Translate this page into:
Synthesis, characterization of inclusion compounds of amygdalin with β-cyclodextrin and sod-like activity and cytotoxicity on hela tumor cells
⁎Corresponding author. narcis.duteanu@upt.ro (Narcis Duteanu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this work is presented the synthesis of the inclusion complexes of amygdalin with β-cyclodextrin by three methods: kneading, co-precipitation and freeze drying and their characterization.
The inclusion compounds were synthesized using a molar ratio of amygdalin: β-cyclodextrin of 1:1. Synthesized compounds were analyzed by FTIR spectroscopy, X-rays, thermal analysis and the results confirm the formation of inclusion compounds by amygdalin with β-cyclodextrin.
The studies carried out have shown the protective effect of amygdalin-β-cyclodextrin compounds obtained by freeze drying, kneading and coprecipitation against free radicals (SOD-like activity in vivo) and the results of in vitro cell cytotoxicity of the compounds on HeLa cell line compared to pure amygdalin. Compound obtained by freeze drying has the best SOD-like activity and cytotoxicity on HeLa tumor cells, so it may be considered as potential therapeutic agent.
Keywords
Amygdalin
β-cyclodextrin
Inclusion compounds
Free radicals
Cytotoxicity
1 Introduction
Amygdalin (AMG) also known as B17 vitamin, or previously called laetrile represent a chemical compound from the largest class of nitrilosides. Such compounds which are found in the prunasian family seeds (seeds of apricots, almonds, peaches, apples, and other rosaceous plants) represent cyanide containing natural substances(Chang et al., 2006). Among the other prunasins, Armeniacae seeds have been used for the treatment of asthma, bronchitis, emphysema, leprosy, colorectal cancer, leucoderma, and pain, throughout time (Hwang et al., 2003; Chang et al., 2005; Jung et al., 2019). Chemical structure of amygdalin consists from two molecules of glucose linked with a molecule of benzaldehyde, which is responsible for its analgesic action, and one of hydrocyanic acid, having an anti-neoplastic action. Apart from the above indications, amygdalin has been used to treat cancers and relieve pain (Ellison et al., 1978; Sim et al., 2000; Fukuda et al., 2003), D-amygdalin (D-mandelonitrile-b-D-gentiobioside) is known to exhibit selective antinoplastic action (Koo et al., 2005).
AMG chemical formula is presented in Fig. 1.a, and the chemical name of amygdalin according to IUPAC is [(6-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy](phenyl)acetonitrile.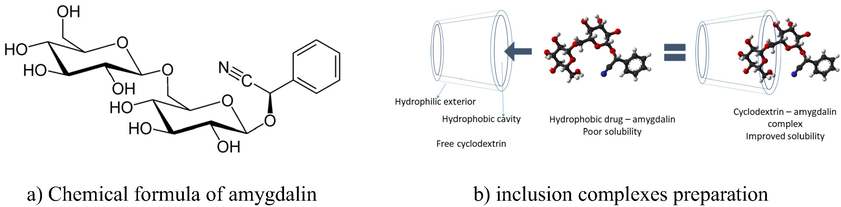
Chemical formula of amygdalin.
As pure compound amygdalin is a slightly hygroscopic product which can be found as white or slightly yellow powder, or as slightly yellow crystals (Chang et al., 2006). From medical point of view, amygdalin’s limited solubility in water, which decreases as temperature rises, represents the main issue. Amygdalin presents a good solubility in alcohol and methanol. A different approach used to increase amygdalin’s solubility concomitant with the increase of its bio-availability can be represented by preparation of his complexes with cyclodextrins.
Cyclodextrins are a class of cyclic oligosaccharides which are prepared from enzymatic starch hydrolysis. The most known and used cyclodextrins consists in 6, 7 or 8 units of D-glucopyranose which are linked together by oxygen bridges. These compounds are designed as α, β, and γ cyclodextrin, which are also known as parental cyclodextrins, because derivative cyclodextirns compounds are obtained by attaching different functional groups at parental cyclodextrin structures. Pharmaceutical usage of cyclodextrins is possible mainly due to their ability to form inclusion stable complexes with different active substances (substances responsible for therapeutic effect). β-cyclodextrin and its derivative as hydroxypropyl – β – cyclodextrin and methyl – β – cyclodextrin are compounds which present a high solubility in water. Due to the higher solubility in water correlated with the ability to form stable inclusion complexes with different active compounds, it can be used β–cyclodextrin to pharmaceutical preparation of new substances with significantly improved bio-availability of active compound along with reduction of different adverse effects. Experimental tests proved that the active compounds encapsulation in cyclodextrins cavity modify only physical properties (solubility, chemical reactivity, odor) having no effects over the therapeutic properties. In real practice the cyclodextrins were used for development and preparation of active compound with controlled release.
β-cyclodextrin is the most studied in its class of compounds proving that it is a safety compound which can be easily metabolized by humans. Long term feeding studies proved that β-cyclodextrin have no toxicology significance when the diet included up to 5% of compound. Also, such tests proved that this cyclodextrin present no toxicity for subject’s reproductive system. Main advantage of β-cyclodextrin usage for drugs encapsulation is represented by its high resistance at the action of salivary or pancreatic α-amylases. Its ring is open in the colon by enzymes from Bacteroides species which lead at the formation of maltoheptaose fragments (Hedges 2009).
Recently different active compounds were used for inclusion of pharmaceutical substances with cyclodextrins: repaglinide (Nicolescu, Arama et al., 2010), **sulphonamidic diuretics (Soica et al., 2006a, 2006b; Aluas et al., 2007; Soica et al., 2007; Şoica et al., 2008), pentacyclic triterpenes (Dehelean et al., 2008), fosinopryl natrium (Sbarcea et al., 2011), erythromycin (Marian et al., 2011).
AMG it is a natural cyanogen glycoside which can be splited by beta-glucosidase producing free cyanide. In present study we aimed to activate amygdalin directly at the tumor site, so the malign cells can be killed without systemic toxicity. In order to achieve this aim the amygdalin molecule has been included into the cyclodextrin cavity and obtained inclusion complexes (Fig. 1b) were tested in vitro and in vivo to prove compounds specificity and cytotoxicity. Other aim of this work was to investigate the molecular encapsulation of amygdalin in the β-cyclodextrin cavity by using FTIR spectrometry, X-ray diffraction (XRD) and differential scanning calorimetry (DSC). In order to prove the molecular encapsulation of amygdalin was studied the protective effect of produced complex against free radicals produced by oxidative agents. Protective effects were evaluated by using a strain of S. Cerevisiae which is known for its ability to delete / insert the SOD1 gene.
2 Materials and methods
All materials were of reagent grade and were used without further purification. Amygdalin and β-cyclodextrin were purchased from Sigma Aldrich GmbH, Germany.
FTIR spectra were recorded using a JASCO 6100 FTIR spectrometer in the 4000–400 cm−1 spectral domain with a resolution of 4 cm−1 by using KBr pellet technique.
X-ray diffractograms were recorded using a Bruker D8 Advance diffractometer in the angular domain 2θ = 2–50° using Cu Kα1 radiation. In order to increase the resolution a monochromator was used to eliminate the Kα2 radiation.
The DSC thermograms were obtained with a DSC-60 Shimadzu differential scanning calorimeter. The samples’ heating was done with a rate of 20 °C/min in the 20–500 °C interval by using sealed Al cells, in nitrogen atmosphere.
2.1 In vivo SOD-like activity
The protective effect of pure AMG and of amygdalin-β-cyclodextrin compounds obtained by freeze-drying, coprecipitation and kneading against free radicals produced by oxidative agents had been determined.
The SOD-like activities of amygdalin-β-cyclodextrin compounds where evaluated using a strain of S. Cerevisiae Δsod1 (ATCC96687) (American Type Culture Collection), which has the ability to delete/insert the SOD1 gene encoding the synthesis of Cu2Zn2SOD. Yeast strains lacking CuZnSOD (Δsod1) were first created by deletion-replacement mutations in the S. cerevisiae CuZnSOD gene. Higher levels of oxidative damage to both cytosolic and mitochondrial proteins in the Δsod1 strain provide evidence that CuZnSOD protects yeast from oxidative stress (Sheng et al., 2014). The characteristics of S. Cerevisiae Δsod1 (ATCC96687) are: MAT aura 3–52 trp1-289 his3-Δ1 leu 2–3 leu 2–112 sod1: URA3). The Cu2Zn2SOD is the main SOD in the cell and is localized in the cytoplasm.
Yeast cells were grown in YPD reach medium (1% yeast extract, 2% peptone and 2% glycerol). The used culture medium does not contain glucose, it contains glycerol instead, because in its presence the levures can breathe. This is determinant, as free radicals are going to be generated during the breath processes taking place in the mitochondria (Sas et al., 2007).
Solid media used for cell culture contained 1.5% agar. Cell density from cultures grown overnight was determined by cell counting in a “Nebauer” hematimetre. 106 cells were resuspended in 15 mL of melted solid YPD media kept at 45 °C. Solutions of pure AMG and amygdalin-β-cyclodextrin compounds in a mixture of DMSO: EtOH (1:4) at increasing concentrations (30, 50, 70 µm, see table 2) were added to the growth medium. Cell suspensions were poured into Petri dishes and allowed to solidify at room temperature. Paper disks measuring 6 mm in diameter (Antibiotica test Blättchen) containing 5 μL of a 5 mM menadione solution in ethanol or 5 μL of 17.5% H2O2 have been used. The diameters of clear zones around the disks, measured after 3 days of incubation at 28 °C, where taken as a quantitative estimate of the protective action (Marian et al., 2018b).
2.2 Cell culture
Two cell lines were used for determining the cytotoxicity of amygdalin-β-cyclodextrin compounds, a human cervical carcinoma line (HeLa) (Sigma) and a normal fibroblastic epithelial cell line (HFL1) (Sigma). The cell lines were maintained in DMEM (Sigma-Aldrich), supplemented with 10% fetal bovine serum (Hyclone), 1 mM glutamine (Sigma-Aldrich), 1% antimycotic antibiotic 100x (Sigma-Aldrich). The cells were cultured at 37 °C in an atmosphere of 5% CO2 and 95% relative humidity (Niculescu et al., 2014; Hangan et al., 2016; Hangan et al., 2017).
2.3 Cytotoxicity assays
For the cell survival both cell lines were plated (1 × 105 cells/ well) in 96-well plates for 24 h in normal propagation media. The culture medium was then replaced with complete medium containing pure amygdalin and three type of amygdalin-β-cyclodextrin compounds obtained by freeze-drying, kneading and coprecipitation, in six different concentrations (100 µM, 50 µM, 25 µM, 12.5 µM, 2 µM and 0.2 µM). The amygdalin-β-cyclodextrin compounds were initially dissolved in DMSO, followed by serial dilutions in media until the final concentration of DMSO was less than 1%. The negative controls were represented by cells lines cultivated in normal expansion medium added with the same amount of diluted DMSO, while the positive control was represented by Cisplatin (Ebewe Pharma Ges.m.b.H. Nfg. KG, Austria) in the same concentration as the amygdalin-β-cyclodextrin compounds. Each experiment was carried out in triplicate. The cellular viability was determined using the MTT assay at 24 and 48 h. The formazan particles were solubilized with dimethyl sulfoxide (DMSO) (Sigma). The absorbance was read at 550 nm using a spectrophotometer (Bio-Rad, Hercules, CA, USA) (Hangan et al., 2012; Boodram et al., 2016; Sevastre et al., 2017).
2.4 Statistics
All the experiments were conducted in triplicates and data are displayed as Mean ± SD, representing the average of independent experiments performed in triplicate. The IC50 values representing the amygdalin-β-cyclodextrin compounds concentration required to inhibit 50% of cell proliferation were calculated from the dose response curve using non-linear regression. Statistical values were generated using GraphPad Prism version 5.0 for Windows, GraphPad Software, San Diego California USA.
3 Results and discussions
3.1 General procedure for the synthesis of the β-cyclodextrin inclusion compound
Kneading method – were triturated together 0,0005 mol of β-cyclodextrin with 0,0005 mol of amygdalin (1:1 M ratio) in a mortar by adding drops of ethanol (0.5 mL each). Obtained paste were triturated till the solvent's evaporation, the process was repeated several times, so that the total time period to be about 40 min.
Coprecipitation method − 0,0005 mol amygdalin is dissolved in 5 mL of ethanol, simultaneously 0,0005 mol β-cyclodextrin are dissolved in 5 mL distilled water (molar ratio 1:1), over cyclodextrin aqueous solution is added drop by drop amygdalin alcoholic solution under continuous stirring, the solvents are then evaporated at 50 °C in a drying stove to obtain the complex powder.
Freeze-dryer – Equimolar amounts of amygdalin and β-cyclodextrin were dissolved in water and mixed for 2 h at 35 °C wrapped in the aluminum foil to protect the solutions from the direct sun light. After the equilibration period of 24 h the clear solutions were frozen and subsequently lyophilized in a freeze-dryer of Alpha 1–2 LD type.
3.2 Physical – chemical characterization of synthesized compounds was realized by using FT-IR spectrometry, XRD diffraction, and thermal analysis.
3.2.1 FTIR spectrometry of amygdalin-β-cyclodextrin inclusion compounds
In Figs. 2–4 are presented the IR spectra recorded for: amygdalin, β–cyclodextrin and prepared complexes of amygdalin with β - cyclodextrin.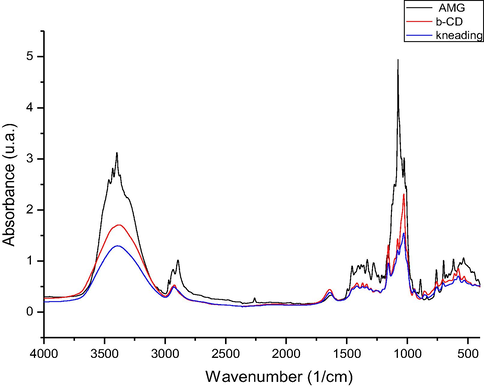
FTIR spectra of the inclusion compound obtained by kneading procedure (kn) on 4000–400 cm−1 spectral domain.
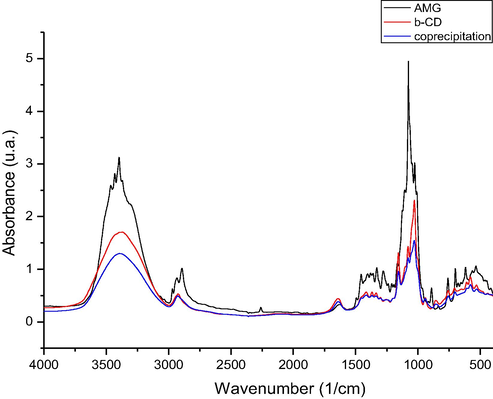
FTIR spectra of the inclusion compound obtained by coprecipitation procedure (co) on 4000–400 cm−1 spectral domain.
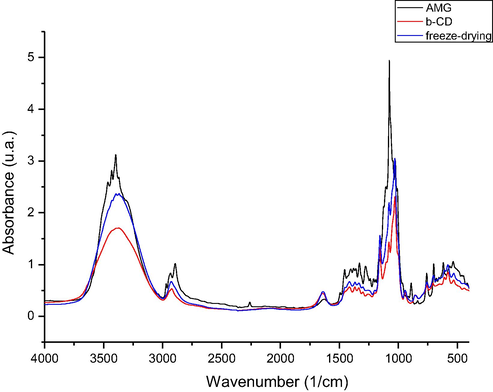
FTIR spectra of the inclusion compound obtained by freeze-drying procedure (fd) on 4000–400 cm−1 spectral domain.
By analyzing the FT-IR spectra recorded were observed the presence of some vibrations located at approximately 3400 cm−1, which are associated with the stretching vibration of —OH groups from unassociated alcohol molecules. Into the spectra recorded for β – cyclodextrin were observed some intense bands located between 3400 and 302 cm−1, bands which are related to the —OH groups stretching, and in region 3000–2900 cm−1 were located the stretching vibrations of —CH2 and —CH groups.
One important vibration which is represented by the stretching vibration of C—O—C group (etheric group), vibration which were presented into the amygdalin spectra (at 1176 cm−1), cyclodextrin spectra (at 1180 cm−1), and into the spectra of prepared complexes (at 1184, 1174, and 1178 cm−1).
Into the spectra recorded for produced complexes were not identified the peaks located at 3435 and 3404 cm−1, peaks which are presented into the amygdalin spectra and which correspond to the stretching vibrations of —OH groups bonded by hydrogen bonds. Absence of these peaks can be related to the formation of complexes, by formation of new bonds between cyclodextrin hydroxyl groups and amygdalin hydroxyl groups.
Other vibration founded in all recorded spectra (amygdalin – 885 cm−1, β – cyclodextrin 860 cm−1, and produced complexes – 855, 853, 848 cm−1) is the stretching vibration of —CH bond from aromatic ring. Into the amygdalin spectra was identified the presence of the stretching vibration of nitrile group located at 2259 cm−1, vibration with a low intensity, which cannot be identified into the spectra recorded for produced complexes. Other vibration related to the stretching of —CH bonds from aromatic ring were identified into the amygdalin spectra at 699 cm−1, also into the prepared complexes at 688, 691 and 691 cm−1.
One vibration with high intensity corresponding to the stretching vibration of C—O and C—C bonds is observed into the amygdalin spectra at 1020 cm−1, these vibrations being located also into the complexes recorded spectra at approximately same wavelength.
3.2.2 X Ray-diffraction of amygdalin-β-cyclodextrin inclusion compounds
Analyzing the diffractograms depicted in Fig. 5 can observe that some of the peaks corresponding to the pure compounds are presented into the produced complexes, being shifted, and having lower intensities. Also were observed that in spectra recorded for produced inclusion complexes some peaks disappear, or present a diminished intensity. Such behavior demonstrates that into the produced compounds exist some interactions between the guest molecule (amygdalin) and the host one (β – cyclodextrin), confirming the formation of new compounds.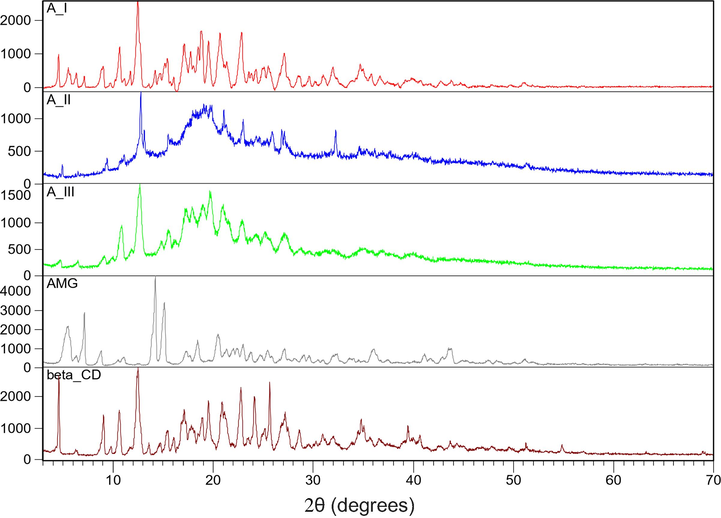
X-ray diffraction patterns of β-cyclodextrin, amygdalin and of the inclusion compounds obtained by coprecipitation (AIII), kneading (AII) and freeze-drying (AI) procedures.
Analyzing the data presented in Fig. 5 can observe that into the patterns recorded for obtained complex the peaks of amygdalin are superposed with the peaks of β-cyclodextrin leading at an increase of height. The X-ray changes represent the proof of complexation between the guest molecule-amygdalin and the host molecule-β-cyclodextrin.
3.2.3 Thermal analysis of amygdalin, β-cyclodextrin, amygdalin-β-cyclodextrin inclusion compounds
Analyzing the TG and DSC (Fig. 6) curves recorded for pure amygdalin were observed a small mass loss (around 1.59%) into the temperature range between 50 and 160 °C, with a maximum when the temperature has the value of 119 °C. This mass loss can be associated with loss of some small quantity of water which was adsorbed from atmosphere during amygdalin manipulation. Into the temperature range 200–500 °C can be observed the presence of two peaks, first located at 226 °C which correspond to the amygdalin melting, followed by his thermal decomposition. During this temperature interval the mass loss is 84.64%, both processes being endothermal one. At temperature of 599.6 °C the residual mass is 13.77% (Marian et al., 2018a).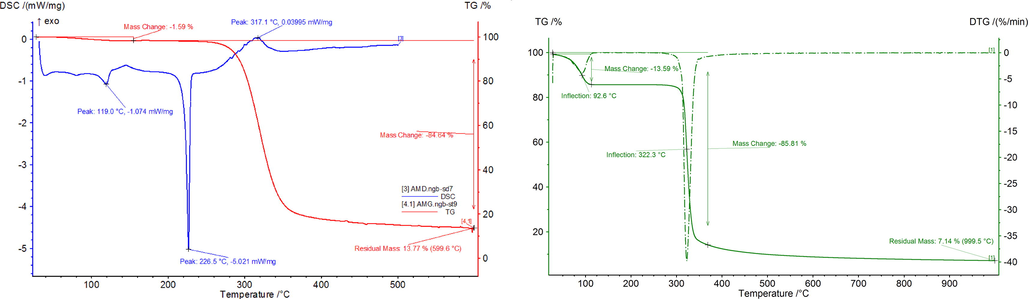
TG and DSC curves of amygdalin and TG and DTG of β-cyclodextrin.
From the TG spectra recorded for β – cyclodextrin (Fig. 6) can observe that the studied compound had a first mass loss between 50 and 114.7 °C, loss which is associated with water loss. During this process 13.59% from initial mass was lost. Into the temperature range 114.7 and 306.5 °C β – cyclodextrin is stable, no mass loss being observed. Into the temperature interval 306.5 and 377.2 °C the thermal decomposition of β – cyclodextrin is taking place, when 72.22% from initial mass were lost. Both processes are endothermic one.
The thermal behavior of the produced complexes is similar. From the TG curves recorded for prepared complexes (Fig. 7) can observe that three different processes had been identified on the curves. Parameters associated with thermal behavior of prepared complexes are presented in Table 1.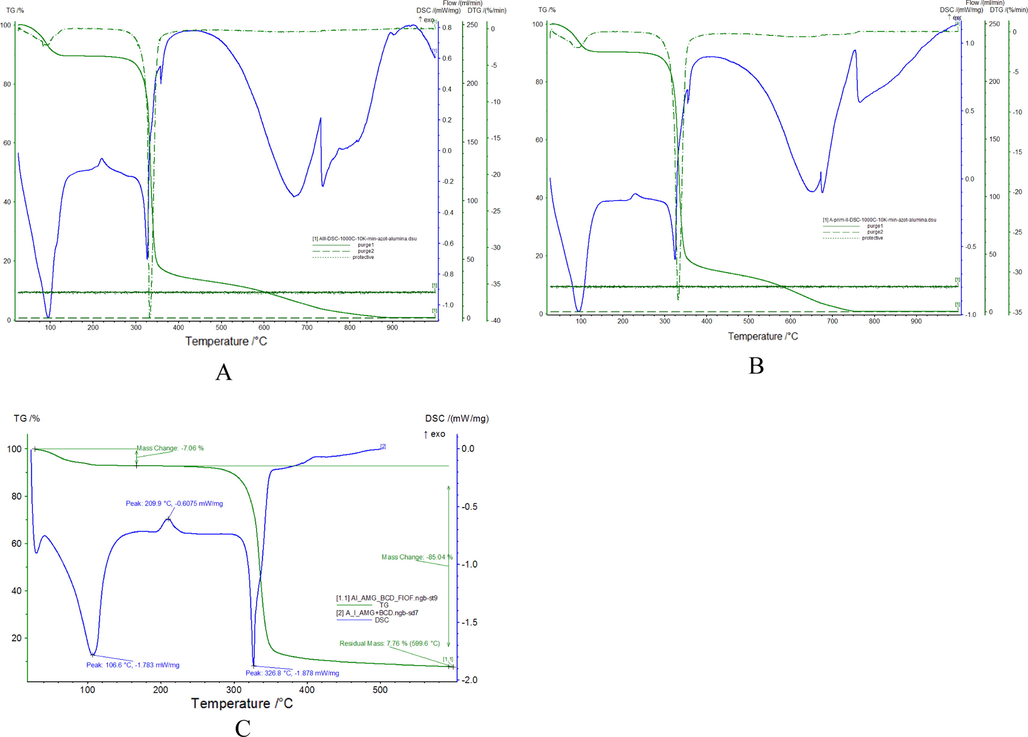
TG, DTA and DSC curves of the inclusion compounds of amygdalin with β-cyclodextrin obtained by kneading (kn) procedures (A), coprecipitation (co) procedures (B) and freeze-drying (fd) procedures (C).
Prepared complex
Thermal decomposition phase
Temperature interval (°C)
Tpic DTG (°C)
Mass loss (%)
Process nature
Residual mass (%)
Kneading complex
I
50–120
90.0
10.00
Endothermic dehydration
1.12
II
120–260
225.0
0.00
Exothermic melting
III
260–400
332.0
75.55
Endothermic decomposition
IV
400–700
680.0
13.33
Endothermic decomposition
Coprecipitation complex
I
50–120
98.0
8.88
Endothermic dehydration
1.12
II
120–260
225.0
0.00
Exothermic melting
III
260–420
334.4
65.64
Endothermic decomposition
IV
420–750
668.0
24.37
Endothermic decomposition
Freeze-drying complex
I
50–150
106.6
7.06
Endothermic dehydration
7.76
II
150–280
209.9
0.00
Exothermic melting
III
280–400
326.8
85.18
Endothermic decomposition
Pure AMG Diameter of the inhibition area (cm)
Freeze-drying (fd) Diameter of the inhibition area (cm)
Kneading (kn) Diameter of the inhibition area (cm)
Coprecipitation (co) Diameter of the inhibition area (cm)
Menadione
H2O2
Menadione
H2O2
Menadione
H2O2
Menadione
H2O2
Control (Menadione 5 mM or H2O2 17.5%)
8
7.5
8
7.5
8
7.5
8
7.5
30
6.2
6.4
4.9
5.4
5.1
5.8
6
6.2
50
6
6.3
4.6
5.3
4.9
5.7
5.8
6.2
70
5.9
6.3
4.6
5.1
4.8
5.6
5.8
6.1
After that was determined the solubility of pure AMG and of prepared inclusion complexes, obtaining for pure AMG a value close to the value from literature (0.09 g mL−1). For inclusion complexes have been obtained values which indicate an increase of the solubility: freeze drying 0.19 g mL−1, 0.17 g mL−1 for coprecipitation and 0.12 g mL−1 for kneading. Obtained values indicate the preparation of desired inclusion complexes.
3.3 In vivo SOD-like activity
The in vivo SOD-like activity of pure AMG and of amygdalin-β-cyclodextrin compounds obtained by freeze-drying, kneading and coprecipitation was quantified by a method based on the protection against free radicals provided by the extracts to the yeast S. Cerevisiae (Gonzalez-Alvarez et al., 2008; Marian et al., 2017). The SOD-mimetic activity of pure AMG and of amygdalin-β-cyclodextrin compounds on cell growth with a Δsod1 mutant treated with menadione or H2O2 had been evaluated. The oxidative stress is produced by two oxidative agents: menadione which toxicity is due to the superoxide radicals’ production and H2O2 which toxicity is due to OH• radicals.
It will be considered that pure AMG and amygdalin-β-cyclodextrin compounds have a SOD-like activity if a decrease of the diameter of the inhibition zone is registered versus the control zone. The efficacy will then be evaluated by comparison of the diameter of the inhibition area for pure AMG, amygdalin-β-cyclodextrin compounds obtained by freeze-drying, kneading, coprecipitation and control.
Fig. 8 shows the results for pure AMG and for amygdalin-β-cyclodextrin compounds obtained by freeze-drying, kneading and coprecipitation on the growth of the Δsod1 mutant against free radicals produces by H2O2 and menadione. In the presence of amygdalin-β-cyclodextrin compounds obtained by freeze-drying, kneading and coprecipitation at 30, 50 and 70 μM concentrations, a significant reduction of the inhibition area is observed when the oxidative stress is produced by both menadione and H2O2. In the presence of pure AMG a reduction of the inhibition area is also observed, but less pronounced than for all the amygdalin-β-cyclodextrin. The diameter of the inhibition area for pure AMG for amygdalin-β-cyclodextrin compounds obtained by freeze-drying, kneading and coprecipitation in different concentrations, using menadione and H2O2 are given in Table 2.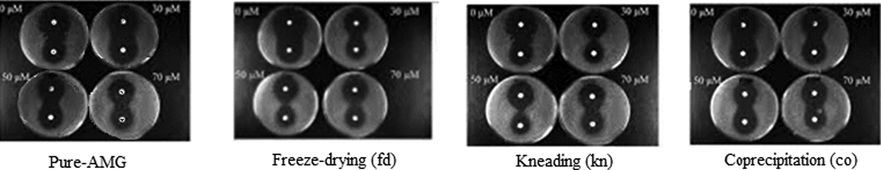
Effect of pure AMG and of amygdalin-β-cyclodextrin compounds obtained by freeze-drying, kneading and coprecipitation, on the growth of the Δsod1 mutant against free radicals produces by H2O2 (disk at the top of each Petri disk) and menadione (disk at the bottom of each Petri disk).
The reduction of the inhibition area is between 39 and 43% for the amygdalin-β-cyclodextrin obtained by freeze-drying, 36–40% for the amygdalin-β-cyclodextrin obtained by kneading and 25–28% for the amygdalin-β-cyclodextrin obtained by coprecipitation, and 23–26% for pure AMG against oxidative stress generated by menadione. The protective activities of pure AMG and of amygdalin-β-cyclodextrin compounds obtained by freeze-drying, kneading and coprecipitation do not seem to be dependent on amygdalin-β-cyclodextrin compounds concentration.
The protection of the amygdalin-β-cyclodextrin compounds against free radicals generated by H2O2 is lower than in the case of free radicals generated by menadione. Menadione-induced superoxide dismutase very quickly to H2O2 which can be easily detected in different systems. Thus, effect of menadione can be related to both superoxide and hydrogen peroxide (Timoshenko et al., 1996). The compound obtained by freeze-drying produce a reduction of the inhibition diameter about 28–32%, the compound obtained by kneading between 23 and 25%, while the compound obtained by coprecipitation only between 17 and 19%, while pure AMG only between 15 and 16%. Nor in this case the protective action produced by pure AMG and by amygdalin-β-cyclodextrin compounds obtained by freeze-drying, kneading and coprecipitation does not depend on amygdalin-β-cyclodextrin compounds concentration.
In conclusion, all three amygdalin-β-cyclodextrin shows a higher SOD-like activity compare with pure AMG. As a conclusion, amygdalin-β-cyclodextrin compound obtained by freeze-drying register a higher SOD-like activity compared with the compounds obtained by kneading and coprecipitation. The lower SOD-like activity was obtained for compound obtained by coprecipitation. At the maximum concentration analyzed (70 µM), a fraction of cells appears to have a higher protection.
The current study suggests that amygdalin-β-cyclodextrin compounds obtained by freeze-drying, kneading and coprecipitation are able to protect efficiently against superoxide anions and they could be considered as promising effective agents against toxicity of superoxide anion, improving significantly the growth of Δsod1 strain. They supply the Cu2Zn2SOD deficiency of the mutant. For this reason, they are potential therapeutic agents in the prevention and treatment of diseases mediated by free radicals. The most active is amygdalin-β-cyclodextrin compound obtained by freeze-drying who owns the best potential.
3.4 Cytotoxicities of the complexes
Amygdalin-β-cyclodextrin compounds obtained by freeze-drying, kneading and coprecipitation were examined for their antiproliferative properties compared to pure amygdalin. The in vitro cytotoxicity of amygdalin-β-cyclodextrin compounds were tested on two human cells, a cervical line (HeLa); and a normal fibroblastic epithelial cell line (HFL1), using Cisplatin as positive control. The response was quantified using 3-(4,5-dimethyl-2-thioazolyl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay. The cells were exposed to various concentrations of each compound (ranging between 0.2 and 100 μM) at 24 and 48 h.
The results revealed a promising profile, the amygdalin-β-cyclodextrin compounds expressing different levels of cytotoxicity. On HeLa cell line, freeze-drying by two folds more potent as compared to coprecipitation, while kneading has an intermediate activity. The difference was already visible after 24 h, their differences became even more visible after 48 h, but they maintained the same trend. Freeze-dying has the highest cytotoxicity because it is the most soluble amygdalin-β-cyclodextrin compounds, as the solubility test indicated. All inclusion compounds were by far more active than pure amygdalin, but the efficiency remained lower than those of Cisplatin (Table 3).
Cells
Compound
IC50 (µM)
24 h
48 h
HeLa cells
Pure AMG
231.19 ± 22.87
51.30 ± 12.78
coprecipitation
113.93 ± 12.65
22.91 ± 8.87
kneading
82.60 ± 15.78
17.24 ± 4.87
Freeze-drying
63.81 ± 9.43
8.32 ± 1.09
Cisplatin
22.13 ± 0.04
5.83 ± 0.15
HFL1 cells
Pure AMG
278.19 ± 24.18
73.08 ± 8.08
Coprecipitation
151.15 ± 11.04
28.82 ± 6.45
Kneading
143.21 ± 8.12
24.66 ± 4.05
Freeze-drying
116.21 ± 5.34
24.28 ± 1.07
Cisplatin
12.96 ± 1.05
2.95 ± 0.17
On normal fibroblast (HFL-1 line), all amygdalin-β-cyclodextrin compounds showed a slightly lower toxicity than the toxicity found on tumor cells, but remarkably they were significantly less toxic than Cisplatin.
The antitumor activity of AMG is widely known, but its mechanism is not fully established. However, several studies already proved that HeLa cells are sensitive to AMG in a dose dependent manner, cell death being triggered by apoptotic mechanism via intrinsic pathway, as suggested by the increasing activity of Caspase-3 activity (Chen, Ma et al., 2013), downregulation of Bcl-2 and upregulation of Bax (Chang et al., 2006). In addition, AMG treated cells exhibit overexpression of JNK/c-Jun pathway, thus the JNK/c-Jun pathway is a possible mechanism of apoptosis in HeLa cells (Chen et al., 2013).
In cervical cancer cells, the effect on cell cycle proliferation was not investigated, but in several prostate cancer cell lines (Makarević et al., 2016) AMG inhibit the proliferation by blocking the tumor cells G0/G1 phase, by down-regulating the expression of cell cycle proteins CKD1, CKD2, cyclin A, and cyclin B. In colon cancer tumor cells, arresting of the cell cycle is done by inhibiting the ATP-binding cassette, exonuclease 1 (EXO1), sub-family F and topoisomerase (DNA) I (TOP1) (Park et al., 2005).
The selective toxicity exhibited by AMG on tumor cells, was previously described and it can be explained by the interaction between amygdaline and β-glucosidase (Song and Xu 2014). This interaction inhibits the cytochrome c oxidase, which prevent the synthesis of adenosine triphosphate and enhance the production of hydrocyanic acid. The hydrocyanic acid exerts selective toxicity on tumor cells, which, unlike normal cells, lack rhodanese, an enzyme able to remove it (Li et al., 2018).
In this study we demonstrated that amygdalin-β-cyclodextrin obtained by freeze-drying has a superior antitumor activity on HeLa cell line comparative with the other inclusion compounds and pure amygdalin.
4 Conclusions
Presence of benzaldehyde molecule into the amygdalin structure induce an analgesic action, and concomitant the presence of hydrocyanic acid induces an anti-neoplastic action. Amygdalin have a low solubility in water and one possibility to increase his bioavailability is represented by his inclusion in cyclodextrins.
Three complexes were synthesized by the inclusion of amygdalin with β-cyclodextrin through three methods (kneading, coprecipitation, freeze-drying) using molar ratio 1:1 and after were characterized with FTIR spectrometry, X Ray-diffraction and differential scanning calorimetry. Solubility test indicate an increase of amygdalin solubility after inclusion into the β-cyclodextrin, being in concordance with the results obtained for in-vivo test.
Amygdalin has a clear pharmacological activity, but there is still little in‑depth research on the pharmacological mechanism of the compound, so it has an important application value to systematically investigate the mechanism of amygdalin pharmacological activity and develop antitumor drugs. Therefore, AMG-β-CD compound provided by fd requires future testing, as a potential selective anticancer agent.
AMG-β-CD compound obtained by freeze-drying register a higher SOD-like activity compared with AMG-β-CD compounds obtained by kneading and coprecipitation. The lower SOD-like activity was obtained for AMG-β-CD obtained by coprecipitation. At the maximum concentration analyzed (70 µM), a fraction of cells appears to have a higher protection. AMG-β-CD compound obtained by freeze-drying has the best SOD-like activity and cytotoxicity on HeLa tumor cells, so it may be considered as potential therapeutic agent.
5 Contributions
Eleonora Marian, Narcis Duteanu, Laura Vicas designed the research, Tunde Jurca, Gerlinde Rusu, Mariana Muresan, Otilia Micle, Adriana Corina Hangan, Roxana Liana Stan, Corina Ionescu, Bogdan Sevastre, Emoke Pall performed experiments, Eleonora Marian and Narcis Duteanu wrote the manuscript.
References
- Physico-chemical analysis of binary complexes of furosemide and randomly methylated beta-cyclodextrin. Rev. Chim.. 2007;58(10):891-894.
- [Google Scholar]
- Breast cancer stem cell potent Copper(II)-non-steroidal anti-inflammatory drug complexes. Angew. Chem.-Int. Ed.. 2016;55(8):2845-2850.
- [Google Scholar]
- Amygdalin induces apoptosis through regulation of bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol. Pharm. Bull.. 2006;29(8):1597-1602.
- [Google Scholar]
- Armeniacae semen extract suppresses lipopolysaccharide-induced expressions of cycloosygenase-2 and inducible nitric oxide synthase in mouse BV2 microglial cells. Biol. Pharm. Bull.. 2005;28(3):449-454.
- [Google Scholar]
- Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol. Immunotoxicol.. 2013;35(1):43-51.
- [Google Scholar]
- Physico-chemical and molecular analysis of antitumoral pentacyclic triterpenes in complexation with gamma-cyclodextrin. Rev. Chim.. 2008;59(8):887-890.
- [Google Scholar]
- Special report on laetrile: the NCI laetrile review. N. Engl. J. Med.. 1978;299(10):549-552.
- [Google Scholar]
- Anti-tumor promoting effect of glycosides from <i>Prunus persica</i> seeds. Biol. Pharm. Bull.. 2003;26(2):271-273.
- [Google Scholar]
- Evaluation of antiproliferative activities and apoptosis induction caused by copper(II)-benzothiazolesulfonamide complexes in Jurkat T lymphocytes and Caco-2 cells. J. Biol. Inorg. Chem.. 2008;13(8):1249-1265.
- [Google Scholar]
- Synthesis of new n-substituted heterocyclic sulfonamides. Farmacia. 2012;60(6):932-938.
- [Google Scholar]
- Synthesis, crystal structures, characterization and antitumor activities of two copper(II) complexes of a sulfonamide ligand. Transition Met. Chem.. 2017;42(2):153-164.
- [Google Scholar]
- Synthesis, crystal structure and characterization of new biologically active Cu(II) complexes with ligand derived from N-substituted sulfonamide. J. Chem. Sci.. 2016;128(5):815-824.
- [Google Scholar]
- Chapter 22 - Cyclodextrins: Properties and Applications. In: BeMiller J., Whistler R., eds. Starch (Third Edition). San Diego: Academic Press; 2009. p. :833-851.
- [Google Scholar]
- Studies on the Allergic asthma effect of Semen Armeniaceae Amarum. Korea J. Herbol.. 2003;18
- [Google Scholar]
- Effects of armeniacae semen and amygdalin on prostaglandin E2 synthesis and nitric oxide production. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol.. 2019;32(3):13-22.
- [Google Scholar]
- Quantitative determination of amygdalin epimers from armeniacae semen by liquid chromatography. J. Chromatogr. B. 2005;814(1):69-73.
- [Google Scholar]
- Chinese medicine amygdalin and β-glucosidase combined with antibody enzymatic prodrug system as a feasible antitumor therapy. Chin. J. Integr. Med.. 2018;24(3):237-240.
- [Google Scholar]
- Amygdalin delays cell cycle progression and blocks growth of prostate cancer cells in vitro. Life Sci.. 2016;147:137-142.
- [Google Scholar]
- Inclusion compounds of erythromycin with beta-cyclodextrin. Rev. Chim.. 2011;62(11):1065-1068.
- [Google Scholar]
- Thermal behaviour and kinetic study of amygdalin. J. Therm. Anal. Calorim.. 2018;134(1):765-772.
- [Google Scholar]
- Salivia Officinalis L. and Verbascum Phiomoides L. Chemical, antimicrobial, antioxidant and antitumor investigations. Rev. Chim.. 2018;69(2):365-370.
- [Google Scholar]
- A comparative study on the biologic activity of centaurea cyanus versus calendula officinalis. Farmacia. 2017;65(6):940-946.
- [Google Scholar]
- Preparation and characterization of inclusion complexes between repaglinide and beta-cyclodextrin, 2-hydroxypropyl-beta-cyclodextrin and randomly methylated beta-cyclodextrin. Farmacia. 2010;58(1):78-88.
- [Google Scholar]
- Novel 2,3-disubstituted 1,4-naphthoquinone derivatives and their metal complexes - Synthesis and in vitro cytotoxic effect against mouse fibrosarcoma L929 cells (vol 700, pg 13, 2012) J. Organomet. Chem.. 2014;761
- [Google Scholar]
- Amygdalin inhibits genes related to cell cycle in SNU-C4 human colon cancer cells. World J. Gastroenterol.: WJG. 2005;11:5156-5161.
- [Google Scholar]
- Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci.. 2007;257(1–2):221-239.
- [Google Scholar]
- Characterization of fosinopril natrium-hydroxypropyl-beta-cyclodextrin inclusion complex. Rev. Chim.. 2011;62(3):349-351.
- [Google Scholar]
- Anticancer activity of euonymus europaeus fruits extract on human melanoma cells. Farmacia. 2017;65(1):56-62.
- [Google Scholar]
- Superoxide dismutases and superoxide reductases. Chem. Rev.. 2014;114(7):3854-3918.
- [Google Scholar]
- Study on toxicity, anti-cancer and NK cell activity of Lateril oil. J. Korean Oriental Oncol.. 2000;6(1):19-27.
- [Google Scholar]
- The influence of polyvinylpyrrolidone on furosemide complexation with randomly-methylated-beta-cyclodextrin. Rev. Chim.. 2006;57(7):726-730.
- [Google Scholar]
- Obtaining and physical-chemical characterization of inclusion complex of a sulphonamidic diuretic with methylated beta-cyclodextrine. Rev. Chim.. 2006;57(4):392-397.
- [Google Scholar]
- Preparation and physico-chemical characterization of chlorthalidone-hydroxypropyl-beta-cyclodextrin binary systems. Rev. Chim.. 2007;58(7):606-611.
- [Google Scholar]
- Complexation with hydroxypropyl-??-cyclodextrin of some pentacyclic triterpenes. characterisation of their binary products. Farmacia. 2008;56:182-190.
- [Google Scholar]
- Advanced research on anti-tumor effects of amygdalin. J. Cancer Res. Ther.. 2014;10(Suppl 1):3-7.
- [Google Scholar]
- Effects of metabolic inhibitors and lectins on the menadione-dependent generation of H2O2 by rat thymocytes. IUBMB Life. 1996;40(6):1149-1158.
- [Google Scholar]







