Translate this page into:
Revealing bacteriophage capabilities: pH and NaCl concentration effects on RSJ2 phage infectivity and stiffness
⁎Corresponding author at: National Science and Technology Development Agency (NSTDA), 111 Thailand Science Park, Phahonyothin Road, Khlong Nueng, Khlong Luang, Pathum Thani 12120, Thailand. udom.sae@biotec.or.th (Udom Sae-Ueng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The urgent necessity of reducing pesticides in the agricultural sector compels us to explore alternative, environmentally friendly approaches for controlling pathogenic bacteria and crop diseases. The usage of bacterial viruses or bacteriophages (phages) to control pathogenic bacteria is still limited due to the lack of understanding of the factors influencing shelf life. We examined the effects of pH and NaCl on the storage buffers on the infectivity of the RSJ2 phage. The RSJ2 phage infects Ralstonia solanacearum, causing bacterial wilt disease in tomatoes. The buffer that retained the highest phage titer (63 % after two months) was at pH 8.3 and 0.5 M NaCl. pH and NaCl concentrations directly affected the phage particles. The phage stiffness was measured with the nanoindentation technique, and a correlation between the phage remaining titers and the stiffness was revealed. The average stiffness associated with the highest remaining titer was 0.07 N/m, which falls into the intermediate stiffness range. The results agree with the previous study on the C22 phage stiffness, revealing an association between intermediate stiffness and phage infectivity. Phage stiffness can become an attribute of phage metastability and can be used to predict the phage shelf life.
Keywords
Bacteriophages
Phage stiffness
Atomic force microscope
Phage infectivity
Phage titer
- AFM
-
atomic force microscope
- CFU
-
colony forming unit
- OD600
-
optical density at the 600-nm wavelength
- PFU
-
plaque forming unit
- R. solanacearum
-
Ralstonia solanacearum
- TEM
-
Transmission Electron Microscope
Abbreviations
1 Introduction
The use of pesticides for crop diseases caused by pathogenic bacteria is seemingly inevitable (Tudi et al., 2021). Realizing nature’s resources can unfold vast possibilities to lessen the excessive usage of chemicals. One of the natural resources is bacterial viruses or bacteriophages (phages), the world’s most abundant forms of living “entity” (Clokie et al., 2011). Phages specifically lyse their target bacteria and take over the bacterial host cell as their factories to manufacture new phages. The progeny phages become the next troops to kill the host cells (Salmond and Fineran, 2015). Since their discovery more than a hundred years ago, the capability of phages has brought about significant findings and research, unraveling ample phage facts. Phage utilization has emerged in all fields, including agriculture (Zia and Alkheraije, 2023).
Despite the expansive effort in phage research for agriculture, the implementation of phages as biological control agents for crop disease is relatively low compared to other agents. The reasons stem from the lack of in-depth understanding of controversial issues in infection and replication cycles, such as possible mutation induction in host cells (Shan et al., 2023). Other reasons derived from the need to decipher the influence of the disrupting factors phages may endure. Such factors include pH, ions, temperature, internal DNA pressure, and mechanical disruption. These factors can degrade the phage structure, which consists of structural proteins and genomes (Ye et al., 2019). It is crucial to understand the phage stability or the ability of phages to retain infectivity while experiencing microenvironments that may harm them. Uncovering the nature of the stability dynamic becomes a key to the success of phage replication and usage (Di Natale et al., 2022; Holtappels et al., 2021).
The dependence of phage stability on external factors has been extensively studied. Two omnipresent elements are pH and salt. They are present everywhere phages inhabit and thus can affect all steps in the phage replication cycle. pH and salt are also shelf-life-determining factors when developing a phage storage formulation (Malik et al., 2017). Phage stability and infection depend on pH and salts (Jończyk-Matysiak et al., 2019). Most phages remain intact and infectious at a modest pH (approximately 5 to 9). The study showed that the P100 phage infected the host cell Listeria monocytogenes well at pH 6, 7, and 8, but the infection was reduced at pH 5 and 9 (Fister et al., 2016). The optimal pH for the T7 phage infecting Escherichia coli was between 6 and 8, but the phage partially lost its activity at pH 4 and 9 (Ranveer et al., 2024). Ps1 and Ps2 long-tailed phages infecting Pseudomonas spp. were stable for 12 h at a pH of 6–8 (EL-Wafai et al., 2022). Acinetobacter baumannii phage Acibel004 and staphylococcal phage ISP were infectious at pH 3.5 to 7.6 (Duyvejonck et al., 2021).
On the other hand, the effects of salts on phage stability vary on individual phages. The lysis of the P100 phage infecting Listeria monocytogenes was reduced by 0.5 M NaCl concentration (Fister et al., 2016). However, phage MS2 was stable in monovalent salt, including NaCl, at a concentration as high as 1 M (Mylon et al., 2010). Substantial studies have been performed on the phage stability dependence on pH and salt conditions. However, the dynamic of phage stability has rarely been discussed (Maghsoodi et al., 2019). Phage dynamics refers to how phage structure and integrity in situ respond or adapt to external triggers. Understanding the stability dynamics allows us to comprehend how phage structural components contribute to stability and shelf life. The knowledge enables us to design pH and salt conditions to maximize phage stability without intensively relying on the trial-and-error approach.
Understanding the in situ dynamic of the phage structure requires the in situ technique for probing the phage particle. One of the few techniques is atomic force microscopy (AFM). AFM utilizes a nanometer-size tip to interact with the exterior of the phage structure to extract the phage morphology and to measure the phage mechanical properties, including stiffness and adhesion, in desired conditions (Müller et al., 2021). Over the years, phage characteristics and properties have been proposed to correlate with phage stability and infection (Bruinsma et al., 2021). Young’s modulus of the phage and viral capsids strengthened by minor proteins was proposed to correlate with the phage survival and infection in the host cells (Evilevitch and Sae-Ueng, 2021; Sae-Ueng et al., 2022). Phage stiffness was shown to play a role in binding to the host cell and phage stability (Sae-Ueng et al., 2022, 2020). The mechanical reinforcement of the internal DNA also contributed to the phage integrity and infection (Liu et al., 2014). Evidence on the association between phage dynamical properties and biological functions is piling up. However, the association between the dynamics and functions of the phages, especially novel phages, remains unclear and requires examination.
In this work, we elucidated the role of phage stiffness on phage infection and its dependence on pH and NaCl concentration. pH and NaCl can affect the phage’s capsid and genomic DNA, thus affecting how the phage can infect the host bacteria. The effect of pH and NaCl concentration was studied on infectivity, adsorption, and stiffness of a short-tailed phage RSJ2 or C19 (National Center for Biotechnology Information (NCBI), n.d.). This soilborne phage infects Ralstonia solanacearum, causing bacterial wilt disease in chili and tomato, thus presenting a good candidate for phage-based biocontrol of bacterial wilt disease (Bhunchoth et al., 2015). A correlation was established between phage infection and the nanomechanical property, namely stiffness. We propose that stiffness can be used to speculate about phage infection and long-term shelf life. Deciphering the role of phage stiffness in phage shelf life can improve phage storage conditions and promote phage adoption for biocontrol applications.
2 Materials and methods
2.1 RSJ2 phage preparation and purification
-
An overnight culture of Ralstonia solanacearum strain RS10/3 (race 1 biovar 3) was shaken at 28 °C and 230 rpm (Bhunchoth et al., 2015). The culture was diluted 100-fold with 200 mL CPG medium containing 0.1 % (w/v) casamino acids (BD, USA), 1 % (w/v) peptone (HiMedia, India), and 0.5 % (w/v) glucose supplemented with 0.01 M MgSO4 (Merck KGaA, Germany) in a 1000-mL flask.
-
RSJ2 phage was added at a multiplicity of infection of 0.1 after the cultures reached an OD600 (optical density of the culture measured at a wavelength of 600 nm) of 0.4–0.6. Clear culture indicated bacterial lysis after an overnight incubation.
-
The culture was incubated for an hour with DNase I (Roche, Germany) and RNase A (Sigma-Aldrich, USA) at 37 °C. The cell debris was removed by centrifuging at 7,500 rpm for 45 min at 4 °C.
-
The supernatant was filtered through a 0.45-µm membrane (GVS, Italy). The phages were collected through centrifugation at 19,500 rpm for 1 h at 4 °C. The phages were resuspended in SM buffer containing 50 mM Tris-HCl pH 7.5 (Merck KGaA, Germany), 100 mM NaCl (Carlo Erba, Germany), and 10 mM MgSO4.
-
The phages were purified by sucrose (Sigma-Aldrich, USA) density gradient (20–50 % sucrose in SM buffer) and subjected to ultracentrifugation at 24,000 rpm at 4 °C for 1 h in swing buckets. The phage band was extracted by puncturing the tube side wall. The phages were diluted with the experimental buffers (Table S1) before being precipitated by ultracentrifugation at 24,000 rpm at 4 °C for 1 h. The phages were resuspended in each buffer and stored at 4 °C.
2.2 Buffers with varying pH and ionic strength
Nine buffers with varying pH and ionic strength were studied for their effect on RSJ2 phage. 2-(N-Morpholino)ethanesulfonic acid or MES (Sigma-Aldrich, USA) at pH 6.0 was used for acidic buffers. Tris-HCl at pH 7.5 was used for neutral buffers. N,N-Bis(2-hydroxyethyl)glycine or Bicine at pH 8.3 was used for basic buffers. The NaCl concentration was adjusted (0, 0.1, and 0.5 M) for ionic strength varying. The buffer compositions are listed in Table S1. pH and NaCl concentration range were chosen to mimic the variation of pH and NaCl in natural soil conditions of the RSJ2 phage. The ranges were also relatively moderate, so the buffer conditions could be modified for future storage formulation.
2.3 Transmission electron microscopy (TEM)
Following the previous method, the RSJ2 phage structure and morphology were investigated using TEM. The TEM grid (EMS, USA) was cleaned with a rotary pumped coater (Quorum, UK) (Ackermann, 2012, 2009). The phages were deposited on the cleaned grid before being rinsed with deionized water and stained with a 2 % uranyl acetate solution (EMS, USA). The sample grid was examined using 80 kV TEM (Hitachi High-Tech, Japan) at magnifications of 50,000 to 100,000 times.
2.4 Plaque assay of RSJ2 phage
R. solanacearum strain RS10/3 cultured overnight at 28 °C and 230 rpm shaking was diluted to OD600 of 0.25 with CPG medium. A 0.01 mL phage sample was added to 0.25 mL of diluted culture. After incubating for 30 min, the sample was mixed with molten 0.45 % agar (PAC, Thailand) in CPG medium and overlaid on a CPG plate containing 1.5 % agar. The number of plaques was recorded after 16–18 h of incubation at 28 °C and converted to plaque forming unit (PFU) per mL. The PFU represented the number of infectious phage particles that can initiate a clear zone or plaque. The titers of the RSJ2 phage in 9 buffers stored at 4 °C were recorded every 7 days for 57 days. The plaque assay was performed in triplicate, and the average titers were calculated and normalized to the phage titers recorded on the first day (termed Day 0) (Abedon, 2018).
2.5 AFM imaging and nanoindentation of RSJ2 phage
The substrate preparation for the phage immobilization was previously described (Sae-Ueng et al., 2020). AFM imaging and measurement were performed with a JPK NanoWizard 3 and NanoWizard ULTRA Speed 2 (Bruker, USA). The AFM micrographs were captured in a liquid milieu in non-contact modes. The nanoindentation data were collected in a liquid milieu using force spectroscopy. The calculation and analysis of the force-distance curve from nanoindentation data were described elsewhere (Ivanovska et al., 2007). A BioLever mini BL-AC40TS-C2 (Olympus, Japan) was used for this study, with the spring constant of 0.03–0.10 N/m determined by thermal tuning. The collection of the images and the stiffness data were described elsewhere (Sae-Ueng et al., 2022, 2020). The RSJ2 phage stiffness in each buffer was collected at Day 0.
2.6 Statistical analysis and data visualization
Statistical analysis was performed using the t-test function and exponential decay model in SciPy Python and LinearGAM in pyGAM Python. The t-test quantifies the difference between the two data sets. The ‘n.s.’ (not significant) denotes a p-value ≥ 0.05. The ‘*’ denotes 0.05 > p-value > 0.01. The ‘**’ denotes 0.01 > p-value > 0.001. The ‘***’ denotes a p-value < 0.001. An exponential decay model was fitted to the relative phage titer data (Table S2). The fitting returned the initial relative phage and the decay rate constant. The plot figures were generated by the Mathplotlib and Seaborn libraries in Python (Python Software Foundation, n.d.).
3 Result
3.1 RSJ2 phage morphology
A soilborne RSJ2 (or C19) phage was isolated from a soil sample in Chiang Mai, Thailand (Bhunchoth et al., 2015). The TEM micrographs (Fig. 1.) exhibited the RSJ2 phage structure consisting of capsid or head, tail, and tail fibers. The phage head contains double-stranded genomic DNA, and the phage tail functions as a delivery pass box for the DNA. The functions of the tail fibers are not only to recognize and attach the binding sites but also to have a role in phage stability by interacting with other components (e.g., tail) (Ouyang et al., 2024). The phage components agree with the morphology of the phages in the Autographiviridae family (National Center for Biotechnology Information (NCBI), n.d.).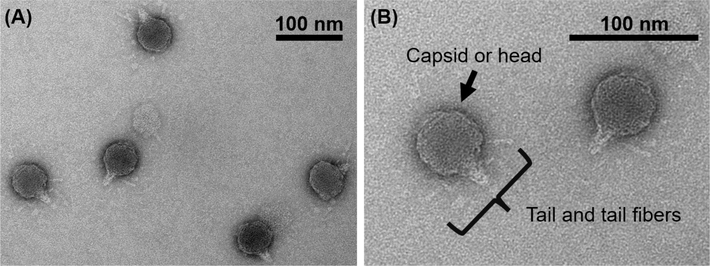
TEM micrographs of the RSJ2 phage. The scale bars in (A) and (B) are 100 nm. Tail and tail fibers of the RSJ2 phage were observed (B).
3.2 RSJ2 phage infectivity in buffers with varying pH and NaCl concentration
Single-step growth experiments were conducted to understand the infection cycle of the RSJ2 phage (Fig.S1). As the number of progeny phage particles increased, the number of host cells decreased. The reduction of the host cells was observed at about four hours after being incubated with the RSJ2 phage. One infection cycle of the RSJ2 phage took about eight hours, and the average burst size was 23 PFU per infected cell. As a soilborne phage in a tropical region like Thailand, RSJ2 is habitually exposed to constantly changing soil environments, such as lowering salt concentration due to rain or increasing salt concentration due to aridity (Arunin and Pongwichian, 2015). Understanding how phage infectivity varies as a function of salts and pH allows us to decipher how phages may survive in the fields. Knowing the infectivity dependence on pH and salts provides valuable insight into developing phage storage formulation. Therefore, we investigated how RSJ2 phage infectivity varies in buffers with varying pH and NaCl, as NaCl is prominently found in soil in Thailand (Sritongon et al., 2022). RSJ2 phage was stored in buffers at varying pH (6.0, 7.5, and 8.3) and NaCl concentration (0, 0.1, and 0.5 M) at 4–10 °C. The titers representing infectivity were recorded every 7 days for 57 days (Fig. 2. and Table S2).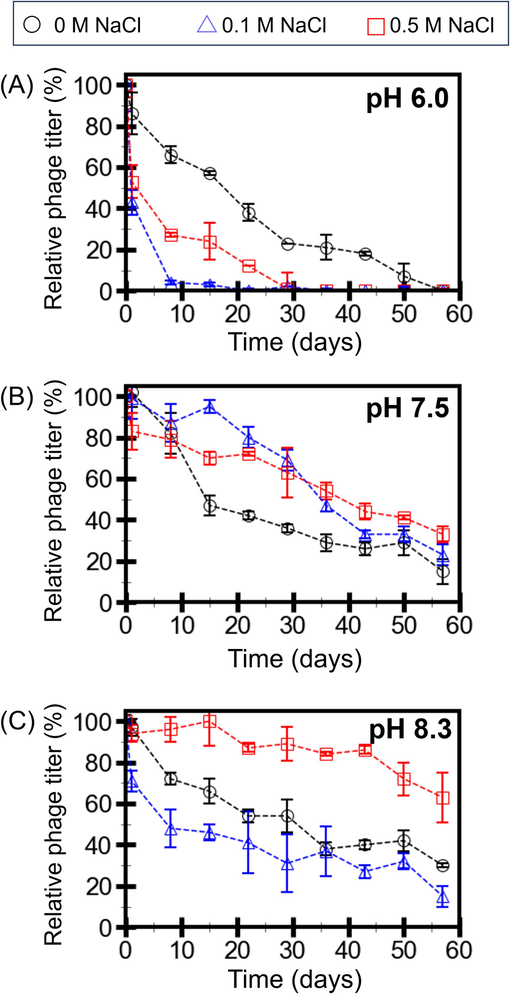
RSJ2 phage titers in buffers at pH 6.0 (A), 7.5 (B), and 8.3 (C) with 0, 0.1, and 0.5 M NaCl. The titers were recorded every 7 days and normalized by the “Day 0” titers. Error bars represent the standard deviations from the triplicate experiments.
The phage titers drastically decreased in the buffers with pH 6.0 (Fig. 2A). Without NaCl, the titers decreased to about 40 % within 3 weeks. At pH 6.0, RSJ2 phage did not favor NaCl since the titers decreased to almost 0 % within 3 weeks and a month for 0.1 and 0.5 M NaCl, respectively. The decay rate constants from fitting the exponential decay model into the data also showed that the decay rates were higher in the buffer with pH 6.0 with 0.1 and 0.5 M NaCl (Fig.S2).
The phage titers gradually decreased in the buffers with pH 7.5 (Fig. 2B). Without NaCl, the titers decreased to about 40 % within 3 weeks, similar to the buffer at pH 6.0 without NaCl. However, the titers decreased to about 60 % within a month for 0.1 and 0.5 M NaCl. The results imply that 0.1 to 0.5 M helps RSJ2 phage preserve the infectivity at pH 7.5 (but not pH 6.0). The decay rates from the buffers with 0.1 and 0.5 M were also lower than those without NaCl (Fig.S2). The results further suggest that pH and NaCl are not independent factors.
At pH 8.3, the phage titers also gradually decreased (Fig. 2C). Without NaCl and at 0.1 M NaCl, the titers decreased to about 10–40 % after two months. With 0.5 M NaCl, the titer remained at 60 % after two months of storage. The data suggest that a high concentration of NaCl (0.5 M) helps retain phage infectivity at the slightly basic pH. The decay rate in this condition was also the lowest among all 9 buffer conditions (Fig.S2). Therefore, RSJ2 phage prefers the buffer at pH 8.3 with 0.5 NaCl the most. pH 6.0, slightly acidic, also harms the RSJ2 phage the most. In other cases where the titers gradually decrease, NaCl may play some role in stabilizing phages, thus preserving phage infectivity.
3.3 Host cell adsorption of the RSJ2 phage in buffers with varying pH and NaCl concentration
The plaque assay result showed that pH and NaCl concentration significantly influenced phage infectivity. However, it is unclear which infection step is affected by pH and NaCl. Plaque formation is the outcome of a successful infection, which consists of a few crucial steps. First, the phage must remain intact before interacting with the host cell. Secondly, phage must successfully bind to the binding sites on the cell membrane. Finally, the progeny phages are synthesized before lysing the cell membrane. In the plaque assay, after the adsorption (binding) step, RSJ2 phage and Rs cells proceeded in the same conditions (e.g., in a CPG medium at the same temperature). In other words, the replication and lysis occurred under the same circumstances. Therefore, the difference in the phage infectivity in 9 buffers resulted from phage adsorption or phage structure before the binding. So, the RSJ2 phage adsorption in 9 buffers was tested. The adsorption test result revealed that the phage adsorption rate was the same in 9 buffers (Table S3). The data suggested that pH and NaCl concentration did not affect the RSJ2 phage binding. Therefore, the infectivity difference was caused by the effect of the pH and NaCl concentrations on the phage structures before the binding step.
3.4 RSJ2 phage stiffness in buffers with varying pH and NaCl concentration
Phage structural components generally respond to varying pH and salts (Mylon et al., 2010; Silva et al., 2014). The collective responses of the structural components, instead of individual elements, affect the properties and functions, including infectivity. Therefore, investigating the collective response is crucial for understanding phage infection. Phage structural responses can only be observed by high-resolution microscopic techniques. A TEM is suitable for studying static structures since the samples are dried, and imaging is collected in a vacuum (Ackermann, 2012). Collecting phage data in buffers is required to capture the in situ dynamic of structural alterations. So, we collected the phage stiffness representing nanomechanical properties in buffers with varying pH and salts with AFM-based nanoindentation. This technique caresses the cantilever with a nanometer-sized tip on the particle. The tip-particle interaction results in an image of the particle (Fig. 3A). The phage capsid and tail were observed in the AFM micrograph. The tail fibers were not observed, probably because the disordering nature of the fibers prevented their immobilization on the substrate. Due to the tip convolution, the particle size must be extracted from the Z-axis profile (Fig. 3B), revealing the capsid diameter to be about 45 nm.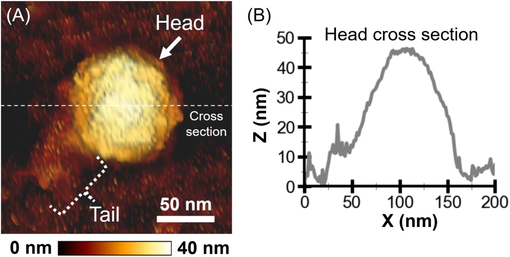
AFM micrograph and cross-section of the RSJ2 phage particle. (A) AFM micrograph of an RSJ2 phage particle in SM buffer. The underneath color gradient scale bar indicates the height (Z direction). The white scale bar (50 nm) indicated the lateral dimensions (X and Y directions). (B) The capsid diameter was extracted from the particle’s cross-section line profile (middle white dashed line in (A)).
After capturing an image of a single phage, the tip was indented into the particle to probe the phage stiffness. The applied force was measured as a function of the cantilever’s vertical displacement. The force-distance curves (Fig.S3) were converted to phage stiffness. We measured the phage stiffness in buffers at varying pH (6.0, 7.5, and 8.3) and NaCl concentration (0, 0.1, and 0.5 M) at 25 °C (Fig. 4 and Table S4). The range of the overall stiffness average was 0.050 – 0.126 N/m. At pH 6.0 (Fig. 4A), the stiffness averages of the RSJ2 phage in the buffer with 0, 0.1, and 0.5 M NaCl were 0.112, 0.126, and 0.099 N/m, respectively. The statistical analysis revealed that the phage stiffness in the buffer with 0 M NaCl was not significantly different from that with NaCl at 0.1 M and 0.5 M. However, the phage stiffness in the buffer with NaCl at 0.1 M significantly differed from that with NaCl at 0.5 M.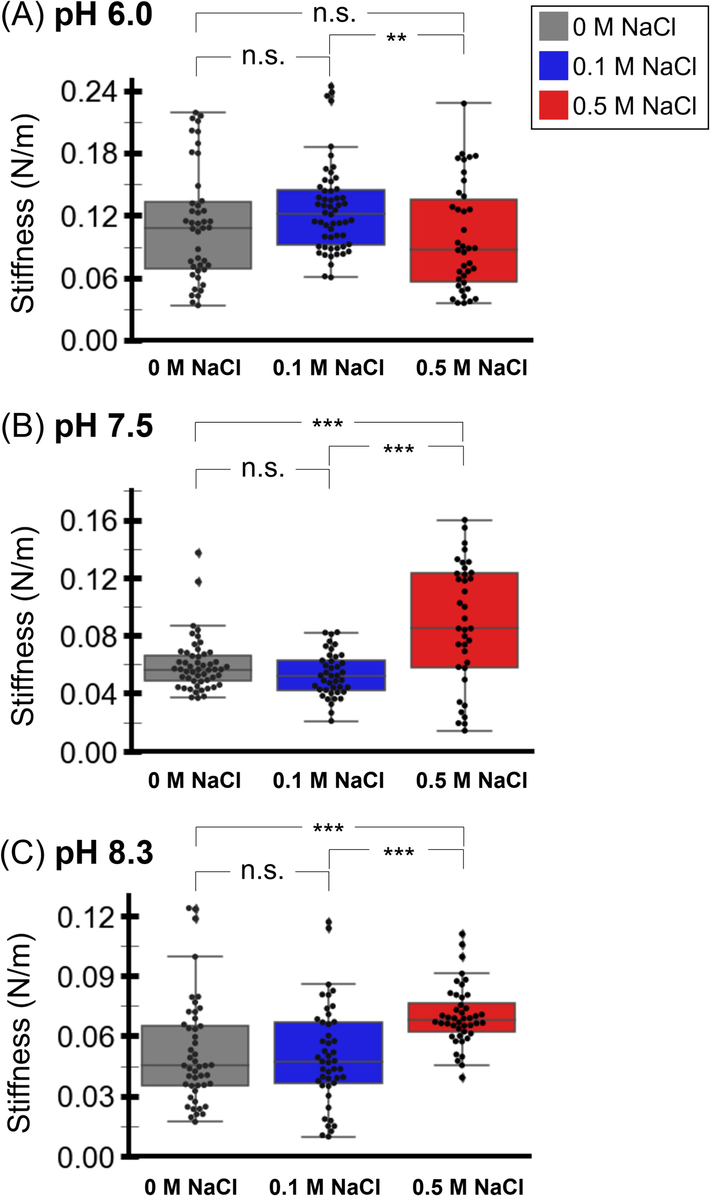
Stiffness of the RSJ2 phage particles in the buffers with pH 6.0 (A), 7.5 (B), 8.3 (C), and varying concentrations of NaCl (0, 0.1, 0.5 M). A statistical analysis of the phage stiffness at the same pH was performed. The ‘n.s.’ (not significant) denotes a p-value ≥ 0.05. The ‘*’ denotes 0.05 > p-value > 0.01. The ‘**’ denotes 0.01 > p-value > 0.001. The ‘***’ denotes a p-value < 0.001.
At pH 7.5 (Fig. 4B), the stiffness averages in the buffer with 0, 0.1, and 0.5 M NaCl were 0.060, 0.053, and 0.088 N/m, respectively. The phage stiffness in these buffers was lower than in the buffers at pH 6.0, implying softer phage particles at pH 7.5. The statistical analysis revealed no significant difference in the phage stiffness between the buffer with 0 M and 0.1 M NaCl. However, the phage stiffness in the buffer with NaCl at 0 and 0.1 M significantly differed from that with NaCl at 0.5 M.
Lastly, at pH 8.3 (Fig. 4C), the phage stiffness averages in the buffer with 0, 0.1, and 0.5 M NaCl were 0.050, 0.052, and 0.070 N/m, respectively. The phage stiffness in the buffers at pH 8.3 was also lower than that in the buffers at pH 6.0. The statistical analysis revealed that the stiffness of the phage in the buffer with 0 M NaCl was not significantly different from that with 0.1 M NaCl. However, the phage stiffness in the buffer with NaCl at 0 and 0.1 M significantly differed from that with NaCl at 0.5 M. The stiffness results show that the phage stiffness strongly depends on the storage buffers’ pH and NaCl concentration.
4 Discussion
In this work, the RSJ2 phage titers were affected by the pH and NaCl concentration of the storage buffer (Fig. 2). The titers rapidly decreased in the buffers at pH 6.0. The titers gradually reduced in the buffers at pH 7.5, remaining at 15–33 % after two months of storage. The titers of RSJ2 phage in the buffers at pH 8.3 depended on the NaCl concentration. RSJ2 phage is the most stable in the buffer at pH 8.3 with 0.5 M NaCl, remaining at about 63 % after two-month storage, while in the buffers with 0 and 0.1 M NaCl, the phage titers remained at 30 and 15 %, respectively. We tested if the buffers may affect the phage adsorption to the host cells (Table S3). The phage adsorption was not altered by pH and NaCl concentration. Therefore, the titer difference is caused by the direct effect of pH and NaCl concentration on the phage particles.
We measured the phage stiffness to investigate the in situ effect of pH and NaCl on the phage particles. The phage stiffness largely depended on the pH and NaCl concentration of the buffers. In addition to statistical analysis of the stiffness data at each pH, each stiffness data set (a total of 9 data sets) was compared by the independent-sample t-test pairwise (Table S5). The analysis result indicated that the phage stiffness in the buffer at pH 8.0 and 0.5 M NaCl was distinctively different from the phage stiffness in the other eight buffers, with a significant difference with p-value < 0.05.
The effect of pH and NaCl concentration causes the difference in phage stability in 9 buffers. pH and NaCl can influence phage stability dynamics. The stability dynamics depend on how phage structures respond to external fluctuations, such as pH, ionic strength, and temperature (Sae-Ueng et al., 2022, 2020). While the phage structure may seem simple, consisting of external protein capsid and internal genomic DNA, the responsive dynamics of each component can be complex. The behaviors of the capsid depend on protein physio-chemical properties, protein–protein interaction, and hydrophobicity-hydrophilicity. A study revealed that a hydrophobic network of capsid proteins supports phage stability (Asija and Teschke, 2019). Another study showed that hydrophobicity and hydrophilicity of phage proteins can regulate the phage structure and infection (Dika et al., 2015). In our study, pH and NaCl gradients can perturb such protein interactions, causing the difference in phage infectivity, shelf life, and stiffness.
Phage stiffness has been recently proposed to be associated with infectivity (Sae-Ueng et al., 2022, 2020). An association between the RSJ2 phage stiffness and its infectivity was examined by statistical analysis of the stiffness and titer with the generalized additive model (Wood et al., 2015). The analysis revealed a large pseudo-r-squared value of 0.9767, indicating a correlated stiffness and titer of the RSJ2 phage. The analysis suggests that the phage stiffness in the buffer at pH 8.0 and 0.5 M NaCl is correlated to the maximum remaining phage titer after two months of storage. The average phage stiffness in this buffer is 0.070 N/m, which is approximately the intermediate stiffness value of the entire RSJ2 stiffness (0.050–0.126 N/m).
Since the phage stiffness results from the phage capsid and packaged DNA reinforcement, the result implies that the phage capsid and DNA are in the intermediate state. Phage capsid protects the phage particle and thus contributes to high-range stiffness. Phage DNA requires fluidity to transfer to the host cell, thus contributing to low-range stiffness (Liu et al., 2014). The intermediate stiffness is the result of balancing between two components. Phages in this state can readily shift to a high-stiffness state to protect themselves or to a low-stiffness state to release the genome. However, since our study did not separately measure the stiffness of the capsid and DNA, the hypothesis on the RSJ2 phage stiffness contribution could not be verified. Different experimental designs to study the effect of pH and NaCl on packed DNA and empty capsids will be needed to deconvolute each contribution. That way, we can truly understand the phage structure and stiffness regulation by pH, NaCl, and other factors.
The stiffness-titer association of the RSJ2 phage agrees with the work of the C22 phage (Sae-Ueng et al., 2020). The study showed that the intermediate stiffness (0.07–0.12 N/m) compared to the entire C22 phage stiffness (0.03–0.17 N/m) is associated with the high remaining titer. The intermediate stiffness of the C22 phage has a broader range than that of the RSJ2 phage. A narrow intermediate RSJ2 stiffness range implies that the RSJ2 phage can only survive in narrowly fluctuating factors, potentially reflecting higher environmental sensitivity than the C22 phage. The higher sensitivity of the RSJ2 phage can be due to different morphology between the two phages. The RSJ2 phage (Autographiviridae family) has a slightly longer tail with tail fibers, while the C22 phage (Podoviridae family) has a shorter tail with no observable fibers. The phage fibers were fragile structures, and losing the fibers resulted in unsuccessful infection. These fibers may contribute to the higher sensitivity and narrower intermediate stiffness range.
Our findings on RSJ2 and C22 phages suggest a link between intermediate stiffness and the metastable state of phage. Viral metastability refers to the virus’s ability to protect itself from harsh surroundings while readily infecting a host cell upon binding (Ramesh et al., 2019). Phage particles in the intermediate-stiffness state may allow them to quickly shift between the protecting state (high stiffness) and the infection state (low stiffness). The correlation between stiffness and phage titer also implies that intermediate stiffness may be one of the attributes of metastability proposed as a key for viral infection.
5 Conclusion
We studied the effects of the buffer pH and NaCl concentration on the survival and the nanomechanics of the RSJ2 phage. Both factors directly alter phage stiffness associated with the remaining titers. The highest remaining titer of the RSJ2 phage (>60 % during the two-month storage) is associated with the intermediate phage stiffness, demonstrated in another short-tailed phage. The role of responsive phage stiffness in phage infectivity was discussed. Deciphering the precise underlying mechanisms of the phage stiffness alternation, such as structural alternation, as a function of external triggering factors requires future studies with complementary techniques. Understanding the stiffness manipulation by external factors will allow us to learn how such factors regulate and control phage survival and shelf life. Determining appropriate pH and salt concentration to extend phage shelf life, which mainly relies on trial-and-error approaches, is laborious and time-consuming. Linking phage stiffness to the phage shelf life under specific buffers can be helpful and improved in predicting phage shelf life, promoting phage use in biocontrol applications.
CRediT authorship contribution statement
Udom Sae-Ueng: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Chooseel Bunsuwansakul: Writing – review & editing, Validation, Methodology, Formal analysis. Kittiya Showpanish: Writing – review & editing, Validation, Methodology, Formal analysis. Namthip Phironrit: Writing – review & editing, Resources, Methodology, Formal analysis. Chaweewan Sapcharoenkun: Writing – review & editing, Resources, Methodology, Formal analysis. Alongkot Treetong: Writing – review & editing, Resources, Methodology, Formal analysis. Jidapa Thadajarassiri: Writing – review & editing, Resources, Methodology, Formal analysis.
Acknowledgment
The RSJ2 phage was obtained from Dr. Orawan Chatchawankanphanich, BIOTEC, NSTDA, Thailand. Ralstoina solanacearum strain RS10/3 (race 1 biovar 3) was obtained from Assoc.Prof.Dr. Chalida Leksomboon, Department of Plant Pathology, Faculty of Agriculture at Kamphaeng Saen, Kasetsart University, Kamphaeng Saen Campus. The authors appreciated access to the TEM facility at the NSTDA Characterization and Testing Service Center (NCTC), NSTDA, Thailand. Furthermore, the authors acknowledged Prof. Dr. Thomas Gutsmann for access to the AFM facility at the Centre for Structural Systems Biology (CSSB), Germany. Moreover, the authors acknowledge the contributions of the research intern students from 2019 to 2023 in the Biomolecular Analysis and Application Laboratory, BIOTEC, NSTDA, Thailand.
Disclosure of Funding
This research has received funding support from the NSRF via the Program Management Unit for Human Resources & Institutional Development, Research, and Innovation (Grant number B16F640116) and National Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Technology Development Agency (NSTDA), Thailand (project number P21-51892). The funders had no role in the study’s design, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Detection of bacteriophages: phage plaques. In: Bacteriophages. Springer International Publishing; 2018. p. :1-32. 10.1007/978-3-319-40598-8_16-1
- [Google Scholar]
- Salt-affected soils and management in Thailand. Bull. Soc. Sea Water Sci. Japan. 2015;69:319-325.
- [Google Scholar]
- A Hydrophobic Network: Intersubunit and Intercapsomer Interactions Stabilizing the Bacteriophage P22 Capsid. J. Virol.. 2019;93
- [CrossRef] [Google Scholar]
- Isolation of Ralstonia solanacearum-infecting bacteriophages from tomato fields in Chiang Mai, Thailand, and their experimental use as biocontrol agents. J. Appl. Microbiol.. 2015;118:1023-1033.
- [CrossRef] [Google Scholar]
- Phages in natureBacteriophage. 2011;1:31.
- [CrossRef]
- Enhancing the stability of bacteriophages using physical, chemical, and nano-based approaches: A review. Pharmaceutics. 2022;14:1936.
- [CrossRef] [Google Scholar]
- Isoelectric point is an inadequate descriptor of MS2, Phi X 174 and PRD1 phages adhesion on abiotic surfaces. J. Colloid Interface Sci.. 2015;446:327-334.
- [CrossRef] [Google Scholar]
- Evaluation of the stability of bacteriophages in different solutions suitable for the production of magistral preparations in Belgium. Viruses. 2021;13:865.
- [CrossRef] [Google Scholar]
- Phenotypic and molecular characterization of two lytic bacteriophages against multidrug resistant Pseudomonas aeruginosa. Saudi J. Biol. Sci. 2022
- [CrossRef] [Google Scholar]
- Mechanical capsid maturation facilitates the resolution of conflicting requirements for herpesvirus assembly. J. Virol. 2021
- [CrossRef] [Google Scholar]
- Influence of environmental factors on phage-bacteria interaction and on the efficacy and infectivity of phage P100. Front. Microbiol.. 2016;7
- [CrossRef] [Google Scholar]
- The future of phage biocontrol in integrated plant protection for sustainable crop production. Curr. Opin. Biotechnol.. 2021;68:60-71.
- [CrossRef] [Google Scholar]
- Internal DNA pressure modifies stability of WT phage. PNAS. 2007;104:9603-9608.
- [CrossRef] [Google Scholar]
- Factors determining phage stability/activity: challenges in practical phage application. Expert Rev. Anti Infect. Ther. 2019
- [CrossRef] [Google Scholar]
- Solid-to-fluid-like DNA transition in viruses facilitates infection. PNAS. 2014;111:14675-14680.
- [CrossRef] [Google Scholar]
- How the phage T4 injection machinery works including energetics, forces, and dynamic pathway. PNAS. 2019;116:25097-25105.
- [CrossRef] [Google Scholar]
- Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017
- [CrossRef] [Google Scholar]
- Atomic Force Microscopy-Based Force Spectroscopy and Multiparametric Imaging of Biomolecular and Cellular Systems. Chem. Rev.. 2021;121:11701-11725.
- [CrossRef] [Google Scholar]
- Influence of salts and natural organic matter on the stability of bacteriophage MS2. Langmuir. 2010;26:1035-1042.
- [CrossRef] [Google Scholar]
- National Center for Biotechnology Information (NCBI), n.d. Ralstonia phage RSJ2 DNA, complete genome - Nucleotide - NCBI [WWW Document]. URL https://www.ncbi.nlm.nih.gov/nuccore/AB920995.1 (accessed 1.3.23).
- Phage fibers and spikes: a nanoscale Swiss army knife for host infection. Curr. Opin. Microbiol.. 2024;77:102429
- [CrossRef] [Google Scholar]
- Python Software Foundation, n.d. PyPI · The Python Package Index [WWW Document]. URL https://pypi.org/ (accessed 4.21.24).
- Uncovering metastability and disassembly hotspots in whole viral particles. Prog. Biophys. Mol. Biol. 2019
- [CrossRef] [Google Scholar]
- Positive and negative aspects of bacteriophages and their immense role in the food chain. npj Sci. Food. 2024;8(1):1-13.
- [CrossRef] [Google Scholar]
- C22 podovirus infectivity is associated with intermediate stiffness. Sci. Rep.. 2020;10(1)
- [CrossRef] [Google Scholar]
- Thermoresponsive C22 phage stiffness modulates the phage infectivity. Sci. Rep.. 2022;12:1-9.
- [CrossRef] [Google Scholar]
- A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015
- [CrossRef] [Google Scholar]
- Influence of environmental variables in the efficiency of phage therapy in aquaculture. J. Microbial. Biotechnol.. 2014;7:401.
- [CrossRef] [Google Scholar]
- The effect of salinity on soil chemical characteristics, enzyme activity and bacterial community composition in rice rhizospheres in Northeastern Thailand. Sci. Rep.. 2022;12:1-12.
- [CrossRef] [Google Scholar]
- Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health. 2021;18:1-24.
- [CrossRef] [Google Scholar]
- Generalized Additive Models for Large Data Sets. J. R. Stat. Soc. Ser. C. Appl. Stat.. 2015;64:139-155.
- [CrossRef] [Google Scholar]
- A review of bacteriophage therapy for pathogenic bacteria inactivation in the soil environment. Environ. Int.. 2019;129:488-496.
- [CrossRef] [Google Scholar]
- Recent trends in the use of bacteriophages as replacement of antimicrobials against food-animal pathogens. Front Vet Sci. 2023;10:1162465.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103344.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







