Translate this page into:
Synthesis and biological evaluation of Schiff’s bases and 2-azetidinones of isonocotinyl hydrazone as potential antidepressant and nootropic agents
⁎Corresponding author. Mobile: +91 9881236220; fax: +91 020 27420261. dypharmachem@yahoo.co.in (Asha B. Thomas)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The synthesis and pharmacological activity of N′-[(1Z)-(substituted aromatic) methylidene] pyridine-4-carbohydrazides (3a–k) and N-[3-chloro-2-(substituted aromatic)-4-oxoazetidin-1-yl]pyridine-4-carboxamides (5a–k) are described. Synthesis of 2-azetidinones was performed by novel methods of stirring and sonication involving the cyclocondensation of the appropriate Schiff’s bases (3a–k) with chloroacetyl chloride, followed by the addition of triethyl amine in the presence of molecular sieves. The compounds were investigated for their antidepressant activity, compounds N′-[(1Z)-(2,5-dimethoxyphenyl)methylidene]pyridine-4-carbohydrazide (3k) and N-[3-chloro-2-(2,5-dimethoxyphenyl)-4-oxoazetidin-1-yl]pyridine-4-carboxamide (5k) with 2,5-dimethoxy substitution on the aryl ring exhibited the highest antidepressant activity. In the elevated plus maze test and passive avoidance test in mice for the evaluation of nootropic activity N′-[(1Z)-(4-nitrophenyl)methylidene]pyridine-4-carbohydrazide (3d) and N-[3-chloro-2-(4-nitrophenyl)-4-oxoazetidin-1-yl]pyridine-4-carboxamide (5d) with para nitro substitution on the aryl ring exhibited the highest activity. All synthesised Schiff’s bases and azetidinone analogues exhibited antidepressant and nootropic activities in a dose dependant manner. The results confirmed the fact that the 2-azetidinone skeleton has potential as a CNS active agent and can be explored for the development of more potent and safe CNS active agents for therapeutic use.
Keywords
2-Azetidinone
Anti-depressant activity
Nootropic activity
Schiff’s bases
Sonication
Stirring
1 Introduction
2-Azetidinones, commonly known as β-lactams, are well-known heterocyclic compounds among the organic and medicinal chemists. The activity of the known antibiotics such as penicillins, cephalosporins and carbapenems are attributed to the presence of 2-azetidinone ring in them. The broad and potent activity of 2-azetidinones has established them as one of the biologically important scaffold (Risi et al., 1999). Recently, other biological activities besides the antibacterial activity have been reported in compounds containing the 2-azetidinone ring (Singh et al., 2007). The recent discoveries of some 1,3,4-trisubstituted-β-lactams as new potent cholesterol absorption inhibitors, human cytomegalovirus protease inhibitors and thrombin inhibitors justified a renewed interest in these compounds. Furthermore, interest in chemistry, synthesis and biology of 2-azetidinone pharmacophore continues to be researched due to their wide range of biological properties, such as antibacterial (Chavan and Pai, 2007; Desai and Desai, 2006; Singh and Mmolotsi, 2005), antihyperglycemic (Leach and Deirdre, 2001), antihyperlipidemic (Wu et al., 1999; Xu, 2007), CNS activity (Goelab et al., 2005), tryptase inhibitory (Bisacchi and Slusarchyk, 2004), human leucocyte elastase inhibitory (Hagmannt et al., 1991), anti-inflammatory (Kumar and Rajput, 2009), vasopressin v1a antagonist (Guillon and Koppel, 2004), and anticancer activity (Banik et al., 2005; Banik et al., 2003).
2-Azetidinones have not been reported so far as CNS active agents; however, the presence of this moiety in a variety of heterocyclic compounds has been reported to increase the CNS activity of the parent structure. Reports of compounds containing a quinazoline ring at N-1 position of 2-azetidinone ring have resulted in compounds with potent activity against oxtremorine induced tremors and as antiparkinson agents. The active compounds have phenyl, 2-chlorophenyl and 2-furyl groups at C-4 position of azetidinone ring (Srivastva et al., 1987). The synthesis of benzopyran-2-one substituted 2-azetidinone derivatives at N-1 position of ring with significant CNS depressant has been reported (Kulkarni et al., 1996). Many researchers have also reported the substitution of heterocyclic rings such as carbazole ring at N-1 position of the 2-azetidinone nucleus (Srivastava et al., 1999) and 1,2,4-triazole derivative of 2-azetidinones (Srivastava et al., 2002) resulting in compounds with anticonvulsant properties. The presence of halogens in the biologically active molecules has shown to play a crucial role in their pharmacological properties. Spiroazetidinones derived from 3-(N-substituted) imino-1-methylindol-2-ones have also been reported as anticonvulsants (Singh et al., 1994). Recently various 2-azetidinones were synthesised which were further evaluated for their CNS modulating activities using the computer software PASS (Prediction of activity spectra for substances) (Goel et al., 2005). Lately azetidinone derivatives have also been reported for the treatment of Alzheimers disease (Davis et al., 2003). It is postulated that azetidinones may exert this effect due to their ability to regulate the production of amyloid-b-peptides (Puglielli et al., 2001).
The Staudinger’s ketene-imine cycloaddition reaction [2+2] is the most common method for the synthesis of azetidinones (Staudinger, 1907) and has been reviewed till date by several researchers. The reactions are carried out thermally (Alcaide et al., 2000) or photochemically (Griesbeck and Heckroth, 2002) using acid chlorides in the presence of triethylamine or α-diazoketones as ketene precursors. However the conventional methods reported for the synthesis of 2-azetidinones require longer reaction times (12–16 h reflux) Barrett et al., 2000; Escalante et al., 2001 at very low temperature (−70 to −90 °C) (Alcaide et al., 2001; Banik and Becker, 2000) with low yields (less than 70%) Penfold et al., 2000. The reactions also involve the use of a Dean-stark water separator for the removal of water from the reaction. The previous decade has also seen the use of microwave radiation in the synthesis of azetidinones. However, the reported microwave methods also required an inert atmosphere of nitrogen gas to enable the completion of the reaction (Bose et al., 1996, 1995).
On the basis of the above findings and considering the need for the development of potent CNS active agents, we were stimulated to explore new simplified 2-azetidinone analogues for their antidepressant and nootropic activities. Here, in this paper, we are reporting the synthesis of N-[3-chloro-2-(substituted aromatic)-4-oxoazetidin-1-yl]pyridine-4-carboxamide analogues by our previously reported green route methods of stirring and sonication and their screening for antidepressant and nootropic activities.
2 Experimental
2.1 General considerations
All research chemicals were purchased from Across organics (NY, USA), Sigma-Aldrich (St. Loius, Missiouri, USA) and used as such for the reactions. Melting points (mp) were determined on a Veego melting point apparatus (VMP PM, 32/1104) and are uncorrected. Reactions were monitored by thin layer chromatography carried out using pre-coated silica gel plates (E. Merck and Co., Darmstadt, Germany). UV studies were carried out on a UV Visible spectrophotometer (Shimadzu 1700) and the λmax of the respective synthesised compounds was determined using ethanol as the solvent. The IR spectra (KBr) were recorded on a FTIR spectrophotometer with Diffuse Reflectance attachment (Shimadzu 8400S). The 1H NMR spectra were obtained on an NMR Spectrophotometer (Bruker Avance II 400 NMR) using dimethyl sulphoxide-d6 as the solvent. Chemical shifts were expressed in parts per million relative to SiMe4 as an internal standard. The mass spectra were obtained on a Hewlett Packard Electron Impact mass spectrometer GCD-1800A (70 eV EI source) using a direct insertion probe and a Quadrapole TOF Mass spectrometer using electrospray ionisation (Positive mode). Microanalyses were performed on a Thermo Finnigan C, H, N analyser.
2.2 Synthesis
2.2.1 General procedure for the synthesis of N′-[(1Z)-(substituted aryl)methylidene] pyridine-4-carbohydrazides (Schiff’s base)
The synthesis of compounds (3a–k) was performed according to our previously reported procedure (Scheme 1) Thomas et al., 2009. Isoniazid (0.01 M) and appropriate aromatic aldehydes (0.01 M) (2a–k) in water were irradiated under microwave using a microwave synthesiser (Make-Raga’s Scientific, India) at power level 3 (240 W, 35% irradiation) in an open vessel until the completion of the reaction. The reaction was monitored by TLC (acetone/ethanol = 4:1). The reaction mixture was then filtered, the residue obtained was washed with water, followed by sodium thiosulphate (Na2S2O3) solution and dried. The crude product upon recrystallisation from alcohol gave the pure hydrazones of INH (3a–k). The synthesised compounds were characterised on the basis of their spectral and analytical data. (UV, IR, 1H NMR, MS, CHN).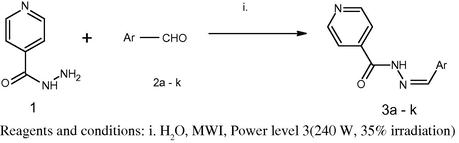
Microwave assisted synthesis of intermediate Schiff’s bases of INH (3a–k).
2.2.1.1 N′-[(1Z)-(2-Hydroxyphenyl) methylidene]pyridine-4-carbohydrazide (3a)
White crystals, yield 95.6, mp 262–264 °C, IR (νmax, cm−1, KBr): 3344 (–OH), 3178 (–NH), 3004 (–CH), 1685 (amide C⚌O), 1566 (imine C⚌N). 1H NMR (400 MHz, DMSO-d6): δ ppm 11 (s, OH), 8.8–8.9 (d, Pyridine 2H), 8.6–8.8 (d, Pyridine 2H), 8.7 (s, CH), 7.5–7.6 (d, Aromatic1H), 8.6 (s, NH), 6.8–7.0 (d, Aromatic 2H), 7.3–7.4 (m, Aromatic1H). MS: m/z 242.5 (M+H)+. Anal. Calcd for C13H11N3O2 (241.25): C, 64.72; H, 4.60; N, 17.42%. Found: C, 64.51; H, 4.73; N, 17.60%.
2.2.1.2 N′-[(1Z)-(4-Hydroxyphenyl)methylidene]pyridine-4-carbohydrazide (3b)
Yellow powder, yield 94.5, mp 264–268 °C, IR (νmax, cm−1, KBr): 3340 (–OH), 3213 (–NH), 3055 (–CH), 1662 (amide C⚌O), 1608 (imine C⚌N). 1H NMR (400 MHz, DMSO-d6): δ ppm 11.5 (s, OH), 8.8–8.5 (d, Pyridine 2H), 8.4–8.3 (d, Pyridine 2H), 7.8 (s, CH), 8.0 (s, NH) 7.5–7.3 (d, Aromatic 2H), 7.0–6.8 (d, Aromatic 2H). MS: m/z 242.5(M+H)+. Anal. Calcd for C13H11N3O2 (241.25): C, 64.72; H, 4.60; N, 17.42%. Found: C, 64.57; H, 4.52; N, 17.36%.
2.2.1.3 N′-[(1Z)-(4-Chlorophenyl)methylidene]pyridine-4-carbohydrazide (3c)
White crystals, yield 98.1, mp 218–221 °C, IR (νmax, cm−1, KBr): 3166 (–NH), 3020 (–CH), 1674 (amide C⚌O), 1593 (imine C⚌N), 698 (–Cl). 1H NMR (400 MHz, DMSO-d6): δ ppm 8.88–8.91 (d, Pyridine 2H), 8.32 (s, CH), 8.65 (s, NH), 7.99–8.05 (d, Pyridine 2H), 7.79–7.80 (d, Aromatic 2H), 7.50–7.51 (d, Aromatic 2H). MS: m/z 260.4 (M+H)+. Anal. Calcd for C13H10ClN3O (259.69): C, 60.12; H, 3.88; N, 16.18%. Found: C, 60.34; H, 3.76; N, 16.13%.
2.2.1.4 N′-[(1Z)-(4-Nitrophenyl)methylidene]pyridine-4-carbohydrazide (3d)
Yellow powder, yield 90.7, mp 255–257 °C, IR (νmax, cm−1, KBr): 3186 (–NH), 3001 (–CH), 1685 (amide C⚌O), 1558 (imine C⚌N), 1334, 1512 (–NO2). 1H NMR (400 MHz, DMSO-d6): δ ppm 9.32–9.35 (d, Pyridine 2H), 8.51–8.55 (d, Pyridine 2H), 8.34 (s, NH), 8.10 (s, CH), 7.89–7.93 (d, Aromatic 2H), 7.60–7.62 (d, Aromatic 2H). MS: m/z 271.4 (M+H)+. Anal. Calcd for C13H10N4O3 (270.24): C, 57.78; H, 3.73; N, 20.73%. Found: C, 58.07; H, 3.68; N, 20.51%.
2.2.1.5 N′-[(1Z)-(2-Nitrophenyl) methylidene] pyridine-4-carbohydrazide (3e)
Orangish yellow powder, yield 99.2, mp 229–231 °C, IR (νmax, cm−1, KBr): 3193 (–NH), 3018 (–CH), 1677 (amide C⚌O), 1552 (imine C⚌N), 1517, 1363 (–NO2). 1H NMR (400 MHz, DMSO-d6): δ ppm 9.04 (s CH), 8.77–8.78 (d, Pyridine 2H), 8.62 (s, NH), 8.27–8.29 (d, Aromatic 1H), 8.04–8.06 (d, Aromatic 1H), 7.88–7.89 (d, Pyridine 2H), 7.70–7.74 (m, Aromatic 1H), 7.59–7.63 (m, Aromatic 1H). MS: m/z 271.5 (M+H)+. Anal. Calcd for C13H10N4O3 (270.24): C, 57.78; H, 3.73; N, 20.73%. Found: C, 57.92; H, 3.66; N, 20.95%.
2.2.1.6 N′-[(1Z)-(4-Flurophenyl)methylidene]pyridine-4-carbohydrazide (3f)
White powder, yield 98.76, mp 189–190 °C, IR (νmax, cm−1, KBr): 3178 (–NH), 3064 (–CH), 1656 (amide C⚌O), 1598 (imine C⚌N), 1301 (–F). 1H NMR (400 MHz, DMSO-d6): δ ppm 8.75–8.76 (d, Pyridine 2H), 8.46 (s, CH), 8.05 (s, NH), 7.83–7.84 (d, Pyridine 2H), 7.77–7.80 (d, Aromatic 2H), 7.08–7.10 (d, Aromatic2H). MS: m/z 245.4 (M+H)+. Anal. Calcd for C13H10FN3O (243.24): C, 64.19; H, 4.14; N, 17.28%. Found: C, 64.83; H, 4.52; N, 18.02%.
2.2.1.7 N′-[(1Z)-(4-Methoxyphenyl)methylidene]pyridine-4-carbohydrazide (3g)
Off white powder, yield 90.98, mp 170–173 °C, IR (νmax, cm−1, KBr): 3153 (–N–H), 3037 (–CH), 2995 (–CH), 1665 (–C⚌O), 1600 (–C⚌N), 1477 (–CH3). 1H NMR (400 MHz, DMSO-d6): δ ppm 8.75–8.85 (d, Pyridine 2H), 8.35 (s, –NH), 7.63–7.70 (d, Pyridine 2H, d, Aromatic 2H), 7.4 (s, ⚌CH), 6.84–6.89 (d, Aromatic 2H), 3.78 (s, –OCH3 3H). MS: m/z 256.5 (M+H)+. Anal. Calcd for C14H13N3O2 (255.27): C, 65.87; H, 5.13; N, 16.46%. Found: C, 65.43; H, 5.64; N, 16.80%.
2.2.1.8 N′-[(1Z)-(4-Hydroxy-3-methoxyphenyl)methylidene]pyridine-4-carbohydrazide (3h)
Yellow powder, yield 94.18, mp 226–228 °C, IR (νmax, cm−1, KBr): 3517 (O–H), 3218 (–N–H), 3064 (–CH), 2985 (Alkane –CH), 1664 (–C⚌O), 1568 (–N–H), 1596 (–C⚌N), 1068 (⚌C–O–C), 810, 881 (1,3,4-tri substituted alkene –CH). 1H NMR (400 MHz, DMSO-d6): δ ppm 9.48 (s, –NH), 9.37 (s, –OH), 8.74–8.75 (d, Pyridine 2H), 8.36 (s, ⚌CH), 7.82–7.83 (d, Pyridine 2H), 7.37–7.38 (d, Aromatic 1H), 7.07–7.09 (d, Aromatic 1H), 6.64–6.68 (d, Aromatic 1H), 3.87 (s, –CH3). MS: m/z 272.4 (M+H)+. Anal. Calcd for C14H13N3O3 (271.27): C, 61.99; H, 4.83; N, 15.49%. Found: C, 62.17; H, 4.92; N, 16.04%.
2.2.1.9 (Z)-N′-((Furan-2-yl)methylene)isonicotinohydrazide (3i)
Brownish powder, yield 92.09, mp 208–212 °C, IR (νmax, cm−1, KBr): 3271 (–NH), 3051 (–CH), 1650 (amide C⚌O), 1620 (imine C⚌N), 1350 (C–O).1H NMR(400 MHz, DMSO-d6): δ ppm 8.91–8.89 (d, Pyridine 2H), 8.32 (s, NH), 8.18–8.13 (d, Pyridine 2H), 7.84 (s, CH), 7.54–7.52 (d, Furan 1H), 6.85–6.55 (m, Furan 2H), 5.97–5.92 (d, Furan 1H). MS: m/z 216.4 (M+H)+. Anal. Calcd for C11H9N3O2 (215.21): C, 61.39; H, 4.22; N, 19.53%. Found: C, 61.27; H, 4.13; N, 19.64%.
2.2.1.10 N′-{(1Z)-[4-(Dimethylamino)phenyl]methylidene}pyridine-4-carbohydrazide (3j)
Yellow powder, yield 97.16, mp 104–106 °C, IR (νmax, cm−1, KBr): 3153 (–N–H), 3045 (–CH), 2961 (–CH), 1664 (–C⚌O), 1604 (–C⚌N). 1H NMR (400 MHz, DMSO-d6): δ ppm 8.73–8.72 (d, Pyridine 2H), 8.35 (s, ⚌CH), 8.07 (s, –NH), 7.82–7.84 (d, Pyridine 2H), 7.62–7.60 (d, Aromatic 2H), 6.69–6.66 (d, Aromatic 2H), 3.07 (s, –CH3 6H). MS: m/z 269.5 (M+H)+. Anal. Calcd for C15H16N4O (268.31): C, 67.15; H, 6.01; N, 20.88%. Found: C, 67.58; H, 6.12; N, 21.13%.
2.2.1.11 N′-[(1Z)-(2,5-Dimethoxyphenyl)methylidene]pyridine-4-carbohydrazide (3k)
Brown crystals, yield 95.2, mp 185–188 °C, IR (νmax, cm−1, KBr): 3190 (–NH), 3061 (–CH), 1654 (amide C⚌O), 1600 (imine C⚌N), 1060 (O–CH3). 1H NMR (400 MHz, DMSO-d6): δ ppm 8.89–8.92 (d, Pyridine 2H), 8.22 (s, CH), 8.48 (s, NH), 8.11–8.15 (d, Pyridine 2H), 7.5–7.8 (d of d, Aromatic 2H), 6.92 (s, Aromatic1H), 3.92–4.12 (m, O–CH3). MS: m/z 286.5 (M+H)+. Anal. Calcd for C15H15N3O3 (285.3): C, 63.15; H, 5.30; N, 14.73%. Found: C, 63.49; H, 5.21; N, 14.84%.
2.2.2 General procedure for synthesis of N-[3-chloro-2-(substituted aryl)-4-oxoazetidin-1-yl] pyridine-4-carboxamide using Schiff’s bases
The 2-azetidinones (5a–k) were synthesised as per our reported methods of stirring and sonication (Thomas et al., 2010). To the appropriate Schiff’s base (3a–k, 0.0025 M) dissolved in dichloromethane, chloroacetyl chloride (0.0037 M), followed by triethyl amine (0.0075 M) was added drop wise with constant stirring at low temperature (0–5 °C). The reaction was carried out in the presence of molecular sieves [MS (1–2 g, 3A × 1.5 mm)]. The reaction mixture was further stirred at room temperature until the completion of reaction. [TLC (toulene/ethanol = 4.5:0.5)]. For the sonication method, the reaction mixture was sonicated on an ultrasonic bath [Local make] until the completion of reaction [TLC (toulene/ethanol = 4.5:0.5)], Scheme 2. The reaction mixture was added into crushed ice and stirred to obtain the crude product. The product obtained was washed with concentrated brine and sodium bicarbonate solution and then dried. The crude product on recrystallisation from alcohol yielded the pure 2-azetidinones of isonicotinoyl hydrazones (5a–k). The synthesised compounds were characterised on the basis of their spectral and analytical data (UV, IR, 1H NMR, MS, CNH).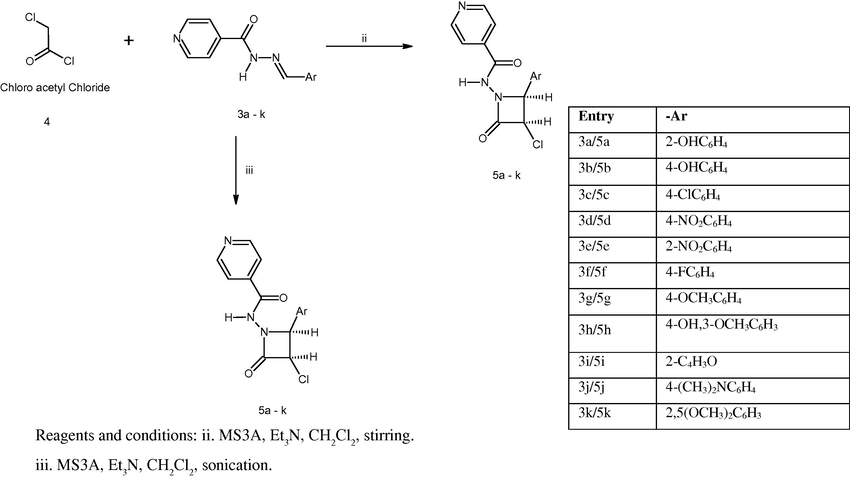
Synthesis of 2-azetidinones (5a–k) using Schiff’s bases.
2.2.2.1 N-[3-Chloro-2-(2-hydroxyphenyl)-4-oxoazetidin-1-yl]pyridine-4-carboxamide (5a)
Yellow powder, yield 91.1, mp 98–101 °C, IR (νmax, cm−1, KBr): 3467 (–OH), 3193 (–NH), 3043 (–CH), 1774 (ring C⚌O), 1677 (amide C⚌O). 1H NMR (400 MHz, DMSO-d6): δ ppm 8.89–8.83 (d, Pyridine 2H), 8.22 (s, OH), 7.77–7.75 (d, Pyridine 2H), 7.36–7.38 (m, Aromatic, 4H), 5.75–5.71 (d, CH Aze ring (C-3) 1H), 4.51–4.42 (d, CH Aze ring (C-4), 4.91 (s, –NH). MS: m/z 318.4 (M+H)+. Anal. Calcd for C15H12ClN3O3 (317.73): C, 56.7; H, 3.81; N, 13.23%. Found: C, 56.96; H, 3.92; N, 13.54%.
2.2.2.2 N-[3-Chloro-2-(4-hydroxyphenyl)-4-oxoazetidin-1-yl] pyridine-4-carboxamide (5b)
Yellow powder, yield 86, mp 102–105 °C, IR (νmax, cm−1, KBr): 3556 (–OH), 3186 (–NH), 3008 (–CH), 1766 (ring –C⚌O), 1685 (amide –C⚌O). 1H NMR (400 MHz, DMSO-d6): δ ppm 8.77–8.79 (d, Pyridine, 2H),8.31 (s, OH 1H), 7.96–7.93 (d, Pyridine, 2H), 7.24–7.23 (d, Aromatic, 2H), 6.86–6.84 (d, Aromatic, 2H), 5.71–5.68 (d, Aze ring H (C-3) 1H), 4.50 (s, NH Secondary amide 1H), 4.19–4.21 (d, CH Aze ring (C-4) 1H)). MS: m/z 318.5 (M+H)+. Anal. Calcd for C15H12ClN3O3 (317.73): C, 56.7; H, 3.81; N, 13.23%. Found: C, 56.38; H, 3.97; N, 13.33%.
2.2.2.3 N-[3-Chloro-2-(4-chlorophenyl)-4-oxoazetidin-1-yl]pyridine-4-carboxamide(5c)
Brown crystals, yield 81.9, mp 92–95 °C, IR (νmax, cm−1, KBr): 3193 (–NH), 3024 (–CH), 1720 (ring C⚌O), 1666 (amide C⚌O), 829 (–Cl). 1H NMR (400 MHz, DMSO-d6): δ ppm 9.38–9.36 (d, Pyridine 2H), 8.30–8.28 (d, Pyridine 2H), 7.70–7.76 (d, Aromatic 2H), 7.35–7.33 (d, Aromatic 2H), 5.81–5.80 (d, CH Aze ring (C-3) 1H), 5.43–5.42 (d, CH Aze ring (C-4) 1H), 4.78 (s, –NH). MS: m/z 337.5 (M+H)+. Anal. Calcd for C15H11Cl2N3O2 (336.17): C, 53.59; H, 3.30; N, 12.50%. Found: C, 53.87; H, 3.24; N, 12.34%.
2.2.2.4 N-[3-Chloro-2-(4-nitrophenyl)-4-oxoazetidin-1-yl]pyridine-4-carboxamide (5d)
Brown crystals, yield 85.8, mp 112–116 °C, IR (νmax, cm−1, KBr): 3035 (–NH), 2974 (–CH), 1735 (ring C⚌O), 1685 (amide C⚌O), 1519, 1338 (–NO2). 1H NMR (400 MHz, DMSO-d6): δ ppm 9.38–9.36 (d, Pyridine 2H), 8.30–8.28 (d, Aromatic 2H), 7.97–7.95 (d, Pyridine 2H), 7.19–7.17 (d, Aromatic 2H), 5.81–5.80 (d, CH Aze ring (C-3) 1H), 5.43–5.42 (d, CH Aze ring (C-4), 1H), 4.91 (s, –NH). MS: m/z 347.4 (M+H)+. Anal. Calcd for C15H11ClN4O4 (346.73): C, 51.96; H, 3.20; N, 16.16%. Found: C, 52.09; H, 3.13; N, 16.23%.
2.2.2.5 N-[3-Chloro-2-(2-nitrophenyl)-4-oxoazetidin-1-yl]pyridine-4-carboxamide (5e)
Pale yellow powder, yield 85.1, mp 193–194 °C, IR (νmax, cm−1, KBr): 3174 (NH), 3016 (CH), 1774 (ring –C⚌O), 1697 (amide –C⚌O), 1666 (NH), 1523, 1353 (NO2), 744 (CH). 1H NMR (400 MHz, DMSO-d6): δ ppm 9.07–9.08 (d, Pyridine, 2H), 8.81 (s, NH Secondary amide 1H), 8.32–8.28 (d, Aromatic 1H), 8.04–8.13 (m, Aromatic 1H), 7.99–8.00 (d, Pyridine 2H), 7.84–7.86 (d, Aromatic 1H), 7.60 – 7.74 (m, Aromatic 1H), 5.79, 5.85 (m, CH Aze ring (C-3) 1H), 5.42, 5.45 (d, CH Aze ring (C-4) 1H). MS: m/z 347.5(M+H)+. Anal. Calcd for C15H11ClN4O4 (346.73): C, 51.96; H, 3.20; N, 16.16%. Found: C, 51.81; H, 3.12; N, 16.24%.
2.2.2.6 N-[3-Chloro-2-(4-fluorophenyl)-4-oxoazetidin-1-yl]pyridine-4-carboxamide (5f)
Yellow powder, yield 95.2, mp 132–135 °C, IR (νmax, cm−1, KBr): 3182 (–NH), 3062 (–CH), 1789 (ring C⚌O), 1662 (amide C⚌O). 1H NMR (400 MHz, DMSO-d6): δ ppm 8.52–8.51 (d, Pyridine 2H), 7.93–7.92 (d, Pyridine 2H), 8.79 (s, –NH), 7.82–7.75 (m, Aromatic 2H), 7.02–7.05 (m, Aromatic 2H), 5.44, 5.43 (d, CH Aze ring (C-3) 1H), 4.99,5.01 (d, CH Aze ring (C-4) 1H). MS: m/z 320.5 (M+H)+. Anal. Calcd for C15H11ClFN3O2 (319.72): C, 56.35; H, 3.47; N, 13.14%. Found: C, 56.18; H, 3.38; N, 13.22%.
2.2.2.7 N-[3-Chloro-2-(4-methoxyphenyl)-4-oxoazetidin-1-yl]pyridine-4-carboxamide (5g)
Pale yellow powder, yield 88.4, mp 103–105, IR (νmax, cm−1, KBr): 3205 (–NH), 3047 (CH), 1770 (ring C⚌O), 1666 (amide C⚌O), 1604 (NH), 1253 (O–CH3), 837 (CH). 1H NMR (400 MHz, DMSO-d6): δ ppm 9.07–9.08 (d, Pyridine 2H), 8.87–8.90 (d, Pyridine 2H), 8.60 (s, NH Secondary amide 1H), 8.19–8.20 (d, Pyridine 2H), 7.70–7.72 (d, Aromatic 2H), 6.93–6.95 (d, Aromatic 2H), 5.74–5.75 (d, CH Aze ring (C-3) 1H), 5.04–5.06 (d, CH Aze ring (C-4) 1H), 3.84 (s, OCH3 3H). MS: m/z 332.4 (M+H)+. Anal. Calcd for C16H14ClN3O3 (331.75): C, 57.93; H, 4.25; N, 12.67%. Found: C, 58.12; H, 4.31; N, 12.55%.
2.2.2.8 N-[3-Chloro-2-(3-methoxy-4-hydroxyphenyl)-4-oxoazetidin-1-yl]pyridine-4-carboxamide (5h)
Brown crystals, yield 89.2, mp 134–135, IR (νmax, cm−1, KBr): 3444 (OH), 3180 (NH), 3010 (CH), 2972 (CH), 1789 (ring C⚌O), 1660 (amide C⚌O), 1577 (NH), 1135 (O–CH3), 813, 844 (CH). 1H NMR (400 MHz, DMSO-d6): δ ppm 8.93–8.95 (d, Pyridine 2H), 8.76 (s, –NH Secondary amide 1H), 8.61 (s, Aromatic –OH 1H), 7.78–7.79 (d, Pyridine 2H), 7.42–6.87 (complex multiplet, Aromatic 3H), 5.74–5.75 (d, CH Aze ring (C-3) 1H), 4.44–4.46 (d, CH Aze ring (C-4) 1H), 4.09 (s, O–CH3 3H). MS: m/z 348.4 (M+H)+. Anal. Calcd for C16H14ClN3O4 (347.75): C, 55.26; H, 4.06; N, 12.08%. Found: C, 55.41; H, 4.17; N, 12.12%.
2.2.2.9 N-[3-Chloro-2-oxo-4-(tetrahydro-2H-pyran-3-yl)azetidin-1-yl]pyridine-4-carboxamide (5i)
Pale brown powder, yield 79.57, mp 174–179 °C, IR (νmax, cm−1, KBr): 3271 (–NH), 3051 (–CH), 1782 (ring C⚌O), 1647 (amide C⚌O). 1H NMR (400 MHz, DMSO-d6): δ ppm 8.48–8.47 (d, Pyridine 2H), 7.58–7.57 (d, Pyridine 2H), 7.99–7.97 (d, Furan ring 1H), 7.86–7.85 (m, Furan ring 1H), 6.53–6.52 (d, Furan ring 1H), 8.79 (s, –NH), 7.82–7.75 (m, Aromatic 2H), 7.02–7.05 (m, Aromatic 2H), 4.55–4.47 (d, CH Aze ring (C-3) 1H), 4.17–4.15 (d, CH Aze ring (C-4) 1H). MS: m/z 292.4 (M+H)+. Anal. Calcd for C13H10ClN3O3 (291.69): C, 53.35; H, 3.46; N, 14.41%. Found: C, 53.58; H, 3.23; N, 14.54%.
2.2.2.10 N-[3-Chloro-2-(4-dimethylaminophenyl)-4-oxoazetidin-1-yl]pyridine-4-carboxamide (5j)
Brown crystals, yield 87.32, mp 127–128 °C, IR (νmax, cm−1, KBr): 3190 (–NH), 3039 (–CH), 2891 (–CH), 1712 (ring C⚌O), 1647 (amide C⚌O). 1H NMR (400 MHz, DMSO-d6): δ ppm 8.79–8.78 (d, Pyridine 2H), 7.97–7.87 (d, Pyridine 2H), 8.44 (s, –NH), 7.68–7.67 (d, Aromatic 2H), 7.35–7.33 (d, Aromatic, 2H), 1H), 5.81–5.72 (d, CH Aze ring (C-3), 1H), 4.60–4.51 (d, CH Aze ring (C-4), 3.09 (s, –CH3 6H). MS: m/z 345.5 (M+H)+. Anal. Calcd for C17H17ClN4O2 (344.8): C, 59.22; H, 4.97; N, 16.25%. Found: C, 59.57; H, 5.01; N, 16.32%.
2.2.2.11 N-[3-Chloro-2-(2,5-dimethoxyphenyl)-4-oxoazetidin-1-yl]pyridine-4-carboxamide (5k)
Pale brown crystals, yield 89.4, mp 140–148 °C; IR (νmax, cm−1, KBr): 3195 (–NH), 2974 (–CH), 1735 (ring C⚌O), 1681 (amide C⚌O), 1073 (O–CH3). 1H NMR (400 MHz, DMSO-d6): δ ppm 9.38–9.36 (d, Pyridine 2H), 7.97–7.84 (d, Pyridine 2H), 4.42 (s, –NH), 6.83–6.86 (d, Aromatic 1H), 6.19 (s, Aromatic 1H), 6.21 (d, Aromatic, 1H), 5.42–5.43 (d, CH Aze ring (C-3), 1H), 4.78–4.90 (d, CH Aze ring (C-4), 1H), 3.77–3.60 (m, O–CH3 6H). MS: m/z 362.5 (M+H)+. Anal. Calcd for C17H16ClN3O4 (361.78): C, 56.44; H, 4.46; N, 11.61%. Found: C, 56.67; H, 4.33; N, 11.42%.
3 Biological activity
3.1 Experimental animals
Swiss albino mice of either sex (20–30 g) were obtained from the National Toxicological Centre (NTC), Pune; Serum Institute, Pune, India and were used for the biological studies. They were housed under standard laboratory conditions, maintained on a natural dark and light cycle and had free access to food and water. Animals were acclimatised to laboratory conditions before the experimentation. All experiments were carried out between 0900 and 1500 h. The screening protocol was approved by the Institutional Ethics Committee and conducted according to the Indian National Science Academy Guidelines for the use and care of experimental animals (RP. No. 048).
3.2 Acute toxicity study
The acute toxicity study for the test drugs was carried out in mice according to OECD guidelines (OECD-423, 2004). Albino mice of either sex weighing between 20 and 25 g were divided into six groups of six animals each. Animals were starved for 24 h with water ad libitum prior to test. On the day of the experiment, test drugs were administered at graded doses up to 2000 mg/kg, p.o. and animals were monitored individually and continuously for 30 min, 2 h and up to 24 h to detect any changes in the autonomic or behavioural responses and also for tremors, convulsions, excessive salivation, diarrhoea, lethargy, sleep and coma. The animals were then monitored for any mortality for the following 14 days. As there was no toxic reaction or mortality observed, the test drugs were found to be devoid of any toxicity up to a maximum dose of 2000 mg/kg body weight.
3.3 Evaluation of nootropic activity
3.3.1 Elevated plus-maze test in mice
The effect of the test drugs on learning and memory was investigated using transfer latency (TL) as the parameter for the acquisition and retention of memory process in the elevated plus-maze test in mice. An elevated plus maze consisting of two open arms (16 × 5 cm) and two enclosed arms (16 × 5 × 12 cm) elevated 25 cm from the floor was used in the present study. Albino mice of either sex weighing between 20 and 25 g were divided into 24 groups of six animals each. Animals were starved for 24 h with water ad libitum prior to test. The animals were placed at the corner of an open arm facing away from the centre of maze and the time taken by each animal to enter the closed arm was noted as transfer latency (TL), on the 7th and 8th day after administering the test drugs for continuous 7 days. The control group received 1 ml of 0.5% carboxy methyl cellulose (CMC) solution. The test compounds 3a–k, 5a–k at two graded doses of 50 and 100 mg/kg were administered orally in the form of suspension in 0.5% CMC solution. Piracetam (400 mg/kg) was used as the standard drug and scopolamine (0.4 mg/kg) was employed as the antagonist in the study.
3.3.2 Passive avoidance test in mice
The nootropic activity was also evaluated using the Passive Avoidance test in mice. Albino mice of either sex weighing between 20 and 25 g were divided into 24 groups of six animals each. Animals were starved for 24 h with water ad libitum prior to the experiment. A rectangular box (50 × 50 cm) with electrifiable grid floor and 35 cm fits over the block was employed for the test. The grid floor was connected to a shock device which delivered scrambled foot shocks (50 Hz; 1.5 mA; 1 s). The step down latency times (SDL) were measured in the training test and after 24 h in the retention test. The test is finished when the mice steps down or remains on the platform (cut-off time: 120 s) (Joshi and Parle, 2006).
3.4 Evaluation of antidepressant activity
3.4.1 Forced swim test in mice
The antidepressant activity of test compounds was investigated by the forced swim in mice. In the forced swim test, the mice were individually forced to swim inside a vertical plexiglass cylinder (height: 40 cm; diameter: 18 cm) containing 15 cm of water maintained at 25 °C. The mice placed in the cylinders for the first time were initially highly active, however after 2–3 min the activity subsided and the phases of immobility increased. After 5–6 min, the duration of immobility reached a plateau where the mice remained immobile for about 80% of the time. After 15 min in the water, the rats were removed, allowed to dry in a heated enclosure (32 °C) and then returned to their home cages. The total duration of immobility was measured during a 5 min test after the animals were placed in the cylinder 24 h later.
3.4.2 Tail suspension test in mice
The antidepressant activity was also assessed by the Tail suspension test in mice. Albino mice of either sex weighing between 20 and 25 g were suspended on the edge of a shelf 58 cm above a table top by an adhesive tape placed approximately 1 cm from the tip of the tail. The duration of immobility of each animal was recorded for a period of 5 min. The test compounds 3a–k, 5a–k (50/100 mg/kg) were administered orally in the form of a suspension in 0.5% CMC solution and the standard Fluoxetine (10 mg/kg) intraperitoneally 1 h before the performance of both tests (Zhang et al., 2001).
3.5 Statistical analysis
All values in the tests were expressed as means ± SEM for n observations, where n represents number of animals studied (n = 6). The data were analysed by ANOVA followed by Dunnett’s test. In all the tests the criterion for statistical significance was p < 0.05 when compared with the control group.
4 Results and discussion
4.1 Chemistry
The N′-[(1Z)-(substituted aromatic) methylidene] pyridine-4-carbohydrazide (3a–k) were prepared in excellent yields in a one-step reaction (Scheme 1) of isonocotinyl hydrazone (1) with various substituted aryl aldehydes (2a–k) in water using our previously reported microwave irradiation method. Isoniazid (INH, 0.01 M) and appropriate aromatic aldehydes (0.01 M, 2a–k) in water on irradiation under microwave at power level 3 (240 W, 35% irradiation) gave N′-[(1Z)-(substituted aromatic)methylidene] pyridine-4-carbohydrazide.
The conventional reaction for the synthesis of Schiff’s bases of isonicotinic acid hydrazide involves the use of glacial acetic acid as a catalyst with longer reaction times (360–420 min reflux) for the completion of the reaction. In contrast, the microwave method required shorter reaction times (7–9 min for completion of reaction) with improved yields (90.7–98.76%). The synthesised Schiff’s base intermediates (3a–k) were characterised by the presence of strong band at 1552–1620 cm−1 for the N⚌C imino group. The 1H NMR spectra also showed a singlet signal equivalent to 1 proton for ⚌CH group between 7.4 and 9.04 ppm, confirming the formation of Schiff’s bases.
The synthesised intermediates were further utilised for the synthesis of N-[3-chloro-2-(substituted aromatic)-4-oxoazetidin-1-yl]pyridine-4-carboxamides (5a–k) containing 2-azetidinone nucleus by our reported methods of stirring and sonication (Scheme 2). The appropriate Schiff’s base (3a–k, 0.0025 M) in dichloromethane on reaction with chloroacetyl chloride (4, 0.0037 M), followed by the addition of triethyl amine (0.0075 M) in the presence of molecular sieves [MS (1–2 g, 3A × 1.5 mm)] gave the 2-azetidinone derivatives in high yields (79.5–95.2%). The IR spectra of the synthesised derivatives were characterised by the presence of a strong band at 1712–1789 cm−1 for the ring carbonyl group, which is considered a strong confirmation for the azetidinone nucleus formation. Another piece of evidence for cyclization is the appearance of a doublet signal equivalent to 1 proton in 1H NMR spectrum between 4.47 and 5.85 ppm (C-3, CH) and a doublet signal equivalent to 1 proton between 4.17 and 5.45 ppm (C-4, CH), which represent the formation of azetidinone nucleus. The structures of the synthesised compounds were further confirmed by mass spectra and elemental analysis.
4.2 Biological activity
All the newly synthesised N′-[(1Z)-(substituted aromatic)methylidene] pyridine-4-carbohydrazide (3a–k) and their N-[3-chloro-2-(substituted aromatic)-4-oxoazetidin-1-yl]pyridine-4-carboxamide derivatives (5a–k) were tested in animal models for their nootropic and antidepressant activities.
The nootropic activity was evaluated by the elevated plus maze and passive avoidance tests in mice at two graded dose levels of 50 mg/kg and 100 mg/kg. All the tested compounds exhibited a significant decrease (*p < 0.05) in the transfer latency (TL) on the eighth day indicating an improvement in the cognitive function. All the tested drugs showed an increase in the activity at the dose level of 100 mg/kg suggesting that the activity is dose dependent. Of the entire newly synthesised N′-[(1Z)-(substituted aromatic) methylidene]pyridine-4-carbohydrazides, the compound 3d with an electron withdrawing nitro substitution at the para position of the aryl ring exhibited the highest activity (TL period: 23.3 s). Compounds 3c, 3e, 3h, and 3k with 4-Cl; 2-NO2; 4-OH, 3-OCH3; and 2,5-dimethoxy substitution on the aryl ring also exhibited significant activity comparable to 3d (TL period: 25.2, 25.1, 24.6, 25.2 s, respectively). However all the tested compounds showed lower activity as compared to the standard drug Piracetam (TL period: 14.0 s at 400 mg/kg). Among the N-[3-chloro-2-(substituted aromatic)-4-oxoazetidin-1-yl]pyridine-4-carboxamide derivatives synthesised, compound 4d with the similar p-nitrophenyl ring substituted at C-4 position of the azetidinone ring exhibited the highest activity (TL period: 22.4 s). Furthermore compounds 4e and 4h also showed activity comparable to 4d. Although, the 2-azetidinones synthesised in the present study did not exhibit substantial activity as compared to piracetam, there has been an enhancement in the nootropic activity (Figs. 1 and 2).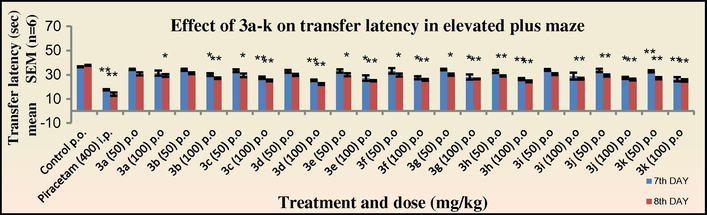
Effect of 3a–k on transfer latency in elevated plus maze in mice. The transfer latency was compared to standard Piracetam.
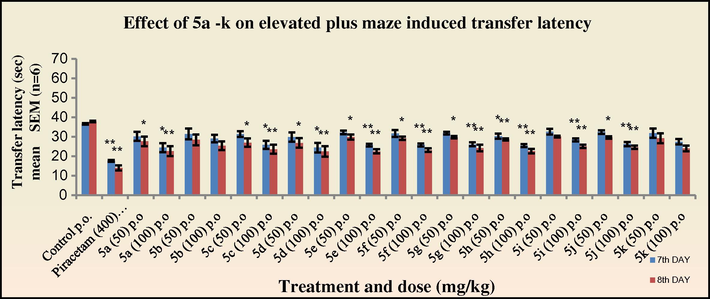
Effect of 5a–k on transfer latency in elevated plus maze in mice. One way ANOVA followed by Dunnett test was employed, *p < 0.05, **p < 0.01 when compared with vehicle treated control group.
In the scopolamine treated group (Figs. 3 and 4), an increase in the transfer latency period was observed. However, all the tested compounds were able to competitively inhibit the antagonistic action of scopolamine. It was observed that the synthesised 2-azetidinones were able to more effectively show a reversal of the scopolamine-induced memory deficit as compared to the intermediate Schiff’s bases.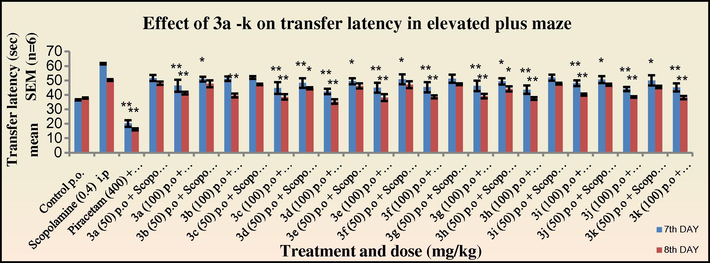
Effect of 3a–k on transfer latency in elevated plus maze in mice in scopolamine treated group.
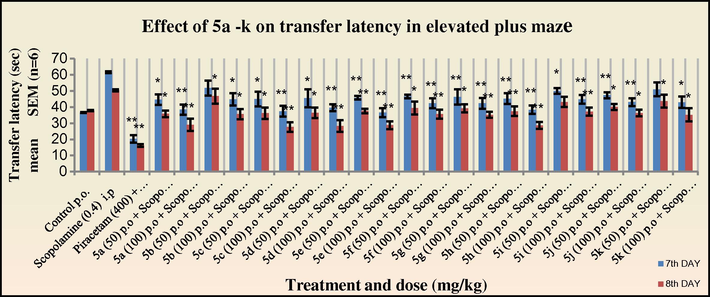
Effect of 5a–k on transfer latency in elevated plus maze in mice (Scopolamine treated group). One way ANOVA followed by Dunnett test was employed; *p < 0.05, **p < 0.01 when compared with vehicle treated control group.
In the passive avoidance test for the evaluation of nootropic activity, all the tested compounds exhibited a significant increase (*p < 0.05) in the step down latency period on the eighth day indicating improvement in the cognitive function. The compounds 3c, 3d, 3e, 3h and 4c, 4d, 4e and 4h exhibited significant activity in concordance to the elevated plus maze test (Figs. 5–8). The results suggest an indirect evidence of the participation of cholinergic and NMDA-receptor blockades in the mechanism of these drugs.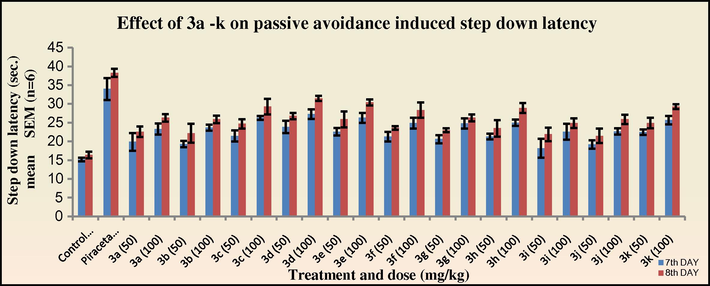
Effect of 3a–k on passive avoidance induced step down latency in mice. The step down latency period was compared with standard Piracetam.
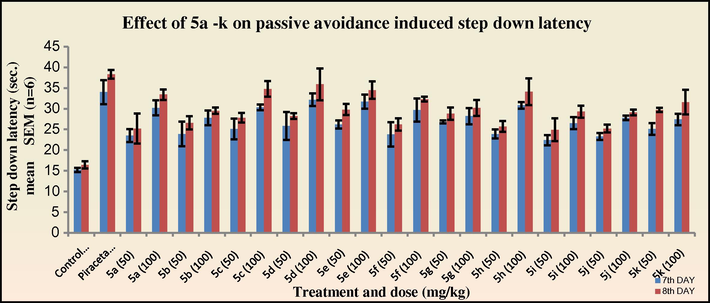
Effects of 5a–k on passive avoidance induced step down latency in mice. One way ANOVA followed by Dunnett test was carried out; *p < 0.05, **p < 0.01 when compared with vehicle treated control group.

Effect of 3a–k on passive avoidance induced step down latency in mice in scopolamine treated group. Results were compared with standard Piracetam and antagonist Scopolamine.
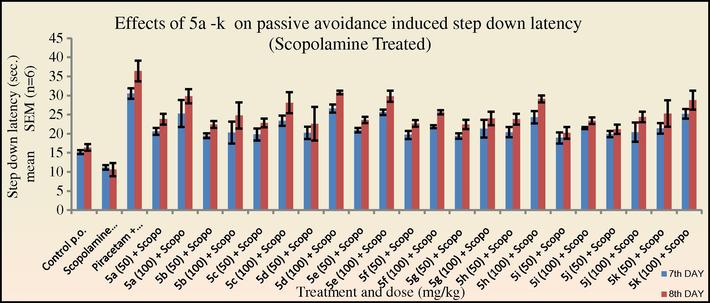
Effects of 5a–k on passive avoidance induced step down latency in mice in the Scopolamine treated group. One way ANOVA followed by Dunnett test was carried out; *p < 0.05, **p < 0.01 when compared with vehicle treated control group.
The antidepressant activity data in both the tail suspension (Figs. 9 and 10) and forced swim test (Figs. 11 and 12) in mice indicated that all the synthesised Schiff’s base (3a–k) and 2-azetidinone analogues (5a–k) exhibited significant activity (*p < 0.05). Anti-depressants activity was observed in a dose dependant manner, showing increased activity at 100 mg/kg dose level. Compounds 3k and 4k with 2,5-dimethoxy substitution on the aryl ring exhibited the highest antidepressant activity (duration of immobility:77.1, 65.6 s, respectively) as compared to the nitro substituted derivative in the cognitive models and similar to the standard Fluoxetine (79.6 s). Compounds 3c, 3d, 3e, 3h; 4c, 4d, 4e, 4h with 4-Cl, 4-NO2; 2-NO2; 4-OH, 3-OCH3 substitutions on the aryl ring also exhibited significant activity (duration of immobility: 88.5, 84.1, 78.0, 83.0 s; 73.6, 71.8, 67.1, 68.5 s, respectively) comparable to 3k and 4k. Surprisingly, the 2-azetidinone analogues exhibited a substantial enhancement in activity when compared to the synthesised intermediates. The behavioural response of individual rats to forced swimming can be correlated with changes in extracellular 5-HT in the lateral septum.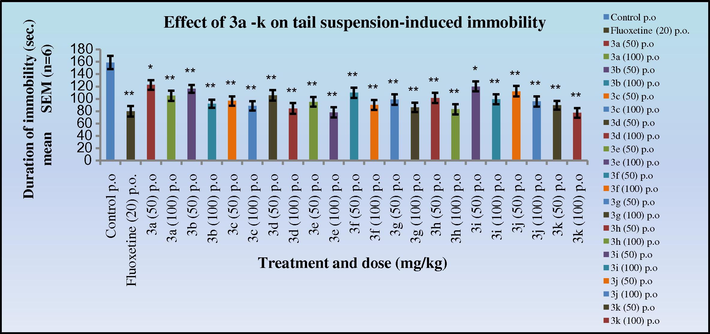
Effect of 3a–k on tail suspension-induced immobility in mice. Duration of immobility was compared to standard fluoxetine .
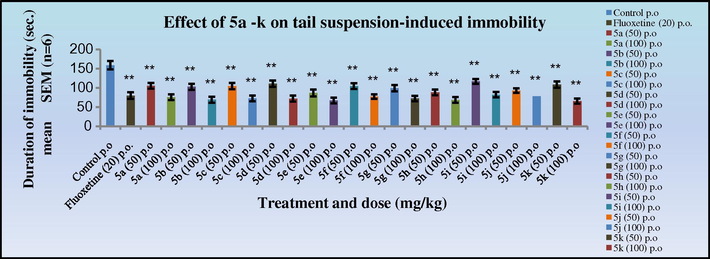
Effect of 5a–k on tail suspension induced immobility in mice. The duration of immobility is compared with standard Fluoxetine. One way ANOVA followed by Dunnett test was carried out; *p < 0.05, **p < 0.01 when compared with vehicle treated control group.
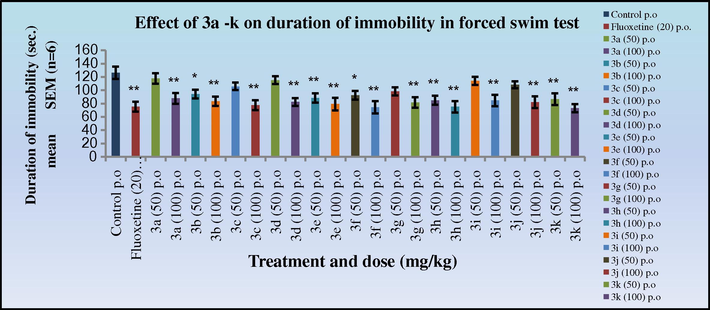
Effect of 3a–k on duration of immobility in forced swim test in mice. Duration of immobility was compared to standard Fluoxetine. 0.5% CMC acts as the vehicle control.
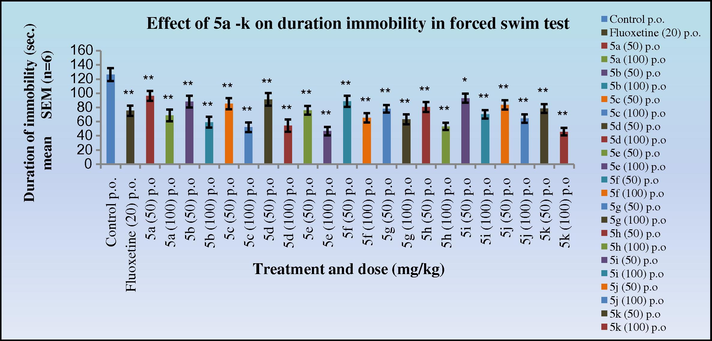
Effect of 5a–k on duration of immobility in forced swim test in mice. Duration of immobility was compared to standard Fluoxetine. One way ANOVA followed by Dunnett test was carried out; *p < 0.05, **p < 0.01 when compared with vehicle treated control group.
The results of the cognitive models for evaluation of the nootropic activity and the antidepressant testing indicate that the nature of the substituent, i.e., their electronic parameters and their position on the 2-azetidinone nucleus might play a significant role in influencing the in vivo activity.
5 Conclusions
In the present study, the synthesis and CNS activity of 2-azetidinone derivatives have been described. The synthesis of the N′-[(1Z)-(substituted aromatic) methylidene]pyridine-4-carbohydrazides and N-[3-chloro-2-(substituted aromatic)-4-oxoazetidin-1-yl]pyridine-4-carboxamides were carried out by us using a new green route method of microwave irradiation and stirring/sonication resulting in improved yield with shorter reaction times. It was observed that the tested compounds containing electron withdrawing (nitro, halogen and dimethoxy) moiety on phenyl ring of Schiff’s bases and 2-azetidinones were found to have significant invivo nootropic and antidepressant activities in the animal models. These results confirm the fact that the 2-azetidinone skeleton has potential as a CNS active agent. In conclusion, it can be stated that the 2-azetidinones deserve further investigation to gain insight into the mechanistic action of the most active compounds to help in the development of more potent and safe CNS active agents for therapeutic use.
Acknowledgements
This work was financially supported by the University of Pune, Pune (India).The authors thank Padm. Dr. D. Y. Patil Institute of Pharmaceutical Science and Research, Pune (India) for providing the necessary infrastructural facilities to carry out this work. The spectral analysis was carried out by SAIF/CIL, Punjab University, Chandigarh (India).
References
- J. Org. Chem.. 2000;65:4453.
- J. Org. Chem.. 2001;66:1612.
- Tetrahedron Lett.. 2000;41:6551.
- J. Med. Chem.. 2003;46:12.
- Bioorg. Med. Chem.. 2005;13:3611.
- J. Org. Chem.. 2000;65:3716.
- Bioorg. Med. Chem. Lett.. 2004;14:2227-2231.
- Tetrahedron Lett.. 1995;36:213.
- Tetrahedron Lett.. 1996;37:6989.
- Molecules. 2007;12:2467.
- Chem. Abstr.. 2003;138:375.
- Bioorg. Med. Chem.. 2006;14:8271.
- Tetrahedron. 2001;57:1883.
- J. Pharm. Pharm. Sci.. 2005;8(2):182.
- J. Pharm. Pharm. Sci.. 2005;8(2):182.
- Synth. Let. 2002:131.
- Bioorg. Med. Chem.. 2004;15:2054.
- Bioorg. Med. Chem. Lett.. 1991;1(10):545.
- J. Trad. CAM.. 2006;3(1):64.
- J. Indian Chem. Soc.. 1996;73:173.
- Eur. J. Med. Chem.. 2009;44:83.
- Il Farmaco. 2001;56:45.
- Tetrahedron Lett.. 2000;41:10347.
- Nat. Cell Biol.. 2001;10:905.
- Tetrahedron Lett.. 1999;4:6995.
- Il Farmaco. 2005;60:727.
- Boll. Chim. Farm.. 1994;133:76.
- Arkivoc. 2007;9:80-90.
- Indian J. Chem. (B). 1999;8:183.
- Indian J. Chem. (B). 2002;41:2357.
- Indian J. Chem. (B). 1987;26:652.
- Anal. Chem.. 1907;51:365.
- Green Chem. Lett. Rev.. 2009;2:23-27.
- Green Chem. Lett. Rev.. 2010;3:293-300.
- Org. Chem.. 1999;64:3714.
- Bioorg. Med. Chem. Lett.. 2007;17:101.
- Chin. Med. J.. 2001;20(20):1792-1796.







