Translate this page into:
Synthesis, characterization and study of antibacterial activity of enaminone complexes of zinc and iron
*Corresponding author umer0101@hotmail.com (Umer Shafique)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 9 June 2010
Abstract
Complexes of enaminones; 4-N,N-diethylamine-pent-3-ene-2-one [HL1], 4-N,N-di n-propylamine-pent-3-ene-2-one [HL2] and 4-N,N-dicyclohexylamine-pent-3-ene-2-one [HL3] with Fe(II) and Zn(II) ions were prepared by reacting the equimolar ethanolic solutions of the ligands (HL1, HL2 and HL3) with ethanolic metal solutions. The complexes formed, were characterized by infrared, ultraviolet and atomic absorption spectroscopy. Ligands and their metal complexes were tested against Escherichia coli and Staphylococcus aureus bacteria to assess their antibacterial action using disc diffusion method. Ligands were completely inactive against bacteria whereas the complex Zn (HL1) has significant action on both bacteria, indicating that it has a good potential as bactericide. Other complexes have normal antiseptic character.
Keywords
Antibacterial activity of enaminone–metal complex
Enaminones
Enaminone–iron complex
Enaminone–zinc complex
1 Introduction
Enaminones consist of an amino group linked by carbon–carbon double bond to a carbonyl group. The carbonyl group eases the preparation, isolation and storage of the complex, by providing required stability to the system. The chemistry of the enamino carbonyl group is an area of considerable scope (Montanile, 2003). Enaminones have received a large attention during last decade. Lot of work has been done to explore new routes for the synthesis of enaminones. Azzoro and his co-workers reported a simple method for the synthesis of enaminones by using amines and 1–3 diketones (Azzoro et al., 1981). In another method, β-chloro vinyl ketone was reacted with amines to synthesize enaminones (Pohland and Benson, 1966). Other methods of preparation include reactions between: formamide dimethyl acetates and ketones (Abdulla and Brinkmeye, 1979); acid chlorides with terminal alkynes and triethylamine (Karpov and Müller, 2003); primary amines and β-dicarbonyl compound catalyzed with copper nano-particles (Kidwai et al., 2009). Imada et al. (1996) has transformed β-amino ketones to enaminones using palladium. Lue and Greenhill (1996) functionalize enaminones by introducing different substituents on nitrogen, α-carbon and β-carbonylic carbon. These derivatives were used extensively, for preserving natural products and analogues.
Enaminones are important organic intermediates used to synthesize various heterocyclic and biologically active analogues. Naringrekar and Stella (1990) investigated the structural stability of enaminones and evaluated their ability as pro drugs of primary amines. Enaminone synthesized by acetyl acetone and diethyl amine was used as universal cation locating agent showing characteristic colors with different metals (Nasir et al., 1996). Eddington et al. (2000) reported synthesis and anticonvulsant evaluations of some enaminones. Enaminones have shown high p-glycoprotein affinity and can act as p-glycoprotein modulators (Salama et al., 2004). Zhuo (1998) studied the 17O NMR spectrums of primary and secondary enaminones.
In coordination chemistry, enaminones are considered “good chelating ligands” for transition metals. The anions produced from enaminone, offer potential isoelectronic alternatives to cyclopentadienyl-based anions and therefore, their transition metal complexes can act as possible alternative catalysts for olefinic polymerization (Markéta et al., 2006). A new ferrocene-containing enaminone [C5H5FeC5H4C(O)CH⚌C–(NHAr)CH3] (Ar = 2-CH3OC6H4) and its copper(II) complex has been synthesized and characterized by elemental analysis, 1H NMR, IR, UV and X-ray crystallography (Shi et al., 2004).
The present work comprises the synthesis of complexes of 4-N,N-diethylamine-pent-3-ene-2-one [HL1], 4-N,N-di n-propylamine-pent-3-ene-2-one [HL2] and 4-N,N-dicyclohexylamine-pent-3-ene-2-one [HL3] ligands with Fe(II) and Zn(II) ions. The ligands were synthesized using reported procedures (Nelson and Joe, 1959). Metal complexes were characterized by infrared, ultraviolet and atomic absorption spectroscopy. Ligands and their metal complexes were examined to assess their antibacterial activity using disc diffusion method.
2 Experimental work
2.1 Material and apparatus
Analytical grade chemicals bought from Sigma–Aldrich, Inc. were used throughout this work. Gallen Kamp melting point apparatus was used to find out melting points. Labomed UV–Visible spectrophotometer (Model UVD-3500, 190–1100 nm) was used to find out λmax and absorbance in various solvents. IR spectrums of ligands and their metal complexes were recorded with FT-IR spectrophotometer (Perkin–Elmer Spectrum RX-I) using nujol mull in range 4000–500 cm−1. Fe(II) and Zn(II) ions, in the complexes were estimated by atomic absorption spectrophotometer (Model AAnalyst-100) using air–acetylene flame.
2.2 Synthesis of ligands
2.2.1 Synthesis of 4-N,N-diethylamine-pent-3-ene-2-one (HL1)
Acetyl acetone (0.1 mol L−1, 10.5 mL) was added to conical flask placed in an ice bath. Subsequently, diethyl amine (0.1 mol L−1, 10.4 mL) was added, dropwise, with continuous stirring. Yellow crystals of HL1 were formed in 15 min. These needle-like crystals were purified by sublimation and were stored in a refrigerator at 4 °C.
2.2.2 Synthesis of 4-N,N-di-n-propylamine-pent-3-ene-2-one (HL2)
Acetyl acetone (0.6 mol L−1, 61.7 mL), di-n-propyl amine (0.6 mol L−1, 83.9 mL), p-toluene sulfonic acid (2.0 g) and benzene (150 mL) were taken in a round bottom flask and refluxed till the complete removal of water, using a Dean–Stark apparatus. Afterwards, benzene was removed under reduced pressure in a rotary evaporator. The contents of the flask were cooled in an ice bath. The yellow liquid obtained was agitated with cold saturated ethanolic sodium bicarbonate solution and extracted with ether, thrice. The ethereal extract was taken into a distillation flask and the fraction boiling at 95 °C was collected.
2.2.3 Synthesis of 4-N,N-dicyclohexylamine-pent-3-ene-2-one (HL3)
Acetyl acetone (0.3 mol L−1, 31.0 mL), dicyclohexylamine (0.3 mol L−1, 58.0 mL), p-toluene sulfonic acid (1.0 g) and benzene (150 mL) were refluxed on heating mantle at 85 °C for 5 h. The water produced in reaction mixture was removed using a Dean–Stark apparatus. Benzene was removed by distillation under reduced pressure and the viscous oily liquid gained yielding shiny needle-like crystals on cooling. The product was recrystallized with ethanol.
2.3 Synthesis of metal complexes
2.3.1 Synthesis of Fe(II) and Zn(II) complexes of HL1
FeSO4·7H2O (0.3 mol L−1, 2.62 g) was added to ethanolic solution of enaminone (HL1) (0.01 mol L−1, 1.55 g). The precipitates formed, were allowed to stand for about 5 h. Afterwards, filtered off and washed with small amount of ethanol and n-hexane, respectively and recrystallized in ethanol. The reddish brown crystals were dried at room temperature and stored in desiccator.
The Zn(II) complex of the ligand (HL1) was prepared by adding aqueous solution of ZnCl2 (0.01 mol L−1, 1.36 g) in ethanolic solution of (HL1) enaminone (0.01 mol L−1, 1.55 g). The reaction mixture was stirred for 10–15 min and allowed to settle. The light brown precipitates of Zn(II) complex formed, were removed by filtration and crystallized with ethanol–tetrahydrofuran (1:1) mixture. Later, dried at room temperature and stored in a desiccator.
2.3.2 Synthesis of Fe (II) and Zn (II) complexes of HL2
Fe(II) and Zn(II) complexes were prepared by adding FeSO4·7H2O (0.01 mol L−1, 2.62 g) and ZnCl2 (0.01 mol L−1, 1.36 g) to cold ethanolic solution of (HL2) enaminone (0.01 mol L−1, 1.83 g), separately. The reaction mixtures were stirred for 10–15 min and allowed to stand for 5 h. The orange red precipitates of iron complex and light brown precipitates of zinc complex were removed by filtration and crystallized with ethanol–tetrahydrofuran mixture. Afterwards, dried at room temperature and stored in a desiccator.
2.3.3 Synthesis of Fe(II) and Zn(II) complexes of HL3
Fe(II) and Zn(II) complexes were prepared by adding FeSO4·7H2O (0.01 mol L−1, 2.62 g) and ZnCl2 (0.01 mol L−1, 1.36 g) to ethanolic solution of (HL3) enaminone (0.01 mol L−1, 2.63 g), separately. The reaction mixtures were stirred for 10–15 min and allowed to stand for 5 h. The reddish brown precipitates of iron complex and light brown precipitates of zinc complex were removed by filtration and crystallized with ethanol–tetrahydrofuran mixture. Afterwards, dried at room temperature and stored in a desiccator.
2.4 Antibacterial studies
The ligands and their metal complexes were tested to assess their antibacterial activity against the following bacterial species.
-
Escherichia coli
-
Staphylococcus aureus
The ligand complex (30 μg in 0.01 mL DMF) was applied to a paper disc with the help of micropipette. The discs were placed in an incubator for 48 h at 37 °C and later, applied to bacteria grown on agar plates. Inoculation was performed with the help of a platinum wire loop, which was made red-hot in flame and used for the application of bacterial strains after cooling. Sterilized forceps were used to apply paper disc on inoculated agar plates. When the discs were applied, they were incubated at 37 °C for 24 h. The zone of inhibition (diameter in mm) was measured around the disc.
3 Results and discussion
In our previous work, synthesis, characterization and antibacterial activity of several isomeric enaminones and their metal complexes with Ca(II) and Mg(II) have been reported (Tariq et al., 2009). In the present work, synthesis, characterization and antibacterial activity of three enaminones and their complexes with zinc and iron have been presented. Syntheses of the enaminones are represented in Schemes 1–3.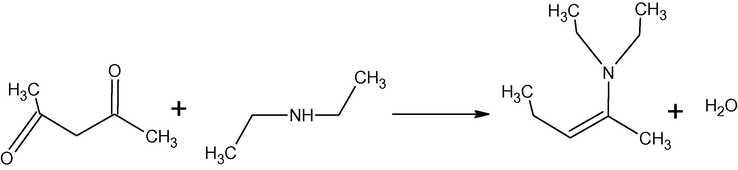
Synthesis of 4-N,N-diethylamine-pent-3-ene-2-one (HL1).
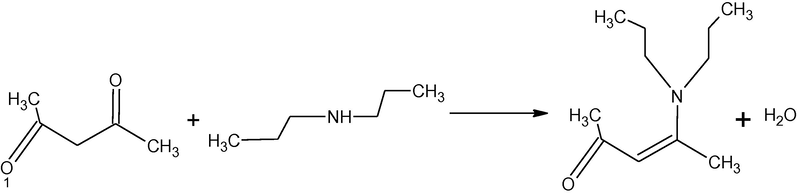
Synthesis of 4-N,N-diethylamine-pent-3-ene-2-one (HL2).
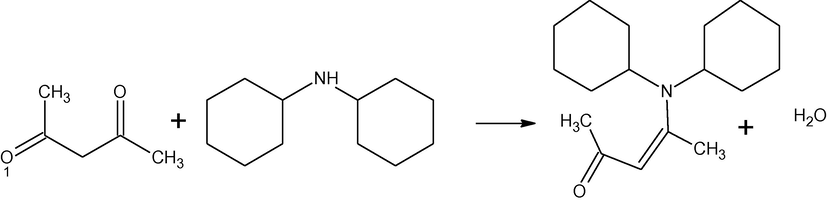
Synthesis of 4-N,N-dicyclohexylamine-pent-3-ene-2-one (HL3).
These enaminones can react with the Fe(II) and Zn(II) ions forming the neutral complexes. Preparation of enaminones involved the removal of water. Anhydrous sodium sulfate, sodium carbonate, anhydrous magnesium sulfate and p-toluene sulfonic acid were used as water removing agents. However, separation of p-toluene sulfonic acid from the reaction mixture was difficult in case of oily products. Water plays an important role as some enaminones were susceptible to hydrolysis by water. HL1 (m.p = 105 °C) and HL3 (m.p = 154 °C) were colorless crystalline solids whereas HL2 (b.p = 95 °C) was viscous liquor. The percentage of nitrogen in these ligands, determined by Kjeldahl’s methods, varies in the range 4.94–8.90%. These enaminone ligands were used to form complexes with Fe(II) and Zn(II). Most of these complexes were crystalline solids with shiny appearance and only few were amorphous solids. Their decomposition points were above 230 °C. Physicochemical characteristics of ligands and their metal complexes are listed in Table 1.
Sr. No.
Compound
Appearance
M.P.(1,3)/B.P.(2)/D.P.(4–9) (°C)
% Yield
% Age of nitrogen
Theoretical
Found
1
HL1
Needles like, shiny crystals
105
66–68
9.03
8.90
2
HL2
Golden yellow viscous liquid
95
65–67
7.60
7.00
3
HL3
Colorless needle like shiny crystals
154
64–65
5.32
4.94
4
Fe(HLI)
Reddish brown shiny crystals
>500
27.02
8.06
7.34
5
Zn(HLI)
Light brown, shiny crystals
>230
26.50
7.45
7.12
6
Fe(HL2)
Dark Green, shiny crystals
500
30.23
6.94
6.20
7
Zn(HL2)
Light brown, shiny crystals
>230
20.55
6.49
5.91
8
Fe(HL3)
Light Green, shiny crystals
>500
28.90
4.97
3.99
9
Zn(HL3)
Light brown, shiny crystals
>230
37.0
4.73
4.71
The solubility of the ligands and their complexes were tested in eight different solvents in both hot and cold states. All the ligands and the complexes were insoluble in n-hexane. Most of these were soluble in water and benzene. Details are given in Table 2. Soluble = Sol, insoluble = Ins, sparingly soluble = Sp. S, soluble in hot = S. Hot, sparingly soluble in hot = Sp. S.H.
Solvent
HL1
HL2
HL3
Fe (HL1)
Zn (HL1)
Fe (HL2)
Zn (HL2)
Fe (HL3)
Zn (HL3)
Distilled water
Sol
Ins
Sol
Sol
Sp. S
Sp. S
Ins
S. Hot
Sp. S
Ethanol
Sol
Sol
Sol
Sp. S
Sp. S.H
Sp. S
Sp. S
Sp. S
Ins
THF
Sol
Sol
Sol
Sol
Ins
Ins
Ins
Sol
Ins
Toluene
S. Hot
S. Hot
S. Hot
Sol
Ins
Sp. S
Ins
Sol
Ins
Benzene
Sol
Sol
Sol
Sp. S
Ins
S. Hot
Ins
S. Hot
Ins
n-Hexane
Ins
Ins
Ins
Ins
Ins
Ins
Ins
Ins
Ins
Acetone
Sol
Sol
S. Hot
Sp. S
Sp. S
S. Hot
Ins
Sp. S.H
Sp. S
CCl4
Sol
Sol
Sol
Sp. S
S. Hot
Sp. S
Ins
Sp. S.H
S. Hot
The spectral data has been summarized in Table 3. The λmax values are 310 nm, 310 nm and 290 nm for HL1, HL2 and HL3 respectively. Probable electronic transition is π–π*. λmax values have shifted down after forming metal complex.
Sr. No.
Compound
π–π* transitions
λmax (nm)
Absorbance (AU)
1
HL1
310
1.722
2
HL2
310
1.315
3
HL3
290
1.039
4
Fe(HL1) Complex
290
1.959
5
Zn(HL1) Complex
295
0.213
6
Fe(HL2) Complex
295
0.476
7
Zn(HL2) Complex
290
0.257
8
Fe(HL3) Complex
290
1.309
9
Zn(HL3) Complex
295
0.476
IR spectra of some enaminones or enamino ketones were reported by Dabrowski (1963). The IR spectra were recorded with nujol mull. Peaks of interest lay in the range 1572–1670 cm−1 and 1427–1542 cm−1. Some enaminone showed absorption at 1612–1620 cm−1, as well. The IR data is given in the Table 4. Strong absorption bands for C–H stretch, C–H bend, C⚌C stretch, C⚌O stretch and C–N stretch are indicative of the formation of enaminones. These peaks were shifted to lower wave numbers after formation of metal complexes.
Bond frequencies (cm−1)
HL1
Fe (HL1)
Zn (HLI)
HL2
Fe (HL2)
Zn (HL2)
HL3
Fe (HL3)
Zn (HL3)
ν (C–H) stretching
2974
2376
2354
2875
2724
2353
2850
2842
2723
ν (C–H) bending
1388
1372
1364
1365
1354
1342
1370
1347
1338
ν (C–C) stretching
1483
1454
1384
1532
1460
1461
1550
1451
1459
ν (C⚌C) stretching
1665
1659
1652
1668.6
1653
1644
1662.5
1655
1648
ν (C–N) stretching
1291
1269
1260
1255
1080
1040
1257
1090
1046
ν (C⚌O) stretching
1698
1675
1670
1706
1660
1671
1694
1642
1670
Metal to ligand ratio was calculated by finding out metal by atomic absorption spectrophotometer. By comparing these values, suitable geometries of these metal complexes were proposed. The ligand (HL1, HL2, and HL3) to Fe(II) ratio is 3:1. The synthesized complexes are proposed to show octahedral geometries. The ligand (HL1, HL2, and HL3) to Zn(II) ratio is 2:1. The synthesized complexes are proposed to show square planar geometries. The proposed structures of the complexes are shown in Figs. 1 and 2.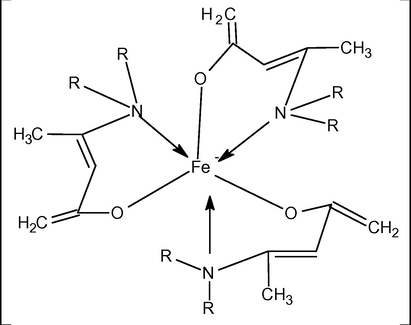
Proposed geometry of the complexes of Fe(II) with HL1, HL2 and HL3, where R = ethyl, n-propyl, cyclohexyl.
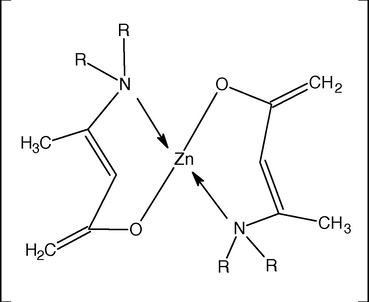
Proposed geometry of the complexes of Zn(II) with HL1, HL2 and HL3, where R = ethyl, n-propyl, cyclohexyl.
The antibacterial activities of the synthesized ligands and their metal complexes were tested against strains of bacteria, S. aureus and E. coli, using disc-diffusion method. All the three ligands were found to be quiet against both strains of bacteria. However, the metal complexes were active against bacterial strains. In general, the complex Zn(HL1) has highest activity against the S. aureus and E. coli, showing that it has a good potential as bactericide. Fe(HL3) has shown good activity as well. The other complexes showed limited activity against S. aureus and E. coli bacteria. The Fe(HL1) complex did not show any activity against the S. aureus. Results are depicted in Table 5. + = Active, ++ = very active, − = inactive.
Sr. No.
Compound
Bacteria
Staphylococcus aureus
Escherichia coli
1
HL1
−
−
2
Fe(HL1) Complex
−
−
3
Zn(HL1) Complex
+
++
4
HL2
−
−
5
Fe(HL2) Complex
−
+
6
Zn(HL2) Complex
−
+
7
HL3
−
−
8
Fe(HL3) Complex
+
+
9
Zn(HL3) Complex
−
+
4 Conclusion
Complexes of enaminones; 4-N,N-diethylamine-pent-3-ene-2-one [HL1], 4-N,N-di n-propylamine-pent-3-ene-2-one [HL2] and 4-N,N-dicyclohexylamine-pent-3-ene-2-one [HL3] with Fe(II) and Zn(II) ions were synthesized. The complexes formed, were characterized by infrared, ultraviolet and atomic absorption spectroscopy. To evaluate anti bacterial character of complexes, these were tested against E. coli and S. aureus bacteria. It was found that complex Zn (HL1) has significant action on both bacterial species while other complexes have normal antiseptic character.
References
- Use of boron trifluoride etherate in the preparation of 2-amino-1-alkenyl ketones from ß-diketones and low-boiling amines. Synthesis. 1981;1981(11):880-881.
- [Google Scholar]
- Infra-red spectra and structure of substituted unsaturated carbonyl compounds—I: enamino ketones with primary amino group. Spectrochimica Acta. 1963;19(2):475-496.
- [Google Scholar]
- Enaminones – ersatile therapeutic pharmacophores. Current Medicinal Chemistry. 2000;7(4):417-436.
- [Google Scholar]
- Palladium-catalyzed double and single carbonylations of β-amino alcohols. selective synthesis of morpholine-2,3-diones and oxazolidin-2-ones and applications for synthesis of α-oxo carboxylic acids. Bulletin of the Chemical Society of Japan. 1996;69(7):2079-2090.
- [Google Scholar]
- Straightforward novel one-pot enaminone and pyrimidine syntheses by coupling–addition–cyclocondensation sequences. Synthesis. 2003;2003(18):2815-2826.
- [Google Scholar]
- A novel method for the synthesis of β-enaminones using Cu-nanoparticles as catalyst. Catalysis Communications. 2009;10(11):1514-1517.
- [Google Scholar]
- Enaminones in heterocyclic synthesis. Advances in Heterocyclic Chemistry. 1996;67:207-343.
- [Google Scholar]
- Novel 5-(4-substituted-phenyldiazenyl)-1,3,2λ4-oxazaborines and their rearrangement to 1, 2,4,3λ4-triazaborines. Organometallics. 2006;25(8):2025-2030.
- [Google Scholar]
- The chemistry of enaminones, diazocarbonyls and small rings: our contribution. Journal of Brazilian Chemical Society. 2003;14(6):945-969.
- [Google Scholar]
- Mechanism of hydrolysis and structure–stability relationship of enaminones as potential prodrugs of model primary amines. Journal of Pharmaceutical Sciences. 1990;79(2):138-146.
- [Google Scholar]
- 4-N,N-diethylamino-3-penten-2-one – a new locating agent for some inorganic cations in paper chromatography. Journal of Chemical Society of Pakistan. 1996;18(4):296-297.
- [Google Scholar]
- Unsaturated amines. XIII. The sites of alkylation and protonation in certain enaminoketones. Substituted trimethinium compounds from o-alkylated enaminoketones1. Journal of the American Chemical Society. 1959;81(3):595-602.
- [Google Scholar]
- The influence of enaminones on the transport and oral bioavailability of p-glycoprotein substrate therapeutic agents. International Journal of Pharmaceutics. 2004;273(1–2):135-147.
- [Google Scholar]
- Syntheses and crystal structures of a ferrocene-containing enaminone and its copper complex. Polyhedron. 2004;23(9):1541-1546.
- [Google Scholar]
- Synthesis, characterization and study of antibacterial activity of enaminone complexes of calcium and magnesium. Journal of Scientific Research. 2009;37(1):1-8.
- [Google Scholar]







