Translate this page into:
Spongisulfins A-C, three S-bridged angucycline dimers from Spongiactinospora rosea LHW63015 and their gut epithelium protective activity in Drosophila melanogaster
⁎Corresponding author. lilei2526@163.com (Lei Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Spongiactinospora rosea is a rare actinomycete derived from sponges belonging to the Streptosporangiaceae family. Genomic analysis of the strain S. rosea LHW63015 revealed that it contains 41 secondary metabolite biosynthetic gene clusters (BGCs), including four cryptic type II polyketide synthases (T2PKS) BGCs. By using a metabolite mining approach in conjunction with LC-MS guided isolation, we identified three previously undescribed sulfur-bridged angucycline dimers, namely spongisulfins A-C (1–3), a configurational isomeric new angucycline monomer, rubiginone A3 (4), as well as three known related analogs (5–7). Comprehensive analyses of 1D/2D NMR, HR-ESIMS, single-crystal X-ray diffraction and ECD calculations were performed to elucidate their structures. Further sequence analyses of the chain length factors (CLF) protein and biosynthetic gene clusters (BGCs) identified the angucycline-type T2PKS BGC spo in the genome. Additionally, a dextran sulfate sodium salt (DSS)-induced Drosophila melanogaster reporter line (gstd1-GFP) model was established to evaluate the gut epithelium protective activities of these isolated compounds. The results demonstrated that spongisulfin A (1), with a concentration of 5 nM, significantly alleviated the high mortality caused by 5 % DSS treatment, and exerted its gut protective activity by modulating the ROS level through alleviation the expression of gstd1 in the gut.
Keywords
Spongiactinospora rosea
Angucycline dimer
Spongisulfins
Biosynthetic gene cluster
Drosophila melanogaster
1 Introduction
Angucyclines, the largest group of type II polyketide synthase (T2PKS) natural products, have gained significant attention due to their unique architecture and remarkable bioactivities (Kharel et al., 2012). The typical angular tetracyclic benz[a]anthracene backbone of angucycline is formed through cyclization and reduction from a decaketide chain, which is biosynthesized from an acyl-CoA starter and nine malonyl-CoA extender units by a heterodimer of a ketosynthase subunit KSα and an inactive subunit KSβ (Hertweck et al., 2007). The tetracyclic intermediate undergoes diverse post-PKS modifications, including methylation, acetylation (Ashrafian et al., 2021), epoxidation (Shen et al., 2021), glycosylation (Galm et al., 2002), halogenate (Guo et al., 2020), hydroxy or carbonyl substitution (Schäfer et al., 2016; Xiao et al., 2020), ring rearrangement (Fischer et al., 2003) or contraction (Jiang et al., 2017) and intermolecular dimerization (Kersten et al., 2013) or hybridization (Wu et al., 2019b), which have led to the discovery of more than 300 structurally diverse angucyclines to date. However, sulfur-containing angucycline dimers are rare, with only seven structurally characterized examples, including donghaesulfins A-B (Bae et al., 2019) and thioangucyclines A-E (Cao et al., 2021).
The application of genomic sequencing and bioinformatic analysis has revolutionized the study of uncharted microbial taxa and has revealed an astonishing array of BGCs (Wei et al., 2021; Hemmerling and Piel, 2022). The KSβ subunit, also termed CLF proteins, is the primary determinant of the carbon chain length of T2PKS products (Hertweck et al., 2007) and has been demonstrated to have a fine phylogenetic correlation with the product carbon number (Hillenmeyer et al., 2015). Recently, CLF was proposed as a reliable marker to predict both chemical class and molecular uniqueness of T2PKS BGC products, with a proposed CLF identity threshold of 0.88 to evaluate whether their products are identical or not (Chen et al., 2022). Additionally, a following large-scale analysis on the distribution and diversity of T2PKS in bacteria indicated the immense potential of rare actinobacteria for new T2PKS (Chen et al., 2022). Undoubtedly, CLF phylogeny alone is insufficient to accurately predict the structure of T2PKS products as their typical non-modular biosynthesis, along with various post-modification encoded at multiple genomic loci. Therefore, comparative analysis of new T2PKS BGC with identified ones from databases (Chopra et al., 2022), grouping them into BGC families (Navarro-Muñoz et al., 2020), rapid finding and comparative analysis (Wang et al., 2004; Gerlt et al., 2015) for tailoring homologs could significantly improve the structural prediction and biosynthesis pathway of these products.
Invertebrate-associated microbes from marine ecosystems offer unmatched chemical and structural diversity and have been known as an unexhausted source of drugs and drug leads (Wilson et al., 2014; Morita and Schmidt, 2018; Maini Rekdal et al., 2019; Zhang et al., 2020; Liu et al., 2021b; Liu et al., 2022). Exploiting actinomycetes from marine sponges have attracted attention for isolating secondary metabolites with diverse biological activities (Abdelmohsen et al., 2014; Yan et al., 2019; Zhang et al., 2021d; Zhang et al., 2012; Yan et al., 2017; Wang et al., 2019). As part of our ongoing efforts to discover bioactive chemicals from marine sponges (Hong et al., 2022), we have recently investigated the species diversity and chemical profiles of actinomycetes derived from marine sponges in the South China Sea (Li et al., 2019a-e; Zhang et al., 2021a; Cheng et al., 2021; Jiao et al., 2018; Zhang et al., 2022). Among them, Spongiactinospora rosea LHW63015, isolated from a Craniella sp. marine sponge collected in Xisha Islands, was identified as a new member of the family Streptosporangiaceae (Li et al., 2019a) and also as the first new actinomycete genus from marine sponges. Members of the family Streptosporangiaceae are known as good producers of new T2PKS natural products, such as cytotoxic S-bridged pyronaphthoquinone dimer hypogeamicin A from Nonomuraea specus (Derewacz et al., 2014), pentangular polyphenols hexaricins with antioxidant activity from marine Streptosporangium sp. (Tian et al., 2016; Gao et al., 2018), antibacterial enduracyclinones from Nonomuraea sp. (Monciardini et al., 2019), and new pentangular polyketide analogs of FR901533 from Streptosporangium roseum (Xu et al., 2020). During the polyphasic taxonomy study, S. rosea LHW63015 showed remarkable characteristics, such as abundant aerial mycelium formation, sporulation, and yellowish-brown pigmentation on tested media (Li et al., 2019a), which are regarded as beacons for secondary metabolism (Liu et al., 2013). Furthermore, the unusually large genome size (9.8 Mb) of S. rosea LHW63015 suggested that it may harbor 40 secondary metabolism biosynthetic gene clusters (SM-BGCs) based on the equation for total SM-BGCs per genome size, as summarized by Baltz (Baltz, 2017). These findings suggest that S. rosea LHW63015 is a promising candidate for the production of secondary metabolites (Li et al., 2019a).
In this study, we mined the metabolites from S. rosea LHW63015 to discover new sulfur-bridged angucycline dimers. Modern spectroscopy was employed to unveil their structures, and their bioactivity was assessed. Through systematic bioinformatic analyses, we also identified and proposed the BGC and pathway responsible for synthesizing these novel metabolites.
2 Materials and methods
2.1 Strain, media, and culture conditions
S. rosea LHW63015T (= DSM 106635T= CCTCC AA 2018019T) was identified, isolated and deposited in public collections as previously described (Li et al., 2019a). Five media were used to explore the potential for secondary metabolites (Table S1). The strain was revitalized on the ISP 2 agar plates (0.4 % Glucose, 0.4 % Yeast extract, 1.0 % Malt extract, pH 7.2–7.4) at 30 °C for 5 days. Phenotypic characteristics such as growth, colony colors and pigmentations on tested media were observed and recorded. Colonies were transferred into 40 mL of seed medium (TSBY: 3 % Tryptone Soya Broth, 0.5 % Yeast extract, 10 % Sucrose, pH 7.2–7.4) in 250 mL flasks and placed on a rotating shaker with glass beads at 200 rpm for 72 h at 30 °C. Then, the seed broths were transferred to 2 L spring-loaded flasks containing 400 mL of different test media at 30 °C and 200 rpm for 8–14 days.
2.2 Secondary metabolism BGCs
The genome of S. rosea LHW63015 (GenBank accession no. QMEY00000000) was previously reported (Li et al., 2019a). Here, BGCs were predicted using antiSMASH version 6.0 with Detection Strictness set to relaxed mode and all Extra features activated as previously described (Blin et al., 2021). Notably, aberrantly oversized BGCs were reexamined, manually separated into individuals, and reanalyzed using antiSMASH.
2.3 Monitoring natural product production
The tested broths or blank medium were extracted thrice with ethyl acetate (EtOAc) in equal volume. The EtOAc layer was concentrated in vacuo to afford the crude extracts. The crude extracts were redissolved in methanol (MeOH) and centrifuged at 15,000 × g for 5 min. Analytical thin-layer chromatography (TLC) systems were performed on silica gel 60 F254 plates. LC-MS spectra were obtained using a Waters HPLC system equipped with a Waters Acquity QDa spectrometer and a Waters XBridge C18 column (Waters, 4.6 mm × 250 mm, 5 μm). HPLC was performed using a Waters 1525 pump equipped with a 2998 photodiode array detector and a YMC-Pack Pro C18 RS (250 × 4.6 mm, Φ 5 µm).
2.4 Extraction and compound isolation
The preparation of medium-pressure liquid chromatography (MPLC) was performed on an Interchim PuriFlash 450 instrument (Interchim). The semi-preparative HPLC (spHPLC) was based on the same analysis HPLC system with the preparative XBridge C18 column (Waters, 19 × 150 , 5 μm).
After the scale-up fermentation in a selected medium, the broth (30 L) was extracted and concentrated as the test broth to obtain an extract (66.7 g). The EtOAc extract A was redissolved in MeOH-H2O and de-oiled by petroleum ether thrice. The MeOH/H2O layer A1 was extracted with dichloromethane (CH2Cl2) to obtain component A1B (6.2 g). The extract was put on a Sephadex LH-20 gel column and eluted with CH2Cl2/MeOH mixture (50:50, v/v) to obtain nine components (A1B1-A1B9). Component A1B3 (258.1 mg) was further purified using ODS-MPLC, and 14 components (A1B3A-A1B3N) were obtained using the gradient of an acetonitrile–water mixture (MeCN-H2O, 10–100%, v/v). Component A1B4G (12.6 mg) was further purified by silica-MPLC, eluted with a CH2Cl2/MeOH equation system (50:1, v/v), flow rate 10 mL/min to obtain components A1B4G1-A1B4G2. Component A1B4G1 was reacted with MeOH-H2O (70:30, v/v) using spHPLC to yield 1 (4.8 mg, tR 14.0 min, 2.5 mL/min). A1B3D (12.4 mg) was purified using spHPLC with MeOH-H2O (65:35, v/v) to yield 2 (1.4 mg, tR 16.8 min, 2.5 mL/min), while A1B3F (24 mg) was purified using spHPLC with MeOH/H2O (55:45, v/v) to yield 3 (4.5 mg, tR 18.2 min, 2.5 mL/min). The fraction A1B4 (2.1424 g) was further purified using ODS-MPLC, and 20 subfractions (A1B4A-A1B4T) were obtained from MeOH/H2O (10–100%, v/v) with the elution gradient. Among them, subfractions A1B4M (106.9 mg) and A1B4P (187.7 mg) were further purified by silica-MPLC, yielding 8 (A1B4M 1–8) and 12 (A1B4P 1–12) components, respectively. Component A1B4M7 (41 mg) was purified by spHPLC with MeCN/H2O (40:60, v/v) to yield 6 (23 mg, tR 13.3 min, 3 mL/min) and 7 (16 mg, tR 12.6 min, 3 mL/min). 4 (40 mg) and 5 were crystallized from A1B4P8 and A1B4M6, respectively.
2.5 Structure elucidation
NMR spectra were conducted on Bruker AV 600 (Bruker), in which the chemical shift (δ) was based on the residual solvent signal in deuterated. Ultraviolet spectra (UV) were recorded using the Hitachi U-3010 spectrophotometer. HRESIMS spectroscopic data were acquired with a Waters Xevo G2-XS QTOF spectrometer. Then, optical rotations were performed at room temperature on a PerkinElmer model 341 polarimeter with a 10 cm length cell. The IR spectra were registered on Nicolet iZ10-iN10 MX (Thermo Fisher Scientific). The melting point (MP) was recorded on the DSC2500 instrument (TA). Crystals data were obtained on Bruker D8 VENTURE diffractometer (Bruker) and visualized using Olex2 (Dolomanov et al., 2009). ECD was measured with a Jasco J-715 spectropolarimeter. The geometry of 1 (3/3′R, 4/4′R) was built based on the X-ray diffraction data (Table S2). Conformational analyses of 3 (3/3′R, 4/4′R, 7/7′S) and 3′ (3/3′R, 4/4′R, 7/7′R) were conducted via random searching in the MacroModel 9.9.22333 software using the MMFF94S force field with an energy cutoff of 5 kcal/mol (MacroModel, Schrödinger LLC, 2012. https://www.schrodinger.com/macromodel). Five and nine lowest energy geometries of 3 and 3′ were obtained, respectively, then subjected to geometry optimization at the B3LYP/6-31G(d) with polarizable continuum model (PCM) for Methanol level (Tables S3-S4). Time-dependent DFT (TDDFT) calculations with a functional B3LYP/TZVP basis set were performed to calculate the spin-allowed excitation energy and rotatory strength. The ECD spectra were generated by applying a Gaussian band shape with a width of 0.3 eV from dipole-length rotational strengths and averaged according to the Boltzmann distribution theory and their relative Gibbs free energy (ΔG). The spectrum of 1′ (3/3′S, 4/4′S) was vertically flipped from that of 1.
2.6 Analysis of putative BGC
The resulting GenBank files of T2PKS BGCs were analyzed using BiG-SCAPE for gene cluster families (GCF) grouping and phylogeny (Navarro-Muñoz et al., 2020), with a distance cutoff value of 0.8. The CLF protein sequences of angucycline T2PKS BGC were treated using MUSCLE (Edgar, 2004) and submitted into MEGA 7 (Kumar et al., 2016) for phylogenetic tree reconstruction with the neighbor-joining (Saitou and Nei, 1987) and maximum-likelihood (Kluge and Farris, 1969) tree-making algorithms. The identity and similarity between homologous proteins from BGCs were calculated using Clinker (Gilchrist and Chooi, 2021). Sequences of coding products extracted from angucycline BGCs were uploaded into EFI-EST (Gerlt et al., 2015) for comparison and sequence similarity network (SSN) construction with an E-Value limit 10. Lastly, the SSN file was transferred into Cytoscape (Shannon et al., 2003) for visualization and analysis.
2.7 Bioactivity assay
2.7.1 Antibacterial assay
Vibrio alginolyticus MVP01 and Pseudomonas plecoglossicida XSDHY-P on LB (1 % Tryptone, 0.5 % Yeast extract, 3 % NaCl, 1.8 % Agar), Edwardsiella piscicida EIB202 and Vibrio parahaemolyticus RIMD2210633 on modified LB (1 % NaCl) were incubated at 37 °C for 24h. Single colonies were picked and inoculated into 15 mL of LB broth as seed, incubated at 37 °C on a rotating shaker overnight. Chloramphenicol was used as a positive control for V. alginolyticus MVP01, V. parahaemolyticus and E. piscicida EIB202. Gentamicin and ampicillin were the positive antibiotics for P. plecoglossicida. The assay system on 96 well plates consisted of 600 μL medium, 30 μL seed broth, 12 μL DMSO solvent of compounds 1–7 or positive drugs for final concentrations 8–64 μg/ mL were incubated at 220 rpm for 12h, then observed every 4 h. The optical density (OD) was recorded at 450 nm using a microplate reader (SpectraMax 190, Molecular Devices), and the inhibition rates were calculated (Chen et al., 2021).
2.7.2 Cytotoxicity assay
The cytotoxicity of compounds 1–7 on human tumor cell lines A2780, HCT-8, NCI-H460, SW480, PC-9 and HepG2 were evaluated in vitro using the Cell Counting Kit-8 (CCK-8). A2780, HCT-8, NCI-H460, SW480 and PC-9 cell lines were grown in RPMI 1640 at 37 °C, while HepG2 cells were cultured in DMEM. The medium was supplemented with 10% fetal bovine serum and antibiotics. The above cell line (3 × 103 cells/ well) was treated with the test compound for 48 h, and then 10 μL CCK-8 solution was added. After incubation at 37 °C (5% CO2) for 1h, the OD was recorded at 450 nm using a microplate reader. Each cell line is measured more than 3 times to calculate the SD value. Cisplatin was used as the positive control against human tumor cell lines A2780, HCT-8, NCI-H460, SW480, PC-9 and HepG2 (Wu et al., 2019a).
2.7.3 Drosophila melanogaster survival assay
The Drosophila melanogaster reporter line (gstd1-GFP) was cultured on a standard cornmeal-yeast medium at room temperature in an incubator with 60 % humidity under a 12 h light/dark cycle. Survival assay in conventional rearing condition (25 degrees) were performed in three parallel, independent replicates with a mixture of 3–5 day-old of 25 females and 10 males in each vial. These flies were starved at 29 degrees in empty vials for 2 h before feeding with 200 μL of positive control solution (5 % DSS) or with two negative control solutions (5% sucrose or 0.1 % DMSO), or with three different experimental solutions (1 nM, 5 nM,10 nM compound + 5 % DSS) in vials contained two layers of filter papers, respectively. Fresh filter papers were changed every day and the number of living females was counted at each transfer for 5 days (Zhou et al., 2016).
3 Results and discussion
3.1 BGCs for secondary metabolites
A total of 41 BGCs were identified using antiSMASH (Table S5), covering the major natural product classes such as ribosomally synthesized and post-translationally modified peptides (RiPPs), polyketides (T1-T3PKs), nonribosomal peptides (NRPs), PKS-NRP hybrids, terpenes, etc. (Fig. 1a). In detail, only four potential BGCs showed high similarity (>80 % of genes showed similarity), three potential BGCs showed moderate similarity (50 %–80 %) with known BGCs, other 34 BGCs shared low similarity (<50 %) to reported BGCs. Out of 34 potential novel BGCs, 12 BGCs showed non-similarity with any known BGCs. The findings suggest that S. rosea LHW63015 could be a potential source for various natural products.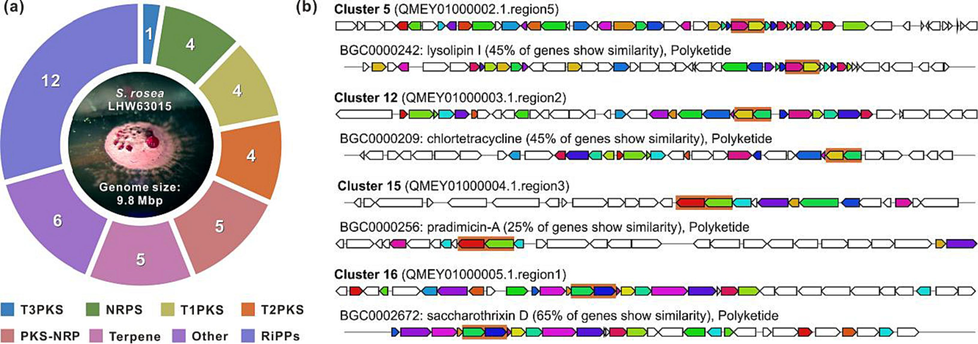
Genome minning of S. rosea LHW63015. a Category distribution of 41 BGCs predicted by antiSMASH. b Four cryptic T2PKSs BGCs with known homologous from MIBiG. Ketosynthase genes were highlighted.
3.2 Fermentation analysis and structure elucidation
S. rosea LHW63015 showed good growth (Fig. S1a) and brown pigmentation in test media M1-M4, while it grew poorly on M5 (Fig. S1a, b). White or pink aerial hyphae were observed, except for colonies on M3 (Fig. S1a). In the extraction of test broths with EtOAc for further analyses, serious emulsification was found in the M3 group and excluded from further analyses. S. rosea LHW63015 in M2 showed the highest metabolic chemical diversity and intensity according to the TLC profile (Fig. S1c), and an attractive mass peak at m/z 703 with characteristic aromatic UV absorption was observed on LC-MS (Fig. S2) compared to the blank M2. The fermentation period was evaluated using HPLC, and 14 d was selected for the metabolic chemical diversity, intensity, and time costs (Fig. S3). Subsequently, the fermentation of S. rosea LHW63015 in M2 was scaled up, followed by extraction, Sephadex LH-20 separation, ODS C18 flash chromatography and spHPLC purification, which led to the discovery of three new sulfur-bridged angucycline dimers, spongisulfins A-C (1–3), a new angucycline monomer isomer, rubiginone A3 (4), together with three known angucycline monomers, namely rubiginone A1 (5), SNA-8073-B (6) and fujianmycin B (7) (Fig. 2).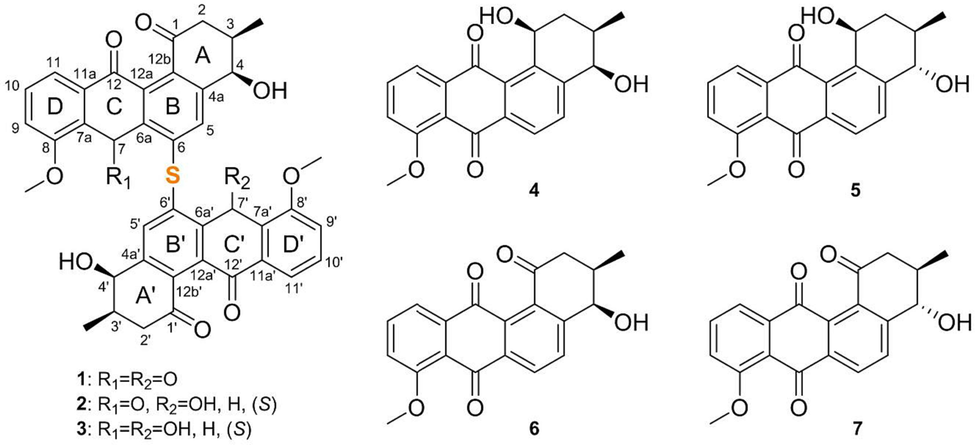
Structure of compounds 1–7.
Spongisulfin A (1) was isolated as orange-yellow needles; [α] 18.86 (c 0.1, MeOH); mp 111.54 °C; UV (MeOH) λmax (log ε), 234 (3.63), 261 (3.71), 375 (3.13) nm (Fig. S15); IR νmax 3451, 2963, 1694, 1669, 1588, 1568, 1472, 1449, 1378, 1353, 1308, 1273, 1243, 1190, 1170, 1117, 1067, 1032, 989, 944, 908, 889, 839, 792, 773, 734, 591, 489, 433, 415; ECD (142 μM, MeOH), λmax (Δε) 225 (−6.63), 243 (+4.67), 270 (+9.66), 311 (+3.62), 380 (−7.70), 459 (+3.27) nm (Fig. S15). The molecular formula of 1 was determined to be C40H30O10S based on the HRESIMS data (m/z 703.1607 [M + H]+)(Fig. S14), while the 13C spectra of 1 (Table 1) revealed 20 carbon signals suggesting it to be a symmetrical structure. Further analysis of the 1D (Table 1, Figs. S7-S9) and HSQC NMR data (Fig. 3a and Fig. S11) revealed that 20 carbon signals comprising three carbonyls (δC 197.3, 180.9, and 184.3), eight non-protonated sp2 carbons (δC 158.8, 149.5, 141.4, 136.2, 136.0, 133.8, 132.9, and 121.4), four aromatic methines (δC/δH:135.5/7.82, 135.3/7.82, 118.4/7.55, and 117.8/7.53), one oxygen-bearing sp3 methine bearing group (δC/δH: 68.9/4.52), one methine (δC/δH: 34.3/2.37), one aliphatic methylene (δC/δH: 42.3/2.82 and 2.51), one methoxy (δC/δH: 56.4/3.90), and one methyl (δC/δH:15.8/0.97) group. Since the above NMR data of 1 accounted for only half of the carbons and protons deduced from its molecular formula, the structure of spongisulfin A (1) was proposed as a dimer. The contiguous sequence of 1H–1H COSY (Fig. S10) correlations from H2-2 to H-3, from H-3 to H-4, from H-9 to H-10, and from H-10 to H-11 showed the presence of two isolated spin-coupling systems, H-2/H-3/H-4 and H-9/H-10/H-11, which were featured in a typical tetracyclic angucycline structure (Cao et al., 2021).
no.
1
3
4
δC, type
δH, (mult, J in Hz)
δC, type
δH, (mult, J in Hz)
δC, type
δH, (mult, J in Hz)
1
197.3, C
197.9, C
64.5, CH
5.45, td (6.5, 5.6)
1-OH
/
/
5.00, d (5.6)
2
42.3, CH2
2.82, dd (16.7, 6.4);
43.1, CH2
2.78, dd (15.7, 7.3);
34.1, CH2
1.85ɑ, m
2.51ɑ, dd (16.7, 8.5)
2.53, dd (15.7, 5.1)
1.90ɑ, m
3
34.3, CH
2.37, m
35.3, CH
2.31, m
31.3, CH
1.86ɑ, m
3-CH3
15.8, CH3
0.97, d (6.9)
16.2, CH3
0.94, d (6.8)
16.7, CH3
1.03, d (6.1)
4
68.9, CH
4.52, dd (3.8, 3.2)
68.4, CH
4.47, dd (5.3, 4.2)
70.0, CH
4.45, s
4-OH
5.58, d (3.8)
5.58, d (5.3)
5.40, d (5.0)
4a
149.5, C
146.5, C
146.2, C
5
135.3, CH
7.61, s
134.9, CH
7.48, s
134.7, CH
7.79, d (8.0)
6
136.2, C
134.6, C
125.5, CH
8.03, d (8.0)
6a
133.8, C
143.3, C
135.6, C
7
180.9, C
59.6, CH
6.33, d (7.4)
181.3, C
7-OH
5.90, d (7.5)
7a
121.4, C
130.6, C
119.9, C
8
158.8, C
157.4, C
159.2, C
8-OCH3
56.4, CH3
3.90, s
56.4, CH3
3.95, s
56.4, CH3
3.94, s
9
118.4, CH
7.55, d (8.4)
116.0, CH
7.38, d (8.4)
118.1, CH
7.53, d (8.4)
10
135.5, CH
7.82, dd (8.0, 7.6)
130.3, CH
7.56, dd (8.1, 7.9)
135.5, CH
7.82, t (8.0)
11
117.8, CH
7.53, d (7.7)
118.0, CH
7.44, d (7.6)
119.0, CH
7.66, d (7.6)
11a
136.0, C
135.5, C
137.3, C
12
184.3, C
186.2, C
186.4, C
12a
141.4, C
142.0, C
132.1, C
12b
132.9, C
133.1, C
141.5, C

Structure elucidation of compounds. a Key COSY, HMBC, and NOESY correlations (asymmetric) of 1–4. b X-ray crystal structure of 1, 2 and 4 (1, Mo Kα radiation, 2, 4, 6, Cu Kα radiation, displacement ellipsoids are drawn at the 30% probability level). c Experimental (exp.) and calculated (cal.) ECD spectra of 1 and 3.
The 1H–1H correlations between H-3 and the methyl doublet at δH 0.97, as well as between the H-4 and the 4-OH at δH 5.58, allowed the identification of the methyl group at C-3 and the 4-OH group at C-4. The HMBC correlations from H2-2 to C-1 (δC 197.3), C-4 (δC 68.9), and C-12b (δC 132.9) and from H-4 to C-4a (δC 149.5) and C-12b assembled the hydroxymethylcyclohexenone moiety (ring A) in 1 (Fig. S12). The HMBC correlations from H-4 to C-5 (δC 135.3) and C-6 (δC 136.2) and from H-5 to C-6, C-6a (δC 133.8), and C-12b formed the aromatic C-4a-C-5-C-6-C-6a-C-12b ring B. In addition, the establishment of another 6-membered aromatic ring (ring D) was confirmed by the HMBC correlations from H-9 to C-8 (δC 158.8), C-7a (δC 121.4), from H-10 to C-8 and C-11a (δC 136.0), and from H-11 to C-7a, as well as the vicinal coupling constants (7.7–8.0 Hz) in the spin system of H-9/H-10/H-11. The placement of one methoxy group at C-8 was deduced based on the HMBC correlation from 8-OCH3 to C-8. Lastly, considering the monomer molecular formula C20H15O5 of 1, the unassigned two carbonyl groups (δC 180.9 and 184.3) and the remaining unsaturation equivalent required the presence of the 1,4-cyclohexanonedione skeleton (ring C) to form the partial structure, thereby finalizing the structure of the monomer of 1. Altogether the symmetrical structure of 1 and the presence of only one sulfur atom in its molecular formula led to the elucidation of the full planar structure of spongisulfin A (1) as a sulfur-bridged anagucycline dimer.
The relative configuration of C-3 and C-4 in 1 was established by analyzing its 3JHH values and ROESY spectroscopic data (Fig. 3a, Fig. S13). The observation of a small 1H–1H coupling constant (3.2 Hz) between H-3 and H-4 indicated that both were cis diaxial hydrogens (Bae et al., 2019; Oka et al., 1990). The H-2β, 4-OH, and 3-CH3 were assigned as β-oriented based on their respective ROESY correlations H-2β/3-CH3 and 3-CH3/4-OH. To validate the above deduction, an X-ray crystallographic experiment was conducted using Mo Kα radiation (Fig. 3b), which confirmed the relative configuration of 1 as 3R*, 4R*, 3′R*, 4′R*. Next, the absolute configurations of 1 as 3R, 4R, 3′R, 4′R was determined by experimented and calculated ECD (Fig. 3c). This was further supported by the similar chemical shifts of 1 to those of the co-isolated monomer ligand SNA-8073-B (6), whose structure was tentatively established by X-ray crystallography (Fig. 3b), along with the biosynthetic consideration of 6 and 1. Accordingly, the structure of spongisulfin A (1) was established.
Spongisulfin B (2) was isolated as orange flake crystals; [α] –32.57 (c 0.1, MeOH); mp 177.76 °C; UV (MeOH) λmax (log ε), 227 (3.98), 317 (3.54) nm (Fig. S24); IR νmax 3451, 2958, 2923, 2852, 1694, 1667, 1588, 1470, 1450, 1379, 1305, 1272, 1244, 1191, 1061, 1033, 990, 793, 771, 733; ECD (199 μM, MeOH), λmax (Δε) 228 (−6.39), 238 (+10.96), 267 (−10.07), 281 (+0.36), 295 (−7.15), 327 (−8.82), 437 (+1.89) nm (Fig. S24). The molecular formula of 2 was determined to be C40H32O10S based on the HRESIMS (m/z 727.1621 [M + Na]+) data (Fig. S23), indicating it contained two more hydrogen atoms than 1. The similar molecular formula and UV behavior revealed that the structure of 2 was analogous to 1. However, the observation of 40 carbon signals in its 13C NMR spectra (Fig. S17) further indicated that 2 might have a heterodimeric anagucycline structure. The detailed comparison of the NMR data (Figs. S16-S22) between 1 and 2 (Table 2) demonstrated that 2 shared a similar structure to 1, albeit with the obvious difference being that a ketone group (δC 180.9, C-7′) in 1 was replaced by a hydroxy group in 2, which was accordance with a 2 mass unit more than that of 1. Additionally, the HMBC correlations confirmed the heterodimeric structure of 2, with a modification on the C ring of the two monomers (Fig. S21). The relative configuration of C-3/3′ and C-4/4′ in 2 was determined to be similar to that of 1 through a comprehensive analysis of its ROESY data as well as 1H–1H coupling constants. Fortunately, a suitable crystal of 2 was obtained from methanol solution and subsequently subjected to X-ray diffraction analysis using Cu Kα radiation[Flack parameter = 0.021(13)], which allowed an unambiguous assignment of the absolute configuration in 2 as 3R, 4R, 7S, 3′R, 4′R.
no.
δC, type
δH, (mult, J in Hz)
no.
δC, type
δH, (mult, J in Hz)
1
197.7, C
1′
197.1, C
2
42.8, CH2
2.85, dd (15.6, 7.4)
2′
42.2, CH2
2.71, dd (16.7, 6.1)
2.60, dd (15.6, 5.1)
2.49ɑ, dd (16.7, 8.4)
3
34.9, CH
2.40, m
3′
34.3, CH
2.27, m
3-CH3
15.9, CH3
1.01, d (6.8)
3′–CH3
15.8, CH3
0.89, d (6.8)
4
68.1, CH
4.64, dd (5.2, 3.6)
4′
69.0, CH
4.34, dd (4.5, 2.8)
4-OH
5.72, d (5.2)
4′–OH
5.42, d (4.5)
4a
146.5, C
4′a
149.1, C
5
135.7, CH
7.86, s
5′
130.6, CH
7.09, s
6
136.0, C
6′
134.8, C
6a
145.7, C
6′a
137.1, C
7
59.0, CH
6.22, d (7.2)
7′
181.4, C
7-OH
5.83, d (7.2)
7′a
120.6, C
7a
130.3, C
8′
159.3, C
8
156.7, C
8′–OCH3
56.5, CH3
3.99, s
8-OCH3
55.9, CH3
3.82, s
9′
118.6, CH
7.60, d (8.6)
9
115.6, CH
7.31, d (8.2)
10′
135.7, CH
7.86, t (8.0)
10
129.8, CH
7.52, t, (7.9)
11′
117.9, CH
7.55, d (7.6)
11
117.6, CH
7.42, d (7.7)
11′a
145.7, C
11a
135.1, C
12′
184.3, C
12
185.9, C
12′a
130.7, C
12a
136.3, C
12′b
131.0, C
12b
134.7, C
Spongisulfin C (3) was isolated as yellow needles; [α] 25.24 (c 0.1, MeOH); UV (MeOH) λmax (log ε), 284 (3.79) nm (Fig. S33); IR νmax 3424, 2957, 2924, 2852, 1694, 1667, 1589, 1573, 1470, 1462, 1452, 1382, 1300, 1274, 1191, 1110, 1071, 1031, 983, 791, 771, 736; ECD (142 μM, MeOH), λmax (Δε) 223 (−14.18),237 (−14.40), 263 (−19.51), 280 (+33.69), 294 (−6.18), 307 (+13.75), 331 (−11.59), 381 (+4.63) nm. The molecular formula of 3 was confirmed to be C40H34O10S based on its HRESIMS (m/z 707.1922 [M + H]+) (Fig. S32), with 11 degrees of unsaturation. The 13C and DEPT spectra of 3 (Table 1, Figs. S26-S27) also revealed 20 resonances, indicating that 3 had a symmetrical structure. The 1D NMR data (Figs. S25-S27) of 3 were similar to those of spongisulfin A (1), which readily revealed that 3 and 1 had the same core skeleton as another sulfur-bridged anagucycline dimer, with the exception that the resonances of two ketone groups at C-7/7′ in 1 were replaced by the resonances of two hydroxy groups in 3. The HMBC correlations also supported this hypothesis from 7-OH to C-7 and C-6a, which was in accordance with a 4 mass unit more than that of 1 (Figs. S28-S30). Additionally, the relative configuration of 3 was determined by interpreting the ROESY spectroscopic. ROESY correlations from H-2β (δH 2.53) to 3-CH3 (δH 0.94) and from 3-CH3 to 4-OH (δH 5.58) suggested that H-2β, 4-OH and 3-CH3 had the same β-orientation. The correlation of 4-OH and 7-OH (δH 5.90) showed that these two groups were on the same plane (Fig. S31). Nevertheless, the limited amount of 3 prohibited the cultivation of a suitable crystal. The absolute configuration of 3 was finally verified via electronic circular dichroism (ECD) calculation at the B3LYP/6-31G(d) level. The calculated ECD curve of 3 demonstrated good agreement with the experimental curve (Fig. 3c, Fig. S33), which confirmed the absolute configuration of 3 as 3R, 4R, 7S, 3′R, 4′R,7′S.
Rubiginone A3 (4) was isolated as a yellowish flake; [α] 296.58 (c 0.1, MeOH); mp 99.12 °C; UV (MeOH) λmax (log ε), 258 (3.22), 375 (2.41) nm (Fig. S42); IR νmax 3457, 2923, 1701, 1667, 1587, 1471, 1444, 1304, 1270, 1190, 1152, 1112, 1060, 1022, 970, 864, 834, 794, 736, 701, 596; ECD (278 μM, MeOH), λmax (Δε) 202 (−9.14), 226 (−4.83), 258 (+1.84), 290 (−1.57), 354 (−0.93) nm (Fig. S42). Its molecular formula was determined to be C20H18O5 through HRESIMS (m/z 361.1054 [M + Na]+) (Fig. S41). Comprehensive analysis of the 1D NMR data of 1 (Table 1, Figs. S34-S36) revealed that 1 had a similar structure to that of rubiginone A1 (5) (Table S6) (Oka et al., 1990), except for the changes of the chemical shifts of C-3 and C-4. Further comparing the NMR data (Table 1 and Table S3; Figs. S37-S40) showed that 4 shared the same planar structure as rubiginone A1 (5), with the only difference being in their relative configurations of C-4. The ROESY correlations of 1-OH (δH 5.00)/3-CH3 (δH 1.03)/4-OH (δH 5.40) suggested that the 1-OH, 4-OH, and 3-CH3 were on the same face with β-orientation. To confirm the absolute configuration, an ideal crystal of 4 was obtained and subjected to the single-crystal X-ray diffraction experiment using Cu Kα radiation (Fig. 3b), which unequivocally secured the absolute configuration of 4 as 1S, 3R, 4R on the basis of the flack parameter of 0.03(11).
Three known compounds were identified as rubiginone A1 (5) (Figs. S43-S46) (Oka et al., 1990), SNA-8073-B (6) (Figs. S47-S50) (Kimura et al., 1997) and fujianmycin B (7) (Figs. S51-S54) (Rickards and Wu, 1985) by comparing their MS and NMR data with those of previously reported data and X-ray diffraction data.
3.3 Proposed BGC and biosynthetic pathway of spongisulfins
There are four T2PKS BGCs as clusters 5, 12, 15 and 16 in the genome of S. rosea LHW63015 (Fig. 1b, Table S5). Based on the product type of similar BGC (Fig. 1b) and confirmation by the CLF protein sequence analyses (Table S7), the four T2PKS could be assigned to four different subclasses, namely pentangular polyketide (cluster 5), tetracycline (cluster 12), anthracycline (cluster 15), and angucycline (cluster 16), respectively. Cluster 16 showed a 65% similarity with the BGC sxn of highly oxygenated anthracycline saccharothrixin D (Shen et al., 2021), 57% similarity with the BGC tac of sulfur-bridged dimeric angucyclines thioangucyclines (Cao et al., 2021), 44% similarity with the BGC lug of rearranged angucycline lugdunomycin (Wu et al. 2019), and 33% similarity with the BGC rub of classic angucycline rubiginone A2 (Zhang et al., 2021b). As expected, the spo cluster was assigned to angucycline GCF and formed a distinct branch with sxn in the clade comprising other similar angucycline BGCs (Fig. 4a). Their close evolutionary relationships were supported by the phylogeny of CLFs (Fig. S5). Therefore, the spo gene cluster was identified as the putative BGC for the biosynthesis of spongisulfins (1–3) and angucycline monomers (4–7).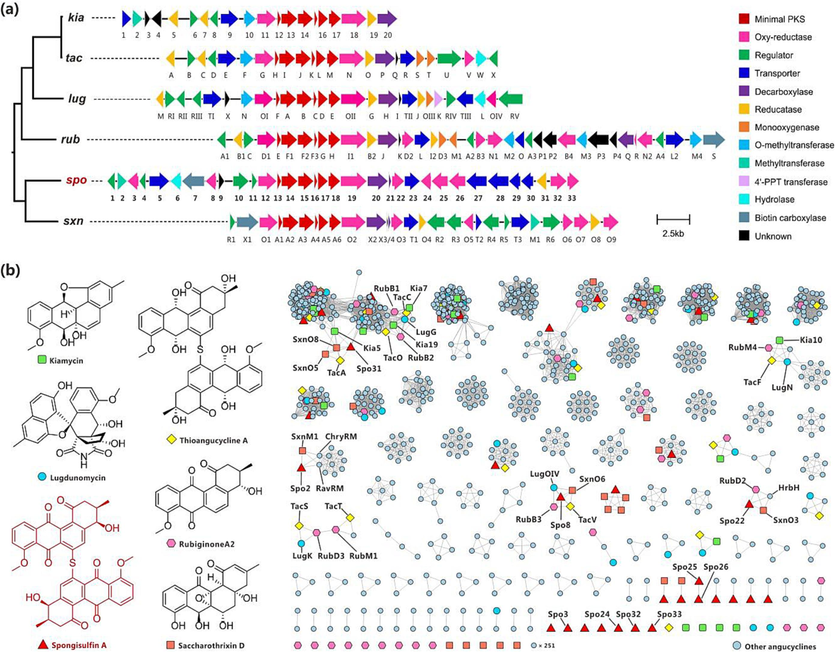
Analysis of BGC of spongisulfin (spo) with related angucyclines. a Organization of spo and phylogenetic relationship in GCF (partial, the complete tree was provided in Fig. S4). b Selected products and sequence similarity network (SSN) of 38 angucycline BGCs. Kiamycin, kia; Thioangucycline A, tac; Lugdunomycin, lug (BGC0002016); Rubiginone A2, rub; Saccharothrixin D, sxn; ChryRM, putative dehydrogenase in chrysomycin BGC chry; HrbH, putative oxygenase in hatomarubigin BGC hrb; RavRM, putative dehydrogenase in ravidomycin BGC rav.
The spo gene cluster was proposed to span 36.5 kb at contig 5 (58,061–94,615), containing 33 open reading frames (ORFs) (Table S8, Fig. 4a). The oxygenase Spo12, minimal polyketide synthase (PKS) Spo13-18, and oxygenase Spo19 shared 51–81% protein identity to their homologs TacG-N, SxnO1-O2, RubD1-I1 and LugOI-OII, and their genes were organized in identical styles in corresponding BGCs (Fig. S6, Fig. 4a). Spo12 showed a 65% identity with TacG, which was proven to be necessary for the earlier stages of angucycline biosynthesis (Wu et al., 2019); LugOII, the counterpart of Spo19 (63% identity) in lug, was found to promiscuously catalyze ketoreductions at both C6 and C1 of angucyclines (Xiao et al., 2020). Outside the cassette comprising genes spo12-19, there were regulator genes spo1, as well as 10–11, reductase gene spo31, methyltransferase gene spo2, and other additional genes with different or unknown functions. Most remarkably, there were seven transporter genes spo4, 5, 23 and 27–30 and eight oxidoreductase genes spo3, 8, 22, 24–26 and 32–33 in gene cluster spo, and both far outnumbered that in the reference BGCs. Spo2, the only putative methyltransferase encoded in the cluster, showed a 63% identity with SxnM1, which was proposed to catalyze methylation on 8-OH of angucyclines (Shen et al., 2021).
The tac cluster was the only BGC associated with sulfur-bridged dimeric angucyclines (Wu et al., 2019), while monomeric angucycline epoxide was proven to be the nascent final product: ketone at C-12 of earlier intermediate 8-O-methyltetrangomycin was reduced by ketoreductase TacA; ring B was epoxidized at C-6a/C-12a and dearomatized by monooxygenases TacS-T; the intermediate was further reduced by ketoreductase TacO at C-7. Following the enzymatic synthesis of the epoxide, the sulfur incorporation and dimerization at C-6 via 1,6-Michael addition were revealed to be non-enzymatic (Wu et al., 2019). Based on similar structures and the same site of sulfur incorporation and dimerization between spongisulfins and thioangucyclines, we postulate that a similar biosynthetic pathway might be shared between them.
An SSN analysis was conducted to find homologs or counterparts of TacA, TacS-T and TacO, which could be responsible for ketoreduction or epoxidation on intermediates for spongisulfins (Fig. 4b). Thirty-seven T2PKS gene clusters reported for angucycline were collected from MIBiG (Terlouw et al., 2023) or GenBank (Table S9), following which a total of 1,222 protein sequences were extracted from the GBK files of spo and the references. We found that Spo31 shared a 46% identity with ketoreductase TacA, but we did not identify any molecular species with a reduced C-12. Although the homolog of C-7 ketoreductase TacO, including Kia19, RubB2, LugG, etc., are widely encoded in different angucycline BGC, none was observed in spo. Furthermore, no homolog of epoxidases TacS-T was found in spo. The four homologs LugOIV (52% identity), RubB3 (48% identity), SxnO6 (52% identity), and TacV (49% identity) of putative oxidoreductase Spo8 were revealed, from which TacV was found to be the only one that had been experimentally explored, and gene deletion test showed that it did not play a role in the biosynthesis pathway of thioangucyclines (Wu et al., 2019). Spo22 shared a 70% identity with SxnO3, which was regarded as the monooxygenase for epoxidation of C-6a/C-12a in intermediate for saccharothrixin D (Shen et al., 2021). Therefore, we proposed Spo22 as the counterpart of TacS-T during the biosynthesis pathway of key intermediates for spongisulfins. However, the remaining six putative oxidoreductase Spo3, Spo24-26 and Spo32-33 lack homologs in known angucycline BGCs.
Based on the above analysis and early biosynthetic studies on typical angucyclines (Hertweck et al., 2007; Yushchuk et al., 2019), we propose a plausible biosynthetic pathway for spongisulfins (Fig. 5). The minimal PKS comprising six proteins Spo13-18 was sufficient to produce the early intermediate UWM6. After redox and aromatization, UWM6 was converted to intermediate I-1. Spo2 represents a class I SAM-dependent methyltransferase and was predicted to catalyze the O-methylation of the C-8 hydroxyl group in I-1 to yield intermediate I-2. Oxidation of the C-4 in I-2 led to 6 and 7, and reduction at C-1 by Spo19 could generate 4 and 5, respectively. Spontaneous oxidation in the air of hydroxyl groups at C-12 in 4 and 5 was detected (data not shown). Keto-reduction at C-7 in 6 yielded the intermediate I-3, and epoxidation of C-6a/C-12a in I-3 led to the nascent final enzymatic product I-4. The electrophilic C-6 in I-4 was attacked by endogenous HS− via a 1,6-Michael addition, leading to the epoxide opening. The thermodynamically unstable intermediate I-5 lost a water molecule and re-aromatized it into pre-spongisulfin (I-6). The sulfide in I-6 attacked another molecule of I-4 to produce 3, which could be non-enzymatically oxidized to 2 and 1.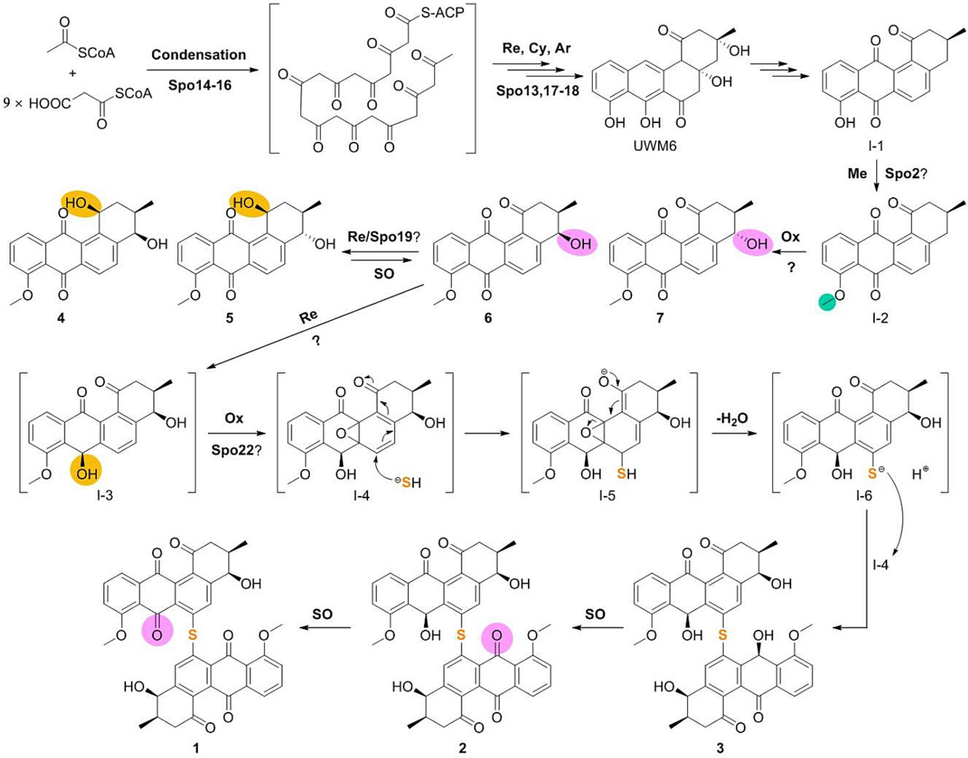
The proposed biosynthetic pathway of spongisulfins. Ar, aromatization; Cy, cyclization; Me, methylation; Ox, oxidation; Re, reduction; SO, spontaneous oxidation. Sites of methylation, oxidation, and reduction were highlight on products with green, pink, and orange, respectively.
3.4 Biological activity
Upon screening the antibacterial activity and cytotoxicity, we found that the isolated compounds did not show any significant inhibitory effects (minimum inhibitory concentrations, MICs > 64 μg/ml, data not shown) against the tested fishery pathogenic bacteria or cytotoxicity on all tested cancer cell lines (p < 0.05). However, a dextran sulfate sodium salt (DSS)-induced Drosophila melanogaster reporter line (gstd1-GFP) model was established to evaluate the gut epithelium protective activities of these isolated compounds. As expected, 5% of DSS treatment group drastically shortened the lifespan of adult female Drosophila melanogaster when compared to 5 % sucrose or 0.1 % DMSO feeding groups. Strikingly, feeding flies with a mixture of 5 nM of compound 1 significantly alleviated the high mortality caused by 5 % DSS treatment (Fig. 6), indicating the protecting potential of compound 1 against chemical damage on flies.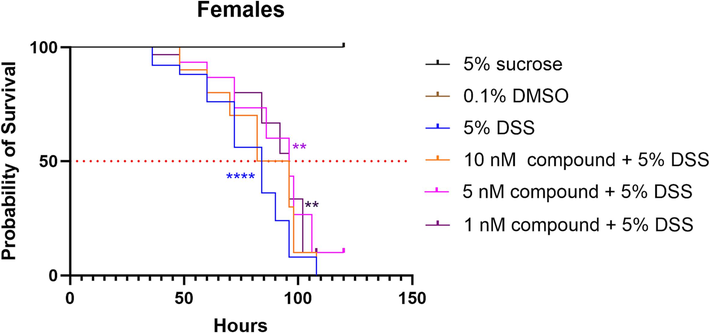
Effects of Spongisulfin A (1) on Drosophila melanogaster survival (**p < 0.01; ****p < 0.0001, Statistical significance: Log rank (Mantel-Cox) test.).
Generally, the activation of the Nrf2-Keap pathway produces high level of ROS (reactive oxygen species) by activation of the expression of its downstream target gene gstd1, causing gut epithelium damage and further adult death. Therefore, the gstd1-gfp reporter line can be used as the read out for ROS generation in the gut. To further evaluate the gut epithelium protective activity of compound 1, the gut RNA from the above groups was extracted and the RT-qPCR experiment was performed to measure the expression level of gstd1, one of the target genes of the Nrf2-Keap1 pathway in the drosophila gut. As shown in Fig. 7, the mRNA of gut gstd1 was significantly increased upon DSS treatment, suggesting the high production of ROS in the gut. Strikingly, the gstd1 expression was greatly decreased in response to DSS treatment when flies fed with 5 nM compound 1, indicating that the compound 1 has the ability to alleviate the DSS induced gut damage by reducing gstd1 expression. Additionally, our immunofluorescence image also confirmed that the ROS level indicated by the gstd1-GFP activity is drastically decreased in the compound 1+DSS treatment group. Taken together, we proposal that the compound 1 may exert its gut protective activity by modulating the ROS level through alleviation the expression of gstd1 in the gut. However, the detail mechanism of this protective action should be further studied.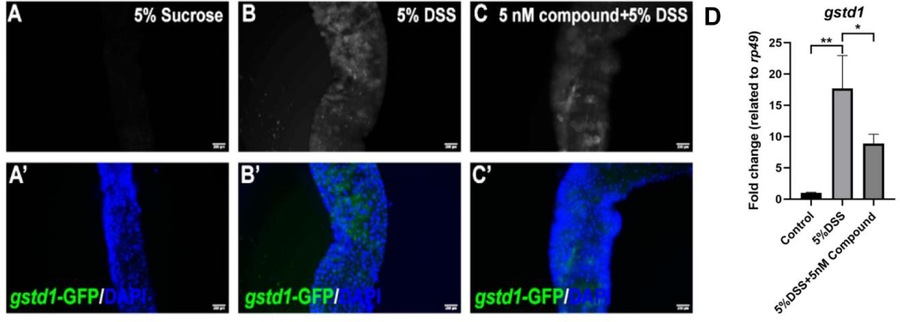
Effects of Spongisulfin A (1) on intestinal ROS production in Drosophila melanogaster.
3.5 Discussion
Rare taxonomic groups of actinomycetes are a source of chemically unique metabolites with diverse activities (Doroghazi et al., 2014). However, the lack of universal and functioning genetic tools hamper mining and further investigation. More than 100 species have been identified in the family Streptosporangiaceae, while few genetic studies on members of limited genera have been reported, such as Nonomuraea (Marcone et al., 2010.) and Streptosporangium (Tian et al., 2016; Xu et al., 2020). The genus Spongiactinospora was established by our group in 2019 (Li et al., 2019a) based on unique phenotypic and genetic characteristics different from members of the family Streptosporangiaceae, and at present, it is known to comprise two species, the type species S. rosea from the marine sponge, and Spongiactinospora gelatinilytica from desert soil (Ay et al., 2020). Similar to the gifted S. rosea, S. gelatinilytica has a large genome (8.0 Mb) as well, and 38 SM-BGCs were predicted by antiSMASH (Saygin et al., 2019). To validate the spo BGC in the biosynthesis of spongisulfins by an in-frame deletion in S. rosea and set the stage for future exploration of other cryptic BGCs in Spongiactinospora, the antibiotic sensitivity and E. coli-Spongiactinospora conjugation are currently being explored.
Angucyclines have diverse biological activities, such as antibacterial, cytotoxicity, enzyme inhibition, and platelet aggregation inhibition (Zhang et al., 2021c). Since the discovery of the first angucycline tetrangomycin from Streptomyces rimosus in 1965 (Dann et al., 1965), the angucycline family has been expanding quickly, and hundreds of angucycline natural products have now been characterized. Relative to the huge amount of monomers, the dimeric angucyclines are reported far lesser, including methylene-linked hatomarubigin D (Hayakawa et al., 1991), C-C coupled lomaiviticins (He et al., 2001) and difluostatins (Yang et al., 2015), sulfur-bridged donghaesulfins (Bae et al., 2019) and thioangucyclines (Cao et al., 2021). Dimerization significantly changes the biological activities of angucyclines, as reported that the heterodimeric lomaiviticin A is more cytotoxic to human cancer cells than its monomers lomaiviticin C and kinamycin C (Colis et al., 2014). Difluostatin A displayed notable antibacterial activities against Klebsiella pneumonia, Aeromonas hydrophila and Staphylococcus aureus, while the monomer fluostatin C showed no antibacterial (Yang et al., 2015). Donghaesulfins displayed quinone reductase inducing or antiangiogenesis activities compared to their monomer rubiginones (Bae et al., 2019). Thioangucyclines have no antibacterial activities against Gram-positive bacteria after dimerization from precursor monomer, which was proposed as an innate detoxification approach for bacteria (Cao et al., 2021). However, none of the spongisulfins and monomers showed significant antibacterial activity and cytotoxicity in this study. Thus, more diverse screening models must be applied in unveiling the bio-potential of the newly discovered sulfur-bridged dimeric angucyclines.
Dimerization is a comprehensive approach for determining the structural complexity and chemical diversity of small molecules, encompassing almost all types of natural products, but most of the mechanisms in biosynthesis are unilluminated (Liu et al., 2021a). Among the limited examples of dimeric angucyclines, the methylene-linked hatomarubigin D has been reported to be generated enzymatically. The putative oxidoreductase HrbY encoded in the hatomarubigin BGC was confirmed as a candidate for catalyzing the methylene bridge formation by gene disruption and hetero-transformation (Izawa et al., 2014; Kawasaki et al., 2010), while the source of the methylene in vivo is still unknown. NmrA-like nicotinamide cofactor-binding regulatory proteins Lom19 and FlsQ1 were implicated as the C-C coupled dimerization enzymes for lomaiviticins and difluostatins (Kersten et al., 2013; Huang et al., 2018), respectively. However, more solid evidence on the functions of NmrA-like proteins is still lacking. Sulfur-bridged dimerization of angucyclines is non-enzymatic, as exemplified by the systematic study on thioangucyclines (Wu et al., 2019), of which the epoxidation at C-6a/C-12a by two monooxygenases TacS-T on the intermediate is critical. As the BGC and biosynthesis pathway of donghaesulfins has not yet been reported, spo of spongisulfins is the only second candidate BGC for sulfur-bridged angucyclines dimers, in which the homolog of TacS-T was not found, indicating a good chance to discover either new enzymes or new mechanism in angucyclines sulfur-bridged dimerization.
4 Conclusions
In summary, this present study reports the discovery of novel sulfur-bridged angucycline dimers, namely spongisulfins A-C (1–3), isolated from the sponge-derived rare actinomycete S. rosea LHW63015, which was guided by the genome sequencing and metabolite profiling. Furthermore, a candidate BGC for the new angucyclines was identified by comprehensive bioinformatic analysis, and the biosynthetic pathway for spongisulfins was proposed based on shared biological chemistry principles of the angucycline family. This study provides evidence that marine sponge-derived rare actinomycetes could be a prolific source of structurally novel scaffolds and offers new insights into the biosynthesis of the natural products of the angucycline family.
CRediT authorship contribution statement
Weizhuo Tang: Methodology, Supervision. Die Zhang: Investigation, Software, Formal analysis, Data curation, Writing – original draft. Jing Xu: Methodology, Data curation. Shuping Wang: Software, Data curation. Bin Wei: Methodology, Supervision. Lei Li: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Acknowledgments
This project is financially supported by National Key Research and Development Program of China (No. 2022YFC2804100), the Natural Science Fund of Hunan Province (No. 2022JJ30638), National Natural Science Foundation of China (Nos. 31600014 and 81903534), the Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZDCX20212702), and the Training Program for Excellent Young Innovators of Changsha (kq2305014). The authors are grateful to State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology for strain.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Diversity, abundance and natural products of marine sponge-associated actinomycetes. Nat. Prod. Rep.. 2014;31(3):381-399.
- [Google Scholar]
- Extracellular vesicles and pasteurized cells derived from Akkermansia muciniphila protect against high-fat induced obesity in mice. Microb. Cell Fact.. 2021;20(1):219.
- [Google Scholar]
- Phylogenomic revision of the family Streptosporangiaceae, reclassification of Desertactinospora gelatinilytica as Spongiactinospora gelatinilytica comb. nov. and a taxonomic home for the genus Sinosporangium in the family Streptosporangiaceae. Int. J. Syst. Evol. Microbiol.. 2020;70(4):2569-2579.
- [Google Scholar]
- Donghaesulfins A and B, dimeric benz[a]anthracene thioethers from volcanic island derived Streptomyces sp. Org. Lett.. 2019;21(10):3635-3639.
- [Google Scholar]
- Gifted microbes for genome mining and natural product discovery. J. Ind. Microbiol. Biotechnol.. 2017;44(4–5):573-588.
- [Google Scholar]
- AntiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res.. 2021;49(W1):W29-W35.
- [Google Scholar]
- Cryptic sulfur incorporation in thioangucycline biosynthesis. Angew. Chem. Int. Ed. Engl.. 2021;60(13):7140-7147.
- [Google Scholar]
- Investigation of the molecular landscape of bacterial aromatic polyketides by global analysis of type II polyketide synthases. Angew. Chem. Int. Ed. Engl.. 2022;61(24):e202202286.
- [Google Scholar]
- Antimicrobial chlorinated carbazole alkaloids from the sponge-associated actinomycete Streptomyces diacarni LHW51701. Chin. J. Chem.. 2021;39(5):1188-1192.
- [Google Scholar]
- Assessing the in vitro binding affinity of protein-RNA interactions using an RNA pull-down technique. Bio Protoc.. 2022;12(23):e4560.
- [Google Scholar]
- The cytotoxicity of (-)-lomaiviticin A arises from induction of double-strand breaks in DNA. Nat. Chem.. 2014;6(6):504-510.
- [Google Scholar]
- Tetrangomycin, a new quinone antibiotic. Antimicrob. Agents Chemother. (Bethesda). 1965;5:832-835.
- [Google Scholar]
- Structure and stereochemical determination of hypogeamicins from a cave-derived actinomycete. J. Nat. Prod.. 2014;77(8):1759-1763.
- [Google Scholar]
- OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst.. 2009;42:339-341.
- [Google Scholar]
- A roadmap for natural product discovery based on large-scale genomics and metabolomics. Nat. Chem. Biol.. 2014;10(11):963-968.
- [Google Scholar]
- MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res.. 2004;32(5):1792-1797.
- [Google Scholar]
- The complete gene cluster of the antitumor agent gilvocarcin V and its implication for the biosynthesis of the gilvocarcins. J. Am. Chem. Soc.. 2003;125(26):7818-7819.
- [Google Scholar]
- Cloning and analysis of the simocyclinone biosynthetic gene cluster of Streptomyces antibioticus Tü 6040. Arch. Microbiol.. 2002;178(2):102-114.
- [Google Scholar]
- Hexaricins, pradimicin-like polyketides from a marine sediment-derived Streptosporangium sp. and their antioxidant effects. J. Nat. Prod.. 2018;81(9):2069-2074.
- [Google Scholar]
- Enzyme function initiative-enzyme similarity tool (EFI-EST): A web tool for generating protein sequence similarity networks. BBA. 2015;1854(8):1019-1037.
- [Google Scholar]
- Clinker & clustermap.js: automatic generation of gene cluster comparison figures. Bioinformatics. 2021;37(16):2473-2475.
- [Google Scholar]
- Gene cluster activation in a bacterial symbiont leads to halogenated angucyclic maduralactomycins and spirocyclic actinospirols. Org. Lett.. 2020;22(7):2634-2638.
- [Google Scholar]
- Studies on the isotetracenone antibiotics. IV. Hatomarubigins A, B, C and D, new isotetracenone antibiotics effective against multidrug-resistant tumor cells. J. Antibiot.. 1991;44(11):1179-1186.
- [Google Scholar]
- Lomaiviticins A and B, potent antitumor antibiotics from Micromonospora lomaivitiensis. J. Am. Chem. Soc.. 2001;123(22):5362-5363.
- [Google Scholar]
- Strategies to access biosynthetic novelty in bacterial genomes for drug discovery. Nat. Rev. Drug Discov.. 2022;21(5):359-378.
- [Google Scholar]
- Type II polyketide synthases: Gaining a deeper insight into enzymatic teamwork. Nat. Prod. Rep.. 2007;24(1):162-190.
- [Google Scholar]
- Evolution of chemical diversity by coordinated gene swaps in type II polyketide gene clusters. PNAS. 2015;112(45):13952-13957.
- [Google Scholar]
- Chemical and biological diversity of new natural products from marine sponges: A review (2009–2018) Mar. Life Sci. Technol.. 2022;4:356-372.
- [Google Scholar]
- Molecular basis of dimer formation during the biosynthesis of benzofluorene-containing atypical angucyclines. Nat. Commun.. 2018;9(1):2088.
- [Google Scholar]
- Functional analysis of hatomarubigin biosynthesis genes and production of a new hatomarubigin using a heterologous expression system. J. Antibiot.. 2014;67(2):159-162.
- [Google Scholar]
- Isolation, structure elucidation and biosynthesis of benzo[b]fluorene nenestatin A from deep-sea derived Micromonospora echinospora SCSIO 04089. Tetrahedron. 2017;73(26):3585-3590.
- [Google Scholar]
- Anti-MRSA actinomycins D1–D4 from the marine sponge-associated Streptomyces sp. LHW52447. Tetrahedron. 2018;74(40):5914-5919.
- [Google Scholar]
- Cloning and characterization of a gene cluster for hatomarubigin biosynthesis in Streptomyces sp. strain 2238-SVT4. Appl. Environ. Microbiol.. 2010;76(13):4201-4206.
- [Google Scholar]
- Bioactivity-guided genome mining reveals the lomaiviticin biosynthetic gene cluster in Salinispora tropica. Chembiochem. 2013;14(8):955-962.
- [Google Scholar]
- Angucyclines: biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep.. 2012;29(2):264-325.
- [Google Scholar]
- SNA-8073-B, a new isotetracenone antibiotic inhibits prolyl endopeptidase. I. Fermentation, isolation and biological properties. J. Antibiot.. 1997;50(4):291-296.
- [Google Scholar]
- MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol.. 2016;33(7):1870-1874.
- [Google Scholar]
- Spongiactinospora rosea gen. nov., sp. nov., a new member of the family Streptosporangiaceae. Int. J. Syst. Evol. Microbiol.. 2019;69(2):427-433.
- [Google Scholar]
- Streptomyces reniochalinae sp. nov. and Streptomyces diacarni sp. nov., from marine sponges. Int. J. Syst. Evol. Microbiol.. 2019;69(1):99-104.
- [Google Scholar]
- Actinomadura craniellae sp. nov., isolated from a marine sponge in the South China Sea. Int. J. Syst. Evol. Microbiol.. 2019;69(4):1207-1212.
- [Google Scholar]
- Geodermatophilus marinus sp. nov., isolated from the marine sponge Leucetta chagosensis. Int. J. Syst. Evol. Microbiol.. 2019;69(10):2966-2971.
- [Google Scholar]
- Micromonospora craniellae sp. nov., isolated from a marine sponge, and reclassification of Jishengella endophytica as Micromonospora endophytica comb. nov. Int. J. Syst. Evol. Microbiol.. 2019;69(3):715-720.
- [Google Scholar]
- Molecular regulation of antibiotic biosynthesis in streptomyces. Microbiol. Mol. Biol. Rev.. 2013;77(1):112-143.
- [Google Scholar]
- Enzymatic dimerization in the biosynthetic pathway of microbial natural products. Nat. Prod. Rep.. 2021;38(8):1469-1505.
- [Google Scholar]
- Divergent syntheses of pyridoacridine alkaloids via palladium-catalyzed reductive cyclization with nitro-biarenes. Chin. J. Chem.. 2021;39:1905-1910.
- [Google Scholar]
- Structural and biological insights into the hot-spot marine natural products reported from 2012 to 2021. Chin. J. Chem.. 2022;40(15):1867-1889.
- [Google Scholar]
- Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364(6445):eaau6323.
- [Google Scholar]
- Methods for the genetic manipulation of Nonomuraea sp. ATCC 39727. J. Ind. Microbiol. Biotechnol.. 2010;37(10):1097-1103.
- [Google Scholar]
- Antibacterial aromatic polyketides incorporating the unusual amino acid enduracididine. J. Nat. Prod.. 2019;82(1):35-44.
- [Google Scholar]
- Parallel lives of symbionts and hosts: chemical mutualism in marine animals. Nat. Prod. Rep.. 2018;35(4):357-378.
- [Google Scholar]
- A computational framework to explore large-scale biosynthetic diversity. Nat. Chem. Biol.. 2020;16(1):60-68.
- [Google Scholar]
- Chemical and biological properties of rubiginone, a complex of new antibiotics with vincristine-cytotoxicity potentiating activity. J. Antibiot.. 1990;43(8):967-976.
- [Google Scholar]
- Fujianmycins A and B, new benz[a]anthraquinone antibiotics from a Streptomyces species. J. Antibiot.. 1985;38(4):513-515.
- [Google Scholar]
- The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol.. 1987;4(4):406-425.
- [Google Scholar]
- Desertiactinospora gelatinilytica gen. nov., sp. nov., a new member of the family Streptosporangiaceae isolated from the Karakum Desert. Antonie Van Leeuwenhoek. 2019;112(3):409-423.
- [Google Scholar]
- Substrate-assisted catalysis in polyketide reduction proceeds via a phenolate intermediate. Cell Chem. Biol.. 2016;23(9):1091-1097.
- [Google Scholar]
- Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res.. 2003;13(11):2498-2504.
- [Google Scholar]
- Genome-guided discovery of highly oxygenated aromatic polyketides, saccharothrixins D-M, from the rare marine actinomycete Saccharothrix sp. D09. J. Nat. Prod.. 2021;84(11):2875-2884.
- [Google Scholar]
- MIBiG 3.0: a community-driven effort to annotate experimentally validated biosynthetic gene clusters. Nucleic Acids Res.. 2023;51(D1):D603-D610.
- [Google Scholar]
- Discovery of pentangular polyphenols hexaricins A-C from marine Streptosporangium sp. CGMCC 4.7309 by genome mining. Appl. Microbiol. Biotechnol.. 2016;100(9):4189-4199.
- [Google Scholar]
- G-PRIMER: greedy algorithm for selecting minimal primer set. Bioinformatics. 2004;20(15):2473-2475.
- [Google Scholar]
- Yangpumicins F and G, enediyne congeners from Micromonospora yangpuensis DSM 45577. J. Nat. Prod.. 2019;82(9):2483-2488.
- [Google Scholar]
- An atlas of bacterial secondary metabolite biosynthesis gene clusters. Environ. Microbiol.. 2021;23(11):6981-6992.
- [Google Scholar]
- An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature. 2014;506(7486):58-62.
- [Google Scholar]
- Fuscasins A-D, cycloheptapeptides from the marine sponge Phakellia fusca. J. Nat. Prod.. 2019;82(4):970-979.
- [Google Scholar]
- Lugdunomycin, an angucycline-derived molecule with unprecedented chemical architecture. Angew. Chem. Int. Ed. Engl.. 2019;58(9):2809-2814.
- [Google Scholar]
- Functional and structural insights into a novel promiscuous ketoreductase of the lugdunomycin biosynthetic pathway. ACS Chem. Biol.. 2020;15(9):2529-2538.
- [Google Scholar]
- Discovery of a novel analogue of FR901533 and the corresponding biosynthetic gene cluster from Streptosporangium roseum No. 79089. Appl. Microbiol. Biotechnol.. 2020;104(16):7131-7142.
- [Google Scholar]
- Genome mining of Micromonospora yangpuensis DSM 45577 as a producer of an anthraquinone-fused enediyne. Org. Lett.. 2017;19(22):6192-6195.
- [Google Scholar]
- Madurastatin D1 and D2, oxazoline containing siderophores isolated from an Actinomadura sp. Org. Lett.. 2019;21(16):6275-6279.
- [Google Scholar]
- Heterologous expression of fluostatin gene cluster leads to a bioactive heterodimer. Org. Lett.. 2015;17(21):5324-5327.
- [Google Scholar]
- Landomycin biosynthesis and its regulation in Streptomyces. Appl. Microbiol. Biotechnol.. 2019;103(4):1659-1665.
- [Google Scholar]
- Dassonmycins A and B, polycyclic thioalkaloids from a marine sponge-derived nocardiopsis dassonvillei SCSIO 40065. Org. Lett.. 2021;23(8):2858-2862.
- [Google Scholar]
- Novel angucycline/angucyclinone family of natural products discovered between 2010 and 2020. Sheng Wu Gong Cheng Xue Bao. 2021;37(6):2147-2165.
- [Google Scholar]
- Rhodococcus spongiicola sp. nov. and Rhodococcus xishaensis sp. nov., from marine sponges. Int. J. Syst. Evol. Microbiol.. 2021;71(7)
- [Google Scholar]
- Genome mining of novel rubiginones from Streptomyces sp. CB02414 and characterization of the post-PKS modification steps in rubiginone biosynthesis. Microb. Cell Fact. 2021;20(1):192.
- [Google Scholar]
- Micromonospora yangpuensis sp. nov., isolated from a sponge. Int. J. Syst. Evol. Microbiol.. 2012;62(Pt 2):272-278.
- [Google Scholar]
- Marinacarboline glucuronide, a new member of β-carboline alkaloids from sponge-derived actinomycete Actinoalloteichus cyanogriseus LHW52806. J. Antibiot.. 2022;75(9):523-525.
- [Google Scholar]
- A marine microbiome antifungal targets urgent-threat drug-resistant fungi. Science. 2020;370(6519):974-978.
- [Google Scholar]
- Identification of the protective effects of traditional medicinal plants against SDS-induced Drosophila gut damage. Exp. Ther. Med.. 2016;12:2671-2680.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105687.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







