Translate this page into:
Spherical NiWO4-reduced graphene oxide nanocomposite for effective visible light driven photocatalytic activity for the decolourisation of organic pollutants
⁎Corresponding authors. bsathish401@gmail.com (Sathish Mohan Botsa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A hybrid nanocomposite of nickel tungstate – reduced graphene oxide (NiWO4-RGO) was prepared using a surfactant free hydrothermal approach. Resulting hybrid nanocomposite powder was grounded for forty minutes to ensure homogeneity and avoid agglomerations of particles. Resulting nanocomposite was characterised by XRD, FTIR, FESEM and EDX analysis. Photocatalytic activities of the characterised catalyst were examined by the decolourisation of Methylene blue (MB) and ortho-nitrophenol (ONP) under visible light irradiation were conducted at room temperature. The results confirmed that 15 wt% NiWO4-RGO composite was degraded almost completely MB (95%) and ONP (82%) within 150 and 240 min respectively under visible light sources. The catalyst was reused and stable for successive six runs with loss of 10% of degradation rate.
Keywords
Hydrothermal
NiWO4-RGO
Photocatalysis
Methylene blue
Ortho nitrophenol
1 Introduction
During few decades, human beings are facing huge challenges in energy calamity and environmental preservation. Due to the use of water combined with different types of dyes as the promising material in various industries, the pollution is getting more environmental problems mainly effect the aquatic life (Chala et al., 2014). Therefore, scientists are admiring to the contraption of new sustainable energy storage methods, among which electrochemical splitting of water with the help of semiconductor photocatalyst is the promising method to remove toxic pollutants from industrial effluents as the degradation method could mineralise organic pollutants completely to CO2, H2O and less and/or non toxic compounds without generating any secondary pollutions. In addition, the operating process does not require high energy consumption (Chatterjee and Dasgupt, 2005). In recent years, vast research has been carried out to explore the novel properties of metal oxide semiconductors, mostly advantageous in solar cells, photovoltaic devices Sunlight-driven photocatalysts such as TiO2 (Thiruvenkatachari, 2008), doped TiO2 (Moradi et al., 2018), Cu2O (Muthukumaran and Gnanamoorthy, 2020), Fe2O3 (Sivaranjani et al., 2019), ZnO (Chen et al., 2017), SnO2 (Prakash et al., 2016) etc. are extensively used. After that, instead of metal oxide photocatalyst, mixed metal oxide nano semiconductors BiFeO3 (Mushtaq et al., 2018), LaFeO3 (Hu et al., 2012), SrTiO3 (Konstas et al., 2018), BiVO4 (Zhou et al., 2011), Fe2Mo3O4 (Suresh et al., 2014), Bi2Mo3O4 (Changsheng et al., 2013), CuWO4 (Jagadeesh Babu et al., 2020), Bi2WO6 (Deepthi et al., 2018), ZnWO4 (Zhang et al., 2010), CuBi2O4 (Paliki et al., 2018) etc. are used as a photocatalyst.
Tungstates has received great attention by researchers due to their wide range applications in sensing applications for Hg, humidity and photocatalyst, photoelectrochemical because of their unique properties (Jagadeesh Babu et al., 2020; Eranjaneya et al., 2019). They reported that NiWO4 were successfully synthesized by decomposition approach, hydrothermal method (Hosseini et al., 2018), electrochemical method (Pourmortazavi et al., 2018), and molten salts route (AlShehri et al., 2017). Hydrothermal route has attracted more interest towards the preparation of various nanomaterials or composites due to the ability of this route to form good, large crystal phase. However, NiWO4 has broad bandgap which struggles to export the electrons and resulting in less photocatalytic activity (Jagadeesh Babu et al., 2020).
Presently, there has been more delight in fabricating and utilizing novel graphene oxide (GO) containing metal tungstate nanocomposites for environmental remediation by the degradation or elimination of hazardous organic contaminants and heavy metals (Botsa and Basavaiah, 2019; Nair Abhinav and Jagadeesh Babu, 2017) Among the utilized nanomaterials in water treatment, GO having a honeycomb-like structure with its interesting 2D carbon framework, has more fascinate interest from the few decade for its unique specific surface area, high charge carrier mobility and electron conductivity (Sree et al., 2020; Mohan et al., 2018). We have deliberated the competence of the NiWO4-RGO composite as induced visible light active photocatalyst for the decolourisation of Methylene blue and Ortho nitrophenol organic pollutants. A probable photocatalytic degradation mechanism is proposed and the superlative photocatalytic efficacy is ascribed to the electron–hole pair separation was achieved by the combination of RGO with NiWO4.
2 Experimental details
2.1 Materials
Nickel nitrate (Ni(NO3)2·6H2O), sodium tungstate dihydrate (Na2WO4·2H2O) were purchased from Sigma Aldrich and Merck Co., were used without further purification for analysis. The selected dye stuffs were collected from Himedia Lab, India and Doubled distilled water from local supplier for the making solution.
2.2 Graphene oxide preparation
Graphene Oxide (GO) was synthesized from graphite powder according to the modified hummers method reported by Hummers and Offeman (Hummers and Offeman, 1958). In brief, 1.0 g of graphite powder and 0.5 g of NaNO3 were added into 23 ml of cooled concentrated H2SO4. Then, gradually added 3 g of KMnO4 with continuous stirring followed by cooling and temperature of the reaction mixture was maintained below 10 °C using an ice bath. Later, the ice bath was removed and the reaction mixture was further stirred for 30 min at 35 °C. The distilled water (46 ml) was added slowly to increase in temperature to 100 °C and it continues further 15 min. The reaction was terminated by the addition of distilled water (140 ml) followed by 30% H2O2 (10 ml) aqueous solution. The resultant solid yield was detached by centrifugation and washed repeatedly with 5% HCl solution until sulphate anion could not be detected with BaCl2. The resultant solid GO was dried in vacuum at 50 °C.
2.3 NiWO4-RGO composite preparation
For the preparation of NiWO4, the equimolar ratio of Ni(NO3)2 and Na2WO4 solutions were prepared with 50 ml milli Q water separately followed by stirring for 30 min to homogeneous nature. In addition five times higher concentration of NaOH solution was added to the Ni(NO3)2 solution and stirred for almost 30 min. Meanwhile, to above mixture suspension, Na2WO4 solution was added slowly drop wise and the blend was transferred into a Teflon-lined stainless-steel autoclave with sealed and employed in hot air stabilizer at heating of 170 °C for 18 h. After, the autoclave was cooled to get room temperature and the obtained product was centrifuged, washed with Milli Q water and ethanol successively. Utmost, the yield was dried in an oven during overnight to get the purest form of NiWO4. We have employed the same procedure for synthesizing the NWR composites by stoichiometry with GO loading to the NiWO4 to form 5%, 10% and 15% NiWO4-RGO composites. The advantage of this synthesis is the materials prepared without using any reducing agents like NaBH4, LiAlH4 and so on.
2.4 Characterisation
In order to study the properties of prepared NiWO4-RGO composite were characterized by various characterization techniques. Initially, X-ray powder diffraction (XRD) pattern of as-prepared nanoparticles samples were taken by a Bruker D8 Advance X-ray diffractometer with Cu-Kα radiation (1.5406 Å, X-ray tube voltage = 35 kV and current = 35 mA) with the scan range of 2θ from 0° to 90°. FESEM images of NiWO4-RGO samples were taken using the instrument FEI (Model-Nova Nano SEM 450) operating at 15 kV and the EDX was used to analyse the chemical composition of as prepared sample using BRUKER EDS detector (Model-XFlash 6/10). Fourier transform infrared spectrum of the NiWO4/RGO samples was recorded by FTIR spectrophotometer (IR prestige 21, Shimadzu) in the range 4000–400 cm−1, spectral output was recorded by transmittance as a function of wave number.
2.5 Photocatalytic activity
Photocatalytic efficiency of characterized NiWO4-RGO was evaluated by the degradation of Methylene Blue (MB) and ortho-nitrophenol (O-NP) in the light irradiation, 400 W metal halide lamp as a light source for irradiation. UV radiation below 350 nm is eliminated by surrounding the sample with a water jacket. 100 mg of the photocatalyst powder was added into 100 ml of the model dye pollutant solution and the suspension mixture was magnetically stirred for 30 min in a dark room to ensure adsorption-desorption thermal equilibrium between photocatalyst surface and dye molecule. The suspension mixture was further exposed to light emanating and 5 ml aliquots were pipette out at regular time intervals. 0.45 µm Millipore filters were utilized for filtration to separate the suspended catalyst. The progress of decolourisation of tested pollutant was examined by using UV–Visible absorption spectrum. Degradation rate of dye is calculated by the following equation (4).
3 Results and discussion
Fig. 1 depicts X-ray diffraction (XRD) Patterns of NiWO4 and Graphene oxide loaded – NiWO4 at various wt% i.e. 5%, 10% and 15% were synthesized by surfactant free hydrothermal method. From the Fig. 1, we can say that all peaks could be indexed to NiWO4 was confirmed with JCPDS card NO 15-0755. Absence of extra peaks or impurity peaks indicates that the sample obtained in phase pure. The strong peak was appeared at 10.6° confirmed the GO was produced. All diffraction peaks of NiWO4-RGO composites are presented as the NiWO4. The pure single crystalline phase was formed without other relevant peaks as the hydrothermal method is facile, easy to operate and enhance the crystal growth and purity of compound. The average crystalline sizes (S) of hydrothermally prepared composites were measured using Debey-Scherrer’s equation (5).
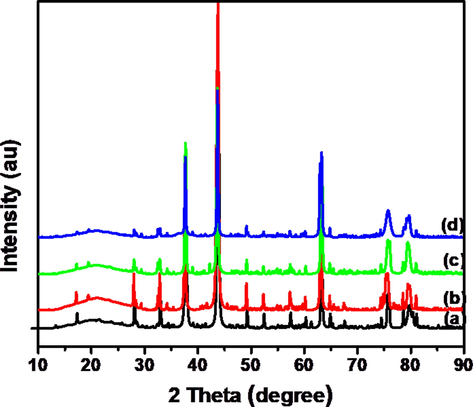
XRD patterns of hydrothermally prepared (a) NiWO4 (b) NiWO4-RGO (5%) (c) NiWO4-RGO (10%) and (d) NiWO4-RGO (15%) composites.
In order to get the information regarding surface morphology of synthesized nanocomposites, field emission scanning electron microscopy (FESEM) has been employed. Fig. 2 depicts the FESEM images of NiWO4 and 15 wt% of NiWO4-RGO composite at different magnifications. Although, the small variation in GO loading amount to obtained desirable composites they possess agglomeration due to overlay of tungstates on RGO layer and exhibited nanomicrospherical shape comprised of uniform sheets with their structure was greatly developed. From the Fig. 2a, it can be seen that pure NiWO4 consists of relatively uniform and smooth surface plate like/rod like microstructures with average length of 1.397 μm and diameter 140.6 nm combined with spherical nanoparticles. Whereas RGO loaded NiWO4 nanoparticles are changing their shapes to spherical structure with porous nature, due to agglomeration of NPs as shown in Fig. 2b. Some kind of uniformity can be seen in the structure of prepared composites.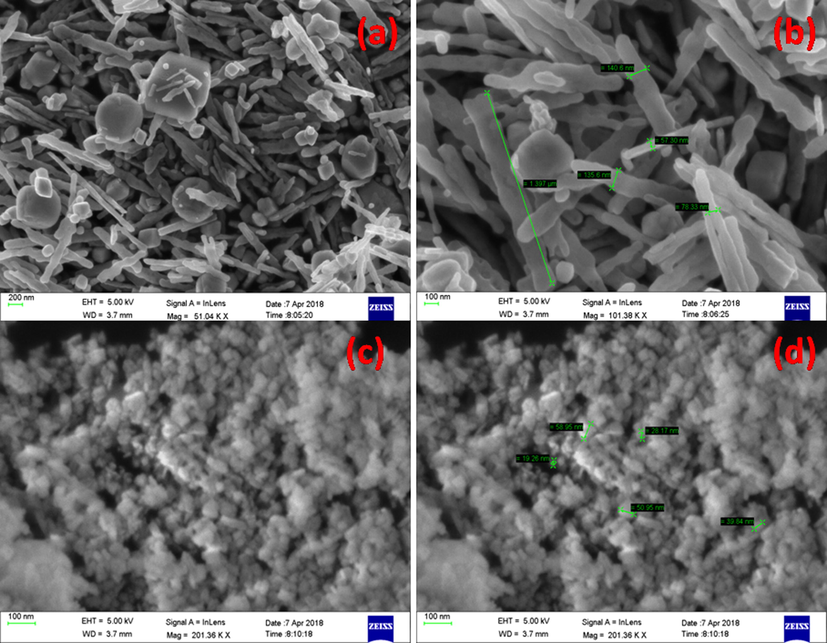
SEM images of pure NiWO4 (a-b) and 15 wt% of RGO loaded NiWO4 (c-d).
The energy dispersive X-ray analysis of hydrothermally synthesised NiWO4 and NiWO4-RGO nanocomposite were carried out is depicted in Fig. 3, in which the characteristic peaks corresponding to Ni (nickel), W (tungsten), C (carbon) and O (oxygen) are seen and there is no traces of precursors which can be concluded that the final products through hydrothermal method were obtained in pure form.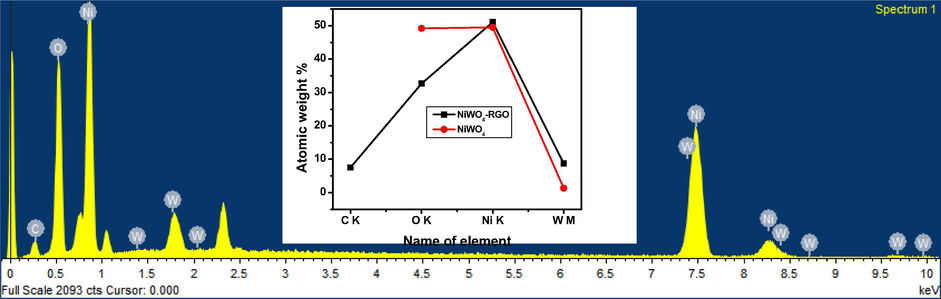
Energy Dispersive X-ray Analysis of pure NiWO4 (a) and NiWO4-RGO 15% (b).
Fig. 4 reveals that FTIR spectra for fabricated tungstates within the range 4000–400 cm−1. From Fig. 4a and 4b, we can conclude that the absorption bands at 3390–3429 cm−1 and 1560–1630 cm−1 indicates the existence of —OH stretching and bending vibrations related to water molecule absorption. The weak absorption bands appeared within the absorption range at 835–850 cm−1 can be evidenced to the stretching mode of W—O bonds in the joints with WO4 tetrahedral. The bands nearly observed at 930 cm−1 related to the stretching mode of the W⚌O bond (Mancheva et al., 2007). In Fig. 4b spectra exhibits the peaks nearly at 1160 cm−1 and 1550 cm−1 corresponding to the stretching frequencies of C—O—H and C⚌C respectively, which suggests that the GO has been effectively reduced during the process (Ossonon and B’elanger, 2017). All of results further confirmed that the synthesized crystalline materials are tungstate composites.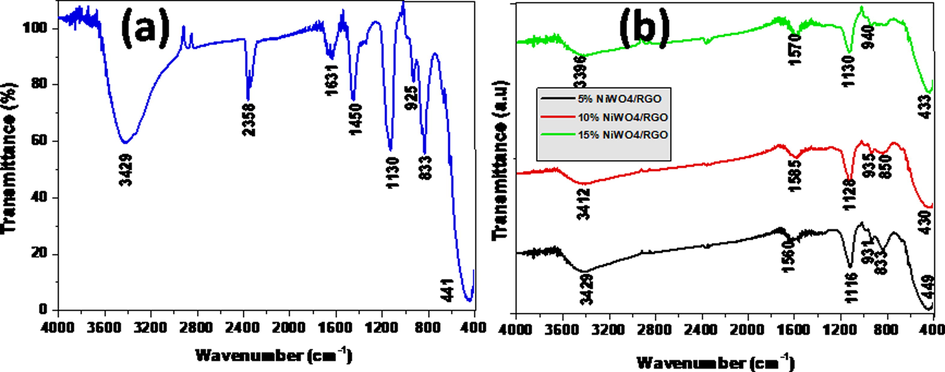
FTIR spectra of (a) NiWO4 and (b) NiWO4-RGO composites.
3.1 Photocatalytic performance
Discoloration of MB and O-NP were taken as model reactions to determine the photocatalytic efficiency of as synthesised tungstates. Temporal variations of spectral changes during photodegradation of MB as a function of irradiation time are shown in Fig. 5a. As expected, the NiWO4 was initially tested on MB dye solution, but it doesn’t show any significant degradation rate (31%). Remarkable degradation rate was noticed using the RGO based NiWO4 composites were studied on same dye at the conditions of 100 mg catalyst quantity and 100 ml of 10 mg/L dye solution at pH-10. RGO has assembled with metal tungstate found to be an excellent in photocatalytic efficiency for the decolourisation of dyes (Upadhyay et al., 2014) and found to be 63% degradation of MB dye using NiWO4-RGO (5%) (Fig S1 in ESI). The effect of RGO loading on activity of composites were studied and optimized threshold mass percent of RGO to attain greater photocatalytic performance (Sree et al., 2020). This leads to increase the GO dose in synthesis of NiWO4-RGO composite and their photocatalytic performance was mostly depends on the interface between NiWO4 and RGO which facilitates the charge separation of photo-electrons and photo-holes, finally decrease the recombination rate and 10% GO loaded NiWO4 composite exhibits better degradation percentage (81%) and NiWO4-RGO (15%) was the most effective for photocatalytic degradation of MB dye using stimulation of visible light (95%). The π-π stacking is formed between aromatic rings of RGO and dye pollutant assists the adsorption of dye pollutants on to the surface of catalyst hence increasing the quenching of dyes.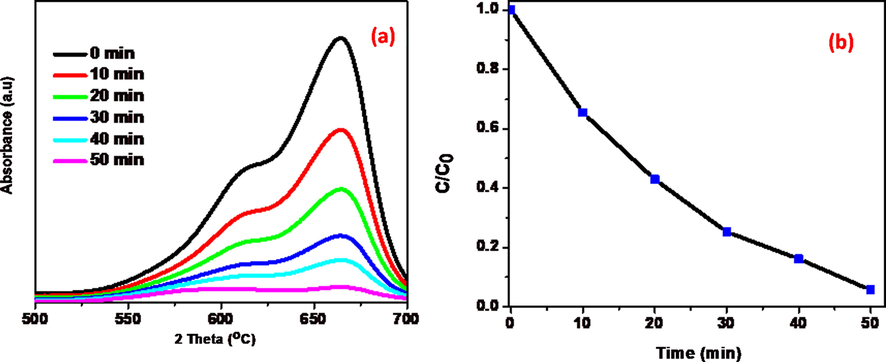
(a) Absorbance spectra of MB in presence of 15 wt% NiWO4-RGO nanocomposite. (b) Plot of C/C0 as a function of irradiation time.
From the Fig. 5a, it can be seen that as increasing visible light exposure on MB aqueous solution contains synthesised NiWO4-RGO nanocomposite decrease in absorption at 665 nm clearly signifies that the chromophoric structure of MB was decomposed completely almost and the hypochromic shift was attributed. Fig. 5b indicates the plots of C/C0 vs exposure of time for the MB aqueous solution. Based on the spectral data, it can be concluded that 95% of 10 ppm MB dye was degraded in presence of 100 mg of 15 wt% RGO loaded NiWO4 photocatalyst under visible light irradiation.
Significantly, the hydrothermal assisted synthesis of NiWO4-RGO composite exhibited better photocatalytic activity towards the MB degradation than similar class of tungstates which reported earlier as shown in table 1.
S. No
Catalyst Name
Catalyst dose (g)
Dye Conc. (ppm)
Light source (lamp)
Degradation rate (%)
Time (min)
Ref. No
1
NiWO4-ZnO-NRGO
0.02
10
250 W Hg
99.3
120
(Sadiq et al., 2017)
2
CaWO4
0.025
10
sunlight
98
180
(Farsi and Barzgari, 2015)
3
CuWO4-GO
0.05
10
Metal Halide
100
60
(Medidi et al., 2018)
4
CoWO4
CuWO4
ZnWO4
1
10
Hg lamp
40
70
95120
120
120(Montinia et al., 2010)
5
Bi2WO6
CdS
PVA assisted Bi2WO6-CdS2 × 2 cm3
20
500 W halogen
86
72
92100
100
100(Rajendran et al., 2018)
6
BaWO4-GO
0.05
10
400 W metal halide
100
90
(Sunitha et al., 2017)
7
Bi2WO6-GO
0.05
10
500 W Xe
88.1
120
(Zhou and Zhu, 2012)
8
WO3/CdWO4
0.1
10
500 W xenon
95
50
(Aslam et al., 2015)
9
SrWO4
1.15 × 10−5
54 W Philips
72
390
(Dirany et al., 2020)
10
Pr2(WO4)3
0.1
25
Hg
99.7
60
(Pourmortazavi and Nasrabadi, 2017)
11
NiWO4
50
10
Heber multi-lamp
100
50
(Saravanakumar et al., 2019)
12
NiWO4-RGO
0.05
10
400 W metal halide
98.5
50
Present study
Based on this experiment, further studies were conducted on photocatalytic degradation of O-NP aqueous solution using 15 wt% NiWO4-RGO composite. Fig. 6a depicts the temporal variation of spectral changes of 100 ml of 10 ppm O-NP solution with 100 mg of NiWO4-RGO nanocomposite. From the Fig. 6a, decrease in absorbance of O-NP as a function of exposure time in under 400 W metal halide lamp as visible light source. Fig. 6b indicates that the plots of C/C0 vs exposure of time for the O-NP solution. Based on the spectral data, it can be concluded that 82% of 10 ppm O-NP was degraded in presence of 100 mg of 15 wt% RGO loaded NiWO4 photocatalyst under visible light irradiation.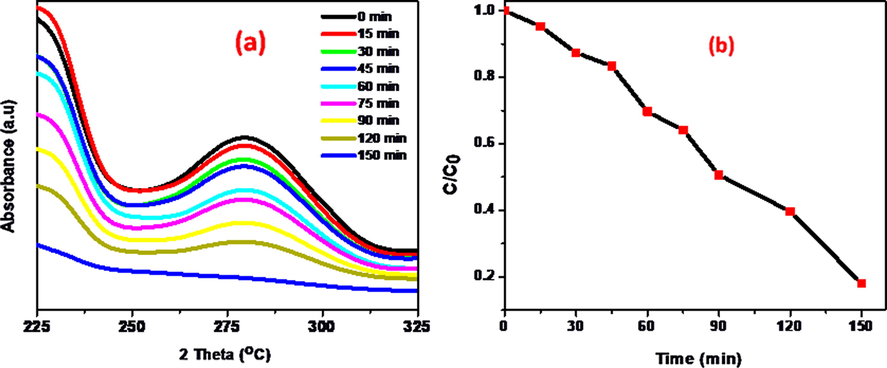
(a) Absorbance spectra of O-NP in presence of 15 wt% NiWO4-RGO nanocomposite and (b) Plot of C/C0 as a function of irradiation time.
The rate constants for degradation of MB and O-NP under visible light irradiation were examined with the help of the following equation (6).
From the linear plot of ln(C/Co) with irradiation time, the observed degradation rates (k) of MB and O-NP in presence of hydrothermally synthesised hybrid nanocomposite were determined to be 1.06 × 10−6 and 1.66 × 10−5 min−1, respectively.
Suggested photocatalytic degradation mechanism of MB and O-NP is given as follows: startlingly, the semiconductor materials (NiWO4-RGO) absorb the energy in the form of photons in presence of visible light irradiation. Electrons are ejected from valance band to conduction band, resulting electron-hole pairs are generated at the photocatalyst surface. Then, photoinduced electrons are transferred from the nanocomposite to the RGO, which could efficiently reduce the electron and holes recombination. Afterwards, the high oxidation potential of the holes is more responsible to degradation of MB and O-NP by the oxidation of pollutants. Schematic representation of MB/O-NP degradation mechanism is shown in Fig. 7.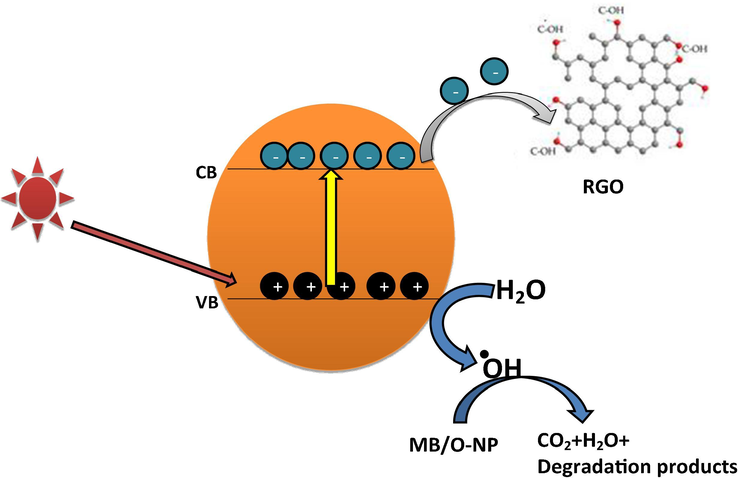
Schematic representation of MB/O-NP degradation mechanism using NiWO4-RGO Nanocomposite.
3.2 Reactive oxygen species assay
In context to photocatalytic degradation mechanism, identification of the reactive oxygen species ROS) such as h+, •OH and •O2− is most important. Hence, such ROS species were identified by using 2-propanol, KI and Benzoquinone for the confirmation of •OH, holes and •O2− radicals. The tested dye molecule reacts with these ROS species and finally produced deminerialized products like H2O and CO2 (Botsa and Basavaiah, 2020). Remarkable removal rate (97.1%) was recorded in absence of scavenger and the photodegradation rate is very less when tested with scavenger (Fig. 8). Hence, NiWO4-RGO (15%) composite possess greater photocatalytic efficacy due to create more active sites on their surface which can accommodate more dye molecules, this may be presence of RGO in composite.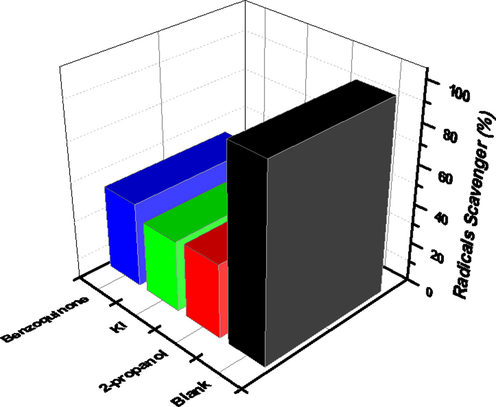
ROS analysis of photodegradation of dye by prepared tungstate composites.
3.3 Reusability and sustainability
The photocatalyst was examined under reusability after photocatalytic process. For that, the catalyst was washed properly and used it in next degradation process. The NiWO4-RGO composite was re-run for six runs continuously and found to be loss of 10% degradation rate (Fig. 9a). In addition, the stability was checked by XRD patterns after six cycles, there is no apparent change in its phase (Fig. 9b).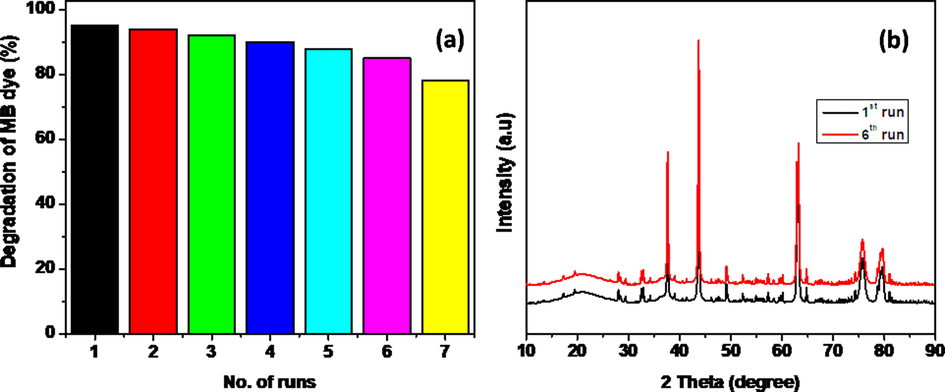
Reusability (a) and sustainability (b) of prepared photocatalyst NiWO4-RGO (15%).
4 Conclusion
NiWO4 and the NiWO4-RGO nanocomposite with different wt% of RGO have been synthesised via hydrothermal method. These materials are characterised by XRD, FESEM, EDX and FTIR. Characterisation study revealed that as synthesised nanocomposite was phase pure, plate like and spherical microstructures with porous nature. These materials degrade MB and O-NP very effectively under visible light irradiation. Photo catalytic degradation of 100 ml aqueous solutions of MB and O-NP (10 mg/L) containing 100 mg of the dispersed catalyst occurred 94% and 82% in 150 and 240 min respectively. The catalyst was reused and stable for successive six runs with loss of 10% of degradation rate.
Acknowledgement
S M Botsa and M J Babu sincerely acknowledge the University Grants Commission (UGC), India for providing Rajiv Gandhi National Fellowship (RGNF).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Enhanced visible-light-response photocatalytic degradation of methylene blue on Fe-loaded BiVO4 photocatalyst. J. Alloys Comp.. 2014;597:129-135.
- [Google Scholar]
- Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol.. 2005;6(2–3):186-205.
- [Google Scholar]
- Saravanamuthu Vigneswaran & Il Shik Moon, A review on UV/TiO2 photocatalytic oxidation process. Korean J. Chem. Eng.. 2008;25:64-72.
- [Google Scholar]
- Significant improvement in visible light photocatalytic activity of Fe doped TiO2 using an acid treatment process. Appl. Surf. Sci.. 2018;427:791-799.
- [Google Scholar]
- Enhanced photocatalytic activity of Cuprous Oxide nanoparticles formalachite green degradation under the visible light radiation. Mater. Res. Express.. 2020;7:015038
- [Google Scholar]
- Photocatalytic activity of Zn-doped Fe2O3 nanoparticles:a combined experimental and theoretical study. Bull. Mater. Sci.. 2019;42:185.
- [Google Scholar]
- Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of Azo dyes. Nanoscale Res. Lett.. 2017;12:143.
- [Google Scholar]
- Controllable synthesis of SnO2 photocatalyst with superior photocatalytic activity for the degradation of methylene blue dye solution. J. Exp. Nanosci.. 2016;11(14):1138-1155.
- [Google Scholar]
- Piezoelectrically enhanced photocatalysis with BiFeO3 nanostructures for efficient water remediation. Iscience. 2018;4:236-246.
- [Google Scholar]
- Photocatalytic activities of LaFeO3 and La2FeTiO6 in p – chlorophenol degradation under visible light. Catal. Commun.. 2012;29:35-39.
- [Google Scholar]
- Development of SrTiO3 photocatalysts with visible light response using amino acids as dopant sources for the degradation of organic pollutants in aqueous systems. Catalysts. 2018;8:528.
- [Google Scholar]
- Synthesis of visible-light sensitive MBiVO4 (M= Ag, Co and Ni) for the photocatalytic degradation of organic pollutants. Seperat. Purif. Technol.. 2011;77:275-282.
- [Google Scholar]
- Rapid photo catalytic degradation of crystal violet and carmine indigo under sun light by Fe2Mo3O12. J. Appl. Chem.. 2014;3(4):1670-1678.
- [Google Scholar]
- Photodegradation of sulfamethazine in an aqueous solution by a bismuth molybdate photocatalyst. Catal. Sci. Technol.. 2013;3:1603-1611.
- [Google Scholar]
- Enhanced photocatalytic degradation of cationic dyes under visible light irradiation by CuWO4-RGO nanocomposite. Adv. Compos. Hybrid Mater.. 2020;3:205-212.
- [Google Scholar]
- Visible light assisted photocatalytic degradation of Methyl orange and Congo red using Bi2WO6 prepared by solid-state metathesis. Asian J. Chem.. 2018;30:998-1002.
- [Google Scholar]
- Controllabla synthesis of Bi2MoO6 and effect of morphology and variation in local structure on photocatalytic activities. Appl. Catal. B. 2010;98:138-146.
- [Google Scholar]
- Catalytic activity of graphene oxide hybridized ZnWO4 for dyes degradation and oxidation of functionalized benzyl alcohols. MOJ Biorg. Org. Chem.. 2018;2(5):230-234.
- [Google Scholar]
- Nickel tungstate nanoparticles: synthesis, characterization and electrochemical sensing of mercury (II) ions. J. Mater. Sci.: Mater. Electron.. 2019;30:3574-3584.
- [Google Scholar]
- Nickel tungstate (NiWO4) nanoparticles/graphene composites: preparation and photoelectrochemical applications. Semicond. Sci. Technol.. 2018;33:5.
- [Google Scholar]
- Evaluation of photocatalytic and supercapacitor potential of nickel tungstate nanoparticles synthesized by electrochemical method. New J. Chem.. 2018;42:19934-19944.
- [Google Scholar]
- Synthesis, characterization, and enhanced photocatalytic properties of NiWO4 nanobricks. New J. Chem.. 2017;41:8178-8186.
- [Google Scholar]
- Removal of Nitrophenols from wastewater by monoclinic CuO/RGO nanocomposite. Nanotechnol. Environ. Eng.. 2019;4(1):1.
- [Google Scholar]
- TiO2 nanosheetgraphene oxide based photocatalytic hierarchical membrane for water purification. Surf. Coating. Technol.. 2017;320:259-262.
- [Google Scholar]
- Enhanced UV-Visible triggered photocatalytic degradation of Brilliant green by Reduced Graphene Oxide based NiO and CuO ternary nanocomposite and their antimicrobial activity. Arab. J. Chem.. 2020;13(4):5137-5150.
- [Google Scholar]
- Fe2O3/RGO nanocomposite photocatalyst: Effective degradation of 4-Nitrophenol. Physica B. 2018;553:190-194.
- [Google Scholar]
- Direct mechanochemical synthesis of nanocrystalline NiWO4. J. Phys. Chem. C.. 2007;111(3):1101-1104.
- [Google Scholar]
- Synthesis and characterization of sulfophenylfunctionalized reduced graphene oxide sheets. RSC Adv.. 2017;7:27224.
- [Google Scholar]
- Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: a review. RSC Adv.. 2014;4:3823.
- [Google Scholar]
- NiWO4-ZnO-NRGO ternary nanocomposite as an efficient photocatalyst for degradation of methylene blue and reduction of 4-nitrophenol. J. Phys. Chem. Solids. 2017;109:124-133.
- [Google Scholar]
- Sunlight-induced photocatalytic activity of nanostructured calcium tungstate for methylene blue degradation. Res. Chem. Intermed.. 2015;41:5463-5474.
- [Google Scholar]
- Visible light photocatalytic degradation of methylene blue and malachite green dyes with CuWO4-GO nanocomposite. Modern Res. Cataly.. 2018;7:2.
- [Google Scholar]
- Synthesis, characterization and photocatalytic performance of transitionmetal tungstates. Chem. Phys. Lett.. 2010;498:113-119.
- [Google Scholar]
- Photocatalytic degradation of metronidazole and methylene blue by PVA-assisted Bi2WO6–CdS nanocomposite film under visible light irradiation. Appl. Nanosci.. 2018;8:61-78.
- [Google Scholar]
- Visible light photocatalytic degradation of methylene blue and malachite green dyes with BaWO4-GO nanocomposite. Int. J. Environ., Agric. Biotechnol.. 2017;2(3):1173-1183.
- [Google Scholar]
- Significant photocatalytic enhancement in methylene blue degradation of Bi2WO6 photocatalysts via graphene hybridization. J. Adv. Ceram.. 2012;1:72-78.
- [Google Scholar]
- A novel Z-scheme WO3/CdWO4photocatalyst withenhanced visible-light photocatalytic activity forthe degradation of organic pollutants. RSCAdv.. 2015;5:6019-6026.
- [Google Scholar]
- Effect of morphology and temperature treatment control on the photocatalytic and photoluminescence properties of SrWO4 crystals. Photochem. Photobiol. Sci.. 2020;19:235-250.
- [Google Scholar]
- Facile and effective synthesis of praseodymium tungstate nanoparticles through an optimized procedure and investigation of photocatalytic activity. Open Chem.. 2017;15(1):129-138.
- [Google Scholar]
- Structural and photocatalytic analysis of NiWO4 materials. Int. J. Eng. Adv. Technol.. 2019;9(1S4):347-351.
- [Google Scholar]
- Fabrication of multifunctional TANI/Cu2O/Ag nanocomposite for environmental abatement. Sci. Rep.. 2020;10:14080.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.09.017.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







