Translate this page into:
Structural properties and antioxidant activities of polysaccharides isolated from sunflower meal after oil extraction
⁎Corresponding author. wangxuede1962@126.com (Xue-De Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Making full use of sunflower seeds, including oil and the polysaccharides extracted from the meals which oil has been extracted, is one way to enhance their industrial value. Such meals contain abundant polysaccharides; however, the application of polysaccharides isolated from sunflower remaining meals after oil extraction has not been investigated. In this study, polysaccharides were isolated by alkali from sunflower meals after different oil extraction processes, and their structural properties and antioxidant activities were compared. The results indicated that these polysaccharides displayed significant variability in monosaccharide composition and molecular weight. Differences in structural properties could result in differences in functional antioxidant properties. The polysaccharide (SPHE-1) obtained from the meals after traditional hexane extraction exhibited the best antioxidant activities, including DPPH free radical-scavenging assay and hydroxyl radical scavenging activity among all the polysaccharide fractions. The research provides valuable information for making efficient use of sunflower seeds in the food industry.
Keywords
Sunflower
Oil extraction process
Polysaccharide
Structural properties
Antioxidant activities
1 Introduction
Sunflower (Helianthus annuus L.), as an important oilseed crop, is widely cultivated all over the world (Flagella et al., 2002; González-Martín et al., 2013). The seeds contain 22–55% oil, and this oil has high amounts of polyunsaturated fatty acids (Akkaya, 2018). The by-products from sunflower oil extraction (namely, sunflower meal or cakes) are a valuable potential source of components, such as carbohydrate, protein and phenols, which could be used to the food industry (Zardo et al., 2019). The sunflower meal or cake remaining after oil extraction contains about 25-50 wt% of protein, 29-52 wt% of carbohydrate and 3 wt% of phenols, with chlorogenic acid being the dominant phenol (Karefyllakis et al., 2018).
Extracting high quality oil is the major objective of commercial oil extraction. Generally, the major methods of plant oil extraction are expeller pressing and conventional organic solvent extraction. There are two types of mechanical expeller extraction, cold pressing and hot pressing. Mechanical extraction typically yields less oil than solvent extraction. Organic solvent extraction, using volatile solvents such as hexane and petroleum ether, achieves high crude oil yield (over 95%) (de Moura et al., 2009). One drawback of the solvent extraction method is that the meal and crude oil should be heated, after extraction, to remove the solvents. Thus, during both solvent extraction and hot pressing processes of oil, the seed materials are usually treated at high temperature, which results in poor quality and darker color of meal/cake because of protein denaturation. In general, this makes the oil and meal unsuitable for human food products (Wang et al., 2017). In recent years, low temperature techniques for extracting oil, such as supercritical CO2 and subcritical propane/butane extraction, has attracted increasing attention due to the high oil yield and high quality of meal (Liu et al., 2015; Barbosa et al., 2014; Mezzomo et al., 2010; Reverchon and Osséo, 1994). However, supercritical CO2 extraction requires high pressure, which limits its application in industry. As compared with supercritical fluid extraction, subcritical propane/butane extraction is operated under lower pressure, which makes it more popular for plant oil extraction, particularly for human food products.
The production of protein from sunflower meal/cake has been widely investigated because it means more of the meal can be used (less wasted), and it can reduce the dependency of the food industry on expensive soy protein (Malik and Singh, 2018). However, as far as we know, there is little literature on the utilization of sunflower meal/cake for polysaccharide extracts. As a natural polymers widely found in plants, microorganisms and animals, polysaccharide has been applied in the medicine and food industries for a long time because of its specific biological activities and high safety profiles (Wang et al., 2012; Yang et al., 2016). Sunflower meal/cake is also a potential source of polysaccharides because of its high amount of fiber. The application of any polysaccharide in the medicine and food industries largely depends on its structural and functional properties. The extraction processes, whether by heat or pressing, influence the physicochemical properties of the meal/cake, and these properties may be directly related to the functional and structural properties of the polysaccharides and their interaction with protein. As far as we know, there is no report on polysaccharides isolated from the meal/cake of sunflower seeds that remains after oil extraction. The aim of this study was to compare the structural and functional properties of polysaccharide fractions isolated from the sunflower seed meal/cake that remains after oil extraction by different processes.
2 Materials and methods
2.1 Materials and chemicals
Raw sunflower seeds were provided by Hangzhou Shanwei Food Coperation (Zhejiang Province of China, Variety of RH318). The sunflower seeds were stored in a desiccator at room temperature. 1,1-diphenyl-2-picrylhydrazyl (DPPH) and ascorbic acid (Vc) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, USA). Other reagents and chemicals used were of analytical grade unless otherwise specified.
2.2 Preparation of the meals
Cold pressing was achieved using a hydraulic oil press (Siwei Co., Ltd., Zhengzhou, China) under the condition of 55 MPa. The squeezing was continued until no oil was drained out of the press. Then the pressed seed meals were crushed into powder by a sealing-type swinging traditional Chinese medicine pulverizer (China) followed by sieving through a 40-mesh screen. The fine powder was placed in a breaker, which was added petroleum ether (w/v = 1:5) for defatting; and the beaker was left for 9 h at room temperature. The defatted meals were separated from the mixture by centrifugating (3000 rpm, 15 min), and placed in the fuming cupboard to remove any remaining solvent.
For hot pressing, the sunflower seeds were roasted at 180 °C for 20 min in a glass laboratory roaster heated by an oil bath. After cooling, the roasted seeds were pressed, defatted and dried according to the methods described for the cold-pressed meal.
Subcritical ether extraction of sunflower seed was conducted using a subcritical fluid extraction system (CBE-5L, HSBT Co., Ltd., Anyang, China) at 40 °C and 0.5 MPa. After the extraction was completed, the remaining ether was removed by air drying at room temperature, leaving ether-free meal. Traditional hexane extraction of sunflower seeds was carried out using hexane with the ratio of 1:5 at 55 °C for 9 h, after centrifugation (3000 rpm, 15 min), the extracted meal was dried in a blast air oven at 60 °C for 30 min, leaving hexane-free meal. The cakes/ meals obtained from sunflower seeds by cold pressing, hot pressing, subcritical ether and traditional hexane extraction methods were defined as SMCP, SMHP, SMSE, and SMHE, respectively.
2.3 Isolation of polysaccharides
The defatted meals were mixed with 2 M NaOH with a ratio of 1:20 (g/mL) at 50 °C for 5 h, the undissolved meal was separated by centrifugating (3500 rpm, 20 min), and refluxed three times by three volumes of distilled water. The supernatants were combined and concentrated, then acidified to pH = 5.5 with 6 M HCl. After completition of the reaction, centrifugation (3500 rpm, 20 min) was carried out to separate sediment from supernatant. The supernatant was concentrated by a rotary evaporator, treated with three volumes of 95% ethanol, and then centrifuged to separate sediment from supernatant. The sediment was washed three times with 70% ethanol, deproteinated by Sevage method (chloroform: n-butylalcohol = 4:1, v/v), and finally freeze-dried. The fractions obtained from SMCP, SMHP, SMSE, and SMHE were defined as SPCP-1, SPHP-1, SPSE-1 and SPHE-1, respectively. The supernatants were treated similarly, first deproteinated by the Sevage method, and finally freeze-dried. The fractions obtained from SMCP, SMHP, SMSE, and SMHE were defined as SPCP-2, SPHP-2, SPSE-2, SPHE-2, respectively.
2.4 Structural characterization
2.4.1 Monosaccharide composition analysis
Monosaccharide compositions of all the polysaccharide fractions were determined using High Performance Anion Exchange Chromatography (Dionex, ICS-3000, USA) consisting of a carbopacTM PA-20 column (4 mm × 250 mm, Dionex), AS50 autosampler and pulsed amperometric detector (PAD). Polysaccharide fractions were hydrolyzed with 2 M H2SO4 for 2.5 h at 105 °C, then filtered through a 0.22 μm filter. The filtered hydrolysate was fed into the autosampler for monosaccharide composition measurement. NaOH isocratic eluent (5 mM) was used to separate neutral sugars and uronic acids, and 5 mM NaOH containing 0–75 mM NaAc followed for 15 min. 200 mM NaOH was applied for 10 min to remove carbonate. The column was re-equilibrated before each injection. Calibration curves were established using standard solutions of D-glucose, L-arabinose, D-xylose, D-mannose, D-glucose, D-galactose, glucuronic acid, and galacturonic acids.
2.4.2 UV and FT-IR analysis
The FT-IR spectroscopic analysis of all fractions was recorded by Fourier transform infrared (FT-IR) spectrometer (Nicolet iS10, Thermo Fisher Scientific, USA) in the frequency range of 4000–400 cm−1. 2 mg polysaccharide fraction was ground with 200 mg potassium bromide (KBr), then pressed into 1 mm pellets to determine IR spectra with a resolution of 4 cm−1 over 32 scans. Spectroscopy analysis of UV was conducted on a UV–Vis spectrophotometer (HACH, Loveland, USA) at absorbance mode of 190–1190 nm−1. Polysaccharide fractions were made into 0.5 mg/mL solutions by dissolving in distilled water. The amount of protein in the samples (C) was determined by UV absorption using the following formula:
Where A260 and A280 are the absorbance values at 260 and 280 nm, respectively.
2.4.3 Molecular weight analysis
The molecular weight of all the polysaccharide fractions was determined by a GPC (Viscotek TDA305max, Malvern, UK) with multiple detectors, the system included an AGuard column, two A6000M columns, a differential refractive index detector (RID), and a right angle laser light scattering detector (RALLS). The eluent was 0.3 M NaNO3 and the temperature of the column was maintained at 40 °C. Velocity of the eluent was set as 0.7 mL/min. The PEO standard was provided by Malvern.
2.4.4 Nuclear magnetic resonance (NMR) analysis
50 mg of SPHE dissolved by 500 μL D2O was used for 1H analysis, 13C analysis, and HSQC analysis. The insoluble matter was removed by centrifugation, then placed in a 5 mm NMR tube. The NMR spectra were determined by Bruker DRX-500 spectrometer (Bruker, Rheinstetten, Germany). The result was processed by standard Bruker Topspin-NMR software.
2.5 Antioxidant analysis
2.5.1 DPPH radical scavenging activity
DPPH radical scavenging activities of all the polysaccharide samples were detected in accordance with the previously reported method with slight modification (Cai et al., 2018). In brief, 100 μL polysaccharide solution dissolved in deionized water, in a series of concentrations (0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.0, 2.5, 3.0 mg/mL), then mixed with 50 μL of 200 μM DPPH-C2H5OH, respectively. The mixtures were thoroughly shaken, then incubated for 0.5 h in the dark. Ascorbic acid (Vc) was regarded as the positive standard. The microplate reader (Multiskan, BIC, USA) was set to measure the absorbance of samples at 517 nm. The DPPH radical-scavenging activity was calculated as follows:
Where A0 is the absorbance of the mixture of 100 μL distilled water and DPPH-C2H5OH; A1 is the absorbance of the mixture of sample and DPPH-C2H5OH; A2 is the absorbance of the mixture of sample and ethanol.
2.5.2 Hydroxyl radical scavenging activity
The antioxidant activities of polysaccharide factions in terms of hydroxyl radical scavenging activity potential were tested using the method of (Shen et al., 2018) with some alterations. Ferrous sulfate (9 mM), H2O2 (10 mM), and salicylic acid (9 mM) were prepared immediately before use. 50 μL of polysaccharide solutions, varying from 0.1 mg/mL to 3.0 mg/mL, were mixed with 50 μL of ferrous sulfate and 50 μL of salicylic acid. The addition of 50 μL of H2O2 was used to initiate the reaction. After incubated at 37 °C for 10 min, the absorbance at 510 nm of the resulting solution was measured using a microplate reader (Multiskan, BIC, USA). Vc was used as the positive control. A blank, in which H2O2 was substituted with deionized water, was also measured. The scavenging ability was calculated as follows:
Where A0 represents the absorbance of the control (substituting sample with deionized water); A1 represents the absorbance of the combination of ferrous sulfate, salicylic acid, H2O2 and sample; A2 represents the absorbance of the blank (substituting H2O2 with deionized water).
2.5.3 Ferrous ion chelating ability
The ferrous iron chelating activity of polysaccharides was detected using the method of (Jridi et al, 2019). FeCl2 solution (2 M) and ferrozine (2 M) were freshly prepared. Briefly, 50 μL polysaccharide solutions of different concentrations were each blended with 100 μL FeCl2 solution, and then the chelating procedure was initiated by adding 50 μL of ferrozine. Na2-EDTA was considered as the positive standard, and a blank was prepared in the same manner using 150 μL deionized water instead of FeCl2 solution and ferrozine. The ferrous ion chelating ability was determined with the following equation:
Where A0 is the absorbance of the control (substituting sample with distilled water); A1 is the absorbance of the mixture of polysaccharide fraction, FeCl2 solution and ferrozine; A2 is the absorbance of the blank (substituting FeCl2 solution and ferrozine with 150 μL distilled water).
2.6 Statistical analysis
The analysis of experimental data was conducted using Origin 9.0 (Origin Lab Corporation, USA). All measurements were carried out independently in triplicate. The data are presented as the mean ± standard deviation (S.D.). The least significant difference test was done to compare means of all data, and a value of p < 0.05 was judged to represent a significant difference.
3 Results and discussion
3.1 Polysaccharide yields and monosaccharide compositions
The yields and monosaccharide compositions of the eight polysaccharides isolated from sunflower meals after the different oil extraction methods were exhibited in Table 1. As can be seen, the yields of polysaccharide fractions extracted from the cold/hot press sunflower meals were much lower than those of the fractions isolated from the chemical extraction methods (hexane and subcritical butane). The yields of SPHP-1 and SPHP-2 fractions isolated from the hot press meal were 3.76% and 4.24%, respectively, which together was the lowest yield (8.00%). However, this result was higher than the yield of polysaccharides isolated from sunflower seed meal using ammonium oxalate solution by Zemnukhova et al. (2007). In general, the yield of the polysaccharide polymers varies depending on the isolation method. In the present study, all the polysaccharide fractions were isolated by alkaline solution under the same conditions and then graded using the same amount of ethanol. Thus, the yields of polysaccharides were significantly affected by the various oil extraction methods used. SEM observation (Fig. 1) revealed that the surface of the meals remaining after the solvent extractions was granular without obvious damage or fissures. In contrast, the surface morphology of the mechanically-pressed sunflower seed showed high agglomeration. It is believed that the reason for the higher yields from the chemical extractions was the higher accessibilities of aqueous alkalis at the meal surface, as shown by the differences in surface texture. For the hot pressing process, the sunflower seeds were roasted at 180 °C for 20 min before mechanical pressing. Heating may have degraded the polysaccharides in the seeds, resulting in low yield of polysaccharides from the alkali isolation process. a Note: SPCP-1, SPHP-1, SPSE-1, and SPHE-1 represent the polysaccharide fractions precipitated by 70% ethanol from cold pressed meal, hot pressed meal, subcritical butane extraction and traditional hexane extraction, respectively; SPCP-2, SPHP-2, SPSE-2, SPHE-2 represent the supernatants from cold pressed meal, hot pressed meal, subcritical butane extraction and traditional hexane extraction, respectively. Abbreviations Abbreviations: Ara, arabinose, Gla, galactose, Glu, glucose, Xyl, xylose, GalA, Galacturonic acid, and GluA, Glucuronic acid
Fractiona
Yields (%)
Monosaccharide components (wt %)
Arab
Galb
Glub
Xylb
GalAb
GluAb
Ara/Xylb
SPCP-1
4.87 ± 0.13
42.39
13.94
14.21
24.56
4.58
0.31
1.73
SPHP-1
3.76 ± 0.33
35.04
15.73
13.46
27.61
7.78
0.38
1.27
SPSE-1
5.52 ± 0.71
32.91
19.83
4.02
29.92
12.6
0.72
1.1
SPHE-1
9.65 ± 0.49
49.04
12.17
8.6
22.52
7.23
0.45
1.64
SPCP-2
4.69 ± 0.63
38.35
13.04
14.38
27.06
3.34
3.83
1.32
SPHP-2
4.24 ± 0.05
41.95
8.235
8.195
28.1
9.32
4.22
1.49
SPSE-2
7.43 ± 0.14
43.71
13.62
13.34
23.63
5.3
0.39
1.85
SPHE-2
6.49 ± 0.08
30.17
20.72
26.84
14.79
2.23
5.23
2.04
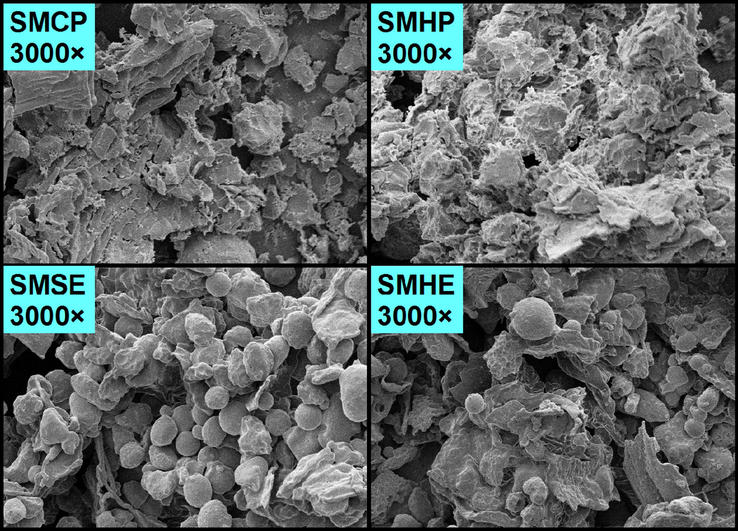
Scanning electron micrographs of the various meals remaining after various oil extraction methods. SMCP, SMHP, SMSE, and SMHE represent meals obtained from sunflower seeds by cold pressing, hot pressing, subcritical ether and traditional hexane extraction methods, respectively.
As shown in Table 1, the monosaccharide compositions of the polysaccharide fractions varied depending on the method of oil extraction. All the polysaccharide fractions contained six kinds of monosaccharides: glucose, galactose, arabinose, xylose, glucuronic acid and galacturonic acid. For the polysaccharides excepted the SPHE-2 fraction, arabinose and xylose were the predominant neutral monosaccharides, accounted for 30–50% and 22–30% of the total sugars, respectively. Arabinose (30.17%), glactose (20.72%), and glucose (26.84%) were the major monosaccharide components of the SPHE-2 fraction. The amounts of glucose in the SPSE-1 and SPHE-1 fractions were lower as compared with the SPCP-1 and SPHP-1 fractions. The SPCP-1 and SPHP-1 fractions in the investigation were isolated from the cakes of sunflower seeds by mechanical pressing. The polysaccharide fractions of SPSE-1 and SPHE-1 were obtained from the meals of the seeds without mechanical pressing. Mechanical pressing may destroy the surface of starch granules (Liu et al., 2019), thereby releasing their contents. Thus, glucan could be solubilized more easily during the alkali isolation. Small amounts of galacturonic acid (2–13%) were also observed in all the polysaccharides, indicating that pectic polysaccharides were present in the cell walls of the sunflower seeds. According to the yield and the monosaccharide composition analyses, the various oil extraction methods had a significant effect on the yields and compositions of the polysaccharides separated by the alkali isolation. The detailed mechanism(s) for these results need(s) to be further explored.
3.2 Average molecular weight analysis
Gel permeation chromatography (GPC) coupled with a differential refractive index detector (RID) and a right angle laser light scattering detector (RALLS) was used to assess the molecular weight distributions of the polysaccharides isolated from the sunflower meal residues after oil extraction. Fig. 2 showed the chromatograms of the various polysaccharide fractions. Clearly, all the ethanol-insoluble polysaccharide fractions contained high molecular weight polysaccharides, which had a retention volume at around 8 mL. In contrast, the chromatograms of the ethanol-soluble polysaccharide fractions exhibited complete absence of relatively high weight fractions, which had a retention volume at around 10 mL. In addition, as seen in Fig. 2, the ethanol-soluble polysaccharide fractions showed relatively narrow molecular weight distributions as compared with the ethanol-insoluble polysaccharide fractions because of the lack of higher molecular weight polysaccharides.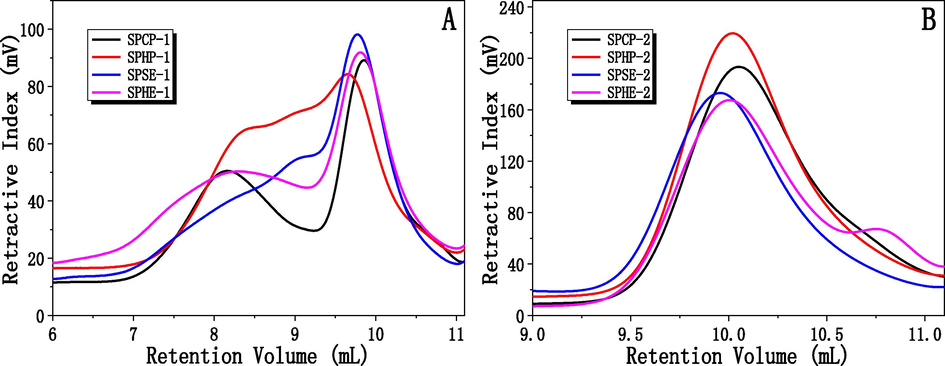
Molecular weight distribution of the polysaccharides (A: SPCP-1, SPHP-1, SPSE-1 and SPHE-1; B: SPCP-2, SPHP-2, SPSE-2, SPHE-2) isolated from sunflower meals remaining after various oil extraction methods.
To quantitatively compare the polysaccharides, the molecular weights (weight-average (Mw), number-average (Mn), and polydispersity (Mw/Mn)) of the various polysaccharides were detected. As displayed in Table 2, SPHE-1 had the highest Mw value (784,525 Da) followed by SPSE-1 (509,463 Da) and SPCP-1 (426,571 Da) while SPHP-1 had the lowest Mw value (236,741 Da) among the four fractions. The major components of plant cell walls are cellulose, hemicelluloses and lignin. In the sunflower meals, the hemicelluloses may have been primarily degraded when the temperature exceeded 180 °C (Acharya et al., 2015; Ciolkosz and Wallace, 2011). Thus, the lower Mw value of SPHP-1 fraction was induced by the thermal degradation during the roasting prior to oil extraction. This interpretation was consistent with the low polysaccharide yields as illustrated in Table 1. Generally, the polysaccharides of lower molecular weight could not be precipitated at lower ethanol concentrations (Liu et al., 2018). Thus, the ethanol-soluble polysaccharides clearly exhibited a lower molar mass compared with the precipitated fractions at 75% ethanol, ranging from 5,216 to 9,603 Da. Furthermore, the polydispersity of the SPCP-2, SPHP-2, SPSE-2 and SPHE-2 fractions was similar, indicating that the polydispersity of the ethanol soluble fractions was not obviously affected by the type of oil extraction process. The findings also indicated that the oil extraction methods resulted in major changes to the chemical structure of the polysaccharides isolated from the meals by alkali extraction. a Note: SPCP-1, SPHP-1, SPSE-1, and SPHE-1 represent the polysaccharide fractions precipitated by 70% ethanol from cold pressed meal, hot pressed meal, subcritical butane extraction and traditional hexane extraction, respectively. SPCP-2, SPHP-2, SPSE-2, and SPHE-2 represent the supernatant from cold pressed meal, hot pressed meal, subcritical butane extraction and traditional hexane extraction, respectively.
Hemicellulosic fractions
Mw
Mn
Mw/Mn
SPCP-1
426,571
36,863
11.6
SPHP-1
236,741
68,162
3.5
SPSE-1
509,463
74,078
6. 9
SPHE-1
784,525
95,928
8.2
SPCP-2
7816
6014
1.3
SPHP-2
5216
4170
1.3
SPSE-2
9603
6363
1.5
SPHE-2
5524
4237
1.4
3.3 Uv and FTIR spectroscopy
The UV spectra of the polysaccharide samples were determined by UV–Vis spectrophotometer, and the results were exhibited in Fig. 3A and B. As shown, all the UV spectra for the polysaccharides have small absorption peaks at 280 nm, which demonstrated the presence of nucleic acid or protein in the various polysaccharide fractions. The amounts of protein in all polysaccharide samples were determined based on the UV spectra, and the results were also presented in Fig. 3A and B. As can be seen, the amounts of protein in the polysaccharides ranged from 0.43% to 0.56%. For the deproteinization of the polysaccharides by the Sevage method, all the samples were reacted with Sevage solution repeatedly until no obvious precipitation was observed in the polysaccharide solutions. Any protein or nucleic acid left after this procedure was assumed to be combined with polysaccharides.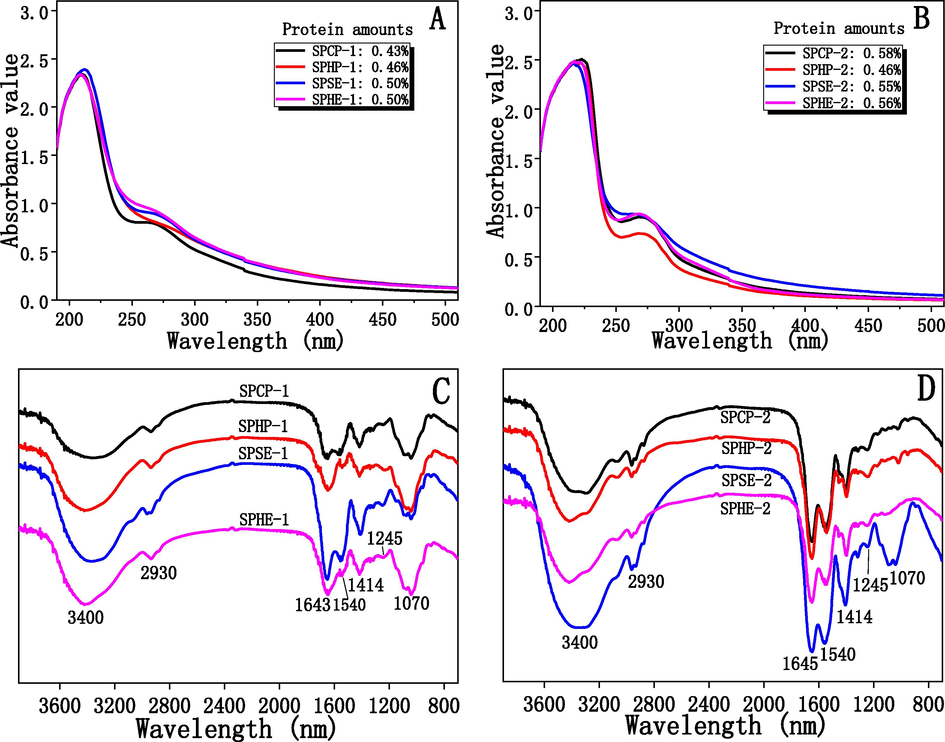
UV spectra (A and B) and FT-IR spectra (C and D) of the polysaccharides isolated from sunflower meals remaining after various oil extraction methods.
The FT-IR spectra of the polysaccharides were showed in Fig. 3C and D. The Figures showed the typical absorption peaks of polysaccharides. As shown in Fig. 3C and D, the IR bands of the polysaccharides were similar; this similarity confirmed that the various oil extraction methods had no significant effect on the structural linkages among the sugars of the polysaccharides. The strong absorption between 3200 cm−1 and 3600 cm−1 was presumably due to the stretching vibration of O-H in the polysaccharides (Zouambia et al., 2017). The stretching vibration of C-H bond falls into the wave number of 2930 cm−1 (Chen et al., 2012). The signal band at about 1643 cm-1 was assigned to C = O asymmetric vibration of carboxyl group; 1414 cm-1 and 1245 cm-1 were the absorption peaks of C-O stretching vibration and O-H bending vibration respectively, indicating the presence of uronic acid in the polysaccharides. This conclusion agreed with the monosaccharide analysis as shown in Table 1 (Fardiyah et al., 2020). In general, the absorption band at about 1730 cm−1 can be used to estimate the esterification degree in uronic acids of polysaccharides (Nep et al., 2016). However, no peak at 1730 cm−1 could be found in the IR spectra of the polysaccharides, which indicated the uronic acids in the polysaccharides were not esterified (Nep et al., 2016). Additionally, the absorption peak at 1540 cm−1 was assigned to the vibration of C-O (Chen et al., 2012), and the band at approximately 1070 cm−1 was assigned to the stretching of C-O-C linkages (Guo et al., 2013).
3.4 Nmr analysis
According to the FT-IR analysis, the types of linkages in the polysaccharides had no obvious differences. Thus, only the SPHE-1 fraction was further investigated by using 1D (1H and 13C) and 2D (HSQC) NMR spectroscopy to further elucidated the structural properties of the major polysaccharides (ethanol-insoluble fractions). The 1H NMR spectrum of SPHE-1 was shown in Fig. 4A. In the 1H NMR spectrum, a strong signal at 4.70 ppm was assigned to D2O. The three small high-field signals at 4.43, 4.47, and 4.50 ppm indicated the SPHE-1 fraction had a β-anomeric configuration because according to the literature, the signals at 4.4–5.0 ppm and 5.0–5.4 ppm represent the anomeric protons of the β-anomeric configuration and α-anomeric configuration, respectively (Zhao et al., 2014). Low intensity of signal around 5.69 ppm indicated that SPHE-1 fraction had little uronic acid (Wang et al., 2015). The result was in agreement with the sugar analysis and FT-IR spectra. No obvious signal at 2.11 ppm suggested that there was no acetyl group binding in the SPHE-1 fraction, which is in agreement with both the literature (Popov et al., 2014; Tamaki et al., 2008) and the IR results here (no signal peak at 1730 cm−1).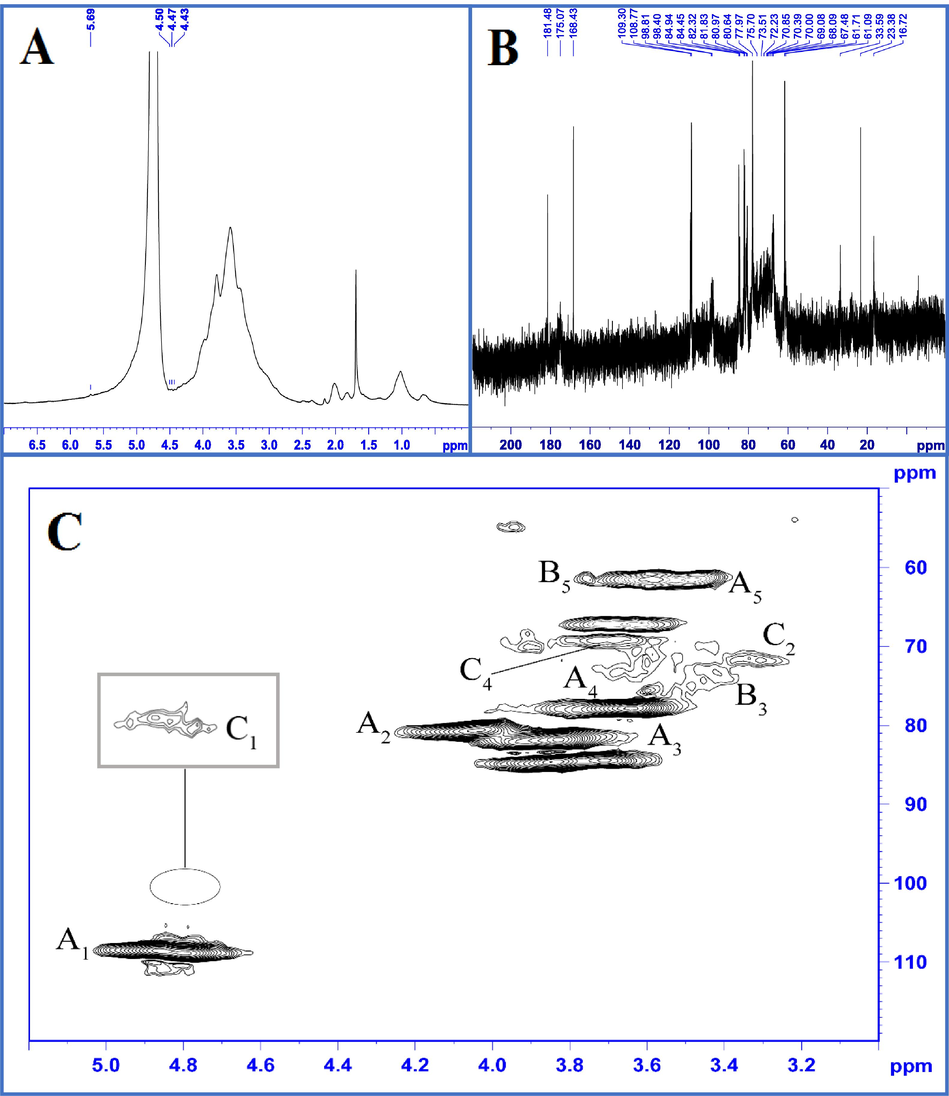
1H, 13C, and 1H–13C HSQC NMR spectra of SPHE-1: (A) 1H NMR spectra of SPHE-1; (B) 13C NMR spectra of SPHE-1; (C) 1H–13C HSQC spectra of SPHE-1; A: α-L-arabinofuranosyl; B: β-1,3,4-linked Xylp; C: β-T-linked Xylp.
Fig. 4B showed the 13C NMR spectrum of the SPHE-2 fraction. As shown in Fig. 4B, δ 60–85 ppm was assigned to the chemical signal peaks of C2 to C6, and δ 90 to 110 ppm was the signal of anomeric carbon area (Chen et al., 2016). SPHE-1 fraction contained four major types of anomeric carbon signals (Fig. 4B). The chemical signal peaks at δ 108–110 ppm were possibly the result of the anomeric carbon of α-L-Araf residues (He et al., 2009; Kang et al., 2011). The lower-field chemical signals in the range of 170–180 ppm indicated the existence of uronic acid in the polysaccharides. Similar structural features have been reported in other sunflower species by Diisterhoft et al. (1992). The overlapped signals of C2-C6 were distributed in the range of 60–80 ppm. Some absorbance at 80–90 ppm was observed in the 13C NMR spectrum, which indicated the presence of furanose in SPHE-1. The result was consistent with the sugar composition analysis. In the 13C NMR spectrum, the peak at 175.07 ppm was assigned to free carboxylic groups in the SPHE-1 fraction, which also suggested that the uronic acid residues in the fraction were predominantly non-esterified (Qin et al., 2020). The result was found in the FT-IR spectrum of the ethanol-insoluble fractions (non-esterified carboxyl groups identified by no signal peak at 1730 cm−1). According to references (Chaikumpollert et al., 2004), the resonance at 181.48 ppm may be caused by the carbonyl resonance from C-6 of the glucuronic acid units in xylan or from the acetyl groups in aliphatic acid. According to the FT-IR analysis, no acetyl group occurred in the fractions. Thus, the chemical signal at 181.48 ppm was attributed to uronic acid. The absence of acetyl groups in the polysaccharides may be due to complete degradation of the facile ester bond during the alkaline extraction.
It was difficult to identify the chemical linkages among the monosaccharides in the polysaccharides because the signal peaks of the fractions overlapped seriously in the 1H and 13C NMR spectra. Thus, HSQC 13C/1H NMR was carried out to further investigate the structural properties of the SPHE-1 fraction. As shown in Fig. 4C and Table 3, five predominant signals were observed in HSQC 13C/1H cross peaks at 108.5/4.99, 81.2/3.95, 82.3/3.83, 77.8/3.64, and 61.6/3.6 ppm, which were attributed to the C1-H1, C2-H2, C3-H3, C4-H4, and C5-H5 of the α-L-arabinofuranosyl residues, respectively (Sun et al., 2013). Some cross-peaks attributed to Xylp units have been also found in the HSQC spectrum. For example, the spectra showed HSQC 13C/1H cross peaks at δ 73.7/3.42 and 61.6/3.78 ppm, which may be caused by C3 and C5 of the β-1,3,4-linked Xylp residues (Yin et al., 2012). The structural elements of the β-T-linked Xylp units appeared in the spectra of HSQC at δ 99.1/4.70 (C1-H1), 72.0/3.31 (C2-H2), and 69.2/3.62 (C4-H4) (Yin et al., 2012). These results suggested that the polysaccharides isolated from sunflower meals had complicated structures. In the present investigation, designation of typical signals in the FT-IR and NMR spectra led to a preliminary deduction regarding the structural properties of the polysaccharides, which were also supported by sugar analysis and previous reports. Ideally, in order to understand the structural features of a polysaccharide accurately, obtaining a pure fraction was a prerequisite; then its structures must be investigated by various analytical methods. Clearly, future investigations are warranted to elucidate in-depth the detailed structural features of the polysaccharides obtained from sunflower meals, possibly by purification first and employing more analytical techniques including methylation analysis, NOESY, COSY, and XHCOR.
Sugar linkage
Chemical shift (ppm)
C1/H1
C2/H2
C3/H3
C4/H4
C5/H5
α-L-arabinofuranosyl
H
108.5
81.2
82.3
77.8
61.6
C
4.99
3.95
3.83
3.64
3.60
β-1,3,4-inked Xylp
H
n.d.
n.d.
73.7
n.d.
61.6
C
n.d.
n.d.
3.42
n.d.
3.78
β-T-inked Xylp
H
99.1
72. 0
n.d.
69.2
n.d.
C
4.70
3.31
n.d.
3.62
n.d.
3.5 Antioxidant activity of polysaccharides
Antioxidant activities can be assigned to different mechanisms and reactions, including radical scavenging activity, binding of transition metal ion catalysts, and prevention of chain initiation (Frankel and Meyer, 2000). In the present investigation, antioxidant activities of the eight polysaccharide fractions isolated from the seed meal samples were evaluated in terms of hydroxyl radical scavenging, DPPH free radical-scavenging, and ferrous ion chelating.
3.5.1 DPPH radical scavenging activity
Fig. 5A and B showed the DPPH radical scavenging rates of the eight polysaccharide fractions and Vc. At 3.0 mg/mL, the activities against the DPPH radical of SPCP-1, SPHP-1, SPSE-1, and SPHE-1 fractions were 89.6%, 94.6%, 92.9%, and 39.7%, respectively. In other words, three of the four showed strong scavenging activities. The scavenging activities of the fractions were strong at high concentrations, although the DPPH radical scavenging activities were lower as compared to Vc. It should be noted that all the ethanol-soluble polysaccharide fractions (SPCP-2, SPHP-2, SPSE-2, and SPHE-2) showed good DPPH radical scavenging, ranging from 87.4% to 93.1% at 3.0 mg/mL. However, the inhibiting ability of the samples also proved to be lower as compared with that of Vc.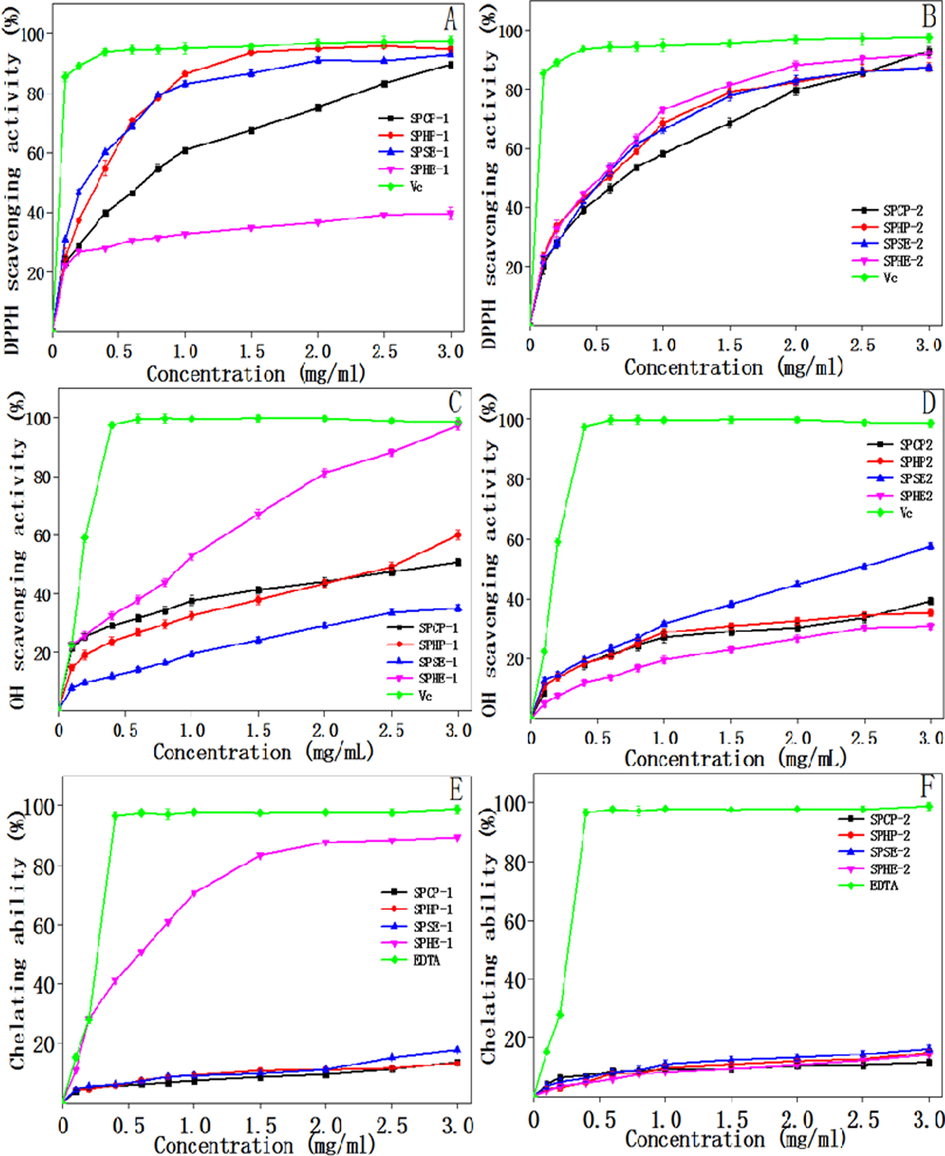
Antioxidant activity of the polysaccharides isolated from sunflower meals after various oil extraction methods: DPPH radical scavenging assay (A and B); Hydroxyl radical scavenging activity (C and D); Ferrous ion chelating ability. (E and F).
3.5.2 Hydroxyl radical scavenging activity
Hydroxyl radicals can cause oxidative damage to bio-molecules (nucleic acids, proteins, and lipids); hence, these radicals are particularly dangerous in organisms. As shown in Fig. 5C and D, at concentrations in the range from 0 to 3.0 mg/mL, all the polysaccharide fractions showed increased scavenging abilities. At 3.0 mg/mL, the hydroxyl radical scavenging activities were 50.7%, 60.5%, 35.0%, 97.4%, and 98.7% for SPCP-1, SPHP-1, SPSE-1, SPHE-1, and Vc, respectively. The highest scavenging activity of the polysaccharides analyzed was SPHE-1, which indicated that this fraction could potentially be used as an antioxidant in industry. Among the ethanol-soluble polysaccharide fractions, the SPSE-2 fraction exhibited obviously higher antioxidant activity than SPCP-2, SPHP-2, and SPHE-2, but less than that of SPHE-1 and Vc.
3.5.3 Ferrous ion chelating ability
Fig. 5E and F exhibited the abilities of the eight polysaccharide samples to chelate Fe2+. As shown, for all the polysaccharide fractions, the chelating abilities depended on the amount within the range of 0–3.0 mg/mL. The SPHE-1 fraction had significantly stronger (82.20%) ferrous ion chelating ability among the polysaccharide fractions at the same concentrations. At the amount of 3.0 mg/mL, however, the ferrous ion chelating ability of other samples ranged from 11.0% to 20.0%, which were lower than those of SPHE-1 and EDTA (98.9%).
These results clearly demonstrated that SPHE-1 was a stronger antioxidant in terms of hydroxyl radical scavenging activity and ferrous ion chelating ability than other fractions. In general, structure, conformation, and molecular size of monosaccharide components, and electron-transfer mechanism are the key factors that affect the antioxidant properties of polysaccharides (Han et al., 2011; Wang et al., 2010; Wang et al., 2011). In the present investigation, the type of oil extraction process had an important effect on the monosaccharide compositions of the polysaccharides; those changes in structural properties caused by oil extraction methods were presumably responsible for the distinct variability in antioxidant properties. Further, investigation was needed to determine the relationships between structural properties and antioxidant activities of the polysaccharides isolated from sunflower seed meal or cake.
4 Conclusions
In this study we compared the structural properties and antioxidant activities of polysaccharides isolated by alkali from sunflower seed meals remaining after various oil extraction processes. The different oil extraction methods significantly affected the yield of the polysaccharides isolated by the alkali isolation process. These polysaccharides displayed significant variability in monosaccharide components and molecular weight. The types of linkages in the polysaccharides had no obvious differences. Differences in yield among the isolated polysaccharides were due to the influences of the oil extraction process on the surface of the meal granules. Hypothetically, these surface changes altered the accessibilities of aqueous alkalis in the meals. During the hot press oil extraction, polysaccharides in the seeds may be degraded by the heat, as these samples showed the lowest polysaccharide yield and the lowest molecular weight. Structural properties are related to functional antioxidant properties. The polysaccharide (SPHE-1) obtained from the meals after traditional hexane extraction exhibited stronger hydroxyl radical scavenging activity and ferrous ion chelating ability than other fractions. NMR spectroscopy indicated that the polysaccharides had complicated structures, these structural features deserved in-depth investigation in the future. This investigation provided information about the structural properties and antioxidant activities of polysaccharides isolated from sunflower meal after oil extraction. This information suggested that the sunflower meal can be a source of polysaccharides and antioxidants for the food and pharmacological industries.
CRediT authorship contribution statement
Hua-Min Liu: Data curation, Methodology, Validation, Supervision. Xiao-Yan Liu: Methodology, Data curation. Yuan-Yuan Yan: Methodology, Data curation. Jing-Hao Gao: Methodology, Data curation, Project administration. Zhao Qin: Methodology. Xue-De Wang: Supervision, Resources.
Acknowledgements
The research was financially supported by the Earmarked fund for Plan for China Agriculture Research System of MOF and MARA and the Doctor Research Fund of Henan University of Technology (2018BS009).
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.







