Translate this page into:
Simple and practical, highly sensitive and responsive recognition of cysteine: Design, synthesis and mechanism study of a novel curcumin fluorescent probe
⁎Corresponding author. organicboron@ujs.edu.cn (Guofan Jin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A novel class of curcumin-derived fluorescent probes was designed. This kind of probe introduces easy leaving groups methylsulfonyl and phenylsulfonyl respectively to achieve the detection effect through the nucleophilic attack of amino acids. At the same time, BF2 group is introduced to increase the emission wavelength of the probe. Probes 4 and 5 can respond quickly with amino acids, but can specifically recognize Cys. In UV detection, the maximum absorption wavelength of the probes can be blue-shifted by 81 nm with the addition of Cys and still show a strong fluorescence signal. The detection limits for compounds 4 and 5 were determined to be 0.40 μM and 0.87 μM, respectively, with a goodness-of-fit of 0.99. In addition, a rapid response of the probe to Cys could be observed with the naked eye within 1 min. These results provide a new method for rapid detection of Cys; And this kind of probe has the drug structure of curcumin, which can provide ideas for the design of drug-probe.
Keywords
Curcumin
Amino acid
Cysteine
Fluorescent probe
Luminescence
1 Introduction
Cysteine (Cys) is an important conditional amino acid in human body and biological thiol molecule, which plays an important role in various physiological activities and is closely related to the integrity of many key proteins (Wang and Qian, 2019; Chan et al., 2021; Tu et al., 2022; Jiang et al., 2018; Yang et al., 2020). At present, many studies have shown that Cys metabolic imbalance can induce a variety of diseases, including weakened immunity, growth retardation, nervous system diseases, cardiovascular diseases, and metabolic abnormalities and so on (Schleyer and Cui, 2021; Rehman et al., 2020). Therefore, efficient and rapid detection of Cys can help in the early prediction and prevention of diseases.
In the past decades, many methods for detecting Cys have been reported, such as electrochemical methods, high performance liquid chromatography, and fluorescent probes (Thota and Ganesh, 2016; Dey et al., 2017; Deáková et al., 2015; Zhao et al., 2020; Zhang et al., 2019; Wang et al., 2019; Zhang et al., 2020). And fluorescent probes are of great interest to research scholars because of their simplicity, convenience, ease of control, high sensitivity and good selectivity (Singh et al., 2021; Hao et al., 2021; Lian et al., 2021; Wang et al., 2021; Xiao et al., 2020; Shao et al., 2022). Curcumin is a natural polyphenol probe, which is cheap and easy to obtain (Ge et al., 2021; Moniruzzaman and Min, 2020; Jiang et al., 2021). It has attracted much attention because of its strong fluorescence and good anti-oxidant stress, anti-inflammatory and anti-tumor effects (Giordano and Tommonaro, 2019; Zoi et al., 2021; Vallée and Lecarpentier, 2020; Xiang et al., 2020; Shi and Zhu, 2020; Liang et al., 2018; Aliabbasi et al., 2021). It is reported that after complexation with boron difluoride, the emission spectrum changes under different substituents, which can be used as electron / charge transfer material for organic light emitting diodes (Selvam et al., 2019); Curcumin boron complexes can also be used as a sensing indicator of pH and anion in mineral water (Tsuchikawa et al., 2017). However, so far, no curcumin-boron complex for selective detection of Cys has been designed.
Sulfonyl groups are oxygen-rich groups with high reactivity and anti-inflammatory and anti-tumor activities, so they are widely used in the structural modification of compounds (Berredjem et al., 2022; Vishwakarma et al., 2018; Shao et al., 2021). For example, the introduction of sulfonyl groups in purines can improve the reactivity of compounds (Zaķis et al., 2020); the introduction of polar groups sulfonyl groups on polysiloxanes to increase their dielectric constants (Dünki et al., 2017); the introduction of it on the parent nucleus of BODIPY can be used as a bio-thiol detection probe (Lv et al., 2020). Based on the previous studies, this group was considered to be introduced into curcumin to improve the recognition activity of Cys, and the hydrophilic oxygen-rich groups are more suitable for the detection of water-soluble amino acids.
In this work, methylsulfonyl and phenylsulfonyl groups were used to replace the phenolic hydroxyl groups on curcumin to increase the recognition of probes and amino acids. Then boron trifluoride was used to increase the emission wavelength of the probe, and finally novel curcumin-derived probes were obtained. Through the rapid reaction with Cys, the maximum UV absorption and emission wavelength are blue shifted to realize the detection of amino acids. In addition, the selective recognition mechanism of cysteine was deeply discussed to prove that these derivatives can be used for simple, rapid and efficient identification and detection of cysteine.
2 Experimental section
2.1 Reagents and instrumentation
All reagents are commercially purchased with high commercial quality and can be used without further purification. The solvents dichloromethane (DCM), methanol, tetrahydrofuran (THF) and dimethyl sulfoxide (DMSO) were analytically pure, and the experimental water was ultrapure water. NMR were recorded by Bruker avance II instrument in deuterated dimethyl sulfoxide (DMSO‑d6, 400 MHz for 1H and 100 MHz for 13C). Chemical shifts are reported in units of parts per million (ppm), versus internal tetramethylsilane (TMS) as a standard. The mass spectrum were obtained at the Thermo LXQ by liquid chromatgraphy-ion trap mass spectrometry. The absorption spectra were recorded on UV-2550 spectrophotometer using quartz cuvettes with a path length of 1 cm. The fluorescence emission spectra were measured with 400 nm excitation wavelength on Shimadzu RF-5301PCS spectrofluorophotometer.
2.2 Synthetic route
((1E,6E)-3,5-dioxohepta-1,6-diene-1,7-diyl)bis(2-methoxy-4,1-phenylene) dimethanesulf-onate (2).
Curcumin (compound 1, 1.0 g, 2.7 mmol) was dissolved in THF (15 mL), methanesulfonyl chloride (2.2 mL, 28.4 mmol) and TEA (4.2 mL, 30.2 mmol) were added, and the reaction was confirmed by TLC after stirring magnetically for 30 min at room temperature. Evaporate THF under reduced pressure and add ultrapure water to precipitate the solid. After stirring the solid with methanol for 30 min, 1.3 g yellow powder 3 (93%) was obtained by suction filtration. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) = 7.65 (d, J = 16.0 Hz, 2H), 7.57 (s, 2H), 7.02 (d, J = 16.0 Hz, 2H), 6.21 (s, 1H), 3.91 (s, 6H), 3.37 (s, 6H). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) = 183.59, 152.24, 139.89, 139.54, 135.39, 125.90, 124.74, 121.78, 113.35, 102.40,56.68, 38.97. ITMS (ESI) calcd for C23H24O10S2 [M + H]+ m/z 525.5629; found 525.2363.
((1E,6E)-3,5-dioxohepta-1,6-diene-1,7-diyl)bis(2-methoxy-4,1-phenylene) dibenzenesulf-onate (3).
1(1.0 g, 2.7 mmol) was dissolved in THF (8 mL), and benzenesulfonyl chloride (0.8 mL, 6.3 mmol) and triethylamine (TEA, 0.9 mL, 6.8 mmol) were added. The reaction was confirmed by thin layer chromatography (TLC) after magnetic stirring at room temperature for 30 min. Evaporate THF under reduced pressure and add ultrapure water to precipitate solid. After stirring the solid with methanol for 30 min, 1.7 g yellow powder 2 (97%) was obtained by suction filtration.1H NMR (400 MHz, DMSO‑d6): δ (ppm) = 7.84–7.78 (m, 6H), 7.67–7.56 (m, 6H), 7.41 (d, J = 1.6 Hz, 2H), 6.96 (d, J = 8.0 Hz, 2H), 3.53 (s, 7H). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) = 152.05, 139.70, 139.13, 135.55, 135.53, 135.33, 129.92, 128.66, 126.02, 124.37, 121.62, 113.16, 102.45, 67.48, 56.25, 25.60. ITMS (ESI) calcd for C33H28O10S2 [M + H]+ m/z 649.7049; found 649.2880.
((1E,3Z,6E)-3-((difluoroboranyl)oxy)-5-oxohepta-1,3,6-triene-1,7-diyl)bis(2-methoxy-4,1-phenylene) dimethanesulfonate (4).
2 (0.2 g, 0.4 mmol) was dissolved in THF (15 mL) and boron trifluoride diethyl etherate (0.5 mL, 4.0 mmol) was added. The reaction was confirmed by TLC after stirring magnetically for 5 h at room temperature·THF solvent was evaporated under reduced pressure, water was added to neutral pH, filtered, and rinsed with a large amount of methanol. The solid was added to DCM and stirred for 30 min, and then filtered to obtain orange solid 4 (0.14 g, 62%). 1H NMR (400 MHz, DMSO‑d6): δ (ppm) = 8.06 (d, J = 15.6 Hz, 2H), 7.72 (s, 2H), 7.52 (d, J = 8.0 Hz, 2H), 7.41 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 16.0 Hz, 2H), 6.66 (s, 1H), 3.92 (s, 6H), 3.40 (s, 6H). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) = 180.66, 152.36, 146.39, 140.70, 135.40, 134.66, 124.94, 124.74, 123.09, 122.98, 114.58, 113.31, 102.90, 56.77, 39.11. ITMS (ESI) calcd for C23H23BF2O10S2 [M + Na]+ m/z 595.3436; found 595.6051.
((1E,3Z,6E)-3-((difluoroboranyl)oxy)-5-oxohepta-1,3,6-triene-1,7-diyl)bis(2-methoxy-4,1-phenylene) dibenzenesulfonate (5).
3 (0.2 g, 0.3 mmol) was dissolved in THF (15 mL), added with boron trifluoride diethyl etherate (0.4 mL, 3.2 mmol) and reacted at room temperature for 5 h. Spin dry THF, add water until pH is neutral, filter and rinse with excess methanol. The solid was stirred with DCM for 30 min and then filtered to give a ginger yellow solid 5 (0.13 g, 60%). 1H NMR (400 MHz, DMSO‑d6): δ (ppm) = 7.99 (d, J = 16.0 Hz, 2H), 7.85–7.79 (q, J = 7.6 Hz, 6H), 7.65 (t, J = 7.6 Hz, 4H), 7.56 (d, J = 8.4 Hz, 2H), 7.28–7.13 (m, 4H), 6.60 (s, 1H), 3.54 (s, 6H). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) = 180.64, 152.16, 146.22, 140.28, 135.47, 134.82, 129.97, 128.66, 126.02, 124.60, 123.11, 122.92, 121.62, 114.42, 113.15, 102.94, 56.35. ITMS (ESI) calcd for C33H27BF2O10S2 [M + Na]+ m/z 737.5008; found 737.6677.
2.3 Selectivity of amino acids
Prepare 100 μM DMSO solutions of compounds 4 and 5; prepare 30 mM and 100 μM solutions of Gly, Trp, Phe, His, Tyr, Cys in 10% DMSO-H2O (DMSO: H2O = 9:1, v/v), respectively. UV and fluorescence tests were performed by adding the corresponding equivalents (eq) of amino acid solutions to 100 μM compounds 4 and 5, respectively. Unless otherwise specified, the fluorescence spectra in this paper were measured under the following conditions (λex = 400 nm, slit width: 10 nm/10 nm).
3 Results and discussion
A new drug-fluorescent probe with Cys recognition function was designed by Scheme 1. Using curcumin as the raw material, the disubstitution reaction with methanesulfonyl chloride and benzenesulfonyl chloride was carried out at room temperature, respectively, and the reaction rapidly produced compounds 2 and 3 with yields higher than 90%. After that, the substituted compounds were dissolved in THF and added with boron trifluoride ether to react to obtain probes 4 and 5.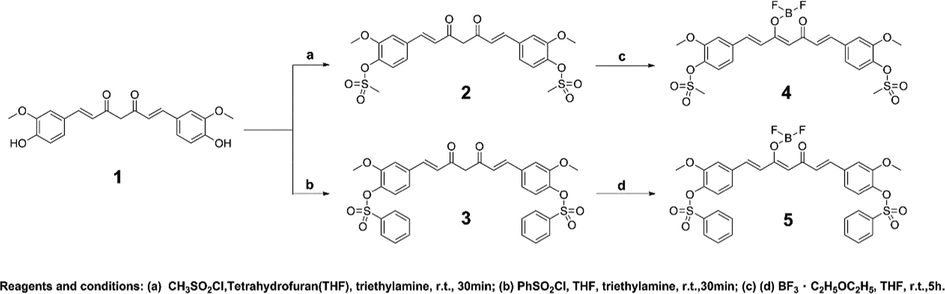
Reaction of bisulfonyl curcumin complexed with BF2 group.
As shown in Fig. 1, the maximum absorption and emission wavelengths of the raw materials, intermediates and products were compared in DMSO solvent. 1–5 had maximum absorption wavelengths of 436, 404, 442 and 466 nm, respectively. Intermediates 2 and 3 were blue-shifted in the UV due to the influence of the electron-absorbing sulfonyl group. The conjugated systems of compounds 4 and 5 increased after BF2 coordination, the energy difference between energy levels decreases, the electron transition energy decreases, the maximum absorption wavelength redshifts compared with the intermediate, and the ultraviolet double peak appears. The maximum emission wavelengths of 1–5 at the same excitation wavelength of 400 nm were 535, 475 and 510 nm, respectively. In addition,4 and 5 were analyzed with 400, 440 and 465 nm excitation respectively (Fig.S1). Obviously, the maximum emission wavelength of 4 and 5 is only changed by 8 nm under this condition, so 400 nm is still used as the excitation wavelength for the subsequent analysis.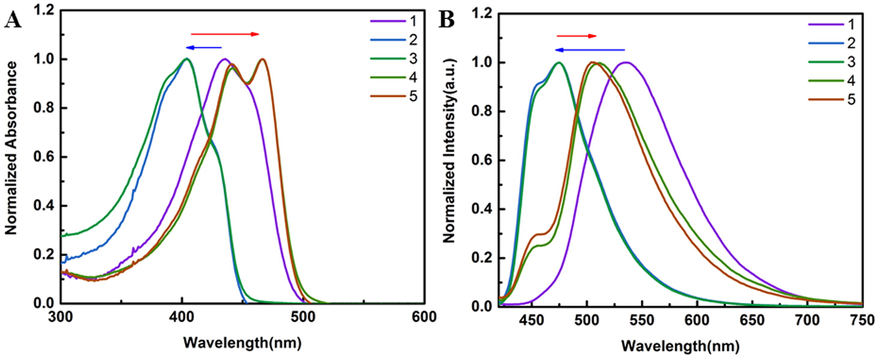
(A) Absorption spectra of 100 μM compounds 1–5 at 300–600 nm. (B) Emission spectra of 100 μM compounds 1–5 at the same excitation wavelength of 400 nm.
The UV–vis spectra of 300 eq Gly, Trp, Phe, His, Tyr, Cys solutions added to 100 μM compounds 4 and 5 (Fig. 2) showed that the addition of different amino acids had a decreasing effect on the UV of the compounds, but Cys had a significant blue shift of the probe, the maximum absorption wavelength blue-shifted from 466 nm to 385 nm. We speculate that the probe will lose small molecules of methanesulfonic acid or benzenesulfonic acid when the amino acids attack the readily departing sulfonyl group upon addition of the amino acids (Kato et al., 2010; Hartwig, 2008). The Cys containing sulfhydryl groups will also undergo Michael addition reaction (Wang et al., 2013) with the compound, which greatly reduces the conjugated structure of the probe and leads to a significant blue shift in the UV spectrum. The addition of 3 eq Cys also blueshifted the maximum absorption wavelength of the compounds to 385 nm (Fig. S2). It indicates that curcumin-derived probes have selective recognition of Cys.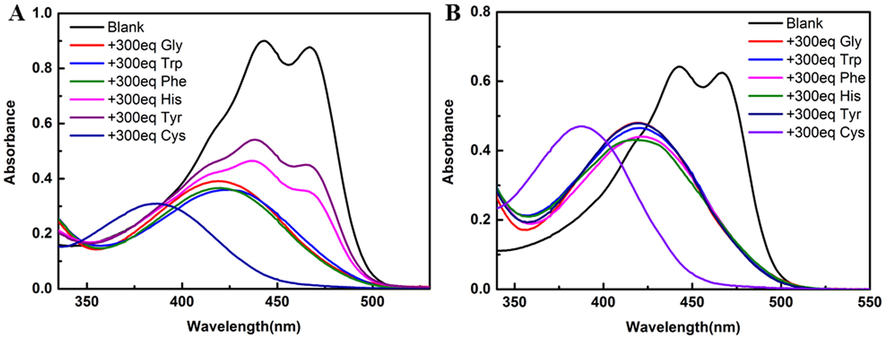
The absorption spectra of compounds 4 and 5 (100 μM in DMSO solution) were determined by adding 300 eq different amino acid solutions (30 mM in 10% DMSO-H2O solution). (A) Compound 4; (B) Compound 5.
The fluorescence spectra of both 4 and 5 with the addition of different amino acids were blue-shifted and the fluorescence intensity decreased (Fig. 3). The solid of 4 is orange yellow and red fluorescence; 5 solid ginger yellow, fluorescence is yellow. The probe interacts with the amino acid, causing a blue shift in its fluorescence. Here, speculate on the recognition mechanism as in Fig. 4. The amino group on the amino acid attacks sulfonyl group and loses the small molecule methanesulfonic acid or benzenesulfonic acid. In contrast, the sulfhydryl group of Cys contains lone pair of electrons and can nucleophilically attack this type of probe and undergo an addition reaction. To verify this mechanism, mass spectra were performed on solutions 4 and 5 with 300 eq Cys added, and on a randomly selected solution 4 containing 300 eq Tyr, respectively (Fig. 5). 4 + Cys: ITMS (ESI) calcd for C30H36BF2N3O10S3 [M + 6H]6+ m/z 749.2092; found 749.1025; 5 + Cys: ITMS (ESI) calcd for C30H36BF2N3O10S3 [M + 2H + K]3+ m/z 784.2763; found 784.1183. 4 + Tyr: ITMS (ESI) calcd for C39H37BF2N2O10 [M + 4H + H2O]4+ m/z 764.2973; found 764.1643. The mass spectrometry analysis is consistent with mechanism diagram, which corroborates our proposed mechanism.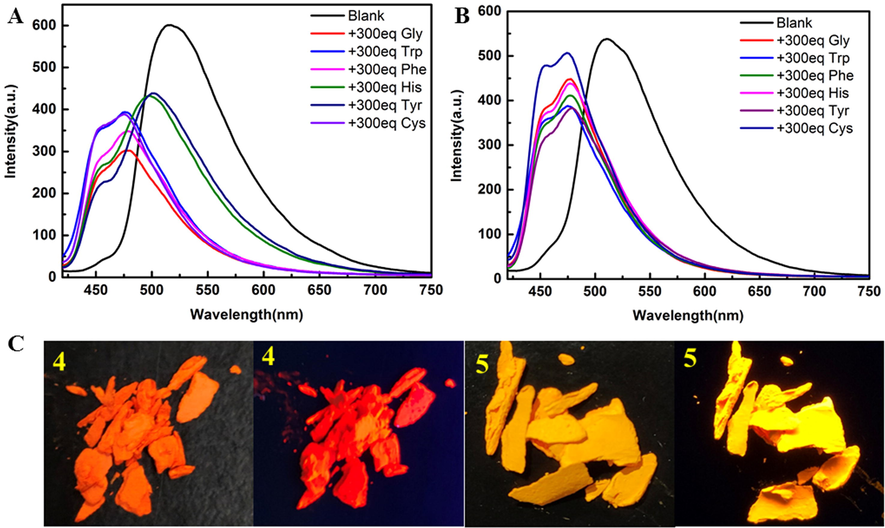
Emission spectra of 4 and 5 (100 μM in DMSO solution) with the addition of different amino acid solutions (30 mM in 10% DMSO-H2O solution, ex = 400 nm), respectively. (A) compound 4; (B) compound 5; Solid images of compounds 4 and 5 in sunlight and 365 nm UV lamps, respectively.
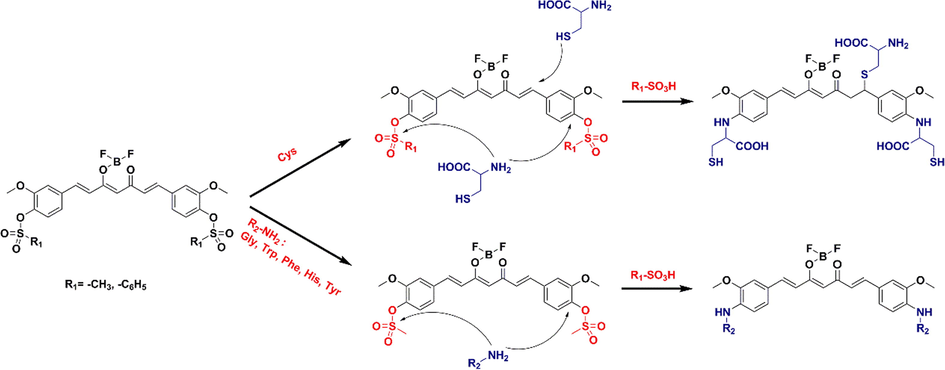
Mechanism of recognition of six amino acids by curcumin-derived probes.
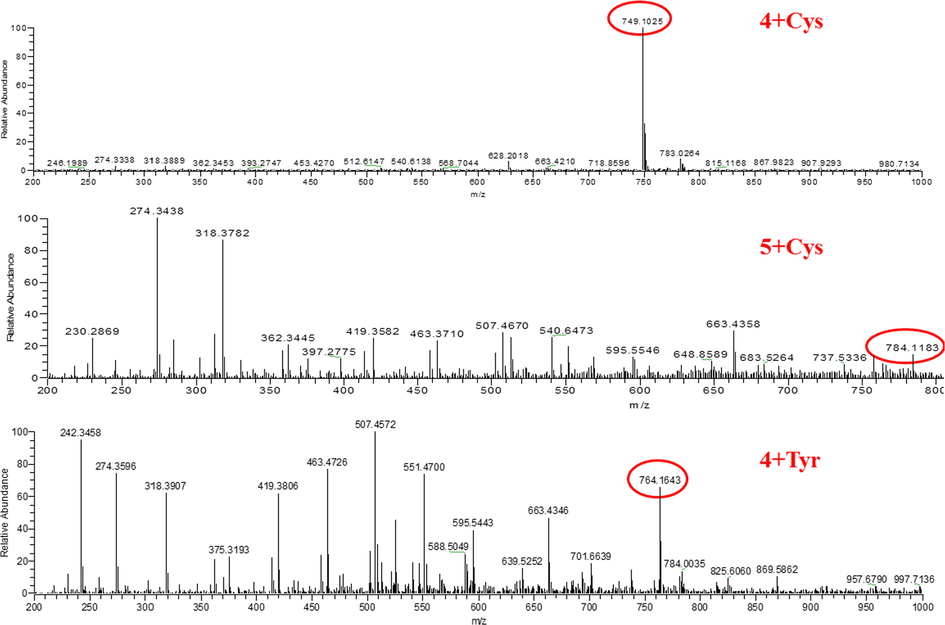
Mass spectral analysis of probes 4 and 5 after incorporation of amino acids.
In the naked eye recognition (Fig. 6), after the addition of Cys to 4 and 5, respectively, the solutions both changed from yellow to colorless and the fluorescence decreased significantly under 365 nm UV light. Whereas, when the other five amino acids were added, the solutions did not show significant changes under daylight and the fluorescence was weakened under ultraviolet lamp.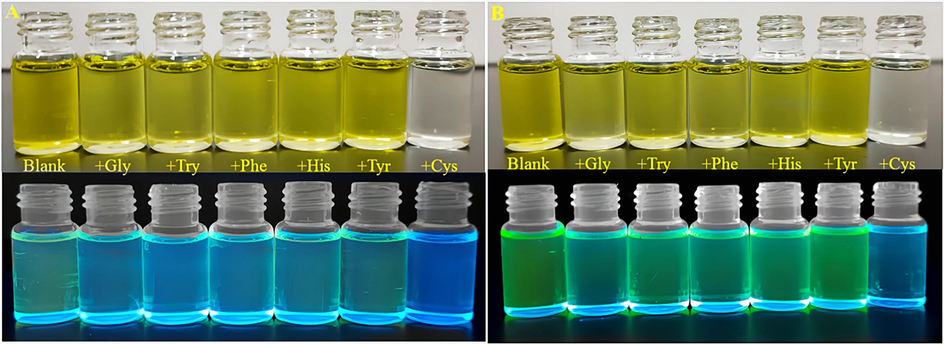
Natural light pictures of compounds 4 and 5 (100 μM in DMSO solution) after addition of 300 eq of different amino acid solutions respectively with 365 nm UV lamp. (A) Compound 4; (B) Compound 5.
As shown in Fig. 7, both UV and fluorescence of the probe decreased rapidly within 1 min and gradually stabilized after the addition of 0.1 eq Cys. In order to better explore the selectivity of curcumin derived probe to Cys, compounds 4 and 5 (100 μM in DMSO) were added to PBS buffer solution (10 mM) with pH = 5.5, 6.0, 6.5, 7.0, 7.4, 8.5, respectively, and 300 eq Cys were added. The changes of photophysical properties in the absence and presence of Cys were analyzed under different pH conditions (Fig. 8). Under different pH conditions, the maximum absorption wavelength of compound 4 blue-shifted to 380 nm, the fluorescence showed double peaks, and the maximum emission was 660 nm. After adding 300 eq Cys, the UV absorbance and fluorescence intensity decreased significantly. The absorbance of compound 5 decreased under different pH conditions, especially after adding Cys. The maximum emission of 5 was red-shifted to about 610 nm at different pH values, and blue-shifted to about 545 nm after adding Cys, but the fluorescence intensity was significantly increased compared with that of blank control. This phenomenon is that the oxygen atom on the compound is easily protonated in the pH environment, thus preventing the charge transfer from the phenyl group on curcumin to the sulfonyl group, resulting in enhanced fluorescence intensity and red-shift of the maximum emission wavelength. After the addition of Cys, the interaction between the sulfhydryl group and the probe leads to the reduction of conjugation, so the emission wavelength is blue-shifted. In addition, under the condition of weak acid (pH = 6.5, 7.0, 7.4), compound 5 showed the strongest fluorescence intensity after adding Cys. This shows that compound 5 still has good detection ability for Cys under weak acid conditions.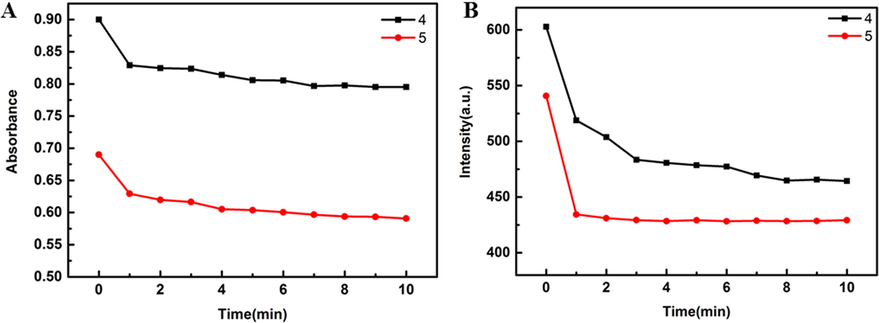
The time-varying absorption and emission spectra of compounds 4 and 5 (3 mL 100 μM in DMSO solution) with 0.1 eq Cys (300 μL 100 μM in 10% DMSO-H2O solution, ex = 400 nm).
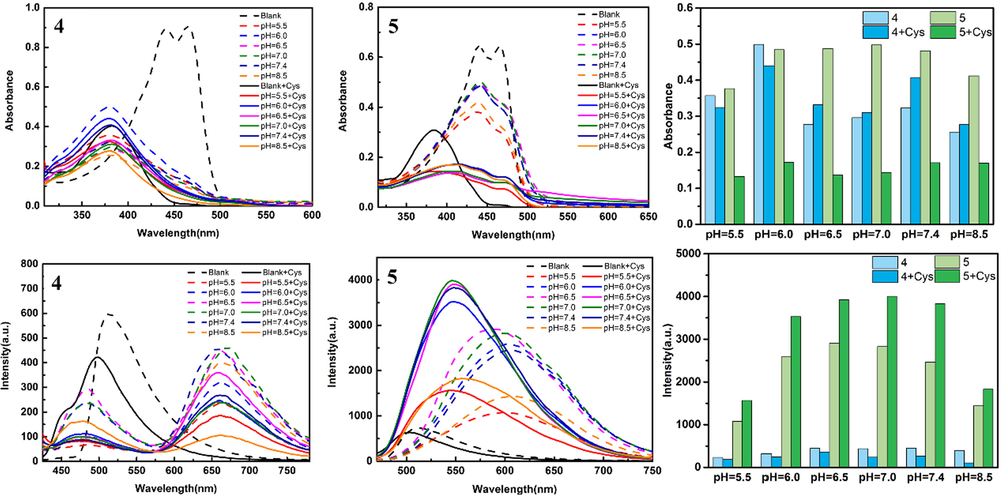
Photophysical properties of compounds 4 and 5 (100 μM) at different pH values; Histogram: Changes of the maximum absorbance and fluorescence intensity of the probe at different pH conditions.
A good linear relationship existed between the curves of Cys concentration and absorption wavelength at 442 nm and fluorescence intensity at 510 nm (Fig. 9). The absorbance of compound 4 satisfied a linear relationship with Cys concentration of y = 0.8975–0.01327x (R2 = 0.9956); the fluorescence satisfied y = 605.4950–14.6963x with a good fit of 0.9933. The absorbance of compound 5 satisfied y = 0.6925–0.00812x (R2 = 0.9952) and the fluorescence satisfied y = 542.7825–6.8245x with goodness of fit 0.9932. According to the formula of 3δ / k (δ: standard deviation of the probe after 11 tests, k: slope of titration curve fitted by fluorescence intensity (Wang and Qian, 2019); δ 4 = 1.97, k4 = 14.6963; δ5 = 1.98, k5 = 6.8245), and the detection limits of probes 4 and 5 were 0.40 μM and 0.87 μM to Cys in the linear range of 1.0–10.0 μM. These results indicate that the probes are more sensitive to subtle changes in Cys.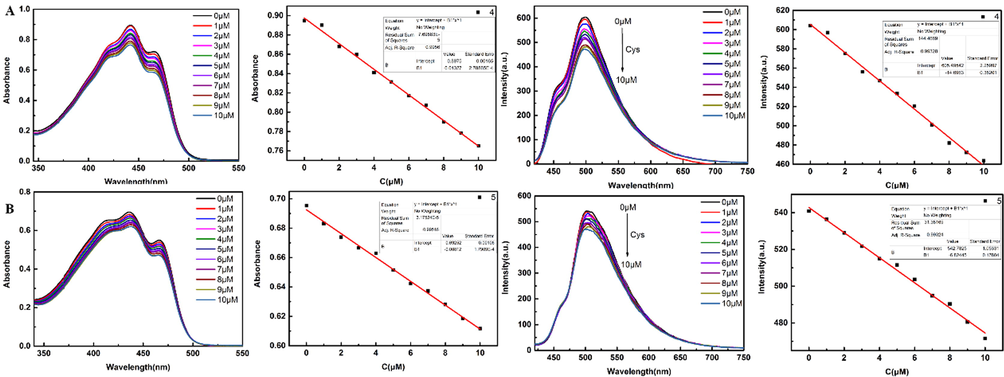
Cys titration was performed in compounds 4 and 5 (3 mL 100 μM in DMSO), respectively. Cys solution (300 μL 100 μM in 10% DMSO-H2O solution, ex = 400 nm) was dropped each time. The linear relationship about UV and fluorescence was plotted.
4 Conclusions
In conclusion, we developed a new curcumin-derived fluorescent probe for the detection of Cys. The probe has a rapid response and selectivity for Cys compared to other amino acids. The introduced sulfonyl group is easily departed upon addition of amino acids. And influenced by the sulfhydryl group of Cys, the probe can undergo an addition reaction with Cys, resulting in a significant blue shift in the UV maximum absorption and emission wavelength of the probe and a color change visible to the naked eye. This study provides a new method for the rapid detection of Cys; and the compounds have the drug structure of curcumin, which can provide a new idea for the design of drug-fluorescence probe.
Acknowledgements
This research reported in this publication was supported by the scientific research foundation of Jiangsu University (Grant No: 17JDG002) and Zhenjiang Social Development Project (Grant No: SH2020025).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Curcumin: A promising bioactive agent for application in food packaging systems. J. Environ. Chem. Eng.. 2021;9:105520
- [CrossRef] [Google Scholar]
- Antitumor activity, X-Ray crystallography, in silico study of some-sulfamido-phosphonates. Identification of pharmacophore sites. J. Mol. Struct.. 2022;1250
- [CrossRef] [Google Scholar]
- A highly selective quinolizinium-based fluorescent probe for cysteine detection. RSC Adv.. 2021;11:33294-33299.
- [CrossRef] [Google Scholar]
- Two-dimensional high performance liquid chromatography for determination of homocysteine, methionine and cysteine enantiomers in human serum. J. Chromatogr. A. 2015;1408:118-124.
- [CrossRef] [Google Scholar]
- Probing adsorptive/desorptive redox processes and detection of cysteine: A voltammetric and scanning electrochemical microscopy study. J. Electroanal. Chem.. 2017;807:119-127.
- [CrossRef] [Google Scholar]
- A facile synthetic strategy to polysiloxanes containing sulfonyl side groups with high dielectric permittivity. Polym. Chem.. 2017;8:715-724.
- [Google Scholar]
- Curcumin complex analogues as near-infrared fluorescent probes for monitoring all Aβ species in the early Alzheimer’s disease model. ACS Chem. Neurosci.. 2021;12:3683-3689.
- [Google Scholar]
- Nitrogen, sulfur, phosphorus, and chlorine co-doped carbon nanodots as an “off-on” fluorescent probe for sequential detection of curcumin and europium ion and luxuriant applications. Microchim. Acta.. 2021;188:16.
- [Google Scholar]
- Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res.. 2008;41:1534-1544.
- [Google Scholar]
- Trapping of acrolein by curcumin and the synergistic inhibition effect of curcumin combined with quercetin. J. Agric. Food Chem.. 2021;69:294-301.
- [Google Scholar]
- Isomeric flavonoid aglycones derived from Epimedii Folium exerted different intensities in anti-osteoporosis through OPG/RANKL protein targets. Int. Immunopharmacol.. 2018;62:277-286.
- [CrossRef] [Google Scholar]
- Aryl(sulfonyl)amino group: A convenient and stable yet activated modification of amino group for its intramolecular displacement. Org. Lett.. 2010;12:4137-4139.
- [Google Scholar]
- A simple, efficient, sensitive and practicability: Polyoxyphenylpropeone fluorescent probes for biological imaging. Mater. Today Commun.. 2021;29:102817
- [CrossRef] [Google Scholar]
- Curcumin reverses tobacco smoke-induced epithelial-mesenchymal transition by suppressing the MAPK pathway in the lungs of mice. Mol. Med. Rep.. 2018;17:2019-2025.
- [Google Scholar]
- Direct sulfonylation of BODIPY dyes with sodium sulfinates through oxidative radical hydrogen substitution at the α-position. Chem. Commun.. 2020;56:15577-15580.
- [Google Scholar]
- Curcumin, curcumin nanoparticles and curcumin nanospheres: A review on their pharmacodynamics based on monogastric farm animal, poultry and fish nutrition. Pharmaceutics.. 2020;12:447.
- [Google Scholar]
- Cysteine and homocysteine as biomarker of various diseases. Food Sci. Nutr.. 2020;8:4696-4707.
- [CrossRef] [Google Scholar]
- Molecular probes for selective detection of cysteine cathepsins. Org. Biomol. Chem.. 2021;19:6182-6205.
- [Google Scholar]
- Effect of substitution on the excited state photophysical and spectral properties of boron difluoride curcumin complex dye and their derivatives: A time dependent-DFT study. J. Photoch. Photobio. B. 2019;199:111595
- [CrossRef] [Google Scholar]
- Design, synthesis and biological activity of bis-sulfonyl-BODIPY probes for tumor cell imaging. Bioorg. Med. Chem. Lett.. 2021;49:128292
- [CrossRef] [Google Scholar]
- Nitrogen-boron eight-ring rigid cis/trans BODIPY-pyrimidine isomers for in vivo and in vitro fluorescence target recognition and evaluation of inhibitory activity. Dyes and Pigments.. 2022;210:110204
- [CrossRef] [Google Scholar]
- Demethoxycurcumin analogue DMC-BH inhibits orthotopic growth of glioma stem cells by targeting JNK/ERK signaling. Aging-US. 2020;12:14718-14735.
- [Google Scholar]
- Advances in BODIPY photocleavable protecting groups. Coord. Chem. Rev.. 2021;449:214193
- [CrossRef] [Google Scholar]
- Simple and facile preparation of silver-polydopamine (Ag-PDA) core-shell nanoparticles for selective electrochemical detection of cysteine. RSC Adv.. 2016;6:49578-49587.
- [Google Scholar]
- Multifunctional organic dyes: anion-sensing and light-harvesting properties of curcumin boron complexes. RSC Adv.. 2017;7:36612-36616.
- [Google Scholar]
- Specific two-photon fluorescent probe for cysteine detection in vivo. Spectrochim. Acta, Part A. 2022;267:120521
- [CrossRef] [Google Scholar]
- Potent Antitumor effects of a combination of three nutraceutical compounds. Sci. Rep.. 2018;8:12163.
- [Google Scholar]
- A novel quinoline-BODIPY fluorescent probe for fast sensing biothiols via hydrogen bonds assisted-deprotonation mechanism and its application in cells and zebrafish imaging. J. Photochem. Photobiol., A 372 2019:122-130.
- [CrossRef] [Google Scholar]
- A multiple acetal chalcone-BODIPY-based fluorescence: synthesis, physical property, and biological studies. Anal. Bioanal. Chem.. 2021;413:2529-2541.
- [Google Scholar]
- A coumarin-based dual optical probe for homocysteine with rapid response time, high sensitivity and selectivity. Talanta. 2019;196:243-248.
- [CrossRef] [Google Scholar]
- A fluorescent sensor bearing nitroolefin moiety for the detection of thiols and its biological imaging. Dyes Pigm.. 2013;96:232-236.
- [Google Scholar]
- Bisdemethoxycurcumin enhances the sensitivity of non-small cell lung cancer cells to icotinib via dual induction of autophagy and apoptosis. Int. J. Biol. Sci.. 2020;16:1536-1550.
- [Google Scholar]
- Acetonitrilated unsymmetric BODIPYs having glycine fluorescence responsive quenching: Design, synthesis and spectroscopic properties. Spectrochim. Acta, Part A. 2020;233:118211
- [CrossRef] [Google Scholar]
- The protein expression profile and transcriptome characterization of Pichia caribbica induced by ascorbic acid under the oxidative stress. Biol. Control. 2020;142:104164
- [CrossRef] [Google Scholar]
- Sulfonyl Group Dance: A Tool for the Synthesis of 6-Azido-2-sulfonylpurine Derivatives. J. Org. Chem.. 2020;85:4753-4771.
- [CrossRef] [Google Scholar]
- Acryl-modified diazabenzo[ghi]perylene for fast discrimination of Cys from GSH and Hcy with high quantum yield. Sens. Actuators B Chem.. 2020;320:128304
- [CrossRef] [Google Scholar]
- A water-soluble near-infrared fluorescent probe for sensitive and selective detection of cysteine. Talanta. 2019;204:747-752.
- [CrossRef] [Google Scholar]
- A novel near-infrared fluorescent probe for intracellular detection of cysteine. Anal. Bioanal. Chem.. 2020;412:7211-7217.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104087.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







