Translate this page into:
Synthesis, characterization, thermal and catalytic properties of a novel carbazole derived Azo ligand and its metal complexes
⁎Corresponding author. Tel.: +90 5385568281. selmadagli9@hotmail.com (Selma Bal),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This work explains the synthesis of a new azo-Schiff base compound, derived from condensation between N-ethylcarbazole-3-carbaldehyde and 1,3-diaminopropane, followed by azo coupling reaction with the diazonium salt of 2-amino-4-methyl phenol. The newly synthesized azo-Schiff base was further reacted with the acetate salts of Copper, Cobalt and Nickel to give three coordination compounds. All synthesized compounds have been characterized through spectral analysis. The coordination compounds have been examined for their thermal and catalytic features. Good and moderate yields were obtained for the oxidation of styrene and cyclohexene. Thermal features of the ligand and its complexes have been explained and the results obtained have supported the proposed structures.
Keywords
Azo-imine ligand
Complex
Thermal
Catalysis
1 Introduction
During the past two decades, considerable attention has been paid to the chemistry of Schiff bases and their metal complexes. Schiff bases are a class of important compounds in medicinal and pharmaceutical field. So far, various azo containing Schiff bases have been synthesized, characterized and examined for their different features such as antimicrobial, antifungal, electronic, and thermal (Ghasemian et al., 2014a,b; Eren et al., 2014; Bal et al., 2014a,b; Menati et al., 2013; Kose et al., 2013; Shaghaghi, 2014).
In addition to above features, the catalysis of alkene oxidation by soluble transition metal complexes is of great interest in both biomimetic and synthetic chemistry (Heshmatpour et al., 2012; Islam et al., 2013; Chen et al., 2013). Because the oxidation reactions are carried out in elevated temperatures, it has become important to synthesize thermally stable Schiff base complexes as catalysts. In the literature various schiff bases had been tested as catalysts toward different oxidation reactions of saturated or unsaturated organic compounds (Huang et al., 2013; Silva et al., 2013; Netalkar et al., 2014). Some azo containing Schiff base complexes had also been used as catalysts in many organic reactions such as oxidations or reductions on many chemical compounds (Mirkhani et al., 2004; Ispir, 2009; Ozdemir, 2014; Uruş et al., 2012; Pardhi et al., 2010; Islam et al., 2010; Bansod and Aswar, 2007). With this work, we have synthesized a new, carbazole derived azomethine Schiff base and its Cu (II), Co (II) and Ni (II) complexes. The ligand and the coordination compounds were fully characterized and examined for their thermal and catalytic features.
2 Experimental
9-Ethylcarbazole, Phosphorus (V) oxychloride, 1,3-diaminopropane, 2-amino-4-methylphenol, acetate salts of Copper (II), Cobalt (II) and Nickel (II) were purchased from Sigma Aldrich. Nuclear Magnetic Resonance spectra were recorded on a Bruker AV 300 MHz spectrometer in the solvent CDCl3. Infrared spectra were obtained using KBr discs on a Shimadzu 8300 FTIR spectrophotometer in the region of 400–4000 cm−1. Ultra-violet spectra were run in ethanol on a Shimadzu UV-160 A spectrophotometer. Mass spectra of the ligand were recorded on a LC/MS APCI AGILENT 1100 MSD spectrophotometer. The oxidation products were analysed with a gas chromatograph (Shimadzu, GC-14B) equipped with a SAB-5 capillary column and a flame ionization detector. Elemental analyses were performed on a LECO CHNS 932 elemental analyser and the metal analyses were carried out on an Ati Unicam 929 Model AA Spectrometer in solutions prepared by decomposing the compounds in aqua regia and subsequently digesting them in conc. HCl. Thermal analyses of synthesized ligand and its metal complexes were carried out on a Perkin-Elmer Thermogravimetric Analyser TG/DTA 6300 instrument under nitrogen atmosphere between the temperature range 30 °C and 988 °C at a heating rate of 10 °C/min. Magnetic measurements were carried out by the Gouy method using Hg[Co(SCN)4] as calibrant. Molar conductances of the Schiff base ligands and their transition metal complexes were determined in MeOH (ca.10−3 M) at room temperature using a Jenway Model 4070 conductivity meter.
2.1 Synthesis of the ligand
Formylation of 9-ethylcarbazole was done by using Vilsmeier formylating agent DMF and POCl3 (Grigoras and Antonoaia, 2005; Yoon et al., 2007; Bal and Bal, 2014). The monoaldehyde was separated by flash chromatography with ethyl acetate/hexane (1:10) as eluent. N-Ethylcarbazole-3-carbaldehyde was then reacted with 1,3-diaminopropane to give the imine compound; (Z,Z)-N,N′-bis[(9-ethyl-9H-carbazol-3-yl)methylene]propane-1,3-diamine, which was previously synthesized and characterized by us (Bal and Bal, 2014). Finally, our ligand was synthesized through azo coupling reaction (Eren et al., 2014; Bal et al., 2014a,b) between diazonium salt of 2-amino-4-methylphenol and (Z,Z)-N,N′-bis[(9-ethyl-9H-carbazol-3-yl)methylene]propane-1,3-diamine. Synthesis scheme can be seen in Fig. 1.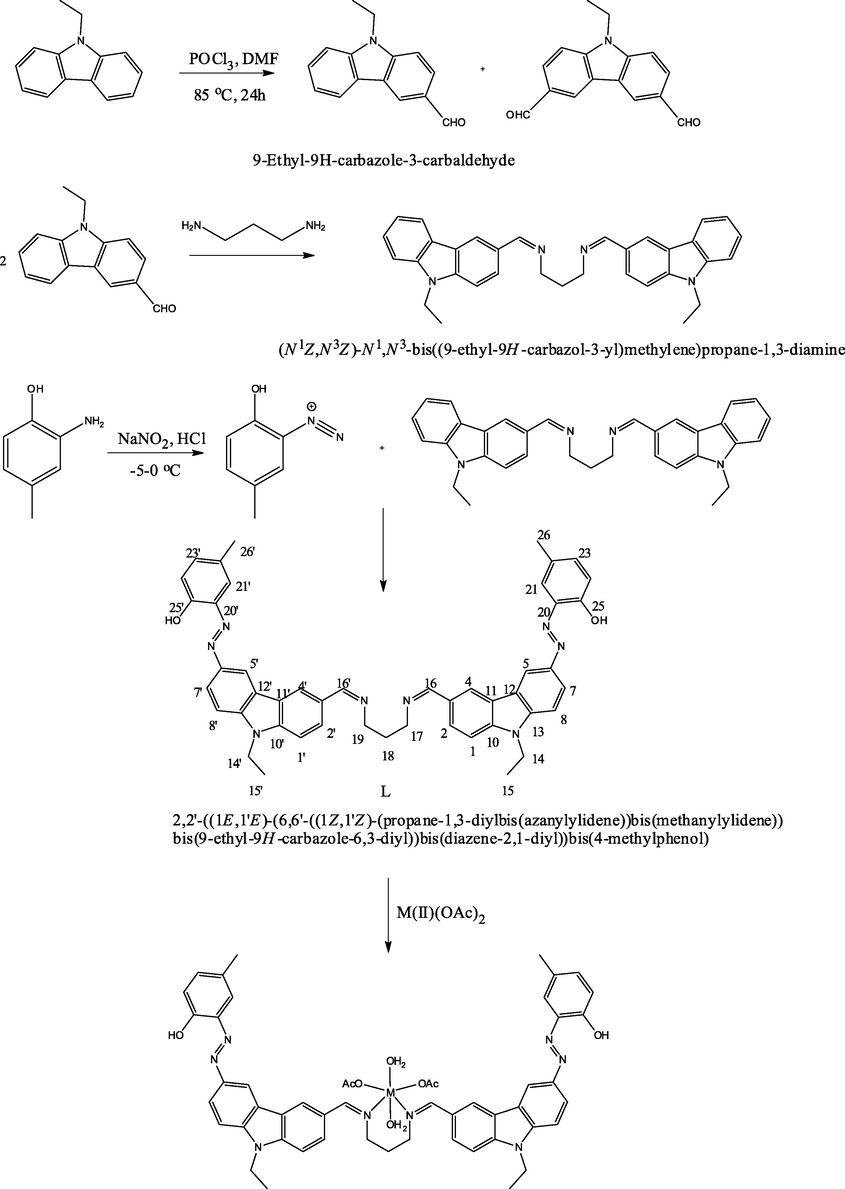
Reaction Scheme for the syntheses and proposed structures of the ligand (L) and its complexes.
Chemical formula: C47H44N8O2.Yield: 70%, m.p.: 185–187 °C, Elemental Analysis found% (calculated%): C 75.21(74.98) H 6.1(5.89) N 14.91(14.88). UV–Vis (ethanol) (λmax, nm) (ε, M−1 cm−1): 265, 358, 448. FT-IR (KBr, cm−1): 3390 (brd) (Ar—OH); 2971 (w), 2847 (w), 2919 (w), 1598 (w) (Ar—H, C—H); 742(s) (Ar—H), 1615(s) (CH—N), 1471(s) (N—N). Mass spectrum (LC/MS APCI): m/z 737.4 [M-CH3]+. 1H NMR (300 MHz,CDCl3): 8.1 (H-5, H-5′, s), 7.91 (H-2, H-2′, d, J = 8.4 Hz), 4.41 (H-14, H-14′, q, J = 7.2), 1.47 (H-15, H-15′, t, J = 7.2), 8.6 (H-16,H-16′, s), 3.84 (H-17&H-19, t, J = 6.8), 2.26 (H-18, pentet, J = 6.8), 7.2 (H-21, H-21′, s), 7.0 (H-23, H-23′, d, J = 7 Hz), 6.9 (H-24, H-24′, d, J = 8.1 Hz), 2.1 (3H-26, 3H-26′, s). 13C NMR (400 MHz,CDCl3): 126.04 (C-1, C-1′), 125.9 (C-2, C-2′), 127.6 (C-3, C-3′), 123.1 (C-4, C-4′), 120.7 (C-5, C-5′), 119.4 (C-6, C-6′), 108.5 (C-7, C-7′), 108.7 (C-8, C-8′), 141.5 (C-10, C-10′), 127.2 (C-11, C-11′), 124.0 (C-12, C-12′), 140.5 (C-13, C-13′′), 37.7 (C-14, C-14′), 13.8 (C-15, C-15′), 162.06 (C-16, C-16′), 59.5 (C-17&C-19), 32.3 (C-18), 135.09 (C-20, C-20′), 120.34 (C-21, C-21′), 136.7 (C-22, C-22′), 120.84 (C-23, C-23′), 120.92 (C-24, C-24′), 150.0 (C-25, C-25′), 29.7 (C-26, C-26′). ɅM (Ω−1 cm2 mol−1): 7.
2.2 Synthesis of complex compounds
Acetate salts (1 mmol) of Cu(II), Co(II) and Ni(II) were mixed with ethanol solution of our ligand (1 mmol). The resulting mixtures were left refluxing overnight. Complex compounds were crystallized from ethanol.
2.2.1 Copper(II) complex of L
CuL(H2O)2(AcO)2 Complex: (C51H54CuN8O8), yield: 62%, m.p.: 220–223 °C, Elemental Analysis found% (calculated%): C 63.77(63.11), H 5.32(5.61), N 11.53(11.55), Cu 6.70(6.55). UV–Vis (ethanol) (λmax, nm): 297, 347, 390, 510. FT-IR (KBr, cm−1): 3600(brd), 3364(brd), 3267(brd) (OH, H2O), 2997(m), 2875(m) (Ar—H, CH), 1552 (s) (CH⚌N), 1590(s) (Ѵas COO), 1417(Ѵs COO), 1498(s) (N⚌N), 470(s) (M—N), 625(s) (M—O). Mass spectrum (LC/MS APCI): m/z 971.3 [M + H]+. ɅM (Ω−1 cm2 mol−1): 19.9. μeff (B.M.): 1.92.
2.2.2 Cobalt(II) complex of L
CoL(H2O)2(AcO)2 Complex: (C51H54CoN8O8), yield: 70%, m.p.: 215–217 °C, Elemental Analysis found% (calculated%): C 63.98(63.41), H 5.91(5.63), N 11.55(11.60), Co 6.34(6.10). UV–Vis (ethanol) (λmax, nm): 285, 351, 516. FT-IR (KBr, cm−1): 3361(brd) (OH, H2O), 2973(m), 2855(m) (Ar—H, CH), 1554 (s)(CH⚌N), 1591 (s)(Ѵas COO), 1410(Ѵs COO), 1495(s)(N⚌N), 472(s) (M—N), 614(s) (M—O). Mass spectrum (LC/MS APCI): m/z 965.2 [M]+. ɅM (Ω−1 cm2 mol−1): 12.7. μeff (B.M.): 4.91.
2.2.3 Nickel(II) complex of L
NiL(H2O)2(AcO)2 Complex: (C51H54NiN8O8), yield: 62%, m.p.: 217–218 °C, Elemental Analysis found% (calculated%): C 63.81(63.43), H 6.11(5.64), N 11.49(11.60), Ni 6.10(6.08). UV–Vis (ethanol) (λmax, nm): 292, 345, 512. FT-IR (KBr, cm−1): 3400(brd) (OH, H2O), 2922(m), 2844(m) (Ar—H, CH), 1557 (s) (CH⚌N), 1590 (s) (Ѵas COO), 1416(Ѵs COO), 1499(s) (N⚌N), 455(s) (M—N), 617(s) (M—O). Mass spectrum (LC/MS APCI): m/z 965.0 [M]+ ɅM (Ω−1 cm2 mol−1): 15.3. μeff (B.M.): 3.11.
2.3 Oxidation procedure
A mixture of 1 ⋅ 10−3 mol catalyst, 20 ml solvent (CH3CN) and 10 mmol cyclohexene/styrene was stirred under nitrogen atmosphere in a 50 ml round-bottom flask. Then 20 mmol hydrogen peroxide (30% in water) was added dropwise. The resulting mixture was vigorously stirred for 8 h at 60 °C. After filtration and washing with solvent, the filtrate was concentrated and then subjected to GC analysis. The yields were recorded as the GC yield based on the starting styrene or cyclohexene. The identity of the oxidation products was confirmed by GC–MS. A blank reaction was also carried out without any catalyst for both oxidation reactions.
3 Results and discussion
3.1 Spectral analysis
1H NMR spectrum of our ligand (Fig. 2) showed the ethyl group protons on the carbazole units at 4.41 ppm (2H-14) as quartet (J = 7.2 Hz) and at 1.47 ppm (3H-15) as triplet (J = 7.2 Hz). The protons on the propane moiety were appeared at δ 3.84 (2H-17 & 2H-19) as triplet (J = 6.8 Hz) and at δ 2.26 (2H-18) as pentet (J = 6.8 Hz). The methyl protons on the two phenolic rings appeared at δ 2.1 and the hydroxyl protons were at around δ 10 ppm (Kose et al., 2013). We had identified the proton signals belonging to the carbazole unit previously (Bal and Bal, 2014). In the proton NMR of our newly synthesized ligand, we were able to see the hydrogens of 4 and 4’ at 8.6 ppm as singlet overlapping with the imine protons. The signal seen as a singlet at 8.1 ppm belongs to H-5 and H-5′ on carbazoles and the doublet observed at δ 7.91 (J = 8.4 Hz) was attributed to H-2 and H-2′. The other carbazole ring protons were seen between 7.3 ppm and 7.7 ppm. Considering the phenolic rings in our compound, we have seen the two isolated hydrogens of 21 at 7.2 as a singlet and the hydrogens of 23 and 24 were seen as doublets at 7 ppm and 6.9 ppm respectively. 13C NMR spectrum (Fig. 3) showed aromatic carbons between 100 ppm and 150 ppm. Among them, the peaks at 108.7 ppm and at 108.4 ppm belong to C-8, C-8′ and C-7, C-7′ on the carbazole units. The quaternary carbons C-10, C-10′ and C-13, C-13′ gave peaks at δ 141.5 and δ 140.4. The imine carbons bridging the two carbazole units appeared at 162.1 ppm. The three quaternary carbons on the phenolic aromatic rings, attached to the carbazole units through azo linkage, appeared at 150 ppm (C-25, C-25′), 135.09, (C-20, C20′) and 136.7 ppm (C-22, C-22′) (Gozel et al., 2014; Kaya et al., 2012). The methyl carbons para to the hydroxyls were seen at 29.7 (Gozel et al., 2014). The ethyl groups attached to Nitrogen on the carbazole units were at 37.7 ppm (C-14, C-14′), and at 13.9 (C-15, C-15′). The carbons belonging to 1,3-diaminopropane between the two carbazoles were observed at δ 59.5 (C-17, C-19); δ 32.3 (C-18). The IR spectra of all the complexes showed a considerable red shift in their imine stretching frequencies compared to that of the ligand itself, which proves that the complexation took place on the imine Nitrogens. Also, it is a common knowledge that the imine Nitrogens are always more likely to undergo complexation in the presence with transition metals. Although it is changeable, depending on the metal (Shindo and Brown, 1965) and the structures of the ligands (e.g. containing electronegative atoms) (Cabaniss and McVey, 1995), it is generally known that the bands between 1380 and 1600 cm−1 are attributed to the symmetric and anti-symmetric stretching modes of carboxylate groups respectively (Finnie et al., 1998). The IR spectra of all complexes showed symmetric and asymmetric carboxylate stretchings, proving the acetate coordinations to the metals, and the difference between the splitting of carboxylate stretching bands (Δ = Ѵas–Ѵs) in our complexes were found to be between 173 and 181 as shown below. These results prove the unidentate pattern of carboxylate coordinations (Finnie et al., 1998).
Complexes
Ѵasymmetric
Ѵsymmetric
Δ (Ѵasym–Ѵsym)
CuL(H2O)2(AcO)2
1590
1417
173
CoL(H2O)2(AcO)2
1591
1410
181
NiL(H2O)2(AcO)2
1590
1416
174
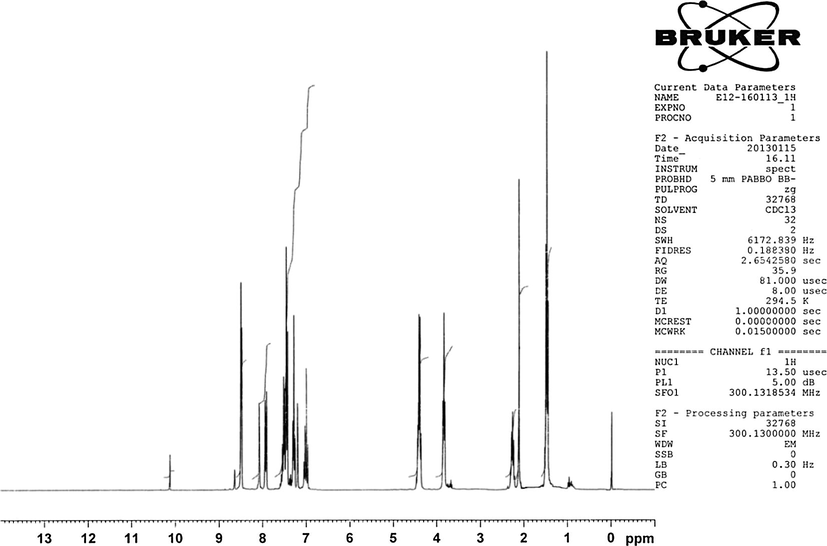
1H NMR spectrum of the ligand (L).
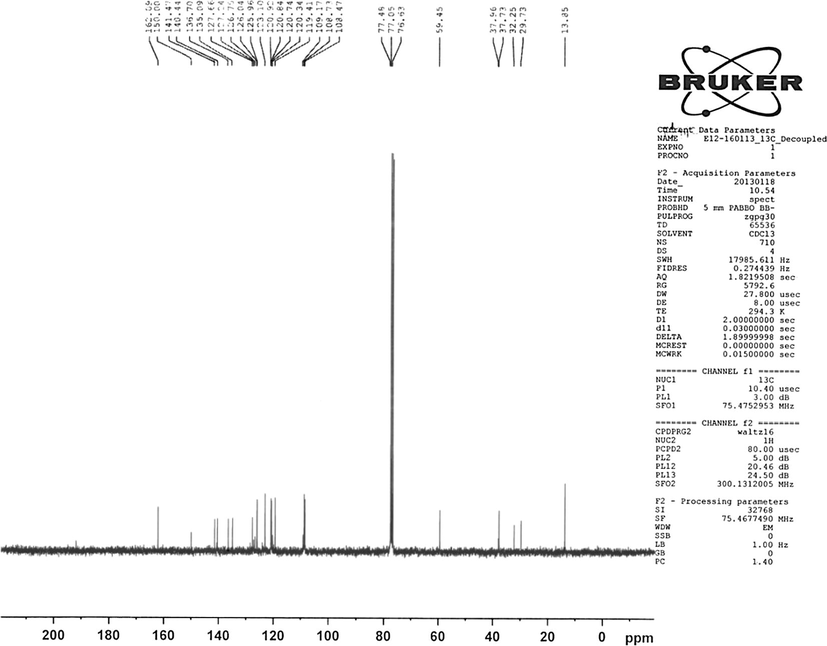
13C NMR spectrum of the ligand (L).
3.2 Thermal analysis
TG traces of the synthesized compounds provided additional information regarding their thermal stabilities and thermal degradation behaviors (Fig. 4). Examination of the thermogram of our ligand shows continuous weight losses, which can be divided somewhat distorted intervals as shown in Table 1. The biggest weight loss was observed for the loss of two carbazole units with a percentage of 47.9 (calc. 47.3). The residual part, however, was around 12.3% (calc. 12.7%) and this percentage can be attributed for the loss of C3H6N2 unit that is the 1,3-diaminopropane unit in the middle of the two carbazoles. TG graphic of the Copper complex of the ligand reveals the loss of two ethyl groups on carbazoles between 100 and 180 °C. The second loss between 220 and 300 °C was assigned to two molecule azo containing phenolic groups with loss of 28.6%. Above 300 °C, the biggest weight loss was recorded as 47.1% (Calc.46.9%), corresponding to two carbazole units, diaminopropane bridge (C3H6N2) and the water molecules in the lattice. Cobalt coordination compound shows two degradation steps, the biggest mass loss happens between 110 °C and 700 °C with a percentage of 75 (Calc. 75.6%) which corresponds to the distinct parts of the whole molecule. The residual parts of all complexes of our ligand gave the masses of the two acetate units and the Copper and Cobalt metallic losses were seen in the residual parts of CuL(H2O)2(AcO)2 and CoL(H2O)2(AcO)2; however, the Nickel metallic loss was found with the losses of two ethyl groups and two water molecules in the first half of the thermal graphic of NiL(H2O)2(AcO)2, the second half of which gave the biggest loss with a mass loss of 70.8% (Calc. 71.1). All thermal analyses were done under nitrogen atmosphere between the temperature range 30 °C and 988 °C at a heating rate of 10 °C/min.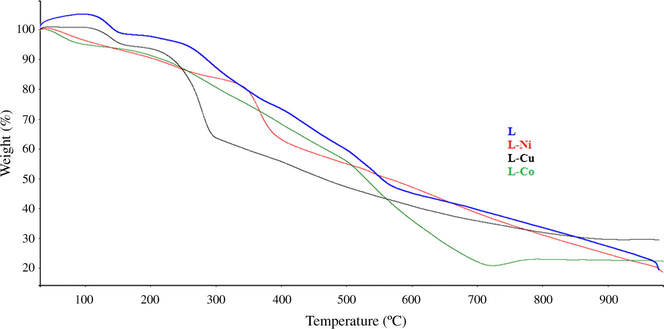
TG plots of L and its complexes recorded under nitrogen atmosphere between the temperature range 30 °C and 988 °C at a heating rate of 10 °C/min.
Compound
M.W.
T/°C
Mass loss/% Found (Calculated)
Assignment
Residual/% Found (Calculated)
L
752.90
130–230
7.4(7.7)
2(CH3CH2)
12.3(12.7)(C3H6N2)
230–420
32.5(32.3)
2(C7H7N2O)
420–900
47.9(47.3)
2(C13H8N)
CuL(H2O)2(AcO)2
970.4
100–180
6(5.9)
2(CH3CH2)
5(6.5)(Cu)
220–300
28.6(28)
2(C7H7N2O)
12(12.1)(2×AcO)
300–900
47.1(46.9)
2(C13H8N)
C3H6N2
2(H2O)
CoL(H2O)2(AcO)2
965.8
30–110
5.9(6)
2(CH3CH2)
8(6)(Co)
110–900
75(75.6)
2(C7H7N2O)
11.7(12.2)(2×AcO)
2(C13H8N)
C3H6N2
2(H2O)
NiL(H2O)2(AcO)2
965.6
30–300
19.8(19.5)
2(CH3CH2)
11.8(12.2)(2×AcO)
2(H2O)
Ni
330–950
70.8(71.1)
2(C7H7N2O)
2(C13H8N)
C3H6N2
3.3 Catalytic activity
Among the complex compounds, Copper (II) coordination compound showed better catalytic activity compared to the other coordination compounds. Both styrene and cyclohexene conversion percentages were 75.3% and 70.2% respectively and the styrene oxide selectivity was recorded as 22.2% while the cyclohexene oxide selectivity was 25.3%. Cobalt complex can be recorded as the second best catalyst in these oxidation reactions with 61.3% styrene and 59.7% cyclohexene conversion. The Nickel (II) complex, however, gave poorer results in both styrene oxidation and cyclohexene oxidation reactions. Catalytic activity results can be seen in Table 2 and Fig. 5 shows the possible oxidation reaction schemes. Reaction conditions: Alkene (10 mmol), H2O2 (20 mmol), catalyst (1 mmol), CH3CN (20 mL) at 60 °C. Others for styrene oxidation: Benzoic acid, Phenylacetaldehyde, 1-phenylethane-1,2-diol. Others for cyclohexene oxidation: 2-Cyclohexene-1-one, 2-cyclohexene-1-hydroperoxide.
Entry
Catalyst
Styrene conversion (%)
Selectivity (%)
Styrene oxide
Benzaldehyde
Others
1
Blank
17.5
5.1
14.2
80.7
2
CuL(H2O)2(AcO)2
75.3
22.2
38.9
38.9
3
CoL(H2O)2(AcO)2
61.3
17.4
33.1
49.5
4
NiL(H2O)2(AcO)2
56.7
13.8
24.5
61.7
Entry
Catalyst
Cyclohexene Conversion (%)
Selectivity (%)
Cyclohexene oxide
2-Cyclohexene-1-one
Others
1
Blank
13.7
4
9.3
86.7
2
CuL(H2O)2(AcO)2
70.2
25.3
42.1
32.6
3
CoL(H2O)2(AcO)2
59.7
17.2
55.4
27.4
4
NiL(H2O)2(AcO)2
51
14.2
41.3
44.5
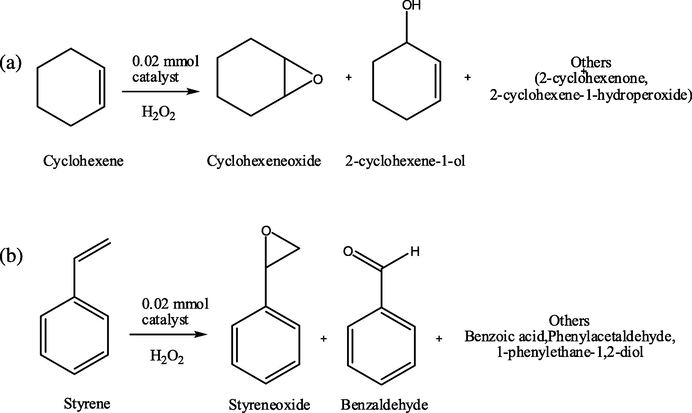
Various oxidation products of cyclohexene (a) and styrene (b).
4 Conclusion
With this work we have synthesized and characterized a new carbazole derived, azo containing Schiff base ligand and its Copper (II), Cobalt (II) and Nickel (II) complexes. Newly synthesized compounds were examined for their thermal and catalytic activity features. Thermal analysis supported the spectral analyses and is in agreement with the proposed structures of the ligand and its complexes. Catalytic activity results showed that in the oxidation reactions of styrene and cyclohexene Copper (II) complex was the most effective complex. This may be related to its thermal stability since the Copper complex was more temperature resistant than the other coordination compounds according to the thermal graphics.
Acknowledgement
We would like to thank K. Maras Sutcu Imam University Research Projects Coordination Unit for the financial support.
References
- Cobalt(II) and manganese(II) complexes of novelschiff bases, synthesis, charcterization, and thermal, antimicrobial, electronic, and catalytic features. Adv. Chem.. 2014;1:1-12.
- [Google Scholar]
- Synthesis, thermal stability, electronic features, and antimicrobial activity of phenolic azo dyes and their Ni(II) and Cu(II) complexes. Cem. Pap.. 2014;68:352-361.
- [CrossRef] [Google Scholar]
- Synthesis and X-ray powder diffraction, electrochemical, and genotoxic properties of a new azo-Schiff base and its metal complexes. Turk. J. Chem.. 2014;38:222-241.
- [CrossRef] [Google Scholar]
- Synthesis, thermal, electrical and catalytic studies of some transition metal polychelates of bis-bidentate schiff base, Chinese. J. Chem.. 2007;25:154-158.
- [Google Scholar]
- Aqueous infrared carboxylate absorbances: aliphatic monocarboxylates. Spectrochim. Acta A. 1995;51:2385-2395.
- [Google Scholar]
- A silica gel supported cobalt(II) Schiff base complex as efficient and recyclable heterogeneous catalyst for the selective aerobic oxidation of alkyl aromatics. Chin. Chem. Lett.. 2013;24:849-852.
- [CrossRef] [Google Scholar]
- In situ room temperature synthesis and characterization of Salicylaldehyde phenylhydrazone metal complexes, their cytotoxic activity on MCF-7 Cell Line, and Their Investigation as Antibacterial and Antifungal Agents. Synth. React. Inorg. Me.. 2015;45:437-446.
- [CrossRef] [Google Scholar]
- A novel azo-aldehyde and its Ni(II) chelate; synthesis, characterization, crystal structure and computational studies of 2-hydroxy-5-{(E)-[4-(propan-2-yl)phenyl]diazenyl}benzaldehyde. J. Mol. Struct.. 2014;1065–1066:191-198.
- [CrossRef] [Google Scholar]
- Vibrational spectroscopic study of the coordination of (2,2′-bipyridyl-4,4′-dicarboxylic acid) ruthenium(II) complexes to the surface of nanocrystalline titania. Langmuir. 1998;14:2744-2749.
- [Google Scholar]
- Spectroscopy and solvatochromism studies along with antioxidant and antibacterial activities investigation of azo–azomethine compounds 2-(2-hydroxyphenylimino)methyl)-4-phenyldiazenyl)phenol. Spectrochim. Acta A.. 2014;124:153-158.
- [CrossRef] [Google Scholar]
- The triazine-based azo–azomethine dyes; spectroscopy, solvatochromism and biological properties of 2,2′-((2,2′-(6-methoxy-1,3,5-triazine-2,4-diyl) bis(oxy) bis(2,1-phenylene))bis(azan-1-yl-1-ylidene) bis(methan-1-yl-1-ylidene))bis(4-phenyldiazenyl)phenol. J. Mol. Liq.. 2014;195:35-39.
- [CrossRef] [Google Scholar]
- Spectral, structural and quantum chemical computational and dissociation constant studies of a novel azo-enamine tautomer. J. Mol. Stuct.. 2014;1074:449-456.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of some carbazole-based imine polymers. Eur. Polym. J.. 2005;41:1079-1089.
- [CrossRef] [Google Scholar]
- Copper(II) Schiff base complexes derived from 2,20-dimethyl-propandiamine: synthesis, characterization and catalytic performance in the oxidation of styrene and cyclooctene. Polyhedron. 2012;31:443-450.
- [CrossRef] [Google Scholar]
- Immobilized complexes of the Salen Schiff’s base with metal as oxidation catalysts. Russ. J. Gen. Chem.. 2013;83(2361):2369.
- [CrossRef] [Google Scholar]
- Use of a new polymer anchored Cu(II) azo complex catalyst for the efficient liquid phase oxidation reactions. J. Inorg. Organomet. Polym.. 2010;20:87-96.
- [CrossRef] [Google Scholar]
- Synthesis, crystal structure and spectroscopic studies of a cobalt(III) Schiff Base complex and its use as a heterogeneous catalyst for the oxidation reaction under mildcondition. J. Mol. Catal. A-Chem.. 2013;380:94-103.
- [CrossRef] [Google Scholar]
- The synthesis, characterization, electrochemical character, catalytic and antimicrobial activity of novel, azo-containing schiff bases and their metal complexes. Dyes Pigm.. 2009;82:13-19.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of polyphenols derived from 4-Fluorobenzaldeyde: the effect of electron-donating group on some physical properties. J. Appl. Polym. Sci.. 2012;125:608-619.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and antimicrobial studies of 2-{(E)-[(2-hydroxy-5-methylphenyl)imino]methyl}-4-[(E)-phenyldiazenyl]phenol as a novel azo-azomethine dye. J. Mol. Struct.. 2013;1053:89-99.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, and electrochemical study of some novel, azo-containing Schiff bases and their Ni(II) complexes. Dyes Pigm.. 2013;98:499-506.
- [CrossRef] [Google Scholar]
- Cytochrome P-450 dependent monooxygenases model system: rapid and efficient oxidation of primary aromatic amines to azo derivatives with sodium periodate catalyzed by manganese (III) Schiff base complexes. Bioorgan. Med. Chem.. 2004;12:4673-4677.
- [CrossRef] [Google Scholar]
- Design, synthesis and characterization of bimetallic palladium complexes for terminal olefin epoxidation. Catal. Lett.. 2014;144:1573-1583.
- [CrossRef] [Google Scholar]
- Catalytic activities of novel silica-supported multifunctional Schiff base ligand & metal complexes under microwave irradiation. Inorg. Chim. Acta.. 2014;421:1-9.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, electrical conductivity, and catalytic studies of some coordination polymers of salentype Schiff base. Russ. J. Coord. Chem.. 2010;36:298-304. ISSN 1070-3284
- [Google Scholar]
- Spectroscopic properties of some new azo–azomethine ligands in the presence of Cu2+, Pb2+, Hg2+, Co2+, Ni2+, Cd2+ and Zn2+ and their antioxidant activity. Spectrochim. Acta A. 2014;131:67-71.
- [CrossRef] [Google Scholar]
- Co(II), Cu(II), Mn(II) and Ni(II) complexes of maleic hydrazide. Inorg. Chim. Acta.. 2015;425:247-254.
- [CrossRef] [Google Scholar]
- Infrared spectra of complexes of l-cysteine and related compounds with zinc (II), cadmium(II), mercury(II), and lead (II) J. Am. Chem. Soc.. 1965;87(9):1904-1909.
- [Google Scholar]
- Oxidation of cyclohexane by transition-metal complexes with biomimetic ligands. Catal. Today. 2013;203:81-86.
- [CrossRef] [Google Scholar]
- Transition metal complexes of bidentate Schiff base ligand. Transit. Metal Chem.. 1999;24:525-532.
- [Google Scholar]
- Chemically modified silica-gel with an Azo-Schiff Ligand and Its Metal Complexes With Cu(II), Co(II), Ni(II) and Mn(II): Applications as Catalysts on the Oxidation of Cyclohexane Under Microwave Power. Synth. React. Inorg. Me.. 2012;42:382-391.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of carbazole derived nonlinear optical dyes. Dyes Pigm.. 2007;75:567-573.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.arabjc.2015.11.012.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







