Translate this page into:
Synthesis and biological evaluation of novel quinazolin-4(3H)-one Schiff base derivatives as nitric oxide synthase inhibitors
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Synthesis of new Schiff base derivatives bearing a 6-nitro-2-ibuprofenyl-4(3H)-quinazolin-4-one skeleton, as well as their in vitro biological evaluation as inhibitors of nitric oxide synthase (iNOS and nNOS) isoforms, is defined. Structures of these novel heterocycles were confirmed based on their spectral, (IR, 1H NMR, 13C NMR and MS) and elemental analyses. In general, the assayed compounds behave as better iNOS than nNOS inhibitors. Quinazolinyl Schiff bases 9a-e are the most active inhibitors of all tested compounds and the most iNOS/nNOS selective of all studied compounds, with expected potential as anti-stroke agents.
Keywords
Quinazolinone
Ibuprofen
Arginine
Inhibition
Schiff Base
1 Introduction
Building of novel pharmaceuticals with enhanced therapeutic characteristics is a common target in medicinal chemistry research. In comparison to the parent medicine, these novel medications should have equivalent activity and fewer adverse effects. Ibuprofen (2-RS-(4-Isobutylphenyl) propanoic acid) is a non-steroidal anti-inflammatory medication (NSAID) that is often used to treat inflammation and pain. Many of these NSAIDs have negative side effects despite their effectiveness in reducing pain and inflammation. Long-term usage of this medication has been linked to gastro-intestinal (GI), ulceration, bleeding, and nephrotoxicity (Kimmey, 1992). Nonselective inhibitors of two isoforms of cyclooxygenase (COX), cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), have been shown to decrease the formation of hormone-like lipid molecules like prostaglandins and thromboxane, which cause inflammation, pain, fever, and platelet aggregation (Flower, 2003). NSAIDs such as aspirin, ibuprofen, and indomethacin, which are preferred COX-1 inhibitors and contain a free carboxylic group, cause the most harm. Masking of the ibuprofen-free carboxylic group, which shifts enzyme selectivity from COX-1 to COX-2, appears to be the primary cause of this reduced topical irritating effect (Selinsky et al., 2001). Certain molecules containing the ibuprofen nucleus have been reported to have strong anti-inflammatory properties, however gastrotoxicity and ulcerogenicity are less severe than with ibuprofen (Elgohary et al., 2011). Quinazolinones feature a one-of-a-kind scaffold with a wide range of medicinal capabilities, and they've been touted for their outstanding pharmacological behavior, including antiproliferative, antiinflammatory, antidiabetic, antimicrobial, antibacterial, antifibrotic, anticoagulant, antioxidant, anticonvulsant, antituberculosis, antifungal, antiviral, antimalarial, antihypertensive, and CNS depressant (Salem et al., 2020). Natural compounds with key pharmacophores make up the Schiff's base family. It can be utilized to develop agrochemicals and medications as excellent lead structures including fungicide, bactericide, antivirals, antioxidants, antiproliferative and antimicrobial drug. Quinazolinone Schiff bases are a type of heterocyclic system that has attracted a lot of attention due to their wide range of biological activities (Chen et al., 2016). Nitric oxide (NO) is created by the oxidation of L-arginine in a NADPH- and O2-dependent process catalyzed by nitric oxide synthase (NOS). There are three distinct isoforms of this enzyme: neuronal (nNOS), endothelial (eNOS), and inducible (iNOS). Overproduction of NO by nNOS has been linked to a variety of illnesses, including neurodegeneration, stroke, migraine, Alzheimer's, and Huntington's. Overproduction of NO by iNOS, on the other hand, has been linked to hypotensive crises in septic shock, arthritis, colitis, tissue damage, neuropathic pain, and a variety of inflammatory conditions. Inhibiting nNOS and iNOS enzymes using synthetic derivatives has emerged as an appealing goal in the therapy of these illnesses since they are therapeutic targets (López Cara et al., 2009).
In our present work we substituted the carboxylic acid group of ibuprofen with extra heterocyclic moieties in our endeavor to create a new and beneficial series of NSAIDs agents for treating disorders. These heterocyclic moieties are anticipated to have an interesting profile of action with a large reduction in their negative effects. From this point of view, a desired hybrid quinazolinyl Schiff base derivatives (Fig. 1) incorporating ibuprofen residues looked to be of interest to be synthesized, with the purpose of testing of its NOS inhibition effect.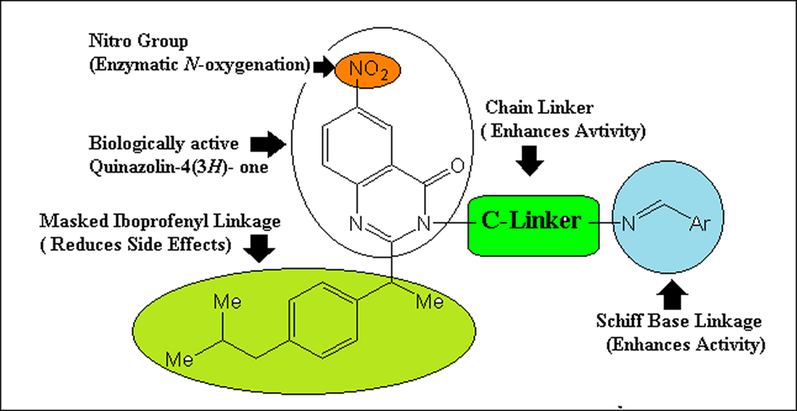
Designing of hybrid quinazolinyl Schiff bases.
2 Results and discussion
2.1 Chemistry
An extension of our group research work in organic synthesis of quinazolinone derivative (Salem et al., 2020), a novel quinazolinyl Schiff base derivatives were developed and investigated as Nitric Oxide Synthase Inhibitors (NOS). Treatment of (RS)-ibuprofen with thionyl chloride yielded 2-(4-isobutylphenyl) propanoyl chloride (1) (Mehta et al., 2010). In dry pyridine, the acid chloride 1 was reacted with the commercially available 2-amino-5-nitrobenzoic acid (2) to afford 2-(2-(4-isobutylphenyl)propanamido)-5-nitrobenzoic acid (3). The anilide 3 structure was confirmed from its spectral and micro analytical data. Hence, its IR spectrum displayed feature absorption stretching bands match with two carbonyl groups of amide and carboxylic acid functions at ν 1644 and 1720 cm−1, respectively. 1HNMR spectrum of the anilide 3 established the existence of two deuterium exchangeable protons at δ 8.70 and 12.66 due to N—H and (OHCarboxylic), respectively. The anilide 3 was intramolecularly dehydrated with acetic anhydride, resulting in cyclization and the formation of 2-(1-(4-isobutylphenyl) ethyl)-6-nitro-4H-benzo[d] [1, 3] oxazin-4-one (4). Benzoxazinone 4 IR and 1H NMR spectra revealed the absence of both carboxylic and amide functionalities. At v 1685 cm−1, a distinct absorption band was found, which is typical of the C⚌O function of 3, 1-benzoxazin-4-ones. A molecular ion peak at m/z 352 was observed in the mass spectrum, which corresponded to the expected molecular formula produced by removing a water molecule from the anilide 3. These findings support the hypothesis that both carboxylic and amidic functionalities were involved in the cyclization process (Scheme 1).![Syntheses of 2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4H-benzo[d][1,3]oxazin-4-one (4), Reagent and conditions: (a) Dry Pyridine, rt, 2hr, 70%; (b) Ac2O, reflux, 2 hr, 65%.](/content/184/2021/14/10/img/10.1016_j.arabjc.2021.103362-fig2.png)
Syntheses of 2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4H-benzo[d][1,3]oxazin-4-one (4), Reagent and conditions: (a) Dry Pyridine, rt, 2hr, 70%; (b) Ac2O, reflux, 2 hr, 65%.
A variety of aminoquinazolinones were obtained through aminolysis with some selected nitrogen nucleophiles. The reactions are expected to be proceeded via the nucleophilic ring opening ring closure (RORC) process with ring oxygen replacement with nitrogen. The attack of a nitrogen nucleophile on the oxazinone ring is accompanied by cyclization on the strongly electrophilic sp2 hybridized carbonyl carbon of the intermediate, yielding our targeted quinazolinones (El-Hashash et al., 2016).
In boiling ethanol, the benzoxazinone 4 was reacted with hydrazine hydrate, arginine, and lysine to give the aminoquinazolinones 5–7 in the desired yields. The spectral results for the amines were all in agreement with the suggested formulas. Amines 5–7 had prominent absorption bands in their infrared spectra within v 3230–3270 cm−1, which is attributed to amino groups. These findings are consistent with 1H NMR spectral data of compounds 5–7, which revealed evident singlet chemical shifts ranged between 6.20 and 6.50 due to deuterium-exchangeable protons in NH2. The molecular ion peaks at m/z 366, 508, and 480, respectively were detected in the mass spectrum of compounds 5–7 verifying the postulated structures (Scheme 2).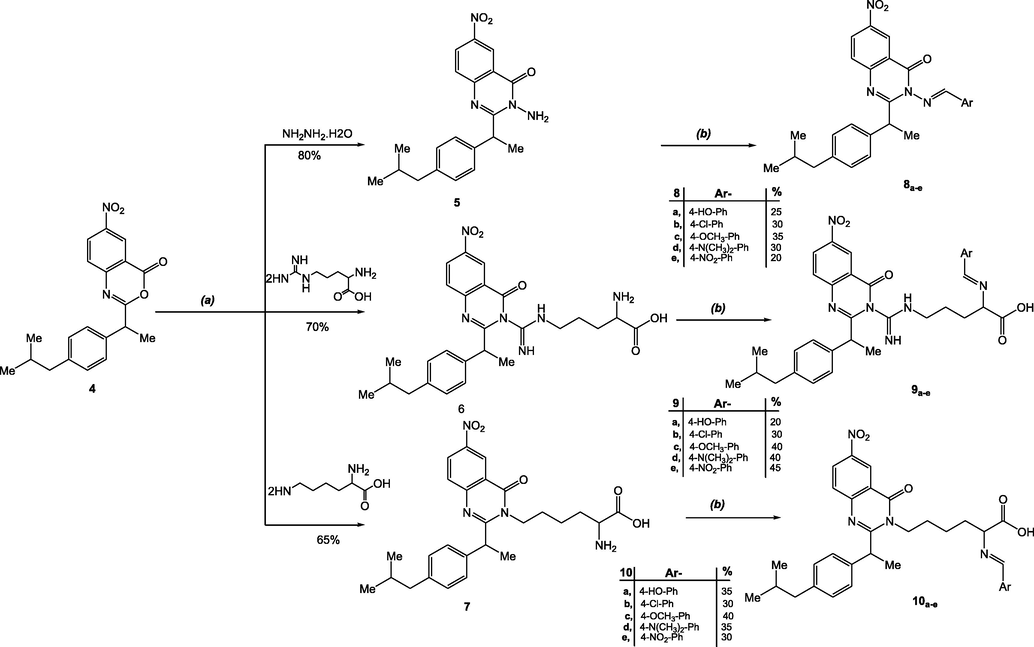
Syntheses of compounds 5–10, Reagent and conditions: (a) Pyridine, Reflux, 4 hr, (b) ArCHO, EtOH, Reflux, 8r.
Refluxing the amino derivatives 5–7 with their equimolar substituted aldehydes namely; 4-hydroxybenzaldehyde, 4-chlorobenzaldehyde, 4- methoxybenzaldehyde, 4-N,N-dimethylbenzaldehyde and 4-nitrobenzaldehyde in dry pyridine for 4 h, afforded the Schiff bases 8–10. For the proposed structures, all of the synthesized compounds provided satisfactory spectral and analytical results. According to the IR spectra, the absence of —NH stretching confirmed the conversion of —NH2 into its Schiff bases feature (N⚌CH) in addition to the existence of C⚌N stretching of compounds 8–10 at v1590–1595 cm−1. The azomethine proton (N⚌CH) resonated as a singlet at 8.85–9.10 ppm in the 1H NMR spectra. The mass spectrometry was an outstanding tool to demonstrate the suggested structures for compounds 8–10 showing their molecular ion peaks in convenience with their proposed molecular formulas. The C, H, and N values for all of the compounds were within ± 0.3 percent of the theoretical values, which was satisfactory.
By the same manner, the benzoxazinone 4 was directed to react with some biologically established amino acids with the aim of incorporating it into the structure to improve the overall biological efficiency of the molecule. Treatment of benzoxazinone 4 with a number of selective amino acids, including glycine, alanine, leucine, and cysteine, in dimethylformamide under reflux yielded quinazolineone carboxylic acid derivatives 11a-d. Absorption stretching bands attributable to C⚌O of both carboxylic acid functions were visible in the IR spectrum of 11a-d at v 1660–1665, respectively. At the convenient chemical shifts, the 1H NMR spectra of compounds 11a- d demonstrated the appearance of the emergence of new singlet’s signals assigned to the characteristic CO2H groups protons where their chemical shift signals anticipated at δ 11.20–11.50 ppm. In addition, mass spectrum represents a good guide to 11a-d structure and showed the molecular ion peaks which corresponds to the suggested molecular formulas.
As acid chlorides are a particularly active intermediate in organic chemistry for achieving transformation to new functional groups. Therefore, we attempted to synthesize a quinazolinonyl acid chloride derivative as a route of obtaining our desired Schiff base via acid chloride aminolysis. The compound 11c was chosen as a starting point for this pathway based on the familiar biological effect of the cysteine derivatives as nitric oxide synthase inhibitors (Fernhoffa et al., 2009; Ellis et al., 2000). The acid chloride derivative 12 was obtained by treating quinazolinonyl acids 11c with thionyl chloride and then removing the excess thionyl chloride under low pressure. The carbonyl chloride 12 that produced as a residue were employed without additional purification in the subsequent processes. New amide derivative 13 was obtained by treating the acid carbonyl chloride 12 with ammonium hydroxide solution in dry THF solution. The structure of amide 13 was validated by its proper analytical and spectral data. The IR spectra revealed strong absorption bands for amide NH2 groups between 3360 and 3339, while the 1H NMR spectrum revealed the typical broad signals associated to NH2 protons around 5.85 ppm. Furthermore, the mass spectrum of compound 13 revealed a molecular ion peak at m/z 454, which is in good agreement with the formula weight confirming the structure's identification.
Under one-pot conditions, an elucidative synthesis of compound 13 was achieved, with improved amide yield generation (Leggio et al., 2016). Interestingly, the chlorinating agent thionyl chloride (SOCl2), which is used to make acyl chlorides 12, could also serve as a coupling agent by activating in situ the acid carboxylic groups. The amine in the reaction medium could react with the reactive intermediate acyl chlorides, allowing the reaction to proceed under one-pot conditions with better amide yield. The spectral and analytical data of the resulted amide are coincident with the data of the previously deduced for the amide 13 (Scheme 3). Recently, a wide range of transformations using catalytic quantities of molecular iodine as catalyst were documented with interesting findings on the role of molecular iodine. In attempt to rationalize the catalytic effect of molecular iodine, three main assumptions are commonly presented in the literature; a) Iodine partial breakdown with creation of the Brønsted acid HI and subsequent Brønsted acid catalysis, b) Lewis acid (or halogen-bond) catalysis by molecular iodine itself, or c) iodine splitting to I+ and I - and subsequent activation by I+ (Breugst and von der Heiden, 2017). Silva Jr. and Quintiliano postulated that the carbonyl undergoes halogen bond activation, followed by iodine splitting and the creation of hypoiodic acid before regeneration of iodine from both HI and HOI (Silva and Quintiliano, 2009). Synthesis of schiff bases by classical condensation between amines and alehydes in refluxing ethanol has notable disadvantages in terms temperature, limited yield, and extensive reaction time. Scheme 4 displays our mechanistic pathway suggestion for molecular iodine's catalytic impact on the formation of our targeted schiff base by condensation between the quinazolinyl amine and the selected carbonyl aromatic aldehydes avoiding the limitations of the classical methods.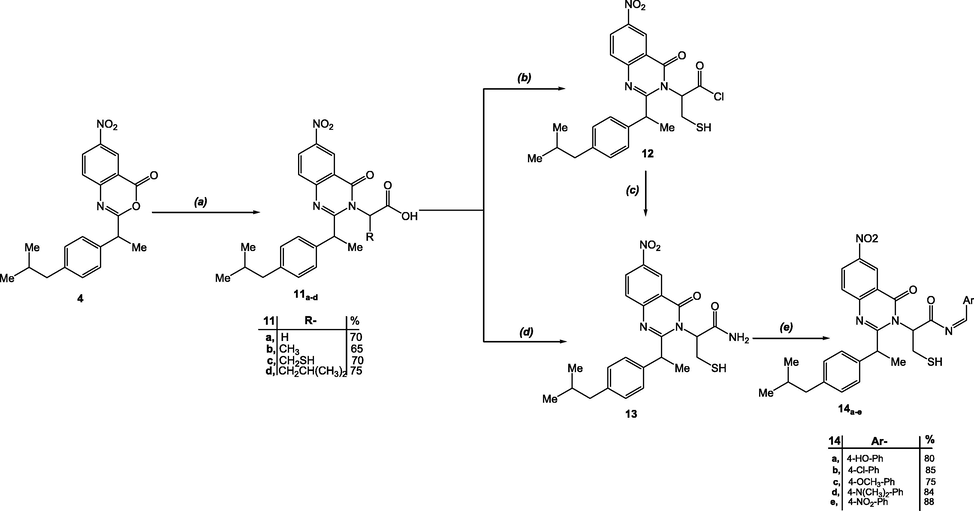
(a) NH2CH(R)COOH, DMF, Reflux, 2hr, (b)SOCl2, Reflux, 4 hr (c) NH4OH, THF, Stirring, 0C, 4hr (d) NH4OH, SOCl2, Et3N, DCM, Stirring, r.t, 20 min. (e) ArCHO, I2/KI, EtOH, Stirring, r.t.
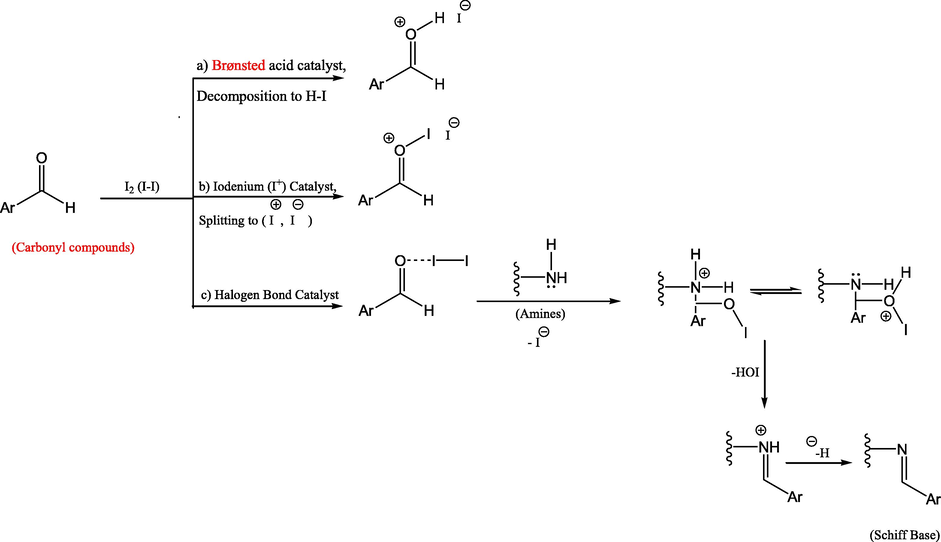
Proposed mechanism for an iodine-catalyzed Prins reaction (Silva and Quintiliano, 2009).
According to our previously published approach for the synthesis of quinazolinyl schiff bases (Elgohary and Ezz El-Arab, 2013) the catalytic condensation via I2/KI aqueous ethanolic solution is described herein to afford the Schiff bases 14a-e in excellent isolated yields at room temperature with minimal reaction time. It can be assumed that in the I2/KI catalytic system, molecular iodine acts as a mild Lewis acid with KI as a water solubilizing agent of iodine. The corresponding Schiff's bases 14a-e were obtained by condensation of 13 with the previously selected aromatic aldehydes, namely; 4-hydroxybenzaldehyde, 4-chlorobenzaldehyde, 4-methoxybenzaldehyde, 4-N,N-dimethylbenzaldehyde and 4-nitrobenzaldehyde. The products' spectral data revealed the absence of the NH2 function, suggesting its inclusion in the condensation reaction. The 1H NMR spectrum revealed unique chemical shift signals of azomethine proton (N⚌CH) at δ 8.80 (±0.03) in addition to its mass spectrum which exhibited all molecular ion peaks confirming the proposed structures Scheme 3.
2.2 nNOS and iNOS inhibition
In vitro tests using recombinant isoenzymes were used to assess the biological activities of the synthesized quinazolinyl Schiff base compounds (8, 9, 10, and 14) as inhibitors of both nNOS and iNOS (Bredt and Snyder, 1990). A preliminary assay was done using 1 mM concentrations of each product to determine the most potent compounds Table 1.
In terms of iNOS inhibition, 9a-e Schiff bases with a guanidine moiety linked to the substrate L-arginine are the best inhibitors of all other Schiff bases studied, displaying more than 50% inhibition. Nonetheless, the experimental data can be used to draw certain inferences. With regards to the azomethine Schiff bas linkage containing the various p-aromatic substituents, the influence of the L-arginine chain on iNOS inhibition activity is vary from a qualitative standpoint. In general, substituted compounds with p-methoxy and p- 4-N, N-dimethyl substituents show more inhibitory action than substituted molecules with P-Hydroxy, and P-chloro substituents. Compounds with P-nitro substituent clearly demonstrate the enzymatic N-Oxygenation effect (Winkler and Hertweck, 2007) with better inhibition values.
Schiff base derivatives 14a-e revealed moderate inhibition values in comparison to 10a-e with less effective inhibition, due to the presence of the thiol functionality in addition to its branched chain structure. With the internal variation in the inhibition values, there is no substantial values of inhibitions of 8a-e isoforms, as expected for it.
Table 1. Also shows the nNOS inhibition values in the presence of a 1 mM concentration of final quinazolinyl Schiff base compounds (8, 9, 10, and 14). The nNOS inhibitory values of Shiff base derivatives 9a-e are the highest of all the compounds studied in this case. Compounds 10c and 10e showed stronger inhibition than thiols 14 a-e, with moderate nNOS inhibition values, which in turn have a better inhibitory effect than compounds 10a, 10b, and 10d. The inhibition values of compounds 8 a-e with shorter chains are lower than those of the other derivatives. bSee Ref. Kilbourn and Griffith (1992).
Compound
% iNOS inhibitiona
% nNOS inhibitiona
8a
10.20 ± 1.21
6.20 ± 0.35
8b
11.33 ± 0.98
8.45 ± 0.62
8c
17.16 ± 1.04
10.04 ± 1.21
8d
18.30 ± 0.52
10.82 ± 1.02
8e
22.90 ± 1.40
14.32 ± 3.65
9a
50.40 ± 1.52
42.31 ± 0.13
9b
52.94 ± 0.41
42.33 ± 0.76
9c
54.62 ± 1.43
54.11 ± 1.05
9d
55.80 ± 1.84
42.80 ± 0.06
9e
58.03 ± 1.50
60.55 ± 0.69
10a
28.08 ± 0.40
15.32 ± 1.58
10b
29.13 ± 1.06
17.46 ± 1.94
10c
30.20 ± 0.58
38.05 ± 0.73
10d
32.53 ± 1.23
15.89 ± 1.23
10e
33.01 ± 0.35
41.08 ± 3.12
14a
35.58 ± 2.33
24.03 ± 0.71
14b
35.76 ± 2.32
24.20 ± 0.71
14c
40.95 ± 2.03
28.01 ± 0.32
14d
42.78 ± 1.10
29.06 ± 2.82
14e
48.41 ± 1.76
29.58 ± 0.87
L-NAME b
77.01 ± 0.96
100.0 ± 1.03
The enzymatic N-Oxygenation may be responsible for the surprising NOS inhibition levels of the p-nitro aromatic Schiff base derivatives (8e, 9e, 10e, and 14e). Because there is a considerable body of research concerning the biodegradation of nitro compounds, especially enzymatic N-oxygenation, amino groups have been discovered to be oxidized by genuine nitro-group-forming N-oxygenases (Winkler and Hertweck, 2007).
Also, Table 1. Shows one of the best known inhibitors of NOS with a guanidine moiety are amino acids related to the substrate L-arginine (L-NAME) (**Kilbourn et al., 1992). Which were used as control to compare with the novel quinazolinyl Schiff base compounds.
Table 2 displays the IC50 values for the most interesting compounds (8e, 9e, 10e, and 14e) in the initial in vitro testing for nNOS and iNOS inhibition. In the case of iNOS, the IC50 values for both 9e and 14e are approximately 22 µM, indicating its highly powerful inhibition. With IC50 values of 18.50 and 28.22 µM, respectively, the quinazolinyl Schiff bases 9e and 10e show the highest efficacy against nNOS, demonstrating that 9e is the most powerful inhibitor of all produced compounds vs nNOS. * Data were obtained by measuring percentage of inhibition with at least five concentrations of inhibitor.
Compound
IC50 iNOS (µM)*
IC50 nNOS (µM) *
8e
43.03 ± 0.90
30.52 ± 0.84
9e
22.05 ± 0.72
18.50 ± 0.49
10e
32.66 ± 0.82
28.22 ± 0.62
14e
22.85 ± 0.73
33.81 ± 0.94
When compared to L-NAME, in general, quinazolinyl Schiff base compounds have a notable potency against iNOS and nNOS, suggesting that the presence of an azomethine Schiff bas linkage bearing various p-aromatic substituents increases NOS inhibition. Some of these compounds have also shown good potency, since the IC50 values against NOS are in the efficient range (Fig. 2).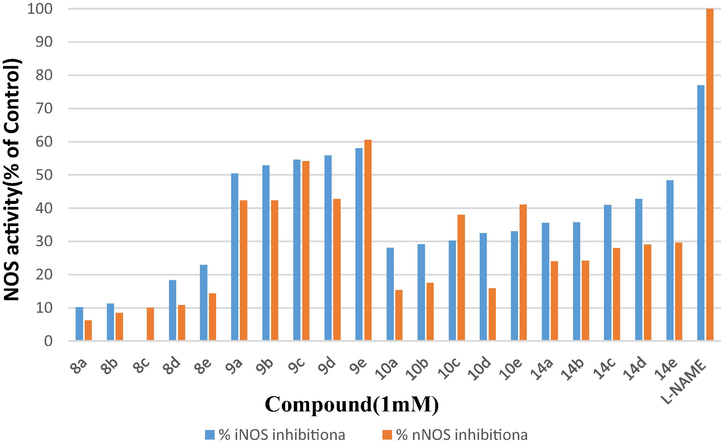
Percentage of the nNOS and iNOS activities in the presence of 1 mM of the Quinazolinyl Schiff bases (compounds 8e, 9e, 10e, and 14e)) compared to control. Each value is the mean of three experiments performed in triplicate using recombinant iNOS or nNOS enzymes.
3 Experimental
3.1 Instruments and Reagent
All the necessary chemicals were obtained from Merck and Aldrich Chemical Company. Precoated aluminium sheets (Silica gel 60 F254, Merck Germany) were utilized for thin-layer chromatography (TLC) and spots were observed under UV light. The melting points were measured using a Veego instrument with the model number REC-22038 A2 and are uncorrected. Elemental studies were carried out on an Elementar Vario analyser, with results that were within ± 0.4% of theoretical values. The IR spectra were recorded using a Bruker FTIR spectrophotometer (KBr). Using DMSO‑d6 as a solvent and trimethylsilane (TMS) as an internal standard, 1H and 13C NMR were recorded on a Bruker Spectrospin DPX 400 MHz and Bruker Spectrospin DPX 75 MHz spectrometer, respectively. Chemical shift measurements are expressed in parts per million (ppm). ESI-MS was used to record mass spectra (AB-Sciex 2000, Applied Biosystem).
3.2 Synthesis
3.2.1 2-(2-(4-isobutylphenyl)propanamido)-5-nitrobenzoic acid (3)
During 10 min, portion-wise additions of 2-(4-isobutylphenyl)propanoyl chloride (1) (2.24 g; 10 mmol) was made to a stirred solution of commercially available 2-amino-5-nitrobenzoic acid (2) (1.80 g; 10 mmol) in dry pyridine (20 mL). The mixture was stirred for 2 h at room temperature before being pour into ice-cold water (30 mL), acidified with hydrochloric acid (2 N), and allowed to precipitate completely.
Recryst. Solvent: Methanol; Yellow solid; Yield: 70%; mp: 120 °C; Anal. Calc. (%) for C20H22N2O5 (3 7 0): C, 64.85; H, 5.99; N, 7.56; found: C, 64.82; H, 5.95; N, 7.54; IR (KBr), ν (cm−1): 3420 (O—H, N—H), 3080 (C—Harom), 2960, 2940, 2880 (C—Haliph), 1720 (C⚌Ocarbonyl), 1644 (C⚌OCarboxylic),1045 (C—N) ; 1H NMR (DMSO‑d6) δ (ppm): 1.15 (d, 6H, CH2CH(CH3)2), 1.50 (d, 3H, CHCH3), 2.20 (m, 1H, CH2CH(CH3)2), 2.45 (d, 2H, CH2CH(CH3)2), 3.35 (q, H, CHCH3), 7.00–8.20 (m, 7H, Harom), 8.70 (bs, 1H exchangeable with D2O, NH) , 12.66 (bs, 1H exchangeable with D2O, OH) ; 13C NMR (DMSO‑d6) δ (ppm): 172.92, 169.60, 149.50, 143.84, 139.60, 133.53, 129.44, 129.40, 128.52, 128.71, 126.66, 125.75, 122.36, 116.86, 45.81, 42.62, 29.40, 22.35, 22.73,14.10; ESI-MS: m/z = 370 (M+).
3.2.2 2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4H-benzo[d][1,3]oxazin-4-one(4)
Anilide 3 (3.70 g, 10 mmol) and acetic anhydride (50 mL) were heated under reflux for 2 h before being concentrated. The benzoxazinone 4 was obtained by crystallizing of the residue from petroleum ether (60–80 °C). Recryst Solvent: Ethanol; White solid; Yield 65%; m. p. 180–182 °C; Anal. Calc. (%) for C20H20N2O4(3 5 2): C, 68.17; H, 5.72; N, 7.95; found: C, 68.15; H, 5.70; N, 7.93; IR (KBr), ν (cm−1): 3060 (C—Harom), 2960, 2940, 2875 (C—Haliph), 1685 (C⚌Oquinazolinone), 1617 (C⚌N), 1160 (C—O—C).1H NMR (CDCl3) δ: 1.04 (d, 6H, CH2CH(CH3)2), 1.45 (d, 3H, CHCH3), 1.85 (m, 1H, CH2CH(CH3)2), 2.50 (d, 2H, CH2CH(CH3)2), 3.35 (q, H, CHCH3), 7.10–8.20 (m, 7H, Harom). 13C NMR (DMSO‑d6) δ (ppm): 169.80, 162.60, 148.60, 144.80, 139.75, 134.56, 129.40, 129.50, 128.70, 128,50, 125.60, 124.75, 120.35, 118.80, 44.85,43.60, 29.50, 23.65, 23.75,16.20; ESI-MS: m/z = 352 (M+).
3.3 General procedures for the synthesis of compounds 5–7
To a cold solution of benzoxazinone (4) (3.53 g, 10 mmol) in anhydrous pyridine (20 mL) was drowsily added a solution of the appropriate amine (10 mmol); namely hydrazine hydrate (0.55 mL, 10 mmol), argnine (1.75 g, 10 mmol), and lysine (1.46 g, 10 mmol), in anhydrous pyridine (10 mL) with continuous stirring. When the addition was complete, the reaction mixture was stirred vigorously for 30 min at room temperature and subsequently heated under reflux for 4 h under anhydrous reaction conditions. The reaction mixture was allowed to cool to room temperature and poured into ice cold water containing diluted hydrochloric acid. The obtained crude precipitates were filtered off, washed repeatedly with water and dried, recrystallized.
3.3.1 3-amino-2-{1-[4-(2-methylpropyl)phenyl]ethyl}-6-nitroquinazolin-4(3H)-one (5)
Recryst Solvent: Ethanol; Yellow solid; Yield: 80%; m. p: 182–184 °C; Anal. Calc. (%) for C20H22N4O3 (3 6 6): C, 65.56; H: 6.05; N: 15.29; found: C: 65.54; H: 6.04; N: 15.27; FT-IR vmax (cm−1): 3230 (NH2), 1672 (C⚌Oquinazolinone), 1628 (C⚌N); 1H NMR (DMSO‑d6) δ (ppm): 1.10 (d, 6H, CH2CH(CH3)2), 1.40 (d, 3H, CHCH3), 1.90 (m, 1H, CH2CH(CH3)2), 2.40 (d, 2H, CH2CH(CH3)2), 3.30 (q, H, CHCH3), 6.20 (s, 2H, D2O-exchangeable, NH2), 7.10–8.00 (m, 7H, Ar-H)); 13C NMR (DMSO‑d6) δ (ppm): 165.01, 161.22, 153.00, 147.25, 137.20, 137.50, 128.11, 128.70, 126.70, 126.50, 125.90, 124.90, 123.70, 121.5, 45.70, 41.50, 28.70, 22.80,22.30, 12.20; ESI-MS: m/z = 366 (M+).
3.3.2 2-amino-5-(2-((R)-1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxo-3,4-dihydroquinazoline-3-carboxamidino)pentanoic acid (6)
Recryst. Solvent: Methanol; Yellow solid; Yield: 70%; mp 210–212 °C; Anal. Calc. (%) for C26H32N4O5 (5 0 8): C, 61.40, H: 6.34, N: 16.52; found: C: 61.39, H: 6.30, N: 16.49; FT-IR vmax (cm−1): 3456–3578 (OH), 3250 (NH2), 1676 (C⚌Oquinazolinone), 1648 (C⚌Ocarboxyl), 1624 (C⚌N); 1H NMR (DMSO‑d6) δ (ppm): 1.09 (d, 6H, CH2CH(CH3)2), 1.44 (d, 3H, CHCH3), 1.50 (m, 2H, NH CH2CH2CH2), 1.70 (m, 2H, NHCH2CH2CH2), 2.5 (m, 2H, NH CH2CH2CH2), 1.95 (m, H, CH2CH(CH3)2), 2.50(d, 2H, CH2CH(CH3)2), 3.33 (q, H, CHCH3), 4.00 (q, H, NCHCOOH), 5.50 (s, H, D2O-exchangeable, C⚌NH), 6.30 (s, 2H, D2O-exchangeable, NH2), 7.20–8.10 (m, 7H, Ar—H)), 9.70 (m, H, D2O-exchangeable, NH), 10.80 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 174.00,170.50, 164.20, 163.90, 153.00, 147.20, 137.30, 137.80 128.50, 128.70, 126.30, 126.70, 125.40, 123.40, 123.90, 121.50, 50.50, 45.70, 37.50, 33.50 ,30.20, 28.10, 22.80, 21.20, 22.80, 12.20; ESI-MS: m/z = 508 (M+).
3.3.3 2-amino-6-(2-((R)-1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)hexanoic acid (7)
Recryst. Solvent: Ethanol, Bale yellow solid; Yield 65%; mp 198–200 °C; Anal. Calc. (%) for C26H32N4O5 (4 8 0): C, 64.98; H, 6.71; N, 11.66; found: C, 64.95; H, 6.69; N, 11.64; FT-IR vmax (cm−1): 3456–3578 (OH), 3270 (NH2), 1677 (C⚌Oquinazolinone), 1645 (C⚌Ocarboxyl), 1624 (C⚌N). 1H NMR (DMSO‑d6) δ (ppm): 1.10 (d, 6H, CH2CH(CH3)2), 1.30 (m, 2H, NHCH2CH2CH2CH2), 1.38 (d, 3H, CHCH3), 1.60 (m, 2H, NHCH2CH2CH2CH2), 1.80 (m, 2H, NHCH2CH2CH2 CH2), 1.95 (m, H, CH2CH(CH3)2), 2.30(d, 2H, CH2CH(CH3)2), 3.20 (m, 2H, NHCH2CH2CH2CH2), 3.50 (q, H, NCHCOOH), 3.35 (q, H, CHCH3), 6.50 (s, 2H, D2O-exchangeable, NH2), 7.20–8.20 (m, 7H, Ar02015H)), 10.80 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 175.10, 164.10, 161.80, 153.90, 147.30, 137.40, 137.10 128.40, 128.20, 126.20, 126.10, 125.40, 123.60, 123.30, 121.20, 55.50, 45.10, 41.20, 34.20, 33.40, 30.00, 27.40, 22.60, 22.60, 21.10, 12.80; ESI-MS: m/z = 480 (M+).
3.4 General procedures for the synthesis of Schiff bases 8–10
An equimolar mixture of compound 5–7 (10 mol) and substituted aldehydes namely; 4-hydroxybenzaldehyde (1.22 g, 10 mmol), 4-chlorobenzaldehyde (1.40 g, 10 mmol), 4- methoxybenzaldehyde (1.36 g, 10 mmol), 4-N,N-dimethylbenzaldehyde (1.49 g, 10 mmol) and 4-nitrobenzaldehyde (1.51 g, 10 mmol) (10 mmol) in absolute ethanol (10 mL) was allowed to be refluxed for 8 h, H2SO4 (0.5 mL) was added slowly to the reaction mixture and the progress of the reaction was monitored through thin layer chromatography. The reaction was allowed to cool to room temperature once it was finished. On standing, the solid crystalline product was filtered, dried, and crystallized.
3.4.1 3-(4-hydroxybenzylideneamino)-2-ibuprofenyl-6-nitro-4(3H)-quinazolin-4ones (8a)
Recryst. Solvent: Methanol; White solid; Yield 25%; mp 220–222 °C; Anal. Calc. (%) for C27H26N4O4 (4 7 0): C, 68.92; H, 5.57; N, 11.91; found: C, 68.90; H,5.53; N, 11.87; FT-IR vmax (cm−1): 3415–3525 (OH), 1669 (C⚌Oquinazolinone), 1629 (C⚌N), 1591 (N⚌CHstreching); 1H NMR (DMSO‑d6) δ (ppm): 1.15 (d, 6H, CH2CH(CH3)2), 1.40 (d, 3H, CHCH3), 1.95 (m, H, CH2CH(CH3)2), 2.45 (d, 2H, CH2CH(CH3)2), 3.33 (q, H, CHCH3),4.90 (s, H, OH), 7.10–8.10 (m, 11H, Ar02015H), 8.90 (s, H, CH⚌N); 13C NMR (DMSO‑d6) δ (ppm): 168.20, 161.30, 161.00, 153.10, 147.35, 143.40, 137.20, 137.30, 130.10, 130.15, 128.50, 128,55, 126.15, 126.10, 126.00, 125.30, 123.20, 123.70, 121.10, 116.30, 116.35, 45.75, 31.50, 28.10, 22.80, 22.85, 12.20; ESI-MS: m/z = 470 (M+).
3.4.2 3-(4-chlorobenzylideneamino)-2-ibuprofenyl-6-nitro-4(3H)-quinazolin-4ones (8b)
Recryst. Solvent: Ethanol, Yellow solid; Yield 30%; mp 330–332 °C; Anal. Calc. (%) for C27H25N4O3Cl (4 8 8): C, 66.32; H,5.15; N, 11.46; found: C, 66.34; H, 5.18; N, 11.48; FT-IR vmax (cm−1): 1669 (C⚌Oquinazolinone), 1629 (C⚌N), 1595 (N⚌CHstreching), 820 (C-Cl); 1H NMR (DMSO‑d6) δ (ppm): 1.10 (d, 6H, CH2CH(CH3)2), 1.55 (d, 3H, CHCH3), 1.90 (m, H, CH2CH(CH3)2), 2.40 (d, 2H, CH2CH(CH3)2), 3.35 (q, H, CHCH3), 7.20–8.10 (m, 11H, Ar02015H), 8.95 (s, H, CH⚌N); 13C NMR (DMSO‑d6) δ (ppm): 167.10, 162.30,162.00, 155.10, 148.30, 143.10, 137.1, 137.10, 131.15, 131.10, 127.45, 127,40, 125.15, 125.10, 125.00, 124.30, 123.10, 123.60, 120.15, 114.30, 114.35, 44.70, 30.40, 28.20, 22.70, 21.80, 12.10; ESI-MS: m/z = 488 (M+).
3.4.3 3-(4-methoxybenzylideneamino)-2-ibuprofenyl-6-nitro-4(3H) -quinazolin-4ones (8c)
Recryst. Solvent: Ethanol, Yellow solid; Yield 35%; mp 230–233 °C; Anal. Calc. (%) for C28H28N4O4 (4 8 4): C, 69.41; H, 5.82; N, 11.56; found: C, 69.39; H, 5.80; N, 11.58; FT-IR vmax (cm−1): 1675 (C⚌Oquinazolinone), 1625 (C⚌N), 1590 (N⚌CHstreching); 1H NMR (DMSO‑d6) δ (ppm): 1.06 (d, 6H, CH2CH(CH3)2), 1.50 (d, 3H, CHCH3), 1.85 (m, H, CH2CH(CH3)2), 2.45 (d, 2H, CH2CH(CH3)2), 3.40 (s, 3H, OCH3), 3.50 (q, H, CHCH3), 7.10–8.20 (m, 11H, Ar02015H), 9.05 (s, H, CH⚌N); 13C NMR (DMSO‑d6) δ (ppm): 164.10, 160.30,160.00, 154.20, 146.30, 141.10, 135.1, 135.10, 130.30, 130.20, 127.30, 127,10, 124.25, 124.20, 124.00, 123.30, 122.10, 122.60, 120.15, 116.20, 116.20, 55.95, 45.60, 31.60, 29.30, 22.40, 22.40, 12.40; ESI-MS: m/z = 484 (M+).
3.4.4 3-(4-(dimethylamino)benzylideneamino)-2-ibuprofenyl-6-nitro-4(3H)-quinazolin-4ones (8d)
Recryst. Solvent: Methanol; pale yellow solid; Yield 30%; mp 185–187 °C; Anal. Calc. (%) for C29H31N5O3 (4 9 7): C: 70.00, H: 6.28, N: 14.07; found: C: 69.98, H: 6.80, N: 14.10; FT-IR vmax (cm−1): 1670 (C⚌Oquinazolinone), 1630 (C⚌N), 1595 (C⚌Nstreching); 1H NMR (DMSO‑d6) δ (ppm): 1.10 (d, 6H, CH2CH(CH3)2), 1.44 (d, 3H, CHCH3), 1.95 (m, H, CH2CH(CH3)2), 2.50 (d, 2H, CH2CH(CH3)2), 2.95 (d, 6H, N(CH3)2), 3.36 (q, H, CHCH3), 7.15–8.20 (m, 11H, Ar02015H), 9.00 (s, H, CH⚌N),; 13C NMR (DMSO‑d6) δ (ppm): 164.20, 160.30, 153.00, 150.10, 147.20, 143.10, 136.35, 136.30, 130.10, 129.15, 128.40, 128,45, 127.15, 126.15, 126.00, 125.30, 123.20, 123.70, 121.15, 114.30, 114.35, 45.75,40.15, 40.10, 30.50, 29.10, 21.70, 21.75, 12.50; ESI-MS: m/z = 497 (M+).
3.4.5 3-(4-nitrobenzylideneamino)-2-ibuprofenyl-6-nitro-4(3H) -quinazolin-4ones (8e)
Recryst. Solvent: Ethanol, Yellow solid; Yield 20%; mp 225–227 °C; Anal. Calc. (%) for C27H25N5O5 (4 9 9): C, 64.92; H, 5.04; N, 14.02, found: C, 64.95; H, 5.09; N, 14.05; FT-IR vmax (cm−1): 1670 (C⚌Oquinazolinone), 1630 (C⚌N), 1595 (C⚌N streching); 1H NMR (DMSO‑d6) δ (ppm): 1.15(d, 6H, CH2CH(CH3)2),1.49 (d, 3H, CHCH3), 2.00 (m, H, CH2CH(CH3)2), 2.45 (d, 2H, CH2CH(CH3)2), 3.30 (q, H, CHCH3),7.10–8.10 (m, 11H, Ar02015H)), 8.88 (s, H, CH⚌N); 13C NMR (DMSO‑d6) δ (ppm): 166.10, 160.30,153.00, 150.10, 147.30, 143.10, 139.10, 136.10, 136.15, 130.45, 130.40, 128.15, 128.10, 125.75, 125.70, 124.20, 123.25, 122.20, 121.35, 121.30, 120.10, 44.50, 32.50, 27.20, 22.75, 22.70, 12.10; ESI-MS: m/z = 499 (M+).
3.4.6 2-(4-hydroxybenzylideneamino)-5-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxo-3,4-dihydroquinazoline-3-carboxamidino)pentanoic acid (9a)
Recryst. Solvent: Methanol; browen solid; Yield 20%; mp 245–247 °C; Anal. Calc. (%) for C33H36N6O6 (6 1 2): C, 64.69; H, 5.92; N, 13.72, found: C, 64.70; H, 5.95; N, 13.75; FT-IR vmax (cm−1): 3456–3578 (OH), 3315 (NH), 1676 (C⚌Oquinazolinone), 1648 (C⚌Ocarboxyl), 1618 (C⚌N); 1H NMR (DMSO‑d6) δ (ppm): δ 1.12 (d, 6H, CH2CH(CH3)2), 1.42 (d, 3H, CHCH3), 1.5 (m, 2H, NH CH2CH2CH2) 1.7 (m, 2H, NH CH2CH2CH2),1.90 (m, H, CH2CH(CH3)2), 2.43 (d, 2H, CH2CH(CH3)2), 2.50 (m, 2H, NH CH2CH2CH2), 3.35 (q, H, CHCH3), 4.00 (q, H, NCHCOOH), 4.40, (s, H, OH), 5.55 (s, H, D2O-exchangeable, C⚌NH), 7.20–8.20 (m, 11H, Ar02015H)), 9.00 (s, H, CH⚌N), 9.50 (s, H, D2O-exchangeable, NH), 10.50 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 13C NMR (DMSO‑d6) δ (ppm): 166.00, 165.40, 164.10, 163.50,160.8, 160.9, 152.40, 146.20, 137.40, 137.70,132.50, 130.40, 130.20, 127.70, 127.50, 125.70, 125.30, 124.40, 123.20, 123.10, 120.50,116.15, 116.10, 51.50, 47.70, 38.50, 32.50, 31.20, 27.10, 22.50, 22.30, 21.00, 12.10; ESI-MS: m/z = 612 (M+).
3.4.7 2-(4-chlorobenzylideneamino)-5-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxo-3,4-dihydroquinazoline-3-carboxamidino)pentanoic acid (9b)
Recryst. Solvent: Ethanol, yellow solid; Yield 30%; mp 218–220 °C; Anal. Calc. (%) for C33H35N6O5Cl (6 3 1): C: 62.80, H: 5.59, N: 13.32; found: C: 62.83, H: 5.61, N: 13.34 ; FT-IR vmax (cm−1): 3456–3578 (OH), 3315 (NH), 1676 (C⚌Oquinazolinone), 1648 (C⚌Ocarboxyl), 1624 (C⚌N); 1H NMR (DMSO‑d6) δ (ppm): δ 1.10 (d, 6H, CH2CH(CH3)2), 1.40 (d, 3H, CHCH3), 1.60 (m, 2H, NH CH2CH2CH2), 1.75 (m, 2H, NH CH2CH2CH2), 1.92 (m, H, CH2CH(CH3)2), 2.45 (d, 2H, CH2CH(CH3)2), 2.62 (m, 2H, NH CH2CH2CH2) , 3.35 (q, H, CHCH3), 4.20 (q, H, NCHCOOH), 5.50 (s, H, D2O-exchangeable, C⚌NH), 7.20–8.10 (m, 11H, Ar02015H)), 9.05 (s, H, CH⚌N), 9.30 (s, H, D2O-exchangeable, NH), 11.50 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 166.50, 165.10, 164.40, 163.10,160.50, 160.90, 151.10, 144.20, 136.10, 136.00,132.10, 130.80, 130.90, 127.30, 127.10, 125.20, 125.10, 124.20, 123.20, 123.00, 120.00, 116.20, 116.10, 50.50, 45.70, 37.50, 31.50, 30.20, 27.20, 22.10, 22.00, 21.00, 12.50; ESI-MS: m/z = 631 (M+).
3.4.8 2-(4-methoxybenzylideneamino)-5-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxo-3,4-dihydroquinazoline-3-carboxamidino)pentanoic acid (9c)
Recryst. Solvent: Ethanol; yellow solid; Yield 40%; mp 205–207 °C; Anal. Calc. (%) for C34H38N6O6 (6 2 6): C: 65.16, H: 6.11, N: 13.41; found: C: 65.12, H: 6.08, N: 13.39 FT-IR vmax (cm−1): 3450–3570 (OH), 3320 (NH), 1670 (C⚌Oquinazolinone), 1640 (C⚌Ocarboxyl), 1615 (C⚌N); 1H NMR (DMSO‑d6) δ (ppm): δ 1.05 (d, 6H, CH2CH(CH3)2), 1.42 (d, 3H, CHCH3), 1.65 (m, 2H, NH CH2CH2CH2), 1.70 (m, 2H, NH CH2CH2CH2), 1.95 (m, H, CH2CH(CH3)2), 2.35 (d, 2H, CH2CH(CH3)2), 2.55 (m, 2H, NH CH2CH2CH2) , 3.33 (q, H, CHCH3), 3.40 (s, 3H, OCH3), 4.10 (q, H, NCHCOOH), 5.30 (s, H, D2O-exchangeable, (C⚌NH), 7.10–8.20 (m, 11H, Ar02015H)) , 8.95 (s, H, CH⚌N), 9.20 (s, H, D2O-exchangeable, NH),11.10 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 167.50, 166.10, 165.35, 163.40,160.90, 16010, 150.10, 145.20, 136.20, 136.10,132.70, 130.30, 130.60, 127.20, 127.10, 125.30, 125.20, 124.10, 123.10, 123.00, 120.50, 115.20, 115.10,55.90, 50.00, 45.30, 37.20, 31.10, 30.00, 27.00, 22.50, 22.20, 21.10, 12.80; ESI-MS: m/z = 626 (M+).
3.4.9 2-(4-(dimethylamino)benzylideneamino)-5-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxo-3,4-dihydroquinazoline-3-carboxamidino)pentanoic acid (9d)
Recryst. Solvent: Ethanol; Pale yellow solid; Yield 40%; mp 250–252 °C; Anal. Calc. (%) for C35H41N7O5 (6 3 9): C: 65.71, H: 6.46, N: 15.33; found: C: 65.69, H: 6.43, N: 15.31; FT-IR vmax (cm−1): 3440–3570 (OH), 3300(NH), 1665 (C⚌Oquinazolinone), 1650 (C⚌Ocarboxyl), 1615 (C⚌N); 1H NMR (DMSO‑d6) δ (ppm): δ 1.10 (d, 6H, CH2CH(CH3)2), 1.40 (d, 3H, CHCH3), 1.60 (m, 2H, NH CH2CH2CH2), 1.75 (m, 2H, NH CH2CH2CH2), 1.92 (m, H, CH2CH(CH3)2), 2.40 (d, 2H, CH2CH(CH3)2), 2.50 (m, 2H, NH CH2CH2CH2) , 2.75 (d, 6H, N(CH3)2), 3.39 (q, H, CHCH3), 4.20 (q, H, NCHCOOH), 5.60 (s, H, D2O-exchangeable, C⚌NH), 7.10–8.20 (m, 11H, Ar02015H)), 9.00 (s, H, CH⚌N), 9.40 (s, H, D2O-exchangeable, NH), 11.20 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 167.00, 166.50, 164.25, 162.40,160.40, 160.10, 149.10, 144.20, 136.00, 136.00,131.70, 130.20, 130.10, 127.10, 127.00, 125.10, 125.00, 124.50, 123.30, 123.10, 120.10, 115.15, 115.10, 50.00, 44.30, 40.35, 40.30, 37.10, 31.50, 30.10, 27.20, 22.20, 22.10, 21.00, 12.30; ESI-MS: m/z = 639 (M+).
3.4.10 2-(4-nitrobenzylideneamino)-5-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxo-3,4-dihydroquinazoline-3-carboxamidino)pentanoic acid (9e)
Recryst. Solvent: Methanol; browen solid; Yield 45%; mp 240–242 °C; Anal. Calc. (%) for C33H35N7O7 (6 4 1): C: 61.77, H: 5.50, N: 15.28; found: C: 61.75, H: 5.48, N: 15.25; FT-IR vmax (cm−1): 3445–3590 (OH), 3310 (NH), 1665 (C⚌Oquinazolinone), 1650 (C⚌Ocarboxyl), 1628 (C⚌N); 1H NMR (DMSO‑d6) δ (ppm): δ 1.11(d, 6H, CH2CH(CH3)2), 1.50 (d, 3H, CHCH3), 1.55 (m, 2H, NH CH2CH2CH2), 1.75 (m, 2H, NH CH2CH2CH2), 1.92 (m, H, CH2CH(CH3)2), 2.45(d, 2H, CH2CH(CH3)2), 2.50 (m, 2H, NH CH2CH2CH2) , 3.32 (q, H, CHCH3), 4.10 (q, H, NCHCOOH), 5.50 (s, H, D2O-exchangeable, C⚌NH), 7.20–8.10 (m, 11H, Ar02015H)), 8.95 (s, H, CH⚌N), 9.30 (s, H, D2O-exchangeable, NH), 10.80 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 13C NMR (DMSO‑d6) δ (ppm): 168.00, 167.40, 165.20, 164.50,162.80, 161.95, 150.40, 144.30, 137.20, 137.10,132.40, 130.50, 130.30, 127.40, 127.10, 125.40, 125.20, 124.00, 123.10, 123.00, 120.10,116.20, 116.10, 50.50, 47.30, 37.50, 33.50, 30.20, 28.10, 24.50, 24.30, 21.50, 12.00; ESI-MS: m/z = 641 (M+).
3.4.11 2-(4-hydroxybenzylideneamino)-6-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)hexanoic acid (10a)
Recryst. Solvent: Methanol; Bale yellow solid; Yield 35%; mp 200–202 °C; Anal. Calc. (%) for C33H36N4O6 (5 8 4): C: 67.79, H: 6.21, N: 9.58; found: C: 67.77, H: 6.18 N: 9.55; FT-IR vmax (cm−1):): 3400–3600 (OH), 1670 (C⚌Oquinazolinone), 1640 (C⚌Ocarboxyl), 1610 (C⚌N). 1H NMR (DMSO‑d6) δ (ppm): 1.09 (d, 6H, CH2CH(CH3)2), 1.35 (m, 2H, NHCH2CH2CH2 CH2), 1.42 (d, 3H, CHCH3), 1.50 (m, 2H, NHCH2CH2CH2CH2), 1.90 (m, 2H, NHCH2CH2CH2 CH2), 2.10 (m, H, CH2CH(CH3)2), 2.50(d, 2H, CH2CH(CH3)2), 3.20 (m, 2H, NHCH2CH2CH2 CH2), 3.30 (q, H, CHCH3), 4.00 (q, H, NCHCOOH), 5.5 (s, H, OH), 7.10–8.00 (m, 11H, Ar02015H)), 8.85 (s, 1H, CH⚌N), 11.40 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 177.20, 165.10, 162.80,160.50, 160.20 155.80, 145.50, 137.30, 137.00, 132.50, 130.80, 130.10, 127.50, 127.20, 125.30, 125.20, 125.00, 122.60, 122.30, 121.00,116.20, 116.00, 53.00, 43.10, 40.20, 32.20, 31.40, 30.00, 28.50, 24.50, 23.80, 22.40, 12.80; ESI-MS: m/z = 584 (M+).
3.4.12 2-(4-chlorobenzylideneamino)-6-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)hexanoic acid (10b)
Recryst. Solvent: Methanol; Bale yellow solid; Yield 30%; mp 195–197 °C; Anal. Calc. (%) for C33H35N4O5Cl (6 0 2): C: 65.72, H: 5.85, N: 9.29; found: C: 65.69, H: 5.82, N: 9.26; FT-IR vmax (cm−1):): 3430–3620 (OH), 1674 (C⚌Oquinazolinone), 1645 (C⚌Ocarboxyl), 1612 (C⚌N). 1H NMR (DMSO‑d6) δ (ppm): 1.10 (d, 6H, CH2CH(CH3)2), 1.30 (m, 2H, NHCH2CH2CH2 CH2), 1.45 (d, 3H, CHCH3), 1.60 (m, 2H, NHCH2CH2CH2 CH2), 1.85 (m, 2H, NHCH2CH2CH2 CH2), 2.00 (m, H, CH2CH(CH3)2), 2.44 (d, 2H, CH2CH(CH3)2), 3.10 (m, 2H, NHCH2CH2CH2CH2), 3.35 (q, H, CHCH3), 3.90 (q, H, NCHCOOH), 7.30–8.10 (m, 11H, Ar02015H)), 8.95 (s, 1H, CH⚌N), 11.60 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 176.10, 166.25, 164.70,162.50, 162.20 160.10, 147.30, 136.30, 136.10, 133.40, 132.60, 132.10, 129.50, 129.20, 126.30, 126.20, 125.00, 122.10, 122.00, 121.60,120.30, 120.10, 50.00, 45.10, 42.20, 35.20, 32.40, 31.00, 29.50, 25.55, 24.85, 21.45, 13.50; ESI-MS: m/z = 602 (M+).
3.4.13 2-(4-methoxybenzylideneamino)-6-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)hexanoic acid (10c)
Recryst. Solvent: Methanol; Bale yellow solid; Yield 40%; mp 200–202 °C; Anal. Calc. (%) for C34H38N4O6 (5 9 8): C: 68.21, H: 6.40, N: 9.36; found: C: 68.19, H: 6.38, N: 9.33; FT-IR vmax (cm−1):): 3410–3650 (OH), 1675 (C⚌Oquinazolinone), 1640 (C⚌Ocarboxyl), 1610 (C⚌N). 1H NMR (DMSO‑d6) δ (ppm): 1.10 (d, 6H, CH2CH(CH3)2), 1.35 (m, 2H, NHCH2CH2CH2 CH2), 1.40 (d, 3H, CHCH3), 1.50 (m, 2H, NHCH2CH2CH2 CH2), 1.75 (m, 2H, NHCH2CH2CH2 CH2), 1.90 (m, H, CH2CH(CH3)2), 2.42 (d, 2H, CH2CH(CH3)2), 3.30 (m, 2H, NHCH2CH2CH2 CH2), 3.35 (q, H, CHCH3), 3.60 (q, H, NCHCOOH), 3.75 (s, 3H, OCH3), 7.20–8.10 (m, 11H, Ar02015H)), 9.10 (s, 1H, CH⚌N), 11.60 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 177.10, 165.20, 163.30,162.80, 162.50, 161.50, 148.30, 137.30, 135.30, 134.45, 133.40, 133.30, 129.10, 129.00, 126.10, 126.00, 124.50, 121.80, 121.50, 121.10,120.10, 120.00, 55.50, 48.00, 44.10, 43.20, 34.20, 31.40, 30.00, 29.20, 26.50, 25.80, 22.40, 12.50; ESI-MS: m/z = 598 (M+).
3.4.14 2-(4-(dimethylamino)benzylideneamino)-6-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)hexanoic acid (10d)
Recryst. Solvent: Methanol; yellow solid; Yield 35%; mp 252–254 °C; Anal. Calc. (%) for C35H41N5O5 (6 1 1): C: 68.72, H: 6.76, N: 11.45; found: C: 68.70, H: 6.73, N: 11.41; FT-IR vmax (cm−1): 3400–3510 (OH), 1675 (C⚌Oquinazolinone), 1655 (C⚌Ocarboxyl), 1610 (C⚌N); 1H NMR (DMSO‑d6) δ (ppm): 1.15 (d, 6H, CH2CH(CH3)2), 1.30 (m, 2H, NHCH2CH2CH2 CH2), 1.45 (m, 2H, NHCH2CH2CH2 CH2), 1.50 (d, 3H, CHCH3), 1.65 (m, 2H, NHCH2CH2CH2 CH2), 2.00 (m, H, CH2CH(CH3)2), 2.40 (d, 2H, CH2CH(CH3)2), 2.55 (d, 6H, N(CH3)2), 3.10 (m, 2H, NHCH2CH2CH2 CH2), 3.40 (q, H, CHCH3), 3.65 (q, H, NCHCOOH), 7.10–8.10 (m, 11H, Ar02015H)), 9.10 (s, 1H, CH⚌N), 11.60 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 176.50, 166.20, 164.50,162.50, 162.10, 160.50, 147.30, 138.10, 135.50, 134.00, 132.10, 132.00, 129.50, 129.20, 125.10, 125.00, 123.50, 122.70, 122.50, 122.10,118.30, 118.10, 47.00, 46.10, 44.20, 40.20, 40.70, 36.20, 33.40, 30.10, 29.50, 27.50, 23.40, 23.40, 12.50; ESI-MS: m/z = 611(M+).
3.4.15 2-(4-nitrobenzylideneamino)-6-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)hexanoic acid (10e)
Recryst. Solvent: Methanol; yellow solid; Yield 30%; mp 248–250 °C; Anal. Calc. (%) for C33H35N5O7 (6 1 3): C: 64.59, H: 5.75, N: 11.41; found: C: 64.62, H: 5.77, N: 11.44; FT-IR vmax (cm−1): 3430–3520 (OH), 1670 (C⚌Oquinazolinone), 1660 (C⚌Ocarboxyl), 1612 (C⚌N); 1H NMR (DMSO‑d6) δ (ppm): 1.11 (d, 6H, CH2CH(CH3)2), 1.25 (m, 2H, NHCH2CH2CH2 CH2), 1.40 (m, 2H, NHCH2CH2CH2 CH2), 1.45 (d, 3H, CHCH3), 1.60 (m, 2H, NHCH2CH2CH2 CH2), 1.95 (m, H, CH2CH(CH3)2), 2.43 (d, 2H, CH2CH(CH3)2), 3.20 (m, 2H, NHCH2CH2CH2 CH2), 3.40 (q, H, CHCH3), 3.70 (q, H, NCHCOOH), 7.15 – 8.10 (m, 11H, Ar02015H)), 9.00 (s, 1H, CH⚌N), 11.30 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 177.50, 165.20, 164.10,163.30, 163.10, 161.50, 145.30, 137.20, 134.50, 134.00, 132.40, 132.20, 129.20, 129.10, 125.50, 125.20, 123.40, 122.20, 122.10, 122.00,114.30, 114.10, 47.30, 46.20, 43.20, 35.20, 32.40, 31.10, 28.30, 25.50, 22.20, 22.10, 13.00; ESI-MS: m/z = 613(M+).
3.5 General procedures for the synthesis of compounds 11a-d
A mixture of benzoxazinone 4 (3.53 g, 10 mmol) and the appropriate amino acids; glycine (0.75 g, 10 mmol), alanine (0.89 g, 10 mmol), cysteine (1.21 g, 10 mmol) and leucine (1.31 g, 10 mmol), in DMF (20 mL) was refluxed for 2 h. After the completion of reaction, the reaction mixture was poured into ice water to give a precipitate that was filtered off and recrystallized from ethanol to give 11a,d.
3.5.1 2-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)acetic acid(11a)
Recryst. Solvent: Methanol; white solid; Yield: 70%; m. p: 270–272 °C; Anal. Calc. (%) for C22H23N3O5 (4 0 9): C: 64.54, H: 5.62, N: 10.26; found: C: 64.50, H: 5.60, N: 10.24; FT-IRvmax (cm−1): 3450–3570 (OH), 1675 (C⚌Oquinazolinone), 1665 (C⚌Ocarboxyl),1612 (C⚌N); 1H NMR (DMSO‑d6) δ (ppm):): δ 1.10 (d, 6H, CH2CH(CH3)2), 1.30 (d, 3H, CHCH3), 1.95 (m, H, CH2CH(CH3)2), 2.50 (d, 2H, CH2CH(CH3)2), 3.40 (q, H, CHCH3), 4.71 (s, 2H, CH2COOH), 7.10–8.20 (m, 7H, Ar02015H)), 11.20 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 173.20, 165.10, 161.30, 154.00, 146.25, 137.60, 137.30, 128.601, 128.40, 126.50, 126.10, 125.70, 124.30, 123.10, 122.50, 45.50, 44.50, 31.10, 28.50, 22.60, 22.10, 12.50; ESI-MS: m/z = 409 (M+).
3.5.2 2-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)propanoic acid (11b)
Recryst. Solvent: Ethanol; white solid; Yield: 65%; m. p: 280–282 °C; Anal. Calc. (%) for C23H25N3O5 (4 2 3): C, 65.24; H, 5.92; N, 9.92; found: C, 65.20; H, 5.95; N, 9.89; FT-IRvmax (cm−1): 3440–3560 (OH), 1670 (C⚌Oquinazolinone), 1660 (C⚌Ocarboxyl), 1610(C⚌N); 1H NMR (DMSO‑d6) δ (ppm):): δ 1.15 (d, 6H, CH2CH(CH3)2), 1.44 (d, 3H, CHCH3), 1.55 (d, 3H, CHCH3COOH), 1.90 (m, H, CH2CH(CH3)2), 2.40 (d, 2H, CH2CH(CH3)2), 3.45 (q, H, CHCH3), 4.75 (q, 1H, CHCH3COOH), 7.20–8.10 (m, 7H, Ar02015H)), 11.45 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 174.10, 164.10, 163.30, 156.00, 147.25, 138.60, 138.30, 129.601, 129.40, 126.30, 126.20, 124.70, 124.30, 123.70, 122.30, 50.50, 45.50, 30.10, 28.10, 22.40, 22.10, 13.20, 12.50; ESI-MS: m/z = 423 (M+).
3.5.3 2-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)-3-mercaptopropanoic acid (11c)
Recryst. Solvent: DMF; Yellowish crystals; Yield: 70%; m. p: 240–242 °C; Anal. Calc. (%) for C23H25N3O5S (4 5 5): C: 60.64, H: 5.53, N: 9.22; found: C: 60.60, H: 5.50, N: 9.20; FT-IRvmax (cm−1): 3400–3500 (OH), 2540 (SH), 1675 (C⚌Oquinazolinone), 1665 (C⚌Ocarboxyl), 1612(C⚌N); 1H NMR (DMSO‑d6) δ (ppm):): δ 1.10 (d, 6H, CH2CH(CH3)2), 1.40 (d, 3H, CHCH3), 1.55 (s, 1H, SH), 1.90 (m, H, CH2CH(CH3)2), 2.45 (d, 2H, CH2CH(CH3)2), 3.35 (q, H, CHCH3), 4.70 (q, 1H, CHCH2SH), 3.15 (d, 2H, CHCH2SH),7.10–8.20 (m, 7H, Ar02015H)), 11.50 (s, H, COOH); 13C NMR (DMSO‑d6) δ (ppm): 175.10, 165.10, 165.30, 153.00, 145.25, 138.50, 138.20, 129.50, 129.20, 126.50, 126.30, 123.50, 123.40, 123.50, 122.70, 55.60, 47.50, 30.40, 28.10, 24.50, 22.10, 22.00, 12.60; ESI-MS: m/z = 455 (M+).
3.5.4 2-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)-4-methylpentanoic acid (11d)
Recryst. Solvent: Ethanol; white solid; Yield: 75%; m. p: 277–280 °C; Anal. Calc. (%) for C26H31N3O5 (4 6 5): C, 67.08;H, 6.71, N: 9.03; found: C: 67.10, H: 6.69, N: 9.00; FT-IRvmax (cm−1): 3430–3550 (OH), 1675 (C⚌Oquinazolinone), 1660 (C⚌Ocarboxyl), 1612(C⚌N); δ 1.15 (d, 6H, CH2CH(CH3)2), 1.20 (d, 6H, CHCH2CH(CH3)2), 1.45 (d, 3H, CHCH3), 1.83 (m, H, HCHCH2CH(CH3)2), 1.70 (t, 2H, HCHCH2CH(CH3)2), 1.95 (m, H, CH2CH(CH3)2), 2.50 (d, 2H, CH2CH(CH3)2), 3.30 (q, H, CHCH3), 3.95 (t, H, CHCH2CH(CH3)2), 7.20–8.20 (m, 7H, Ar02015H), 11.50 (s, H, COOH). 13C NMR (DMSO‑d6) δ (ppm): 174.50, 164.40, 163.60, 158.00, 145.25, 137.60, 137.30, 129.30, 129.10, 126.50, 126.00, 124.50, 124.30, 123.50, 122.20, 50.00, 45.10, 38.20, 31.10, 27.10, 22.90, 22.85, 22.50, 22.40, 22.35, 13.20; ESI-MS: m/z = 465 (M+).
3.5.5 2-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)-3-mercaptopropanoyl chloride (12)
Mixture of both compounds 11c (4.55 g, 10 mmol) and SOCl2 (% 95, 5 mL) was heated for 4 h in an oil bath (80 °C). At 50 °C, the solvents were removed using a rotary evaporator. Ethyl ether (3x15 mL) was used to wash the residue. The product was crystallized from toluene and dried in vacuo at 70 °C.
Recryst. Solvent: Toluene; pale Yellow crystals; Yield: 60%; m. p: 205–207 °C; Anal. Calc. (%) for C23H24N3O4SCl (4 7 3): C, 58.28; H, 5.10; N, 8.87; found: C: 58.25, H: 5.08, N: 8.85; FT-IRvmax (cm−1): 2550 (SH), 1678 (C⚌Oquinazolinone), 1664 (C⚌Ocarboxyl), 1610(C⚌N); 1H NMR (DMSO‑d6) δ (ppm): δ 1.12 (d, 6H, CHCH2CH(CH3)2), 1.38 (d, 3H, CHCH3), 1.50 (s, 1H, SH), 1.90 (m, H, CH2CH(CH3)2), 2.40 (d, 2H, CH2CH(CH3)2), 3.10 (d, 2H, CHCH2SH), 3.35 (q, H, CHCH3), 4.75 (q, 1H, CHCH2SH), 7.10–8.20 (m, 7H, Ar02015H); 13C NMR (DMSO‑d6) δ (ppm): 176.10, 165.30, 164.10, 153.00, 147.20, 138.70, 138.10, 129.30, 129.10, 124.40, 124.30, 123.70, 123.20, 123.10, 121.60, 58.90, 46.40, 33.10, 27.10, 24.20, 22.15, 22.10, 13.00; ESI-MS: m/z = 473 (M+).
3.5.6 2-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)-3-mercaptopropanamide (13)
3.5.6.1 Method A
A solution of compound 12 (4.73 g, 10 mmol) in THF (20 mL) were cooled to 0 °C. The obtained solution was slowly added to ammonium hydroxide solution (1.5 mL, 20 mmol) at 0 °C, stirred, and kept at this temperature for 4 h. The obtained solution was filtered. The solvents were removed on a rotary evaporator at 40 °C. The residue was washed with ether (3 × 15 mL). The product was crystallized from ethyl acetate and dried in vacuo at 70° C.
3.5.6.2 Method B
In dichloromethane, compound 11c (4.55 g, 10 mmol) was mixed with ammonium hydroxide (1.5 mL, 20 mmol) and 3 mmol triethylamine (Et3N), then SOCl2 (% 95, 5 mL) was added at room temperature under continuous stirring for 20 min. Evaporating the solvent under lower pressure was used to recover the reaction product. The resultant residue was dissolved in dichloromethane and washed with 1 N HCl and 1 N NaOH. The organic phase was dried (Na2SO4), and evaporated to dryness to afford the corresponding carboxylic amide.
Recryst. Solvent: Ethyl acetate; White crystals; Yield: Method A, (35%); method B, (80%); m. p: 185–187 °C; Anal. Calc. (%) for C23H26N4O4S (4 5 4): C, 60.77; H, 5.77; N, 12.33; found: C: 60.75, H: 5.74, N: 12.30; FT-IRvmax (cm−1): 3360–3339 (NH2), 2500 (SH), 1675 (C⚌Oquinazolinone), 1645 (C⚌Oamide), 1612(C⚌N); 1H NMR (DMSO‑d6) δ (ppm): δ 1.12 (d, 6H, CH2CH(CH3)2),1.40 (d, 3H, CHCH3), 1.50 (s, 1H, SH), 1.95 (m, H, CH2CH(CH3)2), 2.40 (d, 2H, CH2CH(CH3)2), 3.15 (d, 2H, CHCH2SH), 3.35 (q, H, CHCH3), 4.74 (q, 1H, CHCH2SH), 5.85 (br, s, 2H, NH2), 7.10–8.20 (m, 7H, Ar02015H); 13C NMR (DMSO‑d6) δ (ppm): 175.90, 165.60, 164.30, 154.10, 146.20, 137.70, 137.10, 128.30, 128.10, 124.50, 124.40, 123.60, 123.50, 123.20, 122.60, 57.590, 45.40, 32.10, 26.10, 25.20, 21.15, 21.10, 12.50; ESI-MS: m/z = 454 (M+).
3.6 General procedure synthesis compound (14a-e)
The quinazolinyl amide derivative 13 (4.54, 10 mmol) was stirred at room temperature with the appropriate aldehyde namely; 4-hydroxybenzaldehyde (1.22 g, 10 mmol), 4-chlorobenzaldehyde (1.40 g, 10 mmol), 4- methoxybenzaldehyde (1.36 g, 10 mmol), 4-N,N-dimethylbenzaldehyde (1.49 g, 10 mmol) and 4-nitrobenzaldehyde (1.51 g, 10 mmol) in absolute ethanol (20 mL), for 10 min before adding 0.1 mol/L aqueous solution of iodine/potassium iodide (10 mL). The completion of the reaction was monitored by TLC using chloroform:methanol (7:4) as eluent. The resulting crystalline products were filtered and rinsed with sodium thiosulphate solution 5% and hot water, respectively. The crystalline products was dried and further crystallized from the proper solvent.
3.6.1 (28Z)-N-(4-hydroxybenzylidene)-2-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)-3-mercaptopropanamide (14a)
Recryst. Solvent: Mehanol; Browen crystals; Yield: 80%; m. p: 285–287 °C; Anal. Calc. (%) for C30H30N4O5S (5 5 8): C, 64.50; H, 5.41; N, 10.03; found: C: 64.47, H: 5.39, N: 10.00; FT-IRvmax (cm−1): 3375–3455 (OH), 2530 (SH), 1670 (C⚌Oquinazolinone), 1640 (C⚌Oamide), 1612(C⚌N); 1H NMR (DMSO‑d6) δ (ppm): δ 1.02 (d, 6H, CH2CH(CH3)2), 1.40 (d, 3H, CHCH3), 1.55 (s, 1H, SH), 1.95 (m, H, CH2CH(CH3)2), 2.50 (d, 2H, CH2CH(CH3)2), 3.35 (q, H, CHCH3), 3.43 (d, 2H, CHCH2SH), 4.70 (q, 1H, CHCH2SH), 5.10 (s, H, OH), 7.10–8.20 (m, 11H, Ar02015H), 8.80 (s, H, CH⚌N); 13C NMR (DMSO‑d6) δ (ppm): 175.90, 165.60, 164.30, 163.70, 160.80, 154.10, 146.20, 137.70, 137.10, 130.70, 130.60 128.30, 128.10, 126.40, 124.50, 124.40, 123.60, 123.50, 123.20, 122.60, 116.50, 116.55, 57.50, 45.40, 32.10, 26.10, 25.20, 21.15, 21.10, 12.50; ESI-MS: m/z = 558 (M+).
3.6.2 ((28Z)-N-(4-chlorobenzylidene)-2-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)-3-mercaptopropanamide (14b)
Recryst. Solvent: Mehanol; yellow crystals; Yield: 85%; m. p: 265–267 °C; Anal. Calc. (%) for C30H29ClN4O4S (5 7 7): C, 62.44; H, 5.07; N, 9.71; found: C: 62.40, H: 5.10, N: 9.68; FT-IRvmax (cm−1): 2510 (SH), 1675 (C⚌Oquinazolinone), 1642 (C⚌Oamide), 1610(C⚌N); 1H NMR (DMSO‑d6) δ (ppm): δ 1.10 (d, 6H, CH2CH(CH3)2), 1.43 (d, 3H, CHCH3), 1.50 (s, 1H, SH), 1.90 (m, H, CH2CH(CH3)2), 2.40 (d, 2H, CH2CH(CH3)2), 3.35 (q, H, CHCH3), 3.42 (d, 2H, CHCH2SH), 4.70 (q, 1H, CHCH2SH), 7.20–8.10 (m, 11H, Ar02015H), 8.82 (s, H, CH⚌N); 13C NMR (DMSO‑d6) δ (ppm): 170.80, 165.10, 164.50, 163.8, 155.10, 147.20, 138.20, 138.10,136.10, 132.75, 132.70, 130.20, 130.10, 129.70, 128.60, 128.10, 125.50, 124.50, 123.40, 123.30, 123.20, 122.50, 58.10, 44.40, 33.10, 27.10, 26.20, 22.15, 22.10, 13.00; ESI-MS: m/z = 577 (M+).
3.6.3 ((28Z)-N-(4-methoxybenzylidene)-2-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)-3-mercaptopropanamide (14c)
Recryst. Solvent: Mehanol; yellow crystals; Yield: 75%; m. p: 265–266 °C; Anal. Calc. (%) for C31H32N4O5S (5 7 2): C, 65.02; H, 5.63; N, 9.78; found: C: 65.05, H: 5.66, N: 9.81; FT-IRvmax (cm−1): 2510 (SH), 1675 (C⚌Oquinazolinone), 1642 (C⚌Oamide), 1610(C⚌N); 1H NMR (DMSO‑d6) δ (ppm): δ 1.12 (d, 6H, CH2CH(CH3)2), 1.45 (d, 3H, CHCH3), 1.50 (s, 1H, SH), 2.00 (m, H, CH2CH(CH3)2), 2.45 (d, 2H, CH2CH(CH3)2), 3.30 (q, H, CHCH3), 3.45 (d, 2H, CHCH2SH), 3.50 (s, 3H, OCH3), 4.60 (q, 1H, CHCH2SH), 7.20–8.10 (m, 11H, Ar02015H), 8.83 (s, H, CH⚌N); 13C NMR (DMSO‑d6) δ (ppm): 171.50, 165.30, 164.20, 163.70, 162.50, 156.10, 146.20, 137.10,136.30, 132.70, 132.60, 130.50, 130.40, 129.50, 125.60, 124.60, 123.60, 123.50, 123.10, 122.40,114.50, 114.45, 57.10, 54.50 43.40, 31.10, 27.80, 26.70, 22.30, 22.20, 12.80; ESI-MS: m/z = 572 (M+).
3.6.4 ((28Z)-N-(4-(dimethylamino)benzylidene)-2-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)-3-mercaptopropanamide (14d)
Recryst. Solvent: AcOH; Brown crystals; Yield: 84%; m. p: 250–252 °C; Anal. Calc. (%) for C32H35N5O4S (5 8 5): C, 65.62; H, 6.02; N, 11.96; found: C: 65.60, H: 5.98, N: 11.94; FT-IRvmax (cm−1): 2515 (SH), 1673 (C⚌Oquinazolinone), 1644 (C⚌Oamide), 1615(C⚌N); 1H NMR (DMSO‑d6) δ (ppm): δ 1.10 (d, 6H, CH2CH(CH3)2), 1.38 (d, 3H, CHCH3), 1.53 (s, 1H, SH), 1.95 (m, H, CH2CH(CH3)2), 2.40 (d, 2H, CH2CH(CH3)2), 2.75 (d, 6H, N(CH3)2), 3.30 (q, H, CHCH3), 3.40 (d, 2H, CHCH2SH), 4.50 (q, 1H, CHCH2SH), 7.20–8.10 (m, 11H, Ar02015H), 8.80 (s, H, CH⚌N); 13C NMR (DMSO‑d6) δ (ppm): 170.50, 166.25, 164.70, 163.20, 163.00, 158.30, 147.20, 138.10,137.30, 132.50, 132.40, 130.55, 130.45, 129.80, 125.50, 124.30, 123.50, 123.40, 123.00, 122.50,115.50, 115.45, 56.50, 46.50, 41.50, 41.45, 35.10, 29.30, 25.50, 23.30, 23.20, 12.40; ESI-MS: m/z = 585 (M+, 100).
3.6.5 (28Z)-N-(4-nitrobenzylidene)-2-(2-(1-(4-isobutylphenyl)ethyl)-6-nitro-4-oxoquinazolin-3(4H)-yl)-3-mercaptopropanamide (14e)
Recryst. Solvent: Ethanol; Browen crystals; Yield: 88%; m. p: 280–282 °C; Anal. Calc. (%) for C30H29N5O6S (5 8 7): C, 61.32; H, 4.97; N, 11.92; found: C: 61.30, H: 4.95, N: 11.96; FT-IRvmax (cm−1): 2550 (SH), 1677 (C⚌Oquinazolinone), 1648 (C⚌Oamide), 1610(C⚌N); 1H NMR (DMSO‑d6) δ (ppm): δ 1.05 (d, 6H, CH2CH(CH3)2), 1.40 (d, 3H, CHCH3), 1.50 (s, 1H, SH), 1.95 (m, H, CH2CH(CH3)2), 2.44 (d, 2H, CH2CH(CH3)2), 3.35 (q, H, CHCH3), 3.43 (d, 2H, CHCH2SH),4.75 (q, 1H, CHCH2SH), 7.10–8.20 (m, 11H, Ar02015H), 8.82 (s, H, CH⚌N); 13C NMR (DMSO‑d6) δ (ppm): 170.50, 165.10, 164.10, 163.30, 161.50, 156.10, 147.20, 138.70, 138.10, 131.50, 131.40 130.10, 130.00, 128.10, 128.00, 126.50, 124.20, 124.10, 123.40, 123.30, 123.00, 122.00, 55.50, 45.10, 32.50, 26.40, 25.10, 21.30, 21.20, 12.80; ESI-MS: m/z = 587 (M+).
3.7 Determination of in vitro nNOS and iNOS activities
All required chemicals were provided through Sigma Aldrich. The conversion of L-[3H]-arginine to L-[3H]-citrulline was monitored using the Bredt and Snyder (1990) method to evaluate nNOS activity. The final incubation volume was 100 µl and consisted of 10 µl of an aliquot of recombinant nNOS (specific activity 21.05 nmol/min/mg protein) added to a buffer with a final concentration of 25 mM TriseHCl, 1 mM DTT, 4 µM H4-biopterin, 10 µM FAD, 0.5 mM inosine, 0.5 mg/ml BSA, 0.1 mM CaCl2, 10 µM L-arginine, and 50 nM L-[3H]-arginine, and 10 µg/mL calmodulin (only for nNOS) at pH 7.6 and 7.0 for iNOS and nNOS, respectively. The reaction was initiated by adding 10 µl of NADPH (0.75 mM final concentration) and 10 mL of each quinazolinyl Schiff base derivative to a final concentration of 1 mM in ethanol (20%). The tubes were vortex and incubated at 37 °C for 30 min. Control incubations were accomplished by the omission of NADPH. With the addition of 400 µl of cold 0.1 M HEPES, 10 mM EGTA, and 0.175 mg/ml L-citrulline, pH 5.5, the reaction was halted. The reaction mixture was decanted into a 2 mL column package and eluted with 1.2 mL of water using Dowex-50 W ion-exchange resin (Na+ form). Liquid scintillation spectroscopy was used to quantify L-[3H]-citrulline. L-[3H]-arginine was retained at a rate of more than 98 percent in this method. The control value, which was generally less than 1% of the radioactivity injected, was subtracted to ascertain specific enzyme activity. The activity of nNOS was evaluated in picomoles of L-[3H]-citrulline generated per milligram of protein per minute.The protocol for determining iNOS activity was substantially the same as for nNOS, except that instead of nNOS, an aliquot of 10 µl recombinant iNOS was employed, and the combination was incubated in the absence of calmodulin.
3.8 Statistical analysis
Data are expressed as the mean ± SEM. One-way analysis of variance, followed by the Newmane-Keuls multiple range test was used. A P less than 0.05 was considered statistically significant.
4 Conclusions
In conclusion, a variety of new Schiff base derivatives with the 4(3H)-quinazolinone moiety have been synthesized. We also evaluate the activity of nNOS and iNOS of these novel structures. Although they mostly expose moderate activity, several compounds show promising values. The iNOS inhibitory activity of the studied derivatives is generally higher than that of nNOS, indicating that the insertion of an azomethine Schiff bas linkage with diverse p-aromatic substituents in a new ring is reinforced to the inhibitory activity. Furthermore, we have shown that the presence of guanidine in the heterocyclic ring of derivatives 9a-e results in improved NOS inhibitory efficacy. Because of its enzymatic N-oxygenation, compounds 8e, 9e, 10e, and 14e are extremely powerful NOS inhibitors with a p-nitro-ph skeleton. As a result, our evaluated Schiff base derivatives should be a promising starting point for the development of therapeutic medicines for NO-related disorders involving both isoforms, such as Parkinson's disease and anti-stroke. In light of our study results, which clarified the importance of the biocompatibility of the synthasized compounds, the researchers plan to conduct cytotoxicities of these compounds to ensure their safe use.
Acknowledgments
The authors extend their appreciation to the deputyship for Research & Innovation, Ministry of education in Saudi Arabia for funding this research work through project number IFP-2020-45.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. USA. 1990;87:682-685.
- [Google Scholar]
- Chen, H., Wang, B., Tang, C., Zhang, B., 2016. Synthesis and antibacterial evaluation of novel Schiff base derivatives containing 4(3H)-quinazolinone moiety. Chem. Papers 70 (11), 1521–1528.
- Green and efficient synthesis of some pyrido [2,3-d]pyrimidin-4(3h)-one derivatives via iodine catalyst in aqueous media and evaluation the synthesized compounds as anticancer. Sci. J. Chem.. 2013;1(1):1-6.
- [Google Scholar]
- Synthesis of some quinazolin-4-one derivatives carrying ibuprofenyl moiety and their antiinflammatory activity. Der. Pharma Chemica.. 2011;3(3):1-12.
- [Google Scholar]
- One-Pot Synthesis of Some Dynamic 2-Substituted Benzoxazinones and Their Corresponding Qinazolinones of Anticipated Biological Activity. J. Heterocyclic Chem.. 2016;53:95-101.
- [Google Scholar]
- Deferential actions of L-cysteine on responses to nitric oxide, nitroxyl anions and EDRF in the rat aorta. Br. J. Pharmacol.. 2000;129:315-322.
- [Google Scholar]
- A nitric oxide/cysteine interaction mediates the activation of soluble guanylate cyclase. PNAS. 2009;106(51):21603-26107.
- [Google Scholar]
- Overproduction of nitric oxide in cytokine-mediated and septic shock. L Natl. Cancer Inst.. 1992;84:827-831.
- [Google Scholar]
- Leggio, A., Belsito,E. L., De Luca, G., Di Gioia, M. L., Leotta, V., Romio, E., Sicilianoa, C., Liguori, A., 2016. One-pot synthesis of amides from carboxylic acids activated using thionyl chloride. RSC Adv. 6, 34468–34475.
- López Cara, L.C.M., Camacho, E.M., Carrión, D., Tapias, V., Gallo, M.A., Escames, G., Castroviejo, D.A., Espinosa, A., Entrena, A., 2009. Phenylpyrrole derivatives as neural and inducible nitric oxide synthase (nNOS and iNOS) inhibitors. Eur. J. Med. Chem. 44, 2655–2666.
- Synthesis, pharmacological and toxicological evaluation of amide derivatives of ibuprofen. Inter. J. Chem. Tech.. 2010;2(1):233-238.
- [Google Scholar]
- Synthesis and antiproliferative evaluation of some novel quinazolin-4(3H)-one derivatives. J. Heterocyclic Chem.. 2020;57:3898-3906.
- [Google Scholar]
- Structural analysis of NSAID binding by prostaglandin H2 synthase: time-dependent and time-independent inhibitors elicit identical enzyme conformations. Biochemistry. 2001;40:5172.
- [Google Scholar]
- Silva, Jr, L.F., Quintiliano, A., 2009. An expeditious synthesis of hexahydrobenzo[f]isochromenes and of hexahydrobenzo[f]isoquinoline via iodine-catalyzed Prins and aza-Prins cyclization. Tetrahedron Lett. 50, 2256–2260.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103362.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







