Translate this page into:
Synthesis, biological evaluation and Structure Activity Relationships (SARs) study of 8-(substituted)aryloxycaffeine
⁎Corresponding authors at: College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia. Tel.: +966 1146 70237; fax: +966 1146 76220. ktahir@ksu.edu.sa (Kamal E.H. El-Tahir), afmrahman@ksu.edu.sa (A.F.M. Motiur Rahman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A series of 8-(substituted)aryloxycaffeine were prepared from 8-bromocaffeine and (substituted)phenols by modified Ullmann reaction. In vitro antibacterial activity, inhibitory activity on topoisomerase II and pharmacological activities were evaluated for the synthesized 8-(substituted)aryloxycaffeine. Among the synthesized compounds, 8-(5-chloropyridin-3-yloxy)caffeine (3k) showed strong inhibitory activity (MIC = 15.6 μg/mL) against the tested gram negative (−) bacteria Salmonella enteritidis. 8-(quinolin-8-yloxy)caffeine (3g) showed the strongest inhibitory activity against topoisomerase II. And the compounds 8-(6-methylpyridin-2-yloxy)caffeine (3j) and 8-(3-chloro-6-(trifluoromethyl)pyridin-2-yloxy)caffeine (3m) showed analgesic effect without the central nervous system stimulation.
Keywords
Caffeine
8-Bromocaffeine
8-Aryloxycaffeine
Ullmann reaction
Topoisomerase II
Analgesia
1 Introduction
Caffeine (1) is a xanthine alkaloid found in the seeds, leaves and fruit of some plants. Mainly, coffee beans, tea leaves, cocoa beans and kola nut are the most well known natural sources of caffeine. Caffeine is most commonly consumed worldwide by humans in infusion extracted from the seeds of the coffee plant and the leaves. In addition, caffeine containing soft drinks and energy drinks are also popular in the world. Caffeine is a central nervous system (CNS) and metabolic stimulant (Nehlig et al., 1992) and is used both recreationally and medically to reduce physical fatigue and to restore alertness when drowsiness occurs. It produces increased wakefulness, faster and clearer flow of thought, increased focus, and better general body coordination (Bolton and Null, 1981). It is an important class of adenosine receptor (AR) antagonists (Daly, 2000; Franchetti et al., 1994; Huang et al., 2005; Lobo et al., 1997; Müller and Stein, 1996; Strappaghetti et al., 2001), phosphodiesterase (PDE) inhibitor, DNA intercalating agents and inhibitors of acetyl- and butyrylcholinesterase (Kramer et al., 1977; Müller et al., 2002; Rodríguez-Franco et al., 2005; Shamim et al., 1989; Shimada et al., 1991; Wells et al., 1981).
Studies on caffeine derivatives have shown that 8-(substituted)caffeine (e.g. 3-(3-hydroxypropyl)-7-methyl-1-propargyl-8-[(m-methoxy)styryl]xanthine, MSX-2) is a selective A2A adenosine receptor antagonists (Muller, 2000; Müller et al., 1998), A1 receptor (Corsano et al., 1995) in which 8-(di-substituted amino)caffeine belongs to a pharmacologically important subclass (Scammells et al., 1994) and acetylcholinesterase (AChE) inhibitor (Rodríguez-Franco et al., 2005). 8-Cyclohexylcaffeine, which in binding studies has shown to be 150 times more potent at adenosine A2 receptor in platelets than in A1 receptor in cortical membranes (Shamim et al., 1989). In the past two decades, studies on 8-methoxycaffeine have shown that it possesses a topoisomerase II inhibitory activity (Gellert, 1981; Matsuura and Saito, 1969; Russo et al., 1991, 1997; Tornaletti et al., 1992, 1989; Walther et al., 1989), while caffeine and 8-chlorocaffeine are less potent. Recently, it was reported that (Russo et al., 1997) 8-chlorocaffeine antagonized the A1 adenosine receptor at the rat phrenic-hemidiaphragm. 8-akyloxycaffeine is also reported as phosphodiesterase (PDE) inhibitor (Walther et al., 1989), and it causes DNA unwinding (Tornaletti et al., 1989), where caffeine unwinding potency is less than 8-methoxycaffeine (Tornaletti et al., 1992). It has been found that, 8-alkoxycaffeine is very sensitive to photosensitized oxygenation in MeOH, therefore it causes oxidative damage to DNA and amino acids, but in case of caffeine it easily suffers photosensitized oxygenation in alkaline media (Gellert, 1981) (Chart 1).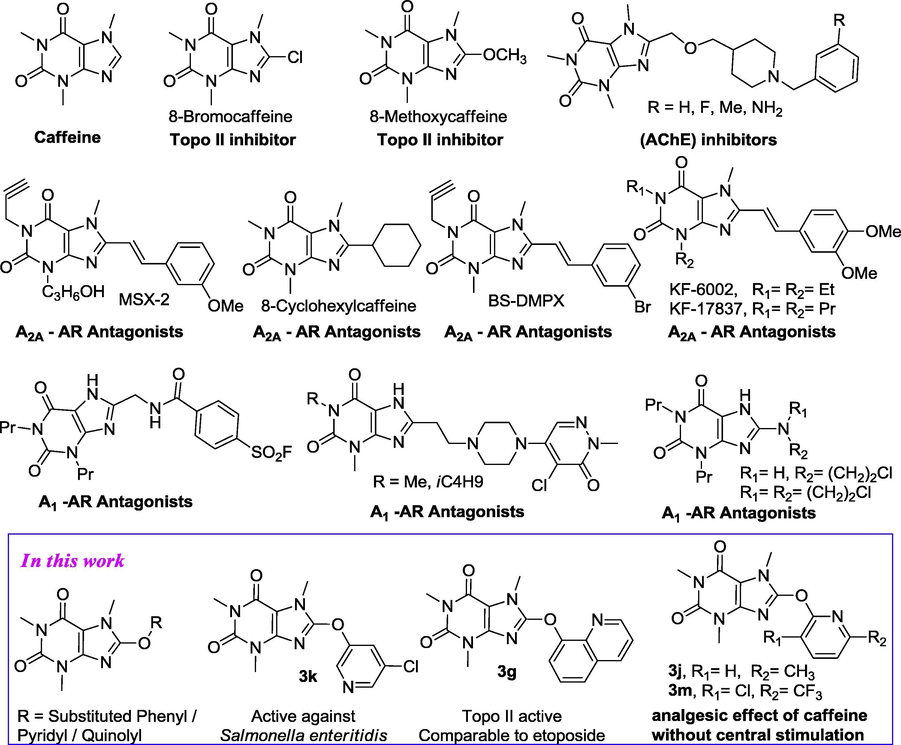
Structure of some potent 8-substituted caffeine derivatives.
Despite its potency, very few methods for synthesizing 8-akyloxycaffeine derivatives have been reported during the last two decades, especially for 8-aryloxycaffeine, not much work has been done so far. Anyway, in 1934, Ralph C. Huston (Huston and Allen, 1934) synthesized 8-substituted (-O-phenyl)caffeine by using Fisher’s method (Fischer, 1884), which was later repeated by W. Wiglicerus (Wislicenus and Körber, 1902), and H. Biltz (Wislicenus and Körber, 1902), consisted simply in heating 8-halocaffeine in methyl or ethyl alcohol solution containing an excess of sodium or potassium alcoholate. Recently, Masakazu Sono et al. (Sono et al., 1994) used electrochemical oxidation of caffeine using KF as electrode in 1.5 V in 2.5 h in MeOH and obtained 43% of 8-methoxycaffeine. Very recently, Hans-Zimmer et al. (Biltz and Bergius, 1918) used sodium metal in THF by refluxing 10 h with moderate yields. However, none of the above methods has been widely applied for the preparation of 8-(substituted)aryloxycaffeine. Since 8-methoxycaffeine is more potent than caffeine and it has one characteristic ether bond in position 8 of caffeine, therefore, 8-(substituted)aryloxycaffeine might be the good candidate for the future development of biological active agents. As a part our study in developing methodology as well as synthesizing natural/unnatural biological active molecule and evaluation of their biological activity, in this article, we report thirteen novel 8-(substituted)aryloxycaffeine (3a–m) via Ullmann reaction and evaluated their antibacterial activity against 12 gram positive/negative bacterial strains, topoisomerase II inhibitory activity and pharmacological actions.
2 Results and discussion
2.1 Chemistry
As a part of our interest (Mohideen et al., 2013; Rahman et al., 2009; Sono et al., 1994; Zimmer et al., 1999) on searching new biological active molecules, a series of 8-(substituted)-aryloxycaffeine (3a–m) were synthesized from 8-bromocaffeine (2). Initially, 8-bromocaffeine (2) was prepared using bromine and sodium acetate in acetic acid at 60 °C from commercially available 1. Modified Ullmann reaction (Ullmann, 1904) condition was applied for the synthesis of 8-(substituted)aryloxycaffeine (3a–m) and obtained good to excellent yields (75–98%) (Scheme 1).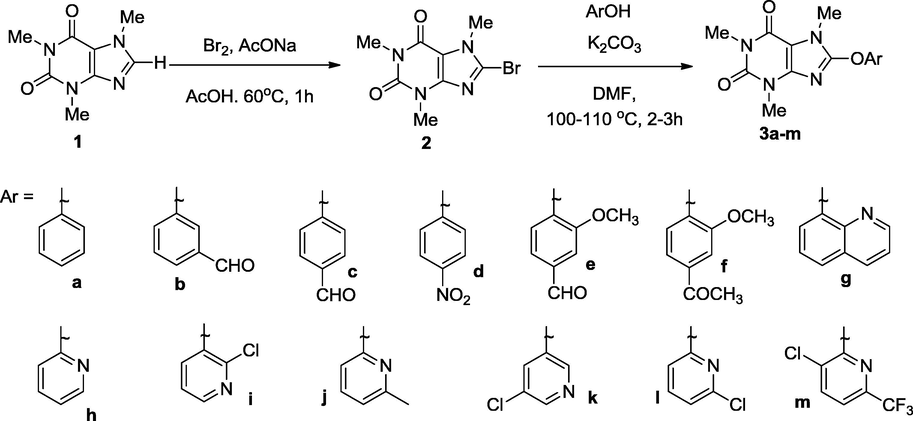
Synthesis of 8-(substituted)aryloxycaffeine (3a–m) from caffeine (1) via 8-bromocaffeine.
2.2 Biology
2.2.1 In vitro antibacterial activity
In vitro antimicrobial activities of synthesized compounds (3a–m) against the microorganisms employed and their activity potentials were qualitatively and quantitatively assessed by the presence or absence of inhibition zones. All the experiments were performed in triplicates and the results were given as the arithmetic mean of these triplicates. As shown in Table 1, 3a–m exhibited a potent inhibitory effect against Staphylococcus aureus (KCTC 1916), Salmonella enteritidis (KCTC 12021), Salmonella typhimurium (KCTC 2515), and Bacillus subtilis ATCC 6633 with the diameter of inhibition zones ranging from 10 to 16 mm whereas Streptomycin (10 μg/disc) showed the diameter of inhibition zones range 14 mm. It should be noted that, 3a–m shows inhibitory effect against all the bacteria tested at 150 μg/disc and the data are summarized in Table 1.
Microorganism
Compoundsa
Antibioticsb
3a
3b
3c
3d
3e
3f
3g
3h
3i
3j
3k
3l
3m
SM
TC
S. aureus (KCTC 1916)
12
14
13
12
11
12
11
13
10
13
13
12
12
14
18
S. typhimurium (KCTC 2515)
13
14
12
14
14
13
12
13
10
12
10
12
13
14
18
S. enteritidis (KCTC 12021)
15
16
16
13
13
14
12
13
14
12
11
12
13
14
19
P. aeruginosa (KCTC 2004)
13
14
11
11
13
12
13
11
13
11
11
10
13
19
20
E. coli 0157:H7 (ATCC 43888)
12
14
13
12
12
13
12
14
12
11
12
11
13
15
19
E. aerogenes (KCTC 2190)
11
13
11
11
12
13
10
12
11
12
09
11
12
15
20
B. subtilis (ATCC 6633)
13
13
14
13
13
15
14
13
10
12
11
12
13
14
18
E. coli (ACTC 8739)
12
14
13
12
09
14
13
10
09
11
12
13
12
15
19
E. coli 0157 (Human)
10
13
12
14
12
15
11
09
10
10
08
10
12
14
20
L. monocytogenes (ACTC 19166)
10
13
12
13
10
12
10
12
10
16
10
12
10
14
21
S. aureus (ATCC 6538)
09
13
12
12
11
13
09
10
12
12
09
11
11
14
21
2.2.2 Minimum inhibitory concentration (MIC)
Table 2 shows the MIC values for the compounds 3a–m against the tested gram (−) bacteria such as S. enteritidis (KCTC 12021), and gram positive (+) bacteria B. subtilis ATCC 6633, which were found in the range of 15.62–125 (μg/mL). Among the synthesized compounds, 3k showed strong inhibitory activity with the value of 15.6 μg/mL against the tested gram negative (−) bacteria S. enteritidis. In addition, compounds 3b, 3i and 3l also show the excellent inhibitory activity with the value of 31.2 μg/mL against the tested gram negative (−) bacteria S. enteritidis and gram positive (+) bacteria B. subtilis.
Microorganism
MIC (in μg/mL)
3a
3b
3c
3d
3e
3f
3g
3h
3i
3j
3k
3l
3m
SM
TC
S. enteritidis (KCTC 12021)
62.5
31.2
62.5
125
62.5
62.5
62.5
31.2
31.2
62.5
15.6
31.2
62.5
15.6
15.6
B. subtilis (ATCC 6633)
125
31.2
62.5
62.5
125
125
31.2
62.5
31.2
125
31.2
31.2
31.2
15.6
15.6
2.2.3 DNA topoisomerase II inhibitory activity
Topo II inhibitory activity of the compounds 3a–m was measured by assessing the relaxation of supercoiled pBR 322 plasmid DNA employing the method previously described (Fukuda et al., 1996). As shown in Table 3, among the caffeine derivatives, 1,3,7-trimethyl-8-(quinolin-8-yloxy)-1H-purine-2,6(3H,7H)-dione (3g) was the most active inhibitor showing 28.42% and 48.92% inhibition comparable to 42.82% and 66.87% of etoposide at the concentrations of 20 μM and 100 μM, respectively. Not only that, but also all of the compounds (3a–m) showed moderate inhibitory activity against Topo II (Table 3).
Compound
Inhibitory activity (%)
Compound
Inhibitory activity (%)
20 μM
100 μM
20 μM
100 μM
3a
10.23
17.22
3h
18.32
24.22
3b
10.56
16.63
3i
20.22
32.22
3c
14.32
24.45
3j
17.46
23.64
3d
12.54
18.37
3k
16.54
23.22
3e
18.21
29.32
3l
18.43
25.45
3f
16.22
26.54
3m
18.25
27.38
3g
28.42
48.92
Etoposide
42.82
66.87
It has been found from the Topo II inhibitory evaluation data that, substitution in any positions with phenyl or pyridyl did not affect on inhibitory activity. Almost similar results were obtained from all the compounds. Only the compound having quinolin substitution (3g) gave different inhibitory activity than the others. Therefore, we can conclude that, main skeleton of caffeine plays an important role for the synthesized 8-(substituted)-aryloxycaffeine and substituent with quinolin moiety increases the inhibitory activity.
2.2.4 Pharmacology
2.2.4.1 Effect of the compounds on motor activity in mice
The locomotion count for 10 min. per group of mice (N = 4) was 435 ± 7 counts. Table 4 shows the percentage change in the count following the treatment of the animals with the different compounds. While, we injected 3a locomotion percentage was increase 5% at 40 mg/kg dosage and increasing of dosage up to 80 mg/kg, locomotion also was increased 17%. Similarly, injection of compound 3h also affected and increased the locomotion percentage. On the other hand, compounds 3e, 3f and 3j decreased the locomotion percentage very much. However, we could not get any effect while we injected compounds 3b–d, 3g, 3i or 3k–m.
Compound
Percentage change in locomotion
40 mg/kg
80 mg/kg
3a
↑5
↑17
3e
↓28.5
↓71.7
3f
↓9
↓23
3h
↑7.3
↑18.3
3j
↓19.2
↓27.3
3b–d, 3g, 3i, 3k–m
No effect
2.2.4.2 Effect of the compounds on the general behaviour at doses of 100/200 mg/kg (i.p)
The results for the effect of the compounds on the general behaviour on albino mice are shown in Table 5. Less shaking (tremor) was observed after injection of 100 mg/kg of 3a/3h but it was increasing depending on increasing of doses. No clear changes was observed with 100 mg/kg doses for 3j/3m but increasing of doses with 200 mg/kg for 3j became calmness and sedation was observed. In case of 3 m (200 mg/kg), calmness then micturition finally defection was observed. Interestingly, for compound 3e with low or high doses severe sedation was observed.
Compound
Doses in mg/kg
Changes in behaviour (during 2 h observation)
3a
100
Minor tremors
3a
200
Tremors and convulsions
3e
100
Severe sedation
3e
200
Severe sedation
3h
100
Minor tremors
3h
200
Tremors and convulsions
3j
100
No clear changes
3j
200
Calmness and sedation
3k
200
Micturition and defecation
3m
100
No clear changes
3m
200
Calmness, micturition and defecation
others
200
No effect
2.2.4.3 Effect of the compounds on thermal pain
In mice treated with caffeine, the reaction time (before treatment) was 6 s. This was increased to 13.5 s, 30 min. after treatment an increase of 125%. In the mice treated with compound 3j, the initial reaction time was 5.6 s. This was increased to 11.2 s 30 min. after treatment an increase of pain threshold by 100%. In the mice treated with compound 3m, the reaction time was increased from 6.1 s to 12.3 s, an increase of 101.6%. There was no significant change in the reaction time in the animals treated with other compounds.
2.2.5 Structure Activity Relationship studies (SARs)
Modification of the caffeine at 8-position by different substituents produced variations in the pharmacological action of the basic molecule (caffeine). Detailed activities of synthesized caffeine derivative are summarized in Table 6.
Substituent (compound)
Activities
Substituent (compound)
Activities
Increase in central nervous system stimulation
Increase in central nervous system stimulation
Loss of central activity
Loss of central nervous system activity
Loss of central activity
Loss of central nervous system activity
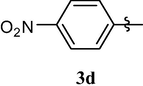
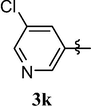
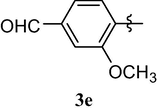
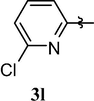
Loss of central nervous system activity
Micturition and defection
Central nervous system depression
Loss of central nervous system activity
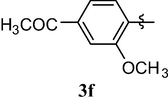
Loss of central nervous system stimulation. Decrease in central nervous system activity
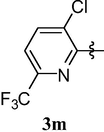
Calmness, Sedation and Analgesia,

Loss of central nervous system activity
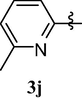
The most interesting finding is that, substitution of 6-methylpyridin-2-yloxy group at position 8 of caffeine namely, 1,3,7-trimethyl-8-(6-methylpyridin-2-yloxy)-1H-purine-2,6(3H,7H)-dione (3j) and substitution of 3-chloro-6-(trifluoromethyl)pyridin-2-yloxy group namely 8-(3-chloro-6-(trifluoromethyl)pyridin-2-yloxy)-1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione (3m) abolished the central nervous system activity of caffeine and left the analgesic effect. Further modification of these structures may provide potent analgesics.
3 Conclusion
In conclusion, caffeine derivatives 3a–m were synthesized in good to excellent yields. Their antimicrobial, topoisomerase II Inhibitory and pharmacological activities have been evaluated. Compound 3k showed strong inhibitory activity (MIC = 15.6 μg/mL) against the tested gram negative (−) bacteria S. enteritidis; 3g showed the strongest inhibitory activity against topoisomerase II comparable to Etoposide; and the compounds 3j and 3m showed the analgesic effect of caffeine without any central stimulation.
4 Experimental
4.1 Chemistry
Melting points were determined using a Fisher-Jones melting point apparatus and were not corrected. NMR spectra were obtained using a Bruker-250 spectrometer 250 MHz for 1H NMR and 62.5 MHz for 13C NMR and are reported as parts per million (ppm) from the internal standard TMS. Chemicals and solvents were commercial reagent grade and used without further purification. Electrospray ionization (ESI) mass spectrometry (MS) experiments were performed on a LCQ advantage-trap mass spectrometer (Thermo Finnigan, San Jose, CA, USA).
4.1.1 Preparation of 8-bromo-1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione (2)
A solution of bromine (3.5 g, 1.1 equiv.) in acetic acid (35 mL) was added dropwise to a solution of caffeine (1, 1.94 g, 10 mmol), sodium acetate (2.2 g, 1.1 equiv.) in acetic acid (50 mL) over 30 min at room temperature. The mixture was stirred for 1 h and the solid formation was collected by filtration, washed with cold acetic acid, dried over vacuum, obtained white powder (92%), mp. 207 °C (lit (Vollmann and Müller, 2002) mp, 206 °C).
4.1.2 General procedure for the preparation of 8-substituted-aryl caffeine ether (3a–m)
A mixture of 8-bromo-1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione (2,100 mg, 0.036 mmol), (substituted) arenols (1 equiv.), anhydrous K2CO3 (1 equiv.) in 10 mL of dry DMF was heated at 100–110 °C for 2–3 h under N2 atmosphere. Upon cooling the reaction mixture at room temperature, water was added, resulting reaction mixture was kept at 4 °C. The solid formation was filtered and washed with cold n-hexane to obtain crystalline solid which was pure enough for analysis.
4.1.3 1,3,7-trimethyl-8-phenoxy-1H-purine-2,6(3H,7H)-dione (3a)
White solid (70%): mp 142–143 °C [lit. (Huston and Allen, 1934) mp: 140–143 °C]. Unreported spectral data are as follows: 1H NMR (CDCl3, 250 MHz) δ 7.41 (t, J = 8.1 Hz, 2H), 7.25 (m, 3H), 3.86 (s, 3H), 3.44 (s, 3H), 3.39 (s, 3H). 13C NMR (CDCl3, 62.5 MHz) δ 154.92, 153.41, 153.33, 151.62, 145.82, 129.75, 125.64, 119.36, 103.77, 30.41, 29.88, 27.81. MS (ESI) Calcd. for C14H14N4O3 [M + H]+ 287.3. Found 287.3.
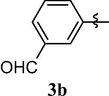
4.1.4 3-(1,3,7-trimethyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yloxy)benzaldehyde (3b)
White solid (87%). mp = 164–65 °C, 1H NMR (CDCl3, 250 MHz): δ 10.00 (s, 1H, —CHO), 7.81 (s, 1H), 7.76 (m, 1H), 7.58 (m, 2H), 3.87 (s, 3H), 3.41 (s, 3H), 3.37 (s, 3H). 13C NMR (CDCl3, 62.5 MHz): δ 190.90, 154.86, 153.80, 152.75, 154.50, 145.57, 137.93, 130.51, 127.43, 125.50, 119.49, 103.90, 30.46, 29.86, 27.81. MS (ESI) Calcd. for C15H14N4O4 [M + H]+ 315.3. Found 315.3.
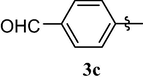
4.1.5 4-(1,3,7-trimethyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yloxy)benzaldehyde (3c)
White solid (87%). mp = 167–68 °C, 1H NMR (CDCl3, 250 MHz): δ 9.99 (s, 1H, —CHO), 7.94 (dm, J = 8.6 Hz, 2H), 7.48 (dm, J = 8.6 Hz, 2H), 3.87 (s, 3H), 3.43 (s, 3H), 3.38 (s, 3H). 13C NMR (CDCl3, 62.5 MHz): δ 190.54, 157.65, 154.89, 152.03, 151.50, 145.51, 133.61, 131.63, 119.59, 103.96, 30.56, 29.89, 27.84. MS (ESI) Calcd. for C14H14N4O3 [M + H]+ 315.3. Found 315.3.
4.1.6 1,3,7-trimethyl-8-(4-nitrophenoxy)-1H-purine-2,6(3H,7H)-dione (3d)
White solid (65%). mp = 207 °C, 1H NMR (CDCl3, 250 MHz): δ 8.30 (dm, J = 9.1 Hz, 2H), 7.52 (dm, J = 9.1 Hz, 2H), 3.88 (s, 3H), 3.44 (s, 3H), 3.38 (s, 3H). 13C NMR (CDCl3, 62.5 MHz): δ 157.52, 154.86, 151.54, 151.45, 145.36, 144.88, 125.68, 119.73, 104.03, 30.61, 29.87, 27.86. MS (ESI) Calcd. for C14H13N5O5 [M + H]+ 332.2. Found 332.2.
4.1.7 3-methoxy-4-(1,3,7-trimethyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yloxy)benzaldehyde (3e)
White solid (86%). mp = 185 °C, 1H NMR (CDCl3, 250 MHz): δ 9.95 (s, 1H, —CHO), 7.51–7.49 (m, 3H), 3.88 (s, 3H), 3.85 (s, 3H), 3.38 (s, 3H), 3.36 (s, 3H). 13C NMR (CDCl3, 62.5 MHz): δ 190.84, 154.94, 153.37, 151.56, 151.36, 146.87, 145.77, 134.93, 124.90, 121.91, 111.21, 103.90, 56.23, 30.52, 29.85, 27.80. MS (ESI) Calcd. for C16H16N4O5 [M + H]+ 345.3. Found 345.3.
4.1.8 8-(4-acetyl-2-methoxyphenoxy)-1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione (3f)
White solid (91%). mp = 218 °C, 1H NMR (CDCl3, 250 MHz): δ 7.59 (s, 1H, H3′), 7.57 (dd, 1H, J = 8.1, 1.8 Hz), 7.35 (d, J = 8.1 Hz, 1H), 3.86 (s, 3H), 3.81 (s, 3H), 3.34 (s, 3H), 3.34 (s, 3H), 2.59 (s, 3H). 13C NMR (CDCl3, 62.5 MHz): δ 196.75, 154.88, 153.56, 151.53, 150.77, 145.82, 145.77, 135.67, 122.06, 121.36, 111.82, 103.81, 56.13, 30.45, 29.80, 27.74, 26.50. MS (ESI) Calcd. for C17H18N4O5 [M + H]+ 359.2. Found 359.2.
4.1.9 1,3,7-trimethyl-8-(quinolin-8-yloxy)-1H-purine-2,6(3H,7H)-dione (3g)
White solid (95%). mp = 184 °C, 1H NMR (CDCl3, 250 MHz): δ 8.79 (dd, 1H, J = 4.3, 1.4 Hz, H2′), 8.18 (dd, 1H, J = 8.4, 1.3 Hz, H4′), 7.74 (dd, 1H, J = 8.0, 0.8 Hz, H5′), 7.64 (dd, 1H, J = 7.5, 0.8 Hz, H7′), 7.54 (t, 1H, J = 8.0 Hz), 7.42 (dd, 1H, J = 8.0, 4.3 Hz, H3′), 4.01 (s, 3H), 3.38 (s, 3H), 3.27 (s, 3H). 13C NMR (CDCl3, 62.5 MHz): δ 155.03, 154.99, 151.63, 150.50, 149.47, 145.95, 140.50, 136.01, 129.73, 126.24, 125.78, 121.94, 119.70, 103.82, 30.78, 29.80, 27.77. MS (ESI) Calcd. for C17H15N5O3 [M + H]+ 338.3. Found 338.3.
4.1.10 ,3,7-trimethyl-8-(pyridin-2-yloxy)-1H-purine-2,6(3H,7H)-dione (3h)
White solid (86%). mp = 287 °C, 1H NMR (CDCl3, 250 MHz): δ 7.47 (dt, 1H, J = 8.0, 6.8, 2.0 Hz, H5′), 7.35 (dd, 1H, J = 6.9, 1.6 Hz, H6′), 6.63 (d, 1H, J = 9.5 Hz, H3′), 6.33 (t, 1H, J = 6.7 Hz, H4′), 3.83 (s, 3H), 3.54 (s, 3H), 3.41 (s, 3H). 13C NMR (CDCl3, 62.5 MHz): δ 161.17, 155.24, 151.41, 146.37, 143.90, 141.48, 136.41, 121.85, 107.83, 107.14, 32.86, 29.83 and 28.06. MS (ESI) Calcd. for C13H13N5O3 [M + H]+ 288.2. Found 288.2.
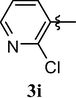
4.1.11 8-(2-chloropyridin-3-yloxy)-1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione (3i)
Pink solid (92%). mp = 174–75 °C, 1H NMR (CDCl3, 250 MHz): δ 8.29 (d, 1H, J = 3.8 Hz, H6′), 7.89 (d, 1H, J = 7.8 Hz, H, H4′), 7.34 (dd, 1H, J = 7.8, 4.8 Hz, H5′), 3.89 (s, 3H), 3.35 (s, 3H), 3.34 (s, 3H). 13C NMR (CDCl3, 62.5 MHz): δ 154.81, 152.27, 151.40, 146.42, 145.74, 145.36, 143.07, 130.40, 123.43, 104.04, 30.51, 29.78, 27.26. (ESI) Calcd. for C13H12ClN5O3 [M + H]+ 322.3. Found 322.3.
4.1.12 1,3,7-trimethyl-8-(6-methylpyridin-2-yloxy)-1H-purine-2,6(3H,7H)-dione (3j)
White solid (80%). mp = 165–66 °C, 1H NMR (CDCl3, 250 MHz): δ 7.67 (t, 1H, J = 7.8 Hz, 1H), 7.01 (d, J = 8.2 Hz, 1H), 6.98 (d, J = 8.6 Hz, 1H), 3.80 (s, 3H), 3.48 (s, 3H), 3.40 (s, 3H), 2.42 (s, 3H). 13C NMR (CDCl3, 62.5 MHz): δ 159.73, 157.84 (C′2), 155.08, 151.59, 145.76, 140.32, 120.56, 108.74, 104.53, 30.87, 29.86, 27.83 and 23.97. (ESI) Calcd. for C14H15N5O3 [M + H]+ 302.3. Found 302.3.
4.1.13 8-(5-chloropyridin-3-yloxy)-1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione (3k)
Pink solid (98%). mp = 193–94 °C, 1H NMR (CDCl3, 250 MHz): δ 8.56 (d, 1H, J = 2.5 Hz, H2′), 8.46 (d, 1H, J = 2.0 Hz, H6′), 7.81 (t, 1H, J = 2.2 Hz, H4′), 3.86 (s, 3H), 3.42 (s, 3H), 3.36 (s, 3H). 13C NMR (CDCl3, 62.5 MHz): δ 154.83, 152.03, 151.41, 149.51, 145.77, 145.27, 139.65, 131.90, 127.23, 103.98, 30.51, 29.86, 27.82. (ESI) Calcd. for C13H12ClN5O3 [M + H]+ 322.2. Found 322.2.
4.1.14 8-(6-chloropyridin-2-yloxy)-1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione (3l)
Pink solid (94%). mp = 169–70 °C, 1H NMR (CDCl3, 250 MHz): δ 7.77 (t, 1H, J = 8.0 Hz, 1H), 7.22 (d, J = 8.0 Hz, 1H), 7.16 (d, J = 8.1 Hz, 1H), 3.83 (s, 3H), 3.48 (s, 3H), 3.40 (s, 3H). 13C NMR (CDCl3, 62.5 MHz): δ 159.47, 155.10, 151.57, 150.60, 149.38, 145.63, 142.28, 121.58, 110.45, 104.73, 30.94, 29.88 and 27.89. (ESI) Calcd. for C13H12ClN5O3 [M + H]+ 322.2. Found 322.2.
4.1.15 8-(3-chloro-6-(trifluoromethyl)pyridin-2-yloxy)-1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione (3m)
Dark-yellow solid (75%). mp = 288–89 °C, 1H NMR (CDCl3, 250 MHz): δ 7.77 (s, 2H, H4′ and H5′), 3.86 (s, 3H), 3.54 (s, 3H), 3.41 (s, 3H). 13C NMR (CDCl3, 62.5 MHz): 156.77, 155.17, 151.28, 146.22, 142.02, 134.80 (3JC–F = 6 Hz), 134.40, 128.60, 122.02 (1JC–F = 250 Hz), 111.26 (2JC–F = 36 Hz), 108.24, 33.23, 28.88 and 28.1δ (ESI) Calcd. for C14H11ClF3N5O3 [M + H]+ 390.2. Found 390.2.
4.2 Biology
4.2.1 Microorganisms
The following microorganisms obtained from the American Type Culture Collection (ATCC), were used in this study: Listeria monocytogenes ATCC 19166, B. subtilis ATCC 6633, S. aureus (ATCC 6538), Escherichia coli ATCC 8739 and E. coli O157:H7 ATCC 43888. Food contaminating Bacillus cereus SCK 11 stain was obtained from College of Veterinary Medicine, Konkuk University, Seoul, Republic of Korea. Other pathogens such as S. aureus (KCTC 1916), S. enteritidis (KCTC 12021), S. typhimurium (KCTC 2515), Enterobacter aerogenes KCTC 2190 and Pseudomonas aeruginosa (KCTC 2004) were obtained from the Korean Collection for Type Cultures (KCTC). Active cultures for experimental use were prepared by transferring a loopful of cells from stock cultures to flasks and inoculated in Luria–Bertani (LB) broth medium (1.0% tryptone, 0.5% yeast extract, 1.0% NaCl, solidified with 1.5% agar when required) at 37 °C for 24 h. Cultures of each bacterial strains were maintained on LB agar medium at 4 °C.
4.2.2 Antibacterial activity assay
The compounds (3a–m) were dissolved in ethanol to a final concentration of 40 mg/mL and sterilized by filtration through 0.45 lm Millipore filters. The antibacterial test was then carried out by agar disc diffusion method (Murray et al., 1995) using 100 μL of standardized inoculum suspension containing 107 CFU/mL of bacteria. To prepare standardized inoculum, bacteria were grown overnight in LB broth that was maintained at 37 °C with constant agitation until the density matched the turbidity of a 0.5 McFarland standard. Tube dilution with sterile saline was carried out to obtain inocula of 107 CFU/mL. Then sterile filter paper discs (6 mm diameter) were impregnated with 10 μL of diluted compounds (3a–m) corresponding to 150 μg/disc separately, using capillary micro-pipette and placed on the inoculated LB agar. Negative controls were prepared by soaking the disc with ethanol only. Standard reference antibiotics, streptomycin and tetracycline (10 μg/disc, each from Sigma–Aldrich Co., St Louis, MO), were used as positive controls for the tested bacteria. The plates were incubated at 37 °C for 24 h. Antibacterial activity was evaluated by measuring the diameter of the zones of inhibition against the tested bacteria. Each assay in this experiment was replicated three times.
4.2.3 Minimum inhibitory concentration (MIC)
Minimum inhibitory concentration (MIC) of the compounds (3a–m) was tested by twofold serial dilution method (Ullmann and Sponagel, 1905). The compounds 3a–m were first dissolved in ethanol, and incorporated into LB broth medium to obtain a concentration of 2000 μg/mL and serially diluted to achieve 1000, 500, 250 125, 62.5, 31.25 and 15.62 μg/mL. The final concentration of ethanol in the culture medium was maintained at 0.1% (v/v). A 10 μL standardized suspension of each tested organism (107 CFU/mL approximately) was transferred to each tube. The control tubes containing only bacterial suspension were incubated at 37 °C for 24 h. The lowest concentration of the test samples, which did not show any growth of tested organism after macroscopic evaluation, was determined as the MIC.
4.2.4 DNA relaxation assay of Topo II
The mixture of 100 ng of supercoiled pBR322 plasmid DNA and 0.2 units of human DNA Topo IIα (Amersham, USA) was incubated without and with the prepared compounds in the assay buffer (10 mM Tris–HCl (pH 7.9) containing 50 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1 mM ATP, and 15 μg/mL bovine serum albumin) for 30 min at 37 °C. The reaction in a final volume of 10 μL was terminated by the addition of 3 μL of 7 mM EDTA. Reaction products are analysed on a 1% agarose gel at 25 V for 4 h with a running buffer of TAE. Gels were stained for 30 min in an aqueous solution of ethidium bromide (0.5 μg/mL). DNA bands were visualized by transillumination with UV light and supercoiled DNA was quantitated using AlphaImagerTM (Alpha Innotech Corporation).
4.2.5 Pharmacology
4.2.5.1 Animals used
Male Swiss albino mice (25 ± 2 g body weight) were used in this study. The animals were provided by the experimental animals care centre, College of Pharmacy, King Saud University, Riyadh, Kingdom Saudi Arabia. The animals were housed in cages under controlled environmental conditions comprising a temperature of 22 ± 2 °C, 12 h light/ dark cycle and a relative humidity of 50 ± 5%. The animals are allowed free access to pulverized standard rodents’ pellets diet and tap water. The experimental protocol followed in the under mentioned experiments was approved by the local Ethical committee.
4.2.5.2 Motor activity: effect of the compounds on motor activity in mice
The motor activity (locomotion) was measured in mice groups (N = 4) (weight was 25 g each) using Apex Activity Meter (Bagneux, France). Initially, each group of mice was placed inside the cage of the activity meter and the locomotion count was recorded for 10 min. The animals were then injected with the test drug in doses of 40 or 80 mg/kg intraperitoneally (1 or 2 mg/mouse). Then the animals were replaced in the same cage of the activity meter and the locomotion count was recorded for 10 min. The percentage change in the locomotion count was then calculated.
4.2.5.3 Behaviour study: effect of the compounds on the general behaviour at doses of 100/200 mg/kg (i.p)
Albino mice (body weight 20 g) were injected with the test compounds 100 and 200 mg/kg (i.p) and they were continuously observed for 2 h for any changes in behaviour and effect on different body systems.
4.2.5.4 Thermal pain: effect of the compounds on thermal pain
Pain was induced in Albino mice (body weight 25 g) using the hot plate method (57 °C) using Columbus Analgesia meter hot plate model 39D (Columbus, Ohio, USA). Initially mice were placed individually on the hot plate and the pain reaction time (in seconds) was recorded. The reaction is defined as lifting of the forelimbs and licking or flowing on them. Then each compound or caffeine was administered (i.p) at 180 mg/kg (i.p). Thirty minutes later the animals were tested for the reaction time on the hot plate.
References
- I. Abkömmlinge der 9-Äthyltrimethylharnsäure. Justus Liebigs Annalen der Chemie. 1918;414:54-67.
- [CrossRef] [Google Scholar]
- Caffeine psychological effects, use and abuse. J. Orthomol. Psychiatry. 1981;10:202-211.
- [Google Scholar]
- Structure-activity relationships in a series of 8-substituted Xanthines as bronchodilator and A1-adenosine receptor antagonists. Arch. Pharm.. 1995;328:654-658.
- [CrossRef] [Google Scholar]
- Ueber die Harnsäure. II. Berichte der deutschen chemischen Gesellschaft. 1884;17:1776-1788.
- [CrossRef] [Google Scholar]
- 8-Azaxanthine derivatives as antagonists of adenosine receptors. J. Med. Chem.. 1994;37:2970-2975.
- [CrossRef] [Google Scholar]
- Synergism between Cisplatin and topoisomerase I inhibitors, NB-506 and SN-38, in human small cell lung cancer cells. Cancer Res.. 1996;56:789-793.
- [Google Scholar]
- Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci.. 2005;8:858-859.
- [Google Scholar]
- Caffeine derivatives. I. The 8-ethers of caffeine. J. Am. Chem. Soc.. 1934;56:1356-1358.
- [CrossRef] [Google Scholar]
- Selective inhibition of cyclic nucleotide phosphodiesterases by analogues of 1-methyl-3-isobutylxanthine. Biochemistry. 1977;16:3316-3321.
- [Google Scholar]
- On the high affinity of 8-cyclohexylcaffeine for the presynaptic inhibitory adenosine receptor present in rat motor nerve terminals. Pharmacol. Toxicol.. 1997;6:295-300.
- [CrossRef] [Google Scholar]
- Photoinduced reactions. XXVI. Photosensitized oxygenation of 8-alkyoxycaffeines and related compounds. Tetrahedron. 1969;25:557-564.
- [Google Scholar]
- Design, synthesis, in vitro cytotoxicity evaluation and structure-activity relationship of Goniothalamin analogs. Arch. Pharm. Res.. 2013;36:812-831.
- [CrossRef] [Google Scholar]
- A2A adenosine receptor antagonists-future drugs for Parkinson’s disease? Drugs Future. 2000;25:1043-1052.
- [Google Scholar]
- A2A-selective adenosine receptor antagonists: development of water-soluble prodrugs and a new tritiated radioligand. Drug Dev. Res.. 1998;45:190-197.
- [CrossRef] [Google Scholar]
- Adenosine receptor antagonists: structures and potential therapeutic applications. Curr. Pharm. Des.. 1996;2:501-530.
- [Google Scholar]
- Imidazo[2,1-i]purin-5-ones and related tricyclic water-soluble purine derivatives: potent A2A- and A3-adenosine receptor antagonists†. J. Med. Chem.. 2002;45:3440-3450.
- [CrossRef] [Google Scholar]
- Manual of Clinical Microbiology (sixth ed.). Washington, USA: Amer Society for Microbiology Press; 1995.
- Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev.. 1992;17:139-170.
- [CrossRef] [Google Scholar]
- ChemInform abstract: synthesis and biological properties of luotonin a derivatives. ChemInform. 2009;40
- [CrossRef] [Google Scholar]
- Synthesis of 8-aryloxycaffeines and their inhibitory activity on topoisomerase II. Yakhak Hoeji. 2011;55:441-445.
- [Google Scholar]
- Design and synthesis of N-benzylpiperidine–purine derivatives as new dual inhibitors of acetyl- and butyrylcholinesterase. Bioorg. Med. Chem.. 2005;13:6795-6802.
- [CrossRef] [Google Scholar]
- Production of protein-associated DNA breaks by 8-methoxycaffeine, caffeine and 8-chlorocaffeine in isolated nuclei from L1210 cells: comparison with those produced by topoisomerase II inhibitors. Carcinogenesis. 1991;12:1781-1790.
- [Google Scholar]
- Pharmacol. Toxicol.. 1997;80:295-300.
- Substituted 1,3-dipropylxanthines as irreversible antagonists of A1 adenosine receptors. J. Med. Chem.. 1994;37:2704-2712.
- [CrossRef] [Google Scholar]
- Effects of 8-phenyl and 8-cycloalkyl substituents on the activity of mono-, di, and trisubstituted alkylxanthines with substitution at the 1-, 3-, and 7-positions. J. Med. Chem.. 1989;32:1231-1237.
- [CrossRef] [Google Scholar]
- 8-(Dicyclopropylmethyl)-1,3-dipropylxanthine: a potent and selective adenosine A1 antagonist with renal protective and diuretic activities. J. Med. Chem.. 1991;34:466-469.
- [CrossRef] [Google Scholar]
- Functionalisation including fluorination of caffeine, guanosine tetraacetate, and uridine triacetate using electrochemical oxidation. Tetrahedron Lett.. 1994;35:9237-9238.
- [CrossRef] [Google Scholar]
- Structure–activity relationships in a series of 8-substituted xanthines as A1-adenosine receptor antagonists. Bioorg. Med. Chem.. 2001;9:575-583.
- [CrossRef] [Google Scholar]
- 8-methoxycaffeine inhibition of Drosophila DNA topoisomerase II. Biochim. Biophys. Acta. 1992;7:30-33.
- [Google Scholar]
- Studies on DNA binding of caffeine and derivatives: evidence of intercalation by DNA-unwinding experiments. Biochim. Biophys. Acta. 1989;23:112-115.
- [Google Scholar]
- Ueber eine neue Darstellungsweise von Phenyläthersalicylsäure. Ber. Dtsch. Chem. Ges.. 1904;37:853-854.
- [CrossRef] [Google Scholar]
- Ueber die phenylirung von phenolen. Ber. Dtsch. Chem. Ges.. 1905;38:2211-2212.
- [CrossRef] [Google Scholar]
- Synthesis of 8-substituted xanthine derivatives by Suzuki cross-coupling reaction. Heterocycles. 2002;57:871-879.
- [CrossRef] [Google Scholar]
- 8-substituted xanthines as phosphodiesterase inhibitors: conformation-dependent lipophilicity and structure-activity relationships. Helv. Chim. Acta. 1989;72:507-517.
- [CrossRef] [Google Scholar]
- Inhibition of separated forms of cyclic nucleotide phosphodiesterase from pig coronary arteries by 1,3-disubstituted and 1,3,8-trisubstituted xanthines. J. Med. Chem.. 1981;24:954-958.
- [CrossRef] [Google Scholar]
- Ueber die umlagerung von lactimäthern in lactame. Ber. Dtsch. Chem. Ges.. 1902;35:1991-1992.
- [CrossRef] [Google Scholar]
- Synthesis of 8-substituted xanthines and their oxidative skeleton rearrangement to 1-oxo-2,4,7,9-tetraazaspiro[4,5]dec-2-ene-6,8,10-triones. Eur. J. Org. Chem.. 1999;1999:2419-2428.
- [CrossRef] [Google Scholar]







