Translate this page into:
Synergism of thiocyanate ions and microinterfacial surface as driving forces for heavy multi-metals extraction
⁎Corresponding authors. capalau_nicoleta@yahoo.com (Nicoleta Liliana Olteanu), maria.mihaly@upb.ro (Maria Mihaly)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A comprehensive study has been carried out to evaluate the extraction of heavy metals mixture (Co2+, Cr3+, Cu2+, Ni2+) from aqueous media by Winsor II non-ionic microemulsion, containing polyoxyethylene (4) lauryl ether as non-ionic surfactant and butyl acetate as organic phase. The extraction mechanism is based on the formation of thiocyanate complexes of metals and their transfer from aqueous to microemulsion phase, either towards the interfacial film of surfactant (Co2+) or into the core of micelles (Cr3+, Cu2+, Ni2+). The value of the distribution coefficient for Co2+ was higher than for the other studied metals and its extraction efficiency was not dependent on the working conditions, showing a maximum value (99.99%) in all cases. By using successive extractions, chromium, nickel and copper ions that remained in the aqueous phase after first extraction were transported into the microemulsion phase, leading to an increase in the extraction efficiency up to 99.99% for chromium and copper, and 85% for nickel. Based on pH influence, a selective extraction of Co2+ and Cr3+ can be achieved, since the cobalt ions were completely extracted into the microemulsion phase at pH = 1, and the chromium ions still remained in the aqueous phase.
Keywords
Nonionic microemulsion
Thiocyanate complexes of heavy metals
Simultaneous heavy metals extraction
Microinterfacial effect
Synergism
1 Introduction
There are several conventional processes that have been used to remove and recover metal ions from aqueous media, including physical, chemical and biological technologies. All these methods have their own capabilities and limitations (Ihsanullah et al., 2016; Wang and Jason Ren, 2014; Barakat, 2011; Gunatilake, 2015; Chang and Wang, 2007; Khosravi and Alamdari, 2009).
A fairly new method, namely oil continuous microemulsion (Winsor II system (WII)), has been introduced as very efficient (Dantas et al., 2004; Mihaly et al., 2010; Al-Ghouti et al., 2013) for heavy metals ions recovery. Winsor II represents a two phases system: water in oil microemulsion with excess of water (W/O, W). This extraction system is very effective for the improvement of extractability as well as for the acceleration of extraction process due to an enormous rise of the microinterfacial surface area in the W/O microemulsion phase (Beltrame et al., 2005; Steytler et al., 2001; Gao et al., 2012). Various types of metallic cations have been extracted by this kind of microemulsion, such as chromium (Castro Dantas et al., 2001; Melo et. al., 2015; Li et al., 2009), copper (Arain et al., 2016), gold (Lu et al., 2011), cobalt (Tao et al., 2006), lanthanide (Zeng et al., 2005; Wang et al., 2012; Xia et al., 2008) and other metals (Castro Dantas et al., 2003; Cadar et al., 2017; de Namora et al., 2012).
The aim of the present work is to study the extraction of heavy metals mixture (HMM) (Co2+, Cr3+, Cu2+, Ni2+) from synthetic aqueous media by WII nonionic microemulsion. The proposed system contains polyoxyethylene (4) lauryl ether (Brij30) as nonionic surfactant, butyl acetate (BuOAc) as organic phase and sodium thiocyanate (NaSCN) as complexing agent. Nonionic surfactant was used since it has low toxicity and exhibits better solubility properties in microemulsion systems (Pillai and Shah, 1996). Moreover, nonionic surfactants have the ability to form microemulsion without the assistance of co-surfactant. Butyl acetate has several advantages: it is manufactured on a large scale for use as a solvent; it has moderate volatility, moderate polarity index (4.0) and the lethal dose (LD50) for oral ingestion: 10.7 g/kg (rat) and dermal: 17.6 g/kg (rabbit), indicating low toxicity.
This approach starts from the following assumptions: (i) the microemulsion is able to transfer simultaneously more than one metal from aqueous to microemulsion phase; (ii) the thiocyanate ion exhibits linkage isomerism, bonding to the metal ion either through its sulphur or nitrogen atom, thus resulting into complexes with known stability constants (Nancollas and Torrance, 1967; Khan et al., 1999); (iii) butyl acetate acts both as the microemulsion organic phase and as a solvent for the thiocyanate complexes of metals.
In order to choose the best extraction system and the optimal conditions for heavy metals extraction, the influence of several factors on the extraction efficiency was studied: surfactant concentration and water to oil volumetric ratio (R), sodium thiocyanate concentration, successive extraction processes, pH and inorganic salt concentration. Kinetic studies and the distribution coefficient of heavy metal ions between aqueous and microemulsion phases were also investigated.
Prior to these studies, the Winsor II compositions for Brij30/BuOAc/Me(SCN)nm− (using sodium thiocyanate (NaSCN) dissolved in both water and a mixture of water:acetone 1:1 (w:w)) were determined.
2 Materials and methods
2.1 Materials
Butyl acetate, BuOAc (99%) was purchased from Sigma-Aldrich and was used as organic phase. Nonionic surfactant, polyoxyethylene (4) lauryl ether, Brij30, with molecular formula C12H25(OCH2CH2)4OH was provided by Acros Organics. The heavy metals solution was prepared by mixing appropriate amounts of metal salts. Cobalt nitrate, Co(NO3)2·6H2O and nickel nitrate, Ni(NO3)·6H2O were supplied by Merck. Chromium nitrate, Cr(NO3)3·9H2O and copper nitrate, Cu(NO3)2·6H2O were purchased from Sigma-Aldrich. Sodium thiocyanate NaSCN was used as complexing agent for heavy metals and was supplied by Merck. Distilled water was used for the preparation of the microemulsion samples. All chemicals were used as received without further purification.
2.2 Methods
2.2.1 Extraction procedure
The microemulsion used in this study was composed by Brij 30, as nonionic surfactant, BuOAc as organic phase, sodium thiocyanate as complexing agent and an aqueous media containing the metal ions solutions corresponding to 9 different compositions (Table 1). Since the aim of the present study is the extraction of the Cr3+, Co2+, Ni2+ and Cu2+ ions as a mixture using the microemulsification technique, the synthetic aqueous phase was obtained by dissolving the corresponding metallic salt in distilled water.
R = Vw/Vo
Brij 30
% w/w
BuOAc
% w/w
HMM aq.sol.*
% w/w
Brij 30
% w/w
BuOAc
% w/w
HMM aq.sol.*
% w/w
39.00
5
2.35
92.65
10
2.25
87.75
19.00
5
4.75
90.25
10
4.50
85.50
9.00
5
9.50
85.50
10
9.00
81.00
5.67
5
14.25
80.75
10
13.50
76.50
4.00
5
19.00
76.00
10
18.00
72.00
3.00
5
23.75
71.25
10
22.50
67.50
2.33
5
28.50
66.50
10
27.00
63.00
1.86
5
33.20
61.80
10
31.50
58.50
1.50
5
38.00
57.00
10
36.00
54.00
Since it is desirable to obtain a ratio between the aqueous and microemulsion phases (F) greater than one, which enables to obtain a high volume of clean water, 5% and 10% surfactant concentrations were used. At higher surfactant concentrations, a microemulsion volume greater than aqueous phase is obtained, which is not prefered from practical point of view.
The extraction experiments were performed in a hermetically sealed glass container in order to preserve the composition of the system, since the organic phase is a volatile compound. The microemulsion was vigorously shaken for about 2 min, and then was allowed to equilibrate at room temperature. Finally, only the samples with two clear phases were considered for heavy metals extraction in which the upper phase was represented by the W/O microemulsion, strongly colored due to its rich content in metal complexes, and the bottom colourless phase represented by the clear aqueous phase. At the end of the procedure, after the thermodynamic equilibrium was achieved, the two phases were collected separately.
2.2.2 Quantification of heavy metals concentration
The concentrations of heavy metals in the aqueous phase were determined by Flame Atomic Absorption Spectroscopy (FAAS) using an Analytik Jena AAS – CONTRAA 700 spectrometer in an air-acetylene flame, equipped with a Xenon lamp and Aspect CS Version 1.5.6.0 software for electronic processing of the results.
2.2.3 Spectrophotometric measurements
After achieving the thermodynamic equilibrium, the microemulsion phase, rich in heavy metal content was collected. The electronic spectra of the studied systems were recorded using a UV–VIS/NIR spectrophotometer type V670, Jasco, in the range 200–800 nm.
2.2.4 Extraction efficiency
The extraction efficiency of the heavy metal ions by the microemulsification technique was calculated using the following formula:
Cin = the heavy metals concentration in the aqueous solution subjected to extraction;
Cfin = the heavy metals concentration remained in the aqueous phase after their extraction in the microemulsion phase.
3 Results and discussion
The extraction efficiency of HMM by WII microemulsion may be dependent on many factors including the transferring agent, kinetics process, equilibrium partition, inorganic salt concentration or the environment.
3.1 Heavy metals extraction promoted by ion association complexes of metal with thiocyanate
The heavy metals extraction from aqueous solution using microemulsification technique is promoted by the conformational changes and stability of the ion association complexes, which they form with the SCN− ion. In Table 2 are presented the electronic transitions and their corresponding wavelength for metal salts and metal thiocyanate complexes in different environments compared to their electronic transitions in microemulsion phase. The electronic spectra are presented in the Supporting Information. *All solvents = Water (W), Water/Acetone (W/Ace), Butyl Acetate (BuOAc), Brij30 0.3 mM (B30 0.3 mM), Brij30 1 mM (B30 1 mM); mE = microemulsion, Oh = Octahedric; Td = Tetrahedric; LMCT = Ligand to metal charge transfer.
Complex
Environment
Geometry
λ (nm)
Electronic transitions
[Co(H2O)6]2+
All solvents
Oh
510
480 (shoulder)
[Co(SCN)4]2−
W, B30 0.3 mM
Oh
510
480 (shoulder)
B30 1 mM
Oh
Td510
626
W/Ace, BuOAc,
mETd
623
586
[Cr(H2O)6]3+
All solvents
Oh
576
410
[Cr(SCN)x]3−
All solvents, mE
Oh
576
410
[Ni(H2O)6]2+
All solvents
Oh
721
657
393
[Ni(SCN)x]3−
All solvents, mE
Oh
721
657
393
[Cu(H2O)6]2+
All solvents
Oh
800
CuSCN
All solvents, mE
–
364
LMCT
The electronic spectra of hydrated metals salt or thiocyanate complexes of Cr3+ and Ni2+, in different solvents and microemulsion phase (see Supporting Information), showed no changes in VIS spectrum, maintaining an octahedral geometry; hence the same two spin-allowed transitions are expected (Table 2), as the Tanabe - Sugano diagram shows (Tanabe and Sugano, 1954).
The charge transfer bands presented in the UV region involve p → d or d → p transitions. The high intensity of these bands is due to the fact that these transitions are both Laporte and spin-allowed.
The VIS spectra of cobalt nitrate hexahydrate, in all the tested solvents, micellar systems, and microemulsion phase (see Supporting Information), presents similar features, while in the case of cobalt thiocyanate complex is no longer noticed the same behaviour.
The electronic spectra of cobalt thiocyanate complex show that in a less polar solvent, the cobalt ions occupy tetrahedral (Td) symmetry sites leading to the formation of [Co(SCN)4]2−. Tetrahedral Co2+ has the same energy level scheme as Cr3+ ion in octahedral symmetry. From T-S diagram, the ground state is a non-degenerated 4A2, and the spin multiplicity is a quartet. The visible ground-state absorption band centered at 623 nm is assigned to the spin- and electric-dipole-allowed 4A2 → 4T1(4P) transition, while the shoulder observed at 586 nm can be assigned to one of the doublet levels arising from the 2G free-ion level (Yumashev et al., 2000). The big difference between the very intense d-d bands in the blue Td complex [Co(SCN)4]2−, compared with the much weaker band in the pink octahedral complex [Co(H2O)6]2+ arises because the Td complex has no center of symmetry, helping to overcome the g ↛ g Laporte selection rule.
The experimental UV–VIS spectra of copper thiocyanate complex, in different solvents showed that the band at around 800 nm disappears, while another band appears at 364 nm. This band can be assigned to a ligand to metal charge transfer transition and the formation of CuSCN, wherein the metal ion was reduced from 2+ to 1+ charge.39 The formation of copper monothiocyanate is also sustained by the high value of stability constant (213 M−1) compared with that for nickel and cobalt monothiocyanate (∼50 M−1) (Giacomelli et al., 2004).
In all octahedral complexes that typify the hydrated metal salts, the absorption band at around 300 nm is due to the π – π∗ transition of uncomplexed nitrate counterions (Sun et al., 2006).
From experimental spectra one can observe that for the formation of tetrahedral [Co(SCN)4]2− it is necessary the presence of a less polar solvent. Hence, we can assume that the tetrahedral complex is formed in the microemulsion phase, more precisely in the interfacial film of surfactant, where the major phase is butyl acetate.
In the case of Cu2+ and SCN− ions, the CuSCN is formed in the aqueous solution, leading to the assumption that its distribution between aqueous and microemulsion phase will take place in the core of the reverse micelles.
For chromium and nickel thiocyanate complexes, the insignificant changes of electronic spectra lead to the supposition that a mixture of less stable complexes is formed. Thus, their distribution between the two phases of the microemulsion system will be conditioned by the repartition of the more stable complexes represented by cobalt and copper thiocyanate.
Based on these observations, the thiocyanate ions play the transferring agent role of the metal ions from the aqueous phase into the microemulsion. Therefore, different mechanisms of extraction for every tested metal will be expected.
3.2 Microemulsion formulations
The extraction of heavy metal ions from aqueous media and their further concentration in the microemulsion phase requires the use of WII microemulsion system. For this purpose, nine samples with water/oil volumetric ratio (R) higher than 1, which are currently used in phase diagram design (Rogozea et al., 2014; Fleancu et al., 2013), were chosen (Table 1) in order to determine the compositions where WII microemulsion are formed.
3.2.1 Phase changes promoted by acetone co-solvent addition
Because the formation of some thiocyanate metals complexes requires a polar or slightly polar environment, the influence of acetone on both phase transitions and heavy metals extraction within microemulsion was firstly studied. In the pseudo-ternary B30/BuOAc/Me(SCN)nm− system the aqueous solution contained NaSCN 5 mol/L and a mixture of Cr3+, Co2+, Ni2+, Cu2+ 0.15 g/L. The two studied systems contains similar components, except the addition of the co-solvent, acetone. The dependence of F volumetric ratio on R values under acetone influence is presented in Fig. 1 (with acetone) and Fig. 2 (without acetone).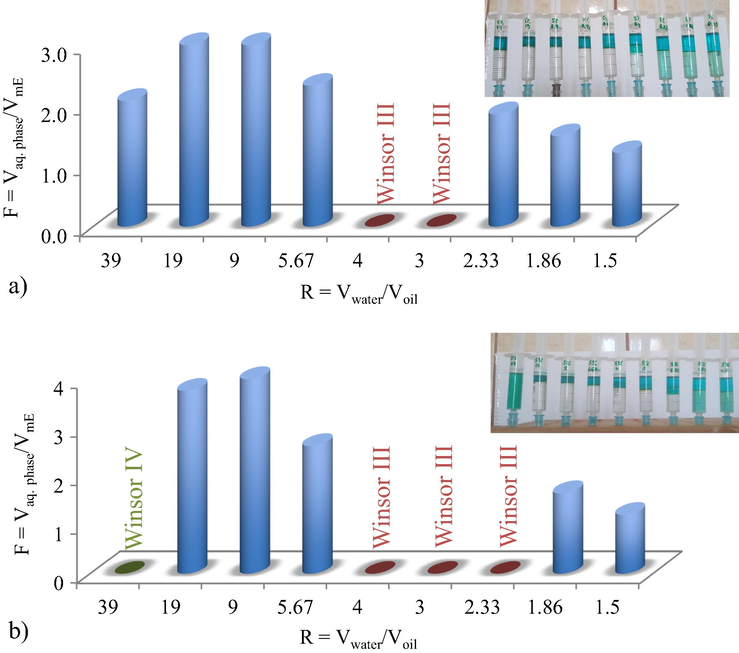
The influence of acetone on volumetric ratio of aqueous phase to microemulsion, F, at 5% Brij 30 concentration and different water/oil volumetric ratio, R, (a) with and (b) without acetone.
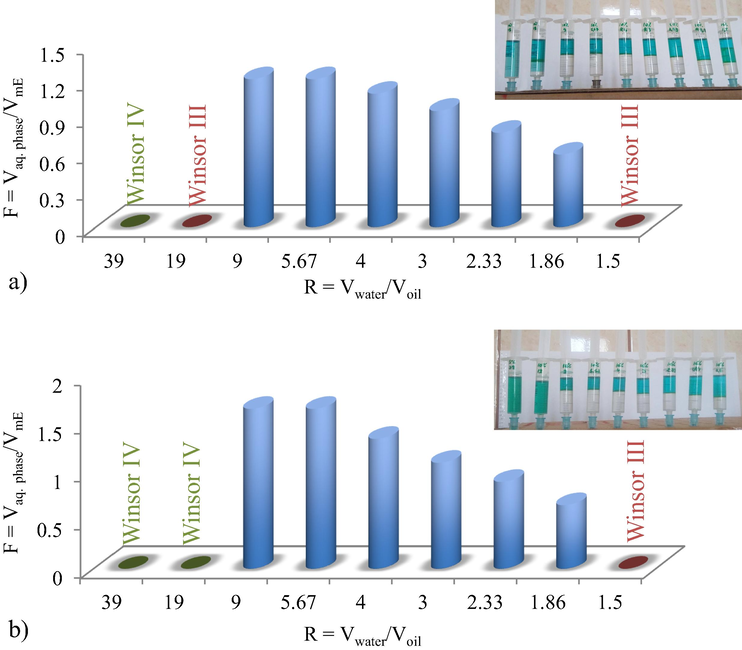
The influence of acetone on volumetric ratio of aqueous phase to microemulsion, F, at 10% Brij 30 concentration and different water/oil volumetric ratio, R, (a) with and (b) without acetone.
By comparing the two systems, one can notice that: (i) Winsor II (WII) microemulsions are formed at both surfactant concentration (5% and 10%) over a large R range; (ii) F values significantly higher than 1 are obtained using 5% surfactant concentration (Fig. 1a and 2a).
But then, some changes are considered: (i) the F volumetric ratio present a decrease tendency by acetone addition, as at 10% surfactant concentration and R < 3, values less than 1 are obtained, which from practical point of view is not preferred (Fig. 1b); (ii) a restriction of the WII microemulsion domain at 5% surfactant is observed in the absence of acetone, for R = 39 and R = 2.33 (Fig. 2); (iii) by acetone addition the partition equilibrium of metal thiocianate complexes between aqueous and microemulsion phases is unfavourable, as for R < 2.33, the aqueous phase is not completely cleaned.
The system without acetone allows microemulsion formulations corresponding to a higher F ratio, which is preferable, both in terms of reducing the reagent consumption, as well as for the concentration of the metal ions in a smallest microemulsion volume.
3.2.2 Phase changes promoted by heavy metals mixture increasing concentration
For this study, the concentration of Cr3+, Co2+, Ni2+ and Cu2+ from synthetic solutions varied in the range 0.05–0.6 g/L. The concentration of sodium thiocyanate was kept constant at 5.0 mol/L.
The increase of metal salt concentration in synthetic aqueous solution reduces the water content of microemulsion leading to an increase of F values for both surfactant concentrations (Fig.3). An explanation for this behavior is that the charge density inside W/O microemulsions increases with enhancing the salt concentration, therefore, it screens the repulsive interactions between surfactant head groups at the interfacial region of the W/O microemulsions. As the polar heads get closer, the water uptake and the size of W/O microemulsions decreases and, therefore the F volumetric ratio increases.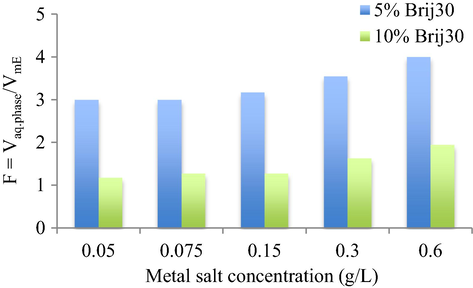
Influence of metal salt concentrations on the volumetric ratio of aqueous phase to microemulsion, F, at 5% and 10% Brij30 concentration.
3.3 Optimization of heavy metals mixture extraction by microemulsion
The influence of environmental parameters on the extraction efficiency is another important issue; thereby the surfactant concentration, the co-solvent addition, the effect of inorganic salt concentrations and pH were investigated. The kinetics of extraction for Cr3+ and Ni2+ were also investigated.
3.3.1 Optimum microemulsion compositions
In order to find out the optimum microemulsion compositions, in terms of the water to oil volumetric ratio, R, on simultaneous HMM extraction, several R values, varying between 19 and 3, at 5% and 10% surfactant concentrations were chosen (Fig. 4).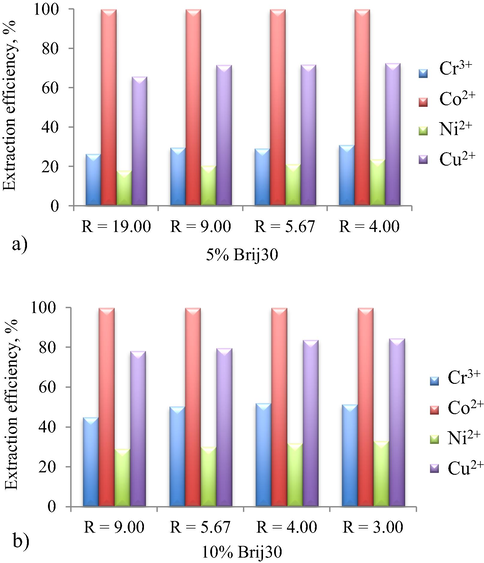
Extraction efficiency of HMM at (a) 5% and (b) 10% Brij30 concentrations and different water/oil volumetric ratio, R.
In the case of Co2+, the extraction efficiency is not affected by the surfactant concentration or by R volumetric ratio, showing a maximum value (99.99%) in all cases. By increasing the surfactant concentration and decreasing the R volumetric ratio an increase in the extraction efficiency of Cr3+, Ni2+ and Cu2+ was noticed.
Thus, two microemulsion compositions would be considered for further investigations: a water/oil volumetric ratio, R = 9, at 5% and, respectively, 10% Brij30 concentrations.
3.3.2 Acetone influence on extraction efficiency
In order to reveal the influence of co-solvent on the extraction efficiencies, two microemulsion systems, at 5% surfactant concentration and water/oil volumetric ratio, R = 9, with or without acetone addition, were investigated for HMM extraction (Fig. 5).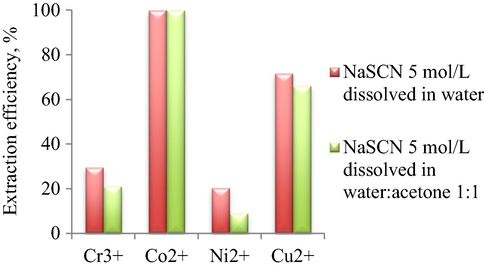
Influence of acetone on extraction efficiency of HMM at 5% Brij30 concentration and water/oil volumetric ratio, R = 9.
In the absence of NaSCN, the extraction of metal ions does not occur; hence no extraction efficiency was calculated. It can be seen that, the system containing aqueous NaSCN corresponds to higher Cr3+, Ni2+ and Cu2+ extraction efficiencies than the slightly polar NaSCN (water:acetone 1:1 (w:w) solvent). The extraction efficiency of Co2+ presented a maximum value in both systems.
Based on these results, the composition containing aqueous sodium thiocyanate was chosen for further studies.
3.3.3 Thiocyanate concentration influence on extraction efficiency
As it has been shown before, the presence of SCN− plays a significant role for the heavy metals extraction using microemulsification technique. Therefore, the influence of sodium thiocyanate concentration (2.5, 5.0 and 7.5 mol/L) on the HMM extraction efficiency, were investigated.
From Fig.6 one can observe a different behavior of the metal ions with the variation of the NaSCN concentration. While in the case of Co2+ the extraction efficiency is not affected by the sodium thiocyanate concentration, one cannot say the same about the other metals. For Cr3+ and Ni2+, the extraction efficiency is enhanced with the increasing of NaSCN concentration, while for Cu2+, a slightly decrease tendency can be observed.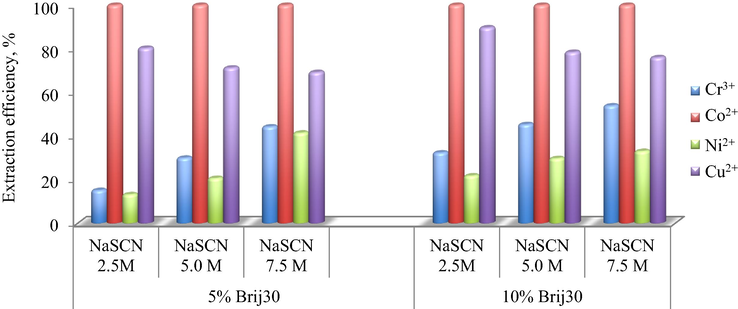
Influence of SCN− concentration on the extraction efficiency of HMM at 5% and 10% Brij30 concentration and water/oil volumetric ratio, R = 9.
A 5.0 mol/L thiocianate aqueous solution seems to be the optimum concentration for simultaneously extraction of all metals, at both surfactant concentrations.
3.3.4 Inorganic salts influence on extraction efficiency
In order to study how the presence of inorganic salts affects the extraction efficiency of heavy metal ions, the concentration of NaCl solution was ranging between 1 and 5%. Due to the fact that the surfactant is nonionic and the thiocyanate complexes have no charge, it was expected that the presence of inorganic salt to have no significant influence on the extraction efficiency.
As illustrated in Fig. 7, the presence of NaCl does not affect the extraction of Co2+ (99.99%). For the other metal ions, the extraction efficiency slightly increases from 30 to 35% in the case of Cr3+, from 73 to 78% for Cu2+ and from 22 to 27% for Ni2+ at 5% Brij30 surfactant concentration. As such, there is no significant improvement on extraction efficiency by adding inorganic salt.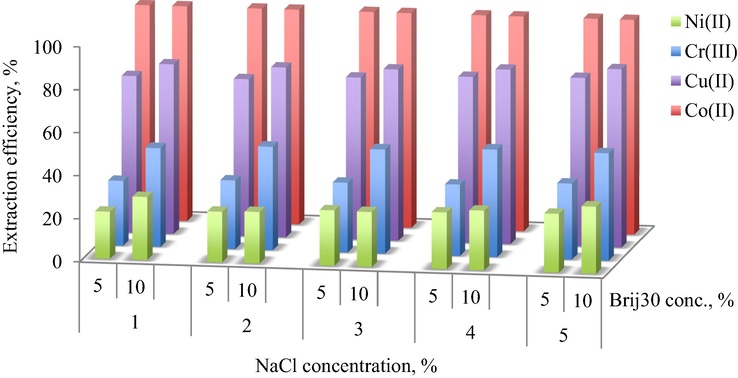
Influence of NaCl concentration on the extraction efficiency of HMM at (a) 5% Brij30, (b) 10% Brij30 concentrations and water/oil volumetric ratio, R = 9.
3.3.5 Selective extraction of heavy metal mixture by pH controlling
The pH of pristine solution containing Cr3+, Co2+, Ni2+ and Cu2+ 0.15 g/L was 3.5. In order to study the influence of pH on the extraction efficiency of the heavy metal ions, a pH range between 1 and 5 was investigated. Above this pH value, the precipitation of the metal hydroxides occurs, which makes impossible the extraction of metal ions using the microemulsification technique. From Fig. 8 it can be seen that Cr3+ presented the largest variation domain of extraction efficiency with the increasing of pH value. The extraction efficiency of Cr3+ increases from 2.6% (pH = 1) to 91.2% (pH = 5) for 5% Brij30 and from 10.2% (pH = 1) to 92.8% (pH = 5) for 10% Brij30 surfactant concentration. Another interesting finding is that the extraction efficiency of Co2+ is not affected by the pH variation, showing a maximum value (99.99%) in all cases. The extraction efficiencies of Cu2+ decreases with the increasing of pH value, while those for Ni2+ present an increasing tendency.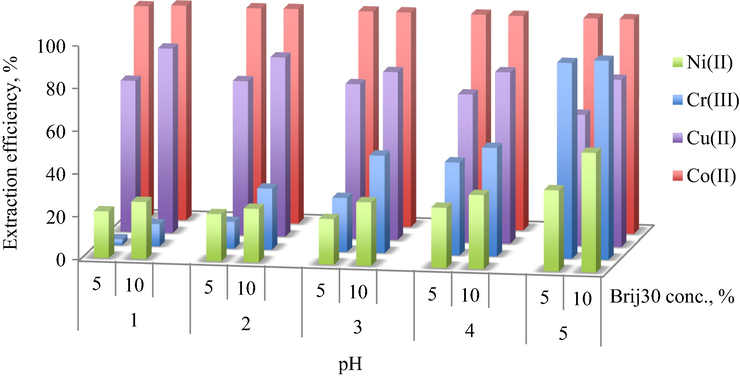
Influence of pH on the extraction efficiency of HMM at 5% and 10% Brij30 concentrations and water/oil volumetric ratio, R = 9.
Different extraction efficiencies for Cr3+, Co2+, Ni2+ and Cu2+ as a function of pH can be attributed to changes in the speciation of the metal complexes as the aqueous phase pH varies. For example, the improvement of Cr3+ extraction efficiency into the microemulsion phase would be explained by its transformation from the kinetically inert aqua complex Cr(H2O)33+ (acidic pH) into the more labile and less hydrated form Cr(H2O)(OH)2+ as illustrated in Scheme 1 (Hanrahan et al., 2003).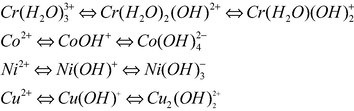
Speciation diagram showing changes in speciation with pH for illustrative purposes only.
Several factors can change metal ion speciation (Hanrahan et al., 2003) making it difficult to definitively assign the proper speciation for each metal. However, it should be apparent that speciation and pH changes might have a significant effect on the extraction efficiency of metal ions. In addition to changes in the binding mechanism caused by speciation, these different ligand-metal complexes (e.g., a Cr(H2O)3+-SCN complex vs a Cr(H2O)(OH)2+-SCN complex) may also have different solubilities in microemulsion phase effecting extraction. Finally, pH and speciation effects may affect the formation and stability of micelles formed in the microemulsion phase.
Regarding these results, a selectively extraction of Cr3+ from Co2+ can be achieved based on pH influence, since at pH = 1 the Co2+ are completely extracted in the microemulsion phase, while most of the Cr3+ still remain in the aqueous phase.
3.3.6 Improved efficiency by successive extractions
It was shown before that the extraction efficiency of Co2+ was 99.99%, while the other metal ions still remain in the aqueous phase. In order to improve the extraction efficiency of Cr3+, Ni2+ and Cu2+ from aqueous media, successive extractions were used. A synthetic solution containing Cr3+, Co2+, Ni2+ and Cu2+ 0.15 g/L and a solution of NaSCN 5 mol/L, dissolved in water were used. The second and the third extractions were achieved by mixing aqueous phase separated after previous extraction with new amounts of surfactant, oil phase and complexing agent.
In Fig. 9 it can be observed that the third extraction process presents an improvement of the extraction efficiency up to 99.99% for Cr3+ and Cu2+ and 8575% for Ni2+, using the composition with 10% Brij30 concentration.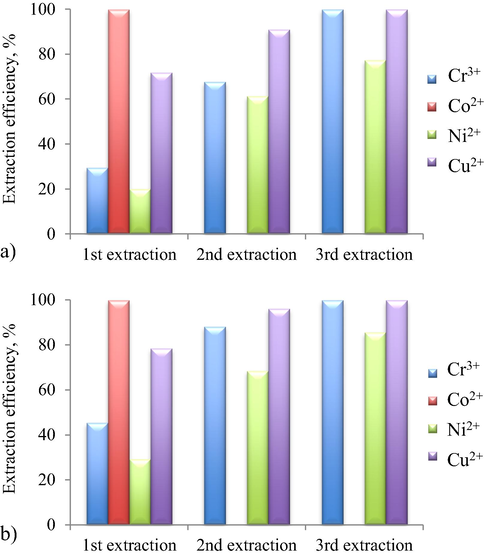
Successive extraction efficiencies of Cr3+, Co2+, Ni2+ and Cu2+ using (a) 5% and (b) 10% Brij30 surfactant concentrations.
3.4 Extraction kinetics of heavy metals mixture in microemulsion
The kinetic models and the equations constants for the three cases presented in Table 3 have been established based on the equations of Nernst-Planck, which apply to the diffusion of two species in almost homogeneous media (Lemos et al., 2008).
Kinetic models
Equations
Eq. no.
Diffusion of the heavy metals from the diluted phase towards the interfacial film of surfactant
(2)
Diffusion of the heavy metals into the core of micelles
(3)
Mass transfer by chemical reaction between the heavy metals and the functional groups of surfactant
(4)
This model was selected for its simplicity and applicability (Priya and Chiranjeevi, 2007; Alguacil and Alonso, 2003).
In this approach, the extraction process is formally considered analogous to a reversible, pseudo-first order chemical reaction: where P is a pollutant entity (heavy metal ions), kf and kr are forward (from the diluted phase to microemulsion phase) and reverse (from microemulsion phase to the diluted phase) constants, which include constant geometric factors, such as the interfacial area and the solution volume.
The extraction by microemulsion must be seen as a liquid–liquid equilibrium, which includes several steps, as shown in Table 3.
Since the extraction efficiencies of Co2+ and Cu2+ are not influenced by the contact time, the kinetics of extraction in microemulsion were studied for Cr3+ and Ni2+.
The application of Equations (2) - (4) for heavy metal ions extraction in microemulsion is exemplified in Fig. 10, using the system with 5% surfactant. The results for all systems investigated are collected in Table 4.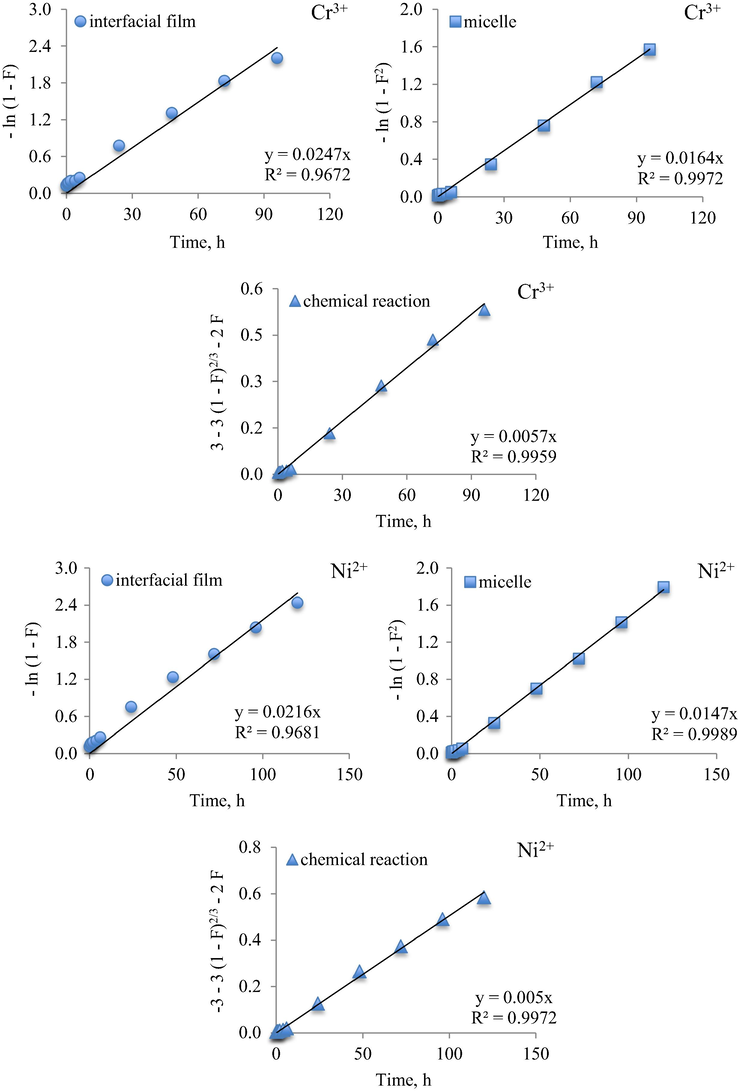
Plot of Eqs. (2)–(4) of Cr3+ and Ni2+ extraction in Brij30/BuOAc/Me(SCN)nm− microemulsion (5% surfactant concentration and water/oil volumetric ratio, R = 9).
Heavy metals
Interfacial film
Micelle
Chemical reaction
Equation
R2
Equation
R2
Equation
R2
Cr3+
y = 0.0212x
0.9098
y = 0.0132x
0.9906
y = 0.0048x
0.9890
Ni2+
y = 0.0220x
0.9121
y = 0.0159x
0.9963
y = 0.0050x
0.9954
In conclusion, that the best-fitted model of both cations is that described by the diffusion of the heavy metal ions into the core of micelles.
3.5 The equilibrium partition of metal ions between aqueous and microemulsion phases
The extraction of Cr3+, Co2+, Ni2+ and Cu2+ (HMM) from aqueous into the microemulsion phase depends on the thermodynamic of equilibrium partition. Assuming that the microemulsion extraction involves the distribution of metal ions between aqueous and microemulsion phases and the separation equilibrium is similar to that of a liquid-liquid extraction process, the distribution coefficient, D, was calculated using Eq. (5) (Fleancu et al., 2013).
The calculation of distribution coefficient for Cr3+, Co2+, Ni2+ and Cu2+ between microemulsion and the aqueous phase was carried out for 5% and 10% Brij30 surfactant concentrations, at 24 and 144 h contact time. The obtained results are presented in Table 5.
Composition
Contact time
DCr(III)
DCo(II)
DNi(II)
DCu(II)
5% Brij30
24 h
2.5
604.6
0.4
4.1
R = 9
144 h
3.8
606.2
3.4
4.7
10% Brij30
24 h
1.5
591.1
0.6
5.4
R = 9
144 h
6.5
582.4
1.4
7.1
From Fig. 11 it can be noticed that the higher distribution coefficient was obtained for Co(II) (D = 604.6), while the lowest was obtained for Ni(II) (D = 0.4). These values are in good agreement with the extraction efficiencies obtained in the experiments presented above, where the extraction efficiency of Co2+ reached maximum, while for Ni2+ was the lowest.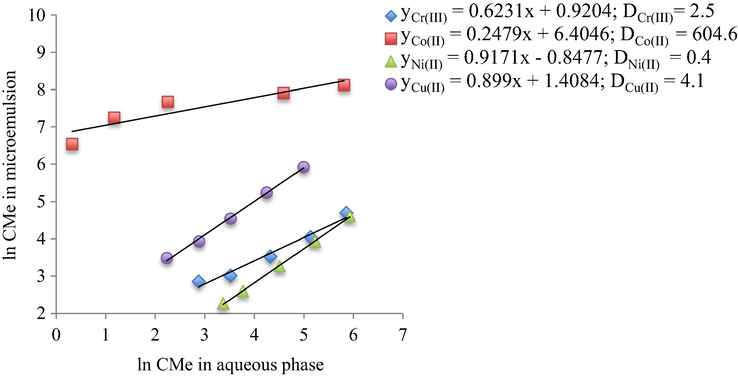
Distribution coefficient of Cr3+, Co2+, Ni2+ and Cu2+ between microemulsion and aqueous phase at 5% Brij30 concentration and 24 h contact time.
4 Conclusions
The ternary system, Polyoxyethylene (4) lauryl ether/Butyl acetate/Me(SCN)nm−, using NaSCN as ion association complexing agent, with no addition of acetone, can be successfully applied for the extraction of heavy metals mixture from aqueous media. The thiocyanto complexes of metals are trapped in the inner aqueous phase microemulsion by their diffusion either towards the interfacial film of surfactant (Co2+) or into the core of micelles (Cr3+, Cu2+, Ni2+). This system presents several advantages:
-
its ability to form Winsor II microemulsion without the assistance of co-surfactant;
-
the properties of the organic phase, since it has low toxicity level and a moderate volatility;
-
low consumption of reagents. It was necessary only 5% surfactant concentration and a water to oil volumetric ratio, R, equal to 19 to obtain a Winsor II microemulsion, meaning that the system has the ability to clean a volume of water by 19 times higher than the volume of the organic phase, leading to a concentration of the heavy metal ions in the microemulsion phase.
In order to obtain a high extraction efficiency of heavy metals mixture, with less reagent consumption, the following conditions should be taken into account: 5% surfactant concentration, water/oil volumetri ratio, R, equal to 9, complexing agent concentration NaSCN, equal to 5 mol/L and pH of heavy metals mixture aqueous solution equal to 5. The results showed that the extraction efficiency of cobalt ions was not dependent on the extraction conditions, showing a maximum value (99.99%) in all cases. For chromium, nickel and copper the extraction efficiency can be improved by successive extractions, leading to 99.99% for chromium and copper and 85.75% for nickel after three successive extractions. Based on the pH specific behaviour, a separation of chromium and cobalt ions can be achieved, since at pH = 1 the cobalt ions are completely extracted in the microemulsion phase, while the chromium ions still remain in the aqueous phase.
The Brij30/BuOAc/Me(SCN)nm− microemulsion systems can be successfully used for extraction of heavy metals ions from aqueous media as the procedure is fast, cheap, easy handle and very efficient compared with other currently used treatment solutions that are time consuming, require expensive equipment and have significant energy requirements.
Acknowledgements
This work was done in the frame of the European Regional Development Fund Grant no 130/23.09.2016, SMIS 105558, with title “Eco-nanotechnologies for water depollution and wastes capitalisation”.
Also, we want to thank to the financial support provided by the project Clean Integrated Nanotechnology for Dyes Removal from Wastewaters (CLIENT-DR), PN3-P3-301/02.05.2017, Contract no. 12/2017.
References
- J. Environ. Manage.. 2013;130:80-89.
- Sci. Technol.. 2003;37:1043-1047.
- Ecotox. Environ. Safe.. 2016;126:186-192.
- Arabian J. Chem.. 2011;4:361-377.
- J. Chem. Technol. Biot.. 2005;80:92-98.
- U.P.B. Sci. Bull. Ser. B. 2017;79(2):13-24.
- Water Res.. 2001;35:2219-2224.
- Chem. Eng. Sci.. 2007;62:4636-4643.
- J. Chem. Technol. Biot.. 2004;79:645-650.
- Fluid Phase Equilib.. 2013;337:18-25.
- Sci. China. Chem.. 2012;55:1712-1718.
- J. Braz. Chem. Soc.. 2004;15(6):818-824.
- J. Multidiscip. Eng. Sci. Stud.. 2015;1(1):12-18.
- Langmuir. 2003;19:3145-3150.
- Sep. Purif. Technol.. 2016;157:141-161.
- J. Inorg. Chem.. 1999;75:79-83.
- J. Hazard. Mater.. 2009;166:695-700.
- Hazard. Mater.. 2008;159:245-251.
- Desalination. 2009;238:158-165.
- J. Hazard. Mater.. 2011;186:2166-2170.
- Braz. J. Chem. Eng.. 2015;32:949-956.
- Int. Multi. Sci. Geoco.. 2010;2:733-740.
- J. Hazard. Mater.. 2012;241:14-31.
- Thermodyn. Ion Assoc.. 1967;6(8):1567-1569.
- Dynamic Properties of Interfaces and Association Structures. Illinois: Ed. AOCS Press Champaign; 1996.
- Hazard. Mater.. 2007;144:152-158.
- Mater. Let.. 2014;132:346-348.
- Langmuir. 2001;17:417-426.
- PCCP. 2006;8:1731-1738.
- J. Phys. Soc. Jpn.. 1954;9(5):753-779.
- J. Radioanal. Nucl. Chem.. 2006;267:401-406.
- Water Res.. 2014;66:219-232.
- J. Radioanal. Nucl. Chem.. 2012;292:1093-1098.
- J. Radioanal. Nucl. Chem.. 2008;275:535-540.
- Appl. Phys. B. 2000;70:179-184.
- J. Radioanal. Nucl. Chem.. 2005;265:419-421.
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.arabjc.2017.11.018.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







