Translate this page into:
Synthesis, biological evaluation, molecular docking and in silico ADMET screening studies of novel isoxazoline derivatives from acridone
⁎Corresponding author. m.aarjane@edu.umi.ac.ma (Mohammed Aarjane)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
A series of new acridone derivatives from isoxazoline (2a-l) were synthesized via 1,3-dipolar cycloaddition reaction between a variety of aryl nitrile oxides and N-allyl acridones using simple and efficient methods. This is the first ever green protocol to synthesize novel isoxazolines derivatives from acridone under microwave condition and offers broad substrate scope with good to excellent yields. The synthesized compounds (2a-l) were tested for their antibacterial potency against four pathogenic bacterial and were found to exhibit moderate to potent antibacterial activity. Two of these compounds 2a and 2k exhibited a significant antibacterial activity against E. coli and P. putida, respectively. The in silico molecular docking results supported the antibacterial activity of the synthesized compounds. ADMET properties of the most synthetized compounds showed an excellent bioavailability, therefore, can be considered as promising drugs candidates for further studies.
Keywords
Acridone
Antibacterial evaluation
Isoxazoline
Molecular docking
ADMET
1 Introduction
Heterocycles compounds have served as valuable synthetic templates for the synthesis of new compounds with interesting biological properties (Jia et al., 2020). Antibacterial activities of heterocycles, particularly those with nitrogen and oxygen as heteroatoms are well documented in the literature (Aarjane et al., 2020c; Banerjee, 2017; Guariento et al., 2018; Naouri et al., 2020; Zorina et al., 2019). Among these heterocycles, acridones have attracted much attention as a result of their divers medicinal applications, including antimalarial (Kumar et al., 2009), antibacterial (Aarjane et al., 2019), anticancer (Oyedele et al., 2020), and anti-inflammatory (Sondhi et al., 2010) (Fig. 1). Moreover, the photophysic properties of acridones allow these kind of compounds to be interesting tools for selective recognition in ecological and biological areas (Aarjane et al., 2020b). On the other hand, isoxazolines are key skeletons of several synthetic and naturally occurring pharmacologically active compounds such as antitumor (Ribeiro et al., 2017), anti-HIV (Srivastava et al., 1999), antibacterial (Zghab et al., 2017) and anticancer (Kaur et al., 2014) (Fig. 1). Numerous methods have been reported for the preparation of these heterocycles, 1, 3-dipolar cycloaddition reactions are typically utilized to construct isoxazoline ring (Bonacorso et al., 2018; Dadiboyena and Nefzi, 2012; Rouf et al., 2017).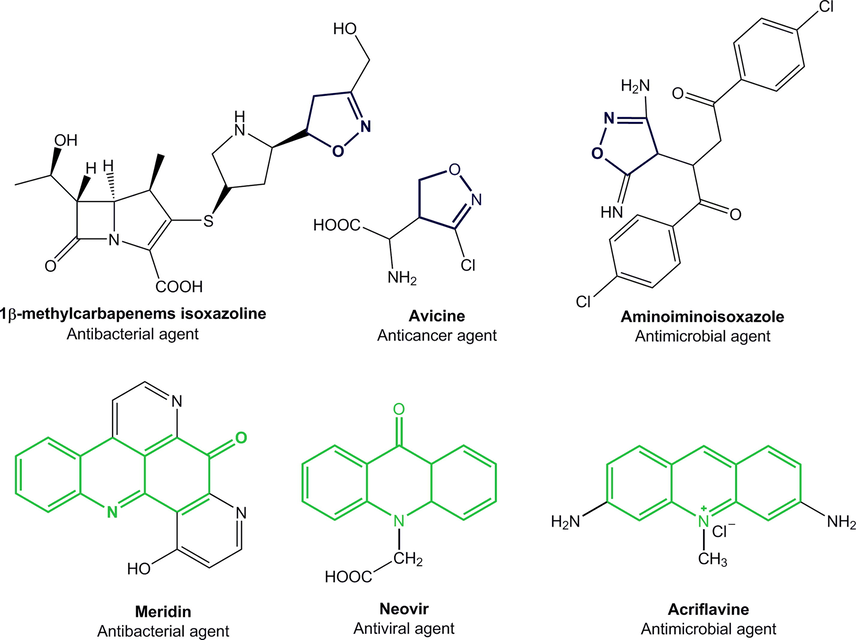
Isoxazolines and acridones used as pharmaceuticals agents.
In recent years, antibiotic resistance has become a major problem to public health security worldwide (Yin et al., 2020) and the occurrence of antibiotic resistance has expected an alarming rate the world over (Botelho and Schulenburg, 2020). Some P. putida shows a high resistance phenotype to diverse xenobiotic and organic compounds such as flavonoids, β-lactam antibiotics and other antimicrobial compounds (Fernandez-Escamilla et al., 2015). The transcriptional regulator (TtgR) enzyme of P. putida is a HTH-type transcriptional repressor that controls expression of the TtgABC efflux pump, which is the main contributor to resistance against several antimicrobials agents in this bacteria (Terán et al., 2003). Moreover, previous studies on the TtgR multidrug binding potential have shown that it binds with moderate to high affinity to plant-derived compounds such as phloretin and naringenin (Alguel et al., 2007b; Choudhury, 2010). All of these compounds are characterized by having an aromatic ring (Daniels et al., 2010). On the other hand, MenB or dihydroxynaphthoic acid synthetase, is involved in the biosynthesis of menaquinone. E. coli utilize menaquinone (vitamin K2), a polyisoprenylated naphthoquinone, as the lipid-soluble redox cofactor in the electron transport chain of bacteria. The enzymes in the biosynthetic pathway of bacterial menaquinone are potential targets for novel antibacterial drug (Sharma et al., 1992). Several new scaffolds have been identified as dihydroxynaphthoic acid synthetase inhibitors (Amzoiu et al., 2020; Evans et al., 2016).
Computational tools in drug discovery become a major role in the development of therapeutically important small compounds (Franchini et al., 2017; Ragusa et al., 2017). These computational methods are relevant in limiting the use of animal models and for aiding the rational design of novel and safe drug candidates (Brogi et al., 2020; Righetti et al., 2020). The in silico molecular docking technique was applied to explain the obtained biological activities by identify the stable poses of synthesized compounds into the studied receptor pocket. During clinical drug development process many drugs candidates can be excluded due to pharmacokinetic problems which affect costs and time investments in drug discovery process (Ghaleb et al., 2020). To overcome this problem an in silico ADMET (Absorption; Distribution; Metabolism, Excretion and Toxicity) study was applied to predict drugs pharmacokinetics.
In view of the promising antibacterial profile of acridones as well as isoxazolines and also relying on our previous studies on acridone derivatives as potent antibacterial agents (Aarjane et al., 2020a), we have synthesized novel compounds of both isoxazoline and acridone pharmacophores, with the expectation of a synergetic effect. The adopted strategy for preparing novel isoxazoline derivatives from acridone was based on the 1,3-dipolar cycloaddition reaction between N-allyl acridone and nitrile oxides using simple and efficient methods. The newly synthesized compounds were then tested for their potential antibacterial activities. Furthermore, molecular docking study have been applied to explain and identify the possible modes of interactions between synthesized compounds and studied receptors. The bioavailability of these drugs candidates has been determined by ADMET screening.
2 Results and discussions
2.1 Synthesis
The aim of this work is preparing novel acridone derivatives bearing isoxazoline moieties. The novel isoxazoline derivatives obtained from acridone (2a–l) were synthesized through a two-step method (Scheme 1).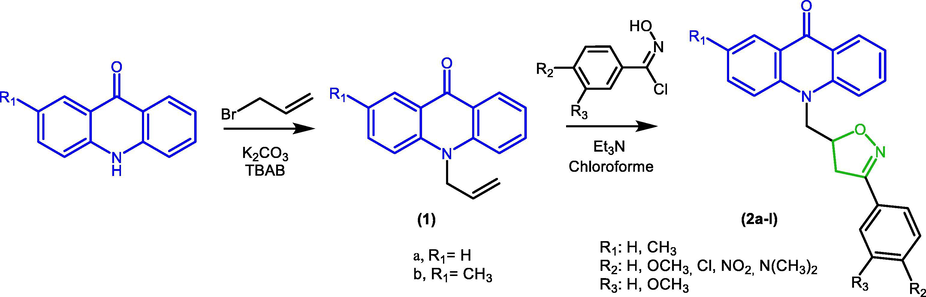
Synthesis route of novel isoxazoline derivatives from acridone (2a-l).
The first step was the preparation of the dipolarophile by N-allytion of acridone using solid–liquid phase transfer catalyst. Stirring acridone with allyl bromide in the presence of tetra-n-butylammonium bromide (TBAB) as catalyst and anhydrous potassium carbonate in DMF at 80 °C give compound 1a-b with good yields.
The 3,5-disubstituted isoxazolines were obtained by 1,3-dipolar cycloaddition reaction between an appropriately substituted nitrile oxides and N-allyl acridones. At the outset, our investigation focused on exploring the 1,3-dipolar cycloaddition reaction conditions between benzaldoxime and the dipolarophiles 1a. For that, we adopted in the first step a conventional protocol based on the generation “in situ” of nitrile oxide in the presence of sodium hypochlorite (NaOCl) at 0 °C in the biphasic mixture H2O/CH2Cl2 (Table 1). The reaction resulted in the formation of the desired 3,5-disubstituted isoxazoline 2a but with very low yield ranging from 10 to 12%. This result could be explained by the low solubility of dipolarophile 1a in water. In order to increase the yield of the 1,3-dipolar cycloaddition reaction, we turned our attention to the development of a process for the synthesis of isoxazoline derivatives based on the generation of nitrile oxide “in situ” from N-hydroxybenzimidoyl chloride as well as adopting a microwave assisted synthesis method. As shown in Table 1, different reaction conditions were probed to improve the yield of compound 2a.
Entry
Base
Solvent
Temp. (°C)
Time
Yield%
1b
NaOCl
CH2Cl2/H2O
0–5 °C
24 h
14
2
Et3N
CH2Cl2
RT
24 h
60
3
Et3N
CHCl3
RT
24 h
68
4
Et3N
CHCl3
50 °C
8 h
75
5
Et3N
Toluene
50 °C
6 h
60
6c
Et3N
CHCl3
50 °C
25 min
89
7c
Et3N
Toluene
70 °C
20 min
80
The results indicated in Table 1 show that the use of alkyne 1a (1 mol equiv) and N-hydroxybenzimidoyl chloride (1.2 mol equiv) in the presence of triethylamine at 50 °C in chloroform afforded 3,5-disubstituted isoxazoline 2a in good yield (75%). Moreover, strong acceleration of the cycloaddition reaction under microwave irradiation was noticed in comparison to conventional conditions that required 6–24 h of agitation against 20–25 min under microwave irradiation.
With the optimized reaction conditions in hand (Table 1, entry 4 and 6), we synthesized novel isoxazoline derivatives 2a-l (Scheme 1, Table 2) by changing the starting substrates. With the different para- or meta-substituted N-hydroxybenzimidoyl chlorides used in this work, the regioselectivity of the reaction was established since no 3,4-disubstituted isoxazole regioisomers were observed. Moreover, electron donating as well as electron withdrawing N-hydroxybenzimidoyl chlorides gave similar results, except in the case of compounds 2e and 2f we obtained mediocre yields. The compounds (2a-l) were purified by column chromatography and/or recrystallization method. Purified compounds were characterized using IR, NMR and Mass spectrometry.
Compounds
R1
R2
R3
Yield% a
Yield% b
2a
H
H
H
70
86
2b
–
OMe
H
79
89
2c
–
Cl
H
63
68
2d
–
NO2
H
71
80
2e
–
N(CH3)2
H
60
66
2f
OH
OMe
65
66
2g
CH3
H
H
76
81
2h
–
OMe
H
70
84
2i
–
Cl
H
68
67
2j
–
NO2
H
72
80
2k
–
N(CH3)2
H
63
64
2l
OH
OMe
60
65
The IR spectra of novel isoxazoline derivatives obtained from acridone (2a-l) showed characteristic absorption bands in the region of 1640–1635 cm−1 corresponding to the vibration of the carbonyl of acridone ring (C⚌O) and imine group of isoxazoline nucleus showed the C⚌N stretching frequency at 1605–1600 cm−1. The 1H NMR spectra of compounds (2a-l) showed aromatic protons between 8.63 and 6.67 ppm. We also noticed the presence of two signals as double doublet between 4.78 and 4.55 ppm attributable to the two protons of the methylene group (N—CH2), as well as two double doublet between 3.56 and 3.27 ppm related to the methylene protons (CH2) of isoxazoline ring, in addition to a multiplet between 5.40 and 5.30 ppm assigned to the proton (C—H) of isoxazoline nucleus. The chemical displacements of the proton (CH) of isoxazoline nucleus are in the order of 5.4 ppm, whereas in the case of 3,4‐disubstituted isoxazoline is expected to be higher values due to the oxygen-attracting effect. These results confirm the regioselectivity of the cycloaddition reaction. 13C NMR spectra of compounds (2a-l) confirm the formation of 3,5‐disubstituted isoxazoline. The chemical displacements observed for the CH carbon of the isoxazoline nucleus between 79 and 78 ppm find their explanation in the oxygen attractor effect of the isoxazolic nucleus. In addition the signals between 50 and 48 ppm and 38 ppm corresponding to the CH2 groups, as well as a signal between 157 and 156 ppm relative to the carbon C⚌N of the isoxazoline ring and another signal between 178 and 176 ppm attributed to the carbonyl (C⚌O) of the acridone nucleus.
2.2 Antibacterial activity
The novel acridones 2a-l have been studied to evaluate their antibacterial activity against Gram (−) bacteria (E. coli, P. putida and K. pneumoniae) and Gram (+) bacteria (S. aureus). The antibacterial activities have been primarily tested by agar diffusion method. Then, minimum inhibitory concentrations (MIC) were determined for the synthesized compounds. Chloramphenicol was used as positive control for antibacterial activities. Results are displayed in Table 3 as minimum inhibitory concentrations (MIC) in µg/mL. The antibacterial activity results indicate that the tested compounds displayed various degrees of inhibition against the four tested bacteria species. The highest antibacterial activity was obtained against S. aureus strains. Compound 2k with methyl on acridone ring and N,N-dimethylamine on the phenyl group showed the best antibacterial activity against P. putida with MIC value 38.57 μg/mL, which is very close to the known commercial antibiotic chloramphenicol with MIC value 37.03 µg/mL. While compound 2a with phenyl and hydrogen at C-2 on the acridone ring on the isoxazoline-acridone moiety, showed good activity against E. coli strains with MIC value 26.95 µg/mL, compared to the standard drug Chloramphenicol with MIC value 22.41 µg/mL. In addition, the compound 2 h with p-methoxy on the phenyl group and methyl at C-2 on the acridone ring exhibited high potential of antibacterial activity against S. aureus with MIC = 24.60 µg/mL. The antibacterial activity against the tested Gram-positive and Gram-negative pathogens indicate that the replacement of the allyl group with isoxazoline nucleus enhanced the antibacterial potential against Gram-negative bacteria and Gram-positive bacteria for compounds 2a-l, whereas no significant differences of the antibacterial activity between the N-allyl acridones and acridones against all bacteria were noticed. The antibacterial activity for the isoxazoline-acridone derivatives was increased significantly, especially for P. putida when the 2-methylacridone-isoxazoline skeleton was substituted by N,N-dimethylamine on the phenyl group.
Compounds
Staphylococcus aureus
Escherichia coli
Klebsiella pneumoniae
Pseudomonas putida
Acridone
122.83
133.41
137.93
156.31
2-methylacridone
118.43
124.22
130.43
145.52
1a
97.10
80.66
97.25
100.95
1b
83.20
70.14
102.20
115.20
2a
39.57
26.95
71.00
88.48
2b
32.15
36.88
65.00
74.64
2c
32.15
42.15
74.64
91.00
2d
35.26
32.75
74.64
61.00
2e
41.78
49.57
65.00
74.64
2f
39.12
50.12
60.10
83.44
2g
34.78
43.23
71.00
81.27
2h
24.60
34.39
65.00
56.55
2i
28.39
30.39
74.64
61.00
2j
30.12-
50.12
80.10
56.55
2k
33.74
35.88
65.00
38.57
2 l
30.12
30.39
76.23
56.55
Chloramphenicol
11.65
22.41
15.38
37.03
DMSO
–
–
–
–
2.3 In silico ADMET prediction and evaluation of lipinski's ‘Rule of 5′
In silico ADMET analysis was performed to evaluate the drug-likeness and pharmacokinetic properties of the synthesized compounds 2a-l. On this purpose, pkCSM online tool (Pires et al., 2015) and DruLito software (“Drug Likeness Tool (DruLiTo 1),” n.d.) were used. Therefore, human intestinal absorption, blood–brain barrier penetration (BBB), acute oral toxicity, skin sensitization, AMES toxicity, HEGR inhibitor and some druglike properties were calculated.
Based on Lipinski rules of 5 compounds with molecular mass less than 500 Da, 5 hydrogen bond donors, 10 hydrogen bond acceptors, and with an octanol–water partition coefficient log P less than 5 are likely absorbed. Table 4 shows that the studied compounds (2a-2 l) have high lipophilicity property (LogP = 3.05–3.86), molecular weight MW (354.14–414.16), H-bond acceptor (<10) and H-bond donor (<5) and total polar surface area (≤140) which confirmed the desirable drug likeness criteria of these synthetized drugs candidates. Drugs with poor bioavailability are considered as inefficient because major portion of a dose never reaches the plasma to exert the pharmacological effect. In general, the predictive model of pKCSM indicates that molecules with predicted values > 0.9 have high Caco2- permeability, human intestinal absorption values less than 30% are poorly absorbed, low values of total clearance means high half-life and molecules with logBB < −1 are poorly distributed to the brain (Ghaleb et al., 2019).
Compound
Property
Lipinski violation
LogP
H-bond acceptor
H-bond donor
Polar surface area (A2)
Rotatable Bonds
Molecular weight
2a
3.47
2
0
41.9
3
354.14
0
2b
3.08
3
0
51.13
4
384.15
0
2c
3.41
2
0
41.9
3
388.1
0
2d
3.05
2
0
85.04
4
399.12
0
2e
3.37
3
0
45.14
4
397.18
0
2f
3.0
4
1
71.36
4
400.14
0
2g
3.86
2
0
41.9
3
368.15
0
2h
3.47
3
0
51.13
4
398.16
0
2i
3.81
2
0
41.9
3
402.11
0
2j
3.45
2
0
85.04
4
413.14
0
2k
3.76
3
0
45.14
4
411.19
0
2l
3.39
4
1
71.36
4
414.16
0
The predicted bioavailability of the synthetized compounds 2b and 2h presented in Table 5 shows an excellent pharmacokinetics property, they present high Caco2-permeability (1.08–1.06) and intestinal absorption (98.64–99.19), they are poorly distributed to the brain (−0.04; −0.06) with total clearance (0.31–0.35). The subtype of cytochrome P450 CYP2D6 indicates that they could not be a substrates or inhibitors of this main subtype, which can decrease the chance of drug-drug interactions. They present no hERG inhibitory, hepatotoxicity or skin sensibilization, while compounds (2d, 2f, 2j, 2l) show low Caco2- permeability and the compounds (2a, 2c, 2e, 2g, 2i, 2k) can exhibit blood barrier permeability. Moreover, the drug candidate 2b shows an AMES toxicity which can be a mutagenic compound and therefore acts as a carcinogen. While the synthetized compound 2h present good pharmacokinetics properties (absorption, distribution, metabolism, excretion and toxicity). In general, most synthetized compounds can be considered as promising drugs candidates for further studies.
Name
Absorption
Distribution
Metabolism
Excretion
Toxicity
Caco2 permeability
Intestinal absorption (human)
Blood brain barrier Permeability
CYP
Total Clearance
hERG inhibitor
Max. tolerated dose (human)
Hepatot-oxicity
Skin Sensitization
AMES
2D6
3A4
1A2
2C19
2C9
2D6
3A4
substrate
inhibitor
(log mol/L)
Numeric (% Absorbed)
(logBB)
Categorical (Yes/No)
Numeric log (ml/min/kg)
(Yes/No)
log(mg/kg/Day)
log(LC50)
(Yes/No)
(Yes/No)
2a
1.06
97.51
0.27
No
Yes
Yes
Yes
Yes
No
Yes
0.31
No
0.40
No
No
No
2b
1.08
98.64
−0.04
No
Yes
Yes
Yes
Yes
No
Yes
0.33
No
0.34
No
No
Yes
2c
1.05
96.10
0.24
No
Yes
Yes
Yes
Yes
No
Yes
0.15
No
0.30
No
No
Yes
2d
0.42
95.74
−0.69
No
Yes
Yes
Yes
Yes
No
Yes
0.33
No
0.14
No
No
No
2e
1.05
98.85
0.19
No
Yes
Yes
Yes
Yes
No
Yes
0.51
No
0.29
No
No
No
2f
0.58
96.06
−0.49
No
Yes
Yes
Yes
Yes
No
Yes
0.34
No
0.18
No
No
No
2g
1.05
98.05
0.27
No
Yes
Yes
Yes
Yes
No
Yes
0.34
No
0.3
No
No
Yes
2h
1.05
99.19
−0.06
No
Yes
Yes
Yes
Yes
No
Yes
0.35
No
0.24
No
No
No
2i
1.04
96.65
0.24
No
Yes
Yes
Yes
Yes
No
Yes
0.11
No
0.19
No
No
No
2j
0.49
96.29
−0.69
No
Yes
Yes
Yes
Yes
No
Yes
0.35
No
0.01
No
No
No
2k
1.04
99.40
0.19
No
Yes
Yes
Yes
Yes
No
Yes
0.53
No
0.18
No
No
No
2l
0.66
96.61
−0.50
No
Yes
Yes
Yes
Yes
No
Yes
0.36
No
0.10
No
No
No
2.4 Molecular docking studies
Molecular docking was used to understand the binding modes and to support the antibacterial activity of the synthesized compounds obtained experimentally, also to elucidate new information for further structural optimization.
The transcriptional regulator (TtgR) enzyme of P. putida is a HTH-type transcriptional repressor that controls expression of the TtgABC efflux pump, which is the main contributor to resistance against several antimicrobials agents (Terán et al., 2003). Moreover, previous studies on the TtgR multidrug binding potential have shown that it binds with moderate to high affinity to plant-derived compounds such as phloretin and naringenin (Alguel et al., 2007b; Choudhury, 2010). Concerning E. coli, MenB or dihydroxynaphthoic acid synthetase is interesting enzyme in the biosynthesis of menaquinone. The enzymes in the biosynthetic pathway of bacterial menaquinone are potential targets for novel antibacterial drug (Sharma et al., 1992). In this work, the molecular docking studies of the synthesized compounds have been applied to determine the different type of interactions and clarify the probable binding modes between acridone derivatives and transcriptional regulator (TtgR) enzyme (PDB ID : 2UXI) of P. putida (Alguel et al., 2007a) and E. coli MenB, OSB-NCoA complex (PDB ID : 3 T88) respectively (Li et al., 2011).
The molecular docking setup was first validated by performing self-docking of the co-crystalized ligands (Phloretin) and (S0N) in the active site of transcriptional regulator (TtgR) enzyme (PDB ID : 2UXI) of P. putida and E. coli MenB, OSB-NCoA (PDB ID : 3T88), respectively. The results of self-docking validation show small RMSD between the docked pose and the experimental co-crystallized inhibitor pose with 0.70 Å for (transcriptional regulator (TtgR) enzyme) and 1.52 Å for (E. coli MenB, OSB-NCoA), which is satisfactory (less than 2 Å). In addition, all the twelve acridones were docked into the binding pocket of transcriptional regulator (TtgR) enzyme and E. coli MenB, OSB-NCoA successfully.
The analysis of the active site of transcriptional regulator (TtgR) enzyme, reveals that all the synthesized compounds 2a-l are making various close contacts with the residues lining the active site of transcriptional regulator (TtgR) enzyme, the interacting amino acids of all compounds are shown at Table 6. The analysis of best scoring pose of compound (2k) (stable conformation) in the transcriptional regulator (TtgR) enzyme pocket of 2XUT showed significant hydrogen bonding as well as hydrophobic interactions between them (Fig. 2). The isoxazoline ring and phenyl group exhibits hydrophobic interactions with the residues His70, Val96, Ala74, Leu66, Leu92 and Leu100. Carbonyl group of acridone nucleus exhibited hydrogen bonding interactions with Asn110. The high docking scores of compounds of 2k, 2j, 2h and 2l reveal that these compounds are well accommodated in the active site of enzyme and they strongly interact within the active site of transcriptional regulator (TtgR) enzyme. Relying on the different interactions of the synthesized compounds presented in Fig. 2, Table 6, it can be concluded that the most active compounds have H-bond interaction with Asn110 and hydrophobic interactions with the receptor indicating the crucial role that play to inhibit the transcriptional regulator (TtgR) enzyme, this results has been demonstrated by previous researches (Choudhury, 2010; Paul and Choudhury, 2010; Zhang et al., 2017). In Bold: H-bonding interaction.
2XUI
3T88
Interacting residues
Binding affinity
Interacting residues
Binding affinity
2a
His67, Met89, Val96, His70, Leu66, Leu92, Val171, Cys137, Ile141, Ala74
−7.03
Thr155, Gly133, Val159, Tyr97, Gly132
−7.04
2b
Asn110, His70, Met89, His67, Val96, Leu92, Val171, Cys137, Ile141, Ala74
−7.58
Val159, Gly133, Gly85, Tyr97, Val108
−6.74
2c
His70, Met89, His67, Val96, Leu100, Ile175, Leu66, Leu92, Val171, Cys137, Ile141, Ala74
−7.27
Val159, Gly164, Asp163, Phe162, Gly133
−6.53
2d
Asn110, His70, Met89, His67, Val96, Leu66, Leu92, Val171, Cys137, Ile141, Ala74
−7.71
Thr155, Gly132, Gly164, Phe162
−6.96
2e
Asn110, His70, His67, Val96, Leu66, Leu92, Cys137, Ala74, Ser77, Glu78, Gly140, Phe168, Val134
−7.91
Phe162, Gly133, Ser84
−6.96
2f
Val134, Cys137, Phe168, His70, Val96, Leu92
−7.34
Asp163, Phe162, Gly164, Gly133
−7.01
2g
His70, His67, Val96, Leu66, Leu92, Cys137, Ala74, Phe168, Met89, Ala144, Val171, Ile141
−7.43
Phe162, Asp163, Gly164, Gly133
−6.69
2h
Asn110, Met89, His70, His67, Val96, Leu92, Cys137, Ala74, Phe168, Ile141, Ala144, Val171
−8.01
Phe162, Ser84, Gly164, Gly133, Gly86
−7.03
2i
Met89, Phe168, His70, His67, Val96, Leu92, Cys137, Ala74, Ile141, Ala144, Val171, Ile175, Leu100
−7.68
Thr155, Val159, Gly132, Ile131, Ala47, Val44, Lys89, Ser84
−6.80
2j
Asn110, Met89, Phe168, His70, His67, Val96, Cys137, Ala74, Ile141, Ala144, Val171, Leu66, Leu92
−8.14
Phe162, Asp163, Gly164, Gly133
−6.92
2k
Asn110, Phe168, His70, His67, Val96, Cys137, Ala74, Ser77, Leu66, Leu92, Glu78, Leu100, Val134, Gly140
−8.33
Thr155, Gly133, Gly132, Ile131, Val159, Ala47, Lys89, Val44,Arg45
−7.01
2l
Asn110, Phe168, His67, Val96, Cys137, Ala74, Leu66, Leu92, Leu100, Val134, Ile141
−7.84
Thr155, Val108, Leu106
−7.04
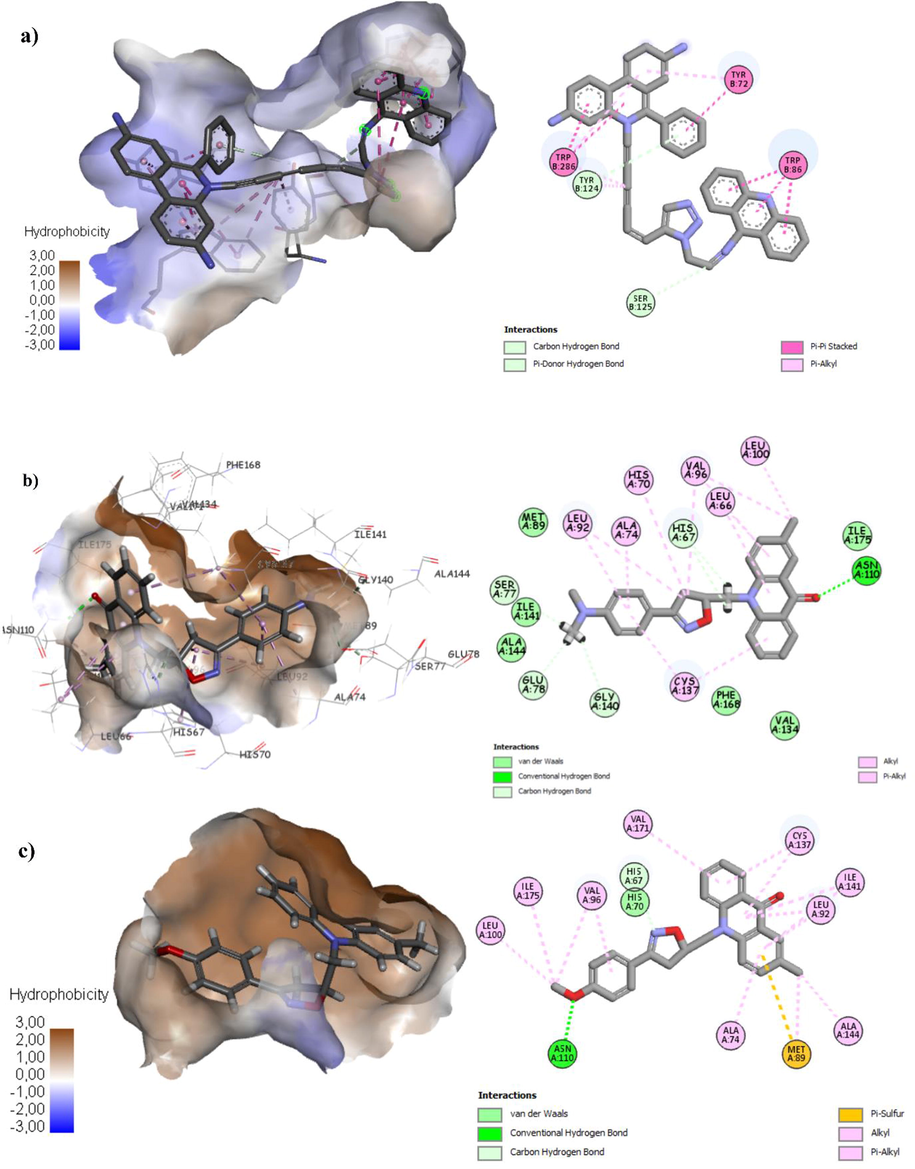
(a) Binding mode of co-crystalized compound with TtgR enzyme. (b) Binding mode of compound most active compound 2k with TtgR enzyme. (c) Binding mode of compound 2h with TtgR enzyme.
Concerning E. coli MenB, OSB-NCoA, the stable pose of the most active compound 2a showed two favorable hydrogen bonds between the nitrogen and oxygen atoms of isoxazoline nucleus and the hydrogen of the side chain of Thr155 and Gly133, respectively. The phenyl and acridone core shows hydrophobic interactions with Val115 and Gly132 (Fig. 3). Also compounds 2d, 2i and 2l showed one favorable hydrogen bond between the nitro group of isoxazoline ring and the hydrogen of the side chain of Thr155, besides hydrophobic interactions. The molecular docking results of the different interactions of the synthesized compounds presented in Table 6, indicate that the most active compounds 2a, 2i, 2d and 2l have H-bond interaction with the hydrogen of the side chain of Thr155 in the active site of E. coli MenB, OSB-NCoA. Moreover, all synthesized compounds 2a-l bind to the active site of E. coli MenB, OSB-NCoA and share largely homogeneous in binding mode specially with Val159, Gly133, Phe162 and Asp163 to several E. coli MenB inhibitors reported in the literature (Fahim and Farag, 2020; Mahmoud et al., 2017). The high docking scores of compounds of 2a, 2i, 2d and 2l reveal that these compounds are well accommodated in the active site of enzyme and they strongly interact within the active site of E. coli MenB, OSB-NCoA. In consequence, molecular docking studies showed strong binding affinity of 2a into the active site of E. coli MenB, OSB-NCoA, which could be responsible for its significant in vitro antibacterial activity especially against E. coli.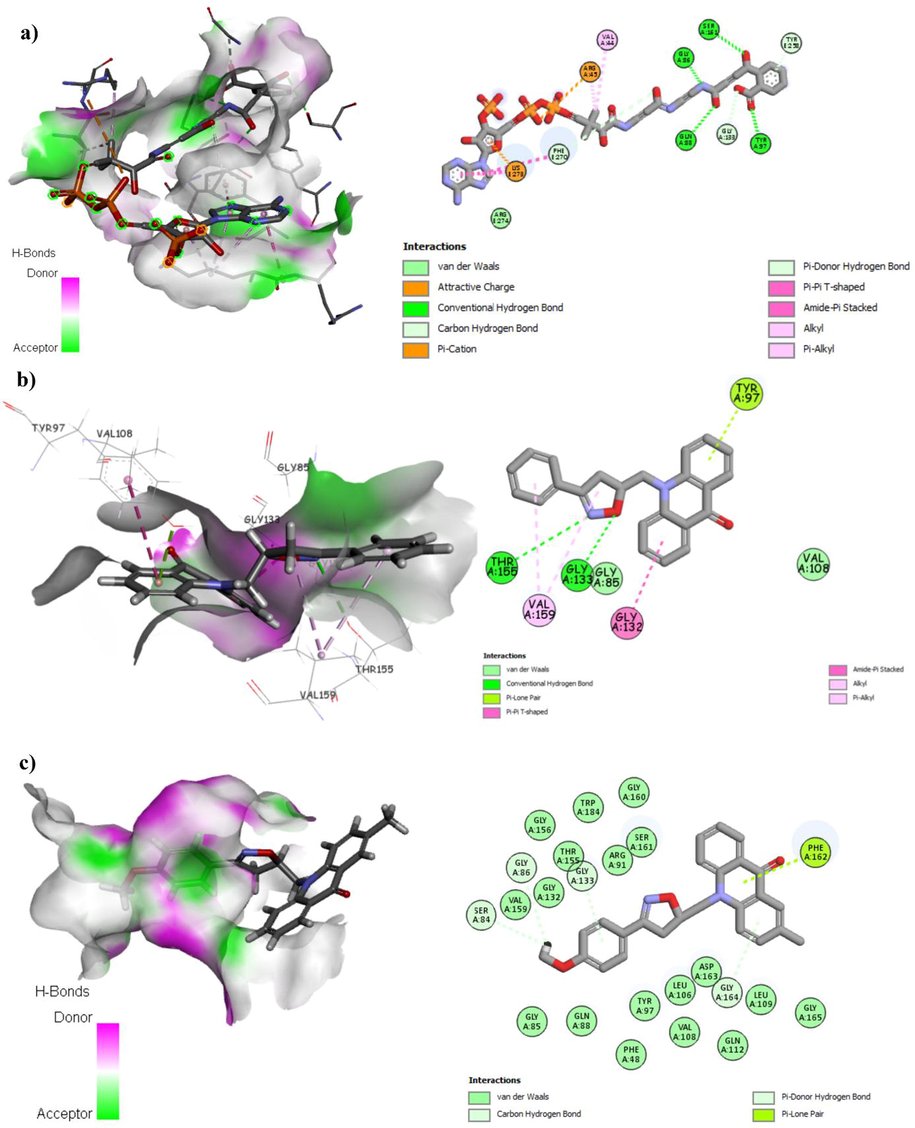
(a) Binding mode of the co-crystalized ligand with E. coli MenB, OSB-NCoA complex, the hydrogen bonds are presented in green dashed lines. (b) Binding mode of compound 2a with E. coli MenB, OSB-NCoA complex, the hydrogen bonds are presented in green dashed lines. (c) Binding mode of compound 2h with Escherichia coli MenB, OSB-NCoA complex.
The molecular docking results presented in the Fig. 2 shows the interactions of most promising derivatives 2k and 2h with TtgR enzyme, the compound 2k is stabilized into the receptor pocket with hydrogen bond, pi-alkyl and Van der-waals interactions. The compound 2h shows hydrogen bond, pi-sulfur and different pi-alkyl interactions, while the co-crystalized compound shows Van der-waals and pi-alkyl interactions. The Fig. 3 presents the different interactions of the most promising compounds (2a and 2h) and the co-crystalized compound (o-succinylbenzoyl-N-coenzyme A) with E. coli MenB, OSB-NCoA complex. The compound 2a is stabilized with two hydrogen bonds, pi-alkyl and Van der-waals interactions, and the compound 2 h shows pi-ion with different Van der-waals interactions, while the co-crystalized compound is stabilized with four hydrogen bonds, pi-cation and pi-alkyl interactions.
3 Conclusion
The synthesis of novel acridone-isoxazoline derivatives 2a-l has been accomplished with a very accessible way under mild conditions through the 1,3-dipolar cycloaddition of various aryl nitrile oxides in situ generated and N-allyl acridones. The molecular structures of the obtained compounds are supported by 1H NMR, 13C- NMR, IR and MS spectra. All the synthesized compounds were evaluated for their antibacterial activity, compounds 2a and 2k were found to be the most potent agents against E. coli and P. putida, respectively. To determine the stable conformation of the synthetized compounds into the receptor pocket and understand receptor-ligands interactions molecular docking was applied. The docking results suggest that compounds 2l, 2h, 2k and 2a are the most potent inhibitor of transcriptional regulator (TtgR) enzyme of P. putida and E. coli MenB, OSB-NCoA, respectively. Moreover, in silico ADMET screening results showed that the synthesized compounds are easily absorbed and present a good bioavailability.
4 Experimental
4.1 Materials
All materials were purchased from commercial suppliers. IR spectra were recorded using JASCO FT-IR 4100 spectrophotometer. The 1H, 13C NMR spectra was recorded with Bruker Avance 300. Mass spectrometric measurements were recorded using Exactive™ Plus Orbitrap Mass Spectrometer. Microwave irradiation was carried out with CEM Discover™.
4.2 Antibacterial activity
The synthesized compounds (2a-l) were tested for their in vitro antibacterial activity against Gram (−) bacteria (E. coli, P. putida and K. pneumoniae) and Gram (+) bacteria (S. aureus). The antibacterial activities have been primarily tested by agar diffusion method, the active compounds were subjected to the determination of the MIC, using the broth microdilution method. The microorganisms used in this study are pathogenic germs isolated from urine samples, collected from patients suspected of urinary tract infection (UTI), provided by Mohammed V Hospital in Meknes. Bacterial inoculums were prepared by subculturing microorganisms into Mueller Hinton broth (MHB) at 37 °C for 18 h and were diluted to approximately 106 CFU mL−1. Initial solution with concentration 0.5 mg/mL of the compounds (2a-l) were prepared in DMSO, further serial dilutions were made in the microplates and 100 μL of MHB containing each test microorganism were added to the microplate (Smith et al., 2018), then incubated at 36 °C for 24 h. After incubation, 20 μL of TTC (0.04 mg/mL) were added to each microplate. The color changes of TTC from colorless to red were accepted as microbial growth (Veiga et al., 2019).
4.3 Docking studies
4.3.1 Preparation of protein and ligands
The Discovery Studio (version 4.5) installed on windows 7 workstations was used to prepare the protein. The crystal structure of E. coli MenB, OSB-NCoA complex (PDB ID :3T88) (Li et al., 2011) and transcriptional regulator (TtgR) enzyme (PDB Code: 2UXI) (Alguel et al., 2007a) were retrieved from the protein data bank (PDB), [https://www.rcsb.org/]. The protein and co-crystallized ligand were isolated from the complex. The protein extracted from the complex was treated by removing all of the substructures, removing all of the water molecules and adding hydrogen atoms. The three-dimensional (3D) structures of ligands were drawn by using the chemical modeling software Avogadro (Hanwell et al., 2012). Geometry optimization tool embedded in Avogadro was used for structural refinement.
4.3.2 Molecular docking
Molecular docking process between ligands and the receptor was carried out by using the AutoDock software (Morris et al., 1998). In this study, AutoDockTools, Autogrid 4.2 and Autodock 4.2 were used to prepare input files, calculate grid maps and docking simulations. Grid-point spacing of 0.375 Å and grid box of 50 × 50 × 50 Å (x, y, and z) points with the xyz-coordinated 42.921, 42.976, and 8.801 was used. After merging all non-polar hydrogen, Kollman charged were added to the receptor. All other values were set as defaults and Lamarckian genetic algorithm (GLA) search for 100 run job were used (Shirgahi Talari et al., 2015). Discovery Studio visualizer version 4.5 was used for visualization. Docking setup was first validated by self-docking of the co-crystallized inhibitor in the enzyme active. The validated molecular docking setup was then used to investigate the ligand-target interactions of the newly synthesized compounds in E. coli MenB, OSB-NCoA complex and transcriptional regulator (TtgR) enzyme to predict their binding pattern and to investigate their ability to satisfy the required structural features for binding interactions.
4.3.3 Lipinski rule of 5 and in silico ADMET prediction:
Computational approaches have improved the success rate of drug development and reduced the experimental trials. In this concept an in silico ADMET and drug likeness studies were applied to the synthetizes compounds, using pkCSM online tool (Pires et al., 2015) and DruLito software (“Drug Likeness Tool (DruLiTo 1),” n.d.). The drug likeness was determined by predicting Log P, H-bond donor and acceptor, molecular weight. The drugs bioavailability is determining different descriptors such as Caco2- permeability, human intestinal absorption, blood brain barrier permeability, subtypes of cytochrome P450, total clearance and AMES toxicity.
4.3.4 Synthesis of acridon-isoxazoline derivatives (2a-l)
To a solution of N-allyl acridone 1 (1.2 g, 5 mmol) in chloroform (15 mL), N-hydroxybenzimidoyl chloride (1 g, 6.4 mmol) and TEA (0.64 g, 6.4 mmol) were added at room temperature, the reaction mixture was stirred at 50 °C for 6 h. Then, water (40 mL) was added and the mixture was extracted with chloroform, the organic layer was evaporated in high vacuum, and the obtained product was purified by recrystallization in DMF or by flash chromatography on silica gel using hexane/diethyl ether (2:5).
4.3.4.1 10-((3-phenyl-4,5-dihydroisoxazol-5-yl)methyl)acridin-9(10H)-one (2a)
Yellow solid; yield: 86%, mp = 185 °C. IR (KBr): 3012, 2975, 1640, 1600, 1596 cm−1. 1H NMR (300 MHz, DMSO‑d6, 25 °C, TMS): 8.40 (d, J = 8.1 Hz, 2H, H1,H8), 8.09–7.66 (m, 6H), 7.65–7.45 (m, 3H), 7.39 (t, J = 7.5 Hz, 2H), 5.28–5.20 (m, 1H), 5.03 (dd, J = 17.0, 9.2 Hz, 1H), 4.72 (dd, J = 17.0, 8.1 Hz, 1H), 3.42 (dd, J = 16.7, 7.1 Hz, 1H), 3.13 (dd, J = 16.7, 7.1 Hz, 1H).. 13C NMR (75 MHz, DMSO‑d6, 25 °C, TMS): 177.72, 156.09, 142.01, 135.46, 134.05, 128.43, 128.08, 122.60, 121.76, 121.47, 121.30, 114.94, 114.31, 78.10, 48.47, 38.24. MS (ESI) for C23H18N2O2 [M+H]+, calcd: 355.1441, found: 355.1440.
4.3.4.2 10-((3-(4-methoxyphenyl)-4,5-dihydroisoxazol-5-yl)methyl)acridin-9(10H)-one (2b)
White solid; yield: 89%, mp = 148 °C. IR (KBr): 3014, 2975, 1635, 1603, 1594 cm−1. 1H NMR (300 MHz, CDCl3, 25 °C, TMS): 8.61 (d, J = 8.1 Hz, 2H, H1,H8), 7.76–7.52 (m, 8H), 7.34 (t, J = 7.3 Hz, 1H), 6.96 (d, J = 8.4 Hz, 1H), 5.35 (m, 1H), 4.78 (dd, J = 16.1, 7.5 Hz, 1H), 4.69–4.45 (dd, J = 16.1, 7.5 Hz, 1H), 3.88 (s, 3H, OCH3), 3.55 (dd, J = 17.0, 9.4 Hz, 1H), 3.4 –3.07 (dd, J = 17.0, 9.4 Hz, 1H). 13C NMR (75 MHz, CDCl3, 25 °C, TMS): 177.92, 161.49, 156.57, 142.31, 135.46, 134.05, 128.43, 128.08, 122.60, 121.76, 121.47, 121.30, 114.94, 114.31, 78.47, 55.41, 48.72, 38.72. MS (ESI) for C24H20N2O3 [M+H]+, calcd: 385.1510, found: 385.1510.
4.3.4.3 10-((3-(4-chlorophenyl)-4,5-dihydroisoxazol-5-yl)methyl)acridin-9(10H)-one (2c)
Yellow solid; yield: 68%, mp = 159 °C. IR (KBr): 3011, 2985, 1639, 1604, 1590 cm−1. 1H NMR (300 MHz, CDCl3, 25 °C, TMS): 8.59 (dd, J = 8.1, 1.7 Hz, 2H, H1,H8), 7.78–7.66 (m, 2H), 7.66–7.51 (m, 4H), 7.42 (d, J = 8.2 Hz, 2H), 7.37–7.29 (m, 2H), 5.42–5.31 (m, 1H), 4.77 (dd, J = 15.4, 7.6 Hz, 1H), 4.57 (dd, J = 16.3, 4.5 Hz, 1H), 3.64 (dd, J = 16.7, 7.1 Hz, 1H), 3.23 (dd, J = 16.7, 7.1 Hz, 1H).13C NMR (75 MHz, CDCl3, 25 °C, TMS): 177.86, 156.11, 142.21, 142.08, 140.29, 136.66, 135.47, 134.06, 133.89, 131.54, 129.17, 128.04, 127.29, 122.42, 121.82, 121.53, 114.84, 79.10, 48.63, 38.29. MS (ESI) for C23H17ClN2O2 [M+H]+, calcd: 389.1019, found: 389.1018.
4.3.4.4 10-((3-(4-nitrophenyl)-4,5-dihydroisoxazol-5-yl)methyl)acridin-9(10H)-one (2d)
White solid; yield: 80%, mp = 198 °C. IR (KBr): 3000, 2987, 1638, 1605, 1598 cm−1. 1H NMR (300 MHz, CDCl3, 25 °C, TMS): 8.64 (dd, J = 8.0, 1.7 Hz, 2H), 8.36–8.22 (m, 2H), 8.03–7.88 (m, 2H), 7.78 (td, J = 8.7, 7.0, 1.7 Hz, 2H), 7.56–7.35 (m, 4H), 5.52–5.41 (m, 1H), 4.77 (dd, J = 15.4, 7.6 Hz, 1H), 4.67 (dd, J = 16.3, 4.5 Hz, 1H), 3.74 (dd, J = 16.7, 7.1 Hz, 1H), 3.33 (dd, J = 16.7, 7.1 Hz, 1H). 13C NMR (75 MHz, CDCl3, 25 °C, TMS): 177.95, 168.76, 161.17, 148.92, 141.80, 134.50, 128.32, 127.77, 124.20, 122.90, 122.35, 114.17, 8010, 49.63, 39.29. MS (ESI) for C23H17N3O4 [M + H]+, calcd: 400,1282, found: 400,1272.
4.3.4.5 10-((3-(4-(dimethylamino)phenyl)-4,5-dihydroisoxazol-5-yl)methyl)acridin-9(10H)-one (2e)
Yellow solid; yield: 66%, mp = 136 °C. IR (KBr): 3000, 2985, 1636, 1603, 1591 cm−1. 1H NMR (300 MHz, CDCl3, 25 °C, TMS): 8.61 (d, J = 7.9 Hz, 2H), 7.73 (dt, J = 9.7, 5.1 Hz, 3H), 7.58 (dq, J = 15.7, 9.6, 9.1 Hz, 4H), 7.40–7.31 (m, 2H), 6.72 (d, J = 8.5 Hz, 1H), 5.30–5.21 (m, 1H), 4.88–4.67 (d, 1H, J = 14.2 Hz, 1H), 4.57 (d, J = 14.3 Hz, 1H), 3.54 (dd, J = 16.6, 6.0 Hz, 1H), 3.24 (dd, J = 16.2, 5.8 Hz, 1H), 3.05 (s, 6H, CH3). 13C NMR (75 MHz, CDCl3, 25 °C, TMS): 176.43, 155.86, 152.23, 142.37, 134.08, 129.32, 128.19, 128.11, 128.03, 126.02, 121.81, 121.72, 119.63, 115.06, 111.72, 77.96, 77.46, 77.03, 76.61, 48.72, 40.13, 38.89. MS (ESI) for C25H23N3O2, [M + H]+, calcd: 398,1862, found: 398.1852.
4.3.4.6 10-((3-(4-hydroxy-3-methoxyphenyl)-4,5-dihydroisoxazol-5-yl)methyl)acridin-9(10H)-one (2f)
Yellow solid; yield: 66%, mp = 200 °C. IR (KBr): 3410, 3010, 2974, 1639, 1601, 1598 cm−1 1H NMR (300 MHz, DMSO‑d6, 25 °C, TMS): 9.50 (s, 1H, OH), 8.41 (dd, J = 8.1, 1.7 Hz, 2H), 7.48–7.41 (m, 2H), 7.30 (dd, J = 7.9, 1.4 Hz, 2H), 7.22–7.12 (m, 3H), 6.81 (td, J = 7.5, 1.5 Hz, 2H), 5.37 (m, 1H), 4.79 (dd, J = 16.1, 7.5 Hz, 1H), 4.65–4.40 (dd, J = 16.1, 7.5 Hz, 1H), 3.89 (s, 3H, OCH3), 3.51 (dd, J = 17.0, 9.4 Hz, 1H), 3.42–3.09 (dd, J = 17.0, 9.4 Hz, 1H).13C NMR (75 MHz, DMSO‑d6, 25 °C, TMS): 178.63, 168.83, 159.04, 148.70, 147.80, 141.33, 131.82, 126.87, 123.32, 121.86, 121.76, 121.55, 116.05, 115.83, 110.50, 79.47, 56.41, 48.79, 38.82. MS (ESI) for C24H20N2O4 [M + H]+, calcd: 401,1483, found: 401,1480.
4.3.4.7 2-methyl-10-((3-phenyl-4,5-dihydroisoxazol-5-yl)methyl)acridin-9(10H)-one (2g)
Yellow solid; yield: 81%, mp = 205 °C. IR (KBr): 3014, 2975, 1635, 1603, 1597 cm−1. 1H NMR (300 MHz, CDCl3, 25 °C, TMS): 8.60 (dd, J = 8.0, 1.7 Hz, 1H, H1), 8.37 (s, 1H, H8), 7.74–7.68 (m, 3H), 7.59–7.51 (m, 3H), 7.49–7.39 (m, 3H), 7.33–7.27 (m, 1H), 5.40–5.30 (m, 1H), 4.75 (dd, J = 16.3, 7.5 Hz, 1H), 4.55 (dd, J = 16.3, 4.4 Hz, 1H), 3.56 (dd, J = 16.7, 10.3 Hz, 1H), 3.27 (dd, J = 16.7, 6.9 Hz, 1H), 2.47 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3, 25 °C, TMS): 177.86, 157.02, 142.12, 140.34, 135.48, 133.88, 131.49, 130.61, 128.88, 128.81, 128.05, 127.33, 126.85, 122.43, 122.39, 121.49, 114.91, 114.80, 78.81, 48.62, 38.45, 20.54. MS (ESI) for C24H20N2O2 [M + H]+, calcd: 369,1560, found: 369,1560.
4.3.4.8 10-((3-(4-methoxyphenyl)-4,5-dihydroisoxazol-5-yl)methyl)-2-methylacridin-9(10H)-one (2h)
Yellow solid; yield: 84%, mp = 165 °C. IR (KBr): 3010, 2970, 1640, 1604, 1590 cm−1. 1H NMR (300 MHz, CDCl3, 25 °C, TMS): 8.61 (dd, J = 8.0, 1.5 Hz, 1H, H1), 8.38 (s, 1H, H8), 7.81–7.76 (m, 2H), 7.63–7.41 (m, 4H), 7.31 (d, J = 6.6 Hz, 1H), 7.12 (dd, J = 8.5, 6.4 Hz, 1H), 6.69 (d, J = 8.6 Hz, 1H), 5.33 (m, 1H), 4.71 (dd, J = 15.2, 6.1 Hz, 1H), 4.54 (dd, J = 15.3 Hz, 1H), 3.59–3.41 (m, 1H), 3.38–3.10 (m, 1H), 3.86 (s, 3H, CH3), 2.48 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3, 25 °C, TMS): 177.89, 161.41, 156.57, 142.31, 135.46, 134.05, 128.43, 128.08, 122.60, 121.76, 121.47, 121.30, 114.94, 114.31, 78.48, 55.51, 48.72, 38.82, 20.56. MS (ESI) for C25H22N2O3 [M + H]+, calcd: 399,1673 , found: 399,1675.
4.3.4.9 10-((3-(4-chlorophenyl)-4,5-dihydroisoxazol-5-yl)methyl)-2-methylacridin-9(10H)-one (2i)
Yellow solid; yield: 67%, mp = 207 °C. IR (KBr): 3000, 2985, 1637, 1604, 1591 cm−1. 1H NMR (300 MHz, CDCl3, 25 °C, TMS): 8.59 (dd, J = 8.1, 1.7 Hz, 1H), 8.37 (s, 1H, H8), 7.72 (td, J = 8.8, 6.9, 1.8 Hz, 1H), 7.66–7.45 (m, 5H), 7.45–7.38 (m, 2H), 7.35–7.27 (m, 1H), 5.51–5.31 (m, 1H), 4.76 (dd, J = 16.4, 7.4 Hz, 1H), 4.58 (dd, J = 16.4, 4.6 Hz, 1H), 3.51 (dd, J = 16.7, 7.1 Hz, 1H), 3.23 (dd, J = 16.7, 7.1 Hz, 1H), 2.48 (s, 3H, CH3).13C NMR (75 MHz, CDCl3, 25 °C, TMS): 177.79, 156.09, 142.08, 140.29, 136.64, 135.47, 133.89, 131.55, 129.16, 128.12, 128.04, 127.40, 127.31, 122.46, 122.43, 121.53, 114.81, 114.70, 79.10, 48.47, 38.24, 20.54. MS (ESI) for C24H19ClN2O2 [M + H]+, calcd: 403,1118, found: 403.1117.
4.3.4.10 2-methyl-10-((3-(4-nitrophenyl)-4,5-dihydroisoxazol-5-yl)methyl)acridin-9(10H)-one (2j)
Yellow solid; yield: 80%, mp = 220 °C. IR (KBr): 3000, 2970, 1638, 1600, 1598 cm−1. 1H NMR (300 MHz, DMSO‑d6, 25 °C, TMS): 8.40 (dd, J = 8.0, 1.5 Hz, 1H), 8.33–8.25 (m, 2H), 8.19 (s, 1H), 8.15–8.07 (m, 2H), 7.96–7.75 (m, 3H), 7.68 (dd, J = 8.9, 2.3 Hz, 1H), 7.38 (td, J = 7.9, 6.3, 1.5 Hz, 1H), 5.42–5.31 (m, 1H), 4.67 (dd, J = 15.4, 7.6 Hz, 1H), 4.57 (dd, J = 16.3, 4.5 Hz, 1H), 3.84 (dd, J = 16.7, 7.1 Hz, 1H), 3.43 (dd, J = 16.7, 7.1 Hz, 1H), 2.45 (s, 3H, CH3). 13C NMR (75 MHz, DMSO‑d6, 25 °C, TMS): 176.54, 169.70, 160.62, 148.35, 141.59, 139.83, 135.67, 134.23, 134.11, 131.12, 127.95, 126.79, 126.02, 124.19, 121.77, 121.59, 115.94, 115.76, 79.92, 49.97, 39.54, 20.64. MS (ESI) for C24H19N3O4 [M + H]+, calcd: 414,1440, found: 414,1431.
4.3.4.11 10-((3-(4-(dimethylamino)phenyl)-4,5-dihydroisoxazol-5-yl)methyl)-2-methylacridin-9(10H)-one (2k)
White solid; yield: 64%, mp = 141 °C. IR (KBr): 3004, 2975, 1639, 1604, 1591 cm−1. 1H NMR (300 MHz, CDCl3, 25 °C, TMS): 8.60 (dd, J = 8.0, 1.5 Hz, 1H, H1), 8.39 (s, 1H, H8), 7.86–7.66 (m, 2H), 7.67–7.44 (m, 4H), 7.32 (d, J = 6.6 Hz, 1H), 7.05 (dd, J = 8.5, 6.4 Hz, 1H), 6.71 (d, J = 8.6 Hz, 1H), 5.31 (m, 1H), 4.73 (dd, J = 16.2, 7.3 Hz, 1H), 4.54 (dd, J = 16.3 Hz, 1H), 3.59–3.42 (m, 1H), 3.31–3.17 (m, 1H), 3.05 (s, 6H, CH3), 2.49 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3, 25 °C, TMS): 177.84, 156.98, 151.76, 142.14, 140.44, 135.47, 133.86, 129.31, 128.18, 128.02, 127.29, 126.02, 122.44, 121.42, 114.93, 111.72, 78.80, 48.70, 40.12, 20.56. MS (ESI) for C26H25N3O2, [M + H]+, calcd: 412,1939, found: 412.1938.
4.3.4.12 10-((3-(4-hydroxy-3-methoxyphenyl)-4,5-dihydroisoxazol-5-yl)methyl)-2-methylacridin-9(10H)-one (2l)
Yellow solid; yield: 65%, mp = 205 °C. IR (KBr): 3420, 3014, 2970, 1638, 1603, 1595 cm−1 1H NMR (300 MHz, DMSO‑d6, 25 °C, TMS): 9.50 (s, 1H, OH), 8.41 (dd, J = 8.1, 1.7 Hz, 1H), 7.78 (d, J = 1.9 Hz, 1H), 7.48–7.41 (m, 1H), 7.33–7.27 (m, 2H), 7.24 (d, J = 8.4 Hz, 1H), 7.22–7.12 (m, 2H), 6.82 (td, J = 7.5, 1.5 Hz, 2H), 5.36 (m, 1H), 4.78 (dd, J = 16.1, 7.5 Hz, 1H), 4.67–4.43 (dd, J = 16.1, 7.5 Hz, 1H), 3.88 (s, 3H, OCH3), 3.53 (dd, J = 17.0, 9.4 Hz, 1H), 3.41–3.1 (dd, J = 17.0, 9.4 Hz, 1H), 2.47 (s, 3H, CH3).13C NMR (75 MHz, DMSO‑d6, 25 °C, TMS): 178.76, 168.83, 158.04, 148.70, 147.80, 141.36, 140.06, 134.16, 132.19, 131.78, 126.88, 124.21, 123.35, 121.79, 121.64, 121.50, 116.05, 115.83, 114.86, 110.50, 110.12, 79.80, 49.70, 41.12, 20.66. MS (ESI) for C25H22N2O4 [M+H]+, calcd: 415,1620, found: 415,1622.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis, antibacterial evaluation, in silico ADMET and molecular docking studies of new N-acylhydrazone derivatives from acridone. Arab. J. Chem.. 2020;13:6236-6245.
- [CrossRef] [Google Scholar]
- Novel highly selective and sensitive fluorescent sensor for copper detection based on N-acylhydrazone acridone derivative. J. Mol. Struct.. 2020;1199:126990
- [CrossRef] [Google Scholar]
- Synthesis, antibacterial evaluation and molecular docking studies of novel series of acridone- 1,2,3-triazole derivatives. Struct. Chem.. 2020;31:1523-1531.
- [CrossRef] [Google Scholar]
- Novel series of acridone-1,2,3-triazole derivatives: microwave-assisted synthesis, DFT study and antibacterial activities. J. Chem. Sci.. 2019;131:85.
- [CrossRef] [Google Scholar]
- Crystal structures of multidrug binding protein TtgR in complex with antibiotics and plant antimicrobials. J. Mol. Biol.. 2007;369:829-840.
- [CrossRef] [Google Scholar]
- Amzoiu, M., Amzoiu, D., Belu, I., Popescu, G.-S., Cristea, O.M., Emin, C., Popescu, D.-F., 2020. Comparative study of antibacterial and antifungal activities of acetamidic derivatives. J. Sci. Arts Year.
- Recent developments on ultrasound-assisted one-pot multicomponent synthesis of biologically relevant heterocycles. Ultrason. Sonochem. 2017
- [CrossRef] [Google Scholar]
- Useful approach for O-functionalization of trifluoromethyl-substituted spirotetracyclic isoxazolines, and their application in the synthesis of 1,2,3-triazole derivatives. J. Fluor. Chem.. 2018;210:142-148.
- [CrossRef] [Google Scholar]
- The role of integrative and conjugative elements in antibiotic resistance evolution. Trends Microbiol. 2020
- [CrossRef] [Google Scholar]
- Editorial: in silico methods for drug design and discovery. Front. Chem.. 2020;8
- [CrossRef] [Google Scholar]
- Molecular docking studies on the activity of naturally occurring pyranochalcones on the transcriptional regulator enzyme of Pseudomonas putida. Open Access Bioinformatics. 2010;61
- [CrossRef] [Google Scholar]
- Solid phase synthesis of isoxazole and isoxazoline-carboxamides via [2+3]-dipolar cycloaddition using resin-bound alkynes or alkenes. Tetrahedron Lett.. 2012;53:2096-2099.
- [CrossRef] [Google Scholar]
- Daniels, C., Daddaoua, A., Lu, D., Zhang, X., Ramos, J.-L., 2010. Domain Cross-talk during Effector Binding to the Multidrug Binding TTGR Regulator *. https://doi.org/10.1074/jbc.M110.113282.
- Drug Likeness Tool (DruLiTo 1) [WWW Document], n.d. URL http://www.niper.gov.in/pi_dev_tools/DruLiToWeb/DruLiTo_index.html (accessed 7.20.20).
- Stereoselective synthesis, docking, and biological evaluation of difluoroindanediol-based MenE inhibitors as antibiotics. Org. Lett. 2016
- [CrossRef] [Google Scholar]
- Synthesis, antimicrobial evaluation, molecular docking and theoretical calculations of novel pyrazolo[1,5-a]pyrimidine derivatives. J. Mol. Struct.. 2020;1199:127025
- [CrossRef] [Google Scholar]
- Molecular binding mechanism of TtgR repressor to antibiotics and antimicrobials. PLoS One. 2015;10
- [CrossRef] [Google Scholar]
- Synthesis, biological evaluation and molecular modeling of 1-oxa-4-thiaspiro- and 1,4-dithiaspiro[4.5]decane derivatives as potent and selective 5-HT1Areceptor agonists. Eur. J. Med. Chem.. 2017;125:435-452.
- [CrossRef] [Google Scholar]
- In silico molecular investigations of pyridine N-Oxide compounds as potential inhibitors of SARS-CoV-2: 3D QSAR, molecular docking modeling, and ADMET screening. J. Biomol. Struct. Dyn.. 2020;1–11
- [CrossRef] [Google Scholar]
- In silico exploration of aryl halides analogues as CheckpointKinase 1 inhibitors by using 3D QSAR, molecular docking study, and ADMET screening. Adv. Pharm. Bull.. 2019;9:84-92.
- [Google Scholar]
- Rational design, chemical synthesis and biological evaluation of novel biguanides exploring species-specificity responsiveness of TAAR1 agonists. Eur. J. Med. Chem.. 2018;146:171-184.
- [CrossRef] [Google Scholar]
- Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform.. 2012;4:17.
- [CrossRef] [Google Scholar]
- Design, diversity-oriented synthesis and biological evaluation of novel heterocycle derivatives as non-nucleoside HBV capsid protein inhibitors. Eur. J. Med. Chem.. 2020;112495
- [CrossRef] [Google Scholar]
- Isoxazoline containing natural products as anticancer agents: A review. Eur. J. Med. Chem. 2014
- [CrossRef] [Google Scholar]
- Synthesis of 9-anilinoacridine triazines as new class of hybrid antimalarial agents. Bioorganic Med. Chem. Lett.. 2009;19:6996-6999.
- [CrossRef] [Google Scholar]
- Mechanism of the intramolecular claisen condensation reaction catalyzed by MenB, a crotonase superfamily member. Biochemistry. 2011;50:9532-9544.
- [CrossRef] [Google Scholar]
- Synthesis, physicochemical characterization, geometric structure and molecular docking of new biologically active ferrocene based Schiff base ligand with transition metal ions. Appl. Organomet. Chem.. 2017;31:e3858
- [CrossRef] [Google Scholar]
- Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem.. 1998;19:1639-1662.
- [Google Scholar]
- Multicomponent and 1,3-dipolar cycloaddition synthesis of triazole- and isoxazole-acridinedione/xanthenedione heterocyclic hybrids: Cytotoxic effects on human cancer cells. J. Mol. Struct.. 2020;1217:128325
- [CrossRef] [Google Scholar]
- Synthesis, biological evaluation and virtual screening of some acridone derivatives as potential anticancer agents. Bioorganic Med. Chem.. 2020;28:115426
- [CrossRef] [Google Scholar]
- Computational analysis of the activity of pongachalcone I against highly resistant bacteria Pseudomonas putida. Bioinformation. 2010;4:473-477.
- [CrossRef] [Google Scholar]
- pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem.. 2015;58:4066-4072.
- [CrossRef] [Google Scholar]
- New pyridazinone-4-carboxamides as new cannabinoid receptor type-2 inverse agonists: Synthesis, pharmacological data and molecular docking. Eur. J. Med. Chem.. 2017;127:398-412.
- [CrossRef] [Google Scholar]
- Spirotriazoline oxindoles: A novel chemical scaffold with in vitro anticancer properties. Eur. J. Med. Chem.. 2017;140:494-509.
- [CrossRef] [Google Scholar]
- New insights into the binding features of F508del CFTR potentiators: a molecular docking, pharmacophore mapping and QSAR analysis approach. Pharmaceuticals. 2020;13:445.
- [CrossRef] [Google Scholar]
- Divergent synthesis of polysubstituted isoxazoles, isoxazoline N-oxides, and dihydroisoxazoles by a one-pot cascade reaction. Tetrahedron. 2017;73:331-337.
- [CrossRef] [Google Scholar]
- Menaquinone (vitamin K2) biosynthesis: Nucleotide sequence and expression of the menB gene from Escherichia coli. J. Bacteriol.. 1992;174:5057-5062.
- [CrossRef] [Google Scholar]
- Potent human telomerase inhibitors: molecular dynamic simulations, multiple pharmacophore-based virtual screening, and biochemical assays. J. Chem. Inf. Model.. 2015;55:2596-2610.
- [CrossRef] [Google Scholar]
- Influence of incubation temperature and time on the precision of MIC and disc diffusion antimicrobial susceptibility test data. Aquaculture. 2018;490:19-24.
- [CrossRef] [Google Scholar]
- Synthesis, anti-inflammatory and anticancer activity evaluation of some novel acridine derivatives. Eur. J. Med. Chem.. 2010;45:555-563.
- [CrossRef] [Google Scholar]
- In search of new chemical entities with spermicidal and anti-HIV activities. Bioorganic Med. Chem.. 1999;7:2607-2613.
- [CrossRef] [Google Scholar]
- Antibiotic-dependent induction of Pseudomonas putida DOT-T1E TtgABC efflux pump is mediated by the drug binding repressor TtgR. Antimicrob. Agents Chemother.. 2003;47:3067-3072.
- [CrossRef] [Google Scholar]
- Colorimetric microdilution assay: Validation of a standard method for determination of MIC, IC50%, and IC90% of antimicrobial compounds. J. Microbiol. Methods. 2019;162:50-61.
- [CrossRef] [Google Scholar]
- Accelerated evolution of bacterial antibiotic resistance through early emerged stress responses driven by photocatalytic oxidation. Appl. Catal. B Environ.. 2020;269:118829
- [CrossRef] [Google Scholar]
- Regiospecific synthesis, antibacterial and anticoagulant activities of novel isoxazoline chromene derivatives. Arab. J. Chem.. 2017;10:S2651-S2658.
- [CrossRef] [Google Scholar]
- Structural basis for the transcriptional repressor NicR2 in nicotine degradation from P seudomonas. Mol. Microbiol.. 2017;103:165-180.
- [CrossRef] [Google Scholar]
- Synthesis, structure and in vitro biological evaluation of new lupane and dammarane triterpenoids fused with pyrazine heterocycle. Mendeleev Commun.. 2019;29:500-502.
- [CrossRef] [Google Scholar]







