Translate this page into:
Structure and physiochemical characteristics of whey protein isolate conjugated with xylose through Maillard reaction at different degrees
⁎Corresponding author at: State Key Laboratory of Food Science and Technology, School of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu 214122, China. liyue@jiangnan.edu.cn (Yue Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The purpose of this study is to prepare Maillard reaction products (MRPs) with both good emulsification and antioxidant activity, and the relationship between the Maillard reaction degree and structural changes of whey protein isolate (WPI) conjugated with xylose through wet-heating was explored. Browning intensity didn’t change while the free amino groups reduced significantly at the initial stage of Maillard reaction (MR). Amino acid analysis indicated that the lysine and arginine reduced significantly. The antioxidant property of the MRPs was significantly improved. The best emulsifying properties could be obtained in the middle degree of MR. The sodium dodecyl sulphate-polyacrylamide gel electrophoresis analysis illustrated that WPI and xylose formed high molecular weight conjugates. The circular dichroism spectra suggested that α-helix and random coil were increased while the β-sheet and β-turns were decreased after the MR. All of the MRPs exhibited a marked red shift in the maximum fluorescence intensity, indicating that the hydrophilicity of MRPs was enhanced.
Keywords
Maillard reaction
Whey protein isolate
Xylose
Antioxidant property
Emulsifying property
Structure
1 Introduction
Maillard reaction (MR) is considered to be the reaction between amines and carbonyls, mainly occurs among the amino groups of proteins and the reducing sugars during food processing and storage, spontaneously (Jing and Kitts, 2002; Qi et al., 2018). It is regarded as an important method to modify protein (Zhang et al., 2020). A variety of early, intermediate, and advanced compounds are produced by MR with different molecular structures, functional properties, odors and colors (Aljahdali and Carbonero, 2019; Starowicz and Zieliński, 2019). The Maillard reaction products (MRPs) take fewer safety problems than chemically modified compounds (Maillard et al., 2007). Consequently, the MRPs could be used as functional ingredients in foods, improving the functionality and reducing digestive incompatibility and lessen potential toxicity (Corzo Martinez et al., 2013; Wang et al., 2019).
Whey protein isolate (WPI) has good emulsifying properties which can reduce the interfacial tension and tightly wraps fat globules to form a more stable oil-water interface (Xi et al., 2020). However, they are unstable under a number of processing conditions, such as heating, lowering in pH, and high concentrations of salt (Wang et al., 2020). Glycosylation through MR could highly improve the solubility, lower the isoelectric point and enhance the thermal stability of WPI (Wang and Ismail, 2012; Bourebaba et al., 2020; Liu et al., 2020). Xylose has the advantages of high availability and low cost, and can also be used as a substrate for Maillard reaction (Chen et al., 2019). The wet Maillard reaction with xylose has the advantages of short reaction time, high solubility and small charge density (Wang et al., 2018).
Moreover, the MRPs formed by MR has powerful antioxidant activity (Zhao et al., 2020). In our previous study, MRPs were prepared through MR between WPI and xylose, dextran G20, dextran G40, or dextrin, respectively, and the highest radical chain-breaking, scavenging of reactive oxygen species and decomposing hydrogen peroxide activity were obtained for WPI-xylose among all the MRPs (supplementary data Fig. S1). Compounds with both higher emulsifying and antioxidant properties are desirable for food application, which can be used as emulsifiers and simultaneously inhibit the oil oxidation (Wang et al., 2013; Zhang et al., 2018; Rammohan et al., 2020).
There have been a lot of studies on MR about the effects of reactant molar ratio, time, temperature or pH on the MRPs (Thomsen et al., 2012; Li et al., 2013; Wang et al., 2013; Sunds et al., 2018). However, the study on structural characteristics of MRPs with both antioxidant and emulsifying properties that prepared from different MR levels is relatively limited. The relationship among the degree of MR, emulsifying, antioxidant properties as well as the structural changes of MRPs during the reaction should be further elucidated.
In this study, a wet-heating MR of WPI and xylose mixture under controlled conditions was carried out. Both emulsifying and antioxidant properties of the resulting conjugates were evaluated. The relationship between the MR degree and structural changes was studied by investigating the grafting degree, non-fluorescent intermediate products, free amino group content, molecular weight distribution, infrared spectrum and fluorescence intensity of the MRPs. The MRPs with both emulsifying and antioxidant activity were prepared by exploring the influence of Maillard reaction process on the structure and functional properties of the product, which provided technical support for the application of MRPs in food as a new and natural antioxidant and emulsifier.
2 Materials and methods
2.1 Chemicals
Ferrozine and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). WPI (90% purity) was provided by Beijing Yin He Road trade co., LTD (Beijing, China). Lecithin was purchased from East China Norm University Chemical Reagent Co., Ltd. (Shanghai, China). The other solvents/chemicals used were of analytical grade and obtained from Shanghai Chemical Reagent Co., Ltd. (Shanghai, China).
2.2 Preparation of Maillard reaction products
WPI and xylose (3:2, w/w) were dissolved in water to make a solution at the concentration of 3% (w/v). Then the solution was adjusted to different pH, and heated at various temperatures and times under refluxing in a water bath. Then it was taken out and quickly cooled in an ice water bath, and stored at −4 °C. The sample of W1 was obtained by heating for 30 min at pH 8.0 and 75 °C, W2 was obtained by heating for 90 min at pH 9.0 and 95 °C and W3 was obtained by heating for 90 min at pH 10.0 and 95 °C. WPI heated at 75 °C for 30 min (WPIH) and a simple mixture of WPIH and xylose heated at 75 °C for 30 min (MIX) were also prepared as the control.
2.3 Measurement of the absorbance
Samples were diluted with 0.1% (w/w) sodium dodecyl sulfate (SDS). The absorbance of the solution diluted to 5 mg mL−1 at 294 nm and the absorbance of the solution diluted to 0.5 mg mL−1 at 420 nm were measured using a spectrophotometer (UNICO UV-2100, Shanghai, China).
2.4 Measurement of free amino group content
Free amino groups (FAG) were measured using the method as described by Dong et al (Dong et al., 2020) with some modifications. The 5 mg/ml sample was mixed with NaHCO3 (pH 8.2) solution and 2,4,6-trinitrobenzene sulfonic acid (TNBS) solution, and heated at 50 °C for 60 min. After the reaction was terminated with 0.1 mol/L HCl solution, the absorbance of the conjugate solution was measured at 340 nm. The FAG content was calculated as follows:
2.5 Amino acid analysis
The sample was hydrolyzed by hydrochloric acid in vacuum. Amino acid detection was performed with an Agilent 1200 (Agilent Technology, Palo Alto, CA, UAS), after derivatization by O-phthalaldehyde with pre-column. Chromatographic conditions: Hypersil ODS C18 column (4.6 mm × 150 mm, 5 μm); column temperature (40 °C); detection wavelength was 338 and 262 nm. The mobile phase was 20 mmol L−1 sodium acetate (A) and 20 mmol L−1 sodium acetate: methanol: acetonitrile = 1:2:2 (v/v) (B). The flow rate was 1.0 mL min−1.
2.6 Antioxidant ability
2.6.1 Determination of reducing power
The reducing power of MRPs samples was determined using the method as described by Lertittikul et al. (2007). Fe3+ was reduced to Fe2+ when contacted with reductant, the absorbance of solution increased at 700 nm. Reducing power was expressed as absorbance at 700 nm.
2.6.2 Determination of DPPH radical-scavenging activity
The DPPH radical-scavenging activity of MRPs samples was determined using the method as described by Liu et al. (2019). The absorbance of supernatant was measured at 517 nm after the MRPs samples reacted fully with DPPH-ethanol solution. Deionized water was used instead of the samples and mixed with DPPH-ethanol solution as the control group, and ethanol instead of DPPH-ethanol was mixed with the samples as blank group. The percentage of DPPH radical-scavenging activity was calculated as follows:
2.6.3 Determination of chelating activity on Fe2+
The chelating activity on Fe2+ of MRPs samples was determined using the method as described by Liu et al. (2010). The MRPs samples were mixed with deionized water and 2.0 mM FeCl2, and then added with 5 mM phenazine (Sigma, St. Louis, MO, USA). The absorbance of supernatant at 562 nm was measured using a UNICO UV-2100 spectrophotometer. The deionized water was used instead of MRP samples as the blank. The percentage of chelating activity was calculated as follows:
2.6.4 Inhibition of lipid peroxidation induced by iron
The inhibition of lipid peroxidation of MRPs samples was determined using the method as described by Gu et al. (2009). Lecithin was suspended in phosphate buffered saline (10.0 mg ml−1), which labeled as LLS. The same amount of MRPs samples solution, LLS, 400 μM FeCl3 and 400 μM ascorbic acid were mixed at 37 °C in dark for 60 min. Then the mixture of 15 g trichloroacetic acid, 0.37 g thiobarbituric acid and 2 mL concentrated hydrochloric acid was added and the mixture was heating at 100 °C for 15 min. The absorbance of the supernatant was determined at 532 nm after centrifugation. The ddH2O was used to substitute MRPs sample as control. The inhibition percentage (%) was calculated as follow:
2.7 Measurement of emulsifying properties
2.7.1 Determination of emulsifying ability and stability
Emulsifying ability (EA) was determined according to the method of Pearce and Kinsella (1978) with some modifications. 50 μL emulsion obtained after sample treatment were taken out at 0 and 10 min respectively, and immediately diluted with 5 mL of 0.1% sodium dodecyl sulfate solution. The absorbance of emulsion was measured at 500 nm. The emulsifying activity was determined from the absorbance measured at 0 min. The emulsion stability (ES) was calculated as follow:
2.7.2 Acceleration stability
The accelerated stability of the emulsions was measured using the LUMiSizer (L.U.M. GmbH, Germany) according to Fang et al. (2013). The sample emulsion was injected into the bottom of test tube. Test temperature was 25 °C and centrifugal speed was 4000 rpm. The characteristic curve of emulsion transmissivity was recorded every 15 s and the total time was 4 h. The integration graph showed the rate of integral transmission. The acceleration stability of the emulsion was characterized by the change of transmittance at a specific position in different time during the high-speed centrifugal process (Petzold et al., 2010). All the measurements were repeated in duplicate.
2.8 Electrophoretic analysis
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was carried out by Bayram et al. (2008) with slightly modified. The gels were dyed with Coomassie Brilliant Blue R-250 and decolorized with mixed solution of isopropanol and acetic acid。
2.9 Far-UV circular dichroism (CD) measurement
CD spectra of MRPs in 10 mm path length quartz cells were determined by a circular dichroism spectropolarimeter (MOS-450, Biologic, France) at 25 °C. The determination spectra range was in the far ultraviolet region (190–250 nm). The scan rate was 100 nm min−1 and the spectral resolution was 1.0 nm. Buffer background was subtracted from the raw spectra, and the Far-UV CD spectra were an average of eight scans.
2.10 Intrinsic fluorescence spectroscopy
The intrinsic fluorescence spectroscopy of samples was carried out by a fluorescence spectrometer (Hitachi F-7000, Japan) at 25 °C. The excitation wavelengths were set to 275 and 295 nm, and the scanning range was from 300 to 450 nm. Both of the excitation slit and the emission slit widths were 5.0 nm.
2.11 Statistical analysis
All experiments and analyses were performed with triplicate samples and the data were presented as means ± standard deviation. Statistical significance (p < 0.05) was determined with the analysis of variance (One-Way ANOVA) using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Graphs were generated using Excel software.
3 Results and discussion
3.1 Browning and grafting analysis
The absorbance at 294 and 420 nm was used to represent the amount of intermediate products of MR and the browning degree of MRPs, respectively (Ajandouz et al., 2001). The browning intensity didn’t change at the initial stage of MR reaction, however, as the Maillard reaction deepened, the browning intensity and the amount of intermediate products of MRPs increased significantly (Fig. 1). Under alkaline condition, Schiff-base forms quickly and promotes the further Maillard reaction. As the reaction progressed, the pH of the reaction system became lower and the rate of formation of the intermediate product decreased, so that the change in absorbance at 294 nm was not significant between W2 and W3 (Liu et al., 2008; Rufián-Henares et al., 2006). Transformation of some intermediate products into brown polymers occurred during final stage of Maillard reaction (Ajandouz et al., 2001), resulting in a deeper browning.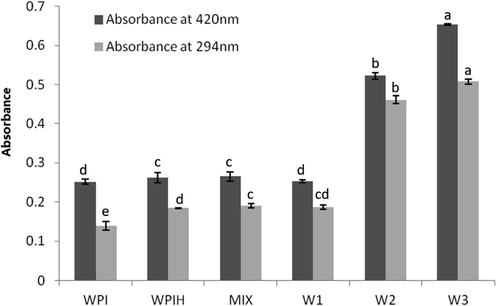
Absorbance (at 294 and 420 nm) of native WPI, WPIH, MIX and Maillard reaction products (MRPs) treated under different conditions. Means with different letters show significant differences (p ≤ 0.05). (WPI = Whey protein isolate; WPIH = WPI heated at 75 °C; MIX = a simple mixture of WPIH and xylose; W1 = the MRPs obtained by heating for 30 min at pH 8.0 and 75 °C; W2 = the MRPs obtained by heating for 90 min at pH 9.0 and 95 °C; W3 = the MRPs obtained by heating for 90 min at pH 10.0 and 95 °C).
MR was a complex reaction firstly occurs between amines and carbonyls (Jing and Kitts, 2002; Liu et al., 2008). The free amino group consumed by the carbonylation reaction mainly comes from the free amino group on the side chain such as lysine and arginine, or the free amino group on the N-terminus of the peptide chain of the protein molecule. The carbonyl condensation between reducing sugar and nucleophilic amino group could be analyzed by the loss of amino acid in the reaction (Li et al., 2013). So the reaction was then monitored by the loss of available —NH2 groups after different degrees of treatments (Fig. 2). About 30% free amino groups were lost owing to the conjugated reaction for all the MRPs. Amino acid component analysis of the products showed that Maillard reaction caused a dramatic loss in Lys and Arg (Table 1). The loss of Tyr and Cys were also significant because of the formation of the dehydroalpropyl side chain, which was combined lysine to form lysinoalanine (Ajandouz et al., 2008). After the carbonylation reaction, there was no significant difference in the free amino group content between W2 and W3 (Fig. 2) due to the rapid consumption of most free amino acids, and the lysine residue tending to protonation. This result was consistent with the absorbance test at 294 nm (Fig. 1).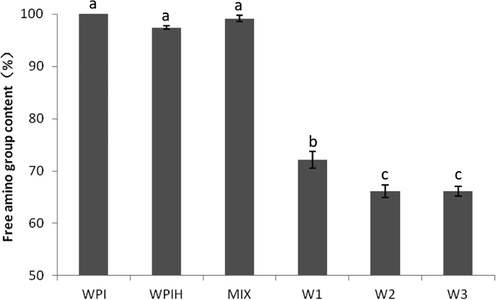
Remained free amino group content of native WPI, WPIH, MIX and MRPs treated under different conditions. Means with different letters show significant differences (p ≤ 0.05).
Amino acids
WPI (%)
WPIH (%)
MIX (%)
W1 (%)
W2 (%)
W3 (%)
Asp
9.702
9.726
9.886
10.034
10.061
10.165
Glu
19.068
18.959
19.286
19.446
19.683
19.797
Ser
3.500
3.700
3.468
3.736
3.349
3.611
His
1.470
1.520
1.527
1.518
1.500
1.555
Gly
1.427
1.429
1.488
1.473
1.491
1.511
Thr
5.989
6.090
5.909
5.745
5.911
6.137
Arg
4.196
4.252
4.211
4.283
3.924
3.966
Ala
5.789
5.703
5.901
5.820
5.801
5.887
Tyr
3.404
3.422
3.406
3.095
2.781
2.886
Cys
0.967
1.006
1.031
0.693
0.747
0.613
Val
6.025
6.003
6.125
6.158
6.267
6.254
Met
2.443
2.458
2.514
2.283
2.371
2.373
Phe
2.722
2.740
2.739
2.765
2.802
2.817
Ile
6.899
6.846
7.029
7.120
7.189
7.155
Leu
10.981
10.940
11.041
11.111
11.218
11.196
Lys
9.355
9.297
9.504
9.120
8.437
7.856
Pro
6.065
5.938
6.003
6.102
6.467
6.120
3.2 Antioxidant properties
The reducing power of all MRPs had a significant increase compared with WPI and ungrafted samples (Fig. 3a). However, the reducing power of W3 was slightly higher than that of W2. The results revealed that higher level of MR was more effective in promoting glycosylation, and MRPs could be used as good electron donors to exert strong antioxidant capacity. The intermediate compounds of MRPs were reported to break the radical chain by donation of a hydrogen atom and hydroxyl groups of advanced MRPs might play a role in reducing activity. These results were in agreement with the previous reports of Delgado-Andrade et al. (2005).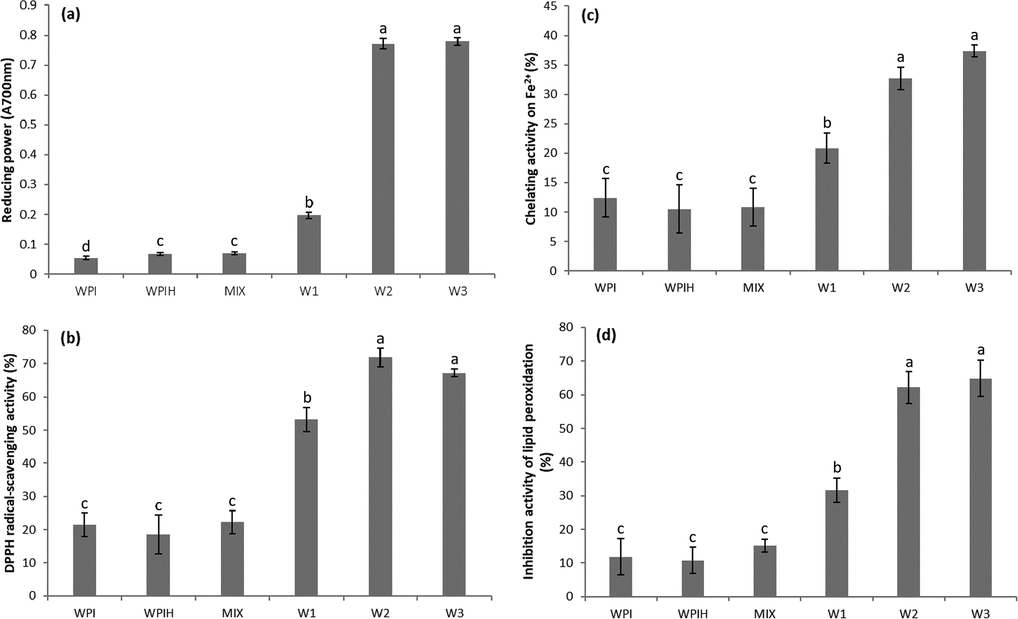
Reducing power (a), DPPH radical-scavenging activity (b), Chelating activity on Fe2+ (c), and Inhibition activity of lipid peroxidation (d) of native WPI, WPIH, MIX and different types of Maillard reaction products (MRPs). Means with different letters show significant differences (p ≤ 0.05).
In Fig. 3b, the DPPH radical-scavenging activity of MRPs enhanced significantly. DPPH radical was scavenged by MRPs by donation of hydrogen to form a stable DPPH-H molecule. The color changed from purple to yellow by acceptance of hydrogen radical from MRPs and it became a stable diamagnetic molecule (Benjakul et al., 2005). Both of intermediates and the final brown polymer could play a role as hydrogen donors, and the caramelization of sugar could also promote the antiradical activity of DPPH detection. However, the increase of DPPH radical-scavenging activity was lower than that of reducing power. This may be due to the fact that the experimental system was hydrophobic but the macromolecular substances tended to be hydrophilic. The MRPs quenched hydrophilic groups more effectively than hydrophobicity (Jing and Kitts, 2002). The higher ability to quench hydrophilic radicals was due to the higher water solubility of melanoproteins.
The chelating activity on Fe2+ of MRPs samples was shown in Fig. 3c. Compared with WPI, chelating activity of W2 and W3 increased from 12.41% to 32.71% and 37.38%, respectively. MRPs have been found to be effective as metal chelating compounds, especially the high molecular weight fractions (Nooshkam et al., 2019). High molecular weight products had certain polyhydroxy or chromophores that provided more hydrogen atoms and the chelating activity could possibly be attributed to hydroxyl groups originating from MRPs.
The result of lipid peroxidation was showed in Fig. 3d. The free radical abstract hydrogen from a fatty acid double bonded to produce fatty acid free radicals, which further reacted with oxygen to produce fatty acid hydroperoxide. The hydroperoxide was unstable and decomposed readily to shorter chain hydrocarbons such as aldehydes, etc. The propagation step might be inhibited and led to lower oxidation in the presence of MRPs. Due to the chelation of iron ions, MRPs could delay lipid oxidation by eliminating its catalytic activity. The higher concentration of MRPs might prevent the oxidation of lecithin through higher iron chelating potential (Benjakul et al., 2005; Li et al., 2014).
The results of antioxidation test showed that the total antioxidant capacity was significantly improved as the degree of MR increased. Based on the four aspects of antioxidant properties of MRPs from WPI-xylose, it can be potentially used as a fine antioxidant for food products.
3.3 Emulsifying properties
It was reported that the properties of protein emulsions could be enhanced by the use of high molecular weight polysaccharides (Zhu et al., 2010). While the electrostatic attraction between negatively charged membrane proteins and positively charged oxidation promoting metal ions could also improve the emulsification ability.
From Fig. 4a, the emulsifying ability of W2 was the strongest among all the MRPs. Heating treatment and excessive MR would destroy the state of WPI in varying degrees, resulting in the imbalance of hydrophobic and hydrophilic groups or polarization of molecular weight, which reducing the emulsifying ability. There was no significant difference in emulsifying stability among samples because WPI was an excellent macromolecular emulsifier in nature (Fig. 4b). It revealed that WPI-xylose conjugate obtained by adjusting the MR degree was capable to prepare a fine protein-stabilized emulsion, promisingly being used as a potential emulsifier in food industry. In the glycoprotein-stabilized emulsions, hydrophobic protein moiety firmly attached to the oil droplet surface and the bulky hydrophilic part of covalently linked polysaccharide highly oriented to the water phase, which was supposed to provide a steric stabilizing layer to inhibit the coalescence of the oil droplets (Setiowati et al., 2017; Chen et al., 2018). Therefore, the appropriate degree of MR could effectively improve the emulsification characteristics of protein.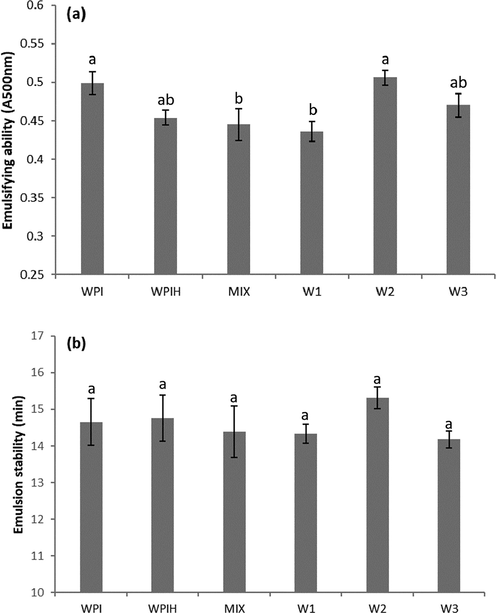
Emulsifying ability (a), Emulsion stability (b) of native WPI, WPIH, MIX and different types of Maillard reaction products (MRPs). Means with different letters show significant differences (p ≤ 0.05).
The emulsifying ability and stability is traditionally measured by the creaming test by measuring the absorbance of the emulsions. The Centrifugal sedimentation method is often used to measure the stability of dispersion (Sobisch and Lerche, 2008). The centrifugal force accelerates the occurrence of instabilities and measures their effects on the light transmission in the whole sample. The more severe change of transmission with the centrifugation resulted in the lowest stability of the emulsion (Fang et al., 2013). The stability of the emulsion was investigated by accelerating changes in the steady state of the emulsion. Fig. 5 showed the rate of change in transmittance of different WPI and MRPs samples during centrifugal sedimentation. At the initial stage of centrifugation, the emulsion was in a stable state, the particles are small and uniform, and the sample was opaque. After centrifugation, the aqueous phase moved to the bottom and increased the transmission, the oil phase moved to the top, while a cream layer was shown as a trough due to the low transparency. The integration graph shows the percentage of light transmission per hour (rate of integral transmission). The higher rate means lower stability. From Fig. 5, W2 exhibited the highest stability of all the samples, and no significant differences were found between WPI, WPIH and MIX.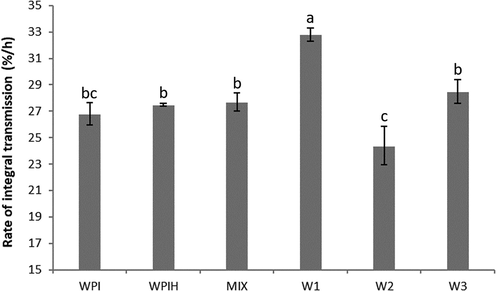
Rate of change in transmittance of WPI-X conjugates. Means with different letters show significant differences (p ≤ 0.05).
3.4 Molecular weight distributions of MRP
Change of molecules for protein as well as the conjugates were evaluated by SDS-PAGE. As shown in Fig. 6, WPI showed four distinct bands including bovine serum albumin (BSA), Immunoglobulin G (IgG), β-lactoglobulin (β-Lg) and α-lactalbumin (α-La). The bands of MIX and WPIH samples were similar to those of WPI, indicating that there was no obvious protein polymerization when WPI was simply mixed with xylose or heated alone. The composition and molecular weight remained basically unchanged. The bands of W1 didn’t change significantly, but the bands of α-La and β-Lg were weakened due to the low degree of maillard reaction. However, the bands of W2 and W3 were significantly different from WPI and W1. All the original protein components decreased or turned to disappear, meanwhile the corresponding larger molecular weight polymers and the middle components (25–37 kDa) formed.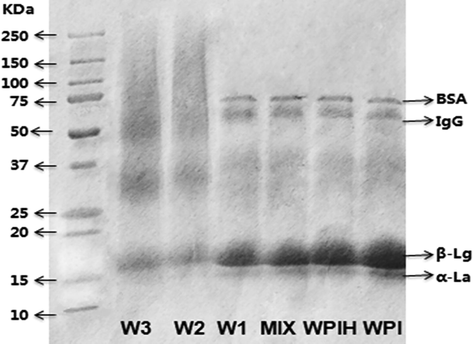
SDS-PAGE patterns of native WPI, WPIH, MIX and different types of Maillard reaction products (MRPs).
Chevalier et al. (2002) revealed that the presence of sugar during heating would make the β-Lg bands became less perceptible with the increase of the glycation degree. The molecular weight distribution of W2 and W3 showed a significant transition to higher molecular weight, and the content of protein polymer larger than 40 kDa increased obviously with the reaction time, which indicated that the degree of protein crosslinking increased.
3.5 Secondary structure analysis
CD spectra were shown in Table 2, which included the detailed data of α-helix, β-sheet, β-turns and random coil content. The secondary structure of MRPs changed significantly compared with the initial WPI. Obviously, α-helix and random coil (especially W2 and W3) were increased while the β-sheet and β-turns were decreased of WPI-xylose after the MR.
Samples
α-Helix (%)
β-Sheet (%)
β-Turns (%)
Random coil (%)
WPI
21.7
25.5
20.5
37.3
WPIH
21.1
25.0
19.7
37.0
MIX
21.2
24.9
19.6
37.0
W1
26.7
21.0
22.4
33.5
W2
26.4
19.4
20.1
39.1
W3
24.6
19.1
19.4
40.6
The structure expansion of protein molecules made it easy to diffuse at the interface, which led to the enhancement of emulsifying properties (Sun et al., 2011). The addition of xylose might lead to the formation of hydrogen bond between WPI and xylose molecules, which could weaken the interaction between molecules and resulted in the reduction of β-sheet β-turns and the increase of random coil. With the increase of random coil, the protein structure was partially extended. The hydrophobic groups of the protein were exposed sufficiently and were easier to disperse in the emulsification interface, which improved the emulsification activity of WPI (Karbasi and Madadlou, 2018). However, the increase in the α-helix content tended to order the protein structure, which was detrimental to the emulsification activity. The combined effect resulted in the final emulsifying properties of the MRPs. Comparing the secondary structure contents of W1, W2 and W3, α-helix, β-sheet and β-turns were decreased while the random coil were increased with the reaction degree deepened. In particular, the random coil of W2 and W3 were much higher than W1, which explained their higher emulsification activities than W1. The random coil content of WPIH and MIX samples without MR modification was lower, which is consistent with its poor emulsification characteristics, further confirming the correlation between protein structure and its functional properties. This result was consistent with Wooster and Augustin (Wooster and Augustin, 2007), who found that the secondary structure of WPI changed after heating with sugar.
3.6 Fluorescence spectroscopic analysis
The hydrophobic environment of whey protein could be studied by the intrinsic fluorescence of tyrosine (Tyr) and tryptophan (Trp). Trp and Tyr have intrinsic fluorescence residues when excited in whey protein at 280 and 295 nm respectively.
As shown in Fig. 7, trends of fluorescence spectra in Fig. 7(a) and (b) is similar. The maximum fluorescence intensity of W2 and W3 were significantly reduced, probably because the access of xylose caused a steric hindrance effect after the MR reaction, and the fluorescent signal of the amino acid was shielded, so that the fluorescence of the copolymer was decreased. The fluorescence intensity of WPIH, MIX and W1 samples was greater than WPI. While all of the MRPs exhibited a marked red shift in the maximum fluorescence intensity, indicating that the hydrophilicity of MRPs was enhanced. This result was caused by the relative reduction of the hydrophilic environment of Trp exposed to the conjugate by thermally induced denaturation, which indicates a stronger hydrophilic microenvironment around Trp.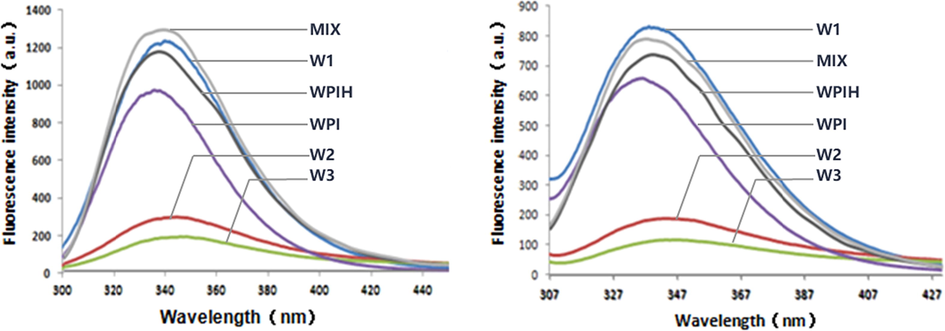
Intrinsic emission fluorescence spectra of native WPI, WPIH, MIX and different types of Maillard reaction products (MRPs) at λex = 280 nm (a) and λex = 295 nm (b).
4 Conclusions
At the initial stage of MR reaction between WPI and xylose, the carbonylation reaction reacted rapidly, consuming a large amount of free amino groups, and the contents of arginine, lysine, tyrosine and cysteine were significantly reduced. The antioxidant properties of WPI were significantly enhanced after Maillard reaction with xylose. The measured increase in antioxidant properties coincided with an increase in the reaction degree that indicated by browning intensity and intermediate products. The middle degree Maillard reaction product (MRP) showed the best emulsifying properties among all the MRPs with different reaction levels. The molecular weight distribution, secondary structure, and hydrophobic environment of WPI were modified by the Maillard reaction. This study revealed that the structural changes were highly related with the changes in the MRP’ properties and thus contributed to an on-going research of MRPs. Future research should be directed at studying the detailed structure and properties relationship of MRPs and their potential application as emulsifier of fine antioxidant properties to encapsulate functional components.
Acknowledgments
The research was supported by the National Science Foundation of China (No. 21676122, 31871794, 31871846), 111 project (B0719028), national first-class discipline program of Food Science and Technology (JUFSTR20180204), and program of “Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province”, China.
Declaration of Competing Interest
The authors confirm that they have no conflicts of interest with respect to the work described in this manuscript.
References
- Effects of temperature and pH on the kinetics of caramelisation, protein cross-linking and Maillard reactions in aqueous model systems. Food Chem.. 2008;107(3):1244-1252.
- [Google Scholar]
- Effects of pH on caramelization and maillard reaction kinetics in fructose-lysine model systems. J. Food Sci.. 2001;66(7):926-931.
- [Google Scholar]
- Impact of Maillard reaction products on nutrition and health: current knowledge and need to understand their fate in the human digestive system. Crit. Rev. Food Sci.. 2019;59(3):474-487.
- [Google Scholar]
- Antioxidant activity of whey protein fractions isolated by gel exclusion chromatography an protease treatment. Talanta. 2008;75:705-709.
- [Google Scholar]
- Antioxidative activity of caramelisation products and their preventive effect on lipid oxidation in fish mince. Food Chem.. 2005;90(1-2):231-239.
- [Google Scholar]
- Phytochemical composition of Ecballium elaterium extracts with antioxidant and anti-inflammatory activities: comparison among leaves, flowers and fruits extracts. Arab J. Chem.. 2020;13(1):3286-3300.
- [Google Scholar]
- Covalent conjugation of bovine serum album and sugar beet pectin through Maillard reaction/laccase catalysis to improve the emulsifying properties. Food Hydrocolloid. 2018;76:173-183.
- [Google Scholar]
- Characterization and antioxidant activity of products derived from xylose-bovine casein hydrolysate Maillard reaction: impact of teaction time. Foods. 2019;8:242.
- [Google Scholar]
- Maillard glycation of β-lactoglobulin induces conformation changes. Mol. Nutr. Food Res.. 2002;46:58-63.
- [Google Scholar]
- In vitro bifidogenic effect of Maillard-type milk protein-galactose conjugates on the human intestinal microbiota. Int. Dairy J.. 2013;31:127-131.
- [Google Scholar]
- Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agr. Food Chem.. 2005;53(20):7832-7836.
- [Google Scholar]
- Study on the antioxidant activity and emulsifying properties of flaxseed gum-whey protein isolate conjugates prepared by Maillard reaction. Int. J. Biol. Macromol.. 2020;153:1157-1164.
- [Google Scholar]
- Impact of high hydrostatic pressure on the emulsifying properties of whey protein isolate-chitosan mixtures. Food Bioprocess Tech.. 2013;6:1024-1031.
- [Google Scholar]
- Characteristics and antioxidant activity of ultrafiltrated Maillard reaction products from a casein–glucose model system. Food Chem.. 2009;117(1):48-54.
- [Google Scholar]
- Chemical and biochemical properties of casein–sugar Maillard reaction products. Food Chem. Toxicol.. 2002;40(7):1007-1015.
- [Google Scholar]
- Interface-related attributes of the Maillard reaction-born glycoproteins. Crit. Rev. Food Sci.. 2018;58(10):1595-1603.
- [Google Scholar]
- Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein–glucose model system as influenced by pH. Food Chem.. 2007;100(2):669-677.
- [Google Scholar]
- Lipid and lipid oxidation analysis using surface enhanced Raman spectroscopy (SERS) coupled with silver dendrites. Food Res. Int.. 2014;58:1-6.
- [Google Scholar]
- Functional properties of Maillard reaction products of rice protein hydrolysates with mono-, oligo- and polysaccharides. Food Hydrocolloid. 2013;30(1):53-60.
- [Google Scholar]
- Pickering emulsions stabilized by amphiphilic anisotropic nanofibrils of glycated whey proteins. Food Hydrocolloid. 2020;101:105503.
- [CrossRef] [Google Scholar]
- Effect of ultrasound assisted heating on structure and antioxidant activity of whey protein peptide grafted with galactose. LWT-Food Sci Technol. 2019;109:130-136.
- [Google Scholar]
- Sensory characteristics and antioxidant activities of Maillard reaction products from soy protein hydrolysates with different molecular weight distribution. Food Bioprocess Tech. 2010;5:1775-1789.
- [Google Scholar]
- Kinetics of color development, pH decreasing, and anti-oxidative activity reduction of Maillard reaction in galactose/glycine model systems. Food Chem.. 2008;108(2):533-541.
- [Google Scholar]
- Free radical scavenging, inhibition of polyphenoloxidase activity and copper chelating properties of model Maillard systems. LWT - Food Sci. Technol.. 2007;40(8):1434-1444.
- [Google Scholar]
- The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem.. 2019;275:644-660.
- [Google Scholar]
- Emulsifying properties of proteins: evaluation of a turbidimetric technique. J. Agr. Food Chem.. 1978;26(3):716-723.
- [Google Scholar]
- Monitoring the stability of nanosized silica dispersions in presence of polycations by a novel centrifugal sedimentation method. J. Appl. Polym. Sci.. 2010;114:696-704.
- [Google Scholar]
- Research progress of Maillard reaction and its application in food industry. Food Industry 2018:248-252.
- [Google Scholar]
- In silico, in vitro antioxidant and density functional theory based structure activity relationship studies of plant polyphenolics as prominent natural antioxidants. Arab J. Chem.. 2020;13(2):3690-3701.
- [Google Scholar]
- Occurrence of acetic acid and formic acid in breakfast cereals. J. Sci. Food Agr.. 2006;86(9):1321-1327.
- [Google Scholar]
- Improved heat stability of whey protein isolate stabilized emulsions via dry heat treatment of WPI and low methoxyl pectin: effect of pectin concentration, pH, and ionic strength. Food Hydrocolloid. 2017;63:716-726.
- [Google Scholar]
- Thickener performance traced by multisample analytical centrifugation. Colloid Surface. A. 2008;331(1–2):114-118.
- [Google Scholar]
- How Maillard reaction influences sensorial properties (color, flavor and texture) of food products? Food Rev. Int.. 2019;35(8):707-725.
- [Google Scholar]
- Properties of whey protein isolate–dextran conjugate prepared using pulsed electric field. Food Res. Int.. 2011;44(4):1052-1058.
- [Google Scholar]
- Maillard reaction progress in UHT milk during storage at different temperature levels and cycles. Int. Dairy J.. 2018;77:56-64.
- [Google Scholar]
- Effect of water activity, temperature and pH on solid state lactosylation of beta-lactoglobulin. Int. Dairy J.. 2012;23:1-8.
- [Google Scholar]
- Conjugation of soybean protein isolate with xylose/fructose through wet-heating Maillard reaction. Food Meas Charact. 2018;12(4):2718-2724.
- [Google Scholar]
- Effect of Maillard-induced glycosylation on the nutritional quality, solubility, thermal stability and molecular configuration of whey proteinv. Int. Dairy J.. 2012;25(2):112-122.
- [Google Scholar]
- A glycated whey protein isolate–epigallocatechin gallate nanocomplex enhances the stability of emulsion delivery of β-carotene during simulated digestion. Food Funct.. 2019;10(10):6829-6839.
- [Google Scholar]
- Physicochemical properties and bioactivity of whey protein isolate-inulin conjugates obtained by Maillard reaction. Int. J. Biol. Macromol.. 2020;150:326-335.
- [Google Scholar]
- Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chem.. 2013;139(1–4):355-361.
- [Google Scholar]
- Rheology of whey protein–dextran conjugate films at the air/water interface. Food Hydrocolloid. 2007;21(7):1072-1080.
- [Google Scholar]
- Effects of pH and different sugars on the structures and emulsification properties of whey protein isolate-sugar conjugates. Food Biosci. 2020;33:100507.
- [CrossRef] [Google Scholar]
- Coating effect of whey protein and xylose Maillard reaction products on walnut lipid peroxidation. Int. J. Food Eng. 2018:58-65.
- [Google Scholar]
- Covalent conjugation of whey protein isolate hydrolysates and galactose through Maillard reaction to improve the functional properties and antioxidant activity. Int. Dairy J.. 2020;102:104584.
- [CrossRef] [Google Scholar]
- Improvement of the oxidative stability of cold-pressed sesame oil using products from the Maillard reaction of sesame enzymatically hydrolyzed protein and reducing sugars. J. Sci. Food Agr.. 2020;100:1524-1531.
- [Google Scholar]
- Physicochemical and emulsifying properties of Whey Protein Isolate (WPI)−dextran conjugates produced in aqueous solution. J. Agr. Food Chem.. 2010;58(5):2988-2994.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.09.034.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







