Translate this page into:
Structure-reactivity relationships on Michael additions of secondary cyclic amines with 3-cyanomethylidene-2-oxindoline derivatives
⁎Corresponding authors at: Department of Chemistry, Faculty of Applied Science, Umm Al-Qura University, 21955 Makkah, Saudi Arabia. naguesmi@uqu.edu.sa (Nizar El Guesmi), amhfarghaly@uqu.edu.sa (Essam M. Hussein)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
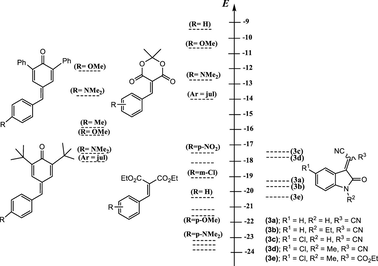
Comparison of the E parameters of the 3-cyanomethylidene-2-oxindoline derivatives with those of the reference electrophiles benzylidenemalonates and analogously substituted benzylidene Meldrum’s acids.
Abstract
A kinetic study of the nucleophilic addition reactions of 3-cyanomethylidene-2-oxindoline derivatives with cyclic amines (namely: piperidine, morpholine and pyrrolidine) in MeCN solution at 20 °C is reported. The second-order rate constants showed of this process fit nicely the Brönsted equation log k1 = βnuc pKa + C, allowing the determination of the βnuc parameter in the range of 0.63 < βnuc < 0.77 that indicates that the degree of formation of N–C bond in the transition state is more half complete. Moreover, the analysis of the kinetic measurements based on a good linear log k (20 °C) = sN (E + N) free enthalpy relationship are used to assess the electrophilic reactivity in term of E parameter of these series of 2-oxindoline derivatives Michael acceptors. Of major interest is that the estimated E values were established to cover a domain of reactivity of ∼3 units of E, ranging from −17.5 for 2-(5-chloro-2-oxindolin-3-ylidene)malononitrile (the most reactive electrophile) to −20.3 for ethyl 2-(5-chloro-1-methyl-2-oxindolin-3-ylidene)-2-cyanoacetate (the least reactive electrophile). The theoretical reactivity indices ω based on the conceptual Density Functional Theory (DFT) explains correctly the experimental electrophilicity E ordering founded in terms of experimental scales.
Keywords
3-Cyanomethylidene-2-oxindolines
Secondary cyclic amines
Electrophilicity
Basicity effect
Structure-reactivity relationships
1 Introduction
Indoline-2,3-dione frequently recognized as isatin (I) and it’s 2-oxindoline derivatives (II-IV) (Fig. 1) have pinch great interest of researchers in the various areas especially in the domain of synthetic organic chemistry as well as of medicinal chemistry (Singh and Desta, 2012; Almeida et al., 2010; Verma et al., 2004; Chiyanzu et al., 2003). The outstanding character of indoline-2,3-dione to be act as an electrophile as well as nucleophile and their facile disponibility have made them precious building blocks in the field of organic synthesis. The origin of the special properties of isatin is that his architecture undergoes electrophilic aromatic substitution (SEAr) at carbons on positions 5 and 7 of the phenyl ring, nucleophilic additions on to the C-3 carbonyl group, in addition, ring-expansions, as well as spiro-annulations. Recent years have witnessed emergence of syntheses of several heterocyclic frameworks employing isatins as substrates such as indoles, quinolines, pyrrolidines, 2-oxindolines presenting biological importance (Quiroga et al., 2011; Kumar et al., 2010; Kumar and Perumal, 2007; Thangamani, 2010; Cheng et al., 2008; Bentabed-Ababsa et al., 2008; Schulz et al., 2007; Elinson et al., 2007; Rehn et al., 2004; Marti and Carreira, 2003; Bergman et al., 2003; Nair et al., 2002; Hussein et al., 2017a; Hussein et al., 2017b; Hussein and Abdel-Monem, 2011; El-Zohry et al., 2009; El-Zohry et al., 2008a; El-Zohry et al., 2008b; El-Zohry et al., 2008c). Isatins revealed many interesting aspects in organic synthesis pathway the most important owing to the greatly reactive C-3 carbonyl group. It is evident that the reactions recorded at the C-3 carbonyl group of isatins whether by nucleophilic additions or spiro-annulation, convert it into 2-oxindole derivatives (Structures II-IV, Fig. 1).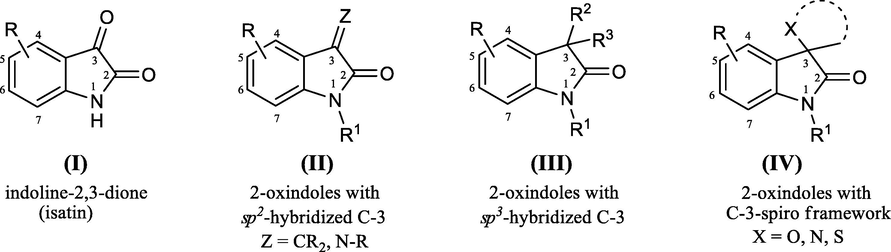
The chemical structure of indoline-2,3-dione and 2-oxindoles derivatives.
An extensive investigation of researchers in organic and medicinal chemistry has unfolded many methods in the design, synthesis and biological assessment of diverse spiro-carbocyclic oxindoles. Indeed, 3-cyanomethylidene-2-oxindolines are used as Michael acceptors precursors for the synthesis of various spiro-carbocyclic-oxindoles in good yields and especially with excellent enantioselectivity (Hussein et al., 2017a; Hussein et al., 2017b; Hussein and Abdel-Monem, 2011; El-Zohry et al., 2009; El-Zohry et al., 2008a; El-Zohry et al., 2008b; El-Zohry et al., 2008c).
We report here the reactions of 3-cyanomethylidene-2-oxindoline derivatives 3a-e with some secondary cyclic amines 4a-c e.g. piperidine, pyrrolidine, morpholine in acetonitrile to investigate the reaction mechanism. In this approach, the relative rate of adduct formation process proved useful in giving an estimation of reactivity of a given chemical.
The obtained values of reactivity electrophilicity parameter E of 2-oxindoline derivatives 3a-e and the effect of basicity of secondary cyclic amines on the rate constants are also discussed. It was the goal of this work to gain further information about other types of Michael acceptors as further reference electrophiles allowing the extension of the electrophilicity scale in the region of compounds at the low-reactivity end and thus offering further electrophiles in the range of −15 > E > −20, in which this part of scale and for this Michael-type electrophile only few reference compounds had been available.
2 Experimental
2.1 General methods
All solvents used purchased from Sigma-Aldrich are spectroscopic grade and used without further purifications. Melting points were determined on a Stuart SMP3 melting point apparatus and are uncorrected. NMR spectra were acquired on a Bruker Avance 500 instrument (500 MHz) in CDCl3 using residual solvent signals as internal standards.
The electronic absorption spectra and kinetic determinations were recorded with a conventional UV–Vis spectrophotometer (Shimadzu model 1800 PC), whose temperature of cell compartments were preserved around 20 ± 0.1 °C. All kinetic-runs were performed no less than three times under conditions of pseudo-first-order with the 3-cyanomethylidene-2-oxindoline derivatives 3a-e concentration of ∼1 × 10−4 mol dm−3 and a concentration of secondary cyclic amines 4a-c (piperidine, morpholine and pyrrolidine) in the range 1.0 × 10−3–1.0 × 10−1 mol dm−3. In all realized experiment, the rates data were found to be reproducible to 2–3%.
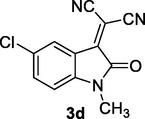
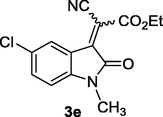
2.2 General procedure for the synthesis of 3-cyanomethylidene-2-oxindolines 3a-e
To a mixture containing isatins (1) (1 mmol) and malononitrile or ethyl cyanoacetate (2) (1 mmol) in water (5 mL), MCM‐SO3H (20 mg) was added and stirred at room temperature for 5–10 min. After completion of the reaction (monitored by TLC), the solid product was dissolved in hot ethanol and then the solid catalyst was removed by filtration. The filtrate was cooled to room temperature to afford the pure product 3a-e as dense red crystals. The physical and spectroscopic data of 3a-e were matched as previously reported (Demchuk et al., 2011; Lashgari et al., 2012). Compound 3e was obtained as a mixture of E-/Z isomers in ratio of 8:1 (Junek et al., 1989).
3 Results and discussion
3.1 Synthesis of the Michael acceptors 3a-e
First of all, five Michael acceptors 3a-e were smoothly synthesized in excellent yields (91–99%) via simple, mild, and efficient Knoevenagel condensation reaction of isatins 1 and malononitrile CH2(CN)2 or ethyl cyanoacetate (NCCH2COOC2H5) 2 in aqueous medium in the presence of sulfonated mesoporous silica (MCM‐SO3H) (Hussein and Ahmed, 2017) as heterogeneous acid catalyst (Scheme 1). The reaction proceeded smoothly at room temperature, in short reaction time (5–10 min) and no byproducts have been detected. After achievement of the reaction (monitored by TLC), the raw product was dissolved subsequently in hot ethanol solution. The precipitate was collected by filtration in which heterogeneous solid catalyst was removed. The obtained pure crystals of products after cooling of the filtrate were directly collected as dark red crystals.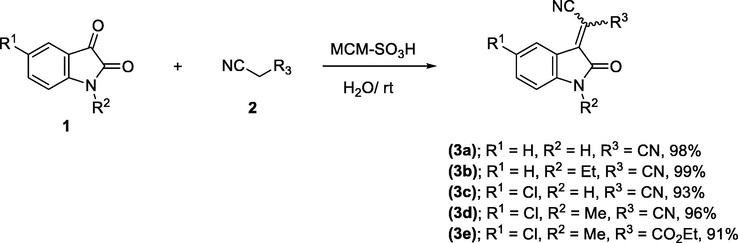
Synthesis of the Michael acceptors 3a-e.
3.2 UV–Vis spectra of the reactions of 3-cyanomethylidene-2-oxindoline derivatives 3a-e with secondary cyclic amines 4a-c
The UV/Vis spectra of compound 3a-e, recorded before time scan measurements, and of Michael adducts of products 8-10a-e resulting from reaction of 3a-e with piperidine (4a), morpholine (4b) and pyrrolidine (4c), respectively (Scheme 2), recorded at the end of time scan measurements, are given in Supporting information Section (Figs. S1-S14 and Tables S1-S8). Unfortunately, we did not succeed the isolation of Michael adducts 8-10a-e, because they are unstable at room temperature. The UV/Vis spectra of the adducts of piperidine, morpholine and pyrrolidine 4a-c and compounds 3a-e lack the absorption maximum at ∼340 and 550 nm, which appears in the UV/Vis spectra of 3a-e, and corresponds to the conjugated double bond chromophore.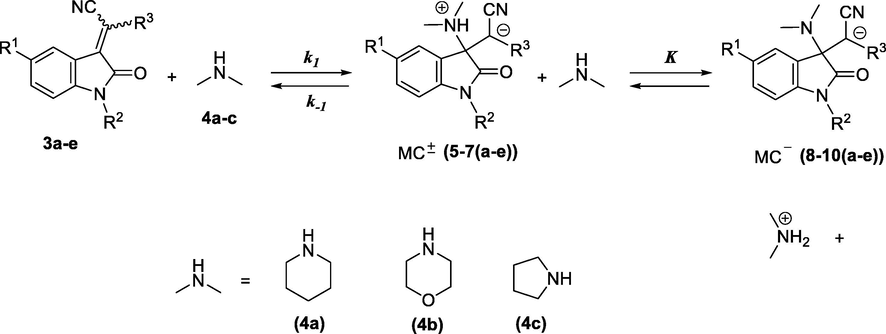
Reactions of 3-cyanomethylidene-2-oxindolines 3a-e with secondary cyclic amines 4a-c.
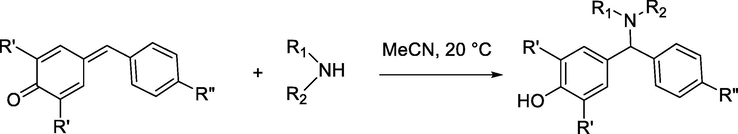
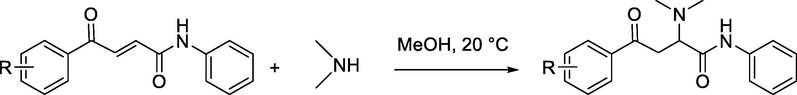
On the other hand, the peak at ∼380 and 440 nm appears in the UV/Vis spectra of adducts, this decrease in wavelength is due to the reduction of the conjugation accompanying the addition of amine. Analogous behavior has been reported for the reactions of secondary amines with quinone methides (Eq. (1)) (Kanzian et al., 2009) and (E)-4-aryl-4-oxo-2-butenoic acid phenylamides (Eq. (2)) (Cvijetić et al., 2014). In our work, the progress of the reaction can monitored at two wavelengths simultaneously, either through the decrease of absorbance at 340 nm as well as 550 nm or increase of absorbance at 380 nm as well as 440 nm (see supporting information section). In this study, according to absorbance intensity, the rate of the reactions were monitored photometrically from the decays of the absorbance of the electrophiles at a wavelength of 340 nm.
3.3 Kinetics of the reactions of 3-cyanomethylidene-2-oxindoline derivatives 3a-e with secondary cyclic amines 4a-c
The kinetic investigations were performed under pseudo-first order conditions with at least a 10-fold excess of secondary cyclic amines 4a-c over 2-oxindoline derivatives 3a-e substrate concentration. Experiments were performed by directly mixing a solution of 1.0 × 10−4 mol/dm3 of 2-oxindoline derivatives 3a-e with the solution of amines 4a-c with concentrations in the range of ([4a-c]o = 1.0 × 10−3 to 1.0 × 10−1 mol/dm3). It therefore appears that all the reactions in this work obeyed first-order kinetics; this undertaken that the pseudofirst-order rate constants (kobs) were determined from the equation ln(A∞−At) = − kobsdt + C. As can be seen for example in Fig. 2, the plot of kobs versus amine concentration relative to reaction of 2-(2-oxindolin-3-ylidene)malononitrile (3a) with piperidine (4a) showed curves upward (Fig. 2(A)). Similar curved plots are demonstrated for the nucleophilic addition of piperidine on 1-(4-nitrophenoxy)-2,4-dinitrobenzene (Um et al., 2014). Such plots indicate a reaction in which a second molecule of amine acts as a general base catalyst (Um et al., 2007; Um et al., 2012; Crampton et al., 2006). Consequently, one might suggest that the Michael Addition reactions of 3a-e keep through a stepwise-type mechanism with one intermediate (MC±) as shown in Scheme 2, the process that is, a catalytic route to form anionic σ-adduct MC− from zwitterionic intermediate MC±. For this reason, it seems reasonable to suggest that addition products 8-10a-e can be formed via the mechanism showed in Scheme 2, involving a nucleophilic addition of secondary cyclic amine and formation of zwitterionic intermediate MC± in the first step. Thus, under the experimental conditions, the observed first-order rate constant (kobs) can be expressed by assuming that low concentration of the zwitterions MC± as follows in Eq. (3) (Ben Salah et al., 2015; Ben Salah et al., 2017).
![Plots of kobs vs [pip] (A) and kobs[pip] vs [pip]2 (B) for the reaction of 2-(2-oxindolin-3-ylidene)malononitrile (3a) with piperidine (4a) in MeCN at 20 °C.](/content/184/2020/13/5/img/10.1016_j.arabjc.2020.03.027-fig5.png)
Plots of kobs vs [pip] (A) and kobs[pip] vs [pip]2 (B) for the reaction of 2-(2-oxindolin-3-ylidene)malononitrile (3a) with piperidine (4a) in MeCN at 20 °C.
Obviously, in agreement with Eq. (3), a good straight line plot (R2 > 0.9933) with negligible intercepts were found when the values of (kobs [NuH]) were plotted versus the square concentration of secondary cyclic amines [NuH]2 as shown in Fig. 2(B) and Fig. S15-S20 (see Experimental section), so that Eq. (3) reduces to Eq. (4). A careful examination of these results confirm the proposed mechanism suggesting that the reactions between our 2-oxindoline derivatives electrophiles 3a-e and secondary cyclic amines nucleophiles 4a-c proceed via a nucleophilic addition mechanism, in which the rate-limiting step involve the formation of the N—C bond. Hence, it could be concluded from Eq. (4) that the slopes of the plots (Fig. 2(B) and Fig. S15-S20 (see Experimental section)) induced the second-order rate constants, k1, which are collected in Table 1.
bvalues measured in acetonitrile at 20 °C.

pKa = 19.58 a
pKa = 18.92 a
pKa = 16.61 a
k1 (mol s−1 dm−3)b
3a
2.73 × 10−1
3.22 × 10−2
1.41 × 10−3
3b
2.22 × 10−1
2.72 × 10−2
1.02 × 10−3
3c
4.63
7.85 × 10−1
4.79 × 10−2
3d
3.06
5.16 × 10−1
2.91 × 10−2
3e
9.97 × 10−2
1.12 × 10−2
3.79 × 10−4
3.4 Effect of basicity of secondary cyclic amines 4a–c on k1: Brönsted relationship
Examination of the rate constants (k1) relative to the couplings of Michael acceptors (3a-e) with secondary cyclic amines 4a–c (values obtained in acetonitrile solution, see Table 1) reveals that the obtained rates relative to this addition-type reactions have been found remarkably sensitive to the effect of basicity of present secondary cyclic amines. On the basis of these findings, the observed effect is illustrated in Fig. 3, which shows clearly the linear dependence of the log k1 with
corresponding to the conjugated acid of secondary cyclic amines 4a–c (
values are measured in acetonitrile solution (Coetzee and Padmanabhan, 1965; Hall-Jr, 1957) and given in Table 1). The following Eqs. . (5)–(9) describes this linear Brönsted-type relationship dependence:
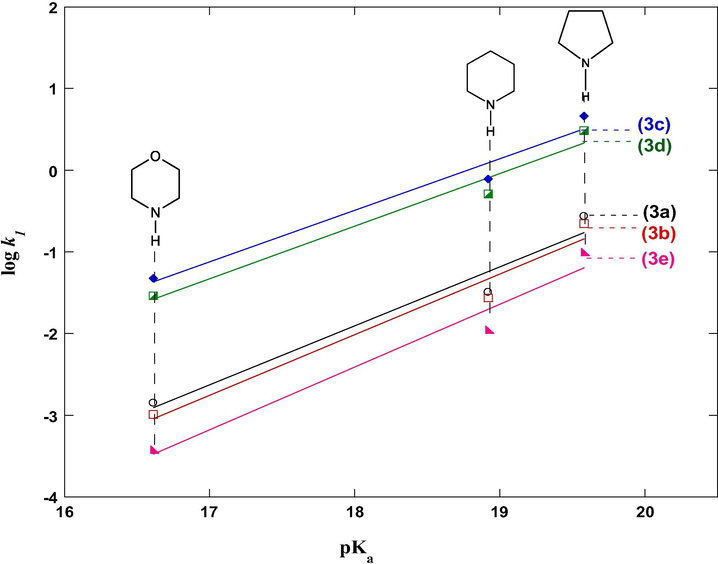
Effect of the secondary cyclic amine basicity on the rate constants (k1) of the coupling reactions of 3-cyanomethylidene-2-oxindoline derivatives 3a-e with amines 4a–c in MeCN at 20 °C.
Our experimental βnuc values of the reactions of 3-cyanomethylidene-2-oxindoline derivatives 3a-e with secondary cyclic amines 4a-c deduced from the slope of log(k1) = f(pKa) plots are in the range of 0.63 and 0.77 (Eqs. (5)–(9)) proves comparable and in accord to those reported in literature for some nucleophilic addition reactions in which a major diagnostic feature of reaction mechanism that the first step is a rate-limiting step that corresponding to nucleophilic attack step (Um et al., 2012; Castro et al., 1997; Bordwell and Hughes, 1986; Dixon and Bruice, 1972; El Guesmi et al., 2010). As mentioned for example for the coupling reactions of secondary cyclic amines with benzoselenadiazolium in MeCN at 20 °C when obtained βnuc value is 0.55 (Ben Salah et al., 2017), also, and in a same condition, similar value of βnuc = 0.52 has been signalized for addition reactions of secondary cyclic amines on 1-iodo-2,4-dinitrobenzene (Um et al., 2012), while for the reactions of a bis(4-nitrophenyl)thionocarbonates with various alicyclic amines in water at 25 °C, βnuc is 0.50 (Castro et al., 1997).
Moreover, it is interesting to note in the present work that the value obtained of βnuc is remarkably higher than βnuc value of 0.41 previously reported for the reaction of benzothiadiazolium cation with various substituted anilines in acetonitrile solution at 20 °C (Ben Salah et al., 2015). We note particularly that the βnuc value of 0.63 < βnuc < 0.77 measured for the coupling of 3-cyanomethylidene-2-oxindoline derivatives 3a-e with amines 4a-c using in this work can be properly interpreted for in terms that the initial Nitrogen-Carbon coupling is rate-limiting step.
Furthermore, the linearity obtained in Brönsted plots (Fig. 3) for the secondary cyclic amines (4a-c) informs us on the same reaction mechanism for all reactions in this study. More importantly, the Brönsted coefficient of 0.63 < βnuc < 0.77 obtained in this work which represent the degree of bond formation in transition state indicates that the nitrogen–carbon bond formation for coupling reactions Amines/Michael acceptors is more than half complete.
3.5 Mayr Relationship: Quantification of reactivity electrophile in term of E parameter of Michael acceptors 3-cyanomethylidene-2-oxindoline derivatives 3a-e
In accordance with numerous kinetic investigations, it has been demonstrated that it is possible that the rate constants for the reactions of some electrophiles such as, carbocations and related quinone methides with n-, σ- and π-nucleophiles can be depicted by an Eq. (10) with three parameters, wherein E is an electrophile dependent parameter and s and N are nucleophile dependent parameters (Mayr and Ofial, 2005; Ofial and Mayr, 2004; Mayr and Ofial, 2004; Mayr et al., 1998a; Mayr et al., 1998b).
Subsequently, nucleophilicity parameters for numerous strong nucleophiles has been assigned, such as carbanions (Phan and Mayr, 2006; Bug et al., 2004; Lucius et al., 2002; Lucius and Mayr, 2000), aliphatic and aromatic amines (Minegishi and Mayr, 2003; Brotzel et al., 2007a; Brotzel et al., 2007b; Brotzel and Mayr, 2007), silyl enol ethers (Dilman and Mayr, 2005; Burfeindt et al., 1998; Patz and Mayr, 1993), enamines (Kempf et al., 2003), and ketene acetals compounds (Tokuyasu and Mayr, 2004) through employing reference electrophiles such as, substituted diarylcarbenium ions and structurally related quinone methides. Consequently, this makes it very reasonable to consider Eq. (10) is also suitable to describe the reactions of electron deficient arenes as well as ordinary Michael acceptors (Seeliger et al., 2007; Berger et al., 2007; Lakhdar et al., 2007; Terrier et al., 2005; Lemek and Mayr, 2003; Remennikov et al., 2003).
In this regard, much attention has been given to Michael-type acceptors involving the initial nucleophilic attack step at the electron deficient double bond. For this purpose, Bernasconi reported the studies of the kinetics of the reactions of various nucleophiles such as, carbanions, amines and alkoxides towards some Michael acceptors, e.g., benzylidene Meldrum’s acids and benzylidene indandiones in DMSO/H2O mixtures (Bernasconi et al., 1998; Bernasconi, 1989; Bernasconi and Panda, 1987; Bernasconi and Murray, 1986; Bernasconi and Leonarduzzi, 1982; Bernasconi and Fornarini, 1980; Bernasconi and Leonarduzzi, 1980). On the other hand, Rappoport have focused mainly on reactions of chloro-substituted benzylidene Meldrum’s acids on which follow nucleophilic vinyl substitutions (Ali et al., 2006; Bernasconi et al., 1998; Rappoport, 1992). In view of recent investigations, other Michael acceptors have been reported (Oh and Lee, 2002; Oh et al., 2003) during the studies of the kinetic of the addition of benzylamines to substituted benzylidene-acetylacetones in acetonitrile.
3.6 Correlation analysis
It thus appears that Eq. (10) is valid for the reactions of the 3-cyanomethylidene-2-oxindoline derivatives 3a-e with secondary cyclic amines 4a-c since the plots of (log k1)/s vs. N exhibit slopes very close to 1.0. The five parallel correlation lines in Fig. 4 describe this linear tendency (Eqs. (11)–(15)).
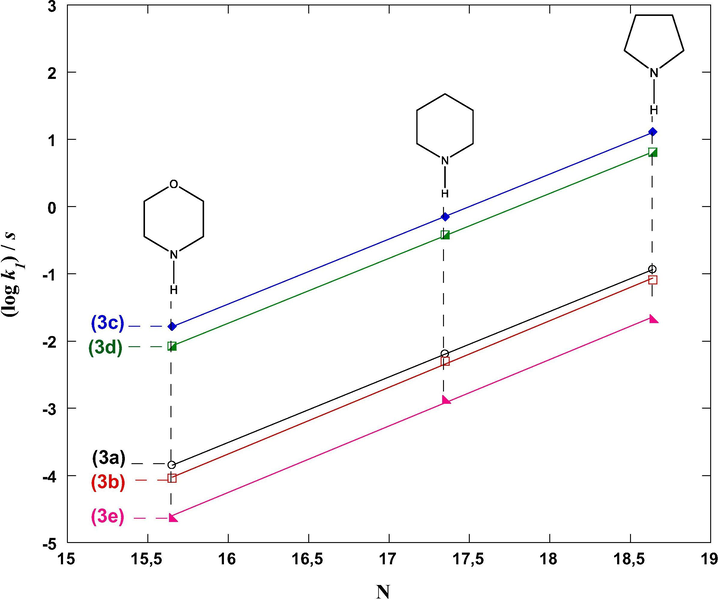
Relationships between (log k1)/s and nucleophilicity parameters N for the reactions of the 3-cyanomethylidene-2-oxindoline derivatives 3a-e with secondary cyclic amines 4a–c in MeCN at 20 °C.
Support for this measure of E parameters for 3a–e is provided by calculation through least squares minimization method [Δ2 = Σ(log k – s(N + E))2] using parameters k1, N and s given in Table 2.

N = 18.64/s = 0.60
N = 17.35/s = 0.68
N = 15.65/s = 0.74
log k1
−0.564
(E = −19.58)−1.492
(E = −19.54)−2.851
(E = −19.50)
−0.654
(E = −19.73)−1.565
(E = −19.65)−2.991
(E = −19.69)
0.665
(E = −17.53)−0.105
(E = −17.50)−1.320
(E = −17.44)
0.486
(E = −17.84)−0.287
(E = −17.78)−1.536
(E = −17.73)
−1.001
(E = −20.31)−1.951
(E = −20.22)−3.421
(E = −20.27)
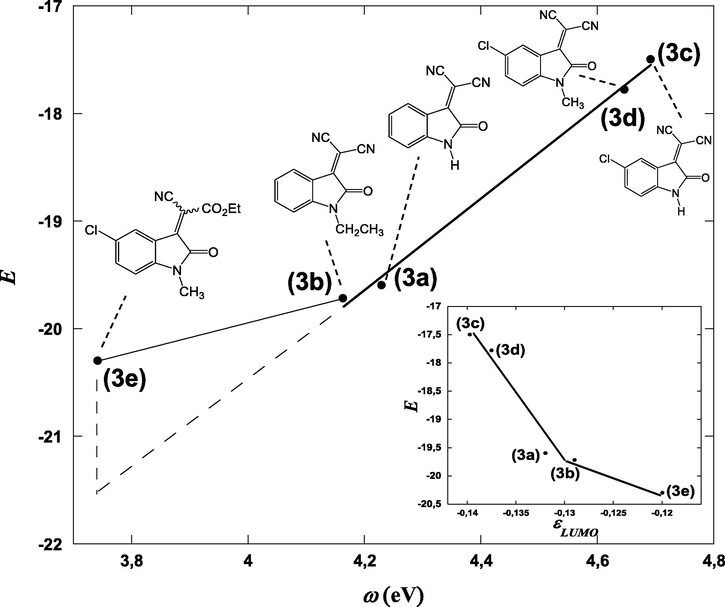
Importantly, Fig. 5 can be employed as a tool for comparing the electrophilicities of the 3-cyanomethylidene-2-oxindoline derivatives 3a-e with some reference electrophiles. It thus appears that the electrophilic reactivity of our five Michael acceptors 3a-e cover a range of almost three orders of magnitude from –17.5 > E > –20.3 (Fig. 5), this seems in good agreement with measured empirical electrophilicity parameters E for series of monoacceptor-substituted ethylenes (H2C = CH-Acc) and styrenes (PhCH = CH-Acc) recently reported by Mayr group (Allgäuer et al., 2017). It is noteworthy that the electrophile 3e is roughly two orders of magnitude of E parameter less reactive comparing than their analogues 3c and 3d. Thus, this represents a nice reflection of a great effect on the electrophilic reactivity of these Michael-type acceptors when one ester groups was fixed on 3d. Additional, Fig. 5 that also summarize substrates previously studied by Mayr and co-workers, unfolds that the most reactive Michael acceptor studied in this work, i.e. (3c) (E = −17.50) exhibits an electrophilic reactivity that compares well with those of diethyl 4-nitrobenzylidenemalonate (E = −17.67), of (E)-3-(4-cyanophenyl)-1-phenyl-prop-2-en-1-one (E = −17.64) and of (E)-3-(4-nitrophenyl)-1-phenyl-prop-2-en-1-one (E = −17.33) indicating a normal electrophilic ranking of 3c in the Mayr’s scale. It is apparent nevertheless that this value of E remains lower than that of p-substituted-benzylidenemalononitril (−9.32 < E < −13.30), of 5-benzylidene-1,3-dimethylbarbituric and -thiobarbituric acids (−9.15 < E < −13.97) and of 2-(p-substituted-benzylidene)-indan-1,3-dione (−10.11 < E < −13.56). On the other hand it’s clear that E values of our neutral Michael acceptor 3a-e remains relatively lower in term of electrophilic reactivity than that of 4,6-dinitrotetrazolopyridine (E = −4.67) which represent one of the neutral compounds with the most electrophilic character known to date. In undertaking the works of Mayr; it is shown recently that 2,3-dichloro-5,6-dicyano-p-benzoquinone (DCQ) represent at the moment, the neutral compound the most electrophile with an value of E of −3.66. A significant feature of 3a-e that the values obtained of our Michael-type E are considerably higher and thus more electrophile than diethyl 4-substituted-benzylidene malonate (−20.55 < E < −23.80). To be noted is that comparison of individual pairs of electron-deficient Michael acceptors substrates emphasizes that our E values quite similar to those measured for (E)-3-(4-substituted-phenyl)-1-phenyl-prop-2-en-1-one (−17.33 < E < −19.39).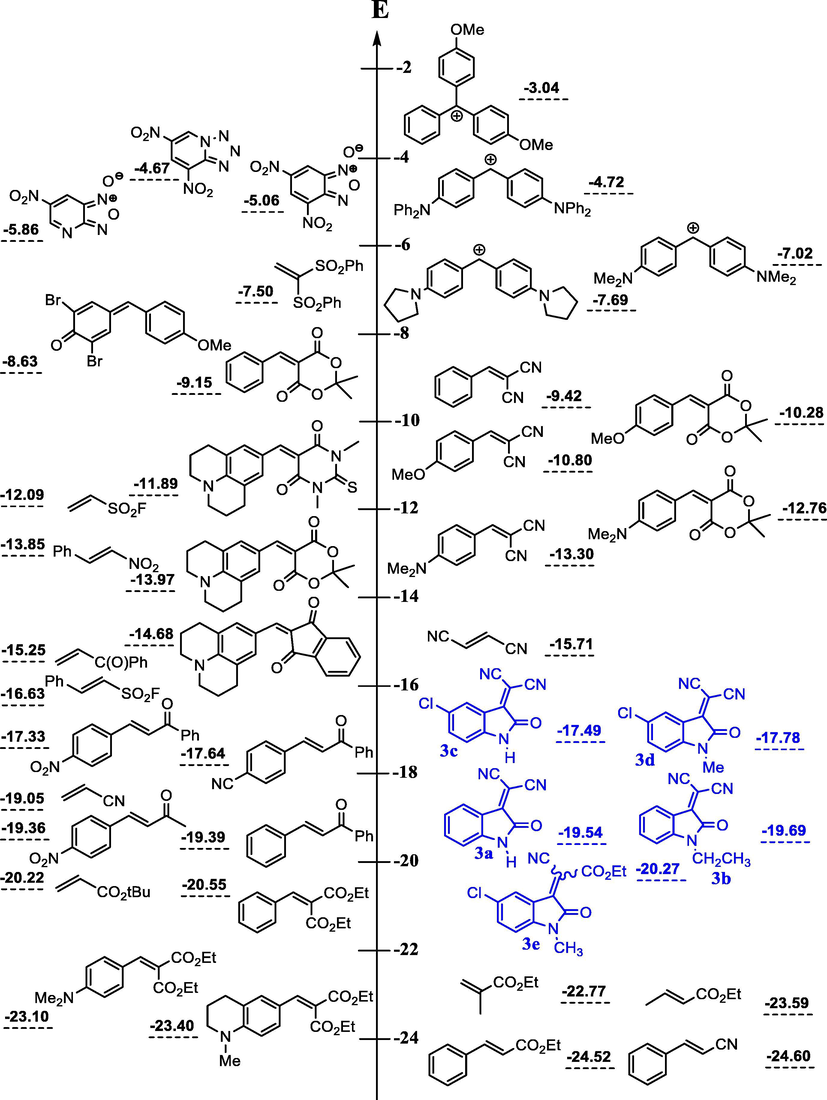
Comparison of the electrophilicity parameters E of 3-cyanomethylidene-2-oxindoline derivatives 3a-e with those of electron-deficient neutral electrophilic substrates.
Comparison of the data obtained in this study makes it reasonable to consider that the substitution of one cyano group (—CN) in the electrophile 3d by a (—CO2Et) group to provide 3e is accompanied by a decrease of ∼3 E unit in the electrophilic reactivity. It follows such an E value remains markedly lower than that caused by the replacement of a cyano group (—CN) by a second CO2Et group into benzylidenemalononitriles to form benzylidenemalonates in which E values are in the range of 9.06–10.48. As an illustration of this behavior can be presumed mainly as reported by Mayr and co-workers (Kanzian et al., 2009) in terms of the reflection of an augmentation of stabilization of the ground state through resonance of benzylidenemalonates that possessing two carbonyl groups (—CO2Et) (resonance structure (I)) compared to their analogous Michael-type acceptors possessing only one carbonyl group (resonance structures (II) and (III)).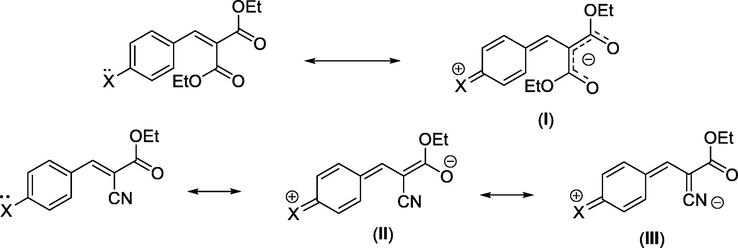
3.7 The electrophilicity ω index Parr
From a theoretical point of view, a comprehensive of the accurate nature of a chemical reactivity descriptor described in terms of the electrophilicity concept has seduced the attention of many quantum chemists (Pérez et al., 2003; Pérez et al., 2002; Parr et al., 1999; Maynard et al., 1998; Roy et al., 1998). These authors have clearly emphasized that the theoretical scales of electrophilicity are based essentially on descriptors of the electronic structure of molecules in its ground state. It therefore appears that these indexes are considered as static reactivity indexes and consequently forming reactivity absolute scales, without however depending on the nucleophile partners.
Indeed, based on the work of Maynard (Maynard et al., 1998) and from the knowledge of Density functional theory (Parr and Yang, 1989), a theoretical definition of electrophilicity ω has been quite successful introduced by Parr and co-workers (Parr et al., 1999), which can be expressed as a measure of the energy stabilization of an electrophilic molecule upon receiving an extra amount of electronic charge from the environment. Consequently, the global electrophilicity ω index may be recast and given into the simple expression:
In Eq. (16), μ ≈ (εH + εL)/2 and η ≈ (εL − εH) reactivity descriptors are the electronic chemical potential and the chemical hardness, respectively. Note that, the calculation of reactivity descriptors can be approached by the frontier HOMO and LUMO energies.
Calculated values μ, η, and ω of relevance for this work are given in Table 3. So, 3-cyanomethylidene-2-oxindoline derivatives 3a-e acts as an electrophilic molecule owing to the relatively great value of its ω (3.742 < ω < 4.692). So, starting from the reference compound 2-(2-oxindolin-3-ylidene)malononitrile (3a) (ω = 4.230 eV), the N-alkylation with ethyl group (–CH2CH3) result in an electrophilic deactivation in compounds 2-(1-ethyl-2-oxindolin-3-ylidene)malononitrile (3b) (ω = 4.164 eV). At the same time, the highest activation effect is produced by introduction of electron withdrawing (–Cl) in compound 2-(5-chloro-2-oxindolin-3-ylidene)malononitrile (3c) (ω = 4.692 eV). In this series, the highest electrophilic deactivation was caused by the substitution of (—CN) group by (—CO2CH2CH3) groups in compound ethyl 2-(5-chloro-1-methyl-2-oxindolin-3-ylidene)-2-cyanoacetate (3e) (ω = 3.742 eV).
εH
εL
μ
η
ω
3a
−0.24805
−0.13195
−0.19000
0.11610
4.230
3b
−0.24047
−0.12897
−0.18472
0.11150
4.164
3c
−0.24966
−0.13973
−0.19470
0.10993
4.692
3d
−0.24406
−0.13750
−0.19078
0.10656
4.647
3e
−0.23360
−0.11996
−0.17678
0.11364
3.742
Fig. 6 summarizes the comparison between the global electrophilicity index ω calculated using Parr model and the electrophilicity E estimated experimentally using Mayr model. The resulting regression equation for the compounds 3a-d presenting 2 cyano group is:
Correlation between experimental E and theoretical ω electrophilicity parameters and gas phase lowest unoccupied molecular orbital energies (εLUMO).
Excellent linear relationship is obtained for 3a-d between both variables (regression coefficient R = 0.997), thereby suggesting that the electrophilicity index ω may be used to properly evaluate the chemical substitution effect on the electrophilic potential of compounds, and consequently it can be further considered as a trustworthy descriptor of reactivity within this class of electrophiles.
On the other hand and as can be seen in Fig. 6, significant deviation is observed for compound 3e from experimental value, this can be explained as previously reported by Bernasconi (Bernasconi, 1989; Bernasconi and Murray, 1986; Bernasconi and Stronach, 1991; Bernasconi, 1987; Bernasconi and Kanavarioti, 1986) and Oh (Oh et al., 2000; Oh and Lee, 2002; Oh et al., 2003) that the transition states of amine additions to 3e are stabilized by hydrogen bridging from NH to the carbonyl group (six membered transition state TS6). This results are in good agreement with those reported by Mayr and coworkers for similar system, the additions of secondary amines to 2-benzylidene-indan-1,3-diones 11 (Berger et al., 2007), benzylidene-Meldrum's acid 12 (Kaumanns and Mayr, 2008) and benzylidenebarbituric and -thiobarbituric acids 13 (Seeliger et al., 2007). In line with this observations, Fig. 6 in this work shows also that correlation obtained when using LUMO energies present the same deviation (see Scheme 3).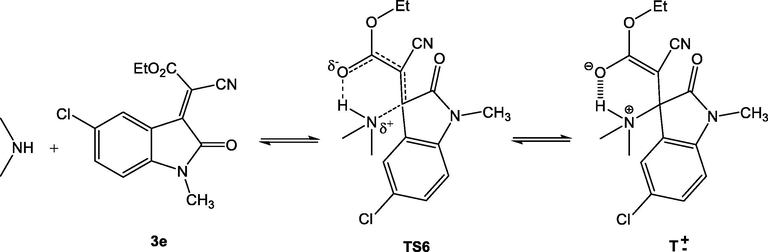
Addition of a secondary amine to ethyl 2-(5-chloro-1-methyl-2-oxindolin-3-ylidene)-2-cyanoacetate (3e) (TS6: six membered transition state, T±: zwitterionic intermediate).
Comparisons with literature data shows that the experimental rate constants are generally 20–120 times greater than the calculated values. Though deviations from experimental values by two orders of magnitude are still within the confidence limit of Eq. (10) (Mayr et al., 2001; Mayr et al., 2003). Our study has shown that additions of secondary amines to 3e are only six times faster than predicted by Eq. (10). It has, therefore, been concluded that H-bridging as indicated in TS6 cannot have a large effect on the transition states of the additions of secondary amines to 3e.
4 Conclusion
By applying the common approach electrophilicity improved by (Mayr et al., 2003), the E-parameters that determine the electrophilic-reactivity of the 3-cyanomethylidene-2-oxindoline derivatives 3a-e in acetonitrile were determined by kinetic investigations of nucleophilic addition reactions involving secondary cyclic amines 4a-c (piperidine, morpholine and pyrrolidine) as the reference nucleophiles. Global reactivity descriptors in terms of theoretical scales of electrophilicity have been proposed and used to gain knowledge about the reactivity of molecules, moreover, they can further furnish worthy information which would lead to an improved understanding of the synthetic scope of this class of compounds.
Acknowledgment
The authors would like to acknowledge the funding provided by King Abdulaziz City for Science and Technology (KACST), Saudi Arabia, for supporting this work through project No. 1649-37-.أط
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kinetics and mechanism of reactions of substituted (methylthio)benzylidene Meldrum's acids with primary amines in aqueous DMSO. J. Phys. Org. Chem.. 2006;19:647-653.
- [Google Scholar]
- Quantification and theoretical analysis of the electrophilicities of Michael acceptors. J. Am. Chem. Soc.. 2017;139:13318-13329.
- [Google Scholar]
- Counter-current chromatography separation of Isatin derivatives using the Sandmeyer methodology. J. Braz. Chem. Soc.. 2010;21:764-769.
- [Google Scholar]
- Kinetic studies on nucleophilic addition reactions of 4-X-substituted anilines to N1-methyl-4-nitro-2,1,3 benzothiadiazolium terafluoroborate in acetonitrile: reaction mechanism and Mayr's approach. Int. J. Chem. Kinet.. 2015;47:461-470.
- [Google Scholar]
- Reactions of N1-Methyl-4-nitro-2,1,3-benzoselenadiazolium tetrafluoroborate with aliphatic and cyclic amines in acetonitrile: kinetic and structure-reactivity correlations. Int. J. Chem. Kinet.. 2017;49:576-583.
- [Google Scholar]
- Polar [3 + 2] cycloaddition of ketones with electrophilically activated carbonyl ylides. Synthesis of spirocyclic dioxolane indolinones. Org. Biomol. Chem.. 2008;6:3144-3157.
- [Google Scholar]
- Electrophilicity parameters for 2-benzylidene-indan-1,3-diones—a systematic extension of the benzhydrylium based electrophilicity scale. Org. Biomol. Chem.. 2007;5:3020-3026.
- [Google Scholar]
- Studies of the reactions between indole-2,3-diones (isatins) and 2-aminobenzylamine. Tetrahedron. 2003;59:1033-1048.
- [Google Scholar]
- Intrinsic barriers of reactions and the principle of nonperfect synchronization. Acc. Chem. Res.. 1987;20:301-308.
- [Google Scholar]
- Nucleophilic addition to olefins.Kinetics and mechanism. Tetrahedron. 1989;45:4017-4090.
- [Google Scholar]
- Nucleophilic addition to olefins. 4. Structure-reactivity relationships in the reactions of amines with substituted benzylidene Meldrum's acids. Evidence for intramolecular proton transfer to carbon. J. Am. Chem. Soc.. 1980;102:5329-5336.
- [Google Scholar]
- Nucleophilic additions to olefins. 19. Abnormally high intrinsic barrier in the reaction of piperidine and morpholine with benzylideneacetylacetone. J. Am. Chem. Soc.. 1986;108:7744-7751.
- [Google Scholar]
- Kinetics of the reactions of methoxybenzylidene Meldrum's acid with thiolate ions, alkoxide ions, OH-, and water in aqueous DMSO. Detection and kinetic characterization of the SNV intermediate. J. Am. Chem. Soc.. 1998;120:7461-7468.
- [Google Scholar]
- Nucleophilic addition to olefins. 2. Reaction of benzylidene Meldrum's acid with water, hydroxide ion, and aryloxide ions. Complete kinetic analysis of the hydrolytic cleavage of the carbon:carbon double bond. J. Am. Chem. Soc.. 1980;102:1361-1366.
- [Google Scholar]
- Nucleophilic addition to olefins. 6. Structure-reactivity relationships in the reactions of substituted benzylidene Meldrum's acids with water, hydroxide ion, and aryloxide ions. J. Am. Chem. Soc.. 1982;104:5133-5142.
- [Google Scholar]
- Nucleophilic addition to olefins. 18. Kinetics of the addition of primary amines and.alpha.-effect nucleophiles to benzylidene Meldrum's acid. J. Am. Chem. Soc.. 1986;108:5251-5257.
- [Google Scholar]
- Nucleophilic addition to olefins. 21. Substituent and solvent effects on the reaction of benzylidene Meldrum's acids with piperidine and morpholine. J. Org. Chem.. 1987;52:3042-3050.
- [Google Scholar]
- Kinetics of amine addition to benzylidene-1,3-indandione and other vinylic.beta.-diketones. Effect of cyclic structure and steric strain on intrinsic rate constants. J. Am. Chem. Soc.. 1991;113:2222-2227.
- [Google Scholar]
- Nucleophilic aromatic substitution reactions with carbanions and nitranions in dimethyl sulfoxide solution. J. Am. Chem. Soc.. 1986;108:5991-5997.
- [Google Scholar]
- Nucleophilicities of primary and secondary amines in water. J. Org. Chem.. 2007;72:3679-3688.
- [Google Scholar]
- Nucleophilicities and carbon basicities of pyridines. Chem. Eur. J.. 2007;13:336-345.
- [Google Scholar]
- Nucleophilicities of amino acids and peptides. Org. Biomol. Chem.. 2007;5:3814-3820.
- [Google Scholar]
- Structure−reactivity correlations in the aminolysis and pyridinolysis of bis(phenyl) and bis(4-nitrophenyl) thionocarbonates. J. Org. Chem.. 1997;62:6568-6574.
- [Google Scholar]
- Determination of the nucleophilicities of silyl and alkyl enol ethers. J. Am. Chem. Soc.. 1998;120:3629-3634.
- [Google Scholar]
- Direct catalytic asymmetric synthesis of cyclic aminals from aldehydes. J. Am. Chem. Soc.. 2008;130:15786-15787.
- [Google Scholar]
- Synthesis and evaluation of isatins and thiosemicarbazone derivatives against cruzain, falcipain-2 and rhodesain. Bioorg. Med. Chem. Lett.. 2003;13:3527-3530.
- [Google Scholar]
- Properties of bases in acetonitrile as solvent. IV. Proton acceptor power and homoconjugation of mono- and diamines. J. Am. Chem. Soc.. 1965;87:5005-5010.
- [Google Scholar]
- Effects of ortho- and para-ring activation on the kinetics of SNAr reactions of 1-chloro-2-nitro- and 1-phenoxy-2-nitrobenzenes with aliphatic amines in acetonitrile. Eur. J. Org. Chem.. 2006;2006:1222-1230.
- [Google Scholar]
- Reactivity of (E)-4-aryl-4-oxo-2-butenoic acid phenylamides with piperidine and benzylamine: Kinetic and theoretical study. Monatsh. Chem.. 2014;145:1297-1306.
- [Google Scholar]
- ‘On water’ Knoevenagel condensation of isatins with malononitrile. Mendeleev Commun.. 2011;21:224-225.
- [Google Scholar]
- Nucleophilic reactivities of silyl ketene acetals and silyl enol ethers containing (C6F5)3SiO and (C6H5)3SiO Groups. Eur. J. Org. Chem.. 2005;2005:1760-1764.
- [Google Scholar]
- alpha. Effect. V. Kinetic and thermodynamic nature of the.alpha. effect for amine nucleophiles. J. Am. Chem. Soc.. 1972;94:2052-2056.
- [Google Scholar]
- Activation of the aromatic system by the SO2CF3 group: Kinetics study and structure–reactivity relationships. Int. J. Chem. Kinet.. 2010;42:203-210.
- [Google Scholar]
- Electrocatalytic multicomponent transformation of cyclic 1,3-diketones, isatins, and malononitrile: Facile and convenient way to functionalized spirocyclic (5,6,7,8-tetrahydro-4H-chromene)-4,3′-oxindole system. Tetrahedron. 2007;63:10543-10548.
- [Google Scholar]
- Synthesis and reactions of some new spiro(indeno(1,2-b) pyran-4, 3'-indolines) Heterocycles. 2008;75:955-963.
- [Google Scholar]
- Synthesis and reactions of some new spiropyranthiazoline derivatives. Phosphorus Sulfur Silicon Relat. Elem.. 2008;183:2095-2107.
- [Google Scholar]
- A facile synthesis of some new 7, 8-dihydrospiro {imidazo [1, 2-a] pyridine-7, 3'-indoline}-2'-one derivatives. Heterocycles. 2008;75:2791-2802.
- [Google Scholar]
- Novel syntheses of some new 3,4-dihydrospiro{benzimidazo[1,2-a]pyridine-3,3′-indolin}-2′-one derivatives. Monatsh. Chem.. 2009;140:265-272.
- [Google Scholar]
- Sterically hindered phenolic buffers. Application to determination of rates of amidation of ethyl chloroformate. J. Am. Chem. Soc.. 1957;79:5439-5441.
- [Google Scholar]
- Regioselective synthesis and anti-inflammatory activity of novel dispiro[pyrazolidine-4, 3′-pyrrolidine-2′, 3″-indoline]-2″, 3, 5-triones. Arkivoc. 2011;10:85-98.
- [Google Scholar]
- An efficient and green synthesis of polyfunctionalized spirothiazolidin-4-ones using sulfonated mesoporous silica as a reusable catalyst. Chem. Heterocycl. Compd.. 2017;53:1148-1155.
- [Google Scholar]
- A convenient regioselective synthesis of spirooxindolinopyrrolizidines incorporating the pyrene moiety through a [3+ 2]-cycloaddition reaction. Heterocycl. Commun.. 2017;23:379-384.
- [Google Scholar]
- 1,3-Dipolar cycloaddition approach to novel dispiro[pyrazolidine-4,3′-pyrrolizidine-2′,3″-indoline]-2″,3,5-triones. J. Chem. Res.. 2017;41:346-351.
- [Google Scholar]
- Synthesen mit Nitrilen, LXXXIV Farbe und Stereochemie von 3-(Cyanmethylen)-2-indolonen. Liebigs Ann. Chem.. 1989;1989:1065-1069.
- [Google Scholar]
- Nucleophilic reactivities of primary and secondary amines in acetonitrile. Eur. J. Org. Chem.. 2009;2009:6379-6385.
- [Google Scholar]
- Electrophilicity parameters of 5-benzylidene-2,2-dimethyl[1,3]dioxane-4,6-diones (benzylidene Meldrum's Acids) J. Org. Chem.. 2008;73:2738-2745.
- [Google Scholar]
- Structure-Nucleophilicity relationships for enamines. Chem. Eur. J.. 2003;9:2209-2218.
- [Google Scholar]
- Novel three-component tandem reactions of cyclic mono ketones, isatin and sarcosine: formation of dispiropyrrolidines. Tetrahedron Lett.. 2007;48:7164-7168.
- [Google Scholar]
- Novel three-component domino reactions of ketones, isatin and amino acids: synthesis and discovery of antimycobacterial activity of highly functionalised novel dispiropyrrolidines. Eur. J. Med. Chem.. 2010;45:411-422.
- [Google Scholar]
- Superelectrophilicity of the nitroolefinic fragment of 4-nitrobenzodifuroxan in Michael-type reactions with Indoles: A kinetic study in acetonitrile. Chem. Eur. J.. 2007;13:8317-8324.
- [Google Scholar]
- Knoevenagel condensation of isatins with malononitrile/ethyl cyanoacetate in the presence of sulfonic acid functionalized silica (SBA-Pr-SO3H) as a new nano-reactor. Eur. J. Chem.. 2012;3:310-313.
- [Google Scholar]
- Electrophilicity parameters for benzylidenemalononitriles. J. Org. Chem.. 2003;68:6880-6886.
- [Google Scholar]
- Kinetic studies of carbocation–carbanion combinations: Key to a general concept of polar organic reactivity. Angew. Chem. Int. Ed.. 2002;41:91-95.
- [Google Scholar]
- Constant selectivity relationships of addition reactions of carbanions. Angew. Chem. Int. Ed.. 2000;39:1995-1997.
- [Google Scholar]
- Construction of spiro[pyrrolidine-3,3′-oxindoles] − Recent applications to the synthesis of oxindole alkaloids. Eur. J. Org. Chem.. 2003;2003:2209-2219.
- [Google Scholar]
- Reactivity of the HIV-1 nucleocapsid protein p7 zinc finger domains from the perspective of density-functional theory. Proc. Natl. Acad. Sci. U.S.A.. 1998;95:11578-11583.
- [Google Scholar]
- Reference scales for the characterization of cationic electrophiles and neutral nucleophiles. J. Am. Chem. Soc.. 2001;123:9500-9512.
- [Google Scholar]
- π-Nucleophilicity in carbon−carbon bond-forming reactions. Acc. Chem. Res.. 2003;36:66-77.
- [Google Scholar]
- Linear free enthalpy relationships: a powerful tool for the design of organic and organometallic synthesis. J. Phys. Org. Chem.. 1998;11:642-654.
- [Google Scholar]
- Mayr, H., Ofial, A.R., 2004. In: Olah, G.A., Prakash, G.K.S. (Eds.), Carbocation Chemistry. Hoboken, NJ, Wiley, pp 331–358.
- Kinetics of electrophile-nucleophile combinations: A general approach to polar organic reactivity. Pure Appl. Chem.. 2005;77:1807-1821.
- [Google Scholar]
- Reactivities and selectivities of free and metal-coordinated carbocations. Pure Appl. Chem.. 1998;70:1993-2000.
- [Google Scholar]
- How constant are Ritchie's “Constant Selectivity Relationships”? A general reactivity scale for n-, π-, and σ-Nucleophiles. J. Am. Chem. Soc.. 2003;125:286-295.
- [Google Scholar]
- Reaction of nitrile ylides with isatins and o-benzoquinones: formation of novel spirooxazoline derivatives. Tetrahedron. 2002;58:3003-3007.
- [Google Scholar]
- Kinetics and mechanism of the addition of benzylamines to benzylidene Meldrum's Acids in acetonitrile. Bull. Korean Chem. Soc.. 2003;24:193-196.
- [Google Scholar]
- Kinetic studies on the nucleophilic addition reactons of vinylic β-diketones. Bull. Korean Chem. Soc.. 2002;23:1459-1462.
- [Google Scholar]
- Kinetics and mechanism of addition of benzylamines to benzylidene-1,3-indandiones in acetonitrile. J. Org. Chem.. 2000;65:5391-5395.
- [Google Scholar]
- Density Functional Theory of Atoms and Molecules. Oxford: Oxford University Press; 1989.
- How nucleophilic are silyl enol ethers? Kinetics of the reactions of electron rich CC-double bonded systems with carbenium ions. Tetrahedron Lett.. 1993;34:3393-3396.
- [Google Scholar]
- Quantitative characterization of the global electrophilicity pattern of some reagents involved in 1,3-dipolar cycloaddition reactions. Tetrahedron. 2003;59:3117-3125.
- [Google Scholar]
- Comparison between experimental and theoretical scales of electrophilicity in benzhydryl cations. J. Org. Chem.. 2002;67:4747-4752.
- [Google Scholar]
- Nucleophilicity parameters for carbanions in methanol. Eur. J. Org. Chem.. 2006;2006:2530-2537.
- [Google Scholar]
- An efficient synthesis of pyrazolo[3,4-b]pyridine-4-spiroindolinones by a three-component reaction of 5-aminopyrazoles, isatin, and cyclic β-diketones. Tetrahedron Lett.. 2011;52:2664-2666.
- [Google Scholar]
- The rapid steps in nucleophilic vinylic addition-elimination substitution. Recent developments. Acc. Chem. Res.. 1992;25:474-479.
- [Google Scholar]
- The three-component reaction between isatin, α-amino acids, and dipolarophiles. Eur. J. Org. Chem.. 2004;2004:413-418.
- [Google Scholar]
- 5-Methoxyfuroxano[3,4-d]pyrimidine: a highly reactive neutral electrophile. J. Phys. Org. Chem.. 2003;16:431-437.
- [Google Scholar]
- Local softness and hardness based reactivity descriptors for predicting intra- and intermolecular reactivity sequences: Carbonyl compounds. J. Phys. Chem. A.. 1998;102:3746-3755.
- [Google Scholar]
- Straightforward stereoselective synthesis of spiro-epoxyoxindoles. Org. Lett.. 2007;9:1745-1748.
- [Google Scholar]
- Electrophilicity of 5-benzylidene-1,3-dimethylbarbituric and -thiobarbituric bcids. J. Org. Chem.. 2007;72:9170-9180.
- [Google Scholar]
- Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev.. 2012;112:6104-6155.
- [Google Scholar]
- Ranking the reactivity of superelectrophilic heteroaromatics on the electrophilicity scale. J. Org. Chem.. 2005;70:6242-6253.
- [Google Scholar]
- Regiospecific synthesis and biological evaluation of spirooxindolopyrrolizidines via [3+2] cycloaddition of azomethine ylide. Eur. J. Med. Chem.. 2010;45:6120-6126.
- [Google Scholar]
- Nucleophilic reactivities of ketene acetals. Eur. J. Org. Chem.. 2004;2004:2791-2796.
- [Google Scholar]
- Mechanistic assessment of SNAr displacement of halides from 1-halo-2,4-dinitrobenzenes by selected primary and secondary amines: Brønsted and Mayr Analyses. J. Org. Chem.. 2012;77:9738-9746.
- [Google Scholar]
- Kinetic study on SNAr reaction of 1-(Y-substituted-phenoxy)-2,4-dinitrobenzenes with cyclic secondary amines in acetonitrile: Evidence for cyclic transition-state structure. J. Org. Chem.. 2014;79:7025-7031.
- [Google Scholar]
- Synthesis and properties of dendrimers possessing the same fluorophore(s) located either peripherally or off-center. J. Org. Chem.. 2007;72:8707-8715.
- [Google Scholar]
- Anticonvulsant activity of Schiff bases of isatin derivatives. Acta Pharm.. 2004;54:49-56.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.03.027.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







