Translate this page into:
Schisandra chinensis: A comprehensive review on its phytochemicals and biological activities
⁎Corresponding author.
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Schisandra chinensis (Turcz.) Baill is a climbing plant widely distributed in the northeastern part of China, Korea, and Japan, and used in traditional Chinese herb as a tonic, antitussive, and sedative agent. This review focuses on the phytochemicals, biological activities and analytical methods, in order to promote further studies on the plant. 202 chemical compounds have been isolated and identified from this plant, and the most important are dibenzocyclooctadiene lignans and triterpenoids. The isolated compounds of S. chinensis were shown to possess anti-cancer, anti-oxidant, neuroprotective, hepatoprotective, anti-inflammatory activities and so on. Further studies should be carried on this plant in order to disclose many more active principles and mechanisms of active components.
Keywords
Schisandra chinensis
Phytochemicals
Triterpenoids
Dibenzocyclooctadiene lignans
Biological activities
1 Introduction
Schisandra chinensis (Turcz.) Baill belongs to Schisandraceae, is distributed in the northeastern part of China, Korea, and Japan. The dried fruit of this plant, have been used in traditional Chinese herb as a tonic, antitussive, and sedative agent under the name of ‘‘wu-wei zi’’, has been historically used for the treatment of hepatitis for over 2000 years (Hancke et al., 1999; Xue et al., 2015). Previous phytochemical investigations have identified a variety of secondary metabolites, such as lignans, triterpenoids, diterpenoids, sesquiterpenoids, monoterpenes, and fatty acids. Modern pharmacological studies have shown that the extracts and compounds of S. chinensis possess a broad range of biological activities, such as anti-cancer, anti-oxidant, neuroprotective, hepatoprotective, anti-inflammatory activities, etc. Due to their great structural diversity and broad relevant bioactivities, S. chinensis have attracted increasing research attention. Sowndhararajan et al. reported the lignan extracts and individual compounds from S. chinensis were summarized in relation to their neuroprotective and cognitive enhancement activities (Sowndhararajan et al., 2018).
In the last decade, there has been a dramatic progress in the chemical constituents and relevant biological activities. However, so far, no comprehensive review has been published. In the present review, we summarize systematically the research advances on the chemical constituents, their biological activities and analytical methods of S. chinensis reported in the literature, as found on Web of Science, Sciencedirect, SpringerLink, ACS, Taylor&Francis, PubMed, and Thieme, with the objective of providing a basis for further research of natural product drug discovery.
2 Chemical constituents
To date, 202 chemical compounds have been identified and isolated from S.chinensis, including eighty-three triterpenoids (1–83), one diterpenoid (84), twelve sesquiterpenoids (85–96), fifteen monoterpenoids (97–111), eighty-six lignans (112–197), five fatty acids (198–202). It can be seen that, lignans and triterpenoids are the dominant chemical constituents in this plant. Their names, structures, and references are summarized in Tables 1–5 and Figs. 1–9.
NO.
Compound class and name
Ref.
Lanostane triterpenoids
1
epi-anwuweizic acid
Zhang et al. (2013)
2
micranoic acid A
Huang et al. (2008)
3
kadsuric acid
Huang et al. (2008)
4
schisanlactone I
Qiu et al. (2018)
Cycloartane triterpenoids
5
cis-3-oxo-cycloart-24-ene-26-oic acid = ganwuweizic acid
Huang et al. (2008)
6
schinalactone D
Qiu et al. (2018)
7
wuweizilactone acid
Huang et al. (2008)
8
kadcoccilactone Q
Xue et al. (2010)
9
kadsuphilactone B
Xue et al. (2010)
10
schinchinenin G
Song et al. (2013)
11
schinchinenin H
Song et al. (2013)
12
henrischinin C
Song et al. (2013)
13
schisanlactone J
Qiu et al. (2018)
14
schisanlactone C
Qiu et al. (2018)
15
henrischinin A
Song et al. (2013)
16
henrischinin B
Song et al. (2013)
17
schinchinenlactone C
= schisphendilactone BSong et al. (2013)Qiu et al. (2018)
18
schinchinenlactone B
Song et al. (2013)
19
schinchinenin D
Song et al. (2013)
20
schinchinenin B
Song et al. (2013)
21
schinchinenin C
Song et al. (2013)
22
schinchinenin E
Song et al. (2013)
23
schinchinenin F
Song et al. (2013)
24
schinchinenin A
Song et al. (2013)
25
schinchinenlactone A
Song et al. (2013)
Nortriterpenoids
Pre-schisanartane nortriterpenoids
26
pre-schisanartanin A
= pre-schisanartaninHuang et al. (2007a)
27
pre-schisanartanin B
Huang et al. (2008)
28
pre-schisanartanin F
Shi et al. (2014)
29
arisanlactone C
Shi et al. (2014)
30
pre-schisanartanin N
Shi et al. (2014)
31
pre-schisanartanin E
Shi et al. (2014)
32
schisdilactone J
Shi et al. (2014)
Schisanartane nortriterpenoids
33
schindilactone C
Huang et al. (2007a)
34
schindilactone I
Shi et al. (2014)
35
schindilactone J
Shi et al. (2014)
36
lancifodilactone I
Xue et al. (2010)
37
schindilactone A
Huang et al. (2007a)
38
schindilactone B
Huang et al. (2007a)
39
schindilactone K
Shi et al. (2014)
40
schindilactone H
Xue et al. (2010)
41
lancifodilactone N
Xue et al. (2010)
42
lancifodilactone D
Xue et al. (2010)
43
lancifodilactone C
Huang et al. (2007a)
44
henridilactone D
Huang et al. (2007a)
45
lancifodilactone L
Xue et al. (2010)
46
schindilactone D
Huang et al. (2008)
47
schindilactone E
Huang et al. (2008)
48
arisanlactone B
Shi et al. (2014)
49
micrandilactone A
Shi et al. (2014)
50
schindilactone F
Huang et al. (2008)
51
schindilactone L
Yang et al. (2018)
52
schindilactone G
Huang et al. (2008)
53
schindilactone M
Yang et al. (2018)
18-Nor-schiartane nortriterpenoids
54
wuweizidilactone A
Huang et al. (2007c)
55
wuweizidilactone B
Huang et al. (2007c)
56
wuweizidilactone I
Xue et al. (2010)
57
wuweizidilactone L
Shi et al. (2014)
58
wuweizidilactone M
Shi et al. (2014)
59
wuweizidilactone N
Shi et al. (2014)
60
wuweizidilactone G
Huang et al. (2008)
61
wuweizidilactone O
Shi et al. (2014)
62
wuweizidilactone H
Huang et al. (2008)
63
wuweizidilactone P
Shi et al. (2014)
64
propindilactone Q
Shi et al. (2014)
65
wuweizidilactone S
Yang et al. (2018)
66
19(R)-hydroxyl-wuweizidilactone H
Li et al. (2017)
18 (13 → 14)-abeo-Schiartane nortriterpenoids
67
wuweizidilactone C
Huang et al. (2007c)
68
wuweizidilactone D
Huang et al. (2007c)
69
wuweizidilactone E
Huang et al. (2007c)
70
wuweizidilactone F
Huang et al. (2007c)
71
wuweizidilactone J
Shi et al. (2014)
72
wuweizidilactone K
Shi et al. (2014)
Wuweiziartane nortriterpenoids
73
schintrilactone A
Huang et al. (2007b)
74
schintrilactone B
Huang et al. (2007b)
75
propintrilactone A
Shi et al. (2014)
76
propintrilactone B
Shi et al. (2014)
Schiartane nortriterpenoids
77
micrandilactone B
Huang et al. (2007a)
78
micrandilactone C
Kim et al. (2015)
Other type of nortriterpenoids
79
schicagenin A
Shi et al. (2011)
80
schicagenin B
Shi et al. (2011)
81
schicagenin C
Shi et al. (2011)
Ursane triterpenoids
82
ursolic acid
Huang et al. (2008)
83
2α,3β,19α-trihydroxy-urs-12-en-28-oic acid
Huang et al. (2008)
NO.
Compound class and name
Ref.
Diterpenoid
84
7-oxocallitrisic acid
Huang et al. (2008)
Sesquiterpenoids
85
guaidiol
Liu et al. (2020)
86
α-iso-cubebene
Lee et al. (2009)
87
α-iso-cubebenol
Lee et al. (2010)
88
α-iso-cubebenol acetate
Guo et al. (2020)
89
(−)-clovane-2,9-diol
Huang et al. (2008)
90
widdaranal A
Venkanna et al. (2014)
91
widdaral B
Venkanna et al. (2014)
92
15-hydroxy-α-cadinol
Liu et al. (2020)
93
β-chamigrenal
Venkanna et al. (2014)
94
(6R)-β-chamigrenic acid
Li et al. (2017)
95
iso-cuparenal
Venkanna et al. (2014)
96
3β-hydroxy-5α,6α-epoxy-β-ionone
Liu et al. (2020)
NO.
Compound class and name
Ref.
97
schisandenoid A
Liu et al. (2020)
98
thymoquinol 5-O-α-L-arabinopyranosyl- (1 → 6)-β-D-glucopyranoside
Liu et al. (2019)
99
thymoquinol 5-O-β-D-glucopyranoside
Liu et al. (2019)
100
thymoquinol 2-O-β-D-glucopyranoside
Liu et al. (2019)
101
thymoquinol 2-O-β-D-apiofuranosyl-(1 → 6)-β-D-glucopyranoside
Yang et al. (2016)
102
thymoquinol 2-O-α-D-arabinofuranosyl-
(1 → 6)-β-D-glucopyranosideYang et al. (2016)
103
thymoquinol 5-O-β-D-apiofuranosyl-(1 → 6)- β-D-glucopyranoside
Yang et al. (2016)
104
thymoquinol 5-O-α-D-arabinofuranosyl- (1 → 6)-β-D-glucopyranoside
Yang et al. (2016)
105
(R)-p-cymene 9-O-α-D-arabinofuranosyl- (1 → 6)-β-D-glucopyranoside
Liu et al. (2020)Liu et al. (2019)
106
(R)-p-cymene 9-O-β-D-apiofuranosyl- (1 → 6)-β-D-glucopyranoside
Liu et al. (2019)
107
(R)-p-cymene 9-O-β-D-glucopyranoside
Liu et al. (2019)
108
cuminic acid 7-O-α-D- arabinofuranosyl- (1 → 6)-β-D-glucopyranosyl ester
Liu et al. (2019)
109
p-cymene 7-O-α-D-arabinofuranosyl-(1 → 6)-β-D-glucopyranoside
Liu et al. (2019)
110
p-methylhydratropic acid 9-O-α-D-arabinofuranosyl-(1→
6)-β-D-glucopyranosyl esterLiu et al. (2019)
111
p-menthane-1, 8, 9-triol 9-O-β-D-
glucopyranosideDai et al. (2005)
NO.
Compound class and name
Ref.
Dibenzocyclooctadiene lignans
R-biphenyl configuration
112
schisandrin A = schizandrin A
Choi et al. (2006)
113
schisandrin
=schizandrol A = schizandrinKochetkov et al. (1961)
114
isoschizandrin
Ikeya et al. (1988a)
115
schizandrin B= (±)-γ-schizandrin
Sovová et al. (2007)
116
gomisin H
Ikeya et al. (1979e)
117
(+)-tigloylgomisin H
Ikeya et al. (1978)
118
angeloylgomisin H
Ikeya et al. (1978);
119
benzoylgomisin H
Ikeya et al. (1978)
120
(+)-gomisin K2
Nakajima et al. (1983)
121
deoxyschizandrin = deoxyschisandrin
Kochetkov et al. (1962a)
122
gomisin A = schisandrol B
Taguchi et al. (1977)
123
schisandroside E
Liu et al. (2020)
124
schisandroside B
Kim et al. (2015)
125
schisandroside A
Kim et al. (2015)
126
14-tigloylschinlignan D
Pel et al. (2017)
127
(−)-gomisin M1
Hu et al. (2014)
128
(−)-neglschisandrin E
Pel et al. (2017)
129
schinlignan D
Xue et al. (2015)
130
(+)-schilignan E
Xue et al. (2015)
131
schinlignan F
Xue et al. (2015)
132
schinlignan G
Xue et al. (2015)
133
schisanchinin B
Hu et al. (2014)
134
neglschisandrin E
Xue et al. (2015)
135
schisanchinin C
Hu et al. (2014)
136
schisanchinin D
Hu et al. (2014)
137
(+)-gomisin M2
Hu et al. (2014)
138
(+)-gomisin K3
Hu et al. (2014)
139
(+)-14-tigloylgomisin K3
Pel et al. (2017)
140
micrantherin A
Li et al. (2017)
141
gomisin T
Ikeya et al. (1988b)
142
schinlignan A
Xue et al. (2015)
143
schischinone
Xue et al. (2015)
S-biphenyl configuration
144
(−)-gomisin K1
Choi et al. (2006)
145
gomisin J
Šmejkal et al. (2010)
146
schisantherin A = gomisin C
Taguchi et al. (1977)
147
gomisin F
Taguchi et al. (1977)
148
(−)-tigloyl-deangeloyl-gomisin F
Šmejkal et al. (2010)
149
angeloylgomisin Q
Ikeya et al. (1979d)
150
benzoylgomisin Q
Piao et al. (2005)
151
gomisin G
Taguchi et al. (1977)
152
schizandrin C = schisandrin C = wuweizisu C
Pel et al. (2017)Choi et al. (2006)
153
gomisin N
Choi et al. (2006)
154
gomisin B
Taguchi et al. (1977)
155
schisandrene
Choi et al. (2006)
156
(−)-gomisin L1
Hu et al. (2014)
157
(−)-gomisin L2
Pel et al. (2017)
158
(−)-rubrisandrin B
Pel et al. (2017)
159
(−)-tigloylgomisin P
Ikeya et al. (1980)
160
(−)-angeloylgomisin P
Ikeya et al. (1980)
161
schinlignan B
Xue et al. (2015)
162
schinlignan C
Xue et al. (2015)
163/164
rubrisandrins A
Xue et al. (2015)
165
methylgomisin O
Xue et al. (2015)
166
wuweilignan E
Xue et al. (2015)
167
schisanchinin A
Hu et al. (2014)
168
(−)-tigloylgomisin Q
Pel et al. (2017)
169
1,2,13,14-tetramethoxydibenzocyclooctadiene 3,12-O-β-D-diglucopyranoside
Yang et al. (2016)
170
3,7-dihydroxy-1,2,13,14-tetramethoxydibenzocyclooctadiene 12-O-β-D -glucopyranoside
Yang et al. (2016)
171
schisandroside C
Kim et al. (2015)
172
schisandroside D
Kim et al. (2015)
173
gomisin R
Ikeya et al. (1982a)
174
schisantherin D
Ikeya et al. (1982a)
175
gomisin O
Ikeya et al. (1979b)
176
epigomisin O
Ikeya et al. (1979b)
177
angeloylgomisin O
Ikeya et al. (1982b)
178
angeloylisogomisin O
Ikeya et al. (1982b)
179
benzoylisogomisin O
Ikeya et al. (1982b)
180
gomisin S
Ikeya et al. (1988b)
181
gomisin E
Ikeya et al. (1979b)
182
gomisin D
Ikeya et al. (1979c)
Other types of lignans
183
D-epigalbacin
Zhang et al. (2013)
184
machilin G
Zhang et al. (2013)
185
chicanine
Zhang et al. (2013)
186
schinlignin A
Xue et al. (2010)
187
schinlignin B
Xue et al. (2010)
188
rel-(7R, 8R, 7′R, 8′R)-manglisin E
Pel et al. (2017)
189
anwulignan
Zhang et al. (2013)
190
schineolignin A
Xue et al. (2010)
191
schineolignin B
Xue et al. (2010)
192
schineolignin C
Xue et al. (2010)
193
pregomisin
Xue et al. (2010)
194
meso-dihydroguaiaretic acid
Xue et al. (2010)
195
8,8′-dihydroxypinoresinol
Liu et al. (2020)
196
8-hydroxypinoresinol
Liu et al. (2020)
197
pinobatol-9-O-β-D-glucopyranoside
Yang et al. (2016)
NO.
Compound class and name
Ref.
198
dimethyl-malate
Pel et al. (2017)
199
methyl-malate
Pel et al. (2017)
200
butyl-1-methyl malate
Pel et al. (2017)
201
1,5-dibutyl-1′-methyl citrate
Pel et al. (2017)
202
1-butyl-1′,5-dimethyl citrate
Pel et al. (2017)
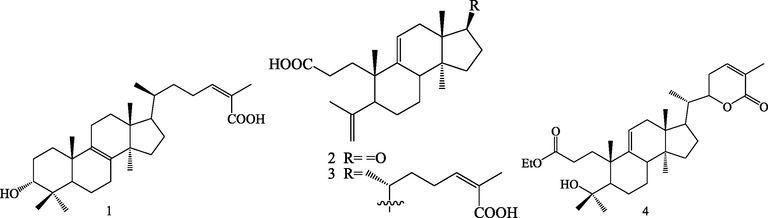
Structures of lanostane triterpenoids (1–4).
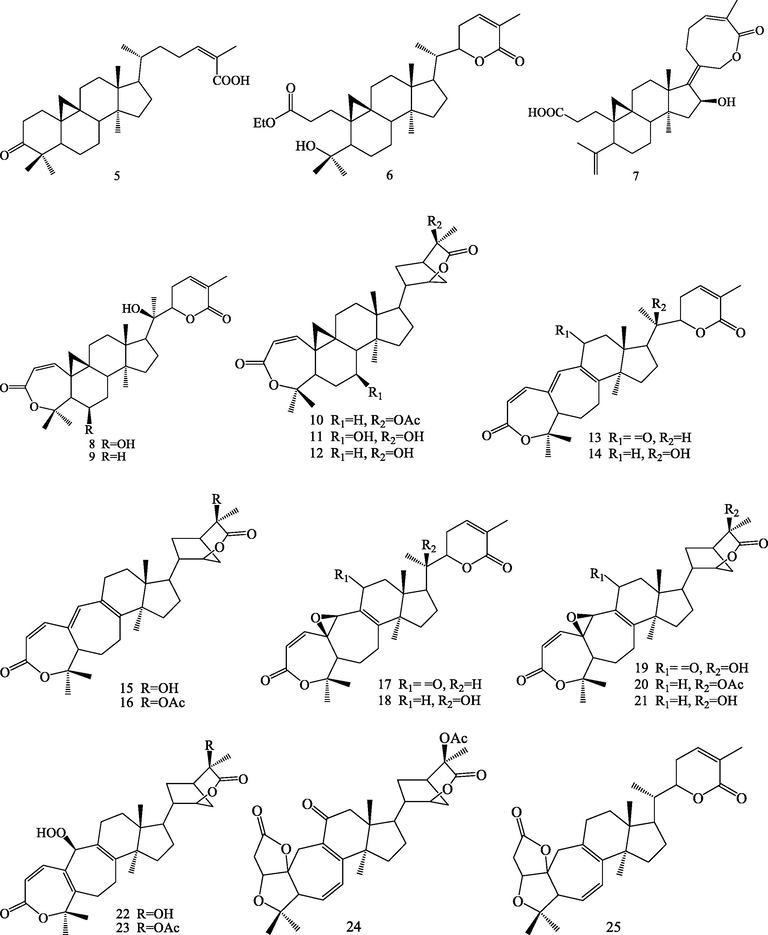
Structures of cycloartane triterpenoids (5–25).
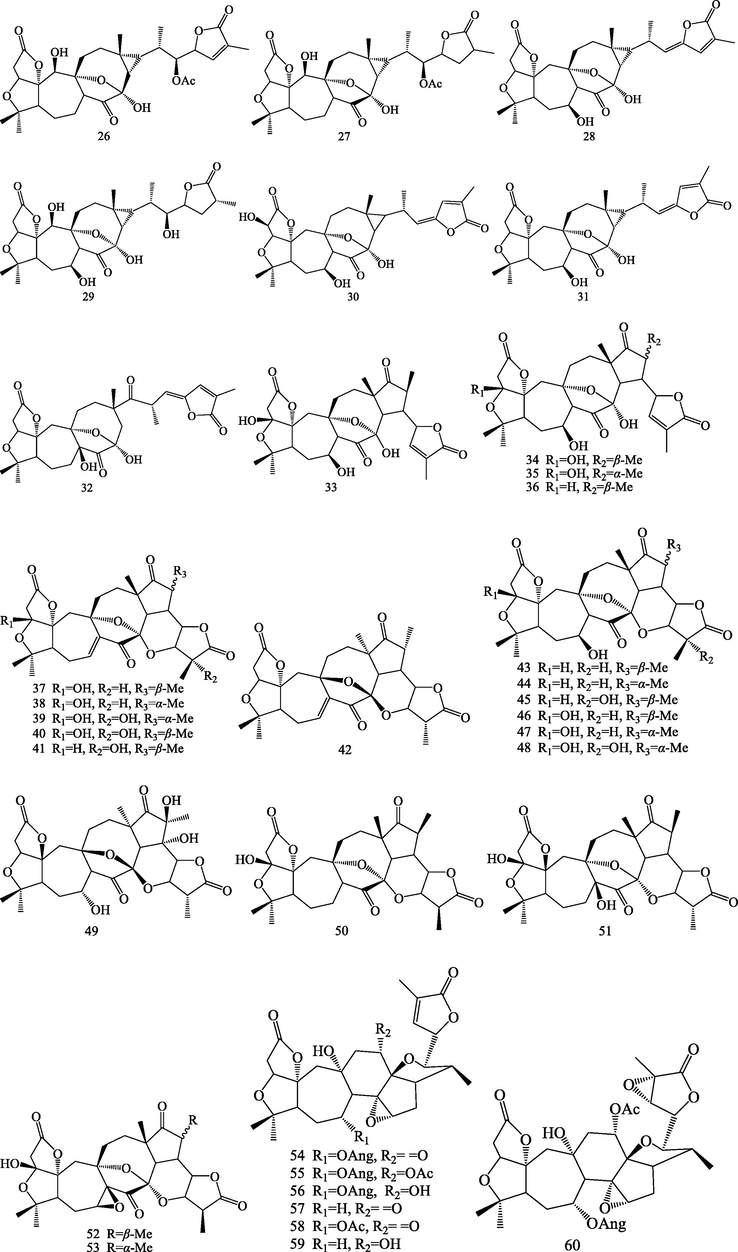
Structures of nortriterpenoids (26–81).
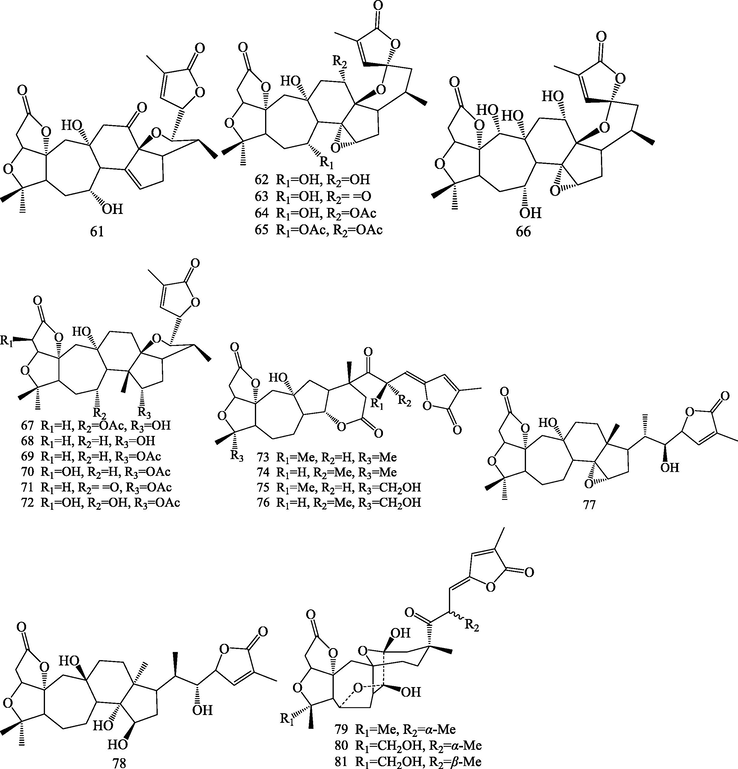
Structures of nortriterpenoids (26–81).
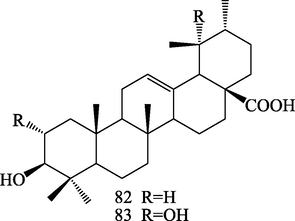
Structures of ursane triterpenoids (82–83).
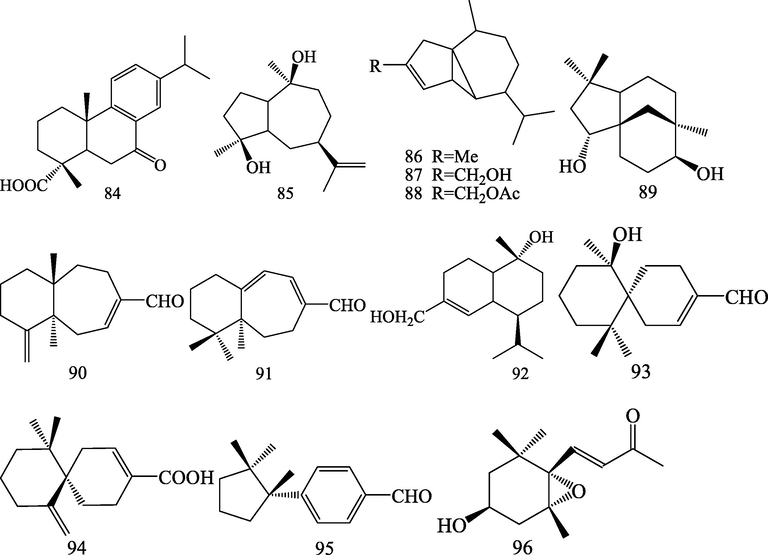
Structures of diterpenoid (84) and sesquiterpenoids (85–96).
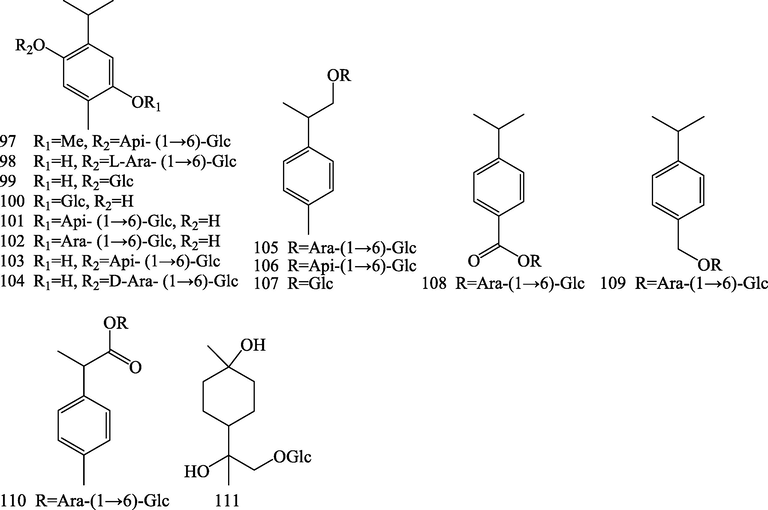
Structures of monoterpenoids (97–111).
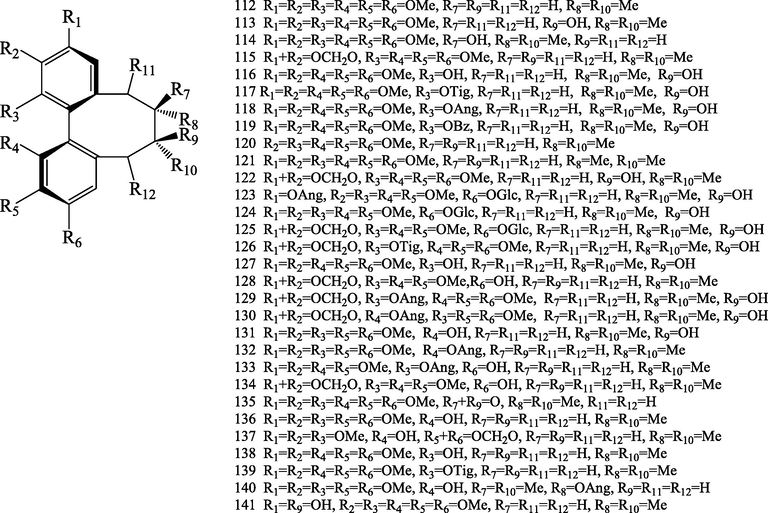
Structures of dibenzocyclooctadienes lignans (112–182).
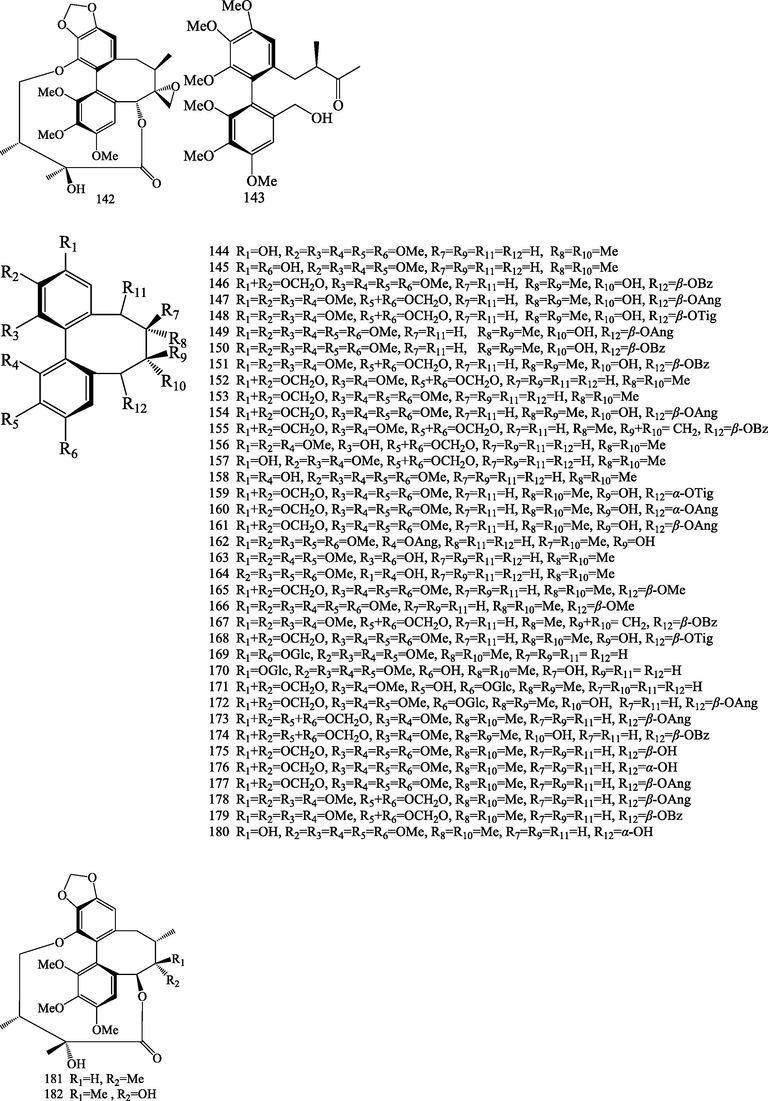
Structures of dibenzocyclooctadienes lignans (112–182).
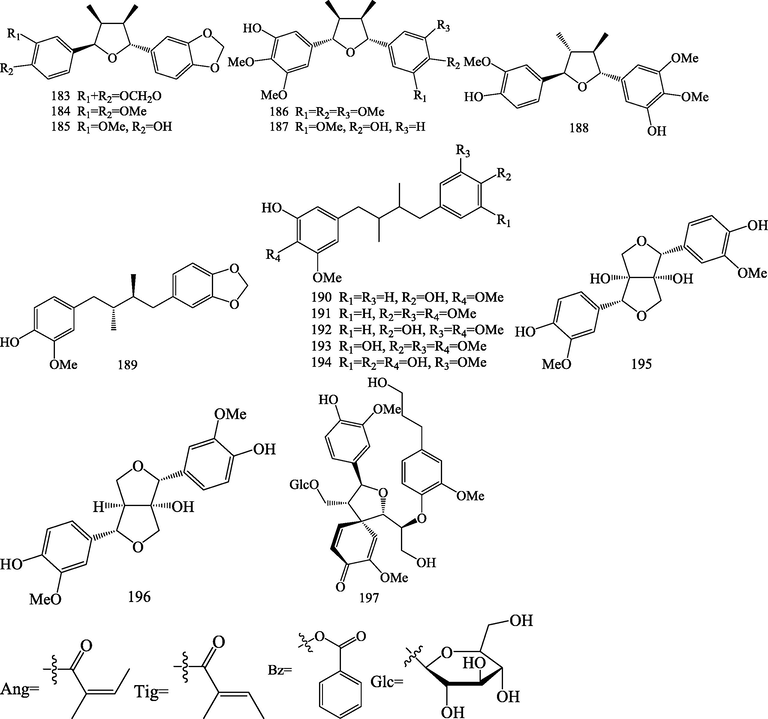
Structures of other types of lignans (183–197).
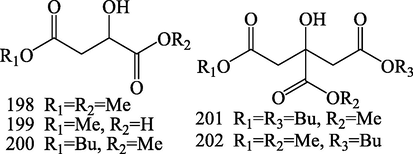
Structures of fatty acids (198–202).
2.1 Triterpenoids
Triterpenoids are kind of important bioactive compounds from S.chinensis. Triterpenoids are formed by six isoprene units via squalene intermediate. It is a structurally very diverse class with nearly 200 different skeletons, which are known to come from natural sources or enzymatic reactions (Wang et al., 2021). Up to now, phytochemical studies led to isolation and identification of 83 triterpenoids from S.chinensis. The triterpenoids were isolated from this plant can be divided into four categories according to their different structural skeletons: lanostane triterpenoids (1–4), cycloartane triterpenoids (5–25), nortriterpenoids (22–81) and ursane triterpenoids (82–83).
2.1.1 Lanostane triterpenoids
Four lanostane triterpenoids (1–4) were isolated from S.chinensis, this class of triterpenoids possess tetracyclic system. Among them, compoud 1 belong to intact lanostane triterpenoid (Zhang et al., 2013). Triterpenoids 2–4 are 3,4-secocycloartane triterpenoids, they are formed by the cleavage of C-3(4) bond, with C-3 usually could be oxidized further to carboxylic acid and carboxylic acid derivatives, and the side chain is 24 (Z)-en-26-acid or 22, 26 lactone ring (Huang et al., 2008; Qiu et al., 2018).
2.1.2 Cycloartane triterpenoids
Twenty-one cycloartane triterpenoids (5–25) have been reported from the plant. Triterpenoid 1 is intact cycloartane triterpenoid, with C-3 being oxidized to carbonyl group, and the side chain is 24 (Z)-en-26-acid (Huang et al., 2008). Triterpenoids (6–25) belong to 3,4-secocycloartane triterpenoids. This class is formed by the cleavage of C-3 (4) bond, with C-3 usually could be oxidized further to carboxylic acid and carboxylic acid derivatives, three-membered ring is formed by dehydrogenation of C-19 methyl and C-9 methine, and the side chain is 24 (Z)-en-26-acid or 22, 26 lactone ring or 3-one-2-oxabicyclo-[3.2.1]-octane. Among them, schinalactone D (6) and wuweizilactone acid (7) possess a novel eight-membered lactone ring between C-21 and C-26 (Huang et al., 2008). Triterpenoids 8–23 all possess seven-membered lactone ring between C-3 and C-4, formed by Baeyer Villiger oxidation, after the cleavage of C-3(4) bond. In addition, triterpenoids 13–25 all possess seven-membered ring, formed by he cleavage of C-9 (10) bond. Schinchinenin E (22) and schinchinenin F (23) is possessing hydroperoxyl group at C-19 (Song et al., 2013). Schinchinenin A (24) and schinchinenlactone A (25) both possess 5/5/7/6/5-fused pentacyclic ring (Song et al., 2013).
2.1.3 Nortriterpenoids
Schisandra nortriterpenoids are a structurally intriguing group of polycyclic, highly oxygenated, fused heterocyclic natural products isolated from S.chinensis. These compounds are showing different carbon frameworks and oxygenated pattern such as pre-schisanartanes (26–32), schisanartanes (33–53), 18-norschiartanes (54–66), 18(13 → 14)-abeo-schiartanes (67–72), wuweiziartanes (73–76), schiartanes (77–78), and other novel skeletons nortriterpenoids (79–81) have been reported from this plant. Among them, Compounds 26–32 belong to pre-schisanartane nortriterpenoids, this class possess a unique 7/8/3 consecutive carbocycle. Compounds 54–66 are 18-norschiartane nortriterpenoids, this type of compound was postulated to originate from precursors that contain schiartane carbon skeletons through a sequence of reactions that involve a 1,2-methyl shift followed by oxidation and decarboxylation of the C-14 methyl group (Huang et al., 2007c). Compounds 67–72 possess an unprecedented 18(13 → 14)-abeo-schiartane skeleton, which have a β-oriented methyl group at the C-14 position. This structural feature corroborates the biogenetic pathway proposed for the formation of 18-norschiartane-type (Huang et al., 2007c; Xue et al., 2010; Shi et al., 2014; Yang et al., 2018). Compounds 73–76 possess five-membered carbon ring (ring D) (Huang et al., 2007b; Shi et al., 2011). Micrandilactones B-C (77–78) were schiartane nortriterpenoids (Huang et al., 2007a; Kim et al., 2015). Three unprecedented nortriterpenoids, schicagenins A-C (79–81) are possessing a tetracyclic oxa-cage motif and C9 side chain. Their structures were determined on the basis of extensive spectroscopic analysis, and the absolute stereochemistries were established by single-crystal X-ray diffraction and CD experiments (Shi et al., 2011).
2.1.4 Ursane triterpenoids
Two ursane triterpenoids, ursolic acid (82) and 2α,3β,19α-trihydroxy- urs-12-en-28-oic acid (83) were isolated from S.chinensis (Huang et al., 2008).
2.2 Diterpenoid and sesquiterpenoids
A abietane diterpenoid, 7-oxocallitrisic acid (84) was isolated from S.chinensis (Huang et al., 2008). Twelve sesquiterpenoids (85–96) were isolated from S.chinensis, including eight new compounds: α-iso-cubebene (86), α-iso-cubebenol (87), α-iso-cubebenol acetate (88), widdaranal A (90), widdaral B (91), β-chamigrenal (93), (6R)-β-chamigrenic acid (94), and iso-cuparenal (95), along with four known compounds: guaidiol (85), (−)-clovane-2,9-diol (89), 15-hydroxy-α-cadinol (92), 3β-hydroxy-5α,6α-epoxy-β-ionone (96) (Huang et al., 2008; Lee et al., 2009; Lee et al., 2010; Venkanna et al., 2014; Liu et al., 2017; Guo et al. 2020; Liu et al., 2020).
2.3 Monoterpenoids
Fifteen monoterpenoid glycosides (97–111) were isolated from S.chinensis, among them, compouds (97–110) belong to aromatic monoterpenoid glycosides (Dai et al., 2005; Yang et al., 2016; Liu et al., 2019; Liu et al., 2020).
2.4 Lignans
Lignans are the most common constituents of S. chinensis, they are a class of secondary plant metabolites produced by oxidative dimerization of two phenylpropanoid units. At present, eighty-six lignans (112–197) have been isolated and identified from S.chinensis. Among them, dibenzocyclooctadienes lignans are the major bioactive constituents of S. chinensis.
2.4.1 Dibenzocyclooctadiene lignans
Dibenzocyclooctadienes lignans were characteristic constituents of Schisandraceae family. So far, seventy-one dibenzocyclooctadiene lignans (112–182) were isolated and identified from S.chinensis. Dibenzocyclooctadienes lignans show skeletal diversity in their chemical structures. Dibenzocyclooctadienes lignans have R-biphenyl and S-biphenyl configuration. This class is fromed by an aryl-aryl bond and an eight-membered ring, the positions of C-1, C-2, C-3, C-12, C-13, and C-14 possess different substituted groups, such as hydroxyl, methoxy, methylenedioxy, and ester group, and methylenedioxy group may existed at C-2 (3) or C-12 (13) and hydroxyl group at C-7 and C-8. Different ester groups, such as, angeloyl, tigloyl, acetyl, and benzoyl may exist at the C-6 of the octatomic ring, and ester group linkage are usually β-configuration (Wang et al., 2021). Compounds 112–143 possess a R-biphenyl configuration (Choi et al., 2006; Hu et al., 2014; Ikeya et al., 1978, 1988a,a,b,e; Kim et al., 2015; Kochetkov et al., 1961, 1962a,b; Li et al., 2017; Liu et al., 2020; Nakajima et al., 1983; Pel et al., 2017; Sovová et al., 2007; Taguchi and Ikeya, 1977; Xue et al., 2015), and compounds 144–182 possess a S-biphenyl configuration (Taguchi et al., 1977; Ikeya et al., 1979b, 1979c, 1979d, 1980, 1982a, 1982b; Piao et al., 2005; Šmejkal et al., 2010; Hu et al., 2014; Zhu et al., 2015; Kim et al., 2015; Xue et al., 2015; Yang et al., 2016; Pel et al., 2017; Choi et al., 2006, 2020). Among them, schisandrosides A (1 2 5), B (1 2 4), C(1 7 1) and D (1 7 2) represent the first example of a dibenzocyclooctadiene lignan glycoside (Kim et al., 2015). Schinlignan A (1 4 2) possess 2-hydroxy-2,3-dimethylbutyryl moiety , and an epoxide ring exist between C-7 and C-18. Schischinone (1 4 3) possess rare 6,7-seco- dibenzocyclooctadiene carbon skeleton (Xue et al., 2015).
2.4.2 Other types of lignans
Fifteen other types of lignans (183–197) were isolated from S.chinensis. Among these compounds, D-epigalbacin (1 8 3), machilin G (1 8 4), chicanine (1 8 5), schinlignin A (1 8 6), schinlignin B (1 8 7), and rel-(7R, 8R, 7′R, 8′R)-manglisin E (1 8 8) belong to terahydrofuran-type lignans (Xue et al., 2010; Zhang et al., 2013; Pel et al., 2017). Anwulignan (1 8 9), schineolignin A (1 9 0), schineolignin B (1 9 1), schineolignin C (1 9 2), pregomisin (1 9 3), and meso-dihydroguaiaretic acid (1 9 4) are dibenzylbutane-type lignans (Xue et al., 2010; Zhang et al., 2013; Zhu et al., 2015; Pel et al., 2017; Liu et al., 2020), 8,8′-dihydroxypinoresinol (1 9 5) and 8-hydroxypinoresinol (1 9 6) are furofuran-type lignans (Liu et al., 2020), pinobatol-9-O-β-D -glucopyranoside (1 9 7) belong to futoenone lignans (Yang et al., 2016).
2.5 Fatty acids
Five fatty acids have been reported from S.chinensis, namely dimethyl-malate (1 9 8), methyl-malate (1 9 9), butyl-1-methyl malate (2 0 0), 1,5-dibutyl-1′-methyl citrate (2 0 1), 1-butyl-1′,5-dimethyl citrate (2 0 2) (Pel et al., 2017).
3 Biological activities
3.1 Anti-cancer activity
Widdaral B (91) and β-chamigrenal (93) showed obvious cytotoxic activity against Caco-2 cell lines, with IC50 values of 17.10 and 16.46 μg/mM, respectively (Venkanna et al., 2014). (+)-Deoxyschisandrin (1 2 1) and (−)-gomisin N (1 5 3) showed anti-proliferative activity against the LoVo cell lines, with EC50 values of 22.6 and 27.4 μg/mL, respectively. And schisandrin (1 1 3) and (−)-tigloyl-deangeloyl-gomisin F (1 4 8) showed little anti-proliferative activity against the LoVo cell lines, with EC50 values of 84.4 and 81.7 μg/mL, respectively (Šmejkal et al., 2010). Schisandrin C (1 5 2) inhibited human leukemia U937 cells growth in a dose dependent manner (Park et al., 2009). Epi-anwuweizic acid (1) exhibited the strongest cytotoxic activity against on prostate cancer cells PC3, with an IC50 of 36.5 μM. chicanine (1 8 5) showed good anti-proliferation, with an IC50 of 44.2 μM (Zhang et al., 2013). Schisandroside E (1 2 3), gomisin F (1 4 7), angeloylgomisin Q (1 4 9), and schisandrin (1 1 3) exhibited strong cytotoxic activities against MGC-803, with an IC50 values of 4.621, 0.050, 0.075, and 4.773 μM, respectively, and showed strong cytotoxic activities against Ishikawa cell lines, with an IC50 values of 0.356, 0.426, 0.567, and 0.437 μM, respectively. Schisandroside E (1 2 3), gomisin F (1 4 7), (−)-tigloyl- deangeloyl-gomisin F (1 4 8), angeloylgomisin Q (1 4 9), and schisandrin (1 1 3) demonstrated strong cytotoxicity against Caco-2 cell lines, with IC50 values of 0.021, 0.572, 0.033, 2.305, and 0.537 μM, respectively (Liu et al., 2020). Dibenzocyclooctadiene lignans (−)-gomisin K1 (1 4 4), gomisin J (1 4 5), gomisin A (1 2 2), and angeloylgomisin H (1 1 8) showed anti-cancer activity against AGS, HeLa and HT-29 cells, especially, angeloyl-gomisin H (1 1 8), concentration dependently suppressed the proliferation and viability against three cancer cells. (Choi et al., 2020). (Table 6).
Biological activities
Compounds
Class of compound
Results
Reference
Anti-cancer
Epi-anwuweizic acid (1)
Triterpenoid
IC50 36.5 μM against prostate cancer cells.
Zhang et al.(2013)
Widdaral B (91)
Sesquiterpenoid
IC50 17.10 μg/mM against Caco2 cell lines.
Venkanna et al.(2014)
β-Chamigrenal (93)
Sesquiterpenoid
IC50 16.46 μg/mM against Caco2 cell lines
Venkanna et al.(2014)
Schisandrin (1 1 3)
Lignan
IC50 4.773, 0.437, 0.537 μM against MGC-803, Ishikawa and Caco-2 cell lines.
Liu et al.(2020)
Angeloylgomisin H (1 1 8)
Lignan
IC50 12.94 ± 0.12, 9.36 ± 0.39, 7.94 ± 0.19 μM against AGS, Hela, HT29 cell lines.
Choi et al. (2020)
(+)-Deoxyschisandrin (1 2 1)
Lignan
EC50 22.6 μg/mL against the LoVo cell lines.
Šmejkal et al.(2010)
Gomisin A (1 2 2)
Lignan
IC50 13.76 ± 0.38 μM against Hela cell lines and IC50 14.81 ± 1.02 μM against AGS cell lines.
Choi et al. (2020)
Schisandroside E (1 2 3)
Lignan
IC50 4.621, 0.356, 0.021 μM against MGC-803, Ishikawa and Caco-2 cell lines.
Liu et al.(2020)
(−)-Gomisin K1 (1 4 4)
Lignan
IC50 5.46 ± 0.24 μM against Hela cell lines.
Choi et al. (2020)
Gomisin J (1 4 5)
Lignan
IC50 6.51 ± 0.26 μM against Hela cell lines.
Choi et al. (2020)
Gomisin F (1 4 7)
Lignan
IC50 0.050, 0.426, 0.572 μM against MGC-803, Ishikawa and Caco-2 cell lines.
Liu et al. (2020)
(−)-Tigloyl- deangeloylgomisin F (1 4 8)
Lignan
IC50 0.033 μM against Caco-2 cell lines.
Liu et al. (2020)
Angeloylgomisin Q (1 4 9)
Lignan
IC50 0.075, 0.567, 2.305 μM against MGC-803, Ishikawa and Caco-2 cell lines.
Liu et al. (2020)
(−)-Gomisin N (1 5 3)
Lignan
EC50 27.4 μg/mL against the LoVo cell lines.
Šmejkal et al. (2010)
Chicanine (1 8 5)
Lignan
IC50 44.2 μM against prostate cancer cells
Zhang et al. (2013)
Anwulignan (1 8 9)
Lignan
IC50 39.3 μM against prostate cancer cells.
Zhang et al. (2013)
Anti-oxidant
Schisandrene (1 5 5)
Lignan
Showed better antioxidant activity than commercial antioxidant Vitamin C and Trolox using a DCFH-DA cellular-based assay.
Choi et al. (2006)
Anwulignan (1 8 9)
Lignan
Showed better DPPH free radicals scavenging activity (IC50 = 11.2 μM), compared to the positive control ascorbic acid (IC50 = 25.3 μM).
Zhang et al. (2013)
Chicanine (1 8 5)
Lignan
Showed potent DPPH free radicals scavenging activity with IC50 value of 26 μM.
Zhang et al. (2013)
Neuroprotective
Ganwuweizic acid (5)
Lignan
Showed 78.05 ± 2.34% inhibition activity on lipopolysaccharide (LPS)-induced NO release at 1 μM.
Hu et al. (2014)
(±)-γ-Schizandrin (1 1 5)
Lignan
Showed 91.78 ± 1.32% inhibition activity on lipopolysaccharide (LPS)-induced NO release at 1 μM.
Hu et al. (2014)
Deoxyschisandrin (1 2 1)
Lignan
Showed significant neuroprotection against glutamate-induced toxicity.
Kim et al. (2004)
Gomisin A (1 2 2)
Lignan
Showed 95.57 ± 1.86% inhibition activity on lipopolysaccharide (LPS)-induced NO release at 1 μM.
Hu et al. (2014)
(−)-Gomisin M1 (1 2 7)
Lignan
Showed 96.06 ± 0.70% inhibition activity on lipopolysaccharide (LPS)-induced NO release at 1 μM.
Hu et al. (2014)
Schisanchinin B (1 3 3)
Triterpenoid
Showed 76.70 ± 1.18% inhibition activity on lipopolysaccharide (LPS)-induced NO release at 1 μM.
Hu et al. (2014)
(+)-Gomisin M2 (1 3 7)
Lignan
Showed 92.77 ± 0.93% inhibition activity on lipopolysaccharide (LPS)-induced NO release at 1 μM.
Hu et al. (2014)
(+)-Gomisin K3 (1 3 8)
Lignan
Showed 96.06 ± 0.70% inhibition activity on lipopolysaccharide (LPS)-induced NO release at 1 μM.
Hu et al. (2014)
Gomisin G (1 5 1)
Lignan
Showed 92.35 ± 0.68% inhibition activity on lipopolysaccharide (LPS)-induced NO release at 1 μM.
Hu et al. (2014)
Wuweizisu C (1 5 2)
Lignan
Showed significant neuroprotection against glutamate-induced toxicity.
Kim et al. (2004)
Gomisin N (1 5 3)
Lignan
Showed significant neuroprotection against glutamate-induced toxicity.
Kim et al. (2004)
(−)-Gomisin L1 (1 5 6)
Lignan
Showed 89.08 ± 0.72% inhibition activity on lipopolysaccharide (LPS)-induced NO release at 1 μM.
Hu et al. (2014)
Schisanchinin A (1 6 7)
Lignan
Showed 83.74 ± 0.47% inhibition activity on lipopolysaccharide (LPS)-induced NO release at 1 μM.
Hu et al. (2014)
1,2,13,14-Tetramethoxydibenzocyclooctadiene 3,12-O-β-D- diglucopyranoside (1 6 9)
Lignan
Showed protecting activity against Aβ- induced toxicity in PC12 cells.
Yang et al. (2016)
3,7-Dihydroxy-1,2,13,14-tetramethoxy- dibenzocyclooctadiene 12-O-β-D- glucopyranoside (1 7 0)
Lignan
Showed protecting activity against Aβ- induced toxicity in PC12 cells.
Yang et al. (2016)
Anti-HIV-1
Pre-schisanartanin(26)
Triterpenoid
Exhibited anti-HIV-1 activity with an EC50 value of 13.81 μg/mL (AZT: EC50 = 2.26 μg/mL)
Huang et al. (2007a)
Wuweizidilactone A (54)
Triterpenoid
Exhibited anti-HIV-1 activity with an EC50 value of 26.81 μg/mL (AZT: EC50 = 2.26 μg/mL)
Huang et al. (2007c)
Wuweizidilactone B (55)
Triterpenoid
Exhibited anti-HIV-1 activity with an EC50 value of 28.86 μg/mL (AZT: EC50 = 2.26 μg/mL)
Huang et al. (2007c)
Schintrilactone A (73)
Triterpenoid
Exhibited anti-HIV-1 activity with an EC50 value of 17.9 μg/mL (AZT: EC50 = 2.26 μg/mL)
Huang et al. (2007b)
Schintrilactone B (74)
Triterpenoid
Exhibited anti-HIV-1 activity with an EC50 value of 36.2 μg/mL (AZT: EC50 = 2.26 μg/mL)
Huang et al. (2007b)
Anti-inflammatory
α-Iso-cubebenol (87)
Sesquiterpenoid
Demonstrated inhibition of nitric oxide (NO) and prostaglandin E2 (PGE2) production in LPS-stimulated macrophages
Lee et al. (2010)
Schisandrin (1 1 3)
Lignan
Demonstrated potenti anti- inflammatory activity.
Guo et al. (2008)
Hepatoprotective
Schizandrin (1 1 3)
Lignan
Demonstrated moderate hepatoprotective activities (survival rate 44.5%) against damage induced by N-acetyl-p- aminophenol.
Li et al. (2017)
Gomisin M2 (1 3 7)
Lignan
Demonstrated moderate hepatoprotective activities (survival rate 43.5%) against damage induced by N-acetyl-p- aminophenol.
Li et al. (2017)
Micrantherin A (1 4 0)
Lignan
Demonstrated moderate hepatoprotective activities (survival rate 44.6%) against damage induced by N-acetyl-p- aminophenol.
Li et al. (2017)
Schisantherin D (1 7 4)
Lignan
Demonstrated significant hepatoprotective activity.
Xu et al. (2020)
Anti-platelet aggregation
Gomisin N (1 5 3)
Lignan
Demonstrated anti-platelet aggregation activity against platelet aggregation induced by AA (IC50 = 153.3 ± 6.8 μM) and PAF (IC50 = 122.4 ± 5.6 μM).
Kim et al. (2010)
Pregomisin (1 9 3)
Lignan
Demonstrated anti-platelet aggregation activity against platelet aggregation induced by AA (IC50 = 96.5 ± 4.7 μM) and PAF (IC50 = 49.3 ± 2.7 μM).
Kim et al. (2010)
Anti-acetylcholinesterase
Preschisanartanin E (31)
Triterpenoid
Exhibited anti-AChE activity, at concentration of 50 μM, with 16.6% inhibition.
Shi et al. (2014)
Schindilactone I (34)
Triterpenoid
Exhibited anti-AChE activity, at concentration of 50 μM, with 12.7% inhibition.
Shi et al. (2014)
Schindilactone A (37)
Triterpenoid
Exhibited anti-AChE activity, at concentration of 50 μM, with 10.7% inhibition.
Shi et al. (2014)
Propindilactone Q (64)
Triterpenoid
Exhibited anti-AChE activity, at concentration of 50 μM, with 32.1% inhibition.
Shi et al. (2014)
Anti-hepatitis B virus
Schinlignan G (1 3 2)
Lignan
Exhibited anti-hepatitis B virus activity against HBV DNA replication, with IC50 value of 5.13 μg/mL.
Xue et al. (2015)
methylgomisin O (1 6 5)
Lignan
Exhibited anti-hepatitis B virus activity against HBV DNA replication, with IC50 value of 5.49 μg/mL.
Xue et al. (2015)
Anti-feedant
Gomisin J (1 4 5)
Lignan
Showed antifeedant activity against Tribolium castaneum adults, at 1500 ppm concentration, with 40.3% antifeeding index percentage.
Guo et al. (2020)
Anti-HSV-2
Henrischinin C (12)
Triterpenoid
Showed inhibitory activities against HSV-2, with SI value of 19.49.
Song et al. (2013)
Henrischinin A (15)
Triterpenoid
Showed inhibitory activities against HSV-2, with SI value of 23.31.
Song et al. (2013)
Henrischinin B (16)
Triterpenoid
Showed inhibitory activities against HSV-2, with SI value of 29.95.
Song et al. (2013)
Anti-adenovirus
Schinchinenin G (10)
Triterpenoid
Showed modest activities against adenovirus, with SI value of 11.43.
Song et al. (2013)
Henrischinin A (15)
Triterpenoid
Showed modest activities against adenovirus, with SI value of 13.67.
Song et al. (2013)
Henrischinin B (16)
Triterpenoid
Showed modest activities against adenovirus, with SI value of 11.45.
Song et al. (2013)
Schinchinenin A (24)
Triterpenoid
Showed modest activities against adenovirus, with SI value of 13.75.
Song et al. (2013)
LDL-cholesterol biosynthesis inhibition
activity(+)-Schisandrol B (1 2 2)
Inhibited PCSK9 mRNA expression, with IC50 value of 1.10 μM.
Pel et al. (2017)
Schinlignan D (1 2 9)
Inhibited PCSK9 mRNA expression, with IC50 value of 0.36 μM.
Pel et al. (2017)
(−)-Schisandrin C (1 5 2)
Inhibited PCSK9 mRNA expression, with IC50 value of 3.85 μM.
Pel et al. (2017)
Rel-(7R,8R,7′R,8′R)-Manglisin E (1 8 8)
Inhibited PCSK9 mRNA expression, with IC50 value of 3.15 μM.
Pel et al. (2017)
3.2 Anti-oxidant activity
Tigloylgomisin H (1 1 7), gomisin K3 (1 3 8), angeloylgomisin H (1 1 8), gomisin J (1 4 5), gomisin G (1 5 1), gomisin B (1 5 4), and schisandrene (1 5 5) showed DCFH-DA cellular-based antioxidant activity (2.8–160.9 μM). Meanwile, the structure–activity relationships of the dibenzocyclooctadiene lignans exhibited that the exocyclic methylene functionality was essential for antioxidant activity, with the benzoyloxy group probably increasing antioxidant activity (Choi et al., 2006). Schisandrin (1 1 3) could ameliorate Aβ1–42-induced memory impairment in mice at least in part by enhancing the activity of the antioxidative defense system and free radical-scavenging activity (Hu et al., 2012). The extract and two compounds of S. chinensis exhibited antioxidant activities on DPPH radical scavenging effects. There were good dose-dependence effects of the extract and compounds anwulignan (1 8 9) and chicanine (1 8 5) with IC50 values of 188 μg/mL, 11.2 and 26.0 μM, respectively. The two lignans anwulignan (1 8 9) and chicanine (1 8 5) showed better antioxidant activities than the extract (Zhang et al., 2013). (Table 6).
3.3 Neuroprotective activity
Deoxyschisandrin (1 2 1), gomisin N (1 5 3), and wuweizisu C (1 5 2) exhibited significantly neuroprotective activity gainst glutamate-induced neurotoxicity (Kim et al., 2004). The neuroprotective activity was tested on PC12 cells with neurotoxicity induced by amyloid-beta 1–42(Aβ1-42). 1,2,13,14-Tetramethoxydibenzocyclooctadiene 3,12-O-β-D-diglucopyranoside (1 6 9), 3,7-dihydroxy-1,2,13,14-tetramethoxy- dibenzocyclooctadiene 12-O-β-D-glucopyranoside (1 7 0) exhibited neuroprotective activity against Aβ-induced toxicity in PC12 cells (Yang et al., 2016). Schisanchinin A (1 6 7) and B (1 3 3), gomisin G (1 5 1), deoxyschizandrin (1 2 1), (±)-γ-schizandrin (1 1 5), gomisin A (1 2 2), (−)-gomisin M1 (1 2 7), (−)-gomisin L1 (1 5 6), (+)-gomisin M2 (1 3 7), (+)-gomisin K3 (1 3 8), and ganwuweizic acid (5) significantly inhibited NO release by LPS-activated microglia in a dose-dependent manner. Among them, schisanchinin B (1 3 3) and ganwuweizic acid (5) showed strong inhibition activities, whereas schisanchinin A (1 6 7), gomisin G (1 5 1), deoxyschizandrin (1 2 1), (±)-γ-schizandrin (1 1 5), (−)-gomisin L1 (1 5 6), and (+)-gomisin M2 (1 3 7) demonstrated moderate inhibition activities, and gomisin A (1 2 2), (−)-gomisin M1 (1 2 7) and (+)-gomisin K3 (1 3 8) exhibited weak inhibition activities, which implied the lignans from the fruit of S. chinensis may be a potential healthy food for anti-AD (Hu et al., 2014). Homogeneous polysaccharides (SCP2-1) of S. Chinensis could improve M1/M2 polarization, especially inhibit M1 polarization, and ameliorate the cognition of mice in Y-maze and NOR test. SCP2-1 play a neuroprotective role through LRP-1 to reverse activation of microglia via suppressing the overactive NF-κB and JNK pathway (Xu et al., 2020). (Table 6).
3.4 Anti-HIV-1 activity
The anti-HIV-1 activities testing showed that pre-schisanartanin(26) demonstrated anti-HIV-1 activity with an EC50 value of 13.81 μg/mL (AZT: EC50 = 2.26 μg/mL), and six triterpenoids schindilactones A-C (33, 37–38), micrandilactone B (77), lancifodilactone C (43), and henridilactone D (44) showed weak anti-HIV-1 activity with EC50 values of > 50 μg/mL (Huang et al., 2007a). Schintrilactones A and B (73–74) demonstrated anti-HIV-1 activity, with EC50 values of 17.9 and 36.2 μg/mL, respectively (Huang et al., 2007b). Wuweizidilactones A and B (54–55) exhibited anti-HIV-1 activity, with EC50 values of 26.81 and 28.86 μg/mL, respectively. Wuweizidilactones C-F (67–70) showed weak anti-HIV-1 activity, with EC50 values of > 50 μg/mL (Huang et al., 2007c). (Table 6).
3.5 Anti-inflammatory activity
Schisandrin (1 1 3) showed the potent inhibition of nitric oxide (NO) production, prostaglandin E2 (PGE2) release, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression in a RAW 264.7 macrophage cell line (Guo et al., 2008). α-Iso-cubebenol (87) exhibited inhibition of nitric oxide (NO) and prostaglandin E2 (PGE2) production in LPS-stimulated macrophages (Lee et al., 2010). (Table 6).
3.6 Hepatoprotective activity
Schizandrin (1 1 3), gomisin M2 (1 3 7), and micrantherin A (1 4 0) showed moderate hepatoprotective activities against damage induced by N-acetyl-p-aminophenol (APAP) in human liver carcinoma (HepG2) cells, at a concentration of 10 µM (Li et al., 2017). S. chinensis-derived lignans (SCDLs) and schisantherin D (1 7 4) have ETBR antagonistic effects, which may protect the normal function of PC and NPC by protecting ER stress and mitochondrial dysfunction, thereby exerting hepatoprotection (Xu et al., 2020). (Table 6).
3.7 Anti-platelet aggregation activity
Gomisin N (1 5 3) and pregomisin (1 9 3) showed anti-platelet aggregation activity against platelet aggregation induced by AA (153.3 and 96.5 μM) and PAF (122.4 and 49.3 μM). Pregomisin and gomisin N were more potent platelet inhibitors than aspirin against PAF (Kim et al., 2010). (Table 6).
3.8 Anti-acetylcholinesterase activity
Preschisanartanin E (31), schindilactone I (34), schindilactone A (37), and propindilactone Q (64) exhibited anti-AChE activity, at concentration of 50 μM, with 16.6, 12.7, 10.7, and 32.1% inhibition, respectively (Shi et al., 2014). (Table 6).
3.9 Anti-hepatitis B virus activity
Schinlignan G (1 3 2) and methylgomisin O (1 6 5) showed potent anti-hepatitis B virus activity against HBV DNA replication, with IC50 values of 5.13 and 5.49 μg/mL, respectively (Xue et al., 2015). (Table 6).
3.10 Other biological activities
Gomisin J (1 4 5) showed antifeedant activity against Tribolium castaneum adults, at 1500 ppm concentration, with 40.3% antifeeding index percentages (Guo et al., 2020). Sesquiterpenoid α-iso-cubebene is a novel natural compound which stimulates intracellular calcium signaling and CXCL8 production, and should be useful for the development of an immune-modulating agent (Lee et al., 2009). Henrischinin A (15), henrischinin B (16), and henrischinin C (12) had selectivity index values of 23.31, 29.95 and 19.49, respectively, exhibited better activities than Schinchinenin A (24), schinchinenin B (20), and schinchinenin G (10) against HSV-2. Schinchinenin A (24), schinchinenin G (10), henrischinin A (15), and henrischinin B (16) showed modest activities against adenovirus, with selectivity index values ranging from 11.43 to 13.75. From a structure–activity relationship viewpoint, it is obvious that the acetyl and hydroxyl groups at C-25 may play different roles in the inhibition of HSV-2 and adenovirus by different types of triterpenoids (Song et al., 2013). rel-(7R, 8R, 7′R, 8′R)-Manglisin E (1 8 8), (−)-schisandrin C (1 5 2), schinlignan D (1 2 9), and (+)-schisandrol B (1 2 2) potently inhibited PCSK9 mRNA expression, with IC50 values of 3.15, 3.85, 0.36, and 1.10 μM, respectively. Furthermore, schinlignan D (1 2 9) and (+)-schisandrol B (1 2 2) suppressed PCSK9 protein expressions, and schinlignan D (1 2 9) deemed to increase low density lipoprotein receptor expression (Pel et al., 2017). (Table 6).
4 Analytical methods
In recent years, researcher have attempted to establish analytical methods focusing on lignans and triterpenoids analysis with different chromatographic equipment. A rapid HPLC-DAD method was described for simultaneous determination of nine lignans, including schisandrin (1 1 3), gomisin J (1 4 5), gomisin A (1 2 2), tigloylgomisin H (1 1 7), angeloylgomisin H (1 1 8), schisandrin A (1 1 2), schisandrin B (1 1 5), gomisin N (1 5 3) and schisandrin C (1 5 2) (Lee and Kim, 2010). A new HPLC-FLD method was described for simultaneous determination schisandrin (1 1 3), gomisin A (1 2 2), schisandrin A (1 1 2), schisandrin B (1 1 5), schisandrin C (1 5 2) in S. chinensis. This method enables routine quality evaluation and standardization of the bioactive lignans from the raw material, extracts or formulations (Xia et al., 2014). A TLC-ESI-MS method for monitoring the quality of the herb was reported. The results showed that gomisin A (1 2 2), schisandrin B (1 1 5), schisandrin (1 1 3), schisantherin A (1 4 6) and schisandrin A (1 1 2) were detected simultaneously, the method possess rapid identification of chemical components and high reliability for the plant extracts (Hu et al., 2015). A simple, environment-friendly and efficiency micro MSPD–MEEKC method was reported, The method was developed to simultaneously analyze schisandrin (1 1 3), gomisin A (1 2 2), schisantherin A (1 4 6), deoxyschisandrin (1 2 1), and schisandrin B (1 1 5) in S.chinensis. This method exhibits good precision, satisfactory recovery, and low detection limits. Moreover, it showed excellent advantages of small samples and sorbent amounts, low consumption of elution solvent and high extraction efficiency compared with conventional MSPD techniques (Chu et al., 2017). An UHPLC-Q-TOF/MS method is widely used for data collection of herbal medicine extracts, because of its high resolution and high mass accuracy (Gao et al., 2019; Liu et al., 2017; Yang et al., 2017; Yu et al., 2019). A supercritical fluid chromatography method were used for separation of lignans in S. chinensis. The determined lignan patterns were typical for S. chinensis, with schisandrin (1 1 3) being the most abundant compound, followed by schisandrin B (1 1 5) or gomisin A (1 2 2) (Onay et al., 2020). These novel methods would be valuable for future development and utilization of S. chinensis.
5 Conclusions
This review summarized the recent advance in the phytochemistry, biological activities and analytical methods of S.chinensis. The phytochemical investigation on S.chinensis resulted in the isolation of many novel triterpenoids and dibenzocyclooctadiene lignans. The biological activities research on the plant components showed that some components exhibit significant biological activities, especially anticancer, anti-HIV-1, neuroprotective, hepatoprotective, antioxidant activities, which supported the use of S.chinensis in traditional medicines.
Nevertheless, there are several aspects that needed to explore and investigate further: (1) work on the stem and leave extracts of S.chinensis to isolate sufficient amount of major as well as minor chemical components to explore their pharmacological activities and mechanism for therapeutic potential; (2) Further studies on the mechanism of actions and the structure–activity relationship are needed, in order to provide a better understanding of the chemical constituents of S.chinensis as potential medicines; (3) >200 compounds have been isolated and identified, whereas only a few have been explored for pharmacological activities and pre-clinical studies. Overall, further studies on the chemical constituents of S.chinensis are needed, in order to obtain novel molecules with new pharmacological potential.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Micro-matrix solid-phase dispersion coupled with MEEKC for quantitative analysis of lignans in Schisandrae Chinensis Fructus using molecular sieve TS-1 as a sorbent. J. Chromatogr. B. 2017;1063:174-179.
- [Google Scholar]
- Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis: structure-antioxidant activity relationships of dibenzocyclooctadiene lignans. J. Nat. Prod.. 2006;69(3):356-359.
- [Google Scholar]
- Dibenzocyclooctadiene lignans from the fruits of Schisandra chinensis and their cytotoxicity on human cancer cell lines. App. Biol. Chem.. 2020;63:39.
- [Google Scholar]
- A new monoterpenoid glyciside from Schisandra chinensis. Chem. J. Chinese U.. 2005;26(9):1659-1661.
- [Google Scholar]
- Characterization of lignans in Schisandra chinensis oil with a single analysis process by UPLC-Q/TOF-MS. Chem. Phys. Lipids. 2019;218:158-167.
- [Google Scholar]
- Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis baill. Eur. J. Pharmacol.. 2008;591(1-3):293-299.
- [Google Scholar]
- Chemical constituents isolated from stems of Schisandra chinensis and their antifeedant activity against Tribolium castaneum. Nat. Prod. Res.. 2020;34(18):2595-2601.
- [Google Scholar]
- Dibenzocyclooctadiene lignans from Schisandra chinensis and their inhibitory activity on NO production in lipopolysaccharide-activated microglia cells. Phytochemistry. 2014;104:72-78.
- [Google Scholar]
- Schizandrin, an antioxidant lignan from Schisandra chinensis, ameliorates Aβ1–42-induced memory impairment in mice. Oxid. Med. Cell. Longev.. 2012;2012:1-7.
- [Google Scholar]
- Thin layer chromatography coupled with electrospray ionization mass spectrometry for direct analysis of raw samples. J. Chromatogr. A. 2015;1415:155-160.
- [Google Scholar]
- Isolation and characterization of biogenetically related highly oxygenated nortriterpenoids from Schisandra chinensis. Org. Lett.. 2007;9(11):2079-2082.
- [Google Scholar]
- Structural characterization of schintrilactone, a new class of nortriterpenoids from Schisandra chinensis. Org. Lett.. 2007;9(21):4175-4178.
- [Google Scholar]
- Wuweizidilactones A-F: novel highly oxygenated nortriterpenoids with unusual skeletons isolated from Schisandra chinensis. Chem. Eur. J.. 2007;13(17):4816-4822.
- [Google Scholar]
- Isolation and characterization of miscellaneous terpenoids of Schisandra chinensis. Tetrahedron. 2008;64(19):4260-4267.
- [Google Scholar]
- The constituents of Schizandra chinensis Baill. the structures of three new lignans, angeloylgomisin H, tigloylgomisin H and benzoylgomisin H, and the absolute structure of schizandrin. Chem. Pharm. Bull.. 1978;26(1):328-331.
- [Google Scholar]
- The constituents of Schizandra chinensis Baill. I. Isolation and structure determination of five new lignans, gomisin A, B, C, F and G, and the absolute structure of schizandrin. Chem. Pharm. Bull.. 1979;27:1383-1394.
- [Google Scholar]
- The constituents of Schizandra chinensis Baill. Ⅴ. the structures of four new lignans, gomisin N, gomisin O, epigomisin O and gomisin E, and transformation of gomisin N to deangeloylgomisin B. Chem. Pharm. Bull.. 1979;27(11):2695-2709.
- [Google Scholar]
- The constituents of Schizandra chinensis Baill. II. the structure of a new lignan, gomisin D. Chem. Pharm. Bull.. 1979;27(6):1395-1401.
- [Google Scholar]
- The constituents of Schizandra chinensis Baill. the cleavage of the methylenedioxy moiety with lead tetraacetate in benzene, and the structure of angeloylgomisin Q. Chem. Pharm. Bull.. 1979;27(10):2536-2538.
- [Google Scholar]
- The constituents of Schizandra chinensis Baill. . the structures of four new lignans, gomisin H and its derivatives, angeloyl-, tigloyl- and benzoyl-gomisin H. Chem. Pharm. Bull.. 1979;27(7):1576-1582.
- [Google Scholar]
- The constituents of Schizandra chinensis Baill. VIII. the structures of two new lignans, tigloylgomisin Pand angeloylgomisin P. Chem. Pharm. Bull.. 1980;28(11):3357-3361.
- [Google Scholar]
- The constituents of Schizandra chinensis baill.Ⅶ. Isolation and structure of a new lignan, gomisin R, the absolute structure of wuweizisu C and isolation of schisantherin D. Chem. Pharm. Bull.. 1982;30(9):3207-3211.
- [Google Scholar]
- Ikeya, Y., Ookawa, N., Taguchi, H., Yosioka, I., 1982b. The constituents of Schizandra chinensis Baill. XI. the structures of three new lignans, angeloylgomisin O, and angeloyl- and benzoylisogomisin O. Chem. Pharm. Bull. 30(9), 3202-3206.
- The constituents of Schizandra chinensis Baill. ⅩⅤ. isolation and structure determination of two new lignans, gomisin S and gomisin T. Chem. Pharm. Bull.. 1988;36(10):3974-3979.
- [Google Scholar]
- Dibenzocyclooctadiene lignans from Schisandra chinensis protect primary cultures of rat cortical cells from glutamate-induced toxicity. J. Neurosci.. 2004;76(3):397-405.
- [Google Scholar]
- Anti-platelet aggregation activity of lignans isolated from Schisandra chinensis fruits. J. Korean Soc. Appl. Bi.. 2010;53(6):740-745.
- [Google Scholar]
- Schisandrosides A-D, dibenzocyclooctadiene lignin glucosides from the roots of Schisandra chinensi. Chem. Pharm. Bull.. 2015;63:746-751.
- [Google Scholar]
- Deoxyschizandrin-structure and total synthesis. Tetrahedron Lett.. 1962;3(9):361-363.
- [Google Scholar]
- Chemical investigation of Schizandra chinensis. Russ. Chem. Bull.. 1962;11(5):792-796.
- [Google Scholar]
- Identification of a novel compound that stimulates intracellular calcium increase and CXCL8 production in human neutrophils from Schisandra chinensis. Biochem. Biophs. Res. Commun.. 2009;379(4):928-932.
- [Google Scholar]
- Lee, H.J., Kim, C.Y., 2010. Simultaneous determination of nine lignans using pressurized liquid extraction and HPLC-DAD in the fruits of Schisandra chinensis Food Chem. 120(4):1224-1228.
- Identification of a novel compound that inhibits iNOS and COX-2 expression in LPS-stimulated macrophages from Schisandra chinensis. Biochem. Biophs. Res. Commun.. 2010;391(4):1687-1692.
- [Google Scholar]
- A new nortriterpenoid, a sesquiterpene and hepatoprotective lignans isolated from the fruit of Schisandra chinensis. Molecules. 2017;22:1931-1939.
- [Google Scholar]
- An integrated strategy using UPLC-QTOF-MSE and UPLC- QTOF-MRM (enhanced target) for pharmacokinetics study of wine processed Schisandra chinensis fructus in rats. J. Pharm. Biomed. Anal.. 2017;139:165-178.
- [Google Scholar]
- Aromatic monoterpenoid glycosides from rattan stems of Schisandra chinensis and their neuroprotective activities. Fitoterapia. 2019;134:108-112.
- [Google Scholar]
- Lignans and Terpenoids from the Leaves of Schisandra chinensis. Chem. Biodivers.. 2020;17(4)
- [CrossRef] [Google Scholar]
- The constituents of Schizandra chinensis Baill. ⅩIII. quantitative analysis of lignans in the fruits of Schizandra chinensis Baill. by high performance liquid chromatography. Yakugaku Zasshi. 1983;103(7):743-749.
- [Google Scholar]
- Rapid analysis of nine lignans in Schisandra chinensis by supercritical fluid chromatography using diode array and mass spectrometric detection. J. Pharmaceut. Biomed.. 2020;185:113254.
- [CrossRef] [Google Scholar]
- Induction of G1 arrest and apoptosis by schisandrin C isolated from Schizandra chinensis Baill in human leukemia U937 cells. Int. J. Mol. Med.. 2009;24(04):495-502.
- [Google Scholar]
- Lignans from the fruits of Schisandra chinensis (turcz.) baill inhibit proprotein convertase subtilisin/kexin type 9 expression. Phytochemistry. 2017;136:119-124.
- [Google Scholar]
- Dibenzocyclooctene lignan compounds isolated from the fruits of Schisandra chinensis baill. Nat. Prod. Commun.. 2005;11(4):248-252.
- [Google Scholar]
- Isolation, structural elucidation of three new triterpenoids from the stems and leaves of Schisandra chinensis (Turcz) Baill. Molecules.. 2018;23:1624-1631.
- [Google Scholar]
- Schicagenins A-C: three cagelike nortriterpenoids from leaves and stems of Schisandra chinensis. Org. Lett.. 2011;13(15):3848-3851.
- [Google Scholar]
- Nortriterpenoids from Schisandra chinensis and their absolute configurational assignments by electronic circular dichroism study. Tetrahedron. 2014;70(4):859-868.
- [Google Scholar]
- Evaluation of cytotoxic activity of Schisandra chinensis lignans. Planta. Med.. 2010;76(15):1672-1677.
- [Google Scholar]
- Supercritical fluid extraction of lignans and cinnamic acid from Schisandra chinensis. J. Supercrit. Fluid.. 2007;42(1):88-95.
- [Google Scholar]
- Eleven new highly oxygenated triterpenoids from the leaves and stems of Schisandra chinensis. Org. Biomol. Chem.. 2013;11(7):1251-1258.
- [Google Scholar]
- An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed. Pharmacother.. 2018;97:958-968.
- [Google Scholar]
- The constituents of Schizandra chinensis Baill. The structures of two new lignans, gomisin F and G, and the absolute structures of gomisin A, B, and C. Chem. Pharm. Bull.. 1977;25(2):364-366.
- [Google Scholar]
- Phytochemical investigation of sesquiterpenes from the fruits of Schisandra chinensis and their cytotoxic activity. Fitoterapia. 2014;95:102-108.
- [Google Scholar]
- A review of the phytochemistry and pharmacology of Kadsura heteroclita, an important plant in tujia ethnomedicine. J. Ethnopharmacol.. 2021;268:113567.
- [CrossRef] [Google Scholar]
- Simultaneous quantification of five dibenzocyclooctadiene lignans in Schisandra chinensis by HPLC separation and fluorescence detection. Anal. Meth.. 2014;6(15):5981-5985.
- [Google Scholar]
- Nortriterpenoids and lignans from the fruit of Schisandra chinensis. Chem. Pharm. Bull.. 2010;58(12):1606-1611.
- [Google Scholar]
- Isolation and anti-hepatitis B virus activity of dibenzocyclooctadiene lignans from the fruits of Schisandra chinensis. Phytochemistry. 2015;116:253-261.
- [Google Scholar]
- Polysaccharide from Schisandra chinensis acts via LRP-1 to reverse microglia activation through suppression of the NF-κB and MAPK signaling. J. Ethnopharmacol.. 2020;256:112798.
- [CrossRef] [Google Scholar]
- Lignans from Schisandra chinensis ameliorate alcohol and CCl4-induced long-term liver injury and reduce hepatocellular degeneration via blocking ETBR. J. Ethnopharmacol.. 2020;258:112813.
- [CrossRef] [Google Scholar]
- New thymoquinol glycosides and neuroprotective dibenzocyclooctane lignans from the rattan stems of Schisandra chinensis. Chem. Biodiversity.. 2016;13(9):1118-1125.
- [Google Scholar]
- Rapid classification and identification of chemical components of Schisandra chinensis by UPLC-Q- TOF/MS combined with data post-processing. Molecules. 2017;22(10):1778.
- [Google Scholar]
- Three new nortriterpenoids from the rattan stems of Schisandra chinensis. Phytochem. Lett.. 2018;24:145-149.
- [Google Scholar]
- Application of characteristic fragment filtering with ultra high performance liquid chromatography coupled with high-resolution mass spectrometry for comprehensive identification of components in Schisandrae chinensis fructus. J. Sep. Sci.. 2019;42(7):1323-1331.
- [Google Scholar]
- Antioxidant and anti-proliferative activities of five compounds from Schisandra chinensis fruit. Ind. Crops and Prod.. 2013;50:690-693.
- [Google Scholar]
- Purification of six lignans from the stems of Schisandra chinensis by using high-speed counter-current chromatography combined with preparative high-performance liquid chromatography. Food Chem.. 2015;186:146-152.
- [Google Scholar]







