Translate this page into:
Synthesis and characterization of CoFe2O4@SiO2-polyethyleneimine magnetic nanoparticle and its application for ultrasonic-assisted removal of disulfine blue dye from aqueous solution
⁎Corresponding author. Hossein.ali_Rasekh@yahoo.com (Hossein Ali Rasekh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present work, a simple synthesis approach was applied for the fabrication of CoFe2O4@SiO2-polyethyleneimine magnetic nanoparticles as an effective sorbent for ultrasonic-assisted removal of disulfine blue dye from an aqueous solution. For identification and characterization of prepared sorbent, different analysis including Fourier transform infrared spectroscopy (FT-IR), Field emission scanning electron microscopy (FE-SEM), Vibrating sample magnetometer (VSM), Energy dispersive X-ray analysis (EDX) and Transmission electron microscopy (TEM) were applied. The effect of effective parameters on the removal of disulfine blue such as pH, sorbent mass, ultrasonic time and disulfine blue concentration were also assessed. The optimum values for investigated parameters were achieved to be as follows: pH of 5.0, sorbent mass of 0.015 g, ultrasonic time of 5.0 min and disulfine blue concentration of 10.0 mg L−1. Different isotherm and kinetic models were used for the evaluation of isotherm and kinetic of adsorption. Results showed that the Langmuir isotherm model was better than other isotherm models as well as the second-order equation model was selected as a kinetic model. The maximum adsorption capacity of the proposed magnetic sorbent was achieved to be 110.0 mg g−1 which shows the applicability of proposed sorbent for removal of disulfine blue dye from aqueous solution.
Keywords
CoFe2O4@SiO2-polyethyleneimine
Magnetic nanoparticles
Removal
Disulfine blue
1 Introduction
Water pollution is one of the most important challenges that the world faces. There are plenty of resources for pollution of water. Industrial and synthetic dyes are one of the main branches of these resources that not only can change the chemical and physical property of water but also can have adverse side effects (Homem et al., 2019; Tanhaei et al., 2019). These dyes can cause chemical changes, consume dissolved oxygen and also generate the carcinogenic and genotoxic problem. So, they are harmful to both human health and the environment. Disulfine blue (DB) (Fig. 1.) is a synthetic dye that has been widely applied in different industries including coloring paper, temporary hair colorant, dyeing cottons, wools and so on (Bagheri et al., 2016b). In spite of its applications and advantages, it can cause some illnesses for humans. So, removing it from water resources is still a vital task. Different methods such as adsorption, membrane separation, flocculation–coagulation and aerobic or anaerobic treatment have been extensively used for removal of dyes from water (Bagheri et al., 2017b; Wang et al., 2020). Compare to other methods, the adsorption technique has been very much considered since it is a simple, easy, low cost and more applicable without the generation of hazardous intermediate products (Bagheri et al., 2017a; Li et al., 2020). Different sorbents have been applied for removal of dyes from wastewater which have main drawbacks in terms of low specific surface area as well as low binding capacity (Oussalah et al., 2019). In this regard, much attention has recently been paid to the use of nano-sized material due to their high surface area, presentation of highly porous structure and high adsorption capacity which can improve the dye adsorption efficiency (Bagheri and Ghaedi, 2020a, 2020b, 2020c; Bidgoli et al., 2019; Uogintė et al., 2019). One of the tedious and non-interesting stages in the adsorption process is the face separation difficulty and arrival of nano-scale based material to different ecosystem following usage. This difficulty and domination simply and efficiently can be overcome by using magnetic nano-scale material that can be separated by exposure to external magnetic fields (Arabi et al., 2020; Bagheri et al., 2019a; Bagheri et al., 2019b; Bagheri and Ghaedi, 2019; Khani et al., 2019). Iron-based magnetic nanostructures have distinguished properties which make them as a good candidate for using in different fields such as separation and removal of dyes from water resources (Yang et al., 2020a; Zangeneh et al., 2019). Nano-sized magnetic compounds have supreme physical and chemical properties due to their mesoscopic effect, small object effect, quantum size effect and surface effect (Arabi et al., 2016; Gholami et al., 2019a; Gholami et al., 2019b; Gholami et al., 2019c; Ostovan et al., 2018a; Ostovan et al., 2018b; Yang et al., 2020c). Recently, metal-oxide nanoparticles have been the subject of much interest because of their unusual optical, electronic and magnetic properties, which often differ from the bulk. Cobalt ferrite is an efficient magnetic compound that has distinguished properties in terms of mechanical hardness, high coercively, high cubic magneto crystalline anisotropy, high physical and chemical stability (Arabi et al., 2017; Guo et al., 2020; Maaz et al., 2007), and appropriate magnetization for separation using an external magnetic field (Ansari et al., 2019; Pastor-Belda et al., 2017). Cobalt (II) ions are more difficult to oxidize than the iron (II) and that consequently, cobalt ferrite nanoparticles are more stable over long times than Fe3O4 particles. Modification of magnetic nanoparticles is essential that is due to enhances their stability in different media as well as prevents the oxidation of them which can improve their performance. For this purpose, SiO2 is a good choice that is due to exceptional properties of SiO2 in terms of high chemical and physical properties and bio-compatibility that can modify magnetic nanoparticles surface completely and increase the physical and chemical stability of magnetic nanoparticles (Yang et al., 2019; Zhang et al., 2019). In addition, to increase the surface area of adsorbent, polyethyleneimine (PEI) is a good choice. PEI is a positive charge polyelectrolyte supplying a large number of nitrogen atoms corresponding to amino groups which contain primary, secondary, and tertiary amino groups in a ratio of approximately 1:2:1 (An and Gao, 2007; Yang et al., 2020b; Zhong and Jia, 2019; Zhou et al., 2016). Their combination with a number of polar groups (amino groups) in PEI affects their adsorption capability towards organic compounds. It will be a good method for modification of cobalt ferrite (CoFe2O4) nanoparticles with PEI and their association can be applied as an effective sorbent for separation and removal of DB from an aqueous solution. In this work, CoFe2O4@SiO2-polyethyleneimine was synthesized as an effective sorbent for the removal of DB from an aqueous solution. It was characterized using different analysis such as FTIR, XRD, FE-SEM, VSM, EDX, and TEM. Also, the effect of influential parameters on DB removal including pH, sorbent mass, ultrasonic time and DB concentration was investigated and optimum levels were achieved. The results showed that prepared sorbent has good magnetic strong and adsorption capacity which make it as a good sorbent for removal of DB from an aqueous solution.
Chemical structure of disulfine blue.
2 Experimental section
2.1 Chemicals, reagents and instrumental
All materials and reagents including DB dye, deionized water, ethanol, NaOH, HCl, NH3 solution, tetraethylorthosilicate, methylbenzene, polyethyleneimine and (3-chloropropyl) trimethoxysilane were purchased from Merck Company. A pH-meter (Model 692, Metrohm AG, Herisau, Switzerland) was used for the pH measurements. Ultrasonic-assisted adsorption of DB was done in an ultrasonic bath (Tecno-GAZ SPA Ultra Sonic System, Parma, Italy) operated at a frequency of 40 kHz and 130 W of power with a useable volume of 3.0 L. The morphology analysis of obtained CoFe2O4@SiO2-polyethyleneimine was carried out by scanning electron microscope (SEM, Hitachi S- 4160, Japan) under an acceleration voltage of 26 kV and transmission electron microscope (TEM, Hitachi H-800 at 200 kV, Japan), X-ray diffraction (XRD, Philips PW 1800 Holland, Netherland, 40 kV and 40 mA) and Fourier transformed infrared spectroscopy (FTIR, RX-IFTIR, Perkin Elmer). The concentration of the DB in the solution after equilibrium adsorption was measured by a double beam UV–Vis spectrophotometer (Shimadzu, Model UV 1601, Japan) at 637 nm.
2.2 Synthesis of CoFe2O4 nanoparticles
An aqueous solution of 0.2 M CoCl2·6H2O and 0.4 M FeCl3·6H2O were mixed under the ultrasonic irradiation for 30 min. Then, 0.2 M oleic acid solution was added to the mixture and subsequently, the 3 M solution of NaOH was added to adjust pH 8. Then, the mixture was heated under 80 °C for 1 h. In the following, the solution was cooled at room temperature, and the obtained precipitation was separated using an external magnetic as well as washed several times by deionized water and ethanol and dried at 60 °C.
2.3 Preparation of CoFe2O4@SiO2
Coating of CoFe2O4 nanoparticles with a layer of silica was attained through the Stöber method which is based on the hydrolysis of tetraethylorthosilicate (TEOS) in the presence of NH3 to obtain CoFe2O4@SiO2 composite (Stöber et al., 1968). In this regard, 0.5 g of CoFe2O4@SiO2 was reacted with 0.5 mL of (3-chloropropyl) trimethoxysilane in the presence of 100 mL methylbenzene. Then, the mixture was stirred at 110 °C for 12 h to obtain CoFe2O4@SiO2-Cl. Then, the obtained CoFe2O4@SiO2-Cl was reacted with 25 mL of an aqueous solution of polyethyleneimine at 90 °C for 6 h. Finally, CoFe2O4@PEI particle was washed with deionized water and ethanol several times and finally dried at 60 °C for 12 h.
2.4 Ultrasound-assisted adsorption method
Ultrasound-assisted experiments were conducted to investigate the effect of different factors on the removal of DB. The batch sorption experiments were carried out in 100 mL Erlenmeyer flasks, where 0.005–0.03 g of the CoFe2O4@PEI and 50 mL of the DB solutions (10–35 mg L−1) were added following pH (2–8) adjustment (using diluted acid or base) and irradiated by ultrasonic irradiation (2–7 min). Then, the CoFe2O4@PEI was separated by an external magnetic field and the concentration of the DB in the solution after equilibrium adsorption was measured at 620 nm. The removal percentage of DB at equilibrium was calculated by:
3 Results and discussion
3.1 Characterization of Co-Fe2O4@PEI
Fig. 2 represents the FTIR spectra of CoFe2O4, CoFe2O4@SiO2, Co-Fe2O4@SiO2-Cl, and CoFe2O4@SiO2@PEI, respectively. The characteristic peak at 585 cm−1 is related to Fe–O stretching which is observed in four stages even after polymer coating samples and chemical modification. However, the intensity of the Fe–O peak reduced comparatively with further modification and approximate formation of shells that show the successful modification of CoFe2O4 nanoparticles. The characteristic peaks at 681 cm−1, 1267 cm−1 and 1424 cm−1 are attributed to N–H, C–N and C–H bonds respectively, while the characteristic peaks at 2977, 2925, and 2856 cm−1 are related to C–H vibrations peaks of C–H in CH2 groups. These characteristic absorptions denote that polyethyleneimine has been successfully grafted onto the silica surface. Fig. 3 shows the EDX analysis of CoFe2O4@PEI which confirms the presence of the Fe, O, Co, C, N, and Si with an approximate mass ratio of 35.6, 22.1, 19.8, 16.1, 2.5, and 3.8% respectively. In should be pointed out that the presence of N and the absence of Cl proves that PEI was formed and 3-chloropropyl removed on the surface of CoFe2O4@SiO2. Fig. 4 represents the XRD pattern of CoFe2O4@PEI in which diffraction peaks located at 2θ = 30.09, 35.44, 37.06, 43.06, 47.15, 53.45, 56.98, 62.59, 65.76, 70.95, 74.01, 75.01, and 78.97 could be well attributed to the (2 2 0), (3 1 1), (2 2 2), (4 0 0), (4 2 2), (5 1 1), (4 4 0), (5 3 1), (6 2 0), (5 3 3), (6 2 2), and (4 4 4). These are related to a cubic unit cell and approve the cubic spinel structure (JCPDS No. 00-022-1086). The expanded XRD lines indicate that the particles are in nano-size range and, according to Scherer Eq., are based on a distinguished peak of (3 1 1) plane: the calculated values of the size corresponding to CoFe2O4-NPs were 18.0 nm. Fig. 5 indicates the FESEM image of Co-Fe2O4@PEI which shows that the spherical shapes of prepared sorbent with a low degree of agglomeration that is corresponds to the magnetic properties of CoFe2O4@PEI. The structure of CoFe2O4@PEI was evaluated by TEM analysis (Fig. 6). TEM image shows that the SiO2@PEI layer is anchored on the surface of CoFe2O4 nanoparticle successfully. The thickness of the SiO2@PEI layer leads to a fast mass transfer of DB, which decreases removal time as well as enhances in the performance and applicability of the proposed sorbent. To investigate the magnetic properties of CoFe2O4, VSM was applied. As can be seen in Fig. 7, saturation magnetization is nearly constant at 60 emu g−1 that shows the strong magnetization property of CoFe2O4 nanoparticles.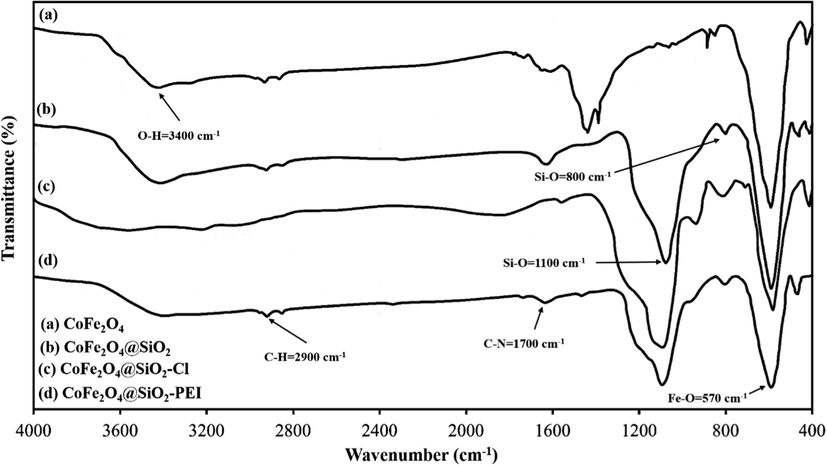
FTIR spectra of CoFe2O4, CoFe2O4@SiO2, CoFe2O4@SiO2-Cl and CoFe2O4@SiO2@PEI.
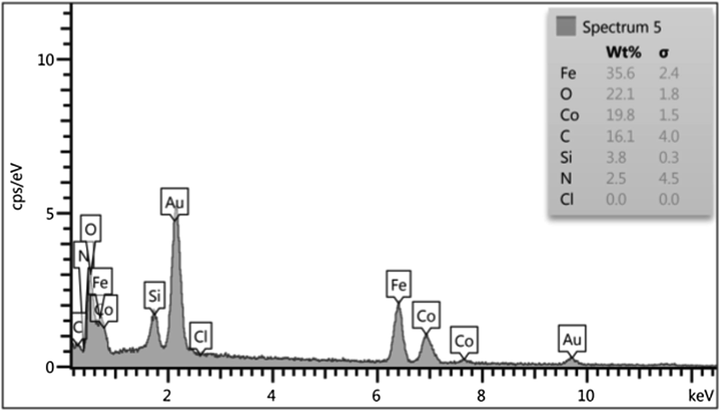
EDX analysis of CoFe2O4@ PEI.
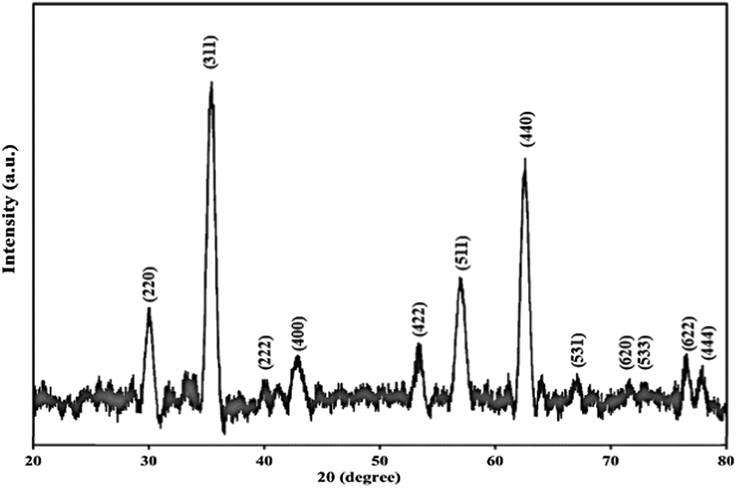
XRD pattern of CoFe2O4@ PEI.
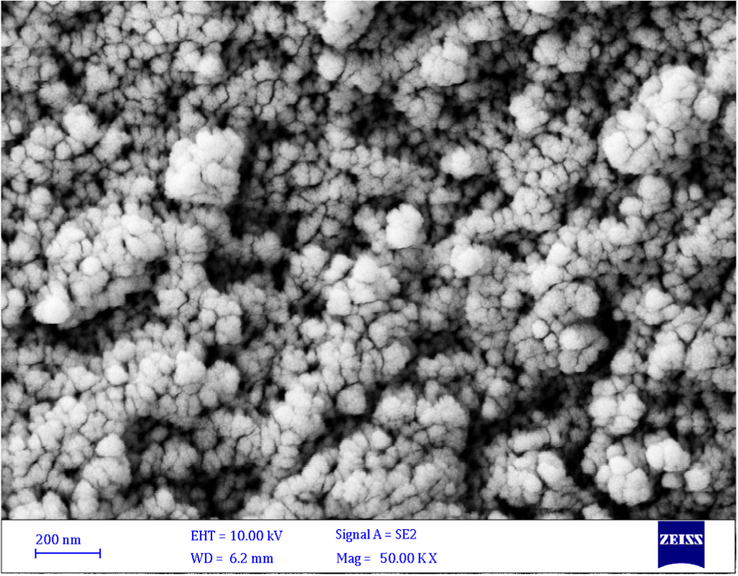
FESEM image of CoFe2O4@ PEI.
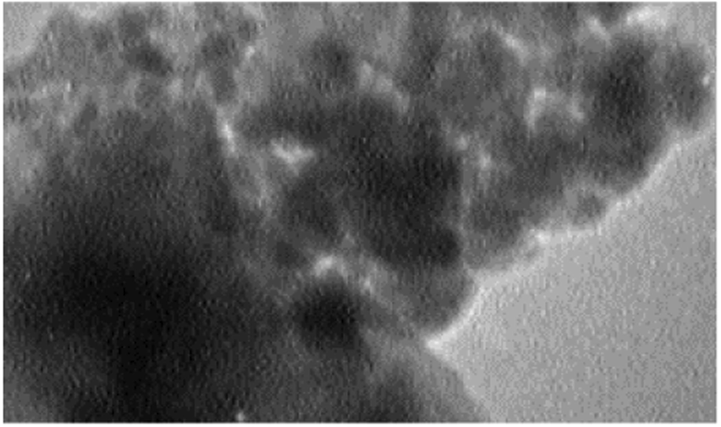
TEM image of CoFe2O4@ PEI.
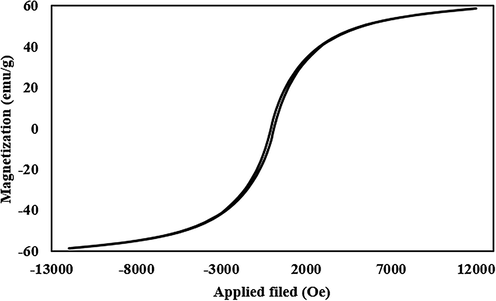
VSM image of CoFe2O4@ PEI.
3.2 Optimization of effective parameters
The effect of four parameters on removal percentage including pH, sorbent mass, ultrasonic time and Disulfine blue concentration were evaluated individually. The solution pH is one of the most important parameters that can affect and change the ionic or neutrality state of DB and Fe2O4@PEI and has a noticeable role in the removal of SY. In this regard, for finding the optimum pH, the effect of pH solution was examined in the range of 2.0–8.0 (Fig. S1). The optimum extraction recovery was achieved at pH 5.0. This phenomenon is probably due to hydrogen bonding interactions between NH+ groups of PEI and SO3− groups of DB at pH 5.0. At pH 5, N-H groups on the surface of Fe2O4@PEI get a positive charge and can interact thought hydrogen bond with SO3− of DB. At pH values lower than 5.0, the removal percentage was decrease that due to change in stability of CoFe2O4 @PEI. On the other hand, under strongly alkaline conditions, PEI cannot be protonated which decreases the electrostatic interaction for adsorption. Therefore, pH 5.0 was selected as optimum pH for the next experiments. Fig. S2 shows the effect of sorbent dosage on removal of DB was evaluated in the range of 0.005–0.03 g. As can be seen in Fig. S2, the maximum removal percentage was achieved at 0.015 g of CoFe2O4@PEI. An adequate amount of sorbent dosage can increase specific surface area and adsorption capacity which subsequently rise the removal percentage, while the inadequate amount of sorbent has the opposite effect and can decrease specific surface area and mass transfer and subsequently decrease removal percentage. Fig. S3 represents the effect of ultrasonic time in the range of 2.0–7.0 min. Results showed that maximum removal percentage was obtained at 5.0 min. Ultrasonic irradiation can enhance the contact surface between DB molecules and CoFe2O4@PEI and then increase the removal of SY molecules. The decrease of removal percentage at a time more than 5 min is probably due to desorption of DB molecules from CoFe2O4@PEI. Also, the effect of DB concentration was assessed in the concentration range of 10.0–35.0 mg L−1. The effect of initial DB concentration on the removal percentage reveals that maximum removal was observed at 10.0 mg L−1 of DB concentrations that is due to a low ratio of solute concentrations to adsorbent sites while in high DB concentrations it has the opposite effect (Fig. S4). Fig. S5 shows the UV–Vis spectra of disulfine blue before and after removal at optimum conditions.
3.3 Adsorption isotherms assessment for removal of DB by CoFe2O4@PEI
The adsorption isotherm is an important aspect of adsorption study that shows the affinity, tendency as well as adsorption mechanism of analyte toward sorbent. Therefore, different isotherm models including Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich were evaluated as listed in Table S1. According to Table S1, qe shows the adsorbed DB at equilibrium, Qm is the maximum adsorption capacity, Ce is the DB concentration in sample solution at equilibrium, KL, RL, KF, 1/n, B1, KT, and K are the Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich constants, respectively (Agarwal et al., 2016). Based on Fig. S6, Qm for was achieved to be 110.0 mg g−1. The R2 for Langmuir model (0.9983) was higher than other models which confirms that the adsorption of DB onto CoFe2O4@PEI can be well described by Langmuir isotherm. The principle of Langmuir isotherm is based on the monolayer adsorption of DB molecules onto the homogenous surface of sorbent while the Freundlich model assumes the multilayer adsorption of DB molecules onto the heterogeneous surface of the sorbent. Considering the previous discussion on the effect of pH, it is speculated that the DB molecules are monolayer adsorbed on the surface of CoFe2O4@PEI mainly through hydrogen interactions. RL is a separation factor that can take values between 0 and 1 which show desire (irreversible) and undesired (reversible) adsorption, respectively. The RL for DB adsorption onto CoFe2O4@PEI was achieved to be 0.017–0.067 that is near to 0 and shows a desire (irreversible adsorption) process. 1/n shows the adsorption intensity. If n take values between 0 and 1 show that the adsorption process is favorable. B is the Temkin constant related to the heat of sorption. Dubinin–Radushkevich represents the physical and/or chemical nature of adsorption. If E values are between 8 and 16 KJ mol−1 chemical mechanism controlled the adsorption process while for E lower than 8 KJ mol−1, the adsorption process occurs through a physical mechanism.
3.4 Adsorption kinetic investigation for adsorption of SY by COFNP
To examine the dynamics of the adsorption process in terms of the order of rate constant, different adsorption kinetics such as First-order-kinetic, Pseudo-second-order-kinetic, Intraparticle diffusion, and Elovich were studied. Based on Table S2, qe and qt are the adsorbed DB at equilibrium and time t, K1, K2, Kdiff, C, β and α are the constants of models, respectively. According to Table S2, R2 value for the Pseudo-second-order-kinetic model (0.9972) was higher than other models which confirms that adsorption kinetic followed by Pseudo-second-order. Also, the theoretical qe(cal) value was close to the experimental qe(exp) value for Pseudo-second-order-kinetic model which implies that the second-order model is in good agreement with experimental data and can be used to favorably explain the DB adsorption on CoFe2O4@PEI.
3.5 Reusability of CoFe2O4@PEI
To investigate the practical aspect of prepared CoFe2O4@PEI, the reusability of the sorbent was examined. In this regard, different adsorption-desorption cycles of CoFe2O4@PEI were completed and results represented that the prepared CoFe2O4@PEI is applicable for at least five times (Fig. S7). Thus, the proposed CoFe2O4@PEI is a good candidate for practical application for the removal of DB from an aqueous solution.
3.6 Comparison of the presented method
The presented method was compared with some other reported methods and results are presented in Table S3. As can be seen from Table S3, the prepared sorbent has high adsorption capacity (110.0 mg g−1) and also short extraction time (5.0 min). The prepared sorbent has a nano-diameter size which has extensive properties in terms of high chemical and physical stability, high contact surface area and also high adsorption capacity. The magnetic property of prepared sorbent eliminates the use of centrifuge, filtration and also precipitation methods for a gathering of the sorbent. In addition, the magnetic property of sorbent decreases removal time and also enhances repeatability of sorbent and also proposed method (Bagheri et al., 2016a; Savari et al., 2019; Wang et al., 2018).
4 Conclusion
In the present study, a novel nano-composite namely CoFe2O4@PEI was prepared and subsequently used as an adsorbent for ultrasonic-assisted removal of disulfine blue dye from aqueous solution. In this study was tried to introduce magnetic nanoparticles for the removal of disulfine blue from water samples. The magnetic property of sorbent accelerates the isolation of sorbent without using filtration, centrifuge and precipitation procedures. Also, it can decrease the removal time and also enhances the contact surface area and repeatability of the proposed method. It was observed that the combination of ultrasonic irradiation with CoFe2O4@PEI is an efficient, fast and applicable adsorption method for the removal of disulfine blue dye. The adsorption capacity of the applied adsorbent was found to be 110.0 mg g−1. The influences of experimental parameters on the DB removal percentage were successfully investigated and the optimum conditions were obtained as follows: pH of 5.0, sorbent dosage of 0.015 g, ultrasonic time of 5.0 min and initial concentration of 10.0 mg L−1. The isotherm models such as Langmuir, Freundlich, and Temkin were evaluated and it was shown that the equilibrium data were best described by the Langmuir model. Also, the process kinetics was successfully fitted to the pseudo-second-order kinetic model. Generally, routine synthesis approaches are faced with main drawbacks in terms of high consumption of organic, toxic and expensive sorbent, multi-step approaches that are time-consuming, low mass transfer and also low adsorption capacity. One of the main plans for future researches is using green and sustainable synthesis approaches for the fabrication of applicable sorbent for removal of hazardous materials from the environment especially water samples without using a hazardous, toxic, organic and expensive solvent. The other main plan is a synthesis and using hollow porous sorbent to increase the mass transfer and also adsorption capacity. The other plan is a synthesis of a selective sorbent like molecularly imprinted polymers for selective removal of one or many compounds from the environment especially complex matrixes. Also, we will try to combinate the molecularly imprinted polymers with excellent sorbents like metal-organic frameworks and also covalent organic frameworks to introduce a selective sorbents with high adsorption capacity.
References
- Rapid adsorption of ternary dye pollutants onto copper (I) oxide nanoparticle loaded on activated carbon: Experimental optimization via response surface methodology. J. Environ. Chem. Eng.. 2016;4(2):1769-1779.
- [Google Scholar]
- Chelating adsorption properties of PEI/SiO2 for plumbum ion. J. Hazard. Mater.. 2007;145(3):495-500.
- [Google Scholar]
- Particle size, morphology, and chemical composition controlled CoFe2O4 nanoparticles with tunable magnetic properties via oleic acid based solvothermal synthesis for application in electronic devices. ACS Appl. Nano Mater.. 2019;2(4):1828-1843.
- [Google Scholar]
- Water compatible molecularly imprinted nanoparticles as a restricted access material for extraction of hippuric acid, a biological indicator of toluene exposure, from human urine. Microchim. Acta. 2017;184(3):879-887.
- [Google Scholar]
- Hydrophilic molecularly imprinted nanospheres for the extraction of rhodamine B followed by HPLC analysis: A green approach and hazardous waste elimination. Talanta 2020 120933
- [Google Scholar]
- Novel strategy for synthesis of magnetic dummy molecularly imprinted nanoparticles based on functionalized silica as an efficient sorbent for the determination of acrylamide in potato chips: optimization by experimental design methodology. Talanta. 2016;154:526-532.
- [Google Scholar]
- Dummy molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. Talanta. 2019;195:390-400.
- [Google Scholar]
- Bagheri, A.R., Arabi, M., Ghaedi, M., Ostovan, A., Wang, X., Li, J., Chen, L.J.T., 2019b. Dummy molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. 195, 390–400.
- Synthesis of chitosan based molecularly imprinted polymer for pipette-tip solid phase extraction of Rhodamine B from chili powder samples. Int. J. Biol. Macromol.. 2019;139:40-48.
- [Google Scholar]
- Application of Cu-based metal-organic framework (Cu-BDC) as a sorbent for dispersive solid-phase extraction of gallic acid from orange juice samples using HPLC-UV method. Arabian J. Chem.. 2020;13:5218-5228.
- [Google Scholar]
- Green preparation of dual-template chitosan-based magnetic water-compatible molecularly imprinted biopolymer. Carbohydrate Polym. 2020 116102
- [Google Scholar]
- Magnetic metal organic framework for pre-concentration of ampicillin from cow milk samples. J. Pharm. Anal. 2020
- [Google Scholar]
- Bagheri, A.R., Ghaedi, M., Asfaram, A., Bazrafshan, A.A., Jannesar, R.J.U.s., 2017. Comparative study on ultrasonic assisted adsorption of dyes from single system onto Fe3O4 magnetite nanoparticles loaded on activated carbon: experimental design methodology. 34, 294–304.
- Modeling and optimization of simultaneous removal of ternary dyes onto copper sulfide nanoparticles loaded on activated carbon using second-derivative spectrophotometry. J. Taiwan Inst. Chem. Eng.. 2016;65:212-224.
- [Google Scholar]
- Bagheri, A.R., Ghaedi, M., Asfaram, A., Hajati, S., Ghaedi, A.M., Bazrafshan, A., Rahimi, M.R.J.J.o.t.T.I.o.C.E., 2016b. Modeling and optimization of simultaneous removal of ternary dyes onto copper sulfide nanoparticles loaded on activated carbon using second-derivative spectrophotometry. 65, pp. 212–224.
- Bagheri, A.R., Ghaedi, M., Asfaram, A., Jannesar, R., Goudarzi, A.J.U.s., 2017. Design and construction of nanoscale material for ultrasonic assisted adsorption of dyes: application of derivative spectrophotometry and experimental design methodology. 35, 112–123.
- Bidgoli, H., Mortazavi, Y., Khodadadi, A.A.J.J.o.h.m., 2019. A functionalized nano-structured cellulosic sorbent aerogel for oil spill cleanup: Synthesis and characterization. 366, 229–239.
- Column packing elimination in matrix solid phase dispersion by using water compatible magnetic molecularly imprinted polymer for recognition of melamine from milk samples. J. Chromatogr. A. 2019;1594:13-22.
- [Google Scholar]
- Application of molecularly imprinted biomembrane for advancement of matrix solid-phase dispersion for clean enrichment of parabens from powder sunscreen samples: optimization of chromatographic conditions and green approach. ACS Omega. 2019;4(2):3839-3849.
- [Google Scholar]
- Preparation of hollow porous molecularly imprinted and aluminum (III) doped silica nanospheres for extraction of the drugs valsartan and losartan prior to their quantitation by HPLC. Microchim. Acta. 2019;186(11):702.
- [Google Scholar]
- Molecular imprinting-based surface-enhanced Raman scattering sensors. ACS Sensors; 2020.
- Homem, N.C., Beluci, N.d.C.L., Amorim, S., Reis, R., Vieira, A.M.S., Vieira, M.F., Bergamasco, R., Amorim, M.T.P.J.A.S.S., 2019. Surface modification of a polyethersulfone microfiltration membrane with graphene oxide for reactive dyes removal.
- Khani, R., Sobhani, S., Yari, T.J.M.J., 2019. Magnetic dispersive micro solid-phase extraction of trace Rhodamine B using imino-pyridine immobilized on iron oxide as nanosorbent and optimization by Box–Behnken design. 146, 471–478.
- Facile approach to the synthesis of molecularly imprinted ratiometric fluorescence nanosensor for the visual detection of folic acid. Food Chem. 2020 126575
- [Google Scholar]
- Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater.. 2007;308(2):289-295.
- [Google Scholar]
- Fabrication of water-compatible superparamagnetic molecularly imprinted biopolymer for clean separation of baclofen from bio-fluid samples: a mild and green approach. Talanta. 2018;179:760-768.
- [Google Scholar]
- Hydrophilic multitemplate molecularly imprinted biopolymers based on a green synthesis strategy for determination of B-family vitamins. ACS Appl. Mater. Interfaces. 2018;10(4):4140-4150.
- [Google Scholar]
- Oussalah, A., Boukerroui, A., Aichour, A., Djellouli, B.J.I.j.o.b.m., 2019. Cationic and anionic dyes removal by low-cost hybrid alginate/natural bentonite composite beads: Adsorption and reusability studies. 124, 854–862.
- Magnetic solid phase extraction with CoFe2O4/oleic acid nanoparticles coupled to gas chromatography-mass spectrometry for the determination of alkylphenols in baby foods. Food Chem.. 2017;221:76-81.
- [Google Scholar]
- Synthesis and characterization of CoFe2O4@ SiO2@ NH-NH2-PCuW as an acidic nano catalyst for the synthesis of 1, 4-benzodiazepines and a powerful dye remover. Polyhedron. 2019;166:233-247.
- [Google Scholar]
- Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci.. 1968;26(1):62-69.
- [Google Scholar]
- Tanhaei, B., Ayati, A., Sillanpää, M.J.I.j.o.b.m., 2019. Magnetic xanthate modified chitosan as an emerging adsorbent for cationic azo dyes removal: Kinetic, thermodynamic and isothermal studies. 121, 1126–1134.
- Uogintė, I., Lujanienė, G., Mažeika, K.J.J.o.h.m., 2019. Study of Cu (II), Co (II), Ni (II) and Pb (II) removal from aqueous solutions using magnetic Prussian blue nano-sorbent. 369, 226–235.
- Core-shelled mesoporous CoFe2O4–SiO2 material with good adsorption and high-temperature magnetic recycling capabilities. J. Phys. Chem. Solids. 2018;115:300-306.
- [Google Scholar]
- Fluorescent nanosensor designing via hybrid of carbon dots and post-imprinted polymers for the detection of ovalbumin. Talanta. 2020;211 120727
- [Google Scholar]
- Disperse ultrafine amorphous SiO2 nanoparticles synthesized via precipitation and calcination. Colloids Surf., A. 2019;568:445-454.
- [Google Scholar]
- Multi-emitting fluorescence sensor of MnO2-OPD-QDs for the multiplexed and visual detection of ascorbic acid and alkaline phosphatase. J. Mater. Chem. C 2020
- [Google Scholar]
- Rational construction of a triple emission molecular imprinting sensor for accurate naked-eye readout of folic acid. Nanoscale 2020
- [Google Scholar]
- Ratiometric fluorescence and colorimetry dual-mode assay based on manganese dioxide nanosheets for visual detection of alkaline phosphatase activity. Sens. Actuators, B. 2020;302 127176
- [Google Scholar]
- Preparation and characterization of a novel photocatalytic self-cleaning PES nanofiltration membrane by embedding a visible-driven photocatalyst boron doped-TiO2SiO2/CoFe2O4 nanoparticles. Sep. Purif. Technol.. 2019;209:764-775.
- [Google Scholar]
- Magnetic adsorbent based on mesoporous silica nanoparticles for magnetic solid phase extraction of pyrethroid pesticides in water samples. J. Chromatogr. A 2019
- [Google Scholar]
- Room temperature preparation of water-soluble polydopamine-polyethyleneimine copolymer dots for selective detection of copper ions. Talanta. 2019;197:584-591.
- [Google Scholar]
- Sensitive determination of As (III) and As (V) by magnetic solid phase extraction with Fe@polyethyleneimine in combination with hydride generation atomic fluorescence spectrometry. Talanta. 2016;156–157:196-203.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.03.022.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







