 and 625 °C was found to produce higher liquid fuel yield. However, the nature of feedstock mixture and other process parameters will alter these temperature values for obtaining the desired product (Wirawan, 2019).
2.2.3 Effect of initial plastic-biomass mixture to catalyst ratio
Several researchers, such as Chen et al. (2020) and Duan et al. (2017) studied the effect of initial plastic-biomass mixture to catalyst ratio, while other studies had focused on catalytic pyrolysis of plastic alone. Gulab et al., 2010 revealed that pyrolysis of high-density polyethylene using zeolite as the catalyst can be done at low catalyst content to reduce the operating cost. The study concluded that decreased catalyst content will reduce the activity of the system but have stressed that this reduction can be compensated by increasing the process temperature. Studies has also shown that amount used catalyst during co-pyrolysis of plastic and biomass affect the product yield and distribution of the co-pyrolysis process through secondary cracking of the pyrolyzates thereby facilitating the generation of polyaromatic hydrocarbons and different hydrogenated bio-oil fractions which are components of convention fuels (Wu et al., 2020a; Zhang et al., 2016a,b).
2.2.4 Effect of initial pressure
Studies on the effect of initial pressure on co-pyrolysis of plastic and biomass wastes are scarcely available in the literature. Pinto et al. (2018) showed that as an operating parameter, pressure has the least effect on the co-pyrolysis of a mixture of pre-treated eucalyptus with polyethylene. Thus, they were able to use relatively low pressures to reduce the capital and operation costs in subsequent experiments. Similar observations were made by Costa et al. (2014) and Filipe et al., (2009), whereby liquid yield at a particular temperature and time were almost the same, regardless of the initial pressure used. Increasing the initial pressure of pyrolysis processes have often resulted in the cracking of the reaction mixture and suppression of evaporated hydrocarbon, leading to a shift to shorter chains in the liquid and gaseous fractions (Schubert et al., 2019). Reaction pressure has a great impact on the evolution process of oil and gas products, as well as the hydrocarbon volatilisation rate during pyrolysis as it can influence the splitting of the C⚌C bonds (Murata et al., 2004). Normally, variations of initial pressure during pyrolysis of plastic at high temperature can also increase product yield (Zhang et al., 2018a,b,c).
2.3 Synergistic effect between plastic and biomass wastes on oil yield and composition during co-pyrolysis
Pyrolysis of combined biomass and plastic feedstock through co-pyrolysis technology may have an unanticipated influence on the composition and distribution of the resultant liquid products. Thus, synergistic interaction is one of the most critical aspects in co-pyrolysis studies. Since plastic and biomass wastes have distinct degradation patterns due to differences in composition, it is imperative to establish whether the two raw materials interact during co-pyrolysis. Some researchers have observed positive synergetic effect during co-pyrolysis of biomass and plastic waste that increased the quality of bio-oil by reducing oxygenated compounds and increasing the alcohol content. The term of ‘synergistic effect’ has been commonly used to describe how thermal co-pyrolysis improves the yield of bio-oil, not the yield of non-polar fraction of bio-oil.
Some interesting effects and benefits of co-pyrolysis of plastic and biomass based on the outcome of several investigations using different pyrolysis systems are presented in Table 4. The studies showed that due to the interaction between plastic and biomass, the co-pyrolysis not only had a promoting effect on the production and distribution of the products, but also influenced a great change in properties. Supramono et al. (2020) and Nguyen et al., (2019) achieved 70% and 47% reduction in oxygenated compounds in the oil produced during the catalytic co-pyrolysis of plastic and biomass. These indicates that co-pyrolysis demonstrated strong deoxidation effect on its derived oil, in comparison to biomass derived oil. This is expected to improve the instability and corrosion of oil obtained from the pyrolysis of these biomasses pyrolysis alone when they are used as liquid fuel. Maximum yield of alcohols and hydrocarbons reached 85.88% Oxygen content reduction from 2.4 to 5.4% 83% MAH content was obtained. Highest oil yield up to 32% was obtained lesser oxygenated compounds obtained 70% Non-oxygenate composition 93% aliphatic hydrocarbons 4.74% increase in phenols 32.1% increase in hydrocarbons 45.7% increase in hydrocarbons 63.1% increase in hydrocarbons 29.3% decrease in nitrogen content
PS bio-oil decreased by 30.4% More aromatic compounds Enhancement of alkane (22.3%) Enhancement of alkene (64.0%). Activation energy was lowered Aliphatic hydrocarbon and oxygenated products repressed No impact on product yield More stable alcohols and esters CO2. gas generation suppressed Stable alcohols formed 47.1% reduction in oxygen content
Co-reactant
Plastic
Plastic content %
Pyrolysis system and applied catalyst
Observed effect in biomass oil
Reference
Waste paper
HDPE
50.0
Fluidized bed
(Chen et al., 2017a,b)
Corn Stover
PS
75.0
Fixed bed, HZSM-5
(Muneer et al., 2019)
Juliflora
LPDE
66.6
Auger reactor
(Somasundaram and k, 2019)
Corn cobs
PP
87.0
Stirred tank
(Supramono et al., 2020)
Frying oil
Plastic waste
66.6
Microwave
(Adibah et al., 2018a,b))
Macro algae
PVC
25.0
Fixed bed
(Cao et al., 2019)
Macroalgae
HDPE
50.0
Fixed-bedFixed-bed, HZSM-5
(Xu et al., 2020)
Micro algae
PVC
90.0
Microwave
(Dai et al., 2018)
Lignin
PP
66.6
Microwave, ZSM-5.
47.8% cycloalkanes obtained
(Duan et al., 2017)
Grape seeds
PS
40.0
Fixed bed
(Veses et al., 2019)
Cellulose
HDPE
75.0
TG-MS, Py-GC/MS
(Yuan et al., 2018)
Paulownia wood
PP, PVC, PET
TGA
(Chen et al., 2017a,b)
Corn stalk
HDPE
TGA-FTIR and MS.
(Kai et al., 2019)
Potato blend
HDPE
TGA.
(Xiong et al., 2015)
Paper rejects
Cable plastics
50.0
GC/MS, ZSM-5
(Johansson et al., 2018)
Xylan
HDPE
75.0
TG-FTIR-MS.
(Gu et al., 2020)
Sugarcane bagasse
HDPE
75.0
Fixed-bed.
(Hassan et al., 2020)
Pine sawdust
PS
75.0
Fluidised.
(Nguyen et al., 2019)
Muneer et al., (2019) found that co-pyrolysis of polystyrene (PS) and Corn Stover (CS) led to increase in oil yield from 47 to 70 wt% as the PS content in the blend increased. This is due to the fact that PS is rich in volatile matter and has negligible fixed carbon as compared to CS. The addition of HZM-5 catalyst increased the selectivity of benzene, toluene and xylene with selectivity of these compounds reaching maximum at 39.6, 33.4 and 7.7% respectively.
Duan et al., (2017), also observed that HZM-5 catalysed co-pyrolysis of PP and lignin increased the formation of cycloalkanes with maximum yield of 47.8% obtained under microwave heating. This value is higher than the result reported for a military jet JP-8 (POSF 5699) fuel which contains about 25–30% (Dryer et al., 2014). Oil derived from biomass alone has not been considered as a suitable alternative to jet turbine fuels partly due to the lack of the aromatic and cycloalkane hydrocarbons in bio-oils. These compounds help to improve the combustion characteristics and material compatibility of traditional jet fuels.
Chen et al. (2017a,b) found that co-pyrolysis of wastepaper with HDPE at feed ratio of 50:50 wt% and 600 °C gave a maximum sum yield of alcohols and hydrocarbons of 85.88%. This result indicates that co-pyrolysis is a promising upstream process which can be used to improve the quality of waste paper derived oil without any catalyzer.
The co-pyrolysis of PVC with biomass is rarely investigated in the literature which is due to the chlorine content of the liquids obtained in pyrolysis of PVC containing plastic wastes. However, (Dai et al., 2018) investigated the microwave-assisted co-pyrolysis of micro algae and PVC and found very interesting results. The increase in PVC proportion in the feed increased the aromatic (29.58% to 92.71%) and total hydrocarbon (51.5% to 99.4%) content of the microalgae derived oil which are desirable fractions for fuel applications. In addition, the nitrogen content significantly decreased from 29.7% to 0.38%, which indicates that the addition of PVC would enhance the ignition characteristic of oil. Researchers have asserted that positive synergistic effects exists during the pyrolysis of biomass and plastic waste when the empirical value is higher than the computed values (Chen et al., 2016, 2017a,b; Hassan et al., 2020; Yuan et al., 2018).
Researchers have also investigated the synergistic effects of different metals and catalysts on the co-pyrolysis of plastic and biomass. Lin et al. (2019a,b), Park et al. (2019a,b), and Xu et al. (2020) studied the co-pyrolysis of cellulose/HDPE using TGA and Py-GC/MS. They discovered that potassium can inhibit the synergistic effect between cellulose and HDPE, by increasing the yields of furans and ketones. A contrary outcome was found in the co-pyrolysis of sugarcane bagasse and polystyrene in the presence of HZSM-5 and MgO/CaO catalysts. These catalysts promoted the formation of aromatic hydrocarbons and suppressed poly-aromatic hydrocarbon (PAH) and oxygenates in liquid yield (Iftikhar et al., 2019).
3 Kinetic analyses and reaction pathways in co-pyrolysis of biomass with plastic wastes
Kinetic analysis is employed to ascertain the rate laws of pyrolysis reactions and to support explanation of the reaction path. Reaction rate of pyrolysis reactions is a vital requirement for the development of efficient and flexible pilot-scale pyrolysis reactors. Several studies (Alam et al., 2020; Kai et al., 2019; Mishra et al., 2019; Özsin & Eren, 2019; Song et al., 2020; Wu et al., 2020b; Xu et al., 2017) conducted kinetic analyses in order to discern the synergy during the co-pyrolysis of biomass and plastic wastes. A summary of these kinetic models is presented in Table 5. In most studies, activation energy is calculated using the Kissinger-Akahira-Sunose (KAS), Ozawa-Flynn-Wall (OFW), and Coats-Redfern (CR) models. Newer models, such as Friedman method (FM), Starink (ST), and Distributed activation energy model (DAEM) (Burra and Gupta, 2018; Xu et al., 2020) have recently been used. During degradation, conversion or weight loss rate,
is assumed to have a constant value. It views the CCP as one overall reaction. It is illogical to consider the overall CCP of plastic and biomass mixtures as a one-step reaction. This model simply requires three sets of experimental kinetic data that can be obtained at different heating rates. ST is a revision of the FWO and KAS based on analyses of their approximation errors. Degradation is assumed to be influenced by the rate of mass loss and not by temperature.
Model
Plotting method
Remark
Reference
F1
Degradation is assumed to happen uniformly through the volume of the particles of the biomass/plastic mixture.
(El-Sayed and Mostafa, 2020)
Vy
Nil
It is not valid at high conversions when mass transfer is reduced
(Vyazovkin, 1997) (Senum and Yang, 1977) (Özsin and Pütün, 2017)
FWO
.
Degradation process and temperature are assumed to be the main factors that determine the rate of reaction at any given degree of conversion.
(Flynn and Wall, 1966) (Bianchi et al., 2008)
KAS
(Kissinger, 1956); (Abdelouahed et al., 2017); (Mong et al., 2019)
CR
(Coats and Redfern, 1964) (Yorulmaz and Atimtay, 2009)
Miura-Maki
(Miura Kouichi, 1998)
ST
(Starink, 1996)
FR
(Friedman, 2007)
DEAM
Degradation process is assumed to involve unlimited parallel reactions, having a distinct value of activation energy.
(Braun and Burnham, 1987)
Several researchers have suggested the use of isoconversional models (El-Sayed and Mostafa, 2020) because this method allows the decomposition rate of a sample to be calculated using its latest temperature (Samuelsson et al., 2015). Nonetheless, the first-order reaction model is still being used by other researchers. According to Guida et al., (2017), the use of first-order reaction models to describe pyrolysis reaction has become almost customary, and has been universally accepted by researchers with no painstaking authentication or enough understanding of their drawbacks. It is important to note that these isoconversional models are multistep models involving a higher number of fitting parameters that may lead to over-fitting of data (Yeo et al., 2019). Furthermore, some of these models could result in wrong estimations of kinetic parameters due to the approximation methods used during derivation. Therefore, it would be better to use a method that has a perfectly linear relationship to describe the TGA data obtained during the CCP of plastic and biomass wastes (Guodong et al., 2018).
As previously noted, the chemical and physical structures of plastic and biomass wastes are extremely complicated (Okolie et al., 2019). Further complications can arise from mineral matters that have a wide range of species differing in reactivity. Consequently, plastic, biomass, and their blends would undergo decomposition in a complicated mechanism involving many parallel or competitive reactions. It might be imperative to look at a new method, known as the Distributed Activation Energy Model (DAEM), which can accurately estimate the volatile amount released from plastic and biomass wastes versus activation energy or time during CCP. In this way, the difference in reactivity of species may be defined by different activation energies, E.
Table 6 presents a summary of the kinetic parameters (activation energies, E and pre-exponential factors, A), that have been obtained during degradation and reactions involved in the pyrolysis and co-pyrolysis of plastic and biomass wastes. Some variations can be seen from the kinetic parameters obtained, which are presented. Although the heating rates could be responsible for these variations (Hoekstra et al., 2012), some fundamental reasons are responsible for this, Firstly, the variations derive from the diverse chemical composition of the plastic and biomass feedstock used in the investigation. Secondly, the sample size and weight of samples within the crucibles used in the thermogravimetric analyser are not the same (Schulze et al., 2017). The Influence of size and weight of samples particles is necessary in order to minimize heat transfer problem. N/A: Not Available, * FWO, KAS, FR, DEAM, Vy, applied; ** KAS, FR, FWO applied.
Plastic type
Co-feed
Model
Catalyst
Feed mass (mg)
Heating rate °C/min
Kinetic parameters
Reference
R2
A (min−1)
E (kJ/mol)
PE
Lignin
F1
Metal
10
10–40
0.9920
1.93 × 10−1
14.08
(Wang et al., 2020)
PP
Cellulose
CR
MCM-41
5
−263.15
N/A
5.0 × 109
115
(Chi et al., 2018)
LDPE
Bamboo sawdust
KAS
N/A
10
5–20
0.982–0.990
–
143–255
(Alam et al., 2020)
LDPE
Cellulose
CR
ZSM-5
5000
20
0.95
6.73 × 105
89.51
(Zhang et al., 2016a,b)
LDPE
Douglas fir sawdust
5000
0.99
5.19 × 103
54.51
Cellulose
FWO
HZSM-5
5–10
10
0.9996
N/A
168.81
(Zheng et al., 2018)
LDPE
Yunnan pine
5–10
0.9984
N/A
185.87
Rice straw
CR
ZSM-5
5
30
0.9685
4.12 × 102
70.58
(Xiang et al., 2018)
PP
Pinewood
DEAM
N/A
−263
N/A
N/A
115.2
(Burra and Gupta, 2018)
PETE
N/A
N/A
104.1
HDPE
Sea weeds
*
HZSM-5
10
10–40
0.9973–0.9994
N/A
108.75–109.09
(Xu et al., 2020)
HDPE
Cornstover
CR
HZSM-5
5
10
0.9817
311.88
96.24
(Wang et al., 2018)
PS
Walnut shell
**
N/A
10
10
0.9947–0.9959
5.03 × 1015
200.2–202.9
(Özsin and Pütün, 2018)
PET
Samanea Saman Seeds
FM
N/A
9
10
0.92–1.0
3.36 × 107-4.93 × 1014
129.51–138.56
(Mishra et al., 2019)
Paulsen et al., (2013) identified the conditions under which cellulose particles can be isothermal during pyrolysis, and the process kinetically limited. The author stated that the particle Biot number must be less than 10-1 and the temperature less than 550 °C. All the test presented in Table 6 used the particle mass that is in the range of 9–5000 mg which does not equate to a particle dimension of less than 10 μm. Also, the temperature range were more than 550 °C which makes it subject to the vagaries of experimental conditions, making any comparisons between the empirical data relatively useless. Therefore, any commercial application would need to simulate the laboratory conditions. Moreover, specific data for feed sizes of different plastic and biomass types to be used in a pyrolysis system is missing from literature.
Catalyst also has a major role to play in the activation energies, E and pre-exponential factors, A, obtained in the co-pyrolysis of plastic and biomass. Several works have investigated how synergistic effect between biomass and plastic lowers the activation energy. However, only few works have further analyzed the vital influence of catalyst in decreasing activation energy during co-pyrolysis of plastic and biomass. For instance, Zhang et al. (2016a,b) conducted a kinetics study on catalytic co-pyrolysis of LDPE and cellulose. It was discovered that E value of LDPE reduced from 213.78 to 134.59 kJ/mol, when cellulose was added to the feed. This indicates that there was a positive synergistic effect between cellulose and LDPE. In the presence of ZSM-5 catalyst, the activation energy (E) decreased to 89.51 kJ/mol. The kinetic analysis confirms the fact that catalyst can significantly reduce energy input. Similar trend were was observed in the work of (Wang et al., 2018) in which HZSM-5 catalyst also caused a decreasing trend for E and A values during the catalytic co-pyrolysis of corn-stover and HDPE. The apparent activation energy (E) of HDPE reduced from 252.52 to 96.24 kJ/mol, when corn stover was added to the feed. The A values also reduced from 252.52 to 96.24 s−1.
The co-pyrolysis of PS-Walnut shell (Özsin and Pütün, 2018) and PET-Samanea seed (Mishra et al., 2019) mixtures had a pre-exponential factor of 5.03 × 1015 and 4.93 × 1014 s−1 respectively. This values of A is more than 1014 s−1 and this indicates a slower and more difficult degradation effect and indicate the necessity of higher molecular collision (Vuppaladadiyam et al., 2019). In such case, the reaction demands more energy or a catalyst and this scenario is in agreement with the activation energy characteristics. The values of pre-exponential factor A for most catalytic co-pyrolysis of plastic and biomass was less than 109 s−1 which indicates surface reaction involving catalyst. The pre-exponential factor (A) of a co-pyrolysis reaction can vary in a wide range from 1010 to 1020 depending on plastic/biomass type and blending ratio of the plastic and biomass in the feed. This reflects the complex nature of plastic and biomass and of the reactions that occur during the process of co-pyrolysis.
4 Reaction mechanism for the co-pyrolysis of plastic and biomass
The thermal pathway of the co-pyrolysis of plastic-biomass mixture, in the absence of catalyst, has mostly been explained as free radical degradation. However, in the presence of catalyst, the reaction pathway is often described using ionic mechanism (Miskolczi and Nagy, 2012). During a catalytic pyrolysis, thermal and catalytic cracking occur concurrently, and follow different reaction pathways.
In thermal cracking, the pathway involves arbitrary chain scission, chain-end scissions or removal of pendant groups. Meanwhile, a catalytic cracking pathway would lead to the absorption of carbenium ions onto the catalyst substance, followed by beta scission and desorption. Consequently, a number of product variants are formed, which would sequentially combine with each other, resulting in numerous potential reaction pathways (Lopez-Urionabarrenechea et al., 2012). According to the literature, hydroxyl groups are produced during the pyrolysis of cellulose, and they react with vinyl groups produced by polyethylene bond cleavage to obtain alcohols (Zhao et al., 2020). A potential reaction pathway for the formation of alcohols and linear hydrocarbons has been suggested in Fig. 5.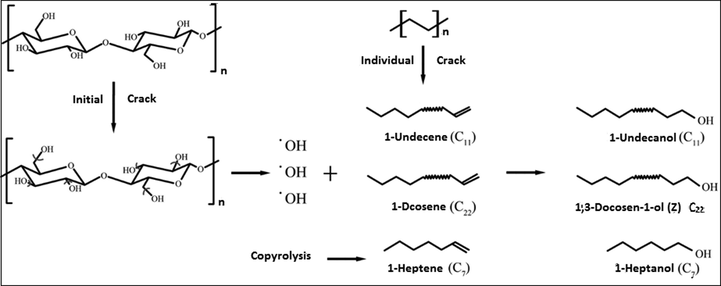
Mechanism of the possible interaction between plastic and biomass for production of liquid fuel rich in long chain alcohols functional group (Chen et al., 2017a,b).
Similarly, furan and its end-products will combine with unsaturated hydrocarbons (generated from polyethylene) via Diels-Alder reaction, and dehydration reactions to form aromatic hydrocarbons (Gu et al., 2020). The alkanes, alkenes, and alkynes released from the degradation of polyethylene supply hydrogen for the oxygenates produced from cellulose, thus, decreasing the amount of coke produced (Xue et al., 2017). A comprehensive formation mechanism of deoxygenation aromatisation for the catalytic co-pyrolysis of walnut shell and LDPE has been proposed, as shown in Fig. 6.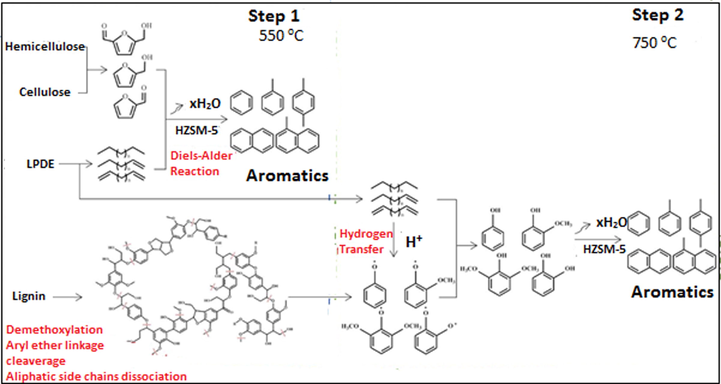
Mechanism of plastic/biomass mixture pyrolysis for the production of liquid fuel enriched with aromatic compounds (Yu et al., 2019).
Thermal degradation of olefins (unsaturated hydrocarbons) will initiate random chain scission, followed by the generation of loose radicals that produce chain and cracking reactions, thus, forming wide-ranging liquid hydrocarbons that contain naphtha and diesel (Donaj et al., 2012). Several factors could impact this process and the most prominent ones are holding time, pyrolysis temperature, and pyrolysis agents. An overview of the suggested mechanism reaction for the co-pyrolysis of grape seeds and polystyrene is presented in Fig. 7.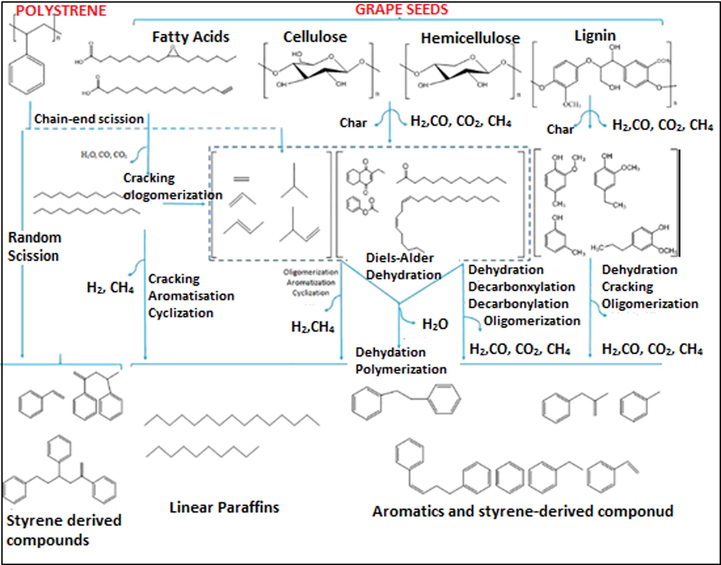
Simplified reaction mechanism proposed for the co-pyrolysis of Grape seeds and PS (Veses et al., 2019).
5 Future direction and challenges
The quality of pyrolytic bio-oil produced from biomass alone may not be sustainable since the liquid product often contains high quantity of oxygenated compounds, which reduce the calorific value of the oil. Research is now geared towards producing high quality fuel from other sources, such as plastics. The simultaneous use of both plastic and lignocellulosic biomass wastes for this goal using the CCP process is showing great promise. However, there are still some fundamental challenges that have impeded its development into a commercialised process.
The design and development of catalysts for the CCP of plastic and biomass wastes to produce high-grade fuel have continued to be a significant challenge in the field of environmental engineering and renewable energy. Some studies have recommended that co-pyrolysis of plastic mixtures (PET, PS, and PVC) with biomass as a co-feed in the presence of functionalized catalyst could improve the quality of the liquid products. The foremost reason for the utilisation of plastic mixtures lies in the enhancement of CCP process, with the objective of scaling up the management of plastics in municipal solid wastes (MSW).
There is also a dire need to develop low-cost catalysts with functionalised groups that can guarantee high sensitivity and selectivity to achieve the desired quality of pyrolytic oil produced from the CCP of plastic and biomass wastes. Currently, the functionalization of catalysts is at the frontier of materials sciences. Such research will promote the development of innovative, low cost, and highly capable catalysts.
There is one notable attribute of functionalised catalyst, namely, their enhanced recyclability, which makes it possible for reutilisation after numerous CCP cycles. Some studies have suggested that functionalised catalyst could be achieved by introducing metallic oxides into the zeolite surface through chemical routes. Others have reported that this can also be realised by impregnating catalyst with transition metals (Ni, Co, Fe, or Mn). Apart from modifying catalyst with either metal or metallic oxides, changing its physical structure through dealumination techniques (heat, chemical agents, etc.) can improve the conversion of plastic and biomass wastes to produce high-grade fuel during CCP. Future research should carefully choose the functionalization route based on the properties of the plastic-biomass feedstock and the developed catalyst, as well as the operating conditions of the CCP process.
6 Conclusion
This review has focused on the catalytic co-pyrolysis of plastic and lignocellulosic biomass wastes to produce liquid fuel. The exploitation of biomass waste as co-feed in the co-pyrolysis of plastic waste will positively influence the quality and quantity of liquid fuel due to the synergetic interaction between the feedstock. This emerging catalytic co-pyrolysis technique could make thermo-chemical transformation of plastic and biomass wastes into liquid fuel a more viable process in the future. Catalyst developed from industrial and agricultural wastes could also enhance the yield and selectivity of desirable hydrocarbons with commercial values. Undoubtedly, catalytic co-pyrolysis has ushered in a new era for future explorations because of its capacity and potential to produce liquid fuel, as well as eliminate mismanaged plastic and biomass wastes in the environment. As this process gradually gains momentum in all respect, it is expected to sustainably produce an alternative for fossil fuel.
Acknowledgment
The authors thankfully acknowledge the support obtained from Lotte Chemical Titan (M) Sdn. Bhd. and Universiti Sains Malaysia (Grant Number: 304/PJKIMIA/6050422/L128), in the form of research grant and facilities which brought forth this article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Comparative investigation for the determination of kinetic parameters for biomass pyrolysis by thermogravimetric analysis. J. Therm. Anal. Calorim.. 2017;129:1201-1213.
- [Google Scholar]
- Co-pyrolysis of palm shell and polystyrene waste mixtures to synthesis liquid fuel. Fuel. 2013;108:311-318.
- [Google Scholar]
- Microwave co-pyrolysis of waste polyolefins and waste cooking oil: Influence of N2 atmosphere versus vacuum environment. Energy Convers. Manage.. 2018;171:1292-1301.
- [Google Scholar]
- Production of value-added liquid fuel via microwave co-pyrolysis of used frying oil and plastic waste. Energy. 2018;162:309-317.
- [Google Scholar]
- Pyrolysis of plastic packaging waste: A comparison of plastic residuals from material recovery facilities with simulated plastic waste. Waste Manage.. 2012;32:826-832.
- [Google Scholar]
- A review on advanced catalytic co-pyrolysis of biomass and hydrogen-rich feedstock: Insights into synergistic effect, catalyst development and reaction mechanism. Bioresour. Technol.. 2020;310:123457
- [Google Scholar]
- Co-pyrolysis of bamboo sawdust and plastic : Synergistic effects and kinetics. Renew. Energy. 2020;149:1133-1145.
- [Google Scholar]
- A review on pyrolysis of plastic wastes. Energy Convers. Manage.. 2016;115:308-326.
- [Google Scholar]
- Plastic waste as a challenge for sustainable development and circularity in the European Union. Ekon. i Prawo. 2020;19:7.
- [CrossRef] [Google Scholar]
- Bengali, S., 2018. How mountains of U.S. plastic waste ended up in Malaysia, broken down by workers for $10 a day - Los Angeles Times [WWW Document]. URL https://www.latimes.com/world/asia/la-fg-malaysia-plastic-2018-story.html (accessed 4.21.20).
- Assessment of Avrami, Ozawa and Avrami-Ozawa equations for determination of EVA crosslinking kinetics from DSC measurements. Polym. Test.. 2008;27:722-729.
- [Google Scholar]
- Pyrolysis of waste plastic and use of plastic oil as an alternate fuel in diesel engine. Int. J. Eng. Res. Technol.. 2020;9:245-254.
- [Google Scholar]
- Borin, D., Sbaizero, O., Scuor, N., 2019. Application of a dielectric barrier discharge plasma for heating plastic materials. Plasma Res. Express 1.
- Borrelle, S.B., Law, K.L., 2020. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. https://doi.org/10.1126/science.aba3656
- Analysis of chemical reaction kinetics using a distribution of activation energies and simpler models. Energy nd Fuels. 1987;1:153-161.
- [Google Scholar]
- Fast pyrolysis of biomass and waste plastic in a fluidized bed reactor Fast pyrolysis of biomass and waste plastic in a fluidized bed reactor. Fuel. 2015;156:40-46.
- [Google Scholar]
- Microwave-assisted co-pyrolysis of microwave torrefied biomass with waste plastics using ZSM-5 as a catalyst for high quality bio-oil. J. Anal. Appl. Pyrolysis. 2018;134:536-543.
- [Google Scholar]
- Catalytic pyrolysis of plastic waste for the production of liquid fuels for engines. RSC Adv.. 2019;9:5844-5857.
- [Google Scholar]
- Kinetics of synergistic effects in co-pyrolysis of biomass with plastic wastes. Appl. Energy. 2018;220:408-418.
- [Google Scholar]
- Synergistic effects of co-pyrolysis of macroalgae and polyvinyl chloride on bio-oil/bio-char properties and transferring regularity of chlorine. Fuel. 2019;246:319-329.
- [Google Scholar]
- Effect of PET functionalization in composites of rubber–PET–HDPE type. Arab. J. Chem.. 2017;10:300-312.
- [Google Scholar]
- Synergistic effect on thermal behavior and char morphology analysis during co-pyrolysis of paulownia wood blended with different plastics waste. Appl. Therm. Eng.. 2017;111:834-846.
- [Google Scholar]
- Chen, W., Lu, J., Zhang, C., Xie, Y., Wang, Y., Wang, J., Zhang, R., 2020. Aromatic hydrocarbons production and synergistic effect of plastics and biomass via one-pot catalytic co-hydropyrolysis on HZSM-5. J. Anal. Appl. Pyrolysis 104800.
- Fast co-pyrolysis of waste newspaper with high-density polyethylene for high yields of alcohols and hydrocarbons. Waste Manage.. 2017;67:155-162.
- [Google Scholar]
- Co-pyrolysis of waste newspaper with high-density polyethylene: Synergistic effect and oil characterization. Energy Convers. Manage.. 2016;112:41-48.
- [Google Scholar]
- Catalytic co-pyrolysis of cellulose and polypropylene over all-silica mesoporous catalyst MCM-41 and Al-MCM-41. Sci. Total Environ.. 2018;633:1105-1113.
- [Google Scholar]
- Study of the experimental conditions of the co-pyrolysis of rice husk and plastic wastes. Chem. Eng. Trans. 2014;39:1639-1644.
- [Google Scholar]
- Microwave-assisted fast co-pyrolysis behaviors and products between microalgae and polyvinyl chloride. Appl. Therm. Eng.. 2018;136:9-15.
- [Google Scholar]
- Dominguez, A., Menendez, J.A., Fernandez, Y., Pis, J.J., Valente J.M., Carrott, P.J.M., Carrott, M.R., 2007. Conventional and microwave induced pyrolysis of coffee hulls for the production of a hydrogen rich gas. J. Anal. Appl. Pyrolysis 79, 128–135.
- Pyrolysis of polyolefins for increasing the yield of monomers’ recovery. Waste Manage.. 2012;32:840-846.
- [Google Scholar]
- Emulating the combustion behavior of real jet aviation fuels by surrogate mixtures of hydrocarbon fluid blends: implications for science and engineering. Energy and Fuels. 2014;28:3474-3485.
- [Google Scholar]
- Bioresource technology ex-situ catalytic co-pyrolysis of lignin and polypropylene to upgrade bio-oil quality by microwave heating. Bioresour. Technol.. 2017;241:207-213.
- [CrossRef] [Google Scholar]
- Dublin, 2020. Global Plastic Recycling Market Size, Market Share, Application Analysis, Regional Outlook, Growth Trends, Key Players, Competitive Strategies and Forecasts, 2019 To 2027 [WWW Document]. GLOBE NEWSWIRE.
- Emeka, Dumbili, Henderson, L., 2020. The challenge of plastic pollution in Nigeria, in: Plastic Waste and Recycling Environmental Impact, Societal Issues, Prevention, and Solutions. Academic Press, pp. 569–583.
- Microwaves in heterogeneous gas-phase catalysis: Experimental and numerical approaches. Chem. Eng. Technol.. 2009;32:1301-1312.
- [Google Scholar]
- Thermal pyrolysis and kinetic parameter determination of mango leaves using common and new proposed parallel kinetic models. RSC Adv.. 2020;10:18160-18179.
- [Google Scholar]
- Thermo-catalytic co-pyrolysis of waste plastic and paper in batch and tubular reactors for in-situ product improvement. J. Environ. Manage.. 2020;269:110741
- [Google Scholar]
- Fernandez, Y., Arenillas, A., Angel, J., 2011. Microwave Heating Applied to Pyrolysis. Adv. Induction Microw. Heat. Miner. Org. Mater.
- Study of the co-pyrolysis of biomass and plastic wastes. Clean Technol. Environ. Policy 2009:115-122.
- [Google Scholar]
- General treatment of the thermogravimetry of polymers. J. Res. Natl. Bur Stand A Phys. Chem.. 1966;70:487-523.
- [Google Scholar]
- Kinetics of thermal degradation of char-forming plastics from thermogravimetry. application to phenolic plastic. J. Polym. Sci. Part C. Polym. Symp.. 2007;6:183-195.
- [Google Scholar]
- RF-ICP thermal plasma for thermoplastic waste pyrolysis process with high conversion yield and tar elimination. Processes. 2020;8:281
- [CrossRef] [Google Scholar]
- DC thermal plasma design and utilization for the low density polyethylene to diesel oil pyrolysis reaction. Energies. 2017;10
- [Google Scholar]
- Goodman, S., 2014. The Microwave Induced Pyrolysis of Problematic Plastics Enabling Recovery and Component Reuse. Civ. Environ. Eng. Imp. Coll. London, UK.
- GreenPeace International, 2020. ‘The Story of Plastic’ is an eye-opener on the global plastic pollution crisis - Greenpeace International [WWW Document]. URL https://www.greenpeace.org/international/story/30102/the-story-of-plastic-is-an-eye-opener-on-the-global-plastic-pollution-crisis/ (accessed 4.22.20).
- Production, use, and fate of all plastics ever made. Science Advances. 2017;3(7):25-29.
- [CrossRef] [Google Scholar]
- Co-pyrolysis of xylan and high-density polyethylene: Product distribution and synergistic effects. Fuel. 2020;267
- [Google Scholar]
- Journal of Analytical and Applied Pyrolysis Operating parameters for bio-oil production in biomass pyrolysis : A review. J. Anal. Appl. Pyrolysis. 2018;129:134-149.
- [Google Scholar]
- Guida, M., Bouaik, H., El Mouden, L., Moubarik, A., Aboulkas, A., El harfi, K., Hannioui, A., 2017. Utilization of Starink Approach and Avrami Theory to Evaluate the Kinetic Parameters of the Pyrolysis of Olive Mill Solid Waste and Olive Mill Wastewater. J. Adv. Chem. Eng. 07.
- Plastic catalytic pyrolysis to fuels as tertiary polymer recycling method : Effect of process conditions. J. Environ. Sci. Heal.. 2010;45:908-915.
- [Google Scholar]
- Jiang, Guodong, Wei, Liping, 2018. Phase change materials and their applications. In: Analysis of Pyrolysis Kinetic Model for Processing of Thermogravimetric Analysis Data. Mohsen Mhadhbi, IntechOpen.
- Hassan, H., Hameed, B.H., Lim, J.K., 2020. Co-pyrolysis of sugarcane bagasse and waste high-density polyethylene: Synergistic effect and product distributions. Energy 191.
- Catalytic co-pyrolysis of sugarcane bagasse and waste high-density polyethylene over faujasite-type zeolite. Bioresour. Technol.. 2019;284:406-414.
- [Google Scholar]
- Future strategies in plastic waste management in Sri Lanka. J. Res. Technol. Eng. 2020:62-71.
- [Google Scholar]
- Fast pyrolysis in a novel wire-mesh reactor: Decomposition of pine wood and model compounds. Chem. Eng. J.. 2012;187:172-184.
- [Google Scholar]
- Production of liquid fuel from co-pyrolysis of polythene waste and rice straw. Energy Procedia. Elsevier Ltd 2019:116-122.
- [Google Scholar]
- Iftikhar, H., Zeeshan, M., Iqbal, S., Muneer, B., Razzaq, M., 2019. Co-pyrolysis of sugarcane bagasse and polystyrene with ex-situ catalytic bed of metal oxides/HZSM-5 with focus on liquid yield. Bioresour. Technol. 289.
- Preparation and properties of novel flame-retardant PBS wood-plastic composites. Arab. J. Chem.. 2018;11:844-857.
- [Google Scholar]
- Solar Pyrolysis: Converting Waste Into Asset Using Solar Energy. In: Clean Energy for Sustainable Development Comparisons and Contrasts of New Approaches. Academic Press; 2017. p. :213-235.
- [Google Scholar]
- Journal of Analytical and Applied Pyrolysis Co-pyrolysis of woody biomass and plastic waste in both analytical and pilot scale. J. Anal. Appl. Pyrolysis. 2018;134:102-113.
- [Google Scholar]
- TG-FTIR-MS study of synergistic e ff ects during co-pyrolysis of corn stalk and high-density polyethylene (HDPE) Energy Convers. Manag.. 2019;181:202-213.
- [Google Scholar]
- Study of the effects of regeneration of USY zeolite on the catalytic cracking of polyethylene. Appl. Catal. B Environ.. 2019;244:704-708.
- [Google Scholar]
- Khaghanikavkani, E., 2013. Microwave Pyrolysis of Plastic. J. Chem. Eng. Process Technol. 04.
- Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stondards. 1956;57:60.
- [Google Scholar]
- Mixing and operability characteristics of mechanically fluidized reactors for the pyrolysis of biomass. Powder Technol.. 2015;274:205-212.
- [Google Scholar]
- Enhancement of bio-oil yield and selectivity and kinetic study of catalytic pyrolysis of rice straw over transition metal modified ZSM-5 catalyst. J. Anal. Appl. Pyrolysis. 2017;128:324-334.
- [Google Scholar]
- Effects of alkali and alkaline earth metals on the co-pyrolysis of cellulose and high density polyethylene using TGA and Py-GC/MS. Fuel Process. Technol.. 2019;191:71-78.
- [Google Scholar]
- Enhancing jet fuel range hydrocarbons production from catalytic co-pyrolysis of Douglas fir and low-density polyethylene over bifunctional activated carbon catalysts. Energy Convers. Manag.. 2020;211:112757
- [Google Scholar]
- Evaluation of zeolite catalysts on product distribution and synergy during wood-plastic composite catalytic pyrolysis. Energy. 2019;189
- [Google Scholar]
- Catalytic stepwise pyrolysis of packaging plastic waste. J. Anal. Appl. Pyrolysis. 2012;96:54-62.
- [Google Scholar]
- Pyrolysis of municipal plastic wastes: Influence of raw material composition. Waste Manag.. 2010;30:620-627.
- [Google Scholar]
- López, A., Marco, I. De, Caballero, B.M., Laresgoiti, M.F., Adrados, A., Aranzabal, A., 2011. Applied Catalysis B : Environmental Catalytic pyrolysis of plastic wastes with two different types of catalysts : ZSM-5 zeolite and Red Mud 104, 211–219.
- In-situ and ex-situ upgrading of pyrolysis vapors from beetle-killed trees. Fuel. 2016;166:367-375.
- [Google Scholar]
- Conventional and microwave-assisted pyrolysis of gumwood: A comparison study using thermodynamic evaluation and hydrogen production. Fuel Process. Technol.. 2019;184:1-11.
- [Google Scholar]
- Marks, D., 2019. Southeast Asia’s plastic waste problem | East Asia Forum [WWW Document]. URL https://www.eastasiaforum.org/2019/06/26/southeast-asias-plastic-waste-problem/ (accessed 4.21.20).
- Mateo, W., Lei, H., Villota, E., Qian, M., Zhao, Y., Huo, E., Zhang, Q., Lin, X., Wang, C., Huang, Z., 2020. Synthesis and characterization of sulfonated activated carbon as a catalyst for bio-jet fuel production from biomass and waste plastics. Bioresour. Technol. 297.
- Plastic waste to liquid oil through catalytic pyrolysis using natural and synthetic zeolite catalysts. Waste Manag.. 2017;69:66-78.
- [Google Scholar]
- Influence of temperature and reaction time on the conversion of polystyrene waste to pyrolysis liquid oil. Waste Manag.. 2016;58:250-259.
- [Google Scholar]
- Pyrolysis kinetics and synergistic effect in co-pyrolysis of Samanea saman seeds and polyethylene terephthalate using thermogravimetric analyser. Bioresour. Technol.. 2019;289:121608
- [Google Scholar]
- Hydrocarbons obtained by waste plastic pyrolysis: Comparative analysis of decomposition described by different kinetic models. Fuel Process. Technol.. 2012;104:96-104.
- [Google Scholar]
- A simple method for estimating f(E) and k0(E) in the distributed activation energy model. Energy Fuels. 1998;12:864-869.
- [Google Scholar]
- Kinetic study of horse manure through thermogravimetric analysis. Chem. Eng. Trans.. 2019;72:241-246.
- [Google Scholar]
- Influence of in-situ and ex-situ HZSM-5 catalyst on co-pyrolysis of corn stalk and polystyrene with a focus on liquid yield and quality. J. Clean. Prod.. 2019;237:117762
- [Google Scholar]
- Effect of pressure on thermal degradation of polyethylene. J. Anal. Appl. Pyrolysis. 2004
- [CrossRef] [Google Scholar]
- Effect of Kaolin as a catalyst on the yield of catalytic pyrolysis of waste plastic effect of Kaolin as a catalyst on the yield of catalytic pyrolysis of waste plastic mixtures. UofKEJ. 2019;8:17-24.
- [Google Scholar]
- Drivers to sustainable plastic solid waste recycling: a review. Procedia Manuf.. 2017;8:649-656.
- [Google Scholar]
- New Straits Times, 2018. Alarm grows as M’sia becomes world’s top dump site for plastic waste [WWW Document]. URL https://www.nst.com.my/news/nation/2018/10/424840/alarm-grows-msia-becomes-worlds-top-dump-site-plastic-waste (accessed 4.21.20).
- Plasma Pyrolysis Technology and its Evolution at FCIPT (Report Number: IPR/TR-364/2016). India: Institute for Plasma Research; 2016.
- Improvement of bio-crude oil properties via co-pyrolysis of pine sawdust and waste polystyrene foam. J. Environ. Manage.. 2019;237:24-29.
- [Google Scholar]
- Optimization and modeling of process parameters during hydrothermal gasification of biomass model compounds to generate hydrogen-rich gas products. Int. J. Hydrogen Energy. 2019;45:18275-18288.
- [Google Scholar]
- Journal of Analytical and Applied Pyrolysis Conventional and microwave-assisted pyrolysis of rapeseed oil for bio-fuel production. J. Anal. Appl. Pyrolysis. 2014;105:131-142.
- [Google Scholar]
- TGA / MS / FT-IR study for kinetic evaluation and evolved gas analysis of a biomass / PVC co-pyrolysis process. Energy Convers. Manag.. 2019;182:143-153.
- [Google Scholar]
- A comparative study on co-pyrolysis of lignocellulosic biomass with polyethylene terephthalate, polystyrene, and polyvinyl chloride: Synergistic effects and product characteristics. J. Clean. Prod.. 2018;205:1127-1138.
- [Google Scholar]
- Insights into pyrolysis and co-pyrolysis of biomass and polystyrene: Thermochemical behaviors, kinetics and evolved gas analysis. Energy Convers. Manag.. 2017;149:675-685.
- [Google Scholar]
- Co-feeding effect of waste plastic films on the catalytic pyrolysis of Quercus variabilis over microporous HZSM-5 and HY catalysts. Chem. Eng. J.. 2019;378:122151
- [Google Scholar]
- Catalytic co-pyrolysis of yellow poplar wood and polyethylene terephthalate over two stage calcium oxide-ZSM-5. Appl. Energy. 2019;250:1706-1718.
- [Google Scholar]
- The role of sample dimension and temperature in cellulose pyrolysis. Energy Fuels. 2013;27:2126-2134.
- [Google Scholar]
- Effect of experimental conditions on co-pyrolysis of pre- treated eucalyptus blended with plastic wastes. Chem. Eng. Trans.. 2018;70:793-798.
- [Google Scholar]
- Novel helical screw-fluidized bed reactor for bio-oil production in slow-pyrolysis mode: A preliminary study. J. Anal. Appl. Pyrolysis. 2019;142:104605
- [Google Scholar]
- Effect of zeolite catalysts on pyrolysis liquid oil. Int. Biodeterior. Biodegrad.. 2017;119:162-175.
- [Google Scholar]
- Recent advances in catalytic co-pyrolysis of biomass and plastic waste for the production of petroleum-like hydrocarbons. Bioresour. Technol.. 2020;310:123473
- [Google Scholar]
- Salvia, L., Silvarrey, D., Phan, A.N., Zhang, K., 2019. Advanced pyrolysis of plastic waste for chemicals, fuel and materials.
- Model-free rate expression for thermal decomposition processes: The case of microcrystalline cellulose pyrolysis. Fuel. 2015;143:438-447.
- [Google Scholar]
- Sarpong, S., 2020. Counting the Cost: Malaysia’s Push-Back Begins over Overseas Waste Dumping. Society 57, 77–84.
- In fl uence of reaction pressure on co-pyrolysis of LDPE and a heavy petroleum fraction. Fuel Process. Technol.. 2019;193:204-211.
- [Google Scholar]
- Heat and mass transfer within thermogravimetric analyser: From simulation to improved estimation of kinetic data for char gasification. Fuel. 2017;187:338-348.
- [Google Scholar]
- Rational approximations of the integral of the Arrhenius function. J. Therm. Anal.. 1977;11:445-447.
- [Google Scholar]
- Shi, K., Yan, J., Menéndez, J.A., Luo, X., Yang, G., Chen, Y., 2020. Production of H2 -rich syngas from lignocellulosic biomass using microwave-assisted pyrolysis coupled with activated carbon enabled reforming. Front. Chem. 8.
- New policy framework with plastic waste control plan for effective plastic waste management. Sustain.. 2020;12
- [Google Scholar]
- Microwave pyrolysis valorization of used baby diaper. Chemosphere. 2019;230:294-302.
- [Google Scholar]
- Microwave vacuum pyrolysis of waste plastic and used cooking oil for simultaneous waste reduction and sustainable energy conversion : Recovery of cleaner liquid fuel and techno-economic analysis. Renew. Sustain. Energy Rev.. 2019;115:109359
- [Google Scholar]
- Somasundaram, M., Midhun Prasad, K., 2019. Co-pyrolysis of Juliflora biomass with low-density polyethylene for bio-oil synthesis. Energy Sources, Part A Recover. Util. Environ. Eff. 0, 1–16.
- Pyrolysis of municipal solid waste with iron-based additives: A study on the kinetic, product distribution and catalytic mechanisms. J. Clean. Prod.. 2020;258:120682
- [Google Scholar]
- A new method for the derivation of activation energies from experiments performed at constant heating rate. Thermochim. Acta. 1996;288:97-104.
- [Google Scholar]
- Synergistic effect on the non-oxygenated fraction of bio-oil in thermal co-pyrolysis of biomass and polypropylene at low heating rate. Processes. 2020;8
- [Google Scholar]
- Microwave assisted co-pyrolysis of biomasses with polypropylene and polystyrene for high quality bio-oil production. Fuel Process. Technol.. 2018;175:64-75.
- [Google Scholar]
- Bioresource Technology E ff ective deoxygenation for the production of liquid biofuels via microwave assisted co-pyrolysis of agro residues and waste plastics combined with catalytic upgradation. Bioresour. Technol.. 2020;302:122775
- [Google Scholar]
- A cleaner production of synthesis gas from glycerol using thermal water steam plasma. J. Clean. Prod.. 2016;130:187-194.
- [Google Scholar]
- Production of synthesis gas from propane using thermal water vapor plasma. Int. J. Hydrogen Energy. 2014;39:2078-2086.
- [Google Scholar]
- Deoxygenation of pyrolysis vapour derived from durian shell using catalysts prepared from industrial wastes rich in Ca, Fe, Si and Al. Sci. Total Environ.. 2020;703:134902
- [Google Scholar]
- Development of plasma pyrolysis/ gasification systems for energy efficient and environmentally sound waste disposal. J. Electrostat.. 2013;71:839-849.
- [Google Scholar]
- Tiseo, I., 2020. Plastic Waste Worldwide - Statistics & Facts [WWW Document]. Statista. URL https://www.statista.com/topics/5401/global-plastic-waste/ (accessed 12.17.20).
- Tripathi, M., Sahu, J.N., Ganesan, P., 2016. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev.
- Products and bioenergy from the pyrolysis of rice straw via radio frequency plasma and its kinetics. Bioresour. Technol.. 2009;100:2052-2061.
- [Google Scholar]
- Co-pyrolysis of polyethylene and sawmills powder: influence of polyethylene onpyrolysis product value. Nevşehir Sci. Technol.. 2017;6:306-313.
- [Google Scholar]
- Drop-in biofuels from the co-pyrolysis of grape seeds and polystyrene. Chem. Eng. J.. 2019;377:120246
- [Google Scholar]
- Ulloa, R.Z., Santiago, M.G.H., Rueda, V.L.V., 2019. The Interaction of Microwaves with Materials of Different Properties, in: Electromagnetic Fields and Waves. IntechOpen, p. 956.
- Uzoejinwa, B.B., He, X., Wang, S., El-Fatah Abomohra, A., Hu, Y., Wang, Q., 2018. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers. Manage.
- Testing a novel, mechanically fluidized bed pilot unit intended for the production of bio-oil and biochar from forest biomass. Ind. Biotechnol.. 2019;15:179-187.
- [Google Scholar]
- Thermogravimetric and kinetic analysis to discern synergy during the co-pyrolysis of microalgae and swine manure digestate. Biotechnol. Biofuels. 2019;12:1-18.
- [Google Scholar]
- A review on ex situ catalytic fast pyrolysis of biomass. Front. Chem. Sci. Eng.. 2014;8:280-294.
- [Google Scholar]
- Thermal and kinetic behaviors of corn stover and polyethylene in catalytic co-pyrolysis. BioResources. 2018;13 4102–4117–4117
- [Google Scholar]
- Wang, X., Ma, D., Jin, Q., Deng, S., Stan, H., Tan, H., 2019. Synergistic effects of biomass and polyurethane co-pyrolysis on the yield, reactivity, and heating value of biochar at high temperatures. Fuel Process. Technol. 194.
- Co-pyrolysis of lignin and polyethylene with the addition of transition metals - Part I : Thermal behavior and kinetics analysis. J. Energy Inst.. 2020;93:281-291.
- [Google Scholar]
- Wirawan, R.P., 2019. Plastic Waste Pyrolysis Optimization to Produce Fuel Grade Using Factorial Design. In: E3S WEb of Conferences, ICENIS.
- Conventional and microwave-assisted pyrolysis of biomass under different heating rates. J. Anal. Appl. Pyrolysis. 2014;107:276-283.
- [Google Scholar]
- Catalytic conversion of carbohydrate-derived oxygenates over HZSM-5 in a tandem micro-reactor system. Green Chem.. 2015;17:557-564.
- [Google Scholar]
- Effects of different conditions on co-pyrolysis behavior of corn stover and polypropylene. Polymers (Basel).. 2020;12
- [CrossRef] [Google Scholar]
- Interactive effect of the sorted components of solid recovered fuel manufactured from municipal solid waste by thermogravimetric and kinetic analysis. Waste Manage.. 2020;102:270-280.
- [Google Scholar]
- Xiang, Z., Liang, J., Marion, H., Jr, M., Liu, Y., 2018. Bioresource Technology Thermal behavior and kinetic study for co-pyrolysis of lignocellulosic biomass with polyethylene over Cobalt modi fi ed ZSM-5 catalyst by thermogravimetric analysis. Bioresour. Technol. 247, 804–811.
- Study on the co-pyrolysis of high density polyethylene and potato blends using thermogravimetric analyzer and tubular furnace. J. Anal. Appl. Pyrolysis. 2015;112:66-73.
- [Google Scholar]
- Synergistic effects of catalytic co-pyrolysis of macroalgae with waste plastics. Process Saf. Environ. Prot.. 2020;137:34-48.
- [Google Scholar]
- Journal of analytical and applied pyrolysis pyrolysis characteristic and kinetics of polyvinylidene fluoride with and without Pine Sawdust. J. Anal. Appl. Pyrolysis. 2017;123:402-408.
- [Google Scholar]
- Synergetic effect of co-pyrolysis of cellulose and polypropylene over an all-silica mesoporous catalyst MCM-41 using thermogravimetry-fourier transform infrared spectroscopy and pyrolysis-gas chromatography-mass spectrometry. Energy Fuels. 2017;31:9576-9584.
- [Google Scholar]
- Co-pyrolysis of polypropylene and biomass. Energy Sources, Part A Recover. Util. Environ. Eff.. 2008;30:1689-1697.
- [Google Scholar]
- Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J. Energy Inst.. 2019;92:27-37.
- [Google Scholar]
- Investigation of combustion kinetics of treated and untreated waste wood samples with thermogravimetric analysis. Fuel Process. Technol.. 2009;90:939-946.
- [Google Scholar]
- Two-step catalytic co-pyrolysis of walnut shell and LDPE for aromatic-rich oil. Energy Convers. Manage.. 2019;198:111816
- [Google Scholar]
- Study of synergistic effects during co-pyrolysis of cellulose and high-density polyethylene at various ratios. Energy Convers. Manage.. 2018;157:517-526.
- [Google Scholar]
- Zhang, B., Zhong, Z., Li, T., Xue, Z., Wang, X., Ruan, R., 2018. Biofuel production from distillers dried grains with solubles (DDGS) co-fed with waste agricultural plastic mulching films via microwave-assisted catalytic fast pyrolysis using microwave absorbent and hierarchical ZSM-5/MCM-41 catalyst. J. Anal. Appl. Pyrolysis.
- Science of the Total Environment Microplastic pollution in China ’ s inland water systems : A review of fi ndings, methods, characteristics, effects, and management. Sci. Total Environ.. 2018;630:1641-1653.
- [Google Scholar]
- High quality H2 -rich syngas production from pyrolysis-gasification of biomass and plastic wastes by Ni e Fe @ Nanofibers / Porous carbon catalyst. Int. J. Hydrogen Energy. 2019;44:26193-26203.
- [Google Scholar]
- Catalytic co-pyrolysis of lignocellulosic biomass with polymers: A critical review. Green Chem. 2016
- [Google Scholar]
- Thermal behavior and kinetic study for catalytic co-pyrolysis of biomass with plastics. Bioresour. Technol.. 2016;220:233-238.
- [Google Scholar]
- Fast microwave-assisted ex-catalytic co-pyrolysis of bamboo and polypropylene for bio-oil production. Bioresour. Technol.. 2018;249:69-75.
- [Google Scholar]
- Synergistic effect of catalytic co-pyrolysis of cellulose and polyethylene over HZSM-5. J. Therm. Anal. Calorim.. 2020;140:363-371.
- [Google Scholar]
- Zheng, Y., Tao, L., Yang, X., Huang, Y., Liu, C., Zheng, Z., 2018. Study of the thermal behavior, kinetics, and product characterization of biomass and low-density polyethylene co-pyrolysis by thermogravimetric analysis and pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis.
- Bioresource Technology Development and application of a continuous fast microwave pyrolysis system for sewage sludge utilization. Bioresour. Technol.. 2018;256:295-301.
- [Google Scholar]







