Translate this page into:
Synthesis and characterization of high wash fastness novel azo reactive dyes incorporating aromatic bridged diamines
⁎Corresponding author. Tel.: +92 51 9064 2128; fax: +92 51 9064 2241. asaeed@qau.edu.pk (Aamer Saeed) aamersaeed@yahoo.com (Aamer Saeed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A new series of azo reactive dyes containing free chlorine atoms have been synthesized. The synthetic methodology involves the diazotization of 4′4-diamino diphenylamine-2-sulfonic acid (2) followed by azo coupling with 1-amino-8-naphthol-3,6-disulfonic acid (3) in alkaline medium to yield intermediate dye (4). Condensation of the latter with 1,3,5-trichlorotriazine afforded the cyanurated dye (5). A number of novel bis aromatic diamines (1a–j) were separately synthesized as bridging groups and were coupled with cyanurated dye (5) at room temperature to provide the target dyes designated as S1–S10 containing triazine and linker in a single molecule. The structures of newly synthesized compounds were confirmed by analytical data and spectroscopic techniques. The synthesized dyes were applied on cotton fibers to assess their light fastness, wash fastness and rubbing fastness and were found to possess medium to high fastness values in different dyes.
Keywords
Azo dye
Coupling reaction
Diazotization
Spectral study
H-acid
FC-acid
1 Introduction
Reactive dyes are the colored compounds having one or two groups capable of further establishing covalent bond between carbon or phosphorus atom of the dyes and oxygen, nitrogen or sulfur atoms of the substrate (Taylor, 2000). These dyes are commonly applied on expensive clothes which are generally mercerized (Freeman and Sokolowska, 1999). Reactive dyes, though a late entry into the field of synthetic dyes, have immediately achieved a commercial status due to their brilliancy, variety of hues, high wet fastness, convenient handling and high applicability (Vickerstaff, 1957). Several new reactive systems have been introduced from time to time, which is the subject of numerous patents and publications. It was exceptional to note that dyeing is carried out by chemical reaction between the dye and the fiber, enabling one to obtain collection of bright, attractive shades of satisfactory fastness with considerable ease of dyeing. It is implicit that dyes with two reactive groups provide a higher fixation yield than those with one reactive group, as if one of the two dye-fiber bonds is hydrolyzed, there is still one left for fixation (Bredereck and Schumacher, 1993; Masaki et al., 1988; Otutu et al., 2011). Various triazine derivatives find an important position among the reactive dyes (Konstantinova and Petrova, 2002). s-Triazine based molecules have been applied in the manufacture of polymers, dyes, drugs, explosives, pesticides and commodity chemicals (Zhang, 2005) as a corollary, theoretical and experimental studies have widely been carried out on such systems (Shufen and Jinzong, 2003; Juozas et al., 2005). The s-triazine ring is recognized as a vital conjugated heterocycle whose electronic properties are expected to show suitable differences from those of benzene due to the alternate replacement of methine groups by nitrogen (Rupin et al., 1970).
In recent years, reactive dyes maintain the largest annual consumption in the world among the dyes used for cellulosic fibers (Wei et al., 2004), which establishes its important status in the dye industry. However problems, such as low dye utilization, large amount of electrolyte used and high volume of wastewater discharged, always hinder the application of reactive dyes (Hauser and Tabba, 2002). Many studies have been devoted to improving the substantivity of cotton fibers for reactive dyes, thus reducing or eliminating the amount of electrolyte used. The surface modification of textile fibers is one of the best routes to achieve high fixation of dyes on fibers (Evans et al., 1984; Lewis and Lei, 1991). Many compounds containing quaternary ammonium groups or amino groups have been used in the following years since 1930 for this application. Different physical and chemical modifications of cotton were employed with diverse compounds involving monomers and polymers (Subramannian et al., 2006), and some of them have been proven effective. Colo-cotto, Cottum and Viewline are some of the brand names of cationic cotton produced by Japanese manufactures, and fabrics from cationic and normal cotton were commercialized to give differential color and cross-dye effect in the dyeing, examples of other compounds include epoxy (Manish et al., 2013), chlorotriazine type quaternary compounds (Lehr, 1990), N-methylolacrylamide (Korkin and Bartlett, 1996; Divyesh et al., 2009) and choline chloride (Divyesh and Keshav, 2011).
Keeping in view the aforementioned facts, the objective of the current investigation was to develop and evaluate tinctorially strong, bright reactive dyes, which could be applied to cotton by exhaust dyeing using low salt (0–30 g/l) quantities. To this end ten novel monochloro-s-triazinyl (MCT) disazo reactive dyes (S1–S10) were synthesized by coupling a series of diazotized intermediates with 1-hydroxy-8-aminonaphthalene-3,6-disulfonic acid [H-acid].
2 Materials and methods
2.1 General conditions
All commercial products were purchased from Sigma–Aldrich. Solvents used were of analytical grade and, when necessary, were purified and dried by the standard methods. Melting points were determined in open capillary tubes on a Stuart melting point apparatus. The FTIR spectra were run in the single beam Nicolet IR 100 (Fourier-Transform); while UV of all the samples were run in water using UV-Genesys spectrophotometer. The 1H NMR and 13C NMR spectra were recorded in D2O using NMR Bruker DPX 400 spectrophotometer operating at 400 MHz. TMS was used as internal standard with the deuterium signal of the solvent as the lock and chemical shifts δ recorded in ppm. The elemental analysis (C, H, N, S) of the compounds was performed using Flash EA 1112 elemental analyzer while the pH was monitored using Portable pH Meter Model PHB4. Compounds were routinely checked by TLC on silica gel G plates using three different eluting solvents depending on the polarity disparity. The solvent systems are petroleum ether:chloroform (9:1, v/v), petroleum ether:chloroform (6:4, v/v) and chloroform:methanol (9:1, v/v). Also, the developed plates were visualized using UV lamp for the presence of spots and Rf values were duly calculated. All crude products were isolated as solids and purified by a combination of column chromatography and recrystallization. Fastness to light was assessed in accordance with BS 1006-1978. Rubbing fastness was checked with an Atlas Crock meter in accordance with AATCC TM 8-1961 and the wash fastness was determined according to ISO: 765-1979 (Patel and Patel, 2009).
2.2 General procedure for the synthesis of 5,5′-methylenebis(3-nitroanilines (1a–j)
Suitably substituted aniline (0.01 mol) was dissolved in water (12.5 ml) and 36.5% hydrochloric acid (2.5 ml) at 50 °C. The reaction mixture was then reacted with 3% aqueous formaldehyde (3.5 ml) solution at 60 °C with stirring for 1 h and neutralized with 10% sodium hydroxide solution. Precipitates obtained, were filtered, washed with hot water, dried and recrystallized from acetic acid (Divyesh et al., 2011).
By adopting the above procedure bis anilines (1a–j) were synthesized as shown in Scheme 1.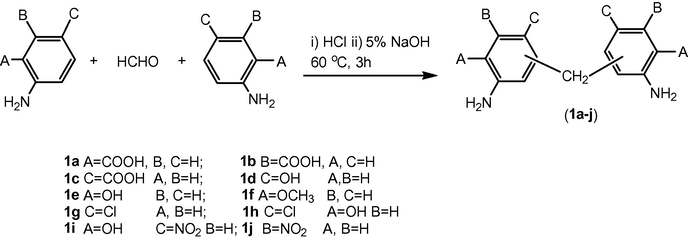
Synthesis of 5,5′-methylenebis(3-nitroanilines).
2.2.1 5,5′-methylenebis(2-aminobenzoic acid)
Yellowish orange solid (80%) m.p. 143–145 °C. Rf = 0.75 (EtOAc: Pet Ether, 4: 1 v/v). FTIR (KBr) cm−1: 3500 (br COOH) 3420, (N–H), 3077(C⚌C–H Aromatic) 2858 (C–H aliphatic), 1701(C⚌O) 1666, 1614, 1586 (C⚌C benzene ring) 1480 (N–H bend.), 781 (o-disubstituted Aromatic ring).
2.2.2 5,5′-methylenebis(3-aminobenzoic acid)
Yellow solid (82%) m.p. 172–173 °C, Rf = 0.73 FTIR (KBr) cm−1: 3600–3500 (br COOH) 3417, (N–H), 3023 (C⚌C–H Aromatic) 2830 (C–H aliphatic), 1700(C⚌O) 1670, 1631, 1587 (CC benzene ring) 1488 (N–H bend.), 896, 835, 746 (C⚌C bending, Aromatic ring).
2.2.3 5,5′-methylenebis(4-aminobenzoic acid)
Dark yellow solid (81.5%) m.p. 245–247 °C, Rf = 0.70 FTIR (KBr) cm−1: 3600–3500 (br COOH) 3417, (N–H), 3023 (C⚌C–H Aromatic) 2840 (C–H aliphatic), 1710 (C⚌O) 1665, 1620, 1590 (C⚌C benzene ring) 1488 (N–H bend.), 890, 828, 740 (C⚌C bending, Aromatic ring).
2.2.4 2,2′-methylenebis(4-aminophenol)
Dark brown Solid (78%) m.p. 220–221 °C, Rf = 0.67 FTIR (KBr) cm−1: 3600–3500 (br COOH) 3474, (N–H), 3023 (C⚌C–H Aromatic) 2825 (C–H aliphatic), 1665, 1603, 1514 (C⚌C benzene ring) 1471 (N–H bend.), 845, 833, (C⚌C bending, Aromatic ring).
2.2.5 4,4′-methylenebis(2-aminophenol)
Orange solid m.p. 348–349, Rf = 0.65, FTIR (KBr) cm−1: 3600–3500 (br COOH) 3385, (N–H), 3190 (C⚌C–H Aromatic) 2745 (C–H aliphatic), 1661, 1601, 1531 (C⚌C benzene ring) 1444 (N–H bend.), 836, 814, (o-disubstituted Aromatic ring).
2.2.6 5,5′-methylenebis(2-methoxyaniline)
Light yellow solid (85%) m.p. 348–349 °C, Rf = 0.67, FTIR (KBr) cm−1: 3418, (N–H), 3050 (C⚌C–H Aromatic) 2836 (C–H aliphatic), 1665, 1606, 1523 (C⚌C benzene ring) 1461 (N–H bend.), 833, 810, (o-disubstituted Aromatic ring).
2.2.7 6,6′-methylenebis(3-chloroaniline)
Light yellow solid (77%) m.p. 172 °C, Rf = 0.76, FTIR (KBr) cm−1: 3418, (N–H), 3030 (C⚌C–H Aromatic) 2830 (C–H aliphatic), 1663, 1606, 1503 (C⚌C benzene ring) 1384 (N–H bend.), 830, 810, (C⚌C bending, Aromatic ring), 683 (C–Cl).
2.2.8 6,6′-methylenebis(2-amino-4-chlorophenol)
Brown solid (74%) m.p. 136–141 °C, Rf = 0.75 IFTR (KBr) cm−1: 3420, (N–H), 3070 (C⚌C–H Aromatic) 2858 (C–H aliphatic), 1662, 1614, 1586 (C⚌C benzene ring) 1455 (N–H bend.), 880, 808, (C⚌C bending, Aromatic ring), 761 (C–Cl).
2.2.9 6,6′-methylenebis(2-amino-4-nitrophenol)
Orange solid (71%) m.p. 189 °C, Rf = 0.80 FTIR (KBr) cm−1: 3417, (N–H), 3023 (C⚌C–H Aromatic) 2825 (C–H aliphatic), 1670, 1631, 1587 (C⚌C benzene ring) 1345 (N–H bend.), 1304 (NO2) 896, 838, (C⚌C bending, Aromatic ring),
2.2.10 5,5′-methylenebis(3-nitroaniline)
Yellow solid (83%,) m.p. 204–205, Rf = 0.68 FTIR (KBr) cm−1: 3418(N–H), 2936 (C–H), 1666, 1523 (C⚌C) 1595 (N–H bend.), 1428 (N–H) 1128 (C–O), 860 (o-disubstituted Aromatic ring).
2.3 General procedure for the synthesis of H-acid based reactive azo dyes
2.3.1 Tetrazotization of 4,4′-diamino diphenylmethane-2-sulfonic acid (FC-acid) and coupling with H-acid
FC-acid (2) (3.36 g, 0.01 mol) was suspended in H2O (80 ml). Hydrochloric acid (15 ml) was added drop wise to this well stirred suspension. The mixture was gradually heated up to 70 °C till clear solution obtained. The solution was cooled to 0–5 °C in an ice bath. A solution of NaNO2 (1 g, 0.014 mol) in H2O (10 ml) previously cooled to 0 °C, was then added over a period of 5 min with stirring. Stirring was continued for an hour maintaining the same temperature, with positive test for nitrous acid with required amount of a solution of a sulfamic acid. The clear tetrazo solution at 0–5 °C was used for subsequent coupling reaction.
To a well-stirred solution of H-acid (3) (6.48 g, 0.02 mol), a freshly prepared solution of tetrazo FC-acid (3.36 g, 0.01 mol) was added drop wise over a period of 10–15 min. The pH was maintained at 7.5–8.5 by simultaneous addition of sodium carbonate solution (10% w/v). During coupling the blue solution was formed. Stirring was continued for 3–4 h, maintaining the temperature below 5 °C. The reaction mixture was heated up to 60 °C and sodium chloride (15 g) added until the coloring material was precipitated. It was stirred for an hour, filtered and washed with a small amount of sodium chloride solution (5% w/v). The solid was dried at 80–90 °C and extracted with DMF. The dye was precipitated by diluting the DMF-extract with excess of chloroform. The blue colored dye (4) was then filtered, washed with chloroform and dried at 60 °C.
2.3.2 Cyanuration of dye
Cyanuric chloride (3.7 g, 0.02 mol) was stirred in acetone (50 ml) at a temperature below 5 °C for a period of an hour. A neutral solution of (4) (6.17 g, 0.01 mol) was then added in small lots in about an hour. Neutral pH was maintained below 5 °C through this reaction. The reaction mass was then stirred at 0–5 °C for further four hours when a clear solution was obtained. The cyanurated dye solution thus formed was salted out from 10% NaCl solution, filtered, washed and dried at 70 °C (5) in 90% yield. Cyanurated dye was used for subsequent condensation with different bis anilines.
2.3.3 Condensation of cyanurated dye with bis anilines
To the vigorously stirred aqueous solution of cyanurated dye (5) (1.5 g, 0.0016 mol) at room temperature, was added a solution of 5,5′-methylenebis(3-nitroaniline), (0.228 g 0.0008 mol) in 15 ml water in acidic medium of HCl. The pH of reaction mixture was kept 4.0 and continued the stirring for 4 h for completion of reaction which was checked by taking paper chromatography of reaction mixture. Dye was salted out from 15% solution of NaCl, filtered and dried in oven at 70 °C keeping overnight. In this way all S1–S10 dyes were prepared by changing the bis anilines and keeping all other conditions identical.
2.3.3.1 S1 [C53Cl2H34N15O21S5]
Bluish crystal, (75.32%) λmax in nm (log ε): 599 (3.63), 396 (3.58), 355 (3.47). FTIR (KBr, cm−1) νmax: 3526–2500 (O–H, COOH, N–H), 1750(C⚌O) 1619, 1591, 1529, (C⚌C aromatic), 1483 (CH2), 724 (Ar–H) 672 (C–Cl). 1H NMR (300 MHz, D2O) δ: 3.37 (s, 2H, CH2), 5.04 (s, 2H, 2OH), 9.6 (s, 2H, 2COOH), 9.8 (s, 2H, 2NH), 10.6 (s, 2H, 2NH), 7.6–8.3 (m, 19H, Ar–H) ppm.
2.3.3.2 S2 [C53Cl2H34N15O21S5]
Bluish crystal,(76.11%) λmax in nm (log ε): 614 (3.54), 396 (3.82), 355 (3.54). FTIR (KBr, cm−1) νmax: 3471 br (OH, NH), 3072 (C⚌C–H) 1711 (C⚌O), 1660, 1590, 1502 (C⚌C aromatic), 723 (Ar–H), 1483 (CH2), 1070 S⚌O 772 (Ar–H) 672 (C–Cl). 1H NMR (300 MHz, D2O) δ: 3.40 (s, 2H, CH2), 5.01 (s, 2H, 2OH), 9.62 (s, 2H, 2COOH), 9.79 (s, 2H, 2NH), 10.62 (s, 2H, 2NH), 7.6–8.3 (m, 19H, Ar–H) ppm.
2.3.3.3 S3 [C53Cl2H34N15O21S5]
Bluish crystal, (75.65%) λmax in nm (log ε): 608 (3.67), 396 (3.49), 355 (3.48). FTIR (KBr, cm−1) νmax: 3443 br (OH, NH), 3071 (C⚌C–H) 2971 (CH2), 1747 (C⚌O), 1660, 1590, 1502 (C⚌C aromatic), 1483 (CH2), 1070, S⚌O 772 (Ar–H) 672 (C–Cl). 1H NMR (300 MHz, D2O) δ: 3.39 (s, 2H, CH2), 5.02 (s, 2H, 2OH), 9.59 (s, 2H, 2COOH), 9.78 (s, 2H, 2NH), 10.55 (s, 2H, 2NH), 7.6–8.3 (m, 19H, Ar–H) ppm.
2.3.3.4 S4 [C51Cl2H34N15O19S5]
Bluish crystal (81.15%) λmax in nm (log ε): 643 (3.65), 396 (3.60), 355 (3.44). FTIR (KBr, cm−1) νmax: 3441 (OH, NH), 3078 (C⚌C–H), 2835 (CH2), 1660, 1590, 1502 (C⚌C aromatic), 1070, S⚌O, 723 (Ar–H), 672 (C–Cl). 1H NMR (300 MHz, D2O) δ: 3.2 (s, 2H, CH2), 5.03 (s, 2H, 2OH), 5.3 (s, 2H, 2OH), 9.5 (s, 2H, 2NH), 10.56 (s, 2H, 2NH), 7.55–8.3 (m, 19H, Ar–H) ppm.
2.3.3.5 S5 [C51Cl2H34N15O19S5]
Bluish crystal (80.72%). λmax in nm (log ε): 635 (3.72), 455 (3.61), 396 (3.60), 355 (3.52). FTIR (KBr, cm−1) νmax: 3448 (OH, NH), 1660, 1590, 1502 (C⚌C aromatic), 1070, S⚌O, 723 (Ar–H), 672 (C–Cl). 1H NMR (300 MHz, D2O) δ: 3.23 (s, 2H, CH2), 5.02 (s, 2H, 2OH), 5.33 (s, 2H, 2OH), 9.76 (s, 2H, 2NH), 10.57 (s, 2H, 2NH), 7.55–8.3 (m, 19H, Ar–H) ppm.
2.3.3.6 S6 [C51Cl2H32N17O23S5]
Bluish crystal (83.22%). λmax in nm (log ε): 609 (3.49), 455 (3.72), 396 (3.67), 355 (3.47). FTIR (KBr, cm−1) νmax: 3422 (OH, NH), 2929 (CH2), 1660, 1590, 1502 (C⚌C aromatic), 1070, S⚌O, 723 (Ar–H), 672 (C–Cl). 1H NMR (300 MHz, D2O) δ: 3.41 (s, 2H, CH2), 5.05 (s, 2H, 2OH), 5.35 (s, 2H, 2OH), 9.71 (s, 2H, 2NH), 10.58 (s, 2H, 2NH), 7.7–8.3 (m, 17H, Ar–H) ppm.
2.3.3.7 S7 [C51Cl4H32N15O19S5]
Bluish crystal (85.71%). λmax in nm (log ε): 615 (3.85), 420 (3.83), 396 (3.82), 355 (3.68). FTIR (KBr, cm−1) νmax: 3440 (OH, NH), 2945 (CH2), 1660, 1585, 1507 (C⚌C aromatic), 1075, S⚌O, 720 (Ar–H), 672 (C–Cl).1H NMR (300 MHz, D2O) δ: 3.38 (s, 2H, CH2), 5.07 (s, 2H, 2OH), 5.31 (s, 2H, 2OH), 9.72 (s, 2H, 2NH), 10.53 (s, 2H, 2NH), 7.82–8.01 (m, 17H, Ar–H) ppm.
2.3.3.8 S8 [C51Cl2H32N17O21S5]
Bluish crystal (78.77%). λmax in nm (log ε): 601 (3.97), 420 (3.99), 396 (3.97), 355 (3.81). FTIR (KBr, cm−1) νmax: 3465 (OH, NH), 2890 (CH2), 1648, 1570, 1510 (C⚌C aromatic), 1072, S⚌O, 735 (Ar–H), 670 (C–Cl). 1H NMR (300 MHz, D2O) δ: 3.55 (s, 2H, CH2), 5.1 (s, 2H, 2OH), 9.8 (s, 2H, 2NH), 10.75 (s, 2H, 2NH), 7.85–8.30 (m, 19H, Ar–H) ppm.
2.3.3.9 S9 [C51Cl2H36N15O19S5]
Bluish crystal (84.57%). λmax in nm (log ε): 638 (3.95), 355 (3.94), 326 (3.95). FTIR (KBr, cm−1) νmax: 3454 (OH, NH), 2929 (CH2), 1642, 1584, 1522 (C⚌C aromatic), 1125, S⚌O, 732 (Ar–H), 672 (C–Cl). 1H NMR (300 MHz, D2O) δ: 3.41 (s, 2H, CH2), 3.9 (s, CH3, OCH3) 5.0 (s, 2H, 2OH), 9.74 (s, 2H, 2NH), 10.53 (s, 2H, 2NH), 7.4–8.2 (m, 19H, Ar–H) ppm.
2.3.3.10 S10 [C51Cl4H32N15O17S5]
Bluish crystal, (80.43%). λmax in nm (log ε): 620 (3.82), 396 (3.86), 355 (3.66). FTIR (KBr, cm−1) νmax: 3442 (OH, NH), 2890 (CH2), 1639, 1560, 1508 (C⚌C aromatic), 1127, S⚌O, 730 (Ar–H), 672 (C–Cl). 1H NMR (400 MHz, D2O) δ: 3.4 (s, 2H, CH2), 5.01 (s, 2H, 2OH), 9.73 (s, 2H, 2NH), 10.65 (s, 2H, 2NH), 7.6–8.2 (m, 19H, Ar–H) ppm.
2.4 Dyeing method
A laboratory model glycerin-bath high-temperature beaker dyeing machine was used. A paste of finely powdered dye (25 mg) was prepared with the dispersing agent EDTA (1 ml, 10% w/v), in a ball mill for 10 min. To this paste, water (15 ml) was added under stirring and the pH was adjusted to 8.5–9.0, using Na2CO3 (1 ml, 10% w/v). This dye suspension (100 ml) was added to a beaker provided with a lid and a screw cap. Before closing the lid and tightening the metal cap over the beaker, a wetted piece of cotton fiber was rolled into the beaker. The beaker was then placed vertically on the rotatory carrier inside the tank. The rotatory carrier was then allowed to rotate in the glycerin-bath, the temperature of which was raised to 80 °C (for cotton fiber) at the rate of 2 °C/min. The dyeing was continued for 1 h under pressure. After cooling for 1 h, the beaker was removed from the bath and washed with water. The pattern was thoroughly washed with hot water at 50 °C, and then with cold water, and finally dried at room temperature.
3 Results and discussion
3.1 Synthesis
The synthesis of bisazomonochlorotriazine (MCT) reactive dyes was accomplished following a two-step procedure. It is crucial to note that only a single type of bisazomonochlorotriazine (MCT) reactive dyes was synthesized having a common coupler, bisazo component and reactive system but different bridging bis anilines.
Numerous literature reports are available in recent years to supplement this paradigm shift in azo dye utilization. In order to create novelty and to increase the fixation of reactive dyes on fibers bis anilines were used as bridging groups. Thus substituted anilines were treated with dilute solution of formaldehyde, at low temperature in order to avoid polymerization. Conc. HCl was used to protonate the NH2 group in differently substituted anilines, to peter out the nucleophilicity of the NH2 group. Short duration of time about 2 h was required for the completion of reaction. Neutralization of reaction mixture was achieved with 10% NaOH solution which resulted in the separation of product, by snatching the proton and making the product insoluble in aqueous medium. The synthetic route to novel bis anilines (1a–j) has been sketched in Scheme 1.
Synthesis of dyes bisazomonochlorotriazine (MCT) reactive dyes S1–S10 has been conducted in accordance with Scheme 2. The rational for selection of these dyes for synthesis, is to acquire various scaffolds of this nature by derivatization which will help in the future structure activity relationship (SAR) study of these compounds. Here S1–S10 reactive dyes have been synthesized containing 1-amino-8-naphthol-3,6-disulfonic acid (H-acid) coupler and 4,4′-diamino diphenylamine-2-sulfonic acid (FC-acid) as bisazo component.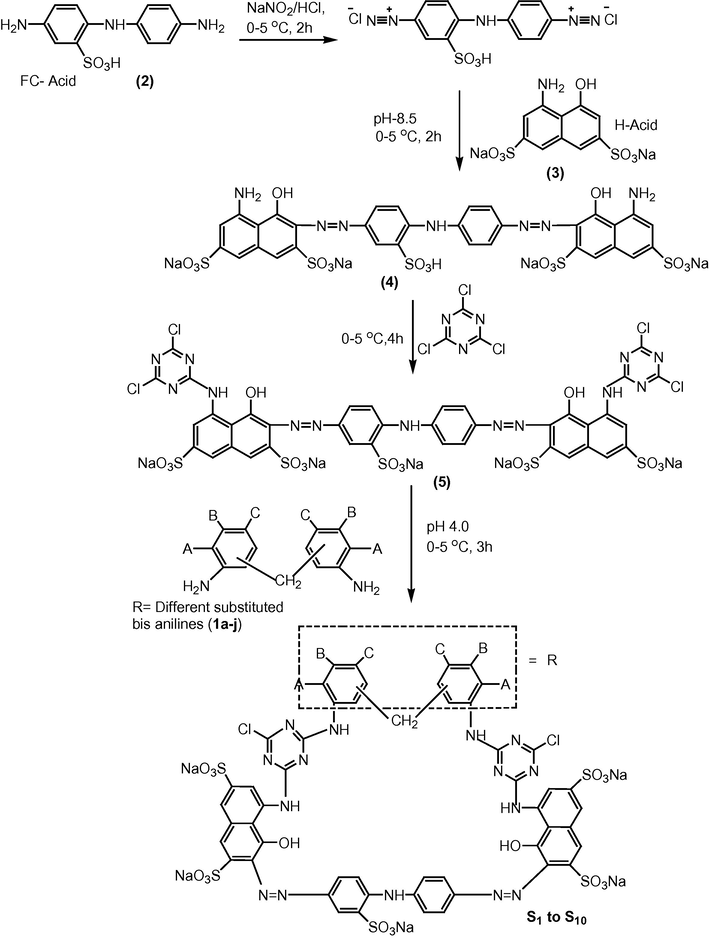
Synthetic route to bisazo MCT reactive dyes (S1–S10).
Accordingly, FC-acid (2) was tetra azotized at low temperature 0–5 °C in order to stabilize the azo compound. Coupling of tetrazo of FC-acid with H-acid (3) was achieved to afford (4) in alkaline medium to accomplish the coupling at position ortho to the hydroxyl group of H-acid at 0–5 °C and the coupling was completed in 3 h. Coupling at ortho position of the hydroxyl group occurs at alkaline pH and in acidic medium coupling ortho to the amino group takes place. Cyanuration of dye (4) was achieved by addition of dye to cyanuric chloride solution in ice bath at pH 2.0–2.5 in 1:2 M ratio followed by filtration, and drying of the dye at 70 °C. Low temperature and highly acidic pH was maintained throughout condensation with cyanuric chloride for the replacement of only one chloro group with the amino group of H-acid coupling component of dye 4 to afford 5. When pH and temperature conditions are varied then chances of substitution at second and third chloro groups are also enhanced. Bis anilines (1a–j) were added to solution of dye at pH 4–5 at room temperature in a molar ratio 1:1 to furnish dyes S1–S10. Different bis anilines were used as bridging component for dyes, which did not affect the λmax too much but increased the substantivity of dye with fiber (Scheme 2).
3.2 Spectral properties of dyes
The absorption maxima (λmax) of the dyes S1–S10 were recorded in water and are shown in Table 1. These dyes show two absorption maxima, one in the UV range due to π–π∗ transition of C⚌C present in the aromatic moiety of dyes common in all dyes and other in the visible region, and is due to π–π∗ transition of azo linkage N⚌N of dyes. All dyes have same chromophoric functionalities but difference is of bridging groups. These bridging groups affect the λmax of dyes but effect is not too high as these are not directly attached with chromophoric groups of dyes.
Dye
Shade on fiber
λmax nm in H2O
Log ε
Exhaustion% (C)
Fixation% (C)
S1
Sky Blue
599
3.63
65.77
91.54
S2
Sky Blue
608
3.54
63.53
92.35
S3
Sky Blue
604
3.67
64.36
91.98
S4
Sky Blue
643
3.65
72.35
81.23
S5
Sky Blue
635
3.72
74.26
80.18
S6
Sky Blue
609
3.49
71.28
82.23
S7
Sky Blue
615
3.85
75.25
79.63
S8
Sky Blue
601
3.97
73.18
81.25
S9
Sky Blue
638
3.95
73.22
81.15
S10
Sky Blue
620
3.82
75.55
79.49
The values of log ε (molar extinction coefficient) are summarized in Table 1 and are in the range 3.54–3.97, due to high absorption intensity of dyes. Intermediate dye (4) has λmax 626 nm which is without bridging anilines.
Dyes S1, S2 and S3 have λmax 599, 608 and 604 nm respectively (Fig. 3.1, Supporting information) as these contain bridging anilines having carboxylic function at ortho, meta and para positions to NH groups. There is a shift of 18–27 nm in λmax because of carboxylic group electron withdrawing nature through resonance and inductive effect. Dye S2 has higher λmax than S1 and S3 because of the carboxylic group at m-position where only inductive effect operates. Dyes S4 and S5 have λmax 640 and 634 nm while S6 and S7 have λmax values 609 nm and 615 nm respectively. Here the introduction of auxochrome like the hydroxyl group in bridging anilines produces bathochromic effect in S4 and S5 and give rise to shift of 8–14 nm λmax from So. S6 and S7 dyes have bridging anilines containing both electron donating as well as electron withdrawing groups which cancel the effect of each other and their values are close to the original dye So. S8 and S10 dyes have λmax lower than original dye So due to electron withdrawing groups NO2 and Cl at m-position to NH and their values are 601 and 620 nm respectively. λmax of S9 is above So and is 638 nm due to the methoxy group at ortho to NH. From the UV analysis of dyes it is observed that the dyes containing electron withdrawing groups in bridging anilines are lower while those containing electron donating groups are higher than original dye without bridging groups (Table S1, Supporting information).
From the FTIR spectra of bis anilines (1a–j), appearance of CH2 stretching and bending vibrations is evidenced which are absent in the respective substituted anilines (Fig. 4.1–4.10, Supporting information). The infrared spectra of the synthesized bisazo MCT reactive dyes showed absorption peaks due to O–H, N–H, Ar–H, C–H, C⚌O, C⚌C, C⚌N, NO2, SO3H, N⚌N and C–Cl stretching and bending vibrations at 3400–3500, 3160–3000, 2929, 1700–1760, 1660,1590, 1502, 1070, 723, and 672 cm−1 as depicted from their FTIR spectra (Fig. 4.11–4.16, Supporting information) Specifically speaking, using FTIR spectrum of S1, S2 and S3 a broad band is observed in the range 3000–3500 cm−1 which is due to H-bonding of OH and COOH groups present in the bridging groups. This broad band is masking the peaks of N–H functionality. A peak is observed in the range 1710–1750 cm−1 which is due to C⚌O functionality of dyes. The absorption bands at 1660, 1660, 1590, 1502 and 750 cm−1 depicted the presence of C⚌C stretching and bending vibrations of aromatic moieties, respectively, C–Cl peak is observed in all dyes at 672–700 cm−1 which confirm the triazine ring system in dyes. Azo linkage is inveterated by the peaks in the range 1540–1510 cm−1.
Dyes S4 and S5 have bridging aniline containing OH groups at o- and p-position to the N–H group of diamines, they show a broad peak at 3448 cm−1which is due to OH and N–H stretching vibrations. Similarly S9 shows a peak at 3410 cm−1due to N–H stretching vibrations. This also shows a prominent peak at 1120 cm−1 due to C–O–C stretching vibrations, as it contains the methoxy group at o-position to N–H. S4, S5 and S9 show the remaining peaks similar to S1, S2 and S3 due to common moieties in all dyes. S6 and S7 have bridging groups containing NO2 and Cl, p-position to the NH group, so a peak is observed for the NO2 group at 1550 cm−1 and a peak for C–Cl is observed at 710 cm−1 in addition to C–Cl absorption of triazine ring system. S8 has NO2 at m-position to NH, this also shows peak for NO2 at 1530 cm−1. S10 has Cl at p-position to NH, so here another peak at 700 cm−1 is observed due to Cl attached to the benzene ring. Peak for C–Cl in triazine ring system is at lower wave number than Cl attached to the benzene ring due to the fact that in the latter case bond is stronger than previous one, so appearing at higher wave number. An absorption band at 2900–2950 cm−1 is common in all dyes. (For representative IR spectra see Fig. 4.4, in Supporting information.)
The 1H NMR spectrum of S1, S2 and S3 showed signals down field at 9.59–9.62 ppm due to COOH groups present in the bridging diamines and in the aromatic region of the TMS scale in between δ 7.60 and 8.3 ppm due to 19 aromatic protons. A broad singlet is observed at 5.0–5.10 ppm because of 2O–H groups attached to the naphthalene ring. At range 3.37–3.40 ppm sharp singlet is observed due to the bridging CH2 group present in bis-anilines. All these dyes S1 to S10 are compounds of a series where difference arises in case of bridging groups and chromophores, coupling component and reactive systems are same. Here peak positions and intensity are varied for O–H and bridging methylene protons. In case of S6, S7, S8 and S10 which contain NO2 and Cl at m- and p-positions to the N–H group of bis anilines, methylene as well as N–H peaks move to downfield. While S4, S5 and S9 have OH and OCH3 groups at o and p- positions to N–H groups, these peaks shifted upfield and extra peaks observed at 5.3–5.40 ppm and 3.9 ppm for 1H and 3H attached to oxygen atoms and benzene rings.
3.3 Dyeing properties of dyes
All the dyes S1–S10 were applied at 2.0% depth (Figs. 1 and 2) on cotton fibers according to the usual procedure (Patel and Dasondi, 2008) in the dye bath containing materials (listed in Table S2, in Supporting information).
Samples of dyes applied on cotton cloth pieces before washing treatments for wash fastness.
Samples of dyes applied on cotton cloth pieces after washing treatments for wash fastness.
3.3.1 Exhaustion and fixation study
Exhaustion and fixation values are determined by the application of dyes at 2% dyeing on cotton fibers. Exhaustion and fixation values are shown in Table 1.
Exhaustion refers to the degree of dye transfer from dye bath to fiber usually expressed as percentage of the amount of dye originally placed in the dye bath. For economic and environmental reasons a high degree of exhaustion and fixation is required. Fixation of dye deals with the amount of dye fixed with the fiber of textile materials. These are two important parameters which contribute a lot in selection of dyes for fiber dyeing. Driving force for exhaustion is concentration of dye in two phases and for fixation is the physical as well as chemical interaction. In order to get high degree of exhaustion auxiliary chemicals like NaCl, Na2SO4 and EDTA were added in the dye bath to improve exhaustion. Salts NaCl and Na2SO4 open the grains of cotton fibers and enhance the dye absorption and exhaustion of dyes. EDTA does so by trapping the calcium, magnesium and zinc ions, and prevents the dye precipitation. Triazine has been used as a reactive component to interact with cellulose fibers. Hydroxyl of cotton fibers (cellulose) in alkaline pH interacts with electron deficient carbon attached to the chloro group and high temperature required to replace the third group and to establish covalent bonding as it is evident from the mechanistic pathway dyes with fibers in Fig. 3.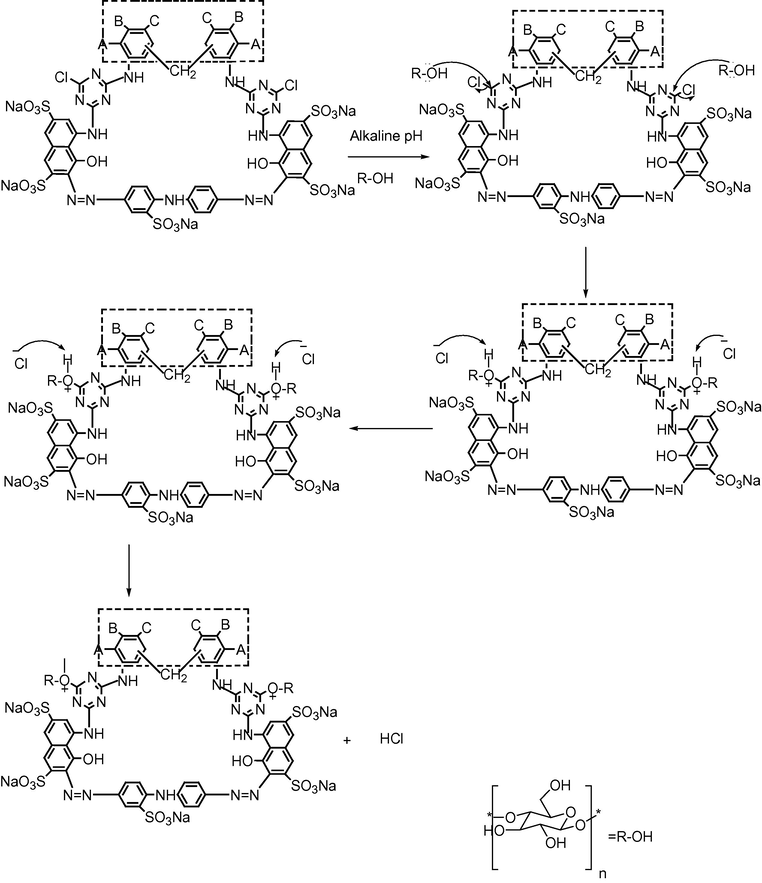
Mechanistic pathway of cotton fiber reaction with synthesized reactive dyes S1–S10.
The percentage exhaustion (Nikhil and Kalpana, 2011) and percentage fixation of 2% dyeing on cotton ranges from 65–75% and 75–92% respectively as it is represented in Table 1. All the dyes have good exhaustion and fixation values which is expected due to the rapid diffusion of the dye molecule within the fabric under dyeing condition and physical as well as chemical interactions of polar groups present in disazo, coupler and bridging components. Reactive component establishes covalent linkages with fiber. Dyes S1, S2 and S10 have high exhaustion and fixation values owing to the presence of carboxylic groups in the bridging anilines. Good exhaustion and fixation values of dyes are in accordance with structure of dyes bearing polar groups which establish physical and chemical interactions with fibers.
3.3.2 Fastness properties
Fastness properties of dyes were assessed after application of 2% dye with respect to cotton fibers as represented in Table 2. These were light fastness, wash fastness and rubbing fastness which provided the clear picture regarding quality of dye.
Dye
Light fastness
Wash fastness
Rubbing fastness
Dry
Wet
S1
6–7
4–5
4
3
S2
6–7
4–5
4
3
S3
6–7
4
5
4
S4
5–6
4–5
4–5
3–4
S5
5–6
4–5
4–5
3–4
S6
5–6
3–4
4–5
3–4
S7
5–6
4
4
3–4
S8
5–6
4
4–5
3–4
S9
5–6
5
4–5
3–4
S10
5–6
4–5
4–5
3–4
Light fastness is the degree to which a dye resists fading due to light exposure. Different dyes have different degrees of resistance to fading by light. Light fastness of all dyes was high in range 4–5. These dyes have little susceptibility to light damage, simply because their strong colors are indications that they absorb the wavelengths that they do not reflect back. Light absorbed by pigmented compounds may serve to degrade them.
Wash fastness is the resistance offered by dyed fibers to retain color when washed by soaps and detergents. In the test, change in color of the textile and also staining of color on the adjacent fabric are assessed. Wash fastness of dyes was in range 5–6.
Color Fastness to rubbing is a main test which is always required for every colored fabric either it is printed or dyed. Rubbing fastness was designed to determine the degree of color which may transfer from the surface of a colored fabric to a specified test cloth for rubbing. Rubbing fastness of all dyes was very high 4–5. Rubbing fastness is an indication of other improved properties like wash fastness, substantively and durability in use. It is obvious from rubbing fastness value that all these dyes have high washing fastness and fixation on cotton fibers.
4 Conclusions
A series of new reactive dyes S1–S10 have been synthesized in high yields from FC-acid and H-acid. Cyanuric chloride was used as a reactive component in these dyes by utilizing its differential reactivity under different temperature and pH conditions. Application of dyes was made on cotton fibers where they showed high light fastness, rub fastness and wash fastness, 6–7, 4–5 and 4–5 respectively. The justification of synthesis of these dyes has been achieved to encompass high exhaustion and fixation values.
References
- Structure reactivity correlations of azo dyes based on H-acid. NMR chemical shift values, pKa values, dyestuff aggregation and dyeing behavior. Dyes Pigm.. 1993;21:23-43.
- [Google Scholar]
- Synthesis and evaluation of a series of symmetrical hot brand bisazo reactive dyes using 4,4′-methylene-bis-metanilic acid on various fiber. J. Saudi Chem. Soc.. 2009;13:279-285.
- [Google Scholar]
- Novel 2-phenyl-3-{4′-[N-(4″ aminophenyl) carbamoyl]-phenyl}-quinazoline-4(3H) one-6-sulphonic acid based mono azo reactive dyes. J. Serb. Chem. Soc.. 2011;76:177-188.
- [Google Scholar]
- Synthesis, characterization and application of novel bisazo reactive dyes on various fibers. Orbital Elec. J. Chem.. 2011;3:57-67.
- [Google Scholar]
- Dyeing behavior of cotton after pretreatment with reactive quaternary compounds. J. Soc. Dyers Colour.. 1984;100:304-315.
- [Google Scholar]
- New methods for improving the dyeability of cellulose fibers with reactive dyes. Rev. Prog. Color.. 1999;29:8-22.
- [Google Scholar]
- Dye migration influences on colour characteristics of wool fabric dyed with acid dye. Fibre Text.. 2005;13:65-69.
- [Google Scholar]
- The synthesis of some bifunctional reactive triazine dyes. Dyes Pigm.. 2002;52:115-120.
- [Google Scholar]
- A new, powerful, high-energy density materials. J. Am. Chem. Soc.. 1996;118:12244-12245.
- [Google Scholar]
- New methods for improving the dyeability of cellulose fibers with reactive dyes. J. Soc. Dyers Color.. 1991;107:102-109.
- [Google Scholar]
- Synthesis and application of reactive dyes with heterocyclic reactive systems. Dyes Pigm.. 1990;14:239-263.
- [Google Scholar]
- Adsorption and thermodynamic study of pigment dyeing on cationised cotton. Inter J. Fibre Text. Res.. 2013;3:6-12.
- [Google Scholar]
- Dye–fibre bond stabilities of dyeings of bifunctional reactive dyes containing a monochlorotriazine and a ß-hydroxyethylsulphonesulphuric acid ester group. J. Soc. Dyers Color.. 1988;104:425-431.
- [Google Scholar]
- Dyeing performance of heterocyclic monoazo dyes based on 3-amino 1H-pyrazolon [3,4-b] quinoline derivatives on various fibers. Arch. Appl. Scie. Res.. 2011;3:359-365.
- [Google Scholar]
- Synthesis and spectral properties of hetarylmonoazo dyes derived from 2-amino-5-nitrothiazole. Oriental J. Chem.. 2011;27:1389-1396.
- [Google Scholar]
- Studies on synthesis and dyeing performance of acid dyes based on 4,7-dihydroxy-1,10-phenanthroline-2,9-dione. Eur. J. Chem.. 2008;5:1033-1036.
- [Google Scholar]
- Synthesis and performance studies of novel bisazo reactive dyes on various fibres. Oriental J. Chem.. 2009;25:927-934.
- [Google Scholar]
- Utilization of reactive epoxy-ammonium quaternaries on cellulose treatment for dyeing with direct and reactive dyes. Textilveredlung. 1970;5:829-838.
- [Google Scholar]
- Current trends in dye chemistry and dyeing technology. Dyestuff Dyeing. 2003;40:185-188.
- [Google Scholar]
- Influence of cationization of cotton fibers on dyeing. J. Text. Apparel Technol. Manag.. 2006;5:1-16.
- [Google Scholar]
- Cationization of cotton and salt-free dyeing them with reactive dyes. J. Dyestuff. Color.. 2004;41:340-345.
- [Google Scholar]
- New developing trends for dyestuff industry home and abroad. Dyes Print.. 2005;4:47-50.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.arabjc.2014.11.010.
Appendix A
Supplementary data
Supplementary data
Supplementary data







