Translate this page into:
Synthesis and in vitro antimycobacterial potential of novel hydrazones of eugenol
⁎Corresponding author. sachinrohane29@gmail.com (Sachin H. Rohane)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
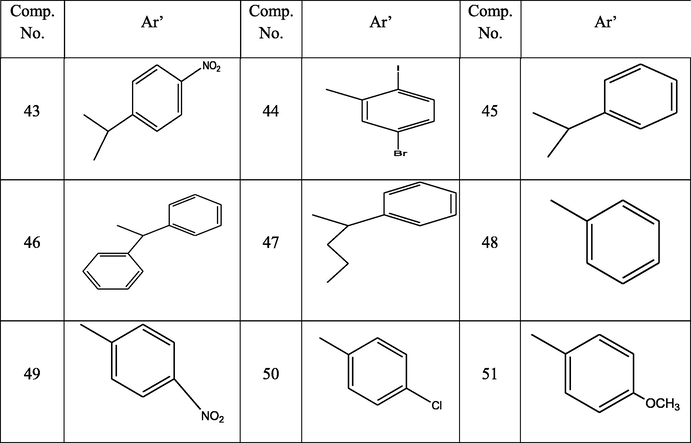
Fifty one hydrazone derivatives of eugenol were designed and docked with 2NSD and 2X22 (enzymes of H37Rv strain) using Schrodinger v7.4. The selective ten hydrazone derivatives (4, 5, 11, 18, 30, 34, 35, 37, 42, and 45) of eugenol were synthesized via esterification, hydrazination and treatment with different aldehydes. Synthesized compounds were characterized by IR, 1H NMR, and LCMS data. The compounds were evaluated for their antitubercular potential against H37Rv using microplate alamar blue assay (MABA). The study revealed that all synthesized compounds were significantly active at concentration 50 and 100 μg/ml, whereas compound 11 exhibited activity at 25 μg/ml. Present study showed that antitubercular activity of novel hydrazone derivatives of eugenol is strongly connected with the position of the substituent on aromatic aldehyde or ketones.
Keywords
Hydrazone
Molecular docking
Antimycobacterial activity
1 Introduction
Current decade noticed tuberculosis as the most common infectious disease in the world that being a leading cause of mortality. Regardless of availability of tuberculosis treatment, yet every year 9 million new cases are reported in those 1.5 million are fatal cases (Pitucha et al., 2019). Tuberculosis (TB) is caused by mycobacterium of the “tuberculosis complex”, including primarily Mycobacterium tuberculosis, Mycobacterium bovis and Mycobacterium africanum (Sensi and Grass, 1996). Drug discovery and development are complicated, time intense and costly method (Pieczonka et al., 2013). It becomes more expensive when safety, efficacy and other issues are raised. In silicon approach of drug design plays a significant role in all stages of drug development from the initial lead design to final stage of clinical aspect of drug (Abdel-Wahab et al., 2011). Reports suggest eugenol and hydrazones to possess significant anti-tubercular potential (de Almeida et al., 2019; More et al., 2018; Krátký et al., 2017). The chemistry of hydrazones always attract the investigators, as incorporation of these moieties in medicinal compounds due to their biological potential. The hydrazones are known to exhibit wide variety of biological activities. They are used as antibacterial agent, anti-tubercular agent, analgesic, anti-inflammatory agent, antiviral agent, antifungal agent, muscle relaxants and antihistamines etc (Yatcheria et al., 2015; Saidugari et al., 2017; Raja et al., 2010; Zheng et al., 2009). In hydrazone, the nitrogen is attached to hydrogen and these hydrazones are stable enough for isolation (March, 1992). However, in some cases, especially with simple alkyl group, they rapidly decompose or polymerizes unless there is at least one aryl group on nitrogen or the carbon (Fuloria et al., 2008). When there is an aryl group the compound are quite stable and these compound are called as Schiff bases. The Schiff reaction is straight forward and proceeds in high yield (Fuloria et al., 2008). Enticed by research evidences, it was thought worthwhile to synthesize some novel hydrazone derivatives of eugenol. We have made an attempt to convert aryloxy moiety into some novel hydrazones via hydrazide and ester intermediates to explore its biological potential. These hydrazones were subjected to molecular docking, synthesis, characterization and antimycobacterial screenings against Mycobacterium tuberculosis.
2 Materials and methods
2.1 General
All chemicals, reagents and solvents were procured from Sigma-Aldrich and Merck Pvt Ltd. and were used without further purification. The reactions were carried out in oven-dried glassware (120 °C) under atmospheric condition. Chemicals and related solvents were procured from Merck Chemicals. From fifty-one in silico docked compounds, the selective ten compounds were subjected to synthesis. The reactions and purity of compounds were monitored by thin layer chromatography (TLC) over percolated plates coated with 0.2 mm Merck 60 F254 silica gel using butanol, acetic acid and methanol (4:3:1, v/v) eluent mixture, and were visualized by UV irradiation (254 nm). The synthesized compounds were purified using column chromatography. The melting points were determined using B-540 melting point apparatus using open capillaries and are uncorrected. The infrared spectra were recorded on a Shimadzu, MIRacle-10, IR Affinity-1 in the range of 400–4000 cm−1. The 1H NMR spectra were recorded in CDCl3 using Agilent VNMRS 400 instrument at 300 MHz with chemical shift 0–10. The chemical shifts, δ are reported in ppm from 0 to 10 using tetramethylsilane (TMS) as internal standard. The mass spectra were performed using LCMS6103 at m/z values: 0–500.
2.2 Molecular docking
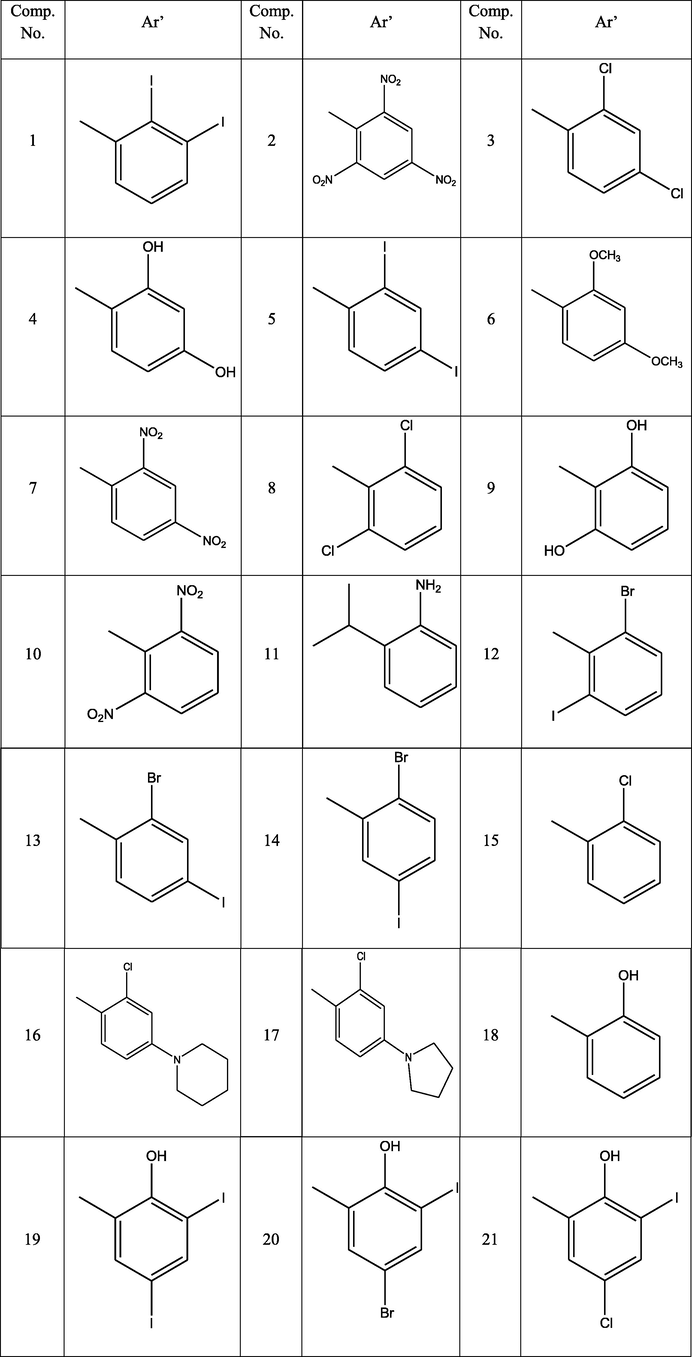
Fifty one compounds (Table 1) were docked in Small-Molecule Drug Discovery Suite of Schrodinger. All compounds were targeted on two enzymes such as 2NSD and 2X22 involved in tuberculosis activity. InhA, the enoyl-ACP reductase in Mycobacterium tuberculosis is an attractive target for the development of novel drugs against tuberculosis. The generated lower energy conformers of all ligands were docked into generated grid of active site of enzymes by XP precision of docking inside Glide-v7.4 (Joshi et al., 2016; Rohane and Makwana, 2019).
2.3 Synthesis of ethyl aryloxy acetate 2
A mixture of compound 1 (eugenol: 0.1 mol), ethyl chloro acetate (0.1 mol) and anhydrous potassium carbonate (0.15 mol) in dried acetone was refluxed for 12 h. Resultant mixture was distilled off and poured on to ice-cold water and stirred. Residue was extracted with ether and the extract was dried over anhydrous sodium sulphate and was purified under reduced pressure to yield compound 2.
2.4 Synthesis of ethylaryloxyacetyl hydrazine 3
A mixture of compound 2 (0.05 mol) and hydrazine hydrate (0.075 mol) in ethanol was refluxed for 4 h and after distilling off the solvent the residue was recrystallized from methanol to yield compound 3.
2.5 Synthesis of hydrazones 4, 5, 11, 18, 30, 34, 35, 37, 42, and 45
A mixture of compound 3 (0.01 mol) and 2, 4-dihydroxy benzaldehyde (0.01 mol) was refluxed for 2 h using acetic acid. The crystals formed were washed with ice-cold water, dried and recrystallized from methanol to yield compound 4. Following the same procedure using respective aldehydes / ketones, other compounds 5, 11, 18, 30, 34, 35, 37, 42, and 45 were synthesized Scheme 1.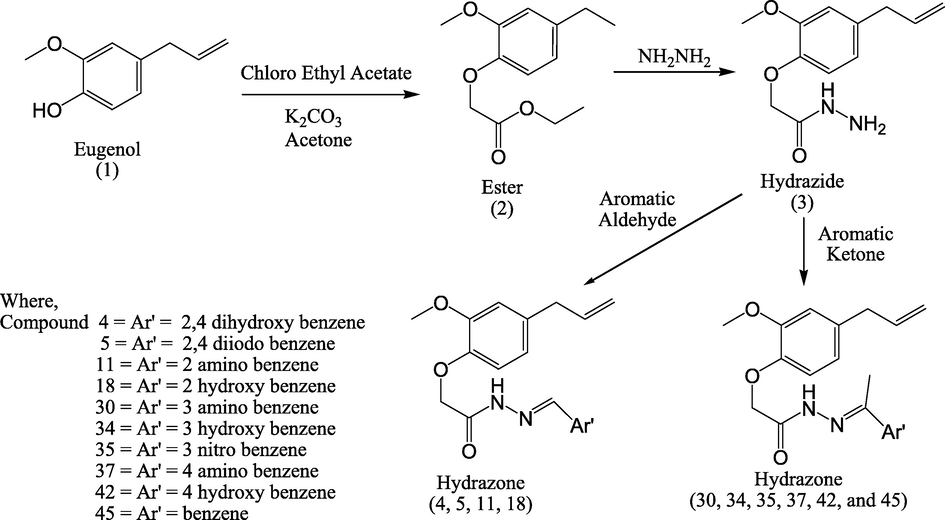
Synthesis of novel hydrazones.
2.6 Anti-tubercular activity
The synthesized compounds (4, 5, 11, 18, 30, 34, 35, 37, 42, and 45) were evaluated for their anti-tubercular potential against standard strain of H37Rv. The method used was microplate Alamar Blue assay (MABA). The final drug concentrations tested were 100–0.2 µg/ml. Plates were covered and sealed with parafilm and incubated at 37 °C for five days. After addition of Alamar Blue reagent and incubating for 24 h, the results were observed. A blue color in the well was interpreted as no bacterial growth, and pink color was scored as growth.
Being a non-toxic method, it has several advantages such as thermal stability of the reagent, and good correlation with BACTEC radiometric method.
3 Result and discussion
3.1 Molecular docking
The Insilco study of all fifty-one compounds was performed using Small-Molecule Drug Discovery Suite of Schrödinger. The compound 11exhibited good docking score and predicted interaction with enzymes. The docking result of novel hydrazone revealed that the binding energies were in the range of −6.097 kcal/mol to −10.393 kcal/mol, with the minimum binding energy of −10.393 kcal/mol (Table 2). The molecules were tested for structure analysis by the visualization tool. The entire compounds protein-ligand complex showed H - bond with the active site residue TYR 158 and PHE 149of 2NSD (Fig. 1) and GLY96 and TYR 158 of X22 (Fig. 2). Note-: Sign ‘ -- ’ indicates compound does not show any Gscore.
Title
2NSD
Title
2X22
XP GScore
Title
XP GScore
XP GScore
Title
XP GScore
Isoniazid
−3.682
26
−8.15
Isoniazid
−5.451
26
−4.74
1
−8.747
27
−7.37
1
−5.959
27
–
2
−6.847
28
−8.101
2
−4.009
28
−7.486
3
–
29
−6.097
3
−8.007
29
−2.37
4
−10.393
30
−9.021
4
−8.426
30
−7.769
5
−7.919
31
−7.804
5
−6.09
31
−6.092
6
−7.247
32
−6.384
6
–
32
−4.919
7
−7.179
33
−6.955
7
−6.67
33
−6.071
8
−8.387
34
−10.13
8
−5.981
34
−7.448
9
−8.525
35
−9.813
9
−7.538
35
–
10
−7.099
36
−6.538
10
–
36
−6.226
11
−9.5
37
−9.632
11
−7.726
37
−9.092
12
−6.942
38
−9.587
12
−4.965
38
−7.932
13
−8.921
39
−6.818
13
–
39
−5.289
14
−8.216
40
−6.921
14
−8.073
40
−7.674
15
−7.428
41
−6.98
15
−6.721
41
–
16
−7.815
42
−9.747
16
−2.124
42
−8.527
17
−7.266
43
−9.256
17
−6.04
43
−6.551
18
−7.632
44
−8.898
18
−6.625
44
−5.356
19
−7.681
45
−9.049
19
−5.954
45
−6.262
20
−7.466
46
−9.593
20
−4.423
46
−7.758
21
−6.799
47
−9.485
21
−7.037
47
−7.556
22
−7.005
48
−6.469
22
−5.353
48
−4.896
23
−7.867
49
−8.072
23
–
49
−5.725
24
−7.883
50
−6.338
24
−4.742
50
−6.666
25
−8.657
51
−6.175
25
−6.098
51
−4.293
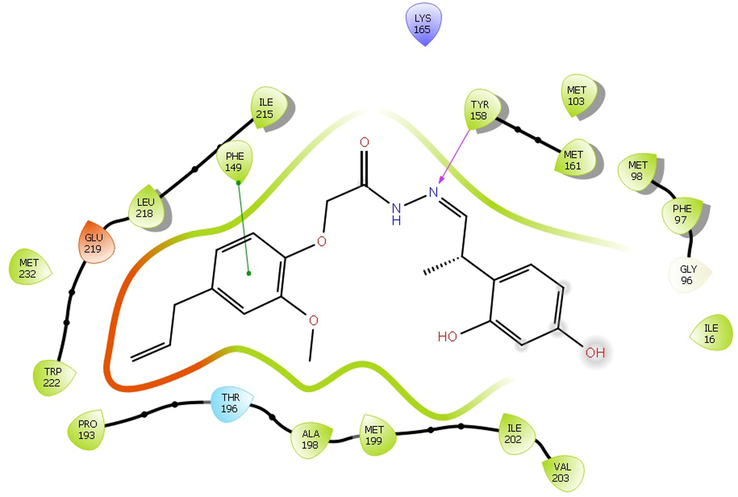
The Interaction between the compound 4 with the active site of PDB 2NSD.Abbreviation: PDB, protein data bank.
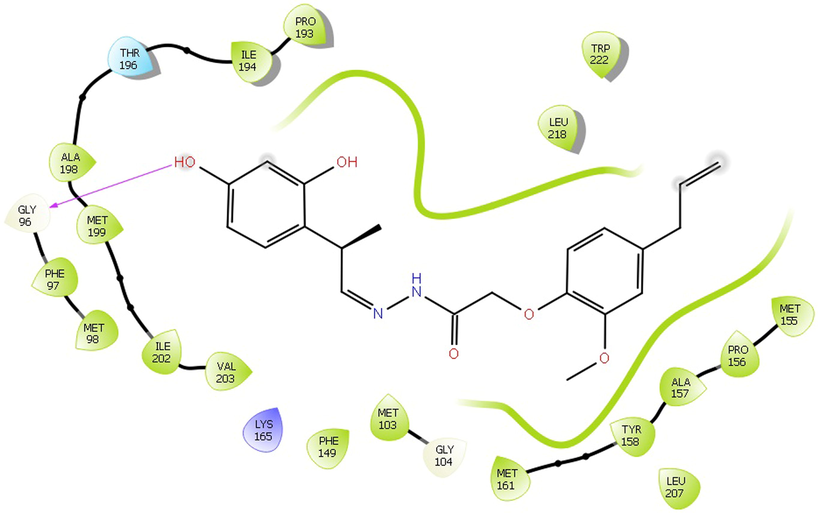
The Interaction between the compound 4 with the active site of PDB 2X22.
3.2 Synthesis and characterization of synthesized compounds
The ten hydrazone compounds 4, 5, 11, 18, 30, 34, 35, 37, 42 and 45 were selected for synthesis based on their in-silico docking results. The derivatives were synthesized by condensation of arylhydrazide with various aromatic aldehydes or ketones using ethanol. Physical data of all synthesized compounds are given in Table 3 and characterization data mentioned in supplementary file. In the IR spectra, all hydrazone derivatives displayed characteristic band from 1700 to 1650 cm−1 attributed to C⚌O stretching vibration. The N—H stretching vibration of the compounds exhibited a band at near 3150 cm−1. The stretching bands for C⚌C and C⚌N groups were observed at 1610–1490 cm−1. In general, the IR stretching frequencies for –OH groups varied for the compounds 4, 18, 34 and 42 in the region 3200–3650 cm−1. In the 1H NMR spectra of all the compounds, the aromatic and aliphatic protons were observed at the specified ppm scale. Aromatic protons were observed at about δ 6.15–7.78 ppm. Synthesized hydrazones (4, 5, 11, 18, 30, 34, 35, 37, 42 and 45) displayed characteristic NMR signals for —OH, —NH, —CH⚌N— protons as coupled peaks at δ value of 4.90–5.10 ppm, 7.10–6.90, and 3.35–2.53 respectively.
Compound- No
Ar’
Mol. Formula
Mol. Weight
MP
oCYield
%
4
C21H24N2O5
384.19
220–221
73.08
5

C19H18I2N2O3
576.18
235–236
62.00
11
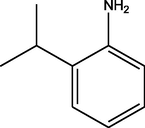
C21H25N3O3
367.44
191–192
72.03
18
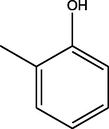
C19H20N2O4
340.37
229–230
75.00
30
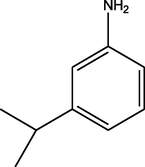
C21H25N3O3
367.44
190–191
59.15
34
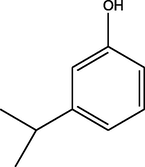
C21H24N2O4
368.43
217–218
74.08
35
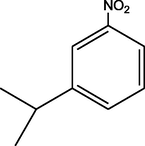
C21H23N3O5
397.42
235–236
60.08
37
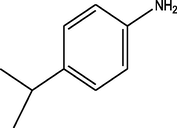
C21H25N3O3
367.44
190–191
72.03
42
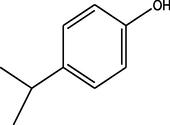
C21H24N2O4
368.43
219–220
76.90
45
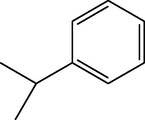
C21H24N2O3
352.43
184–185
65.55
3.3 Antitubercular activity
The compounds 4, 5, 11, 18, 30, 34, 35, 37, 42 and 45 were evaluated for in vitro antimycobacterial activity against M. tuberculosis H37Rv by microplate alamar blue assay (MABA) method, using isoniazid as standard. The results expressed in minimum inhibitory concentration (MIC) are given in Table 4 and the colour change observed during assay method is shown in Fig. 3. The obtained results indicate the biological potential and varying activity depending on the type of substituent on hydrazide nucleus. The results of anti-tubercular activity revealed all newer hydrazones possess inhibitory potential against Mycobacterium tuberculosis. Whereas compound 11 possess highest sensitivity against M. tuberculosis H37Rv at 25 µg/ml level. The antibacterial activity is strongly connected with the position of the electronegative substituent on phenyl ring of aldehydes in relation to the hydrazone skeleton (Fuloria et al., 2017). NOTE: S - Sensitive R- Resistant.
Compound
100 µg/ml
50 µg/ml
25 µg/ml
12.5 µg/ml
6.25 µg/ml
3.12 µg/ml
1.6 µg/ml
0.8 µg/ml
4
S
S
R
R
R
R
R
R
5
S
S
R
R
R
R
R
R
11
S
S
S
R
R
R
R
R
18
S
S
R
R
R
R
R
R
30
S
R
R
R
R
R
R
R
34
S
S
R
R
R
R
R
R
35
S
S
R
R
R
R
R
R
37
S
S
R
R
R
R
R
R
42
S
S
R
R
R
R
R
R
45
S
S
R
R
R
R
R
R
Isoniazid
S
S
S
S
R
R
R
R
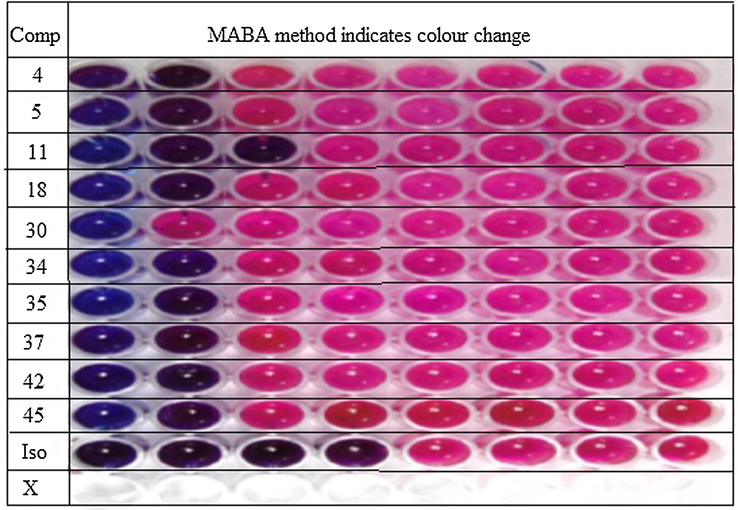
Assessment of Microplate Alamar blue assay result.
4 Conclusion
The molecular docking studies investigating hydrazone derivatives using the enzyme 2NSD and 2X22 as their potential biological target indicate that the amino, azide, hydroxyl and phenyl nucleus of hydrazone derivatives spacer play an important role in interactions with the active site such as TYR 158, ILE 215, GLU 219, PHE 97 and PHE 149 as the most active amino acid residues. Present study establishes the synthesis of newer hydrazone derivatives 4, 5, 11, 18, 30, 34, 35, 37, 42 and 45 by Schiff’s reaction of aryloxy hydrazide with appropriate aldehyde or ketone. The structures of synthesized compounds were confirmed by spectroscopic methods. In the prepared hydrazone all compounds showed significant anti-tubercular action where as compound 11, exhibited highest anti-tubercular activity.
References
- Synthesis, antimicrobial, antioxidant, anti-hemolytic and cytotoxic evaluation of new imidazole-based heterocycles. Eur. J. Med. Chem.. 2011;46:1505-1511.
- [Google Scholar]
- Eugenol and derivatives activity against Mycobacterium tuberculosis, nontuberculous mycobacteria and other bacteria. Future Microbiol.. 2019;14:331-344.
- [Google Scholar]
- Synthesis, characterization and biological studies of novel imines and azetidinones derivatives of haloaryloxy moiety. Asian J. Chem.. 2008;20:4891-4900.
- [Google Scholar]
- Synthesis, characterization and biological studies of new Schiff bases and azetidinones derived from propionic acid derivatives. Asian J. Chem.. 2008;20:6457-6462.
- [Google Scholar]
- Synthesis and discerning of antimicrobial potential of novel oxadiazole derivatives of chloroxylenol moiety. Acta Pol. Pharm.. 2017;74:1125-1130.
- [Google Scholar]
- Molecular docking, synthesis, and antimycobacterial activities of pyrrolylhydrazones and their copper complexes. Res. Rep. Med. Chem.. 2016;6:1-14.
- [Google Scholar]
- Sulfadiazine salicylaldehyde-based Schiff bases: Synthesis, antimicrobial activity and cytotoxicity. Molecules. 2017;22:1573-.
- [Google Scholar]
- Advanced organic chemistry (fourth ed.). New York: John Willey & Sons; 1992.
- Synthesis, characterization and in vitro antitubercular and antimicrobial activities of new aminothiophene Schiff bases and their Co (II), Ni (II), Cu (II) and Zn (II) metal complexes. Oriental J. Chem.. 2018;34:800-812.
- [Google Scholar]
- Synthesis and evaluation of antimicrobial activity of hydrazones derived from 3-oxido-1H-imidazole-4-carbohydrazides. Eur. J. Med. Chem.. 2013;64:389-395.
- [Google Scholar]
- Synthesis, In vitro screening and docking studies of new thiosemicarbazide derivatives as antitubercular agents. Molecules. 2019;24:251-266.
- [Google Scholar]
- Antibacterial and antitubercular activities of some diphenylhydrazones and semicarbazones. Indian J. Chem.. 2010;49:1384-1388.
- [Google Scholar]
- In silico study for the prediction of multiple pharmacological activities of novel hydrazone derivatives. Indian J. Chem.. 2019;58:387-402.
- [Google Scholar]
- Synthesis, characterization and antibacterial activity of (E)-4-((3-methyl-4-(methylsulfonyl)pyridin-2-yl)methoxy)-N'-(substitutedbenzylidene)benzohydrazide derivatives. Indian J. Chem.. 2017;56:177-182.
- [Google Scholar]
- Burger’s medicinal chemistry and drug discovery (5th ed.). New York, NY: John Wiley and Sons; 1996.
- Synthesis, characterization and antibacterial activity of some new 3-(3-(trifluoromethyl)-phenyl)-3-(2-hydroxy-5-methylphenyl)-propanehydrazones. Indian J. of Chem.. 2015;54:1162-1167.
- [Google Scholar]
- Synthesis of novel substituted pyrazole-5-carbohydrazide hydrazone derivatives and discovery of a potent apoptosis inducer in A549 lung cancer cells. Bioorg. Med. Chem.. 2009;17:1957-1962.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.09.004.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







