Translate this page into:
Removal of Pb(II) from aqueous solutions by using steelmaking industry wastes: Effect of blast furnace dust’s chemical composition
⁎Corresponding authors. masanchez@uaslp.mx (Marco Antonio Sánchez-Castillo), raul.carrillo@uadec.edu.mx (Francisco Raul Carrillo-Pedroza)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Slags, sludges, and dust from steelmaking industries have been probed as adsorbents for Pb(II) removal from synthetic water solutions, and these residues have showed good potential in the treatment of industrial effluents contaminated with this heavy metal. Adsorption and precipitation of Pb(II) have been postulated as the main mechanisms to remove Pb(II) from aqueous solutions by using steelmaking residues. The significant effect of pH on Pb(II) removal has been well studied but few studies explicitly address the effect of chemical and mineralogical composition of steelmaking residues and a better understanding of this key parameter is still needed for full elucidation and optimization of the Pb(II) removal process. In this study, samples obtained from different sections of a dust collector system (BFD) in a steelmaking factory, were used to evaluate the effect of BFD sample’s chemical composition on the removal of Pb(II) from synthetic aqueous solution. BFD samples were characterized to determine their chemical composition, particle size distribution and isoionic point. Equilibrium and transient experiments of Pb(II) removal from aqueous solutions were conducted a 25 °C and initial pH = 5.0. Results showed that CaO and MgO, as well as metallic Fe and FeO had a positive linear effect BDF samples Pb(II) adsorption capacity. Pb(II) removal process may take place by ion exchange with CaO and MgO, and by precipitation on the surface of metallic Fe and FeO. MgO and FeO promoted the Pb(II) removal in lesser extend that CaO and metallic Fe, respectively, because the surface ion exchange with MgO and FeO are less thermodynamic favourable and their lower composition in the sample. Bimolecular ion exchange process between Pb(II) ions and Ca and Fe species was supported by results from equilibrium and transient adsorption studies. Results of this work clearly showed that removal of Pb(II) from aqueous solutions is a strong function of the chemical composition of BFD samples and it provided further insight to promote the valorisation and optimization of steelmaking residues as heavy-metal adsorbents.
Keywords
Metallurgical residues
Steelmaking wastes valorisation
Pb(II) removal
Sustainable processes.
1 Introduction
Lead is listed as one of the top 10 chemicals of public health concern by the World Health Organization Chemical Safety Agenda (PAHO, 2020). Disposal of untreated wastewaters effluents on processing industries such as acid battery manufacturing, metal plating and finishing, tetraethyl lead manufacturing, ceramic and glass industries and environmental clean-up services is one of the major sources of water pollution by lead (Vijayakumar et al., 2012; Arbabi et al., 2015; Vu et al., 2019). The permissible concentration limit for Pb(II) in industrial wastewater effluents, set by the Environmental Protection Agency (EPA), is 0.05 mg/L; however, in many wastewater effluents Pb(II) concentration is the range of 200–500 mg/L. For this reason, it is imperative to reduce Pb(II) concentration to a level of 0.05–0.10 mg/L before the wastewater effluent be discharged to municipal sewage systems (Arbabi et al., 2015; WHO, 2020).
Several methods have been used to remove Pb from wastewater effluents, including chemical precipitation, electrochemical reduction, ion exchange, reverse osmosis, membrane separation, and adsorption (Arbabi et al., 2015; Bădescu et al., 2018). These technologies have proven to be effective for Pb removal, but they also exhibit some disadvantages such as continuous input of chemicals, high cost, toxic sludge generation or incomplete Pb removal. To date, adsorption technology still stands as a suitable alternative for the treatment of heavy metal polluted wastewaters because of recent developments of more selective adsorbents with increased adsorption capacity and, also, because the technology is simple and it may save operating costs if an appropriate adsorbent regeneration process is developed (Al-Asheh et al., 2000; Zahra and Pak, 2012).
Several smart materials have recently been explored as highly efficient adsorbents for removal of Pb(II) ions from aqueous solutions. Different types of nanoparticles and nanocomposites (Fe, Zn, SiO2, Ti, SiC, Mo, Ce, graphene) have been reported for the removal of Pb(II) from aqueous media, but technological and economic issues still limit their practical applications (Ata et al., 2018; Alghamdi et al., 2019; Lucaci et al., 2019). For this reason, there is an ongoing effort to improve the adsorption capacity and selectivity of conventional low-cost adsorbents including activated carbon, bentonite, chitosan and iron oxides such as magnetite, hematite and maghemite (naturals or synthetized), which are capable of adsorbing lead and other heavy metal ions from aqueous solutions (Olowoyo and Garuba, 2012; Roy and Bhattacharya, 2012; Zhu et al., 2012; Xiong et al., 2015; Hosseinzadeh et al., 2016). In addition, development of low-cost adsorbents based on the use of industrial solid wastes, such as red mud, fly ash, dusts and slags also have also been studied as potential Pb(II) adsorbents (Ghaedi and Mosallanejad, 2013; Chung et al., 2016; Juned et al., 2016; Soliman and Moustafa, 2020). This approach of reuse industrial residues than may cause health and environmental risks is particularly useful in the current context of circular economy, as it has beneficial impacts in terms of developing sustainable industrial processes.
In particular, slags, sludges, and dusts from steelmaking industry have been investigated as promising feedstock for several applications. A large availability of these industrial residues is foreseen given the fact that steelmaking industry steel has sustained a significant average growth rate over the last decade and also because steelmaking residues amount up for 1–4% of hot metal production (Jalkanen et al., 2005; Andersson et al., 2017; Naidu et al., 2020). Given the fact that it is impractical to recycle steelmaking residues due to operational difficulties in the blast furnace (Sarkar and Mazumder, 2015), they are being reused in an increasing number of innovative applications such as construction materials, soil enrichment and treatment, environmental remediation (including waste stabilization, carbon capture and storage and mitigation on landfill emissions) and water treatment of industrial effluents. Very importantly, the beneficial impact of steelmaking residues in a given application is highly dependent on their physical and chemical properties (Fisher and Barron, 2019; Naidu et al., 2020). Blast furnace (BF) and Basic Oxygen Furnace (BOF) dusts and slags have been used to remove heavy metals cations, anions or organic toxic compounds from synthetic water solutions and from polluted water effluents (Huy et al, 2020: Kennedy and Arias-Paić, 2020; Naidu et al., 2020; Vu et al., 2021). In particular, literature shows that BF and BOF dusts and slags are low-cost adsorbents to remove Pb(II) ions from aqueous solutions over a wide range of experimental conditions (Srivastava et al., 1997; Lopez-Delgado et al., 1998; Bhatnagar and Jain, 2006; Liu et al., 2010; Chung et al., 2016; Bouabidi et al., 2018; Yang et al., 2019; Zhan et al., 2019; Huy et al, 2020; Mercado-Borrayo et al., 2020; Kim et al., 2021). As it could be expected, the efficiency of heavy metals removal from aqueous solutions by using steelmaking residues is determined by the physical and chemical properties of the residues and the set of experimental conditions during the metal removal process. The initial metal concentration, temperature, pH and ionic strength of the metal aqueous solution determine the nature and distribution of the ionic species in solution. In the other hand, the physical properties, such as surface area and porosity, regulate the accessibility of the ionic species to the adsorption or active sites, and the surface chemical properties play a major role to control the nature, the equilibrium and the kinetics of the surface processes taking place to remove the metal ions from the aqueous solution (Yiacoumi and Tien, 1995; Cooney, 1998). Adsorption and precipitation of Pb(II) have been postulated as the main mechanisms to remove Pb(II) from aqueous solutions by using steelmaking residues. It has been reported that pH of Pb(II) solution plays a major role to determine extent of each Pb(II) removal mechanism (Nassar, 2010; Yang et al., 2019: Huy et al, 2020; Soliman and Moustafa, 2020; Kim et al, 2021). However, very few studies explicitly address the effect of chemical composition of steelmaking residues in Pb(II) removal from aqueous solutions (Lopez-Delgado et al., 1998; Nilforoushan and Otroj, 2008), and a better understanding of this key parameter is still needed for full elucidation of the Pb(II) removal process. This information will facilitate the valorisation of steelmaking residues as Pb(II) adsorbents or catalysts in the remediation of Pb(II) polluted aqueous effluents.
In this study, four dust samples collected in different section of a blast furnace (BFD samples), were used to determine the effect of BFD’s chemical composition in the removal capacity of Pb(II) from aqueous solutions. To this purpose, composition of BFD samples was characterized by atomic adsorption spectroscopy and X-Ray fluorescence; the particle size and the isoionic point of BFD samples were also determined. Experimental test of Pb(II) removal capacity from aqueous solutions were conducted in a batch system at 25 °C and pH = 5.0. The effect of BFD’s chemical composition in Pb(II) removal capacity, as well as in the Pb(II) removal mechanism under the conditions of the study were rationalized based on previous findings in the literature.
2 Experimental
2.1 BFD characterization
Four BFD samples were collected from a dust collector system installed in a major integrated steelmaking plant in Mexico. As schematically shown in Fig. 1, sample A (SA) was collected from the dust catcher, sample B (SB) from the dust cyclone, sample C (SC) from the wet scrubber, and sample D (SD) was obtained from the sludge dam. The samples were only shredded (to break up lumps or agglomerates) and dried at 105 °C for 24 h, to obtain representative samples.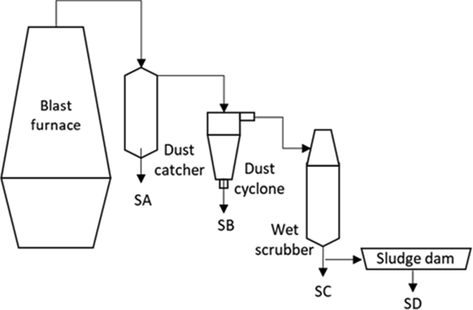
Schematic representation of a blast furnace dust collector system.
Chemical analysis of the BFD samples was carried out by atomic absorption spectroscopy (AAS) using a Thermo Electron Solaar S4 spectrophotometer. Quantification of each cation was performed using the corresponding ASTM method. For Ca, K and Na (reported as CaO, K2O and Na2O, respectively), a 0.2% w/v Cl3La solution was added to prevent signal reduction caused by aluminium and a 0.1%w/v CsCl solution was added as an ionization buffer. Mg and Al (reported as MgO and Al2O3) were measured under more oxidative conditions using a N2O/acetylene gas mixture. SiO2 retained in the solid was determined by gravimetry (by oxidation with HClO4). Sulphur and total carbon were determined by using a LECO CS-244 analyser.
Complementary, chemical analyses were also performed by using X-Ray fluorescence (XRF) using a Brucker AXS S4 PIONEER spectrophotometer. Iron phases composition were determined by X-ray diffraction (XRD) using a Brucker D8 diffractometer equipped with monochromatized CuKα radiation (generator tension = 40 kV, current = 30 mA). XRD pattern was recorded from (2θ) 5 to 70°, with step size of 0.02° and a counting time of 0.4 s per step. Rietveld method was used to quantify iron phases in BFD samples.
Particle size distributions for BFD samples were determined by using Tyler sieves. In addition, the isoionic point (IIP) of BFD slurries was determined by potentiometric titration. Experiments were performed using 1 g of BFD and 160 mL of an ionic strength regulated solution (NaNO3 = 10−3 M). The evolution of the slurry pH, initially acid or alkaline, was measured during titration with HCl or NaOH until the slurry pH remained constant, as an indication that an equilibrium condition had been reached.
2.2 Pb(II) removal tests
Experiments to evaluate the effect of BFD samples chemical composition in the removal of Pb(II) cations were carried out with synthetic Pb(II) aqueous solutions, which were prepared by using lead nitrate (Aldrich, chemical grade) and distilled water. Experiments were conducted in a 200 mL batch reactor (borosilicate conical bottles) with different Pb(II) concentration (obtained by dissolution from a stock solution with initial Pb(II) concentration of 538 mg/L) and initial pH = 5, which was fixed by using HNO3 analytical grade reactive. This initial pH was selected to avoid the precipitation of Pb(II) as Pb(OH)2, which occurs around pH = 5.5. In each experiment, a given load of BFD (1 g) was added to 100 mL of a Pb(II) aqueous solution; then, the adsorption process took place at atmospheric pressure, 25 °C and under stirring condition at 300 rpm, for up to 6 h. Afterwards, the resulting suspension was filtered. The concentration of lead in the aqueous solution was quantified before and after the adsorption test by atomic absorption spectroscopy (AAS, Perkin Elmer 3100), using an appropriate lead calibration curve. The effects of contact time, BFD load and lead initial concentration on the Pb(II) removal process were also studied in similar sets of experiments.
3 Results and discussion
3.1 BFD characterization
Table 1 shows the chemical composition of the BFD samples used in this study. Iron species were the major component in all BFD samples. In addition, a significant amount of C was also measured in all samples. The higher C content was found in catcher’s sample (SA) and cyclone’s sample (SB) (16.2 and 36.8%, respectively). Basic oxides such as CaO, SiO2 and MgO were also found. Wet scrubber sample (SC) and sludge dam sample (SD) showed the higher CaO (26.93 and 24.45%, respectively) and MgO (4.39 and 3.74%) content. Residual quantities of Zn (0.25–1.6%) and Al2O3 (less than 1%) were also quantified in all samples. The presence of iron oxides and basic oxides have been reported in the literature as active sites or species to promote the removal of Pb(II) from aqueous solutions (Ugwu and Igbokwe, 2019).
Sample
Fe
CaO
C
SiO2
Al2O3
MgO
Zn
SA
48.08
6.01
16.20
6.57
0.94
1.44
0.25
SB
35.78
14.22
32.80
8.72
0.91
1.17
0.28
SC
41.16
26.93
6.00
4.33
0.91
4.39
0.44
SD
46.19
24.45
4.39
3.45
0.50
3.74
1.56
In closer detail, Table 2 includes the specific iron phases found in the BFD samples. In addition, phase percentages were calculated based on the amount of total iron distributed in each phase Clearly, all BFD samples contained the following iron phases: hematite (Fe2O3), magnetite (Fe3O4), wustite (FeO) and metallic or zero-valence iron (Fe0). Smaller amounts of franklinite (Fe2ZnO4) were also found in BFD samples, in agreement with the chemical analysis that showed the presence of a residual amount of Zn. Iron phase distribution was clearly different in the BFD samples. For SA and SB, iron was predominantly found as hematite and magnetite, followed by wustite and metallic iron. In the other hand, for SC and SD the main iron phases were metallic iron and wustite. The main difference in the composition of SC and SD was the franklinite content as a result that SD was collected from the sludge dam, which is used in the steelmaking factory to collect dusts from other steelmaking operations.
Sample
Fe2O3
Fe3O4
FeO
Fe0
Fe2ZnO4
Sum of Fe
SA
15.04
12.53
11.03
9.02
0.45
48.08
SB
13.77
11.80
7.87
1.97
0.37
35.78
SC
2.13
6.40
14.92
17.06
0.66
41.16
SD
1.42
7.05
16.46
18.82
2.44
46.19
Table 3 includes the particle size of BFD samples, given as D90 (90% of the particles pass or have a size less that the corresponding mesh). SA had a D90 = 150 µm indicating that particles with a D less than 150 µm were collected by the dust catcher. According to chemical and phase analysis, the particles that are dragged and trapped by the catcher have undergone little decomposition or reduction (or oxidation in the case of coal). The particles with smaller size that were not collected in the catcher, went to the dust cyclone, which collected particles (SB) with a D90 = 75 µm, but larger than 20 µm. The dust trapped by the cyclone also had a low degree of reduction and high carbon content. In a subsequent stage, smaller particles still dragged by the gases were trapped in the last collector equipment, the wet scrubber. The characteristic size of these particles (SC) was D90 = 20 µm. In this stage, particles had suffered the greatest reduction (and calcination in the case of limestone). The dust discharged from the wet scrubber was deposited on a sludge dam, which also contain dusts from others BF and BOF; the particle size of a sample taken from the sludge dam (SD) was D90 = 15 µm. In the other hand, Table 3 also includes the isoionic points (IIP) of the BFD samples, which varied in a relatively close range, from pH = 5–0 to 6.4, suggesting a slightly surface acid character. It should be noticed that the IIP of BFD samples was in close agreement with the IIP reported in the literature for similar industrial residues, and also for the IIP of pure hematite and magnetite species (Rezaei and Vione, 2018; Kosmulski, 2020). The IIP of BFD samples is helpful to elucidate the removal mechanism of Pb(II) from aqueous solutions.
Sample
Particle size, D90, μm
Isoionic point, pHIIP
SA
150
5.0
SB
75
6.4
SC
20
5.7
SD
15
5.5
BFD
CaO + MgO, %
Iron phases, %
Fe0, %
D90, μm
pHIIP
Pb(II) removal, %
Qe, mg/g
SA
7.45
39.05
9.02
150
5.0
43.6
45.1
SB
15.39
33.82
1.97
75
6.4
50.7
59.6
SC
31.32
24.11
17.06
20
5.7
92.9
107.6
SD
28.19
27.37
18.82
15
5.5
96.1
112.8
3.2 Effect of the chemical composition and particle size in Pb(II) removal from aqueous solutions
The effect of chemical composition of BFD samples on Pb(II) removal from aqueous solutions was determined by comparing the maximum Pb(II) removal percentage by SA, SB, SC and SD, at 25 °C, initial pH = 5. To this purpose, for each sample, a series of experiments were conducted by varying the initial Pb(II) concentration, Co, from 54 to 538 mg/L and using a sample load of 1 g. The corresponding equilibrium Pb(II) concentration in the aqueous solution, Ce (mg/L), was obtained when Pb(II) concentration did not change over time. Pb(II) removal percentage (Pb(II)removal) was calculated by using the following equation:
Fig. 2 shows Pb(II)removal as a function of Ce for the BFD samples used in this study. The Ce corresponding to the maximum Pb(II)removal reached in each sample (Ce, max) was used to calculate the maximum Pb(II) adsorption capacity per gram of adsorbent, Qe (mg/g), by using the following equation:
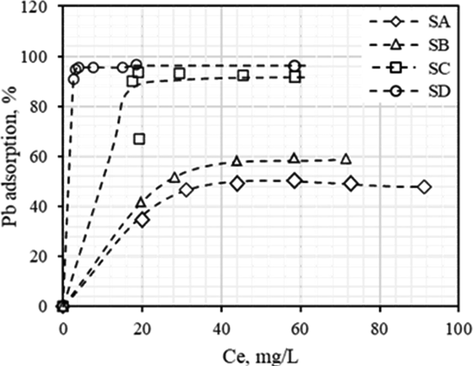
Pb(II)removal as a function of Ce for SA, SB, SC and SD, at 25 C and initial pH = 5.0.
The main results of Fig. 2 are summarized in Table that 4 includes of the values of Pb(II)removal and Qe for each of sample. Under the conditions used in the study, the maximum Pb (II)removal was 43.6, 50.7, 92.9 and 96.1% for the SA, SB, SC and SD samples, respectively. Similarly, the Qe value for these samples was 45.1, 59.6, 107.6, and 112.8 mg/g.
In the other hand, Table 5 qualitatively compares the Qe values determined for the BFD samples used in this study with Qe values reported in the literature for the removal of Pb(II) from aqueous solutions by using similar steelmaking residues and similar experimental conditions (temperature = 20–25 °C and pH = 5 and 5.5). It must be noticed that the experimental conditions used in each data set were different and, therefore, the comparison of the maximum Pb(II) removal capacity should only be made in qualitatively basis. Taking into account this fact, SC and SD showed Qe of 107.6 and 112.8 mg/g, respectively, which were within the range of Qe values reported in Table 5 for residues such as BF sludge and ladle furnace steel dust.
Material
Particle size
Qe, mg/g
Reference
Furnace slag
na
4.93
Chung et al., 2016
Cyclone steel dust
−600 nm
39.8
Bouabidi et al., 2018
Ladle furnace steel dust
−600 nm
208.9
Bouabidi et al., 2018
Blast furnace sludge
−40 μm
64.42
Lopez-Delgado et al., 1998
High carbon content sludge
150 μm
55.04
Lopez-Delgado et al., 1998
Steel slag
49–149 μm
6.6
Mercado-Borrayo et al., 2020
Blast furnace slag
150–200 μm
2.27
Srivastava et al., 1997
Blast furnace sludge
na
227
Bhatnagar and Jain, 2006
Blast furnace dust
na
142
Bhatnagar and Jain, 2006
Blast furnace slag
na
25
Bhatnagar and Jain, 2006
Blast furnace SA
150 μm
45.1
This work
Blast furnace SB
75 μm
59.6
This work
Blast furnace SC
20 μm
107.6
This work
Blast furnace SD
15 μm
112.8
This work
na = no available
In the condition used in the study, BFD samples particle size varied from D90 = 150 μm (SA) to D90 = 15 μm (SD). It was expected that particle size could affect the Qe value. In fact, Fig. 3 shows that Qe had a linear dependence with the ln of particle size (ln D90). Clearly, SD (D90 = 15 μm) led to a higher Qe. These results could be explained in terms that a smaller particle size les to a larger specific surface area. In this condition, a larger number of adsorption or active sites were available for Pb(II) removal from aqueous solution. This results might also suggest that no internal transport effects were limiting the surface phenomena responsible for Pb(II) removal.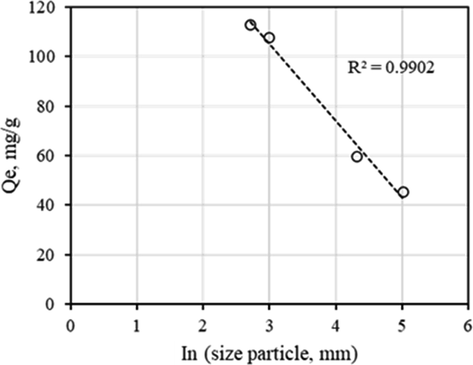
Effect of ln particle size (ln D90) in Qe.
Based on these results, it was evident that, under the conditions used in this study, Pb(II) removal was influenced by the chemical composition of BFD samples. As discussed, each sample had different distribution of their main chemical constituents: iron species (hematite, magnetite, wustite and metallic iron), basic oxides (CaO, SiO2, MgO) and carbon species. To elucidate if there was a trend between the chemical composition and Qe, Pearson’s correlation coefficients were calculated to determine which chemical species had significant effect on Qe. Pearson's correlation coefficient is the covariance of the two variables divided by the product of their standard deviations (Asuero et al., 2006). A correlation value of 0.7 or higher between two variables would indicate that a significant and positive relationship exists between the two (Boslaugh and Watters, 2008). Fig. 4a) shows that Qe increased with the content of CaO and MgO. Similarly, Fig. 4b) shows that an increase in metallic iron and wustite enhanced Qe. In the other hand, Fig. 4c) indicates that an increasing content of more oxidized iron species (hematite and magnetite) led to a decrease in Qe. As a summary, Fig. 4d) includes Pearson’s correlation coefficients for a), b) and c), as a measure of the strength of a linear association between the chemical phases content in BFD samples and Qe. In all cases Pearson’s correlation coefficients were higher than 0.72. Positive values suggested a beneficial effect of a given chemical species on Qe, and a negative coefficient indicated a detrimental effect of chemical composition on Qe Thus, under the conditions of this study, it could be postulated that Pb(II) removal was enhanced by the content of Fe and FeO, as well as CaO and MgO. Based on the Pearson’s correlation coefficients, the basic oxides seem to have a stronger effect on Pb(II) removal. In the other hand, analysis of Pearson’s correlation coefficients also suggested that the content of more oxidized ion species such hematite and magnetite, as well as C and SiO2 had a detrimental effect in Pb(II) removal from aqueous solutions.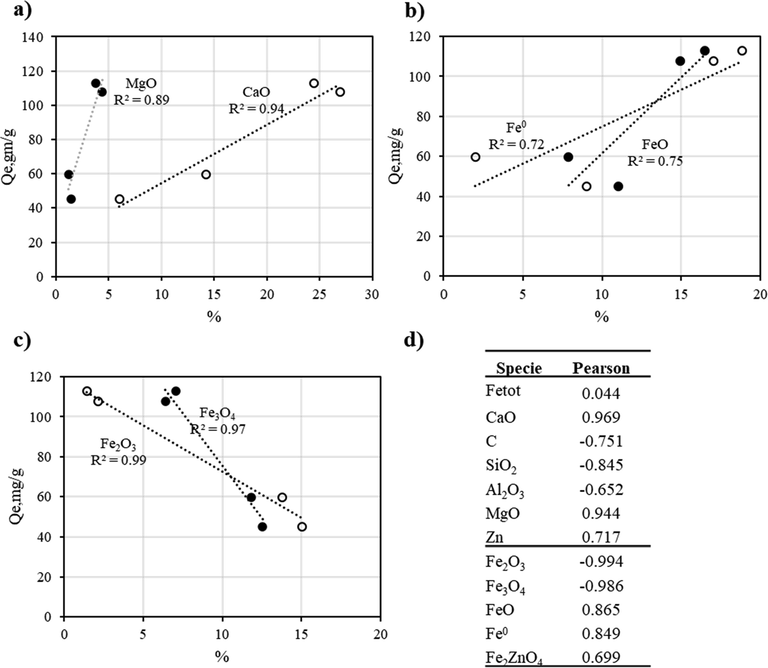
Effect of the content of: a) CaO and MgO, b) Fe0 and FeO, and c) Fe3O4 and Fe2O3 on Qe; d) Summary of Pearson’s correlation coefficients for the chemical species in BFD samples.
With respect to the surface phenomena responsible for Pb(II) removal from aqueous solutions by using steelmaking residues, several authors have suggested the occurrence of two competitive processes: Pb(II) adsorption and Pb precipitation. The prevalence and the extent of each surface process has been found highly dependent on the experimental set up: the adsorbent’s structure and surface composition as well as the operating conditions: temperature, initial pH, ionic strength, initial Pb(II) concentration, adsorbent load and contact time, among the most relevant parameters (Yang et al., 2019: Huy et al, 2020; Soliman and Moustafa, 2020; Kim et al, 2021). Related to the effect of chemical composition, iron species and basic oxides has been indicated to play a key role in Pb(II) removal from aqueous solutions when using steelmaking residues as adsorbents (Yiacoumi and Tien, 1995). The effect of iron species composition on Pb(II) removal have been related to the number of surface Fe OH– groups formed when an iron oxide is immersed in aqueous solution, which favours an adsorption process by electrostatic attraction. In this case, the adsorbent IIP and the ionic species distribution in the aqueous solution are key parameters to determine if such adsorption process is taking place (Cooney, 1998). As it is well known, the IIP determines the adsorbent’s surface species that are formed as a function of the solution pH. When an adsorbent is placed in a solution which pH > IIP, the adsorbent will develop positively-charged surface groups. In the other hand if the solution pH > IIP, the adsorbent will develop negatively-charged surface groups. Under these conditions a solution with pH > IIP is preferential to promote the adsorption of positively-charged cations by electrostatic attraction. In agreement with the chemical composition of the BDF samples used in this study, and based on calculations made with HSC 6.1 (Roine, 2006), iron species may undergo the following protonation and deprotonation surface reactions:
-
Zero valent iron
-
(b)
Wustite, FeO
-
(c)
Magnetite, Fe3O4:
-
(d)
Hematite, Fe2O3:
As it is reported in the literature, the following reactions may also be taking place as a function of solution pH (Kosmulski, 2020):
As shown in Table 4, the IIP of the BFD samples of this study were in the range of 5.0–6.4. Therefore, when these samples are placed in a solution with pH = 5.0, the formation of negatively-charged surface groups might be expected. However, the extent of the reactions forming these surface groups might be limited due to the small gradient between the initial solution pH and the IIP of samples SB, SC and SD.
In the other hand, a lead species distribution diagram was calculated by using Medusa software (Puigdomenech, 2010) under the same conditions used for the experimental work: initial Pb(II) concentration = 538 mg/L, 1 atm and 25 °C. The lead species distribution diagram shown in Fig. 5 indicates that at pH = 5 the most stable lead species is Pb(II) ion. This cation could de adsorbed by electrostatic attraction if enough negatively-charged surface groups such as FeO- exist on the BFD samples. In this scenario, Pb(II) adsorption may be related to the number of surface FeOH– groups formed when the BFD samples is immersed in aqueous solution. However, the small differences in the pHIIP observed form the BFD samples suggested that the number of potential surface FeOH– groups available for Pb(II) adsorption was about the same. As a result, the differences in Qe observed for the various BFD samples could not be explained by Pb(II) adsorption, thus suggesting that other surface process must be taking place in parallel to promote Pb(II) removal, as a function of the chemical composition of the samples.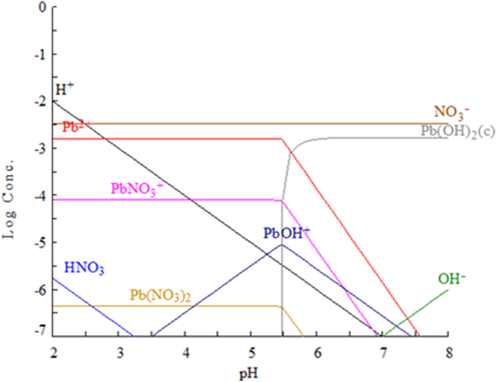
Lead species distribution diagram calculated for the experimental conditions used in this work.
It should be noticed that Fig. 4d) showed that Pb adsorption decreased with the Fe2O3 and Fe3O4 content. This observation suggested that equations (8), 10 and 12 were favoured because pH was acid (i.e. pH < pHIIP) and, as a result, [H+] > [OH−]. On the other hand, Fig. 4c) showed that the amount of adsorbed Pb increased with the Fe0 and FeO content. Due to the fact that the standard reduction potential of Fe2+/Fe0 is −0.44 V, which is lower than that of Pb2+/Pb (−0.13 V), then lead ions could react with metallic iron as an electron donor and precipitate (Rezaei and Vione, 2018), following reduction into insoluble metal forms, according to the following reaction:
In the case of FeO, Pb could be removed by ion exchange, like CaO, in agreement with the following reaction:
In the other hand, as it is shown in Table 4 and Fig. 4a), Qe was clearly a function of CaO + MgO content in BFD samples. This trend was in agreement with the recent reports in the literature. In fact, Zhan et al. (2019) reported that in solutions with pH < 7, Pb2+ can be adsorbed by ion exchange with Ca ions, resulting of the hydrolysis of CaO (or calcium and magnesium aluminosilicates) contained in the samples. It should be pointed out that in this study the solution pH could have slightly increased by the alkaline nature of BFD samples. According to the lead distribution diagram shown in Fig. 6, for pH above 5.5 Pb could have been hydrolysed in the form of Pb(OH)+ and Pb(OH)2. Based on these premises, a feasible mechanism of Pb(II) removal under the conditions of this study could be started by Pb(OH)+ adsorption, followed by complexation to Pb(OH)2, and Pb(OH)2 precipitation. In the other hand, it may be suggested that Pb2+ could be removed from the aqueous solution by an ion exchange process according to the following reactions (Gibbs energy obtained from HSC 6.1 software (Roine, 2006)):
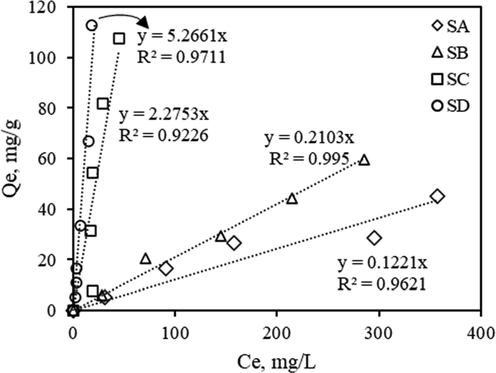
Comparison of Pb(II) adsorption experimental data with predictions from Henry’s adsorption isotherm for SA, SB, SC and SD samples.
Similar reactions may also occur for MgO. However, taking in consideration the larger composition of CaO with respect to MgO in the BFD samples, CaO was considered as the most important contribution.
Taking into account the change in Gibbs free energy for the chemical reactions occurring in the system and the composition of CaO, Fe and FeO in BFD samples, it could be postulated that the removal of Pb(II) from aqueous solution, under the conditions used in this study, may include two routes: i) Pb(II) is removed by ion exchange with CaO and MgO, ii) Pb(II) is removed by precipitation on the surface of iron phases. These two routes may be occurring to different extent in parallel mode. The predominance or extent of each Pb(II) removal processes is determined by the change in Gibbs free energy of the surface reactions (14) to (17), as also by the relative composition of basic oxides (CaO an MgO) and two of the iron species (Fe and FeO) in the BFD samples. Thus, the removal of Pb(II) from aqueous solutions is a strong function of the chemical composition of BFD samples; in fact, it is mostly enhanced by the content of CaO and MgO through an ion exchange process, and by Fe and FeO species through a precipitation process.
3.3 Adsorption isotherm analysis
Henry, Langmuir, Freundlich, Tempkin and Dubinin-Radushkevich adsorption isotherm models have been used to describe the adsorption process (Limousin et al., 2007; Ayawei et al., 2017). Henry’s isotherm is the simplest adsorption isotherm model, and it represents the thermodynamic adsorption limit at low surface coverage, assuming that there is no interaction among the adsorbed molecules (Silva da Rocha et al., 1998). In agreement with Henry’s model, Qe is proportional to the Ce:
In the other hand, Langmuir isotherm model assumes that adsorbate molecules adsorb with uniform energy on the adsorbent external surface, progressively filling the available adsorption sites; the limiting adsorption condition is the formation of a monolayer of adsorbed molecules (Ayawei et al., 2017). In this model, Qe is a function of Ce as given by the following equation (Al-Ghouti and Da'ana, 2020).
The empirical Freundlich adsorption model is generally used to describe adsorption process on heterogeneous surfaces, according to the following equation (Ezzati, 2020):
In the other hand, Tempkin’s adsorption model assumes that heat of adsorption would decrease linearly with surface coverage (Aharoni and Ungarish, 1997) and it includes a factor that explicitly considers the adsorbent–adsorbate interactions:
Finally, Dubinin–Radushkevich isotherm model has been successfully used to fit high solute activities and the intermediate range of concentration data (Hutson and Yang, 1997; Hu and Zhang, 2019).
The indicated adsorption models were used to fit the experimental adsorption data of Pb (II) removal from aqueous solutions by using BFD samples. The set of parameters that better fitted the experimental data to each adsorption model was summarized in Table 6, which also includes the correlation coefficient ratio (r2) as the error function to determine the best-fitting relationship (Foo and Hameed, 2010). Henry’s isotherm model showed the best linear fit for SA, SB and SD, with r2 > 0.96. Only SC had a slightly lower r2 value. Fig. 6 shows the agreement between experimental data and the predictions of the Henry’s adsorption model. KH values varied for BFD samples in the following order SD > SC≫SB > SA, thus resembling the same order experimentally observed for Pb(II) adsorption capacity (Qe).
Isotherm model
Parameter
Samples
SA
SB
SC
SD
Henry
r2
0.962
0.995
0.923
0.971
KH
0.1221
0.2103
2.2753
5.2661
Freundlich
r2
0.975
0.943
0.850
0.985
n
1.062
1.255
0.887
0.833
Kf
0.293
0.395
1.589
2.994
Langmuir
r2
0.943
0.99
0.777
0.974
Qo
105
137
222
147
KL
3 × 10−3
3 × 10−3
−8 × 10−3
−2 × 10−2
RL
0.496
0.498
0.511
0.532
Tempkin
r2
0.917
0.878
0.950
0.874
A
1 × 10−4
1 × 10−3
3 × 10−4
1 × 10−3
bt
116
176
33
50
B
21.3
14
74.6
49.8
Dubinin-Radushkevich
r2
0.766
0.878
0.922
0.938
Kad
8 × 10−4
3 × 10−4
6 × 10−5
4 × 10−6
qs
49.58
30.08
133.77
77.51
E
0.025
0.042
0.085
0.341
Langmuir isotherm model also made a reasonable fit of the experimental adsorption data for SA, SB and SD, showing r2 > 0.94. In the other hand, Freundlich adsorption model was less adequate than Henry’s and Langmuir’s adsorption models to fit the experimental data (r2 > 0.85). However, Freundlich’s adsorption parameters, Kf and 1/n, related to the adsorption capacity and to the strength of the adsorption process (Foo and Hameed, 2010), respectively, clearly showed that the Pb(II) adsorption was more favourable in SC and SD. Based on the fitting of Tempkin’s adsorption, B constant value for SD and SC was at least twice the B values for SA and SB, suggesting stronger interactions between the adsorbate and the surface. Finally, Dubinin–Radushkevich adsorption model showed that constant qs, related to heat of adsorption, varied in the following order: SC≫SD > SA > SB, while the free energy E values varied in the order SD≫SC > SB > SA. These trends suggested that Pb(II) removal could be related to the ion exchange processes described in the previous sections. In brief, the best fit of equilibrium adsorption data for all BFD samples was obtained by using Henry’s adsorption model. Complementary, the other adsorption models also described the higher adsorption capacity of SC and SD, and they provided the basis to support that Pb(II) adsorption by ion exchange (mainly due to the CaO content and favoured by a smaller particle size) may be a predominant mechanism in the BFD samples used in this study.
3.4 Kinetics analysis of Pb(II) removal
Kinetics of Pb(II) removal process was studied by using SC. As it was indicated SC comes from a single equipment (wet scrubber), so it would have better traceability for quality purposes and, its addition, it displayed a large Qe. Kinetic studies of Pb(II) removal were conducted at atmospheric pressure, 25 °C and using an aqueous solution with initial Pb(II) concentration of 120 mg/L. Fig. 7a showed that Pb(II) concentration in the aqueous solution continuously decreased as a function of contact time. The fastest Pb(II) removal took place over the first 5 min. A Pb(II) removal of about 90% was reached after 20 min. The fast adsorption of Pb(II) observed at short contact times may be attributed to external surface reactions, such as ion exchange or precipitation reactions, suggested in this study as the most feasible Pb(II) removal mechanisms. In this way, Pb(II) ions could easily reach active sites due to a relatively high CaO and Fe and FeO surface concentration, thus resulting in a rapid Pb(II) uptake. As the reaction progressed, the rate of Pb(II) removal decreased as the availability of Ca and Fe surface species suitable for the surface reactions processes became scarce and Pb(II) ions must diffuse through the sample pores to find new active sites.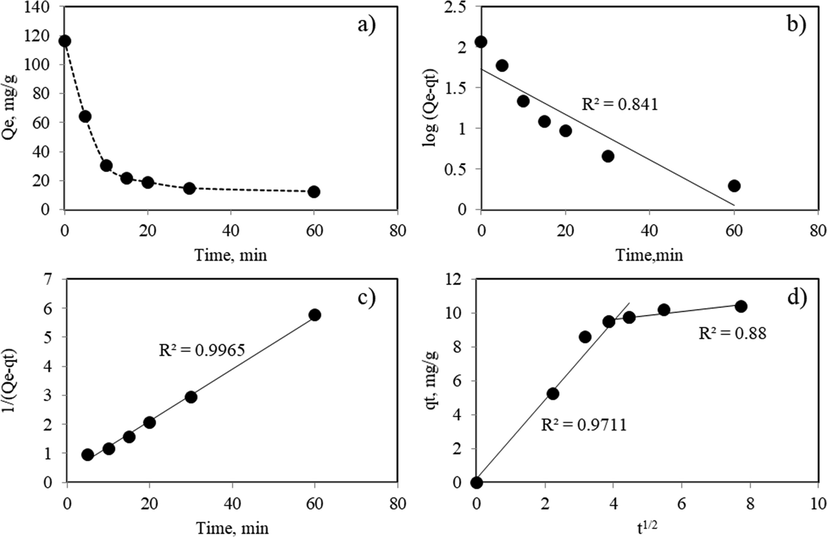
a) Pb(II) removal by sample SC under dynamic conditions (1 g BDF in 100 mL solution with initial Pb(II) concentration = 538 mg/L, pH = 5 and 25 °C). Fit of experimental data by using: b) Pseudo-first order kinetic model, c) Pseudo-second order kinetic model, d) Intraparticle diffusion model.
Data of the kinetic experiment were fitted by models typically used to describe transient metal removal processes (Fulbert, 2019; Wang and Guo, 2020). A pseudo-first order kinetic model, based on the Lagergren equation, was initially used:
A pseudo-second order kinetic model, which is described by the following equation, was also used:
Finally, an intraparticle diffusion model was also used to fit the experimental data. This model assumes that adsorption rate is controlled by internal or intraparticle diffusion of Pb(II) ions in the sample, the governing rate of Pb(II) removal is given by the following equation (Li et al., 2013):
Results of the fitting of the experimental data with the kinetic models previously described and kinetics parameters were summarized in Fig. 7 and Table 7, respectively.
Kinetic model
Parameters
r2
a) Pseudo-1st order
Qe
Kad
0.841
53.33
0.064
b) Pseudo-2nd order
Qe
K2
h
0.996
11.15
0.025
3.159
c) Intraparticle diffusion
BL effect
Kid
0.989
0.24
2.32
Clearly, the pseudo first order kinetic model did not fit the kinetic data (r2 = 0.841). Similarly, the intraparticle diffusion model was not able to correlate the experimental data. Finally, the pseudo-second-order model showed a good fit of the kinetic data (r2 = 0.9926) and it was assumed as the best model to describe the transient Pb(II) removal process. This finding may support that Pb(II) removal occur through bimolecular reactions such as ion exchange or the precipitation of Pb(II) with either CaO or Fe species, respectively.
3.5 Practical implications of using BFD for removal of Pb(II) ions in aqueous solutions
Results in previous sections clearly showed that it is possible to use BFD samples, collected from a dust collector system installed in a steelmaking plant, to remove Pb(II) from aqueous solutions at 25 °C and initial pH = 5.0. Results of this study confirm that chemical composition of BFD samples play a key role for Pb(II) removal. In more detailed, both CaO and MgO content in BFD samples had a positive linear correlation with Pb removal and Qe. As suggested by Zhang et al. [18], it was shown that removal of Pb(II) may occur through an ion exchange process with Ca ions. The analysis suggested that removal of Pb(II) by MgO followed a similar mechanism; however, MgO promoted the Pb(II) removal in lesser extend because it is less thermodynamic favourable and because its composition is 10 times lower than CaO. In a similar way, it was shown that iron species had a promotional effect for Pb(II) removal from aqueous solutions. It was evident that less oxidized iron species (i.e., Fe0 and FeO) favoured Pb(II) removal. Under the conditions used in this study, it was postulated that iron species remove Pb though an ion exchange process. The beneficial effect of Fe species was explained in terms of a lower change of Gibbs free energy, being the of the exchange process with Fe more favourable than FeO. These bimolecular ion exchange process between Pb(II) ions and Ca and Fe species was further supported by results obtained from equilibrium and transient adsorption process.
The Pb(II) removal performance of the samples collected form a dust collector system of in a steelmaking plant showed that those residues collected at the end of the collector system, SC (from the wet scrubber) and SD (from the sludge dam) exhibited higher Pb(II) adsorption capacity, 107.6 and 112.8 mg/g, respectively. This range of Pb(II) adsorption capacity of these BFD samples is similar to Qe values reported in the literature for analogous residues (furnace sludge and ladle furnace steel dust). It is noteworthy that the BFD samples used in this study were no subjected to any pre-treatment and, therefore, must be taken as a residue of the steelmaking process that can be directly valorised for the removal of heavy metals such as Pb(II) ions, with potential economic benefits in the treatment of polluted industrial effluents. Finally, based on the results of this study, a set of guidelines could be set to improve the Pb(II) adsorption capacity of the BFD samples. However, these improvements, such as the tuning of the particle size, the adjustment of T and initial pH, or the chemical treatment of the BFD, may have economic implications for the practical viability of the heavy metal removal process.
4 Conclusions
In the context of circular economy, the re-use of metallurgical wastes has an increasing relevance, and a number of innovative applications are under development. One of the most promising applications is water treatment of industrial effluents due to its high availability, low cost, high chemical affinity for heavy metals and magnetic properties that facilitate their recovery after treatment. Several studies have addressed the effect of experimental conditions on the removal of heavy metals such as lead from aqueous solutions. This study focused on the effect of chemical composition of steelmaking residues on the maximum Pb(II) adsorption capacity (Qe) from synthetic aqueous solutions, by using samples collected from different sections of a dust collector system in a steelmaking factory. Chemical composition of the BFD samples used in the study showed the presence of iron species (Fe, FeO, Fe2O3 and Fe3O4), basic oxides (CaO, MgO, SiO2) and C species as the main components. Equilibrium and transient experiments of Pb(II) removal from aqueous solutions by BFD samples were conducted a 25 °C and initial pH = 5.0. Results showed that CaO and MgO, as well as metallic Fe and FeO had a positive linear effect in Pb(II) removal and Qe. Under the experimental conditions used in this study, Pb(II) removal process may take place by two parallel routes; Pb(II) is removed by ion exchange with CaO and MgO, and Pb(II) is also removed by precipitation on the surface of iron phases. A thermodynamics analysis of the surface chemistry suggested that MgO and FeO promoted the Pb(II) removal in lesser extend that CaO and metallic Fe, respectively, because the surface ion exchange with MgO and FeO are less thermodynamic favourable and their lower composition in the sample. These bimolecular ion exchange process between Pb(II) ions and Ca and Fe species was further supported by results obtained from equilibrium and transient adsorption process. Results of this work clearly showed that removal of Pb(II) from aqueous solutions is a strong function of the chemical composition of BFD samples and it provided further insight to promote the valorisation of steelmaking residues as heavy-metal adsorbents, contributing to future development of sustainable processes.
Acknowledgements
Authors thank the International Center for Nanotechnology and Advanced Materials - Kleberg Advanced Microscopy Center - at the University of Texas at San Antonio (ICNAM-UTSA) for technical characterization assistance.
Declaration of Competing Interest
Authors declare no conflicts of interest.
References
- Kinetics of activated chemisorptions. Part 2 Theoretical models. J. Chem. Soc. Faraday Trans.. 1997;1, 73:456-464.
- [CrossRef] [Google Scholar]
- Predictions of Binary Sorption Isotherms for the Sorption of Heavy Metals by Pine Bark Using Single Isotherm Data. Chemosphere. 2000;41(5):659-665.
- [CrossRef] [Google Scholar]
- Efficient adsorption of lead (ii) from aqueous phase solutions using polypyrrole-based activated carbon. Materials. 2019;12:12.
- [CrossRef] [Google Scholar]
- Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazardous Mater.. 2020;393:122383.
- [CrossRef] [Google Scholar]
- Characterization and upgrading of a low zinc-containing and fine blast furnace sludge – a multi-objective analysis. ISIJ Int.. 2017;57(2):262.
- [CrossRef] [Google Scholar]
- Removal of lead ions from industrial wastewater: A review of removal methods. Int. J. Epidemiologic Res.. 2015;2(22):105-109.
- [Google Scholar]
- The Correlation Coefficient: An Overview. Crit. Rev. Anal. Chem.. 2006;36:41-59.
- [CrossRef] [Google Scholar]
- Lead remediation using smart materials. A review. Zeitschrift für Physikalische Chem.. 2018;223:10.
- [CrossRef] [Google Scholar]
- Modelling and Interpretation of Adsorption Isotherms. J. Chem.. 2017;2017
- [CrossRef] [Google Scholar]
- Valorisation possibilities of exhausted biosorbents loaded with metal ions - A review. J. Environ. Manag.. 2018;224:288-297.
- [CrossRef] [Google Scholar]
- Removal of Lead Ions from Aqueous Solutions by Different Types of Industrial Waste Materials: Equilibrium and Kinetic Studies. Sep. Sci. Technol.. 2006;41:1881-1892.
- [CrossRef] [Google Scholar]
- Steel-making dust as a potential adsorbent for the removal of lead (II) from an aqueous solution. Chem. Eng. J.. 2018;334:837-844.
- [CrossRef] [Google Scholar]
- Boslaugh, S., Watters, P., 2008. Statistics in a Nutshell: A Desktop Quick Reference, ch. 7. Sebastopol, CA: O'Reilly Media. ISBN-13: 978-0596510497.
- Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. J. Sci. Technol.. 2016;54:314-320.
- [CrossRef] [Google Scholar]
- Cooney, D.O., 1998. Adsorption Design for Wastewater Treatment, CRC Press, ISBN 9781566703338.
- Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J.. 2020;392:123705.
- [CrossRef] [Google Scholar]
- Developing the new kinetics model based on the adsorption process: From fitting to comparison and prediction. Sci. Total Env.. 2020;725:138490.
- [CrossRef] [Google Scholar]
- The recycling and reuse of steelmaking slags — A review Resources. Conserv. Recycl.. 2019;146:244-255.
- [CrossRef] [Google Scholar]
- Insights into the modelling of adsorption isotherm systems. Chem. Eng. J.. 2010;156(1):2-10.
- [CrossRef] [Google Scholar]
- Modelling adsorption mechanism of paraquat onto Ayous (Triplochiton scleroxylon) wood sawdust. Appl. Water Sci.. 2019;9:1.
- [CrossRef] [Google Scholar]
- Removal of heavy metal ions from polluted waters by using of low-cost adsorbents: Review. J. Chem. Health Risk. 2013;3(1):07-22.
- [Google Scholar]
- Removal of cadmium and lead ions from aqueous solution by nanocrystalline magnetite through mechanochemical activation. J. Ultrafine Grained Nanostruct. Mater.. 2016;49(2):72-79. 10.7508.jufgnsm/2016.02.03
- [Google Scholar]
- Application of Dubinin-Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq.. 2019;277(1):646-648.
- [CrossRef] [Google Scholar]
- Removal mechanisms of cadmium and lead ions in contaminated water by stainless steel slag obtained from scrap metal recycling. J. Water Process Eng.. 2020;36:101369.
- [CrossRef] [Google Scholar]
- Theoretical basis for the Dubinin-Radushkevith (D-R) adsorption isotherm equation. Adsorption. 1997;3(3):189-195.
- [CrossRef] [Google Scholar]
- Recycling of steelmaking dusts – the radust concept. J. Mining Metall.. 2005;41:1-16.
- [CrossRef] [Google Scholar]
- A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng.. 2016;10:39-47.
- [CrossRef] [Google Scholar]
- Adsorption phenomenon and its application in removal of lead from wastewater: a review. Int. J. Hydro.. 2017;1(2):38-47.
- [Google Scholar]
- Application of powdered steel slag for more sustainable removal of metals from impaired waters. J. Water Process Eng.. 2020;38:101599
- [CrossRef] [Google Scholar]
- Identification of pH-dependent removal mechanisms of lead and arsenic by basic oxygen furnace slag: Relative contribution of precipitation and adsorption. J. Clean. Prod.. 2021;279:123451.
- [CrossRef] [Google Scholar]
- The pH dependent surface charging and points of zero charge. Adv. Colloid Interface Sci.. 2020;275:1-18.
- [CrossRef] [Google Scholar]
- Adsorbent for lead removal based on graphene oxide functionalized with magnetic cyclodextrin–chitosan. Coll. Surf. B Biointerf.. 2013;107:76-83.
- [CrossRef] [Google Scholar]
- Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem.. 2007;22(2):249-275.
- [CrossRef] [Google Scholar]
- Adsorption intrinsic kinetics and isotherms of lead ions on steel slag. J. Hazard. Mater.. 2010;173(1–3):558-562.
- [CrossRef] [Google Scholar]
- Sorption of heavy metals on blast furnace sludge. Wat. Res.. 1998;32(4):989-996.
- [CrossRef] [Google Scholar]
- Lucaci, A., Dimbu, R., Bulgariu, D., Bulgariu, L., 2019. Use of Iron Nanoparticles Functionalized with Alginate for the Adsorption of Pb(II) Ions from Aqueous Media. 2019 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 1-4, https://doi.org/10.1109/EHB47216.2019.8969913.
- Optimisation of the removal conditions for heavy metals from water: A comparison between steel furnace slag and CeO2 nanoparticles. Arabian J. Chem.. 2020;13(1):1712-1719.
- [CrossRef] [Google Scholar]
- Absorption of Lead Ions by Various Types of Steel Slag. Iran. J. Chem. Chem. Eng.. 2008;27(3):69-75.
- [Google Scholar]
- Basic oxygen furnace slag: Review of current and potential uses. Minerals Eng.. 2020;149:106234.
- [CrossRef] [Google Scholar]
- Rapid removal and recovery of Pb(II) from wastewater by magnetic nanoadsorbents. J. Hazardous Mater.. 2010;184(1–3):538-546.
- [CrossRef] [Google Scholar]
- Adsorption of cadmium ions using activated carbon prepared from Coconut shell. Global Adv. Res. J. Food Sci. Technol.. 2012;1(6):81-84.
- [Google Scholar]
- Panamerican Health Organization Site, https://www.paho.org/hq/index.php?option=com_content&view=article&id=8206:2013-lead-contamination&Itemid=39800&lang=en (accessed July 2020).
- Puigdomenech, I., 2010. MEDUSA Software, Royal Institute of Technology, Stockholm, Sweden.
- Effect of pH on zero valent iron performance in heterogeneous Fenton and Fenton-Like processes: A review. Molecules. 2018;23:31-127.
- [CrossRef] [Google Scholar]
- Roine, A., 2006. HSC Chemistry 6.0, Outotec Research Oy, Pori, Finland.
- Removal of Cu (II), Zn (II) and Pb (II) from water using microwave-assisted synthesized maghemite nanotubes. Chem. Eng. J.. 2012;211:493-500.
- [CrossRef] [Google Scholar]
- Solid waste management in steel industry - challenges and opportunities. Int. J. Social, Behav. Educ. Econ., Bus. Ind. Eng.. 2015;9(3):978-981.
- [CrossRef] [Google Scholar]
- Industrial solid waste for heavy metals adsorption features and challenges; a review. J. Mater. Res. Technol.. 2020;9(5):10235-10253.
- [CrossRef] [Google Scholar]
- Henry's Law as a Limit for an Isotherm Model Based on a Statistical Mechanics Approach. J. Colloid Interface Sci.. 1998;208(1):211-215.
- [CrossRef] [Google Scholar]
- Removal of lead and chromium by activated slag-a blast-furnace waste. J. Environ. Eng.. 1997;123:461-468.
- [CrossRef] [Google Scholar]
- Sorption of Heavy Metals on Clay Minerals and Oxides: A Review. Advanced Sorption Process Applications, Serpil Edebali, IntechOpen 2019
- [CrossRef] [Google Scholar]
- Sustainable treatment for sulfate and lead removal from battery wastewater. Sustainability. 2019;11:3497.
- [CrossRef] [Google Scholar]
- Phosphorus removal from aqueous solution by steel making slag – Mechanisms and performance optimisation. J. Cleaner Prod.. 2021;284:124753.
- [CrossRef] [Google Scholar]
- Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater.. 2020;390:122156.
- [CrossRef] [Google Scholar]
- WHO Guidelines for Drinking-water Quality, https://www.who.int/water_sanitation_health/dwq/chemicals/lead.pdf (accessed July 2020).
- Investigation on the efficiency and mechanism of Cd (II) and Pb (II) removal from aqueous solutions using MgO nanoparticles. J. Hazard. Mater.. 2015;299:664-674.
- [CrossRef] [Google Scholar]
- Kinetics of Metal Ion Adsorption from Aqueous Solutions: Models, Algorithms, and Applications. US: Springer; 1995. ISBN 978-1-4615-2319-2
- The stability of the compounds formed in the process of removal Pb(II), Cu(II) and Cd(II) by steelmaking slag in an acidic aqueous solution. J. Environ. Manage.. 2019;231:41-48.
- [Google Scholar]
- Lead removal from water by low cost adsorbents: A review. Anal. Environ. Chem.. 2012;13:1-8.
- [Google Scholar]
- Removal of Pb(II) from Acid Mine Drainage with Bentonite-Steel Slag Composite Particles. Sustainability. 2019;11:4476.
- [CrossRef] [Google Scholar]
- Competitive adsorption of Pb (II), Cu (II) and Zn (II) onto xanthate-modified magnetic chitosan. J. Hazard. Mater.. 2012;221:155-161.
- [CrossRef] [Google Scholar]







