Translate this page into:
Structural characterization of chlorogenic acid-metal complexes derived from the aqueous extracts of medicinal plants and their DNA cleavage and antibacterial activities
⁎Corresponding authors at: Engineering Center of Jiangxi University for Fine Chemicals, School of Pharmacy, Jiangxi Science and Technology Normal University, No. 605, Fenglin Avenue, Nanchang 330013, PR China. pengliang8036@163.com (Liang Peng), Lzmin3026@163.com (Zhimin Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The discovery of organic components with pharmacological activities in medicinal plants is crucial for new drug development. We examined chlorogenic acid (CGA) using high-performance liquid chromatography to analyze the aqueous and methanolic extracts of medicinal plants dissolved in methanol or methanol containing 15% formic acid. We found that for the aqueous extract, the peak areas of various components dissolved in 15% formic acid methanol were significantly larger than those dissolved in methanol. For the differences in the methanolic extract, the peak areas of all chromatographic peaks were relatively small between the samples dissolved in the two solvents. Electrospray ionization time-of-flight mass spectrometry showed that CGA could react in hot water with the eight most common plant metal ions (Ca2+, Cu2+, Fe3+, Mg2+, Mn2+, Ni2+, Sr2+, and Zn2+) to form complexes with different coordination forms. The bioactivity experiments showed that CGA-Cu complexes could cause DNA cleavage through oxidative damage. CGA-Ni, CGA-Mn, and CGA-Zn showed significantly stronger inhibition against Staphylococcus aureus than CGA and the corresponding metal ions. These results suggest that, during the decoction process of medicinal plants, CGA forms complexes with metal ions, which are likely to be one of the active ingredients in medicinal plants. Subsequently, further research is needed on the antibacterial mechanism of chlorogenic acid-metal complexes not mentioned in this article.
Keywords
Medicinal plant
Chlorogenic acid
Metal complexing
DNA cleavage
Antibiotics
- CGA
-
chlorogenic acid
- HPLC
-
high-performance liquid chromatography
- FA
-
formic acid
- UV–Vis
-
Ultraviolet–visible spectrophotometry
- ESI-TOF MS
-
electrospray ionization time-of-flight mass spectrometry
- ICP-MS
-
inductively coupled plasma mass spectrometer
- DMSO
-
dimethyl sulfoxide
- TSB
-
tryptic soy broth
- SC
-
super-coiled conformation
- NC
-
nicked circular
- L
-
linear
Abbreviations
1 Introduction
The discovery of bioactive lead compounds in plants and their subsequent structural modification is an effective strategy for the research and development of innovative drugs, such as paclitaxel and vincristine for treating cancer; artemether and artemotil for treating malaria, and galantamine for treating Alzheimer’s disease. The organic components in medicinal plants, which contain functional groups such as phenolic hydroxyl, sulfhydryl, and amine groups, are likely to form complexes with metal ions during extraction in medicinal plants. For example, Ruzik and Kwiatkowski (2018) detected the formation of numerous zinc (Zn) complexes with organic components in goji berry extract. Wojcieszek and Ruzik (2016) and Wojcieszek et al. (2017) detected the formation of various copper (Cu) complexes with phenolic compounds in acai and bilberry extracts. Borowska et al. (2020) detected the formation of Zn and Cu complexes with polyphenolic ingredients in chokeberry extract. Furthermore, previous studies have also shown that complexation between organic components and metal ions may enhance pharmacological activity. For example, Rutin-Zn (Ikeda et al., 2015), emodin-manganese (Mn) (Yang et al., 2014) and emodin-Cu (Mandal et al., 2017) complexes all have anticancer activities obviously stronger than their corresponding ligands and metal ions. The Rutin-nickel (Ni) complex not only has stronger antioxidant activity than free Rutin but also produces DNA cleavage effects absent from Rutin or Ni2+ alone (Raza et al., 2017). Furthermore, quercetin-iron complexes showed more potent antioxidant, antibacterial, and anti-obesity activities than quercetin alone (Imessaoudene et al., 2016), whereas also producing DNA cleavage activity that was absent when quercetin or iron ions acted alone (Raza et al., 2016). These findings suggest that the metal complexes formed between the organic components and the metal ions of plants during the extraction process show good bioactivity and warrant further investigation.
Chlorogenic acid (CGA) is a widely distributed phenolic compound in plants, mainly in roots, stems, flowers, leaves, and fruits. Modern pharmacological studies have found that CGA has antibacterial, antiviral, anticancer, antioxidant activities (Cai et al., 2019a), and is a potential treatment for Parkinson's disease (Singh et al., 2018; Singh et al., 2020). The structure of CGA (Fig. S1) comprises O-diphenol hydroxyl and carboxyl groups, which have relatively large conjugated systems and can easily form complexes with metal ions. In this study, high-performance liquid chromatography (HPLC) was time for the first implemented to analyze the aqueous and methanolic extracts of medicinal plants dissolved in methanol and 15% formic acid (FA) + methanol to confirm the presence of CGA-metal complexes in the aqueous extracts of plants. Ultraviolet–visible spectrophotometry (UV–Vis) and electrospray ionization time-of-flight mass spectrometry (ESI-TOF MS) were used to derive the structural characterization of the products of the reactions between CGA and the eight most common metal ions in plants (Ca2+, Cu2+, Fe3+, Mg2+, Mn2+, Ni2+, Sr2+, and Zn2+) in hot water. Finally, the DNA cleavage and anti-Staphylococcus aureus activities of the reaction products were investigated. The search for lead compounds from natural medicinal plants mainly focuses on the organic components. This study indicates that there are various metal complexes in the aqueous extracts of natural medicinal plants, which may have good biological activities. Thus, this study will help identify new pharmacodynamic components of natural medicinal plants.
2 Materials and methods
2.1 Materials and reagents
Jin Yin Hua (JYH), Yu. Xing Cao (YXC), Du Zhong (DZ), and Sang Ye (SY) were purchased from Kangmei Pharmaceutical Co., Ltd. (Guangdong, China). Chinese herbs were identified by the Department of Pharmaceutical Botany, School of Pharmacy, Jiangxi Science and Technology Normal University (Nanchang, China). JYH was identified as dried buds or the first blooming flowers of Lonicera japonica (Caprifoliaceae). YXC was identified as the dried aerial part of Houttuynia cordata Thunb. (Saururaceae). DZ was identified as the dried bark of Eucommia ulmoides Oliver (Eucommiaceae). SY was identified as the dried leaf of Morus alba L. (Moraceae).
CGA (purity > 98%) was purchased from Chengdu Desite Biotechnology Co., Ltd. (Chengdu, China). Methanol (MS-grade) was purchased from Merck KGaA (Darmstadt, Germany). FA (LC grade) was purchased from Aladdin Co., Ltd. (Shanghai, China). Anhydrous calcium chloride, copper chloride dihydrate, ferric chloride hexahydrate, magnesium chloride hexahydrate, manganese chloride tetrahydrate, nickel dichloride hexahydrate, strontium chloride hexahydrate, and anhydrous zinc chloride were purchased from Xilong Chemical Co., Ltd. (Guangdong, China). The pBR322 DNA was purchased from Sangon Biotech (Shanghai, China). S. aureus ATCC 25923 was purchased from the Henan Engineering Research Center of Industrial Microbial Strains (He Nan, China).
2.2 Instruments and equipment
The following instruments and equipment were used in this study: UV-2550 UV–Vis (Shimadzu Corp., Kyoto, Japan); Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, CA, USA); Xevo G2-XS ESI-TOF-MS (Waters Corp., Milford, MA, USA); XS203S electronic precision balance (Mettler Toledo GmbH, Columbus, OH, USA); Hei-VAP rotary evaporator (Heidolph Instruments, Schwabach, Germany); Agilent 7700 inductively coupled plasma mass spectrometer (ICP-MS); DF-101S heat-collecting, constant-temperature magnetic stirrer (Henan Gongyi Yuhua Instrument Co., Ltd., Henan, China) with heating (Henan Gongyi Yuhua Instrument Co., Ltd., Henan, China); Mini-PROTEAN4 electrophoresis system (with PowerPac Basic power supply) (Bio-Rad Laboratories, Hercules, CA, USA); JY04s-3c gel imaging analysis system (Beijing Junyi Electrophoresis Co., Ltd., Beijing, China); LDZH-50KBS vertical pressure steam sterilizer (Shanghai ShenAn Medical Instrument Factory, Shanghai, China); and SPX-150 biochemical incubator (Shanghai Ke Heng Industrial Co., Ltd., Shanghai, China).
2.3 Confirming the presence of CGA-metal complexes in plant extracts
Four kinds of medicinal plants with high CGA content were selected: JYH, YXH, DZ, and SY. They were crushed and passed through a 20-mesh sieve, dried in an oven at 50 °C to a constant weight, and distilled water was added in a solid-to-liquid ratio of 1:20. The mixture was then soaked at 25 °C for 30 min, refluxed at 100 °C for 3 h, and filtered under reduced pressure. The filtrate was accurately divided into two aliquots and concentrated to dryness under reduced pressure to obtain the aqueous extract. The aqueous extract was then dissolved in an equal volume of methanol or 15% FA-methanol, ultrasonicated for 2 min, filtered with a 0.22 µm filter membrane, and subjected to HPLC.
The same methods were applied to prepare the methanol extracts of the four medicinal plants, which were then dissolved in methanol or 15% FA-methanol (extraction temperature: 70 °C) and subjected to HPLC.
The HPLC conditions were as follows: chromatography column, Agilent Eclipse XDB-C18 (4.6 × 250 mm, 5 µm); detection wavelength, 327 nm; column temperature, 30 °C; flow rate, 1.0 mL/min; injection volume, 10 μL; mobile phase A for 0.1% FA-H2O and C for methanol. The gradient elution conditions for the JYH, DZ, and SY samples were as follows: 0–90 min, 10% C → 100% C; 90–100 min, 100% C. The gradient elution conditions for the YXC samples were as follows: 0–5 min, 20% C; 5–10 min, 20–30% C; 10–15 min, 30% C; 15–70 min, 30% B–100% C; 70–80 min, 100% C.
Where PAFM is the peak area of samples dissolved in 15% FA-methanol, and PAM is the peak area of samples dissolved in methanol.
2.4 Measurement of the metal-ion content in the aqueous extracts of the four medicinal plants
The aqueous extracts of the four medicinal plants were prepared according to the methods described in Section 3.1. After drying in an oven at 50 °C to a constant weight, 100.00 mg of the samples were accurately weighed, dissolved in methanol or 15% FA-methanol using an ultrasonicator, centrifuged at 5,000 × g, and concentrated until dryness under reduced pressure. The dried samples were transferred to the container using 8 mL of aqua regia, to which 2 mL of hydrogen peroxide was added. Subsequently, they were well mixed and heated at 150 °C for 40 min. Subsequently, the samples were cooled to 25 °C and filtered, and the filtrate was diluted with ultrapure water to 50 mL for ICP-MS detection.
ICP-MS was performed under the following conditions: radio frequency power, 1.5 kW; plasma gas flow rate, 15.0 L/min; auxiliary gas flow rate, 1.00 L/min; and atomizer flow rate, 0.10 L/min.
2.5 Structural characterization of CGA-metal complexes
2.5.1 UV–vis detection
UV–vis absorption spectra of chlorogenic acid and chlorogenic acid-metal complexes were determined by a UV–Vis spectrophotometer (Cai et al., 2019b). CGA (0.02 mmol) was accurately weighed and placed with 0.01 mmol of anhydrous calcium chloride, magnesium chloride hexahydrate, anhydrous zinc chloride, ferric chloride hexahydrate, copper chloride dihydrate, nickel dichloride hexahydrate, manganese chloride tetrahydrate, or strontium chloride hexahydrate in a round-bottom flask. Then, 20 mL of distilled water was added and mixed well, which reacted at 100 °C while heating under reflux and magnetic stirring for 3 h. Two aliquots (2 mL each) were obtained from the reaction solution, while hot and diluted 10 times with distilled water and 15% FA-H2O solution preheated to 75 °C. After mixing, the solution was centrifuged at 6,000 × g for 8 min, and the supernatant was obtained, the UV–Vis spectrum was measured at the wavelength range of 240 to 700 nm. The same methods were used to measure the UV–Vis absorption spectrum of CGA.
2.5.2 ESI-TOF MS analysis of CGA-metal complexes
The reaction solution (2 mL) was centrifuged at 6,000 × g for 8 min and the resulting supernatant was subjected to ESI-TOF MS analysis. ESI was performed in the sample injection positive-ion mode, the sample injection infusion method was adopted for MS, the injection flow rate was 10 µL/min, and the mass detection range was 100–1,500. The drying gas flow rate was 800 L/h, and the temperature was 500 °C (Cai et al., 2019b).
2.5.3 Identification of ferrous ions
Distilled water (20 mL) was added to 0.02 mol of CGA, 0.01 mol of FeCl3, 0.01 mol of FeCl2, and the mixture of 0.02 mol of CGA and 0.01 mol of FeCl3 and reacted under the conditions described in Section 3.3.1. Then, 2 mL of the reaction solution was centrifuged at 6,000 × g for 8 min, followed by the addition of 1 mL of 1,10-phenanthroline solution with a mass fraction of 0.1% (first dissolved with a little ethanol, and then diluted with distilled water) and 1 mL acetic acid-sodium acetate buffer solution (pH 4.5) to the supernatant. After well mixing, the solution was left to stand for 150 s and color changes were observed.
2.6 DNA cleavage activity of CGA-metal complexes
2.6.1 DNA cleavage activity
Reactions were performed according to the conditions described previously (Wang J. et al., 2010). The reaction solution was concentrated to dryness under reduced pressure and dissolved in dimethyl sulfoxide (DMSO) to prepare a 4 mmol/L solution (calculated based on the molar mass of CGA) for later use. In addition, equal amounts of CGA and eight types of metal salts were obtained to prepare solutions of equal concentrations using DMSO.
Eight microliters of distilled water, 10 µL of Buffer 1, 1 µL of sample solution, and 1 µL of pBR322 DNA were well mixed and allowed to react at 37 °C for 24 h. Once the reaction was complete, 4 µL of Buffer 2 was added, mixed well, and 5 µL of the resulting solution was loaded into the wells of agarose gel (0.32 g agarose, 40 mL Buffer 3) for electrophoresis at 120 mV for 1 h. The gel was then stained with 0.5 mg/L of Gel Red solution for 2 h, and the results were observed and recorded using a gel imager.
2.6.2 Mechanism of DNA cleavage by CGA-Cu complexes
Ten microliters of Buffer 1, 1 µL of CGA and Cu2+ reaction products in DMSO solution (CGA final concentration: 100 μmol/L, Cu2+ final concentration: 50 μmol/L), 1 µL pBR322 DNA, and 8 µL of distilled water were mixed, followed by the addition of NaN3 (final concentration: 100 mmol/L), EDTA (final concentration: 5 mmol/L), TEMPO (final concentration: 10 mmol/L), mannitol (final concentration: 100 mmol/L), catalase (CAT, final concentration: 4 U/µL), or superoxide dismutase (SOD, final concentration: 4 U/µL). After well mixing well and reacting at 37 °C for 1 h, 4 µL of Buffer 2 was added, and gel electrophoresis was performed according to the methods described in Section 3.4.1.
-
Buffer 1: 10 mmol/L KH2PO4, 10 mmol/L NaCl, pH 7.2
-
Buffer 2: 0.05% bromophenol blue, 36% glycerol, and 30 mmol/L of Na2H2 EDTA
-
Buffer 3: 89 mmol/L tris–borate, 2 mmol/L Na2H2 EDTA
2.7 Antibacterial activity of CGA-metal complexes
Reactions were performed using 0.12 mmol of CGA and 0.06 mmol each of the eight metal salts according to the reaction conditions described previously. Briefly, the reaction solution was concentrated to dryness under reduced pressure, and distilled water containing 20% DMSO (V/V) was used to prepare a 24 mmol/L drug solution (calculated based on the molar mass of CGA) for later use.
Staphylococcus aureus was inoculated into fresh tryptic soy broth (TSB), incubated at 37 °C and 220 rpm for 12 h, and then transferred to fresh TSB at an inoculum size of 0.1% volume fraction for later use.
A set of seven sterilized test tubes were prepared for each group, 1 mL of drug solution was added to the first test tube, 500 μL of distilled water was added to the 2nd–7th test tubes, and the 7th test tube was used as the blank control. Then 500 μL of drug solution was obtained from the first test tube, added to the second test tube, and mixed well. Two-fold serial dilutions were performed in this manner until the sixth test tube, from which 500 μL of solution was obtained and discarded. A bacterial solution (2 mL) was added to each of the seventh test tubes, then incubated at 37 °C for 18 h. Next, 10 μL of the solution was obtained in the test tube, smeared on prepared solid medium, and incubated at 37 °C for another 18 h, and the results were recorded.
3 Results
3.1 Confirming the presence of CGA-metal complexes in plant extracts
3.1.1 Investigation on linear relationships and precision
Methanol was used to prepare a 3.2 mmol/L CGA stock solution and diluted twice to arrive at sample concentrations of 1.6, 0.8, 0.4, 0.2, 0.1, 0.05, 0.025, and 0.0125 mmol/L. Quantitative measurement of the CGA peak area was performed based on the HPLC conditions described in Section 2.3. Using the CGA peak area as the y-axis and the sample concentration as the x-axis, we obtained the regression equation for CGA: y = 8.559x − 121.39 (R2 = 0.9993). This implies that CGA shows a good linear relationship within the range of 0.0125–3.2 mmol/L.
Chlorogenic acid control solutions of 0.0125, 0.8, and 3.2 mmol/L were injected six times and analyzed according to HPLC conditions in Section 3.1. The relative standard deviation of the peak area was less than 2%, suggesting that the instrument had good precision for high-, medium-, and low-concentration samples.
3.1.2 Effect of different extraction solvents on the formation of CGA-metal complexes
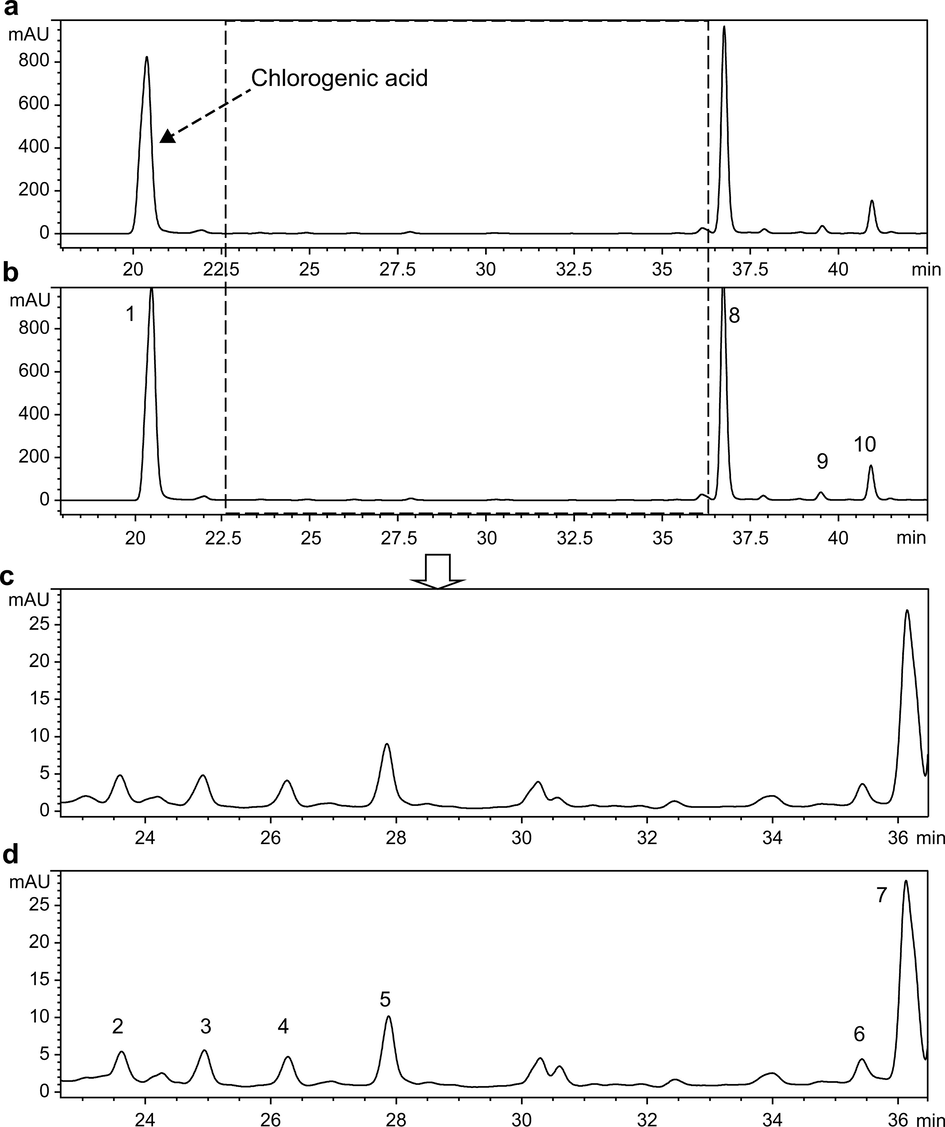
CGA dissolved in methanol showed maximum absorbance at 243, 296, and 327 nm. To reduce the interference of impurities, 327 nm was selected as the detection wavelength for HPLC. The HPLC chromatograms of aqueous and methanolic extracts of JYH dissolved in equal volumes of 15% FA-methanol and methanol are shown in Figs. 1 and 2, whereas the detection results are shown in Tables 1. The peak area increase of CGA in the plant extract samples dissolved in two solvents is shown in Fig. 3.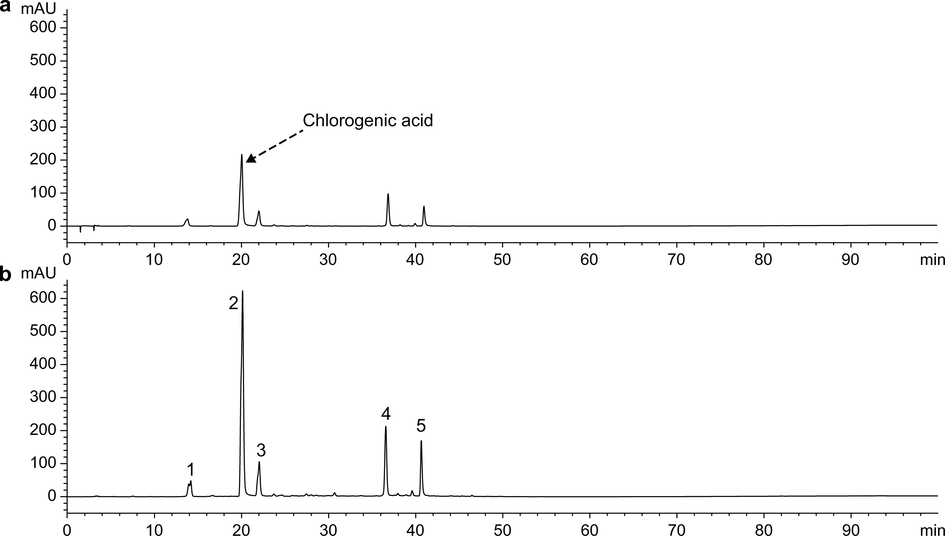
HPLC spectra of aqueous extracts of JYH dissolved in two solvents (327 nm). (a) Samples dissolved in methanol. (b) Samples dissolved in FA-methanol.
HPLC spectra of methanol extracts of JYH dissolved in two solvents (327 nm). (a) Samples dissolved in methanol. (b) Samples dissolved in FA-methanol.
Sample
Peak no.
Methanol
FA-methanol
Increase in peak area (%)
Retention time (min)
Peak area
Retention time (min)
Peak area
aqueous extracts
1
13.8 ± 0.39
550.8 ± 38.56
14.17 ± 0.99
599.4 ± 38.36
8.82
2
20.04 ± 0.46 (CGA)
4,687.7 ± 285.95
20.11 ± 1.39
12,116.7 ± 654.3
158.48
3
22.01 ± 0.22
904.2 ± 57.87
22.03 ± 1.41
1964.9 ± 117.89
117.31
4
36.64 ± 0.81
1,508.4 ± 104.08
36.56 ± 2.52
3,307.5 ± 198.45
119.27
5
40.96 ± 0.9
825.4 ± 46.22
40.64 ± 2.56
2,136.4 ± 134.59
158.83
methanol extracts
1
20.38 ± 1.3 (CGA)
17,954.7 ± 1,167.06
20.52 ± 1.4
18,604.2 ± 948.81
3.62
2
23.60 ± 0.82
51.45 ± 14.86
23.62 ± 1.32
58.47 ± 15.24
13.64
3
24.92 ± 1.29
68.95 ± 37.29
24.94 ± 1.88
69.84 ± 29.82
1.29
4
26.26 ± 1.56
54.40 ± 12.47
26.28 ± 0.94
56.90 ± 9.64
4.60
5
27.85 ± 2.47
127.61 ± 23.85
27.88 ± 1.99
132.68 ± 19.75
3.97
6
36.15 ± 1.99
432.58 ± 23.79
36.13 ± 1.81
442.02 ± 28.73
2.18
7
36.76 ± 2.35
12,583.2 ± 805.32
36.74 ± 1.98
12,982.5 ± 714.04
3.17
8
37.9 ± 2.39
270.46 ± 13.52
37.87 ± 2.65
276.47 ± 18.8
2.22
9
39.54 ± 2.14
459.46 ± 29.86
39.5 ± 2.61
455.41 ± 30.51
−0.88
10
40.95 ± 2.33
2,020.61 ± 105.07
40.92 ± 2.5
2,075.59 ± 105.86
2.72
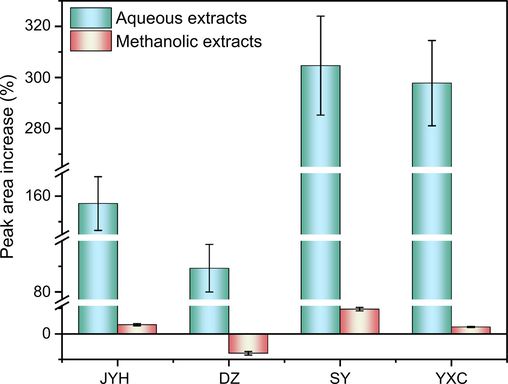
Peak area increase (%) of CGA in the four plant extracts dissolved in two solvents (n = 3).
As shown in Figs. 1 and 2, the number and retention time of the chromatographic peaks were essentially the same for aqueous and methanolic extracts of JYH dissolved in methanol and 15% FA-methanol. This indicates that adding an appropriate amount of FA to methanol did not lead to changes in the types of chemical compounds. Based on the data in Table 1, the samples of aqueous extracts dissolved in 15% FA-methanol showed significantly higher peak areas for various compounds, including CGA, and the peak area for CGA increased by 158.4%. In contrast, the methanolic extract samples dissolved in the two solvents showed increases in the peak area of less than 10%, of which the peak area of CGA in samples dissolved with 15% FA-methanol increased by only 3.62%. The HPLC results of extracts from the other three medicinal plants were like those of JYH (HPLC spectra and results for the other three extracts are shown in Figs. S2–S7 and Tables S1–S3).
3.2 Measurement of metal-ion content in the aqueous extracts of the four medicinal plants
Previous studies have found that samples of aqueous plant extracts dissolved in 15% FA-methanol showed varying degrees of increase in the peak areas of the organic components compared to samples dissolved in methanol. Therefore, ICP-MS was performed to determine the contents of eight metal ions (Ca2+, Cu2+, Fe3+, Mg2+, Mn2+, Ni2+, Sr2+ and Zn2+) in the two types of samples. As shown in Fig. 4, the contents of the eight metal elements in the aqueous extract of JYH dissolved in 15% FA-methanol increased to different degrees compared to samples dissolved in methanol. This suggests that the addition of an appropriate amount of FA to methanol can promote the decomposition of some metal complexes that are insoluble in methanol, increasing the content of metal elements. Furthermore, among the eight metal elements, Mn showed the largest increase (1,300%), whereas Zn showed the smallest increase (12.31%), reflecting the varied abilities of different metals to form complexes with organic components or substantial differences in the stabilities of the metal complexes (the contents of the eight metal ions in four aqueous extracts dissolved in different solvents are shown in Table S4).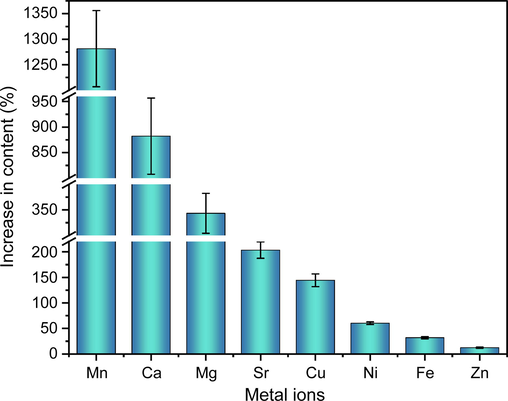
Content increase (%) of the eight metal ions in JYH aqueous extract dissolved in two solvents (n = 3).
3.3 Structural characterization of CGA-metal complexes
3.3.1 UV–vis analysis
The UV–Vis absorption spectra of the CGA and CGA-Cu complexes dissolved in water and 15% FA-H2O are shown in Fig. 5. The figure shows that the absorption spectra of CGA were essentially consistent between water and 15% FA-H2O. The maximum absorption wavelength was 327 nm, and the absorbance increased from 1.39 (water) to 1.46 (15% FA-H2O), which suggests that adding an appropriate amount of FA did not affect the UV–Vis spectra of CGA. The UV–Vis absorption spectrum of the CGA and Cu2+ reaction supernatant showed notable changes compared to that of the CGA. Although the maximum absorption wavelength remained at 327 nm, the absorbance at this wavelength decreased by 69.09%, which implies that new products were formed by the reaction between CGA and Cu2+ in hot water. Furthermore, the addition of FA to the reaction solution caused the absorbance at 327 nm to increase from 0.43 to 0.61, which may be because of the decomposition of some CGA-Cu complexes into CGA and Cu2+ in the FA-H2O solution. The UV–Vis absorption spectra of the CGA and Fe3+ reaction supernatants (Fig. S8-d) showed changes similar to those presented in Fig. 5. After CGA reacted with the other six metal ions (Fig. S8), the maximum absorption wavelength was also at 327 nm; however, the absorbance at this wavelength increased to varying degrees compared to that of CGA. Adding an appropriate amount of FA led to different changes in absorbance. Specifically, the absorbance of the reaction supernatant for CGA with Ca2+, Mg2+, and Ni2+ decreased to different extents, that with Zn2+ basically remained unchanged, and that with Mn2+ and Sr2+ increased to different extents. Thus, CGA can form complexes with all eight metal ions in hot water, and these complexes can be partially decomposed in an acidic solution.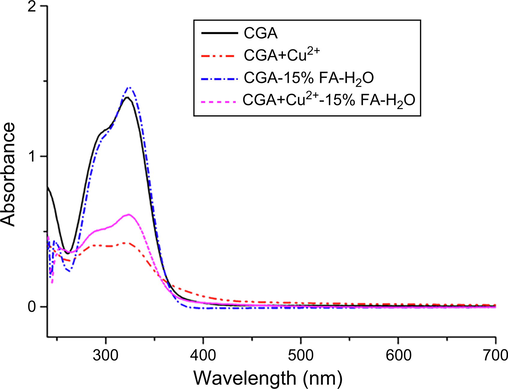
UV–Vis absorption spectra of CGA and CGA-Cu complexes in different solvents.
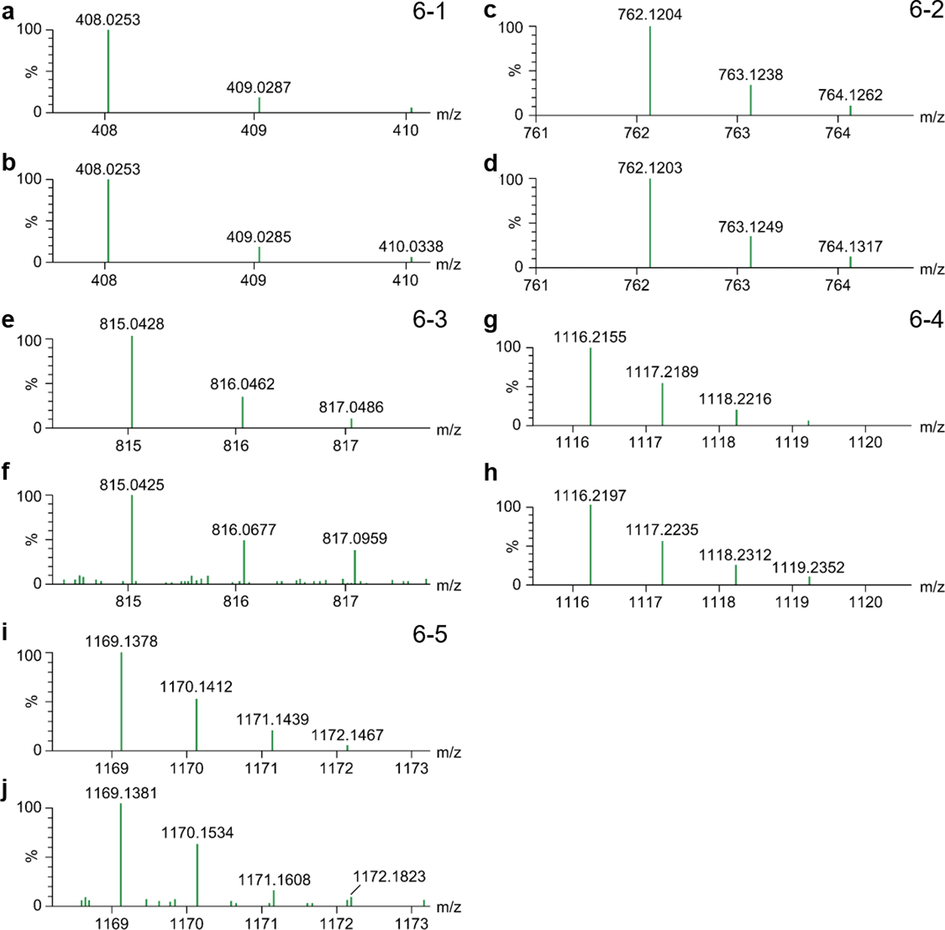
Theoretical (a) and measured (b) isotopic distributions of compound C16H17O9Mn. Theoretical (c) and measured (d) isotopic distributions of compound C32H35O18Mn. Theoretical (e) and measured (f) isotopic distributions of compound C32H33O18Mn2. Theoretical (g) and measured (h) isotopic distributions of compound C48H53O27Mn. Theoretical (i) and measured (j) isotopic distributions of compound C48H51O27Mn2.
3.3.2 ESI-TOF MS analysis of CGA-metal complexes
The ESI-TOF MS results of the supernatant from the reaction of CGA with the eight metal ions in hot water are shown in Table 2. The table shows that the eight metal ions selected for this study formed complexes with CGA in hot water in various ratios. Using the reaction products of CGA and Mn2+ as an example, in the positive-ion mode of ESI, unreacted CGA (m/z = 355.1031) and compounds with m/z of 408.0253, 762.1203, 815.0424, 1,116.2197, and 1,169.1381 were detected in the reaction supernatant. Based on the comparisons of accurate molecular weights and isotopic distributions (Fig. 6-1–5), we speculated that these five compounds were quasi-molecular ions of M+ or [M+H]+ complexes formed by CGA and Mn2+ at ratios of 1:1, 2:1, 2:2, 3:1, and 3:2.
Source of reaction supernatant
m/z
Molecular formula
Possible structure
Comparison of the theoretical and measured isotopic distributions
Theoretical value
Measured value
All samples
355.1029
355.1031
C16H19O9
[L + H]+
CGA and Ca2+
747.1450
747.1452
C32H35O18Ca
[2L + Ca2+-H]+
Fig. S9a, b
1,101.2400
1,101.2443
C48H53O27Ca
[3L + Ca2+-H]+
Fig. S9c, d
1,139.1870
1,139.1869
C48H51O27Ca2
[3L + 2Ca2+–3H]+
Fig. S9e, f
CGA and Mg2+
1,085.2625
1,085.2649
C48H53O27Mg
[3L + Mg2+-H]+
Fig. S10a, b
1,107.2318
1,107.2305
C48H51O27Mg2
[3L + 2 Mg2+–3H]+
Fig. S10c, d
CGA and Zn2+
417.0164
417.0164
C16H17O9Zn
[L + Zn2+-H]+
Fig. S11a, b
771.1115
771.1116
C32H35O18Zn
[2L + Zn2+-H]+
Fig. S11c, d
CGA and Cu2+
416.0168
416.0168
C16H17O9Cu
[L + Cu2+-H]+
Fig. S12a, b
770.1119
770.1119
C32H35O18Cu
[2L + Cu2+-H]+
Fig. S12c, d
831.0259
831.0255
C32H33O18Cu2
[2L + 2Cu2+–3H]+
Fig. S12e, f
CGA and Ni2+
411.0226
411.0355
C16H17O9Ni
[L + Ni2+-H]+
Fig. S13a, b
765.1177
765.1177
C32H35O18Ni
[2L + Ni2+-H]+
Fig. S13c, d
821.0374
821.0380
C32H33O18Ni2
[2L + 2Ni2+–3H]+
Fig. S13e, f
1,175.1324
1,175.1332
C48H51O27Ni2
[3L + 2Ni2+–3H]+
Fig. S13g, h
CGA and Mn2+
408.0253
408.0253
C16H17O9Mn
[L + Mn2+-H]+
Fig. 6a, b
762.1204
762.1203
C32H35O18Mn
[2L + Mn2+-H]+
Fig. 6c, d
815.0428
815.0425
C32H33O18Mn2
[2L + 2Mn2+–3H]+
Fig. 6e, f
1,116.2155
1,116.2197
C48H53O27Mn
[3L + Mn2+-H]+
Fig. 6g, h
1,169.1378
1,169.1381
C48H51O27Mn2
[3L + 2Mn2+–3H]+
Fig. 6i, j
CGA and Sr2+
440.9930
440.9929
C16H17O9Sr
[L + Sr2+-H]+
Fig. S14a, b
795.0883
795.0883
C32H35O18Sr
[2L + Sr2+-H]+
Fig. S14c, d
880.9785
880.9789
C32H33O18Sr2
[2L + 2Sr2+–3H]+
Fig. S14e, f
1,149.1836
1,149.1864
C48H53O27Sr
[3L + Sr2+-H]+
Fig. S14g, h
CGA and Fe3+
408.0144
408.0147
C16H16O9Fe(III)
[L + Fe3+–2H]+
Fig. 7
409.0222
409.0231
C16H17O9Fe(II)
[L + Fe2+-H]+
762.1095
762.1100
C32H34O18Fe(III)
[2L+Fe3+-2H]+
763.1174
763.1174
C32H35O18Fe(II)
[2L + Fe2+-H]+
1,116.2047
1,116.2051
C48H52O27Fe(III)
[3L+Fe3+-2H]+
1,117.2125
1,117.2130
C48H53O27Fe(II)
[3L + Fe2+-H]+
Besides Fe3+, CGA formed complexes with the other six metal ions similar to those formed with Mn2+. CGA formed complexes with Ni2+ at ratios of 1:1, 2:1, 2:2, and 3:2, with Sr2+ at ratios of 1:1, 2:1, 2:2, and 3:1; with Ca2+ at ratios of 2:1, 3:1, and 3:2, with Mg2+ at ratios of 3:1 and 3:2; with Zn2+ at ratios of 1:1 and 2:1; and with Cu2+ at ratios of 1:1, 2:1, and 2:2.
In the CGA and Fe3+ reaction supernatant, CGA formed complexes with m/z = 408.0149, 409.0227, 762.1095, 763.1184, 1,116.2052, and 1,117.2120. Among them, m/z = 408.0149 was close to the theoretical molecular weight of C16H18O9Fe, and the theoretical isotopic distributions of the two were essentially consistent (Fig. 7a and c). Therefore, we speculated that this was a [L + Fe3+–2H]+ compound formed by CGA and Fe3+ in a 1:1 ratio. Because [L + Fe3+–2H]+ carries one positive charge, it was detected as M+ in the positive-ion mode of ESI. Furthermore, when analyzing the measured isotopic distribution, m/z = 409.0227 was close to the theoretical molecular weight of C16H18O9Fe(II), and the isotopic distributions of the two were essentially consistent (Fig. 7b and c). CGA is an antioxidant, whereas Fe3+ has strong oxidizing properties. Therefore, by combining the properties of the two, we speculated that a portion of Fe3+ was reduced to Fe2+ during the reaction process and then formed complexes with CGA in a ratio of 1:1 to yield C16H18O9Fe(II). To determine whether Fe2+ was present in the reaction supernatant, 1,10-phenanthroline, which is a common reagent to identify Fe2+, was used to perform chromogenic assays. The reaction results are shown in Fig. S16. The figure shows that no color changes were detected after the addition of 1,10-phenanthroline to the CGA and FeCl3 solutions, whereas a reddish-orange appearance was detected after 1,10-phenanthroline was added to the FeCl2 solution and the reaction supernatant for CGA and FeCl3. This implies that Fe2+ was produced in the reaction between CGA and Fe3+ in hot water, showing that CGA had formed complexes with Fe(II). From this we can infer that the compounds with m/z = 762.1095, 763.1184, 1,116.2052, and 1,117.2120 are complexes formed by CGA with Fe3+ and Fe2+ in 2:1 and 3:1 ratios, respectively.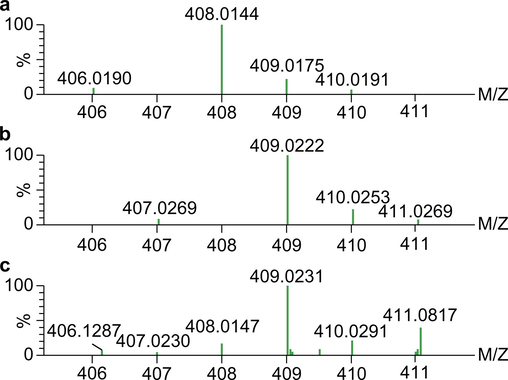
Theoretical isotopic distributions of compounds C16H16O9Fe(III) (a) and C16H17O9Fe(II) (b) and measured isotopic distributions (c).
3.4 Effect of CGA-metal complexes on DNA cleavage activity
3.4.1 In vitro DNA cleavage activity
As it was still impossible to obtain pure products of CGA-metal complexes, experiments were conducted using reaction solutions of CGA and different metal ions dried under reduced pressure and dissolved in DMSO to facilitate the comparison of DNA cleavage activity among different CGA-metal complexes.
pBR322 DNA is a super-coiled double-stranded closed-loop molecule in three forms. Intact DNA usually exhibits a super-coiled conformation (SC), which has the most compact structure, and thus the fastest rate of migration in gel electrophoresis. When a nick occurs on one strand (i.e., a single-strand break), it converts to the nicked circular (NC) form, which has the slowest rate of migration in gel electrophoresis due to its loose structure. When breaks occur at the same position on both strands, it converts to the linear (L) form, and its rate of migration in gel electrophoresis is between those of SC DNA and NC DNA. Fig. S17 shows that at the doses applied in this study, 5% DMSO, the eight metal ions, CGA, as well as the reaction products of CGA with Ca2+, Fe3+, Mg2+, Sr2+, and Zn2+ did not produce significant cleavage of pBR322 DNA after incubation for 24 h. After incubation with the reaction products of CGA and Cu2+, all DNA bands disappeared, indicating that the CGA-Cu complexes had a strong DNA cleavage effect. When the CGA reaction products with Ni2+ and Mn2+ were incubated with pBR322 DNA for 24 h, the SC DNA disappeared completely, and the NC DNA increased significantly, whereas a little L DNA appeared. This indicates that the CGA-Ni and CGA-Mn complexes also have a certain level of DNA cleavage activity. Based on this experiment, we explored the effect of time (Fig. S18) and concentration (Fig. S19) on the pBR322 DNA cleavage activity of the CGA and Cu(II) reaction products.
As shown in Fig. S18, when the reaction product of CGA and Cu(II) was reacted with PBR322 DNA for 0 h, a little NC DNA appeared. After incubation for 10 min, all SC DNA was cleaved into NC DNA, and a little L DNA appeared. As the incubation time increased, NC DNA gradually decreased, whereas L DNA showed an initial increase, followed by a decrease. At 2 h, most of the L DNA was cleaved into even smaller DNA fragments; therefore, no clear DNA bands could be observed on the electropherogram.
Fig. S19 shows that when the Cu(II) concentration reached 30 μmol/L in the reaction system, all SC DNA was cleaved into NC DNA, and L DNA emerged. When the Cu(II) concentration was > 20 μmol/L, the NC DNA gradually decreased and virtually disappeared completely at 70 μmol/L. In summary, these findings indicate the complexes formed by CGA and Cu(II) play a key role in DNA cleavage activity and their cleavage effect is critically related to concentration and time.
3.4.2 Mechanism underlying the in vitro DNA cleavage activity of CGA-Cu complexes
To explore the possible mechanisms underlying the cleavage of pBR322 DNA by CGA-Cu complexes, we added EDTA and different types of free radical scavengers to the reaction system of the DNA cleavage assay. As shown in Fig. 8, when DNA was incubated with the reaction products of CGA and Cu2+ alone for 2 h, only a little L DNA remained. The addition of NaN3 (a singlet oxygen scavenger) did not lead to significant changes in the experimental results. The addition of the metal-ion chelating agent, EDTA, led to the disappearance of DNA cleavage activity. The addition of TEMPO (a carbon-free radical scavenger), mannitol (a hydroxyl radical scavenger), CAT (decomposition of H2O2), and SOD (superoxide anions scavenger) led to a significant increase in NC DNA and L DNA bands. CAT and SOD essentially caused the disappearance of L DNA, which implies that the DNA cleavage activity of CGA-Cu complexes was inhibited.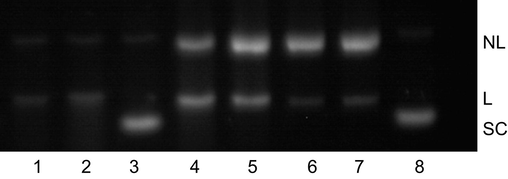
Effect of free radical inhibitors on the DNA cleavage activity of CGA and Cu(II) reaction products. Lane 1, CGA and Cu(II) reaction products; lanes 2–7, addition of NaN3, EDTA, TEMPO, mannitol, catalase, and SOD, respectively, to the reaction system; lane 8: DNA blank control. In lanes 1–7, the final concentration of CGA was μmol/L, and the incubation time was 2 h.
3.5 Antibacterial activity of CGA-metal complexes
The inhibitory effects of CGA, eight metal ions, and the reaction products of CGA and the eight metal ions in hot water S. aureus were explored. As shown in Fig. 9, all eight metal ions had no antibacterial activity at 2.4 mmol/L. CGA showed antibacterial activity at 4.8 mmol/L, and the antibacterial effect disappeared after reacting with Ca2+, Mg2+, Cu2+, Fe3+, and Sr2+. The reaction products of CGA with Ni2+ and Mn2+ showed significantly stronger antibacterial effects than CGA. The reaction products of CGA and Mn2+ still exhibited bactericidal activity when the final CGA concentration was 1.2 mmol/L (final Mn2+ concentration: 0.6 mmol/L). These results indicate that the properties of metal ions have a crucial impact on the activity of the complexes.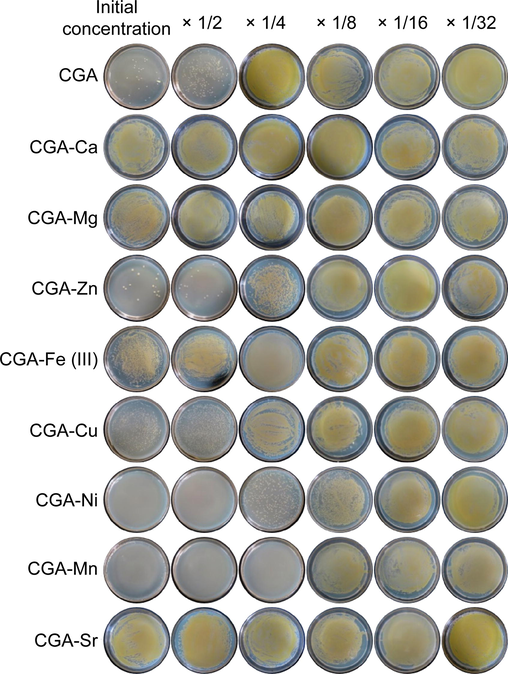
Inhibitory effects of CGA and its metal complexes on Staphylococcus aureus. The initial concentration of CGA was 4.8 mmol/L, and those of the metal ions were 2.4 mmol/L. All eight metal ions showed no antibacterial activity at this concentration.
4 Discussion
Current research on the formation of complexes between organic components and trace metal elements in natural medicine shows that the choice of reaction solvent significantly affects the coordination between them. For example, quercetin is an extensively studied organic compound. Quercetin and Cu2+ form complexes in a methanolic solution in a 1:1 ratio (Vimalraj et al., 2018). However, in an alkaline aqueous solution with a pH of 10, coordination cannot occur at 4 = O and 5-OH between the two, and the complexes are formed in a quercetin/Cu2+ 2:1 ratio. Coordination can also occur at 3′–OH and 4′–OH to form complexes at a ratio of 1:1 (Torreggiani et al., 2005). Furthermore, quercetin and Co2+ formed complexes in a methanolic solution in a quercetin/Co2+ ratio of 1:2 (Birjees Bukhari et al., 2008). However, in ethanol, the complexes formed in a 2:1 ratio (Zhou et al., 2001). Most medicinal plants are decocted in water for consumption, whereas studies on the chemical components of medicinal plants often use methanol as an extraction solvent. Therefore, in this study, we first examined whether the aqueous and methanolic extracts of four CGA-rich medicinal plants contained metal complexes. To minimize errors during the weighing and extraction of medicinal materials, the same extract sample was divided into two aliquots, dried under reduced pressure, and dissolved in methanol and 15% FA-methanol. Our experimental findings revealed that the aqueous extract dissolved in 15% FA-methanol showed significantly higher peak areas for all chromatographic peaks. Possible reasons for this phenomenon may include the following:
Compounds with significantly higher peak areas were alkaline, and their solubility improved substantially in an acidic solution.
These compounds are aglycone components, and their corresponding glycoside components can be hydrolyzed in an acidic solution, increasing the aglycone content.
These compounds act as ligands that form complexes with metal ions during aqueous extraction, and these complexes were wholly or partially decomposed in acidic solution, increasing the content of the compounds.
Considering that aqueous extracts dissolved in methanol and 15% FA-methanol showed the same number and retention time of chromatographic peaks in the HPLC spectra, minimal changes in peak areas were observed between compounds in methanolic extracts dissolved in methanol and 15% FA-methanol. In addition, FA is a moderately strong acid that cannot hydrolyze glycosides. The increased peak area in the HPLC spectra for aqueous extracts dissolved in 15% FA-methanol is due to the third reason. These experimental findings suggest that hot water is a suitable solvent for the formation of complexes between organic components and metal ions in medicinal plants.
Complexes of CGA with metal ions, such as Cu2+ (Kiss et al., 1989), Mn2+ (Améziane et al., 1996), Zn2+ (Kalinowska et al., 2020), Fe3+ (Mahal et al., 2005), V4+ (Naso et al., 2014), heavy metals (Hg2+, Pb2+, Cu2+, and Cd2+) (Milić et al., 2011), and alkali metals (Li+, Na+, K+, Ru+, and Cs+) have been previously synthesized (Kalinowska et al., 2018). Their results showed different solvents might cause CGA and metal ions to form different complexes. For example, in a buffer solution of pH 7.5, CGA could be complexed with Cu2+ at 1:1 and 1:2 but not with Zn2+ (Milić et al., 2011). In an aqueous solution, CGA could be complexed with Cu, Mn, Zn, and Fe in ratios of 1:1, 1:2, or 1:3, respectively; in a nitric acid solution, it formed complexes with Cu and Fe in a ratio of 1:1, whereas in an aqueous solution of pH > 10, it formed complexes with Zn in a ratio of 2:1 (Améziane et al., 1996). In this study, UV–Vis spectroscopy was used to confirm that CGA had complexed with the eight metal ions in hot water. ESI-TOF MS was performed to detect the CGA-metal complexes found in the reaction solution. Our results showed complexes formed by CGA and metal ions in various ratios could be found simultaneously in the reaction solution. When attempting to obtain monomers of the CGA-metal complexes through recrystallization and column chromatography, only CGA could be detected in the elution product of column chromatography (silica gel, polyamide, Sephadex LH-20, and RP-18) and not the CGA-metal complexes; this may have occurred because these metal complexes had undergone decomposition on the chromatographic media, or they were too tightly bound to the chromatographic media and therefore could not be easily eluted. The ESI-TOF MS results of recrystallized products dissolved in hot water consistently showed the simultaneous presence of CGA and its metal complexes. This may have occurred because the complexes comprising CGA and metal ions were insufficiently stable, and some may decompose into CGA and metal ions once the metal complexes are dissolved in hot water.
DNA is a crucial target for many anticancer drugs (Samsonowicz and Regulska, 2017), and the effect of metal complexes on DNA structure has been extensively researched (Ahmadi et al., 2011; Tan et al., 2009; Wang et al., 2010; Yamashita et al., 1999). Therefore, in this study, we used the double-stranded closed-loop pBR322 DNA molecule as the basis to measure the DNA cleavage activity of different CGA-metal complexes. These findings indicated EDTA could inhibit DNA cleavage; in contrast, TEMPO, mannitol, CAT, and SOD partially inhibited DNA cleavage; this implies that Cu(II) plays a significant role in DNA cleavage, while binding of CGA-Cu complexes with DNA produces carbon-free radicals, hydroxyl radicals, H2O2, and superoxide anions. Thus, CGA-Cu complexes can promote DNA cleavage via oxidative damage, similar to quercetin-Cu (Tan et al., 2009; Yamashita et al., 1999) and Rutin-Cu complexes (Cai et al., 2019b).
Antibiotics have been effective against bacterial infections. However, their overuse has accelerated the rate of resistance to bacterial drugs. The rapid increase in carbapenem-resistant bacteria (carbapenems are one of the last few classes of antibiotics available) is considered a harbinger of the imminent arrival of a “public health nightmare” and “catastrophic threat.” (Mckenna, 2013). Therefore, the development of new antibiotics is an urgent requirement. The antibacterial mechanisms of metal complexes differ from those of traditional antibiotics: (1) After ligands with antibacterial activity form complexes with essential metal ions, they can enter bacteria via pathways related to the absorption, transport, and storage of essential metal ions as “Trojan horses,” killing the bacteria (Kali et al., 2016). (2) Complexes composed of non-essential metals can inhibit the absorption of essential metals in bacteria through competition, depleting essential metals in the bacteria and eventual death (Amidani et al., 2021). (3) Metal ions and ligands can enhance antibacterial activity through synergistic effects (Amidani et al., 2021; Song et al., 2009; Wanninger et al., 2015). Therefore, some researchers believe that searching for organic components or metal complexes with metal-ion binding ability in natural drugs is an effective way to discover new antibiotic candidates (Dandawate et al., 2019). Because CGA has a broad spectrum of antibacterial activity (Bajko et al., 2016; Cai et al., 2019a; Lou et al., 2011; Santana-Gálvez et al., 2017; Wang et al., 2015), we previously selected S. aureus, Escherichia coli, and Pseudomonas aeruginosa to examine the antibacterial activity of CGA and its various metal complexes. Our findings indicated that, at the maximum concentration used in this study, CGA and its various metal complexes only produced significant inhibitory activity against S. aureus.
5 Conclusions
Metal complexes are important active components of medicinal plants. There are limited reports on organic component-metal complexes found in medicinal plant extracts because of their wide variety, low content, lack of separation and purification methods, and easy formation only in hot water. In this study, we have established a method for discovering bioactive metal complexes rapidly and systematically from aqueous extracts of medicinal plants. Using this method, we successfully obtained chlorogenic acid-metal complexes with DNA cleavage and antibacterial activity from water extracts of medicinal plants.
Funding
This work was supported by the Natural Science Foundation of Jiangxi Province [grant number 20212BAB206005], Science and technology research project of Education Department of Jiangxi Province [grant number GJJ211119], Open Project of Engineering Center of Jiangxi University for Fine Chemicals [grant number JFCEC-KF-2102], and Jiangxi Science and Technology Normal University Undergraduate Research Fund Project [grant number 202111318018]. The funding sources had no role in the study design, collection, analysis, or interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
CRediT authorship contribution statement
Kangkang Zheng: Methodology. Yafeng Liu: Conceptualization, Methodology. Liang Peng: Conceptualization, Writing – original draft. Zhimin Li: Writing – original draft. Wenbin Xu: .
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Study binding of al–curcumin complex to ds-DNA, monitoring by multispectroscopic and voltammetric techniques. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2011;79:1466-1474.
- [CrossRef] [Google Scholar]
- Thermodynamic stability of copper(II), manganese(II), zinc(II) and iron(III) complexes with chlorogenic acid. Bull. Soc. Chim. Fr.. 1996;133:243-249.
- [Google Scholar]
- Amidani, L., Vaughan, G.B.M., Plakhova, T.V., Romanchuk, A.Y., Gerber, E., Svetogorov, R., Weiss, S., Joly, Y., Kalmykov, S.N., Kvashnina, K.O., 2021. Front cover: The application of HEXS and HERFD XANES for accurate structural characterisation of actinide nanomaterials: The case of ThO2. Chem. Eur. J. 27, 1–1. https://doi.org/.
- 5-O-caffeoylquinic acid: a spectroscopic study and biological screening for antimicrobial activity. LWT Food Sci. Technol.. 2016;65:471-479.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and investigation of antioxidant activity of cobalt–quercetin complex. J. Mol. Struct.. 2008;892:39-46.
- [CrossRef] [Google Scholar]
- Estimation of the chelating ability of an extract from Aronia melanocarpa L. berries and its main polyphenolic ingredients towards ions of zinc and copper. Molecules. 2020;25:1507.
- [CrossRef] [Google Scholar]
- Antibacterial activity and mechanism of cinnamic acid and chlorogenic acid against Alicyclobacillus acidoterrestris vegetative cells in apple juice. Int. J. Food Sci. Technol.. 2019;54:1697-1705.
- [CrossRef] [Google Scholar]
- ESI-TOF MS analysis and DNA cleavage activity of rutin–metal complexes in aqueous extracts of medicinal plants. Inorg. Chem. Front.. 2019;6:3184-3195.
- [CrossRef] [Google Scholar]
- Discovery of natural products with metal-binding properties as promising antibacterial agents. Expert Opin. Drug Discov.. 2019;14:563-576.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and biological evaluation of Rutin–zinc(II) flavonoid -metal complex. Chem. Biol. Interact.. 2015;239:184-191.
- [CrossRef] [Google Scholar]
- Beneficial effects of quercetin–iron complexes on serum and tissue lipids and redox status in obese rats. J. Nutr. Biochem.. 2016;29:107-115.
- [CrossRef] [Google Scholar]
- Antibacterial synergy of curcumin with antibiotics against biofilm producing clinical bacterial isolates. J. Basic Clin. Pharm.. 2016;7:93-96.
- [CrossRef] [Google Scholar]
- The study of anti-/pro-oxidant, lipophilic, microbial and spectroscopic properties of new alkali metal salts of 5-O-caffeoylquinic acid. Int. J. Mol. Sci.. 2018;19:463.
- [CrossRef] [Google Scholar]
- Zn(II) complex of plant phenolic chlorogenic acid: antioxidant, antimicrobial and structural studies. Materials (Basel). 2020;13:3745.
- [CrossRef] [Google Scholar]
- Complexes of 3, 4-dihydroxyphenyl derivatives—X. Copper(II) complexes of chlorogenic acid and related compounds. Polyhedron. 1989;8:2345-2349.
- [CrossRef] [Google Scholar]
- Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci.. 2011;76:M398-M403.
- [CrossRef] [Google Scholar]
- Radical scavenging and catalytic activity of metal–phenolic complexes. J. Phys. Chem. B. 2005;109:24197-24202.
- [CrossRef] [Google Scholar]
- Cu II Complex of emodin with improved anticancer activity as demonstrated by its performance on HeLa and Hep G2 cells. RSC Adv.. 2017;7:41403-41418.
- [CrossRef] [Google Scholar]
- A polarographic study of chlorogenic acid and its interaction with some heavy metal ions. Electroanalysis. 2011;23:2935-2940.
- [CrossRef] [Google Scholar]
- Promising antioxidant and anticancer (human breast cancer) oxidovanadium (IV) complex of chlorogenic acid. synthesis, characterization and spectroscopic examination on the transport mechanism with bovine serum albumin. J. Inorg. Biochem.. 2014;135:86-99.
- [CrossRef] [Google Scholar]
- Quercetin-iron complex: synthesis, characterization, antioxidant, DNA binding, DNA cleavage, and antibacterial activity studies. J. Fluoresc.. 2016;26:2023-2031.
- [CrossRef] [Google Scholar]
- Rutin–nickel complex: synthesis, characterization, antioxidant, DNA binding, and DNA cleavage activities. Biol. Trace Elem. Res.. 2017;178:160-169.
- [CrossRef] [Google Scholar]
- Application of CE-ICP-MS and CE-ESI-MS/MS for identification of Zn-binding ligands in goji berries extracts. Talanta. 2018;183:102-107.
- [CrossRef] [Google Scholar]
- Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal Hydroxyflavonol complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2017;173:757-771.
- [CrossRef] [Google Scholar]
- Chlorogenic acid: recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules. 2017;22
- [CrossRef] [Google Scholar]
- Effect of chlorogenic acid supplementation in MPTP-intoxicated mouse. Front. Pharmacol.. 2018;9:757.
- [CrossRef] [Google Scholar]
- Neuroprotective effect of chlorogenic acid on mitochondrial dysfunction-mediated apoptotic death of DA neurons in a Parkinsonian mouse model. Oxid. Med. Cell Longev. 2020:6571484.
- [CrossRef] [Google Scholar]
- Syntheses, characterization and biological activities of rare earth metal complexes with curcumin and 1,10-phenanthroline-5,6-dione. J. Inorg. Biochem.. 2009;103:396-400.
- [CrossRef] [Google Scholar]
- DNA binding and oxidative DNA damage induced by a quercetin copper(II) complex: potential mechanism of its antitumor properties. J. Biol. Inorg. Chem.. 2009;14:727-739.
- [CrossRef] [Google Scholar]
- Copper(II)–quercetin complexes in aqueous solutions: spectroscopic and kinetic properties. J. Mol. Struct.. 2005;744–747:759-766.
- [CrossRef] [Google Scholar]
- Mixed-ligand copper(II) complex of quercetin regulate osteogenesis and angiogenesis. Mater. Sci. Eng. C Mater. Biol. Appl.. 2018;83:187-194.
- [CrossRef] [Google Scholar]
- The therapeutic effect of chlorogenic acid against Staphylococcus aureus infection through sortase A inhibition. Front. Microbiol.. 2015;6:1031.
- [CrossRef] [Google Scholar]
- An effective approach to artificial nucleases using copper (II) complexes bearing nucleobases. Dalton Trans.. 2010;39(8):2128-2136.
- [CrossRef] [Google Scholar]
- Oxidative damage to DNA by 1,10-phenanthroline/L-threonine copper (II) complexes with chlorogenic acid. BioMetals. 2010;23:265-273.
- [CrossRef] [Google Scholar]
- Metal complexes of curcumin–synthetic strategies, structures and medicinal applications. Chem. Soc. Rev.. 2015;44:4986-5002.
- [CrossRef] [Google Scholar]
- Speciation analysis and bioaccessibility evaluation of trace elements in goji berries (Lycium barbarum, L.) J. Chromatogr. A. 2017;1492:70-78.
- [CrossRef] [Google Scholar]
- Enzymatic extraction of copper complexes with phenolic compounds from açaí (Euterpe oleracea mart.) and bilberry (Vaccinium myrtillus L.) fruits. Food Anal. Methods. 2016;9:2105-2114.
- [CrossRef] [Google Scholar]
- Mechanism of oxidative DNA damage induced by quercetin in the presence of Cu(II) Mutat. Res.. 1999;425:107-115.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, and anti-cancer activity of emodin-Mn(II) metal complex. Chin. J. Nat. Med.. 2014;12:937-942.
- [CrossRef] [Google Scholar]
- Antioxidative and antitumour activities of solid quercetin metal (II) Transit. Met. Chem.. 2001;26:57-63.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104835.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







