Translate this page into:
Synthesis, characterization, and application of griseofulvin surface molecularly imprinted polymers as the selective solid phase extraction sorbent in rat plasma samples
⁎Corresponding author. fuqiang@mail.xjtu.edu.cn (Qiang Fu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In present study, an investigation was carried out to develop and validate an analytical method for the selective extraction and determination of griseofulvin (GSF) from plasma samples. For this purpose, a rational approach was made to synthesize and characterize the surface molecularly imprinted polymers (SMIPs). The SMIPs were utilized as solid phase extraction (SPE) sorbents. The SMIPs were prepared by using GSF as template molecule on the surface of modified silica particles through a non-covalent technique. The particles demonstrated high adsorption capacity (119.1 µg/mL), fast adsorption equilibrium time (30 min) and good recognition selectivity for the template drug. The scanning electron microscopy and infrared spectroscopy were used to explain the structural and morphological characteristics of the SMIPs and surface non-imprinted polymers. The SPE method was combined with HPLC for plasma analysis. The method validation results demonstrated that the established method possessed good linearity for GSF ranging from 0.1 to 50 µg/mL (R2 = 0.997). The limit of detection for this method was 0.02 µg/mL for rat plasma samples. The recoveries of GSF from spiked plasma samples were (90.7–97.7%) and relative standard deviations were (0.9–4.5%). Moreover, the SMIPs as selective SPE sorbent can be reused more than 8 times which is a clear advantage over commercial SPE sorbents. Finally, the usefulness of the proposed strategy was assessed by extraction and detection of GSF in real rat plasma samples.
Keywords
Griseofulvin
HPLC
Solid phase extraction
Surface molecularly imprinted polymers
Plasma
- AIBN
-
2,2′-azobisisobutyronitrile
- APTES
-
3-aminopropyltriethoxysilane
- EDMA
-
Ethylene glycol dimethacrylate
- FT-IR
-
Fourier transform infrared
- GSF
-
griseofulvin
- IF
-
Imprinting factor
- MAA
-
Methacrylic acid
- MIPs
-
molecularly imprinted polymers
- RSD
-
Relative standard deviation
- SEM
-
Scanning electron microscope
- S/N
-
signal to noise ratio
- SMIPs
-
Surface molecularly imprinted polymers
- SNIPs
-
Surface non-molecularly imprinted polymers
- SMISPE
-
Surface molecularly imprinted solid phase extraction
Abbreviations
1 Introduction
Griseofulvin (GSF) is originated from fungus Penicillium griseofulvum. Now some synthetic or semi-synthetic ways are applied to manufacture it. GSF is first oral anti-mycotic drug and effectively cured the dermatophytosis for 50 years (Di Santo, 2010; Huang et al., 2004; Petersen et al., 2014). It's a drug of choice for the treatment of Tinea capitis (Finkelstein et al., 1996). Currently; some new therapeutic applications i.e. an-anti-mitotic and anti-proliferative are revealed through research. These studies indicate its anti-tumor activities against the mammalian’s tumor cells. (Ho et al., 2001; Mauro et al., 2013; Raab et al., 2012; Rathinasamy et al., 2010; Rebacz et al., 2007). Thus, it may be a potential chemotherapeutic agent along-with antifungal use (Dumontet and Jordan, 2010; Pasquier and Kavallaris, 2008; Rønnest et al., 2012; Singh et al., 2008). This is considered a very cost-effective medicine. (Develoux, 2001). Although GSF have many clinical benefits but some adverse or side-effects are associated with its therapy. Pre-clinical toxicity studies and safety profile show that GSF causes liver and thyroid toxicity, abnormal germ cell maturation, teratogenicity and embryo toxicity among various species (Knasmüller et al., 1997; K. Liu et al., 2015).
The extraction and estimation in biological fluids such as plasma, serum and urine have been focused mainly for GSF analysis. The reported plasma concentrations in man vary between 0.5 and 2.4 µg/mL (Barrett and Bianchine, 1975). Different analytical methods like thin layer chromatography (Fischer and Riegelman, 1966; Garceau et al., 1980), liquid chromatography (LC) (Meyer and Raghow, 1979; Zia et al., 1980), and gas chromatography (Kamimura et al., 1979) were applied. Extraction and estimation of GSF in some other sample media i.e molds, aquatic strains, food and feeds was performed and its bioavailability also analyzed.
For the analytical advancement, a reverse phase LC method was also developed and validated for the analysis of GSF and its impurities (Kahsay et al., 2013). An HPLC method based on liquid extraction in rat plasma sample was developed and validated by using a fluorescence detector and warfarin as an internal standard (Wei et al., 2008). The extraction methods applied in previous studies were laborious, non-selective and less specific. Some of these methods based on liquid-liquid extraction for plasma samples. The liquid-liquid extraction offered clear and good recoveries but recoveries cannot quantify and causes protein precipitation and ion suppression. Mistri and his colleagues developed and validated an electrospray ionization LC-MS method to overcome this problem (Mistri et al., 2007). The LC-MS method was expensive and used non-selective commercial solid phase extraction (SPE) cartridge. The major drawback associated with these sorbents are, low selectivity and recovery for target compounds (Luo et al., 2014). Therefore; a selective and practical strategy to extract GSF and its high recovery in plasma samples by HPLC is needed.
Selectivity is a basis for an analytical method. It's essential to investigate the trace compounds in real complex samples, biological, environmental, and food samples. To address this issue, pre-sample treatment with advanced adsorbent material (Liu et al., 2019) is utilized to extract, analyze the highly pure trace drug in biological samples (Speltini et al., 2016). SPE is widely employed as a pre-concentration and pre-sample treatment procedure during analytical investigations. The SPE has many advantages over liquid extraction such as; elimination of the sample treatment steps, reduction of the amount of the sample, strong reductions in consumption of hazardous reagents and energy also maximizes safety for operators and environment, the avoidance of the use of big amount of organic solvents, form the basis for green sample preparation and analytical methods (Filippou et al., 2017). SPE related divisions such as stir-bar sportive extraction, dispersive SPE, magnetic SPE, and solid-phase micro-extraction are replacing the outdated extraction techniques (Beyazit et al., 2016; Ma et al., 2019).
Molecular imprinted polymers (MIPs) are smart in generation, powerful, and artificial selective sorbent materials. These are artificial receptors able to bind specifically the template in the presence of structurally related molecules (Gokulakrishnan and Prakasam, 2016). The advantage of MIPs is that the imprinted polymers give enough binding surfaces similar to those of natural receptors, and this is the reason they are likewise alluded to as “plastic antibodies” (Martín-Esteban, 2016). MIPs have attracted increasing interest as selective SPE sorbents and have been applied for isolation and enrichment of drugs, small organic molecules, bio-macromolecules, etc., (Bagheri et al., 2019; P. Guo et al., 2018). But, the MIPs prepared by bulk polymerization often have poor site accessibility and low capacity. Surface molecularly imprinted polymers (SMIPs) preparation method is applied to overcome these drawbacks associated with bulk polymerization method. Surface imprinting method is relatively simple, cheap and easy (Hu et al., 2016). The SMIPs has more recognition sites at the surface of material, which facilitates in fast mass transfer, rapid recognition and good recoveries (Xie et al., 2018; Yin et al., 2012). The prepared polymers exhibit good stability at a wide range of pH values, great mechanical strength at various temperatures, and better robustness in different solvents (Luo et al., 2014).
The objective of this study was to prepare and characterize the GSF imprinted polymers that can be used effectively for extraction, analysis, separation, and detection of this drug from complex plasma samples. According to the best of our knowledge, this is the first report of using GSF as a template molecule for the preparation of SMIPs, and for the first time selective SPE method was designed for the extraction from real plasma samples. The method of preparation and SPE procedure is shown in Fig. 1. The prepared polymers were chemically and morphologically evaluated by scanning electron microscope (SEM) and Fourier transform infrared (FT-IR) spectra. The adsorption characteristics, surface recognition abilities and optimization of SPE were studied comprehensively. Finally, an analytical method was established for the simple and selective extraction of GSF in rat plasma samples based on SMIPs and SPE (SMISPE) combined with HPLC.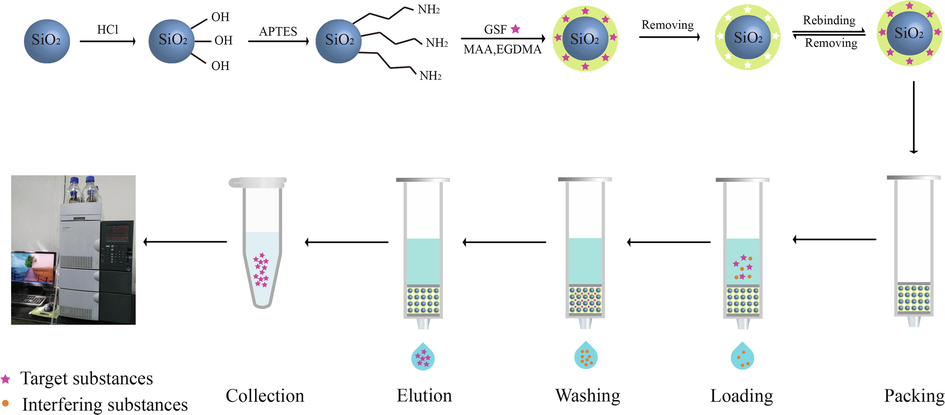
Method of preparation and SPE procedure.
2 Experimental
2.1 Materials
GSF, fluconazole, ketoconazole, clotrimazole, dexamethasone purchased from Aladdin Co. (Beijing, China). The 3-aminopropyltriethoxysilane (APTES), and naproxen were supplied from J&K Chemical Co. (Beijing, China). Dehydrogriseofulvin was purchased from Toronto Research Chemicals Inc. (Toronto, Canada). Silica gel (diameter: 50 μm, average pore radius: 43.54 nm) was supplied by Tokyo Chemical Co. (Tokyo, Japan). Methacrylic acid (MAA) was purchased from Tianjin Chemical Reagent Plant (Tianjin, China). Ethylene glycol dimethacrylate (EDMA) was obtained from Sigma-Aldrich (New Jersey, USA). 2,2′-Azobisisobutyronitrile (AIBN) was purchased from Shanghai No. 4 Reagent Factory (Shanghai, China). SPE cartridges (3 mL) were purchased by Biocomma limited (Shenzhen, China). Commercial C-18 SPE cartridge (100 mg, 3 mL) was purchased from Sepax Technologies, Inc (Newark, USA). Ultra-pure water was purified with Molement 1805b (Shanghai, China). Methanol and acetonitrile were of HPLC grade. All other chemicals used in this study were supplied from local suppliers and were of analytical grade.
2.2 Instruments
The HPLC analysis was performed with a Shimadzu LC-2010AHT series HPLC (Kyoto, Japan) and a Lab-Solution workstation. FT-IR spectra (4000–500 cm−1) were recorded in a Thermo Nicolet Nexus 330 FT-IR spectrometer (Madison, USA). The SEM images were obtained by a TM-1000 Scanning Microscope (Hitachi, Japan). The shaking was performed by a SHA-C Vapour-bathing Constant Temperature Vibrator (Changzhou, China).
2.3 Preparation of surface molecularly and non-molecularly imprinted polymers
The silica particles were first activated and then activated silica particles were modified as amino modified silica particles (denoted as NH2-SiO2) by using APTES. The silica particles activation and modification were performed according to a previous method (Ge et al., 2017). The GSF SMIPs preparation method is as follows. Firstly, GSF (0.5 mM) as a template drug was dissolved in 20 mL of toluene. After that, the functional monomer (MAA, 2 mM) was added in the above mixture. Further, 0.5 g amino modified silica was added in the above solution. The above mixture was pre-polymerized with constant stirring at 30 °C for 2 hrs. After that the cross-linker (EDMA, 10 mM) and an initiator (AIBN, 16.4 mg) were added. The solvents were mixed on a sonication apparatus for 10 min, and then nitrogen was purged for 15 min to remove oxygen. The polymerization flask was sealed for 12 h. under a nitrogen atmosphere and the temperature was maintained at 60 °C. After polymerization, the polymers were subsequently washed and extracted by a mixture of methanol-acetic acid (4:1, v/v) to remove unreacted reagents and template molecules. At the same time, the preparation method of surface non-molecularly imprinted polymers (SNIPs) was the same as that of the imprinted polymers except that no template molecule was added. Graphical abstract Fig. 1 illustrates the scheme for the synthesis of SMIPs.
2.4 Adsorption and selectivity experiment
The adsorption quantities of GSF polymers were assessed by adsorption equilibrium and adsorption kinetics experiments. The analysis was performed by using following HPLC-UV conditions; the column was C18 (Promosil 250 mm × 4.6 mm i.d., 5 µm); mobile phase was methanol-water (70:30, v/v), the flow rate was 1.0 mL/min, the detection wavelength was 291 nm, and the column oven temperature was 30 °C.
For the adsorption equilibrium experiment, 10 mg of SMIPs and SNIPs were added in different test flasks. Each test flask contained 10 mL of drug solution of concentrations (10, 50, 100, 200, 300, 400, 500, 600 µg/mL). The reaction flasks were constantly stirred on the mechanical shaker for 2 h., at 150 revolutions per minute (rpm). The adsorption quantities Qe (µg/mg) of SMIPs and SNIPs and imprinting factor (IF) were estimated by the following equations (Ge et al., 2017).
Furthermore, IF was calculated by the following equation.
The kinetic adsorption tests were performed by using 10 mg each of SMIPs and SNIPs polymers. The concentration of GSF drug solution was kept 400 µg/mL. The mixtures were continuously vibrated on a mechanical shaker at 150 rpm at 25 °C. The concentration of the drug was measured at different time intervals (0, 5, 10, 15, 30, 45, and 60 min). The adsorption amounts and IF were calculated by Equations (1) and (2).
The selectivity experiment was performed by using therapeutic and structural analogues such as fluconazole, clotrimazole, ketoconazole, dehydrogriseofulvin, naproxen, and dexamethasone. For this purpose, 5 mg of polymers were added into the 5 mL of each drug solution. The concentration of each drug solution was 100 μg/mL, and the incubation time was 60 min. at room temperature. Finally the adsorption amounts measured and IF of these drugs were determined.
2.5 Drug standards, spiked plasma, and real plasma sample preparations
A stock solution (1000 µg/mL) of GSF was prepared in methanol. Further the working standard solutions of 0.1, 1, 10, 25, 50, 75, 100, 200, 500 µg/mL, were prepared by serially diluting with methanol-water (70:30, v/v).
Blood samples were collected form untreated rats with heparin. The blood collected from untreated rats was subjected to centrifugation at 12000 rpm for 3 min. The lower layer was removed and clear plasma was collected and stored at −20 °C until used. Plasma samples were spiked with GSF working standard solutions to yield calibration standards in plasma at concentration of 0.1, 1, 10, 25, 50, 75, 100, 200 µg/500 µL. For 0.1 (10 µL of 10 µg/mL of working standard solution plus 490 µL of blank plasma), 1 (10 µL of 100 µg/mL of working standard solution and 490 µL of blank plasma), 10 (100 µL of 100 µg/mL of working standard solution plus 400 µL of blank plasma), 25 (250 µLof 100µg/mL of working standard solution plus 250 µL of blank plasma), 50 (250 µL of 200 µg/mL of working standard solutions and 250 µL of blank plasma), 75 (150 µL of 500 µg/mL of working standard solution and 350 µL of blank plasma), 100 (200 µL of 500 µg/mL of working standard solution and 300 µL of blank plasma) and for 200 (400 µL of 500 µg/mL working standard solution and 100 µL of blank plasma). After spiking a calibration curve was constructed. The spiked plasma samples stored at −20 °C until used.
For real plasma samples, a dose of 50 mg/kg as aqueous suspension was given to two adult male Sprague-Dawley rats. The blood samples were collected after 5 hrs., from the jugular vein, centrifuged at 12000 rpm for 3 min (Wei et al., 2008). The plasma was separated and stored at −20 °C until further analysis. The animal experiments were performed according to the International guidelines of experimental animal care and approval by the ethical committee of Xi’an Jiaotong University (certificate no. 22-9601018). All frozen plasma (blank, spiked and real) samples were thawed at room temperature before use.
2.6 SPE procedure and plasma samples analysis
The SPE cartridge (3 mL) was packed with SMIPs. After that, plasma sample was loaded, and then washing step was performed to wash and remove the impurities. After washing elution solvent was employed. The eluents were collected and evaporated to complete dryness under nitrogen stream at room temperature. After evaporation 1 mL methanol was added in the residual volume and an aliquot of 10 μL was analyzed under HPLC conditions.
For the analysis of plasma samples, a C18 column (Promosil, 250 mm × 4.6 mm, i.d., 5 µm) was used. The mobile phase was a mixture of 20 mM aqueous solution of potassium dihydrogen phosphate and acetonitrile (55:45, v/v). The final pH of the mobile phase was 3.5, which was adjusted with phosphoric acid. The flow rate was 1.0 mL/min, the detection wavelength was 291 nm, and the column oven temperature was set at 30 °C.
3 Results and discussion
3.1 Optimization of surface molecularly imprinted polymers
The amount of template, functional monomer and cross-linking agent commonly affect the morphology, structure, adsorption behavior, and selectivity performance of the polymers. The amount of functional monomer is linked with the adsorption performance of polymer. If, the amount of functional monomer is less, it cannot offer enough functional groups, which ultimately result in less adsorption and if the concentration of functional monomer is more, then non-specific adsorption sites are formed which result in decreased adsorption amount (Chen et al., 2016). In our investigation we used 1:4 M ratios of the template and functional monomer. At this optimized molar ratio, the adsorption amount was higher as compared with other molar ratios (Table S1). In the meantime, the molar quantity of cross-linking agent also disrupted the three-dimensional assembly of the imprinted surface. Due to an increase in the amount of cross linker, a rigid polymer structure was formed, which obstruct the template molecule to reach its recognition site. The mass transfer became difficult and resulted in a decreased adsorption amount. So, a molar quantity of 10 mM of EDMA provided enough sites for binding of the drug (Table S1).
The adsorption behavior and structure of polymers are also reliant on the nature of solvents. The solvents such as toluene, acetonitrile, and chloroform are applied for the synthesis of non-covalent based polymers. Toluene is a less polar solvent and provided good imprinting factor, high adsorption capacity and uniform structure to the polymers (Chen et al., 2016). So, different solvents were used in this experiment. Toluene showed high adsorption amount and IF in comparison to other solvents (Fig. S.2).
3.2 Characterization
3.2.1 SEM analysis
The SEM images of activated silica particles, SNIPs and SMIPs are shown in (Fig. 2a,b,c). The activated silica particles presented a smoother surface as compared to the surfaces of SMIPs and SNIPs. The SMIPs surface was comparatively rougher than the surface of SNIPs and this was the only remarkable difference between these particles. These distinctive features in morphology may ensure the formation of MIP film on the surface of modified silica particles.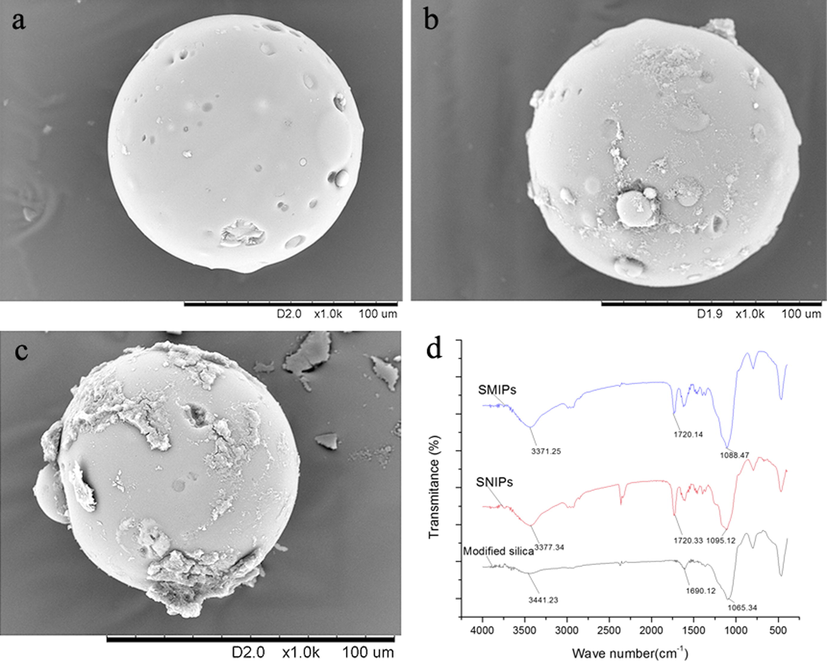
Characterization of silica particles, SMIPs, SNIPs. (a): SEM of Activated silica; (b): SEM of SNIPs; (c): SEM of SMIPs; (d): FT-IR spectra.
3.2.2 FT-IR analysis
The FT-IR spectra of the modified silica particles, SMIPs and SNIPs are displayed in Fig. 2d. The SMIPs and SNIPs spectra displayed different characteristic bands when compared with the modified silica spectrum. The bands at about 3371 cm−1, 1720 cm−1, and 1088 cm−1 of SMIPs and 3377 cm−1, 1720 cm−1 and 1095 cm−1 of SNIPs attributed to the stretching vibrations of —OH, C⚌O, C⚌C, respectively. FT-IR spectra of SMIPs and SNIPs proposed that functional groups were effectively formed on the surface of silica particles through the polymerization of MAA and EGDMA.
3.3 Evaluation of the adsorption and selectivity characteristics
3.3.1 Adsorption isotherm experiment
The adsorption of the drug by SMIPs and SNIPs gradually increased as the concentration of drug in solution increased until it reached equilibrium concentration level. The equilibrium concentration level was 400 µg/mL (Fig. 3a). At this concentration level, the adsorption amount for SMIPs and SNIPs were 119.1 and 48.8 µg/mg respectively. At this point, the recognition surfaces for the drug molecule were saturated and no more drugs adsorbed after this equilibrium level. The adsorption amount of SNIPs was lower than the adsorption amount of SMIPs. The SMIPs have both the specific binding sites and the non-specific binding sites. However, the SNIPs just have non-specific binding sites. To further elucidate this process, two adsorption models were used to evaluate the adsorption process, Langmuir model and Freundlich model.
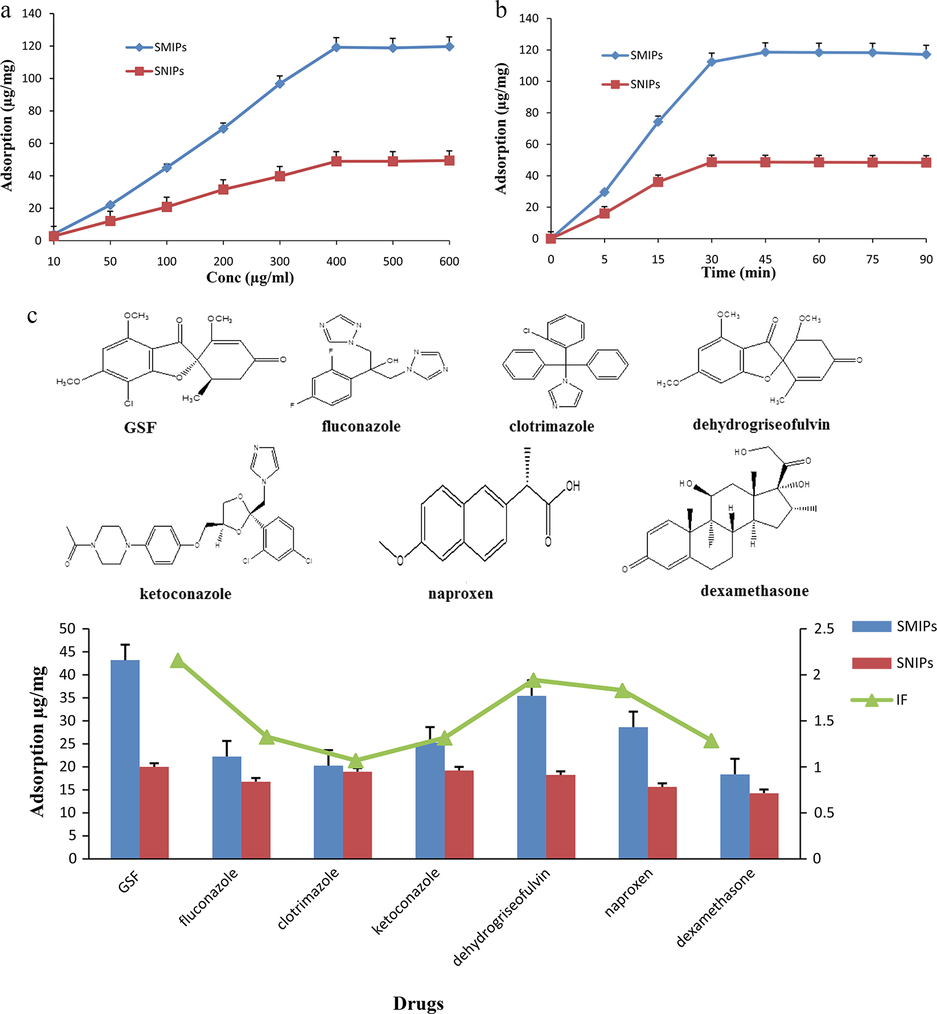
Adsorption experiments (a): adsorption isotherm curves of SMIPs and SNIPs; (b): adsorption kinetics curves of SMIPs and SNIPs; (c): adsorption selectivity of SMIPs and SNIPs.
According to the data, the linear correlation coefficient (R2) value of Langmuir model (0.9902) was higher than Freundlich model (0.9534). This result indicated that the adsorption process belonged to the Langmuir adsorption process and was a monolayer adsorption process.
3.3.2 The adsorption kinetics experiment
The adsorption amount of GSF on SMIPs and SNIPs increased as the time of incubation increased until it reached the equilibrium time level. The equilibrium time levels for SMIPs and SNIPs were 30 and 45 min respectively (Fig. 3b). At this equilibrium time, the recognition cavities on the SMIPs surface were fully saturated with template drug molecules. There was no facilitated diffusion of drug molecules towards the recognition sites after this point. The adsorption of SNIPs was comparatively slower than the SMIPs. This phenomenon was due to the only surface binding mechanism of silica particles (Pengqi Guo, Xu, et al., 2017).
3.3.3 Selectivity experiment
The selective adsorption experiment was performed by using therapeutic (fluconazole, clotrimazole, ketoconazole), structural (dehydrogriseofulvin) and non-structural drugs (naproxen, and dexamethasone). The presence of ‘imprinted sites’ is usually verified by IF. The IF is the ratio of the distribution ratio for a particular analyte, under a particular set of conditions, on the imprinted polymer, to the distribution ratio for the same analyte, under identical conditions. The IF should have a value greater than 1, the higher the value the greater the difference between the imprinted and non-imprinted case (Baggiani et al., 2012). The IF for GSF, fluconazole, clotrimazole, ketoconazole, dehydrogriseofulvin, naproxen, and dexamethasone were 2.2, 1.3, 1.0, 1.3, 1.9, 1.8, and 1.2, respectively (Fig. 3c). The SMIPs had a certain recognition capability for structural analogue (dehydrogriseofulvin). The SMIPs had relatively low recognition for other therapeutic and non-structural drugs, which suggested that the prepared polymers recognition ability are selective, and has a good capability of identifying the template molecule from complex mixtures. For therapeutic analogues and non-structural drugs, the structural differences make them incapable to match specific structures (Pengqi Guo, Luo, et al., 2017) thus resulted in a lower IF than that of GSF. This experiment further assured that the imprinted material showed good selectivity for the template drug.
3.4 SPE optimization
A 25 μg/mL of spiked plasma solution was used in this experiment to optimize the SMISPE process. The amount of adsorbent material is a major contributing factor responsible for good recoveries. A high amount of adsorbent material assures more adsorption sites are available for target analyte. Firstly, we explored different amounts of the polymers such as 10, 50, 100 and 150 mg of polymer with 0.5 mL volume of the sample. Fig. 4a shows that 100 mg of the polymer resulted in a maximum recovery about 81.9%. While the lower or higher amount of polymers did not achieve maximum recovery. So, 100 mg SMIPs was packed in SPE cartridge. In washing step, solvents such as water, water: methanol (7:3, v/v), water: methanol (5:5, v/v) and methanol were optimized. The volume of each washing solvent used in washing step was 2 mL and a recovery of 85.4% was achieved by water: methanol (7:3, v/v) (Fig. 4b). Washing step was carried out to reduce the interference of substances and to achieve a good clean recovery. Subsequently, different types of elution solvents were applied such as methanol-acetic acid (4:1, v/v), methanol-acetic acid (9:1, v/v), methanol-1 M hydrochloric acid (4:1, v/v), methanol-0.1 M hydrochloric acid (4:1, v/v), and ethanol-1 M hydrochloric acid (9:1, v/v). The hydrophobic GSF absorbed on the SMIPs was supposed to be desorbed in the organic solvents. So we used less toxic organic solvents like methanol and ethanol as elution solvents in this step. The maximum recovery 89.4% was achieved by use of methanol and acetic acid (4:1, v/v) (Fig. 4c) In the last step, volume of elution solvent was also optimized. The results demonstrated that by using 2 mL volume of the elution solvent a maximum recovery was achieved (Fig. 4d).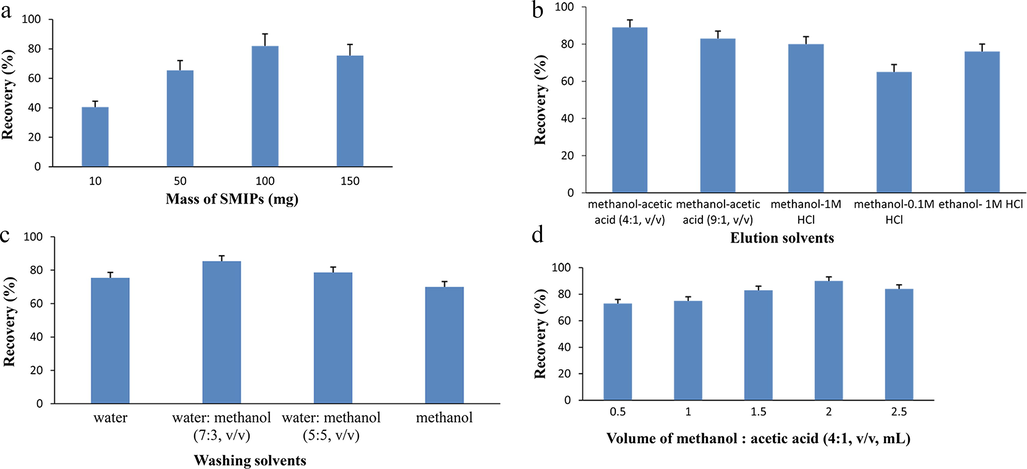
Optimization of SMISPE procedure. (a): Optimization of Mass of SMIPs; (b): optimization of washing solvents; (c): elution solvents (d): Volume of elution solvent.
So, 0.5 mL loading sample, 100 mg polymers, 2 mL water: methanol (7:3, v/v) as washing solvent to reduce interferences and 2 mL of methanol-acetic acid (4:1, v/v) used as an elution solvent were chosen as the optimized quantities for the procedure.
3.5 Method validation
The practicality of SMISPE method coupled with HPLC was assessed at the optimum conditions. A linear calibration curve of GSF spiked samples were achieved for the concentration range of 0.1–50 μg/mL. The correlation coefficient of linear calibration curve was (R2 = 0.997). The method’s limit of detection and limit of quantification were 0.02 μg/mL and 0.05 μg/mL respectively when estimated as three to ten times signal to noise ratio (S/N). The experimental accuracy (intra-day and inter-day) were determined in terms of percentage recoveries by three repeated experiments at four concentration levels (0.1, 1, 10, 25 μg/mL) of spiked plasma samples. Finally, the precision at concentration levels (0.1, 1, 10, 25 μg/mL) was calculated as intra-day and inter-day mean percentage of relative standard deviations (RSD). The RSD % of intra-day and inter-day was less than 4.5%. The SMISPE-HPLC had good recoveries (90.7–97.7%) at the four concentration levels (Table 1). The Fig. 5 shows the recovery chromatogram of the drug standard (10 μg/mL), spiked plasma sample after SMISPE procedure (10 μg/mL) and blank plasma sample. After SMISPE method, the interference of impurities was reduced in plasma samples chromatogram, relative content of the drug was improved and a recovery of 97.7% was achieved.
Samples
Spiked drug Conc (µg/mL)
Accuracy (Recovery % n = 3)
Precision (RSD %, n = 3)
Intra-day
Inter-day
Intra-day
Inter-day
Plasma
0.1
91.7
93.7
1.6
2.3
1.0
91.6
90.7
0.9
1.7
10
97.7
96.8
1.2
2.8
25
94.0
92.9
2.6
4.5
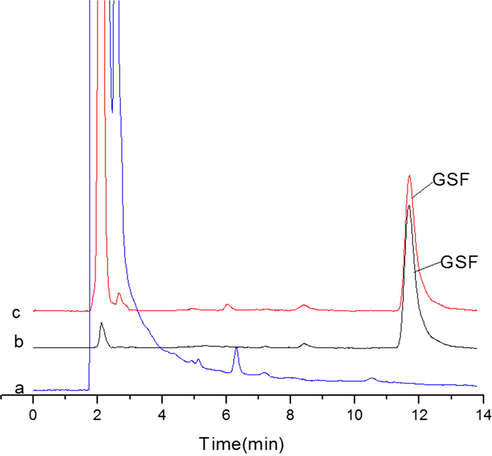
Recovery from spiked plasma. (a): Blank plasma; (b): Drug standard 10 µg/mL; (c): Recovery from spiked plasma (10 µg/mL) after SMISPE.
3.6 Detection of GSF in real plasma samples
The above established method was applied to real plasma samples in order to detect and separate the GSF. The blood plasma concentration was 0.8 μg/mL (after 5 hrs. drug dosing interval). The chromatogram of real plasma samples (Fig. 6) showed that after the SMISPE method the GSF was extracted from real plasma samples. The interference of impurities decreased and the quantity of GSF increased as compared to the chromatogram obtained by using SNIPs and C-18 SPE conventional SPE cartridge. The results demonstrated that the established method can meet the analytical requirements of GSF in plasma samples and can be used as a promising sample treatment technique for the determination and estimation of GSF in real plasma samples.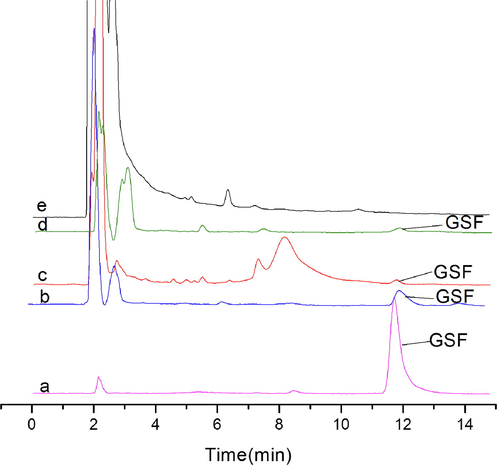
Detection of GSF in real plasma samples. (a): Griseofulvin standard (10 µg/mL); (b): plasma sample collected at 5 hrs., after a 50 mg/kg oral dose of griseofulvin, treated with SMISPE; (c): plasma sample collected at 5 hrs., after a 50 mg/kg oral dose of griseofulvin, treated with SNISPE; (d): plasma sample collected at 5 hrs., after a 50 mg/kg oral dose of griseofulvin, treated with C-18, SPE; (e): blank plasma.
3.7 Reusability of SMIPs
The % recovery was determined in order to evaluate the performance of SMISPE after 8 repeated experiments. The results of reusability experiment (Fig. S.4) demonstrated that SMIPs showed good recoveries and stability even after it was recycled eight times. The recoveries after eight cycles were ≥70.1%, which was still significant for reuse. The results assured that the prepared polymer has good durability and can be reused 8 times with relatively low performance loss.
3.8 Comparison with different extraction methods
The SMISPE-HPLC method was compared in terms of extraction efficiency and sensitivity with previously reported methods. The results (Table 2) demonstrated that the developed SMISPE method has better recoveries as compared with previously reported SPE methods. Additionally, the extraction process is highly selective, accurate, specific, simple, and reproducible. However, some limitations are also associated with this method such as low yield of specific binding sites and high non-specific binding sites, tedious optimization method and slow evaporation of eluents.
Method
Sample
Sample volume used (mL)
Precision
Limit of detection (ng/mL)
Recoveries (%)
References
GLC, LLE
Human plasma
1
NAa
6
97–107
(Schwarz et al., 1976)
LC-MS/MS,SPE
Human Plasma
0.5
7.5%
20 (LLOQ)b
87.36
(Mistri et al., 2007)
HPLC-Fluorescence, LLE
Rat plasma
0.1
3.0–7.5%
1
99.20
(Wei et al., 2008)
HPLC-UV, SMISPE
Rat plasma
0.5
0.9–4.5%
20
97.7
Current work
Considering the advantages like cost, easy availability of instrument, selectivity, good recovery, high precision, less organic solvent consumption, less amount of sample and sorbent applied and reusability of sorbent material for multiple analysis best suited our SMISPE-HPLC method for pre-clinical and pharmacokinetic evaluation in plasma samples.
4 Conclusion
We have successfully synthesized, characterized and applied SMIPs for the selective extraction of GSF from plasma samples. The SMISPE extraction procedure is simple, highly selective, cost effective and reproducible as compared with previous extraction methods. SMISPE method coupled with HPLC provided significant recoveries with less matrix interference. In addition, the method requires a small amount of sample and small volumes of solvent, with a significant reduction in sample manipulation, cost of materials, and analysis time. Moreover the SMIPs can be reused for 8 times which is a clear advantage over single use sorbent materials. This method has practical applications in pre-clinical studies and pharmacokinetic evaluation of this drug in human and animal plasma samples. The prospect of this study is the application for enrichment and extraction of GSF from environmental and pharmaceutical samples.
Acknowledgements
We gratefully acknowledge the financial support by National Natural Science Foundations of China (Grant Nos. 81773689, 81573391). We are also thankful for the generous support and scholarship provided by China Scholarship Council.
Declaration of Competing Interest
The authors declared no conflict of interest.
References
- Dummy molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. Talanta. 2019;195:390-400.
- [Google Scholar]
- A connection between the binding properties of imprinted and nonimprinted polymers: a change of perspective in molecular imprinting. J. Am. Chem. Soc.. 2012;134:1513-1518.
- [Google Scholar]
- The bioavailability of ultramicrosize griseofulvin (Gris- PEG) tablets in man. Curr. Ther. Res. Clin. Exp.. 1975;18:501-509.
- [Google Scholar]
- Molecularly imprinted polymer nanomaterials and nanocomposites by controlled/living radical polymerization. Prog. Polym. Sci.. 2016;62:1-21.
- [Google Scholar]
- Molecular imprinting: perspectives and applications. Chem. Soc. Rev.. 2016;45:2137-2211.
- [Google Scholar]
- Natural products as antifungal agents against clinically relevant pathogens. Nat. Prod. Reports.. 2010;27:1084-1098.
- [Google Scholar]
- Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat. Rev. Drug Discov.. 2010;9:790.
- [Google Scholar]
- Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B.. 2017;1043:44-62.
- [Google Scholar]
- Quantitative determination of griseofulvin and griseofulvin-4'-alcohol in plasma by fluorimetry on thin layer chromatograms. J. Chromatogr.. 1966;21:268-274.
- [Google Scholar]
- TLC determination of griseofulvin in plasma and 6-demethylgriseofulvin in urine. J. Pharm. Sci.. 1980;69:561-563.
- [Google Scholar]
- Selective analysis of aristolochic acid I in herbal medicines by dummy molecularly imprinted solid-phase extraction and HPLC. J. Sep. Sci.. 2017;40:2791-2799.
- [Google Scholar]
- Preparation and evaluation of molecularly imprinted polymer liquid chromatography column for the separation of ephedrine enantiomers. Arabian. J. Chem.. 2016;9:S528-S536.
- [Google Scholar]
- Development of molecular imprinted column-on line-two dimensional liquid chromatography for selective determination of clenbuterol residues in biological samples. Food. Chem.. 2017;217:628-636.
- [Google Scholar]
- On-Line two dimensional liquid chromatography based on skeleton type molecularly imprinted column for selective determination of sulfonylurea additive in Chinese patent medicines or functional foods. J. Pharm. Biomed. Anal.. 2017;146:292-301.
- [Google Scholar]
- Dummy-surface molecularly imprinted polymers as a sorbent of micro-solid-phase extraction combined with dispersive liquid-liquid microextraction for determination of five 2-phenylpropionic acid NSAIDs in aquatic environmental samples. Anal. Bioanal. Chem.. 2018;410:373-389.
- [Google Scholar]
- Griseofulvin potentiates antitumorigenesis effects of nocodazole through induction of apoptosis and G2/M cell cycle arrest in human colorectal cancer cells. Int. J. Cancer.. 2001;91:393-401.
- [Google Scholar]
- Novel surface dummy molecularly imprinted silica as sorbent for solid-phase extraction of bisphenol A from water samples. Talanta. 2016;148:29-36.
- [Google Scholar]
- Therapy of common superficial fungal infections. Dermatol. Therapy.. 2004;17:517-522.
- [Google Scholar]
- Development and validation of a reversed phase liquid chromatographic method for analysis of griseofulvin and impurities. J. Pharma. Biomed. Anal.. 2013;80:9-17.
- [Google Scholar]
- Simultaneous determination of griseofulvin and 6-desmethylgriseofulvin in plasma by electron-capture gas chromatography. J. Chromatogr. B: Biomed. Sci. Appl.. 1979;163:271-279.
- [Google Scholar]
- Toxic effects of griseofulvin: disease models, mechanisms, and risk assessment. Crit. Rev. Toxicol.. 1997;27:495-537.
- [Google Scholar]
- A metabolomic perspective of griseofulvin-induced liver injury in mice. Biochem. Pharmacol.. 2015;98:493-501.
- [Google Scholar]
- Facile room-temperature synthesis of a spherical mesoporous covalent organic framework for ultrasensitive solid-phase microextraction of phenols prior to gas chromatography-tandem mass spectrometry. Chem. Eng. J.. 2019;369:920-927.
- [Google Scholar]
- Preparation of surface molecularly imprinted polymers as the solid-phase extraction sorbents for the specific recognition of penicilloic acid in penicillin. Anal. Methods. 2014;6:7865-7874.
- [Google Scholar]
- Development of dispersive solid-phase extraction with polyphenylene conjugated microporous polymers for sensitive determination of phenoxycarboxylic acids in environmental water samples. J. Hazard. Mater.. 2019;371:433-439.
- [Google Scholar]
- Recent molecularly imprinted polymer-based sample preparation techniques in environmental analysis. Trends. Environ. Anal. Chem.. 2016;9:8-14.
- [Google Scholar]
- The anti-mitotic drug griseofulvin induces apoptosis of human germ cell tumor cells through a connexin 43-dependent molecular mechanism. Apoptosis. 2013;18:480-491.
- [Google Scholar]
- High-pressure liquid chromatographic assay for griseofulvin in plasma. J. Pharm. Sci.. 1979;68:1127-1130.
- [Google Scholar]
- Electrospray ionization LC–MS/MS validated method to quantify griseofulvin in human plasma and its application to bioequivalence study. J. Chromatogr. B.. 2007;850:318-326.
- [Google Scholar]
- Boronate affinity-based surface molecularly imprinted polymers using glucose as fragment template for excellent recognition of glucosides. J. Chromatogr. A.. 2016;1474:8-13.
- [Google Scholar]
- GF-15, a novel inhibitor of centrosomal clustering, suppresses tumor cell growth in vitro and in vivo. Cancer. Res.. 2012;72:5374-5385.
- [Google Scholar]
- Griseofulvin stabilizes microtubule dynamics, activates p53 and inhibits the proliferation of MCF-7 cells synergistically with vinblastine. Bmc. Cancer.. 2010;10:213.
- [Google Scholar]
- Identification of griseofulvin as an inhibitor of centrosomal clustering in a phenotype-based screen. Cancer. Res.. 2007;67:6342-6350.
- [Google Scholar]
- Disparate SAR data of griseofulvin analogues for the dermatophytes Trichophyton mentagrophytes, T. rubrum, and MDA-MB-231 cancer cells. J. Med. Chem.. 2012;55:652-660.
- [Google Scholar]
- GLC determination of griseofulvin in human plasma. J. Pharm. Sci.. 1976;65:370-372.
- [Google Scholar]
- Microtubule assembly dynamics: an attractive target for anticancer drugs. IUBMB Life. 2008;60:368-375.
- [Google Scholar]
- Recent trends in the application of the newest carbonaceous materials for magnetic solid-phase extraction of environmental pollutants. Trends. Environ. Anal. Chem.. 2016;10:11-23.
- [Google Scholar]
- Development and validation of a HPLC method to determine griseofulvin in rat plasma: application to pharmacokinetic studies. Anal. Chem. Insights.. 2008;3:103-109.
- [Google Scholar]
- Preparation of nicotine surface molecularly imprinted polymers for selective solid-phase extraction of nicotine from zero-level refill liquids of electronic cigarettes. Anal. Methods.. 2018;10:3637-3644.
- [Google Scholar]
- Dummy molecularly imprinted polymers on silica particles for selective solid-phase extraction of tetrabromobisphenol A from water samples. J. Chromatogr. A.. 2012;1220:7-13.
- [Google Scholar]
- Chromatographic analysis of griseofulvin and metabolites in biological fluids. J. Chromatogr.. 1980;181:77-84.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.06.007.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







