Translate this page into:
Structural, functional, molecular, and biological evaluation of novel triterpenoids isolated from Helichrysum stoechas (L.) Moench. Collected from Mediterranean Sea bank: Misurata- Libya
⁎Corresponding author at: Department of Pharmacognosy, Faculty of Pharmacy, Misurata University, Misurata, Libya. sarfarajpharma@gmail.com (Md. Sarfaraj Hussain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Helichrysum stoechas (L.) Moench (Family Compositae) is a medicinal herb endowed with several pharmacological activities. Ethanolic extract of the aerial parts of the plant was used for the isolation of lignoceric acid (HS-02), lanost-5- en-3β-ol- 26-oic acid (HS-03), and lanost-5-en-26-oic acid-3β-olyl palmitate (HS-04). All molecules were screened for anti-inflammatory and analgesic activities at 5 and 10 mg/kg body weight doses, and the TEST program assessed their toxicity. The molecular interaction profile with numerous anti-inflammatory drug targets was investigated by molecular docking. Compounds HS-03 and HS-04 showed a significant reduction in paw volume compared to the control group challenged with carrageenan in the rats, and prolongation of the paw licking/jumping and reduction in the number of writhes was noted after the injection of acetic acid in mice. In a hot plate test, all compounds showed significant pain inhibition. These findings might aid in the development of anti-inflammatory and anti-analgesic therapies.
Keywords
Helichrysum stoechas (L.) Moench
Compositae
Lanostane triterpenoids
Anti-inflammatory
Analgesic
Molecular docking
1 Introduction
Natural medicinal plants are utilized for medicinal purposes for centuries and persist to be a medicine for various diseases even with the dramatic rise in antibiotics and other synthetic medicine within the current scientific world (Hussain et al., 2009, 2016, 2019, 2020a). Helichrysum stoechas (L.) Moench, syn. Gnaphalium citrinum Lam. and G. stoechas L. (Family Compositae), referred to as shrubby everlasting, God's flower, Gold flower, Golden stoechas, and Goldylocks, is common within the Mediterranean region, north-western Africa, eastern Turkey, southern India, Sri Lanka, and Australia on crude land and alongside the road. This plant better grows on soils with acidic, neutral, or basic pH and very much depleted sites (Hussain et al., 2020b; Tutin et al., 1980; Giuliani et al., 2016; Sobhy and El-Feky, 2007). The plant is perennial, evergreen, rugged, spreading, fragrant spice, growing up to 60 cm; with woolly stem, simple leaves, alternate, linear, with entire margins; flowers umbels of yellow many-stellate foliage. It has anti-inflammatory, antifungal and antioxidant, deobstruent, expectorant, laxative, and sudorific properties; used to treat hypersensitivities, bronchitis, normal colds, influenza, respiratory issues, and sinusitis. The aerial parts of the plant are commonly used as condiments for cooking soups, rice and meat dishes. Its volatile oil has healing, anti-aging, anti-inflammatory, hemostatic, lipolytic, and regenerating properties. The flowers are taken as a diaphoretic and discutient (Lourens et al., 2008; Ascensão et al., 2001). The aerial parts of the plant contain essential oils, composed mainly of α-pinene, limonene, α-bisabolol, β-caryophyllene, α-humulene, pinocampheol, β-elemene, benzyl benzoate, allo-aromadendrene, and epi-α-bisabolol, (Ascensão et al., 2001; Chinou et al., 1997a, 1997b; Lourens et al., 2008; Maggio et al., 2016; Roussis et al., 2002; Tsoukatou et al., 1999; Vernin and Poite, 1998). In addition, isomers of caffeoylquinic and dicaffeoyl quinic acids, apigenin glucoside, quercetin, kaempferol, (Lourens et al., 2008), neo-chlorogenic, chlorogenic and crypto-chlorogenic acids, naringenin, tetrahydroxychalcone-glucoside, (Carini et al., 2001), arzanol, 5,7-dihydroxy-3,6,8-trimethoxyflavone, quercetagetin-7-O-glucopyranoside and santinol B are also present (Lavault and Richomme, 2004; Les et al., 2017). A new acylated flavonoid glycoside helichrysoside (3,5-dihydroxy-6,7,8-trimethoxyflavone) was isolated from the golden yellow flower heads of Helichrysum kraussii (Candy et al., 1975).
In-silico approaches such as molecular docking is considered as one of the fundamental elements of drug designing and discovery paradigms aimed at elucidating ligand-receptor interaction mechanisms and assisting lead optimization (Azam et al., 2012, 2020). This technique is routinely used to accelerate the recognition and investigation of novel drug candidates (Shushni et al., 2013).
In this study, several experimental techniques were implemented to isolate, characterize and scrutinize anti-inflammatory and analgesic potencies of lanostane triterpenoid glycosides from ethanolic extract of H. stoechas. The molecular docking study was employed to ascertain the binding mechanism of isolated compounds HS-02, HS-03, and HS-04 with numerous anti-inflammatory drug targets intended to underscore the structural requirements for intermolecular interactions. Mutagenic potential and LD50 values were also estimated through computational methods. Diclofenac, a standard drug, was also included for comparative analysis in the in-silico studies.
2 Materials and methods
2.1 Collection and authentication of plant material
We collect 3.5 kg weight of the fresh plant sample from the basin of the Mediterranean Sea, Misurata (latitude-32.377533, longitude-15.092017 and altitude-0.10 m), Libya in March 2015, which was authenticated by Dr. Huda Elgubbi, Department of Botany, College of Science, Misurata University, Misurata, Libya. A voucher specimen no. HC 55/01 has been submitted within the herbarium, Department of Botany, College of Science, Misurata University, Misurata, Libya for the proceedings.
2.2 Extraction and isolation
The aerial parts of plant H. stoechas 3.5 kg were shade dried, crudely pulverized, and 500 g powder extracted thoroughly with 95% ethanol using the Soxhlet apparatus. The ethanol extract was then placed on a water bath and dried under reduced pressure to yield 87.9 g of dark brown mass. Numerous components were separated from the extract using a chemical screening method. (Arif & Fareed 2010). For isolation of phytoconstituents, about 80 g of the extract were chromatographed using silica gel (60–120) eluting with solvent mixtures of increasing polarities such as petroleum ether, chloroform, and methanol, were used in various ratios (petroleum ether : chloroform − 9:10, 3:1, 1:1, 1:3 v/v and chloroform: methanol − 99:1, 98:2, 97:3, 24:1, 19:1, 9:1, 3:1, and 1:1, 1:3 v/v) (Ansari et al., 2016). Elution of the column with chloroform gave colorless crystals of HS-02, 102 mg (0.314 % yield). Elution of the column with chloroform: methanol (18:02) mixture yielded colorless crystalline compound HS-03, 115 mg (0.19 % yield) and straw color compound HS-04, 123 mg (0.311% yield).
2.3 Instrumentation, chemicals, and drugs
Melting point apparatus (Perfit), ultraviolet (UV) spectra (Lambda Bio 20 Spectrophotometer Shimadzu-U Singapore) scanned in chloroform, infrared (IR) spectra (Win IR FTS 135 instrument, Biorad, USA), 1H nuclear magnetic resonance (NMR) 300 MHz and 13C NMR 75 MHz spectra in CD3OD (Brucker Spectrometer, Brucker, USA), MS (DART dry Helium, JEOL-Accu TOF JMS-T100LC), plethysmometer (UGO Basil, Italy). Carrageenan was procured from Sigma Chemical Co., St. Louis, MO. Standard drugs Indomethacin, Diclofenac, and Tramadol as a gift sample from Ranbaxy labs, New Delhi. The solvents for isolation were acquired from Merck Germany. Silica gel (60–120) mesh, Merck, Germany) for column chromatography and thin-layer chromatography, silica gel G coated TLC plates (Merck, Germany) was utilized. Spots were visualized by exposure to iodine vapors, UV Lamp 254 nm, and by spraying with anisaldehyde sulphuric acid reagents or with iodine vapors. The percentage yields of the isolated compound were calculated based on dried plant material (500 g) is used for extraction (Hussain et al., 2019).
2.4 Animals
Healthy Wistar albino rats of both sexes weighing between 140 and 160 g were preferred for anti-inflammatory activity and adult Swiss albino mice of every sex (25–30 g) for analgesic activity. They were maintained in clean, sterile, polypropylene cages at room temperature (21 ± 2 °C) in 12 h dark/light control and fed with commercial pellet and water ad libitum. After randomization into various groups, the mice were quarantined for a week for environmental and handling acclimatization before the initiation of experiments. The experimental procedure was permitted by Institutional Ethical Committee, Faculty of Pharmacy, Misurata University, Misurata, Libya, and their guidelines were followed for the studies (Phar-01/2015).
2.4.1 Safety profile study
An acute toxicity study was conceded out for the assurance of LD50 by carrying out the plan (Annexure 2d) of CPCSEA, OECD guideline No. 423. Swiss albino mice of each sex were alienated into seven groups with six animals in each group. Isolated compounds HS-02, HS-03, and HS-04 from ethanolic extract of H. stoechas at various dose levels of 50, 100, and 150 mg/kg were administered orally as a single dose to mice. The animals were observed periodically for the indication of toxicity and death for 24 h and afterward consistently for 14 days (Hussain et al., 2016).
2.4.2 Administration of drugs
The isolated compounds HS-02, HS-03, and HS-04 at doses 05 and 10 mg/kg were administered in all the experimental models. Indomethacin 10 mg/kg was utilized as a standard anti-inflammatory drug, Tramadol 0.1 ml (40 mg/kg. s.c) was used as pain inhibiting drug in hot plate technique, and Diclofenac 5 mg/kg was use d as pain inhibiting drug in acetic acid persuade writhing in mice. All the test and standard medications were formulated into an emulsion using 0.3% Carboxy Methyl Cellulose (CMC) to obtain the desired dose on a bodyweight basis (mg/kg) of the animal and administered orally utilizing a ball finished taking care of needle. The animals were permitted free admittance to water and food in the wake of dosing (Iqbal et al., 2016).
2.4.3 Carrageenan-induced rat hind paw edema
Acute inflammation was incited by injection of 0.1 ml of 1% freshly prepared suspension of carrageenan in normal saline in the sub-plantar region of the right hind paw of all teams of animals (Mukherjee et al., 1997). At 1, 2, 3, 4, and 5 hr spans the volume of the injected paws was measured using a plethysmometer. The animals were premedicated with vehicle (0.3% CMC p.o.), isolated compounds HS-02, HS-03, and HS-04 at doses 05 and 10 mg/kg, and standard drug indomethacin (10 mg/kg) 1hr before carrageenan challenge (Sachan et al., 2011). The percentage inhibition of inflammation was meant consistent with the subsequent formula: % inhibition = 100 (1-Vt/Vc), Where ‘Vc’ stands for inflammation volume in control and ‘Vt’ inflammation volume in the group treated with tested drugs.
2.4.4 Analgesic activity
Analgesic activity of the isolated compounds HS-02, HS-03, and HS-04 was determined by both chemical (acetic acid-induced writhing response) and thermal method (hot plate reaction time).
2.4.4.1 Hot plate test
The pain-relieving (analgesic) test was done by Eddy's hot plate maintained at a temperature of 55 ± 1 °C. The basal response time of all mice towards thermal heat was verified first, then they were medicated with vehicle (0.3% CMC p.o.), isolated compounds HS-02, HS-03, and HS-04 at dose 05 and 10 mg/kg and standard medication Tramadol 0.025 ml (10 mg/kg. s.c). Following an hour of the test and standard medication dosing, the mice in all groups were individually placed to the hot plate maintained at 55 °C. The snapshot of time required in seconds for paw licking or bouncing was noted as reaction time. A remove phase of 30 s is kept up to dodge the paw’s harm. The pain inhibition percentage (PIP) was determined by the accompanying equation (Delporte et al., 2005). T1 is post-drug latency and T0 is pre-drug latency time.
2.4.4.2 Acetic acid-induced writhing test
The animals were pre-medicated with vehicle (0.3% CMC p.o.), HS-02, HS-03, and HS-04 at doses 05 and 10 mg/kg and standard medication diclofenac (5 mg/kg). Acetic acid (1% v/v) at the dose of 1 ml/kg body weight was injected intra-peritoneally to all the groups of animals 1hr after the dosing of test and standard drugs. Writhing was recorded by counting the number of writhes following the injection of acetic acid for a time of 30 min. The writhe is signified by abdominal constriction and full extension of the hind limb (Sajad et al., 2009).
2.5 Molecular docking
Chemical structures of isolated compounds HS-02, HS-03, and HS-04 were drawn using the ChemDraw program and converted to their three-dimensional coordinates in Chem3D. Structural coordinates of diclofenac was obtained from PubChem database in sdf format. Each ligand was subjected to energy minimization by the MM2 method and saved in pdb format. The three-dimensional structures of several drug targets associated with inflammatory cascade were retrieved from the Protein Data Bank (http://www.rcsb.org/pdb/home/home.do) and handled in Biovia Discovery Studio Visualizer 2020 program for checking any missing residue/atom and deleting co-crystallized molecules such as cofactors, inhibitors, and water. MGL Tools 1.5.6 was used for assigning Gasteiger charges on ligands and receptor proteins after merging non-polar hydrogens and saved as pdbqt format. The binding site in each receptor was defined according to the co-crystallized inhibitors. A grid box with dimensions of 30, 30, and 30 points in x, y, and z directions was built with a grid spacing of 1 Å. Molecular docking was performed using AutoDock Vina (Trott and Olson) with default parameters, and the exhaustiveness value was set to 12. Each docking involves ten independent runs keeping rigid protein and flexible ligand. Finally, upon successful completion of docking simulation, the best poses of each ligand were screened by inspecting binding energy (ΔGbinding, kcal/mol). Molecular interactions of ligand–protein complexes were studied using Biovia Discovery Studio Visualizer 2020 and PyMol 2.4.1 programs.
2.5.1 In silico toxicity
The toxicity of the isolated compounds HS-02, HS-03, and HS-04 was evaluated by T.E.S.T. software, Version 5.1.1, a program developed by the US Environmental Protection Agency (Martin 2016). The robust predictiveness of T.E.S.T. relies on innovative quantitative structure–activity relationship computations for toxicity measurement of synthetic as well as natural compounds based on respective molecular frameworks.
2.6 Statistical analysis
A one-way analysis of variance followed by Dunnett’s post-hoc test was performed by using Graph Pad Prism V2.01 (GraphPad Software, Inc., San Diego, California, USA). The data were expressed as the mean ± standard error of the mean and P < 0.05 and < 0.01 was considered as statistically substantial.
3 Results and discussion
3.1 Structural revelation
Compound HS-02- Transparent crystals; yield 102 mg (0.314 % mg/g); RF 0.37 (CH3OH: CHCl3, 1:3); mp 83–84 °C; UV–visible λmax (CH3OH): 283 nm; IR νmax (KBr): 3409, 2923, 2856, 1321, 1176, 1148, 1037, 1003, 889, 724 cm−1; 1H NMR (300 Hz, CH3OD);13C NMR (75 Hz, CH3OD) [Data: Table 1]; MS DART m/z (relative intensity) : 368 [M+] (C24H48O2) (2.8). Compound HS-03 (lignoceric acid) is saturated fatty acid, found as transparent crystals using CHCl3 and CH3OH (9:1) as eluent. Its carboxylic group was confirmed by sodium bicarbonate test: Exhibited effervescence. IR spectroscopy confirmed the presence of OH (3409 cm−1), COOH (1690 cm−1), and aliphatic sequence (724 cm−1). Fragmentation pattern of HS-02 showed parent ion peak on m/z 368; this confirms the presence of C24 fatty acid, C24H48O2. Proton NMR spectrum of HS-02 exhibited a 2H triplet at δH 2.37 (J: 7.2 Hz) to confirm the existence of H-2 vinylic protons. Three two-proton multiplets at 2.06, 1.63, and 1.53 and one six proton multiplets at 1.31. A thirty-proton broad singlet at 1.25 were credited to methylene H’s contiguous to the carboxylic group. A 3H triplet at δH 0.80 (J: 6.5 Hz), integrated 3H’s to display the finale (C-24)1° methyl group. 13C NMR spectra of HS-02 demonstrated the C-1 (δ177.21) as carboxylic carbon and C-24 (14.33) methyl carbons. The methylene carbons echoed in the array of δH 33.85 to 22.91. The spectral data concluded that compound HS-02 be long-chain saturated fatty acid lignoceric acid (n-tetracosanoic acid) (Fig. 1- HS-02). Coupling constants in Hz are provided in parentheses.
Position of proton
Nature of proton
δH (ppm)
δC (ppm)
α
β
C1
C
–
–
177.21
C2
CH2
2.37 (t, J = 7.2)
2.28
33.85
C3
CH2
2.06
1.98 (m)
32.15
C3
CH2
1.63
1.58 (m)
29.92
C4
CH2
1.53
1.47 (m)
29.90
C5
CH2
1.31
–
29.88
C6
CH2
1.25
1.29 (brs)
29.86
C7
CH2
1.25
1.29 (brs)
29.85
C8
CH2
1.25
1.29 (brs)
29.84
C9
CH2
1.25
1.29 (brs)
29.83
C10
CH2
1.25
1.29 (brs)
29.81
C11
CH2
1.25
1.29 (brs)
29.80
C12
CH2
1.25
1.29 (brs)
29.79
C13
CH2
1.25
1.29 (brs)
29.78
C14
CH2
1.25
1.29 (brs)
29.76
C15
CH2
1.25
1.29 (brs)
29.72
C16
CH2
1.25
1.29 (brs)
29.69
C17
CH2
1.25
1.29 (brs)
29.67
C18
CH2
1.25
1.29 (brs)
29.58
C19
CH2
1.25
1.29 (brs)
29.30
C20
CH2
1.25
1.29 (brs)
27.81
C21
CH2
1.28
1.31
26.27
C22
CH2
1.28
1.31
24.98
C23
CH2
1.30
1.34
22.91
C24
CH3
0.83 (t, J = 6.5)
14.33
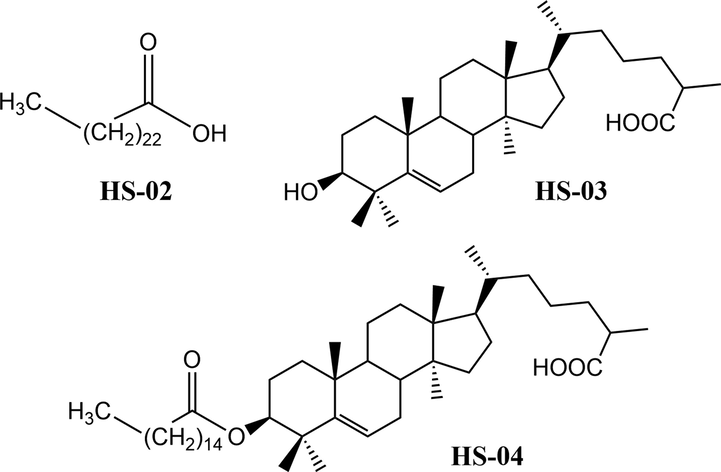
Chemical structures of isolated compound lignoceric acid (HS-02), lanost-5- en-3β-ol- 26-oic acid (HS-03). lanost-5-en-26-oic acid-3β-olyl palmitate (HS-04).
Compound HS-03- Lanost-5-en-3α-ol- 26-oic acid, acquired as transparent crystals using CHCl3 and CH3OH (18:02) as eluents, yield 115 mg (0.19 % w/w); RF 0.49 (CHCl3: Pet ether, 7.5:2.5); mp 90–92 °C; UV–visible λmax (CH3OH): 290 nm; IR νmax (KBr): 3215, 2923, 2856, 1690, 1635, 1461, 1383, 1277, 1193, 1037, 1003, 953 cm−1; 1H NMR (300 Hz, CH3OD) and 13C NMR (75 Hz, CH3OD) [Data: Table 2]; MS DART m/z (relative intensity) : 458 [M]+(C30H50O3) (5.2), 440 (1 0 0), 412 (10.1), 315 (21.2), 297 (41.3) (Fig. 2). It’s confirmatory tests for terpenoids and IR absorption bands for OH (3215 cm−1), COOH (1690 cm−1), unsaturation (1635 cm−1), and aliphatic sequence (953 cm−1). Fragmentation pattern indicated the molecular ion peak of HS-03 at m/z 458; this reinforced the molecular formula of triterpenic moiety C30H50O3. It may be due to the loss of CO2 molecule during the ionization of the compound in position C-26 the molecular ions found in the mass spectra were corresponding to the mass peak at m/z 412. The compound is fragmented into two parts between C11 and C12 and between C8 and C14. These fragments have m/z 191 and 248 with molecular formula C14H22 and C18H32 respectively. 1H NMR spectra of HS-03 exhibited 1H multiplet at δH 5.25 ppm (C-6) and 1H double doublet’s at δH 3.22 (J: 4.5, 5.4 Hz) endorsed vinylic H-6 and oxygenated methine H-3α protons, correspondingly. Two 3H doublet at δH 0.90 (J = 6.1 Hz) with 0.87 (J = 6.3 Hz) credited as methyl C-21 and C-27, correspondingly. The five three-proton broad singlets at δH 1.08, 0.98, 0.93, 0.78, and 0.76 ascribed tertiary C-30, C-29, C-19, C-28, and C-18 methyl protons, and all methyl functionalities were attached to the saturated carbons. The 13C NMR spectrum of HS-03 displayed signals for vinylic carbons at δC 138.81 (C-5) and 119.71 (C-6), carboxylic carbons at δC 176.89 (C-26), oxygenated methine carbon δC 78.26 (C-3) and the other carbon appears in the range of δC 55.06 to 14.03. The 1H and 13C NMR spectral data of the lanostene unit were compared with related triterpenoids (Arif et al., 2013; Hussain et al., 2020b). Based on these results the structure of HS-03 has been formulated as Lanost-5- en-3α-ol- 26-oic acid (Fig. 1-HS-03). This is a new triterpene phytoconstituents of H. stoechas.
Position of proton
Nature of proton
δH (ppm)
δC (ppm)
α
β
C1
CH2
1.23 (brs)
1.27
34.73
C2
CH2
1.38 (brs)
1.45
27.68
C3
CH
3.22 (dd, J = 5.4)
78.17
C3
CH
4.65
OH
–
C4
C
–
–
39.51
C5
C
–
–
138.81
C6
CH
5.25 (m)
119.72
C7
CH2
2.27
2.31(m)
29.26
C8
CH
2.14 (d, J = 6.0)
42.05
C9
CH
2.11
48.28
C10
C
–
–
38.65
C11
CH2
2.17
2.20
21.08
C12
CH2
1.98
2.02
23.21
C13
C
–
48.38
C14
C
–
55.96
C15
CH2
1.87 (brs)
1.90
35.92
C16
CH2
1.62 (brs)
1.68
48.13
C17
CH
1.81(m)
55.27
C18
CH3
0.76 (brs)
14.02
C19
CH3
0.93 (brs)
17.03
C20
CH
2.49 (d, J = 5.7)
30.42
C21
CH3
0.90 (d, J = 6.1)
–
15.49
C22
CH2
1.17
35.42
C23
CH2
1.13
1.08
33.68
C24
CH2
1.03
31.16
C25
CH
1.54 (d)
29.52
C26
COOH
0.78
176.89
C27
CH3
0.87 (d, J = 6.3)
22.18
C28
CH3
0.78 (brs)
20.21
C29
CH3
0.98 (brs)
28.03
C30
CH3
1.08 (brs)
14.08
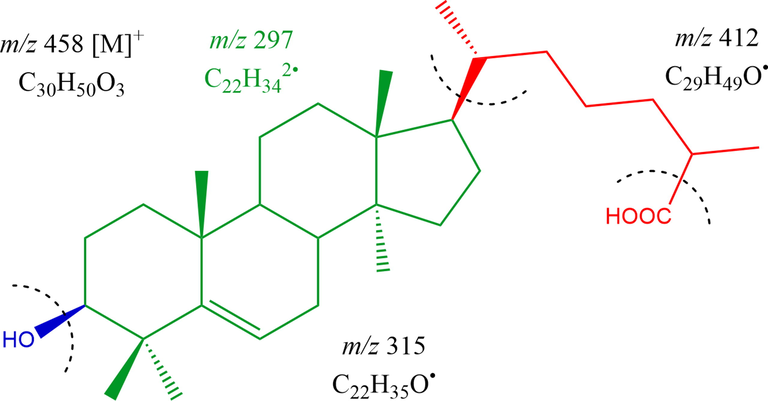
Mass fragmentation pattern of Lanost-5- en-3β-ol- 26-oic acid (HS-03).
Compound HS-04- Lanost-5-en-26-oic acid-3β-olyl palmitate-was acquired as straw colour crystals using CHCl3 and CH3OH (18:2) as eluants; 123 mg (0.311% yield); RF 0.53 (CH3OH: CHCl3, 9:1); mp 100–105 °C; UV–visible λmax (CH3OH): 278 nm; IR νmax (KBr): 2956, 2856, 1725, 1690, 1635, 1461, 1388, 1262, 1061, 953, 730 cm−1; 1H NMR (300 Hz, CH3OD) and 13C NMR (75 Hz, CH3OD) [Data: Table 3]; MS DART m/z (relative intensity) : 696 [M]+ (C46H80O4) (2.2), 457 (21.6), 440 (1 0 0), 256 (10.4), 239 (8.6). It displayed confirmatory tests for glycosides and IR absorption bands for COOR (1725 cm−1), COOH (1690 cm−1), unsaturation (1635 cm−1), and aliphatic sequence (730 cm−1). Mass spectra demonstrated the molecular ion peak of HS-04 at m/z 696 to confirm the triterpene ester moiety C46H80O4 (Fig. 3). 1H NMR spectra of HS-04 exhibited a 1 H doublet at δH 5.35 (J = 5.7 Hz), 2H double doublet at δH 4.19 (J = 5.4, 8.7 Hz) and two 3H proton doublet at δH 0.90 (J = 6.2 Hz) and δH 0.87(J = 6.3 Hz) endorsed as vinylic H-6, methyl C-27, and C-21, respectively. A 2H triplet’s appeared at δH 2.32 (J = 7.2 Hz), one three-proton triplet at δH 0.84 (J = 6.1 Hz), and a two proton multiplet’s ascribed as methene (C-2′) (C-7) and methyl (C-16′) respectively. The six 3H broad singlet’s seemed at δH 1.09, 0.97, 0.94, 0.82, 0.80 and 0.77, ascribed as methyl C-30, C-29, C-19, C-27, C-28 and C-18, respectively. The ester Hs appear in the array of δH 2.12 to 1.28. 13C NMR spectra of HS-04 exhibited vinylic carbons at δC 145.34 (C-5) and 123.27(C-6), COOH carbons at δC 177.63 (C-26), oxygenated methine carbon δC 79.85 (C-3) and the other carbon appears in the range of δC 56.89 to 16.47. The ester carbon appears at δC 166.03 (C-1′) and other aliphatic carbon ranges in δC 33.72 to 14.99. 1H along with 13C NMR spectral features of the lanostene unit exhibited similarity with related triterpenoids (Hussain et al, 2020a). The structural features of HS-04 confirmed it to be Lanost-5-en-26-oic acid-3β-olylpalamitate (Fig. 1- HS-04). This is a new triterpenoid ester of palmitic acid of H. stoechas
Position of proton
Nature of proton
δH (ppm)
δC (ppm)
Α
β
C1
CH2
1.27 (brs)
1.32
34.10
C2
CH2
1.53 (brs)
1.57
27.68
C3
CH
4.19 (dd, J = 5.4, 8.7)
79.85
C3
OH
5.01
–
C4
C
–
–
43.03
C5
C
–
–
145.34
C6
CH
5.35 (d, J = 5.0.74)
123.27
C7
CH2
2.27
2.31(m)
29.17
C8
CH
2.34 (d, J = 6.0)
40.88
C9
CH
2.13
50.11
C10
C
–
–
38.24
C11
CH2
2.17
2.20
23.87
C12
CH2
1.98
2.02
26.24
C13
C
–
47.39
C14
C
–
56.96
C15
CH2
1.87 (brs)
1.90
35.10
C16
CH2
1.62 (brs)
1.68
39.98
C17
CH
1.81(m)
54.50
C18
CH3
0.85 (brs)
16.18
C19
CH3
0.94 (brs)
19.62
C20
CH
2.49 (d, J = 5.7)
28.91
C21
CH3
0.87 (d, J = 6.3)
17.75
C22
CH2
1.17
1.12
34.15
C23
CH2
1.13
1.08
33.21
C24
CH2
1.03
0.98
31.75
C25
CH
1.54 (d)
28.29
C26
COOH
0.78
177.63
C27
CH3
0.90 (d, J = 6.2)
24.27
C28
CH3
0.95 (d, J = 7.8)
21.73
C29
CH3
0.97 (brs)
29.91
C30
CH3
1.09 (brs)
16.47
C1′
C
–
166.03
C2′
CH2
2.32 (t, J = 7.2)
33.72
C3′
CH2
2.30 (m)
33.21
C4′
CH2
2.28 (m)
30.90
C5′
CH2
2.28 (m)
30.88
C6′
CH2
2.26 (m)
30.88
C7′
CH2
2.24 (m)
30.90
C8′
CH2
2.18 (m)
30.90
C9′
CH2
2.16 (m)
30.75
C10′
CH2
2.14 (m)
30.61
C11′
CH2
2.11 (m)
30.36
C12′
CH2
1.98 (brs)
26.24
C13′
CH2
1.48 (brs)
25.47
C14′
CH2
1.32 (brs)
24.14
C15′
CH2
1.22 (brs)
22.68
C16′
CH3
0.84 (t, J = 6.1)
14.99
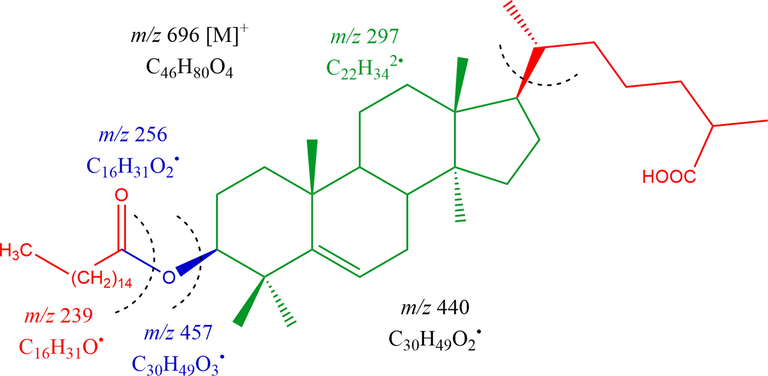
Mass fragmentation pattern of Lanost-5-en-26-oic acid-3β-olyl palmitate (HS-04).
3.2 Safety profile study
It was seen that the dosing of isolated compounds HS-02, HS-03, and HS-04 up to 150 mg/kg orally to the mice didn't incite drug-related harmfulness (toxicity) and mortality. The mice endured the drug well and showed normal behavior up to 150 mg/kg orally. All animals were vigilant with normal spruce, touch, and pain response, and there was no indication of accommodation and vocalization.
3.3 Carrageenan induced rat paw edema
Anti-inflammatory effect of isolated compounds HS-02, HS-03, and HS-04 at a dose (05 & 10 mg/kg) and Indomethacin at a dose (10 mg/kg) on carrageenan disturbed rat paw is displayed in (Fig. 4). The anti-inflammatory effects of all tested drugs were seen from 60 min. after carrageenan challenge. Paw edema in rats accomplished its highest at 4 hrs following carrageenan challenge and animals treated with Indomethacin showed a significant (P < 0.05) decrease in paw volume of rats from 2 hr. Treatment with Indomethacin and HS-04 at (10 mg/kg) showed a significant reduction (P < 0.05) of paw volume in rats incited by carrageenan. The impact of HS-04 at portion 10 mg/kg was discovered favored in lessening the swollen paw volume than a higher portion of Indomethacin (10 mg/kg). Aggravation is associated with a ton of pathophysiologies of various clinical conditions like joint inflammation, malignant growth, gout, and vascular sicknesses. A lot of therapeutic plants are utilized in the scope of conventional clinical frameworks for the help of caution indication of torment and irritation. In this investigation, the lignoceric acid (HS-02), lanost-5- en-3β-ol- 26-oic acid (HS-03), and lanost-5-en-26-oic acid-3β-olyl palmitate (HS-04) isolated from H. stoechas extract showed calming pain-relieving action at dose 05 and 10 mg/kg. Carrageenan is gone about as a phlogistic factor and increased the prostaglandins and bradykinins combination at different periods (He et al., 2005). All tested drugs showed a decrease in paw edema volume from 1hr to 5hr and prolong its anti-inflammatory effect after the 3hr. This investigation unmistakably shows that the impact of all tested drugs may cooperate with the prostaglandins spurt. Curiously both dose levels of HS-04 exhibited a similar pattern in reducing carrageenan-induced paw edema from 1hr to 5hr. Carrageenan-induced inflammation is caused by the commencement of prostaglandins, platelet-activating factors (PAF), and other inflammatory mediators. The primary stage (0-1hr) is supported by the arrival of histamine, 5-HT, and kinin, though the subsequent stage (3–5 hr) is associated with the release of prostaglandin and bradykinin. Even though the calming response found in 10 mg/kg was superior to the 05 mg/kg. This result straightforwardly correlated with the results observed in carrageenan-induced paw edema where both doses of isolated compounds significantly alter the carrageenan initiated prostaglandins intervened inflammatory response (Mukherjee et al., 1997).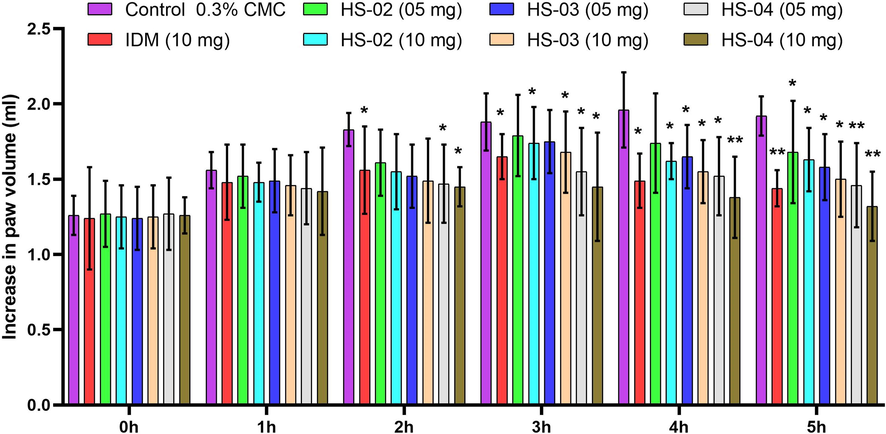
Effect of of isolated compounds HS-02, HS-03 and HS-04 and Indomethacin (IDM) on carrageenan induced rat paw edema. Each value is expressed in Mean ± S.E.M. one way ANOVA followed by Dunnett’s test. P: *p < 0.05 and **p < 0.01 compare to respective control group.
3.3.1 Hot plate test
HS-03 and HS-04 at dose 10 mg/kg and Tramadol significantly (P < 0.01) augmented the response time of animals towards the thermal source with various periods (Fig. 5). In hot plate test of isolated compounds HS-02, HS-03 and HS-04 at dose (10 mg/kg p.o.) and Tramadol (10 mg/kg. s.c) displayed a pain inhibition percentage (PIP) of 36.67%, 44.93%, 72.97% and 79.21%.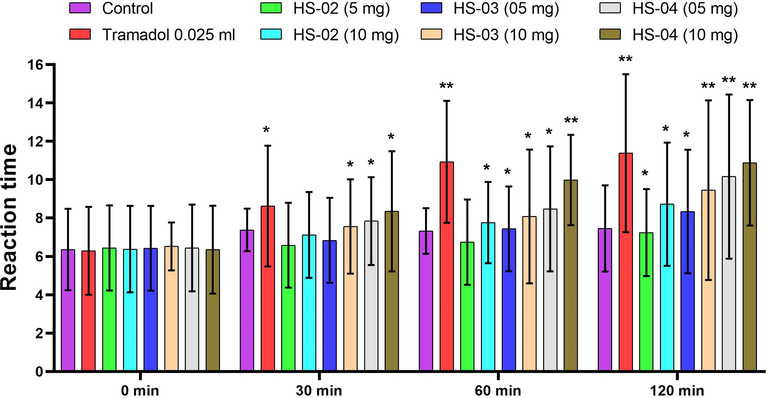
Effect of isolated compounds HS-02, HS-03, HS-04 and Tramadol (Standard) on reaction time of mice exposed to hot plate. Each value is expressed in Mean ± S.E.M. one way ANOVA followed by Dunnett’s test. P: *p < 0.05 and **p < 0.01 compare to respective control group.
3.3.2 Acetic acid-induced writhing methods
The analgesic activity of HS-02, HS-03, and HS-04 at doses 05 and 10 mg/kg and Diclofenac (5 mg/kg) was assessed by acetic acid-induced writhing test in mice. Both HS-04 and Diclofenac significantly (P < 0.001) lowered the quantity of abdominal constriction and draw out of hind limbs provoked by the injection of acetic acid (Fig. 6). The percentage of lowering the quantity of abdominal constriction in HS-02, HS-03, and HS-04 at 10 mg/kg was 48.71%, 65.38%, and 84.61%, whereas standard drug Diclofenac (5 mg/kg) showed 71.79%. The abdominal constrictions observed after the challenge of acetic acid are associated with the sensitization of nociceptive receptors to prostaglandins. So subsequently conceivable outcomes of the concentrates apply their pain-relieving impacts maybe by hindering the amalgamation of prostaglandins.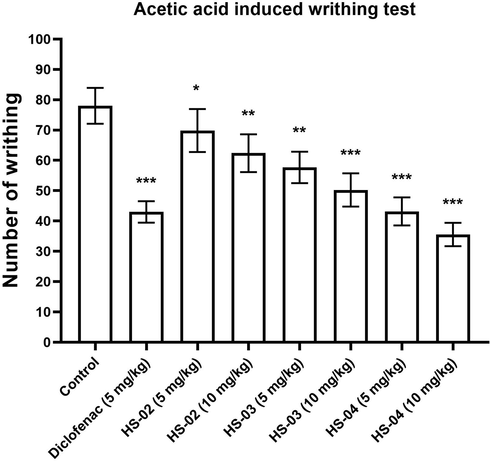
Effect of isolated compounds HS-02, HS-03, HS-04 and Diclofenac (Standard) on acetic acid induced writhing in mice. Each value is expressed in Mean ± S.E.M. one way ANOVA followed by Dunnett’s test. P: *p < 0.05, **p < 0.01 and ***p < 0.001compare to respective control group.
3.4 Molecular docking
All the isolated compounds HS-02, HS-03, and HS-04 were docked with numerous receptors known to be associated with inflammatory pathways, namely cyclooxygenase-2 (PDB id: 3LN1), tumor necrosis factor-α (PDB id: 2AZ5; He et al., 2005), inducible nitric oxide synthase (iNOS) (PDB id: 4NOS; Fischmann et al., 1999) and galectin-3 (PDB id: 1P8D; Williams et al., 2003). The docking protocol implemented in AutoDock Vina was validated by redocking all co-crystallized ligands with respective proteins. The docked poses were compared with crystal conformation of each ligand exhibiting root-mean-square deviation (RMSD) of ≤ 2.0 Å which confirms the reliability of the opted docking methodology (Ahmed et al., 2012; Azam et al., 2019; 2015).
According to the docking results presented in Fig. 7, HS-03 showed the highest docking scores of −12.50, −9.10, and −7.20 kcal/mol against galectin-3, iNOS, and TNF-α receptors, respectively. However, HS-04 was observed as most active against the COX-2 enzyme displaying −8.0 kcal/mol docking predicted binding energy. Interestingly, HS-02 was noted as the least active molecule against all docked targets confirming that aliphatic carboxylic acid appended with a long unbranched carbon chain failed to afford favorable occupation in the binding sites. However, both HS-03 and HS-04 constitute lanostanol-based steroidal nucleus, suitable for interacting with most of the receptors (Fig. 8). Standard drug diclofenac was also included in the docking study for comparative analysis, which exhibited docking score of −8.50, −8.40, −8.30, and −5.70 kcal/mol against galectin-3, iNOS, COX-2, and TNF-α receptors, respectively. The binding interactions of diclofenac were almost similar to other docked compounds, following the native co-crystallized ligands of respective proteins. Intermolecular interactions constituting both hydrophilic and hydrophobic contacts of all docked compounds are presented in Table 4. These outcomes are supposed to assist lead optimization and drug development for the treatment of inflammatory disorders.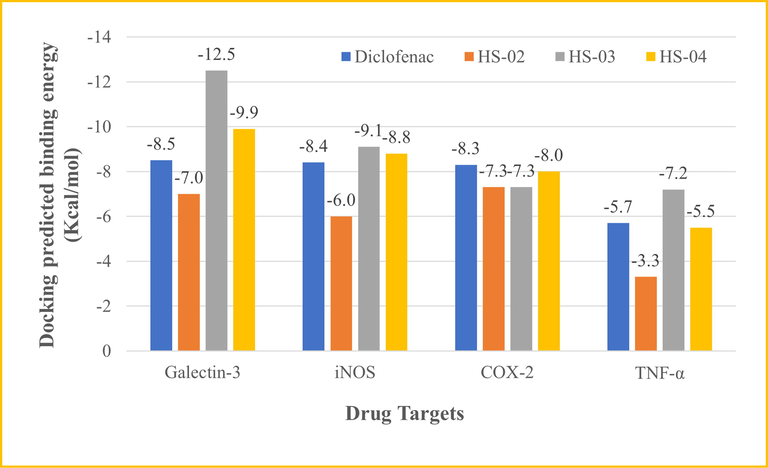
Docking predicted binding energy of isolated compounds and standard drug, diclofenac, plotted against protein targets such as galectin-3, iNOS, COX-2, and TNF-α.
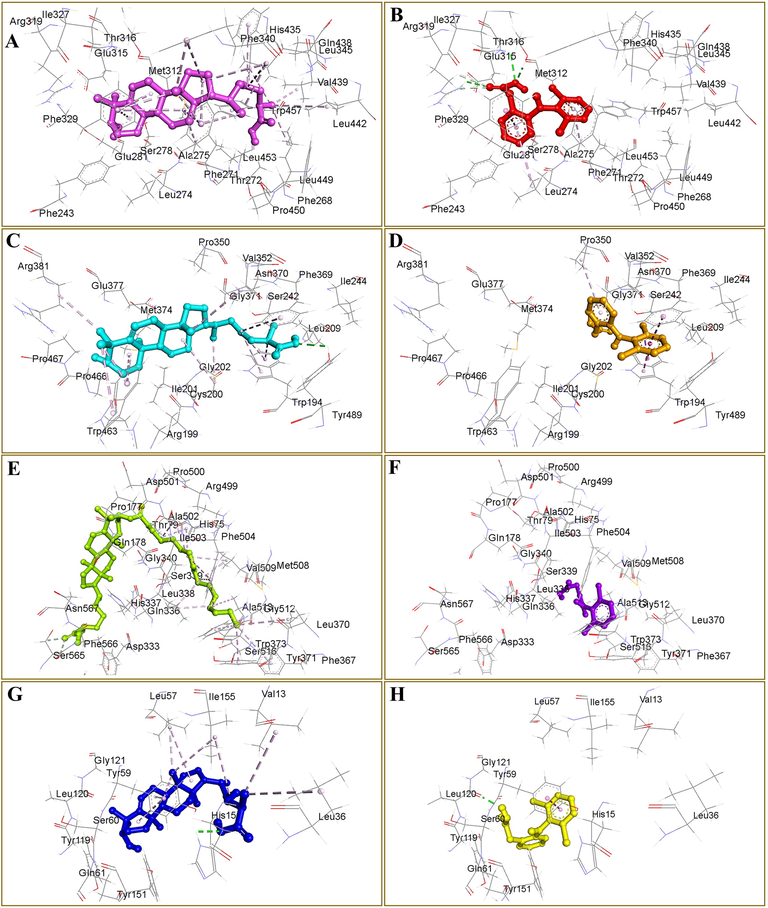
Three-dimensional conformations of the docked compound HS-03 (A, C, E and G) and diclofenac (B, D, F and H) against galectin-3 (A-B), iNOS (C-D), and TNF-α (E-F). Docked compounds HS-04 and diclofenac in complex with COX-2 are presented in panels G and H, respectively. All docked compounds are shown as ball and stick style whereas binding site residues are represented as lines. Non-bond interactions are displayed as broken green (hydrogen bonds) and purple (hydrophobic) lines.
3.4.1 In-silico toxicity prediction
The unique molecular framework of chemical compounds is usually exploited for the generation of advanced mathematical models for quantitative structure–activity relationship (QSAR) studies aimed at prediction of biological activity as well as toxicity. In particular, the toxicity potential of several compounds was successfully predicted by implementing these models (Claeys et al., 2013; Li et al., 2020; Sripriya et al., 2019). The acute toxicity of isolated compounds HS-02, HS-03, and HS-04 was assessed by using the TEST program which predicts mutagenicity and oral lethal dose for 50% of test rats (LD50). This program uses three diverse methods for prediction, namely, consensus, hierarchical clustering, and nearest neighbor. The computed values of LD50 and mutagenicity have been tabulated in Table 5. All tested compounds HS-02, HS-03 and HS-04 exhibited negative mutagenicity scores. However, a positive mutagenicity score was recorded for diclofenac according to consensus and nearest neighbor methods whereas hierarchical clustering method indicated negative result by the TEST program. Several bioassay data show that diclofenac lacks toxicity risks such as carcinogenicity in rodents. However, additional research has recently been published that demonstrates that diclofenac has a genotoxic potential in newer or modified in vitro test systems (Hartmann et al., 2021) (see Table 5).
Target; PDB code
Residues involved in interactions
H-bonds
Hydrophobic
HS-02
HS-03
HS-04
Diclofenac
HS-02
HS-03
HS-04
Diclofenac
Galectin-3; 1P8D
Met312
–
–
Thr316, Arg319
Phe243, Phe271, Leu274, Ala275, Met312, Leu313, Phe329, Ile353, Phe340, Leu345
Phe271, Ala275, Phe329, Phe340, Leu345, Phe349, His435, Val439, Leu442, Leu449, Trp457
Leu236, Phe271, Ala275, Ile282, Phe285, Ile311, Arg319, Phe329, Phe340, Leu345, Phe349, Ile374, His435, Val439, Leu442, Leu449, Trp457
Leu274, Ala275, Met312, Phe329
Inducible Nitric Oxide Synthase; 4NOS
Arg199, Tyr490, Tyr491
Tyr489
Gly202, Gln492
Trp372
Trp194, Ala197, Pro198, Arg199, Cys200, Pro350, Phe369
Trp194, Cys200, Leu209, Val352, Phe369, Met374, Arg381, Trp463
Leu125, Trp194, Ala197, Pro198, Arg199, Cys200, Pro350, Val352, Phe369, Trp463, Phe488, Tyr489, Tyr491
Trp194, Pro350, Phe369
TNF-α; 2AZ5
Ser60
His15
Gly121
Leu120
Leu57, Tyr59
Val13, Leu36, Leu57, Tyr59, Ile155
Leu57, Tyr59, Tyr119
Tyr59
COX-2; 3LN1
Leu338
Thr79, Asp333
Ser565
–
Val102, Val335, Leu338, Leu345, Leu370, Tyr371, Trp373, Phe504, Met508, Val509, Ala513
His80, His342, Pro500
His75, Leu338, Trp373, Ala502, Phe504, Val509
Val335, Ala513, Leu517
Prediction methods
Toxicity parameters
Toxicity results
HS-02
HS-03
HS-04
Diclofenac
Consensus method
Oral rat LD50 mg/kg
20861.82
477.13
326.66
244.02
Mutagenicity value
0.00
0.30
0.18
0.53
Mutagenicity result
Negative
Negative
Negative
Positive
Hierarchical clustering method
Oral rat LD50 mg/kg
13878.45
104.79
146.60
228.56
Mutagenicity value
0.00
0.26
0.03
0.40
Mutagenicity result
Negative
Negative
Negative
Negative
Nearest neighbor method
Oral rat LD50 mg/kg
31359.08
2172.48
727.88
260.53
Mutagenicity value
0.00
0.33
0.33
0.67
Mutagenicity result
Negative
Negative
Negative
Positive
4 Conclusion
The present study suggested that HS-03 and HS-04 at 10 mg/kg showed significant anti-inflammatory, analgesic activity. Carrageenan incited edema is the principle pull for the actuation of prostaglandins, platelet initiating factors (PAF), and other fiery mediators. In conclusion Lanost-5- en-3β-ol- 26-oic acid (HS-03) and Lanost-5-en-26-oic acid-3β-olyl palmitate (HS-04) isolated from H. stoechas extract showed protection against inflammation; pain and cytokine-like pro-inflammatory mediators release might be beneficial in the treatment of endotoxin associated systemic inflammation. To our knowledge, the current study has been conducted for the first time concerning the occurrence of lanostane triterpenoid in ethanolic extract of H. stoechas. The study also affirms that these potent isolated compounds may be a valuable marker for the Helichrysum genus. These outcomes are relied upon to be essential in lead to augment and fostering the potent molecules HS-03 and HS-04 for the treatment of inflammation-mediated diseases.
Acknowledgements
The authors are thankful to the Honorable President, Misurata University Misurata, Libya, for providing necessary facilities in University premises for this research. Dr. Huda Elgubbi, Department of Botany, College of Science, Misurata University, Misurata, Libya is kindly acknowledged for assisting in the authentication of plant material. The authors are thankful to the Deanship of Scientific Research, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the Research Group Project under grant number (RGP.1/48/42).
Compliance with ethical standards
Ethical statement: The experimental protocols were approved by the Institutional Ethical Committee of Faculty of Pharmacy, Misurata University, Misurata, Libya, and their guidelines were followed for the studies (Phar-01/2015).
Author Contributions
Md. Sarfaraj Hussain designed and supervised the entire work and wrote the chemistry part of the manuscript, Faizul Azam performed and wrote the results of the docking study, Hanan Ahmed Eldarrat isolated and identified lignoceric acid, lanost-5- en-3β-ol- 26-oic acid and lanost-5-en-26-oic acid-3β-olyl palmitate, Muhammad Arif & Mohammed Ali interpreted the spectroscopic data and revised the manuscript, Mohd. Zaheen Hassan & Anzarul Haque provided the carrageenan and helped in the anti-inflammatory, analgesic study, Irfan Ahmad, Gaffar Zaman, Nadiyah M. Alabdallah, and Mohd. Saeed designed and revised the submitted manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- ‘Structure-based design, synthesis, molecular docking, and biological activities of 2-(3-benzoylphenyl) propanoic acid derivatives as dual mechanism drugs. J. Pharm. Bioallied. Sci.. 2012;4(1):43-50.
- [Google Scholar]
- Antiplatelet activity and isolation of triterpenoid glycoside from ethanolic extract of Nepeta hindostan. Ori. Pharm & Expt. Med.. 2016;16(4):333-337.
- [Google Scholar]
- Pharmacognostic investigation and authentication of potentially utilized fruit Spondias mangifera (Willd) Int. J. Curr. Pharmaceut. Clin. Res.. 2010;2(1):31-35.
- [Google Scholar]
- Adaptogenic activity of lanostane triterpenoid isolated from Carissa carandas fruit against physically and chemically challenged experimental mice. Pharmacog. J.. 2013;5:216-220.
- [Google Scholar]
- Glandular trichomes and essential oils of Helichrysum stoechas. Israel J. Plant Sci.. 2001;49:115-122.
- [Google Scholar]
- An In-silico analysis of ivermectin interaction with potential SARS-CoV-2 targets and host nuclear importin α. J. Biomol. Struc. Dyn. 2020
- [CrossRef] [Google Scholar]
- Rutin as promising drug for the treatment of Parkinson’s disease: an assessment of MAO-B inhibitory potential by docking, molecular dynamics, and DFT studies. Mol. Simul.. 2019;45(18):1563-1571.
- [Google Scholar]
- ‘Molecular interaction studies of green tea catechins as multitarget drug candidates for the treatment of Parkinson's disease: computational and structural insights. Network.. 2015;26(3–4):97-115.
- [Google Scholar]
- ‘Structure-based design, synthesis and molecular modeling studies of thiazolyl urea derivatives as novel anti-parkinsonian agents. Med. Chem.. 2012;8(6):1057-1068.
- [Google Scholar]
- The crystal and molecular structure of helichrysoside, a new acylated flavonoid glycoside from Helichrysum kraussii. Tetrahedron Lett.. 1975;16(14):1211-1214.
- [Google Scholar]
- LC coupled to ion-trap MS for the rapid screening and detection of polyphenol antioxidants from Helichrysum stoechas. J. Pharm. Biomed. Anal.. 2001;24(3):517-526.
- [Google Scholar]
- Chemical and biological studies on four Helichrysum species of Greek origin. In: Franz C.h., Máthé Á., Buchbauer G., eds. Essential Oils: Basic and Applied Research. Carol Stream, IL: Allured Publishing Corp; 1997. p. :45-48.
- [Google Scholar]
- Chemical and antibacterial studies of two Helichrysum species of Greek origin. Planta Med.. 1997;63(2):181-183.
- [Google Scholar]
- Development and validation of a quantitative structure-activity relationship for chronic narcosis to fish. Environ. Toxicol. Chem.. 2013;32(10):2217-2225.
- [Google Scholar]
- Analgesic-anti-inflammatory properties of Proustia pyrifolia. J. Ethnopharmacol.. 2005;99:119-124.
- [Google Scholar]
- Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat. Struct. Biol.. 1999;6(3):233-242.
- [Google Scholar]
- A volatolomic approach for studying plant variability: the case of selected Helichrysum species (Asteraceae) Phytochem.. 2016;130:128-143.
- [Google Scholar]
- Comprehensive review of genotoxicity data for diclofenac. Mutat. Res. Genet. Toxicol. Environ. Mutagen.. 2021;866:503347
- [CrossRef] [Google Scholar]
- Chemical constituents from the leaves of Carya illinoinensis, aerial parts of Helichrysum stoechas and hulls of Oryza sativa. Euro. J. Pharm. Med. Res.. 2020;7(7):723-731.
- [Google Scholar]
- Free radical scavenging capacity of natural terpenes from Hygrophila auriculata (Schum) Heine. Asian J. Trad. Med.. 2009;4(5):179-187.
- [Google Scholar]
- Anti-endotoxin effects of terpenoids fraction from Hygrophila auriculata in lipopolysaccharide-induced septic shock in rats. Pharm. Bio.. 2016;54(4):628-636.
- [Google Scholar]
- New aliphatic ester constituents from Hygrophila auriculata (K.Schum) Heine from the basin area of Koshi River. Ori. Pharm and Exp. Med.. 2019;19(3):251-258.
- [Google Scholar]
- Anti-inflammatory, analgesic activity and molecular docking studies of Lanostanoic acid 3-O-α-D-glycopryranoside isolated from Helichrysum stoechus against chemically challenged experimental animal. Arabian J. Chem.. 2020;13(12):9196-9206.
- [Google Scholar]
- Protection of hepatotoxicity using Spondias pinnata by prevention of ethanol induced oxidative stress, DNA-damage and altered biochemical markers in Wistar rat. Integr. Med. Res.. 2016;5:267-275.
- [Google Scholar]
- Constituents of Helichrysum stoechas variety olonnense. Chem. Nat. Comp.. 2004;40(2):118-121.
- [Google Scholar]
- Everlasting flower (Helichrysum stoechas Moench) as a potential source of bioactive molecules with antiproliferative, antioxidant, antidiabetic and neuroprotective properties. Indus. Crops and Prod.. 2017;108:295-302.
- [Google Scholar]
- Degradation of fluopyram in water under ozone enhanced microbubbles: Kinetics, degradation products, reaction mechanism, and toxicity evaluation. Chemosphere. 2020;258:127216.
- [Google Scholar]
- South African Helichrysum species: A review of the traditional uses, biological activity and phytochemistry. J. Ethnopharmacol.. 2008;119:630-652.
- [Google Scholar]
- Contribution to a Taxonomic Revision of the Sicilian Helichrysum Taxa by PCA Analysis of Their Essential-Oil Compositions. Chem. Biodivers.. 2016;13(2):151-159.
- [Google Scholar]
- Studies on the Anti-inflammatory activity of Rhizomes of Nelumbo nucifera. Planta Med.. 1997;63:367-369.
- [Google Scholar]
- Composition and antibacterial activity of the essential oils of two Helichrysum stoechas varieties growing in the Island of Crete. J. Essen. Oil Res.. 2002;14(6):459-461.
- [Google Scholar]
- Anti-inflammatory, analgesic and antioxidant potential of the stem bark of Spondias mangifera Willd. Arch. Biol. Sci. Belgrade.. 2011;63(2):413-419.
- [Google Scholar]
- ‘Hippocampal neurodegeneration in experimental autoimmune encephalomyelitis (EAE): potential role of inflammation activated myeloperoxidase. Mol. Cell Biochem.. 2009;328:183-188.
- [Google Scholar]
- Oxasetin from Lophiostoma sp. of the Baltic Sea: identification, in silico binding mode prediction and antibacterial evaluation against fish pathogenic bacteria. Nat. Prod. Commun.. 2013;8(9):1223-1226.
- [Google Scholar]
- Chemical constituents and antimicrobial activity of Helichrysum stoechas. Asian J. Plant Sci.. 2007;6(4):692-695.
- [Google Scholar]
- Sripriya, N., M, R.K., N, A.K., S, B., N.K., U.P., 2019. In silico evaluation of multispecies toxicity of natural compounds. Drug Chem. Toxicol. 1–7.
- Chemical composition of the essential oils and headspace samples of two Helichrysum species occurring in Spain. J. Essen. Oil Res.. 1999;11(4):511-516.
- [Google Scholar]
- 1980. Flora Eurpaea: Cambridge University Press; 1980.
- GC/MS analysis of volatile components of everlasting (Helichrysum stoechas L.) essential oil. J. Essential Oil Res.. 1998;10(5):553-557.
- [Google Scholar]
- X-ray crystal structure of the liver X receptor beta ligand binding domain: regulation by a histidine-tryptophan switch. J. Biol. Chem.. 2003;278(29):27138-27143.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103818.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







