Translate this page into:
Simultaneous removal of SO2 and NOx by potassium-modified carbide slag
⁎Corresponding authors. LeegyerKM@163.com (Lijuan Jia), FJY_DX3906@163.com (Jiayu Feng), xmwbboy@163.com (Mingwu Xiang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The treatment of solid waste carbide slag (CS) poses an urgent challenge in terms of its resolution. This study presented an efficient and cost-effective method for simultaneous desulfurization and denitrification at low temperatures using different modified potassium compounds (KOH, K2CO3, and KHCO3) as adsorbents. The experimental results revealed the significant impact of potassium modification on the performance of the CS, leading to a significant improvement in its denitrification activity while maintaining the same desulfurization effect. The denitrification rates of the modified CS demonstrated increases by 30 % (KOH), 25 % (K2CO3), and 40 % (KHCO3), respectively, compared to the unmodified CS at 200 ℃. The NO adsorption capacities were 2.48 (KOH), 2.24 (K2CO3), and 3.05 (KHCO3) times that of CS, respectively. Subsequent investigations suggested that the potassium modification process induced changes in the microstructure of CS, augmenting the abundance of oxygen vacancies, KOx, C = O, and Oads, thereby enhancing the intensity and quantity of basic sites. This reduced the activation energy of the CS during simultaneous desulfurization and denitrification. In addition, it was observed that certain byproducts formed during the desulfurization and denitrification processes, such as sulfate, sulfite, and nitrate, accumulated on the surface and within the inner pores of the adsorbents, ultimately resulting in a decline in catalytic activity. This study aims to embrace the “treat waste with waste” approach and is expected to provide guidance for the advancement of simultaneous desulfurization and denitrification technologies targeting solid waste CS.
Keywords
Carbide slag
Potassium
Simultaneous desulfurization and denitrification
Activation energy
1 Introduction
Carbide slag (CS) represents a solid waste generated within the chloralkali industry, that can produce calcium carbide slag and acetylene following the hydrolysis of calcium carbide. This byproduct is referred to as poor-quality Ca(OH)2 and manifests as a white powder, with slight solubility in water and alkaline. China currently holds the position of the world's leading CS producer, which annually discharges large quantities of CS from both the polyvinyl chloride (PVC) and chloralkali industries. In 2020, China's CS production reached 27.58 million tons, accounting for over 90 % of the global production, signifying a year-on-year increase of 6.8 % (Gong et al., 2022). The high alkalinity of CS renders conventional disposal methods, such as landfills, inadequate due to their potential to generate severe environmental issues, including soil alkalization and contamination of surface and groundwater(Ma et al., 2016). Moreover, CS is characterized by an elevated Ca(OH)2 content, pronounced particle dispersion, expansive specific surface area, and substantial pore structure. Following appropriate treatment, CS can serve as an outstanding secondary calcium-based resource, capable of substituting limestone in flue gas desulfurization processes, consequently presenting a broad range of applications (Mathieu et al., 2013, Huang et al., 2019). The utilization of CS as an adsorbent exhibits the remarkable advantage of diminishing both raw materials and processing costs, reducing the treatment load and pollution associated with CS, and ultimately realizing the comprehensive utilization of secondary resources.
Among the available desulfurization options, CS is regarded as a cost-effective desulfurization agent. Gong et al. (2022) and Wu et al. (2016) explored the adsorption performance of CS for SO2, revealing a superior adsorption capacity compared to limestone with larger particle sizes. Furthermore, using a fixed bed reactor, Cheng et al. (2009) investigated the desulfurization capacity of three industrial wastes containing sodium calcium, namely white lime mud, CS, and salt cement. Compared to the other two industrial wastes, CS-derived CaO demonstrated the highest number of SO2 diffusion channels and largest specific surface area, which facilitated the effective removal of SO2. Bian et al. (2020) investigated the performance of Al-Mg-modified CS for SO2 removal at high temperatures with the utilization of a double fixed bed reactor. The Al- and Mg-modified CS exhibited significantly higher SO2 removal capacity and cycling stability than CS due to the presence of beneficial compounds, such as Ca3Al2O6 and MgO. In addition, the field has progressed beyond simple desulfurization, and now seeks to achieve simultaneous desulfurization and denitrification. Wang et al. (2018, 2020) developed a method for producing pellets from CS, bagasse pellets, CS, and coal coke via an extrusion rounding process. The prepared pellets demonstrated 100 % efficiency in the removal of SO2/NO within the optimum reaction temperature range of 850–875 °C. Meng et al. (2022) conducted research on the synergistic preparation of hybrid pellets with the application of CS and sludge, evaluating the SO2/NO removal capacity in depth. The addition of sludge to the CS pellets yielded a satisfactory denitrification efficiency of up to 70 % at 550 °C. Furthermore, Wang et al. (2020) prepared a composite adsorbent using modified rice husk ash and CS. The results revealed that the synthetic adsorbent exhibited an increase in the removal of NO and SO2 by 44 % and 2 %, respectively, at 700 °C. However, these methods require high temperature and complex material preparation techniques. It is imperative to propose strategies that can further reduce the reaction temperature while achieving cost-effective and highly efficient desulfurization and denitrification.
Numerous studies have demonstrated the superior efficacy of alkali metal-modified materials for the removal of gaseous SO2/NO. In particular, the utilization of potassium for SO2/NO removal has garnered considerable research attention (Fortier et al., 2007, Gao et al., 2018, Severa et al., 2018, Yang et al., 2019). Impregnating the material surface with potassium creates a robust interaction effect, significantly enhancing the adsorption capacity of the material (Lee et al., 1998). Tang et al. (2015) introduced potassium into manganese cobalt oxides to augment their low-temperature NO oxidation activity. Qie et al. (2020) employed potassium-assisted catalytic activation to prepare activated coke with a high specific surface area and stratified pore structure, which exhibited outstanding SO2 and NO adsorption capacities. Furthermore, adsorbents containing potassium exhibited the best performance in terms of NO adsorption and played a crucial role in preserving surface oxygen, thus increasing the occurrence of oxygen vacancies, which can be an essential factor for catalytic performance (Cheng et al., 2018).
This study focused on the modification of CS using different potassium sources to investigate its performance in desulfurization and denitrification. Additionally, the activation energy of different modified CS samples was calculated. The materials were thoroughly characterized using several techniques including X-ray fluorescence spectroscopy (XRF), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), CO2-temperature programmed desorption (CO2-TPD), scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS). Building on these findings, a possible mechanism was proposed, which provides a promising strategy for the future utilization of waste resources.
2 Experimental
2.1 Adsorbent preparation
In this experiment, the modified CS was prepared by ultrasonic impregnation, and its visual preparation flowchart is shown in Fig. 1(a). The CS samples were immersed in the solutions of KOH, K2CO3, and KHCO3, each at a concentration of 1.0 mol·L-1, respectively, and sonicated at 100 W and 59 Hz for 15 min. Subsequently, the resulting solution was dried in a blast drying oven at 100 °C for 12 h. Finally, the prepared modified CS was sieved using a particle size range of 40–60 mesh. The initial carbide slag was recorded as CS, the potassium-modified calcium carbide slag was recorded as KCS, and the different potassium-modified carbide slags were recorded as CS-KOH, CS-K2CO3, and CS-KHCO3. Following the reaction, the modified carbide slags were further recorded as UCS-KOH, UCS-K2CO3, and UCS-KHCO3.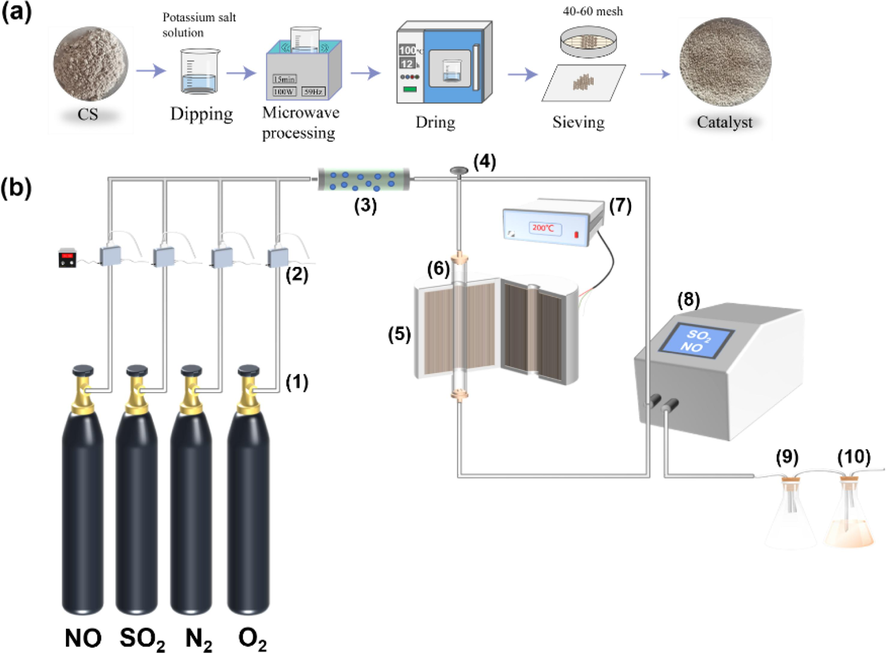
(a) Preparation process of adsorbents; (b) schematics of the experimental setup: (1) Gas cylinder; (2) mass flowmeter;(3) gas mixing tank; (4) control valve; (5) resistance tube furnace; (6) quartz tube; (7) temperature controller; (8) flue gas analyzer; (9) K2MnO4 solution; (10) NaOH solution.
2.2 Activity evaluation
Desulfurization and denitrification reactions were conducted in a fixed bed using 1.0 g of modified CS within a quartz tube reactor with an inner diameter of 6 mm. The performance of the modified CS in terms of SO2/NO removal was assessed using the experimental setup illustrated in Fig. 1(b). To simulate real conditions, a dynamic gas distribution was adopted, setting the flow rate of the simulated flue gas was 200 mL·min−1, and the gas hour space velocity (GHSV) at 8488 h−1. The flue gas composition consisted of 1500 mg·m−3 of SO2, 700 mg·m−3 of NO, and 5 % of O2, with N2 serving as the equilibrium gas. To monitor the SO2/NO concentration in the flue gas, an online MRU infrared flue gas analyzer was employed. Any escaping SO2/NO from the experiment was subsequently oxidized and absorbed by an absorption solution comprising NaOH and K2MnO2 before discharge. Eqs. (1) and (2) represent the removal efficiency and adsorption capacity of SO2/NO, respectively. In accordance with the national standard (Emission standard, 2014), a denitrification rate of 45 % and desulfurization rate of 75 % were employed as benchmarks for measuring effective removal. Denitrification efficiency was evaluated using TNO45%, indicating the duration of denitrification with a denitrification rate exceeding 45 %. Similarly, TSO275% was utilized to evaluate the desulfurization efficiency signifying the duration of desulfurization with a desulfurization rate above 75 %.
where η denotes the SO2/NO removal rate (%), Cin denotes the SO2/NO concentration before the reaction (mg·m−3), Cout denotes the SO2/NO concentration after the reaction (mg·m−3), qe denotes the adsorption capacity of SO2/NO (mg·g−1), Q is the gas flow rate (mL·min−1), t0 and tb denotes the onset and end time of adsorption (min), respectively, and m denotes the material mass (g).
2.3 Characterization methods
The detailed characterization methods are shown in Supplementary Text.
3 Results and discussion
3.1 Results of adsorbent activity evaluation
In the conducted experiment, the reaction temperature emerged as a critical factor affecting the activity of the modified CS. The impact of different reaction temperatures on the simultaneous desulfurization and denitrification activities of the modified CS is presented in Fig. 2. The adsorption capacities of potassium-modified CS for SO2/NO at different temperatures are shown in Fig. 3. As shown in Figs. 2 and 3, the SO2/NO removal activity and adsorption capacity of different modified CS exhibited temperature dependent variations. Notably, the denitrification efficiency of the modified CS was significantly higher than that of CS alone. The highest denitrification rate of CS-KHCO3 was 62 %, whereas the denitrification performance of CS-KOH and CS-K2CO3 was improved to 50 % and 48 %, respectively. From the data in Fig. 2(a), the effect of the reaction temperature on the NO removal rate of the CS exhibited relatively minor fluctuations. In the temperature range of 100–250 °C, the NO removal rate remained below 45 %, and the NO adsorption capacity was limited. As the reaction temperature increased from 100℃ to 200 °C, the value of TNO45 % increased with increasing temperature at 120 min for CS-KOH, 75 min for CS-K2CO3, and 120 min for CS-KHCO3. During this period, the NO adsorption capacities of CS-KOH, CS-K2CO3, and CS-KHCO3 were 2.48, 2.24, and 3.05 times higher than those of CS. However, as time progressed, NO removal decreased, possibly due to the coverage of active sites by the products after SO2/NO adsorption (Guo et al., 2022). However, when the reaction temperature reached 250 °C, the NO removal rate of different modified CS decreased. This is because SO2 has a higher boiling point than NO, making NO more prone to diffusion than SO2 at lower temperatures (100 °C) (Silas et al., 2018). Molecules with higher boiling points have stronger van der Waals forces, whereas those with lower boiling points exhibit weaker intermolecular interactions. Consequently, NO can be readily displaced and desorbed by SO2 at higher temperatures. Therefore, the feasible denitrification temperature of the modified CS was 200 °C.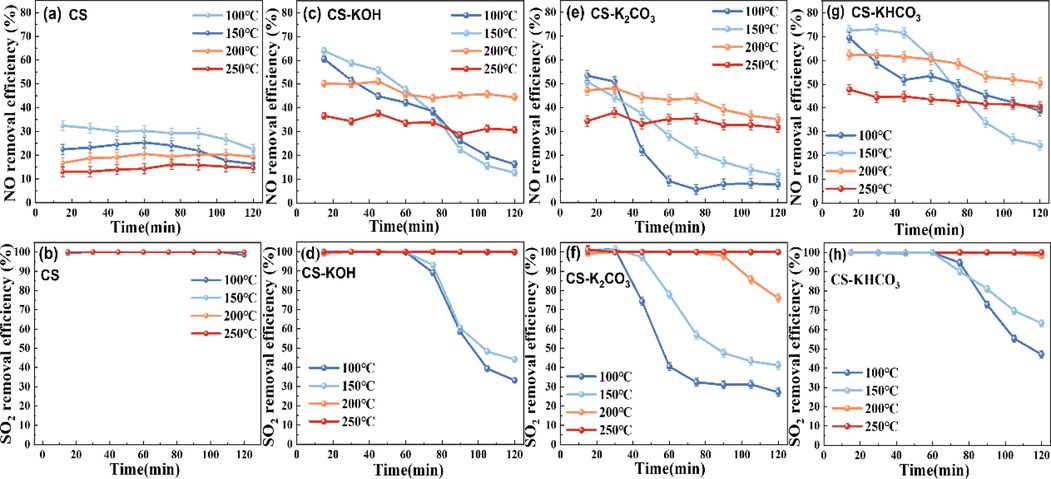
Efficiency of SO2/NO removal at different temperatures: (a)(b) CS; (c)(d) CS-KOH; (e)(f) CS-K2CO3; (g)(h) CS-KHCO3. Reaction conditions: gas flow rate of 200 mL·min−1; initial concentrations of SO2 at 1500 mg·m−3, NO at 700 mg·m−3, and O2 at 5 %.
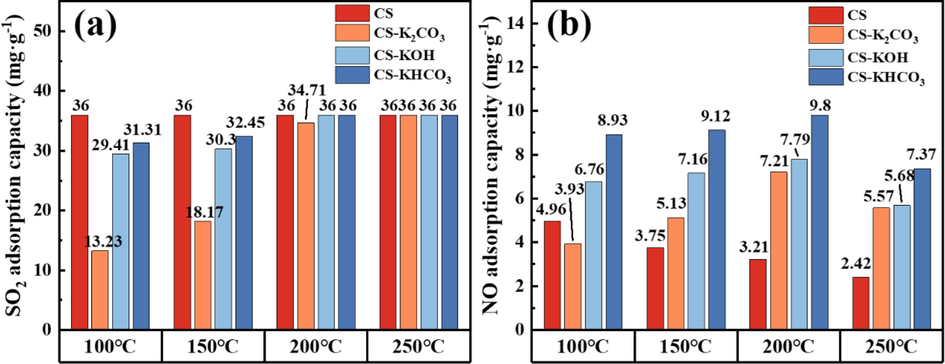
Adsorption capacity of potassium-modified CS for SO2/NO at various temperatures.
As illustrated in Fig. 2(b) and 3(a), the desulfurization rate of CS exhibited relatively minor fluctuations within the range of 100–250 °C, which proved that the effect of reaction temperature on the performance of CS for SO2 removal was not predominantly evident. The optimal performance of CS in terms of SO2 removal remained consistent with a 100 % desulfurization rate within 120 min, and its SO2 adsorption capacity reached 36 mg·g−1. From Fig. 2(d), (f), (h), and 3(a), the removal performance of the modified CS for SO2 decreased slightly at temperatures below 200 °C. However, the TSO275 % values for all three modified CS continued to increase with increasing in temperature. At 200 °C, the SO2 adsorption capacities of the three materials are essentially equal. This can be attributed to the gradual increase in temperature, which reduced the mass transfer resistance and accelerated the reaction rate, leading to an enhanced SO2 adsorption capacity (Li et al., 2021).
Table 1 summarizes the optimal reaction temperatures, removal efficiencies, and durations of the potassium-modified calcium carbide slag. It is evident that potassium-modified cs at 200 °C showed the most efficient and stable SO2/NO removal. In summary, based on the desulfurization and denitrification rates, the removal capacity of the modified CS can be derived as follows: CS-KHCO3 > CS-KOH > CS-K2CO3 > CS.
Sample
Temperature(℃)
ηSO2(%)
TSO275 %(min)
ηNO(%)
TNO45 %(min)
CS
100
100
120
30 %
0
CS-KOH
200
100
80
50
120
CS-K2CO3
200
100
120
48
45
CS-KHCO3
200
100
90
62
120
3.2 Activation energy of adsorbents
From the above experimental results, the desulfurization performance of the different modified CS exhibited slight variations. However, the denitrification performance was significantly different. Therefore, the reaction activation energy of the modified CS for SO2/NO removal was determined. The reaction rate constant k in the rate equation of the catalytic oxidation can be expressed using the Arrhenius equation (Dou et al., 2020).
as follows:
where k denotes the reaction rate constant (mol·s−1·g−1); A is denotes the pre-exponential factor; R denotes the gas constant (8.314 J·mol−1·K−1); T denotes the reaction temperature (K); and Ea denotes the activation energy (kJ·mol−1).
The rate equation of the NO catalytic oxidation of the modified CS is represented by Equation (4). Subsequently, Equation (4) is incorporated into Equation (3), resulting in Equation (5). Simultaneously, the logarithm of both sides of Equation (5) is taken, yielding Equation (6). The conversion rate of NO at different temperatures was measured while maintaining the inlet concentration constant, and the reaction rate r was calculated using Equation (7) (Lei et al., 2020). Using Equation (6), a plot can be constructed with T−1 as the x-axis and lnr as the y-axis. This plot facilitates the derivation of a linear regression equation. The reaction activation energy (Ea) was determined by multiplying the slope of the fitted regression equation by with gas constant R (Zheng et al., 2018).
where r denotes the reaction rate (mol·s−1·g−1); ηNO denotes the NO conversion rate (%); CNO denotes the NO inlet concentration (mg·m−3); Q denotes the total gas flow (L·s−1); mdenotes the mass of the adsorbents (g); and Vm denotes the molar volume of the gas.
As shown in Fig. 4(a)–(d), the correlation coefficients R2 of the linear regression equations for the NO activation energies of CS, CS-K2CO3, CS-KOH, and CS-KHCO3 were 0.9692, 0.9385, 0.9429, and 0.9554, respectively. The calculated activation energies of NO for CS, CS-K2CO3, CS-KOH, and CS-KHCO3 were −7.2446 kJ·mol−1, 15.9048 kJ·mol−1, 8.7301 kJ·mol−1, and 6.7838 kJ·mol−1, respectively. These results demonstrated a significant reduction in the activation energy of the modified CS, indicating higher reaction rates and enhanced catalytic oxidation of NO (Lei et al., 2020). In addition, the negative activation energy of CS suggested a decrease in the reaction rate r with increasing temperature, leading to a weakening of the catalytic oxidation of NO (Valverde 2015, Jayakumar et al., 2017, Joshi et al., 2018). These findings suggest that modified CS materials promote favorable reactions by effectively reducing energy barriers. Hence, the catalytic mechanism of the modified CS was further changed to improve the catalytic efficiency of NO. As shown in Fig. 4(e)–(h), the activation energy for the SO2 reaction in CS- K2CO3 was 10.10 kJ·mol−1, and it remained at 0 kJ·mol−1 for all other materials. This indicates that K2CO3 modification slightly increased the activation energy for CS desulfurization, leading to a reduced reaction rate. Combined with Fig. 2, it became evident that the lower activation energy of the modified CS reaction promoted easier occurrence of the catalytic process and improved desulfurization and denitrification. The catalytic activities of the different modified CS materials can be ranked as follows: CS-KHCO3 > CS-KOH > CS-K2CO3, which is consistent with their respective SO2/NO removal performance.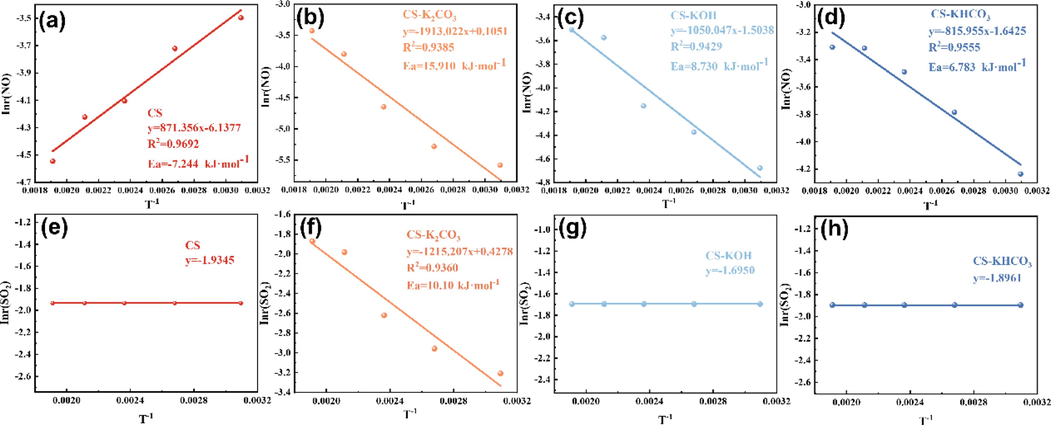
Linear regression diagram of SO2/NO surface activation energy for different materials: (a) (e) CS; (b) (f) CS-K2CO3; (c) (g) CS-KOH; (d) (h) CS-KHCO3.
3.3 Characterization of materials
According to the XRF characterization results, the main compositions of CS are shown in Table S1 (as oxides, wt %), such as CaO (96.96 %), K2O (0.05 %), SiO2 (1.20 %), Fe2O3 (0.80 %), and Al2O3 (0.44 %). To facilitate the comparison, the K2O content was used to express the total potassium oxide (KOx) content. Loading potassium onto the CS surface increased its potassium content, with K2O reaching 22.92 %, 10.34 %, and 12.20 % for CS-K2CO3, CS-KOH, and CS-KHCO3, respectively, whereas SiO2 and Al2O3 experienced slight decreases. Combining these findings with Fig. 2, the addition of potassium can promote the oxidation efficiency observed in NO, which likely explained the increased denitrification efficiency observed in the modified CS. However, CS-K2CO3 exhibited poor effectiveness in desulfurization and denitrification, which can be attributed to the excessive blockage of pore channels and surface adsorption sites on the surface caused by excessive KOx concentration resulting from K2CO3 addition(Yang et al., 2019). The SEM images in Fig. 5(a-d) illustrated the significant changes in the microstructure of CS before and after modification. Ultrasonic impregnation disrupted the CS structure, facilitating accelerated potassium loading on the CS surface. In addition, different potassium modifications yielded distinct microstructures. Fig. 5(a) displayed layered bulk particles of CS with a rough surface, few cracks, and no noticeable pore structure. Fig. 5(b) and 5(c) indicated irregular porous layered surfaces and bulk structures of CS-KOH and CS-K2CO3, respectively, after ultrasonic impregnation and potassium modification. The CS-KOH particles exhibited small honeycomb pores on the surface and a significant number of channels within the particles. In contrast, CS-K2CO3 displayed fewer small surface pores than CS-KOH, potentially due to pore channel blockage caused by excess KOx (Yang et al., 2019). The microstructure of CS-KHCO3 shown in Fig. 5(d) revealed a unique flower cluster-like ordered structure at high resolution, characterized by a loose and uniform appearance. This flower cluster-like microstructure could provide abundant volume and active sites to facilitate the mass transfer of desulfurization and denitrification reaction intermediates (Peng et al., 2021). These microstructural observations were closely linked to CS composition modifications and adherence of potassium to the adsorbent surface (Ding and Liu 2020).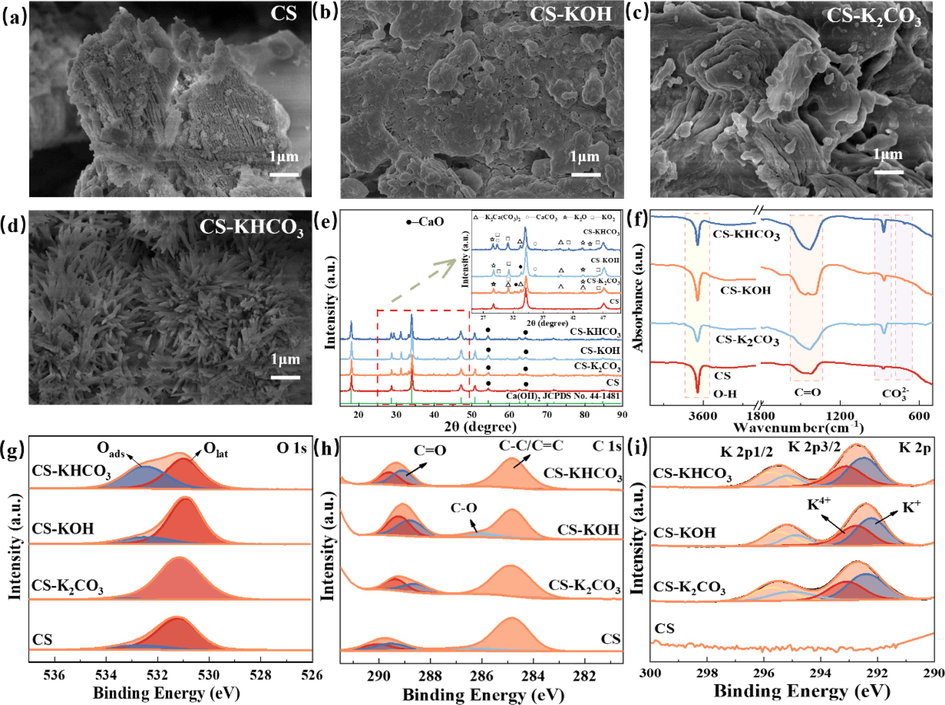
(a–d) SEM, (e) XRD, (f) FTIR spectra, (g) O 1 s, (h) C 1 s, and (i) K 2p spectra of CS, CS-K2CO3, CS-KOH, and CS-KHCO3.
In Fig. 5(e), the XRD spectra of different potassium-modified CS exhibited characteristic peaks of Ca(OH)2 and some CaCO3. The modified CS also indicated certain diffraction peaks corresponding to K2Ca(CO3)2, K2O, and KO2. From Fig. 5(e), the intensity of the Ca(OH)2 diffraction peaks in CS-KHCO3 and CS-K2CO3 was significantly reduced, whereas the intensity of the Ca(OH)2 diffraction peaks in CS-KOH was higher than that in CS. This observation confirmed that the addition of KHCO3 and K2CO3 reduced the crystallinity of CS, resulting in a more dispersed Ca(OH)2 in the modified CS (Tang et al., 2015). Moreover, Ca(OH)2 in CS played a crucial role in the reaction process and promoted the formation of desulfurization and denitrification products (Guo et al., 2022). However, the doping of KOH improved the crystallinity of CS. Notably, the XRD spectra in Fig. 5(e) revealed that the characteristic peaks of KOx in CS-KHCO3 were significantly more than those of CS-K2CO3 and CS-KOH, indicating that doped KHCO3 can facilitate the formation of more KOx, which played a critical role in the denitrification process of desulfurization (Li et al., 2022). However, in the modified CS, CaCO3 easily combined with KOx, to form a stable potassium phase containing calcium carbonate and K+. This interaction affected the activity of KOx, thereby reducing the catalytic capacity of KOx and the adsorption capacity of Ca(OH)2 on sulfur oxides (Li et al., 2022). In Fig. 5(e), CS-K2CO3 exhibited numerous characteristic peaks of K2Ca(CO3)2, resulting in a reduction in KOx content, which may explain the poor desulfurization and denitrification performance of CS-K2CO3. As shown in Fig. 5(f), the FTIR spectra were employed to investigate the surface functional groups of CS before and after modification(CS, CS-KOH, CS-K2CO3, and CS-KHCO3). The FTIR absorption peaks of the modified CS did not exhibit new appearances or disappearances compared to CS; instead, only the intensity of the modified CS peaks changed in the infrared spectrum, without the formation of new functional groups. A strong narrow peak at 3643 cm−1 was observed, representing the O–H bond created by the vibrational pulse. This peak is attributable to the stretching vibration of O–H caused by surface hydroxyl groups and chemically adsorbed potassium water on the adsorbent surface (Cong and Mei 2021). The stretching vibration of the carbonyl C=O surface functional group was represented by an absorption peak at 1430 cm−1 (Tang et al., 2013, Ding and Liu 2020). The O-CO- band in CO32-, demonstrated peaks at 710 cm−1 and 869 cm−1, corresponding to its in-plane and out-of-plane bending vibrations, respectively (Li and Yi 2020, Ma et al., 2021). After potassium modification, the intensity of the C = O peaks increased due to the activation and oxidation properties of potassium, resulting in the generation of numerous oxygen-containing functional groups (Ding and Liu 2020). Zhao et al. (1994), demonstrated that an increase in oxygen-containing functional groups improved the adsorption capacity of SO2 and NO. Interestingly, although CS and modified CS shared similar characteristics, CS-KOH and CS-KHCO3 exhibited a stronger C=O stretching pattern than CS and CS-K2CO3, which indicated a higher concentration of oxygen functional groups in CS-KOH and CS-KHCO3. In summary, the adsorption capacity of modified CS was closely related to the concentration of its surface organic functional groups.
The high-resolution XPS spectra of O 1 s, C 1 s, and K 2p for CS, CS-KOH, CS-K2CO3, and CS-KHCO3 are shown in Fig. 5(g)–5(i). Table S2 lists the corresponding binding energies and their relative contents. In Fig. 5(g), the O 1 s spectra of the modified CS and the corresponding fitted curves were illustrated, which can be deconvoluted into two peaks. A unique peak at a binding energy of 531 eV corresponds to the formation of lattice oxygen (denoted as Olat, such as O2–) by the defective oxides present in the modified CS. Surface adsorbed oxygen (denoted as Oads, such as O22-, O-, OH–, CO32-) in modified CS exhibited a characteristic peak in the range of 531.20 eV–532.90 eV (Panov et al., 2006, Liu and He 2010). It is widely recognized that Oads possesses higher mobility and activity than Olat, thereby accelerating the oxidation process through redox reactions (Liu et al., 2021). In addition, low coordination Oads greatly facilitated the formation of surface oxygen groups, such as carbonyl (–COOH), surface-active chemisorbed oxygen (O2-, O-, etc.), and surface hydroxyl groups (–OH). Table S2 illustrates that the Oads content of CS was 14.93 %, and the loading of potassium resulted in higher peak area ratios of Oads than CS for CS-KOH and CS-KHCO3, which reached 18.54 % and 47.47 %, respectively. In contrast, the Oads content of CS-K2CO3 was only 2.69 %, which could be attributed to the fact that K2Ca(CO3)2 covered the CS surface and blocked the pore channels, consistent with the SEM results of CS-K2CO3. The content of Oads followed the order: CS-KHCO3 > CS-KOH > CS > CS-K2CO3, with CS-KHCO3 showing the highest percentage of Oads. This indicated that CS-KOH and CS-KHCO3 possessed a higher content of surface hydroxyl and/or surface active chemisorbed oxygen. The increased Oads content enhanced the oxidation ability of the modified CS at low temperatures, which aligned with the previous analysis (Ding and Liu 2020). Tiwari et al. (2018) concluded that Oads indicated alkaline properties; thus, high levels of Oads can be more favorable for the adsorption of acidic gases. The measured C 1 s spectra of the modified CS are shown in Fig. 5(h). The C 1 s peak was deconvoluted into three functional groups, including graphitic carbon (C–C, 284.8 ± 0.1 eV), carbonyl (C=O, 287.7 ± 0.2 eV), and carboxyl and/or ester groups (–COO, 289.6 ± 0.2 eV) (Li et al., 2021, Li et al., 2022). As shown in Table S2, CS- KHCO3, CS-KOH, and CS-K2CO3 demonstrated lower C-O contents than CS, but higher C=O contents. The C=O content of CS was 11.17 %, and after modification, the C=O contents of CS-KOH, CS-K2CO3, and CS-KHCO3 increased to 23.46 %, 14.41 %, and 21.35 %, respectively. This increase may be attributed to the basicity of potassium, which introduces defective or unpaired electrons on the CS surface. These electrons promote the formation of surface functional groups via the absorption of oxygen from the environment (Liu et al., 2014). This was particularly evident on the CS-KOH surface. Combining these observations with Fig. 2, it was evident that the adsorption capacities of CS-KHCO3, CS-KOH, and CS-K2CO3 for SO2/NO were superior to that of CS. This can be attributed to the higher C = O content in CS-KHCO3, CS-KOH, and CS-K2CO3, which was favorable to improve the adsorption capacity of SO2/NO. According to the literature(Zhang et al., 2017), the essential property of C = O Brønsted is the active center of SO2/NO oxidation. As shown in Fig. 5(i), the K 2p spectrum presented two components at 292.72 eV and 295.42 eV, confirming the presence of K ions. The peaks of K 2p1/2 were observed at 295.40 eV and 295.71 eV, while the peaks of K 2p3/2 appeared at 292.47 eV and 293.14 eV, respectively, with evident differences in the relative intensities. The binding energies of the different valence states of K cations (K4+ and K+) with K 2p electrons were different. As observed in Fig. 5(d) and Table S2, the relative K4+ content on the surface of CS-KOH and CS-KHCO3 was higher than that of CS-K2CO3. This is in accordance with the XRD results, indicating that CS-KOH doped with KHCO3 promoted the formation of more KO2. KHCO3 loading led to the generation of oxygen vacancies on the modified CS surface and increased the Oads content by absorbing oxygen from the environment, which was consistent with the Oads content in the O 1 s spectrum. This may account for the higher effectiveness of CS-KHCO3 in desulfurization and denitrification. It can be inferred that a certain amount of K4+ can potentiate the oxidation of NO and SO2 during the redox process, thereby promoting the redox reaction and increasing the adsorption activity of CS. These results are consistent with those presented in Figs. 2 and 3.
3.4 Mechanism analysis
To gain deeper understanding of the mechanisms underlying desulfurization and denitrification, the reaction materials were examined by XRD, CO2-TPD, and XPS. The obtained results are presented in Fig. 6. Fig. 6(a) displayed the XRD patterns of several modified CS samples after desulfurization and denitrification. Following these processes, multiple distinctive diffraction peaks corresponding to K2SO4, KNO3, Ca(NO3)2, CaSO4, and CaSO3 were observed (Guo et al., 2022). The production of Ca(NO3)2, CaSO4, and CaSO3 suggested the participation of Ca(OH)2 in the denitrification reaction during desulfurization. In comparison with Fig. 4(e), the intensity of the diffraction peaks related to KOx decreased after desulfurization and denitrification, accompanied by K2SO4 and KNO3 diffraction peaks, indicating the involvement of KO2 in the reaction. Meanwhile, a new K2Ca(CO3)2 diffraction peak was detected, indicating the formation of stable K2Ca(CO3)2 during the desulfurization and denitrification. This formation can lead to a decline in the activity of CS after modification. Especially, the intensity of the diffraction peak of K2Ca(CO3)2 followed the CS-K2CO3 reaction, which generated a substantial amount of K2Ca(CO3)2. The combined analysis in Fig. 2 further demonstrated that K2Ca(CO3)2 hindered the denitrification and desulfurization.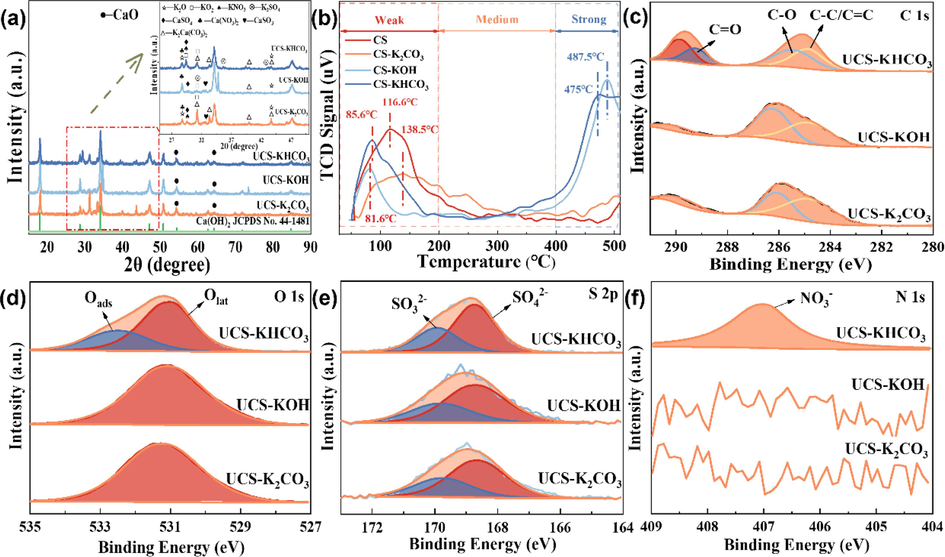
(a) XRD patterns, (b) CO2-TPD profiles, (c) C 1 s, (d) O 1 s, (e) S 2p, and (f) N 1 s spectra of UCS-KHCO3, UCS-KOH, and UCS-K2CO3.
To observe the variation in the alkaline sites on the surfaces of the materials, CO2-TPD experiments were conducted on both the pre-modified and post-modified materials. The experimental results, according to the experimental findings illustrated in Fig. 6(b), revealed the presence of distinct desorption peaks at weak basic sites (0–200 °C), medium basic sites (200–400 °C), and strong basic sites (>400 °C) (de Oliveira et al., 2022). Compared with CS, the modified CS exhibited the following changes: (i) a slight reduction in the intensity of weakly basic sites, (ii) relatively unchanged medium basic sites, and (iii) a significant increase in the intensity of strongly basic sites for CS-KOH and CS-KHCO3. This further indicated that potassium addition had a substantial impact on the distribution of the basic sites in the CS. From Fig. 6(b) and 2, CS exhibited a larger peak area in the weakly basic site, implying a greater number of basic sites and enhanced desulfurization efficiency. This indicated that the removal of SO2 occurred mainly through chemisorption and an acid-base neutralization reaction. However, the weak base sites of the modified CS were reduced, whereas the denitrification effect of the modified adsorbents was significantly enhanced, which can be attributed to the promotion of denitrification by KOx.
The C 1 s spectrum of modified CS obtained after the reaction is shown in Fig. 6(c). Upon comparison with Fig. 4(h), it was evident that the surface functional groups underwent significant changes following the utilization of the modified CS in the desulfurization and denitrification processes. The contents of C = O in UCS-KOH, UCS-K2CO3, and UCS-KHCO3 were reduced by 23.46 %, 14.41 %, and 11.09 %, respectively. Conversely, the C-O content in UCS-KOH increased to 45.5 %, and typical C-O (285.0 ± 0.5 eV) peaks were observed for UCS-K2CO3 and UCS-KHCO3 with 55.13 % and 33.89 %, respectively. This indicated the involvement of C = O in the oxidation of SO2/NO and its consumption in the SO2/NO removal process. The presence of C = O, influenced the adsorption capacity of the modified CS surface by facilitating electron transfer to the adsorbed oxygen species (Fang et al., 2017). Considering the analysis in Figs. 2 and 4(h), C = O, serving as the main active site for acid gas chemisorption (Li et al., 2003, Wang et al., 2020), may play a crucial role in the effective simultaneous desulfurization and denitrification achieved by the modified CS. The O 1 s spectra of UCS-KOH, UCS-K2CO3, and UCS-KHCO3, and their fitted curves are presented in Fig. 6(d). Comparing Tables S2 and S3, a significant decline of the Oads content on UCS-KHCO3 was observed, dropping from 47.47 % to 38.34 %. Only Olat characteristic peaks existed for UCS-KOH and UCS-K2CO3, with the disappearance of the characteristic peaks of Oads. The results indicated a significant decrease in the value of Oads after the reaction, which further determined the involvement of adsorbed oxygen in the reaction and possibly played a key role in the oxidation reaction of the modified CS. Fig. 6(d) illustrated the XPS results of S 2p after desulfurization and denitrification of different modified CS. The S 2p in the XPS spectrum demonstrated two peaks at 168.67 eV and 169.81 eV for sulfate SO42- and sulfite SO32-2-3 (Li et al., 2016, Lian et al., 2017, Arfaoui et al., 2018, Li et al., 2018), respectively. This suggested that Ca2+ readily formed sulfur-containing substances with SO42-and SO32- on the surface of the modified CS. These sulfates and sulfites adhered to the material surface, obstructing the pore channels and covering the active sites, which ultimately led to the deactivation of the modified CS due to SO2 poisoning(Wu et al., 2016, Silas et al., 2018). The formation of NO3- was responsible for the peak in the N 1 s XPS spectrum of UCS-KHCO3 at 407 eV (Hao et al., 2017), as shown in Fig. 6(f). In contrast, UCS-KOH and UCS-K2CO3 failed to form characteristic peaks, probably due to the partial occupation of surface-active sites by the formation of sulfate, resulting in a low level of surface-bound N. The XPS data further confirmed the production of sulfate, sulfite, and nitrate during the reaction, which is consistent with Fig. 6(a).
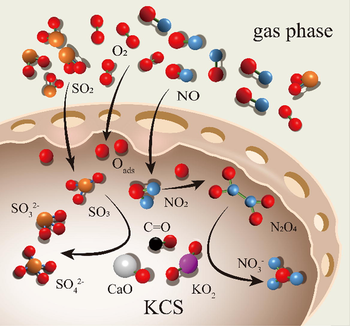
Based on the above process, the successive steps involved in the desulfurization of the modified CS can be postulated through the following equations (Eq. (8) to Eq. (13)) (Li et al., 2022). Firstly, SO2 in the flue gas was converted to the adsorbed state SO2(ads). The Brønsted basic sites on the Ca(OH)2 surface react with SO2(ads) to form CaSO3. Furthermore, the modified CS containing KO2, which has potent oxidizing properties, supplied active oxygen (O*) and facilitated the desulfurization process. As a result, O* originating from the modified CS and O2 in the flue gas oxidized SO2(ads) into SO3(ads). Finally, the formed SO3(ads) was combined with Ca(OH)2 and K2O on the modified CS to form CaSO4 and K2SO4. Meanwhile, the reaction mechanism of the denitrification process was speculated and summarized as the following equations (Eq. (14) to Eq. (18)). In the process of denitrification, NO was converted to the adsorbed state and subsequently oxidized by O* to generate NO2(ads), and NO2(ads) dimerizes to N2O4(ads). In addition, the formation of N2O4 was also promoted by O2. N2O4 isomerizes to NO+NO3– at low temperatures (Wu et al., 2012) and then reacts with Ca(OH)2 in the presence of O* to form Ca(NO3)2 according to an acid-base reaction (Liu et al., 2011). The reaction intermediate products NO2(ads) and K2O combine to form KNO3. Deactivation of the modified CS after the reaction was due to the production of sulphate, sulphite, and nitrate. These substances covered the surface of the modified CS, obstructing the access of SO2/NO to the active sites, thus resulting in a decrease in the adsorption activity of the modified CS(Li et al., 2022).
4 Conclusions
In this study, potassium modification of CS significantly improved its desulfurization and denitrification performance. At 200 °C, TSO275% of CS-KOH, CS-K2CO3, and CS-KHCO3 were maintained at 120 min, and TNO45 % of denitrification rates were for 120 min, 75 min, and 120 min, respectively. The surface characteristics of the modified CS particles varied after potassium modification: CS-KOH displayed small honeycomb-like pores on the surface, CS-K2CO3 exhibited a laminar surface, and CS-KHCO3 formed a unique flower cluster-like structure. The results indicated that the NO catalytic activation energy of the modified CS was reduced, which lowered the reaction barriers of CS. The contents of KOx, C = O, and Oads in different modified CS were responsible for the variations in the activity effects, while the doping of KOH and KHCO3 could effectively increase the contents of C = O and Oads. The presence of Ca(OH)2 in the modified CS presented excellent SO2 trapping ability, and the presence of K4+ facilitated the conversion of NO to NO3- and SO2 to SO42-. Desulfurization and denitrification reactions are hampered by K2Ca(CO3)2, which can be generated during the modification process. Moreover, sulfates, sulfites, and nitrates produced by the reactions can potentially deactivate the activation sites in the modified CS, which can further result in reduced adsorption activity.
CRediT authorship contribution statement
Fang Wang: Conceptualization, Supervision, Funding acquisition. Shaojun Luo: Investigation, Visualization, Writing – original draft. Hui Li: Data curation. Jiyun Gao: Data curation, Methodology. Futing Xia: Data curation, Formal analysis. Shuo Cui: Project administration, Resources. Lijuan Jia: Supervision, Project administration, Funding acquisition. Jiayu Feng: Project administration, Validation. Mingwu Xiang: Conceptualization, Formal analysis.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51968075).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Novel V2O5-CeO2-TiO2-SO42− nanostructured aerogel catalyst for the low temperature selective catalytic reduction of NO by NH3 in excess O2. Appl Catal B. 2018;224:264-275.
- [CrossRef] [Google Scholar]
- SO2 removal performances of Al- and Mg-modified carbide slags from CO2 capture cycles at calcium looping conditions. J. Therm. Anal. Calorim.. 2020;144:1187-1197.
- [CrossRef] [Google Scholar]
- Comparative study of coal based catalysts for NO adsorption and NO reduction by CO. Fuel. 2018;214:230-241.
- [CrossRef] [Google Scholar]
- Cheng, J., J. Zhou, J. Liu, et al., 2009. Physicochemical characterizations and desulfurization properties in coal combustion of three calcium and sodium industrial wastes. 23, https://doi.org/10.1021/ef8007568. Journal Name: Energy and Fuels; Journal Volume: 23; Journal Issue: 5.
- Using silica fume for improvement of fly ash/slag based geopolymer activated with calcium carbide residue and gypsum. Constr. Build. Mater.. 2021;275
- [CrossRef] [Google Scholar]
- Sodium and potassium silicate-based catalysts prepared using sand silica concerning biodiesel production from waste oil. Arab. J. Chem.. 2022;15
- [CrossRef] [Google Scholar]
- Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel. 2020;260:116382-116391.
- [CrossRef] [Google Scholar]
- Desulfurization Performance and Kinetics of Potassium Hydroxide-Impregnated Char Sorbents for SO2 Removal from Simulated Flue Gas. ACS Omega. 2020;5:19194-19201.
- [CrossRef] [Google Scholar]
- 2014. Emission standard of air pollutants for boiler, National Standard of the People's Republic of China. GB 13271-2014.
- Influence of textures, oxygen-containing functional groups and metal species on SO2 and NO removal over Ce-Mn/NAC. Fuel. 2017;202:328-337.
- [CrossRef] [Google Scholar]
- SO2 adsorption capacity of K2CO3-impregnated activated carbon as a function of K2CO3 content loaded by soaking and incipient wetness. Appl. Surf. Sci.. 2007;253:3201-3207.
- [CrossRef] [Google Scholar]
- NiO-Modified Coconut Shell Based Activated Carbon Pretreated with KOH for the High-Efficiency Adsorption of NO at Ambient Temperature. Ind. Eng. Chem. Res.. 2018;57:16593-16603.
- [CrossRef] [Google Scholar]
- Recycling and utilization of calcium carbide slag - current status and new opportunities. Renew. Sustain. Energy Rev.. 2022;159:112133
- [CrossRef] [Google Scholar]
- Microwave-assisted industrial wastes of fly ash and carbide slag as adsorbents for simultaneous desulfurization and denitrification. Sep. Purif. Technol.. 2022;303:122176-122187.
- [CrossRef] [Google Scholar]
- An advanced wet method for simultaneous removal of SO2 and NO from coal-fired flue gas by utilizing a complex absorbent. Chem. Eng. J.. 2017;307:562-571.
- [CrossRef] [Google Scholar]
- Feasibility Study of Using Carbide Slag as In-Bed Desulfurizer in Circulating Fluidized Bed Boiler. Appl. Sci.. 2019;9
- [CrossRef] [Google Scholar]
- Kinetic Behavior of Solid K2CO3 under Postcombustion CO2 Capture Conditions. Ind. Eng. Chem. Res.. 2017;56:853-863.
- [CrossRef] [Google Scholar]
- New insights into the mechanism of NH3-SCR over Cu- and Fe-zeolite catalyst: Apparent negative activation energy at high temperature and catalyst unit design consequences. Applied Catalysis b: Environmental.. 2018;226:565-574.
- [CrossRef] [Google Scholar]
- NO2 and NO Adsorption Properties of KOH-Treated γ-Alumina. Ind. Eng. Chem. Res.. 1998;37:3375-3381.
- [CrossRef] [Google Scholar]
- Application of surfactant-modified cordierite-based catalysts in denitration process. Fuel. 2020;268
- [CrossRef] [Google Scholar]
- Synergetic utilization of microwave - assisted fly ash and carbide slag for simultaneous desulfurization and denitrification: High efficiency, low cost and catalytic mechanism. Chem. Eng. J.. 2022;437
- [CrossRef] [Google Scholar]
- Importance of activated carbon's oxygen surface functional groups on elemental mercury adsorption☆. Fuel. 2003;82:451-457.
- [CrossRef] [Google Scholar]
- Selective catalytic reduction of NO by NH3 over CuO–CeO2 in the presence of SO2. Cat. Sci. Technol.. 2016;6:1719-1725.
- [CrossRef] [Google Scholar]
- O3 oxidation excited by yellow phosphorus emulsion coupling with red mud absorption for denitration. J. Hazard. Mater.. 2021;403:123971
- [CrossRef] [Google Scholar]
- Use of carbide slag from acetylene industry for activation of ground granulated blast-furnace slag. Constr. Build. Mater.. 2020;238
- [CrossRef] [Google Scholar]
- Ho-modified Mn-Ce/TiO2 for low-temperature SCR of NO with NH3: Evaluation and characterization. Chin. J. Catal.. 2018;39:1653-1663.
- [CrossRef] [Google Scholar]
- Effect of calcite on desulfurization and denitration performance of activated coke and its mechanism. J. Fuel Chem. Technol.. 2021;49:554-563.
- [CrossRef] [Google Scholar]
- Improvement of Nb Doping on SO2 Resistance of VOx/CeO2 Catalyst for the Selective Catalytic Reduction of NOx with NH3. J. Phys. Chem. C. 2017;121:7803-7809.
- [CrossRef] [Google Scholar]
- Desulfurization performance of iron supported on activated carbon. Fuel. 2014;123:93-100.
- [CrossRef] [Google Scholar]
- Structure−Activity Relationship of Iron Titanate Catalysts in the Selective Catalytic Reduction of NOx with NH3. J. Phys. Chem. C. 2010;114:16929-16936.
- [CrossRef] [Google Scholar]
- Synergistic reaction between SO2 and NO2 on mineraloxides: a potential formation pathway of sulfate aerosol†. PCCP 2011
- [CrossRef] [Google Scholar]
- Highly efficient MnOx/biochar catalysts obtained by air oxidation for low-temperature NH3-SCR of NO. Fuel. 2021;283
- [CrossRef] [Google Scholar]
- Preparation of feed grade calcium formate from calcium carbide residue. Clean Techn. Environ. Policy. 2016;18:1905-1915.
- [CrossRef] [Google Scholar]
- Synergistic mechanisms of steelmaking slag coupled with carbide slag for CO2 mineralization. Int. J. Greenhouse Gas Control. 2021;105
- [CrossRef] [Google Scholar]
- Adsorption of SOx by oxide materials: A review. Fuel Process. Technol.. 2013;114:81-100.
- [CrossRef] [Google Scholar]
- Utilization of sludge templated carbide slag pellets for simultaneous desulfurization and denitrification: Low and moderate temperatures and synergetic mechanism. J. Clean. Prod.. 2022;379:134663-134675.
- [CrossRef] [Google Scholar]
- Active oxygen in selective oxidation catalysis. Catal. Today. 2006;117:148-155.
- [CrossRef] [Google Scholar]
- Separated growth of Bi-Cu bimetallic electrocatalysts on defective copper foam for highly converting CO2 to formate with alkaline anion-exchange membrane beyond KHCO3 electrolyte. Appl Catal B. 2021;288
- [CrossRef] [Google Scholar]
- A facile trace potassium assisted catalytic activation strategy regulating pore topology of activated coke for combined removal of toluene/SO2/NO. Chem. Eng. J.. 2020;389
- [CrossRef] [Google Scholar]
- Comparative studies of low concentration SO2 and NO2 sorption by activated carbon supported [C2mim][Ac] and KOH sorbents. J. Environ. Chem. Eng.. 2018;6:718-727.
- [CrossRef] [Google Scholar]
- Breakthrough studies of Co3O4 supported activated carbon monolith for simultaneous SO2/NO removal from flue gas. Fuel Process. Technol.. 2018;180:155-165.
- [CrossRef] [Google Scholar]
- Effect of potassium-precursor promoters on catalytic oxidation activity of Mn-CoOx catalysts for no removal. Ind. Eng. Chem. Res.. 2015;54:9116-9123.
- [CrossRef] [Google Scholar]
- Improved phenol decomposition and simultaneous regeneration of granular activated carbon by the addition of a titanium dioxide catalyst under a dielectric barrier discharge plasma. Carbon. 2013;53:380-390.
- [CrossRef] [Google Scholar]
- Adsorption of CO2 on KOH activated, N-enriched carbon derived from urea formaldehyde resin: kinetics, isotherm and thermodynamic studies. Appl. Surf. Sci.. 2018;439:760-771.
- [CrossRef] [Google Scholar]
- On the negative activation energy for limestone calcination at high temperatures nearby equilibrium. Chem. Eng. Sci.. 2015;132:169-177.
- [CrossRef] [Google Scholar]
- Desulfurization and Denitrification Performance of Modified Rice Husk Ash-Carbide Slag Absorbent. Materials (basel).. 2020;14:68-80.
- [CrossRef] [Google Scholar]
- Simultaneous SO2/NO removal performance of carbide slag pellets by bagasse templating in a bubbling fluidized bed reactor. Fuel Process. Technol.. 2018;180:75-86.
- [CrossRef] [Google Scholar]
- Simultaneous SO2 and NO removal by pellets made of carbide slag and coal char in a bubbling fluidized-bed reactor. Process Saf. Environ. Prot.. 2020;134:83-94.
- [CrossRef] [Google Scholar]
- Sulfur poisoning and regeneration of MnOx-CeO2-Al2O3 catalyst for soot oxidation. J. Rare Earths. 2012;30:659-664.
- [CrossRef] [Google Scholar]
- Simultaneous CO2/SO2adsorption performance of carbide slag in adsorption/desorption cycles. Can. J. Chem. Eng.. 2016;94:33-40.
- [CrossRef] [Google Scholar]
- The mechanism of Pd, K co-doping on Mg–Al hydrotalcite for simultaneous removal of diesel soot and NOx in SO2-containing atmosphere. Fuel. 2019;240:244-251.
- [CrossRef] [Google Scholar]
- Multi-stage semi-coke activation for the removal of SO2 and NO. Fuel. 2017;210:738-747.
- [CrossRef] [Google Scholar]
- Influences of surface functional groups on catalytic activity over activated carbon catalysts for sulfur dioxide removal from flue gases. Appl Catal B. 1994;3:229-238.
- [CrossRef] [Google Scholar]
- Amino-Modified Fe-Terephthalate Metal-Organic Framework as an Efficient Catalyst for the Selective Oxidation of H2S. Inorganic Chemistry. 2018;57:10081-10089.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105363.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







