Translate this page into:
Synthesis and antiproliferative activity studies of new functionalized pyridine linked thiazole derivatives
⁎Corresponding author. amqahtani@uqu.edu.sa (Alaa M. Alqahtani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Ten new pyridine linked various substituted thiazole hybrids through (hydrazonomethyl)phenoxy-acetamide spacer were synthesized. The synthetic strategy was based on the reaction of the precursor 2-(4-((2-carbamothioylhydrazono)methyl)phenoxy)-N-(pyridin-2-yl)acetamide (3) with various α-halogenated carbonyl compounds (namely; phenacyl bromides, ethyl bromoacetate, diethyl bromomalonate and 3-chloropentane-2,4-dione). Moreover, the cytotoxicity properties of the synthesized compounds have been studied against liver carcinoma (HepG2), laryngeal carcinoma (Hep-2), prostate cancer (PC3), breast cancer (MCF-7) and normal fibroblast cells (WI38). The pyridine-thiazole compounds 7 and 10 revealed promising anticancer activity against MCF-7 and HepG2 cell lines with IC50 values in the range 5.36–8.76 μM compared to the activity of 5-fluorouracil. Docking study provided valuable insights for binding sites of the synthesized compounds with Rho-associated protein kinase (ROCK-1).
Keywords
Pyridine
Thiosemicarbazone
Phenacyl bromide
Cytotoxicity
Molecular docking
1 Introduction
The pyridine ring system represents the main skeleton in numerous pharmacological natural and synthetic substances of significant interest (Chiacchio et al., 2019; Altaf et al., 2015). Therefore, substituted pyridines have attracted the attention of researchers world and the interest in their synthesis continues undiminished (Reddy et al., 2006; Cocco et al., 2005). A large number of pyridine compounds have been reported in the literature due to their wide range of biological activities, such as anti-tumor (Hassan et al., 2020), cardiotonic (Ravinder et al., 2012), antituberculosis (Ng et al., 2015), antibacterial (Eryılmaz et al., 2020), and antihepatitis B virus (Lv et al., 2010). The pyridine ring is found in many natural compounds, such as nicotinic acid (vitamin B3) and pyridoxine (vitamin B6). Nicotinic acid is essential for the biosynthesis of the redox coenzyme nicotine adenine dinucleotide (NAD+), while pyridoxine is a coenzyme in transaminases (Joule and Mills, 2000). Nicotinic acid is used as a therapeutic agent to raise the levels of high-density lipoprotein and that way diminish the hazard of cardiovascular disease (Gille et al., 2008). Nicotine, whose toxicity has a defensive function in nature (Steppuhn et al., 2004), is widely used for smoking cessation. Pyridine is also found in a vast number of drugs like Isoniazid (Timmins et al., 2004) and Ethionamide (Vannelli et al., 2002) (enoyl-acyl carrier protein reductase inhibitor; tuberculosis). Moreover, azoles occupy a prominent place in current medicinal chemistry due to their wide range of applications in the fields of drug design and discovery (Sharma et al., 2019). Especially, thiazole has been extensively used in the design of effective biologically active agents (Pricopie et al., 2019; Sharma et al., 2017). Thiazole derivatives are amongst most active classes of compounds that are known for their broad spectrum of activity e.g. antibacterial activity (Abdel-Latif et al., 2019), antifungal activity (Lino et al., 2018), antimalarial activity (Bueno et al., 2016), antitubercular activity (Andreani et al., 2001), antiviral activity (Dawood et al., 2015), anti-inflammatory activity (Jacob and Manju, 2020), antidiabetic activity (De Resende et al., 2019), anthelmintic activity (Amnerkar and Bhusari, 2011), anticonvulsant activity (Siddiqui and Ahsan, 2010), antioxidant activity (Kurt et al., 2015), and anticancer activity (Sharma et al., 2020). Many thiazole scaffolds have been found to possess significant antitumor activity, such as the marketed anti-cancer drugs Dasatinib (Lombardo et al., 2004) and Dabrafenib (Dhillon, 2016) with potent anti-proliferative activity.
Furthermore, the pyridine-thiazole hybrids have marked their presence as potent anticancer agents in many published papers. The anticancer activity of nine imidazo[1,2-a]pyridine-thiazole derivatives was screened and showed that hybrid (I) was the most potent inhibitor of NF-κB activity with IC50 value of 6.5 ± 0.6 μM (Vasu et al., 2017). The anti-proliferative effect of pyridine-thiazole hybrid (II) was evaluated against different human carcinoma cell lines (Amin et al., 2017) (Fig. 1). It exhibited significant anti-proliferative activity in hepatocyte carcinoma cell line HEPG2 (IC50 = 23.8 μg/mL). The pyridine-thiazole hybrids (III) were subjected to anti-inflammatory tests that indicated good activity when (R = 3-OMe) and moderate activity when (R = 4-F) (Krishna et al., 2014). Combining pyridine with five-membered heterocycle containing nitrogen and sulfur (thiazole core) in a single hybrid structure promote interesting structural and biological characteristics (Dos Santos Silva et al., 2017; Xie et al., 2018; Bondock et al., 2013). In view of these reports, we therefore envisaged that integrating pyridine and thiazole moieties through phenoxy-acetamide spacer in one molecular platform could potentially produce new compounds with significant synergistic anticancer properties. This spacer enables both the rings to be in a suitable conformation to interact with target protein without any restrictions, in addition to the ability of carbonyl oxygen for hydrogen bonding with the target protein. In order to investigate these hybrids, a series of pyridine linked thiazole nucleus was synthesized with various substitution. The synthetic strategy involves the application of modified Hantzsch synthesis (Kumar et al., 2005; Prakash et al., 2007; Pundeer et al., 2011; Kiran et al., 2020) for the formation of thiazole nucleus through the reaction of thiosemicarbazone derived from 2-(4-formylphenoxy)-N-(pyrid-2-yl)acetamide with α-functionalized carbonyl compounds like phenacyl bromides, ethyl bromoacetate, ethyl bromomalonate and/or 3-chloroacetylacetone. The target pyridine analogues bearing thiazole moiety were prepared to verify their efficacy as potential anticancer agents.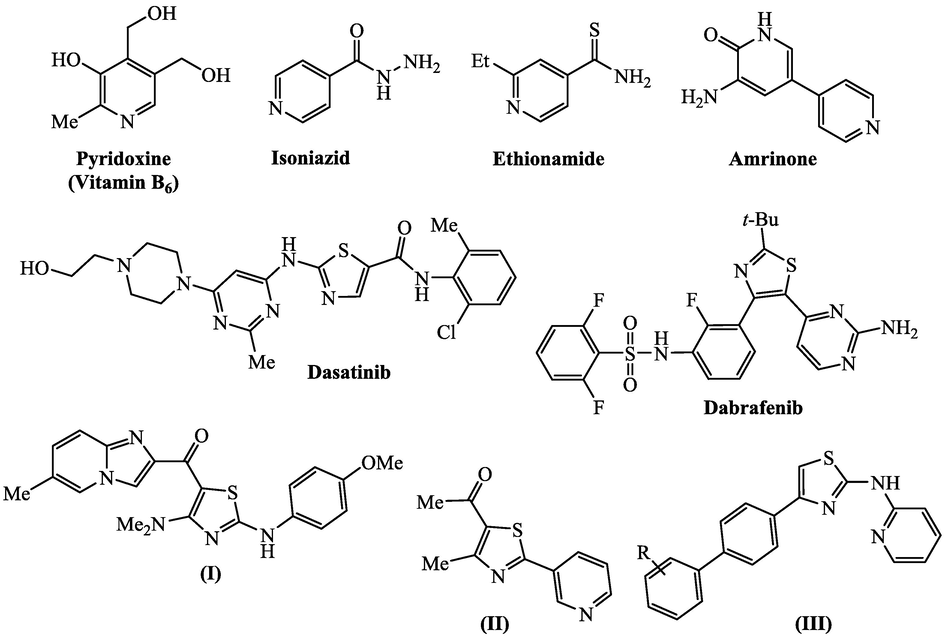
Selected biologically active thiazole and/or pyridine structures.
2 Experimental
2.1 Chemistry
Melting points were determined on Gallenkamp electric device and are uncorrected. The infrared spectra (potassium bromide discs) were registered on Thermo Scientific Nicolet iS10 FTIR spectrometer. The 1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were acquired on JEOL’s 500 MHz NMR spectrometer. The mass spectra were determined on Quadrupole GC-MS (DSQII) mass spectrometer at 70 eV. Elemental analyses of carbon, hydrogen and nitrogen were estimated on Perkin Elmer 2400 analyzer.
2.1.1 Synthesis of 2-(4-formylphenoxy)-N-(pyrid-2-yl)acetamide (2)
To a suspension of 2-chloro-N-(pyrid-2-yl)acetamide (1) (1.70 g, 0.01 mol) and 4-hydroxybenzaldehyde (1.22 g, 0.01 mol) in dimethyl sulfoxide (30 mL), anhydrous sodium carbonate (1.38 g, 0.01 mol) was added. The reaction mixture was stirred at room temperature for 6 h and then diluted with 30 mL cold water. The precipitate was collected, dried and recrystallized from ethyl alcohol. Paige crystals, yield 65%, m.p. = 147–148 °C. IR (KBr): 3215 (N—H), 1717, 1682 cm−1 (C⚌O). 1H NMR (CDCl3): 4.81 (s, 2H, CH2), 7.11 (d, J = 8.50 Hz, 2H, Ar-H), 7.22 (t, J = 7.50 Hz, 1H, pyridine-H5), 7.77–7.79 (m, 1H, pyridine-H4), 7.91 (d, J = 8.50 Hz, 2H, Ar-H), 8.11 (d, 1H, J = 8.00 Hz, pyridine-H3), 8.34 (d, J = 8.00 Hz, 1H, pyridine-H6), 9.86 (s, 1H, CH⚌O), 10.64 ppm (s, 1H, NH). 13C NMR (CDCl3): 66.44, 115.13 (2C), 116.15, 123.62, 131.64 (2C), 133.20, 138.07, 146.38, 151.72, 162.70, 169.47, 191.27 ppm. MS m/z (%): 256 (M+, 38.99), 192 (21.33), 160 (50.91), 128 (54.52), 96 (32.36), 73 (24.76), 69 (36.85), 64 (100.00). Analysis for C14H12N2O3 (256.26): Calcd: C, 65.62; H, 4.72; N, 10.93%. Found: C, 65.71; H, 4.70; N, 10.84%.
2.1.2 Synthesis of 2-(4-((2-carbamothioylhydrazono)methyl)phenoxy)-N-(pyridin-2-yl)-acetamide (3)
A 100 mL round-bottomed flask was charged with 2-(4-formylphenoxy)-N-(pyrid-2-yl)acetamide (2) (1.28 g, 0.005 mol) and thiosemicarbazide (0.46 g, 0.005 mol), 40 mL ethanol and 1 mL acetic acid were added. The reaction components were refluxed for 4 h and then allowed to cool. The solid that separated was collected by filtration and recrystallized from dioxane to afford the thiosemicarbazone compound 3. Yellow crystals, yield 71%, m.p. = 202–204 °C. IR (KBr): 3367, 3248, 3171 (NH2 and NH), 1680 cm−1 (C⚌O). 1H NMR (DMSO‑d6): 4.78 (s, 2H, CH2), 7.14 (d, J = 8.00 Hz, 2H, Ar-H), 7.24–7.27 (m, 1H, pyridine-H5), 7.61–7.65 (m, 1H, pyridine-H4), 7.87 (d, J = 8.00 Hz, 2H, Ar-H), 8.01 (d, J = 7.50 Hz, 1H, pyridine-H3), 8.11 (d, J = 7.50 Hz, 1H, pyridine-H6), 8.25 (s, 1H, NHa), 8.38 (s, 1H, CH⚌N), 8.66 (s, 1H, NHb), 10.27 (s, 1H, NH), 10.79 ppm (s, 1H, NH). 13C NMR (DMSO‑d6): 66.47, 114.89 (2C), 116.18, 123.37, 126.40, 129.58 (2C), 138.05, 145.90, 148.13, 151.85, 160.18, 168.87, 178.69 ppm. MS m/z (%): 329 (M+, 32.16), 233 (15.06), 217 (9.18), 201 (10.91), 173 (9.57), 147 (14.47), 145 (18.27), 121 (1 0 0), 93 (53.59), 65 (38.82). Analysis for C15H15N5O2S (329.38): Calcd: C, 54.70; H, 4.59; N, 21.26%. Found: C, 54.84; H, 4.53; N, 21.17%.
2.1.3 Synthesis of 2-(4-((2-(4-arylthiazol-2-yl)hydrazono)methyl)phenoxy)-N-(pyridin-2-yl)-acetamides 4a and 4b
A mixture of thiosemicarbazone compound 3 (0.66 g, 0.002 mol) and phenacyl bromide or 4-chlorophenacyl bromide (0.002 mol) was refluxed for 2 h in 30 mL ethanol and triethylamine (0.1 mL). The reaction mixture was cooled to 20–25 °C and the solid product was filtered and dried to furnish the corresponding pyridine-thiazole hybrids 4a and 4b, respectively.
2.1.3.1 2-(4-((2-(4-Phenylthiazol-2-yl)hydrazono)methyl)phenoxy)-N-(pyridin-2-yl)acetamide (4a)
Yellow solid, yield 64%, m.p. = 244–245 °C. IR (KBr): 3217, 3174 (N—H), 1678 cm−1 (C⚌O). 1H NMR (DMSO‑d6): 4.72 (s, 2H, CH2), 7.12–7.18 (m, 3H, 2Ar-H and pyridine-H5), 7.33–7.39 (m, 6H, 5Ar-H and thiazole-H5), 7.74 (t, J = 8.00 Hz, 1H, pyridine-H4), 7.87 (d, J = 8.00 Hz, 2H, Ar-H), 8.02 (d, J = 8.10 Hz, 1H, pyridine-H3), 8.17 (s, 1H, CH⚌N), 8.38 (d, J = 8.10 Hz, 1H, pyridine-H6), 10.71 (s, 1H, NH), 11.42 ppm (s, 1H, NH). 13C NMR (DMSO‑d6): 66.47, 104.35, 114.38 (2C), 116.18, 123.10, 126.48, 127.36 (2C), 128.17, 129.26 (2C), 129.77 (2C), 133.22, 138.45, 145.58, 146.88, 148.17, 153.32, 159.73, 168.62, 170.21 ppm. MS m/z (%): 429 (M+, 21.28), 321 (41.87), 158 (100.00), 106 (9.49), 76 (12.60). Analysis for C23H19N5O2S (429.50): Calcd: C, 64.32; H, 4.46; N, 16.31%. Found: C, 64.15; H, 4.38; N, 16.20%.
2.1.3.2 2-(4-((2-(4-(4-Chlorophenyl)thiazol-2-yl)hydrazono)methyl)phenoxy)-N-(pyridin-2-yl)acetamide (4b)
Yellow solid, yield 58%, m.p. = 257–258 °C. IR (KBr): 3231, 3192 (N—H), 1680 cm−1 (C⚌O). 1H NMR (DMSO‑d6): 4.76 (s, 2H, CH2), 7.18–7.22 (m, 5H, 4Ar-H and pyridine-H5), 7.32 (s, 1H, thiazole-H5), 7.49 (d, J = 8.00 Hz, 2H, Ar-H), 7.68–7.71 (m, 1H, pyridine-H4), 7.87 (d, J = 8.00 Hz, 2H, Ar-H), 8.02 (d, J = 7.50 Hz, 1H, pyridine-H3), 8.21 (s, 1H, CH⚌N), 8.36 (d, J = 7.50 Hz, 1H, pyridine-H6), 10.53 (s, 1H, NH), 11.80 ppm (s, 1H, NH). 13C NMR (DMSO‑d6): 66.44, 106.09, 114.37 (2C), 115.88, 123.12, 126.41, 128.30 (2C), 129.28 (2C), 129.70 (2C), 132.36, 134.52, 137.88, 145.58, 147.05, 148.15, 152.32, 160.08, 168.82, 170.79 ppm. MS m/z (%): 463 (M+, 12.92), 410 (12.83), 379 (19.43), 338 (14.71), 244 (13.64), 192 (19.79), 175 (24.06), 164 (27.90), 148 (32.71), 137 (64.62), 121 (24.96), 109 (48.04), 95 (100.0), 81 (35.65), 69 (62.12). Analysis for C23H18ClN5O2S (463.94): Calcd: C, 59.54; H, 3.91; N, 15.10%. Found: C, 59.33; H, 3.82; N, 15.13%.
2.1.4 Synthesis of 2-(4-((2-(4-oxo-4,5-dihydrothiazol-2-yl)hydrazono)methyl)phenoxy)-N-(pyridin-2-yl)acetamide (5)
A mixture of thiosemicarbazone compound 3 (0.66 g, 0.002 mol) and ethyl bromoacetate (0.33 mL, 0.002 mol) was refluxed for 4 h in 25 mL ethanol and anhydrous sodium acetate (0.5 g). After cooling to 20–25 °C, the mixture was diluted with 20 mL cold water. The crude solid product was filtered, dried and recrystallized from EtOH-DMF mixture (5:1) to afford the pyridine-thiazole hybrid 5. Yellow crystals, yield 62%, m.p. = 229–230 °C. IR (KBr): 3316, 3240 (N—H), 1708, 1678 cm−1 (C⚌O). 1H NMR (DMSO‑d6): 4.11 (s, 2H, CH2), 4.72 (s, 2H, CH2), 7.08 (d, J = 8.00 Hz, 2H, Ar-H), 7.21 (t, J = 7.5 Hz, 1H, pyridine-H5), 7.63–7.65 (m, 1H, pyridine-H4), 7.81 (d, J = 8.00 Hz, 2H, Ar-H), 8.04 (d, J = 8.10 Hz, 1H, pyridine-H3), 8.23 (s, 1H, CH⚌N), 8.37 (d, J = 8.10 Hz, 1H, pyridine-H6), 10.63 (s, 1H, NH), 11.78 ppm (s, 1H, NH). 13C NMR (DMSO‑d6): 33.65, 66.53, 114.73 (2C), 115.76, 123.55, 126.45, 129.82 (2C), 138.71, 147.63, 149.40, 152.43, 159.55, 160.88, 169.14, 171.48 ppm. MS m/z (%): 369 (M+, 51.33), 294 (14.86), 250 (70.26), 204 (100.00), 176 (25.06), 149 (21.17), 132 (22.30), 105 (33.01), 91 (53.45), 77 (32.80). Analysis for C17H15N5O3S (369.40): Calcd: C, 55.28; H, 4.09; N, 18.96%. Found: C, 55.39; H, 4.14; N, 19.05%.
2.1.5 Synthesis of 2-(4-((2-(5-arylidene-4-oxo-4,5-dihydrothiazol-2-yl)hydrazono)-methyl)-phenoxy)-N-(pyridin-2-yl)acetamides 6a-c
The thiazolin-4-one derivative 5 (0.74 g, 0.002 mol) was dissolved in hot ethanol (30 mL), then 0.002 mol of the appropriate aldehyde (namely; 4-touladehyde, 4-anisaldehyde and/or 4-chlorobenzaldehyde) and 0.15 mL piperidine were added. The reaction components were refluxed for 3 h and then allowed to cool. The solid crystals that obtained in each case was collected by filtration, washed with cold ethanol and dried.
2.1.5.1 2-(4-((2-(5-(4-Methylbenzylidene)-4-oxo-4,5-dihydrothiazol-2-yl)hydrazono)methyl)-phenoxy)-N-(pyridin-2-yl)acetamide (6a)
Yellow crystals, yield 76%, m.p. = 273–274 °C. IR (KBr): 3341, 3276 (N—H), 1698, 1673 cm−1 (C⚌O). 1H NMR (DMSO‑d6): 2.33 (s, 3H, CH3), 4.68 (s, 2H, CH2), 7.15–7.17 (m, 3H, 2Ar-H and pyridine-H5), 7.31 (d, J = 9.00 Hz, 2H, Ar-H), 7.55 (d, J = 9.00 Hz, 2H, Ar-H), 7.65–7.68 (m, 1H, pyridine-H4), 7.78 (s, 1H, CH⚌C), 7.86 (d, J = 8.00 Hz, 2H, Ar-H), 8.01 (d, J = 8.10 Hz, 1H, pyridine-H3), 8.27 (s, 1H, CH⚌N), 8.39 (d, J = 8.10 Hz, 1H, pyridine-H6), 10.62 (s, 1H, NH), 11.92 ppm (s, 1H, NH). 13C NMR (DMSO‑d6): 21.11, 66.60, 114.85 (2C), 115.88, 123.51, 126.41, 128.83 (2C), 129.55 (2C), 129.93 (2C), 130.42, 130.64, 136.33, 138.46, 148.07, 148.86, 150.40, 152.23, 158.72, 160.29, 165.72, 169.11 ppm. MS m/z (%): 471 (M+, 28.55), 303 (13.06), 239 (43.76), 172 (14.10), 156 (14.16), 135 (14.69), 105 (100.00), 76 (39.40). Analysis for C25H21N5O3S (471.54): Calcd: C, 63.68; H, 4.49; N, 14.85%. Found: C, 63.79; H, 4.44; N, 14.77%.
2.1.5.2 2-(4-((2-(5-(4-Methoxybenzylidene)-4-oxo-4,5-dihydrothiazol-2-yl)hydrazono)-methyl)phenoxy)-N-(pyridin-2-yl)acetamide (6b)
Orange crystals, yield 82%, m.p. = 281–282 °C. IR (KBr): 3308, 3254 (N—H), broad centered at 1691 cm−1 (C⚌O). 1H NMR (DMSO‑d6): 3.78 (s, 3H, OCH3), 4.70 (s, 2H, CH2), 6.89 (d, J = 9.00 Hz, 2H, Ar-H), 7.12–7.15 (m, 1H, pyridine-H5), 7.26 (d, J = 9.00 Hz, 2H, Ar-H), 7.55 (d, J = 9.00 Hz, 2H, Ar-H), 7.65–7.67 (m, 1H, pyridine-H4), 7.76 (s, 1H, CH⚌C), 7.85 (d, J = 8.00 Hz, 2H, Ar-H), 7.99 (d, J = 8.10 Hz, 1H, pyridine-H3), 8.26 (s, 1H, CH⚌N), 8.39 (d, J = 8.10 Hz, 1H, pyridine-H6), 10.58 (s, 1H, NH), 11.66 ppm (s, 1H, NH). 13C NMR (DMSO‑d6): 55.41, 66.52, 114.59 (2C), 114.91 (2C), 115.89, 123.55, 125.88, 126.54, 129.33 (2C), 130.27, 131.85 (2C), 138.40, 148.03, 149.19, 150.56, 152.27, 158.66, 160.15, 160.63, 165.81, 169.14 ppm. MS m/z (%): 487 (M+, 36.44), 309 (100.00), 280 (54.41), 218 (20.31), 198 (40.62), 172 (47.58), 156 (57.45), 148 (27.24), 135 (21.62), 90 (51.72), 76 (45.93). Analysis for C25H21N5O4S (487.53): Calcd: C, 61.59; H, 4.34; N, 14.37%. Found: C, 61.45; H, 4.41; N, 14.31%.
2.1.5.3 2-(4-((2-(5-(4-Chlorobenzylidene)-4-oxo-4,5-dihydrothiazol-2-yl)hydrazono)methyl)-phenoxy)-N-(pyridin-2-yl)acetamide (6c)
Orange crystals, yield 71%, m.p. = 265–266 °C. IR (KBr): 3321, 3260 (N—H), 1695, 1674 cm−1 (C⚌O). 1H NMR (DMSO‑d6): 4.68 (s, 2H, CH2), 7.14 (d, J = 8.50 Hz, Ar-H), 7.23–7.26 (m, 1H, pyridine-H5), 7.58–7.63 (m, 5H, 4Ar-H and pyridine-H4), 7.78 (s, 1H, CH⚌C), 7.87 (d, J = 8.50 Hz, 2H, Ar-H), 8.02 (d, J = 8.10 Hz, 1H, pyridine-H3), 8.24 (s, 1H, CH⚌N), 8.34 (d, J = 8.10 Hz, 1H, pyridine-H6), 10.43 (s, 1H, NH), 11.70 ppm (s, 1H, NH). 13C NMR (DMSO‑d6): 66.52, 114.78 (2C), 116.17, 123.55, 125.57, 128.83 (2C), 129.78 (2C), 131.40, 131.82 (2C), 133.07, 134.47, 138.06, 148.73, 149.35, 151.01, 151.13, 158.79, 160.32, 166.04, 168.49 ppm. MS m/z (%): 493 (M+ + 2, 10.16), 491 (M+, 32.71), 236 (9.22), 149 (51.05), 129 (96.61), 111 (48.89), 81 (68.19), 69 (100.00). Analysis for C24H18ClN5O3S (491.95): Calcd: C, 58.60; H, 3.69; N, 14.24%. Found: C, 58.43; H, 3.61; N, 14.13%.
2.1.6 Synthesis of ethyl 4-oxo-2-(2-(4-(2-oxo-2-(pyridin-2-ylamino)ethoxy)benzylidene)-hydrazinyl)-4,5-dihydrothiazole-5-carboxylate (7)
A mixture of thiosemicarbazone compound 3 (0.66 g, 0.002 mol) and diethyl bromomalonate (0.48 mL, 0.002 mol) was refluxed for 4 h in 25 mL ethanol and anhydrous sodium acetate (0.5 g). After cooling to 20–25 °C, the mixture was diluted with 20 mL cold water. The crude solid product was filtered, dried and recrystallized from dioxane to afford the pyridine-thiazole hybrid 7. Orange crystals, yield 68%, m.p. = 216–218 °C. IR (KBr): 3283, 3188 (N—H), broad at 1735 (C⚌O), 1684 cm−1 (C⚌O). 1H NMR (DMSO‑d6): 1.25 (t, J = 7.25 Hz, 3H, CH3), 4.16 (q, J = 7.25 Hz, 2H, —OCH2), 4.31 (s, 1H, thiazole-H5), 4.71 (s, 2H, CH2), 7.08 (d, J = 8.00 Hz, 2H, Ar-H), 7.22 (t, J = 7.50 Hz, 1H, pyridine-H5), 7.63–7.66 (m, 1H, pyridine-H4), 7.85 (d, J = 8.00 Hz, 2H, Ar-H), 8.03 (d, J = 8.10 Hz, 1H, pyridine-H3), 8.22 (s, 1H, CH⚌N), 8.35 (d, J = 8.10 Hz, 1H, pyridine-H6), 10.67 (s, 1H, NH), 11.60 ppm (s, 1H, NH). 13C NMR (DMSO‑d6): 14.57, 55.78, 59.04, 66.57, 114.78 (2C), 115.91, 123.55, 126.48, 129.34 (2C), 138.40, 148.03, 149.19, 152.27, 158.66, 160.48, 164.19, 169.14, 172.83 ppm. MS m/z (%): 441 (M+, 53.58), 368 (34.67), 348 (47.23), 167 (27.65), 149 (100.00), 69 (55.14). Analysis for C20H19N5O5S (441.46): Calcd: C, 54.41; H, 4.34; N, 15.86%. Found: C, 54.28; H, 4.29; N, 15.78%.
2.1.7 Synthesis of 2-(4-((2-(5-acetyl-4-methylthiazol-2-yl)hydrazono)methyl)phenoxy)-N-(pyridin-2-yl)acetamide (8)
A mixture of thiosemicarbazone compound 3 (1.65 g, 0.005 mol) and 3-chloropentane-2,4-dione (0.67 mL, 0.002 mol) was refluxed for 6 h in 30 mL ethanol and triethylamine (0.2 mL). The reaction mixture was cooled to 20–25 °C and the solid product was filtered and dried to furnish the corresponding pyridine-thiazole hybrid 8. Yellow solid, yield 56%, m.p. = 245–246 °C. IR (KBr): 3290, 3221 (N—H), broad centered at 1688 cm−1 (C⚌O). 1H NMR (DMSO‑d6): 2.58 (s, 3H, COCH3), 2.76 (s, 3H, CH3), 4.68 (s, 2H, CH2), 7.11 (d, J = 9.00 Hz, 2H, Ar-H), 7.17–7.20 (m, 1H, pyridine-H5), 7.67–7.69 (m, 1H, pyridine-H4), 7.84 (d, J = 9.00 Hz, 2H, Ar-H), 8.00 (d, J = 8.10 Hz, 1H, pyridine-H3), 8.17 (s, 1H, CH⚌N), 8.33 (d, J = 8.10 Hz, 1H, pyridine-H6), 10.73 (s, 1H, NH), 11.81 ppm (s, 1H, NH). 13C NMR (DMSO‑d6): 16.46, 28.97, 66.64, 115.08 (2C), 116.12, 123.38, 124.53, 126.41, 129.61 (2C), 138.66, 145.36, 147.34, 152.48, 155.73, 160.10, 168.13, 169.55, 188.17 ppm. MS m/z (%): 409 (M+, 28.79), 366 (55.14), 316 (22.03), 194 (14.65), 149 (37.35), 106 (35.66), 91 (1 0 0), 69 (42.41). Analysis for C20H19N5O3S (409.46): Calcd: C, 58.67; H, 4.68; N, 17.10%. Found: C, 58.85; H, 4.76; N, 17.24%.
2.1.8 Synthesis of 2-(4-((2-(5-(3-(dimethylamino)acryloyl)-4-methylthiazol-2-yl)-hydrazono)-methyl)phenoxy)-N-(pyridin-2-yl)acetamide (9)
A mixture of 5-acetylthiazole compound 8 (0.82 g, 0.002 mol) with DMF-DMA (0.48 mL, 0.004 mol) in 15 mL dry xylene was refluxed for 4 h. The solid that precipitated upon cooling was filtered to afford thiazole-enaminone compound 9 after recrystallization from dioxane. Yellowish brown solid, yield 46%, m.p. = 197–198 °C. IR (KBr): 3272, 3185 (N—H), broad centered at 1678 cm−1 (C⚌O). 1H NMR (DMSO‑d6): 2.58 (s, 3H, CH3), 3.12 (s, 3H, CH3), 3.28 (s, 3H, CH3), 4.71 (s, 2H, CH2), 6.16 (d, J = 12.50 Hz, 1H, —COCH⚌C—), 7.04 (d, J = 9.00 Hz, 2H, Ar-H), 7.14–7.16 (m, 1H, pyridine-H5), 7.67–7.69 (m, 1H, pyridine-H4), 7.85 (d, J = 9.00 Hz, 2H, Ar-H), 7.98 (d, J = 8.10 Hz, 1H, pyridine-H3), 8.12 (s, 1H, CH⚌N), 8.33 (d, J = 8.10 Hz, 1H, pyridine-H6), 8.54 (d, J = 12.50 Hz, 1H, C⚌CH—N), 10.68 (s, 1H, NH), 11.77 ppm (s, 1H, NH). 13C NMR (DMSO‑d6): 17.30, 44.63, 46.21, 66.61, 93.14, 114.71 (2C), 116.34, 122.94, 124.15, 126.80, 129.44 (2C), 138.90, 144.91, 147.81, 150.64, 152.83, 154.29, 159.74, 166.86, 169.47, 183.36 ppm. MS m/z (%): 464 (M+, 20.28), 234 (1 0 0), 218 (18.17), 170 (16.62), 154 (60.38), 127 (17.43). Analysis for C23H24N6O3S (464.54): Calcd: C, 59.47; H, 5.21; N, 18.09%. Found: C, 59.20; H, 5.14; N, 18.17%.
2.1.9 Synthesis of 2-(4-((2-(4-methyl-5-(1H-pyrazol-3-yl)thiazol-2-yl)hydrazono)methyl)-phenoxy)-N-(pyridin-2-yl)acetamide (10)
To a solution of thiazole-enaminone compound 9 (0.93 g, 0.002 mol) in 20 mL ethanol, hydrazine hydrate (0.2 mL, 0.004 mol) was added and heated under reflux for 6 h. The red solid that formed upon cooling was filtered and dried to furnish the pyrazolyl pyridine-thiazole hybrid 10. Red solid, yield 52%, m.p. = 271–272 °C. IR (KBr): 3308, 3258, 3173 (N—H), 1676 cm−1 (C⚌O). 1H NMR (DMSO‑d6): 2.26 (s, 3H, CH3), 4.68 (s, 2H, CH2), 6.64 (d, J = 4.00 Hz, 1H, pyrazole-H4), 7.12–7.17 (m, 3H, 2Ar-H and pyridine-H5), 7.63–7.67 (m, 1H, pyridine-H4), 7.78 (d, J = 4.00 Hz, 1H, pyrazole-H-5), 7.85 (d, J = 9.00 Hz, 2H, Ar-H), 8.02 (d, J = 8.10 Hz, 1H, pyridine-H3), 8.15 (s, 1H, CH⚌N), 8.34 (d, J = 8.10 Hz, 1H, pyridine-H6), 10.62 (s, 1H, NH), 11.55 (s, 1H, NH), 12.26 ppm (s, 1H, NH). 13C NMR (DMSO‑d6): 16.47, 66.58, 103.16, 114.65 (2C), 116.31, 118.16, 122.80, 126.84, 129.49 (2C), 131.64, 137.26, 139.51, 144.87, 147.65, 149.07, 153.19, 160.04, 164.57, 169.32 ppm. MS m/z (%): 433 (M+, 17.97), 281 (100.00), 200 (18.91), 172 (22.98), 125 (39.58). Analysis for C21H19N7O2S (433.49): Calcd: C, 58.19; H, 4.42; N, 22.62%. Found: C, 58.35; H, 4.50; N, 22.72%.
2.2 MTT cytotoxicity assay
The technical method of MTT assay depends on the reduction of yellow 3-(4,5-methyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) into purple formazan product, mainly by mitochondrial reductase activity inside the living cells. The cells used in cytotoxicity assay were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum. Cells suspended in the medium (2 × 104 cells/mL) were plated in 96-well culture plates and incubated at 37 °C in a 5% CO2 incubator for 12 h. The tested sample (2 μL) was added to the cells (2 × 104) in 96-well plates and cultured at 37 °C for 3 days. The cultured cells were mixed with 20 μL of MTT solution and incubated for 4 h at 37 °C. The supernatant was carefully removed from each well and 100 μL of DMSO were added to each well to dissolve the formazan crystals, which were formed by the cellular reduction of MTT. After mixing with a mechanical plate mixer, the absorbance of each well was measured by a microplate reader using a test wavelength of 570 nm. The results were expressed as the IC50 values, which inducing 50% inhibition of cell growth of the treated cells when compared to the growth of control cells.
3 Results and discussion
3.1 Chemistry
Initially, the nucleophilic substitution of chlorine form 2-chloro-N-(pyrid-2-yl)acetamide (1) by the interaction with 4-hydroxybenzaldehyde was successfully carried out in the presence of DMSO and potassium carbonate at room temperature to furnish 2-(4-formylphenoxy)-N-(pyrid-2-yl)acetamide (2) (Ma et al., 2011) (Scheme 1). The key compound, 2-(4-formylphenoxy)-N-(pyrid-2-yl)acetamide (2), was utilized as a precursor for the construction of pyridine-thiazole hybrids through building of thiazole ring systems at the formylphenoxy part. Thus, the synthetic plan of the present part starts by condensation of the formyl group in the key 2 with thiosemicarbazide. The condensation proceeded by heating in ethanol in the presence of glacial acetic acid to afford the corresponding thiosemicarbazone derivative 3 with 71% yield. The target pyridine-thiazole compounds 4a and 4b were obtained by the reaction of equimolar quantities of thiosemicarbazone derivative 3 with phenacyl bromide and/or 4-chlorophenacyl bromide, respectively. The IR, 1H NMR, 13C NMR and mass spectral analyses were utilized to elucidate the structures of these hybrids 4a and 4b.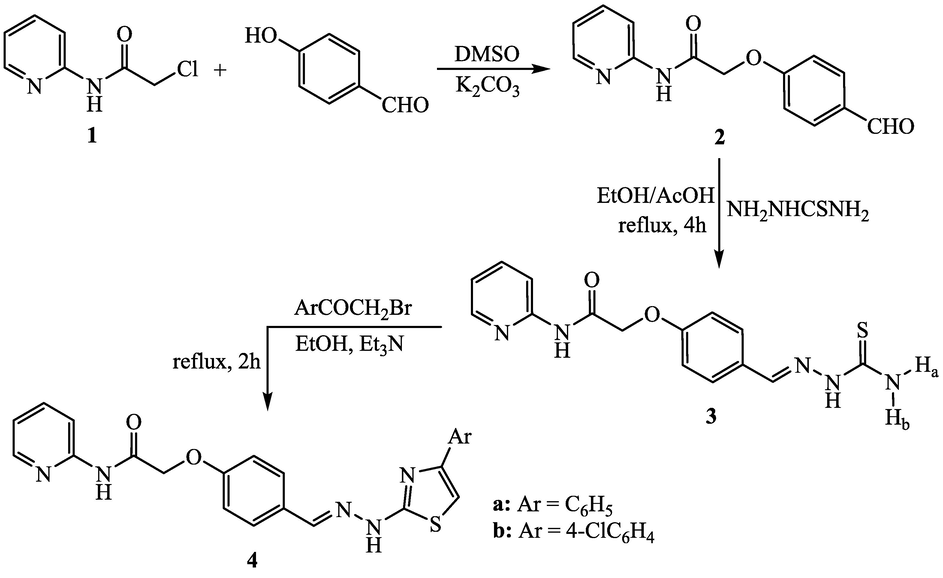
Synthesis of pyridine-thiazole hybrids 4a and 4b.
The cyclocondensation of thiosemicarbazone derivative 3 with ethyl bromoacetate was performed in boiling ethanol in the presence of sodium acetate to produce 2-(4-((2-(4-oxothiazolin-2-yl)hydrazono)methyl)phenoxy)-N-(pyrid-2-yl)acetamide (5), which undergoes Knoevenagel condensation with three different benzaldehyde derivatives (namely; 4-tolualdehyde, 4-anisaldehyde and/or 4-chlorobenzaldehyde). Such condensation was proceeded by refluxing the reaction mixture in ethanol and piperidine to afford the conforming 5-arylidene-thiazolin-4-one derivatives 6a-c (Scheme 2). The structures of pyridine-thiazole hybrids 5 and 6 were established based on their correct spectral analyses. For example, the IR spectrum of pyridine-thiazolin-4-one derivative 5 displayed absorption at 1708 cm−1 for the carbonyl group of the new constructed thiazole moiety. The lower absorption of this carbonyl group (1691–1698 cm−1) in compounds 6a-c is attributed to the conjugation with exocyclic double bond. The 1H NMR spectrum of 5 showed the protons of methylene (thiazolin-4-one moiety) as singlet at 4.11 ppm. The 1H NMR spectra of compounds 6a-c clearly indicated the lack of this signal and instead displayed a singlet signal of the olefinic proton in the region from 7.76 to 7.78 ppm. The reaction of 3 with diethyl bromomalonate was performed in boiling ethanol and sodium acetate to yield ethyl 4-oxo-2-(2-(4-(2-oxo-2-(pyrid-2-ylamino)ethoxy)-benzylidene)hydrazinyl)thiazolin-5-carboxylate (7). The plausible reaction mechanism for the formation of 7 can be initiated by replacement the bromine atom of diethyl bromomalonate by sulfur atom of thiosemicarbazide to form an open chain-sulfide. This subsequently undergoes in situ elimination of ethanol molecule to give final thiazole ring system 7 in the light of modified Hantzsch-Thiazole synthesis.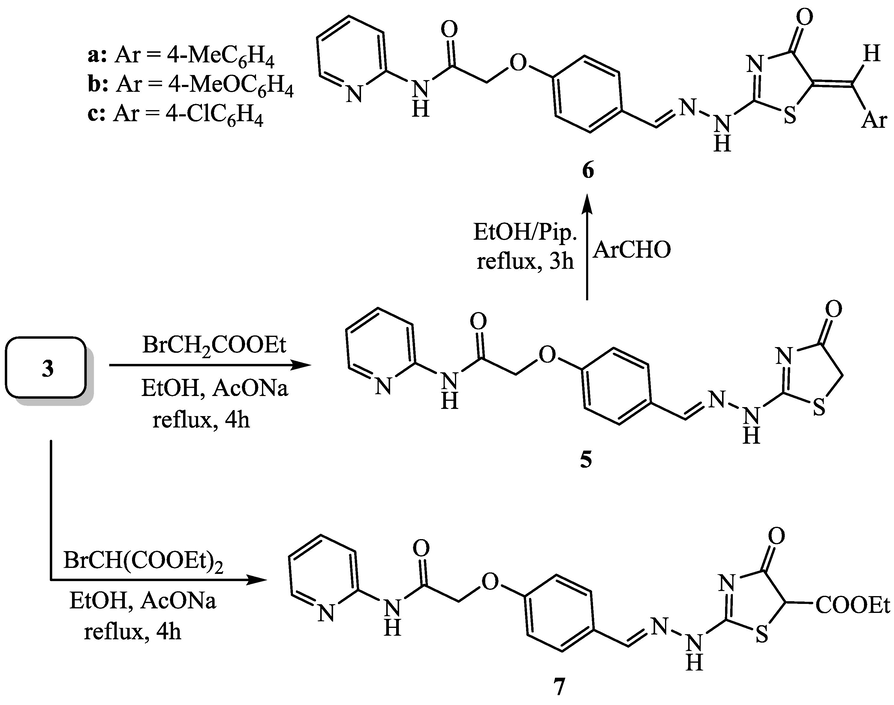
Synthesis of pyridine-thiazole hybrids 5, 6 and 7.
The synthesis of 5-acetylthiazole derivative 8 was achieved via treatment of thiosemicarbazone 3 with 3-chloropentane-2,4-dione in refluxing ethanol and triethylamine (Scheme 3). The structure of pyridine-thiazole hybrid 8 was established because of its agreement spectral data. The 1H NMR spectrum of 8 showed two singlet signals for the protons of methyl groups (thiazole-COCH3 and thiazole-CH3) at 2.58 and 2.76 ppm, respectively. The details of NMR signals were described in the experimental section. The acetyl group of pyridine-thiazole hybrid 8 was successfully converted into to the conjugated thiazole-enaminone derivative 9 by heating with dimethylformamide-dimethylacetal (DMF-DMA) in xylene. The enaminone part of pyridine-thiazole hybrid 8 was utilized as a precursor for the synthesis of pyrazole ring system through treatment with hydrazine hydrate in refluxing ethanol to furnish the corresponding thiazolyl-pyrazole compound 10. The spectroscopic tools (IR, 1H NMR and 13C NMR) were employed to confirm the structures of thiazole-enaminone 9 and its corresponding thiazolyl-pyrazole 10. The IR spectrum of thiazole-enaminone 9 showed the conjugated carbonyl absorption band at 1687 cm−1 as well as the absorption bands at 3272, 3185 cm−1 for the NH groups. Moreover, 1H NMR spectrum of thiazole-enaminone 9 showed the characteristic signals for the conjugated enaminone part as two singlet signals at 3.12 and 3.28 ppm (two methyl groups) and singlet for one proton (C⚌CH-N) at 8.12 ppm. These signals were completely disappeared from the 1H NMR spectrum of compound 10 and instead showed the characteristic doublet signals of the pyrazole ring system at 6.64 ppm (pyrazole-H4) and 7.78 ppm (pyrazole-H5), respectively, with coupling constant = 4.00 Hz.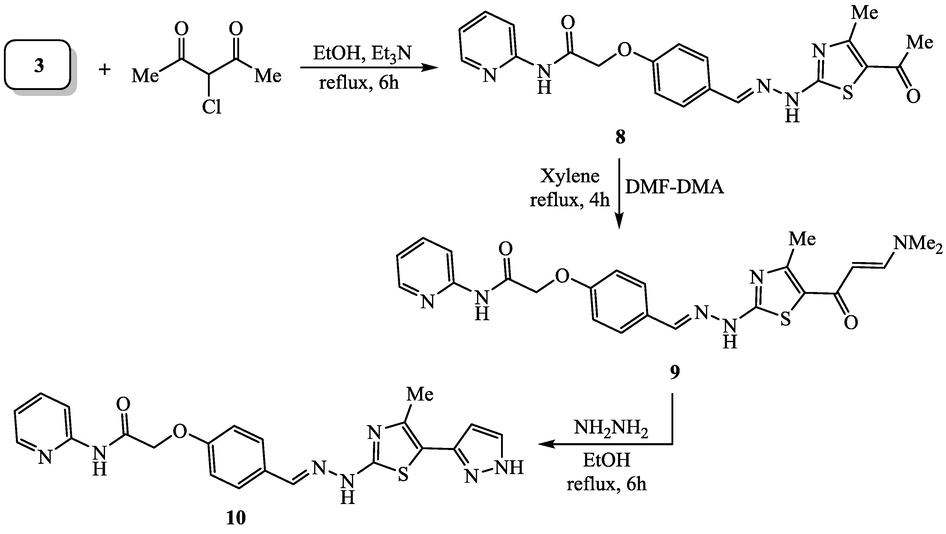
Synthesis of pyridine-thiazole hybrids 8, 9 and 10.
3.2 In vitro anticancer activity
The cytotoxicity of ten pyridine linked thiazole derivatives and their precursors was screened against four cancer cell lines; liver carcinoma (HepG2), laryngeal carcinoma (Hep-2), prostate cancer (PC3), breast cancer (MCF-7) and normal fibroblast cells (WI38) by the MTT assay (Mohamed et al., 2017; Abd El-Meguid et al., 2019) at the National Cancer Institute (Egypt). The results of cytotoxicity are listed (Table 1) in terms of IC50 values and compared with the known anticancer reference drug 5-fluorouracil (5-Fu). Some of the synthesized pyridine-thiazole compounds 7 and 10 exhibited good cytotoxicity with pronounced activity to inhibit the growth of MCF-7 and HepG2 cancer cells. Structure activity relationship (SAR) results of the tested pyridine-thiazole hybrids gives an insight concerning the structural features related to anticancer activity. Firstly, cyclization of the thiosemicarbazone 3 with ethyl bromomalonate to produce compound 7 in which the thiazolin-4-one ring system is substituted at the fifth position with ethyl carboxylate group enhances the cytotoxic effectiveness toward MCF-7 and HepG2 cancer cells with IC50 values 5.36 and 6.78 μM, respectively. Its activity is higher than the anticancer reference drug 5-fluorouracil that exhibit potency with IC50 values 6.14 and 7.20 μM for MCF-7 and HepG2 cancer cells, respectively. The pyridine-thiazole hybrid 10 that substituted with pyrazole moiety at the fifth position of thiazole ring system exhibited the next remarkable activity against the same cancer cells with IC50 = 5.84 and 8.76 μM, respectively. For the pyridine-4-arylidenethiazolin-4-one derivatives 6a-c, it was observed that compound 6c (Ar = 4-ClC6H4) revealed strong cytotoxicity with IC50 = 10.63 μM toward against breast cancer cell line. This is higher than that of compound 6b (Ar = 4-MeOC6H4) which exhibited moderate inhibition with IC50 = 12.68 μM, although the latter compound 6b (Ar = 4-MeC6H4) exhibited sensible inhibition toward MCF-7 cell line with IC50 = 15.42 μM. These results find support from the order of pharmacological activity that follow the arrangement of substituents (Abbas and Abd El-Karim, 2019; Al-Anazi et al., 2019): 4-Cl > 4-OCH3 > 4-CH3. The pyridine-thiazole hybrids 4, 5 and 8 showed acceptable cytotoxic activities towards MCF-7 and HepG2 cell lines to announce IC50 values in the range 18.51–27.16 μM. The introduction of enaminone moiety in compound 9 caused dramatic decrease in the cytotoxic activity towards MCF-7 and HepG2 cell lines (IC50 = 27.63 and 30.36 μM) when compared with the activity of all the other synthesized hybrids. Further examination of the synthesized pyridine-thiazole hybrids was performed to explore their cytotoxicity on the normal cell line (WI38). The results indicated that most of these compounds affected lesser extent of cytotoxicity, their IC50 ranged from 22.82 to 77.14 μM. Fortunately, the promising selectivity of compounds 7 and 10 as cytotoxic agents for MCF-7 and HepG2 cell lines, they showed weak cytotoxic effects on normal cell lines (WI38).
Compound
Cytotoxicity IC50 (μM)a
HepG2
Hep-2
PC3
MCF-7
WI38
2
40.33 ± 0.19
>50
>50
42.95 ± 0.37
15.14 ± 1.29
3
34.04 ± 0.46
43.36 ± 0.20
42.31 ± 0.18
33.21 ± 0.11
10.35 ± 0.88
4a
27.16 ± 0.52
39.98 ± 0.24
36.28 ± 0.45
25.75 ± 0.48
>50
4b
24.66 ± 0.29
32.24 ± 1.07
35.56 ± 0.25
22.84 ± 0.26
>50
5
21.73 ± 0.17
40.59 ± 1.26
37.17 ± 0.36
18.51 ± 0.17
34.38 ± 0.62
6a
18.12 ± 0.23
31.61 ± 0.21
34.44 ± 0.64
15.42 ± 0.23
38.29 ± 0.84
6b
14.82 ± 0.38
29.65 ± 0.43
32.64 ± 0.27
12.68 ± 0.16
22.82 ± 0.27
6c
10.25 ± 0.37
26.98 ± 0.14
20.62 ± 0.14
10.63 ± 0.41
>50
7
6.78 ± 0.18
17.71 ± 0.56
18.36 ± 0.19
5.36 ± 0.23
>50
8
24.15 ± 0.47
>50
40.11 ± 0.36
23.19 ± 0.33
31.26 ± 0.60
9
30.36 ± 0.19
37.44 ± 0.49
27.26 ± 0.03
27.63 ± 0.40
28.41 ± 0.52
10
8.76 ± 0.24
31.81 ± 0.37
26.90 ± 0.19
5.84 ± 0.17
>50
5-Fub
7.20 ± 0.45
5.35 ± 0.23
8.78 ± 0.60
6.14 ± 0.31
10.12 ± 0.62
3.3 Molecular docking
The molecular docking (Al-Dawood et al., 2016) was employed to discuss the binding mode of synthesized pyridine-thiazole derivatives through Rho-associated protein kinase (ROCK-1) via utilizing MOE v10.2015.10 program. The Rho-associated kinase (ROCK-1) is critical for cancer cell migration and invasion, suggesting it may be useful therapeutic target (Patel, et al., 2012). The protein structure of PDB ID: 3TWJ was selected from protein data bank (PDB). One of the famous anti-cancer chemotherapy drugs for colon and breast cancer is Fluorouracil, which was utilized as reference ligand to undertake the molecular docking studies toward ROCK-1 protein. Fluorouracil is a pyrimidine analog that is a nucleoside metabolic inhibitor for the treatment of breast cancer (Choi and Kim, 2009). The interactions of the newly designed hybrids with active sites are summarized in Table 2.
Cpd.
S (energy score) (Kcal/mol)
Rmsd (refine unit)
Interaction with ligand
Types of Interactions
Distance (Å)
2
−5.8000
1.1835
Amidic NH with Ser 108
H-donor
2.81
N of pyridine ring with Phe 110N of pyridine ring with Phe 110
H-acceptor
3.41
O of amidic group with Glu 379
H-acceptor
2.90
O of aldehydic group with Arg 84
H-acceptor
3.02
Benzene ring with Glu 88
π-π interaction
4.27
3
−6.7686
0.6768
NH of thiosemicarbazone with Asp 374
H-donor
2.92
NH2 of thiosemicarbazone with Asp 374
H-donor
3.21
NH2 of thiosemicarbazone with Arg 84
H-acceptor
3.10
4a
−6.8745
1.3217
S of thiazole ring with Ser 108
H-donor
3.20
O of Amidic group with Ala 86
H-acceptor
3.00
Pyridine ring with Ala 86
π-π interaction
3.65
4b
−7.1059
1.2637
S of thiazole ring with Ser 108
H-donor
3.09
S of thiazole ring with Arg 147
H-donor
3.15
O of amidic group with Ala 86
H-acceptor
3.04
Hydrazo N1 with Glu 379
H-acceptor
3.08
5
−6.6614
1.4464
Amidic NH with Asp 347
H-donor
2.94
Pyridine ring with Arg 84
π-π interaction
3.40
6a
−7.4179
1.5616
Amidic NH with Asp 347
H-donor
3.58
Pyridine ring with Arg 84
π-π interaction
4.09
Benzene ring with Glu 379
π-π interaction
4.19
Thiazolinone ring with Thr 380
π-π interaction
4.18
6b
−8.0093
1.9965
S of thiazolinone ring with Glu 111
H-donor
3.92
Pyridine ring with Phe 381
π-π interaction
3.96
6c
−7.7215
2.3986
Amidic NH with Ser 108
H-donor
3.06
Hydrazo N1 with Phe 381
H-donor
3.27
Hydrazo N2 with Phe 381
H-acceptor
3.16
S of thiazolinone ring with Phe 381
H-donor
4.34
7
−7.2041
1.2968
Hydrazo N1 with Asp 374
H-donor
2.96
S of thiazolinone ring with Asp 374
H-donor
3.59
O of thiazolinone ring with Glu 379
H-acceptor
3.27
8
−7.1284
2.3076
Amidic NH with Asp 374
H-donor
3.22
Thiazole ring with Thr 380Thiazole ring with Thr 380
π-π interaction
4.444.44
Benzene ring with Glu 379
π-π interaction
3.96
9
−7.0980
1.8481
S of thiazole ring with Glu 397
H-donor
4.16
Hydrazo N2 with Glu 397
H-acceptor
3.27
Pyridine ring with Gln 391
π-π interaction
3.52
10
−7.5528
2.2258
NH of pyrazole ring with Ala 86
H-donor
3.18
S of thiazole ring with Glu 111
H-donor
3.90
2-(4-Formylphenoxy)-N-(pyrid-2-yl) acetamide derivative 2 displayed small binding over two different types of interactions. Four intermolecular hydrogen bonds between the nitrogen atom of amidic group with Ser 108 (2.81 Å), N-atom of pyridine ring with Phe 110 (3.41 Å), and O-atom of amide function with Glu 379 (2.90 Å). Two π-π interactions between both of O atom of Aldehydic group with Arg 84 and Benzene ring with Glu 88 through intermolecular distance (3.02 Å), (4.27 Å), respectively. This aldehyde precursor 2 exhibited binding energy score, S = −5.8000 kcal/mol (Fig. S1). Thiosemicarbazone derivative 3 displayed three intermolecular hydrogen bonds. The first intermolecular hydrogen bond was displayed between NH of Thiosemicarbazone moiety with Asp 374 (2.92 Å) (Fig. S2). Where, the second and third hydrogen bonds are formed between NH2 of thiosemicarbazone moiety with both of Asp 374 and Arg 84 through intermolecular distances (3.21 Å) and (3.10 Å), respectively. All of these interactions presented good binding energy score, S = −6.7686 kcal/mol.
The pyridine-thiazole compound 4a delivered binding through formation of two intermolecular hydrogen bonds in addition to one π-π interaction (Fig. S3). One hydrogen bond was formed between the sulfur atom of the thiazole ring and Ser 108 (3.20 Å), second intermolecular hydrogen bond was displayed between oxygen atom of the amide moiety and Ala 86 (3.00 Å), and π-π interaction was exhibited between the pyridine ring with Ala 86 (3.65 Å). It is noticeable that derivative 4a was revealed a better interaction energy score (S = −6.8745 kcal/mol). The second derivative 4b presented four intermolecular hydrogen bonds. The first and the second interactions were existed between the S-atom of the thiazole ring and both of Ser 108 (3.09 Å), and Arg 147(3.15 Å) of amino acids of 3TWJ. Meanwhile, the third hydrogen bond was raised between the O-atom of amidc group with Ala 86 via intermolecular distance (3.04 Å), and the fourth was arisen between Glu 379 and the nitrogen atom of the hydrazo moiety (3.08 Å) (Fig. S4). Derivative 4b was shown a score of binding with the amino acids of 3TWJ (S = −7.1059 kcal/mol).
Meanwhile, compound 5 revealed two dissimilar types of interactions (Fig. 2). The first was an intermolecular hydrogen bond between the nitrogen atom of the amide function with Asp 347 (2.94 Å), and π-π interactions were exhibited between the pyridine ring with Arg 84 (3.40 Å). Compound 5 was exhibited reasonable binding score with the amino acids of 3TWJ (S = −6.6614 kcal/mol). The pyridine-thiazole derivative 6a displayed three π-π interactions in addition to one intermolecular hydrogen bond. The first π-π interaction was demonstrated between pyridine ring with Arg 84 (4.09 Å). The second and the third hydrogen bonds were arisen between the benzene ring with Glu 379, and thiazolin-4-one ring with Thr 380 through two intermolecular distances (4.19 Å) and (4.18 Å), respectively. Meanwhile, the intermolecular hydrogen bond was exhibited H-donor on the amidic nitrogen with between Asp 347 (3.58 Å). All of these intermolecular forces were displayed binding energy score, S = −7.4179 kcal/mol (Fig. 3). In addition, pyridine-thiazole derivative 6b demonstrated two types of interactions. The first was exhibited intermolecular hydrogen bond between the S- atom of thiazolin-4-one moiety with Glu 111 (3.92 Å), the second π-π interaction was arisen between Pyridine ring with Phe 381 via intermolecular forces (3.96 Å). All of these bindings were revealed better binding energy score, S = −8.0093 kcal/mol (Fig. 4). Moreover, the pyridine-thiazole derivative 6c displayed four intermolecular hydrogen bonds. The first was displayed between the hydrogen donor on the amidic nitrogen with Ser 108 (3.06 Å). Where, the rest of hydrogen bonds were displayed between both of the two nitrogen atoms of hydrazo moiety, and S atom of thiazolin-4-one ring with Phe 381through intermolecular distance (3.27 Å), (4.34 Å), and (3.16 Å), respectively. Derivative 6c was showed given binding energy score, S = −7.7215 kcal/mol (Fig. 5).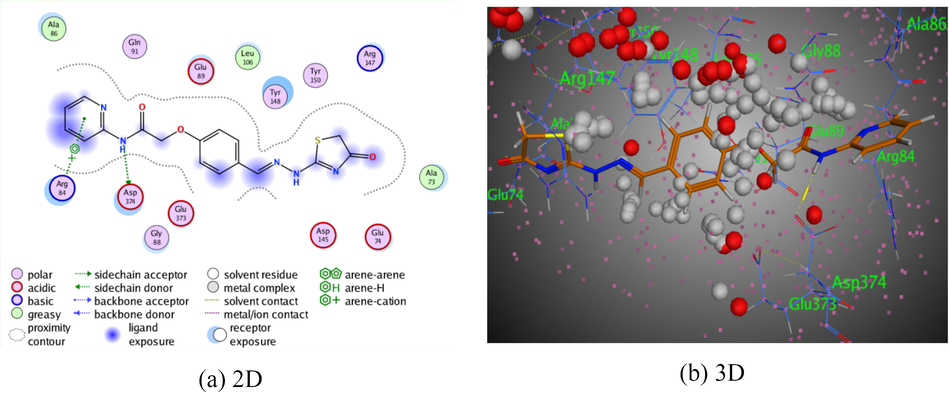
The interaction of pyridine-thiazole hybrid 5 with Rho-associated protein kinase 1.
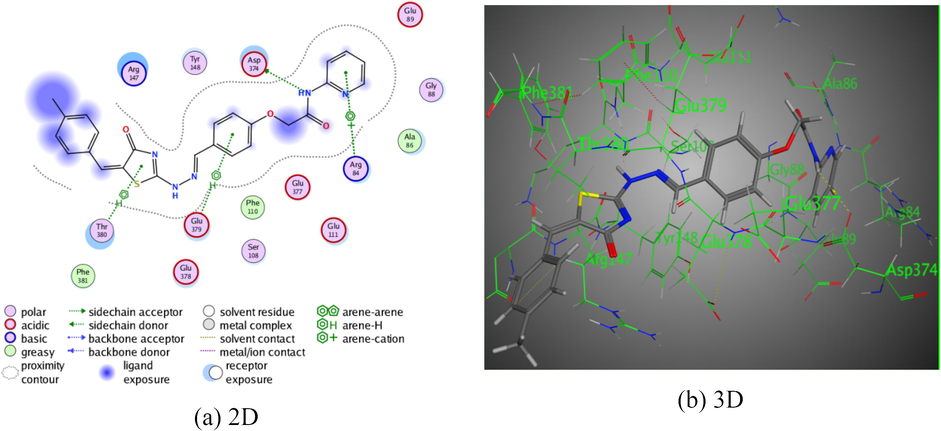
The interaction of pyridine-thiazole hybrid 6a with Rho-associated protein kinase 1.
The interaction of pyridine hybrid 6b with Rho-associated protein kinase 1.
The interaction of pyridine-thiazole hybrid 6c with Rho-associated protein kinase 1.
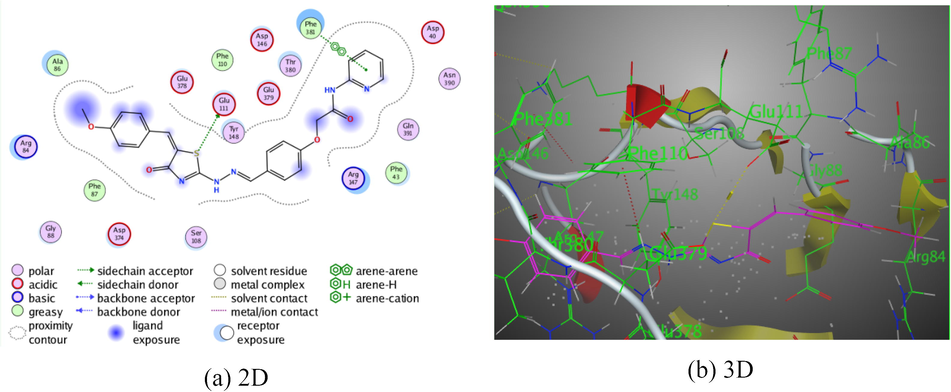
The pyridine-thiazole derivative 7 presented three intermolecular hydrogen bonds. The first and the second intermolecular hydrogen bonds were demonstrated between the hydrogen donor of both of hydrazo N1 and S-atom of thiazolidinone ring with Asp 374 through intermolecular distance (2.96 Å) and (3.59 Å), respectively. While, the third hydrogen bond was established between the oxygen atom of the thiazolin-4-one ring with Glu 379 (3.27 Å). All of these bindings were displayed binding energy score, S = −7.2041 kcal/mol (Fig. 6).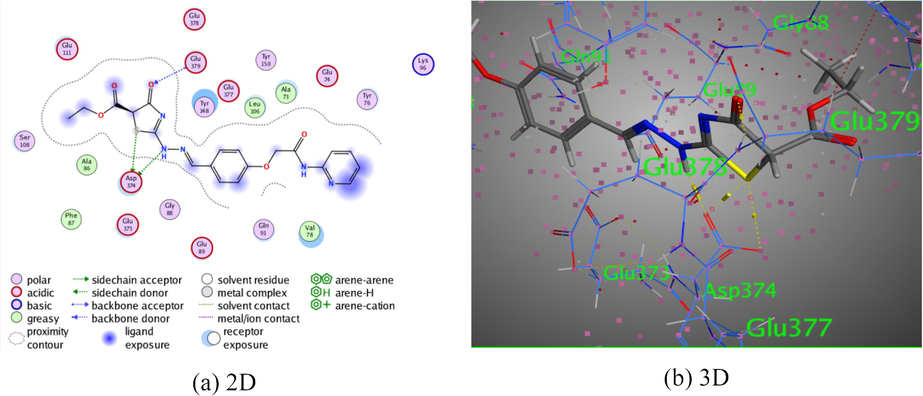
The interaction of pyridine-thiazole hybrid 7 with Rho-associated protein kinase 1.
Furthermore, pyridine-thiazole compound 8 exhibited one intermolecular hydrogen bond in addition to two π-π interactions. The hydrogen bond was demonstrated between the hydrogen donor on the nitrogen atom of the amidic moiety with the Asp 374 (3.04 Å), and the two π-π interactions were risen between both of the thiazole ring with Thr 380 and the benzene ring with Glu 379 through intermolecular distances (4.44 Å) and (3.96 Å), respectively. All of these interactions were revealed binding energy score, S = −7.1284 kcal/mol (Fig. S5). Meanwhile, enaminone compound 9 revealed two intermolecular hydrogen bonds and one π-π interaction. The two hydrogen bonds were demonstrated between both of the nitrogen atom of the hydrazo group and the S-atom of the thiazole ring with Glu 379 through intermolecular distances (3.27 Å) and (4.16 Å), respectively. However, the π-π interaction was displayed between the pyridine ring with the Gln 391 through intermolecular distance (3.52 Å). Somewhere, these bindings were disclosed binding energy score, S = −7.0980 kcal/mol (Fig. S6).
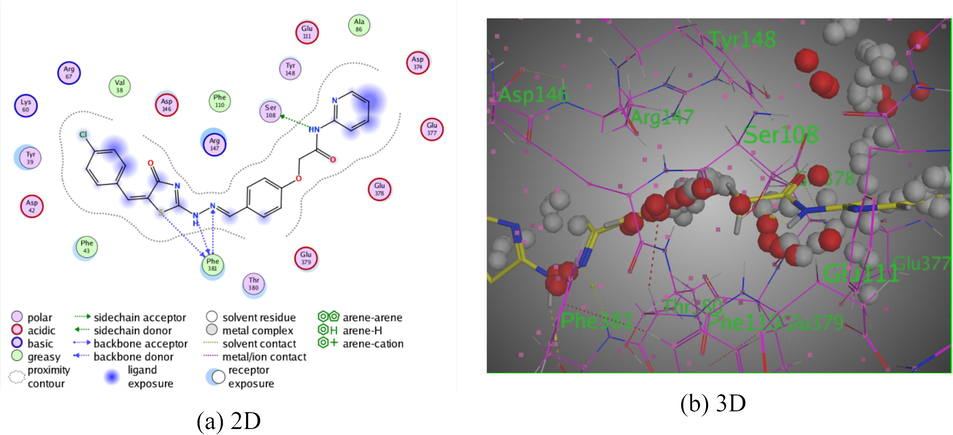
Moreover, derivative 10 presented two intermolecular hydrogen bonds. The first one was shown between the hydrogen donor of NH of pyrazole ring with Ala 86 through intermolecular distance (3.18 Å). Where, the second hydrogen donor was exhibited between S-atom of the thiazole ring with Glu 111 I intermolecular distance (3.90 Å). All of these bindings were disclosed binding energy score, S = −7.5528 kcal/mol (Fig. 7).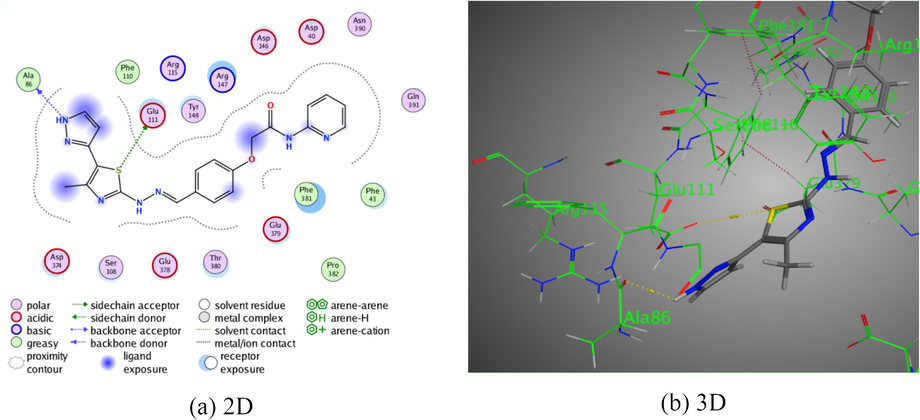
The interaction of pyridine-thiazole hybrid 10 with Rho-associated protein kinase 1.
The mechanism of antitumor action. Key components of the Rho/Rho-associated coiled-coil containing protein kinase (ROCK) signalling pathway. Various extracellular stimuli (growth factors and hormones) bind to cell membrane receptors, which subsequently act upon guanine-nucleotide-exchange factors (GEFs) and GTPase-activating proteins (GAPs) to regulate activation of Rho GTPase proteins. Once in its GTP-bound active state, Rho GTPase binds to ROCK (ROCK1/2) to stimulate key downstream effectors (Chin et al., 2015).
4 Conclusion
In conclusion, the synthesis of ten new pyridine-thiazole compounds was achieved through an inexpensive synthetical protocol based on a modified Hantzsch synthesis when thiosemicarbazone of 2-(4-formylphenoxy)-N-(pyrid-2-yl)acetamide subject to cyclization with different α-halogenated reagents. In addition, the synthesized compounds 7 and 10 showed good cytotoxicity and selectivity towards MCF-7 and HepG2 cancer cells with weak cytotoxic effect on WI38 normal cells. The presence of electronegative chlorine atom in the compound 6c enhances the cytotoxicity against cancer cells more than their corresponding derivatives 6b and 6a that substituted with methoxy and methyl groups, respectively. Moreover, compound 6c does not show high cytotoxicity against WI38 normal cells with an IC50 > 50 μM. The stimulating reasonable docking investigation is to confirm what level the adaptation of the ligand interactions upon interaction with diverse analogues. All the synthesized ten derivatives of pyridine linked various substituted thiazole hybrids that frames by hydrogen bonds network with the chemically aromatic moiety that form dissimilar bindings with the amino acids of 3TWJ. Most of the synthesized analogues were docked in the suitable pocket size of 3TWJ, which can be depicted as a forked bounded by ordinarily little polar residues, with hydrophobic residues in the binding receptors (Ser 108, Phe 110, Arg 84, Asp 374, Ala 86, Glu 379, Phe 381) as recorded in the three-dimensional figures. Furthermore, the docking studies for the synthesized derivatives 10, 7 and 6a-c were recorded the highest binding score against the amino acids of 3TWJ rather than the rest of the derivatives and this is agreed with the results of the cytotoxic activities.
Acknowledgements
The authors thank the Deanship of Scientific Research at Umm Al-Qura University for the continuous support. This work was supported financially by the Deanship of Scientific Research at Umm Al-Qura University (Grant No: 17-MED-1-03-0007 to Alaa Alqahtani).
Declaration of Competing Interest
The authors have declared no conflicts of interest.
References
- Design, synthesis and anticervical cancer activity of new benzofuran–pyrazol-hydrazono-thiazolidin-4-one hybrids as potential EGFR inhibitors and apoptosis inducing agents. Bioorganic Chem.. 2019;89:103035.
- [Google Scholar]
- Synthesis of new 1,3,4-oxadiazole-benzimidazole derivatives as potential antioxidants and breast cancer inhibitors with apoptosis inducing activity. Russ. J. Gen. Chem.. 2019;89:348-456.
- [Google Scholar]
- Synthesis and antibacterial evaluation of some new thiazole-based polyheterocyclic ring systems. J. Heterocycl. Chem.. 2019;56:1978-1985.
- [Google Scholar]
- 2-Amino-5-arylazothiazole-based derivatives: In vitro cytotoxicity, antioxidant properties, and bleomycin-dependent DNA damage. ChemistrySelect. 2019;4:5570-5576.
- [Google Scholar]
- Molecular docking and DFT studies on some nano-meter binuclear complexes derived from hydrazine-carbothioamide ligand, synthesis, thermal, kinetic and spectral characterization. J. Mol. Liq.. 2016;220:311-323.
- [Google Scholar]
- A review on the medicinal importance of pyridine derivatives. J. Drug Des. Med. Chem.. 2015;1:1-15.
- [Google Scholar]
- A thiazole analogue exhibits an anti-proliferative effect in different human carcinoma cell lines and its mechanism based on molecular modeling. Adv. Biol. Chem.. 2017;7:76-87.
- [Google Scholar]
- Synthesis of some thiazolyl aminobenzothiazole derivatives as potential antibacterial, antifungal and anthelmintic agents. J. Enzyme Inhib. Med. Chem.. 2011;26:22-28.
- [Google Scholar]
- Synthesis and antitubercular activity of imidazo[2,1-b]thiazoles. Eur. J. Med. Chem.. 2001;36:743-746.
- [Google Scholar]
- Synthesis of some new 2-(3-pyridyl)-4,5-disubstituted thiazoles as potent antimicrobial agents. Eur. J. Med. Chem.. 2013;62:270-279.
- [Google Scholar]
- Design, synthesis and antimalarial evaluation of novel thiazole derivatives. Bioorg. Med. Chem. Lett.. 2016;26:3938-3944.
- [Google Scholar]
- Pyridine and pyrimidine derivatives as privileged scaffolds in biologically active agents. Curr. Med. Chem.. 2019;26:7166-7195.
- [Google Scholar]
- Rho-associated kinase signalling and the cancer microenvironment: novel biological implications and therapeutic opportunities. Expert Rev. Mol. Med.. 2015;17:e17.
- [Google Scholar]
- 5-Fluorouracil combined with apigenin enhances anticancer activity through induction of apoptosis in human breast cancer MDA-MB-453 cells. Oncol. Rep.. 2009;22:1533-1537.
- [Google Scholar]
- Synthesis and antiproliferative activity of 2,6-dibenzylamino-3,5-dicyanopyridines on human cancer cell lines. Eur. J. Med. Chem.. 2005;40:1365-1372.
- [Google Scholar]
- Synthesis and antiviral activity of some new bis-1,3-thiazole derivatives. Eur. J. Med. Chem.. 2015;102:266-276.
- [Google Scholar]
- Assessment of anti-diabetic activity of a novel hydrazine-thiazole derivative: in vitro and in vivo method. Braz. J. Pharm. Sci.. 2019;55:e18218.
- [Google Scholar]
- A review in advanced melanoma with a BRAF V600 mutation. Target. Oncol.. 2016;11:417-428.
- [Google Scholar]
- Anti-liver cancer activity in vitro and in vivo induced by 2-pyridyl 2,3-thiazole derivatives. Toxicol. Appl. Pharm.. 2017;329:212-223.
- [Google Scholar]
- Derivatives of pyridine and thiazole hybrid: Synthesis, DFT, biological evaluation via antimicrobial and DNA cleavage activity. Bioorg. Chem.. 2020;95:103476.
- [Google Scholar]
- Nicotinic acid: pharmacological effects and mechanisms of action. Annu. Rev. Pharmacol. Toxicol.. 2008;48:79-106.
- [Google Scholar]
- Synthesis and antitumor evaluation of some new derivatives and fused heterocyclic compounds derived from thieno[2,3-b] pyridine: Part 2. J. Heterocycl. Chem.. 2020;57:694-715.
- [Google Scholar]
- Identification and development of thiazole leads as COX-2/5-LOX inhibitors through in-vitro and in-vivo biological evaluation for anti-inflammatory activity. Chem: Bioorg; 2020. p. :103882.
- Heterocyclic Chemistry (4th ed.). Oxford, UK: Blackwell Publishing; 2000.
- Dibromoketone precursors in the synthesis of some new thiazole derivatives: Thiazol-2-yl hydrazonobutanoates, thiazol-2-yl pyrazole-4-carboxylates and acids. J. Heterocycl. Chem.. 2020;57:2173-2183.
- [Google Scholar]
- Synthesis, characterization, molecular docking and evaluation of antimicrobial, antiproliferative, and anti-inflammatory properties of new 4-biphenyl substituted thiazolyl-pyridin-2-amine derivatives. Der Pharma Chem.. 2014;6:233-243.
- [Google Scholar]
- Synthesis of 3β, 3β, 5-tribromoacetyl-4-hydroxy-6-methyl-2H-pyran-2-one by bromination of dehydroacetic Acid. Synth. Commun.. 2005;35:461-464.
- [Google Scholar]
- Synthesis, antioxidant and anticholinesterase activities of novel coumarylthiazole derivatives. Bioorg. Chem.. 2015;59:80-90.
- [Google Scholar]
- Synthesis, molecular modeling studies and evaluation of antifungal activity of a novel series of thiazole derivatives. Eur. J. Med. Chem.. 2018;151:248-260.
- [Google Scholar]
- Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J. Med. Chem.. 2004;47:6658-6661.
- [Google Scholar]
- Design, synthesis, and antihepatitis B virus activities of novel 2-pyridone derivatives. J. Med. Chem. 2010:660-668.
- [Google Scholar]
- Synthesis and biological activity of novel barbituric and thiobarbituric acid derivatives against non-alcoholic fatty liver disease. Eur. J. Med. Chem.. 2011;46:2003-2010.
- [Google Scholar]
- Synthesis and anticancer activity of novel 2-substituted pyranopyridine derivatives. Res. Chem. Intermed.. 2017;43:437-456.
- [Google Scholar]
- Structure activity relationships of 4-hydroxy-2-pyridones: A novel class of antituberculosis agents. Eur. J. Med. Chem.. 2015;106:144-156.
- [Google Scholar]
- RKI-1447 is a potent inhibitor of the Rho-associated ROCK kinases with anti-invasive and antitumor activities in breast cancer. Cancer Res.. 2012;72:5025-5034.
- [Google Scholar]
- α, α-Dibromoketones: A superior alternative to α-Bromoketones in Hantzsch thiazole synthesis. Synth. Commun.. 2007;37:2501-2505.
- [Google Scholar]
- Design and synthesis of novel 1,3-thiazole and 2-hydrazinyl-1,3-thiazole derivatives as anti-Candida agents: in vitro antifungal screening, molecular docking study, and spectroscopic investigation of their binding interaction with bovine serum albumin. Molecules. 2019;24:3435.
- [Google Scholar]
- An efficient synthesis of N-(1-arylethylidene)-N’-(4-arylthiazol-2-yl)hydrazones from α,α-dibromoacetophenones and N-(1-arylethylidene) thiosemicarbazones. Der Pharma Chem.. 2011;3:109-114.
- [Google Scholar]
- Synthesis and evaluation of novel 2-pyridone derivatives as inhibitors of phosphodiesterase3 (PDE3): A target for heart failure and platelet aggregation. Bioorg. Med. Chem. Lett.. 2012;22:6010-6015.
- [Google Scholar]
- Library design, synthesis, and screening: pyridine dicarbonitriles as potential prion disease therapeutics. J. Med. Chem.. 2006;49:607-615.
- [Google Scholar]
- A brief literature and review of patents on thiazole related derivatives. Curr. Bioact. Compd.. 2019;15:304-315.
- [Google Scholar]
- Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem.. 2020;188:112016.
- [Google Scholar]
- Novel fluoroquinolone derivatives bearing N-thiomide linkage with 6-substituted-2-aminobenzothiazoles: synthesis and antibacterial evaluation. Arab. J. Chem.. 2017;10:S568-S575.
- [Google Scholar]
- Triazole incorporated thiazoles as a new class of anticonvulsants: Design, synthesis and in vivo screening. Eur. J. Med. Chem.. 2010;45:1536-1543.
- [Google Scholar]
- Nitric oxide generated from isoniazid activation by KatG: source of nitric oxide and activity against Mycobacterium tuberculosis. Antimicrob. Agents Chemother.. 2004;48:3006-3009.
- [Google Scholar]
- The antituberculosis drug ethionamide is activated by a flavoprotein monooxygenase. J. Biol. Chem.. 2002;277:12824-12829.
- [Google Scholar]
- Imidazo[1,2-a]pyridines linked with thiazoles/thiophene motif through keto spacer as potential cytotoxic agents and NF-κB inhibitors. Bioorg. Med. Chem. Lett.. 2017;27:5463-5466.
- [Google Scholar]
- Design, synthesis and biological evaluations of novel pyridone-thiazole hybrid molecules as antitumor agents. Eur. J. Med. Chem.. 2018;145:35-40.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.11.020.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







