Translate this page into:
Sol-gel derived mesoporous 45S5 bioactive glass containing Mg and Zr ions: Synthesis, characterization, and in vitro biological investigation
⁎Corresponding author. g.dini@sci.ui.ac.ir (Ghasem Dini)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Bioactive glass is one interesting type of material for bone repair. In this work, a new powder (MBG2) was synthesized by a sol–gel method, in which 5 mol.% Mg and 5 mol.% Zr were incorporated into the structure, to investigate the combined effects of the simultaneous addition of these ions on the biological properties of 45S5 mesoporous bioactive glass. Then, the outcomes of various characterization techniques and in vitro biological analyses performed on this powder were compared with those of pure powder (MBG1). For instance, the XRF investigation proved that Zr and Mg ions were present in powder MBG2′s composition. Additionally, XRD, SEM, and BET tests of the powders MBG1 and MBG2 indicated their amorphous structure and spherical morphology with specific surface areas of 620 and 710 m2/g and average particle sizes of about 30 and 20 nm, respectively. The development of an apatite layer on the surfaces of disc-shaped samples produced from both powders were immersed in phosphate-buffered saline (PBS) and simulated body fluids (SBF), and the behavior of the samples' degradation were observed for 28 days. Furthermore, it was assessed how the Zr and Mg ions in the composition affected the MG-63 cells' survival, growth, and adhesion through in vitro biocompatibility testing. From the obtained results, it can be concluded that 45S5 mesoporous bioactive glass powder with minimal cytotoxicity was entitled to be a safe biomaterial due to the coexistence of 5 mol.% Mg and 5 mol.% Zr in the composition, which successfully enhanced bioactivity, cell proliferation, and biocompatibility.
Keywords
Mesoporous bioactive glass
Sol-gel
Magnesium
Zirconium
Bioactivity
Bone repair
1 Introduction
A third generation of bioceramic materials by Hench was introduced in 1971 with 45S5 bioactive glasses (BGs) containing 46.1 SiO2, 26.9 CaO, 24.4 Na2O, and 2.5 P2O5 (all in mol.%) (Solgi, 2015). BGs have caught the interest of many researchers due to their excellent bone induction properties (i.e. osteoconductivity ability) that have been approved by the FDA, high bioactivity, the capability to bond with soft and hard tissues without side effects, release of therapeutic soluble ions, and antimicrobial properties (Goudarzi et al., 2019; Kumar et al., 2020; Spirandeli et al., 2020; Massera, 2016). Since then, BGs have been researched to replace, repair, and encourage bone tissue regeneration. They have a wide range of applications in both research and commercial products, including mineralization elements in toothpaste and cosmetics, drug delivery, embolization treatment, and ophthalmic applications (Spirandeli et al., 2020; Vichery and Nedelec, n.d.). Also, BGs are suitable materials for clinical applications in periodontology, endodontology, and orthopedics (Kesse et al., 2020). The first clinical application of BGs for restoring hearing loss and regeneration of the middle ear bones was reported by Merwin in 1989 (Moghanian et al., 2020). BGs are largely regarded as appealing because of their capacity to chemically connect with host tissues, which is directly related to their atomic structure (Vichery and Nedelec, n.d.). This connection is so strong that shattering the bone is required to separate them (Himanshu et al., 2016).
Bioactivity is closely associated with the glass dissolution rate and its morphology. That is, the more the specific surface area, or the surface interaction between the substance and physiological fluids, the greater the glass’s bioactivity. In light of this issue, it is possible to enhance the specific surface area by increasing porosity and reducing the size of the synthesized BGs. Therefore, BG nanoparticles (20 to 500 nm) are more desirable than micrometric particles due to their higher specific surface area (higher surface-to-volume ratio) and higher surface energy (Vichery and Nedelec, n.d.). Also, mesoporous BG nanoparticles (MBGs) can cause the synergy of biologically active ions and loaded biomolecules (such as antibiotics, and growth factors). This mechanism works through their local release in a controlled mode to ameliorate therapeutic operation (Zheng, et al., 2020).
MBGs showed better osteogenic properties and bioactivity compared to traditional non-porous BGs (Zhu et al., 2011; Shoaib, et al., 2021). Furthermore, other factors like the synthesis process and glass composition are related to bioactivity. Therefore, finding the best synthesis method has always been a challenging issue (Moghanian et al., 2020). BGs are made in different ways (Moghanian et al., 2021). Among the various methods, advances in sol–gel chemistry have led to the creation of BG nanoparticles with controlled morphology (Zheng, et al., 2020). The sol–gel technique is not only due to the synthesis of particles of various sizes and shapes, but also due to benefits like creating porosity and high specific surface area, executable at room temperature, higher purity and homogeneity, greater product bioactivity, product quality, and ease of the process of interest to researchers (Moghanian et al., 2021; Faure, 2015). Sol-gel processing in the fabrication of BG materials was first reported in 1991 (Lukowiak et al., 2013). Sol-gel derived BGs have often shown higher bioactivity with higher SiO2 content, higher dissolution rate, and a higher specific surface area rich in silanol groups (nucleation sites for apatite crystal formation) compared to BGs derived from traditional melt quenching process (Pirayesh and Nychka, 2013; Tabia et al., 2019; Naghizadeh, 2015). As a result of these characteristics, the rate of hydroxycarbonate apatite layer formation and degradation is increased, which improves the osteogenesis property (Lukowiak et al., 2013; Wang et al., 2019). BG nanoparticles synthesized by this method are used in bone repair, wound healing, and nanomedicine (Zheng, et al., 2020).
Also, incorporating different ions into the composition of BGs provides additional properties such as angiogenesis, increased osteogenic ability, increased biological activity, bone conductivity, and antibacterial effects (Shoaib, et al., 2021; Sharifianjazi et al., 2017; Nawaz, 2018). For this reason, several glass compounds have been synthesized by doping therapeutically active ions such as cerium (Ce), gallium (Ga), cobalt (Co), copper (Cu), lithium (Li), niobium (Nb), boron (B), silver (Ag), magnesium (Mg), aluminum (Al), zinc (Zn), strontium (Sr), fluorine (F), potassium (K), and zirconium (Zr) (Sharifianjazi et al., 2017). Zr and Mg are essential for the growth, maintenance, and repair of human bones (Zhu et al., 2011). Mg, with the function of stimulating osteoblast proliferation, is considered one of the important elements in the composition of bone tissues (Shoaib, et al., 2021). Therefore, researchers prefer biomaterials doped with Mg because of its presence in bone, enamel, and muscle structures and as a result of evaluating its beneficial effects in bone regeneration (Shoaib, et al., 2021; Moghanian et al., 2021). Mg contributes to the enhancement of mechanical properties and the acceleration of the formation of the hydroxyapatite (HA) layer both in vitro and in vivo (Moghanian et al., 2021). According to Tabia et al. (Tabia et al., 2019), MBGs made by the sol–gel process with an 85SiO2-10CaO-5MgO composition provide the greatest results because of their higher surface area and pore size. MgO has been replaced by CaO in biological apatite, and higher osteoconductivity and resorption have been reported in hydroxyapatite (Zhu et al., 2011). The synthesis of MBGs in the SiO2-B2O3-CaO-MgO-ZnO system was carried out in another study, in which the cytotoxicity assay showed increased cell viability for samples containing MgO without inducing intracellular oxidative stress (Damian-Buda et al., 2022). Also, the synthesis of melt-derived Mg-doped BG with the composition of 45SiO2-3P2O5-26CaO-15Na2O-7MgO-4K2O which is used in bone regeneration leads to the stimulation of osteogenesis in vitro and in vivo. Additionally, the Mg-doped BGs were able to enhance the ability of human umbilical vein endothelial cells to migrate in vitro and stimulate the formation of bone nodules (Kargozar et al., n.d.). On the other hand, according to in vitro tests, Zr improves the mechanical and biological properties of BGs when it is mixed into their composition (Himanshu et al., 2016; Zheng, et al., 2020; Zhu et al., 2011; Shoaib et al., 2021; Moghanian et al., 2021). Zr has a higher electronegativity and a larger propensity to form covalent bonds than Ca-O bonds, which contributes to the compaction of the BG structure upon its addition. Moghanian et al. (Moghanian et al., 2021) investigated the effect of 5 mol.% of Zr incorporated into 58S BGs along with the addition of Mg (0, 1, 3, 5, and 7 mol.%) instead of CaO through the sol–gel method from the point of view of laboratory and mechanical biology. The modified samples in their report improved alkaline phosphatase (ALP) activity, MC3T3 osteoblast cell proliferation, and antibacterial performance. Additionally, they demonstrated the remarkable performance of BG powders with 5 mol.% Zr, and 5 mol.% Mg. Also, they reported that the combination of Mg with Zr improved the mechanical properties of the synthesized BGs. As they reported, the optimal percentage of Zr in BGs is 5 mol.% (Moghanian et al., 2020).
However, limited studies have been done on MBGs containing Mg and Zr. Even considering that Mg and Zr have been investigated separately in the synthesis of BGs, but still, as far as we know, the successful synthesis and improved bioactivity of 45S5 BGs doped with Mg and Zr simultaneously from the sol–gel method is not well validated. In the present study, the bioactivity, cell viability, and other physical, structural, and biological properties of mesoporous 45S5 BG nanopowder by simultaneous incorporation of 5 mol.% Mg and 5 mol.% Zr into the composition through modified sol–gel have been investigated. The new MBG powder synthesized in this research confirmed the hypothesis that MBGs doped with appropriate amounts of Mg and Zr ions have a preferable ability to be used in bone regeneration.
2 Materials and methods
2.1 Materials
Tetraethyl orthosilicate (TEOS, Si(OC2H5)4, >99 %), triethyl phosphate (TEP, PO(C2H5)3, >99 %), sodium nitrate (NaNO3), hexadecyltrimethylammonium bromide (CTAB, C19H42BrN, >98 %), calcium nitrate tetrahydrate (Ca(NO3)2·4H2O), magnesium nitrate hexahydrate (Mg(NO3)2·6H2O), and zirconium oxynitrate hydrate (ZrO(NO3)2·xH2O) as starting materials for the synthesis of mesoporous 45S5 BG powder were purchased from Merck and Sigma-Aldrich. Based on stoichiometric amounts appropriate to the chemical composition of 45S5 BG, all of these components were employed without additional purification. The relative ratio of each chemical was computed using the freely available software Glass Panacea (Siqueira, 2017). Furthermore, ammonium hydroxide 25 %, distilled water, nitric acid, and ethyl alcohol 96 % were used.
2.2 Synthesis of mesoporous BG powder containing Mg and Zr ions
BG powders with and without Zr and Mg ions were produced using a modified sol–gel method (Siqueira, 2017; Kumar et al., 2017; Liang et al., 2015; Thomas and Bera, 2016; Ranga et al., 2020). Amounts of Zr and Mg ions were chosen from recently published articles (Ranga et al., 2020; Fan et al., 2014). To synthesize 2.5 g of pure mesoporous 45S5 BG powder (labeled as MBG1), first, 0.92 g CTAB was dissolved in distilled water/ethanol solution by stirring for 1 h, where the H2O:TEOS and ethanol:TEOS molar ratios were set to 20, and 8 respectively (Fiume; Bahniuk et al., n.d.). Moreover, 300 μL nitric acid (2 M) was added to maintain pH between 1 and 2. After the solution became clear, 1.677 g sodium nitrate and 1.789 g calcium nitrate were sequentially added to the solution in intervals of 1 h, followed by continuous magnetic stirring at room temperature. Then, while the solution was being continuously stirred, 0.381 g of TEP was added dropwise. A further 30 min of vigorous stirring was followed by the addition of 3.898 g of TEOS to the mixture after 5 min of stirring. The resultant mixture was then stirred under gentle stirring for a further 1.5 h. The reaction was then continued at 40 °C by dropping 400 μL of aqueous ammonia solution to reduce the gelation time while vigorously stirring the mixture (Lukowiak et al., 2013). Then, the mixture was allowed to stir vigorously for 1 h so that gel was formed. To complete the gelation process, the prepared sol was lidded with cellophane and kept sealed motionless in an electric oven at 60 °C for 3 days as the artificial aging time. Thereafter, the product underwent a drying process at 85 °C for 24 h to allow the gradual evaporation of the alcoholic liquid phase. The gels thus formed were then ground in a mortar. Eventually, BG powder was obtained after removing the CTAB, organics, and nitrates as well as condensing the silanol groups by calcination at 670 °C using a heating rate of 2 °C/min in an air atmosphere for 5 h, in an electric tubular furnace (Fig. S1 in Supplementary Information). The 45S5 BG powder containing Mg and Zr ions (labeled as MBG2) was synthesized by changing the Ca2+ mole fraction in MBG1 composition. In the new composition, 5 mol.% Mg and 5 mol.% Zr were substituted for Ca2+. Then, powder MBG2 was synthesized using the same method as described above.
2.3 Characterizations
Using X-ray fluorescence spectroscopy (XRF, S4 PIONEER, Bruker, Germany), the elemental composition of the produced powders MBG1 and MBG2 was determined. With the help of the X-ray diffraction method (XRD, D8 ADVANCE, Cu-Kα Bruker, Germany), the phase identification of the synthesized powders was evaluated. For low-angle tests, the angular range was 0.1-10° and the size of slits was 1, 1, and 0.05 mm for divergence slit, scattering slit, and receiving slit, respectively. For wide-angle tests, the 2 theta range was 10-80°, and the slit configuration was set as 1, 1, and 0.3 mm. All XRD patterns were recorded at a scan speed of 0.05°/s and the voltage and current of the diffractometer were fixed at 40 kV and 40 mA, respectively. To study the particle size and morphology of powders, a scanning electron microscope (SEM, Carls Zeiss 35 VP Supra) with a resolution of ∼ 2 nm at 30 kV and magnification in the range of 50,000 to 150,000 × and a transmission electron microscope (TEM, ZEISS EM10C) with a resolution of ∼ 0.5 nm at 100 kV were used. N2 adsorption–desorption using the Brunauer–Emmett–Teller method (BET, ASAP2460, Micrometrics Instrument) at 77 K was used to measure the surface area and porosity of the products.
2.4 Degradation and apatite mineralization ability assays
Powders MBG1 and MBG2 were shaped as 90 mg disks under a pressure of 10 MPa in a hand-pressing machine using a die with a diameter of 5 mm and then were sintered at 670 °C for 2 h. The disks were exposed to UV light for 30 min. Then, the disks were immersed in 5 and 10 mL of phosphate buffered saline (PBS) and simulated body fluid (SBF) solution, respectively, and kept sealed in an incubator at 37 °C for periods of 0, 7, 14, 21, and 28 days (Ahmadi et al., 2022). The SBF-soaked disks MBG1 and MBG2 were taken out, and the remaining SBF solution was used for elemental analysis using an inductively coupled plasma atomic emission spectrometer (ICP-OES, Analytik Jena, PQ 9000). Calculations were made for the variations in Ca, P, Si, Mg, and Zr ion concentrations in the SBF solution over time. Disk-shaped specimens were also dried and the HA layer on their surface was investigated by XRD and SEM analyses. Additionally, the pH values of PBS and weight loss of disks during the immersing period were monitored.
2.5 Biological assessments
2.5.1 Cell viability
The MG-63 cell line obtained from Royan Institute, Iran was used to study the cytotoxicity of the prepared disks via the MTT test. To do this, first, the UV-sterilized disks were placed in a 48-well plate. Then, 20,000 cells/ml of MG-63 cells were seeded on each disk. The control group included cells and a culture medium. The cell-disk complexes were covered with a proliferation medium. After incubating the plate for 1, 3, and 5 days at 37 °C in a humidified 5 % CO2 atmosphere, the disks were washed three times with PBS to remove cells that did not adhere to the disks. Each well received an addition of MTT solution at the end of each period, and the plate underwent a 4 h incubation at 37 °C. MTTs were converted to formazan and the results were calculated at 492 nm by reading the absorbance of each well along with the optical density value of the control wells.
2.5.2 Fluorescent staining
To evaluate the number of osteoblast-like cells attached to the disks after 24 h of cell seeding, staining of the nucleus and actin filaments were done with 4,6-diamidino-2-phenylindole (DAPI, blue) and TRITC-labeled phalloidin (red), respectively. Cell-seeded disks were fixed with 4 % paraformaldehyde in PBS, pH = 7, at 27 °C for 20 min. Then, they were washed with PBS 3 times to remove any likely non-adherent cells. Following that, the cells were gently washed with PBS after being permeabilized with a 0.2 % (v/v) Triton X-100/PBS solution for 20 min. The disks were first stained with phalloidin at 37 °C for 2 h, followed by 5 min in the dark at room temperature with 1 mg/ml of DAPI/PBS solution (1:100 dilution of stock in PBS). PBS was then used to wash the disks. Finally, the attached cells were seen using a fluorescence microscope with optical filters with excitation and emission wavelengths of 360 and 460 nm.
2.5.3 Cell adhesion
The morphology and spreading degree of MG-63 cells on the disks after 24 h of seeding were investigated by SEM. The cell-seeded disks were fixed by 2.5 % glutaraldehyde in PBS, pH = 7.4, for 2 h at 4 °C. Then, they were washed with PBS 4 times. Dehydration occurred by gradually increasing the series of ethanol levels from 50 to 100 % each for 15 min. The fixed disks underwent drying, gold sputter coating, and SEM analysis.
2.6 Statistical analysis
At least 3 repetitions were used to show the findings of the selected quantitative tests as mean ± standard deviation. To analyze the quantitative significance (p < 0.05) of results, a two-way ANOVA via GraphPad program, Prism was used.
3 Results and discussion
3.1 Characterization of the synthesized powders
The composition of the synthesized powders MBG1 and MBG2 was determined using XRF, and the results are given in Table 1, showing that the composition of the powder MBG1 is close to the composition of 45S5 BG. Therefore, it can be concluded that the described modified sol–gel process used in this work is a suitable alternative method for producing powder with the desired composition. Also, by adding 5 mol.% of each elements Mg and Zr in the lattice of powder MBG1, the weight percentage of CaO has decreased, indicating the successful replacement of Ca ions with Mg and Zr ions in the lattice of powder MBG2 (Table 1). The results of phase identification and crystalline study of powders MBG1 and MBG2 are displayed in Figs. 1, 2. The wide-angle XRD patterns (Fig. 1) confirm that both powders have an amorphous structure, according to the expected structure for BGs. On the other hand, the results of the low-angle XRD analysis (Fig. 2), which is used to confirm the presence of nanometer pores, indicate the porous structure of the particles.
Bioactive glass
Label
Composition (W/W %)
SiO2
Na2O
CaO
P2O5
ZrO2
MgO
45S5
MBG1
46.4
24.7
24.2
4.7
–
–
45S5 + 5 mol.% ZrO2 + 5 mol.% MgO
MBG2
46.3
23.8
17.6
4.3
3.9
4.1
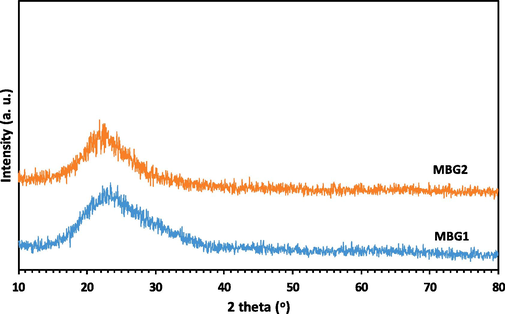
Wide-angle XRD patterns for powders MBG1 and MBG2.
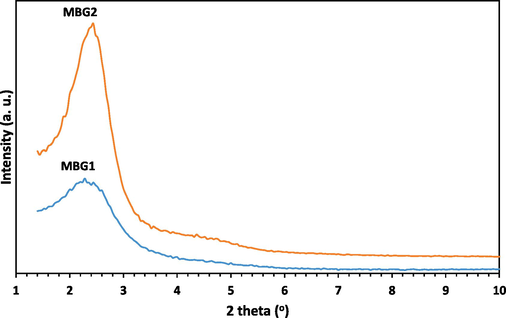
Low-angle XRD patterns for powders MBG1 and MBG2.
Fig. 3 depicts the morphology and particle size distribution of powders MBG1 and MBG2 which were obtained by SEM and TEM analyses. All particles exhibit a spherical morphology with no obvious aggregation. Powder MBG1 shows an average particle size of about 30 nm (Fig. 3a, 3c, and 3e), while powder MBG2 has an average particle size of about 20 nm (Fig. 3b, 3d, and 3f).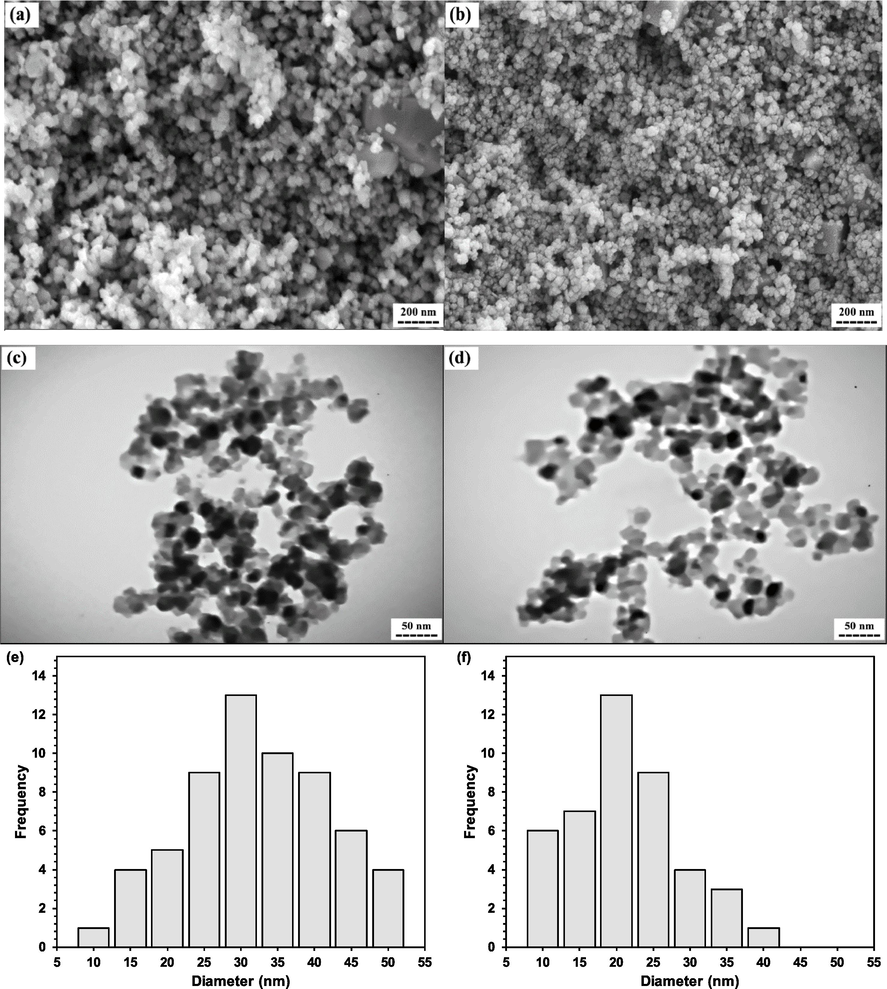
a, and b) sem images, c, and d) tem images, and e, and f) corresponding particle size distribution of synthesized powders mbg1, and mbg2, respectively.
In addition, N2 adsorption–desorption isotherms of both powders, as well as related quantitative data, are presented in Fig. 4 and Table 2, respectively. The results of BET analysis show that the specific surface area, total pore volume, and average pore diameter for powders MBG1 and MBG2 are nearly similar (Table 2). Also, the findings demonstrate that both powders are mesoporous and have a high specific surface area. The obtained specific surface area values are the median of the findings provided by Wang et al. (Wang et al., 2019) for hollow mesoporous BG nanoparticles under CTAB concentration of 4 mM with a SBET = ∼ 592 m2g−1 and hollow mesoporous BG nanoparticles under CTAB concentration of 6 mM with a SBET = ∼ 795 m2g−1.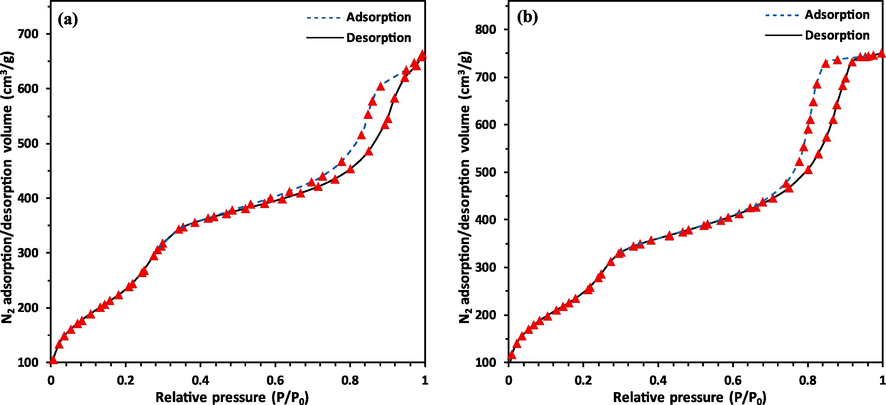
N2 adsorption–desorption isotherms for powders, a) MBG1, and b) MBG2.
Powder
Specific surface area (m2/g)
Average pore diameter (nm)
Total pore volume (cm3/g)
MBG1
620
4.96
1.02
MBG2
710
4.08
1.16
3.2 Biodegradability and bioactivity evaluation of the synthesized powders
By interacting bioceramics with the physiological fluid, the apatite layer binds to the surface of the fabricated bioceramic substance. The ability of the apatite layer formation is therefore investigated for a bioceramic substance in order to forecast its bone formation and its capacity for regeneration (Shoaib, et al., 2021). In this study, the presence of a crystalline HA layer that can be formed on the surface of specimens after immersing in SBF was first investigated using the XRD method. Fig. 5 displays the XRD patterns obtained from the surface of the disks made of synthesized powder MBG1 (Fig. 5a) and powder MBG2 (Fig. 5b) before and after immersion in the SBF solution. Before immersing the disks in SBF, a broad peak in the range of 15 to 35° has appeared, which is related to the Si-O-Si network of amorphous silica. After being immersed in SBF for 14 days, the XRD pattern of the disk made of powder MBG2 (Fig. 5b) revealed some strong peaks of HA structure (ICDD PDF no. 00–009-0432) in comparison with its counterpart made of powder MBG1. The appearance of these peaks confirmed the formation of HA after 14 days on the surfaces of both samples. As increasing immersion period from 14 to 28 days, the intensity of the diffraction peaks in both patterns increased. Fig. 5 clearly depicts the development of crystalline HA on the surfaces of both disks after immersion in SBF for 28 days. However, by adding Zr and Mg ions into the composition of powder MBG2, the XRD peaks belonging to the formed HA became sharper than those for powder MBG1. In the study conducted by Yadav et al. (Yadav, 2017), by immersing the ZrO2 doped BGs into SBF, the diffraction patterns of all BGs showed the hydroxycarbonate apatite crystalline phases, which confirms the formation of the HA layer. They showed that with increasing ZrO2 content, the peaks that belong to HA were getting sharper and the number of peaks became more. In another study on 58S BG containing 0, 5, and 10 mol.% ZrO2, by increasing the amount of Zr up to 5 mol.%, the intensity of the diffraction peaks of HA increased as the immersion period elongated (Moghanian et al., 2020). Moghanian et al. (Moghanian et al., 2018), and Tabia et al. (Tabia et al., 2019) stated that the formation of HA particles was delayed until the 7th day of immersion in SBF, but according to the results of Moghanian et al. (Moghanian et al., 2021), the simultaneous presence of Mg and Zr in the composition promotes the formation of primary HA particles.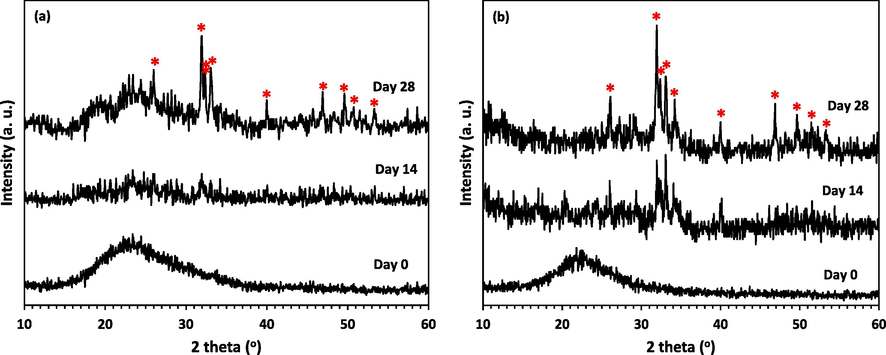
XRD patterns of the disk surface, a) MBG1, and b) MBG2 after immersing in SBF for different days (*=HA).
Moreover, to study the morphology of the formed apatite layer, the surfaces of immersing disks in SBF after drying were investigated by SEM. Fig. 6 shows the SEM micrographs of the surfaces of samples MBG1 and MBG2 before and after the bioactivity assay in SBF for 7, 14, 21, and 28 days. After 7 days of immersion, nucleation, and growth of new crystals were observed in some parts of the surfaces of both disks. After 14 days, the surface of the samples was more covered by irregular particles. XRD analyses proved that the apatite layer formed after 14 days of immersion in SBF. The creation of an HA layer on the surface of Zr-BGs after 7 days immersed in SBF solution has also been demonstrated by previous research (Moghanian et al., 2021). As the immersion period increased, more HA particles were visible than on day 14 of the experiment. The continuation of this process until the 28th day is accompanied by the observation of a dense coating and thicker apatite layers. These results confirm the mineralization of the surface of samples MBG1 and MBG2 and consequently their bioactivity.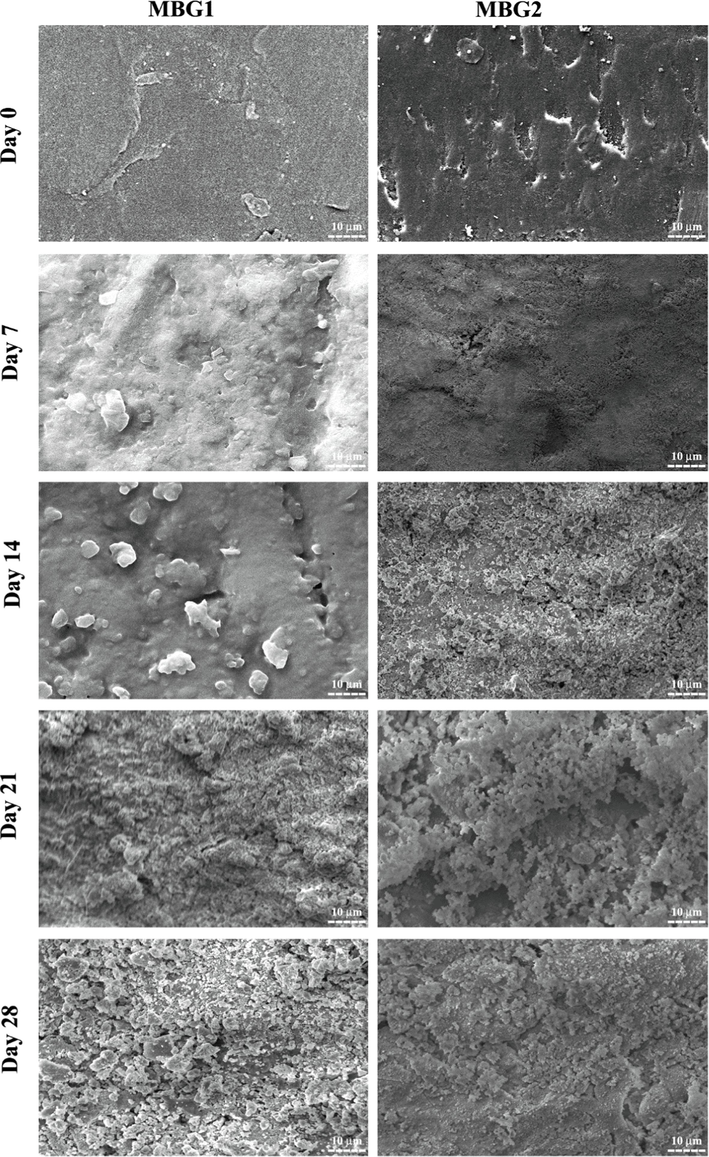
SEM images of the disk surface of powders MBG1 and MBG2 before and after immersing in SBF for different days.
The rate of HA formation and morphological changes of the samples depend on the chemical composition, surface topography, and immersion time. It seems that during 28 days of immersion, sample MBG2 had a faster rate of HA formation than sample MBG1, which is believed to be due to the presence of Mg and Zr ions in it. The findings of the current study are consistent with previous studies in which, with the increase of Zr in the samples, a crystalline phase with a uniform shape and a structure similar to HA was created during 15 days of immersion in SBF (Yadav, 2017). On the other hand, the study of Moghanian et al. (Moghanian et al., 2021) claimed that the presence of Mg did not slow down the formation of HA, which is contrary to the study conducted by Tabia et al. (Tabia et al., 2019). However, in the present study, due to the simultaneous integration of Mg and Zr, it is not possible to compare the effect of Mg or Zr alone on the rate of HA formation. However, according to the obtained results, it can be said that the simultaneous integration of Mg and Zr has increased the rate of HA formation.
In addition, it must be noted that mesoporous BG nanoparticles can degrade over time in physiological fluids (Zheng, 2020; Wang et al., 2019). According to the type and concentration of ions released by these materials during decomposition, certain cellular activities are induced (Zheng, et al., 2020; Hoppe et al., 2011). In this study, the dissolution of Si, Ca, P, Zr, and Mg ions was investigated. Fig. 7 shows the cumulative ion release of samples MBG1 and MBG2 after immersing in SBF for 28 days. As can be seen in Fig. 7a, changes in Ca ion concentration show an upward trend and then a downward trend. MBG1 appears to release a higher concentration of Ca ions compared to MBG2, which is consistent with the fact that the Ca concentration is reduced due to the presence of Mg and Zr in MBG2. Also, the changes in P concentration have been decreasing in the entire period (Fig. 7b). The reason for the decrease in the concentration of Ca and P ions can be attributed to the formation of the HA layer, which is also confirmed by the XRD and SEM results (Figs. 5 and 6). The concentration of Si, Mg, and Zr ions is presented in Fig. 5c to 5e has an increasing trend, which can be justified considering that they don’t participate in the formation of hydroxyapatite. It is understood that MBG2 can release Mg and Zr ions according to their concentration in its chemical composition. The increasing and then decreasing trend of Ca and the decreasing trend of P in other studies are almost consistent with the results obtained in the present work. Although in the study of Nawaz et al. (Nawaz, 2018), P had an increasing trend, in the present study and another research (Moghanian et al., 2021), it had a decreasing trend, which can be attributed to the formation of the HA layer. On the other hand, in another study (Moghanian et al., 2021), Si increased at first and then decreased, but in the present study, the trend is increasing and consistent with Nawaz's study (Moghanian et al., 2021,; Nawaz, 2018). As the immersing time increased, the concentration of Mg and Zr ions in the solution containing MBG2 increased, but Zr showed a slower dissolution rate than Mg. The concentration of Mg and Zr ions had an upward trend similar to other studies (Moghanian et al., 2020; Moghanian et al., 2021; Moghanian et al., 2018). The particles' bioactivity is enhanced by the release of biologically active ions. According to the Si and Ca ion release from MBG1 and MBG2, which probably originates from their high pore content and small particle size, it can be said that these glasses are degradable and can be used for bone healing and regeneration (Nawaz, 2018; Hench, 2006; Jones, 2015). Also, adjusting the bioactive glass chemistry causes a controlled release of various ions like Mn, Si, Ca, and P. Furthermore, the nucleation, growth, and crystallization of the apatite layer are significantly influenced by the textural characteristics, particularly the microstructure of the BGs. The mesoporous structures created in MBG1 and MBG2 and the co-doping of MBG2 with Mg and Zr ions enhanced the microstructural characteristics, resulting in a higher specific surface area for MBG1 and MBG2.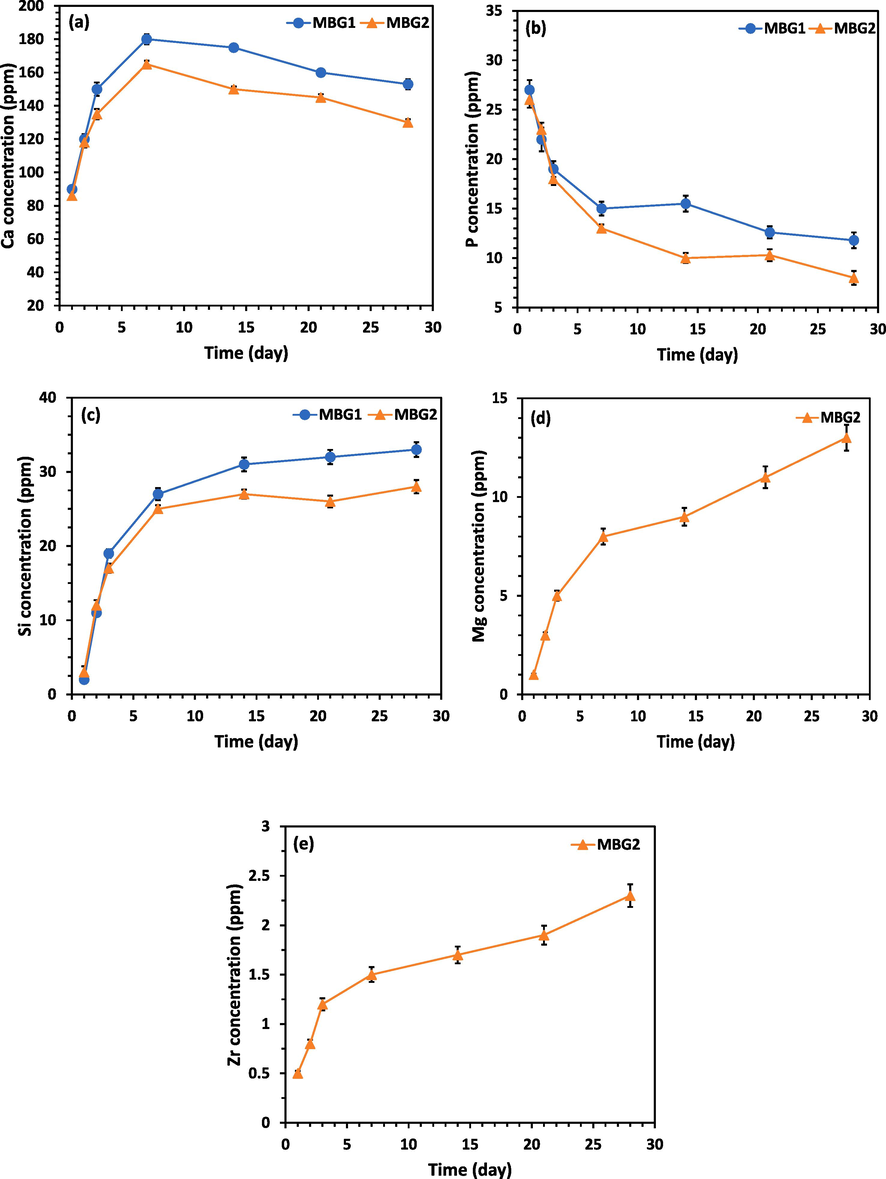
a) ca, b) p, c) si, d) mg, and e) zr concentrations released from samples mbg1, and mbg2 in sbf solutions for various periods.
Moreover, as a result of the dissolution of the ions in samples MBG1 and MBG2, when they are placed in biological environments, the pH changes, which is effective in cell growth and bone integration ability. Fig. 8a shows the pH changes after soaking in PBS. The change of pH in both samples is upward with a high slope in the first days, and then this trend continues with a gentle slope. This outcome is consistent with what Zhu et al. found (Zhu et al., 2011). It seems that the initial upward slope is due to the release of Ca2+ and Na+ ions in samples MBG1 and MBG2. According to Fig. 8a, it can be said that the pH values of PBS solution containing MBG2 after 28 days of soaking are higher than those for MBG1. The higher pHs in MBG2 can be explained by the fact that BG containing Mg (i.e. MBG2) is more susceptible to dissolution and they show more pH change than Mg-free BG (i.e. MBG1) (Vallet-Regí et al., 2007). This pH change is related to the amount of Mg in the BG composition (Soulié et al., 2009). As Tabia et al. (Tabia et al., 2019) also stated, this issue can be justified by the fact that glasses containing more Mg have a higher specific surface than glasses without Mg, which causes more ion exchange between the surface of the glass and PBS solution. This was explained by the fact that voids between particles are created because Mg2+ has a smaller radius (0.72 Å vs. 1.00 Å, respectively) than Ca2+. In addition, the presence of Zr in MBG2 can be mentioned as another reason for the higher pH of related PBS. Probably, Zr can increase pH values for two reasons. First by the alkalinity of Zr and then by increasing the dissolution rate of Ca2+ and Na+ ions from the surface of the sample, which is in accordance with the previous findings (Moghanian et al., 2020; Yadav, 2017). After a few days, the slope of the curves becomes gentle. With the interpretation that in the first stage, the release of Ca2+ and Na+ ions from the surface of the samples increases the pH, then following the replacement of H+ ions with cations, the concentration of OH in the solution increases. This leads to an attack on the silica network and the formation of silanol and a decrease in pH. On the other hand, the formation of HA decreases the pH. Come out of the three things mentioned, it could have caused the reduction of the upward slope after a few days in Fig. 8a.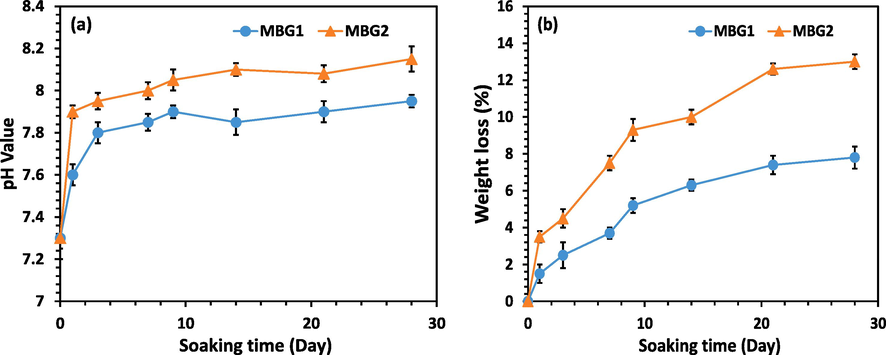
a) ph variations, and b) weight loss during the biodegradation tests of samples MBG1, and MBG2 in PBS.
In Fig. 8b, the weight loss percentage of samples MBG1 and MBG2 is shown. Accordingly, it is inferred that after soaking the sample MBG2 in PBS, a greater percentage of weight loss was seen compared to sample MBG1. This further weight loss is related to the release of Ca2+ and Na+ ions in PBS and the increase in pH. Based on studies, by increasing the amount of Zr, the rate of dissolution in glasses should increase (Yadav, 2017). Also, BGs containing more Mg are prone to dissolution (Vallet-Regí et al., 2007). Therefore, sample MBG2 containing Zr and Mg showed a higher percentage of weight loss than sample MBG1. Similar results have been observed in weight loss percentage in a SiO2, Na2O, CaO, K2O, P2O5, and MgO glass containing 2.0 wt% Zr (Yadav, 2017).
One of the important requirements before the development of any substance that is considered for use in the human body is to evaluate the in vitro cytotoxicity of that substance. Therefore, in this work powders MBG1 and MBG2 were examined in terms of cell compatibility using the MTT method. Fig. 9 shows the results of the viability of MG-63 cells for powders MBG1 and MBG2 during 1, 3, and 5 days. After cultivation for 1, 3, and 5 days, there was a statistically significant difference in increasing cell viability on MBG1 and MBG2 compared to the control. This difference indicated the absence of any leachable toxic substances in the samples (*p < 0.05, and **p < 0.01). According to Fig. 9, cell viability values considerably increase when the culture period is increased for each sample. On all days, MBG2 has more cell viability and less toxicity than MBG1. As a result, it can be concluded that MBG2 shows optimal cell viability. Probably, the addition of Mg and Zr ions in MBG2 caused cell proliferation. Therefore, it is confirmed that the synthesized MBG1 and the addition of Zr and Mg in it (MBG2) not only did not have any toxicity but also increased cell growth. According to studies, cell proliferation, and differentiation have increased with the lengthening of the culture period, and the addition of Mg has been shown to improve the performance of BGs containing Zr ions when the optimal Zr amount (5 mol.%) is used (Moghanian et al., 2021). Another study (Moghanian et al., 2020) reported that the viability of BGs containing Zr increased with the time after the BGs were cultured with osteoblast-like cell lines for 0, 7, and 14 days. Synthesized BGs containing Mg with and without drug were also considered safe and effective biomaterials for in vivo applications by Shoaiba et al. (Shoaib et al., 2021) because they did not cause significant cell death.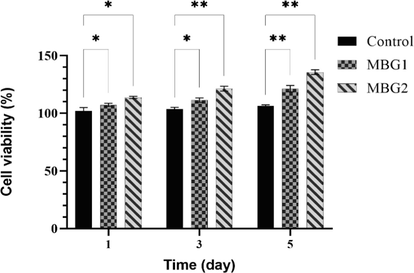
Viability results after exposure of powders MBG1 and MBG2 with MG-63 cells for 1, 3, and 5 days (*p < 0.05 and **p < 0.01).
Also, fluorescent staining of actin filaments in the cytoskeleton and nucleus after 24 h of culture was done by phalloidin and DAPI, respectively. As seen in Fig. 10, the actin filaments in the cytoskeleton are represented by the red color in both samples and the cell nucleus by the blue color. According to the obtained results, the viability of MG-63 cells was confirmed on MBG1 and MBG2. Therefore, MBG1 and MBG2 have biocompatibility and can increase MG-63 cell proliferation.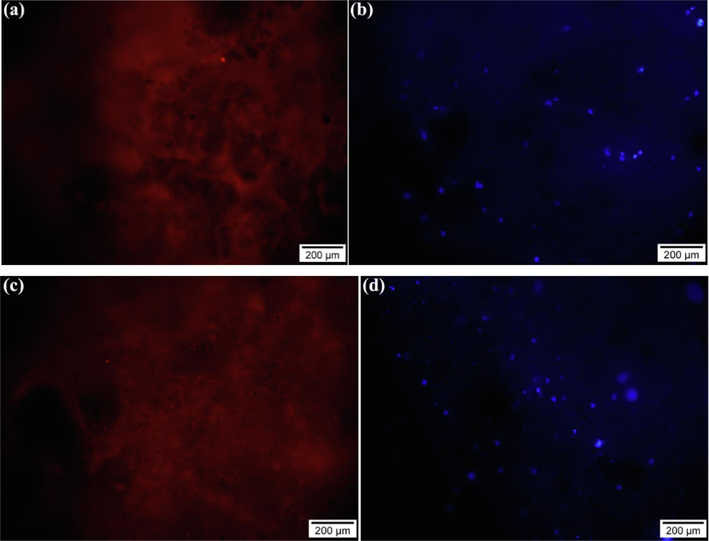
a, and c) red and b, and d) blue colors showing the fluorescent staining of the actin filaments (phalloidin) and nucleus (dapi) after 24 h of MG-63 cells culture on the surface of samples MBG1 and MBG2, respectively.
Finally, SEM analysis was applied to investigate the MG-63 cell morphology and adhesion on the surface of samples MBG1 and MBG2. As seen in Fig. 11, after 24 h of cultivation, MG-63 cells were seen in close contact with the surface of both samples. Also, the cells were not in a homogeneous layer, but appeared as single cells, which is like the results of Ahmadi et al. (Ahmadi et al., 2022; Rezaei et al., 2014), and Rezaei et al. (Ahmadi et al., 2022; Rezaei et al., 2014). There was no discernible distinction between the SEM results of MBG1 and MBG2 after 24 h of cultivation. It can be said that both samples showed good biocompatibility so that the cells adhered to the surface and multiplied. These results are consistent with the cell viability test.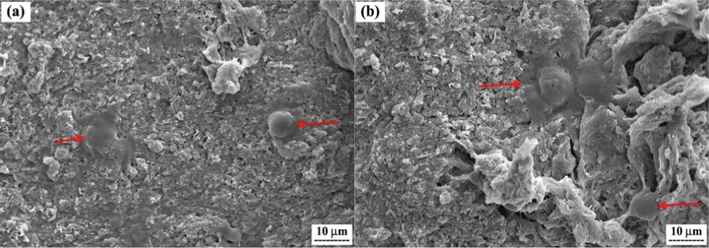
SEM images showing the attachment of MG-63 cells on the surfaces of samples, a) MBG1, and (b) MBG2 after culturing for 24 h.
4 Conclusion
In the present work, the modified sol–gel derived mesoporous 45S5 BG nanoparticles (MBG2) containing 5 mol.% Mg, and 5 mol.% Zr as well as standard mesoporous 45S5 BG nanoparticles (MBG1) were successfully synthesized. The chemical composition, spherical morphology, nanoscale particle size, amorphous, and mesoporous structure of the synthesized powders MBG1 and MBG2 were confirmed by XRF, SEM, XRD, and BET, respectively. Although the addition of Zr and Mg ions to MBG2 instead of Ca ions did not change the morphology, particle size, and textural properties of MBG2, it had remarkable effects on the physicochemical and biological characteristics of powder MBG2. The ability of disk-shaped specimens made of both calcined powders at 670 °C after 28 days of immersion in SBF under in vivo conditions to form HA was confirmed by the results of pH trend, SEM, and XRD analysis. The faster and more sustained HA formation in MBG2 can be attributed to the presence of Zr and Mg ions in the composition of this powder. It is possible to say that the substitution of these ions in powder MBG1 impacts apatite nucleation and growth in addition to enhancing bone-forming capacity. The disks made from powder MBG2 also had the maximum weight loss (2.7 %) after 28 days of immersion in SBF. The results of the ICP tests revealed that MBG2 was more soluble and dissolved quickly due to Mg and Zr incorporation into the glass network. Additionally, the cell viability data demonstrated that the presence of Zr and Mg in powder MBG2 helped the MG-63 cells proliferate. Therefore, powder MBG2 containing 5 mol.% Zr and 5 mol.% Mg ions due to the synergistic effect of both ions through in vitro evaluations, as a reliable, safe, and effective material for future in vivo applications to achieve multifunctional BGs for tissue engineering were introduced.
Acknowledgments
The authors would like to thank the University of Isfahan for making all the necessary resources available for this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis, characterization, and bioactivity evaluation of biphasic calcium phosphate nanopowder containing 5.0 mol% strontium, 0.6 mol% magnesium, and 0.2 mol% silicon for bone regeneration. J. Mater. Res.. 2022;37(11):1916-1928.
- [CrossRef] [Google Scholar]
- Bioactive glass 45S5 powders: effect of synthesis route and resultant surface chemistry and crystallinity on protein adsorption from human plasma. Biointerphases. 2012;7(1):41.
- [Google Scholar]
- Development of mesoporous borosilicate bioactive glass nanoparticles containing Mg2+ and Zn2+: biocompatibility, bioactivity and antibacterial activity. J. Non Cryst. Solids. 2022;594:121819
- [CrossRef] [Google Scholar]
- In vitro response of human osteoblasts to multi-step sol–gel derived bioactive glass nanoparticles for bone tissue engineering. Mater. Sci. Eng. C. 2014;36:206-214.
- [CrossRef] [Google Scholar]
- A new sol–gel synthesis of 45S5 bioactive glass using an organic acid as catalyst. Mater. Sci. Eng. C. 2015;47:407-412.
- [CrossRef] [Google Scholar]
- E. Fiume, C. Migneco, E. Verné, and F. Baino, “Comparison between Bioactive Sol-Gel and Melt-Derived Glasses/Glass-Ceramics Based on the Multicomponent SiO2–P2O5–CaO–MgO–Na2O–K2O System,” Materials, vol. 13, no. 3, doi: 10.3390/ma13030540.
- Formation of hydroxyapatite on surface of SiO2– P2O5–CaO–SrO–ZnO bioactive glass synthesized through sol-gel route. Ceram. Int.. 2019;45(15):19323-19330.
- [CrossRef] [Google Scholar]
- The story of Bioglass®. J. Mater. Sci. - Mater. Med.. 2006;17(11):967-978.
- [CrossRef] [Google Scholar]
- Studies on preparation and characterization of 45S5 bioactive glass doped with (TiO2+ ZrO2) as bioactive ceramic material. Bioceram. Dev. Appl.. 2016;6(2):pp.
- [Google Scholar]
- A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757-2774.
- [CrossRef] [Google Scholar]
- Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater.. 2015;23:S53-S82.
- [CrossRef] [Google Scholar]
- S. Kargozar et al., “Osteogenic Potential of Magnesium (Mg)-Doped Multicomponent Bioactive Glass: In Vitro and In Vivo Animal Studies,” Materials, vol. 15, no. 1, doi: 10.3390/ma15010318.
- Unravelling the impact of calcium content on the bioactivity of sol–gel-derived bioactive glass nanoparticles. ACS Appl. Bio Mater.. 2020;3(2):1312-1320.
- [CrossRef] [Google Scholar]
- A. Kumar, S. Murugavel, A. Aditya, and A. R. Boccaccini, “Mesoporous 45S5 bioactive glass: synthesis, in vitro dissolution and biomineralization behavior,” Journal of Materials Chemistry B, 10.1039/C7TB01738C vol. 5, no. 44, pp. 8786-8798, 2017, doi: 10.1039/C7TB01738C.
- Fabrication and characterization of ZrO2 incorporated SiO2–CaO–P2O5 bioactive glass scaffolds. J. Mech. Behav. Biomed. Mater.. 2020;109:103854
- [CrossRef] [Google Scholar]
- A facile synthesis of novel mesoporous bioactive glass nanoparticles with various morphologies and tunable mesostructure by sacrificial liquid template method. Mater. Lett.. 2015;148:45-49.
- [CrossRef] [Google Scholar]
- A. Lukowiak, J. Lao, J. Lacroix, and J.-M. Nedelec, “Bioactive glass nanoparticles obtained through sol–gel chemistry,” Chemical Communications, 10.1039/C3CC00003F vol. 49, no. 59, pp. 6620-6622, 2013, doi: 10.1039/C3CC00003F.
- J. Massera, “Bioactive glass in tissue engineering: progress and challenges,” 2016.
- The effect of magnesium content on in vitro bioactivity, biological behavior and antibacterial activity of sol–gel derived 58S bioactive glass. Ceram. Int.. 2018;44(8):9422-9432.
- [CrossRef] [Google Scholar]
- The effect of zirconium content on in vitro bioactivity, biological behavior and antibacterial activity of sol-gel derived 58S bioactive glass. J. Non Cryst. Solids. 2020;546:120262
- [CrossRef] [Google Scholar]
- Sol-gel derived silicate-based bioactive glass: Studies of synergetic effect of zirconium and magnesium on structural and biological characteristics. J. Non Cryst. Solids. 2021;554:120613
- [CrossRef] [Google Scholar]
- Rice husk derived bioactive glass-ceramic as a functional bioceramic: Synthesis, characterization and biological testing. J. Non Cryst. Solids. 2015;427:54-61.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of manganese containing mesoporous bioactive glass nanoparticles for biomedical applications. J. Mater. Sci. - Mater. Med.. 2018;29(5):64.
- [CrossRef] [Google Scholar]
- Sol–gel synthesis of bioactive glass-ceramic 45S5 and its in vitro dissolution and mineralization behavior. J. Am. Ceram. Soc.. 2013;96(5):1643-1650.
- [CrossRef] [Google Scholar]
- Antibacterial efficiency of Zn, Mg and Sr doped bioactive glass for bone tissue engineering. J. Nanosci. Nanotechnol.. 2020;20(4):2465-2472.
- [Google Scholar]
- Synthesis, characterization, and in vitro bioactivity of sol-gel-derived SiO2–CaO–P2O5–MgO-SrO bioactive glass. Synth. React. Inorg., Met.-Org., Nano-Met. Chem.. 2014;44(5):692-701.
- [CrossRef] [Google Scholar]
- Synthesis and characteristics of sol-gel bioactive SiO2-P2O5-CaO-Ag2O glasses. J. Non Cryst. Solids. 2017;476:108-113.
- [CrossRef] [Google Scholar]
- M. Shoaib et al., “Magnesium doped mesoporous bioactive glass nanoparticles: A promising material for apatite formation and mitomycin c delivery to the MG-63 cancer cells,” 02/03 2021.
- Bioglass® and resulting crystalline materials synthesized via an acetic acid-assisted sol–gel route. J. Sol-Gel Sci. Technol.. 2017;83(1):165-173.
- [CrossRef] [Google Scholar]
- Sol-gel synthesis and characterization of SiO2-CaO-P2O5-SrO bioactive glass. In vitro study. Key Eng. Mater.. 2015;631:30-35.
- [CrossRef] [Google Scholar]
- J. Soulié, J. M. Nedelec, and E. Jallot, “Influence of Mg doping on the early steps of physico-chemical reactivity of sol–gel derived bioactive glasses in biological medium,” Physical Chemistry Chemical Physics, 10.1039/B913771H vol. 11, no. 44, pp. 10473-10483, 2009, doi: 10.1039/B913771H.
- Evaluation of colloidal and polymeric routes in sol-gel synthesis of a bioactive glass-ceramic derived from 45S5 bioglass. Ceram. Int.. 2020;46(12):20264-20271.
- [CrossRef] [Google Scholar]
- Mesoporous bioactive glass nanoparticles doped with magnesium: drug delivery and acellular in vitro bioactivity. RSC Adv.. 2019;9(22):12232-12246.
- [Google Scholar]
- Sol–gel synthesis and in vitro bioactivity of glass–ceramics in SiO2–CaO–Na2O–P2O5 system. J. Sol-Gel Sci. Technol.. 2016;80(2):411-416.
- [CrossRef] [Google Scholar]
- Mesoporous materials for drug delivery. Angew. Chem. Int. Ed.. 2007;46(40):7548-7558.
- [Google Scholar]
- C. Vichery and J.-M. Nedelec, “Bioactive Glass Nanoparticles: From Synthesis to Materials Design for Biomedical Applications,” Materials, vol. 9, no. 4, doi: 10.3390/ma9040288.
- A mini review on carbon quantum dots: preparation, properties, and electrocatalytic application. Front. Chem.. 2019;7:671.
- [Google Scholar]
- The preparation of hollow mesoporous bioglass nanoparticles with excellent drug delivery capacity for bone tissue regeneration (in English) Front. Chem. Original Res.. 2019;7
- [CrossRef] [Google Scholar]
- Development of zirconia substituted 1393 bioactive glass for orthopaedic application. Orient. J. Chem.. 2017;33(6):2720.
- [Google Scholar]
- Antioxidant mesoporous Ce-doped bioactive glass nanoparticles with anti-inflammatory and pro-osteogenic activities. Mater. Today Bio. 2020;5:100041
- [CrossRef] [Google Scholar]
- K. Zheng et al., “Incorporation of Boron in Mesoporous Bioactive Glass Nanoparticles Reduces Inflammatory Response and Delays Osteogenic Differentiation,” Particle & Particle Systems Characterization, https://doi.org/10.1002/ppsc.202000054 vol. 37, no. 7, p. 2000054, 2020/07/01 2020, doi: https://doi.org/10.1002/ppsc.202000054.
- Y. Zhu, X. Li, J. Yang, S. Wang, H. Gao, and N. Hanagata, “Composition–structure–property relationships of the CaO–MxOy–SiO2–P2O5 (M = Zr, Mg, Sr) mesoporous bioactive glass (MBG) scaffolds,” Journal of Materials Chemistry, 10.1039/C1JM10838G vol. 21, no. 25, pp. 9208-9218, 2011, doi: 10.1039/C1JM10838G.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105374.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







