Translate this page into:
Study on the reuse process of hydrolysate from γ-polyglutamic acid fermentation residues
⁎Corresponding author at: School of Municipal and Environmental Engineering, Shandong Jianzhu University, JiNan 250101, China. zhangmeili8292@sina.com (Chao Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Waste biomass hydrolysate could replace tryptone for γ-PGA production. The addition of waste biomass hydrolysate reduced the amount of glutamate in the medium. The process could offset the disposal cost of waste biomass. The waste biomass could be recycled for 2 times.
Abstract
In order to study the feasibility of using γ-polyglutamic acid (γ-PGA) waste biomass hydrolysate as nitrogen source of culture medium, waste biomass was treated with nitric acid to obtain hydrolysate. Through the orthogonal optimization experiment, the optimal conditions of biomass hydrolysis were obtained as follows: 2% nitric acid, solid-liquid ratio 4:10, 110 °C, and 24 h. Under the optimal conditions, the degree of hydrolysis reached 77.08 ± 0.48%. The fermentation medium was further optimized: biomass hydrolysate 60 mL, glucose 38 g/L, L-glutamate 25 g/L. In the optimal medium, γ-PGA yield was 30.69 ± 0.42 g/L, which was 4.49% higher than that in tryptone medium (29.37 ± 0.43 g/L). The results showed that the waste biomass hydrolysate could be used as tryptone for γ-PGA production. Moreover, the addition of waste biomass hydrolysate reduced the amount of glutamate in the medium. In addition, the waste biomass could be recycled for 2 times. This study provided a new idea for the cleaner production technology of γ-PGA.
Keywords
Waste biomass
Reuse
γ-polyglutamic acid
1 Introduction
γ-polyglutamic acid (γ-PGA) is a kind of water-soluble polymer material synthesized by microbial fermentation, which is polymerized from L-glutamic acid and D-glutamic acid via γ-amide bond (Zhang et al., 2018). Due to its non pollution to the environment, excellent biodegradability, film-forming, fiber-forming, water retention and other special physical and chemical properties, it is widely used in medicine, agriculture, environmental protection, cosmetics, food and other industries (Zhang et al., 2019a). In recent years with the deepening of the research on γ-PGA, the application field of γ-PGA has gradually expanded. Attracted by the market prospect, Chinese enterprises have participated in the layout, and the scale of γ-PGA industrial production has been expanding. In 2019, China's γ-PGA production is close to 1700 tons, with a year-on-year growth of 13.8% (Tao et al., 2020). Large scale γ-PGA production enterprises in China include Shandong Furida, Wuhan Guanghua times, Wanheng biology, etc.
However, the domestic production of γ-PGA by fermentation also faces serious problems of resource waste and environmental pollution (Luo et al., 2016). In the process of microbial fermentation, the biomass at the end of fermentation is not the target product. So a large number of waste biomass residue is produced. Its treatment method is to decompose or burn the solid substance of waste biomass residue in the sewage treatment plant, which not only pollutes the environment and destroys the ecology, but also causes serious waste of resources and energy (Zhang et al., 2020). At present, most γ-PGA production enterprises sell bacterial protein as feed in order to save resources. Although it has achieved certain economic benefits, its application value has not been fully explored. Through the analysis of nutritional components of bacterial protein, it is found that the protein content is rich, the amino acid composition is complete, and it is rich in vitamins, nucleic acids, polysaccharides and so on (Li et al., 2020). Therefore, it is of great significance to explore and develop by-products with higher value by using γ-PGA bacterial protein as raw material. At present, the research in this field mainly focuses on the development of bacterial protein as biological fertilizer, condiment, extraction of oligopeptide, extraction of nucleic acid, etc. (Shih and Van, 2001). Only a few scholars try to use the reuse technology of fermentation waste biomass. For example, Peng et al. used hydrolysate of L-ornithine fermentation residues as the organic nitrogen source to produce L-ornithine. Finally, 37.47 g/L of L-ornithine was obtained, which was no significant difference compared with the control group (Peng, 2015). So far, no scholars have studied the reuse technology of γ-PGA waste biomass. Similar studies only use other fermentation waste biomass as nitrogen source to produce γ-PGA. For instance, Liu et al. used the hydrolysate of waste biomass from glutamate fermentation as a nitrogen source to produce γ-PGA (Liu et al., 2018).
In this study, γ-PGA waste biomass was treated by chemical method. It was developed as waste biomass hydrolysate to replace the nitrogen source in the process of γ-PGA production. It opened up a new way to utilize waste biomass for production enterprises, and reduced the cost of raw materials in the process of γ-PGA production.
2 Experimental
2.1 Strain
Bacillus subtilis W-17 (CICC 10260) was stored in our laboratory. The strain was maintained on slant medium at 5℃. It was used for γ-PGA fermentation.
2.2 Preparation of waste biomass
γ-PGA biomass was produced in 5-L bioreactor (Baoxing Corp., Shanghai, China) containing 3 L fermentation medium. The bioreactor was inoculated with 10% inoculum, and cultured at 37 °C and 200 rpm for 48 h. The waste biomass was collected by centrifugation at 10,000 r/min for 10 min.
In the experiment of waste biomass reuse, only the composition of the culture medium was different, and the preparation steps of the waste biomass were the same as above.
2.3 Preparation of waste biomass hydrolysate
The wet bacteria was taken after fermentation and centrifugation. It was then hydrolyzed with 2% nitric acid. The hydrolysis conditions were as follows: solid-liquid ratio 4:10, 110 °C, and 24 h. NaOH was used to adjust the pH of hydrolysate to 7.5.
2.4 Media
Slant medium (SM), in g/L: glucose, 20; yeast extract, 10; L-glutamate, 20; NaCl, 5; agar 18. The pH was adjusted to 7.0 by HCl or NaOH.
Seed medium (SM), in g/L: glucose, 20; yeast extract, 10; L-glutamate, 20; K2HPO4·3H2O, 2; MgSO4, 0.1; MnSO4, 0.03.
The optimal fermentation medium without waste for B. subtilis W-17, in g/L: glucose, 36; tryptone, 9; L-glutamate, 28; K2HPO4·3H2O, 2; MgSO4, 0.25 (Zhang et al., 2019b).
Acid hydrolysate medium (AM): waste biomass hydrolysate, 60 mL; glucose, 38 g/L; L-glutamate, 25 g/L; K2HPO4·3H2O, 2 g/L; MgSO4, 0.25 g/L. The pH was adjusted to 7.0 by HCl or NaOH.
All media were autoclaved for 20 min at 121℃.
2.5 Culture method of B. subtilis W-17
The γ-PGA production was conducted in 5-L bioreactor (Baoxing Corp., Shanghai, China) containing 3 L fermentation medium. The bioreactor was inoculated with 10% inoculum, and cultured at 37 °C and 200 rpm for 60 h. Three replicates were carried out for each experiment.
2.6 Analytical methods
The content of amino acid nitrogen in hydrolysate was determined by formaldehyde titration (Tamborini et al., 2019). The total nitrogen content was determined by Automatic Kjeldahl nitrogen analyzer. The content of glutamate was determined by SBA-40C biosensor. The degree of hydrolysis was calculated as follows:
The γ-PGA yield was measured by gel permeation chromatography (GPC) system following the method reported previously (Wu et al., 2006).
3 Results and discussion
3.1 Composition and characteristics of γ-PGA waste biomass
The process diagram of the whole experiment is shown in Fig. 1. The γ-PGA waste biomass was separated by centrifugation. The composition of protein and glutamate were shown in Table 1. This study tried to use γ-PGA waste biomass instead of tryptone for γ-PGA production. Therefore, the content of each component in tryptone was also detected for comparison.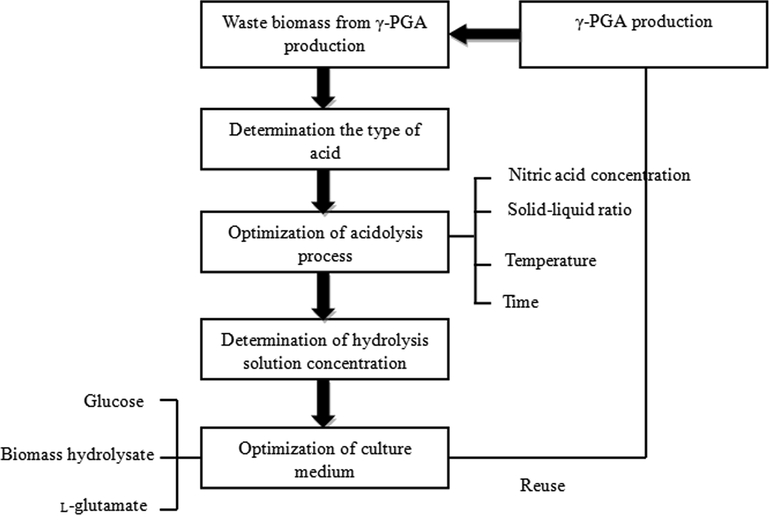
Flow chart.
Parameter
γ-PGA waste biomass
Tryptone
Total nitrogen (g/100 g)
5.76 ± 0.11
11.02 ± 0.35
Crude protein (g/100 g)
35.80 ± 0.37
76.43 ± 0.74
Amino acid nitrogen (g/100 g)
4.48 ± 0.11
4.25 ± 0.12
Glutamate (g/100 g)
2.75 ± 0.09
0.35 ± 0.01
It could be seen from Table 1 that the contents of total nitrogen and crude protein in γ-PGA waste biomass were significantly lower than those in tryptone, which were only 52.27% and 46.84% of the latter, respectively. However, this did not affect γ-PGA waste biomass to replace tryptone as nitrogen source. Because the concentration difference could be compensated by increasing the amount of γ-PGA waste biomass. In addition, the content of glutamate in waste biomass was 7.86 times of that in tryptone. Because a large amount of glutamate was needed in γ-PGA fermentation medium, using γ-PGA waste biomass instead of tryptone as nitrogen source could save the amount of glutamate.
3.2 Effect of different acids on hydrolysis efficiency
Hydrolysis efficiency of nitric acid was compared with other acids at the same condition (solid-liquid ratio 1:2, 90 °C, and 18 h). In terms of degree of hydrolysis and γ-PGA yield, nitric acid was more efficient than other acids (Table 2).
Forms
Level(%)
Degree of hydrolysis (%)
γ-PGA yield (g/L)
HCl
0.10
36.68 ± 0.46
15.54 ± 0.26
0.15
42.41 ± 0.40
16.32 ± 0.24
0.20
47.32 ± 0.56
15.43 ± 0.28
H2SO4
0.10
41.24 ± 0.41
13.32 ± 0.19
0.15
45.78 ± 0.31
14.08 ± 0.20
0.20
50.14 ± 0.34
12.36 ± 0.16
HNO3
0.10
50.94 ± 0.63
20.02 ± 0.13
0.15
59.45 ± 0.42
23.01 ± 0.36
0.20
62.22 ± 0.72
24.05 ± 0.41
H3PO4
0.10
25.24 ± 0.28
11.65 ± 0.10
0.15
28.43 ± 032
13.17 ± 0.18
0.20
32.53 ± 0.42
16.01 ± 0.25
The hydrolysis efficiency of phosphoric acid was too low to further investigation. Although the hydrolysis efficiencies of hydrochloric acid and sulfuric acid were lower than nitric acid, their hydrolysis parameters might be not at optimal conditions. So hydrochloric acid and sulfuric acid could not abandon and required further study. Table 2 showed the effect of hydrochloric acid and sulfuric acid on γ-PGA yield.
The γ-PGA yields were very low after hydrolysis by sulfuric acid and hydrochloric acid. The results showed that sulfur and chlorine could inhibit the γ-PGA production. Although dilute sulfuric acid and hydrochloric acid were widely used in biomass hydrolysis, the hydrolysates after treatment contained a lot of sulfate and chloride ions. The results showed that sulfate could inhibit the process of methanogenesis. This inhibition was accomplished by sulfate reducing bacteria in two ways. One was that sulfate reducing bacteria competed with methanogens in anaerobic fermentation system to utilize substrates such as acetic acid, propionic acid and butyric acid, thus reducing methane production (Sarchami and Rehmann, 2015; Kalyuzhnyi et al., 1998). The other was that sulfate was reduced to H2S by sulfate reducing bacteria, which was toxic to many microorganisms. The main reason was that H2S could diffuse into the cells and denaturate the proteins in the cytoplasm (Sam-Soon et al., 1991). In addition, Table 2 showed that sulfur and chloride ions could inhibit γ-PGA fermentation. Therefore, this study used nitric acid to hydrolyze γ-PGA waste biomass.
The result was similar to those that had been well documented. Gurgel et al. (2012) found that hydrolysis of sugar cane bagasse with nitric acid was more effective than other more common acids, in terms of short treatment time and reduced generation of inhibitors. Nitrate ( ), the neutralized species of nitric acid, is known to promote cell growth and likely so in view of the typical biological way of nitrogen assimilation. Sung et al. (2014) also reported that nitric acid was the best acid catalyst, which efficiently hydrolyzed inulin with the limited production of toxicants and at the same time served as a nitrogen source for the yeast growth.
3.3 Effect of nitric acid concentration on degree of hydrolysis
The conditions of biomass hydrolysis were as follows: solid-liquid ratio 1:2, 90 °C, and 18 h. If the waste biomass was dried first and then hydrolyzed by adding acid. It not only had many processes, but also caused energy waste. Therefore, the waste biomass was hydrolyzed directly without drying. Fig. 2(a) shows the effect of nitric acid concentration on the degree of hydrolysis of waste biomass.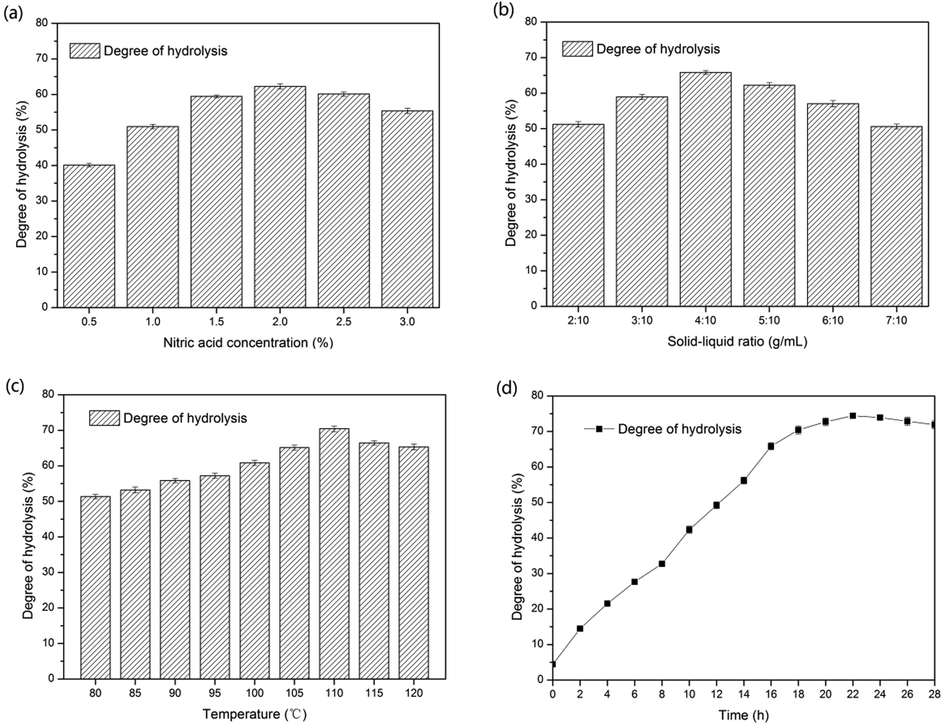
Effect of acid hydrolysis conditions on degree of hydrolysis. (a) nitric acid concentration; (b) solid-liquid ratio; (c) temperature; (d) time.
Too much nitric acid increased the side reaction under high acid. In addition, alkaline substances should be used to adjust the pH value of the hydrolysate. So the amount of nitric acid should be reduced as much as possible. But if the dosage was too small, the hydrolysis was not complete. Moreover, the product concentration was too low, which was not conducive to fermentation production. It can be seen from Fig. 2(a) that the hydrolysis effect was the best when the nitric acid concentration was 2%.
3.4 Effect of solid-liquid ratio on degree of hydrolysis
The effect of solid-liquid ratio on the degree of hydrolysis is shown in Fig. 2(b). When the solid-liquid ratio was low, the degree of hydrolysis was low. With the increase of solid-liquid ratio, the degree of hydrolysis increased gradually. When the solid-liquid ratio was 4:10, it reached the highest value. It can be seen from Fig. 2(b) that the hydrolysis effect is the best when the solid-liquid ratio is 4:10.
3.5 Effect of temperature on degree of hydrolysis
The effect of temperature on the degree of hydrolysis was investigated under the conditions of material water ratio of 4:10, acid content of 2% and hydrolysis time of 18 h. The experimental results are shown in Fig. 2(c).
With the increase of temperature, the degree of hydrolysis increased. However, too high temperature caused decarboxylation reaction of amino acid nitrogen, reduced the content of free amino acid nitrogen and the degree of hydrolysis. It can be seen from Fig. 2(c) that the hydrolysis effect is the best when the temperature is controlled at 110 °C.
3.6 Effect of time on degree of hydrolysis
With the increase of hydrolysis time, the degree of hydrolysis increased to the maximum. After further hydrolysis, not only the amino acid nitrogen increased slightly, but also some amino acid nitrogen was destroyed under acidic conditions. It reduced the concentration of amino acid nitrogen in the hydrolysate and increased the energy consumption. In the process of hydrolysis, the Maillard reaction between glucose and amino acid nitrogen took place at high temperature, which made the product dark and consumed the amino acid nitrogen in the hydrolysate, thus reducing the content of amino acid nitrogen in the product. However, if the hydrolysis time was too short, the degree of hydrolysis would be affected. Therefore, when the amino acid nitrogen accumulated to the maximum concentration, hydrolysis should be stopped to avoid side reactions. It can be seen from Fig. 2(d) that the best hydrolysis effect is 22 h.
3.7 Optimization of acidolysis process
The range of each factor is determined by single factor experiment, but there may be interaction among various factors. In order to further investigate the influence of various factors in the process of acid treatment on the hydrolysis of waste biomass, according to the results of single factor experiment, the orthogonal design L9 (43) with 4 factors and 3 levels was used to optimize the hydrolysis parameters (Altiner, 2019). The orthogonal experimental design and results were shown in Table 3, and the range analysis was shown in Table 4.
Trial no.
A
Nitric acid (%)B
Solid-liquid ratio (g/mL)C
TemperatureD
Time(h)Degree of hydrolysis (%)
1
1:5
3:10
105
20
61.98 ± 0.52
2
1:5
4:10
110
22
73.25 ± 0.47
3
1:5
5:10
115
24
66.63 ± 0.56
4
2
3:10
110
24
74.43 ± 0.64
5
2
4:10
115
20
74.17 ± 0.73
6
2
5:10
105
22
65.13 ± 0.54
7
2.5
3:10
115
22
67.19 ± 0.61
8
2.5
4:10
105
24
69.38 ± 0.49
9
2.5
5:10
110
20
67.51 ± 0.54
k1
67.287
67.867
65.497
67.887
k2
71.243
72.267
71.730
68.523
k3
68.027
66.423
69.330
70.147
R
3.956
5.844
6.233
2.260
Factors
SS
df
F
F0.05
Significant
A
26.550
2
8.258
19.000
B
55.588
2
14.822
19.000
C
159.309
2
19.279
19.000
*
D
8.148
2
1.000
19.000
Error
8.15
2
The larger the R value was, the greater the influence of this factor on the test results was. It could be seen from Table 4 that the order of influence of various factors on the hydrolysis effect of waste biomass was: temperature (C) > solid-liquid ratio (B) > nitric acid concentration (A) > time (D). Therefore, the temperature was the main factor, followed by the solid-liquid ratio and nitric acid concentration. The hydrolysis time had little effect on the degree of hydrolysis. Combined with the results of range analysis and variance analysis, the optimal process conditions were obtained. The optimal combination was A2B2C2D3, that was, nitric acid concentration was 2%, solid-liquid ratio was 4:10, the temperature was 110 °C, and time was 24 h.
It could be seen from Table 4 that temperature (C) had a significant effect on the degree of hydrolysis (P < 0.05). Solid-liquid ratio (B), nitric acid concentration (A) and time (D) had no significant effect on the degree of hydrolysis. The optimal combination was A2B2C2D3, but there was no experiment of this combination in Table 3. Therefore, the verification test was carried out according to this combination. Under the optimal conditions, the hydrolysis rate reached 77.08 ± 0.48%.
3.8 Determination of hydrolysis solution concentration
In this study, biomass hydrolysate was tried to replace tryptone. Therefore, only 44.55 mL biomass hydrolysate was needed to replace 9 g tryptone theoretically. But in fact, due to the complexity of the composition of waste biomass, the addition of nitric acid also provided a part of inorganic nitrogen source. So further experiment was needed to determine the amount of biomass hydrolysate.
It can be seen from Fig. 3 that the optimal amount of biomass hydrolysate is 55 mL. The reason why the theoretical value was higher might be that the protein and polypeptide in biomass hydrolysate were not as easy to be absorbed and utilized as the protein in tryptone.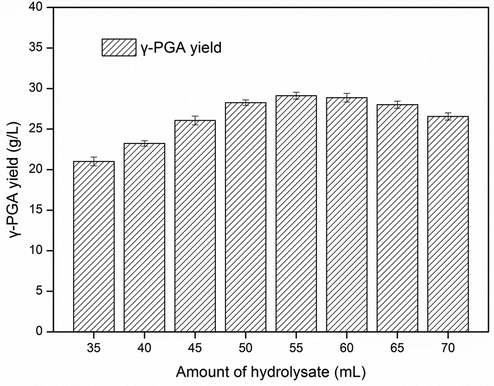
Effect of hydrolysate concentration on γ-PGA production.
3.9 Optimization of culture medium
In this study, biomass hydrolysate was used as a substitute for tryptone. Because the components of hydrolysate and tryptone were quite different, the medium needed to be re-optimized. The orthogonal design L9 (43) with 4 factors and 3 levels was used to optimize the medium composition (Sohrabi et al., 2017). One of the variables was set to a blank column. The experimental results were shown in Table 5 and Table 6.
Trial no.
E
Glucose (g/L)F
Biomass hydrolysate (mL)G
L-glutamate (g/L)γ-PGA yield (g/L)
1
34
50
25
23.55 ± 0.51
2
34
55
28
25.55 ± 0.54
3
34
60
31
27.32 ± 0.62
4
36
50
28
24.77 ± 0.58
5
36
55
31
27.56 ± 0.56
6
36
60
25
29.56 ± 0.42
7
38
50
31
24.60 ± 0.54
8
38
55
25
28.21 ± 0.53
9
38
60
28
30.01 ± 0.43
k1
25.473
24.307
27.107
k2
27.297
27.107
26.777
k3
27.607
28.963
26.493
R
2.134
4.656
0.614
Factors
SS
df
F
F0.05
Significant
E
7.972
2
23.868
19.000
*
F
32.972
2
98.719
19.000
*
G
0.565
2
1.692
19.000
Error
8.15
2
According to the range analysis in Table 5, the effect of three factors of medium on γ-PGA yield was F > E > G, that was, the amount of hydrolysate had the most significant effect on γ-PGA yield. The best combination was E3F3G1 by range analysis. Analysis of variance showed that glucose and hydrolysate had significant effects on γ-PGA yield. The theoretical optimal formula of medium (E3F3G1) was verified, and the γ-PGA yield was 30.69 ± 0.42 g/L, which was 4.49% higher than that in tryptone medium (29.37 ± 0.43 g/L).
3.10 Comparison of different media on γ-PGA production
The γ-PGA yield in different media was investigated. Fig. 4(a) shows that the γ-PGA yield in the hydrolysate medium is not different from that in the optimal medium, indicating that the biomass hydrolysate can replace tryptone in the medium.
Comparison of different media on γ-PGA production and effect of reuse times of waste biomass. (a) Fermentation curve in different media; (b) effect of reuse times of waste biomass.
The γ-PGA yield in hydrolysate medium was even slightly higher than that in the optimal medium (30.69 ± 0.42 g/L VS 29.37 ± 0.43 g/L), which might be due to the fact that the amount of components in the optimal medium had not reached the optimal value and there was still room for improvement. At the same time, the effect of reuse of waste biomass on γ-PGA yield was investigated. The results are shown in Fig. 4(b). The results showed that the γ-PGA yield decreased with the increase of cycle times. The reason might be that the fermentation inhibitors began to accumulate gradually in the circulation process, and the effect on γ-PGA yield gradually increased. However, the γ-PGA yield in the first two cycles decreased slightly. Compared with the yield in the optimal medium, the γ-PGA yield of second cycle had little difference (29.01 ± 0.65 g/L VS 29.37 ± 0.43 g/L). In the third cycle, the γ-PGA yield was significantly different from that in the optimal medium(27.71 ± 0.73 g/L VS 29.37 ± 0.43 g/L). So the waste biomass could be recycled for 2 times. In the later research, the types of inhibitors and the methods to eliminate inhibitors were further studied, and constantly improved the biomass recycling technology.
Different media were compared in Table 7. Compared with the medium of traditional technologies 1, the cost of nitrogen source in this study was lower, and there were no costs of purchase and transportation (Liu et al., 2018). In summary, this medium had the following advantages: (1) the cost of tryptone in the medium was saved; (2) the environmental pollution caused by γ-PGA waste biomass was reduced; (3) because there was a lot of L-glutamate in the hydrolysate, the amount of L-glutamate was reduced in the medium; (4) The waste biomass could be reused. Although there was an accumulation of inhibitors in the hydrolysate, the inhibition was low when the number of cycles was 2, and the γ-PGA yield was ideal. In addition, the amount of glucose increased by 2 g. However, compared with tryptone and glutamate, the price of glucose was very low, and the money saved far exceeded the increased cost. This study opened up a new way to utilize waste biomass for production enterprises, and reduced the cost of raw materials in the process of γ-PGA production.
Forms
Nitrogen source
γ-PGA (g/L)
Nitrogen source price (dollar/kg)
Is waste biomass reused/ reused times ?
L-glutamate dosage
Remarks
Reference
Traditional technologies 1
Glutamate waste biomass
23.77
0.1–0.2(Purchase and transportation costs)
Yes/once
60
There was still the problem of γ-PGA waste biomass treatment.
Liu et al., 2018
Traditional technologies 2
Tryptone
29.37 ± 0.43
1.8–2.0
No
28
This study
This study
γ-PGA waste biomass
30.69 ± 0.42
0
Yes/twice
25
Compared with technology 2, the consumption of glucose was increased by 2 g, and the costs of nitrogen source and glutamate acid were reduced.
So the total cost was still reduced.
4 Conclusion
In order to reduce the cost of γ-PGA medium and solve the pollution problem of γ-PGA waste biomass, the biomass hydrolysate was used to replace tryptone in the medium. In terms of degree of hydrolysis and γ-PGA yield, nitric acid was more efficient than other acids. So the waste biomass was treated with nitric acid to obtain hydrolysate. Through the orthogonal optimization experiment, the optimal conditions of biomass hydrolysis were obtained as follows: 2% nitric acid, solid-liquid ratio 4:10, 110℃, and 24 h. Under the optimal conditions, the degree of hydrolysis reached 77.08 ± 0.48%. The fermentation medium was further optimized: biomass hydrolysate 60 mL, glucose, 38 g/L, L-glutamate 25 g/L. In the optimal medium, γ-PGA yield was 30.69 ± 0.42 g/L, which was 4.49% higher than that in tryptone medium(29.37 ± 0.43 g/L). The results showed that the waste biomass hydrolysate could be used as tryptone for γ-PGA production. Moreover, the addition of waste biomass hydrolysate reduced the amount of glutamate in the medium. In addition, there was a problem of inhibitor accumulation in the reuse process of waste biomass. Due to the small amount of inhibitors in the early cycle, the waste biomass could be reused twice. In a word, it was feasible for waste biomass hydrolysate to replace tryptone in γ-PGA production. This study provided a new idea for the cleaner production technology of γ-PGA.
Acknowledgments
This research was financially supported by State Key Laboratory of Microbial Technology (M2012-14), Shandong University.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Altiner, M., 2019. Use of Taguchi approach for synthesis of calcite particles from calcium carbide slag for CO 2 fixation by accelerated mineral carbonation. Arab. J. Chem. 12, 531–540. https://doi.org/10.1016/j.arabjc.2018.02.015
- Gurgel, L.V.A., Marabezi, K., Zanbom, M.D., Curvelo, A.A.D.S., 2012. Dilute acid hydrolysis of sugar cane bagasse at high temperatures: A Kinetic study of cellulose saccharification and glucose decomposition. Part I: Sulfuric acid as the catalyst. Ind. Eng. Chem. Res. https://doi.org/10.1021/ie2025739
- Kalyuzhnyi, S., Fedorovich, V., Lens, P., Hulshoff Pol, L., Lettinga, G., 1998. Mathematical modelling as a tool to study population dynamics between sulfate reducing and methanogenic bacteria. In: Biodegradation. https://doi.org/10.1023/a:1008339018423
- Microbial synthesis of poly-γ-glutamic acid (γ-PGA) with fulvic acid powder, the waste from yeast molasses fermentation. Biotechnol. Biofuels. 2020
- [CrossRef] [Google Scholar]
- Hydrolysis of glutamic acid fermented waste bacteria and substitution of yeast extract for production of polyglutamic acid. Chinese J. Environ. Eng.. 2018;12:2403-2411.
- [CrossRef] [Google Scholar]
- Microbial synthesis of poly-γ-glutamic acid: Current progress, challenges, and future perspectives. Biotechnol. Biofuels. 2016
- [CrossRef] [Google Scholar]
- Peng, G.X.L.J., 2015. Study on Reuse Process of Hydrolysis Solids of L-ornithine Fermentation Residues 92–95. https://doi.org/10.3969/j.issn.1005-6521.2015.20.025
- Effect of sulphate on pelletisation in the UASB system with glucose as substrate. Water SA. 1991
- [Google Scholar]
- Optimizing acid hydrolysis of Jerusalem artichoke-derived inulin for fermentative butanol production. Bioenergy Res 2015
- [CrossRef] [Google Scholar]
- Biodiesel production from yeast Cryptococcus sp. using Jerusalem artichoke. Bioresour. Technol. 2014
- [CrossRef] [Google Scholar]
- The production of poly-(γ-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 2001
- [CrossRef] [Google Scholar]
- Sohrabi, M.R., Khavaran, A., Shariati, Shahab, Shariati, Shayan, 2017. Removal of Carmoisine edible dye by Fenton and photo Fenton processes using Taguchi orthogonal array design. Arab. J. Chem. 10, S3523–S3531. https://doi.org/10.1016/j.arabjc.2014.02.019
- Tamborini, L.H., Militello, M.P., Balach, J., Moyano, J.M., Barbero, C.A., Acevedo, D.F., 2019. Application of sulfonated nanoporous carbons as acid catalysts for Fischer esterification reactions. Arab. J. Chem. 12, 3172–3182. https://doi.org/10.1016/j.arabjc.2015.08.018
- Tao, L., Tian, L., Zhang, X., Huang, X., Long, H., Chang, F., Li, T., Li, S., 2020. Effects of γ-polyglutamic acid on the physicochemical properties and microstructure of grass carp (Ctenopharyngodon idellus) surimi during frozen storage. LWT. https://doi.org/10.1016/j.lwt.2020.109960
- Biosynthesis of poly(γ-glutamic acid) in Bacillus subtilis NX-2: Regulation of stereochemical composition of poly(γ-glutamic acid) Process Biochem 2006
- [CrossRef] [Google Scholar]
- Zhang, C., Ren, H.X., Zhong, C.Q., Wu, D., 2020. Biosorption of Cr(VI) by immobilized waste biomass from polyglutamic acid production. Sci. Rep. https://doi.org/10.1038/s41598-020-60729-5
- Zhang, C., Wu, D., Qiu, X., 2018. Stimulatory effects of amino acids on γ-polyglutamic acid production by Bacillus subtilis. Sci. Rep. https://doi.org/10.1038/s41598-018-36439-4
- Economical production of agricultural Γ-polyglutamic acid using industrial wastes by Bacillus subtilis. Biochem. Eng. J. 2019
- [CrossRef] [Google Scholar]
- Fishmeal wastewater as a low-cost nitrogen source for γ-polyglutamic acid production using Bacillus subtilis. Waste Biomass Valorization 2019
- [CrossRef] [Google Scholar]







