Studies on chemical profiling and pharmacokinetics of traditional Chinese medicine Formula Kang Shuai Lao Pian
⁎Corresponding author. miaomiaojiang@tjutcm.edu.cn (Miaomiao Jiang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Kang Shuai Lao Pian (KSLP) is a traditional Chinese medicine (TCM) preparation used to delay aging. However, due to the lack of research on the chemical composition and pharmacokinetic behavior of KSLP, its material basis and in vivo components with high exposure remain ambiguous. The UPLC/Q-Orbitrap-MS/MS was performed to identify the preliminary chemical profile of KSLP. A total of 138 compounds, including ginsenosides, phenylethanol glycosides, iridoids, alkaloids, ionones and others, were identified in accordance with their retention times, accurate masses and characteristic MS/MS fragment patterns. Moreover, considering the active components and characteristic components of KSLP, the extraction process of KSLP was optimized, and the quantitative analysis by UPLC/QQQ-MS/MS of 13 compounds in KSLP was established. The method was stable and sensitive, and could be used for the quality control of KSLP. Then, the pharmacokinetic study was carried out by further refining the components of KSLP. Besides, quantitative method for 6 compounds in rat plasma was validated and developed by UPLC/QQQ-MS/MS. The established approach was successfully applied to characterize the pharmacokinetic features of components in KSLP and it was found that the absorption and elimination of ginsenosides in KSLP was slow. Altogether, this study laid a solid foundation and provided theoretical guidance for further clarification of bioactive components of KSLP.
Keywords
Kang Shuai Lao Pian
UPLC/Q-Orbitrap-MS/MS
UPLC/QQQ-MS/MS
Qualitative analysis
Quantitative analysis
Pharmacokinetics analysis
- KSLP
-
Kang Shuai Lao Pian
- TCM
-
traditional Chinese medicine
- MRM
-
multiple reaction monitoring
- QC
-
quality control
- CE
-
collision energy
- CV
-
cone voltage
- LOD
-
limit of detection
- LOQ
-
limit of quantification
- S/N
-
single to noise ratios
- IS
-
internal standard solution
- LLOQ
-
lower limit of quantification
- BPI
-
base peak ion chromatograms
- PPD
-
protopanaxadiol
- PPT
-
protopanaxatriol
Abbreviations
1 Introduction
Aging refers to the process of degenerative changes in various tissues and organs as the body ages after reaching maturity in growth and development, which is affected by genetic, lifestyle and environmental factors (Antell and Taczanowski, 1999, Lee et al., 2016, Noroozi et al., 2021). Population aging is reportedly one of the most significant trends of the 21st century. Therefore, there is a growing need for aging management, and healthy aging is the main goal of aging intervention and research. In recent years, although new drugs have been developed to improve aging, most of them are expensive and have side effects, so it is particularly important to find safe and effective drugs to delay aging (Blagosklonny, 2007, Li et al., 2013). Clinical practice has proved that traditional Chinese medicine (TCM) has the advantages of multiple targets and few side effects, and has played a pivotal role in maintaining human healthcare (Zhao et al., 2020).
Kang Shuai Lao Pian (KSLP) is a famous TCM formulated from a court prescription of the Ming Dynasty. It is prepared from Rehmanniae radix, Ginseng radix et rhizoma rubra, Asparagi radix, Ophiopogonis radix, Lych cortex and Poria in the weight ratio of 409, 167, 26, 26, 26, 77 (Gong et al., 2020a, 2020b). In China, it has been widely accepted as a health care product for delaying senescence (Gong et al., 2020a, 2020b). Obviously, the identification and detection of the main components in KSLP is the premise and key to reveal its active ingredients. The chemical constituents of each crude drug in KSLP have been reported in previous studies, but little attention has been paid to the integral chemical composition of KSLP. In the process of drug development, pharmacokinetic research is an indispensable part of the process, which has important reference value for drug development, evaluation and clinical application (Zhang et al., 2017). However, to the best of our knowledge, no pharmacokinetic study on KSLP has been reported. Thus, the establishment of comprehensive qualitative, quantitative and pharmacokinetic methods for KSLP is urgently needed.
In recent years, with the development of analytical technology, UPLC/Q-Orbitrap-MS/MS has been widely used for the analysis of TCM due to its fast separation speed and high sensitivity (Han et al., 2015). The UPLC/QQQ-MS/MS technology is a powerful tool for high-throughput quantitative analysis of TCM owing to its high-selective simultaneous detection of multiple compounds with a multiple reaction monitoring (MRM) mode (Liu et al., 2017, Ren et al., 2022). The integration of UPLC/Q-Orbitrap-MS/MS and UPLC/QQQ-MS/MS is a potentially effective approach for in-depth chemical profiling and pharmacokinetic of KSLP.
In this study, the UPLC/Q-Orbitrap-MS/MS analysis method was established for the global characterizations of chemical composition in KSLP. Besides, 13 compounds were further quantitatively analyzed with UPLC/QQQ-MS/MS considering the representative components of KSLP, the abundance and activity of chemicals. The extraction and refining process of components in KSLP was optimized to maximize the content of compounds, and then the UPLC/QQQ-MS/MS method was applied to study the pharmacokinetics of refined components in KSLP. Through the chemical analysis of KSLP, the material basis was clarified and a reference was provided as its quality evaluation. Pharmacokinetic studies were conducted to reveal the changes of refined components in KSLP in vivo and provided a basis for their clinical application.
2 Materials and methods
2.1 Chemicals and materials
Acetonitrile (chromatographic purity) was purchased from Fisher company (USA), formic acid (MS grade) was obtained from ACS company (USA). Reference standards of acteoside, isoacteoside, echinacoside, jionoside A1, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re, ginsenoside Rf, ginsenoside Rg1, ginsenoside Rg2, ginsenoside Rg3 and digoxin were acquired from Sichuan Weikeqi Biochemical Co., Ltd. (Sichuan, China). KSLP (batch number: 2207001) was supplied by Chiatai Qingchunbao Medicine Co., Ltd. (Hangzhou, China).
2.2 Qualitative analysis by UPLC/Q-Orbitrap-MS/MS
2.2.1 Sample solutions preparation
0.1 g KSLP powder was added to 10 mL of 50 % ethanol, and ultrasonically extracted for 60 min. The solution was centrifuged at 15,000 g for 10 min, and the supernatant was used for UPLC/Q-Orbitrap-MS/MS qualitative analysis.
2.2.2 Standard solutions preparation
The standards of acteoside, isoacteoside, echinacoside, jionoside A1, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re, ginsenoside Rf, ginsenoside Rg1, ginsenoside Rg2 and ginsenoside Rg3 were weighed accurately and dissolved in 50 % ethanol to 1 mg/mL single standard solutions. 50 µL of each standard solutions were added to 350 µL of 50 % ethanol, and the mixed standard solution was collected after vortex mixing 1 min and the following centrifugation (12000 g, 10 min, 4℃).
2.2.3 Chromatographic and mass spectrographic conditions
The UPLC separation was performed on Ultimate 3000 UPLC system (Thermo Fisher Scientific, USA) with an ACQUITY UPLC HSS T3 (2.1 × 100 mm, 1.7 μm, Waters) maintained at 40℃. The mobile phase was acetonitrile (A) and water with 0.1 % formic acid (B) at a flow rate of 0.3 mL/min, and injection volume of samples was 4 μL. The gradient elution procedure was as follows: 0 ∼ 2 min, 3 % (A); 2 ∼ 5 min, 3 ∼ 21 % (A); 5 ∼ 7 min, 21 ∼ 22 % (A); 7 ∼ 9 min, 22 ∼ 30 % (A); 9 ∼ 13 min, 30 ∼ 33 % (A); 13 ∼ 18 min, 33 ∼ 34 % (A); 18 ∼ 23 min, 34 ∼ 55 % (A); 23 ∼ 28 min, 55 ∼ 70 % (A); 28 ∼ 33 min, 70 ∼ 98 % (A).
The mass spectrometry analysis was completed under Q-Orbitrap MS system (Thermo Fisher Scientific, USA). The optimized MS parameters were: ESI positive and negative (+/-) mode; spray voltage, 3.5kv; capillary temperature, 350℃; aux gas heater temperature, 350 ℃; sheath gas was nitrogen and its flow was 35 L/h; auxiliary gas was nitrogen and its flow was 10 L/h; full scan mode, m/z 100–1500.
2.3 Quantitative analysis by UPLC/QQQ-MS/MS
2.3.1 Sample solutions preparation
In this study, the effects of four single factors (extraction method, extraction solution, extraction time and solid-to-liquid ratio) on the contents of compounds were investigated. The maximum total content of the 13 target compounds was used as the criterion to determine the optimal extraction process.
The levels of the four single factors were as follows: ultrasonic and reflux for extraction method; 30 % ethanol, 50 % ethanol, 70 % ethanol and 90 % ethanol for extraction solution; 30, 60, 90 and 120 min for extraction time; 1:20, 1:50, 1:100 and 1:200 g/mL for solid-to-liquid ratio. When the influence of any single factor was investigated on the total content of 13 target compounds, 50 % ethanol, ultrasonic, 90 min and 1:100 g/mL were selected for the other three factors.
The samples were extracted according to each extraction process, diluted with the corresponding solutions, and finally 100 µg/mL of sample solutions were obtained for UPLC/QQQ-MS/MS quantitative analysis.
2.3.2 Standard solutions preparation
The stock solutions of acteoside, isoacteoside, echinacoside, jionoside A1, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re, ginsenoside Rf, ginsenoside Rg1, ginsenoside Rg2 and ginsenoside Rg3 were prepared in 50 % ethanol. Working standard solutions containing each of the 13 compounds were prepared by diluting the stock solutions with 50 % ethanol to a series of proper concentrations. Similarly, the working solutions of quality control (QC) with low, medium and high concentrations were prepared. QC samples contained acteoside, isoacteoside, echinacoside, jionoside A1, ginsenoside Rg2 and ginsenoside Rg3 of 1, 10 and 80 ng/mL, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re, ginsenoside Rf and ginsenoside Rg1 of 10, 100 and 800 ng/mL.
2.3.3 Chromatographic and mass spectrographic conditions
The chromatographic separation was achieved on an ACQUITY UPLC Ultra Performance Liquid Chromatograph (Waters, American) with an ACQUITY UPLC HSS T3 (2.1 × 100 mm, 1.7 μm, Waters) maintained at 40℃. The mobile phase was acetonitrile (A) and water with 0.1 % formic acid (B) at a flow rate of 0.3 mL/min, and injection volume of samples was 4 μL. The gradient elution was performed as follows: 0 ∼ 2.5 min, 20 ∼ 30 % (A); 2.5 ∼ 10 min, 30 ∼ 60 % (A); 10 ∼ 10.5 min, 60 ∼ 98 % (A).
MS detection was carried on Waters Xevo TQ-S Triple Quadrupole Mass Spectrometer (Waters, American), and the mass spectrometer was operated in a MRM mode. The optimized MS parameters: capillary voltage, 3.0 KV (positive ion mode)/ 2.0 KV (negative ion mode); solvent removal temperature, 350℃. The remaining specific parameters, such as parents ion (Parents), daughters ion (Daughters), collision energy (CE) and cone voltage (CV), were optimized and summarized in Table S1.
2.3.4 Method validation
According to the above optimal analysis conditions and extraction conditions, the selectivity, linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, precision, stability and recovery of 13 compounds were determined.
The selectivity of the method was evaluated by comparing the chromatograms of each analyte in the blank solutions, standard solutions and sample solutions. Linearity was evaluated by plotting the calibration curves, and the curves were plotted with the concentration X (ng/mL) of the standard as the abscissa and the corresponding peak area Y of each standard as the ordinate. For each target compound, LOD and LOQ were determined at single to noise ratios (S/N) of 3 and 10 by continuous dilution of standard solutions. The QC samples of low, medium and high concentrations were analyzed for three consecutive days, accuracy was obtained from the relative error expressed as percentage (RE%), and precision was calculated using the relative standard deviation (RSD%). The stability of QC samples of low, medium and high concentrations was studied after being placed at room temperature for 0, 2, 4, 8, 12, 24 h. The test solutions and the standard solutions were added at a ratio of 1:1 to calculate the recovery.
2.4 Pharmacokinetics analysis by UPLC/QQQ-MS/MS
2.4.1 KSLP preparation
KSLP powder was repeatedly extracted twice under the optimal extraction process conditions, and the extract was concentrated and freeze-dried to obtain crude components of KSLP. The equal crude components of KSLP were further eluted with water and different proportions of ethanol/ethanol/methanol by resin D101/ resin AB-8/ODS, the water-eluting fractions were discarded, other fractions were combined respectively, concentrated and freeze-dried to obtain refined components of KSLP.
0.1 g powders of KSLP, crude components of KSLP and refined components of KSLP were added to 10 mL of 50 % ethanol, and ultrasonically extracted for 90 min. Extracts of KSLP and its crude components were centrifuged and diluted to obtain 100 µg/mL sample solutions. Due to the large difference of the compound content in the refined components, 1000 µg/mL and 10 µg/mL of sample solutions were obtained, The UPLC/QQQ-MS/MS quantitative analysis of sample solutions was the same as in part 2.3. The refined components of KSLP obtained by the optimal refining process were used for pharmacokinetic studies.
2.4.2 Animals and experimental procedure
A total of 6 male Sprague-Dawley (SD) rats (200–220 g) were purchased from Beijing Huafukang Bio-Technology Co., Ltd. All of the rats were kept in pathogen-free animal laboratory in a 12 h light/dark cycle, with a feeding temperature of 26 ℃ and relative humidity of 50 %. Animal experiments were consistent with the Animal Ethics Committee of Tianjin University of Traditional Chinese Medicine, and the animal ethics approval number was TCM-LAEC2022124.
Rats were acclimatized for 7 days to laboratory environments before the experiment was directed. Diet was prohibited for 12 h before the experiment, but the water was freely available. Appropriate refined components of KSLP were dispersed in distilled water as a suspension with a concentration of 0.4 g/mL, and the oral administration dose was set at 10 mL/kg of rat weight. The blood samples were collected through canthus into heparinized tubes before administration and at 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 36, 48, 60 and 72 h after oral administration of refined components of KSLP (4 g/kg). Plasma samples were obtained by centrifuging the blood samples immediately at 1600g for 10 min and stored at −80℃ until analysis.
2.4.3 Plasma sample preparation
100 μL of each sample was processed by adding 300 μL of acetonitrile, 100 μL of internal standard solution (IS) and 100 μL of 50 % acetonitrile. The supernatant was collected after vortex mixing 1 min and the following centrifugation (15000 g, 10 min, 4℃). Then, the supernatant was transferred and dried under a gentle stream of nitrogen gas at room temperature. The resulting residues were redissolved in 100 μL of 50 % acetonitrile, and 5 μL was injected into the UPLC/QQQ-MS/MS system.
2.4.4 Standard solutions preparation
The stock solutions of ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re and ginsenoside Rg1 were prepared in 50 % acetonitrile, which were then diluted with 50 % acetonitrile to obtain mixed standard solutions of the 6 compounds at different concentrations. Digoxin was weighed accurately, dissolved in 50 % acetonitrile and diluted gradually to 100 ng/mL as the internal standard solution. 100 μL of blank plasma was processed by adding 300 μL of acetonitrile, 100 μL of IS and 100 μL of mixed standard solutions. Similar to post-administration plasma processing, a range of working standard solutions were obtained for analysis. The QC samples were prepared in a similar manner at three different concentrations (10, 100 and 800 ng/mL) of ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re and ginsenoside Rg1.
2.4.5 Chromatographic and mass spectrographic conditions
The conditions of chromatographic and mass spectrographic were the same as in part 2.3.3, the remaining specific parameters of 7 compounds were shown in Table S2.
2.4.6 Method validation
According to the above optimal analysis conditions, the selectivity, linearity, lower limit of quantification (LLOQ), accuracy, precision, extraction recovery, matrix effect and stability were validated.
The chromatograms of blank plasma, standard spiked plasma and sample plasma were used to assess the selectivity of the method. Linear regression equations were obtained by least squares linear regression on the ratio Y of the compound peak area to IS peak area as the ordinate, the compound concentration X (ng/mL) in plasma as the abscissa, and LLOQ was the lowest concentration of linearity. Intra-day and inter-day accuracy and precision were estimated by analyzing a calibration curve and QC samples of low, medium and high concentrations on three days. The extraction recovery was evaluated through the ratio of the mean concentration between regularly prepared QC samples of low, medium and high concentrations and spike-after-extraction plasma samples. Similarly, the matrix effect was assessed through the ratio of concentration between post-extraction samples spiked with analytes and ultrapure water spiked with analytes at the same concentration. The stability of each analyte at different conditions (autosampler for 24 h and three freeze–thaw cycles from −80℃ to room temperature) was assessed by analyzing at QC levels.
2.5 Data processing and analysis
Data was expressed as mean ± standard deviation, pharmacokinetic parameters were statistically calculated using the pharmacokinetic software (DAS version 2.0).
3 Results
3.1 Qualitative analysis of chemical constituents in KSLP by UPLC/Q-Orbitrap-MS/MS
The base peak ion chromatograms (BPI) of KSLP samples and standards were shown in Fig. 1, according to the experimental conditions described in part 2.2, combined with standard comparison and literature comparison, a total of 138 compounds were inferred (Table 1), including 79 ginsenosides, 14 phenylethanol glycosides, 10 iridoids, 10 alkaloids, 5 ionones and 20 others, 13 compounds were further identified by comparison with standards.
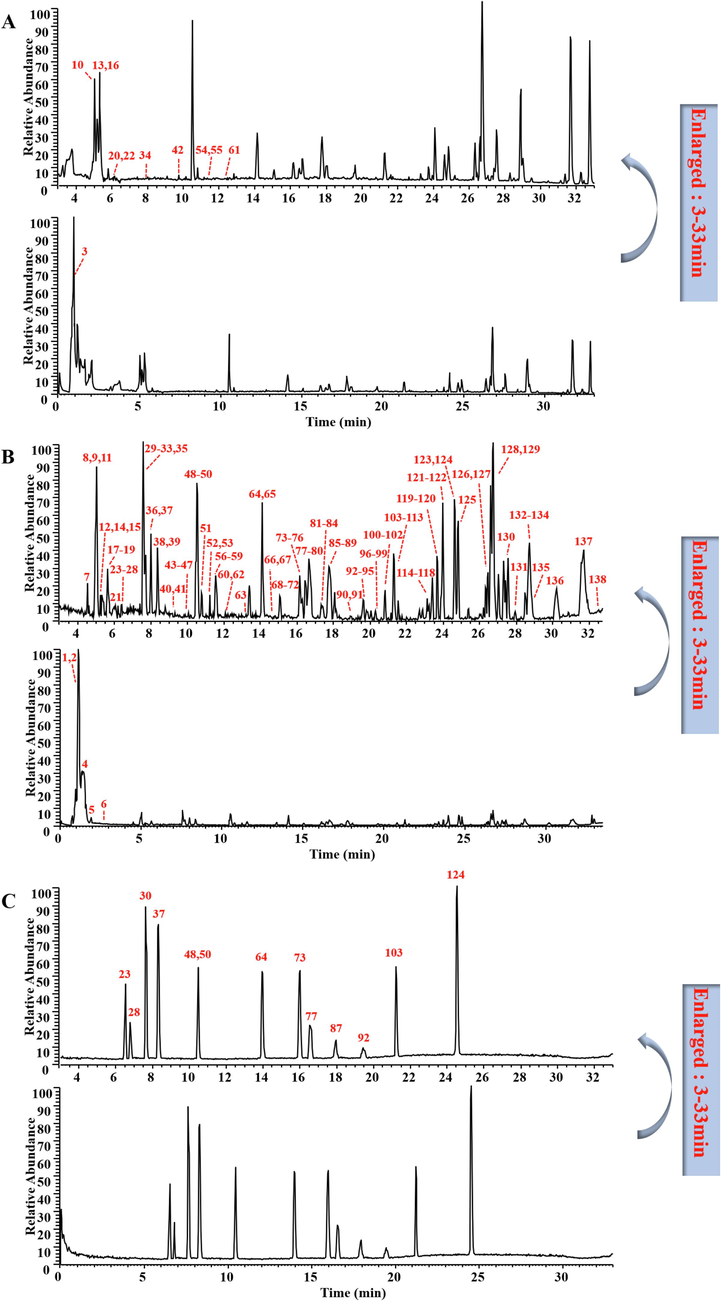
- The base peak ion chromatograms of KSLP samples and standards (A: the sample under positive ion mode; B: the sample under negative ion mode; C: the standards under negative ion mode).
| NO. | Observed (m/z) |
Formula | tR (min) | Mass error(ppm) | Adducts | Identification | Source | ESI-MS2 |
|---|---|---|---|---|---|---|---|---|
| 1 | 407.1197 | C15H22O10 | 0.94 | 0.493 | [M + COOH]- | Monomelittoside | RG | 361.1140 199.0605 169.0497 |
| 2 | 191.0191 | C6H8O7 | 0.96 | −0.626 | [M−H]- | Citric Acid | RG | 173.0441 127.0388 87.0074 85.0282 |
| 3 | 268.1043 | C10H13N5O4 | 1.13 | 1.006 | [M + H]+ | Adenosine | PG | 136.0618 119.0353 |
| 4 | 191.0191 | C6H8O7 | 1.46 | −0.626 | [M−H]- | 3-carboxy-2,3-dideoxy-1-hydroxypropan-1,2,3-tricarboxylic acid | RG | 173.0083 129.0180 111.0075 87.0074 85.0282 |
| 5 | 361.1144 | C15H22O10 | 1.83 | 1.052 | [M−H]- | Catalpol | RG | 199.0604 181.0497 169.0497 |
| 6 | 125.0232 | C6H6O3 | 2.86 | −9.737 | [M−H]- | 5-hydroxymethylfurfural | AC | 97.0281 87.9239 81.0333 69.0332 |
| 7 | 731.2261 | C27H42O20 | 4.38 | 1.305 | [M + COOH]- | Rehmannioside D | RG | 505.1034 341.1085 179.0553 |
| 8 | 373.1141 | C16H22O10 | 4.62 | 0.08 | [M−H]- | Geniposidic acid | RG | 211.0605 193.0499 179.0554 167.0704 123.0439 |
| 9 | 167.0343 | C8H8O4 | 4.91 | −4.083 | [M−H]- | Vanillic acid | RG | 152.0105 137.0230 124.0755 93.0332 |
| 10 | 251.1393 | C13H18N2O3 | 5.02 | 1.119 | [M + H]+ | N-caffeoyl putrescine | LC | 234.1125 190.4467 163.0389 135.0441 |
| 11 | 373.1142 | C16H22O10 | 5.07 | 0.18 | [M−H]- | Gardoside | RG | 211.0606 149.0597 123.0439 |
| 12 | 347.1349 | C15H24O9 | 5.21 | 0.416 | [M−H]- | Ajugol | RG | 317.1079 235.1352 225.1024 167.0707 123.0802 |
| 13 | 531.3179 | C28H42N4O6 | 5.26 | 0.355 | [M + H]+ | Kukoamine A | LC | 513.3179 367.2735 293.1857 222.1124 165.0547 |
| 14 | 461.1669 | C20H30O12 | 5.31 | 0.977 | [M−H]- | Decaffeoyl verbascoside | RG | 315.1082 297.0962 161.0446 135.0439 |
| 15 | 375.1294 | C16H24O10 | 5.38 | −0.72 | [M−H]- | Mussaenosidic acid | RG | 213.0761 195.0652 179.0553 169.0860 |
| 16 | 531.318 | C28H42N4O6 | 5.5 | 0.543 | [M + H]+ | Kukoamine B | LC | 513.3069 293.1858 222.1124 165.0547 |
| 17 | 345.1178 | C14H20O7 | 5.52 | −3.783 | [M + COOH]- | Salidroside | RG | 179.0552 119.0488 101.0230 |
| 18 | 345.1556 | C16H26O8 | 5.52 | 0.316 | [M−H]- | Rehmapicroside | RG | 179.0552 165.0907 119.0488 |
| 19 | 487.1462 | C21H28O13 | 5.56 | 0.998 | [M−H]- | Isomer of 3-O-(6-Deoxy-α-L-mannopyranosyl)-4-O-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]-D-glucose | RG | 179.0341 161.0234 |
| 20 | 474.2602 | C25H35N3O6 | 6.11 | 0.712 | [M + H]+ | N1, N10-bis (dihydro-caffeoyl) spermidine | LC | 457.2328 293.1862 236.1283 222.1126 165.0547 |
| 21 | 487.1462 | C21H28O13 | 6.24 | −0.998 | [M−H]- | 3-O-(6-Deoxy-α-L-mannopyranosyl)-4-O-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]-D-glucose | RG | 179.0341 161.0234 |
| 22 | 472.2451 | C25H33N3O6 | 6.25 | 1.88 | [M + H]+ | N5-caffeoyl-N10-caffeoylspermidine | LC | 454.2336 310.2125 293.1858 220.0968 163.0389 |
| 23# | 785.2518 | C35H46O20 | 6.45 | 0.947 | [M−H]- | Echinacoside | RG | 623.2195 477.1616 459.1498 392.5579 315.1089 179.0342 161.0235 |
| 24 | 785.2513 | C35H46O20 | 6.55 | 0.424 | [M−H]- | Purpureaside C | RG | 623.2187 459.1502 161.0234 |
| 25 | 283.055 | C15H24O5 | 6.63 | −0.343 | [M−H]- | Hydroxy-acetic acid | RG | 183.1018 153.0909 139.1116 |
| 26 | 451.2189 | C19H34O9 | 6.67 | 0.902 | [M + COOH]- | Oxyrehmaionoside B | RG | 243.1595 213.1493 179.0556 161.0447 |
| 27 | 403.1248 | C17H24O10 | 6.74 | 0.534 | [M−H]- | Gardenoside | RG | 371.0966 223.0605 165.0546 |
| 28# | 799.2673 | C36H48O20 | 6.84 | 0.855 | [M−H]- | Jionoside A1 | RG | 623.2185 605.2119 477.1590 459.1524 422.5963 |
| 29 | 435.2236 | C19H34O8 | 7.38 | 0.068 | [M + COOH]- | Rehmannioside B | RG | 179.0557 161.0443 |
| 30# | 623.1985 | C29H36O15 | 7.66 | 0.572 | [M−H]- | Acteoside | RG | 461.1662 315.1082 161.0234 |
| 31 | 183.1019 | C10H16O3 | 7.73 | −4.192 | [M−H]- | (25R)-5β-Spirostane-1β,3β,25-triol | RG | 139.1116 137.0959 |
| 32 | 813.283 | C37H50O20 | 7.8 | 0.901 | [M−H]- | Jionoside B1 | RG | 637.2353 619.2266 491.1768 175.0391 |
| 33 | 435.2236 | C19H34O8 | 7.95 | 0.068 | [M + COOH]- | Rehmaionoside A/B | RG | 389.2188 179.0557 161.0442 |
| 34 | 193.0497 | C10H8O4 | 7.96 | 0.853 | [M + H]+ | Scopoletin | LC | 178.0262 165.1270 149.1328 133.1013 |
| 35 | 193.0499 | C10H10O4 | 7.98 | −3.792 | [M−H]- | Ferulic acid | AC | 178.0263 149.0596 134.0361 |
| 36 | 435.2236 | C19H34O8 | 8.16 | 0.068 | [M + COOH]- | Rehmaionoside A/B | RG | 389.2184 179.0552 161.0445 |
| 37# | 623.1984 | C29H36O15 | 8.3 | 0.412 | [M−H]- | Isoacteoside | RG | 461.1658 315.1091 161.0234 |
| 38 | 497.1667 | C23H30O12 | 8.48 | 0.504 | [M−H]- | 6-O-vanillate ajugol | RG | 317.1029 167.0341 152.0105 123.0439 |
| 39 | 623.1984 | C29H36O15 | 8.52 | 0.412 | [M−H]- | Forsythoside A | RG | 461.1689 161.0234 |
| 40 | 637.2142 | C30H38O15 | 9.24 | 0.638 | [M−H]- | Jionoside C | RG | 526.9341 461.1667 315.1086 193.0498 175.0392 |
| 41 | 1007.5448 | C48H82O19 | 9.42 | 1.556 | [M + COOH]- | 20-Glc-Ginsenoside Rf | PG | 961.5378 799.4826 637.4280 619.4224 475.3815 391.2862 |
| 42 | 874.3734 | C42H51N9O12 | 9.73 | 0.464 | [M + H]+ | Lyciumin A | LC | 856.3506 486.1976 468.1875 442.1821 |
| 43 | 977.5332 | C47H80O18 | 9.75 | 0.544 | [M + COOH]- | Notoginsenoside R1/Fp1/Ginsenoside Re4 | PG | 931.5276 799.4855 637.4318 475.3791 391.2839 |
| 44 | 1007.5446 | C48H82O19 | 9.82 | 1.357 | [M + COOH]- | Notoginsenoside N | PG | 961.5381 799.4863 637.4366 475.3795 391.2864 |
| 45 | 977.5338 | C47H80O18 | 9.96 | 1.158 | [M + COOH]- | Notoginsenoside R1/Fp1/Ginsenoside Re4 | PG | 931.5278 799.4862 637.4304 475.3769 391.2843 |
| 46 | 1007.5447 | C48H82O19 | 10.14 | 1.457 | [M + COOH]- | Ginsenoside Re3 | PG | 961.5371 799.4874 637.4311 475.3807 391.2851 |
| 47 | 977.5339 | C47H80O18 | 10.18 | 1.26 | [M + COOH]- | Notoginsenoside R1/Fp1/Ginsenoside Re4 | PG | 931.5272 799.4841 637.4321 475.3808 391.2865 |
| 48# | 991.5497 | C48H82O18 | 10.47 | 1.394 | [M + COOH]- | Ginsenoside Re | PG | 945.5430 799.4824 783.4907 637.4328 475.3794 |
| 49 | 651.2297 | C31H40O15 | 10.67 | 0.394 | [M−H]- | Martynoside/Isomartynoside | RG | 475.1817 329.1227 175.0391 |
| 50# | 845.4914 | C42H72O14 | 10.67 | 1.172 | [M + COOH]- | Ginsenoside Rg1 | PG | 799.4850 637.4317 475.3789 |
| 51 | 312.1242 | C18H19NO4 | 10.96 | 0.22 | [M−H]- | N-trans-feruloyltyramine | OJ | 190.0502 176.0345 148.0519 |
| 52 | 1031.5449 | C51H84O21 | 11.18 | 1.617 | [M−H]- | Malonylfloralginsenoside Re1 | PG | 945.5433 799.4834 783.4877 637.4315 475.3795 391.2852 |
| 53 | 651.2301 | C31H40O15 | 11.21 | 1.008 | [M−H]- | Martynoside/Isomartynoside | RG | 505.1704 475.1822 339.8273 175.0392 |
| 54 | 897.3893 | C44H52N10O11 | 11.27 | 0.358 | [M + H]+ | Lyciumin B | LC | 879.3740 689.3036 468.1874 442.2082 |
| 55 | 344.1492 | C19H21NO5 | 11.31 | −0.143 | [M + H]+ | N-feruloyl-3-methoxytyramine | LC | 177.0546 145.0284 117.0337 |
| 56 | 887.5012 | C44H74O15 | 11.36 | 0.255 | [M + COOH]- | Acetyl-Ginsenoside Rg1 | PG | 841.5014 781.4736 637.4306 475.3818 161.0446 |
| 57 | 1079.5288 | C50H82O22 | 11.5 | 0.763 | [M + COOH]- | Parisaponin I | OJ | 1033.5266 901.4800 887.4641 755.4255 688.1104 |
| 58 | 183.1019 | C10H16O3 | 11.64 | −4.192 | [M−H]- | 1-hydroxy-2,6,6-trimethylcyclohex- 2-enecarboxylicacid |
RG | 137.096 |
| 59 | 815.4808 | C41H70O13 | 11.79 | 1.172 | [M + COOH]- | Pseudoginsenoside Rt3 | PG | 769.4763 475.3808 |
| 60 | 815.4808 | C41H70O13 | 12.01 | 1.172 | [M + COOH]- | Notoginsenoside A3 | PG | 637.4323 475.3784 391.2861 |
| 61 | 964.4206 | C49H57N9O12 | 12.21 | 0.68 | [M + H]+ | Lyciumin C | LC | 558.2376 477.3723 389.1823 |
| 62 | 535.2554 | C28H40O10 | 12.33 | 0.989 | [M−H]- | Neorehmannioside C | RG | 163.0390 145.0283 117.0332 |
| 63 | 887.502 | C44H74O15 | 13.17 | 1.156 | [M + COOH]- | Notoginsenoside Rt | PG | 841.4960 799.4748 781.4729 637.4343 619.4168 475.3756 |
| 64# | 845.4911 | C42H72O14 | 14 | 0.817 | [M + COOH]- | Ginsenoside Rf | PG | 799.4850 637.4315 475.3798 391.2856 |
| 65 | 799.4843 | C42H72O14 | 14.04 | −0.788 | [M−H]- | Ginsenoside La | PG | 637.4324 475.3801 457.3684 391.2860 |
| 66 | 1285.6432 | C59H100O27 | 14.29 | −0.2 | [M + COOH]- | Notoginsenoside R4 | PG | 1239.6381 1107.5948 945.5484 783.4958 621.4349 459.3830 |
| 67 | 1325.6381 | C62H102O30 | 14.77 | −0.162 | [M−H]- | Malonylnotoginsenoside R4 | PG | 1239.6378 1221.6266 1077.5881 763.5875 459.3802 |
| 68 | 887.5019 | C44H74O15 | 14.91 | 1.044 | [M + COOH]- | Vinaginsenoside R1 | PG | 841.4951 799.4790 637.4281 475.3795 391.2851 |
| 69 | 815.4808 | C41H70O13 | 14.98 | 1.172 | [M + COOH]- | Notoginsenoside R2 | PG | 769.4744 637.4310 475.3793 391.2857 |
| 70 | 815.481 | C41H70O13 | 15.2 | 1.417 | [M + COOH]- | Ginsenoside F5 | PG | 769.4747 637.4305 475.3791 391.2854 |
| 71 | 267.1603 | C15H24O4 | 15.2 | 0.44 | [M−H]- | Aeginetic acid | RG | 205.1593 153.0911 |
| 72 | 815.4808 | C41H70O13 | 15.38 | 1.172 | [M + COOH]- | Ginsenoside F3 | PG | 769.4753 637.4321 475.3788 391.2855 |
| 73# | 829.4965 | C42H72O13 | 16.21 | 1.212 | [M + COOH]- | 20(S)-Ginsenoside Rg2 | PG | 783.4904 637.4402 475.3792 457.3681 391.2857 |
| 74 | 1255.6327 | C58H98O26 | 16.28 | −0.108 | [M + COOH]- | Ginsenoside Ra2 | PG | 1209.6273 1077.5823 915.5300 783.4872 621.4367 459.3846 375.2906 |
| 75 | 683.438 | C36H62O9 | 16.33 | 0.606 | [M + COOH]- | 20(S)-Ginsenoside Rh1 | PG | 637.4297 475.3807 161.0449 |
| 76 | 1285.6429 | C59H100O27 | 16.43 | −0.389 | [M + COOH]- | Ginsenoside Ra3 | PG | 1239.6377 1107.5944 945.5367 783.4913 621.4368 459.3864 |
| 77# | 1153.6017 | C54H92O23 | 16.5 | 0.484 | [M + COOH]- | Ginsenoside Rb1 | PG | 1107.5952 945.5409 783.4985 621.4354 459.3831 |
| 78 | 683.4879 | C36H62O9 | 16.54 | 0.46 | [M + COOH]- | 20(R)-Ginsenoside Rh1 | PG | 637.4308 475.3776 243.5933 161.0444 |
| 79 | 989.5336 | C48H80O18 | 16.58 | 0.942 | [M + COOH]- | Quinquefoloside Le/Ld | PG | 943.5275 781.4734 618.6047 355.3042 |
| 80 | 829.4962 | C42H72O13 | 16.61 | 0.705 | [M + COOH]- | 20(R)-Ginsenoside Rg2 | PG | 783.4894 637.4335 475.3798 391.2856 |
| 81 | 1325.6384 | C62H102O30 | 17.15 | 0.065 | [M−H]- | Malonylginsenoside Ra3 | PG | 1239.6387 1221.6277 1107.5898 1077.5800 945.5466 789.2095 621.4324 375.2910 |
| 82 | 1153.6022 | C54H92O23 | 17.16 | 0.918 | [M + COOH]- | Ginsenoside Rb4/Re8 | PG | 1107.5955 945.5380 459.3853 |
| 83 | 683.4376 | C36H62O9 | 17.4 | 0.021 | [M + COOH]- | Ginsenoside F1 | PG | 637.4374 475.3787 391.2866 359.4645 |
| 84 | 1193.5973 | C57H94O26 | 17.55 | 1.042 | [M−H]- | Malonylginsenoside Rb1 | PG | 1107.5951 1089.5854 945.5410 783.4872 621.4354 537.3389 459.3838 |
| 85 | 955.491 | C48H76O19 | 17.7 | 0.206 | [M−H]- | Ginsenoside Ro | PG | 793.4349 613.3726 569.3881 523.3810 455.3516 |
| 86 | 1209.6276 | C58H98O26 | 17.98 | 0.202 | [M−H]- | Ginsenoside Ra1 | PG | 1077.5856 945.5435 783.4880 621.4350 459.3854 |
| 87# | 1123.5918 | C53H90O22 | 18.09 | 1.089 | [M + COOH]- | Ginsenoside Rc | PG | 1077.5848 945.5372 783.4900 621.4371 459.3848 375.2893 |
| 88 | 1209.6277 | C58H98O26 | 18.12 | 0.284 | [M−H]- | Notoginsenoside Fp2/Fc/FZ | PG | 1077.5834 945.5252 915.5354 783.4905 621.4402 459.3853 |
| 89 | 1193.5962 | C57H94O26 | 18.2 | 0.121 | [M−H]- | Malonylfloralginsenoside Rb1/Rb2 | PG | 1107.5944 1089.5839 987.5690 945.5416 783.4865 459.3839 |
| 90 | 1163.5859 | C56H92O25 | 18.82 | 0.351 | [M−H]- | Malonylginsenoside Rc | PG | 1077.5844 945.5419 915.5381 783.4935 621.4328 459.3820 |
| 91 | 1255.6333 | C58H98O26 | 18.86 | 0.37 | [M + COOH]- | Notoginsenoside Fp2/Fc/FZ | PG | 1209.6272 1077.5790 908.5573 621.4341 459.3869 |
| 92# | 1123.5919 | C53H90O22 | 19.55 | 1.178 | [M + COOH]- | Ginsenoside Rb2 | PG | 1077.5853 945.5428 783.4907 621.4377 459.3840 |
| 93 | 1193.5956 | C57H94O26 | 19.62 | −0.382 | [M−H]- | Malonylfloralginsenoside Rb1/Rb2 | PG | 1107.5959 1048.1158 945.5400 783.4918 459.3834 |
| 94 | 925.4812 | C47H74O18 | 19.91 | 1.039 | [M−H]- | Pseudoginsenoside Rt1 | PG | 763.4271 613.3738 455.3530 |
| 95 | 1123.5916 | C53H90O22 | 20.09 | 0.911 | [M + COOH]- | Ginsenoside Rb3 | PG | 1077.5848 945.5406 915.5377 783.4989 621.4362 459.3835 |
| 96 | 1163.5867 | C56H92O25 | 20.24 | 1.039 | [M−H]- | Malonylginsenoside Rb2 | PG | 1077.5848 945.5446 915.5311 783.4886 621.4409 459.3847 |
| 97 | 1255.6327 | C58H98O26 | 20.35 | −1.108 | [M + COOH]- | Notoginsenoside Fp2/Fc/FZ | PG | 1209.6271 1077.5830 945.5359 783.4948 621.4354 459.3856 |
| 98 | 989.5336 | C48H80O18 | 20.57 | 0.942 | [M + COOH]- | Quinquefoloside Le/Ld | PG | 943.5278 781.4705 457.3697 373.2743 |
| 99 | 1327.6542 | C61H102O28 | 20.71 | 0.177 | [M + COOH]- | Vesanchinoside J | PG | 1281.6461 1239.6394 1221.6276 1107.5925 945.5411 745.9520 564.1544 |
| 100 | 1195.6125 | C56H94O24 | 20.97 | 0.664 | [M + COOH]- | Quinquenoside R2/Yesanchinoside F | PG | 1149.6060 1107.5952 1089.5842 945.5493 783.4852 621.4340 459.3822 |
| 101 | 821.3975 | C42H62O16 | 21.08 | 1.207 | [M−H]- | Glycyrrhizic acid | LC | 561.8256 351.0570 193.0345 |
| 102 | 1163.5867 | C56H92O25 | 21.18 | 1.039 | [M−H]- | Malonylginsenoside Rb3 | PG | 1077.5851 945.5137 915.5367 783.4911 621.4338 459.3853 |
| 103# | 991.5497 | C48H82O18 | 21.22 | 1.394 | [M + COOH]- | Ginsenoside Rd | PG | 945.5433 783.4912 621.4348 459.3830 375.2910 |
| 104 | 1297.6437 | C60H100O27 | 21.22 | 0.231 | [M + COOH]- | Ginsenoside Ra5 | PG | 1251.6377 1191.6173 1077.5901 945.5460 915.5298 783.4849 621.4379 459.3847 |
| 105 | 793.4388 | C42H65O14 | 21.22 | 1.034 | [M−H]- | Chikusetsusaponin Ⅵa | PG | 631.3864 569.3839 455.3526 |
| 106 | 1165.6019 | C55H92O23 | 21.39 | 0.651 | [M + COOH]- | Acetylginsenoside Rc | PG | 1119.5957 1077.5850 945.5319 783.4935 621.4355 459.3840 |
| 107 | 1031.5448 | C51H84O21 | 21.51 | 1.52 | [M−H]- | Malonylfloralginsenoside Rd5 | PG | 945.5433 783.4907 621.4376 459.3843 |
| 108 | 991.5499 | C48H82O18 | 21.61 | 1.596 | [M + COOH]- | Notoginsenoside K | PG | 945.5428 783.4898 621.4308 459.3827 |
| 109 | 1165.6019 | C55H92O23 | 21.69 | 0.651 | [M + COOH]- | Acetylginsenoside Rb2 | PG | 1119.5962 1077.5852 945.5417 783.4894 621.4349 459.3828 |
| 110 | 1195.6127 | C56H94O24 | 21.72 | 0.831 | [M + COOH]- | Quinquenoside R4/Yesanchinoside F | PG | 1149.6067 1107.5955 945.5417 783.4963 621.4375 459.3866 |
| 111 | 1165.6021 | C55H92O23 | 21.9 | 0.823 | [M + COOH]- | Acetylginsenoside Rb3 | PG | 1119.5956 1077.5846 945.5437 783.4910 621.4347 459.3874 |
| 112 | 1221.6277 | C58H96O24 | 21.98 | 0.282 | [M + COOH]- | Ginsenoside Ra6 | PG | 1175.6254 1107.5951 945.5496 740.3268 459.3838 |
| 113 | 1165.6023 | C55H92O23 | 22.09 | 0.994 | [M + COOH]- | Ginsenoside Rs1/Rs2 | PG | 1119.5957 1077.5852 1059.5737 945.5395 915.5432 783.4889 621.4313 459.3859 |
| 114 | 1165.6021 | C55H92O23 | 22.42 | 0.832 | [M + COOH]- | Ginsenoside Rs1/Rs2 | PG | 1119.5957 1077.5847 1059.5741 945.5426 915.5307 783.4898 765.4787 621.4352 459.3841 |
| 115 | 797.47 | C41H68O12 | 22.89 | 0.903 | [M + COOH]- | Notoginsenoside LY | PG | 751.4631 619.4218 |
| 116 | 811.486 | C42H70O12 | 23.07 | 1.319 | [M + COOH]- | Ginsenoside Rg4/Rg6/Rz1 | PG | 765.4791 619.4196 537.1358 |
| 117 | 797.4699 | C41H68O12 | 23.18 | 0.778 | [M + COOH]- | Notoginsenoside T5 | PG | 751.4630 619.4222 457.3689 |
| 118 | 811.486 | C42H70O12 | 23.29 | 1.319 | [M + COOH]- | Ginsenoside Rg4/Rg6/Rz1 | PG | 765.4789 619.4229 471.2258 313.2404 |
| 119 | 811.4861 | C42H70O12 | 23.5 | 1.442 | [M + COOH]- | Ginsenoside Rg4/Rg6/Rz1 | PG | 765.4792 619.4230 |
| 120 | 665.4278 | C36H60O8 | 23.62 | 1.17 | [M + COOH]- | Ginsenoside Rk3 | PG | 619.4216 161.0446 |
| 121 | 665.4275 | C36H60O8 | 24.04 | 0.719 | [M + COOH]- | Ginsenoside Rh4 | PG | 619.4204 161.0446 |
| 122 | 793.4384 | C42H65O14 | 24.05 | 0.53 | [M−H]- | Zingibroside R1 | PG | 613.3741 569.3845 455.3533 |
| 123 | 829.4968 | C42H72O13 | 24.69 | 1.574 | [M + COOH]- | Ginsenoside F2 | PG | 783.4902 621.4359 459.3830 375.2095 |
| 124# | 829.4969 | C42H72O13 | 24.87 | 1.694 | [M + COOH]- | 20(S)-Ginsenoside Rg3 | PG | 783.4899 621.4357 459.3828 375.2894 |
| 125 | 829.4965 | C42H72O13 | 25.05 | 1.005 | [M + COOH]- | 20(R)-Ginsenoside Rg3 | PG | 783.4897 621.4373 459.3828 375.2913 |
| 126 | 341.1028 | C19H18O6 | 26.47 | −0.262 | [M−H]- | Methylophiopogonanone A | OJ | 206.0576 178.0626 163.0388 |
| 127 | 293.2119 | C18H30O3 | 26.51 | −1.085 | [M−H]- | HOTrE | LC | 275.2014 171.1015 |
| 128 | 811.4854 | C42H70O12 | 27.15 | 0.579 | [M + COOH]- | Ginsenoside Rk1 | PG | 765.4797 603.4258 319.0105 |
| 129 | 811.486 | C42H70O12 | 27.33 | 1.319 | [M + COOH]- | Ginsenoside Rg5 | PG | 765.4791 603.4272 453.0886 |
| 130 | 667.4434 | C36H62O8 | 27.73 | 1.092 | [M + COOH]- | 20(S)-Ginsenoside Rh2 | PG | 621.4393 459.3858 |
| 131 | 667.4432 | C36H62O8 | 28.1 | 0.792 | [M + COOH]- | 20(R)-Ginsenoside Rh2 | PG | 504.3103 |
| 132 | 485.3641 | C31H50O4 | 28.72 | 0.692 | [M−H]- | Tumulosic acid | PC | 423.3291 331.3891 180.2139 |
| 133 | 853.4957 | C44H72O13 | 28.75 | 0.241 | [M + COOH]- | Ginsenoside Rs5 | PG | 807.4891 765.4779 603.4246 |
| 134 | 485.3274 | C30H46O5 | 28.75 | 0.314 | [M−H]- | Poricoic acid G | PC | 441.3332 423.3265 351.2659 254.0528 |
| 135 | 853.4957 | C44H72O13 | 29.17 | 0.241 | [M + COOH]- | Ginsenoside Rs4 | PG | 807.4896 765.4786 603.4275 |
| 136 | 481.3328 | C31H46O4 | 30.63 | 0.97 | [M−H]- | Polyporenic acid C | PC | 403.9587 253.2169 152.9946 |
| 137 | 455.3533 | C30H48O3 | 32.24 | 0.508 | [M−H]- | Oleanic acid | PC | 261.8651 246.0458 201.1751 146.1933 129.5593 |
| 138 | 527.3739 | C33H52O5 | 32.5 | −0.565 | [M−H]- | Pachymic acid | PC | 444.3078 371.6741 228.7377 |
RG, Rehmanniae radix; PG, Ginseng radix et rhizoma rubra; AC, Asparagi radix; OJ, Ophiopogonis radix; LC, Lych cortex; PC, Poria.
3.1.1 Identification of ginsenosides in KSLP
Ginsenosides were the main active components of Ginseng radix et rhizoma rubra, which were mainly divided into protopanaxadiol (PPD), protopanaxatriol (PPT) and others according to their structural differences (Yang et al., 2014). The sapogenin fragments at m/z 459.38 (PPD) and m/z 475.38 (PPT) could be used as the diagnostic product ions to rapidly characterize ginsenosides of these two subtypes (Qiu et al., 2015, Li et al., 2021a, 2021b). Taking ginsenoside Rd as an example to illustrate the cleavage pathway of PPD, the excimer ion peak of m/z 991.5497 [M + COOH]− was easily generated in the negative ion mode. Typical neutral losses (NL) of HCOOH (46 Da) and Glu (162 Da) produced fragments of m/z 945.5433 [M−H]−, m/z 783.4912 [M−H−Glu]−、m/z 621.4348 [M−H−2Glu]− and m/z 459.3830 [M−H−3Glu]−. Taking the PPT type ginsenoside Re as an example, the quasimolecular ion peak of m/z 991.5497 [M + COOH]− was easily generated in the negative ion mode. Due to NL of HCOOH (46 Da), Glu (162 Da) and Rha (146 Da), fragments of m/z 945.5433 [M−H]−, m/z 799.4824 [M−H−Rha]−、m/z 783.4907 [M−H−Glu]− and m/z 637.4328 [M−H−Glu−Rha]− and m/z 475.3794 [M−H−2Glu−Rha]− were detected.
3.1.2 Identification of phenylethanol glycosides in KSLP
Phenylethanol glycosides, one of the bioactive components from Rehmanniae radix, possessed various pharmacological activities for human health. The fragmentation pathway was mainly related to breaking of ester bond or C-O bond, resulting in a series of degradation products. Taking acteoside for a witness, the excimer ion peak of m/z 623.1985 [M−H]− was easily generated in the negative ion mode. Acteoside produced ions at m/z 461.1662 [M−H−caffeoyl]− and m/z 315.1082 [M−H−caffeoyl−Rha]− through the loss of caffeoyl and Rha moiety. The caffeoyl moiety was produced ion at m/z 161.0234 [Caffeoyl-H-H2O]− by the loss of H2O (Qi et al., 2013).
3.2 Quantitative analysis of multi-components in KSLP by UPLC/QQQ-MS/MS
Combined with the qualitative analysis of chemical constituents in KSLP, and comprehensively considering its active components and characteristic components, the UPLC/QQQ-MS/MS method for the simultaneous determination of acteoside, isoacteoside, echinacoside, jionoside A1, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re, ginsenoside Rf, ginsenoside Rg1, ginsenoside Rg2 and ginsenoside Rg3 in KSLP was established, in order to provide reference for the quality control of KSLP.
3.2.1 Validation of analytical method
The chromatograms of blank solutions, standard solutions and sample solutions were displayed in Fig. S1. It could be seen from the chromatograms that 13 compounds had good peak shapes and no endogenous interference, indicating that the method was suitable. The linear regression equations, correlation coefficients (R2), linear ranges, LOD and LOQ of the 13 target compounds were listed in Table 2. The R2 of analytes were greater than 0.999, indicating good linearity, the LOD and LOQ of the 13 target compounds were the range of 0.02 ∼ 0.6 ng/mL and 0.08 ∼ 2.0 ng/mL, indicating that the method had high sensitivity. The test results of accuracy, precision and stability were summarized in Table 3. Intra-day and inter-day accuracy (RE%) ranged from −4.78 % to 4.74 %, intra-day and inter-day precision (RSD%) were within 7.16 % and stability (RSD%) was less than 4.75 %, indicating that the method was reproducible and accurate for the determination of analytes. The sample recovery rate was showed in Table S6, and it was between 90.27 ∼ 108.22 % (RSD% less than 5.54 %), indicating that no significant loss of analyte occurred during the analysis.
| Compound | Regression equations | R2 | Linear ranges(ng/mL) | LOD(ng/mL) | LOQ(ng/mL) |
|---|---|---|---|---|---|
| Acteoside | y = 734.92x + 57.755 | 0.9999 | 0.4 ∼ 100 | 0.02 | 0.08 |
| Isoacteoside | y = 937.50x − 6.9475 | 0.9999 | 0.4 ∼ 100 | 0.02 | 0.08 |
| Echinacoside | y = 139.96x + 62.214 | 0.9994 | 0.4 ∼ 100 | 0.06 | 0.20 |
| Jionoside A1 | y = 142.74x + 55.328 | 0.9996 | 0.4 ∼ 100 | 0.06 | 0.20 |
| Ginsenoside Rb1 | y = 29.248x + 99.106 | 0.9994 | 4 ∼ 1000 | 0.06 | 0.20 |
| Ginsenoside Rb2 | y = 39.981x + 7.6629 | 0.9999 | 4 ∼ 1000 | 0.06 | 0.20 |
| Ginsenoside Rc | y = 19.805x − 1.3026 | 0.9998 | 4 ∼ 1000 | 0.60 | 2.00 |
| Ginsenoside Rd | y = 26.910x − 119.27 | 0.9997 | 4 ∼ 1000 | 0.24 | 0.80 |
| Ginsenoside Re | y = 24.074x + 22.586 | 0.9995 | 4 ∼ 1000 | 0.60 | 2.00 |
| Ginsenoside Rf | y = 112.71x + 165.65 | >0.9999 | 4 ∼ 1000 | 0.60 | 2.00 |
| Ginsenoside Rg1 | y = 14.476x − 49.816 | >0.9999 | 4 ∼ 1000 | 0.24 | 0.80 |
| Ginsenoside Rg2 | y = 111.03x + 40.289 | 0.9997 | 0.4 ∼ 100 | 0.24 | 0.80 |
| Ginsenoside Rg3 | y = 87.747x + 17.923 | 0.9998 | 0.4 ∼ 100 | 0.24 | 0.80 |
| Compound | QC (ng/mL) | Intra-day | Inter-day | Stability | |||||
|---|---|---|---|---|---|---|---|---|---|
| ‾X ± S (ng/mL) | RE (%) | RSD (%) | ‾X ± S (ng/mL) | RE (%) | RSD (%) | ‾X ± S (ng/mL) | RSD (%) | ||
| Acteoside | 1 | 0.96 ± 0.06 | −3.70 | 7.16 | 0.96 ± 0.04 | −3.59 | 4.76 | 0.97 ± 0.02 | 2.40 |
| 10 | 9.52 ± 0.11 | −4.78 | 1.47 | 9.54 ± 0.09 | −4.65 | 1.00 | 10.08 ± 0.39 | 4.75 | |
| 80 | 82.77 ± 1.06 | 3.46 | 1.57 | 83.3 ± 1.53 | 4.13 | 2.01 | 81.97 ± 2.34 | 3.50 | |
| Isoacteoside | 1 | 0.96 ± 0.04 | −4.42 | 4.94 | 0.96 ± 0.03 | −4.47 | 3.32 | 0.99 ± 0.03 | 3.20 |
| 10 | 9.56 ± 0.05 | −4.37 | 0.65 | 9.61 ± 0.07 | −3.93 | 0.84 | 9.52 ± 0.05 | 0.63 | |
| 80 | 79.83 ± 0.60 | −0.22 | 0.93 | 78.92 ± 1.12 | −1.35 | 1.56 | 79.28 ± 0.75 | 1.15 | |
| Echinacoside | 1 | 0.97 ± 0.02 | −3.23 | 2.50 | 0.97 ± 0.02 | −3.33 | 2.02 | 0.95 ± 0.01 | 1.12 |
| 10 | 9.55 ± 0.05 | −4.47 | 0.64 | 9.70 ± 0.32 | −3.02 | 3.57 | 10.15 ± 0.09 | 1.04 | |
| 80 | 80.12 ± 1.42 | 0.14 | 2.17 | 81.53 ± 2.91 | 1.91 | 3.91 | 80.76 ± 1.55 | 2.36 | |
| Jionoside A1 | 1 | 0.96 ± 0.02 | −3.66 | 2.71 | 0.97 ± 0.03 | −3.18 | 2.96 | 0.99 ± 0.03 | 3.92 |
| 10 | 10.47 ± 0.36 | 4.74 | 4.21 | 10.31 ± 0.38 | 3.06 | 4.00 | 10.28 ± 0.20 | 2.42 | |
| 80 | 81.67 ± 2.02 | 2.09 | 3.03 | 82.12 ± 2.33 | 2.66 | 3.11 | 80.24 ± 0.86 | 1.32 | |
| Ginsenoside Rb1 | 10 | 9.57 ± 0.05 | −4.28 | 0.68 | 9.58 ± 0.10 | −4.17 | 1.11 | 9.67 ± 0.13 | 1.69 |
| 100 | 97.13 ± 3.50 | −2.87 | 4.41 | 96.33 ± 2.66 | −3.67 | 3.03 | 102.65 ± 1.42 | 1.70 | |
| 800 | 814.85 ± 12.49 | 1.86 | 1.88 | 811.38 ± 10.06 | 1.42 | 1.36 | 806.27 ± 3.25 | 0.49 | |
| Ginsenoside Rb2 | 10 | 9.80 ± 0.18 | −2.01 | 2.27 | 9.84 ± 0.15 | −1.58 | 1.72 | 9.70 ± 0.21 | 2.70 |
| 100 | 96.76 ± 3.10 | −3.24 | 3.92 | 96.82 ± 3.49 | −3.18 | 3.95 | 104.31 ± 0.59 | 0.69 | |
| 800 | 810.68 ± 10.44 | 1.34 | 1.58 | 807.40 ± 9.54 | 0.93 | 1.29 | 801.42 ± 1.43 | 0.22 | |
| Ginsenoside Rc | 10 | 9.85 ± 0.22 | −1.49 | 2.74 | 9.73 ± 0.24 | −2.73 | 2.71 | 9.63 ± 0.26 | 3.35 |
| 100 | 102.35 ± 1.82 | 2.35 | 2.17 | 102.44 ± 2.39 | 2.44 | 2.56 | 104.61 ± 1.23 | 1.44 | |
| 800 | 814.97 ± 4.95 | 1.87 | 0.74 | 809.70 ± 6.46 | 1.21 | 0.87 | 808.47 ± 3.03 | 0.46 | |
| Ginsenoside Rd | 10 | 10.46 ± 0.15 | 4.59 | 1.76 | 10.32 ± 0.23 | 3.21 | 2.41 | 10.35 ± 0.20 | 2.36 |
| 100 | 96.07 ± 1.25 | −3.93 | 1.59 | 98.97 ± 3.65 | −1.03 | 4.04 | 100.17 ± 2.74 | 3.35 | |
| 800 | 804.42 ± 6.53 | 0.55 | 0.99 | 806.89 ± 6.41 | 0.86 | 0.87 | 805.46 ± 8.82 | 1.34 | |
| Ginsenoside Re | 10 | 9.59 ± 0.24 | −4.14 | 3.12 | 9.65 ± 0.22 | −3.53 | 2.47 | 9.57 ± 0.11 | 1.47 |
| 100 | 96.19 ± 4.05 | −3.81 | 5.15 | 96.6 ± 3.29 | −3.40 | 3.73 | 103.49 ± 1.00 | 1.18 | |
| 800 | 815.46 ± 6.46 | 1.93 | 0.97 | 812.48 ± 8.61 | 1.56 | 1.16 | 800.94 ± 15.22 | 2.33 | |
| Ginsenoside Rf | 10 | 9.61 ± 0.29 | −3.85 | 3.74 | 9.78 ± 0.34 | −2.18 | 3.75 | 9.75 ± 0.16 | 2.07 |
| 100 | 97.70 ± 3.09 | −2.30 | 3.87 | 96.55 ± 3.56 | −3.45 | 4.04 | 102.97 ± 1.29 | 1.53 | |
| 800 | 823.39 ± 2.63 | 2.92 | 0.39 | 816.5 ± 7.94 | 2.06 | 1.07 | 804.55 ± 2.31 | 0.35 | |
| Ginsenoside Rg1 | 10 | 10.27 ± 0.15 | 2.70 | 1.77 | 10.18 ± 0.17 | 1.76 | 1.82 | 10.37 ± 0.38 | 4.50 |
| 100 | 101.86 ± 3.81 | 1.86 | 4.58 | 102.36 ± 3.78 | 2.36 | 4.04 | 102.11 ± 1.65 | 1.98 | |
| 800 | 803.33 ± 0.70 | 0.42 | 0.11 | 805.61 ± 4.82 | 0.70 | 0.65 | 812.23 ± 5.56 | 0.84 | |
| Ginsenoside Rg2 | 1 | 0.98 ± 0.02 | −2.15 | 2.19 | 0.98 ± 0.02 | −2.25 | 2.24 | 0.94 ± 0.03 | 3.85 |
| 10 | 9.83 ± 0.30 | −1.70 | 3.72 | 9.72 ± 0.35 | −2.78 | 3.99 | 10.63 ± 0.24 | 2.74 | |
| 80 | 83.29 ± 2.07 | 4.11 | 3.05 | 82.56 ± 2.00 | 3.20 | 2.65 | 80.69 ± 0.54 | 0.81 | |
| Ginsenoside Rg3 | 1 | 0.97 ± 0.04 | −3.03 | 4.67 | 0.97 ± 0.03 | −3.22 | 3.32 | 0.96 ± 0.03 | 3.22 |
| 10 | 9.63 ± 0.36 | −3.71 | 4.54 | 9.92 ± 0.39 | −0.83 | 4.26 | 9.66 ± 0.27 | 3.41 | |
| 80 | 82.56 ± 2.50 | 3.21 | 3.70 | 81.52 ± 2.14 | 1.90 | 2.88 | 81.41 ± 1.92 | 2.89 | |
3.2.2 Quantitative analysis of multi-components in KSLP
The effects of various extraction conditions on the yield of target compounds were represented in Table S3 and Table S4. The yield of crude components extracted by ultrasonic and reflux was not much different, and the total content of target compounds extracted by ultrasonic was slightly higher than that of reflux. With the increase of the ethanol content, the crude components yield showed a trend of first increasing and then decreasing. When the ethanol concentration reached 50 %, the total content of target compounds was higher than that of other concentration group. The yield of crude components increased slowly with increasing extraction time, eventually reached stability at 90 min, indicating that extraction time has a significant positive effect on yield when it is below 90 min. There was no significant change in the yield when extraction time was longer than 90 min. There was a gradual increase in the yield of crude components with increasing solid-to-liquid ratio. The yield increased slightly when the solid-to-liquid ratio was greater than 1:100 g/mL, however, a large solid-to-liquid ratio caused solvent wastage, 1:100 g/mL was selected as the optimal solid-to-liquid ratio. Therefore, 50 % ethanol, ultrasonic, 90 min and 1:100 g/mL were the optimal process for extracting crude components of KSLP based on the criterion of the maximum total content of the 13 target compounds, combined with economic considerations.
According to the established UPLC/QQQ-MS/MS quantitative analysis method and extraction process conditions, the content of 13 compounds in KSLP was determined. Among 13 analytes, the compounds with higher content (>1000 μg/g) were ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Re and ginsenoside Rg1.
3.3 Pharmacokinetic analysis of multi-components in KSLP by UPLC/QQQ-MS/MS
The composition of TCM is complex, and the blood concentration in the body is low, which is not easy to detect. In the previous pilot experiment, the rats were gavaged with the largest dose of KSLP, and no compounds could be detected in the plasma. Therefore, KSLP was further refined to obtain the refined components in formal experiments. According to the UPLC/QQQ-MS/MS quantitative analysis method established in part 2.3, the effects of different fillers on the yield of refined components could be seen in Table S5. After being refined by resin D101, resin AB-8 and ODS, the total contents of the 13 target compounds were not much different in the refined components of KSLP, and the yields of refined components were 13.19 %, 10.10 % and 10.69 % respectively. Although resins D101 and AB-8 retained the ginsenosides well, most of the phenylethanol glycosides were lost due to its large polarity when eluted with water. ODS not only enriched the ginsenosides but also retained the phenylethanol glycosides with greater polarity. Therefore, ODS was selected to refine the crude components of KSLP. The total content of the 13 target compounds in the refined components was 5 times higher than that of the crude components. The UPLC/QQQ-MS/MS was used to determine plasma after administration of refined components of KSLP, and a total of 6 compounds were detected, including ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re and ginsenoside Rg1.
3.3.1 Validation of analytical method
The chromatograms of blank plasma solutions, standard plasma solutions and sample plasma solutions were plotted in Fig. S2, all the peaks of the analytes and IS were detected with excellent resolutions as well as shapes. The endogenous substances in the plasma did not interfere with the determination of each compound and IS, suggesting that the method developed in this study had good selectivity. The 6 target compounds had a good linear relationship within the corresponding concentration range, and its LLOQ were 4.0 ng/mL. The statistical parameters of calibration were listed in Table S7. As exhibited in Table 4, intra-day and inter-day accuracy (RE%) was between −12.22 ∼ 11.89 %, intra-day and inter-day precision (RSD%) were within 7.22 %, the extraction recovery and matrix effect of 6 compounds ranged from 88.02 % to 109.78 %, it showed that the instrument had good precision, the method had high repeatability. The stability results in Table S8 indicated that the 6 compounds in rat plasma were stable with RSD < 14.61 % for autosampler for 24 h, three freeze–thaw cycles.
| Compound | QC (ng/mL) | Intra-day | Inter-day | Extraction recovery and matrix effect | |||||
|---|---|---|---|---|---|---|---|---|---|
| ‾X ± S (ng/mL) | RE (%) | RSD (%) | ‾X ± S (ng/mL) | RE (%) | RSD (%) | Extractionrecovery (%) | Matrixeffect (%) | ||
| Ginsenoside Rb1 | 10 | 11.19 ± 0.48 | 11.89 | 4.32 | 11.09 ± 0.40 | 10.92 | 3.56 | 98.68 ± 3.47 | 100.46 ± 5.83 |
| 100 | 88.18 ± 3.88 | −11.82 | 4.40 | 89.03 ± 3.67 | −10.97 | 4.13 | 89.80 ± 3.65 | 107.69 ± 5.53 | |
| 800 | 766.31 ± 26.96 | −4.21 | 3.52 | 762.72 ± 22.43 | −4.66 | 2.94 | 89.68 ± 3.09 | 103.81 ± 5.55 | |
| Ginsenoside Rb2 | 10 | 10.44 ± 0.59 | 4.45 | 5.66 | 10.20 ± 0.54 | 1.97 | 5.32 | 95.60 ± 3.30 | 105.43 ± 1.89 |
| 100 | 88.65 ± 2.97 | −11.35 | 3.35 | 87.78 ± 3.11 | −12.22 | 3.54 | 93.18 ± 5.65 | 106.33 ± 4.15 | |
| 800 | 766.08 ± 28.71 | −4.24 | 3.75 | 754.13 ± 33.48 | −5.73 | 4.44 | 89.93 ± 3.93 | 99.72 ± 3.97 | |
| Ginsenoside Rc | 10 | 10.28 ± 0.70 | 2.82 | 6.79 | 10.25 ± 0.67 | 2.46 | 6.51 | 91.93 ± 3.33 | 108.45 ± 3.07 |
| 100 | 91.32 ± 0.86 | −8.68 | 0.95 | 90.75 ± 3.21 | −9.25 | 3.54 | 97.20 ± 5.10 | 103.49 ± 8.33 | |
| 800 | 750.49 ± 11.51 | −6.38 | 1.99 | 749.00 ± 14.89 | −6.19 | 1.53 | 97.87 ± 8.87 | 102.47 ± 8.38 | |
| Ginsenoside Rd | 10 | 10.21 ± 0.31 | 2.08 | 3.03 | 10.58 ± 0.16 | 5.79 | 1.53 | 92.09 ± 4.69 | 107.14 ± 6.85 |
| 100 | 94.43 ± 6.82 | −5.57 | 7.22 | 94.26 ± 5.57 | −5.74 | 5.91 | 88.95 ± 1.19 | 102.00 ± 8.56 | |
| 800 | 749.05 ± 10.39 | −6.37 | 1.39 | 743.23 ± 14.4 | −7.10 | 1.94 | 91.94 ± 6.50 | 109.78 ± 5.44 | |
| Ginsenoside Re | 10 | 10.60 ± 0.42 | 6.02 | 3.95 | 10.48 ± 0.51 | 4.81 | 4.89 | 99.07 ± 5.81 | 89.97 ± 1.18 |
| 100 | 93.42 ± 3.89 | −6.58 | 4.16 | 96.24 ± 2.44 | −3.76 | 2.53 | 92.99 ± 4.65 | 105.30 ± 6.42 | |
| 800 | 778.67 ± 21.17 | −2.67 | 2.72 | 775.07 ± 18.73 | −3.12 | 2.42 | 96.45 ± 11.02 | 95.03 ± 5.54 | |
| Ginsenoside Rg1 | 10 | 10.25 ± 0.61 | 2.47 | 5.95 | 10.73 ± 0.19 | 7.26 | 1.78 | 102.09 ± 3.93 | 88.02 ± 3.57 |
| 100 | 97.27 ± 1.59 | −2.73 | 1.63 | 98.67 ± 3.49 | −1.33 | 3.53 | 90.93 ± 7.16 | 97.20 ± 6.47 | |
| 800 | 778.68 ± 14.15 | −2.67 | 1.82 | 776.02 ± 12.72 | −3.00 | 1.64 | 90.34 ± 4.77 | 95.87 ± 1.17 | |
3.3.2 Determination of the content of KSLP in plasma and pharmacokinetic parameter fitting
The plasma drug concentration and time curve of refined components of KSLP after intragastric administration for 72 h with a single dose in rats was presented in Fig. 2, and the pharmacokinetic parameters were summarized in Table 5. The maximum plasma concentration (Cmax) of ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc and ginsenoside Rd was about 100 ng/mL, while the Cmax of ginsenoside Re and ginsenoside Rg1 was about 40 ng/mL. All compounds took long time to their elimination t1/2, indicating that the elimination rate of the target compounds in vivo was slow. Also, the AUC0∼∞ of ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc and ginsenoside Rd was greater than 4500 ng/ml·h, while the AUC0∼∞ of ginsenoside Re and ginsenoside Rg1 was about 2500 ng/ml·h.
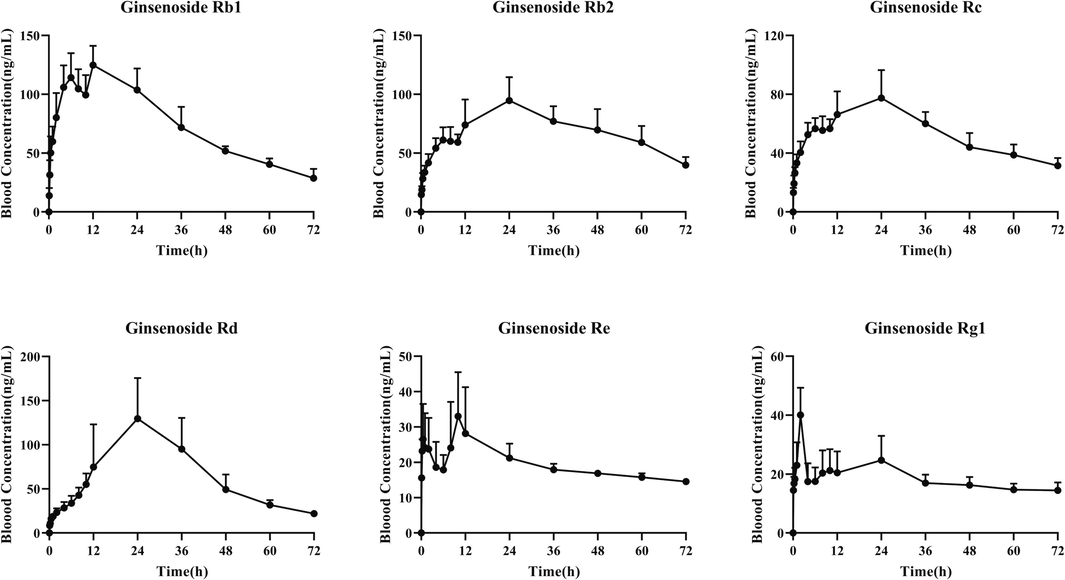
- Plasma drug concentration and time curve of refined components of KSLP in rats after intragastric administration (n = 6).
| Compound | Ginsenoside Rb1 | Ginsenoside Rb2 | Ginsenoside Rc | Ginsenoside Rd | Ginsenoside Re | Ginsenoside Rg1 |
|---|---|---|---|---|---|---|
| T1/2 (h) | 31.672 ± 17.692 | 47.631 ± 10.293 | 37.464 ± 5.061 | 22.997 ± 10.598 | 148.83 ± 64.087 | 67.019 ± 38.726 |
| Tmax (h) | 8.333 ± 3.445 | 24 ± 0 | 22 ± 4.899 | 24 ± 0 | 10.333 ± 1.506 | 2 ± 0 |
| Cmax (ng/mL) | 131.342 ± 13.055 | 94.667 ± 19.846 | 79.147 ± 15.999 | 129.622 ± 46.008 | 41.023 ± 12.471 | 40.079 ± 9.236 |
| AUC0-t (ng/mL*h) | 5297.488 ± 559.59 | 4935.666 ± 876.227 | 3840.482 ± 578.394 | 4700.077 ± 1334.759 | 1402.143 ± 178 | 1342.658 ± 185.234 |
| AUC0-∞ (ng/mL*h) | 6746.532 ± 1047.45 | 8285.218 ± 2023.524 | 5523.503 ± 759.415 | 5445.909 ± 1401.581 | 4500.245 ± 1315.926 | 2539.865 ± 574.874 |
| Vz/F (L/kg) | 26065.292 ± 11112.873 | 33789.23 ± 5310.405 | 40093.334 ± 9621.342 | 25723.978 ± 12221.349 | 184037.526 ± 29842.507 | 144175.903 ± 51476.184 |
| CLz/F (L/h/kg) | 604.993 ± 93.639 | 512.833 ± 153.38 | 738.339 ± 124.228 | 778.744 ± 212.756 | 967.909 ± 334.351 | 1643.17 ± 367.034 |
| MRT0-t (h) | 28.101 ± 0.832 | 34.367 ± 1.5 | 32.2 ± 1.353 | 31.718 ± 2.194 | 32.356 ± 2.351 | 32.517 ± 1.426 |
| MRT0-∞ (h) | 48.737 ± 19.743 | 68.882 ± 14.101 | 61.403 ± 6.679 | 42.874 ± 8.795 | 210.355 ± 87.169 | 113.409 ± 57.93 |
4 Discussion
With its rich resources and unique curative effects, TCM has attracted the attention of many countries around the world, and has been gradually accepted, researched, developed and utilized. The composition of TCM is complex and diverse, quality control is difficult, and traditional analysis methods can’t longer meet the requirements of quality analysis and evaluation of TCM (Zhao et al., 2018). The chemical composition of KSLP was comprehensively characterized, and the results showed that it contained a large number of ginsenosides, phenylethanol glycosides and iridoids. Ginsenosides were the main chemical components of Ginseng radix et rhizoma rubra, and studies have shown that total ginsenosides of Ginseng radix et rhizoma rubra could produce an anti-aging effect by intervening in the lipid metabolism and correcting the amino acid metabolism disorders in aging rats (Sun et al., 2018). Phenylethanol glycosides and iridoids were the most abundant compounds in Rehmanniae radix. Studies have shown that echinacoside and catalpol could delay aging and prevent the development of age-related diseases (Zhang et al., 2008, Shen et al., 2017, Chen et al., 2018).
The complexity of TCM components determines that it is difficult to comprehensively characterize the quality of TCM in the determination of only a single compound (Luo et al., 2013, Xiong et al., 2020). The quantitative analysis of multi-components has become the development direction of quality evaluation of TCM (Wang et al., 2020, Li et al., 2021a, 2021b). The quality evaluation of TCM containing ginsenosides has always been difficult. Ginsenosides lack functional groups with strong ultraviolet absorption, resulting in inaccurate quantitative results of samples, so the detection has certain limitations. Therefore, the UPLC/QQQ-MS/MS technology equipped with electrospray ionization was used to establish a method for simultaneous detection of multi-components in KSLP. The process of extraction is the core link in the field of pharmacodynamic substances and quality control of TCM (Zhang et al., 2018). Optimizing the parameters of extraction of TCM is the premise of efficient and sufficient extraction of pharmacodynamic substances and ensuring clinical effectiveness (Tang et al., 2022, Chang et al., 2023). Under the optimal extraction conditions, 13 represenative compounds of KSLP were simultaneously quantified by UPLC/QQQ-MS/MS.
By further refining the refined components in KSLP, it was used for the study of highly exposed in vivo components. The UPLC/Q-Orbitrap-MS/MS technology was used for the determination of plasma after administration, and the results showed that no compounds were detected. We speculate that ginsenosides have the characteristics of large molecular weight, poor membrane permeability, and unstable chemical structure that can be degraded by hydrolases in the gastrointsteinal tract, these characteristics lead to its low bioavailability (Xiong et al., 2009, Li et al., 2011, Dai et al., 2016). MRM monitoring mode can detect and analyze specific compounds with strong specificity and high sensitivity (Ren et al., 2022). The MRM acquisition method of UPLC/QQQ-MS/MS technology was used to detect plasma after administration, and a total of 6 compounds were detected, including ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re and ginsenoside Rg1. Studies have found that ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re and ginsenoside Rg1 account for more than 90 % of the total ginsenosides, and were called non-rare ginsenosides because of their higher content (Wang et al., 2006). The pharmacokinetic results showed that the absorption and elimination of ginsenosides in the refined components of KSLP were relatively slow. This may be related to the complexity of the components of TCM and their interaction transformation in vivo (Akao et al., 1998, Bae et al., 2002).
In the present study, a total of 138 compounds were identified, which preliminarily clarified the material basis of KSLP. Subsequently, 13 representative compounds of KSLP were simultaneously quantified, which could be beneficial to improve the quality control. Finally, a specific and sensitive method was established for the simultaneous quantification of 6 compounds in the rat plasma after gavage administration of refining components of KSLP, which provided a reference for its clinical application.
CRediT authorship contribution statement
Chengjuan Liu: Conceptualization, Methodology, Investigation, Writing – original draft. Qibao Jiang: Supervision, Data curation, Resources. Zhirong Zhou: Supervision, Data curation, Resources. Peng Lei: Supervision, Data curation, Resources. Peng Zhang: Data curation. Xin Chai: Data curation. Guixiang Pan: Data curation. Yuefei Wang: Data curation. Miaomiao Jiang: Project administration, Funding acquisition, Writing – review & editing.
Acknowledgements
This study was supported by Science and Technology Project of Haihe Laboratory of Modern Chinese Medicine (Nos. 22HHZYJC00003 and 22HHZYJC00007).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb-1 from Panax ginseng. J. Pharm. Pharmacol.. 1998;50:1155-1160.
- [CrossRef] [Google Scholar]
- How environment and lifestyle choices influence the aging process. Ann. Plast. Surg.. 1999;43:585-588.
- [CrossRef] [Google Scholar]
- Metabolism of ginsenoside R-c by human intestinal bacteria and its related antiallergic activity. Biol. Pharm. Bull.. 2002;25:743-747.
- [CrossRef] [Google Scholar]
- An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug Discov. Today. 2007;12:218-224.
- [CrossRef] [Google Scholar]
- Optimization of extraction process of Dioscorea nipponica Makino saponins and their UPLC-QTOF-MS profiling, antioxidant, antibacterial and anti- inflammatory activities. Arab. J. Chem.. 2023;16
- [CrossRef] [Google Scholar]
- Echinacoside, a phenylethanoid glycoside from Cistanche deserticola, extends lifespan of Caenorhabditis elegans and protects from A beta-induced toxicity. Biogerontology. 2018;19:47-65.
- [CrossRef] [Google Scholar]
- Ginsenoside nanoparticle: a new green drug delivery system. J. Mater. Chem. B. 2016;4:529-538.
- [CrossRef] [Google Scholar]
- Profiling the mid-adult cecal microbiota associated with host healthy by using herbal formula Kang Shuai Lao Pian treated mid-adult mice. Chin. J. Nat. Med.. 2020;18:90-102.
- [CrossRef] [Google Scholar]
- Traditional Chinese medicine formula Kang Shuai Lao Pian improves obesity, gut dysbiosis, and fecal metabolic disorders in high-fat diet-fed mice. Front. Pharmacol.. 2020;11
- [CrossRef] [Google Scholar]
- MS-based metabolite profiling of aboveground and root components of Zingiber mioga and officinale. Molecules. 2015;20:16170-16185.
- [CrossRef] [Google Scholar]
- The influence of subjective health status, post-traumatic growth, and social support on successful aging in middle-aged women. Journal of Korean Academy of Nursing.. 2016;46:744-752.
- [CrossRef] [Google Scholar]
- Identification of 20(S)-Protopanaxadiol Metabolites in Human Liver Microsomes and Human Hepatocytes. Drug Metab. Dispos.. 2011;39:472-483.
- [CrossRef] [Google Scholar]
- Advances and challenges in screening traditional Chinese anti-aging materia medica. Chin. J. Integr. Med.. 2013;19:243-252.
- [CrossRef] [Google Scholar]
- Integrating chemical profiling and network pharmacology analysis based on anti-inflammatory effects for quality control of Scutellaria barbata. Phytochem. Anal. 2021;32:1141-1151.
- [CrossRef] [Google Scholar]
- Ultra-high performance liquid chromatography/ion mobility time-of-flight mass spectrometry-based untargeted metabolomics combined with quantitative assay unveiled the metabolic difference among the root, leaf, and flower bud of Panax notoginseng. Arab. J. Chem.. 2021;14
- [CrossRef] [Google Scholar]
- Qualitative and quantitative analysis of major constituents from Dazhu Hongjingtian capsule by UPLC/Q-TOF-MS/MS combined with UPLC/QQQ-MS/MS. Biomed. Chromatogr.. 2017;31
- [CrossRef] [Google Scholar]
- dTGS: method for effective components identification from traditional Chinese medicine formula and mechanism analysis. Evid. Based Complement. Alternat. Med.. 2013;2013
- [CrossRef] [Google Scholar]
- DNA methylation-based age clocks: from age prediction to age reversion. Ageing Res. Rev.. 2021;68
- [CrossRef] [Google Scholar]
- Identification of acteoside and its major metabolites in rat urine by ultra-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. b.. 2013;940:77-85.
- [CrossRef] [Google Scholar]
- A green protocol for efficient discovery of novel natural compounds: Characterization of new ginsenosides from the stems and leaves of Panax ginseng as a case study. Anal. Chim. Acta. 2015;893:65-76.
- [CrossRef] [Google Scholar]
- Qualitative and quantitative analysis of multi-components in Xing-Su-Ning Capsules for quality improvement. Arab. J. Chem.. 2022;15
- [CrossRef] [Google Scholar]
- Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol.. 2017;174:1395-1425.
- [CrossRef] [Google Scholar]
- Anti-ageing effect of red ginseng revealed by urinary metabonomics using RRLC-Q-TOF-MS. Phytochem. Anal. 2018;29:387-397.
- [CrossRef] [Google Scholar]
- Ultrasound-assisted extraction of Cordyceps cicadae polyphenols: Optimization, LC-MS characterization, antioxidant and DNA damage protection activity evaluation. Arab. J. Chem.. 2022;15
- [CrossRef] [Google Scholar]
- Revealing the mechanisms and the material basis of Rubia cordifolia L. on abnormal uterine bleeding with uniting simultaneous determination of four components and systematic pharmacology approach-experimental validation. J. Pharm. Biomed. Anal.. 2020;189
- [CrossRef] [Google Scholar]
- Simultaneous determination of ginsenosides in Panax ginseng with different growth ages using high-performance liquid chromatography-mass spectrometry. Phytochem. Anal. 2006;17:424-430.
- [CrossRef] [Google Scholar]
- Active absorption of ginsenoside Rg1 in vitro and in vivo: the role of sodium-dependent glucose co-transporter 1. J. Pharm. Pharmacol.. 2009;61:381-386.
- [CrossRef] [Google Scholar]
- Discovery of quality-marker ingredients of Panax quinquefolius driven by high-throughput chinmedomics approach. Phytomedicine. 2020;74
- [CrossRef] [Google Scholar]
- Saponins in the genus Panax L. (Araliaceae): a systematic review of their chemical diversity. Phytochemistry. 2014;106:7-24.
- [CrossRef] [Google Scholar]
- Novel mathematic models for quantitative transitivity of quality-markers in extraction process of the Buyanghuanwu decoction. Phytomedicine. 2018;45:68-75.
- [CrossRef] [Google Scholar]
- Recent advances in pharmacokinetics approach for herbal medicine. RSC Adv.. 2017;7:28876-28888.
- [CrossRef] [Google Scholar]
- Further pharmacological evidence of the neuroprotective effect of catalpol from Rehmannia glutinosa. Phytomedicine. 2008;15:484-490.
- [CrossRef] [Google Scholar]
- Anti-aging role of Chinese herbel medicine: an overview of scientific evidence from 2008 to 2018. Ann. Palliat. Med.. 2020;9:1230-1248.
- [CrossRef] [Google Scholar]
- Advanced strategies for quality control of Chinese medicines. J. Pharm. Biomed. Anal.. 2018;147:473-478.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105398.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







