Studies on effective catalytic conversion of xylose to furfural using green sulfonated carbon catalysts: Process optimization by Taguchi approach
⁎Corresponding author at: Department of Chemical and Petroleum Engineering, College of Engineering, Afe Babalola University, km 8.5, Afe Babalola Way, Ado-Ekiti, Ekiti State, Nigeria. yusuffas@abuad.edu.ng (Adeyinka Sikiru Yusuff)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Herein, furfural was synthesized via dehydration of xylose with γ-valerolactone (GVL) in the presence of sulfonated carbon catalysts prepared from eucalyptus activated carbon (EAC) and sulfonating agents (H2SO4 and TsOH). Influences of pure and mixed sulfonating agents on physicochemical attributes of the obtained sulfonated carbon catalysts were explored using different characterization techniques (BET, FTIR, NH3-TPD, CHNS, XRD, TGA, SEM and total acid density). The process input variables (temperature, solvent/substrate ratio, time and catalyst loading) influencing the dehydration process were optimized using Taguchi design approach. The best H2SO4 (H)/TsOH (T) molar ratio for the mixed acid sulfonated EAC (EAC-H-T) material to catalyze xylose conversion to furfural was 3:2. The total acid density (0.79 ± 0.08 mmol/g), specific surface area (711.9 m2/g) and sulfur concentration (9.77%) of the EAC-3H-2T catalyst were higher compared to other sulfonated EAC materials. Taguchi optimization approach revealed that highest furfural yield (74.61 ± 0.05 %) was achieved at the optimum conditions of 180 °C dehydration temperature, 1.5 wt% catalyst loading, 3.0 h dehydration time and 3 mL/g GVL/xylose ratio. 1H- and 13C NMR analyses conducted on isolated product obtained under optimum conditions confirmed the formation of furfural. In addition, the EAC-3H-2T catalyst exhibited sustained activity after it was reused for six times.
Keywords
Xylose
Carbon-based acid catalyst
Furfural
Dehydration
Optimization
Sulfonation
1 Introduction
Various chemicals and fuels are being produced from fossil resources (crude oil, coal and natural gas) for society use, but the non-renewable products may not be able to meet market demand in the near future due to fossil fuel depletion, over population and increase in energy consumption. The utilization of renewable raw materials has gained much interest in recent times as it promotes sustainable fine chemical synthesis and clean energy supply (Naik et al., 2010; Ragauskas et al., 2006). Currently, lignocellulosic-based biomasses, which are the most abundant renewable materials, are being used as alternative raw materials to produce both fine chemicals and fuels through bioconversion/biorefinery technologies (Xu et al., 2021). Some of the compounds that can be made from lignocellulosic biomass include furfural, levulinic acid, 5-hydroxymethylfurfural and ethanol (Xu et al., 2021; Melikoglu et al., 2016; Liu et al., 2013; Morone et al., 2015).
Due to its potential platform to produce renewable fuels, furfural is regarded as one of the most promising chemicals that may be obtained from biomass rich in hemicellulose (Chung et al., 2021). Furfural, a naturally-occurring furan aldehyde, is synthesized via acid-catalyzed dehydration of C5 hydrocarbon (monosaccharide of the aldopentose type such as xylose). Industrially, furfural is made by sequentially dehydrating hemicellulose, which is the main constituent of biomass, in one step with a mineral acid (HCl or H2SO4) as catalyst. However, synthesis and separation of furfural forms a huge quantity of gas, residue and wastewater, leading to equipment corrosion and high cost of maintenance (Xu et al., 2021). Additionally, catalyst reusability is practically impossible since the liquid catalyst is miscible with reactant and solvent. Some researchers suggest that heterogeneous catalysts, which can prevent corrosion, promote environmentally friendly processes, facilitate easy separation of products from reaction mixtures and enable catalyst reusability, could help solve those aforementioned drawbacks (Jia et al., 2019). In the process of producing furfural through heterogeneous catalyzed reaction, homogeneous Lewis and bronsted acids are typically doped with structured supports including zeolite, clay, and ion exchange resins to improve the solid acid catalyst's activity, stability, and recyclability (Liang et al., 2010; Xiao et al., 2010; Wang et al., 2018; Zhang et al., 2016). Unfortunately, a lot of these supported solid catalysts have high costs and need laborious formulation processes.
The utilization of biomass-based sulfonated carbon catalysts in the dehydration of xylose has recently increased because they are mechanically and chemically stable, have large surface area and adjustable surface functional groups, offer high product yield and reduce production cost, thus making them as good replacement for homogeneous catalysts (Gabriel et al., 2020; Liang et al., 2021; Zhang et al., 2022; Xu et al., 2023). For example, an acid-activated sugarcane bagasse carbon catalyst was used for the xylose dehydration to make furfural (Silva et al., 2023), a carbon-based solid acid obtained from pectin was used as a catalyst for furfural synthesis (Xu et al., 2021), H2SO4-modified mesoporous carbon was used for catalytic dehydration of xylose to produce furfural (Wang et al., 2022) and p-TSA modified water hyacinth leave biochar was employed to catalyze the furfural synthesis from xylose (Laohapornchaiphan et al., 2017). Furthermore, the production of furfural from the dehydration of corncob and xylose with different solvents (water, acetone, DMSO, GVL, 1, 4-dioxane, ethanol, methanol and DMF) over heterogeneous sulfanilic acid-derived carbon (S-CG) catalysts obtained from calcium-gluconate (biomass material) has been studied and reported. The furfural yields of 80.2, 73.5, 38.7, 10.1 and 7.3 % were obtained when GVL, 1, 4-dioxane, acetone, DMSO and water were used as solvents, respectively, whereas no xylose conversion was noticed with DMF, methanol and ethanol as solvents (Xu et al., 2023). Additionally, corncob conversion of 52.9 % was achieved with 1, 4-dioxade as a solvent over S-CG catalyst at 180 °C with 200 mg corncob, 10 mL solvent volume and 100 mg catalyst dosage after 70 min of the dehydration reaction (Xu et al., 2023). Corn straw conversion to furfural over the carbon acid catalyst formulated via impregnation of tin on sulfonated pollen was studied. The solid acid heterogeneous catalyst resulted in 77.82 % furfural yield at 190 °C for 3 h (Teng et al., 2020). Bifunctional-SO3H/sucralose catalyst was used to catalyze corncob conversion to furfural with DMSO as solvent, and a furfural yield of 90.8 % was achieved (Zhang et al., 2019). The favourable attributes of carbon-based support, such as mechanical and chemical stability, high specific surface area (SSA), adjustable surface functional groups and porous structure, have formed the motivations for further research (Teng et al., 2020; Ma et al., 2019; Millán et al., 2019). The use of activated carbon derived from eucalyptus bark as a catalyst support is an exciting aspect of green technology. Furthermore, eucalyptus activated carbon (EAC) has a large surface area, well porous structured and high adsorption capacity (Yusuff, 2022). The eucalyptus tree is common in China and Nigeria and grows yearly (Martini et al., 2020). To make sulfonated carbon catalyst, EAC is modified with bronsted acids to improve its acidic attributes as unmodified carbon has low activity (Yusuff, 2022; Yusuff et al., 2022). Therefore, enhancement of activated carbon from biomass via surface modification is necessary to enrich its acidic attributes and outwit the disadvantages of biomass with respect to the relatively low catalytic activity.
More importantly, the quest for facile and more efficient dehydration reaction process has attracted further studies on optimization of xylose conversion to furfural. So many reported studies showed that conventional methods were applied to examine the impact of operating variables on dehydration process. In these traditional techniques, experiments were conducted by changing systematically the independent variable and holding constant the others. This procedure should be repeated for each of the influencing factors, leading to an unpredictable number of experimental runs. The implementation of experimental design methods (EDMs) can be helpful in optimizing the effective parameters with the fewest experiments necessary. Taguchi optimization approach is one of EDMs used to control a process and optimize the process procedures in order to offer the favourable optimum conditions (Yusuff and Onobonoje, 2023). But, there is lack of information on the optimization of xylose conversion to furfural over solid acid catalyst using Taguchi experimental design approach.
The utilization of green solvent, such as GVL, in the dehydration of aldopentose (C5 hydrocarbon) will make the process eco-friendly. Therefore, the present study dwelled on the conversion of xylose by enriched eucalyptus activated carbon with the –SO3H sites as catalyst for the dehydration of xylose with GVL to produce furfural. The Influence of type and composition of the sulfonating agents on the characteristics and the catalyst reactivity was explored. The process input variables (temperature, catalyst dosage, time and solvent/substrate ratio) affecting the dehydration process were optimized using Taguchi optimization approach. Moreover, the catalyst reusability was investigated to examine its stability during reuse.
2 Materials and methods
2.1 Materials
Eucalyptus globulus barks (EBs) were collected from a garden at HUST, Wuhan, China. Xylose (98%, Energy Chemical, sourced from straw), sulfuric acid (H2SO4, 98%, Sigma-Aldrich), p-Toluene sulfonic acid monohydrate (TsOH, 98%, Energy Chemical), phosphoric acid (H3PO4, 85%, Sigma-Aldrich), furfural (98.5%, Energy Chemical) used as standard for quantification, γ-valerolactone (GVL, 98%, Energy Chemical), acetophenone (99.7%, Sigma-Aldrich), ethyl acetate (99.9%, Sigma-Aldrich), dichloromethane (DCM, 99.5%, Sigma-Aldrich) and phenolphthalein (97%) were purchased from Chemical Stores in Wuhan, China.
2.2 Solid acid catalysts synthesis procedures
2.2.1 Preparation of activated carbon from eucalyptus bark
The collected barks were washed with tap water until the leachate was clear and thereafter, the washed barks were dried at 110 °C for 5 h, crushed with pestle and mortar and then screened through 50-mesh sieve. 50 g of eucalyptus bark powder was weighed into 70 mL H3PO4 solution (85% weight), mixed at ambient temperature for 1 h for proper activation and then dried in a vacuum dryer at 60 °C for 12 h. Thereafter, the dried sample was carbonized in a furnace (OTF-1200X) at 600 °C for 1.5 h under N2 gas flow (150 mL/min STP) at 10 °C/min heating rate. The carbonized product was then rinsed in warm distilled water until washed water’s pH was around 7.0 in order to remove residual acid. The washed material was dried at 80 °C for 8 h and then pulverized to obtain uniform particle size powder. The product obtained will henceforth referred to as eucalyptus activated carbon (EAC).
2.2.2 Sulfonated EAC catalysts synthesis
Sulfonated catalysts were formulated by following the procedures reported by Dechakhumwat et al. (2020) with little adjustment. Apart from the adopted procedures, preliminary studies were carried out to determine the optimal operating parameters for catalyst preparation. Eucalyptus activated carbon was sulfonated using H2SO4, TsOH or H2SO4-TsOH mixture at varying mixing ratio as follows: Typically, 3.0 g of EAC was mixed with 30 mL of H2SO4 and sulfonated at 180 °C for 10 h in a closed glass reactor. For TsOH sulfonation, 3.0 g of EAC was added to 7.0 g of TsOH dissolved in 20 mL of distilled water in a glass reactor and heated at 180 °C for 10 h. Furthermore, sulfonation of EAC (3.0 g) using mixture of H2SO4 and TsOH at different H2SO4: TsOH molar ratios of 1:3, 3:1, 2:3 and 3:2 was carried out in a tightly covered glass reactor at 180 °C for 10 h. Thereafter, all the sulfonated materials were washed with distilled water until filtrate become neutral, and the residues were dried at 80 °C overnight and then placed in a desiccator to prevent moisture contamination. The samples sulfonated by H2SO4, TsOH and H2SO4/TsOH were denoted as EAC-H, EAC-T and EAC-
2.3 Characterization of sulfonated carbon catalyst samples
The measurement of SSA and pore sizes of the prepared sulfonated carbon samples was conducted under N2 gas at −196 °C (77 K) using Micromeritics porosity analyzer (ASAP2460, USA). Each sample was degased at 230 °C for 5 h to remove adsorbed gases and moisture from its surface. Surface functional groups of the formulated solid acid catalysts were evaluated using Fourier transform infrared (FTIR) spectrophotometer (Thermofisher Scientific, USA) within 4000–400 cm−1 wavenumber. Before FTIR analysis, each sample was gently pulverized and blended with 0.2 wt% KBr. The phase and crystallinity of the sulfonated samples were examined by X-ray diffraction (XRD) using a Bruker D8 Advance diffractometer. A Cu-kα radiation (
Scanning electron microscope (SEM-Sigma 300, Xplore 3D) was utilized to view the morphological attributes of the sulfonated carbon catalysts. Thermal stability of the catalyst samples was characterized using Thermogravimetric analyzer (TGA, NETZSCH TG 209F1). 3.0 mg of each sample was weighed and analyzed under N2 carrier flow rate of 180 mL/min, 15 °C/min heating rate and temperature range from 30 °C to 900 °C. Moreover, the acid strength of the sulfonated carbon catalysts was examined by temperature-programmed desorption of ammonia gas (NH3-TPD) via the Japan Bayer BELCAT-A TPD/TPR/TRO analyzer. Furthermore, the compositions of atomic carbon, nitrogen, sulfur and hydrogen were determined by CHNS analyzer. Additionally, total acid density of each sulfonated sample was estimated by acid/base titration technique. Briefly, 0.1 g of solid material was suspended in 25 mL of NaCl solution (2.0 M) and agitated for 20 min to allow ions exchange between solid acid catalyst and sodium salt. Thereafter, the stirred solution was centrifuged, and the clear solution was titrated against 0.05 M NaOH with phenolphthalein (indicator). The total acid density (
2.4 Catalytic activity evaluation
2.4.1 Dehydration of xylose
As illustrated in scheme 1, transformation of xylose to furfural via dehydration with GVL as solvent in the presence of sulfonated EAC catalysts (EAC-H, EAC-T or EAC-
- Chemical reaction equation for the catalytic dehydration of xylose to furfural.
2.4.2 Dehydration product analysis
The furfural content in the dehydration product was analyzed by GC-FID instrument (GC9790II) with capillary column (4000 J&W DH-5HT; dimension: 30 m
2.5 Optimization of xylose dehydration over the active sulfonated carbon catalyst
In this study, four input variables were considered at four factor-three level L9 orthogonal of Taguchi design approach to model and optimize the xylose dehydration process. The selection of levels was based on the preliminary experiments carried out and previous reported studies. The established limits were deposited into Design-Expert software (Version 12, Stat Ease) which suggested 9 experimental runs as indicated in Table 1. Furfural yield (%) was considered as a response factor while dehydration temperature, T (oC), catalyst loading, C (wt.%), dehydration time, t (h) and solvent/substrate ratio, S (mL/g) as input parameters.
| Factors | Description | Levels | ||
|---|---|---|---|---|
| L1 | L2 | L3 | ||
| T | Dehydration temperature (oC) | 170 | 180 | 190 |
| C | Catalyst loading (wt.%) | 0.5 | 1.0 | 1.5 |
| t | Dehydration time (h) | 1.0 | 2.0 | 3.0 |
| S | Solvent/solid ratio (mL/g) | 3.0 | 4.0 | 5.0 |
Furthermore, the signal/noise (S/N) ratio, a logarithmic parameter used to compare the response to the desired value (Yusuff and Onobonoje, 2023), was estimated based on values of furfural yields obtained. The S/N ratio is of three categories, namely minimizing the output factor (the smaller the better), maximizing the output factor (the larger the better) and getting a target value (target is better). The experimental values obtained from the dehydration of xylose were analyzed using the larger the better (LB) goal (Eq. (3) to evaluate optimum process variables and to study the contribution of each parameter that influences the xylose conversion process.
2.6 Isolation and analysis of furfural produced under optimum condition
After the dehydration reaction conducted under optimum conditions, the catalyst was separated from product through centrifugation, and the supernatant was purified through a preparative thin-layer chromatography plate using a mixture of DCM and ethyl acetate (ratio 30:1) as eluting solvent to isolate desired product which was then analyzed to examine its chemical structure and purity using 1H and 13C NMR spectrometer (Bruker Avance IIITM 500-MHz) with deuterated chloroform (CDCl3) as solvent. The obtained NMR data were calibrated and analyzed by using MestReNova-14.12-25024 software.
2.7 Catalyst stability study
After each reaction, the spent solid acid catalyst was recovered from product mixture through centrifugation at 7000 rpm for 5 min and rinsed with dichloromethane for three times to remove the physisorbed molecules and finally heated at 80 °C for 9 h to reactivate its catalytic activity before being used for subsequent dehydration experiments. Furthermore, leaching test was conducted by mixing the fresh catalyst with GVL at 70 °C for 4 h. The GVL was retrieved through centrifugation and employed for dehydration of xylose at 190 °C for 2 h and GVL to xylose ratio of 5:1 mL/g. Furthermore, a control dehydration experiment without catalyst was conducted with xylose and pure GVL under the identical conditions.
3 Results and discussion
3.1 Catalysts characterization
In order to investigate the physicochemical attributes of the formulated sulfonated EAC materials, they were characterized by BET, FTIR, NH3-TPD, CHNS, XRD, TGA, SEM and total acid density as follows:
3.1.1 Textural characteristics analysis
The SSA, pore diameter and pore volume of the EAC and sulfonated EAC samples are presented in Table 2. The SSA of the eucalyptus activated carbon was much larger than those of the sulfonated carbon catalysts, which is in excellent agreement with the porous structure observed via SEM analysis. The TsOH sulfonation was more effective at synthesis of a porous catalyst compared to the sulfonation by H2SO4 as the SSA of EAC-T (449.3 m2/g) was higher than that of EAC-H (331.3 m2/g). This observed trend agreed with the study reported by Dechakhumwat et al. (2020). Utilization of H2SO4-TsOH mixture for sulfonation of EAC further promoted the textural and morphological features of the sulfonated materials, as affirmed by the significant increase in surface area (>450 m2/g) and well developed pores observed in the EAC-
| Sample | Ultimate analysis (%)a | Textural properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | C/H | Surface area (m2/g) | Pore volume (cm3/g) | Average pore diameter (Å) | Total acid densityb (mmol/g) | Furfural yield (%)c | |
| REB | 48.7 | 7.32 | 0.91 | 0.38 | 6.65 | − | − | − | − | − |
| EAC | 75.7 | 5.04 | 3.36 | − | 15.1 | 826.1 | 0.72 | 3.69 | − | − |
| EAC-T | 66.4 | 3.79 | 0.35 | 8.50 | 17.5 | 449.3 | 0.23 | 58.2 | 0.28 ± 0.10 | 48.4 |
| EAC-H | 60.7 | 3.21 | 0.6 | 8.00 | 18.9 | 331.3 | 0.21 | 14.5 | 0.96 ± 0.24 | 67.1 |
| EAC-1H-3T | 71.4 | 3.36 | 0.15 | 7.19 | 21.3 | 498.7 | 0.36 | 5.87 | 0.52 ± 0.07 | 54.9 |
| EAC-3H-1T | 75.9 | 3.17 | 0.25 | 7.42 | 23.9 | 608.6 | 0.38 | 6.29 | 0.64 ± 0.83 | 64.8 |
| EAC-2H-3T | 64.8 | 3.43 | 0.41 | 8.96 | 18.9 | 596.2 | 0.27 | 15.1 | 0.51 ± 0.24 | 57.5 |
| EAC-3H-2T | 62.6 | 3.95 | 1.05 | 9.77 | 15.8 | 711.9 | 0.43 | 27.7 | 0.79 ± 0.08 | 71.2 |
| Spent EAC-3H-2T | 77.1 | 5.03 | 0.07 | 4.91 | 15.3 | 518.4 | 0.38 | 12.6 | 0.63 ± 1.06 | 51.6 |
3.1.2 CHNS composition analysis
The CHNS analysis was done to evaluate the chemical elements (carbon (C), hydrogen (H), nitrogen (N) and sulfur (S)) composition in the prepared samples as presented in Table 2. The contents of C and H in REB sample were 48.7% and 7.32% respectively, but C increased to 75.7% while H decreased to 5.04% after activation and carbonization of REB to obtain EAC due to dehydration and deoxygenation effects. However, upon sulfonation of EAC by different sulfonating agents, C, H and N contents reduced, suggesting that the carbonization and dehydration processes as well as volatilization of molecules occurred during the acid treatment (sulfonation) process. The increased sulfur content in sulfonated carbon catalyst suggested successful incorporation of –SO3H active sites into EAC, similar to results reported elsewhere (Chen et al., 2018). In terms of C/H ratio, sulfonated samples exhibited higher ratios as compared to that of non-sulfonated sample, which suggested that acid loading on EAC increased hydrogen- and oxygen-containing functional groups and facilitated the retention of H and O contents. Also, the hydrophilicity of the sulfonated carbon catalysts would be improved, thus enhancing the reactant adsorption onto active sites of the catalyst surface (Yusuff, 2022; Shokrolahi et al., 2012). Furthermore, the increased S content in the sulfonated samples suggested successful doping of –SO3H active sites with EAC, similar to results reported elsewhere (Xu et al., 2021; Chen et al., 2018). The S contents in the solid acid samples were in the range of 7.19–9.77%, where S content in EAC-3H-2T (9.77%) was 25.7 times higher than the S content in the raw eucalyptus bark (0.38%). It is important to note that EAC sample contained no sulfur due to the carbonization at high activation temperature which increased the C-H3PO4 and C-N2 reaction rates, resulting in increasing devolatilization which developed the pore structure in EAC. This observation was similar to findings documented by Nda-Umar et al. (2020).
3.1.3 XRD analysis
Fig. 1 presents the XRD patterns of the EAC and sulfonated EAC samples. The broad diffraction band at around 2
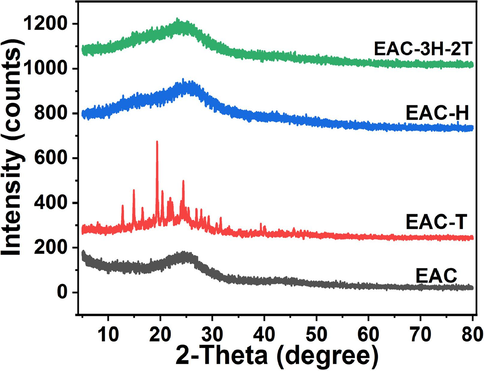
- XRD pattern of EAC and sulfonated EAC (EAC-T, EAC-H and EAC-3H-2T) catalysts.
3.1.4 FTIR analysis
The FTIR spectra of raw eucalyptus bark (REB), eucalyptus activated carbon (EAC) and sulfonated EAC samples (EAC-T, EAC-H and EAC-3H-2T) are displayed in Fig. 2. The spectra of REB, EAC and solid acid catalysts exhibited the presence of O–H group associated with alcohols, carboxylic acids and phenols and adsorbed moisture at around 3350–3530 cm−1. The band broadness of REB reduced after carbonization which suggested dehydration and deoxygenation, thereby forming aromatic carbon sheet (Nda-Umar et al., 2020). The peak at 2908 cm−1 on the REB’s spectrum, which is attributed to the C–H stretching of methyl group, disappeared after carbonization and acid treatment (sulfonation) processes, indicating the biomass transformation into a polycyclic aromatic hydrocarbon framework due to the influence of the sulfonating/activating agent and activation/sulfonation temperature (Xu et al., 2021). The transmittance pattern in the FTIR analysis revealed the presence of –NO2 aromatic nitro compound, in-plane-OH bending, NH stretching, bonded C=C and C=O stretching at 1315 cm−1, 1438–1441 cm−1, 1533 cm−1, 1608 cm−1 and 1675 cm−1, respectively, which was in line with work reported by Thanh et al. (Thanh et al., 2019), while O=S=O asymmetric and symmetric stretching in –SO3H group at around 1060–800 cm−1 and 1200–1100 cm−1 as illustrated in Fig. 2b. The presence of –SO3H group in the sulfonated samples affirmed the successful doping of acid on the eucalyptus activated carbon, forming C-SO3H structure (Dechakhumwat et al., 2020). It is worthy of note that the peaks associated with O=S=O functional groups in EAC-H and EAC-3H-2T samples were more intense as compared to EAC-T sample, thus confirming the strong reactivity of H2SO4.
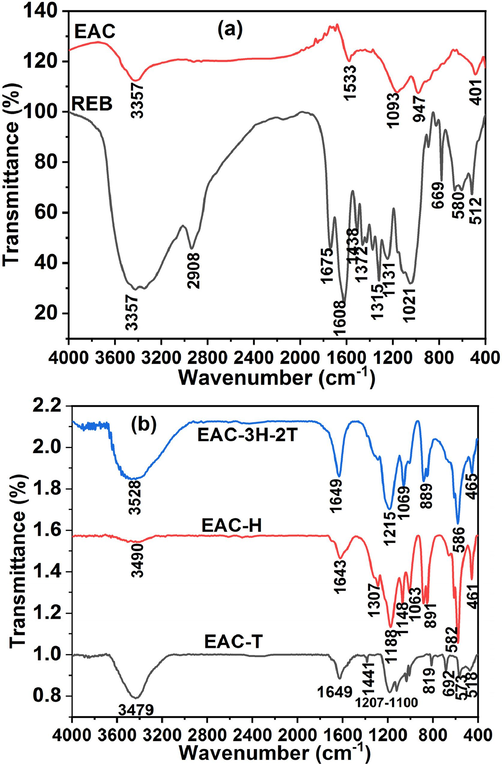
- FTIR spectra of (a) REB and EAC and (b) sulfonated EAC (EAC-T, EAC-H and EAC-3H-2T) catalysts.
3.1.5 NH3-TPD analysis and acidity property
The –SO3H group is a well-known acidic functional group that serves as active center on the solid acid catalyst. The NH3-TPD profiles for the as-synthesized carbon-based catalysts are shown in Fig. 3. The acidic site density was estimated by integration of area under the curve. From the NH3-TPD analysis, the carbon-based catalysts exhibited different desorption characteristics. EAC-T and EAC-H exhibited single desorption peak at 449 °C and 387 °C respectively, while EAC-3H-2T exhibited two desorption peaks at 207 °C and 338 °C. According to Zhang et al. (2004), the desorption peak occurred at around 300 °C, 300–550 °C and > 550 °C are attributed to the weak, medium to strong and strong acid sites on the catalyst surface, respectively. Therefore, the broad desorption peak exhibited by each of EAC-H and EAC-T affirmed the presence of –SO3H as acid sites on them. The two desorption peaks exhibited by EAC-3H-2T could be attributed to weak acid site and medium to strong acid site, respectively, confirming the presence of carboxylic (COOH) and sulfonic (−SO3H) groups on the catalyst surface as the active centers during the xylose dehydration to furfural. However, based on NH3-TPD results, the EAC-T, EAC-H and EAC-3H-2T possessed acidic sites with acidic quantity of 183 µmol/g, 177 µmol/g and 257 µmol/g, respectively. The higher total acid site exhibited by the EAC-3H-2T catalyst as compared to the EAC-T and EAC-H samples could be attributed to the high surface area of the former which promoted the spacing of the –SO3H groups, making it more denser in terms of total acid sites and accessible by the substrate and consequently enhances the reaction product yield (Dosuna-Rodriguez et al., 2011).
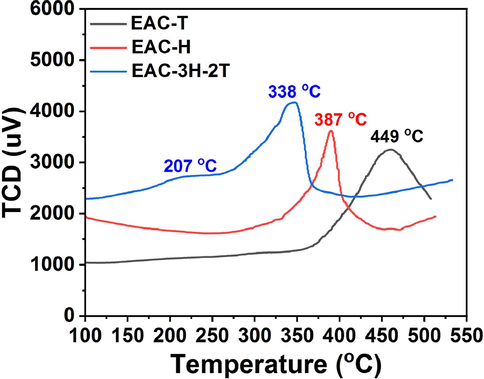
- NH3-TPD profile of EAC-T, EAC-H and EAC-3H-2T catalysts.
The total acid densities for the tested sulfonated carbon catalysts, which were determined via titration method, ranged from 0.28 to 0.96 mmol/g as illustrated in Table 1, where EAC-T, EAC-H and EAC-3H-2T had total acid density of 0.28 ± 0.10, 0.96 ± 0.24 and 0.79 ± 0.08 mmol/g, respectively. The prepared EAC-3H-2T catalyst possessed higher acid density than other sulfonated carbon catalysts prepared via H2SO4-TsOH sulfonation process and EAC-T but had lower acid density as compared to EAC-H sample. This observation could be attributed to the fact that the titration method could not account for the oxygen-containing functional groups (ether and carbonyl) present on the catalyst surface (Konwar et al., 2015). These observed trends were in line with the work of Dechakhumwat et al. (2020).
3.1.6 SEM analysis
The SEM micrographs of eucalyptus activated carbon and sulfonated carbon catalysts are displayed in Fig. 4. The EAC exhibited a relatively rough surface with some flake-like shapes, and it is essentially porous due to the effects of devolatilization and evaporation of phosphoric acid during carbonization, in excellent agreement with results reported by Nda-Umar et al. (2020). Deep and wide pores appeared on the surfaces of the sulfonated carbon samples, which is an excellent possibility for xylose adsorption during dehydration process. It is worthy of note that acid treatment of EAC with H2SO4/TsOH mixture transformed the structure of the catalyst better than the solid acid catalysts prepared either by pure H2SO4 or pure TsOH.

- SEM micrographs of (a) EAC, (b) EAC-T, (c) EAC-H and (d) EAC-3H-2T samples.
3.1.7 TGA analysis
The thermal stability of the REB, EAC and sulfonated EAC samples was examined via TGA analysis, and the obtained TGA profiles are displayed in Fig. 5. From the results, it was obvious that raw eucalyptus bark showed three major stages of degradation. The initial weight loss took place between 40 °C and 125 °C, attributing to the evaporation of water, while the decomposition that occurred between 125 °C and 310 °C was due to depolymerization of hemicellulose. The third weight loss that occurred between 310 °C and 410 °C was as a result of degradation of cellulose in form of adsorbed gases (such as CO2 and NH3), similar to observation reported by Liu et al. (Liu et al., 2013). For the EAC sample, two decomposition stages were noticed with first stage occurred between 35 °C and 100 °C which suggested adsorbed water loss, whereas the second stage presented no significant weight loss (<2%) presumably due to its low amount of cellulose (Burhenne et al., 2013). These observations suggested the reduction in thermal stability of EAC due to carbonization effect, which was in line with the results of Silva et al. (2023) who synthesized acid-activated carbon from sugarcane bagasse for xylose conversion to furfural. As also seen in Fig. 5, the sulfonated carbon catalysts exhibited three weight loss stages but different decomposition patterns due to their chemical compositions. The initial decomposition stage, which took place between 35 and 195 °C for EAC-T, 35 and around 205 °C for EAC-H and 35 and 210 °C for EAC-3H-2T, was attributed to the adsorbed water evaporation (Nagasundaram et al., 2020). The second stage of decomposition that happened from 195 to 302 °C for EAC-T, 205 to 312 °C for EAC-H and 210 to 303 °C for EAC-3H-2T was pointing to the decomposition of sulfonic (–SO3H) groups present in the precursor (EAC) (Silva et al., 2023). The third stage exhibited weight loss between > 300 and 800 °C for the three samples which signified decomposition of the carbonic framework. These TGA data suggested that the sulfonated carbon catalysts were thermally stable as a result of carbon moiety as corroborated by the CHNS and FTIR results, and it is in line with the studies reported elsewhere (Xu et al., 2023; Laohapornchaiphan et al., 2017; Nda-Umar et al., 2020).
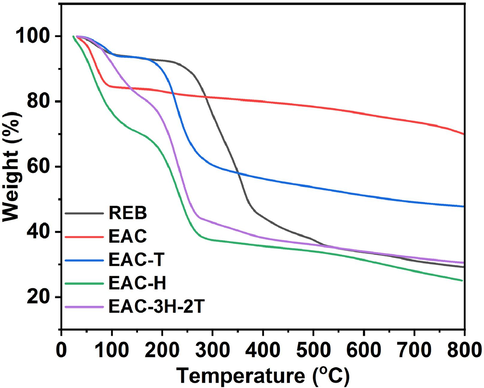
- TGA analysis of REB, EAC and sulfonated EAC materials.
3.2 Influence of the sulfonating agent on furfural yield
The prepared solid acid samples were utilized to catalyze the dehydration of xylose with GVL to produce furfural, with the corresponding product yields presented in Table 2. The SSA and the total acid density of the sulfonated carbon catalysts ranged from 331.3-711.9 m2/g and 0.28–0.96 mmol/g, respectively, and offered a furfural yield ranging from 48.4-71.2%. The furfural yield was corresponded to specific surface area, where EAC-3H-2T catalyst offered the highest dehydration product yield (71.2%) and EAC-1H-3T gave the least furfural yield (51.9%) among the catalysts prepared via EAC sulfonation by H2SO4-TsOH mixture, indicating that large surface area could enhance reactant adsorption onto catalyst surface and promote the dehydration mechanism of xylose to furfural (Silva et al., 2023). Moreover, the total acid density seemed to have played a crucial role in the conversion of xylose to furfural as the sulfonated carbon catalyst with high total acidity gave high product yield as noticed in the case of EAC-H and EAC-3H-2T samples, implying that more and stronger acid sites would enhance the performance of the catalyst. Notably, the obtained furfural yields proved that the performance of the catalysts depends on both surface and acidity properties. Note that the catalyst prepared through pure H2SO4 sulfonation (EAC-H) gave a higher furfural yield despite having a lower sulfur concentration as compared to catalyst obtained via TsOH sulfonation (EAC-T) which had higher surface area and high sulfur concentration, thus suggesting that the catalytic activity of the EAC-H was related to its total acid density, which was in excellent agreement with similar studies reported elsewhere (Xu et al., 2023; Zhang et al., 2016; Zhang et al., 2019).
3.3 Optimization of dehydration process by Taguchi design approach
The impacts of dehydration process conditions (dehydration temperature, catalyst loading, dehydration time and solvent/substrate ratio) on furfural yield were examined using Taguchi design method. Table 3 presents the obtained results from dehydration experiments, which comprised of L9 orthogonal design matrix, furfural yields and S/N ratio values. As listed in the Table 3, the maximum furfural yield of 72.83 % was gotten at 180 °C for 1.0 h with GVL/xylose ratio of 4.0 mL/g and 1.5 wt% EAC-3H-2T catalyst loading.
| Run No. | Xylose dehydration process parameters | Furfural yield | ||||
|---|---|---|---|---|---|---|
| T (oC) | C (wt.%) | t (h) | S (mL/g) | Experimental value (%) | S/N ratio | |
| 1 | 180 | 1.5 | 1.0 | 4.0 | 72.83 ± 0.94 | 37.25 |
| 2 | 170 | 1.5 | 3.0 | 5.0 | 70.44 ± 1.41 | 36.96 |
| 3 | 190 | 1.5 | 2.0 | 3.0 | 65.20 ± 2.05 | 36.28 |
| 4 | 180 | 1.0 | 3.0 | 3.0 | 68.40 ± 0.03 | 36.70 |
| 5 | 170 | 0.5 | 1.0 | 3.0 | 38.63 ± 0.61 | 31.74 |
| 6 | 190 | 0.5 | 3.0 | 4.0 | 29.61 ± 3.05 | 29.43 |
| 7 | 170 | 1.0 | 2.0 | 4.0 | 41.9 ± 0.06 | 32.44 |
| 8 | 180 | 0.5 | 2.0 | 5.0 | 53.14 ± 0.35 | 34.51 |
| 9 | 190 | 1.0 | 1.0 | 5.0 | 39.02 ± 0.71 | 31.83 |
Furthermore, the combined plot of S/N ratio against various dehydration process parameters is shown in Fig. 6. Fig. 6a reveals that the high furfural yield was obtained at middle level of temperature (180 °C). A higher temperature promotes the diffusion of reactant and dispersion of the solid catalyst particles. High temperature also facilitates interaction between the catalyst and solvent, followed by rearrangement and ion transfer, leading to high xylose conversion to furfural (Laohapornchaiphan et al., 2017; Thanh et al., 2019). The decline in the furfural yield at high level of temperature (190 °C) might be attributed to the vaporization of GVL at high temperature and prolong time, which promoted secondary reaction and consequently reduced the desired product yield, in excellent agreement with observation reported elsewhere (Qin et al., 2020). Furthermore, with reaction temperature increasing from 180 °C to 190 °C, the yield of furfural decreases and the colour of the reaction mixture changes from light yellow to dark yellow. This is attributed to the polymerization of furfural with increasing reaction temperature to form humin as byproduct (Xu et al., 2023). As shown in Fig. 6b, the furfural yield increased with increasing EAC-3H-2T loading, and the reason for this observation was that higher catalyst dosage offers more acid sites for dehydration reaction which promoted the formation of furfural and facilitated the condensation and degradation of the reaction products (Silva et al., 2023). These observations implied that 1.5 wt% catalyst loading was adequate in achieving a higher furfural yield. This finding implied that 1.5 wt% EAC-3H-2T loading was appropriate for achieving a maximum furfural yield. Furthermore, the highest furfural yield was obtained at high level of dehydration time (3.0 h) as seen in Fig. 6c. A sufficient reaction time is critical in achieving a high furfural yield in dehydration reaction because higher reaction time facilitates strong interaction between reactant and catalyst, thereby leading to more frequent collision and consequently promoting the xylose conversion (Xu et al., 2021). In addition, the longer reaction time resulted in the increase in furfural yield, suggesting the inhibition of byproduct (humins) under these reaction conditions. According to illustrated result in Fig. 6d, the furfural yield decreased by increasing the solvent/substrate ratio, indicating that solvent volume at low level (3.0 mL) was capable of distributing monosaccharide isotopes and increasing the amount of active isomers, which promoted the xylose conversion to furfural (Zhang et al., 2019). Therefore, the optimum conditions for xylose conversion to furfural were 180 °C dehydration temperature, 1.5 wt% EAC-3H-2T loading, 3.0 h dehydration time and 3:1 mL/g GVL/xylose ratio, and at these conditions, the predicted furfural yield was 72.71% (S/N ratio = 37.23). Further xylose dehydration experiments were conducted to validate the predicted dependent parameter, and the average observed furfural yield was obtained to be 74.61 ± 0.05 % with corresponding S/N ratio estimated to be 37.44.
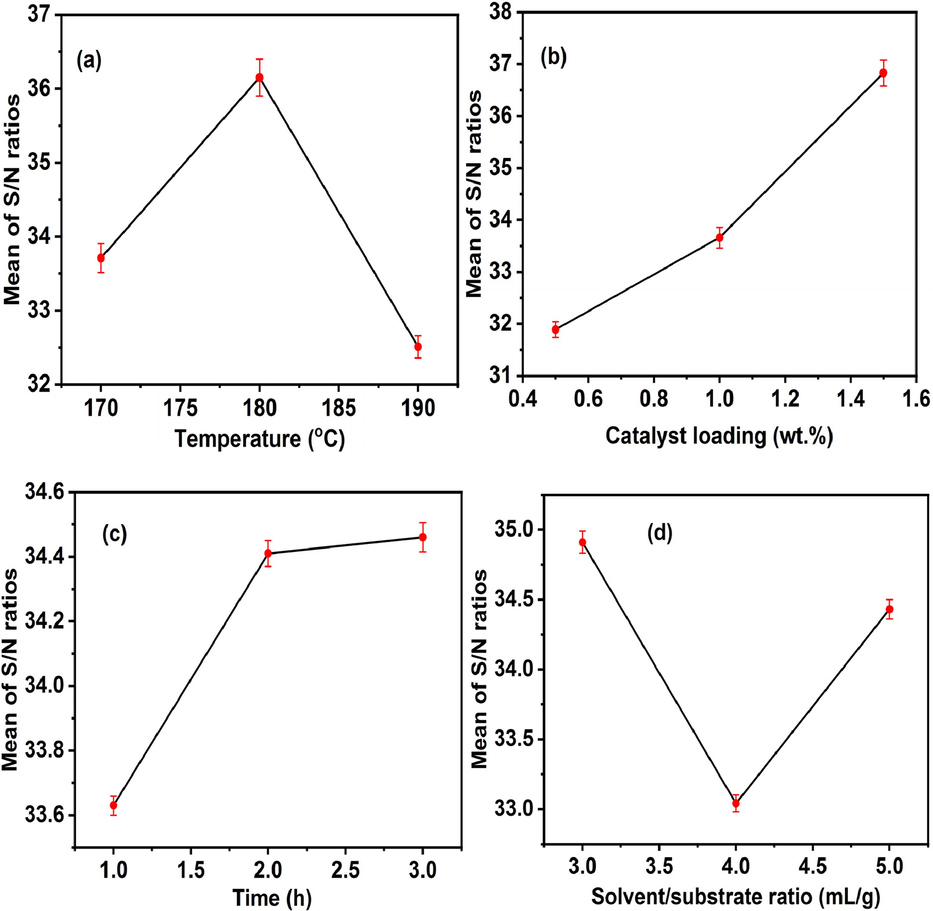
- Mean S/N ratio for the optimization of variables using the Taguchi technique.
3.3.1 Analysis of variance for dehydration of xylose over EAC-3H-2T catalyst
In a bid to ascertain the significance of each of dehydration process variables, analysis of variance (ANOVA) was used as a statistical tool to achieve it. Table SM1 illustrates the ANOVA results for the furfural yield, which include p-value, F-value and contribution factor (CF). According to the results, the parameter with high CF value (estimated by Eq.4) has significant influence on process output variable. In this case, catalyst loading had most significant influence on xylose conversion, followed by dehydration temperature and solvent/substrate ratio. However, time had the least influence on furfural production from xylose. This finding was corroborated by Table SM2 which presents the S/N ratio ranges for the independent parameters considered. The range suggests the rank of the parameter, and the obtained values of range indicated that catalyst loading (with 1st rank) had the significant impact on sulfonated carbon catalyzed dehydration reaction, followed by temperature, solvent/substrate ratio, while the dehydration time had the least impact on furfural synthesis from xylose.
3.4 Investigation on EAC-3H-2T stability
The spent EAC-3H-2T catalyst was reused for the dehydration of xylose in order to evaluate its stability, and the dehydration reaction over the reused catalyst was run under the optimum conditions. As displayed in Fig. 7, the furfural yield reduced from 51.6% to 42.3% as the cycle number increased which signified reduction in the amount of catalyst active sites as a result of insoluble byproducts deposition on catalyst surface and in the pores during dehydration process (Xu et al., 2021). It could also be that –SO3H functional groups reacted with byproducts to generate insoluble residues or leached during reaction, thereby resulting in acid concentration reduction on the catalyst surface. This observation was corroborated by the results of FTIR analysis conducted on spent EAC-3H-2T catalyst (see Fig. 8), which revealed low intensity of peaks associated with –SO3H as well as disappearance of O=S=O stretching in sulfonic acid group earlier seen in fresh catalyst, thus affirming the acid sites loss. This is one of the numerous factors that caused a decline in furfural yield (Xu et al., 2023). To investigate the reason why the furfural yield declined after the catalyst was reused, leaching test was conducted as explained in section 2.7. The dehydration of xylose with used GVL without catalyst gave 3.08% furfural yield, suggesting the leaching of active ingredient in the solid acid catalyst during reaction. However, no dehydration of xylose occurred when pure GVL was used without catalyst, which was affirmation of active sites leaching. Therefore, the decrease in furfural yield noticed during successive reaction cycles was attributed to the loss of active centers during dehydration process or catalyst recovery operation. The observed trend was also supported by the values of total acid density for fresh EAC-3H-2T, reused EAC-3H-2T for the first cycle and reused EAC-3H-2T for the sixth cycle obtained to be 0.79 ± 0.08, 0.63 ± 1.06 and 0.38 ± 0.21 mmol/g, respectively. This observed trend is in excellent agreement with results reported by Xu et al. (2023) who observed a reduction in acid density of S-800-CG (carbon-based acid catalyst) from 1.29 to 0.98 mmol/g after the fourth time reuse for furfural synthesis. Nevertheless, the EAC-3H-2T catalyst showed a great potential to be employed to catalyze furfural production as it exhibited good stability and recyclability for xylose dehydration with less than 10% loss of its activity during the catalytic reaction.
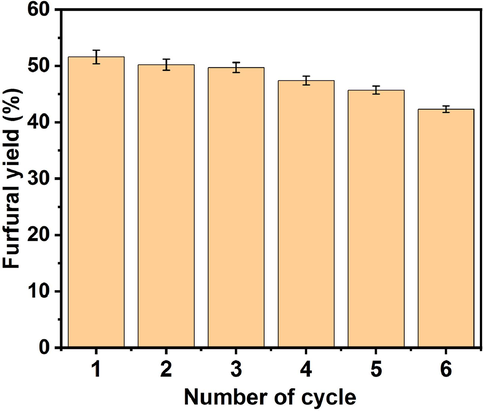
- Reusability effect of EAC-3H-2T catalyst on dehydration of xylose at 180 °C for 3 h with 1.5 wt% EAC-3H-2T loading and 3:1 mL/g GVL/xylose ratio.
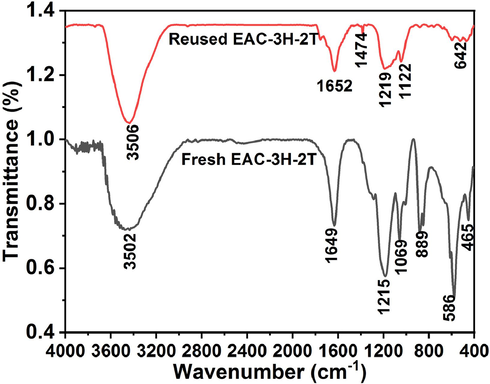
- FTIR spectra of fresh and reused EAC-3H-2T samples.
3.5 Characterization of reused EAC-3H-2T catalyst
In order to gain insight into transformation experienced by the EAC-3H-2T catalyst after it was reused for dehydration reaction, various spectroscopic analyses were conducted. Firstly, FTIR analysis was carried out on the reused EAC-3H-2T sample, and the obtained data were presented in form of a spectrum in Fig. 8. By comparing the spectra of fresh and reused EAC-3H-2T samples, it appeared that there were disappearance and shifting of some peaks earlier observed in the fresh sample as well as reduction in the intensity of peaks that are associated with acid sites (−SO3H) functional groups. For instance, the peak at 889 cm−1 that is associated with symmetric stretching of O = S = O in –SO3H disappeared after reusability, while the absorption bands at the range of 1069–1215 cm−1 in the spectrum of fresh EAC-3H-2T sample demonstrated asymmetric stretching of O = S = O in –SO3H groups, and that after reusability, the bands shifted to 1122–1219 cm−1 and their intensity reduced due to the formation of strong bond between the carbonyl ions in furfural molecules and sulfonic group in the solid acid catalyst sample (Xu et al., 2023; Laohapornchaiphan et al., 2017). These observations implied that the sulfonic groups, which acted as active centers on the catalyst surface, actively participated in the xylose dehydration to produce furfural and maintained their activity after being reused. The SEM micrographs of the fresh and reused EAC-3H-2T samples are displayed in Fig. 9. It appears that there was a change in the morphological framework of the fresh sulfonated carbon catalyst as flake-like, rough surface and irregular morphology was observed after it was reused (see Fig. 9b). Thus, furfural yield declined as the number of reusability cycle increased. Nonetheless, the reused catalyst still appeared to be porous as numbers of pores were observed on its surface, thereby suggesting good possibility for reactant adsorption onto catalyst surface and hence substantial amount of furfural is expected to be formed. This was corroborated by the results obtained through BET surface area analysis conducted on spent EAC-3H-2T sample (see Table 2), where its specific surface area and pore volume were found to be 518.4 m2/g and 0.38 cm3/g, respectively, compared to fresh EAC-3H-2T sample whose surface area and pore volume were 711.9 m2/g and 0.43 cm3/g respectively, pointing to the blockage of the pores on the catalyst surface by the molecules of xylose or furfural (Silva et al., 2023). Nevertheless, the spent catalyst still exhibited better textural attributes as its surface area and pore volume were higher which confirmed the presence of active sites on its surface, and it could eliminate the mass transfer limitation and provide better flow channel (Tan et al., 2015). These results were in line with CHNS analysis results for spent EAC-3H-2T sample (see Table 2) in which the sulfur content in the sample was 4.91% as compared to that of fresh catalyst sample (9.77%), affirming the reduction in number of active sites on the surface of the sulfonated carbon catalyst due to the active participation of the –SO3H groups in the dehydration reaction, similar to findings reported by Xu et al. (2023).
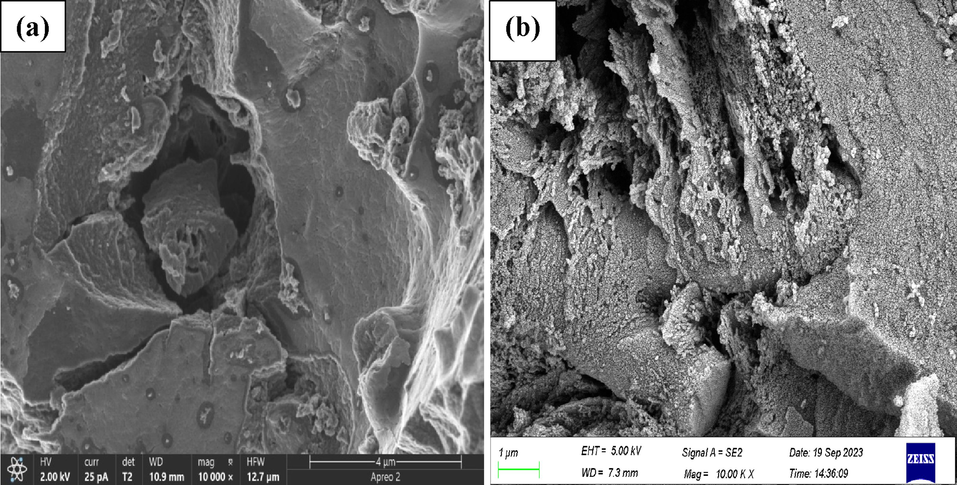
- SEM micrographs of (a) fresh and (b) reused EAC-3H-2T samples.
3.6 Analysis of furfural obtained under optimum conditions
The chemical composition of the dehydration reaction product obtained under the optimum conditions was examined by 1H NMR and 13C NMR analyses, and the results are illustrated in Fig. SM1. Based on the 1H NMR spectrum (Fig. SM1a), the signal relative to a proton associating with carbon atom bearing the aldehyde (–COH) group appeared at chemical shift,
3.7 Comparison between EAC-3H-2T and other sulfonated catalysts for furfural synthesis from xylose
This present research study was compared with the previously reported studies on xylose conversion to furfural over solid acid catalyst as illustrated in Table 4. The furfural yield obtained herein (74.61 ± 0.05 %) was relatively high, indicating that EAC-3H-2T was a promising material for converting C5 hydrocarbon to fine chemical like furfural, particularly when compared with solid acid materials derived from sugarcane (48.63%) (Silva et al., 2023), ZSM-5 (34.1%) (Sato et al., 2019); Acacia mangium wood sawdust (42%) (Thanh et al., 2019) and water hyacinth leaves (64%) (Laohapornchaiphan et al., 2017). It is worthy of note that the obtained yield of furfural from xylose in the current study was higher than the furfural yield reported by He et al. (2017). In their work, furfural yield of 44% was obtained at 170 °C for 20 min with catalyst (
| Catalyst | Dehydration experimental conditions | Solvent used | Furfural yield | Reference | |||
|---|---|---|---|---|---|---|---|
| T (oC) | C (wt.%) | t (h) | S (mL/g) | ||||
| S-800-CG | 140 | 1.0 | 4.0 | 10 | 1,4-dioxane | 73.5 | (Xu et al., 2023) |
| SP-170 | 170 | 0.05 | 1.0 | 5 | GVL-H2O | 80.4 | (Xu et al., 2021) |
| AC-S/CuCl2 | 180 | 5.0 | 2.0 | 15 | H2O | 55.96 | (Silva et al., 2023) |
| WH-PTSA-220 | 170 | 2.0 | 3.0 | 5.0 | GVL | 64 | (Laohapornchaiphan et al., 2017) |
| OMC-SO3H | 200 | 2.0 | 0.75 | H2O | 2.50 | (Wang et al., 2022) | |
| Si-12Nb | 160 | 4.0 | H2O/toluene | 33.60 | (García-Sancho et al., 2014) | ||
| AC-S/NiCl2 | 180 | 5.0 | 2.0 | 15 | H2O | 48.63 | (Silva et al., 2023) |
| AC-S/ZnCl2 | 180 | 5.0 | 2.0 | 15 | H2O | 31.27 | (Silva et al., 2023) |
|
|
180 | 3.6 | 0.3 | − | H2O | 57.07 | (Xue et al., 2018) |
| Sn-DAT-SS | 170 | 0.5 | 10:90 | ChCl: ethylene glycol | 52.4 | (Ni et al., 2021) | |
|
|
170 | 3.5 | 0.5 | 1:2 | Toluene/H2O | 74.3 | (He et al., 2017) |
|
|
180 | 3.6 | 0.25 | ChCl-maleic acid/toluene | 82.6 | (Gong et al., 2022) | |
| EAC-3H-2T | 180 | 1.5 | 3.0 | 3.0 | GVL | 74.61 ± 0.05 % | Present study |
4 Conclusions
In this study, the catalytic activity of sulfonated eucalyptus activated carbon as solid acid catalyst for furfural synthesis from xylose was examined. Both pristine and mixed sulfonating agents (H2SO4, TsOH and H2SO4-TsOH) can be used for the synthesis of carbon-based solid acid catalysts with large amount of acid sites. However, based on catalytic performance in the dehydration of xylose with GVL, the sulfonated carbon (EAC-3H-2T) prepared via doping of EAC with mixture of H2SO4 and TsOH at molar ratio of 3:2 gave the highest furfural yield. The catalytic activity of the sulfonated carbon catalysts solely relied on their textural properties (specific surface area and pore diameter) and acidic strength. The surface area and pore diameter of EAC-3H-2T were 711.9 m2/g and 27.7 Å, respectively, which promoted easy diffusion of xylose in GVL into the pores of the catalyst and dehydrate at the catalytically active centers. Optimization results revealed that catalyst loading had most significant influence on xylose conversion, followed by dehydration temperature and solvent/substrate ratio. However, reaction time had the least influence on furfural production from xylose. The EAC-3H-2T material showed sustained catalytic activity and was relatively stable after being recycled and reused for six times, demonstrating that it could be considered a promising catalyst for hemicellulose conversion to furfural with better reusability to minimize production cost.
5 Author agreement
I, Dr. Yusuff Adeyinka and my co-author had read all the terms and conditions regarding paper publication in your reputable journal. We strongly agreed with your copyright statements. We affirm that this manuscript is original, not under consideration with any other journal and has not been published at all. All the authors read and approved the manuscript. We all agreed that the corresponding author is the contact person for the editorial process. He would be responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
CRediT authorship contribution statement
Adeyinka Sikiru Yusuff: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Conceptualization. Yanlong Gu: Writing – original draft, Resources, Project administration, Funding acquisition, Formal analysis.
Acknowledgment
Professor Yanlong Gu is grateful for financial support from the National Key Research and Development Project (2022YFE0124100) and the Innovation and Talent Recruitment Base of New Energy Chemistry and Device (B21003). The support of Belt and Road Innovation Research at Huazhong University of Science and Technology, Wuhan, China through the Talent Exchange Project No. DL2022154006L is highly appreciated by Dr. Adeyinka Sikiru Yusuff.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Acetylation of glycerol over sulfated Al2O3: reaction parameter study and optimization using response surface methodology. Energy Fuel. 2016;30:584-593.
- [Google Scholar]
- The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrolysis. 2013;101:177-184.
- [Google Scholar]
- Magnetically recyclable cellulose-derived carbonaceous solid acid catalyzed the biofuel 5-ethoxymethylfurfural synthesis from renewable carbohydrate. Fuel. 2018;219:344-352.
- [Google Scholar]
- Comparative study on the conversion of Acacia mangium wood sawdust-derived xylosecontaining acid hydrolysate to furfural by sulfonated solid catalysts prepared from different lignocellulosic biomass residues. Wood Sci. Technol.. 2021;55:659.
- [Google Scholar]
- Direct Diels-Alder reactions of furfural derivatives with maleimides. Green Chem.. 2021;23:367-373.
- [Google Scholar]
- Preparation of sulfonated carbon-based catalysts from murumuru kernel shell and their performance in the esterification reaction. RSC Adv.. 2020;10:20245-20256.
- [Google Scholar]
- Catalytic activity of heterogeneous acid catalysts derived from corncob in the esterification of oleic acid with methanol. Renew. Energy. 2020;148:897-906.
- [Google Scholar]
- Glycerol acetylation on sulphated zirconia in mild conditions. Catal. Today. 2011;167:56-63.
- [Google Scholar]
- New Approach to dehydration of xylose to 2-furfuraldehyde using a mesoporous niobium-based catalyst. ACS Omega. 2020;5:21392-21400.
- [Google Scholar]
- Mesoporous Nb2O5 as solid acid catalyst for dehydration of D-xylose into furfural. Catal. Today. 2014;234:119-124.
- [Google Scholar]
- Highly efficient conversion of sunflower stalk-hydrolysate to furfural by sunflower stalk residue-derived carbonaceous solid acid in deep eutectic solvent/organic solvent system. Bioresour. Technol.. 2022;351:126945
- [Google Scholar]
- One-pot conversion of biomass-derived xylose to furfuralcohol by a chemo-enzymatic sequential acid-catalyzed dehydration and bioreduction. Green Chemistry. 2017;19(16):3844-3850.
- [Google Scholar]
- Chemical-enzymatic conversion of corncob-derived xylose to furfuralalcohol by the tandem catalysis with SO42-/SnO2-kaoline and E.coli CCZU-T15 cells in toluene-water media. Bioresour. Technol.. 2017;245:841-849.
- [Google Scholar]
- Synthesis of Furfural from Water Hyacinth (Eichornia croassipes) IOP Conference. Series. Mater. Sci. Eng.. 2017;172:012027
- [Google Scholar]
- Synthesis and Spectroscopic Characterization of Furan-2-Carbaldehyde-d. Molbank 2023:M1654.
- [Google Scholar]
- Production of furfural from xylose and hemicelluloses using tin-loaded sulfonated diatomite as solid acid catalyst in biphasic system. Bioresource Technol. Rep.. 2019;6:145-151.
- [Google Scholar]
- Shape selectivity and acidity effects in glycerol acetylation with acetic anhydride : selective synthesis of triacetin over Y-zeolite and sulfonated mesoporous carbons. J. Catal.. 2015;329:237-247.
- [Google Scholar]
- One-step preparation of carbon-based solid acid catalyst from water hyacinth leaves for esterification of oleic acid and dehydration of xylose. Chem. - an Asian J.. 2017;12:3178-3186.
- [Google Scholar]
- Synthesis of sulfonated carbon catalyst from waste orange peel for cost effective biodiesel production. Bioresource Technol. Rep.. 2018;2:69-76.
- [Google Scholar]
- One-step synthesis of carbon functionalized with sulfonic acid groups using hydrothermal carbonization. Carbon. 2010;48(6):1844-1848.
- [Google Scholar]
- Valorization of waste lignocellulose to furfural by sulfonated biobased heterogeneous catalyst using ultrasonic-treated chestnut shell waste as carrier. Processes. 2021;9:2269.
- [Google Scholar]
- Efficient conversion of carbohydrates into 5-ethoxymethylfurfural in ethanol catalyzed by AlCl3. Fuel. 2013;113:625-631.
- [Google Scholar]
- Efficient catalytic conversion of corn stalk and xylose into furfural over sulfonated graphene in gamma-valerolactone. RSC Adv.. 2019;9(19):10569-10577.
- [Google Scholar]
- Modified eucalyptus bark as a sorbent for simultaneous removal of COD, oil and Cr(III) from industrial wastewater. Alex. Eng. J.. 2020;59:1637-1648.
- [Google Scholar]
- Melikoglu M, Singh V, Leu SY, Webb C, Lin CSK. Biochemical production of bioalcohols, Handbook of biofuel production, Second Edition, Processes and Technology, 2016 237-258.
- Furfural production in a biphasic system using a carbonaceous solid acid catalyst. Appl. Catal. A. 2019;585:117180
- [Google Scholar]
- Levulinic acid production from renewable waste resources: bottlenecks, potential remedies, advancements and applications. Renew. Sustain. Energy Rev.. 2015;51:548-565.
- [Google Scholar]
- Lalitha A (2020) SO3H@carbon powder derived from waste orange peel: an efficient, nano-sized greener catalyst for the synthesis of dihydropyrano[2,3-c]pyrazole derivatives. Adv. Powder Technolol.. 2020;31(4):1516-1528.
- [Google Scholar]
- Production of first and second generation biofuels: a comprehensive review. Renew. Sustain. Energy Rev.. 2010;14(2010):578-587.
- [Google Scholar]
- Synthesis and characterization of sulfonated carbon catalysts derived from biomass waste and its evaluation in glycerol acetylation. Biomass Convers. Biorefin. 2020
- [CrossRef] [Google Scholar]
- Highly efficient chemoenzymatic cascade catalysis of biomass into furfuryl amine by a heterogeneous shrimp shell-based chemocatalyst and w-transaminase biocatalyst in deep eutectic solvent-water. ACS Sustain. Chem. Eng.. 2021;9(38):13084-13095.
- [Google Scholar]
- Green sulfonation of carbon catalysts via gas–liquid interfacial plasma for cellulose hydrolysis. ACS Sustain. Chem. Eng.. 2020;8:5837-5846.
- [Google Scholar]
- Sadasivuni KK, Al-Dhabi NA, Kumar S, Kumar B. Reduced metal nanocatalysts for selective electrochemical hydrogenation of biomass-derived 5-(hydroxymethyl)furfural to 2, 5-bis(hydroxymethyl)furan in ambient conditions. Frontiers in Chemistry, 11 (2023), 1-8, Article 120046.
- Effect of extraction on furfural production by solid acid-catalyzed xylose dehydration in water. J. Supercrit. Fluids. 2019;144:14-18.
- [Google Scholar]
- Preparation of ordered sulfonated mesoporous polymer (OMP-TsOH) from p-toluenesulfonic acid and application in esterification reaction of fatty acids. J. Brazil. Chem. Soc.. 2012;23:1186-1192.
- [Google Scholar]
- Synthesis and characterization of acid-activated carbon prepared from sugarcane bagasse for furfural production in aqueous media. Catalysts. 2023;13:1372.
- [Google Scholar]
- Waste ostrich- and chicken-eggshells as heterogeneous base catalyst for biodiesel production from used cooking oil: catalyst characterization and biodiesel yield performance. Appl. Energy. 2015;160:58-70.
- [Google Scholar]
- Tin-loaded sulfonated rape pollen for efficient catalytic production of furfural from corn stover. Indust. Crop. Product.. 2020;151:112481
- [Google Scholar]
- Preparation of carbonaceous solid acid catalyst from Acasia mangium wood sawdust for conversion same source into 5-hydroxymethylfurfural. Energy Source, Part a: Recovery, Utilization, and Environmental Effects 2019
- [CrossRef] [Google Scholar]
- Carbon-based solid acid derived from lignin and polyvinyl chloride for conversion of xylose and crop wastes to furfural. Mol. Catal.. 2022;524:112329
- [Google Scholar]
- Selectively convert fructose to furfural or hydroxymethylfurfural on beta zeolite: the manipulation of solvent effects. Appl. Catal. B-Environ.. 2018;235:150-157.
- [Google Scholar]
- Synthesis of sulfonated lignin-derived ordered mesoporous carbon for catalytic production of furfural from xylose. Int. J. Biol. Macromol.. 2022;187:232-239.
- [Google Scholar]
- One-step synthesis of a novel carbon-based strong acid catalyst through hydrothermal carbonization. Monatsh. Chem.. 2010;141:929-932.
- [Google Scholar]
- Conversion of xylose to furfural catalyzed by carbon-based solid acid prepared from pectin. Energy Fuels. 2021;35:9961-9969.
- [Google Scholar]
- Efficient conversion of biomass derivatives to furfural with a novel carbon-based solid acid catalyst. Catal. Commun.. 2023;175:106608
- [Google Scholar]
- One-pot chemo-enzymatic conversion of D-xylose to furfuralalcohol by sequential dehydration with oxalic acid plus tin-based solid acid and bioreduction with whole-cells. Bioresour. Technol.. 2018;268:292-299.
- [Google Scholar]
- Kinetic and thermodynamic study on the esterification of oleic acid over SO3H-functionalized eucalyptus tree bark biochar catalyst. Sci. Rep.. 2022;12(1):8653.
- [Google Scholar]
- Biodiesel production from transesterified yellow grease by ZSM-5 zeolite-supported BaO catalyst: process optimization by Taguchi’s experimental design approach. Mater. Renew. Sustain. Energy. 2023;12(3):199-208.
- [Google Scholar]
- Sulfonated biochar catalyst derived from eucalyptus tree shed bark: synthesis, characterization and its evaluation in oleic acid esterification. RSC Adv.. 2022;12:10237-10248.
- [Google Scholar]
- Catalytic conversion of xylose and and corn stalk into furfural over carbon solid acid catalyst in γ-valerolactone. Bioresour. Technol.. 2016;209:108-114.
- [Google Scholar]
- Transformation of corncob into furfural by a bifunctional solid acid catalyst. Bioresources Technol.. 2019;276:60-64.
- [Google Scholar]
- Biodiesel production via esterification of oleic acid catalyzed by chlorosulfonic acid modified zirconia. Appl. Energy. 2004;116:191-198.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105892.
Appendix A
Supplementary material
The following are the Supplementary material to this article:







