Translate this page into:
Synthesis and investigation of anti-inflammatory and anticonvulsant activities of novel coumarin-diacylated hydrazide derivatives
⁎Corresponding authors at: Department of Chemistry, Arts and Science Faculty, Çukurova University, Adana 01330, Turkey (E. S. Giray). m.cam@ucl.ac.uk (Muhammet Emin Çam), esgiray@cu.edu.tr (Elife Sultan Giray)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A number of novel diacylhydrazide- coumarin derivatives synthesized. Anti-inflammatory and anticonvulsant activities were investigated. N′-(2-hyroxybenzoyl)-2-oxo-2H-chromene-3-carbohydrazide was found the most efficient molecule for both tests.
Abstract
A number of novel coumarin derivatives synthesized by the reaction of 3-carbonyl chloride coumarin with some substituted aryl acid hydrazides to investigate their anti-inflammatory and anticonvulsant activities. Carrageenan (0.1 ml of 1%, w/v) was injected subplantarly in the right paw of rats to induce an acute model of inflammation. Anti-inflammatory efficacy was evaluated for 5 hours at 3 different dosages 5, 10, 25 mg/kg. After that, the changes in the level of paw edema volumes and percentage inhibition of all groups were observed and the most effective coumarin derivative was found as N'-(2-hyroxybenzoyl)-2-oxo-2H-chromene-3-carbohydrazide. In addition, N’-(2-oxo-2H-chromene-3-carbonyl)nicotinohydrazide, (E)-N’-(3-(4-hydroxyphenyl)acryloyl)-2-oxo-2H-chromene-3-carbohydrazide, and N’-(5-amino-2-hydroxybenzoyl)-2-oxo-2H-chromone-3-carbohydrazide showed their anti-inflammatory effects in a dose-dependent manner. On the other hand, pentylenetetrazole (PTZ, 80 mg/kg, i.p.)-induced seizure model was used to investigate the anticonvulsant activities of six newly synthesized coumarin derivatives in mice. Hybrid compound of salicylic acid hydrazide and 3-carbonyl chloride coumarin (8d) was found the most promising anticonvulsant agent among all treatment groups according to the onset of seizure and survival rate. Moreover, (E)-N'-cinnamoyl-2-oxo-2H-chromene-3-carbohyrazide (8b) and (E)-N'-(3-(4-hyroxyphenyl)acryloyl)-2-oxo-2H-chromene-3-carbohydrazide (8c) has potential anticonvulsant efficiency in low doses (30 mg/kg). The anticonvulsant effect of these coumarin derivatives may be through enhanced GABA-mediated inhibition in the brain.
Keywords
Anti-inflammatory activity
Anticonvulsant activity
Coumarin-diacylated hydrazide
Pentylenetetrazole-induced seizures
Carrageenan-induced paw edema
1 Introduction
Epilepsy is a serious chronic neurologic disorder which characterized by spontaneously occurring seizures. It can be seen among people of all ages. Currently available antiepileptic drugs (AEDs) are able to successfully control seizures with in most patients with epilepsy, however, 30–40% of all epileptic patients still suffer from some side effects and seizure resistance to the current AEDs (Sørensen and Kokaia, 2013). Therefore, researchers keep to continue to develop novel anticonvulsant compounds to cover seizures to treat epilepsy.
Inflammation is the natural defense mechanism of the body against irritants, trauma, pathogens, or microbial invasion. It occurs in the human body with symptoms such as pain, swelling, redness, heat, and disturbance of function (Aghasafari et al., 2019; Minhas et al., 2017; Pahwa and Jialal, 2019). Migration and activation of leukocytes cause tissue destruction and enhanced blood vessel permeability. As a result, inflammation could be occur by activation of several enzymes (lipoxygenases and cyclooxygenases (COX)), production of inflammatory mediators (leukotrienes and prostaglandins), and reactive oxygen species (Abdel-Lateff et al., 2020).
The development of hybrid molecules via the combination of two or more different pharmacophore units in one frame is an attractive area in the design of new drugs. Hence, these new molecules may show interesting biological activities. Due to multifunctional features of coumarin among the new hybrid molecules, extensive efforts have been made on design and synthesis of coumarin derivatives, which was coupled with different bioactive molecules (Sashidhara et al., 2011; Sashidhara et al., 2010).
Coumarins are widely reported that they have several biological activities. They have been used to treat diseases in a wide range from cancer, burns, brucellosis to cardiovascular and rheumatic diseases (Kennedy and Thornes, 1997; Hoult and Payá, 1996; Ma et al., 2008; Kostova, 2005; Musa and Cooperwood, 2008). In addition, various coumarin derivatives are reported that they have anti-inflammatory and antiepileptic activities. They also able to inhibit the lipoxygenase and cyclooxygenase enzymes (Piller, 1975). Coumarin compounds, especially substituted with hydroxyl or other electron releasing groups were found to inhibit lipid peroxidation and to scavenge hydroxyl radicals and superoxide anions (Payá et al., 1992). α-lipoic acid derivatives of coumarin-3-carboxamides were also reported as potent antioxidant and anti-inflammatory agents (Kontogiorgis and Hadjipavlou-Litina, 2005; Melagraki et al., 2009; Sashidhara et al., 2011; Vardhan Reddy et al., 2016).
Diacylhydrazines have been reported as a powerful nonsteroidal insect growth regulators and the first commercialized product RH-5992 (tebufenozide) was reported by Rohm&Haas Company (Heller et al., 1992). Later, replacement of one of aryl units with a benzoheterocycle containing oxygen units gave the products ANS-118 and JS-118 (Li et al., 2010; Zhang et al., 2001). CUI et al synthesized a series of novel diacylhydrazine derivatives containing a furan ring and investigated their anti-tumor activity. They reported that some of the furan-N,N′-diacylhidrazites have potential activity against some human cancer cell lines, such as human leukemia cell line HL-60, human gastric carcinoma cell line (Cui et al., 2008).
After all mentioned findings, novel diacyl hydrazide derivatives containing biaryl unites which one of them is coumarin have been designed to evaluate their anti-inflammatory and anticonvulsant activities. During the design of the molecules, keeping in mind the synergistic effects of two biologically active units in one frame, we considered two hydrogen bonding and two electron donor group (—NHCO—) and two hydrophobic aryl groups (coumarin and aryl acid) as key pharmacophores for anticonvulsant and anti-inflammatory activities (Fig. 1). Six novel N,N′-diacyl hydrazide derivatives (8a-f) were prepared by the reaction of 2-oxo-2H-chromen-3-carbonyl chloride (7) with some substituted aryl acid hydrazides (3a-f) (Scheme 1).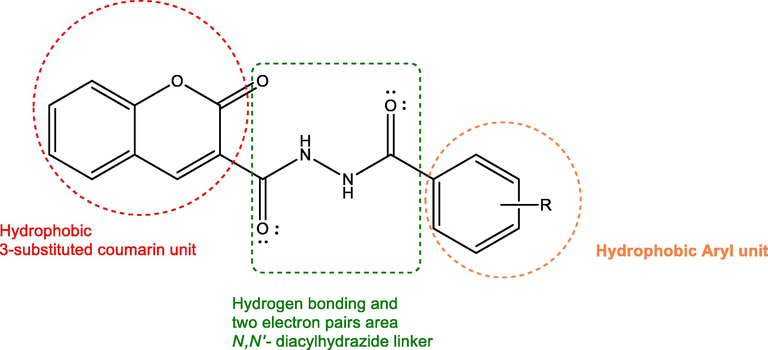
Key pharmacophore units of novel coumarin-diacylhydrazide hybrids.
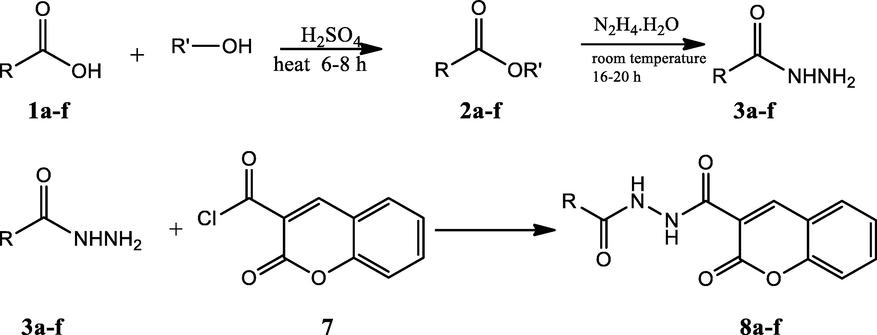
Synthesis of diacylated hydrazides hybrids.
2 Materials and methods
2.1 Chemistry
All the reagents were obtained from different commercial sources. Unless noted otherwise, all of compounds were used as provided without further purification. Nicotinic acid (≥98%), salicylaldehyde (≥98%), trans-cinnamic acid (≥99.%), p-coumaric acid (≥98.%), salicylic acid (ACS reagent ≥ 99.%), 5-amino-salicylic acid (95–98.%), gallic acid (97%), hydrazine hydrate (reagent grade, 50–60%), malonic acid (99.%), thionyl chloride (reagent grade, 97%), sulphuric acid (99.999%), acetic acid glacial (reagent grade (≥99.%), tetrahydrofuran (≥99.%), methanol (anhydrous, 99.8%) and ethanol (99.8%) were purchased from Sigma Aldrich. λ-carrageenan, pentylenetetrazole (PTZ), indomethacin, and carbamazepine were purchased from Sigma-Aldrich (Poole, UK). Follow up of the reactions and checking the purity of the compounds were made by TLC on silica gel-precoated aluminium sheets (Type 60, F254, Merck, Darmstadt, Germany) using hexane/ethyl acetate 80–20 (4:1, v/v) and the spots were detected by exposure to UV lamp at λ254 nanometer for few seconds. Melting points were determined using an Electrothermal 9100 instrument in open capillary tubes. IR spectra were recorded on a Perkin-Elmer 55,148 spectrometer using KBr pellets. 1H and 13C NMR spectra were recorded in CDCI3 or DMSO4-d6 [using the solvent peak as internal reference (DMSO-d6: δ H 2.50; δ C 39.51 and CDCl3 at 7.27 ppm for 1H and 77.0 ppm for 13C) on a Bruker Avance III 400 MHz spectrometer operating at 400 MHz and 100 MHz, respectively. All chemical shift values are quoted in ppm and coupling constants quoted in Hz. Multiplicities are indicated, s (singlet), d (doublet), t (triplet), q (quartet), sept (septet), m (multiplet), br s (broad singlet). Elemental analyses were measured on a Thermo Flash 2000 Organic Elemental Analyzer. The structural data for 2aErharuyi et al. (2015); 2bSteele et al. (2020); 2cLee et al. (2013); 2dPetrus and Sobota, 2013; 2eFiuza et al. (2004); 2fBailey et al. (2004): 3aVimal Kumar Varma and Amareshwar (2011); 3bKatritzky et al. (2001); 3cSaritha and Rajitha (2019); 3dPattan et al. (2009); 3eTaha et al. (2019); 3fErsan et al. (2008); 6Patil et al. (2018); 7Zobaydi et al. (2016) and 8dJesumoroti et al. (2019) is in accordance with the results reported in the literature.
2.1.1 Synthesis of carboxylic acid ester derivatives (2a-2f) (Fiuza et al., 2004)
For the esterification reaction, the reaction was carried out with carboxylic acid and methanol taken at a ratio of 1:10 under reflux for about 6 h at about 60–80 °C. After completion of the reaction, excess of methyl alcohol was distilled and the solid formed was washed with cold water and purified from impurities. The solid was dissolved in ethanol, water was added until the cloudy color was achieved and allowed to crystallize. The product was identified using FT-IR and NMR spectroscopy.
2.1.1.1 Methyl nicotinate (2a) (Erharuyi et al., 2015)
white solid; yield 1.165 g (85%); mp (°C): 42–44; FT-IR (KBr) 3030 (CH, aromatic), 2967 (CH, aliphatic), 1710 (C⚌O), 1674 (C⚌O) 1606, 1567 (C⚌C, aromatic), 1281 (C—O); δH (400 MHz, DMSO-d6) 9.79 (s, 1H), 7.05 (d, J = 8 Hz, 1H), 6.87–6.83 (m, 1H), 6.72 (d, J = 8 Hz, 1H), 3.86 (s, 3H); δC (100 MHz, DMSO-d6) 169.68, 151.81, 140.66, 123.30, 117.61, 113.12, 52.22; Anal. Calcd. for C7H7NO2: C, 61.31; H, 5.14; N, 10.21 Found: C, 61.50; H, 5.11; N, 10.17.
2.1.1.2 Methyl cinnamate (2b) (Steele et al., 2020)
Pale yellow solid; yield 1.525 g (94%); mp (°C): 125–127; FT-IR (KBr) 3111 (CH, aromatic), 2952 (CH, aliphatic), 1682 (C⚌O), 1632 (C⚌C, aliphatic), 1597, 1514 (C⚌C, aromatic), 1193 (C—O), 984 (C⚌C, aliphatic-trans), 831 (C—H, p-substitue); δH (400 MHz, DMSO-d6) 7.38 (d, J = 16 Hz, 1H), 7.21 (d, J = 16 Hz, 1H), 7.10–6.96 (m, 2H), 6.74–6.64 (m, 3H), 3.90 (s, 3H); δC (100 MHz, DMSO-d6) 163.97, 156.66, 134.15, 132.23, 131.18, 127.87, 116.58, 63.75; Anal. Calcd. for C10H10O2: C, 74.06; H, 6.21; Found: C, 74.21; H, 6.17.
2.1.1.3 (E)-methyl 3-(4-hydroxyphenyl)acrylate (2c) (Lee et al., 2013)
Yellow solid; yield 1.337 g (65%); mp (°C) : 143–145; FT-IR (KBr) 3356 (O—H), 3039 (CH, aromatic), 2952 (CH, aliphatic), 1682 (C⚌O) 1632 (C⚌C, aliphatic), 1597, 1514 (C⚌C, aromatic), 1328 (C—O), 984 (C⚌C, aliphatic-trans), 830 (C—H, p-substitue); δH (400 MHz, DMSO-d6) 8.96 (s, 1H), 7.21 (d, J = 16 Hz, 1H), 7.08 (d, J = 16 Hz, 1H), 6.97 (d, J = 8 Hz, 2H) 6.65 (d, J = 8 Hz, 2H), 3.85 (s, 3H); δC (100 MHz, DMSO-d6) 170.93, 155.44, 145.54, 131.19, 128.99, 115.01, 114.88, 61.56; Anal. Calcd. for C10H10O3: C, 67.41; H, 5.66; Found: C, 67.57; H, 5.62.
2.1.1.4 Methyl 2-hydroxybenzoate (2d) (Petrus and Sobota, 2013)
clear liquid; yield 1.370 g (90%); FT-IR (KBr) 3152 (CH, aromatic), 2955 (CH, aliphatic), 1674 (C⚌O), 1614, 1487 (C⚌C, aromatic), 1302 (C—O), 754, 699 (C—H, o-substitue); δH (400 MHz, DMSO-d6) 10.55 (s, 1H), 7.78 (d, J = 8 Hz, 1H), 7.53 (t, J = 8 Hz, 1H), 6.92–6.89 (m, 2H), 3.88 (s, 3H); δC (100 MHz, DMSO-d6) 169.31, 160.14, 135.59, 129.87, 119.29, 117.31, 112.76, 52.33; Anal. Calcd. for C8H8O3: C, 63.15; H, 5.30; Found: C, 63.29; H, 5.27.
2.1.1.5 Methyl 3,4,5-trihydroxybenzoate (2e) (Fiuza et al., 2004)
white solid; yield 1.842 g (99,99%); mp (°C) : 201–203; FT-IR (KBr) 3355 (O—H), 3045 (C—H, aromatic), 2952 (C—H, aliphatic), 1674 (C⚌O), 1614, 1585 (C⚌C, aromatic), 1300 (C—O); δH (400 MHz, DMSO-d6) 9.30 (s, 2H), 8.31 (s, 1H), 6.79 (s, 2H), 3.74 (s, 3H); δC (100 MHz, DMSO-d6) 166.45, 145.40, 136.40, 123.17, 106.23, 54.45; Anal. Calcd. for C8H8O5: C, 52.18; H, 4.38; Found: C, 52.39; H, 4.35.
2.1.1.6 Methyl 5-amino-2-hydroxybenzoate (2f) (Bailey et al., 2004)
white solid; yield 1.504 g (90%); mp (°C): 95–97; FT-IR (KBr) 3409 (O—H), 3326 (N—H), 3027 (C—H, aromatic), 2962 (C—H, aliphatic), 1670 (C⚌O), 1613, 1594 (C⚌C, aromatic), 1340 (C—N), 1207 (C—O), 890 (C—H, 1,2,4-tri substitue); δH (400 MHz, DMSO-d6) 11.13 (s, 2H), 9.01 (s, 1H), 7.70 (d, J = 8 Hz, 1H), 7.41 (t, J = 8 Hz, 1H), 6.99 (d, J = 8 Hz, 1H), 3.34 (s, 3H); δC (100 MHz, DMSO-d6) 168.53, 157.94, 134.45, 128.03, 120.21, 117.90, 113.53, 54.39; Anal. Calcd for C8H9NO3: C, 57.48; H, 5.43; N, 8.38; Found: C, 57.64; H, 5.40; N, 8.33.
2.1.2 Synthesis of carboxylic acid hydrazide derivatives (3a-3f) (Ersan et al., 2008)
The ester compounds (5 mmol) synthesized in the first step were dissolved in methanol (50 mmol, 2.02 ml) and an equivalent amount of hydrazine hydrate was added thereto. The reaction was carried out by mixing the mixture at room conditions for 16–20 h. The solid obtained as a result of the reaction was removed from the impurities by washing with methanol in the filtration apparatus. The crude product was recrystallized from ethanol to obtain pure product. The product was identified using FT-IR and NMR spectroscopy.
2.1.2.1 Nicotinohydrazide (3a) (Vimal Kumar Varma and Amareshwar, 2011)
white solid; yield 0.460 g (67%); mp (°C): 200–202; FT-IR (KBr) 3367 (N—H), 3279 (NH2), 3029 (C—H, aromatic), 2909, 2867 (C—H, aliphatic), 1710 (C⚌O), 1612, 1566 (C⚌C, aromatic), 1226 (C—N), 756 (C—H, m-substitue); δH (400 MHz, DMSO-d6) 9.36 (s, 1H),7.98 (s, 1H), 7.05 (d, J = 8 Hz, 1H), 6.87 (d, J = 8 Hz, 1H), 6.84–6.71 (m, 1H), 4.33 (s, 2H); δC (100 MHz, DMSO-d6) 168.48, 150.23, 138.95, 121.46, 116.43, 112.13; Anal. Calcd. for C6H7N3O: C, 52.55; H, 5.14; N, 30.64; Found: C, 52.76; H, 5.10; N, 30.58.
2.1.2.2 Cinnamohydrazide (3b) (Katritzky et al., 2001)
Brown solid; yield 0.617 g (76%); mp (°C): 114–116; FT-IR (KBr) 3267 (NH2), 3187 (N—H), 3011 (C—H, aromatic), 2973 (C—H, aliphatic), 1650 (C⚌O), 1636 (C⚌C, aliphatic), 1597, 1514 (C⚌C, aromatic), 1279 (C—N), 1248 (C—O) 965 (C⚌C, aliphatic-trans), 826 (C—H, p-substitue; δH (400 MHz, DMSO-d6) 8.30 (s, 1H), 7.72 (t, J = 8 Hz, 4H), 7.42 (d, J = 16 Hz, 1H), 7.30 (d, J = 8 Hz, 1H), 6.82 (d, J = 16 Hz, 1H), 3.83 (s, 2H); δC (100 MHz δC (100 MHz, DMSO-d6) 163.89,154.36, 140.89, 132.95, 128.46, 118.08, 115.85; Anal. Calcd. for C9H10N2O: C, 66.65; H, 6.21; N, 17.27; Found: C, 66.84; H, 6.17; N, 17.22.
2.1.2.3 (E)-3-(4-hydroxyphenyl)acrylohydrazide (3c) (Saritha and Rajitha, 2019)
white solid; yield 0.642 g (72%); mp (°C): 143–145; FT-IR (KBr) 3267 (O—H), 3187 (N—H), 3011 (C—H, aromatic), 2943 (C—H, aliphatic), 1650 (C⚌O), 1637 (C⚌C, aliphatic), 1585, 1515 (C⚌C, aromatic), 1279 (C—N), 1248 (C—O) 965 (C⚌C, aliphatic-trans), 825 (C—H, p-substitue); δH (400 MHz, DMSO-d6) 8.96 (s, 1H), 8.17 (s, 1H), 7.20 (d, J = 16 Hz, 1H), 7.09 (d, J = 16 Hz, 1H), 6.97 (d, J = 8 Hz, 2H), 6.68 (d, J = 8 Hz, 2H), 4.46 (s, 2H); δC (100 MHz, DMSO-d6) 168.67, 153.43, 147.86, 130.36, 128.73, 118.29, 116.08; Anal. Calcd. for C9H10N2O2: C, 60.66; H, 5.66; N, 15.72; Found: C, 60.85; H, 5.63; N, 15.66.
2.1.2.4 2-hydroxybenzohydrazide (3d) (Pattan et al., 2009)
white solid; yield 0.555 g (73%); mp (°C): 149–151; FT-IR (KBr) 3340 (—OH), 3190 (N—H), 1656 (C⚌O), 1531, 1457 (C⚌C, aromatic), 1332 (C—N), 1291 (C—O), 769, 698 (C—H, o-substitue); δH (400 MHz, DMSO-d6) 8.96 (s, 1H), 8.17 (s, 1H), 7.22–6,95 (m, 2H), 6.80–6,64 (m, 2H), 4.44 (s, 2H); δC (100 MHz, DMSO-d6), 163.97, 155.43, 134.08, 131.18, 128.98, 127.87,114.92; Anal. Calcd. for C7H8N2O2: C, 55.26; H, 5.30; N, 18.41; Found: C, 55.45; H, 5.27; N, 18.36.
2.1.2.5 3,4,5-trihydroxybenzohydrazide (3e) (Taha et al., 2019)
white solid; yield 0.875 g (95%); mp (°C): 283–285; FT-IR (KBr) 3424 (O—H), 3390 (N—H), 3297 (NH2), 3143 (C—H, aromatic), 1656 (C⚌O), 1602, 1539 (C⚌C, aromatic), 1338 (C—N), 1204 (C—O); δH (400 MHz, DMSO-d6) 9.33 (s, 1H), 8.38 (s, 3H), 6,75 (s, 2H), 4.41 (s, 2H); δC (100 MHz, DMSO-d6) 166.46, 145.48, 136.40, 123.30, 106.40; Anal. Calcd. for C7H8N2O4: C, 45.66; H, 4.38; N, 15.21; Found: C, 45.83; H, 4.34; N, 15.15.
2.1.2.6 5-amino-2-hydroxybenzohydrazide (3f) (Ersan et al., 2008)
white solid; yield 0.610 g (73%); mp (°C): 190–192; FT-IR (KBr) 3337 (O—H), 3196 (N—H), 3027 (C—H, aromatic), 1585 (C⚌O), 1539, 1470 (C⚌C, aromatic), 1315 (C—N), 1217 (C—O), 900 (C—H, 1,2,4-tri substitue); δH (400 MHz, DMSO-d6) 11.41 (s, 2H), 10.23 (s, 1H), 9.32 (s, 1H), 7.04–6,73 (m, 3H), 4.71 (s,2H); δC (100 MHz, DMSO-d6) 170.92, 155.43, 145.53, 134.17, 131.19, 128.99, 115.03; Anal. Calcd. for C7H9N3O2: C, 50.29; H, 5.43; N, 25.14; Found: C, 50.49; H, 5.40; N, 25.09.
2.1.3 Synthesis of 2-oxo-2H-chromene-3-carboxylic acid (6) (Karade et al., 2007)
Malonic acid (10 mmol) (4), salicylaldehyde (10 mmol) (5) and L-proline (10% by weight) were added to the 50 ml round-bottom flask and the reaction was carried out by mixing with a magnetic stirrer at 80–90 °C for 30 min. The solid formed was filtered and washed away with purified water to remove impurities. The crude product was recrystallized from ethanol to obtain pure product. The product was identified using FT-IR and NMR spectroscopy.
2.1.3.1 2-oxo-2H-chromene-3-carboxylic acid (6) (Patil et al., 2018)
white solid; yield 1.711 g (90%); mp (°C): 185–187; FT-IR (KBr) 3057 (O—H), 3005 (C—H, aromatic), 2912 (C—H, aliphatic), 1737 (C⚌O), 1606, 1557 (C⚌C, aromatic), 1226 (C—O); δH (400 MHz, DMSO-d6) 13.69 (s, 1H), 8.71 (s, 1H), 7.87 (d, J = 8 Hz, 2H), 7.69 (t, J = 8 Hz, 1H), 7.36 (t, J = 8 Hz, 1H); δC (100 MHz, DMSO-d6) 163.74, 156.68, 154.37, 148.32, 134.19, 130.10, 124.72, 118.12, 117.87, 116.01; Anal. Calcd. for C10H6O4: C, 63.16; H, 3.18; Found: C, 63.37; H, 3.15.
2.1.4 Synthesis of 2-oxo-2H-chromene-3-carbonyl chloride (7) (Zobaydi et al., 2016)
3-carboxy coumarin (10 mmol) and thionyl chloride (20 mmol) were added to the 50 ml round bottom flask and mixed under reflux for 6–8 h in solvent-free medium. The solid formed was filtered and washed away with purified water to remove impurities. The crude product was recrystallized from ethanol to obtain pure product. The product was identified using FT-IR and NMR spectroscopy.
2.1.4.1 2-oxo-2H-chromene-3-carbonyl chloride (7) (Zobaydi et al., 2016)
yellow solid; yield 1.817 g (95%); mp (°C): 155–157; FT-IR (KBr) 3058 (C—H, aromatic), 1769 (C⚌O), 1605, 1556 (C⚌C, aromatic), 1364 (C—Cl), 1287 (C—O), 758 (C—Cl); δH (400 MHz, DMSO-d6) 8.71 (s, 1H), 7.89 (d, J = 8 Hz, 1H), 7.85 (d, J = 8 Hz, 1H), 7.72–7.34 (m, 2H); δC (100 MHz, DMSO-d6) 160.30, 159.33, 153.11, 147.90, 136.19, 128.13, 125.03, 120.35, 118.61, 115.22; Anal. Calcd. for C10H5ClO3: C, 57.58; H, 2.42; Cl, 17.00 Found: C, 57.79; H, 2.39; Cl, 16,54.
2.1.5 Synthesis of coumarin hydrazide hybrid derivatives (8a-8f)
Carboxylic acid hydrazide derivative (10 mmol) was taken into a 50 ml round bottom flask and 3–4 drops of acetic acid was added by dissolving with THF and mixed. 2-oxo-2H-chromene-3-carbonyl chloride (3b) (10 mmol) was added on it and mixed under reflux for 6 h. After the reaction was completed, the excess of THF was distilled and removed. The remaining solid was neutralized with aqueous Na2CO3.The crude product was recrystallized from ethanol to obtain pure product. The product was identified using FT-IR and NMR spectroscopy.
2.1.5.1 N′-(2-oxo-2H-chromene-3-carbonyl)nicotinohydrazide (8a)
white solid; yield 2.784 g (90%); mp (°C): >300; FT-IR (KBr) 3365, 3282 (N—H), 3060 (C—H, aromatic), 2923, 2853 (C—H, aliphatic), 1737, 1710, 1643 (C⚌O), 1612, 1566 (C⚌C, aromatic), 1314 (C—N), 1226 (C—O), 766 (C—H, m-substitue), 756 (C—H o-substitue); δH (400 MHz, DMSO-d6) 9.79 (s, 1H), 9.14 (s, 1H), 8.73 (s, 1H), 8.41–8.22 (m, 3H), 6.87–6.71 (m, 5H);δC (100 MHz, DMSO-d6) 163.93, 156.65, 154.41, 148.33, 134.22, 130.13, 127.69, 124.75, 118.25, 117.92, 116.06,112.84; Anal. Calcd. for C16H11N3O4: C, 62.14; H, 3.58; N, 13.59; Found: C, 62.35; H, 3.55; N, 13.53.
2.1.5.2 (E)-N′-cinnamoyl-2-oxo-2H-chromene-3-carbohydrazide (8b)
yellow solid; yield 2.842 g (85%); mp (°C): >300; FT-IR (KBr) 3367 (N—H), 3267 (NH2), 3061 (C—H, aromatic), 2952, 2838 (C—H, aliphatic), 1710 (C⚌O), 1637 (C⚌C, aliphatic), 1611, 1514 (C⚌C, aromatic), 1248 (C—N), 1171 (C—O) 965 (C⚌C, aliphatic-trans), 826 (C—H, p-substitue); δH (400 MHz, DMSO-d6) 9.01 (s, 1H),8.60 (s, 1H), 7.88 (d, J = 16 Hz, 1H), 7.74–7.69 (m, 5H), 7.43–7.36 (m, 5H), 7.14 (d, J = 16 Hz, 1H); δC (100 MHz, DMSO-d6) 165.87, 158.47, 152.50, 140.21, 136.62,132.58, 126.32,119.90, 117.04, 115.28; Anal. Calcd. for C19H14N2O4: C, 68.26; H, 4.22; N, 8.38; Found: C, 68.48; H, 4.19; N, 8.32.
2.1.5.3 (E)-N′-(3-(4-hydroxyphenyl)acryloyl)-2-oxo-2H-chromene-3-carbohydrazide (8c)
brown solid; yield 2.452 g (70%); mp (°C): >300; FT-IR (KBr) 3634 (O—H), 3324 (N—H), 3200 (NH2), 3028 (C—H, aromatic), 2955, 2897 (C—H, aliphatic), 1653 (C⚌O), 1620 (C⚌C, aliphatic), 1603, 1508 (C⚌C, aromatic), 1260 (C—N), 1242 (C—O) 997 (C⚌C, aliphatic-trans), 825 (C—H, p-substitue); δH (400 MHz, DMSO-d6) 13.27 (s, 1H), 8.73 (s, 2H), 8.45 (s, 1H), 7.88 (d, J = 16 Hz, 1H), 7.74–7.69 (m, 2H), 7.43–7.36 (m, 4H), 7.13 (d, J = 16 Hz, 1H); δC (100 MHz, DMSO-d6) 163.93,156.64, 154.421, 148.33, 134.21, 130.13, 124.75, 118.25, 117.92, 116.06; Anal. Calcd. for C19H14N2O5: C, 65.14; H, 4.03; N, 8.00; Found: C, 65.35; H, 4.00; N, 7.94.
2.1.5.4 N′-(2-hydroxybenzoyl)-2-oxo-2H-chromene-3-carbohydrazide (8d) (Jesumoroti et al., 2019)
yellow solid; yield 2.919 g (90%); mp (°C): >300; FT-IR (KBr) 3361 (OH), 3265 (N—H), 3053 (C—H, aromatic), 1698 (C⚌O), 1609, 1564 (C⚌C, aromatic), 1287 (C—N), 1221 (C—O), 769, 698 (C—H, o-substitue); δH (400 MHz, DMSO-d6) 11.14 (s, 1H), 9.01 (s, 1H), 7.70 (d, J = 8 Hz, 2H), 7.44–7.38 (m, 2H), 6.99 (d, J = 8 Hz, 4H); δC (100 MHz, DMSO-d6), 162.75, 158.61, 154.23, 141.56, 133.24, 130.80, 128.38, 124.75, 119.59, 118.17, 116.51; Anal. Calcd. for C17H12N2O5: C, 62.96; H, 3.73; N, 8.64; Found: C, 63.20; H, 3.69; N, 8.57.
2.1.5.5 2-oxo-N′-(3,4,5-trihydroxybenzoyl)-2H-chromene-3-carbohydrazide (8e)
yellow solid; yield 3.385 g (95%); mp (°C): >300; FT-IR (KBr) 3536 (O—H), 3115 (N—H), 3053 (C—H, aromatic), 2930 (C—H, aliphatic), 1687 (C⚌O), 1605, 1556 (C⚌C, aromatic), 1348 (C—N), 1207 (C—O); δH (400 MHz, DMSO-d6) 10.57 (s, 1H), 10.44 (s, 1H), 9.19 (s, 2H), 8.91 (s, 1H), 8.83 (s, 1H), 8.03 (d, J = 8 Hz, 1H), 7.78 (t, J = 8 Hz, 1H), 7.55–7.45 (m, 2H); δC (100 MHz, DMSO-d6) 165.04, 159.82, 159.93, 153.94, 147.98, 145.48, 137.05, 134.36, 130.33, 125.19, 122.07, 116.21, 118.40, 118.27, 107.11; Anal. Calcd. for C17H12N2O7: C, 57.31; H, 3.39; N, 7.86; Found: C, 57.50; H, 3.36; N, 7.80.
2.1.5.6 N′-(5-amino-2-hydroxybenzoyl)-2-oxo-2H-chromene-3-carbohydrazide (8f)
brown solid; yield 2.714 g (80%); mp (°C): >300; FT-IR (KBr) 3382 (O—H), 3265 (N—H), 3052 (C—H, aromatic), 1698 (C⚌O), 1608, 1583 (C⚌C, aromatic), 1287 (C—N), 1220 (C—O), 922 (C—H, 1,2,4-tri substitue); δH (400 MHz, DMSO-d6) 10.88 (s, 1H), 9.08 (s, 1H), 8.92 (s, 1H), 8.75 (s, 1H), 8.03 (d, J = 8 Hz, 1H), 7.77 (d, J = 8 Hz, 1H), 7.58–7.45 (m, 4H); δC (100 MHz, DMSO-d6) 163.33, 159.71, 159.69, 153.95, 152.26, 148.54, 147.92, 135.26, 134.35, 130.33, 128.32, 125.19, 123.55, 118.45, 118.29, 116.22; Anal. Calcd. for C17H13N3O5: C, 60.18; H, 3.86; N, 12.38; Found: C, 60.39; H, 3.82; N, 12.32.
2.2 In vivo animal test
Experimental protocol of in vivo animal tests was approved by Marmara University Animal Experiments Local Ethics Committee (permission number: 57.2019.mar). Adult Sprague-Dawley rats of either sex at the age of 3–4 month and weighing 300–450 g for anti-inflammatory activity test, adult BALB/c mice of either sex at the age of 2–3 month and weighing 20–30 g for anticonvulsant activity were obtained from The Experimental Animal Implementation and Research Center (DEHAMER) of Marmara University. The rats and mice were maintained in controlled temperature (20 ± 2 °C), humidity (40–60%), and 12 h dark/light cycle-regulated rooms, with free access to food and top water. All experiments were performed at the fixed hours between 09:00–19:00 during the light phase of the day. All necessary precautions were taken before the experiment and the factors that would adversely affect the parameters were minimized during the study. The experimental groups were chosen randomly after the adaptation period of 2 days for mice.
2.2.1 Experimental design
2.2.1.1 Experimental design for anti-inflammatory activity
Anti-inflammatory and anticonvulsant activities for six different coumarin derivatives were evaluated and compared with in vivo animal test. The experimental rats for anti-inflammatory activity test were divided into 20 groups each consisting of 6 rats of either sex (equal ratio). All synthesized coumarin derivatives were suspended in 0.5% methylcellulose (vehicle for all coumarin derivatives) and given using oral gavage for once (5 ml/kg). Group A is model control group was given vehicle; Group B is positive control group was given indomethacin (10 mg/kg (Cong et al., 2015) suspended in 0.5% methylcellulose. All coumarin derivatives were given to rats in three different dosages (5, 10, and 25 mg/kg (Witaicenis et al., 2014) (Hemshekhar et al., 2013) for evaluating their anti-inflammatory activities. Group 8a1-8a3 were given treatment 1 in low, middle, and high doses, respectively. In the same way, group 8b1-8b3 were given treatment 2, group 8c1-8c3 were given treatment 3, group 8d1-8d3 were given treatment 4, group 8e1-8e3 were given treatment 5, and group 8f1-8f3 were given treatment 6 in three different doses from low to high, respectively.
2.2.2 Experimental design for anticonvulsant activity
The experimental mice for anticonvulsant activity test were divided into 20 groups each consisting of 7–10 mice of either sex (equal ratio). All synthesized coumarin derivatives were suspended in 0.5% methylcellulose and given using oral gavage for once (10 ml/kg). Group A is model control group was given vehicle; Group B is positive control group was given carbamazepine (100 mg/kg (Bhat and Al-Omar, 2011; Alrohaimi et al., 2014) suspended in 0.5% methylcellulose. All coumarin derivatives were given to mice in three different dosages (30, 100, and 300 mg/kg (Alrohaimi et al., 2014) for evaluating their anticonvulsant activities. Treatment groups were entitled with the same group name as in the anti-inflammatory activity test such as Group 8a1-8a3 are given treatment 1 in low, middle, and high doses, respectively.
2.3 Induction of inflammation and assessment of anti‐inflammatory activity
The acute anti-inflammatory activities of six different coumarin derivatives were investigated and compared in rats with carrageenan-induced paw edema method (Taşkın et al., 2019). Briefly, rats were fasted for 12 h with free access to water until the experiment starts and then, different groups were treated with 6 new coumarin derivatives at 3 different dosages (5, 10, 25 mg/kg, p.o.), indomethacin (10 mg/kg, p.o.), and vehicle (0.5% methylcellulose; 5 ml/kg, p.o.) 30 min before the administration of carrageenan (Taskin et al., 2018). In order to induce inflammation, 0.1 ml of 1% (w/v) carrageenan in saline was injected subplantarly in the right paw of Sprague-Dawley rats (Taskin et al., 2020). The volume of the edema development and its duration was determined for 5 h using a digital plethysmometer at times 0, 1, 2, 3, 4, and 5 h after carrageenan injection (Tamrat et al., 2017). The percent inhibition of edema was measured in comparison to the control animals and was calculated according to the following formula (Taskin et al., 2020): % Inhibition = [(Vt − Vo) control − (Vt − Vo)]/[(Vt − Vo) control] × 100. Where Vt is the paw volume at time t, Vo is the paw volume before carrageenan injection, (Vt − Vo) is edema in paw after time ‘t’.
2.4 Induction of epileptic seizure and assessment of anticonvulsant activity
The anticonvulsant activities of six different coumarin derivatives were investigated and compared by PTZ-induced seizures model in mice (Kamiński et al., 2020). This rodent model is widely utilized as a standard method for predicting protection against tonic-clonic seizures in humans (Keshavarz and Yekzaman, 2018). Effective dose 50 (ED50) value for PTZ (80 mg/kg) was calculated by the method of Litchfield and Wilcoxon (Litchfield and Wilcoxon, 1949). Firstly, mice were fasted overnight and then, carbamazepine (100 mg/kg, p.o.), vehicle (0.5% methylcellulose; 5 ml/kg, p.o.), and all coumarin derivatives at 3 different dosages (30, 100, and 300 mg/kg (Arshad et al., 2014)) were administered to different groups 30 min prior to the administration of PTZ (80 mg/kg, i.p. (Pithadia et al., 2013)). After PTZ injection, each mice was immediately placed in a separate observation box and their behaviors were observed for 30 min for frequency of convulsions, latency to the onset of seizures, percentage of grades, and mortality (Khoshnood-Mansoorkhani et al., 2010). The animals that survived after that period of time were considered to be protected. Moreover, each seizure was graded according to a modified Racine scale as follows: 1-No movements; 2-Head nodding and myoclonic jerks (MKJ); 3-Forelimb clonus; 4-Rearing; 5-Falling and generalized convulsions with tonic extension (Racine, 1972).
2.5 Statistical analysis
All data were expressed as mean ± standard error of mean (SEM). Statistical analysis was performed using GraphPad Prism 6.0 software (GraphPad Software, Inc., San Diego, CA). Groups of data were compared with analysis of variance (ANOVA) and Dunnet post-hoc test. Values were considered significantly different at p < 0.05.
3 Results And Discussion
3.1 Chemistry
The synthesis of the diacyl hydrazide-coumarin derivatives 8 a-f (Table 1) was performed by the reaction of 3-carbonyl chloride coumarin 7 with some substituted aryl acid hydrazides 3a-f as indicated in Scheme 1. To synthesis the aryl acid hydrazides at first aryl acids 1a-f were converted into methyl esters by refluxing with methanol in sulfuric acid in good to excellent yields (65–99%), and then to corresponding hydrazides 3a-f were obtained by stirring at room conditions for 16–20 h (67–95%) via hydrazinolysis. The acid chloride precursor 7 prepared in two steps. At first 2-oxo- 2H-chromene-3-carboxylate 6 was synthesized by Knoevenagel condensation of salicylic aldehyde and diethylmalonate in basic media with 90% yield and then acylated with thionyl chloride to get the key starting molecule 7 with excellent yield (95%). Finally, the target compounds, diacyl hydrazide-coumarin derivatives 8 a-f were synthesized in good–excellent yields (70–95%) from the reaction of acid hydrazides (15–20) and 2-oxo-2H-chromen-3-carbonyl chloride (25). All these molecules are novel except 8d (Jesumoroti et al., 2019). However, anti-inflammatory and anticonvulsant activities of these all compounds were investigated in this study, as a first time.
Entry
Aryl acid
Corresponding hybrid
Yield (%)
1
90
2
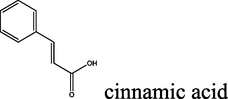
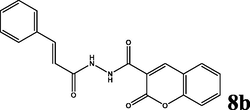
85
3
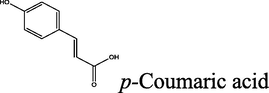
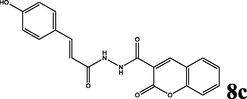
70
4
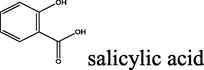
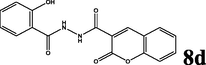
90
5
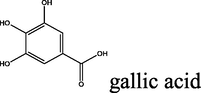
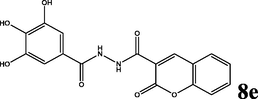
95
6

80
3.2 In vivo animal test
3.2.1 Anti-inflammatory activity
Inflammation is the natural defense mechanism of the body against irritants, trauma, pathogens, or microbial invasion. It occurs in the human body with symptoms such as pain, swelling, redness, heat, and disturbance of function (Aghasafari et al., 2019; Minhas et al., 2017; Pahwa and Jialal, 2019). Migration and activation of leukocytes cause tissue destruction and enhanced blood vessel permeability. Hence, inflammation could be occur by activation of several enzymes (lipoxygenases and cyclooxygenases (COX)), production of inflammatory mediators (leukotrienes and prostaglandins), and reactive oxygen species (Abdel-Lateff et al., 2020). In the present study, 0.1 ml of 1% (w/v) carrageenan that is commonly used to induce an acute model of inflammation was injected subplantarly in the right paw of rats. Carrageenan shows its paw edema effects in two phases: The initial phase (0–1 h) is related to the release of serotonin, bradykinin, and histamine; and the second phase (from the 2nd hour) is derived polymorphonuclear leucocytes infiltration and high production of prostaglandins. Amounts of free radicals, tumor necrosis factor-α, reactive oxygen species, interleukin-1β, and nitric oxide also increase because of releasing polymorphonuclear leucocytes (Chauhan et al., 2018; Pasqua et al., 2019). Indomethacin that is a nonsteroidal anti-inflammatory drug (NSAID) was used as being the positive control group in this study. The mechanism of effect of this drug is to reduce the production of prostaglandins and prevent the production of prostaglandins from arachidonic acid (Lucas, 2016).
In order to screen the anti-inflammatory profile of the synthesized diacylhydrazide-coumarin derivatives of aryl acids (8a-f) were tested for 5 h at 3 different dosages (5, 10, 25 mg/kg). The changes in the level of paw edema volumes and percentage inhibition of all groups were shown in Fig. 3 According to the results, the anti-inflammatory effects of all tested compounds were dose dependent. At 1st h, the maximum percentage inhibition of carrageenan-induced paw edema volumes was shown by the compound 8c with the highest dose (25 mg/g dose; 57.14%) while the compound 8d with the lowest dose (5 mg/kg) was more efficient at 2nd, 3rd, 4th, and 5th hours (65.75%; 52.38%; 36.60% and 45.29%, respectively) when compared to the control group (Fig. 2).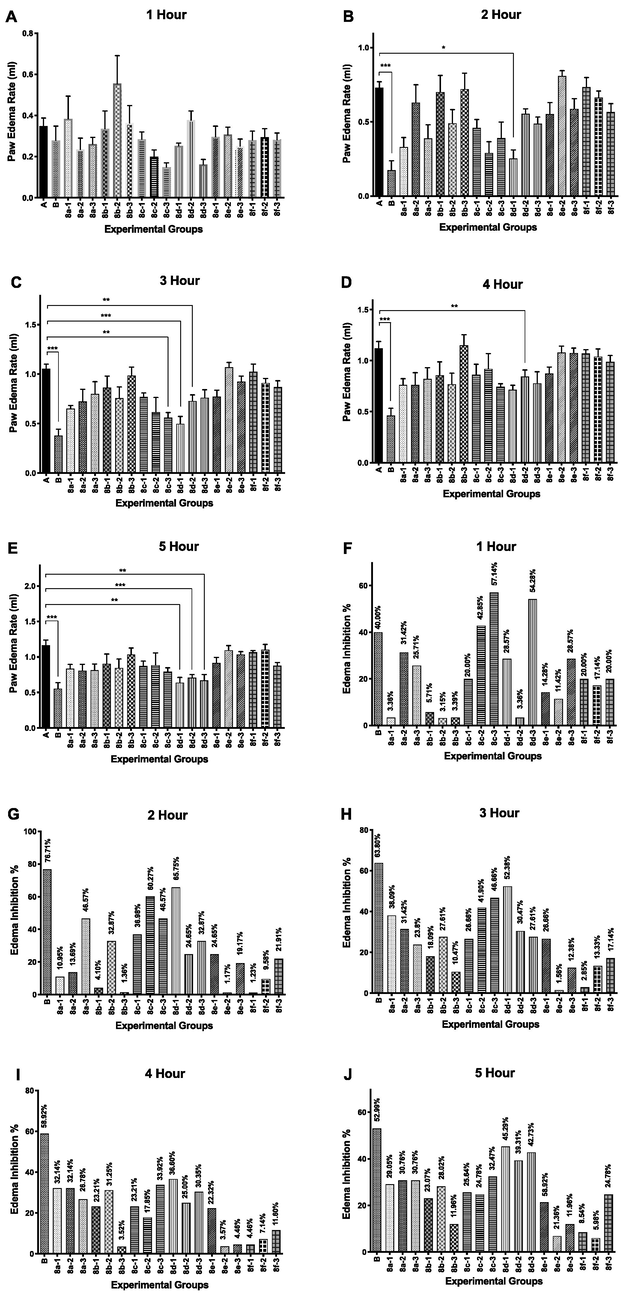
Paw edema rate (ml) and % inhibition of paw edema on carrageenan-induced inflammation model (n = 6). The paw edema volumes were the paw volumes’ difference measured between at different time and at zero hour: (A) 1h, (B) 2h, (C) 3h, (D) 4h, and (E) 5h. The edema inhibitions % were analyzed at determined different times by comparing to the control group: (F) 1h, (G) 2h, (H) 3h, (I) 4h, and (J) 5h. Values were expressed in Mean ± SEM (n = 6 animals for each group). Two-way ANOVA was carried out followed by post hoc Dunnet multiple comparison test. Compounds were compared to the control group and statistical significance is expressed as *p < 0.05, **p < 0.01, ***p < 0.001.
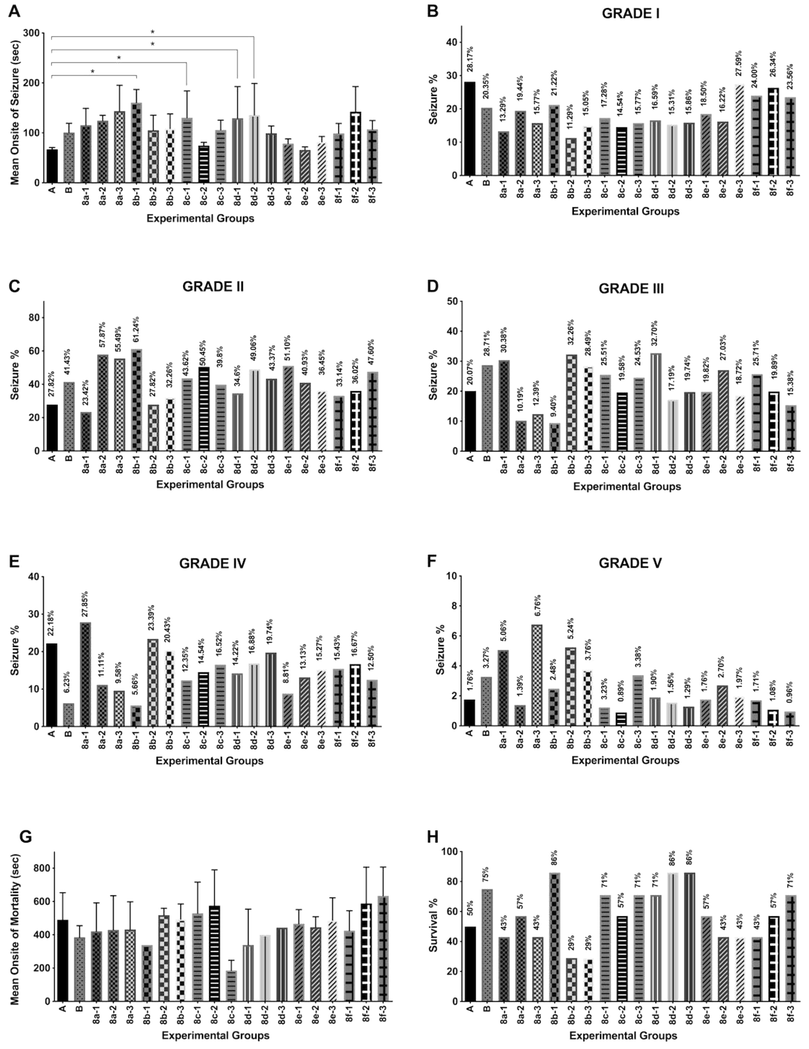
Effects of coumarin derivates on pentylenetetrazole (PTZ)- induced seizures in mice (n = 7–10). The mean onset of seizure (sec) (A); seizures % (grades 1–5) of experimental groups: (B) Grade I, (C) grade II, (D) grade III, (E) grade IV, and (F) grade V; the mean onset of mortality (sec) (G) and survival % (H) were analyzed. Values were expressed in Mean ± SEM (n = 7–10 animals for each group). One-way ANOVA was carried out followed by post hoc Dunnet multiple comparison test. Compounds were compared to the control group and statistical significance is expressed as *p < 0.05.
Indomethacin significantly caused inhibition of carrageenan-induced paw edema volume at 1st, 2nd, 3rd, 4th, and 5th hours, (40.00%, 76.71%, 63.80%, 58.92%, 52.99%, respectively) compared to the control group. The results indicate that at 1st h, the anti-inflammatory effect of 8c with dose 25 mg/kg (57.14%) was greater than the anti-inflammatory effect of indomethacin (40.00%). However, among the tested compounds, 8d with the lowest dose has the maximum average inhibition of paw edema volume during five hours. Furthermore, the anti-inflammatory effects of 8c, and 8f increased with an increase in applied dose according to percentage inhibition of paw edema volumes. When we consider about structure and activity relationship, it’s clearly seen that hydrophilic substituents on aryl sub-units of diacyl-coumarin molecules significantly decreases the inhibition of carrageenan induced paw edema volume. Hence, as we expected, compounds 8e and 8f were non-efficient molecules for the percentage inhibition of carrageenan-induced paw edema volumes.
3.2.2 Anticonvulsant activity
Within the tests utilized for the assessment of anticonvulsant activity, the PTZ test is of predictive relevance about the clinical spectrum of activity of experimental compounds, because the PTZ test is assumed to identify anticonvulsant drugs effective against human generalized tonic-clonic seizures (Khoshnood-Mansoorkhani et al., 2010). PTZ is a noncompetitive antagonist of GABAA receptors that acts through the tert-butyl-bicyclo-phosphorothionate site of the receptor and decreases its activity. Another assumption for the mechanism of PTZ is to change the potassium and calcium channel conductance (Asadi-Shekaari et al., 2014). GABA is a major inhibitory neurotransmitter in the brain and the inhibition of GABA is the underlying factor in epilepsy (Gowda et al., 2012).
Currently used anticonvulsant drugs are effectively control epileptic seizures in almost 75% of the patients. Moreover, treatment difficulty is caused by undesirable side effects from the drugs used clinically; so new antiepileptic drugs have greater potency than more established anticonvulsant drugs. Therefore, newly synthesized diacyl hydrazine- coumarin derivatives are being seriously investigated for their antiepileptic potentials (Alrohaimi et al., 2014; Khoshnood-Mansoorkhani et al., 2010; Zhu et al., 2014). Carbamazepine, as a standard anticonvulsant drug, shows its antiepileptic effects via enhancing of sodium channel inactivation by reducing high-frequency repetitive firing of action potentials and action on synaptic transmission (Tolou-Ghamari et al., 2013). It has been widely used as standard drug in anticonvulsant activity studies and we also used carbamazepine as reference anti-epileptic drug in our study.
In this study, the anticonvulsant potential of six newly synthesized diacyl hydrazine- coumarin derivatives with three different doses was investigated by PTZ model. Compounds (E)-N′-cinnamoyl-2-oxo-2H-chromene-3-carbohyrazide (8b) and (E)-N′-(3-(4-hyroxyphenyl)acryloyl)-2-oxo-2H-chromene-3-carbohydrazide (8c) in low doses and N′-(2-hyroxybenzoyl)-2-oxo-2H-chromene-3-carbohydrazide (8d) in all doses have significant anticonvulsant potential against PTZ-induced convulsions (Fig. 3). PTZ-induced seizure model in the discovery of new antiepileptic drugs has advantages such as using of intact rodents as easy models that detect anticonvulsant effects regardless of the mechanisms of action. PTZ test can be used in high throughput screening according to the National Institutes of Health Anticonvulsant Screening Program. Besides, PTZ model can provide insight into pharmacokinetic–pharmacodynamic relations, which are so valuable for human studies (Löscher, 2011; Rho and White, 2018).
PTZ (80 mg/kg, i.p.) induced tonic-clonic seizures in all the animals used. All experimental groups were compared with the model control group. Mice pretreated with the diacyl hydrazide-coumarin derivatives at three different doses of 30, 100, and 300 mg/kg p.o. were compared for the onset of convulsion, percentage of grades, and mortality. Compounds 8b, 8c and 8d with the lowest dose (30 mg/kg) and 8d with medium dose (100 mg/kg), significantly delayed the onset of convulsion (p < 0.05) compared to control group (1.07 ± 0.04). The results are given in Fig. 3; 8b-1 (2.41 ± 0.26), 8c-1 (2.10 ± 0.54), 8d-1 (2.09 ± 1.03), and 8d-2 (2.16 ± 1.03). On the other side, survival was significantly increased by group B (75%); 8b-1 (86%); 8c-1 and 8c-3 (71%); 8d-1, 8d-2, and 8d-3 (71%, 86%, 86%, respectively), and 8f-3 (71%) compared to the control group (50%). The standard anti-epileptic drugs, carbamazepine (100 mg/kg) inhibited the severe tonic-clonic convulsions, especially considerably decreased the grade IV to 6.23% compared to control group (22.18%) and mortality in mice (Fig. 3) although it could not delay the onset of convulsion. It was also observed that although the survival rate of carbamazepine is 75%, half of animals in this group had long-term grade 5 activity during more than half of observation period. Compound 8b which was donated with two hydrophobic aryl units, showed a strong anticonvulsant effect in low dose but this effect was reversed in higher doses. The survival ratio decreased to 29% in middle and high doses. When we look at overall, the only compound 8d has the anticonvulsant potential in a dose-dependent manner. The mean onsite of mortality increased in a dose-dependent manner. Also, compounds 8b and 8c may have anticonvulsant potential in low dose while compound 8f has a potential in a dose-dependent manner according to the mean onsite of mortality and survival ratio. In these treatment groups, the ratio of severe grades and mortality decreased while the onset of seizure and mortality increased. The anticonvulsant activity of these treatment groups may be explain by inhibited and/or attenuated PTZ-induced seizures of the mice by enhancing GABA-mediated inhibition and/or, decreasing the excitatory neurotransmission and/or, by blocking the sodium channels and/or by neutralizing the PTZ binding site. However, the exact mechanism(s) in anticonvulsant and anti-inflammatory effects will have to be elucidated in future studies. From these data, ideas for future molecular modification leading to compound with greater favorable pharmacological properties may be derived.
4 Conclusion
We have successfully synthesized and evaluated a number of novel diacylated hydrazide-coumarin derivatives as anti-inflammatory and anticonvulsant agents. Among these molecules, N′-(2-hyroxybenzoyl)-2-oxo-2H-chromene-3-carbohydrazide founded has a potential anti-inflammatory effect against carrageenan-induced paw edema model and anticonvulsant efficacy against the tonic-clonic seizure type in rodents. However, further studies are needed to bring out more clearly the facts concerning the exact mechanisms in which N′-(2-hyroxybenzoyl)-2-oxo-2H-chromene-3-carbohydrazide attenuates the carrageenan-induced paw edema and PTZ-induced seizures.
Acknowledgement
The authors are greatly thankful to support of Scientific Research Committee of Çukurova University (FYL-2016-7591 and FBA-2019-12352).
References
- Euryops arabicus displays anti-inflammatory activities in experimental models. J. Ethnopharmacol.. 2020;247
- [CrossRef] [Google Scholar]
- A review of inflammatory mechanism in airway diseases. Inflamm. Res.. 2019;68(1):59-74.
- [CrossRef] [Google Scholar]
- Anticonvulsant and neurotoxicity evaluation of some newly synthesized thiazolyl coumarin derivatives. Am. J. Pharmacol. Toxicol.. 2014;9:132-138.
- [CrossRef] [Google Scholar]
- Anticonvulsant and neurotoxicity evaluation of some newly synthesized thiazolyl coumarin derivatives. American Journal of Pharmacology and Toxicology. 2014;9(2):132-138.
- [CrossRef] [Google Scholar]
- Potential mechanisms involved in the anticonvulsant effect of walnut extract on pentylenetetrazole-induced seizure. Med. Principles Practice. 2014;23(6):538-542.
- [CrossRef] [Google Scholar]
- A novel anti-oxidant and anti-cancer strategy: A peptoid anti-inflammatory drug conjugate with SOD mimic activity. Biochem. Biophys. Res. Commun.. 2004;317:1155-1158.
- [CrossRef] [Google Scholar]
- Coumarin incorporated triazoles: a new class of anticonvulsants. Acta Pol. Pharm.. 2011;68(6):889-895. ISSN 0001-6837
- [Google Scholar]
- Evaluation of toxicity studies and anti-inflammatory activity of Terminalia Bellerica in carrageenan-induced paw edema in experimental rats. J. Nat. Sci. Biol. Med.. 2018;9(2):169-174.
- [CrossRef] [Google Scholar]
- Rat paw oedema modeling and NSAIDs: timing of effects. Int. J. Risk Safety Med.. 2015;27(Suppl 1):S76-S77.
- [CrossRef] [Google Scholar]
- Synthesis and bioactivity of novel N,N′-diacylhydrazine derivatives containing furan(I) Chin. J. Chem.. 2008;26:919-922.
- [CrossRef] [Google Scholar]
- Synthesis and antinociceptive activity of methyl nicotinate. J. Pharm. Bioresour.. 2015;12:54.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial activities of 2-[(alpha-methylbenzylidene)-hydrazino]benzoxazoles, 2008; 32:147–155. Turkey J. Chem.. 2008;32:147-155.
- [Google Scholar]
- Phenolic acid derivatives with potential anticancer properties - A structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorganic Med. Chem.. 2004;12:3581-3589.
- [CrossRef] [Google Scholar]
- K, Evaluation of anticonvulsant activity of ethanolic leaves extract of Desmodium triflorum in mice. Revista Brasileira de Farmacognosia. 2012;22:649-656.
- [CrossRef] [Google Scholar]
- Field evaluation of RH 5992 on lepidopterous pests in Europe. Brighton Crop Protect. Conf. Pests Dis.. 1992;1:59-65.
- [Google Scholar]
- Antiarthritic and antiinflammatory propensity of 4-methylesculetin, a coumarin derivative. Biochimie. 2013;95(6):1326-1335.
- [CrossRef] [Google Scholar]
- Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol.. 1996;27:713-722.
- [CrossRef] [Google Scholar]
- Evaluation of novel N′-(3-hydroxybenzoyl)-2-oxo2H-chromene-3-carbohydrazide derivatives as potential HIV-1 integrase inhibitors. Med. Chem. Commun.. 2019;10:80-88.
- [CrossRef] [Google Scholar]
- N-Benzyl-(2,5-dioxopyrrolidin-1-yl)propanamide (AS-1) with hybrid structure as a candidate for a broad-spectrum antiepileptic drug. Neurotherapeutics. 2020;17(1):309-328.
- [CrossRef] [Google Scholar]
- L-proline catalyzed solvent-free knoevenagel condensation for the synthesis of 3-substituted coumarins. Chin. J. Chem.. 2007;25:1686-1689.
- [CrossRef] [Google Scholar]
- Coumarins. Biology, Applications and Mode of Action. New York: Wiley; 1997. ISBN: 978-0-471-96997-6
- Amelioration of pentylenetetrazole-induced seizures by modulators of sigma, N-methyl-D-aspartate, and ryanodine receptors in mice. Iran J. Med. Sci.. 2018;43(2):195-201.
- [Google Scholar]
- Anticonvulsant activity of teucrium polium against seizure induced by PTZ and MES in Mice. Iran J. Pharm. Res.. 2010;9(4):395-401.
- [Google Scholar]
- Synthesis and antiinflammatory activity of coumarin derivatives. J. Med. Chem.. 2005;48:6400-6408.
- [CrossRef] [Google Scholar]
- Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem.. 2005;5(1):29-46.
- [CrossRef] [Google Scholar]
- Coumaroyl quinic acid derivatives and flavonoids from immature pear (Pyrus pyrifolia nakai) fruit. Food Sci. Biotechnol.. 2013;22:803-810.
- [CrossRef] [Google Scholar]
- Synthesis and bioactivity of novel N,N′NDiacylhydrazine derivatives containing furan (II) Chin. J. Chem.. 2010;28:1233-1239.
- [CrossRef] [Google Scholar]
- A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Therapeutics. 1949;96(2):99-113.
- [Google Scholar]
- Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure: J. Br. Epilepsy Assoc.. 2011;20:359-368.
- [CrossRef] [Google Scholar]
- The pharmacology of indomethacin. Headache: J. Head Face Pain. 2016;56(2):436-446.
- [CrossRef] [Google Scholar]
- Highly suppressing wild-type HIV-1 and Y181C mutant HIV-1 strains by 10-chloromethyl-11-demethyl-12-oxo-calanolide a with druggable profile. J. Med. Chem.. 2008;51:1432.
- [CrossRef] [Google Scholar]
- Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. Eur. J. Med. Chem.. 2009;44:3020-3026.
- [CrossRef] [Google Scholar]
- Benzoxazole-coumarin derivatives: potential candidates for development of safer anti-inflammatory drugs. Der Chemica Sinica. 2017;8(1):146-157. ISSN: 0976-8505 CODEN (USA): CSHIA5
- [Google Scholar]
- A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem.. 2008;15(26):2664-2679.
- [CrossRef] [Google Scholar]
- Synthesis and Characterization of 3 - Substituted Coumarin. Baghdad Sci. J.. 2016;13:89-96.
- [CrossRef] [Google Scholar]
- Pahwa, R., Jialal, I., 2019. Chronic inflammation. Statpearls Publishing Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK493173/.
- Pharmacological study of anti-inflammatory activity of aqueous extracts of Mikania glomerata (Spreng.) and Mikania laevigata (Sch Bip. ex Baker) J. Ethnopharmacol.. 2019;231:50-56.
- [CrossRef] [Google Scholar]
- Clean synthesis of coumarin-3-carboxylic acids using water extract of rice straw husk. Green Mater.. 2018;6:143-148.
- [CrossRef] [Google Scholar]
- Synthesis and evaluation of some novel substituted 1,3,4-oxadiazole and pyrazole derivatives for antitubercular activity. Indian J. Chem. - Sect. B Org. Med. Chem.. 2009;48:1453-1456.
- [CrossRef] [Google Scholar]
- Interactions of a series of coumarins with reactive oxygen species. Biochem. Pharmacol.. 1992;44(2):205-214.
- [CrossRef] [Google Scholar]
- Zinc complexes supported by methyl salicylato ligands: Synthesis, structure, and application in ring-opening polymerization of l-lactide. Dalt. Trans.. 2013;42:13838-13844.
- [CrossRef] [Google Scholar]
- A comparison of the effectiveness of some anti-inflammatory drugs on thermal oedema. Br. J. Exp. Pathol.. 1975;56:554-559.
- [Google Scholar]
- Reversal of experimentally induced seizure activity in mice by glibenclamide. Ann. Neurosci.. 2013;20(1):10-12.
- [CrossRef] [Google Scholar]
- Modification of seizure activity by electrical stimulation. I. After-discharge threshold. Electroencephalogr. Clin. Neurophysiol.. 1972;32(3):269-279.
- [Google Scholar]
- Brief history of anti-seizure drug development. Epilepsia Open. 2018;3(Suppl 2):114-119.
- [CrossRef] [Google Scholar]
- Synthesis, Antimicrobial and Antioxidant Activity of N-Dimethylaminophenylallylidene Cinnamide Derivatives. Int. Res. J. Pharm.. 2019;9:120-127.
- [CrossRef] [Google Scholar]
- Synthesis and antihyperlipidemic activity of novel coumarin bisindole derivatives. Bioorg. Med. Chem. Lett.. 2010;20:6504-6507.
- [CrossRef] [Google Scholar]
- Synthesis and anti-inflammatory activity of novel biscoumarin–chalcone hybrids. Bioorg. Med. Chem. Lett.. 2011;21:4480-4484.
- [CrossRef] [Google Scholar]
- Transmutation of Scent: An Evaluation of the Synthesis of Methyl Cinnamate, a Commercial Fragrance, via a Fischer Esterification for the Second-Year Organic Laboratory. J. Chem. Educ. 2020
- [CrossRef] [Google Scholar]
- Synthesis of 3,4,5-trihydroxybenzohydrazone and evaluation of their urease inhibition potential. Arab. J. Chem.. 2019;12:2973-2982.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and analgesic activities of solvent fractions of the leaves of Moringa stenopetala Bak. (Moringaceae) in mice models. BMC Complement. Alternat. Med.. 2017;17(1):473.
- [CrossRef] [Google Scholar]
- Antioxidant and anti-inflammatory activities of Phlomis pungens and Coridothymus capitatus. Marmara Pharmaceut. J.. 2018;22(1):80-85.
- [Google Scholar]
- In vitro and In vivo biological activities and phenolic characterization of Thymus praecox subsp. skorpilii var. skorpilii. J. Food Meas. Charact.. 2019;13(1):536-544.
- [CrossRef] [Google Scholar]
- The in vitro and in vivo investigation of biological activities and phenolic analysis of Helichrysum plicatum subsp. plicatum. Brazilian J. Pharm. Sci.. 2020;56
- [CrossRef] [Google Scholar]
- Phenolic compounds, biological activities and trace elements of Capparis ovata var. canescens. Rev. Biol. Trop.. 2020;68(2):590-600.
- [CrossRef] [Google Scholar]
- quick review of carbamazepine pharmacokinetics in epilepsy from 1953 to 2012. J. Res. Med. Sci.. 2013;18(Suppl 1):S81-S85.
- [Google Scholar]
- Synthesis of biheterocycles based on quinolinone, chromone, and coumarin scaffolds by palladium-catalyzed decarboxylative couplings. J. Org. Chem.. 2016;81:424-432.
- [CrossRef] [Google Scholar]
- Curr. Pharma Res. 2011;1:300-305.
- Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomed. Int. J. Phytotherapy Phytopharmacol.. 2014;21(3):240-246.
- [CrossRef] [Google Scholar]
- Guo L Preparation of diacylhydrazines insecticides and their intermediates. Chem Abstr.. 2001;137:294865
- [Google Scholar]
- Medicinal compounds with antiepileptic/anticonvulsant activities. Epilepsia. 2014;55(1):3-16.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.10.034.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







