Sesamol as a potential small molecule in inhibiting the induction of neurotoxic amyloids fibrils involved in the pathophysiology of alzheimer's disease
⁎Corresponding author at: Department of Neurosurgery and Laboratory of Neurosurgery, Lanzhou University Second Hospital, No.82, Cuiyingmen, Chengguan District, Lanzhou City 730000, Gansu Province, China. pyw@lzu.edu.cn (Ya-Wen Pan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
The presence of possibly toxic forms of intracellular α-synuclein, binding of aggregated α-synuclein and monomeric Aβ, and the potential co-localization of α-synuclein with intracellular neurofibrillary tangles as well as Aβ plaques in the brain could be the main causes in serious clinical symptoms mostly developed in Alzheimer’s disease (AD) patients. Amyloid formation of α-synuclein can also induce neurotoxic effects through stimulation of endoplasmic reticulum stress (ERS). Therefore, the inhibition of protein aggregation can be of great importance for the control of pathophysiology of AD. Here, we assessed the use of sesamol as bioactive small molecule in inhibition of α-synuclein fibrillogenesis and neurotoxicity by different biophysical [ThT/Nile red/Congo red (CR)/circular dichroism (CD)] and cellular (MTT and qRT-PCR) analyses. From the spectroscopic analyses, we found that sesamol displays potential effect on the inhibiting the α-synuclein fibril formation. Also, sesamol showed no significant neurotoxicity and co-incubation of α-synuclein with sesamol apparently mitigated the ERS-mediated apoptosis induced by α-synuclein amyloids through regulation of IRE1, PERK, ATF6, and caspase-3 mRNA. Overall, sesamol-based compounds can be further developed and assessed for the regulation of pathophysiology of AD in which α-synuclein amyloids are predominantly involved.
Keywords
α-Synuclein
Sesamol
Amyloid
Inhibition
Neurotoxicity
1 Introduction
A significant number of human disorders, including nervous system diseases, are associated with the formation of stable protein aggregates known as amyloid fibrils (Ross and Poirier, 2004). One of these disorders is a neurodegenerative disease called synucleinopathy, which is associated with the formation of amyloid accumulations of the α-synuclein in neurons, neurofibers, or glial cells known as Lewy bodies (Fujiwara et al., 2002; Jeon et al., 2020). The three main types of these diseases are Parkinson's disease (PD), dementia with Lewy body disease (LBD), and multiple system atrophy (MSA) (Twohig and Nielsen, 2019). It has been revealed that α-synuclein contributes in the pathophysiology of Alzheimer’s disease (AD), whereas it was reported that Lewy-related pathology, mainly has α-synuclein, is existed in a most of autopsied brains suffering from AD (Twohig and Nielsen, 2019).
AD is known as one the most common types of dementia in people over 65 years of age (Jalbert et al., 2008). Pathological studies have suggested the formation of fibrous plaques and accumulated masses of α-synuclein in the central nervous system as one of the causes of pathophysiology of AD and inhibition of this process as an effective treatment for this type of disease (Twohig and Nielsen, 2019). It has been well reported that protein misfolding results in multiple assembly pathways through transiently formation of oligomers and protofibrils, which assemble into polymorphic fibrils after longer incubation (Goldsbury et al., 2005).
α-Synuclein as a protein with 140 amino acids belongs to the group of proteins with random coil structure (Lashuel et al., 2013). This protein has an amino terminal part, which contains a repetitive sequence with α-helix structure in the vicinity of the membrane, a middle part, which is completely hydrophobic and plays a major role at the beginning of the aggregation process, and carboxyl terminal part, with an acidic sequence plays a protective role against aggregations (Lashuel et al., 2013). In fact, α-synuclein contains one histidine and one tyrosine in the part which forms β-sheet-reach core of the fibril consisted mostly of the aliphatic amino acids (Lashuel et al., 2013).
Indeed, it has been shown that small molecules can prevent the amyloid formation of α-synuclein through regulation of aggregation kinetic parameters (Marchiani et al., 2013). Also, it has been reported that amyloid structures can interact with cell membranes and induce significant cytotoxicity through different signaling pathways and organelle stress which accelerate the induction of neurodegenerative disease (Marchiani et al., 2013; Pena-Diaz et al., 2020). For example, it has been displayed that α-synuclein amyloid fibrils triggered calcium release, generation of free radicals and mitochondrial deficit during neurodegeneration (Angelova and Abramov, 2017). Also, it has been reported the caspase activation through endoplasmic reticulum stress (ERS) is mediated by amyloid-β (Nakagawa et al., 2000).
Aggregated species of proteins with their unique features, widely produced in central nervous system, have adverse effects on biological systems. Various mechanisms have been proposed to justify the harmful processes of amyloid species, but increasing the levels of reactive oxygen species (ROS) which interact directly with DNA, proteins, and cellular lipids, disrupting the vital function of cellular components such as the nucleus, mitochondria, lysosomes, and ER (Li et al., 2021). The ER is involved in regulating protein production, toxin neutralization, carbohydrate metabolism, lipid production, and calcium homeostasis. Amyloid species-induced ERS leads to mitochondrial dysfunction and cell apoptosis (Li et al., 2021).
Therefore, finding new and potential small molecules with promising anti-amyloid properties to control the protein aggregation-induced cytotoxicity through regulation of ERS can be of great importance in the treatment of neurodegenerative diseases including AD.
Sesamol as a natural organic compound the main active compound of Sesame (Sesamum indicum L.) seeds has demonstrated a wide range of pharmacological perspectives (Bosebabu et al., 2020). For example, it has been reported that sesamol can show protective effects against memory and cognitive impairments (Ren et al., 2018), depression (Kumar et al., 2011), and AD in rat model (Liu et al., 2021).
Therefore, the main novelty of this work is to explore the inhibitory effect of sesamol against α-synuclein amyloid production and associated cytotoxicity against neuron-like cells (PC-12). Also, the mechanism of cytotoxicity mediated by α-synuclein aged in the amyloidogenic buffer with or without sesamol through ERS was aimed to be evaluated.
2 Materials and method
2.1 Materials
α-Synuclein, Thioflavin T (ThT), Congo red (CR), Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) were obtained from Sigma-Aldrich Co. (USA). Fetal bovine serum (FBS) and antibiotics were purchased from Gibco Co. (USA).
2.2 Amyloid preparation
α-Synuclein concentration after centrifugation (100,000 g for 15 min) was calculated spectrophotometrically at 275 nm with an extinction coefficient of 5120 M−1 cm−1 (Meng et al., 2010). The formation of α-synuclein amyloid fibrils by dissolving α-synuclein monomers (100 µM) in the amyloidogenic buffer (20 mM sodium phosphate pH 7.4) was performed at 37 °C (Maqbool et al., 2020) supplemented with 5, 50, and 100 µM sesamol prepared in DMSO, where the final concentration of DMSO was not exceed than 1% (v/v). As a control, DMSO was added to the buffer. For all assays, α-synuclein samples aged for 60 h were withdrawn and used for further analyses.
2.3 ThT fluorescence assay
The fluorescence analysis was done with a fluorescence spectrophotometer (Cary Eclipse VARIAN, Australia) at 25 °C. To explore the inhibitory effect of sesamol with different concentrations of 5, 50, and 100 µM on the formation of α-synuclein amyloid fibril after 60 h, 15 µL of α-synuclein samples (100 µM) were added to 895 µL of 10 µM ThT solution, vortexed, and incubated for 7 min. The fluorescence emission data were then detected at excitation of 440 nm and the emission was read in the range of 460–550 nm. The excitation and emission slit widths were both set at 5 nm. All data were corrected against sesamol fluorescence intensity along with inner filter effect (Nielsen et al., 2001).
2.4 Nile red fluorescence assay
The set up for Nile red fluorescence analysis was similar as reported for ThT assay. The excitation wavelength was set at 550 nm and emission spectra were read between 580 and 760 nm. Both excitation and emission slit widths were set at 5 nm.
2.5 Congo red (CR) absorption assay
Aliquots of α-synuclein samples (10 μM), previously co-incubated with or without various concentrations of sesamol for 60 h, were mixed with CR solution (960 μL, 20 μM). After 45 min of incubation at 25 °C, CR absorbance was read between 440 and 600 nm using a UV–Vis spectrophotometer (Hitachi U 2000).
2.6 Circular dichroism (CD) assay
Far-UV CD (190–260 nm) spectra of different α-synuclein samples aged alone or with different concentrations of sesamol for 60 h were detected using a spectropolarimeter (Aviv Associates, USA), equipped with a 1 mm path cell at 25 °C. The protein concentration was set at 10 µM.
2.7 MTT assay
PC-12 cells (5 × 103 cells/well) were cultured into 96-well plates in DMEM/F12 cell culture medium supplemented with FBS (10%) and antibiotics (1%). After 24 h, the cells were replaced and added by the same medium containing α-synuclein samples or mixture of protein (10 µM) and sesamol with final concentrations of 0.5, 5 and 10 µM (aged for 60 h) for 24 h. The medium was then replaced with 5 mg/mL MTT solution and incubated for another 3.5 h at 37 °C. The MTT solution was then gently removed and then 150 μL of DMSO was poured into each well for 5 min and the optical density was read at 570 nm employing a microplate reader (SpectraMax M5, USA). Cells without any protein or sesamol incubation used as the control sample and the data were reported as a percentage of control.
2.8 Quantitative real time PCR (qRT-PCR) assay
After seeding and treating the cells with the different solutions of α-synuclein fibrillation products (10 µM) for 24 h, total RNA was extracted with the TRIzol reagent (Invitrogen Life Technologies, USA) and cDNA was synthesized employing the relevant RT kit (Invitrogen Life Technologies, USA) based on the manufacturers' instructions. SYBR-Green PCR master mix (Invitrogen Life Technologies, USA) was used for qRT-PCR analysis. The program and data analysis were set up based on the previous reports (Livak and Schmittgen, 2001; Tu et al., 2013).
2.9 Statistical analysis
The data were reported as mean ± standard error of five independent assays. Student’s t-test and one-way ANOVA were performed to assess the statistical difference (P-value < 0.05 using SPSS software.
3 Results
3.1 Determining α-synuclein fibrillization with or without sesamol
The progress of α-synuclein fibrillization (100 μM) was revealed by using ThT at 25 °C by reading fluorescence emission in the range of 460–550 nm. To examine the inhibitory effect of sesamol, α-synuclein fibrillization was performed in the presense of different concentrations of sesamol. As displayed in Fig. 1, sesamol resulted in an apparent decrease in the ThT fluorescence in a concentration-dependent manner. It can be suggested that ThT fluorescence is probably related to the level of amyloid fibrillization and the decrease in the ThT fluorescence can be determined to assess the inhibitory potential of sesamol (Naiki et al., 1989; Jameson et al., 2012). An apparent decline in ThT fluorescence would suggest that sesamol can prevent the α-synuclein fibrillization.
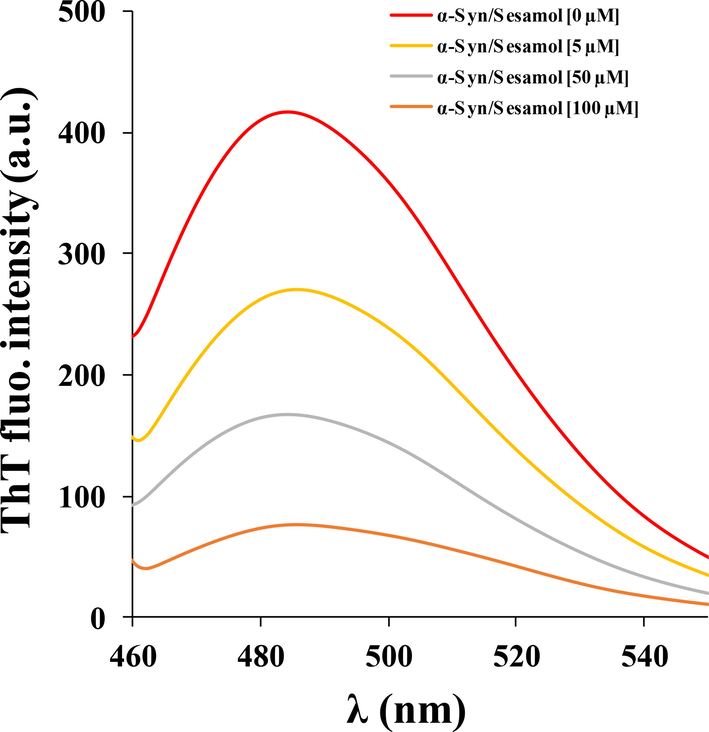
- ThT fluorescence analysis of α-synuclein aged for 60 h in the aggregation buffer with or without different concentrations of sesamol (5, 50, 100 µM).
3.2 Nile read fluorescence assay
Based on probable conformational changes of proteins that may occur during fibrillation, it has been well-established that different stimuli and aggregation buffers can trigger the formation of denatured structures in protein, characterized by appearance of relatively mobile hydrophobic patches on the protein surface (Gilan et al., 2019; Pang et al., 2021). Nile red fluorescence analysis as a hydrophobic reporter dye, showed formation of apparent hydrophobic groups on the surface of the α-synuclein amyloid (Fig. 2). As exhibited in Fig. 2, the presence of sesamol declined the Nile red fluorescence intensity along with a significant red shift, indicating the prevention of the formation of hydrophobic moieties in α-synuclein amyloid structure by sesamol in a probable concentration- dependent manner. Taking these data together, it seems quite likely that sesamol inhibits α-synuclein amyloid fibrillization by preventing the structural changes of protein.
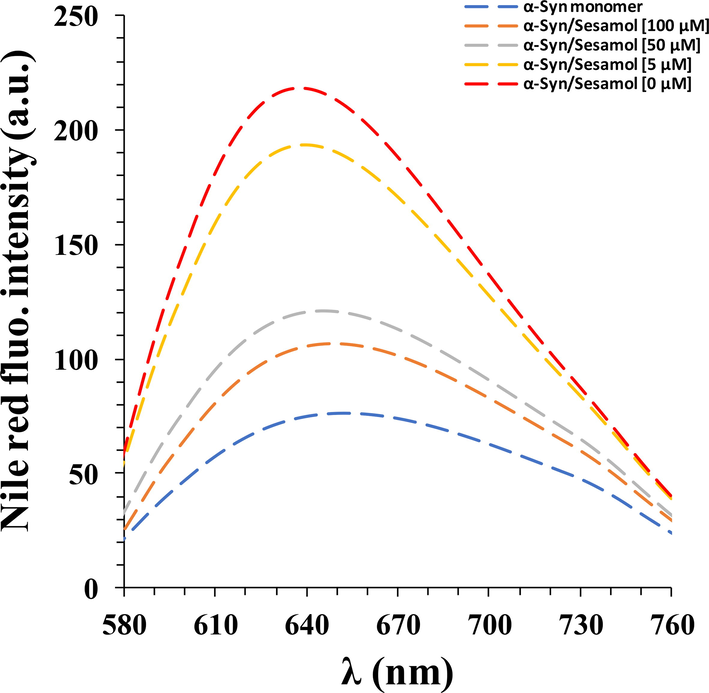
- Nile red fluorescence analysis of α-synuclein aged for 60 h in the aggregation buffer with or without different concentrations of sesamol (5, 50, 100 µM).
3.3 CR analysis
To further investigate whether sesamol influences the process of α-synuclein fibrillization, CR absorption assay was done. It was seen that although α-synuclein fibrillization resulted in a significant increase along with a blue shift in CR absorbance, sesamol was effective in inhibiting amyloid fibril formation of α-synuclein in a concentration dependent manner, as evidenced by the apparent reduction in both CR absorbance and blue shift (Fig. 3).
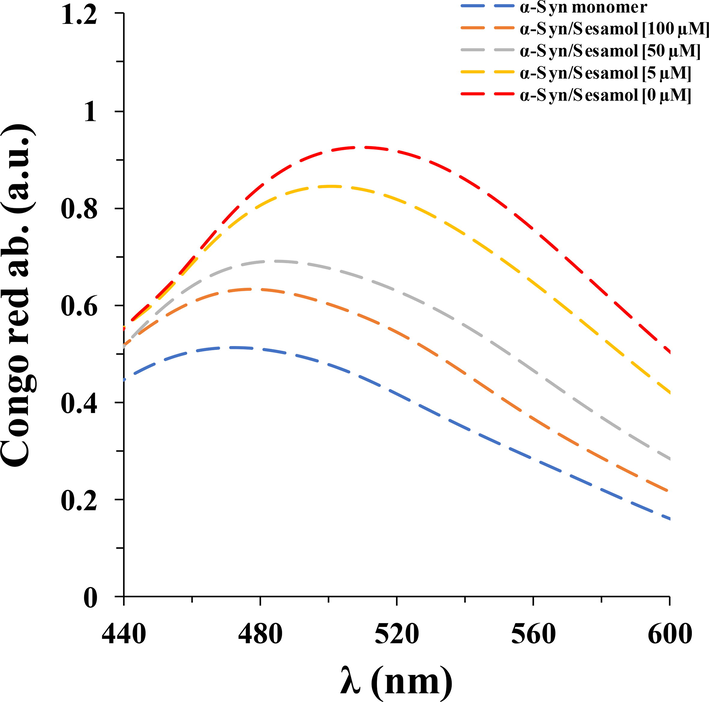
- CR absorption analysis of α-synuclein aged for 60 h in the aggregation buffer with or without different concentrations of sesamol (5, 50, 100 µM).
3.4 Far-UV CD analysis
To investigate whether secondary conformational alterations of α-synuclein induced by the aggregation buffer could be inhibited by sesamol, far-UV CD analysis of the α-synuclein was performed for different α-synuclein samples at the end of fibrillation process. As manifested in Fig. 4, the far-UV CD band of α-synuclein, after incubation in an aggregation buffer, alters from showing a minimum around 199 nm, features of the random coil conformation of the α-synuclein monomer, to a deep band around 218 nm, as reported for intermolecular β-sheet structures (Zand et al., 2019; Khodabandeh et al., 2020). However, sesamol with the studied concentrations afforded apparent inhibition on the formation of intermolecular β-sheet structures. Also, based on the characteristic alteration of the bands, namely the reduction of the intensity of minimum around 218 nm, this reduction in ellipticity intensity was further induced in the presence of higher concentrations of sesamol than that of lower concentrations.
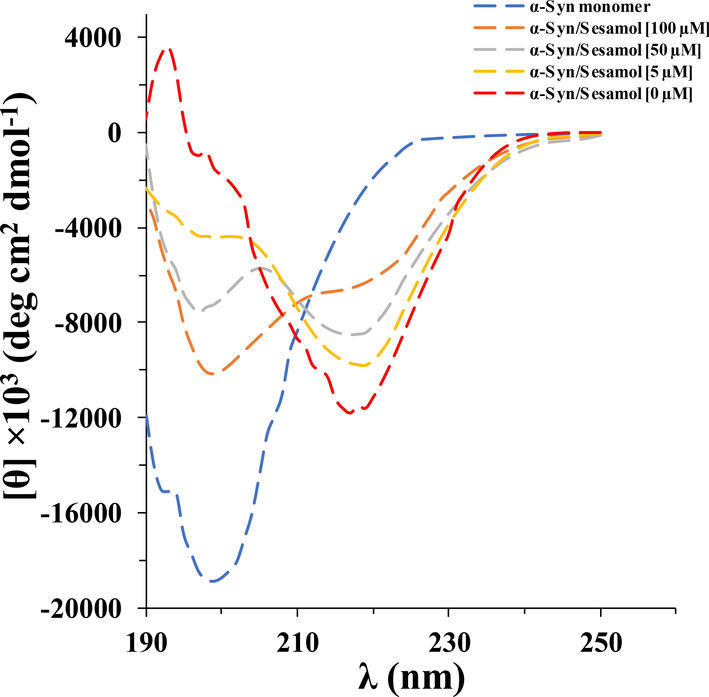
- Far-UV CD analysis of α-synuclein aged for 60 h in the aggregation buffer with or without different concentrations of sesamol (5, 50, 100 µM).
As revealed by the analysis indicated above, sesamol protects α-synuclein against secondary structural alterations.
It has been well-reported that amyloid species as intermediates products in the early stage of fibrillization process, may be dominantly responsible for amyloid-induced cytotoxicity, rather than the mature amyloid fibrils (Yang et al., 2020). Based on this fact, it is of great importance to assess whether the kinds of aggregates formed in the presence of sesamol are less cytotoxic products or not.
3.5 MTT assay
The cytotoxicity of the α-synuclein (100 µM) species formed in the aggregation buffer with or without different concentrations of sesamol (5, 50, and 100 µM) was assessed using the MTT assay (Fig. 5). For MTT assay, the protein samples and corresponding sesamol concentrations were diluted 10-fold. Therefore, PC-12 were cultured with different samples for 24 h and MTT assay was analyzed. The cytotoxicity of sesamol and α-synuclein monomers with the concentration of 10 µM was found to be negligible as compared to the control group. Notably, the cytotoxicity of α-synuclein amyloid without sesamol was significantly higher than that of α-synuclein amyloid with the similar procedure in the presence of varying concentrations of sesamol. Also, it was shown the inhibitory effect of sesamol on the mitigation of α-synuclein amyloid formation and associated cytotoxicity was based on a concentration-dependent manner. This data suggests that the presence of a therapeutic sesamol can inhibit the α-synuclein aggregation and underlying neurotoxicity without a significant injury to neurons.
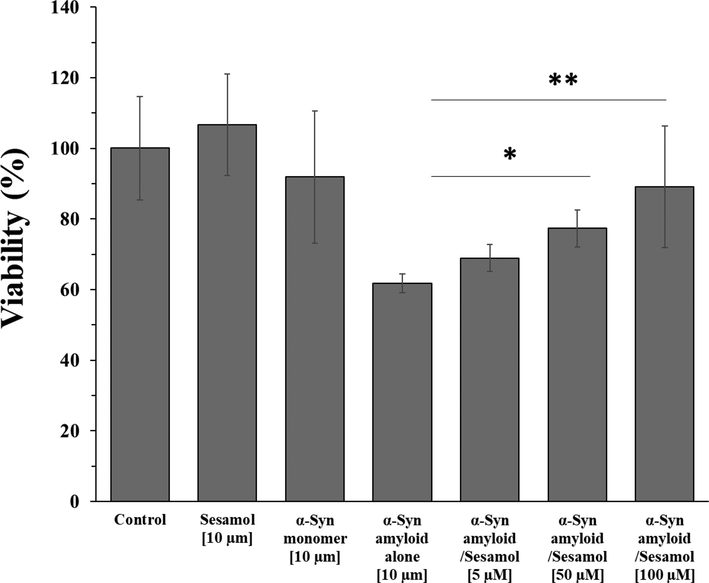
- MTT assay of PC-12 cells after incubation with α-synuclein amyloid aged for 60 h in the aggregation buffer without or with different concentration of sesamol after 24 h. *P < 0.05 and **P < 0.01 relative to control samples.
3.6 qRT-PCR assay
Different external stimuli including protein amyloids, can stimulated ERS which led to abnormal protein folding, i.e, unfolded protein response (UPR) comprised the important signaling pathways through inositol-requiring enzyme 1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6) (Kaneko et al., 2017; Amen et al., 2019). We then assessed the expression of these well-known ERS markers, to explore the impacts of α-synuclein amyloid aged for 60 h in the aggregation buffer with or without sesamol on ERS in PC-12 cells at 24 h. It was shown that α-synuclein amyloid alone apparently triggered overexpression of IRE1(Fig. 6a), PERK (Fig. 6b), ATF6 (Fig. 6c), which results in induction of apoptosis through caspase-3-dependant pathway (Fig. 6d). However, α-synuclein amyloid aged in the presence of sesamol induced downregulation of all studied genes relative to α-synuclein amyloid alone. These data supported that α-synuclein amyloids lead to ERS and associated apoptosis induction in PC-12 cells and this kind of stress can be mitigated after incubation of cells with α-synuclein amyloid aged with sesamol in a concentration-dependent manner.
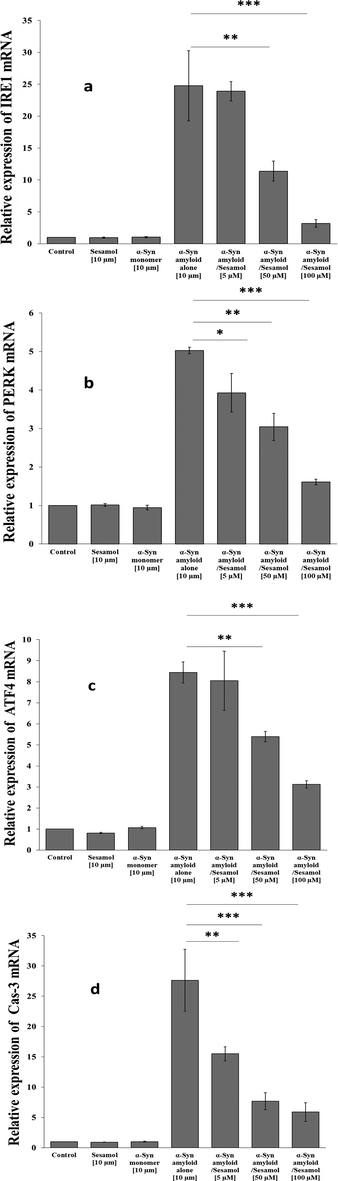
-
(a) IRF1 mRNA expression, (b) PERK mRNA expression, (c) ATF6 mRNA expression, (d) caspase-3 mRNA expression in PC-12 cells after incubation with α-synuclein amyloid aged for 60 h in the aggregation buffer without or with different concentration of sesamol after 24 h. *P < 0.05, **P < 0.01, ***P < 0.001: relative to control samples.
4 Discussion
In this study, we explored the protective effects of sesamol on the formation of α-synuclein fibrillization and associated neurotoxicity in vitro. To the best of our knowledge, this is the first study to show that the neurotoxicity stimulated by α-synuclein amyloids can be probably alleviated by co-incubation of α-synuclein samples with sesamol in an aggregation buffer though inhibiting the ERS-mediated apoptosis.
AD is a recognized global health priority because it imposes high costs on health care and economic systems (Jalbert et al., 2008). AD is a neurodegenerative disease known as the one of the common forms of dementia that cognitive and behavioral disorders are its symptoms and one of the important symptoms of its pathology is the destruction and degeneration of neurons (Warren et al., 2023).
Pathological markers of AD include the presence of amyloid plaques in extracellular and intracellular of neurons. Small molecules can activate cellular pathways such as the ubiquitin protease system, autophagy, apoptosis, and chaperones to protect cells against protein amyloid-induced neurotoxicity (Pourhanifeh et al., 2019).
Interactions of amyloid species of proteins with cells and deregulation of autophagy, apoptosis, inflammation and oxidative stress are important factors in the onset of neurodegenerative diseases (Pourhanifeh et al., 2019). Organized studies on the biological effects of amyloid species of proteins and their mechanism result in developing potential platforms for treatment of neurodegenerative diseases. So far, little information has been obtained about the toxicity mechanism of amyloid species of proteins. In this paper, in addition to characterization of amyloid fibrils formed in the presence of sesamol, we also examined the neurotoxicity of the aggregated species through ERS-mediated signaling pathway.
It has been indicated that ERS is considered as a crucial factor in the development of AD (Santos and Ferreira, 2018). Homeostasis of folded proteins in the ER is critical to cell function, and the presence of extracellular and intracellular abnormalities including protein amyloid fibrils cause accumulated proteins and incorrectly folded proteins in the ER (Huang et al., 2007). ERS has been observed in AD (Santos and Ferreira, 2018) which results in the activation of transcriptional protein response pathways, including the transcription factor 6 activator, inositol bound to protein 1, and the R-like protein kinase of the endoplasmic reticulum (Mahdi et al., 2016).
The role of ERS‐induced apoptosis in neurovegetative diseases has been well-reported (Xiang et al., 2017). Apoptosis can develop the onset and progression of many disorders including neurodegenerative diseases (Mattson, 2000). ERS-induced apoptosis is a process responsible for the destruction of damaged neurons (Huang et al., 2007).
It has been well-documented that formation of protein amyloids leads to ERS-induced apoptosis in vitro (Askanas and Engel, 2011) and some small molecules including Epigallocatechin gallate (Du et al., 2018) as well as bioactive compounds of kimchi (Woo et al., 2018) and syringic acid (SA) (Li et al., 2021) mitigate protein aggregated species‐triggered neurotoxicity through inhibiting ERS‐associated apoptosis.
Actually, it has been recently reported that SA can inhibit the fibrillation of tau protein as another important protein in the onset of neurodegenerative diseases through controlling amyloid kinetic parameters and inhibition of structural changes of protein verified by a number of biophysical and imaging techniques (Li et al., 2021). Furthermore, the protein species that appeared in the presence of SA showed lower adverse effects on the induction of ERS than amyloid spices alone by regulation of ATF-6, caspase-8 and caspase-3 genes (Li et al., 2021), which was in good agreement with the data found in this paper. In conclusion, it can be proposed that small molecules can be used as potential compounds in the development of effective platforms against protein aggregation. In fact, small molecules like sesamol can interact with monomeric states of proteins via different forces and inhibit the protein–protein interaction as a critical step in the protein aggregation pathway through delaying the rate constant of protein fibrillation. Also, it should be noted that some limitations of this study including the lack of exploring the direct interaction of sesamol with α-synuclein as well as the disaggregation of preformed amyloid species in the presence of sesamol, should be explored in the future studies in vitro and in vivo. However, based on this study we can claim that interaction of sesamol with monomeric species of α-synuclein are likely to inhibit the formation of amyloid fibrils and relevant neurotoxicity (ERS and apoptosis) evidenced by ThT/Nile red fluorescence, CR absorbance, CD, and cellular analyses.
Indeed, small molecules due to their unique structures can prevent the formation of amyloid fibrils due to potential interactions with proteins and resultant protein stability (Porat et al., 2006; Gazit, 2002). In fact, protein–protein interactions can play a key role in the formation of amyloid fibrils and any molecules that can target these forces, can be used as a potential anti-amyloid agent (Stains et al., 2007). Also, it should be noted that the iron in varying concentrations (Ganguly et al., 2020) can play a key role in implications in the pathogenesis of neurodegenerative diseases through induction of mitochondrial dysfunction mediated via different signaling pathways (Park et al., 2020).
5 Conclusion
In this study we found that sesamol can prevent the formation of α-synuclein fibrillization and underlying ERS-mediated apoptosis in a concentration dependent manner. Our findings suggest that sesamol can be used as a potential candidate for prevention of α-synuclein amyloids involved in the pathophysiology of AD and relevant regulation of ER stress-associated apoptosis in neurons.
Funding
This study was supported by Open Project of Gansu Neurology Clinical Medical Research Center (NO.20JR10FA663), General Project of Cuiying Plan of Second Hospital of Lanzhou University (NO.CY2019-MS01), and Talent Innovation and Entrepreneurship Project of Lanzhou (NO.2020-RC-87).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Endoplasmic reticulum stress activates unfolded protein response signaling and mediates inflammation, obesity, and cardiac dysfunction: therapeutic and molecular approach. Front. Pharmacol.. 2019 Sep;10(10):977.
- [Google Scholar]
- Alpha-synuclein and beta-amyloid–different targets, same players: calcium, free radicals and mitochondria in the mechanism of neurodegeneration. Biochem. Biophys. Res. Commun.. 2017 Feb 19;483(4):1110-1115.
- [Google Scholar]
- Sporadic inclusion-body myositis: conformational multifactorial ageing-related degenerative muscle disease associated with proteasomal and lysosomal inhibition, endoplasmic reticulum stress, and accumulation of amyloid-β42 oligomers and phosphorylated tau. Presse Med.. 2011 Apr 1;40(4):e219-e235.
- [Google Scholar]
- An Appraisal of current pharmacological perspectives of sesamol: a review. Mini Rev. Med. Chem.. 2020 Jul 1;20(11):988-1000.
- [Google Scholar]
- Epigallocatechin gallate reduces amyloid β-induced neurotoxicity via inhibiting endoplasmic reticulum stress-mediated apoptosis. Mol. Nutr. Food Res.. 2018 Apr;62(8):1700890.
- [Google Scholar]
- α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol.. 2002 Feb;4(2):160-164.
- [Google Scholar]
- Interaction of α-synuclein and Parkin in iron toxicity on SH-SY5Y cells: implications in the pathogenesis of Parkinson's disease. Biochem. J. 2020 Mar 27;477(6):1109-1122.
- [Google Scholar]
- A possible role for π-stacking in the self-assembly of amyloid fibrils. FASEB J.. 2002 Jan;16(1):77-83.
- [Google Scholar]
- α-synuclein interaction with zero-valent iron nanoparticles accelerates structural rearrangement into amyloid-susceptible structure with increased cytotoxic tendency. Int. J. Nanomed.. 2019;14:4637.
- [Google Scholar]
- Multiple assembly pathways underlie amyloid-β fibril polymorphisms. J. Mol. Biol.. 2005 Sep 16;352(2):282-298.
- [Google Scholar]
- Induction of endoplasmic reticulum stress-induced β-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. American Journal of Physiology-Endocrinology and Metabolism.. 2007 Dec;293(6):E1656-E1662.
- [Google Scholar]
- Dye-binding assays for evaluation of the effects of small molecule inhibitors on amyloid (Aβ) self-assembly. ACS Chem. Nerosci.. 2012 Nov 21;3(11):807-819.
- [Google Scholar]
- The Role of Glial Mitochondria in α-Synuclein Toxicity. Front. Cell Dev. Biol.. 2020 Nov;11(8):1297.
- [Google Scholar]
- ER stress and disease: toward prevention and treatment. Biol. Pharm. Bull.. 2017 Sep 1;40(9):1337-1343.
- [Google Scholar]
- Silybin as a potent inhibitor of a-synuclein aggregation and associated cytotoxicity against neuroblastoma cells induced by zinc oxide nanoparticles. J. Mol. Liq.. 2020 Jul;15(310):113198
- [Google Scholar]
- Neuropsychopharmacological effect of sesamol in unpredictable chronic mild stress model of depression: behavioral and biochemical evidences. Psychopharmacology (Berl). 2011 Apr;214(4):819-828.
- [Google Scholar]
- The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat. Rev. Neurosci.. 2013 Jan;14(1):38-48.
- [Google Scholar]
- Syringic acid demonstrates promising protective effect against tau fibrillization and cytotoxicity through regulation of endoplasmic reticulum stress-mediated pathway as a prelude to Alzheimer's disease. Int. J. Biol. Macromol.. 2021 Sep;29(1):1-6.
- [Google Scholar]
- Sesamol Attenuates amyloid peptide accumulation and cognitive deficits in APP/PS1 mice: the mediating role of the gut–brain axis. J. Agric. Food Chem.. 2021 Oct 20;69(43):12717-12729.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods.. 2001;25(4):402-408.
- [Google Scholar]
- Role of endoplasmic reticulum stress and unfolded protein responses in health and diseases. Indian J. Clin. Biochem.. 2016 Apr 1;31(2):127-137.
- [Google Scholar]
- Diphenyl triazine hybrids inhibit α-synuclein fibrillogenesis: Design, synthesis and in vitro efficacy studies. Eur. J. Med. Chem.. 2020 Dec;1(207):112705
- [Google Scholar]
- Small molecules interacting with α-synuclein: antiaggregating and cytoprotective properties. Amino Acids. 2013 Aug 1;45(2):327-338.
- [Google Scholar]
- Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol.. 2000 Nov;1(2):120-130.
- [Google Scholar]
- Effects of various flavonoids on the-synuclein fibrillation process. Parkinson’s Disease.. 2010 Oct;1:1-10.
- [Google Scholar]
- Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavine T. Anal. Biochem.. 1989 Mar 1;177(2):244-249.
- [Google Scholar]
- Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000 Jan;403(6765):98-103.
- [Google Scholar]
- Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism. Biochemistry. 2001 May 22;40(20):6036-6046.
- [Google Scholar]
- Acceleration of α-synuclein fibril formation and associated cytotoxicity stimulated by silica nanoparticles as a model of neurodegenerative diseases. Int. J. Biol. Macromol.. 2021 Feb;1(169):532-540.
- [Google Scholar]
- Alpha-synuclein-induced mitochondrial dysfunction is mediated via a sirtuin 3-dependent pathway. Mol. Neurodegener.. 2020 Dec;15:1-9.
- [Google Scholar]
- Small molecules to prevent the neurodegeneration caused by α-synuclein aggregation. Neural Regen. Res.. 2020 Dec;15(12):2260.
- [Google Scholar]
- Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des.. 2006 Jan;67(1):27-37.
- [Google Scholar]
- The effect of resveratrol on neurodegenerative disorders: possible protective actions against autophagy, apoptosis, inflammation and oxidative stress. Curr. Pharm. Des.. 2019 May 1;25(19):2178-2191.
- [Google Scholar]
- Protective effects of sesamol on systemic oxidative stress-induced cognitive impairments via regulation of Nrf2/Keap1 pathway. Food Funct.. 2018;9(11):5912-5924.
- [Google Scholar]
- Protein aggregation and neurodegenerative disease. Nat. Med.. 2004 Jul;10(7):S10-S17.
- [Google Scholar]
- Crosstalk between endoplasmic reticulum stress and brain inflammation in Alzheimer's disease. Neuropharmacology. 2018 Jul;1(136):350-360.
- [Google Scholar]
- Molecules that target beta-amyloid. ChemMedChem: Chemistry Enabling. Drug Discovery.. 2007 Dec 10;2(12):1674-1692.
- [Google Scholar]
- Vincristine induces cell cycle arrest and apoptosis in SH-SY5Y human neuroblastoma cells. Int. J. Mol. Med.. 2013 Jan 1;31(1):113-119.
- [Google Scholar]
- α-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener.. 2019 Dec;14(1):1-9.
- [Google Scholar]
- Cognitive and behavioral abnormalities in individuals with Alzheimer’s disease, mild cognitive impairment, and subjective memory complaints. Curr. Psychol.. 2023;13:1.
- [Google Scholar]
- Bioactive compounds of kimchi inhibit apoptosis by attenuating endoplasmic reticulum stress in the brain of amyloid β-injected mice. J. Agric. Food Chem.. 2018 Apr 30;66(19):4883-4890.
- [Google Scholar]
- The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis. 2017 Jan;22(1):1-26.
- [Google Scholar]
- Amelioration of aggregate cytotoxicity by catalytic conversion of protein oligomers into amyloid fibrils. Nanoscale. 2020;12(36):18663-18672.
- [Google Scholar]
- Cerium oxide NPs mitigate the amyloid formation of α-synuclein and associated cytotoxicity. Int. J. Nanomed.. 2019;14:6989.
- [Google Scholar]







