Translate this page into:
Semisynthetic derivatives of haemanthamine and their in vitro antiproliferative activity evaluation against a panel of human cell lines
⁎Corresponding authors at: Department of Pharmaceutical Botany, Faculty of Pharmacy, Charles University, Akademika Heyrovskeho 1203, 500 05 Hradec Kralove, Czech Republic (L. Cahlikova). havelekr@lfhk.cuni.cz (Radim Havelek), cahlikova@faf.cuni.cz (Lucie Cahlíková)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
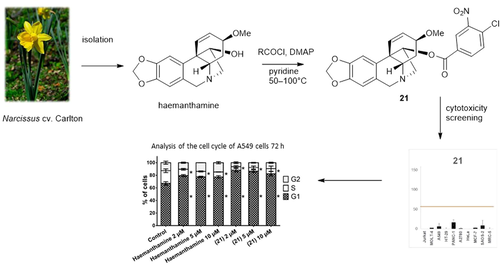
Abstract
In this study, a new series of aliphatic, cyclic, and heterocyclic derivatives of haemanthamine was designed and synthesized to enhance its inhibitory effect on the proliferation and viability of cancer cells. A library of haemanthamine derivatives was subjected to 10 μM single-dose cytotoxicity screening against a panel of human cell lines of various histotypes. Initial cytotoxicity evaluation of the parent haemanthamine (1) and a series of twenty-nine (2–30) semisynthetic analogues showed that for some of the newly formed derivatives, a certain cytotoxic effect was observed, in one case even higher than that of the parent compound. Specifically, 11-O-(4-chloro-3-nitrobenzoyl)haemanthamine (21) showed an enhanced antiproliferative effect, where the mean growth percent (GP) value was 5% compared to haemanthamine, leading to a decrease in the GP to 25%. Among ten cell lines tested, derivative 21, bearing a substituted aromatic ester bond via C-11 of haemanthamine, had excellent activity for inhibiting the growth of HeLa (IC50 = 0.2 ± 0.1 μM), A549 (IC50 = 1.7 ± 0.1 μM) and HT-29 (IC50 = 2.2 ± 0.1 μM) cells. When evaluating response kinetics, we found that 21 and haemanthamine dose- and time-dependently suppressed the proliferation of A549 cells. In contrast to haemanthamine (1), Trypan blue and lactate dehydrogenase (LDH) release assay revealed that 21 was capable of reducing the survival of A549 cells.
Keywords
Haemanthamine
Amaryllidaceae alkaloids
11-O-(4-chloro-3-nitrobenzoyl)haemanthamine
Antiproliferative activity
Cytotoxicity
In vitro
- AAs
-
Amaryllidaceae alkaloids
- DMSO
-
dimethyl sulfoxide
- DPBS
-
Dulbecco's phosphate-buffered saline
- ECACC
-
European Collection of Authenticated Cell Cultures
- GP
-
growth percent
- IC50
-
half maximal inhibitory concentration
- LDH
-
lactate dehydrogenase
- SAR
-
structure-activity relationship
- SD
-
standard deviation
Abbreviations
1 Introduction
Plants of the Amaryllidaceae family produce a large variety of structurally unique isoquinoline alkaloids (also known as Amaryllidaceae alkaloids), many of which have been reported as promising anticancer candidates, which could be considered for further development of new anticancer drugs. The first scientific evidence for the existing anticancer potential of Amaryllidaceae plants and their constituents dates back to at least the fourth century BC when Hippocrates of Cos used oil from the daffodil Narcissus poeticus L. for the treatment of cancer (Kornienko and Evidente, 2008). To date, more than 600 of these compounds have been isolated, including narciclasine, pancratistatin, haemanthamine, haemanthidine, montanine, bulbispermine, and distichamine (Fig. 1), which are, together with lycorine, of interest due to their promising anticancer properties. Narciclassine and lycorine have been intensively studied for their potent anti-tumor effects, both in vitro and in vivo, in various preclinical models of human cancers (Zhao et al., 2021; Gopalakrishnan et al., 2020; Cao et al., 2018; Fürst, 2016; Gao et al., 2021; Xin et al., 2020; Hu et al., 2020).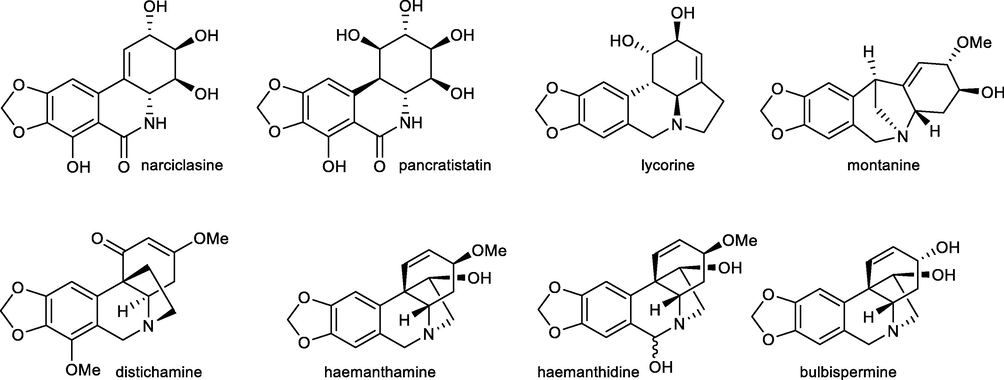
Amaryllidaceae alkaloids with interesting cytotoxic activity.
This study focuses on semisynthetic structural modifications of haemanthamine (1), an α-crinane-type alkaloid of plants from the Amaryllidaceae family, and its effect on biological activities. Haemanthamine (1) has been shown to have cytotoxic, antioxidant, antimicrobial, antiviral, antimalarial, and anti-inflammatory effects (Havelek et al. 2014; Habartová et al. 2016; Kohelová Peřinová, et al. 2019; Sener, Orhan, and Satayavivad 2003; Cedrón et al. 2012). Many studies indicated the anticancer potential of haemanthamine (1), especially as a substance with a growth-inhibitory, cytotoxic and proapoptotic effect on a wide variety of tumor cell lines (Pellegrino et al., 2018; Havelek et al., 2014; Havelek et al., 2017; Cedrón et al., 2015; Van Goietsenoven et al., 2010). Furthermore, possible anticancer activity was monitored in vivo on Erhlich's tumor, where haemanthamine (1) proved ineffective, which may indicate inappropriate selection of tumor tissue or reduced efficacy against the selected tumor type (Havelek et al., 2017).
The exact mechanisms of all the documented biological activities of haemanthamine (1) are not precisely known, but for the cytostatic effect of this substance, current knowledge has revealed its ability to bind to the eukaryotic 80S ribosome and subsequently inhibit proteosynthesis (Pellegrino et al., 2018). Another study, focusing on the anticancer potential of haemanthamine (1), showed inhibition of the growth of the ovarian cancer cell line A2780 in combination with sodium butyrate. The identified underlying molecular mechanisms of the inhibition were based on the reduction of phosphorylated checkpoint kinases Chk1 and Chk2 and the subsequent downregulation of p21 (Seifrtová et al., 2017). Haemanthamine (1), with a significant in vitro cytotoxic effect against different types of cancer cell lines (Habartová et al., 2016), has a high potential to function as an antineoplastic precursor. Therefore, its molecule is a promising starting material for structural optimization to provide a potential anticancer drug with enhanced antiproliferative and cytotoxic activity. Furthermore, this alkaloid is one of the most abundant AAs that can be easily isolated from plant material in gram-scale quantities.
Based on the inhibitory activity of haemanthamine (1) against cancer cells and its unique 5,10b-ethanophenanthridine chemical scaffold, structural modifications were carried out on the initial hit compound to explore structure-activity relationships (SAR), as well as acquire new potent anticancer candidates. In this study, new derivatives incorporating C-1 and C-2 hydrogenated haemanthamine (1), pentenoyl-, halo-substituted benzoyl-, naphthoyl- and furoyl- esters of haemanthamine (1) were prepared. The synthetized compounds were modified by binding aliphatic, cyclic, or heterocyclic ligands to the C-11 hydroxyl group of haemanthamine (1). All newly formed haemanthamine derivatives (2, 10, 11, 16–30), and haemanthamine derivatives (3–9, 12–15) synthesized within previous studies (Kohelová, Perinova, et al., 2019; Peřinová et al., 2020), were tested, together with haemanthamine (1), using an in vitro single-dose screening cytotoxicity test. We investigated the effect of alkaloids on the proliferation using the WST proliferation assay and by determining growth percentage in 9 tumor cell lines (Jurkat, MOLT-4, A549, HT-29, PANC-1, A2780, HeLa, MCF-7, SAOS-2) and one non-tumor line (MRC-5). The most growth-inhibitory derivative of all those tested was shown to be 11-O-(4-chloro-3-nitrobenzoyl)haemanthamine (derivative 21). Thus, the subsequent aim of the study was to assess the derivative itself and its possible cellular mechanism of action, including quantification of cell death, cell proliferation, and cell cycle analysis.
2 Material and methods
2.1 General experimental procedures
All solvents were treated by using standard techniques before use. All reagents and catalysts were purchased from commercial sources (Sigma Aldrich, Czech Republic) and used without purification. The NMR spectra were acquired at ambient temperature on a VNMR S500 (Varian) spectrometer operating at 500 MHz for 1H and 125.7 MHz for 13C. Chemical shifts were recorded as δ values in parts per million (ppm) and were indirectly referenced to tetramethylsilane (TMS) via the solvent signal (CDCl3 - 7.26 ppm for 1H and 77.0 ppm for 13C). Coupling constants (J) are given in Hz. ESI-HRMS were obtained with a Waters Synapt G2-Si hybrid mass analyzer of a quadrupole-time-of-flight (Q-TOF) type, coupled to a Waters Acquity I-Class UHPLC system. The temperature program was: 100–180 °C at 15 °C/min, 1 min hold at 180 °C, and 180–300 °C at 5 °C /min and 5 min hold at 300 °C; detection range m/z 40–600. The injector temperature was 280 °C. The flow-rate of the carrier gas (helium) was 0.8 mL/min. A split ratio of 1:15 was used. TLC was carried out on Merck precoated silica gel 60 F254 plates. Compounds on the plate were observed under UV light (254 and 366 nm) and visualized by spraying with Dragendorff's reagent.
2.2 Isolation of haemanthamine (1)
Haemanthamine has been previously isolated in gram amount from fresh bulbs of Narcissus pseudonarcissus cv. Dutch Master within our phytochemical study (Hulcová et al., 2019).
2.3 Preparation of haemanthamine derivatives
2.3.1 Hydrogenation of haemanthamine (1) to 1,2-dihydrohaemanthamine (2)
70 mg (0.23 mmol) of compound 1 dissolved in 5 mL of THF were hydrogenated in the presence of catalytic amounts of Pd/C. The reaction mixture was stirred for 5 days. The solution was filtered through Celite and the solvent evaporated and the residue was purified by preparative TLC (To:Et2NH 95:5) to yield 20 mg (87%) of 2 as an amorphous white solid. The NMR and MS data were in accordance with published data (Cedrón et al., 2012). Yield 20 mg (20%); amorphous white solid; [α]24D = +39.8 (c = 0.101, CHCl3).
2.3.2 General procedure for preparation of haemanthamine esters (10, 11, and 16–30)
Newly synthesized esters 10, 11, 16–30 (Fig. 1) have been prepared according to a procedure described previously (Kohelová, Peřinová, et al., 2019; Peřinová et al., 2020). The spectroscopic data for already described derivatives (3–9, 12–15) can be found in the following literature (Kohelová, Peřinová, et al., 2019; Peřinová et al., 2020; Maafi et al., 2021).
2.3.3 11-O-(3,5-Dimethylbenzoyl)haemanthamine (10)
Yield 24 mg (80%); amorphous white solid; [α]24D = +36.7° (c 0.120, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 7.53 (2H, s), 7.19 (1H, bs), 6.96 (1H, s), 6.50 (1H, s), 6.43 (1H, d, J = 10.0 Hz), 6.12 (1H, dd, J = 10.0 Hz, J = 4.9 Hz), 5.92–5.90 (2H, m), 5.19 (1H, dd, J = 6.8 Hz, J = 3.4 Hz), 4.40 (1H, d, J = 16.6 Hz), 3.88–3.83 (1H, m), 3.78 (1H, d, J = 16.6 Hz), 3.57–3.41 (3H, m), 3.36 (3H, s), 2.36 (6H, s), 2.18–2.04 (2H, m); 13C NMR (125 MHz, CDCl3) δ: 165.8, 146.7, 146.5, 138.0, 134.7, 134.3, 130.0, 129.4, 127.7, 127.0, 126.5, 106.6, 103.9, 100.9, 80.8, 72.5, 62.9, 61.2, 60.9, 56.5, 49.3, 28.6, 21.2; ESI–HRMS m/z calcd for C26H28NO5+ [M+H]+ 434.1962 found 434.1968.
2.3.4 11-O-(2,3-Dimethylbenzoyl)haemanthamine (11)
Yield 21 mg (70%); amorphous white solid; [α]24D = +67.7 (c 0.130, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 7.48 (1H, d, J = 7.8 Hz), 7.29 (1H, d, J = 7.8 Hz), 7.12 (1H, t, J = 7.8 Hz), 6.99 (1H, s), 6.50 (1H, s), 6.49 (1H, d, J = 10.0 Hz), 6.16 (1H, dd, J = 10.0 Hz, J = 4.9 Hz), 5.91 (2H, s), 5.19 (1H, dd, J = 6.9 Hz, J = 3.9 Hz), 4.40 (1H, d, J = 17.0 Hz), 3.88–3.83 (1H, m), 3.77 (1H, d, J = 17.0 Hz), 3.53 (1H, dd, J = 14.2 Hz, J = 7.3 Hz), 3.49–3.40 (2H, m), 3.36 (3H, s), 2.43 (3H, s), 2.32 (3H, s), 2.13–2.08 (1H, m), 2.04 (1H, td, J = 13.2 Hz, J = 3.9 Hz); 13C NMR (125 MHz, CDCl3) δ: 167.4, 146.7, 146.5, 138.1, 137.8, 134.5, 133.2, 130.7, 129.6, 128.0, 127.2, 126.6, 125.1, 106.6, 104.0, 100.9, 80.9, 72.5, 62.9, 61.2, 60.8, 56.5, 49.3, 28.5, 20.5, 16.6; ESI-HRMS m/z calcd for C26H28NO5+ [M+H]+ 434.1962 found 434.1970.
2.3.5 11-O-(3,5-Diethoxybenzoyl)haemanthamine (16)
Yield 19 mg (63%); amorphous white solid; [α]24D = +38.4° (c 0.125, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 7.04 (2H, d, J = 2.4 Hz), 6.96 (1H, s), 6.64 (1H, t, J = 2.4 Hz), 6.51 (1H, s), 6.43 (1H, d, J = 10.2 Hz), 6.14 (1H, dd, J = 10.2 Hz, J = 4.8 Hz), 5.94–5.91 (2H, m), 5.18 (1H, dd, J = 6.8 Hz, J = 3.7 Hz), 4.42 (1H, d, J = 16.8 Hz), 4.05 (4H, q, J = 6.9 Hz), 3.88–3.85 (1H, m), 3.80 (1H, d, J = 16.8 Hz), 3.57–3.43 (3H, m), 3.36 (3H, s), 2.20–2.14 (1H, m), 2.08 (1H, td, J = 4.2 Hz), 1.43 (6H, t, J = 6.9 Hz); 13C NMR (125 MHz, CDCl3) δ: 165.3, 160.0, 146.8, 146.6, 134.2, 131.8, 129.6, 127.6, 107.5, 106.7, 106.5, 104.0, 101.0, 80.8, 72.4, 63.8, 63.0, 61.1, 60.8, 56.5, 49.3, 28.4, 14.7; ESI-HRMS m/z calcd for C28H32NO7+ [M+H]+ 494.2174 found 494.2181.
2.3.6 11-O-(4-Pentenoyl)haemanthamine (17)
Yield 24 mg (80%); amorphous white solid; [α]24D = ─90.5° (c 0.190, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 6.91 (1H, s), 6.47 (1H, s), 6.35 (1H, d, J = 10.0 Hz), 6.16 (1H, dd, J = 10.0 Hz, J = 4.9 Hz), 5.90 (2H, s), 5.86–5.76 (1H, m), 5.06 (1H, d, J = 17.0 Hz), 5.02 (1H, d, J = 10.0 Hz), 4.98 (1H, dd, J = 7.1 Hz, J = 3.4 Hz), 4.36 (1H, d, J = 17.0 Hz), 3.87–3.82 (1H, m), 3.73 (1H, d, J = 17.0 Hz), 3.45–3.29 (3H, m, overlap), 3.37 (3H, s, overlap), 2.38–2.29 (1H, m, overlap), 2.32 (3H, s, overlap), 2.06 (1H, dd, J = 13.6 Hz, J = 4.1 Hz), 1.94 (1H, td, J = 13.6 Hz, J = 4.1 Hz); 13C NMR (125 MHz, CDCl3) δ: 172.0, 146.7, 146.4, 136.5, 134.3, 129.5, 127.8, 126.5, 115.5, 106.6, 103.9, 100.9, 80.3, 72.6, 62.8, 61.2, 60.7, 56.5, 49.1, 33.7, 28.7, 28.4; ESI-HRMS m/z calcd for C22H26NO5+ [M+H]+ 384.1806 found 384.1810.
2.3.7 11-O-(3,4-Dichlorobenzoyl)haemanthamine (18)
Yield 22 mg (73%); amorphous white solid; [α]24D = +50.9° (c 0.110, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 7.99 (1H, d, J = 2.0 Hz), 7.75 (1H, dd, J = 8.3 Hz, J = 2.0 Hz), 7.53 (1H, d, J = 8.3 Hz), 6.95 (1H, s), 6.51 (1H, s), 6.41 (1H, d, J = 10.1 Hz), 6.14 (1H, dd, J = 10.1 Hz, J = 4.9 Hz), 5.94–5.91 (2H, m), 5.19 (1H, dd, J = 7.0 Hz, J = 3.4 Hz), 4.41 (1H, d, J = 17.0 Hz), 3.88–3.84 (1H, m), 3.78 (1H, d, J = 17.0 Hz), 3.55 (1H, dd, J = 14.4 Hz, J = 7.0 Hz), 3.48–3.42 (2H, m), 3.37 (3H, s), 2.15 (1H, dd, J = 13.7 Hz, J = 4.4 Hz), 2.01 (1H, dd, J = 13.7 Hz, J = 4.4 Hz); 13C NMR (125 MHz, CDCl3) δ: 163.6, 146.8, 146.6, 137.8, 134.1, 133.1, 131.3, 130.7, 130.0, 129.8, 128.3, 127.6, 126.7, 106.7, 103.9, 100.9, 81.5, 72.4, 62.9, 61.3, 60.9, 56.6, 49.3, 27.7; ESI-HRMS m/z calcd for C24H22Cl2NO5+ [M+H]+ 474.0870 found 474.0872.
2.3.8 11-O-(2-Iodobenzoyl)haemanthamine (19)
Yield 18 mg (60%); amorphous white solid; [α]24D = +12.6° (c 0.190, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 7.99 (1H, dd, J = 7.7 Hz, J = 1.2 Hz), 7.63 (1H, dd, J = 7.7 Hz, J = 1.7 Hz), 7.39 (1H, td, J = 7.7 Hz, J = 1.2 Hz), 7.15 (1H, td, J = 7.7 Hz, J = 1.7 Hz), 6.99 (1H, s), 6.48 (1H, s, overlap), 6.48 (1H, d, overlap, J = 10.5 Hz), 6.17 (1H, dd, J = 10.5 Hz, J = 5.0 Hz), 5.91 (2H, s), 5.22 (1H, t, J = 5.0 Hz), 4.39 (1H, d, J = 16.9 Hz), 3.85–3.81 (1H, m), 3.77 (1H, d, J = 16.9 Hz), 3.52 (2H, d, J = 5.0 Hz), 3.43 (1H, dd, J = 13.4 Hz, J = 4.5 Hz), 3.35 (3H, s), 2.09 (1H, dd, J = 13.7 Hz, J = 4.5 Hz), 2.02 (1H, td, J = 13.7 Hz, J = 4.5 Hz); 13C NMR (125 MHz, CDCl3) δ: 165.5, 146.7, 146.5, 141.4, 134.7, 134.2, 132.7, 130.4, 129.7, 127.9, 127.8, 126.6, 106.6, 104.0, 100.9, 94.3, 81.5, 72.4, 62.8, 61.2, 60.5, 56.5, 49.4, 28.6; ESI-HRMS m/z calcd for C24H23INO5+ [M+H]+ 532.0616 found 532.0622.
2.3.9 11-O-(4-Iodobenzoyl)haemanthamine (20)
Yield 20 mg (67%); amorphous white solid; [α]24D = ─3.7° (c 0.215, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 7.83–7.77 (2H, m, AA′BB′), 7.65–7.59 (2H, m, AA′BB′), 6.94 (1H, s), 6.50 (1H, s), 6.41 (1H, d, J = 10.1 Hz), 6.11 (1H, dd, J = 10.1 Hz, J = 5.0 Hz), 5.94–5.89 (2H, m), 5.19 (1H, dd, J = 7.1 Hz, J = 3.8 Hz), 4.40 (1H, d, J = 16.9 Hz), 3.87–3.81 (1H, m), 3.77 (1H, d, J = 16.9 Hz), 3.53 (1H, dd, J = 14.0 Hz, J = 7.1 Hz), 3.49–3.38 (2H, m), 3.36 (3H, s), 2.13 (1H, dd, J = 14.0 Hz, J = 4.0 Hz), 2.03 (1H, td, J = 14.0 Hz, J = 4.0 Hz); 13C NMR (125 MHz, CDCl3) δ: 164.9, 146.7, 146.5, 137.8, 134.1, 130.7, 129.6, 129.6, 127.6, 126.6, 106.6, 103.9, 100.9, 81.1, 72.4, 62.9, 61.2, 60.9, 56.5, 49.3, 28.6; ESI-HRMS m/z calcd for C24H23INO5+ [M+H]+ 532.0616 found 532.0626.
2.3.10 11-O-(4-Chloro-3-nitrobenzoyl)haemanthamine (21)
Yield 23 mg (77%); amorphous yellow solid; [α]24D = +31.7° (c 0.240, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 8.38 (1H, d, J = 1.9 Hz), 8.04 (1H, dd, J = 8.3 Hz, J = 1.9 Hz), 7.65 (1H, d, J = 8.3 Hz), 6.93 (1H, s), 6.51 (1H, s), 6.40 (1H, d, J = 10.3 Hz), 6.15 (1H, dd, J = 10.3 Hz, J = 4.9 Hz), 5.93–5.91 (2H, m), 5.21 (1H, dd, J = 6.8 Hz, J = 2.9 Hz), 4.40 (1H, d, J = 16.6 Hz), 3.88–3.85 (1H, m), 3.77 (1H, d, J = 17.1 Hz), 3.56 (1H, dd, J = 14.2 Hz, J = 6.8 Hz), 3.45 (2H, dd, J = 14.2 Hz, J = 3.9 Hz), 3.37 (3H, s), 2.16 (1H, dd, J = 13.7 Hz, J = 4.2 Hz), 1.97 (1H, td, J = 13.7 Hz, J = 4.2 Hz); 13C NMR (125 MHz, CDCl3) δ: 162.6, 147.9, 146.8, 146.7, 133.8, 133.3, 132.4, 131.9, 130.1, 130.0, 127.4, 126.6, 126.4, 106.7, 103.8, 101.0, 81.8, 72.3, 62.9, 61.2, 60.8, 56.6, 49.2, 28.6; ESI–HRMS m/z calcd for C24H22ClN2O7+ [M+H]+ 485.1112 found 485.1110.
2.3.11 11-O-(4-Methyl-3-nitrobenzoyl)haemanthamine (22)
Yield 26 mg (87%); amorphous white-yellow solid; [α]24D = +38.4° (c 0.125, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 8.46 (1H, d, J = 1.3 Hz), 8.02 (1H, dd, J = 7.8 Hz, J = 1.3 Hz), 7.44 (1H, d, J = 7.8 Hz), 6.92 (1H, s), 6.49 (1H, s), 6.40 (1H, d, J = 10.3 Hz), 6.14 (1H, dd, J = 10.3 Hz, J = 4.9 Hz), 5.92–5.89 (2H, m), 5.20 (1H, dd, J = 6.8 Hz, J = 3.4 Hz), 4.39 (1H, d, J = 17.0 Hz), 3.90–3.86 (1H, m), 3.77 (1H, d, J = 17.0 Hz), 3.58–3.51 (1H, m), 3.48–3.42 (2H, m), 3.36 (3H, s), 2.66 (3H, s), 2.18–2.12 (1H, m), 2.06–1.99 (1H, m); 13C NMR (125 MHz, CDCl3) δ: 163.4, 149.1, 146.7, 146.6, 138.6, 133.9, 133.2, 133.1, 129.9, 129.4, 127.4, 126.6, 125.6, 106.7, 103.8, 100.9, 81.4, 72.3, 62.8, 61.2, 60.8, 56.6, 49.2, 28.5, 20.6; ESI-HRMS m/z calcd for C25H25N2O7+ [M+H]+ 465.1657 found 465.1659.
2.3.12 11-O-(3-Bromo-5-nitrobenzoyl)haemanthamine (23)
Yield 25 mg (83%); amorphous white-yellow solid; [α]24D = +100.0° (c 0.120, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 8.66–8.63 (1H, m), 8.58–8.55 (1H, m), 8.37–8.35 (1H, m), 6.91 (1H, s), 6.49 (1H, s), 6.40 (1H, d, J = 9.8 Hz), 6.19 (1H, dd, J = 9.8 Hz, J = 4.9 Hz), 5.93–5.87 (2H, m), 5.19 (1H, dd, J = 6.9 Hz, J = 3.4 Hz), 4.38 (1H, d, J = 17.1 Hz), 3.91–3.87 (1H, m), 3.76 (1H, d, J = 17.1 Hz), 3.55 (1H, dd, J = 14.2 Hz, J = 6.9 Hz), 3.48–3.42 (2H, m), 3.36 (3H, s), 2.16 (1H, dd, J = 13.7 Hz, J = 4.4 Hz), 1.98 (1H, td, J = 13.7 Hz, J = 4.4 Hz); 13C NMR (125 MHz, CDCl3) δ: 162.2, 148.8, 146.9, 146.8, 138.0, 133.7, 133.3, 130.6, 130.2, 127.3, 126.5, 123.2, 122.8, 106.8, 103.8, 101.0, 81.9, 72.3, 62.9, 61.2, 60.8, 56.7, 49.3, 28.6; ESI-HRMS m/z calcd for C24H22BrN2O7+ [M+H]+ 529.0605 found 529.0606.
2.3.13 11-O-(2-Chloro-4-nitrobenzoyl)haemanthamine (24)
Yield 18 mg (60%); amorphous white-yellow solid; [α]24D = +41.7° (c 0.115, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 8.30 (1H, d, J = 2.5 Hz), 8.15 (1H, dd, J = 8.8 Hz, J = 2.5 Hz), 7.85 (1H, d, J = 8.8 Hz), 6.96 (1H, s), 6.50 (1H, s), 6.45 (1H, d, J = 10.3 Hz), 6.17 (1H, dd, J = 10.3 Hz, J = 4.9 Hz), 5.93–5.90 (2H, m), 5.23 (1H, dd, J = 6.8 Hz, J = 3.9 Hz), 4.40 (1H, d, J = 17.0 Hz), 3.82–3.78 (1H, m, overlap), 3.78 (1H, d, overlap, J = 17.0 Hz), 3.55 (1H, dd, J = 14.5 Hz, J = 6.8 Hz), 3.50 (1H, dd, J = 14.5 Hz, J = 3.9 Hz), 3.44 (1H, dd, J = 13.6 Hz, J = 4.4 Hz), 3.35 (3H, s), 2.10 (1H, dd, J = 13.6 Hz, J = 4.4 Hz), 1.95 (1H, td, J = 13.6 Hz, J = 4.4 Hz); 13C NMR (125 MHz, CDCl3) δ: 163.2, 149.4, 146.8, 146.7, 135.5, 134.7, 133.7, 131.8, 130.1, 127.4, 126.4, 126.1, 121.5, 106.7, 103.9, 101.0, 82.2, 72.3, 62.8, 61.1, 60.4, 56.6, 49.4, 28.3; ESI-HRMS m/z calcd for C24H22ClNO5 [M+H]+ 485.1110 found 485.1111.
2.3.14 11-O-(4-Fluoro-3-methylbenzoyl)haemanthamine (25)
Yield 25 mg (83%); amorphous white solid; [α]24D = ─45.2° (c 0.115, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 7.82–7.78 (1H, m), 7.76–7.71 (1H, m), 7.05 (1H, t, J = 8.8 Hz), 6.95 (1H, s), 6.51 (1H, s), 6.43 (1H, d, J = 10.0 Hz), 6.12 (1H, dd, J = 10.0 Hz, J = 4.9 Hz), 5.93–5.91 (2H, m), 5.18 (1H, dd, J = 6.9 Hz, J = 3.4 Hz), 4.40 (1H, d, J = 16.9 Hz), 3.88–3.84 (1H, m), 3.78 (1H, d, J = 16.9 Hz), 3.54 (1H, dd, J = 14.0 Hz, J = 6.9 Hz), 3.48–3.41 (2H, m), 3.37 (3H, s), 2.32 (3H, s), 2.17–2.11 (1H, m), 2.06 (1H, td, J = 14.0 Hz, J = 4.4 Hz); 13C NMR (125 MHz, CDCl3) δ: 164.8, 164.4 (d, J = 252.8 Hz), 146.7, 146.5, 134.3, 133.2 (d, J = 6.7 Hz), 129.5, 129.0 (d, J = 9.4 Hz), 127.8, 126.7, 126.0 (d, J = 3.8 Hz), 125.3 (d, J = 20.5 Hz), 115.2 (d, J = 23.9 Hz), 106.7, 103.9, 100.9, 81.0, 72.5, 62.9, 61.3, 61.0, 56.5, 49.2, 28.7, 14.5 (d, J = 3.0 Hz); ESI-HRMS m/z calcd for C25H25FNO5+ [M+H]+ 438.1711 found 438.1715.
2.3.15 11-O-(6-Chloro-2-fluoro-3-methylbenzoyl)haemanthamine (26)
Yield 21 mg (70%); amorphous white solid; [α]24D = +14.5° (c 0.110, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 7.22–7.14 (1H, m), 7.13–7.07 (1H, m), 7.01 (1H, s), 6.53–6.45 (2H, m), 6.24–6.16 (1H, m), 5.92 (2H, s), 5.25–5.17 (1H, m), 4.40 (1H, d, J = 16.9 Hz), 3.84–3.73 (2H, m), 3.59–3.39 (3H, m), 3.36 (3H, s), 2.27 (3H, s), 2.08–1.88 (2H, m); 13C NMR (125 MHz, CDCl3) δ: 162.7, 158.1 (d, J = 252.8 Hz), 146.7, 146.6, 134.4, 133.1 (d, J = 5.7 Hz), 130.1, 129.5 (d, J = 4.4 Hz), 127.3, 126.7, 125.1 (d, J = 3.7 Hz), 124.3 (d, J = 21.0 Hz), 121.4 (d, J = 20.8 Hz), 106.6, 104.1, 100.9, 82.3, 72.6, 63.0, 61.3, 60.7, 56.5, 49.2, 28.3, 14.3 (d, J = 2.7 Hz); ESI-HRMS m/z calcd for C25H24ClFNO5+ [M+H]+ 472.1322 found 472.1328.
2.3.16 11-O-(1-Naphtoyl)haemanthamine (27)
Yield 22 mg (73%); amorphous white solid; [α]24D = +71.4° (c 0.185, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 8.90 (1H, d, J = 7.9 Hz), 8.03 (2H, d, J = 7.9 Hz), 7.90 (1H, d, J = 7.9 Hz), 7.62 (1H, t, J = 7.9 Hz), 7.55 (1H, t, J = 7.9 Hz), 7.49 (1H, t, J = 7.9 Hz), 7.03 (1H, s), 6.55 (1H, d, J = 10.0 Hz), 6.53 (1H, s), 6.16 (1H, dd, J = 10.0 Hz, J = 5.4 Hz), 5.95–5.92 (2H, m), 5.33 (1H, dd, J = 7.0 Hz, J = 3.7 Hz), 4.43 (1H, d, J = 16.7 Hz), 3.90–3.84 (1H, m), 3.81 (1H, d, J = 16.7 Hz), 3.58 (1H, dd, overlap, J = 14.3 Hz, J = 7.0 Hz), 3.55 (1H, dd, overlap, J = 14.3 Hz, J = 3.4 Hz), 3.48 (1H, dd, J = 11.8 Hz, J = 5.9 Hz), 3.36 (3H, s), 2.18–2.07 (2H, m); 13C NMR (125 MHz, CDCl3) δ: 166.3, 146.7, 146.5, 134.4, 133.8, 133.5, 131.3, 129.6, 129.6, 128.5, 127.9, 127.8, 126.9, 126.6, 126.3, 125.6, 124.4, 106.6, 104.0, 100.9, 81.1, 72.5, 62.9, 61.2, 60.9, 56.5, 49.4, 28.6; ESI-HRMS m/z calcd for C28H26NO5+ [M+H]+ 456.1805 found 456.1810.
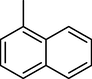
2.3.17 11-O-(2-Naphtoyl)haemanthamine (28)
Yield 19 mg (63%); amorphous white solid; [α]24D = +76.2° (c 0.105, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 8.50 (1H, s), 7.98–7.93 (2H, m), 7.91–7.87 (2H, m), 7.61 (1H, t, J = 7.6 Hz), 7.57 (1H, t, J = 7.6 Hz), 6.99 (1H, s), 6.52 (1H, s), 6.48 (1H, d, J = 9.9 Hz), 6.13 (1H, dd, J = 9.9 Hz, J = 4.9 Hz), 5.94–5.91 (2H, m), 5.29 (1H, dd, J = 6.4 Hz, J = 3.9 Hz), 4.43 (1H, d, J = 16.8 Hz), 3.92–3.87 (1H, m), 3.80 (1H, d, J = 16.8 Hz), 3.62–3.52 (2H, m), 3.48 (1H, t, J = 9.2 Hz), 3.37 (3H, s), 2.21–2.16 (2H, m); 13C NMR (125 MHz, CDCl3) δ: 165.6, 146.7, 146.5, 135.5, 134.3, 132.4, 130.9, 129.6, 129.3, 128.4, 128.3, 127.8, 127.8, 127.4, 126.7, 126.6, 124.8, 106.6, 104.0, 100.9, 81.1, 72.5, 62.9, 61.3, 61.0, 56.5, 49.4, 28.7; ESI-HRMS m/z calcd for C28H26NO5+ [M+H]+ 456.1805 found 456.1814.
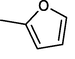
2.3.18 11-O-(2-Furoyl)haemanthamine (29)
Yield 23 mg (77%); amorphous white-yellow solid; [α]24D = +43.4° (c 0.175, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 7.57 (1H, s), 7.06 (1H, d, J = 3.2 Hz), 6.94 (1H, s), 6.51–6.46 (2H, m), 6.40 (1H, d, J = 10.0 Hz), 6.11 (1H, dd, J = 10.0 Hz, J = 4.9 Hz), 5.91 (1H, bs), 5.90 (1H, bs), 5.17 (1H, dd, J = 6.3 Hz, J = 3.4 Hz), 4.38 (1H, d, J = 16.9 Hz), 3.87–3.82 (1H, m), 3.76 (1H, d, J = 16.9 Hz), 3.54–3.38 (3H, m), 3.35 (3H, s), 2.15–2.01 (2H, m); 13C NMR (125 MHz, CDCl3) δ: 157.5, 146.7, 146.5, 144.6, 134.0, 129.8, 127.3, 126.5, 117.7, 111.8, 106.6, 104.0, 100.9, 80.6, 72.5, 62.9, 61.2, 60.7, 56.5, 49.3, 28.4; ESI-HRMS m/z calcd for C22H22NO6+ [M+H]+ 396.1442 found 396.1442.
2.3.19 11-O-(3-Furoyl)haemanthamine (30)
Yield 24 mg (80%); amorphous white-yellow solid; [α]24D = +32.0° (c 0.200, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 7.91 (1H, s), 7.41 (1H, s), 6.92 (1H, s), 6.64 (1H, s), 6.48 (1H, s), 6.40 (1H, d, J = 10.0 Hz), 6.16–6.08 (1H, m), 5.92–5.86 (2H, m), 5.15–5.09 (1H, m), 4.37 (1H, d, J = 16.9 Hz), 3.87–3.80 (1H, m), 3.74 (1H, d, J = 16.9 Hz), 3.53–3.45 (1H, m), 3.43–3.36 (2H, m), 3.34 (3H, s), 2.13–1.94 (2H, m); 13C NMR (125 MHz, CDCl3) δ: 161.8, 147.4, 146.6, 146.5, 143.8, 134.2, 129.5, 127.6, 126.5, 119.4, 109.5, 106.6, 103.9, 100.8, 80.3, 72.4, 62.8, 61.2, 60.9, 56.4, 49.1, 28.5; ESI-HRMS m/z calcd for C22H22NO6+ [M+H]+ 396.1442 found 396.1444.
2.4 In vitro experiments
2.4.1 Cell culture and culture condition
Selected human tumor and non-tumor cell lines, Jurkat (acute T cell leukemia), MOLT-4 (acute lymphoblastic leukemia), A549 (lung carcinoma), HT-29 (colorectal adenocarcinoma), PANC-1 (pancreas epithelioid carcinoma), A2780 (ovarian carcinoma), HeLa (cervix adenocarcinoma), MCF-7 (breast adenocarcinoma), SAOS-2 (osteosarcoma) and MRC-5 (normal lung fibroblasts) were purchased from European Collection of Authenticated Cell Cultures (ECACC, Salisbury, UK) and cultured according to the provideŕs culture method guidelines. All cell lines were maintained at 37 °C in a humidified 5% carbon dioxide and 95% air incubator. Cells in the maximum range of either 10 passages for non-tumor primary cell line (MRC-5), or in the maximum range of 20 passages for cancer cell lines (Jurkat, MOLT-4, A549, HT-29, PANC-1, A2780, HeLa, MCF-7 and SAOS-2) and in an exponential growth phase were used for this study.
2.4.2 Screening for antiproliferative activity, growth percent and IC50 value calculation
In starting experiments, each cell line was seeded at previously established optimal density (1.103 to 50.103 cells per well) in a 96-well plate (TPP, Trasadingen, Switzerland) and cells were allowed to settle overnight. Cells were treated for 48 h with alkaloids in either a final concentration of 10 µM or in a broad concentration range of 0.1–100 µM (determination of IC50 values). Doxorubicin (Sigma-Aldrich, St. Louis, USA), at a concentration of 1 µM, was used as a positive control. At the end of the cultivation period, the WST-1 proliferation assay (Sigma-Aldrich, St. Louis, USA) was performed according to the manufacturer's protocol and the absorbance was measured using Tecan Spark (Tecan, Männedorf, Switzerland). Each value is the mean of three independent experiments and represents the percentage of proliferation of 0.1% DMSO mock treated control cells (100%). The growth percent (GP) value was calculated for each alkaloid tested. GP represents the mean of the proliferation decrease in percent of all the 10 cell lines treated with the same alkaloid. IC50 values were calculated based on data obtained from proliferation determined by a WST-1 assay and were processed using GraphPad Prism 7 biostatistics (GraphPad Software, San Diego, CA, USA) software. Drug concentrations were plotted against the percentage of cell proliferation/viability and the IC50 values were determined using nonlinear regression.
2.4.3 Cell proliferation, viability, and lactate dehydrogenase (LDH) cytotoxicity assay
A549 cells were seeded in 96-well plates (500 cells/well) and allowed to settle overnight at 37 °C in 5% CO2. Cells were exposed to haemanthamine (1) and to 11-O-(4-chloro-3-nitrobenzoyl)haemanthamine (derivative 21) in a concentration range from 1 to 10 µM for 24, 48 and 72 h. To establish the cell proliferation - WST-1 proliferation reagent (Sigma-Aldrich, St. Louis, USA) was used according to the manufacturer’s instruction. At the end of the incubation period absorbance was measured using the microplate reader Tecan Spark (Tecan, Männedorf, Switzerland). Data represent the mean value of three independent experiments and are expressed in percent as the ratio of each sample absorbance related to the absorbance of untreated control cells (100%).
Cell proliferation and viability of A549 cells were monitored 24, 48 and 72 h after treatment with either haemanthamine (1) at 1, 2, 5 and 10 μM, or 21 at 1, 2, 5 and 10 μM. Cells treated with 1 µM doxorubicin were used as a positive control. Cell membrane integrity was determined using the Trypan blue exclusion technique – mixing 10 μL of 0.4% Trypan blue and 10 μL of cell suspension. Cell counts were carried out using a Bürker chamber and a Nikon Eclipse E200 light microscope (Nikon, Tokyo, Japan).
To establish the cytotoxicity of haemanthamine (1) and derivative 21, A549 cells were seeded in 96-well plates (1 000 cells/well) and allowed to settle overnight at 37 °C in 5% CO2. Cells were exposed to haemanthamine (1) and derivative 21 in concentrations ranging from 2 to 10 µM for 24, 48 and 72 h. A cytotoxicity Detection KitPLUS (LDH) (Roche, Basel, Switzerland) was used according to the manufacturer’s instruction. LDH release corresponds to the absorbance detected using the microplate reader Tecan Spark (Tecan, Männedorf, Switzerland).
2.4.4 Cell cycle distribution analysis
Cells were harvested by trypsinization, collected, washed with ice-cold Dulbecco's phosphate-buffered saline (DPBS) (Sigma-Aldrich, St. Louis, USA) and fixed with 70% ethanol. The cells were centrifuged to remove ethanol and washed again with ice-cold DPBS. To detect low-molecular-weight fragments of DNA, the cells were incubated for 5 min at room temperature in a buffer (192 mL of 0.2 M Na2HPO4 + 8 mL of 0.1 M citric acid, pH 7.8) and then labelled with propidium iodide in Vindelov’s solution for 1 h at 37 °C. DNA content was determined by using the flow cytometer CytoFLEX LX Flow Cytometer (Beckman Coulter, Miami, FL, USA) with an excitation wavelength of 488 nm. The list mode data were analyzed by Kaluza Analysis 2.1 software (Beckman Coulter, Miami, FL, USA).
2.4.5 Statistical analysis
The descriptive statistics of the results were calculated, and the charts were made using either Microsoft Office 365 Excel (Microsoft, Redmond, WA, USA) or GraphPad Prism 7 biostatistics (GraphPad Software, La Jolla, CA, USA) software. In this study, all the values were expressed as arithmetic means with SD of triplicates, unless otherwise noted. For quantitative data, normality testing was performed to assess whether parametric or nonparametric tests should be used. For experiments with parametric variables, significant differences between the groups were analyzed using the Student's t-test and a P value ≤ 0.05 was considered significant.
3 Results and discussion
3.1 Design and synthesis of haemanthamine derivatives 10, 11, 16–30
As mentioned above, 1 demonstrates promising cytotoxic activity and can be isolated from plants in gram amounts. Thus, it has been selected from the AAs for structure modification in order to increase the cytotoxic potential of 1. To study details of SAR, a series of aliphatic, aromatic, and heterocyclic esters of haemanthamine (10, 11, 16–30) has been synthesized, structurally supplementing the previously synthesized library (Kohelová, Perinova, et al., 2019; Peřinová et al., 2020). The substitutions were performed on the hydroxyl group at C-11 of 1, using either aliphatic or differently substituted benzoyl chlorides affording the corresponding esters 10, 11, 16–30 (Fig. 2, Table 1). Moreover, 1,2-dihydrohaemanthamine (2) has been prepared to study the role of the double bond in the structure of haemanthamine for its cytotoxic effect. Hydrogenation on palladium black furnished 2 in only 20% yield. On the other hand, all acyl derivatives were obtained in very good to excellent yield (60–95%).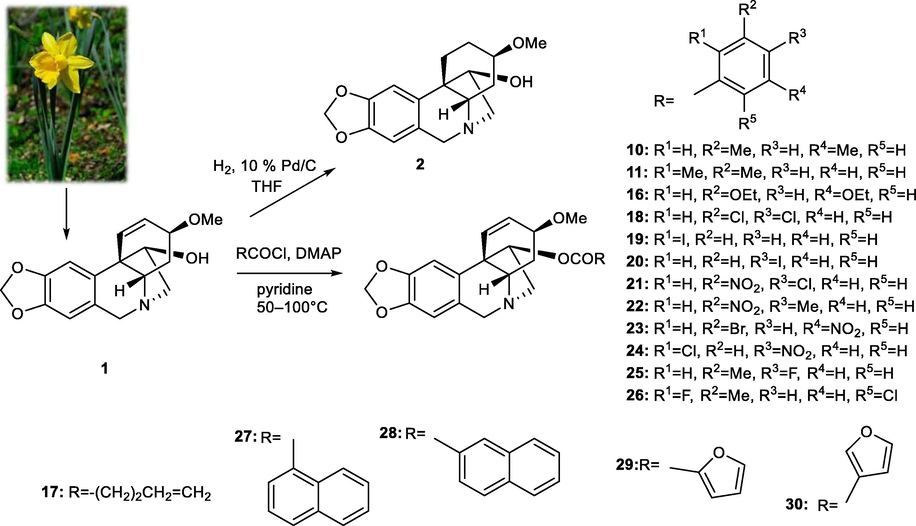
Design and synthesis of new haemanthamine derivatives.
R1
R2
R3
R4
R5
11-O-(2-chlorobenzoyl)haemanthamine (3)
Cl
H
H
H
H
11-O-(4-chlorobenzoyl)haemanthamine (4)
H
H
H
Cl
H
11-O-(2-nitrobenzoyl)haemanthamine (5)
NO2
H
H
H
H
11-O-(3-nitrobenzoyl)haemanthamine (6)
H
NO2
H
H
H
11-O-(3,5-dinitrobenzoyl)haemanthamine (7)
H
NO2
H
NO2
H
11-O-(3-Methylbenzoyl)haemanthamine (8)
H
Me
H
H
H
11-O-(4-Methylbenzoyl)haemanthamine (9)
H
H
Me
H
H
11-O-(3,5-Dimethylbenzoyl)haemanthamine (10)
H
Me
H
Me
H
11-O-(2,3-Dimethylbenzoyl)haemanthamine (11)
Me
Me
H
H
H
11-O-(2-Methoxylbenzoyl)haemanthamine (12)
OMe
H
H
H
H
11-O-(3-Methoxybenzoyl)haemanthamine (13)
H
OMe
H
H
H
11-O-(3,4-Dimethoxybenzoyl)haemanthamine (14)
H
OMe
OMe
H
H
11-O-(3,5-Dimethoxybenzoyl)haemanthamine (15)
H
OMe
H
OMe
H
11-O-(3,5-Diethoxybenzoyl)haemanthamine (16)
H
OEt
H
OEt
H
11-O-(3,4-Dichlorobenzoyl)haemanthamine (18)
H
Cl
Cl
H
H
11-O-(2-Iodobenzoyl)haemanthamine (19)
I
H
H
H
H
11-O-(4-Iodobenzoyl)haemanthamine (20)
H
H
I
H
H
11-O-(4-Chloro-3-nitrobenzoyl)haemanthamine (21)
H
NO2
Cl
H
H
11-O-(4-Methyl-3-nitrobenzoyl)haemanthamine (22)
H
NO2
Me
H
H
11-O-(3-Bromo-5-nitrobenzoyl)haemanthamine (23)
H
Br
H
NO2
H
11-O-(2-Chloro-4-nitrobenzoyl)haemanthamine (24)
Cl
H
NO2
H
H
11-O-(4-Fluoro-3-methylbenzoyl)haemanthamine (25)
H
Me
F
H
H
11-O-(6-Chloro-2-fluoro-3-methylbenzoyl)haemanthamine (26)
F
Me
H
H
Cl
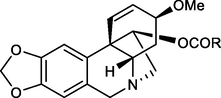
R
11-O-(4-Pentenoyl)haemanthamine (17)
–CH = CHCH2CH2CH3
11-O-(1-Naphtoyl)haemanthamine (27)
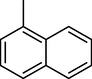
11-O-(2-Naphtoyl)haemanthamine (28)
11-O-(2-Furoyl)haemanthamine (29)
11-O-(3-Furoyl)haemanthamine (30)
3.2 Derivative 21 showed the most pronounced antiproliferative effect among the newly synthesized derivatives of haemanthamine
In the next step, the cytotoxic and antiproliferative activity of all haemanthamine derivatives (2–30), together with haemanthamine itself, were quantified in a panel of selected cancer and non-cancer cell lines. Ten different cell lines, representing eight human tumor types (leukemia, lung, colon, ovarian, breast, pancreas, cervix, and bone), were exposed to 10 μM of 2–30 for 48 h, and the decrease in cell proliferation compared to untreated control was determined by WST-1 assay. The effect of parent haemanthamine (1) corresponds with its similar inhibitory effect on cancer cells observed in other in vitro studies (Havelek et al., 2014). Among the derivatives tested, two analogues (7 and 21) of 1 demonstrated promising cytotoxic potential at the screened concentration of 10 μM (Fig. 3). Both are 3-nitro derivatives, but differ in additional substituents of the benzoyl moiety. In the case of 21, in 7 of the 10 cell lines tested, the proliferation dropped to less than 5% of the control cells. As depicted in Supplementary Fig. S1, even doxorubicin-resistant cell lines such as HT-29, A549, and PANC-1 were sensitive to the growth inhibitory activity of 21. To exclude the effect of the possible product of hydrolysis from the ester 21, 4-chloro-3-nitrobenzoyl chloride was screened for cytotoxic activity, but did not show any effect alone at a 10 μM concentration during 48 h of treatment.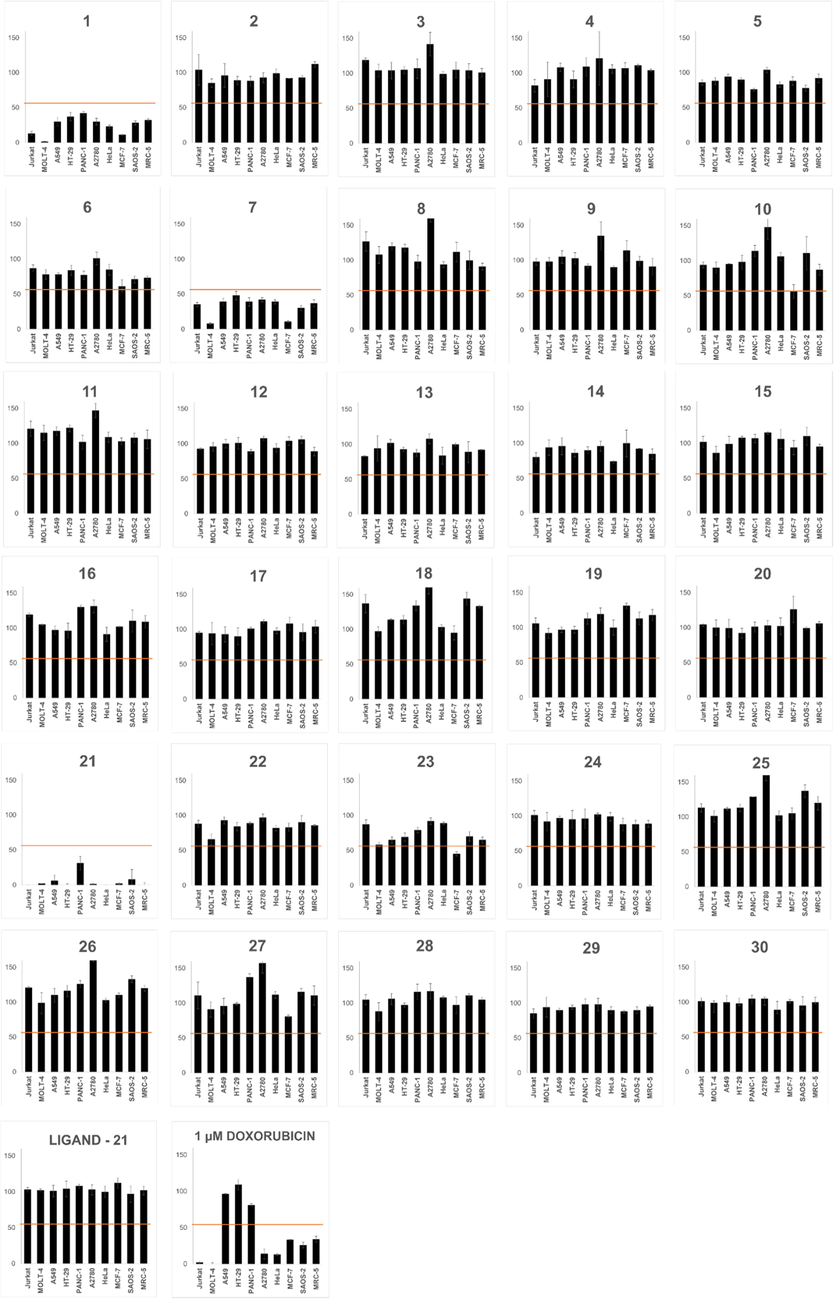
Antiproliferative activity of the tested compounds (1–30, C-11-bound ligand of derivative 21; and positive control doxorubicin). Each graph represents one compound and its effect on proliferation of 10 cell lines. Cells were treated with a concentration of 10 µM for 48 h, and their proliferation was established by WST-1 assay and expressed as a percentage of control cells (0.1% DMSO treated, proliferation 100%). Each value represents the mean ± standard deviation of three independent experiments. The horizontal line borders the 50% value. Doxorubicin was tested at 1 µM.
Interestingly, analogue 22, which differs from 21 only in the presence of a methyl group instead of chlorine in position 3 of the benzoyl group, demonstrated only weak cytotoxic activity. It could be concluded that comparison of the biological activity of haemanthamine (1) and 1,2-dihydrohaemanthamine (2) showed that the C1-C2 double bond is critical for cytotoxic activity. The one-dose treatment data of all screened compounds 1–30 for all ten human cell lines are presented in Supplementary Table S1.
The mean GP value, representing the mean proliferation of all tested cell lines compared to untreated controls, of haemanthamine (1) was 25%, with a range from 1% (MOLT-4) to 42% (PANC-1). Among the haemanthamine (1) derivatives tested, 21 showed the most pronounced overall antiproliferative activity. The mean GP dropped to 5% (compared to control cells), with a range from 0 for the most sensitive cell lines (Jurkat, HeLa, HT-29, MRC-5) to 31% for the least sensitive cell line (PANC-1). Moreover, 21, at a concentration of 10 µM, demonstrated not only higher overall antiproliferative activity compared to haemanthamine (1) as expressed by GP, but its activity was more pronounced against every single cell line (Table 2). Although 21 was highly potent and effective in inhibition of cell proliferation, this derivative did not elicit considerable level of selective antiproliferative activity against cancer cells by avoiding its growth-inhibitory activity towards non-cancer human lung fibroblast cell line MRC-5. Mean growth percent values, the range of proliferation decrease, as well as the most sensitive cell lines for each compound tested are summarized in Table S2. The mean sensitivity for unaltered haemanthamine, 1,2-dihydrohaemanthamine and C-11-substituented semisynthetic haemanthamine analogues across all cell lines tested can be found in Supplementary Material (Fig. S2).
Cell line
Haemanthamine (1)
2
7
21
Doxorubicin
Jurkat
13 ± 3
104 ± 22
35 ± 3
0 ± 0
2 ± 1
MOLT-4
1 ± 1
85 ± 6
8 ± 1
2 ± 0
0 ± 2
A549
30 ± 6
96 ± 17
39 ± 5
6 ± 8
96 ± 1
HT-29
37 ± 6
89 ± 6
48 ± 6
0 ± 2
109 ± 7
PANC-1
42 ± 3
88 ± 7
39 ± 6
31 ± 10
81 ± 2
A2780
30 ± 5
93 ± 7
42 ± 3
1 ± 1
14 ± 7
HeLa
23 ± 2
99 ± 6
39 ± 3
0 ± 0
13 ± 1
MCF-7
11 ± 1
92 ± 0
11 ± 1
2 ± 1
33 ± 1
SAOS-2
28 ± 3
93 ± 3
30 ± 4
8 ± 14
26 ± 4
MRC-5
32 ± 2
112 ± 4
37 ± 5
0 ± 3
34 ± 4
After the initial single-dose screening, the IC50 values of 21 and the parent compound haemanthamine (1) were measured for each tested cell line and are shown in Table 3. The IC50 values for 21 ranged from 0.2 ± 0.1 μM in the case of HeLa to 10.1 ± 0.6 μM in the case of PANC-1 cells. Clearly, the IC50 values for haemanthamine (1) were in a narrow-range from 1.2 ± 0.2 for MOLT-4 to 2.5 ± 0.6 μM for PANC-1 and MRC-5 cells. According to these determinations, derivative 21 has shown the most satisfactory potency among the haemanthamine derivatives and 21 reduced the survival of the determined cancer cell lines down to 50% at a concentration lower than 10 μM. These results confirmed the strong antiproliferative activity of 21 shared across a panel of human cancer cell lines with different doxorubicin sensitivity. Therefore, compound 21 was selected for further mechanistic experiments, and the A549 cell line, which demonstrated some degree of doxorubicin insensitivity, was used as a model. aResults are expressed in µM. bResults are the mean values ± standard deviations of at least three independent replications.
Cell line
Haemanthamine (1)
21
Jurkat
2.4 ± 0.3
5.3 ± 0.1
MOLT-4
1.2 ± 0.2
5.0 ± 0.2
A549
1.4 ± 0.5
1.7 ± 0.1
HT-29
2.4 ± 0.5
2.2 ± 0.1
PANC-1
2.5 ± 0.6
10.1 ± 0.6
A2780
2.2 ± 0.2
7.2 ± 0.3
HeLa
2.1 ± 0.3
0.2 ± 0.1
MCF-7
1.3 ± 0.2
2.6 ± 0.3
SAOS-2
2.3 ± 0.5
8.3 ± 0.4
MRC-5
2.5 ± 0.6
2.3 ± 0.2
3.3 Both haemanthamine (1) and derivative 21 decrease proliferation of A549 cancer cells, but only 21 reduces the cell viability and displays a cytotoxic effect
To compare the effect of haemanthamine (1) and 21 on A549 lung carcinoma cells, we primarily tested their influence on cell proliferation. A549 cells were treated with haemanthamine (1) and 21 for up to 72 h with increasing concentrations ranging from 1 to 10 μM. Both substances reduced the proliferation of A549 cells. The decline was time and dose dependent. The process was slightly different for haemanthamine (1) and 21 exposed cells. The proliferation of haemanthamine (1) treated cells dropped gradually with increasing concentration and time, whereas the effect of 21 was lesser for lower concentrations (1 and 2 μM), but more pronounced at higher concentrations (5 and 10 μM). Derivative 21 applied at 10 μM for 72 h caused a drop to 2% of control cells (as compared to 12% after haemanthamine) (Fig. 4A).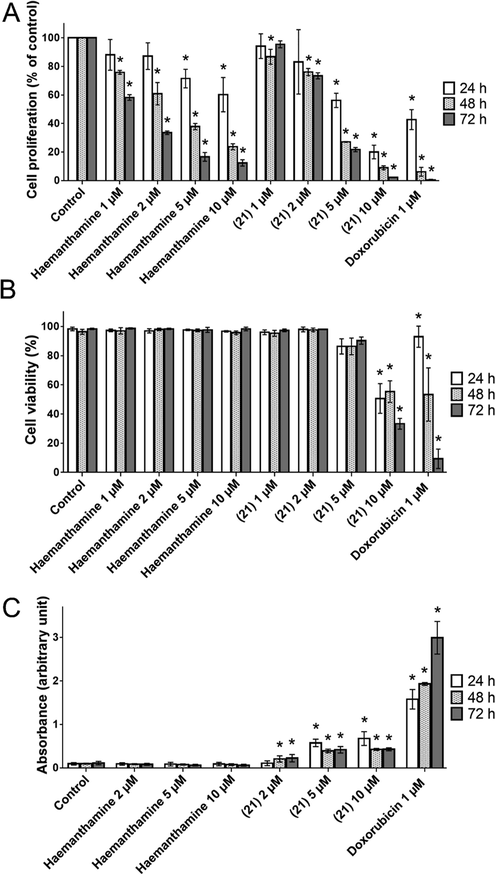
(A) Proliferation of A549 cells determined by WST-1 assay. Cells were treated with increasing concentrations of haemanthamine (1) and its derivative 21 up to 72 h. (B) Viability of A549 determined by Trypan blue exclusion technique. Cells were treated with increasing concentrations of haemanthamine (1) and its derivative 21 up to 72 h. (C) Cytotoxicity of haemanthamine (1) and its derivative 21 was measured by LDH release from A549 cells treated up to 72 h with increasing concentrations of both the alkaloids. All data are presented as mean ± standard deviation from three independent experiments. *P ≤ 0.05 was taken as a significant difference from the controls.
In order to ascertain whether haemanthamine (1) and 21-induced activity was dependent on cell death, we investigated the impact on cell viability. Unexpectedly, here we observed different mechanisms of action of these two compounds tested. Haemanthamine (1) alone did not show any cytotoxic effect, since the percentage of viable cells measured by the Trypan blue exclusion technique remained constant during the whole 72 h interval of treatment with concentrations ranging from 1 to 10 μM. In contrast, derivative 21, at concentrations of 5 and 10 μM, caused a significant decrease in viability after 24 h of treatment, to 86% and 51%, respectively. After 72 h exposure to 5 μM and 10 μM 21, the viability dropped to 90% and 33%, respectively (Fig. 4B). The cytotoxic effect of this derivative was confirmed by a LDH release detection kit. As shown in Fig. 4C, derivative 21, at concentrations of 5 and 10 μM, was proved to have a cytolytic effect linked with a significant increase in LDH activity in the culture medium. These findings correspond to cell damage and correlates with decreased viability of A549 after treatment with 21 in concentrations of 5 and 10 μM. Haemanthamine (1) had no effect on LDH release; this is in agreement with the previous Trypan Blue live/dead stain results, where haemanthamine (1) did not affect the cell viability, but only inhibited cell proliferation. To summarize these findings, while both haemantamine (1) and 21 significantly inhibited proliferation of A549 with comparable efficiency, 21 also exhibited a significant cytotoxic effect against A549, documented by loss of cell membrane integrity and an increased percentage of dead cells. Our observations correlate well with the described ability of 10 μM haemanthamine to induce a decrease in proliferation of ovarian carcinoma A2780 cells in a time-dependent fashion within 72 h (Seifrtová et al., 2017). Another earlier study conducted on peripheral blood-derived human leukemic Jurkat cells had reported apoptosis-mediated cell death and significant reduction of cell viability following haemanthamine treatment, but at higher doses ranging from 10 to 20 μM (Havelek et al., 2014).
3.4 Exposure to haemanthamine and 21 induced accumulation of A549 cells in the G1 phase of the cell cycle
In the WST-1 proliferation assay, haemanthamine (1) and 21 exhibited antiproliferative activity in a dose- and time-dependent manner. Cell cycle arrest is a major cause of cell proliferation inhibition. To determine whether haemanthamine (1) and 21 modulate cell cycle progression, we explored their effects on A549 cell cycle distribution after 24 and 72 h of treatment using flow cytometry. After 24-h of treatment, only the percentage of 10 µM haemanthamine (1)-exposed cells in the G1 phase was significantly increased to 70% (0.1% DMSO mock treated control 64%), with a concomitant decrease in the percentage of S-phase cells to 13% (0.1% DMSO mock treated control 19%). In the case of all other treatments, after a 24-h incubation interval, no significant difference was found compared with the 0.1% DMSO mock treated control group (Fig. 5A). In line with the observed time-dependence of the antiproliferative activity, haemanthamine (1) and 21 triggered significant (P ≤ 0.05) cell cycle redistribution at the G1 and S phase after 72 h of treatment with 2, 5 and 10 µM concentrations (Fig. 5B). On the other hand, this trend did not change considerably with the increase in dose of haemanthamine (1) or 21 at 72 h. For example, the percentage of cells in the G1 phase increased from 67% in the 0.1% DMSO mock treated controls to 82% and 77% in cells treated for 72 h with 10 µM of 21 and 10 µM of haemanthamine (1), respectively. In addition, the number of A549 cells in the S phase showed a reduction from 20% in the 0.1% DMSO mock treated control cells to 8% and 8% after 10 µM treatment with 21 and haemanthamine (1), respectively. In summary, both haemanthamine (1) and 21 cause significant and comparable cell cycle arrest in G1 by 72 h after exposure. Consistent with these results, 24-h exposure of T-leukemic Jurkat cells to haemanthamine at 5 and 15 µM resulted in cell cycle redistribution with an elevated percentage of cells in G1 and G2, while the percentage of cells in the S phase was reduced (Havelek et al., 2014).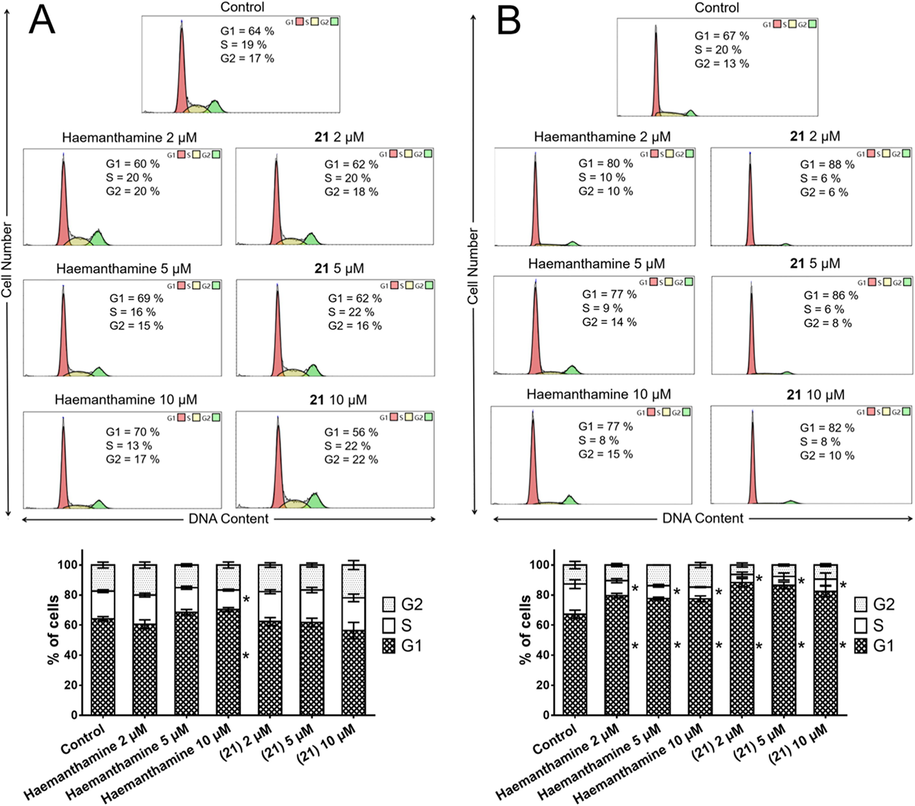
Analysis of the cell cycle of A549 cells 24 h (A) and 72 h (B) after the application of either haemanthamine (1) or 21. The figure shows representative histograms of human A549 lung carcinoma cells with the mean percentage of cells cycling through phases G1, S, and G2 from flow cytometry measurement of three separate treatments. The bar graph summarizes cumulative data on the percentage of cells in each phase of the cell cycle. Data are presented as mean values ± SD, n = 3. *Significantly different to control (P ≤ 0.05).
4 Conclusion
Haemanthamine (1) is reported as a compound with promising anticancer activity. Moreover, it can be isolated from different Amaryllidaceae plants in gram amounts, and thus a series of its derivatives has been developed to study the effect of derivatization on cytotoxic activity. Within 29 screened haemanthamine derivatives, the most effective was 21 (11-O-(4-chloro-3-nitrobenzoyl)haemanthamine). This showed a greater growth inhibition of cancer cell lines, which finally lead to a lower growth percent value than in the case of the parent haemanthamine (1). Based on this finding, the C-11 haemanthamine-bound ligand was tested separately for cytotoxicity. The analyzes were negative, and the ligand alone did not considerably affect the proliferation of the determined human tumor and non-tumor cells. During follow-up experiments aimed at the evaluation of cell viability after treatment with 21, haemanthamine (1), and the positive control doxorubicin, 21 induced a significant toxic effect and was very effective, even against resistant tumor lines, such as A549 non‐small cell lung carcinoma.
Acknowledgements
The skilful technical assistance of Mrs Nadezda Mazankova and Mrs Bozena Janska is greatly acknowledged. The authors thank prof. Gerald Blunden for proof-reading the manuscript. This project reg. No. CZ.02.1.01/0.0/0.0/18_069/0010046: the Pre-application research into innovative medicines and medical technologies project is co-funded by the European Union. This study was also supported in part by the Grant Agency of Charles University Progres/UK Q40/01, SVV 260 543 and SVV 260 548 of the Charles University.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Narciclasine induces autophagy-dependent apoptosis in triple-negative breast cancer cells by regulating the AMPK-ULK1 axis. Cell Prolif.. 2018;51:e12518.
- [Google Scholar]
- Synthesis and antimalarial activity of new haemanthamine-type derivatives. Bioorg. Med. Chem.. 2012;20:5464-5472.
- [Google Scholar]
- Antiproliferative and Structure Activity Relationships of Amaryllidaceae Alkaloids. Molecules. 2015;20:13854-13863.
- [Google Scholar]
- Narciclasine - an Amaryllidaceae Alkaloid with Potent Antitumor and Anti-Inflammatory Properties. Planta Med.. 2016;82:1389-1394.
- [Google Scholar]
- Identification of molecular anti-metastasis mechanisms of lycorine in colorectal cancer by RNA-seq analysis. Phytomedicine. 2021;85:153530.
- [Google Scholar]
- Narciclasine, an isocarbostyril alkaloid, has preferential activity against primary effusion lymphoma. Sci. Rep.. 2020;10:5712.
- [Google Scholar]
- The Biological Activity of Alkaloids from the Amaryllidaceae: From Cholinesterases Inhibition to Anticancer Activity. Nat. Prod. Commun.. 2016;11:1587-1594.
- [Google Scholar]
- Anticancer potential of Amaryllidaceae alkaloids evaluated by screening with a panel of human cells, real-time cellular analysis and Ehrlich tumor-bearing mice. Chem. Biol. Interact.. 2017;275:121-132.
- [Google Scholar]
- The effect of Amaryllidaceae alkaloids haemanthamine and haemanthidine on cell cycle progression and apoptosis in p53-negative human leukemic Jurkat cells. Phytomedicine. 2014;21:479-490.
- [Google Scholar]
- Lycorine Induces autophagy-associated apoptosis by targeting MEK2 and enhances vemurafenib activity in colorectal cancer. Aging. 2020;12:138-155.
- [Google Scholar]
- Amaryllidaceae alkaloids from Narcissus pseudonarcissus L. cv. Dutch Master as potential drugs in treatment of Alzheimer's disease. Phytochemistry. 2019;165:112055.
- [Google Scholar]
- Derivatives of the β-Crinane Amaryllidaceae Alkaloid Haemanthamine as Multi-Target Directed Ligands for Alzheimer's Disease. Molecules. 2019;24
- [Google Scholar]
- Derivatives of the β-Crinane Amaryllidaceae Alkaloid Haemanthamine as Multi-Target Directed Ligands for Alzheimer’s Disease. Molecules. 2019;24:1307.
- [Google Scholar]
- Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev.. 2008;108:1982-2014.
- [Google Scholar]
- Derivatives of montanine-type alkaloids and their implication for the treatment of Alzheimer's disease: Synthesis, biological activity and in silico study. Bioorg. Med. Chem. Lett.. 2021;51:128374.
- [Google Scholar]
- Pellegrino, S., Meyer, M., Zorbas, C., Bouchta, S.A., Saraf, K., Pelly, S.C., Yusupova, G., Evidente, A., Mathieu, V., Kornienko, A., Lafontaine, D.L.J., Yusupov, M., 2018. 'The Amaryllidaceae Alkaloid Haemanthamine Binds the Eukaryotic Ribosome to Repress Cancer Cell Growth', Structure, 26, 416-25.e4.
- Functionalized aromatic esters of the Amaryllidaceae alkaloid haemanthamine and their in vitro and in silico biological activity connected to Alzheimer's disease. Bioorg. Chem.. 2020;100:103928.
- [Google Scholar]
- Haemanthamine alters sodium butyrate-induced histone acetylation, p21. Phytomedicine. 2017;35:1-10.
- [Google Scholar]
- Antimalarial activity screening of some alkaloids and the plant extracts from Amaryllidaceae. Phytother. Res.. 2003;17:1220-1223.
- [Google Scholar]
- Amaryllidaceae alkaloids belonging to different structural subgroups display activity against apoptosis-resistant cancer cells. J. Nat. Prod.. 2010;73:1223-1227.
- [Google Scholar]
- Effect of lycorine on the structure and function of hepatoma cell membrane in vitro and in vivo. Biotechnol. Biotechnol. Equip.. 2020;34:104-114.
- [Google Scholar]
- Narciclasine inhibits LPS-induced neuroinflammation by modulating the Akt/IKK/NF-κB and JNK signaling pathways. Phytomedicine. 2021;85:153540.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103746.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







