Translate this page into:
Structural effects and thermal decomposition kinetics of chalcones under non-isothermal conditions
⁎Corresponding author. Tel.: +91 4144 239523, mobile: +91 9488476098. pvta1998@yahoo.co.in (V. Thanikachalam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Two chalcones namely, 1,5-bis(4-hydroxy-3-methoxyphenyl)pentan-1,4-dien-3-one (BHMPD) and 2,5-bis(4-hydroxy-3-methoxybenzylidene)cyclopentanone (BHMBC) have been synthesised and characterized by microanalysis, FT-IR, mass spectra and NMR (1H and 13C) techniques. The thermal decomposition of these compounds was studied by TGA and DTA under dynamic nitrogen atmosphere at different heating rates of 10, 15 and 20 K min−1. The kinetic parameters were calculated using model-fitting (Coats–Redfern, CR) and model-free methods (Friedman, Kissinger–Akahira–Sunose, KAS and Flynn–Wall–Ozawa, FWO). The decomposition process of BHMPD and BHMBC followed a single step mechanism as evidenced from the data. Existence of compensation effect was noticed for the decomposition of these compounds. Invariant kinetic parameters are consistent with the average values obtained by Friedman and KAS isoconversional method in both compounds.
Keywords
Cyclopentanone
Dienone
Thermal decomposition
Kinetic parameters
Model-fitting
Model-free analysis
1 Introduction
Curcumins have several biological activities, such as anti-inflammatory, antioxidative, antibacterial, antihepatotoxic, hypertensive and hypocholesterolemic properties (Chang et al., 2003; Sharma, 1976; Sharma et al., 1972, Gafner et al., 2004; Bennett, 1986). Curcumin inhibits in vitro lipid peroxide formation by liver homogenates of endemic mice (Tonnesen, 1986). It is used for the synthesis of bioactive pyrimidine compounds (Das et al., 2006) and also finds applications in the preparation of liquid–crystalline polymers (Gangadhara and Kishore, 1995). Thermotropic liquid crystalline behavior of polymeric materials containing cyclopentanone moiety linked with polyethylene spacers is of considerable current interest, not only because of their potential as high-strength fibers, plastics, moldings, etc. (Griffin and Johnson, 1984; Blumstein, 1985; Chapoy, 1986), but also of their applications in non-linear optical materials (Kawamata et al., 1996). The benzylidene derivatives are intermediates in various pharmaceuticals, agrochemicals and perfumes (Ogawa et al., 1988). Curcumin demonstrated a great ability in chelating essential metal ions such as Cu(II) (John et al., 2002) and the complexes showed a higher scavenging ability than curcumin. The aim of this work is to determine the thermal stability and decomposition mechanism of chalcones.
In this paper, we report the synthesis of 1,5-bis(4-hydroxy-3-methoxyphenyl)pentan-1,4-dien-3-one (BHMPD) and 2,5-bis(4-hydroxy-3-methoxybenzylidene)cyclopentanone (BHMBC) and their thermal decomposition in non-isothermal dynamic nitrogen atmosphere condition. The kinetic and thermodynamic parameters are determined using model-fitting and model-free methods.
2 Experimental
2.1 Materials
All the chemicals were of AnalaR grade, purchased from Sd fine chemicals and were used as such without further purifications.
2.2 Instruments
Elemental analyzes were performed on a Carlo Analyzer at Central Drug Research Institute (CDRI), Lucknow, India. FT-IR spectra were recorded in a KBr-pellet on an Avatar-330 spectrometer (with resolution 2 cm−1). The mass spectra were recorded on a JEOL Gcmate, at the Indian Institute of Technology, Chennai, India. The NMR spectra were recorded on a Bruker AMX 400 MHz for 1H and 100 MHz for 13C NMR with TMS as an internal standard at the Indian Institute of Science, Bangalore, India. The simultaneous TGA and DTA curves were obtained with the thermal analysis system model Perkin Elmer TAC 7/DX Thermal Analysis Controller TAC-7. The TG and DTA analyzes of BHMPD and BHMBC were carried out under dynamic nitrogen atmosphere (100 mL min−1), in an alumina crucible with a sample mass around 10 mg with heating rates of 10, 15 and 20 K min−1 from 35 to 700 °C. The kinetic parameters Ea and ln A were calculated using Microsoft® Excel Software. The sample temperature, which is controlled by a thermocouple, did not exhibit any systematic deviation from the preset linear temperature program.
2.3 Synthesis of BHMPD
Dry HCl gas was passed through a solution containing vanillin (1 mmol) and acetone (0.5 mmol) in dry methanol. The solution turned dark pink and yellow precipitate was obtained after the addition of water. It was purified by crystallization from methanol medium (m.p. = 99 °C; lit. = 98–99 °C) (Du et al., 2006).
Elemental analysis: Calculated = C, 70.13; H, 5.19. Found = C, 70.48; H, 5.17. FT-IR (KBr disk, cm−1): , , and . 1H NMR (CDCl3, δ ppm): 3.82 (methoxy proton), 6.94–7.38 (aromatic protons), 7.40, 9.73 (vinyl protons of the α,β-unsaturated arylidene system), 10.26 (phenolic —OH). 13C NMR (CDCl3, δ ppm): 55.46 (methoxy carbon), 110–148 (aromatic carbons and vinyl carbons), 152.97 (C—O carbons of the α,β-unsaturated arylidene system, 190.86 (carbonyl carbon of arylidene keto moiety).
2.4 Synthesis of BHMBC
Vanillin (1 mmol) and cyclopentanone (0.5 mmol) were heated in a water bath (45–50 °C) until a clear solution was obtained, concentrated hydrochloric acid was then added followed by 2 h stirring. After standing overnight, the mixture was treated with cold aqueous acetic acid (1:1) and filtered. The solid material was washed first with cold ethanol, then with hot water and dried in vacuum. The yellow substance was recrystallized from ethanol (m.p. = 212 °C; lit. = 212–214 °C) (Du et al., 2006).
Elemental analysis: Calculated = C, 71.59; H, 6.68. Found: C, 71.23; H, 6.57. FT-IR (KBr disk, cm−1): and . 1H NMR (CDCl3 and DMSO-d6, δ ppm): 7.31 (2H, —OH protons), 6.97–6.98 (2H, aromatic hydrogen), 7.52 (2H, —CH⚌), 3.94 (6H, OCH3 protons), 2.15–2.17 (4H, —H2C—CH2—). 13C NMR (CDCl3, δ ppm): 195.89 (C⚌O), 148.06–147.32 (CH, C⚌C, C—O), 113–134.66 (aromatic carbons), 55.77 (OCH3), 26.32 (CH2).
3 Theoretical background
3.1 Model fitting method
The integral method of Coats and Redfern has been most successfully used for studying the kinetics of dehydration and decomposition of different solid substances (Horowitz and Metzger, 1963; Coats and Redfern, 1964; Wendlandt, 1974; Sestak, 1984). The kinetic parameters can be derived from modified Coats and Redfern equation:
3.2 Model free methods
Friedman method (Friedman, 1963) is a different method and was one of the first isoconversion methods. The logarithm of the non-isothermal rate law:
The plots of ln (βdα/dT) Vs 1/T (Eq. (3)), ln β Vs 1/T (Eq. (4)) and ln (β/T2) Vs 1/T (Eq. (5)) have been shown to give the values of apparent activation energies for the decomposition of BHMPD and BHMBC at different values of α. According to these equations, the reaction mechanism and shape of g(α) function do not affect the values of the activation energies of the decomposition stage.
3.3 Invariant kinetic parameters (IKP) method
The invariant kinetic parameters were obtained by the method of Lesnikovich and Levchik (1983). The straight lines obtained for the plots of ln Aβ Vs Eβ for several constant heating rates should intersect at a point (Lesnikovich and Levchik, 1985) which corresponds to the true values of activation energy and pre-exponential factor, which are called invariant kinetic parameters (Einv, Ainv). The evaluation of the invariant kinetic parameters is performed using the supper correlation equation:
The plot of aβ Vs bβ, obtained for three constant heating rates, is a straight line (Flynn, 1997) whose parameters allow the determination of ln Ainv and Einv.
3.4 Thermodynamic parameters
The kinetic parameters, energy of activation and pre-exponential factor obtained from Kissinger single point (Kissinger, 1957, 1956; Patel and Chaudhri, 1978; Whelan et al., 1984) kinetic method uses the Eq. (7):
Based on the values of activation energy and pre-exponential factor for the decomposition stage, the values of
for the formation of activated complex from the reactant were calculated according (Malek, 1989; Bamford and Tipper, 1980; Cordes, 1968) to, equations:
4 Results and discussion
4.1 Non-isothermal TG and DTA
The TG and DTA thermograms of pure BHMPD and BHMBC were recorded in a dynamic nitrogen atmosphere at different heating rates of 10, 15 and 20 K min−1 and presented in Figs. S1 and S2 and show those two distinct endothermic peaks on account of melting and decomposition. The thermal decomposition process of BHMPD and BHMBC in one stage was observed from the TG curves. The decomposition processes of BHMPD and BHMBC are completed 99.2% and 67% with endothermic peaks, respectively. The decomposition process of BHMPD starts from 180 °C and ends at 280 °C whereas that for BHMBC starts from 300 °C and ends at 480 °C.
4.2 Non-isothermal decomposition reaction kinetics
The heating rates 10, 15 and 20 K min−1 were selected to study the decomposition process of chalcones. The values of α ranging from 0.22 to 0.80 for BHMPD and 0.20 to 0.70 for BHMBC with an increment of 0.02 were chosen for kinetic calculations.
The values of energy of activation corresponding to the values of α for the decomposition procedure obtained by Friedman (Friedman, 1963), Kissinger–Akahira–Sunose (Kissinger, 1957; Akahira and Sunose, 1971) and Flynn–Wall–Ozawa (Flynn and Wall, 1966; Ozawa, 1965) are shown in Fig. 1. It is clear that the value of Ea increases with α values and remains constant from 0.20 to 0.80 for BHMPD and 0.2 to 0.70 for BHMBC the increases with increasing α indicating removal of gaseous products from solid mass. Therefore, the decomposition stage could be recorded as a single step process (Vyazovkin and Wight, 1997). The apparent activation energy varied with increasing conversions and the average value is 100.71 ± 0.14 kJ mol−1 for BHMPD and 128.99 ± 0.25 kJ mol−1 for BHMBC (Friedman method). The meager dependence of activation energy on the extent of conversion suggests that the decomposition took place in a single step for BHMPD and BHMBC. BHMBC compound has higher energy of activation than BHMPD, which indicates cyclic compound thermally more stable than acyclic compound. The mass spectral data of these compounds show that compounds are decomposed instead of sublimation.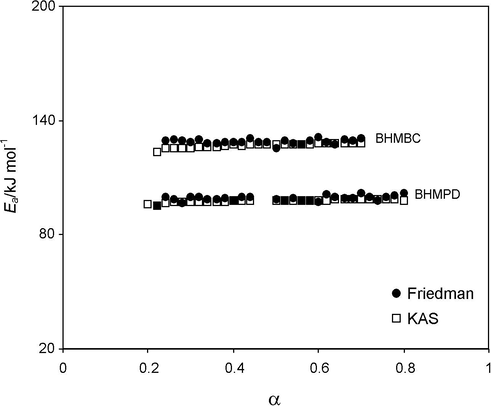
Ea versus α plot for the decomposition of BHMPD and BHMBC under non-isothermal condition.
4.3 Invariant kinetic parameters (IKP) analysis
The invariant kinetic parameter method was applied to the data calculated for the heating rates of 10, 15 and 20 K min−1. The evaluation of the kinetic parameters was performed using Coats–Redfern method. For these kinetic models in the range 0.2 ⩽ α ⩽ 0.8 for BHMPD and 0.2 ⩽ α ⩽ 0.7 for BHMBC, the straight lines corresponding to Coats–Redfern method is characterized by correlation coefficient values close to unity. The values of Ea and ln A depend on the kinetic model as well as on the heating rate, as shown in Tables 1 and 2.
Kinetic model
β = 10 K min−1
β = 15 K min−1
β = 20 K min−1
Ea/kJ mol−1
ln A A/s−1
−r
Ea/kJ mol−1
ln A A/s−1
−r
Ea/kJ mol−1
ln A A/s−1
−r
P2
26.63
3.62
0.999
26.31
3.83
0.999
26.37
4.01
0.999
P3
14.86
0.45
0.999
14.61
0.71
0.999
14.61
0.91
0.999
P4
9.01
−1.33
0.999
8.80
−1.05
0.999
8.76
−0.84
0.999
F1
89.88
19.42
0.996
88.36
19.14
0.993
88.22
19.04
0.991
F2
126.30
28.59
0.985
123.27
27.84
0.980
122.55
27.47
0.978
F3
170.40
39.55
0.972
165.40
38.19
0.967
163.92
37.50
0.963
D1
143.26
38.51
0.999
142.37
38.24
0.999
143.14
38.17
0.963
D2
148.42
31.84
0.999
146.96
31.40
0.998
147.40
31.21
0.999
D3
167.92
35.19
0.998
165.76
34.52
0.996
165.92
34.19
0.995
D4
164.42
34.13
0.999
162.87
33.61
0.998
163.54
33.41
0.997
A2
40.64
7.46
0.995
39.82
7.50
0.992
39.69
7.59
0.989
A3
23.92
3.14
0.994
23.35
3.29
0.990
23.21
3.43
0.987
A4
16.01
0.95
0.992
15.55
1.14
0.987
15.42
1.30
0.983
R2
74.84
14.89
0.999
73.87
14.79
0.997
73.95
14.79
0.996
R3
70.86
14.04
0.999
69.65
13.99
0.998
69.79
14.02
0.998
Kinetic model
β = 10 K min−1
β = 15 K min−1
β = 20 K min−1
Ea/kJ mol−1
ln A A/s−1
−r
Ea/kJ mol−1
ln A A/s−1
−r
Ea/kJ mol−1
ln A A/s−1
−r
P2
50.36
6.83
0.995
47.08
6.35
0.992
49.86
7.08
0.991
P3
29.97
2.72
0.994
27.69
2.48
0.990
29.39
3.08
0.989
P4
19.84
0.52
0.992
18.05
0.39
0.987
19.31
0.94
0.985
F1
133.50
22.87
0.999
133.74
22.82
0.999
140.76
24.23
0.999
F2
158.69
27.95
0.999
168.23
29.59
0.999
177.41
31.35
0.999
F3
186.90
33.59
0.999
208.33
37.40
0.995
220.09
39.57
0.996
D1
248.62
51.27
0.997
236.40
48.47
0.995
247.40
50.47
0.994
D2
247.21
42.67
0.997
238.48
40.51
0.996
250.16
42.62
0.996
D3
262.38
44.16
0.998
258.27
42.83
0.998
271.14
45.13
0.998
D4
289.86
49.12
0.995
275.59
45.76
0.994
288.33
48.02
0.993
A2
61.41
9.18
0.999
61.38
9.33
0.999
64.84
10.20
0.999
A3
36.91
4.29
0.999
36.79
4.50
0.998
39.05
5.19
0.998
A4
25.36
1.85
0.998
25.20
2.09
0.997
26.88
2.70
0.998
R2
122.0
19.86
0.998
118.72
19.15
0.996
124.81
20.41
0.997
R3
118.57
19.42
0.997
114.22
18.53
0.997
120.04
19.75
0.995
For several groups of apparent activation parameters, obtained by different kinetic models, we tried to establish the best combination (r → 1), a better resolution in determining the invariant kinetic parameters and the closest value to the mean isoconversional activation energies (Budrugeac and Segal, 2007; Vyazovkin and Lesnikovich, 1988; Pratap et al., 2007). For (all kinetics models) AKM–{F2; F3; D1; D2; D3; D4}, the plot of ln A Vs Ea has the highest correlation coefficient (r = 0.988) and is a true straight line for BHMPD whereas for AKM–{F2; F3; D1; D2; D3; D4; R2; R3}, the plot of ln A Vs Ea has the highest correlation coefficient (r = 0.999) and is a true straight line for BHMBC. Depending on the choice of kinetic models, the compensation effect parameters are obtained with different accuracies, their values and derived invariant activation parameters varying substantially.
For AKM–{F2; F3; D1; D2; D3; D4}, the invariant kinetic parameters are Einv = 91.02 kJ mol−1 and ln Ainv = 19.41 (Table S1) obtained with r = 0.988 for BHMPD and AKM–{F2; F3; D1; D2; D3; D4; R2; R3}, the invariant kinetic parameters are Einv = 128.75 kJ mol−1 and ln Ainv = 22.05 (Table S2) obtained with r = 0.999 for BHMBC, respectively. For these groups, the invariant activation energy is slightly above 103.06 kJ mol−1, close to 99.06 ± 0.28 kJ mol−1, 97.69 ± 0.14 kJ mol−1 obtained by Friedman and KAS method for BHMPD and for BHMBC is slightly above 128.77 kJ mol−1, close to 126.80 ± 0.24 kJ mol−1 obtained by KAS method (Tables S1 and S2). The higher Ea value for BHMBC may be due to the ring system that influences the stability of the compound and also BHMBC is thermally more stable, because of decomposition that starts at >320 °C at all heating rates (Fig. S2).
The most probable kinetic model for decomposition process of BHMPD and BHMBC is R2 and F1 models, respectively. By introducing the derived reaction model for BHMPD, g(α) = [1 − (1 − α)1/2], the following Eq. (11) is obtained:
The plot of [1 − (1 − α)1/2] against (Ea p(x)/Rβ) at the different heating rates is constructed in Fig. S3, and for BHMBC is, g(α) = −ln (1 − α), the following Eq. (12) is obtained:
The plot of −ln (1 − α) against (Ea/Rβ) p(x) at different heating rates is constructed and is similar to Fig. S3.
By using the above equations, the A value was determined from the slope of the fitted line shown in Fig. S3. For contracting area model (R2) and Ea = 99.06 ± 0.28 kJ mol−1, the pre-exponential (frequency) factor was found to be A = 8.92 × 108 min−1 (ln A = 20.60) for BHMPD and for power law (F1) and Ea = 128.99 ± 0.25 kJ mol−1, the pre-exponential (frequency) factor was found to be A = 3.94 × 109 min−1 (ln A = 22.09) for BHMBC, respectively. The obtained value of ln A is in good agreement with average value of Friedman isoconversional intercept ln [A f(α)] = 20.92 for BHMPD and 21.47 for BHMBC, respectively.
4.4 Thermodynamic parameters
From the DTA curves, the peak temperatures for BHMPD are 542.6, 548.3 and 557.6 K and for BHMBC are 676.58, 686.31 and 696.21 K. These peak temperatures are used to evaluate single point kinetic parameters. The obtained values are 103.71 and 124.32 kJ mol−1 for BHMPD and BHMBC, respectively. The obtained values are in good agreement with values obtained by Friedman method for both compounds.
The thermodynamic parameters, , and were calculated at the peak temperature Tm in the DTA curves for the corresponding stage, since the temperature characterizes the higher rate of decomposition and therefore, it is an important parameter.
As can be seen from the Table 3, the value of
for the decomposition stage is negative for both the compounds. It means that the corresponding activated complexes were with high degree of arrangement than the initial state. The positive values of
and
for both the compounds show that they are connected with absorption of heat and they are non-spontaneous processes (Criado et al., 2005; Sokolskii and Druz, 1981). The obtained Ea values are coincided with invariant parameters. Energy of activation of BHMBC has a higher value than that of BHMPD which indicates that BHMBC is thermally more stable than BHMPD.
Parameter
BHMPD
BHMBC
Ea/kJ mol−1
103.71
124.32
ln A A/s−1
22.14
20.98
ΔS≠/J K−1 mol−1
−64.84
−74.45
ΔH≠/kJ mol−1
99.71
120.32
ΔG≠/kJ mol−1
130.88
156.12
r
−0.969
−0.995
5 Conclusions
The studied compounds decomposed in a single stage with the absorption of heat. The model for the decomposition mechanism is R2 for BHMPD and F1 for BHMBC, respectively. The thermal stability of BHMPD is less than that of BHMBC. Since the energy of activation for the former is lesser than the cyclopentanone derivative, which indicates that the ring structure influenced the thermal stability of the compound. Mass spectra show that these compounds are decomposed instead of sublimation.
Acknowledgments
The authors thank Dr. N. Jayachandramani, Professor and Head, Department of Chemistry, Pachaiappa’s College, Chennai for his fruitful suggestions and The Head, Indian Institute of Technology, RISC, Chennai for TGA–DTA measurements.
References
- Inst. Technol.. 1971;16:22.
- Comprehensive chemical kinetics. Reactions in the Solid State. Vol Vol. 22. Amsterdam: Elsevier; 1980.
- Prog. Lipid Res.. 1986;25:539.
- Polymeric Liquid Crystals. New York: Plenum Press; 1985.
- J. Therm. Anal. Calorim.. 2007;88:703.
- Biochem. Pharmacol.. 2003;65:2009.
- Recent Advances in Liquid Crystalline Polymers. London: Elsevier; 1986.
- Nature (London). 1964;201:68.
- J. Phys. Chem.. 1968;72:2185.
- J. Therm. Anal. Calorim.. 2005;82:671.
- J. Mol. Catal. A. 2006;247:182.
- Eur. J. Med. Chem.. 2006;41:213.
- Thermochim. Acta. 1997;300:83.
- J. Res. Nat. Bur. Stand. A. 1966;70:487.
- J. Polym. Sci. C. 1963;6:183.
- Phytochemistry. 2004;65:2849.
- Polymer. 1995;36:1903.
- Liquid Crystals and Ordered Fluids. New York: Plenum Press; 1984.
- Anal. Chem.. 1963;35:1464.
- J. Exp. Clin. Cancer Res.. 2002;21:219.
- Chem. Phys. Lett.. 1996;249:29.
- J. Res. Natl. Bur. Stand.. 1956;57:217.
- Anal. Chem.. 1957;29:1702.
- J. Therm. Anal.. 1983;27:89.
- J. Therm. Anal.. 1985;30:237.
- Thermochim. Acta. 1989;138:337.
- Ogawa, M., Ishiim, Y., Nakno, T., Irifune, S., 1988. Jpn. Kohai Tokyo JP 63192446, A2 (Chem. Abstr. 63, 238034).
- Bull. Chem. Soc. Jpn.. 1965;38:1881.
- Thermochim. Acta. 1978;25:247.
- J. Therm. Anal. Calorim.. 2007;89:399.
- Thermodynamical Properties of Solids. Prague: Academic; 1984.
- Biochem. Pharmacol.. 1976;25:1811.
- Biochem. Pharmacol.. 1972;21:1210.
- Introduction in Theory Heterogeneous Catalysis. Moscow: Vyshaya Shkola; 1981. (in Russian)
- Tonnesen, H.H., 1986. Ph.D. Thesis. Institute of Pharmacy, University of Oslo, Norway.
- Thermochim. Acta. 1988;128:297.
- Annu. Rev. Phys. Chem.. 1997;48:125.
- Thermal Methods of Analysis. New York: John Wiley and Sons Inc.; 1974.
- Thermochim. Acta. 1984;80:149.
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.arabjc.2011.06.029.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1
Supplementary tables and figures







